Abstract
Sensitive skin (SS) is increasingly recognized as a complex syndrome characterized by discomfort and heightened sensitivity to otherwise harmless stimuli, such as environmental changes, physical contact, and cosmetic products. This condition poses challenges in both diagnosis and treatment due to its variable presentation and subjective nature. The pathophysiological features of SS include neurogenic inflammation and small fiber neuropathy, largely driven by the hyperactivation of sensory nerves. This hyperactivation is closely associated with transient receptor potential (TRP) channels, particularly TRPV1, which contribute to the exaggerated sensory responses seen in SS. Furthermore, psychological factors like stress and anxiety, along with environmental stressors such as pollution and ultraviolet exposure, play significant roles in exacerbating symptoms. The diverse and individualized responses to stimuli make it difficult to establish standardized diagnostic criteria for SS, necessitating a combination of subjective diagnostic tools (e.g., the Sensitive Scale-10) and objective assessments (e.g., transepidermal water loss and lactic acid sting test) to accurately identify and assess SS. This paper provides a comprehensive review of SS, covering its definition, prevalence, pathogenesis, diagnostic challenges, and management strategies, and highlights the importance of personalized care in effectively managing SS and improving patient quality of life.
Keywords: Skin diseases, Skin irritancy tests, Skin physiological phenomena, Pruritus, Pathophysiology, Sensitive Scale-10, Sensitive skin
INTRODUCTION
Sensitive skin (SS) is increasingly recognized as a complex syndrome marked by heightened and often painful sensory responses to typically innocuous stimuli, such as environmental changes, physical contact, or cosmetic products. This condition affects not only physical well-being but also significantly impacts patients’ mental health and quality of life, frequently causing distress and anxiety. The multifaceted nature of SS poses challenges in both diagnosis and treatment, primarily due to the subjective nature of its symptoms and the lack of universally observable clinical signs1.
Recent advances in dermatological research underscore the role of neurogenic components in SS. Specifically, SS is now understood to involve neurogenic inflammation and may be associated with neuropathic characteristics similar to those observed in small-fiber neuropathy2. Neurogenic inflammation in SS arises from the overactivity of sensory nerve fibers, particularly in relation to C-fibers, which are responsible for transmitting sensations of pain, heat, and itch. Additionally, transient receptor potential (TRP) channels, especially TRPV1, have been implicated in the exaggerated sensory responses of SS, linking this condition to an intrinsic hypersensitivity of sensory nerve endings in the skin3. Moreover, psychological and environmental stressors play a crucial role in exacerbating SS symptoms. Psychological factors, such as anxiety and stress, have been shown to lower the sensory threshold in SS patients, making them more susceptible to external triggers. Environmental factors, including pollution, ultraviolet (UV) exposure, and seasonal changes, are also well-established as aggravators of SS, contributing to the activation of sensory receptors and the initiation of neurogenic inflammatory pathways in the skin4. This interplay of neurogenic, psychological, and environmental factors highlights the importance of a comprehensive approach to understanding SS and underscores the need for personalized management strategies.
Therefore, this review aims to provide an in-depth examination of the definition, diagnostic challenges, and appropriate management techniques for SS. By integrating recent findings from neurobiology, psychodermatology, and environmental dermatology, this discussion will advance the understanding of SS as a distinct neurodermatological syndrome, necessitating an interdisciplinary approach in both research and clinical practice.
DEFINITION OF SS
SS is a complex syndrome that is characterized by hypersensitivity to stimuli, and is subjective and diverse depending on the individual’s age, gender, race, etc., and is difficult to diagnose and manage due to the absence of clinical signs4. Frosch and Kligman introduced the concept of skin sensitivity1. In experiments involving lactic acid sensitivity tests on various skin areas, it was confirmed that skin sensitivity varies based on gender and race, with the face being the most common site.
Since 1987, SS has been termed ‘Cosmetic Intolerance Syndrome,’ denoting instances where discomfort arises upon cosmetic application5,6. The definition has progressively broadened to encompass sensations induced by factors beyond cosmetics7. It is now understood that SS can be triggered by various factors beyond cosmetics, including environmental stressors, temperature changes, hormonal fluctuations, and psychological stress8. These triggers, which vary widely across individuals, contribute to the difficulty in establishing a universal diagnostic criterion for SS.
In 2017, the International Forum for the Study of Itch (IFSI) redefined SS as a syndrome causing discomfort in response to typically non-irritating stimuli, often linked to specific skin diseases9. SS is acknowledged as a heterogeneous and complex syndrome lacking fully objective diagnostic criteria; while skin lesions may appear normal or present with erythema, symptoms are quantified through subjective assessment tools, such as the SS-102.
Meanwhile, in Fitzpatrick’s Dermatology, Dr. Bauman categorizes SS into 4 types: allergic type, rosacea type, acne type, and stinging type10. This supports the argument that SS itself is associated with chronic skin conditions on the face. In other words, when defining SS, cases where a specific skin condition exists as a current symptom should be excluded from the category of SS11. This suggests that individuals with frequent abnormal skin sensations may be prone to certain skin conditions, making it essential to distinguish between symptoms of chronic skin conditions and those solely due to sensitivity12.
In summary, SS is characterized by exaggerated responses to typically non-irritating stimuli, influenced by environmental, psychological, and genetic factors, with its complex and subjective nature necessitating ongoing revisions and personalized approaches for accurate definition and treatment.
EPIDEMIOLOGY OF SS
SS affects a significant portion of the global population, with prevalence estimates varying widely across studies. Survey-based studies indicate that approximately 50%–60% of women and 30%–40% of men are reported to have SS13. According to a 2019 meta-analysis, self-reported prevalence of SS varies by region due to survey methods and cultural perceptions, with 71% reporting some degree of SS and 40% reporting moderate to severe SS globally.
Gender and age differences affect SS prevalence, with women and younger individuals (especially under 35) reporting SS more often, likely due to hormonal factors, skin thickness, cosmetic usage, and generational awareness of sensitivity8. Environmental factors like pollution, humidity, UV exposure, and psychological stress exacerbate SS symptoms, especially in urban areas, by compromising the skin barrier and triggering inflammation, highlighting the complex factors influencing SS prevalence and severity14.
In summary, SS is a common but diverse condition influenced by both biological and environmental factors, requiring regionally and culturally tailored approaches to diagnosis and management.
CLINICAL SYMPTOMS OF SS
SS is characterized by subjective sensory symptoms like stinging, burning, and itching in response to benign stimuli, often without visible signs, making diagnosis challenging, and is influenced by external triggers (e.g., temperature, pollution, cosmetics) and internal factors like psychological stress. (Table 1)15,16. In Table 1, higher odds ratios (ORs) suggest stronger associations between specific triggers and the presence of SS. For example, cosmetics (OR, 7.12; 95% confidence interval, 3.98–12.72) represent the most significant trigger, indicating individuals exposed to cosmetics are over 7 times more likely to report SS symptoms compared to those not exposed.
Table 1. Triggers and ORs of sensitive skin according to meta-analysis10 .
| Factor | OR | 95% CI |
|---|---|---|
| Cosmetics | 7.12 | 3.98–12.72 |
| Wet air | 3.83 | 2.48–5.91 |
| Air conditioning | 3.60 | 2.11–6.14 |
| Temperature variation | 3.53 | 2.69–4.63 |
| Heat | 3.50 | 2.56–4.77 |
| Water | 3.46 | 2.82–4.25 |
| Pollution | 3.18 | 2.37–4.27 |
| Dry air | 3.04 | 2.22–4.16 |
| Cold | 2.73 | 1.94–3.84 |
| Wind | 2.33 | 1.69–3.22 |
| Sun | 1.81 | 1.61–2.04 |
| Emotion | 1.77 | 1.44–2.17 |
OR: odds ratio, CI: confidence interval.
Site-specific and demographic variability in symptoms
SS most commonly affects the face, especially areas with thinner skin like the cheeks and forehead, with higher intensity in East Asian populations potentially due to genetic or structural facial skin differences17.
Gender and age also influence symptom severity and frequency
Women and younger individuals report SS more frequently, likely due to factors such as thinner skin, hormonal changes, cosmetic use, environmental stressors, and greater skincare awareness8.
Evaluating symptoms and severity
The SS-10 scale helps quantify SS severity by evaluating symptoms like stinging and burning over recent days, providing a standardized yet still subjective assessment of symptom intensity18,19.
PATHOPHYSIOLOGY OF SS
The pathophysiology of SS involves a multifaceted interaction among impaired barrier function, inflammatory responses, neurosensory dysregulation, and genetic predispositions Fig. 1. These factors collectively contribute to the heightened reactivity of SS, where otherwise benign stimuli provoke intense sensory symptoms. Each mechanism plays a crucial role in the development and persistence of SS symptoms4,20,21.
Fig. 1. Schematic illustration of the pathophysiology of sensitive skin, including key mechanisms such as impaired barrier function, neurosensory dysregulation, inflammation, and genetic susceptibility.
GPCR: G protein-coupled receptor, TRP: transient receptor potential, KC: keratinocyte, DC: dendritic cell, NO: nitric oxide, ATP: adenosine triphosphate.
Impaired skin barrier function
The skin barrier, particularly the stratum corneum (SC), is crucial for protecting against external irritants and preventing transepidermal water loss (TEWL). SS is often associated with a thinner SC and increased TEWL1,22, which allows for the penetration of irritants and allergens, exacerbating sensitivity to external stimuli. Our previous study also demonstrated a significant difference in TEWL between the sensitive and control groups23. Studies have shown that individuals with SS have reduced levels of natural moisturizing factors and lipids such as ceramides, leading to decreased skin hydration and compromised barrier integrity22. Additionally, conditions like atopic dermatitis (AD), where the barrier function is already compromised, often overlap with SS, suggesting a synergistic effect between AD-induced barrier impairment and SS24,25,26,27. However, limited studies on the effect of barriers exist, and some investigations suggest no clear association between barrier conditions and SS24,26.
Inflammatory responses
Individuals with SS often show low-grade inflammation without visible signs, marked by elevated levels of pro-inflammatory cytokines like CCL17 and interferon-γ, sustaining mild skin inflammation.28 This chronic inflammation sensitizes the skin to external triggers, further lowering the threshold for irritation and increasing reactivity to stimuli such as temperature changes and certain chemicals.
Neurosensory dysregulation
SS is considered a neurogenic disorder involving skin barrier and sensory nerve dysfunction, characterized by reduced C-fiber density and overstimulated TRP channels like TRPV1, which amplify sensory reactions and increase sensitivity2,29.
Studies have shown that reduced intraepidermal nerve fiber density (IENFD) is linked to hyperesthesia in SS, resembling small fiber neuropathy30,31. Reduced IENFD in SS patients supports this pattern, with research identifying a decreased number of peptidergic C-fibers in SS, likely contributing to heightened reactivity in the remaining nerve endings32. The overstimulation of nociceptive channels on these nerve endings by neuropeptides, such as calcitonin gene-related peptide (CGRP), may lead to neurogenic inflammation in the skin. Activation of these receptors often results in sensations like burning, tingling, and stinging, frequently reported by SS patients33. Excessive CGRP release in SS overstimulates pain receptors via TRP channels, leading to neuropathic inflammation and sensitivity, mirroring features of small-fiber neuropathy like reduced nerve fiber density and abnormal heat-pain thresholds30,34,35,36,37.
Recent studies suggest SS pathophysiology resembles neuropathic pruritus in small-fiber neuropathy, with a proposed condition called neurogenic rosacea exhibiting rosacea traits alongside neurological symptoms like burning and stinging, resistant to conventional therapies but partially responsive to anticonvulsants and antidepressants, supporting a neuropathic basis shared with SS38. In a recent study, some SS patients displayed typical neurogenic symptoms, as confirmed through responses to the SS-10 questionnaire15. Von Frey Filament Test assessments reveal decreased mechanical pain thresholds in SS-affected areas, like the cheeks, suggesting peripheral sensory neuropathy as a key factor in SS pathogenesis, which is also linked to neuropathic disorders like irritable bowel syndrome and sensitive eyes39.
Sensations in SS are influenced by nerve fibers as well as keratinocytes and other epidermal cells expressing sensory proteins like TRP channels. Overexpression of channels such as TRPV1 and ASIC3 in SS leads to calcium influx and neurogenic inflammation upon activation by stimuli like acidic pH, heat, and chemicals, explaining SS’s heightened sensitivity to environmental triggers40.
Transcriptomic studies reveal reduced PIEZO2 mRNA levels in SS patients, suggesting PIEZO2 dysfunction may contribute to SS hypersensitivity. TRPV1 channels, involved in mechanotransduction in Merkel cells, further link to this sensitivity, as genetic deletion of Merkel cells induces allokinesis, highlighting the roles of both PIEZO2 and Merkel cells in SS pathophysiology41,42. Moreover, the dedicator of cytokinesis nine gene (DOCK9), which encodes a protein regulating dendrite growth, is downregulated in SS, correlating with decreased nerve fibers and increased sensitivity.
SS pathophysiology parallels that of complex regional pain syndrome (CRPS), a disorder involving peripheral sensory neuropathy. In CRPS, repeated exposure to irritants causes sensory nerve damage and neurogenic inflammation, leading to peripheral neuronal sensitization with lowered sensory thresholds and increased excitability through the activation of protein kinases A and C, which phosphorylate sensory-specific sodium channels43. Over time, the desensitization of nociceptive neurons through phosphorylation of G-protein-coupled receptors and ion channels can gradually induce central sensitization, leading to persistent paresthesia.
Recent transcriptomic research shows that patients with SS exhibit upregulated genes associated with inflammatory pathways, revealing a complex interplay between skin barrier dysfunction and neuropathic pruritus29. This dual neurological and dermatological dysfunction places SS within a spectrum of neuropathic skin disorders, similar to neuropathic pruritus.
Genetic and epigenetic contributions
Genetic factors may predispose individuals to SS, with variants in genes related to skin barrier proteins like filaggrin and neurosensory receptors like TRPV1, affecting skin structure and sensory perception and potentially explaining symptom variability across populations8. Additionally, environmental influences, including pollution and lifestyle factors, may cause epigenetic modifications that impact the skin barrier and neurosensory pathways, further contributing to SS.
DIAGNOSIS OF SS
Diagnosing SS presents unique challenges due to the subjective nature of its symptoms and the frequent lack of visible lesions. Patients often report abnormal sensations, such as stinging and burning, triggered by common external factors like temperature changes or cosmetic products. These sensations generally occur without any accompanying clinical signs, complicating the diagnostic process. Thus, a comprehensive diagnostic approach combining both subjective assessments and objective measurements is essential to accurately identify SS44.
Subjective diagnostic tools
The SS-10 is a self-administered questionnaire consisting of 10 items, each rated on a scale from 0 (no symptom) to 10 (extreme symptom intensity), assessing common symptoms such as stinging, burning, and tightness experienced over the past 3 days. The total score ranges from 0 to 100. A score ≥20 is typically indicative of SS, while scores above 50 may represent severe cases requiring intensive management. A cutoff of 12.7 has shown 72.4% sensitivity and 90.3% specificity for SS diagnosis (Fig. 2). In SS research, an SS-10 score of 20 typically defines SS, with 50 indicating severe cases needing intensive treatment, while a cutoff of 12.7 has demonstrated 72.4% sensitivity and 90.3% specificity in distinguishing SS cases45.
Fig. 2. The Sensitive Scale-10 (SS-10): a validated questionnaire developed by Misery et al.18 to quantify sensitive skin symptoms. It includes 10 items rated from 0–10, with a maximum score of 100.
Objective diagnostic tools
Although there are often no clinical signs, diagnostic tests such as the lactate challenge test (Lactic Acid Sting Test [LAST]) and the capsaicin test are used to assess skin sensitivity44. Several methods exist to test SS, with the LAST being the most representative1,11. This test entails applying a 10% lactic acid solution to the nasolabial fold and cheek area, followed by rubbing the skin after 2 minutes 30 seconds, and 5 minutes to assess sensitivity46. Patients scoring 3 or more out of 4 are classified as having SS, but due to inconsistent results in tests like the Lactic Acid Stinging Test, Sodium Lauryl Sulfate (SLS) Occlusion Test, and Capsaicin Test, SS is recognized as a heterogeneous group with limitations arising from subjective interpretation and result variability. Currently, there are subjective and objective methods employed in testing patients with SS, as perceived by the subjects in terms of skin irritation. Subjective measurement methods include tests that induce sensations such as stinging test using lactic acid1 or eliciting a burning sensation with a mixture of chloroform and methanol47. Objective tests involve applying surfactants like SLS48 to measure erythema index and trans-epidermal water loss, as well as using dimethyl sulfoxide49 to induce swelling and erythema, and exposing the skin to ammonium hydroxide solution50 to measure blistering time.
Existing research has struggled to standardize and objectify measurement methods, with studies using the Hilltop chamber to apply 0.1% lactic acid to the malar area51. This method assesses the stinging sensation over 10 minutes by comparing the onset and peak intensity, with studies showing that increasing lactic acid concentration up to 10% raises stinging intensity, after which it plateaus, while earlier onset and additional symptoms like erythema and itching appear51. Another study reported that the LAST is the most effective method for distinguishing sensitive from non-SS based on a survey52. Tests like LAST, SLS occlusion, and capsaicin rely on subjective assessment or show inconsistent results, highlighting the heterogeneity of SS and the need for objective indicators. Recent diagnostic advancements include TEWL measurements and the ANTERA 3D® system for quantitative evaluation of skin texture, redness, and hydration, used alongside clinical photography for long-term SS monitoring53. TEWL measurement, in particular, has proven useful in correlating skin sensitivity with barrier function abnormalities, such as increased water loss and reduced hydration54.
Combining diagnostic methods
Combining SS-10 with objective measures like TEWL or LAST offers a more reliable SS diagnosis, especially for complex cases, such as those with heightened sensitivity from prolonged mask usage during the coronavirus disease 2019 (COVID-19) pandemic.
Investigation into related skin diseases
When skin sensitivity is detected, further investigation into associated skin diseases is essential. Patch testing remains crucial for diagnosing irritant and allergic contact dermatitis in SS, identifying triggers like allergens or irritants, especially for patients whose symptoms worsen with specific skincare products55 During COVID-19, patients reporting increased skin sensitivity from mask-wearing may have conditions like irritant or allergic contact dermatitis, folliculitis, seborrheic dermatitis, or worsened rosacea. Previous studies showed high sensitivity to fragrance mix 1 in SS patients through patch testing23. Coexisting skin conditions like neurogenic rosacea and AD in SS patients are assessed through facial imaging, TEWL, blood tests, patch tests, and Demodex density tests, aiding in accurate treatment, identifying SS causes, and preventing recurrence.
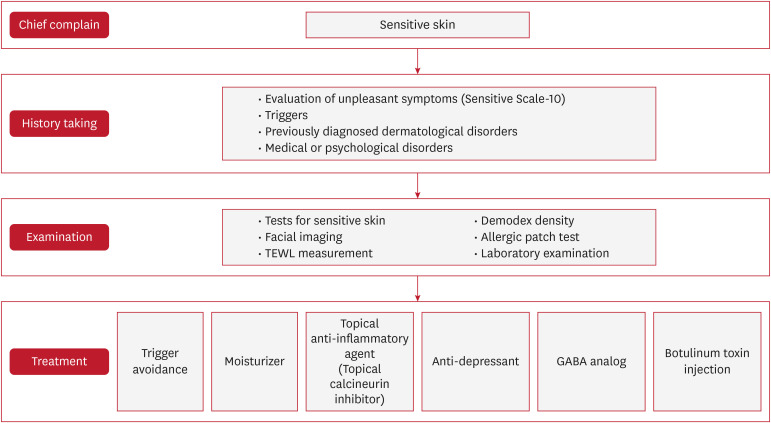
MANAGEMENT OF SS
The treatment of SS requires a personalized approach due to the variability in symptoms and triggers across individuals (Fig. 3). Effective SS treatment plans should address underlying skin conditions, symptom duration, severity, and specific triggers, aiming to relieve sensations and visible issues while strengthening the skin barrier. Treatments should focus on barrier repair and neurosensory regulation, with gradual tapering of medication to avoid rebound symptoms, even if lesions are absent.
Fig. 3. Proposed stepwise management algorithm for sensitive skin. Emphasizes trigger avoidance, barrier repair, subjective/objective assessment, and personalized pharmacologic strategies.
TEWL: transepidermal water loss, GABA: gamma-aminobutyric acid.
Identification and avoidance of triggers
Effective SS management involves identifying and minimizing triggers through patient history and using patch tests to detect allergens. Patients with AD are advised to avoid fragrances and irritants due to their compromised skin barrier56.
Emollients and barrier repair
Moisturization is key in SS treatment, but frequent changes in products can worsen sensitivity. Patients should test new products on the forearm Repeat Open Application Test for 2 weeks, and for recurring reactions, an “as-is” patch test can confirm compatibility. TEWL is a key indicator of barrier integrity and sensitivity; elevated TEWL suggests barrier dysfunction. Barrier-repair products, like ceramide moisturizers, enhance resilience, while anti-inflammatory emollients (e.g., with colloidal oatmeal or niacinamide) help reduce discomfort and support recovery53,54.
Pharmacologic options
If symptom relief through avoidance and barrier repair is insufficient, tailored pharmacologic treatments may be used. Antihistamines help with itching, beta-blockers reduce flushing, and serotonin-norepinephrine reuptake inhibitors and gamma-aminobutyric acid analogs (e.g., pregabalin, gabapentin) relieve SS symptoms by reducing sensitization and nociceptive signaling57. Topical calcineurin inhibitors, like pimecrolimus and tacrolimus, help manage SS by reducing inflammation and neurosensory sensitivity without causing skin thinning, suitable for long-term use. Intradermal botulinum toxin injections also show promise by inhibiting neurotransmitter release and reducing pain.
Additional therapies: oxidative stress and neuromodulators
Oxidative stress worsens SS by weakening the skin barrier and increasing inflammation. Antioxidant treatments, such as topical vitamins C and E or resveratrol, help reduce oxidative damage and aid barrier recovery, supporting SS management. Emerging SS treatments include topical neuromodulators like capsaicin, which desensitizes TRPV1 channels to reduce burning and stinging, and cannabinoids, which may relieve inflammation and pain. Neuropathic pain management options like anticonvulsants and botulinum toxin also offer targeted relief for severe symptoms.
Comprehensive and personalized care
Managing SS requires a comprehensive, patient-centered approach that combines subjective (SS-10) and objective (TEWL, patch testing) assessments. Personalized plans including barrier repair, trigger avoidance, and tailored treatments improve outcomes, especially for patients affected by environmental triggers. Integrating new insights enhances treatment effectiveness, quality of life, and reduces symptom recurrence.
CONCLUSION
SS is a complex and multifaceted condition influenced by diverse physiological, environmental, and genetic factors. This syndrome, characterized by heightened sensory responses to otherwise non-irritating stimuli, presents unique challenges in both diagnosis and management. Due to the subjective nature of symptoms and the absence of universal clinical signs, SS diagnosis relies on a combination of subjective assessments, such as the SS-10, and objective measures like the LAST and TEWL to confirm sensitivity and skin barrier integrity. Individualized treatment approaches are essential, focusing on mitigating skin barrier dysfunction, reducing neurosensory hypersensitivity, and avoiding known irritants. Recent studies emphasize the importance of tailored strategies to manage SS, especially in patients who experience worsening symptoms from common allergens, preservatives, and environmental stressors.
The pathophysiology of SS underscores the importance of treating both the sensory discomfort and skin barrier impairment unique to each patient. By integrating recent advancements in SS assessment and management, clinicians can improve outcomes through personalized care plans that incorporate avoidance therapy, barrier repair, and pharmacologic options. As research continues to elucidate the underlying mechanisms of SS, a multidisciplinary approach to both prevention and management will remain key in addressing this condition’s complexity and improving patient quality of life.
Footnotes
FUNDING SOURCE: This study was funded by the National Research Foundation of Korea (NRF) (grant number NRF-2022R1A2C2007739), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HP23C0201) and, by Hallym University Research Fund.
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Frosch PJ, Kligman AM. A method for appraising the stinging capacity of topically applied substances. J Soc Cosmet Chem. 1977;28:197–209. [Google Scholar]
- 2.Misery L, Bataille A, Talagas M, Le Gall-Ianotto C, Fouchard M, Huet F, et al. Sensitive skin syndrome: a low-noise small-fiber neuropathy related to environmental factors? Front Pain Res (Lausanne) 2022;3:853491. doi: 10.3389/fpain.2022.853491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marek-Jozefowicz L, Nedoszytko B, Grochocka M, Żmijewski MA, Czajkowski R, Cubała WJ, et al. Molecular mechanisms of neurogenic inflammation of the skin. Int J Mol Sci. 2023;24:5001. doi: 10.3390/ijms24055001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Do LHD, Azizi N, Maibach H. Sensitive skin syndrome: an update. Am J Clin Dermatol. 2020;21:401–409. doi: 10.1007/s40257-019-00499-7. [DOI] [PubMed] [Google Scholar]
- 5.Fisher AA. “Status cosmeticus”: a cosmetic intolerance syndrome. Cutis. 1990;46:109–110. [PubMed] [Google Scholar]
- 6.Maibach HI. The cosmetic intolerance syndrome. Ear Nose Throat J. 1987;66:29–33. [PubMed] [Google Scholar]
- 7.Misery L, Loser K, Ständer S. Sensitive skin. J Eur Acad Dermatol Venereol. 2016;30(Suppl 1):2–8. doi: 10.1111/jdv.13532. [DOI] [PubMed] [Google Scholar]
- 8.Goh CL, Wu Y, Welsh B, Abad-Casintahan MF, Tseng CJ, Sharad J, et al. Expert consensus on holistic skin care routine: focus on acne, rosacea, atopic dermatitis, and sensitive skin syndrome. J Cosmet Dermatol. 2023;22:45–54. doi: 10.1111/jocd.15519. [DOI] [PubMed] [Google Scholar]
- 9.Misery L, Ständer S, Szepietowski JC, Reich A, Wallengren J, Evers AW, et al. Definition of sensitive skin: an expert position paper from the special interest group on sensitive skin of the International Forum for the Study of Itch. Acta Derm Venereol. 2017;97:4–6. doi: 10.2340/00015555-2397. [DOI] [PubMed] [Google Scholar]
- 10.Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359–373. doi: 10.1016/j.det.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Bieber AK, Steuer AB, Melnick LE, Wong PW, Pomeranz MK. Autoimmune and dermatologic conditions associated with lichen sclerosus. J Am Acad Dermatol. 2021;85:228–229. doi: 10.1016/j.jaad.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Bauman D, Fortunel C, Delhaye G, Malhi Y, Cernusak LA, Bentley LP, et al. Tropical tree mortality has increased with rising atmospheric water stress. Nature. 2022;608:528–533. doi: 10.1038/s41586-022-04737-7. [DOI] [PubMed] [Google Scholar]
- 13.Misery L, Boussetta S, Nocera T, Perez-Cullell N, Taieb C. Sensitive skin in Europe. J Eur Acad Dermatol Venereol. 2009;23:376–381. doi: 10.1111/j.1468-3083.2008.03037.x. [DOI] [PubMed] [Google Scholar]
- 14.Misery L, Reaux-Le Goazigo A, Morisset S, Seite S, Delvigne V, Cochener B, et al. Association of sensitive eyes with sensitive skin: a worldwide study of 10,743 subjects. Skin Pharmacol Physiol. 2022;35:148–155. doi: 10.1159/000522056. [DOI] [PubMed] [Google Scholar]
- 15.Brenaut E, Barnetche T, Le Gall-Ianotto C, Roudot AC, Misery L, Ficheux AS. Triggering factors in sensitive skin from the worldwide patients’ point of view: a systematic literature review and meta-analysis. J Eur Acad Dermatol Venereol. 2020;34:230–238. doi: 10.1111/jdv.15985. [DOI] [PubMed] [Google Scholar]
- 16.Reinheimer T, Azmi R, Binder JR. Polymerizable ceramic ink system for thin inkjet-printed dielectric layers. ACS Appl Mater Interfaces. 2020;12:2974–2982. doi: 10.1021/acsami.9b18610. [DOI] [PubMed] [Google Scholar]
- 17.Chan MKT, Sayag M, Chavagnac M, Taieb C, Misery L. Sensitive skin in China: characteristics and burden. J Eur Acad Dermatol Venereol. 2021;35:e436–e439. doi: 10.1111/jdv.17100. [DOI] [PubMed] [Google Scholar]
- 18.Misery L, Jean-Decoster C, Mery S, Georgescu V, Sibaud V. A new ten-item questionnaire for assessing sensitive skin: the Sensitive Scale-10. Acta Derm Venereol. 2014;94:635–639. doi: 10.2340/00015555-1870. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira MS, Sousa Lobo JM, Almeida IF. Sensitive skin: active ingredients on the spotlight. Int J Cosmet Sci. 2022;44:56–73. doi: 10.1111/ics.12754. [DOI] [PubMed] [Google Scholar]
- 20.Richters R, Falcone D, Uzunbajakava N, Verkruysse W, van Erp P, van de Kerkhof P. What is sensitive skin? A systematic literature review of objective measurements. Skin Pharmacol Physiol. 2015;28:75–83. doi: 10.1159/000363149. [DOI] [PubMed] [Google Scholar]
- 21.Misery L, Maibach HI. Editorial: pathophysiology of sensitive skin. Front Med (Lausanne) 2020;7:159. doi: 10.3389/fmed.2020.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berardesca E, Cespa M, Farinelli N, Rabbiosi G, Maibach H. In vivo transcutaneous penetration of nicotinates and sensitive skin. Contact Dermat. 1991;25:35–38. doi: 10.1111/j.1600-0536.1991.tb01770.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim JC, Choi MG, Park JS, Lee SY, Park CW, Chung BY, et al. Sensitive skin is associated with contact sensitization and decreased nociceptive threshold. J Eur Acad Dermatol Venereol. 2024;38:e125–e127. doi: 10.1111/jdv.19398. [DOI] [PubMed] [Google Scholar]
- 24.Rerknimitr P, Otsuka A, Nakashima C, Kabashima K. The etiopathogenesis of atopic dermatitis: barrier disruption, immunological derangement, and pruritus. Inflamm Regen. 2017;37:14. doi: 10.1186/s41232-017-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yatagai T, Shimauchi T, Yamaguchi H, Sakabe JI, Aoshima M, Ikeya S, et al. Sensitive skin is highly frequent in extrinsic atopic dermatitis and correlates with disease severity markers but not necessarily with skin barrier impairment. J Dermatol Sci. 2018;89:33–39. doi: 10.1016/j.jdermsci.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Proksch E, Weidinger S. New insights into the pathogenesis of sensitive skin. Hautarzt. 2011;62:900–905. doi: 10.1007/s00105-011-2209-7. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt J, Langan S, Williams HC European Dermato-Epidemiology Network. What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol. 2007;120:1389–1398. doi: 10.1016/j.jaci.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Uehara Y, Inoue T, Ota N, Ikeda S, Murase T. Non-invasive evaluation of subjective sensitive skin by transcriptomics using mRNA in skin surface lipids. Exp Dermatol. 2022;31:172–181. doi: 10.1111/exd.14459. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura Y. Biomarkers for immune checkpoint inhibitor-mediated tumor response and adverse events. Front Med (Lausanne) 2019;6:119. doi: 10.3389/fmed.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huet F, Dion A, Batardière A, Nedelec AS, Le Caër F, Bourgeois P, et al. Sensitive skin can be small fibre neuropathy: results from a case-control quantitative sensory testing study. Br J Dermatol. 2018;179:1157–1162. doi: 10.1111/bjd.17082. [DOI] [PubMed] [Google Scholar]
- 31.Buhé V, Vié K, Guéré C, Natalizio A, Lhéritier C, Le Gall-Ianotto C, et al. Pathophysiological study of sensitive skin. Acta Derm Venereol. 2016;96:314–318. doi: 10.2340/00015555-2235. [DOI] [PubMed] [Google Scholar]
- 32.Lefaucheur JP. The “paradox” of neuropathic pain associated with small-fiber lesions in the context of fibromyalgia. Pain. 2016;157:1364–1365. doi: 10.1097/j.pain.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 33.Mohammed A, Garg R, Trakroo S, Singh A, Sanaka MR. Long term outcomes of sporadic large fundic gland polyps: a single-center experience. Scand J Gastroenterol. 2021;56:1391–1395. doi: 10.1080/00365521.2021.1968032. [DOI] [PubMed] [Google Scholar]
- 34.Misery L, Bodere C, Genestet S, Zagnoli F, Marcorelles P. Small-fibre neuropathies and skin: news and perspectives for dermatologists. Eur J Dermatol. 2014;24:147–153. doi: 10.1684/ejd.2013.2189. [DOI] [PubMed] [Google Scholar]
- 35.Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131:1912–1925. doi: 10.1093/brain/awn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154:1807–1819. doi: 10.1016/j.pain.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 38.Kim HO, Kang SY, Kim KE, Cho SY, Kim KH, Kim IH. Neurogenic rosacea in Korea. J Dermatol. 2021;48:49–55. doi: 10.1111/1346-8138.15629. [DOI] [PubMed] [Google Scholar]
- 39.Misery L, Shourick J, Reychler G, Taieb C. Association between chronic idiopathic cough and sensitive skin syndromes is a new argument in favor of common neuropathic pathways: results from a survey on 4050 subjects. Sci Rep. 2021;11:16976. doi: 10.1038/s41598-021-96608-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talagas M, Misery L. Neurophysiological mechanisms of sensitive skin. Front Med (Lausanne) 2019;6:108. doi: 10.3389/fmed.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang L, Lyu L, Wu W, Lei D, Tu Y, Xu D, et al. Genome-wide identification of long non-coding RNA and mRNA profiling using RNA sequencing in subjects with sensitive skin. Oncotarget. 2017;8:114894–114910. doi: 10.18632/oncotarget.23147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szczot M, Pogorzala LA, Solinski HJ, Young L, Yee P, Le Pichon CE, et al. Cell-type-specific splicing of Piezo2 regulates mechanotransduction. Cell Rep. 2017;21:2760–2771. doi: 10.1016/j.celrep.2017.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol. 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding DM, Tu Y, Man MQ, Wu WJ, Lu FY, Li X, et al. Association between lactic acid sting test scores, self-assessed sensitive skin scores and biophysical properties in Chinese females. Int J Cosmet Sci. 2019;41:398–404. doi: 10.1111/ics.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu P, Li N, Gao L. Inadvertent ocular perforation caused by traditional acupuncture. JAMA Ophthalmol. 2022;140:e221823. doi: 10.1001/jamaophthalmol.2022.1823. [DOI] [PubMed] [Google Scholar]
- 46.Chen SY, Yin J, Wang XM, Liu YQ, Gao YR, Liu XP. A new discussion of the cutaneous vascular reactivity in sensitive skin: a sub-group of SS? Skin Res Technol. 2018;24:432–439. doi: 10.1111/srt.12446. [DOI] [PubMed] [Google Scholar]
- 47.Frosch PJ. In: Textbook of contact dermatitis. 3rd ed. Rycroft RJG, Menne T, Frosch PJ, Lepoittevin JP, editors. Berlin: Springer; 2001. Clinical aspects of irritant contact dermatitis; pp. 337–347. [Google Scholar]
- 48.Seidenari S, Francomano M, Mantovani L. Baseline biophysical parameters in subjects with sensitive skin. Contact Dermat. 1998;38:311–315. doi: 10.1111/j.1600-0536.1998.tb05764.x. [DOI] [PubMed] [Google Scholar]
- 49.Agner T, Serup J. Quantification of the DMSO-response--a test for assessment of sensitive skin. Clin Exp Dermatol. 1989;14:214–217. doi: 10.1111/j.1365-2230.1989.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 50.Hamami I, Marks R. Structural determinants of the response of the skin to chemical irritants. Contact Dermat. 1988;18:71–75. doi: 10.1111/j.1600-0536.1988.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 51.Christensen M, Kligman AM. An improved procedure for conducting lactic acid stinging tests on facial skin. J Soc Cosmet Chem. 1996;47:1–11. [Google Scholar]
- 52.Oh BM, Kim DW, Lee SJ, Na GY, Jung SL. Comparison of results of several lactic acid sting tests on sensitive skin. Korean J Dermatol. 2003;41:569–577. [Google Scholar]
- 53.Anqi S, Xiukun S, Ai’e X. Quantitative evaluation of sensitive skin by ANTERA 3D® combined with GPSkin Barrier® . Skin Res Technol. 2022;28:840–845. doi: 10.1111/srt.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnesen EK, Thorisdottir B, Lamberg-Allardt C, Bärebring L, Nwaru B, Dierkes J, et al. Protein intake in children and growth and risk of overweight or obesity: a systematic review and meta-analysis. Food Nutr Res. 2022;66 doi: 10.29219/fnr.v66.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta A, de Menezes SL. Red in the face: approach to diagnosis of red rashes on the face. Aust J Gen Pract. 2024;53:203–209. doi: 10.31128/AJGP-08-23-6930. [DOI] [PubMed] [Google Scholar]
- 56.Kassa GM, Arowojolu AO, Odukogbe AA, Yalew AW. Adverse neonatal outcomes of adolescent pregnancy in Northwest Ethiopia. PLoS One. 2019;14:e0218259. doi: 10.1371/journal.pone.0218259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh R, McCain S, Feldman S, Strowd L. A qualitative study on dupilumab’s impact on atopic dermatitis among adolescent and adult patients. J Drugs Dermatol. 2023;22:148–153. doi: 10.36849/JDD.7053. [DOI] [PubMed] [Google Scholar]