Abstract
BACKGROUND
Idiopathic normal pressure hydrocephalus (iNPH) typically manifests with the classic Hakim-Adams triad of gait disturbance, cognitive impairment, and urinary incontinence. While akinetic mutism represents a rare and severe neurological presentation characterized by profound reduction in voluntary movement and speech, its association with iNPH remains underrecognized in clinical practice. This case illustrates both the diagnostic challenges and remarkable therapeutic potential when encountering this unusual manifestation of iNPH.
OBSERVATIONS
A previously healthy 52-year-old female developed progressive gait instability, urinary incontinence, and cognitive decline over 6 months, followed by 3 months of akinetic mutism. Examination demonstrated characteristic features of iNPH including paratonic rigidity, magnetic gait, and frontal release signs. Neuroimaging revealed moderate ventriculomegaly with an Evans index of 0.38 and hyperdynamic CSF flow dynamics (peak velocity 7.4 cm/sec, stroke volume 95 µL). The patient exhibited dramatic clinical improvement within 1 week of ventriculoperitoneal shunt placement, with complete resolution of akinetic mutism and significant recovery of other symptoms sustained at the 6-month follow-up.
LESSONS
This case underscores that akinetic mutism, while uncommon, may represent a severe but treatable manifestation of iNPH. The rapid reversal of akinetic mutism following CSF diversion highlights the importance of considering iNPH even in atypical presentations. Quantitative CSF flow analysis emerges as a valuable diagnostic tool in such challenging cases, while the striking clinical response reinforces the potential for complete functional recovery with timely intervention.
Keywords: idiopathic normal pressure hydrocephalus, akinetic mutism, ventriculoperitoneal shunt, cerebrospinal fluid dynamics, hydrocephalus biomarkers, reversible dementia
ABBREVIATIONS: iNPH = idiopathic NPH, mRS = modified Rankin Scale, NPH = normal pressure hydrocephalus
Idiopathic normal pressure hydrocephalus (iNPH) is a potentially reversible neurological disorder characterized by the classic Hakim-Adams triad of gait disturbance, cognitive impairment, and urinary incontinence, typically affecting adults older than 60 years of age.1 The pathophysiology involves impaired CSF dynamics, leading to ventriculomegaly without elevated intracranial pressure. While the exact mechanism remains debated, hypotheses include reduced CSF absorption, altered compliance of brain tissue, and microvascular dysfunction contributing to periventricular white matter damage.2
Akinetic mutism, a rare but striking manifestation of iNPH, is characterized by profound reduction in voluntary movement and speech despite preserved wakefulness. In a study of 101 NPH patients, approximately 6% presented with akinetic mutism, classified as a modified Rankin Scale (mRS) score of 6, while Krauss et al. reported that movement disorders, including akinetic symptoms, were observed in 75% of adult hydrocephalus patients, with the highest prevalence (86%) in idiopathic NPH cases, making it an underrecognized feature.3,4 The pathophysiology may involve disruption of frontal-subcortical circuits due to mechanical compression of the supplementary motor area and cingulate gyrus by distended ventricles, compounded by ischemic changes in thalamocortical pathways.5,6 Alternatively, hyperdynamic CSF flow may exacerbate periaqueductal dysfunction, disrupting arousal and motivation networks.7
This case highlights an atypical presentation of iNPH with akinetic mutism, underscoring the importance of recognizing rare symptoms in diagnostic evaluations. The dramatic response to shunt placement further supports the reversibility of such deficits when treated promptly.
Illustrative Case
A 52-year-old female with no significant prior medical history presented with a 6-month progressive decline characterized by worsening gait instability, urinary incontinence, and cognitive slowing. Her gait disturbance began as mild imbalance but progressed to a broad-based shuffling pattern with frequent freezing episodes, requiring 28 steps and 22 seconds to complete a 10-m walk. Urinary symptoms evolved from urgency to complete incontinence, while cognitive decline manifested as forgetfulness, apathy, and profound slowing of mental processing. Formal testing revealed severe deficits: recall of only 2 of 10 words after 5 minutes, digit span limited to 4 forward and 2 backward, Trail Making Test Part A completed in 150 seconds, and finger tapping reduced to 32 taps in 10 seconds with the dominant hand. Over the final 3 months, the patient developed akinetic mutism, exhibiting complete absence of spontaneous speech and movement despite preserved visual tracking and wakefulness.
Neurological examination demonstrated a hypomimic face with reduced blink rate, normal cranial nerves, and no focal motor deficits. Paratonic rigidity was present in the lower limbs. Gait assessment revealed severe apraxia with short steps, en bloc turning, and retropulsion. Frontal release signs including grasp and palmomental reflexes were elicited.
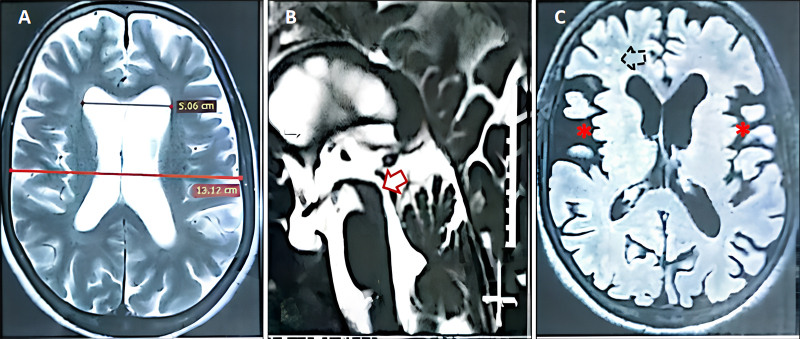
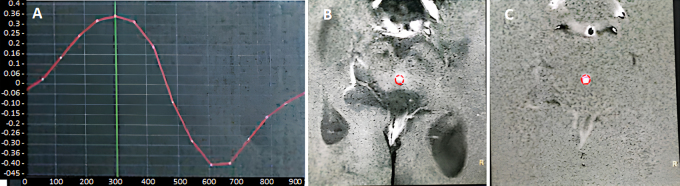
MRI of the brain demonstrated moderate ventriculomegaly with an Evans index of 0.38, patent cerebral aqueduct, and diffuse sulcal prominence with chronic small-vessel ischemic changes (Fig. 1). Quantitative CSF flowmetry showed hyperdynamic circulation with peak velocity of 7.4 cm/sec and elevated stroke volume of 95 µL (Fig. 2).
FIG. 1.
Preoperative MRI findings in iNPH with akinetic mutism. A: Axial T2-weighted MR image demonstrating moderate bilateral supratentorial ventriculomegaly (Evans index 0.38; normal < 0.3), with blunting of the frontal horns and rounding of the third ventricular recesses, consistent with communicating hydrocephalus. B: Sagittal T2-weighted MR image confirming patency of the cerebral aqueduct (arrow) without obstruction, ruling out aqueductal stenosis. C: Axial FLAIR sequence revealing diffuse sulcal prominence (asterisks) and scattered periventricular/subcortical white matter hyperintensities (dotted arrow), indicative of chronic small-vessel ischemic changes. The absence of transependymal CSF seepage argues against acute hydrocephalus. These findings collectively support a diagnosis of iNPH with secondary microvascular injury.
FIG. 2.
Quantitative CSF flowmetry at the cerebral aqueduct. Phase-contrast MRI during peak systole shows hyperdynamic CSF flow dynamics as demonstrated in the velocity-time curve (A), which shows CSF in both diastole (above the baseline) and systole (below the baseline): peak velocity (7.4 cm/sec; normal range 2–5 cm/sec) and stroke volume (95 µL; pathological threshold > 42 µL), measured at the level of the cerebral aqueduct (circles) in axial T2-weighted (B) and phase (C) images, respectively. Phase shifts across the aqueduct are converted to velocity values (in cm/sec), and velocities are averaged across the aqueductal area to reduce noise. The exaggerated flow pulsatility reflects impaired ventricular compliance and altered CSF redistribution, characteristic of iNPH. These hydrodynamic abnormalities likely contribute to periventricular shear stress and dysfunction of adjacent mesencephalic arousal pathways, potentially exacerbating akinetic mutism.
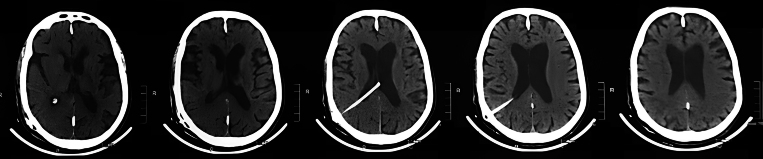
Following ventriculoperitoneal shunt placement (Fig. 3), dramatic improvement occurred within 1 week. The akinetic mutism resolved completely, with restoration of spontaneous speech and movement. Three months later, repeat neuropsychological testing demonstrated remarkable recovery: 10-word recall improved to 8 of 10, digit span normalized to 6 forward and 4 backward, the Trail Making Test Part A was completed in 65 seconds, and finger tapping increased to 48 taps in 10 seconds. Gait velocity improved to 15 steps in 12 seconds for 10 m. At the 6-month follow-up, the patient maintained complete independence in activities of daily living with sustained resolution of all symptoms.
FIG. 3.
Postoperative shunt placement and immediate anatomical outcome. Postoperative day 1 noncontrast axial CT scans confirming optimal placement of the ventriculoperitoneal shunt catheter via the right Keen’s point. The catheter tip is positioned in the occipital horn of the right lateral ventricle, with no immediate complications (e.g., hemorrhage or malposition). Early reduction in ventricular size is not expected at this stage; follow-up imaging at 6 months (not shown) demonstrated sustained decompression correlating with clinical improvement.
Informed Consent
The necessary informed consent was obtained in this study.
Discussion
This case exemplifies the diagnostic and therapeutic challenges of iNPH presenting with the rare feature of akinetic mutism, while demonstrating the remarkable reversibility of symptoms with timely intervention. The patient’s presentation with the classic Hakim-Adams triad (gait disturbance, cognitive decline, and urinary incontinence) coupled with akinetic mutism underscores the phenotypic spectrum of iNPH and emphasizes the importance of recognizing atypical manifestations in clinical practice.
Observations
Diagnostic and Therapeutic Insights
The rapid resolution of akinetic mutism within 1 week after shunt placement is particularly noteworthy, contrasting sharply with the slower recovery typically seen for gait and cognitive symptoms in iNPH. Notably, a Dutch study of NPH identified akinetic mutism in about 6% of their cohort (mRS score of 6), but it did not report specific outcomes for these patients following ventriculoperitoneal shunt placement, leaving a critical gap in understanding the potential for recovery in severe cases.3 Our patient’s rapid resolution of akinetic mutism within 7 days after shunt placement—coupled with sustained functional independence at 6 months—provides compelling evidence that even profound neurological deficits in iNPH may be reversible. This underscores the paramount importance of our case report in bridging this knowledge gap and advocating for aggressive intervention in akinetic mutism associated with iNPH.
Pathophysiological Considerations
The striking reversal of akinetic mutism in our case, occurring earlier than typical improvements in gait or cognition, suggests distinct mechanistic pathways for these symptoms in iNPH. The pathogenesis of akinetic mutism in iNPH involves complex interactions between altered CSF dynamics, mechanical compression of neural structures, and secondary ischemic changes. As highlighted in recent literature, akinetic mutism represents a frontal-subcortical circuit disconnection syndrome, with the anterior cingulate cortex serving as a critical hub for motivated behavior.8 Ventricular enlargement in iNPH exerts direct pressure on these frontal-subcortical circuits, particularly the supplementary motor area and anterior cingulate cortex, which are crucial for initiating voluntary movement and motivated behavior.5 The mechanical distortion of these structures by distended ventricles may explain the profound reduction in spontaneous activity characteristic of akinetic mutism. Compression of dopaminergic mesolimbic and nigrostriatal pathways by ventricular enlargement could further disrupt the “energizing factor” for goal-directed behavior.8 In our case, the observed hyperdynamic CSF flow (peak velocity 7.4 cm/sec, stroke volume 95 µL) suggests additional hydrodynamic stress on periventricular tissues, particularly the periaqueductal gray matter, which plays a key role in arousal and motivation. This hyperdynamic state may exacerbate dysfunction in mesencephalic arousal systems, compounding the effects of mechanical compression.
The white matter changes frequently observed in iNPH, as seen in our patient, likely contribute to symptoms through disruption of thalamocortical connections. Chronic CSF dynamic alterations impair periventricular perfusion, leading to microvascular ischemia that preferentially affects frontal-subcortical pathways.9 This mechanism is shared across conditions like normal pressure hydrocephalus (NPH) and idiopathic chronic adult hydrocephalus, in which reduced CSF turnover and aberrant cerebral blood flow are central pathophysiological features.9 Additionally, venous hypertension may further disrupt intracranial compliance and CSF dynamics, potentially interfering with the intracranial windkessel mechanism.10 In NPH, ischemic injury typically begins in the periventricular white matter and extends to cortical and thalamic regions, resulting in neuronal dysfunction.11
Compromised CSF dynamics also impair critical CNS functions, including cerebral metabolite clearance, neuroendocrine signaling, and neural stem cell regulation.12 These disturbances, combined with thalamocortical dysregulation—potentially mediated by GPi-induced inhibition of thalamic targets due to striatal input loss8—may explain incomplete recovery in some patients after shunt placement. However, the rapid resolution of akinetic mutism in our case suggests that mechanical compression of frontal-mesencephalic circuits (e.g., by distended ventricles) played a dominant role. The differential symptom response—akinetic mutism resolving faster than gait or cognitive deficits—highlights the unique vulnerability of these circuits to acute CSF pressure changes.
Notably, akinetic mutism in obstructive hydrocephalus has shown responsiveness to dopamine agonists (e.g., bromocriptine and levodopa) when shunt placement alone is insufficient.13,14 This parallels the dopaminergic dysfunction implicated in other akinetic mutism etiologies, in which mesolimbic and nigrostriatal pathway disruption impairs motivational circuitry.8 In iNPH, ventricular enlargement may mechanically distort these pathways or alter extracellular dopamine kinetics, although further investigation is needed. Our patient’s rapid improvement after CSF diversion underscores the reversibility of such deficits with timely intervention, reinforcing the importance of early recognition and treatment in severe iNPH presentations.
Akinetic Mutism in Obstructive Hydrocephalus: A Parallel Phenomenon?
While our case focuses on iNPH, akinetic mutism in obstructive hydrocephalus has been reported to respond to dopamine agonists when shunt placement alone is insufficient.13,–,15 These cases suggest shared dopaminergic pathway disruption, possibly due to mesencephalic or thalamic compression. Although the pathophysiology differs from iNPH, this parallel highlights the need for individualized therapeutic strategies when akinetic mutism persists after shunt placement.
Clinical Implications
1. Akinetic mutism, although rare, represents a treatable manifestation of iNPH and should prompt evaluation for ventriculomegaly and CSF dynamics.
2. Ventriculoperitoneal shunt placement can produce dramatic and rapid reversal of akinetic mutism, even when other symptoms improve more gradually.
3. Quantitative CSF flowmetry provides valuable diagnostic and prognostic information, particularly in atypical presentations.
Limitations
While the short-term outcomes are excellent, longer follow-up beyond 2 years would help determine the durability of response, as 20%–30% of iNPH patients with shunts experience gradual symptom recurrence.16 The lack of standardized preshunt neuropsychological testing limits quantitative assessment of cognitive recovery, although the clinical improvement remains unambiguous. Additionally, the lack of subgroup analysis for akinetic mutism outcomes in the Dutch study limits comparative insights, further emphasizing the novelty of our report.
Lessons
This case expands the recognized phenotypic spectrum of iNPH by demonstrating that even severe and rare manifestations like akinetic mutism can be fully reversible with timely CSF diversion. The rapid resolution of akinetic mutism—within 1 week of ventriculoperitoneal shunt placement—highlights the critical importance of early recognition and intervention in such atypical presentations. Key takeaways include the following:
1. Akinetic mutism as a treatable feature of iNPH: While uncommon, akinetic mutism should prompt evaluation for ventriculomegaly and CSF dynamics, as it may represent a severe but reversible manifestation of iNPH.
2. Differential symptom response to shunt placement: The rapid reversal of akinetic mutism contrasts with the slower recovery of gait and cognitive symptoms, suggesting distinct pathophysiological mechanisms. Mechanical compression of frontal-subcortical circuits (e.g., anterior cingulate cortex and supplementary motor area) and hyperdynamic CSF flow may play a predominant role in akinetic mutism, while other symptoms may involve additional microvascular or neurodegenerative components.
3. Diagnostic utility of quantitative CSF flowmetry: Elevated peak velocity and stroke volume in CSF flow analysis emerged as valuable biomarkers in atypical cases, supporting both diagnosis and therapeutic decision-making.
4. Therapeutic implications: The dramatic response to shunt placement underscores the potential for complete functional recovery, advocating for aggressive intervention even in severe cases. Persistent symptoms after shunt placement may warrant adjunctive therapies (e.g., dopamine agonists), as suggested by analogous responses in obstructive hydrocephalus.
5. Long-term considerations: While short-term outcomes are promising, extended follow-up is needed to assess durability, as symptom recurrence occurs in 20%–30% of iNPH patients with shunts. Future research should focus on identifying predictive biomarkers for akinetic mutism reversibility and optimizing patient selection for shunt placement.
This case reinforces the necessity of maintaining high clinical suspicion for iNPH in patients with movement disorders or cognitive impairment, regardless of atypical features. By bridging gaps in the understanding of akinetic mutism outcomes, it advocates for a proactive diagnostic and therapeutic approach to maximize recovery in this debilitating but treatable condition.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Mansour, Mostafa. Acquisition of data: Mansour, Refaat, Kamer-Eldawla, Zohney, El-Batanony, Mostafa. Analysis and interpretation of data: Mansour, Zohney. Drafting the article: Mansour, Refaat. Critically revising the article: Mansour, Refaat, Kamer-Eldawla, El-Batanony, Elshaer, Mostafa. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Mansour. Statistical analysis: Mansour. Administrative/technical/material support: Mansour, Zohney, Elshaer, Mostafa. Study supervision: Mansour, Refaat, Kamer-Eldawla, El-Batanony, Elshaer, Mostafa. Direct patient care: Mansour, Refaat, Selim. Operating surgeon: Mansour.
Correspondence
Moustafa A. Mansour: Nasser Institute for Research and Treatment, Cairo, Egypt. moustafa.medavatar@gmail.com.
References
- 1.Relkin N Marmarou A Klinge P Bergsneider M Black PM.. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(3suppl):S2-S16. [DOI] [PubMed] [Google Scholar]
- 2.Mori E, Ishikawa M, Kato T.Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir (Tokyo). 2nd ed. 2012;52(11):775-809. [DOI] [PubMed] [Google Scholar]
- 3.Boon AJ, Tans JT, Delwel EJ.Dutch normal pressure hydrocephalus study: baseline characteristics with emphasis on clinical findings. Eur J Neurol. 1997;4(1):39-47. [DOI] [PubMed] [Google Scholar]
- 4.Krauss JK Regel JP Droste DW Orszagh M Borremans JJ Vach W.. Movement disorders in adult hydrocephalus. Mov Disord. 1997;12(1):53-60. [DOI] [PubMed] [Google Scholar]
- 5.Yamada S, Ishikawa M, Ito H.Cerebrospinal fluid dynamics in idiopathic normal pressure hydrocephalus on four-dimensional flow imaging. Eur Radiol. 2020;30(8):4454-4465. [DOI] [PubMed] [Google Scholar]
- 6.Abekura M.. Akinetic mutism and magnetic resonance imaging in obstructive hydrocephalus. Case illustration. J Neurosurg. 1998;88(1):161. [DOI] [PubMed] [Google Scholar]
- 7.Williams MA Malm J.. Diagnosis and treatment of idiopathic normal pressure hydrocephalus. Continuum (Minneap Minn). 2016;22(2 Dementia):579-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnts H van Erp WS Lavrijsen JCM van Gaal S Groenewegen HJ van den Munckhof P.. On the pathophysiology and treatment of akinetic mutism. Neurosci Biobehav Rev. 2020;112:270-278. [DOI] [PubMed] [Google Scholar]
- 9.Martín-Láez R Valle-San Román N Rodríguez-Rodríguez EM Marco-de Lucas E Berciano Blanco JA Vázquez-Barquero A.. Current concepts on the pathophysiology of idiopathic chronic adult hydrocephalus: are we facing another neurodegenerative disease? Neurologia (Engl Ed). 2018;33(7):449-458. [DOI] [PubMed] [Google Scholar]
- 10.Beggs CB.. Venous hemodynamics in neurological disorders: an analytical review with hydrodynamic analysis. BMC Med. 2013;11:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura M Tanaka A Yoshinaga S.. Significance of periventricular hemodynamics in normal pressure hydrocephalus. Neurosurgery. 1992;30(5):701-705. [PubMed] [Google Scholar]
- 12.Johanson CE Duncan JA III Klinge PM Brinker T Stopa EG Silverberg GD.. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson B.. Relief of akinetic mutism from obstructive hydrocephalus using bromocriptine and ephedrine. Case report. J Neurosurg. 1992;76(1):152-155. [DOI] [PubMed] [Google Scholar]
- 14.Kim MS Rhee JJ Lee SJ Kwon SJ Lee CH.. Akinetic mutism responsive to bromocriptine following subdural hematoma evacuation in a patient with hydrocephalus. Neurol Med Chir (Tokyo). 2007;47(9):419-423. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Li P, Zhang J.Case report: levodopa-responsive parkinsonism with akinetic mutism after ventriculo-peritoneal shunt. Front Neurol. 2023;14:1184713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marmarou A Bergsneider M Relkin N Klinge P Black PM.. Development of guidelines for idiopathic normal-pressure hydrocephalus: introduction. Neurosurgery. 2005;57(3suppl):S1-S3. [DOI] [PubMed] [Google Scholar]