Abstract
CRISPR–Cas-based genome imaging opened a new era of genome visualization in living cells. While genomic loci with repetitive sequences, such as centromeres and telomeres, can be reliably imaged, applying the technique to nonrepetitive genomic loci has remained challenging. Recent advancements in the design of CRISPR RNAs and Cas proteins, the development of novel fluorophores and the combination of CRISPR–Cas with other molecular machinery amplified target-specific signals and suppressed background signals, revolutionizing this unique genome imaging technique and enabling the tracking of genomic loci with a small number of CRISPR–Cas complexes, down to a single complex. Here we review the latest advancements in CRISPR–Cas-based genome imaging techniques and their application to imaging nonrepetitive genomic loci. The challenges that these techniques are currently facing are the cellular toxicity and genomic instability induced by the expression of CRISPR–Cas and its interference with DNA metabolism, which impacts DNA replication and genome maintenance. Recently reported adverse effects of CRISPR–Cas-based genome labeling are discussed here, along with perspectives on how to overcome the problem.
Subject terms: Chromatin structure, Super-resolution microscopy
Enhancing genome visualization with CRISPR technology
Understanding how our DNA is organized in three dimensions and changes over time is important for studying how genes work. This Review highlights the use of CRISPR–Cas9, a tool originally for gene editing, now adapted for live-cell imaging of DNA. Researchers use a catalytically dead Cas9 and, by attaching fluorescent proteins to the complex, label specific DNA regions in live cells. This technique faces challenges in imaging nonrepetitive DNA regions, which requires transfection with multiple guide RNAs. New techniques amplify signal and reduce background, making it possible to visualize a target even with a single CRISPR complex. Emerging evidence suggests that CRISPR–Cas9 for imaging interferes with genomic processes. The authors suggest that future research should focus on refining these methods to minimize side effects and improve delivery systems for CRISPR components.
This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
Introduction
Visualizing the three-dimensional (3D) organization of genome and its dynamic changes is crucial to understanding the regulation of genomic processes1–4. Hi-C techniques provide genome-wide information on the hierarchical organization of chromosomal domains with kilobase resolution, and fluorescence in situ hybridization (FISH) techniques show the spatial arrangement of genomic regions5–7. These techniques, however, are applicable mostly to fixed cells and do not show how genome organization changes over time, despite several recent works that applied FISH techniques to living cells, but under harsh conditions questioning cell viability8–10. Reliable live-cell chromatin imaging is essential for capturing the spatiotemporal behavior of genome and providing insights into the dynamic interaction of genome with nuclear components. These insights elucidate the mechanisms of chromatin compartmentalization11, cell differentiation12,13, development14 and diseases15,16, where genome organization plays important roles.
CRISPR–Cas9, originally adopted for genome editing, has expanded its scope of applications to many areas, including chromatin imaging17,18. The development of a nuclease-deactivated variant of Cas9 (dCas9), which retains the ability to recognize and bind target DNA, was crucial19. By fusing dCas9 with fluorescent proteins such as eGFP or mCherry, CRISPR has been harnessed for live genome imaging with high target specificity, marking the beginning of a new era in chromatin imaging20–26.
Despite these advances, imaging an arbitrary genomic region is still challenging. CRISPR–Cas-based genome imaging often targets genomic regions with repetitive sequences such as centromeres, alpha satellites and telomeres, which are easier to visualize than nonrepetitive regions owing to the abundance in CRISPR targets with a single kind of guide RNA (gRNA)27,28. Imaging genomic loci with nonrepetitive sequences requires the incorporation of a large number of gRNAs and optimization of the CRISPR design and imaging conditions to maximize signal-to-noise ratios (SNRs)20,26. Varying conditions and microscopy techniques make it difficult to compare the reported methods and find applicable solutions. This Review aims to provide a comprehensive overview of the current CRISPR–Cas-based genome imaging methods, their performances in imaging nonrepetitive genomic loci and the side effects of CRISPR–Cas-based genome imaging on cell viability and genomic processes, to aid future research in adopting these methods.
Recent developments in CRISPR–Cas-based genome imaging
Overview of CRISPR–Cas-based genome imaging
CRISPR–Cas-based imaging leverages the unique properties of Cas proteins to bind a double-stranded DNA with a sequence defined by the CRISPR RNA (crRNA), which is typically linked to another trans-encoded RNA (tracrRNA) to make a single guide RNA (sgRNA), which forms a complex with a Cas protein 20. Cas9 protein is mutated to be nuclease-deficient (dCas9) so that it binds to the target DNA without cleaving the DNA, and fluorescent proteins are fused to dCas9 or engineered to bind the gRNA scaffold20,21,23–26. Orthologous CRISPR systems from multiple bacterial species were exploited to image multiple targets simultaneously21,22. dCas9 of each species recognizes its matching sgRNA and a unique protospacer adjacent motif sequence on the DNA.
Initial designs using dCas9 fused with a fluorophore experienced high background levels and aggregation. This was mitigated by avoiding the placement of labels directly on dCas9 and by using split fluorophores. Split fragments of the fluorescent protein GFP formed full fluorophores on assembled CRISPR complexes and greatly reduced nonspecific background signals29. Multilocus imaging was also achieved by incorporating various RNA aptamers such as MS2 and PP7 motifs into sgRNA scaffolds and using fluorescent proteins linked to MCP and PCP, which bind these motifs22,25. sgRNAs with protein-binding scaffolds were also used for signal amplification, as repeated protein-binding motifs allowed the recruitment of multiple fluorescent proteins and amplified signals23,24,26,30–32. RNA-binding proteins such as MCP, PP7, Com and lambdaN were utilized for orthogonal labeling of sgRNA23,31,33–35. Another method for signal amplification is the SunTag system, which uses a GCN4 peptide array to recruit scFv fused to superfolder GFP (sfGFP)36. In this work, dCas9 fused to 24-repeat SunTag enhanced the signal brightness 19-fold compared with dCas9–EGFP in HEK293 cells. The development of various CRISPR–Cas-based imaging systems has led to significant breakthroughs in understanding chromatin dynamics and nuclear architecture. Long-term live tracking of genomic loci has enabled precise analysis of their diffusion behaviors, which are influenced by factors such as location in the nucleus, cell cycle, metabolic state and DNA damage20,35,37–39.
Novel genome imaging techniques based on CRISPR–Cas
Despite the advancements in CRISPR–Cas-based genome imaging, most applications of the technique focused on targets with repetitive sequences, such as centromeres, satellites and telomeres25,30,40,41. Genomic regions containing repetitive sequences are easier to target owing to the high abundance of target sequences, allowing efficient imaging with a minimal set of sgRNAs. Targeting nonrepetitive regions is challenging as the simultaneous transfection with multiple plasmids is difficult20,26. Transfecting the cell with a plasmid containing multiple sgRNAs provides a plausible solution to this, but the binding of CRISPR–Cas complexes to a group of distinct targets is not as efficient as that for repetitive targets42.
Recent developments of CRISPR–Cas systems for improving the labeling efficiency and coverage are summarized in Table 1. CRISPR-Sirius enhances signal strength and stability by inserting repeated RNA aptamer sequences such as MS2 and PP7 into the tetraloop of sgRNA43. The design of the insert was optimized by randomizing the linker between the RNA aptamers, making synonymous mutations to avoid long repeats in the sequence and minimizing undesired RNA secondary structures. This design improves the stability of sgRNA and allows higher-resolution imaging of genomic loci. Splitting sfGFP into three fragments and integrating these with the SunTag system and extended sgRNA scaffolds greatly reduced background noise and facilitated fluorescence recovery by the frequent disassembly–reassembly of the sfGFP fragments, thereby allowing reliable long-term imaging35. CRISPR/Casilio uses an sgRNA containing multiple Pumilio/FBF (PUF) binding sites and PUF fused with fluorescent proteins such as Clover, iRFP670 and mRuby2 to amplify the signals, enabling high-resolution, multiplexed imaging of chromatin interactions44.
Table 1.
Novel CRISPR–Cas-based genome imaging techniques.
| Live/fixed cells | Name of technology | Type of Cas | Composition of CRISPR–Cas complex | Type of target region | Features | Reference |
|---|---|---|---|---|---|---|
| Live cells | CRISPR-Sirius | dCas9 | dCas9, aptamers inserted into the tetraloop of sgRNA | repetitive | Improved gRNA stability by modifying RNA scaffolds, better signal amplification than conventional methods | Ma et al.43 |
| Live cells | Tripartite sfGFP | dCas9 | GFP1-9, scFv-GFP10, MCP-mCherry-GFP11, dCas9-24X GCN4, sgRNA 12XMBS | Repetitive/nonrepetitive | Enhanced SNR by decreasing background level and amplifying signals using tripartite GFP and SunTag | Chaudhary et al.35 |
| Fixed cells | Genome oligopaint via local denaturation (GOLD) FISH | Cas9 nickase | Cas9 nickase, gRNA, superhelicase, Cy5-FISH probe | Repetitive/nonrepetitive | Increased specificity and amplified signal by local DNA denaturation for FISH probe access | Wang et al.45 |
| Live cells | CRISPR FISHer | dCas9 | dCas9, sgRNA-multi PP7, Foldon-GFP-PCP | Repetitive/nonrepetitive | Signal amplification mediated by phase separation in live cells | Lyu et al.46 |
| Live cells | CRISPR/Casilio-based imaging | dCas9 | dCas9, gRNA-PBS, PUF-fluorescent protein (FP) | Nonrepetitive | Amplifiable and multiplexable imaging by integrating PUF binding sequences in sgRNA | Clow et al.44 |
| Live cells | CRISPR-SunTag with LEXY | dCas9 | dCas9-SunTag, scFv-sfGFP-LEXY | Repetitive | Improved SNR by optically controlled export of untargeted fluorescent proteins to the cytoplasm | Hou et al.47 |
| Fixed cells | CasSABER | dCas9 | dCas9/sgRNA, primer-exchange reaction (PER) probes, imager-fluorescent tag | Nonrepetitive | Signal amplification by branching primer-exchange reaction probes | Li et al.48 |
| Live cells | Fluorogenic CRISPR (fCRISPR) | dCas9 | dCas9, sgRNA with Pepper aptamers, FP–tDeg | repetitive | Enhanced SNR by using degradable fluorogenic proteins to be stabilized by target binding | Zhang et al.49 |
| Live cells | CRISPR/Pepper-tDeg | dCas9 | dCas9, sgRNA with the degron binding Pepper aptamers, split FP–tDeg | Repetitive/nonrepetitive | Enhanced SNR by combining tDeg-based fluorogenic CRISPR and MS2-MCP system | Chen et al.50 |
| Live cells | CRISPRdelight | dCas12a | dCas12a, engineered CRISPR array for multiplexed imaging | Nonrepetitive | Signal amplification and multiplexing by processing CRISPR arrays with dCas12a | Yang et al.57 |
Unconventional ways to amplify target CRISPR signals have been reported. GOLD FISH eliminates the need for multiple CRISPR complexes targeting a single genomic region by using a Cas9 nickase and a superhelicase to unwind target DNA regions and labeling them with conventional FISH probes, which requires the fixation of cells45. This method allows precise labeling of both repetitive and nonrepetitive sequences with enhanced specificity and expands the targeting capability of CRISPR imaging. Another method, CRISPR FISHer, exploits phase separation to mediate the amplification of target-specific fluorescence signals, using an engineered sgRNA scaffold coupled with a trimeric foldon–GFP fusion protein46. It showed a 246-fold improvement in SNR compared with the conventional dCas9–eGFP design, facilitating real-time tracking of chromosomal events, such as chromatin dissociation and intra- and interchromosomal rejoining induced by DNA double-strand breaks. An optogenetically controlled system, which integrates a light-inducible nuclear export tag (LEXY) with a CRISPR-SunTag system, showed a ~2–2.5 fold improvement of SNR by selective removal of untargeted fluorescent proteins from the nucleus47. A signal amplification method based on primer-exchange reaction, CRISPR–Cas-mediated signal amplification by exchange reaction (CasSABER), enhances fluorescent signals by multiple rounds of branching hybridization48.
Recently developed CRISPR labeling methods, fCRISPR and CRISPR/Pepper-tDeg, used a novel fluorogenic protein49,50. Fusing a fluorescent protein with a degron domain derived from Tat peptide (tDeg), which is protected from degradation only when binding an RNA aptamer, Pepper, untargeted fluorophores were eliminated and a much higher SNR was achieved. The CRISPR/Pepper-tDeg technique also fused tDeg with a tandem repeat of a split GFP fragment, GFP11, to amplify signals by assembling this with separately expressed GFP1–10 fragments50. Cas12a has also been exploited for genome imaging51–54. Unlike dCas9, dCas12a can process pre-crRNAs into multiple mature crRNAs, providing a solution to transfect cells with multiple sgRNAs55,56. The CRISPRdelight technique used a CRISPR–dCas12a array for efficient multiplexed imaging of genomic loci57.
Imaging nonrepetitive genomic loci
Repetitive genomic regions offer an advantage in CRISPR–Cas-based imaging due to the redundancy of targets, but many important biological problems involve the reorganization of nonrepetitive genomic regions. It is challenging to image nonrepetitive genomic loci, because it is required to insert many kinds of sgRNA and, even when they are successfully expressed, each sgRNA needs to find a single binding target. In addition, off-target binding needs to be addressed for each sgRNA.
Recently developed techniques have advanced CRISPR–Cas-based imaging by eliminating background signals, amplifying target-specific signals and improving the targeting accuracy of CRISPR–Cas. Table 2 lists the studies that report successful imaging of nonrepetitive genomic loci using these techniques. Several studies reported imaging a genomic locus with a single CRISPR–Cas complex bound to it44,46,48,50. Novel designs of CRISPR systems in these studies all achieved the visualization of nonrepetitive genomic loci using a single sgRNA, but through distinct amplification strategies: Casilio uses Pumilio-mediated recruitment of multiple fluorescent proteins, FISHer exploits protein phase separation, CasSABER leverages iterative primer exchange reaction, and Pepper-tDeg relies on background suppression by target-dependent activation of degron and split-GFP. While Casilio and FISHer provide relatively straightforward designs for signal amplification, CasSABER offers high programmability at the cost of complex probe design and the limited application to fixed cells, and Pepper-tDeg offers high SNR without assembling large number of proteins by degrading fluorescent proteins in the background. As these techniques rely on a single CRISPR complex correctly binding the target, they may face challenges from off-target binding of CRISPR and need finely tuned expression control. Given the varying efficiency of CRISPR editing on different genomic loci, the performance of these methods may also vary depending on the target. The applicability and reliability of these methods for varying target sites need to be addressed in future studies.
Table 2.
Imaging low-repeat and nonrepetitive genomic loci using CRISPR–Cas.
| Labeling strategy | Fluorescent tag | Target site | (Low-repeat targets) Number of gRNAs | (Nonrepetitive targets) Number of gRNAs used/target size | Cell line | Microscope | Reference |
|---|---|---|---|---|---|---|---|
| MPC, PCP motif | MCP–EGFP, PCP–mCherry | Low-repeat (Igh, Akap6 locus) | Igh: 5–18 locations for each 13 sgRNA, Akap6: 87 locations for a sgRNA | - | Mouse 3T3 fibroblast cells | Wide-field microscope, 3D z stack, 3D deconvolution | Fu et al.30 |
| MCP, PCP motif, sgRNA 2.0 16x-MS2 | MCP–YFP, MCP–mCherry | Low-repeat (MUC4, locus ~#1–4), nonrepetitive (the first intron of the MUC4) | MUC4: 84 repeats for a sgRNA, locus ~#1–4: 8, 15, 21 and 33 repeats, respectively | Intron of MUC4: ~4–30 sgRNAs across 5 kb | HeLa, U2OS and RPE1 cells | Confocal microscope, lattice light-sheet microscope, 3D z stack | Qin et al.26 |
| MCP, PCP motif, sgRNA-Sirius-8xMS2 | MCP–Halo, PCP–GFP, RNA aptamers at tetraloop of the sgRNA scaffold | Low-repeat (C19-1, C19-2, intron 10 in FBN3 and 26 loci in Ch19) | C19-1 (36 copies), C19-2 (45 copies), 22 (for FBN3), 26 loci (≥20 copies) | - | U2OS cells | Leica DM IRB microscope with EMCCD camera and 100× oil lens | Ma et al.43 |
| SHACKTeR (short homology and CRISPR–Cas9-mediated knock-in of a TetO repeat) | TetR–EGFP | Nonrepetitive (10 different loci including HSP70 locus), inserted short repeat | 48-mer and 96-mer TetO repeats (~3–4.6 kb for TetO repeat integration) | - | HCT116 cells, HEK293T | Wide-field microscope, deconvolution, structured illumination | Tasan et al.82 |
| CRISPR/dual-FRET MB (dual molecular beacons for FRET) | Donor MB-Atto550, acceptor MB-Atto647N | Nonrepetitive (MUC4 gene, MUC1 gene, intergenic region) | - | 3 unique sgRNAs for each loci/~220–770 bp depending on the locus | HeLa, U2OS cells | Wide-field microscope, 3D z stack, deconvolution | Mao et al.28 |
| SunTag tripartite sfGFP | GFP1-9, scFv–GFP10, MCP–mCherry–GFP11 | Low-repeat (X-114 locus) | 13 copies | – | AD-293 cell | Confocal microscope, 63× 1.4 NA lens, 3D z stack | Chaudhary et al.35 |
| CRISPR FISHer (use Foldon trimerization, sgRNA-8xPP7) | Foldon–GFP–PCP | Nonrepetitive (PPP1R2 gene) | – | sgRNA, single binding site | U2OS, HeLa, HepG2 | Nikon Eclipse Ti-E with Andor Sona 4BV6U Camera, Plan APO λ 100× / 1.45 oil objective, 3D z stack | Lyu et al.46 |
| CRISPR/Casilio (use 15× PUF domain and PUF binding site) | PUF-Clover/iRFP670/mRuby2 | Nonrepetitive (MUC4 gene, MASP1–BCL6 loop, IER5L promoter–super enhancer loop) | – | sgRNA, single binding site | U2OS, ARPE-19, HCT116/RAD21-mAID, HAP1 | Confocal microscope, 3D z stack, 3D drift correction, deconvolution | Clow et al.44 |
| CasSABER (use primer exchange reaction probe) | Cy3/Cy5/AF488 - imager | Low-repeat (MUC4 intron, HTT gene), nonrepetitive (MUC4 gene) | MUC4 intron (90 copies), HTT gene (17 copies) | 1, 3 and 6 gRNAs | MCF-7, HeLa | Confocal microscope, 3D z stacks | Li et al.48 |
| CRISPRdelight (use dCas12a and engineered CRISPR array) | dCas12a–EGFP/StayGold | Nonrepetitive (CCAT1 locus, S100A10, EFNA1, GPBP1, H3C1, MYC, NFIL3, HSPH1) | – | Arrays with 12, 24, 36 and 48 crRNAs | HeLa, U2OS, HCT116 | Wide-field microscope, deconvolution | Yang et al.57 |
| fCRISPR (use degron binding pepper aptamers) | FP–tDeg | Low-repeat (MUC4 intron 1, Chromosomes 3, 9, 13 and 19 regions) | MUC4 intron 1 (90 copies), Chr19 (30 copies), Chr3 (25 copies), Chr9 (17 copies), Chr13 (14 copies) | - | HEK293T, HeLa, Huh7, LO2, U2OS | Confocal microscope | Zhang et al.49 |
| CRISPR/Pepper-tDeg (use degron-binding pepper aptamers at sgRNA) | Split GFP–tDeg | Low-repeat (IDR1, IDR3, FBN3), nonrepetitive (MUC4, IL-1B) | IDR1 (61 copies), IDR3 (45 copies), FBN3 (22 copies) | sgRNA, single binding site | HEK293T | Confocal microscope | Chen et al.50 |
FRET Förster resonance energy transfer.
Adverse effects of CRISPR–Cas-based genome labeling
Increasing evidence suggests that dCas9 binding causes unintended effects on cellular processes. Although dCas9 does not directly cleave the target DNA, it can modulate the accessibility of the DNA to other proteins and interfere with genomic processes such as replication, repair and transcription. Recent reports on the effect of CRISPR–dCas9 binding are summarized in Table 3 and discussed below.
Table 3.
Adverse effects of dCas9 expression and target binding.
| Keyword | Features | Organism/system | Reference |
|---|---|---|---|
| DNA replication | dCas9 binding blocks DNA replication proteins | In vitro (viral, bacterial, eukaryotic) | Whinn et al.58 |
| dCas9 binding destabilizes targeted array and showed copy number variation | Yeast cells | Doi et al.59 | |
| dCas9 binding delays replication timing and sister chromatin resolution | Mammalian cells | Xiong et al.60 | |
| R-loop formation | dCas9 induces mutations due to dCas9-induced R-loop | Yeast cells | Laughery et al.64 |
| dCas9 induced R-loop inhibits the initiation of BER on both strands of the DNA | In vitro | Antony et al.65 | |
| Chromatin accessibility | dCas9 binding opens chromatin inducing accessibility | Mouse embryonic stem cells (mESC) | Barkal et al.66 |
| dCas9 expression | High-level expression of dCas9 induces abnormal cell morphology | E. coli | Cho et al.67 |
| dCas9 induces fitness defects depending on dCas9 concentration | E. coli | Cui et al.68 | |
| dCas9 causes strong growth inhibition in the absence of sgRNA | C. trachomatis | Wurihan et al.69 | |
| Cell cycle | dCas9 causes TP53-dependent cell cycle arrest | Human cells | Geisinger et al.70. |
Replication blockage and genomic instability
Several studies reported that dCas9 binding on DNA hinders DNA replication across different biological systems. In vitro experiments confirmed that the CRISPR–dCas9 complex can obstruct the progression of DNA replication complex from viruses, bacteria and eukaryotic cells58. Remarkably, at high concentrations, dCas9 alone was shown to inhibit the assembly of replisomes. In Saccharomyces cerevisiae, dCas9 binding interferes with DNA replication and generates structural variation59. dCas9-bound CUP1 locus, which is a tandem repeat array, showed copy number variation. dCas9 was also shown to impede replication fork progression and accumulate replication intermediates in cultured cells, as revealed by neutral–neutral 2D gel electrophoresis and Southern blot hybridization59.
Another study reported a reduced formation of doublet foci, which indicate active DNA replication, during early- and mid-S phases in CRISPR–Cas-expressing cells60. The overall duration of DNA replication, from the onset of S phase to its completion, was extended upon CRISPR–Cas-based labeling, compared with TetR-based labeling. Even LacI- or TetR-based labeling can induce replication fork stalling and recruit DNA damage response (DDR) proteins, such as γH2AX, TOPBP1 and 53BP1, indicating the presence of double-strand breaks and subsequent activation of the DDR61. CRISPR–Cas-based labeling presumably causes stronger blockage of the replication fork and triggers stronger DDR and genomic instability at the targeted locus.
R-loop formation
R-loops are three-stranded nucleic acid structures consisting of an RNA–DNA hybrid and a displaced single-stranded DNA, and they have emerged as a key factor in genomic instability62,63. CRISPR–dCas9 binding induces the formation of ~20-base-long R-loops. CRISPR–Cas-induced R-loops promote spontaneous cytosine deamination at the exposed single-stranded DNA, leading to mutations64. They have also been shown to inhibit base excision repair in vitro, especially uracil lesions65. The activity of uracil-DNA glycosylase, a key enzyme in the BER pathway that cleaves uracil-containing DNA, was reduced by 2.6-fold in the presence of CRISPR–dCas9. This suggests that CRISPR–Cas-induced R-loops not only create opportunities for the formation of mutagenic lesions, but also hinder the repair machinery for these lesions, promoting genomic instability.
Chromatin accessibility and gene expression level
dCas9 binding also alters chromatin accessibility. It was reported that dCas9 binding induces chromatin opening and facilitates the binding of DNase and retinoic acid receptors, thus enhancing the ability of retinoic acid to induce GFP expression66. However, the effects and extent of dCas9 binding on gene expression remain contentious. A different study reported that dCas9 binding does not affect the subnuclear location of the labeled loci or the expression of genes at adjacent regions58. These observations suggest that the influence of dCas9 on gene expression may depend on the genomic region owing to the varying genomic context and response of regulatory elements.
Cellular toxicity
The expression level of dCas9 has been shown to correlate with the impact on cellular physiology in various organisms. In Escherichia coli, overexpression of dCas9, even without gRNA, causes abnormal cell morphology and a notable reduction in growth rate to approximately 50% of that of wild-type cells67. RNA sequencing analysis revealed that 574 genes were differentially transcribed under high dCas9 expression, with particularly large impact on cell division and membrane-associated proteins. In addition, gRNA-sequence-dependent blocking of gene transcription in E. coli is observed. This effect produces fitness defects or even kills E. coli, depending on the concentration of dCas968. In Chlamydia trachomatis, high-level expression of dCas9 from Streptococcus pyogenes caused pronounced growth inhibition, in the absence of gRNA69. dCas9 from Staphylococcus aureus also exhibited strong toxicity when expressed with a nontargeting gRNA scaffold. dCas9 binding can also arrest the cell cycle in a TP53-dependent manner70. Imaging applications typically require higher dCas9 concentrations compared with gene editing, increasing the risk of unintended cellular stress or toxicity. These findings suggest that dCas9 expression should be carefully regulated, especially when using it as an imaging tool to study chromatin dynamics.
Conclusions and perspectives
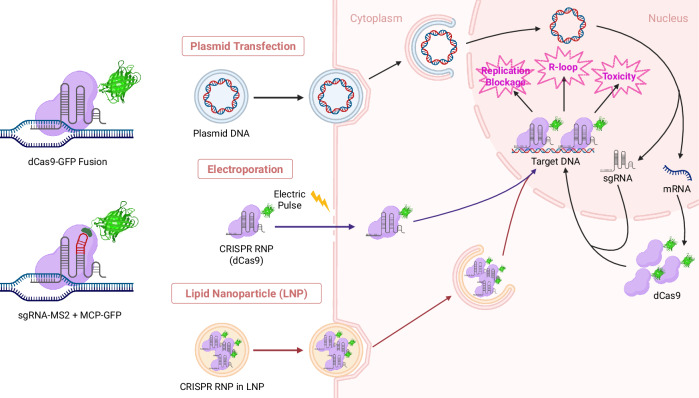
CRISPR–Cas-based chromatin imaging techniques have greatly advanced, expanding our understanding of chromatin dynamics during cellular processes. Although further refinements are required for their application to diverse genomic loci and tissue-level studies, recent technical developments have brought us closer to the labeling of nonrepetitive genomic loci with a single sgRNA. One big challenge the techniques are facing is the delivery of CRISPR complexes. High-level expression of dCas9 is essential for effective genome labeling, but excessive expression of dCas9 is detrimental to cell health. Thus, delivering a controlled amount of dCas9 to the nucleus is essential for reliable imaging of genomic loci. Also, artificial organic fluorophores offer many advantages over fluorescent proteins, if they can be delivered to target sites. Currently, three types of approaches are primarily used to deliver CRISPR–Cas complex to the cell (Fig. 1). Plasmid transfection is the most commonly used technique for genome imaging30,38,40,60,71. Inducible expression systems have been used to mitigate the overexpression problem68,69.
Fig. 1. Schematic showing the delivery dCas9 and gRNA delivery into cell nucleus for chromatin imaging.
Created with BioRender.com. Three dCas9/gRNA delivery methods are illustrated. (1) Plasmid transfection. Plasmid DNA is delivered to the cell cytoplasm via endocytosis. Upon entry into the nucleus, the plasmid is transcribed to produce sgRNAs and mRNAs to be translated to produce dCas9 proteins in the cytoplasm, which then translocate back to the nucleus using their nuclear localization signal and form dCas9–sgRNA complexes to target genomic loci. (2) Electroporation. Preassembled dCas9–sgRNA RNP complexes are delivered into the cell through membrane pores transiently formed by an electric pulse. (3) Lipid nanoparticle. Preassembled dCas9–sgRNA RNP complexes are encapsulated in lipid nanoparticles and delivered by endocytosis.
More precise control of dCas9 and sgRNA levels can be achieved by delivering preassembled ribonucleoprotein (RNP) complexes of CRISPR–Cas. RNP delivery into the nucleus is performed primarily by two methods. One is electroporation or nucleofection, which creates temporary pores on the membrane using electric pulses. The pores enable RNPs to enter the cytoplasm and eventually the nucleus72–74. However, this technique requires a recovery period to diminish the shock and stress that the electric pulses caused, and the cell survival is generally inefficient72,74. Alternatively, lipid nanoparticles are used to encapsulate and deliver CRISPR RNP complexes via endocytosis75–77. The technique uses stable, well-characterized lipid nanoparticle formulations. This method is widely used in CRISPR–Cas-based gene editing, which generally requires lower levels of Cas proteins than chromatin labeling does. Delivering sufficient quantities of Cas proteins for chromatin imaging without compromising cellular functions still remains challenging. Advances in RNP delivery methods are anticipated to revolutionize CRISPR–Cas-based genome imaging. One promising approach uses cryo-shocked cells in combination with lipid nanoparticles for CRISPR–Cas delivery78. The study demonstrated successful dCas9 plasmid delivery to mouse lung tissue using cryo-shocked tumor cells, maintaining the structural integrity of cells while avoiding pathogenicity. Addressing these issues will be instrumental not only for the field of CRISPR–Cas-based imaging, but also for epigenomic gene regulation and prime editing using dCas979–81. With ongoing progress, CRISPR–Cas-based genome imaging holds promise for broadening our understanding of genome dynamics.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flyamer, I. M. et al. Single-nucleus Hi-C reveals unique chromatin reorganization at oocyte-to-zygote transition. Nature544, 110–114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su, J.-H., Zheng, P., Kinrot, S. S., Bintu, B. & Zhuang, X. Genome-scale imaging of the 3D organization and transcriptional activity of chromatin. Cell182, 1641–1659 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nozaki, T. et al. Dynamic organization of chromatin domains revealed by super-resolution live-cell imaging. Mol. Cell67, 282–293 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Boettiger, A. & Murphy, S. Advances in chromatin imaging at kilobase-scale resolution. Trends Genet.36, 273–287 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer-Safer, P. R., Levine, M. & Ward, D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc. Natl Acad. Sci. USA79, 4381–4385 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauman, J., Wiegant, J., Borst, P. & Van Duijn, P. A new method for fluorescence microscopical localization of specific DNA sequences by in situ hybridization of fluorochrome-labelled RNA. Exp. Cell Res.128, 485–490 (1980). [DOI] [PubMed] [Google Scholar]
- 7.Van Berkum, N. L. et al. Hi-C: a method to study the three-dimensional architecture of genomes. J. Vis. Exp.39, e1869 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman, A. P. & Kool, E. T. Quenched autoligation probes allow discrimination of live bacterial species by single nucleotide differences in rRNA. Nucleic Acids Res.33, 4978–4986 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batani, G., Bayer, K., Böge, J., Hentschel, U. & Thomas, T. Fluorescence in situ hybridization (FISH) and cell sorting of living bacteria. Sci. Rep.9, 18618 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amann, R. & Fuchs, B. M. Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat. Rev. Microbiol.6, 339–348 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Amiad-Pavlov, D. et al. Live imaging of chromatin distribution reveals novel principles of nuclear architecture and chromatin compartmentalization. Sci. Adv.7, eabf6251 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.May, D. et al. Live imaging reveals chromatin compaction transitions and dynamic transcriptional bursting during stem cell differentiation in vivo. eLife12, e83444 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricci, M. A., Cosma, M. P. & Lakadamyali, M. Super resolution imaging of chromatin in pluripotency, differentiation, and reprogramming. Curr. Opin. Genet. Dev.46, 186–193 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Kishi, Y. & Gotoh, Y. Regulation of chromatin structure during neural development. Front. Neurosci.12, 874 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youmans, D. T., Schmidt, J. C. & Cech, T. R. Live-cell imaging reveals the dynamics of PRC2 and recruitment to chromatin by SUZ12-associated subunits. Genes Dev.32, 794–805 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi, K., Chen, X., Oji, A., Hiratani, I. & Defossez, P.-A. Large-scale chromatin rearrangements in cancer. Cancers14, 2384 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhary, N., Im, J.-K., Nho, S.-H. & Kim, H. Visualizing live chromatin dynamics through CRISPR-based imaging techniques. Mol. Cells44, 627–636 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Staalduinen, J., van Staveren, T., Grosveld, F. & Wendt, K. S. Live-cell imaging of chromatin contacts opens a new window into chromatin dynamics. Epigenet. Chromatin16, 27 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi, L. S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell152, 1173–1183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, B. et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell155, 1479–1491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma, H. et al. Multicolor CRISPR labeling of chromosomal loci in human cells. Proc. Natl Acad. Sci. USA112, 3002–3007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, B. et al. Expanding the CRISPR imaging toolset with Staphylococcus aureus Cas9 for simultaneous imaging of multiple genomic loci. Nucleic Acids Res.44, e75 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, H. et al. Multiplexed labeling of genomic loci with dCas9 and engineered sgRNAs using CRISPRainbow. Nat. Biotechnol.34, 528–530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao, S. et al. Long-term dual-color tracking of genomic loci by modified sgRNAs of the CRISPR/Cas9 system. Nucleic Acids Res.44, e86 (2016). e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, S., Su, J.-H., Zhang, F. & Zhuang, X. An RNA-aptamer-based two-color CRISPR labeling system. Sci. Rep.6, 26857 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin, P. et al. Live cell imaging of low-and non-repetitive chromosome loci using CRISPR–Cas9. Nat. Commun.8, 14725 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knight, S. C., Tjian, R. & Doudna, J. A. Genomes in focus: development and applications of CRISPR–Cas9 imaging technologies. Angew. Chem. Int. Ed.57, 4329–4337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu, X., Mao, S., Ying, Y., Krueger, C. J. & Chen, A. K. Progress and challenges for live-cell imaging of genomic loci using CRISPR-based platforms. Genomics Proteom. Bioinform.17, 119–128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamiyama, D. et al. Versatile protein tagging in cells with split fluorescent protein. Nat. Commun.7, 11046 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu, Y. et al. CRISPR–dCas9 and sgRNA scaffolds enable dual-colour live imaging of satellite sequences and repeat-enriched individual loci. Nat. Commun.7, 11707 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maass, P. G. et al. Spatiotemporal allele organization by allele-specific CRISPR live-cell imaging (SNP-CLING). Nat. Struct. Mol. Biol.25, 176–184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shechner, D. M., Hacisuleyman, E., Younger, S. T. & Rinn, J. L. Multiplexable, locus-specific targeting of long RNAs with CRISPR-Display. Nat. Methods12, 664–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larson, D. R., Zenklusen, D., Wu, B., Chao, J. A. & Singer, R. H. Real-time observation of transcription initiation and elongation on an endogenous yeast gene. Science332, 475–478 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu, B., Chao, J. A. & Singer, R. H. Fluorescence fluctuation spectroscopy enables quantitative imaging of single mRNAs in living cells. Biophys. J.102, 2936–2944 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhary, N. et al. Background-suppressed live visualization of genomic loci with an improved CRISPR system based on a split fluorophore. Genome Res.30, 1306–1316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanenbaum, M. E., Gilbert, L. A., Qi, L. S., Weissman, J. S. & Vale, R. D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell159, 635–646 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knight, S. C. et al. Dynamics of CRISPR–Cas9 genome interrogation in living cells. Science350, 823–826 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Mehra, D., Adhikari, S., Banerjee, C. & Puchner, E. M. Characterizing locus specific chromatin structure and dynamics with correlative conventional and super-resolution imaging in living cells. Nucleic Acids Res.50, e78 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma, H. et al. Cell cycle- and genomic distance-dependent dynamics of a discrete chromosomal region. J. Cell Biol.218, 1467–1477 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takata, H., Masuda, Y. & Ohmido, N. CRISPR imaging reveals chromatin fluctuation at the centromere region related to cellular senescence. Sci. Rep.13, 14609 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dreissig, S. et al. Live-cell CRISPR imaging in plants reveals dynamic telomere movements. Plant J.91, 565–573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurata, M. et al. Highly multiplexed genome engineering using CRISPR/Cas9 gRNA arrays. PLoS ONE13, e0198714 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma, H. et al. CRISPR-Sirius: RNA scaffolds for signal amplification in genome imaging. Nat. Methods15, 928–931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clow, P. A. et al. CRISPR-mediated multiplexed live cell imaging of nonrepetitive genomic loci with one guide RNA per locus. Nat. Commun.13, 1871 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, Y. et al. Genome oligopaint via local denaturation fluorescence in situ hybridization. Mol. Cell81, 1566–1577 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyu, X.-Y. et al. CRISPR FISHer enables high-sensitivity imaging of nonrepetitive DNA in living cells through phase separation-mediated signal amplification. Cell Res.32, 969–981 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou, Y. et al. Optogenetic control of background fluorescence reduction for CRISPR-based genome imaging. Anal. Chem.94, 8724–8731 (2022). [DOI] [PubMed] [Google Scholar]
- 48.Li, Y. et al. CasSABER for programmable in situ visualization of low and nonrepetitive gene loci. Anal. Chem.95, 2992–3001 (2023). [DOI] [PubMed] [Google Scholar]
- 49.Zhang, Z. et al. Fluorogenic CRISPR for genomic DNA imaging. Nat. Commun.15, 934 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen, M. et al. CRISPR/Pepper-tDeg: a live imaging system enables non-repetitive genomic locus analysis with one single-guide RNA. Adv. Sci.11, 2402534 (2024). [DOI] [PMC free article] [PubMed]
- 51.Zhang, X. et al. Multiplex gene regulation by CRISPR–ddCpf1. Cell Discov.3, 1–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knoot, C. J., Biswas, S. & Pakrasi, H. B. Tunable repression of key photosynthetic processes using Cas12a CRISPR interference in the fast-growing cyanobacterium Synechococcus sp. UTEX 2973. ACS Synth. Biol.9, 132–143 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Choi, S. Y. & Woo, H. M. CRISPRi-dCas12a: a dCas12a-mediated CRISPR interference for repression of multiple genes and metabolic engineering in cyanobacteria. ACS Synth. Biol.9, 2351–2361 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Wu, Y. et al. CRISPR–dCas12a-mediated genetic circuit cascades for multiplexed pathway optimization. Nat. Chem. Biol.19, 367–377 (2023). [DOI] [PubMed] [Google Scholar]
- 55.Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR–Cas system. Cell163, 759–771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fonfara, I., Richter, H., Bratovič, M., Le Rhun, A. & Charpentier, E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature532, 517–521 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Yang, L.-Z. et al. CRISPR-array-mediated imaging of non-repetitive and multiplex genomic loci in living cells. Nat. Methods21, 1646–1657 (2024). [DOI] [PubMed] [Google Scholar]
- 58.Whinn, K. S. et al. Nuclease dead Cas9 is a programmable roadblock for DNA replication. Sci. Rep.9, 13292 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doi, G. et al. Catalytically inactive Cas9 impairs DNA replication fork progression to induce focal genomic instability. Nucleic Acids Res.49, 954–968 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiong, X. et al. Imaging method using CRISPR/dCas9 and engineered gRNA scaffolds can perturb replication timing at the HSPA1 locus. ACS Synth. Biol.12, 1424–1436 (2023). [DOI] [PubMed] [Google Scholar]
- 61.Beuzer, P., Quivy, J.-P. & Almouzni, G. Establishment of a replication fork barrier following induction of DNA binding in mammalian cells. Cell Cycle13, 1607–1616 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Templeton, C. W. & Laimins, L. A. HPV induced R-loop formation represses innate immune gene expression while activating DNA damage repair pathways. PLoS Pathog.20, e1012454 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li, F. et al. R-loops in genome instability and cancer. Cancers15, 4986 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laughery, M. F., Mayes, H. C., Pedroza, I. K. & Wyrick, J. J. R-loop formation by dCas9 is mutagenic in Saccharomyces cerevisiae. Nucleic Acids Res.47, 2389–2401 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antony, J. S., Roberts, S. A., Wyrick, J. J. & Hinz, J. M. dCas9 binding inhibits the initiation of base excision repair in vitro. DNA Repair109, 103257 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barkal, A. A., Srinivasan, S., Hashimoto, T., Gifford, D. K. & Sherwood, R. I. Cas9 functionally opens chromatin. PLoS ONE11, e0152683 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cho, S. et al. High-level dCas9 expression induces abnormal cell morphology in Escherichia coli. ACS Synth. Biol.7, 1085–1094 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Cui, L. et al. A CRISPRi screen in E. coli reveals sequence-specific toxicity of dCas9. Nat. Commun.9, 1912 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wurihan, W., Huang, Y., Weber, A. M., Wu, X. & Fan, H. Nonspecific toxicities of Streptococcus pyogenes and Staphylococcus aureus dCas9 in Chlamydia trachomatis. Pathog. Dis.77, ftaa005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geisinger, J. M. & Stearns, T. CRISPR/Cas9 treatment causes extended TP53-dependent cell cycle arrest in human cells. Nucleic Acids Res.48, 9067–9081 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao, Y., Han, M., Shang, S., Wang, H. & Qi, L. S. Interrogation of the dynamic properties of higher-order heterochromatin using CRISPR–dCas9. Mol. Cell81, 4287–4299 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pi, W. et al. Electroporation delivery of Cas9 sgRNA ribonucleoprotein-mediated genome editing in sheep IVF zygotes. Int. J. Mol. Sci.25, 9145 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richardson, C. D., Ray, G. J., Bray, N. & Corn, J. Non-homologous DNA increases gene disruption efficiency by altering DNA repair outcomes. Nat. Commun.7, 12463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rathbone, T. et al. Electroporation-mediated delivery of Cas9 ribonucleoproteins results in high levels of gene editing in primary hepatocytes. CRISPR J.5, 397–409 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geng, Y. & Pertsinidis, A. Simple and versatile imaging of genomic loci in live mammalian cells and early pre-implantation embryos using CAS-LiveFISH. Sci. Rep.11, 12220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Im, S. H., Jang, M., Park, J.-H. & Chung, H. J. Finely tuned ionizable lipid nanoparticles for CRISPR/Cas9 ribonucleoprotein delivery and gene editing. J. Nanobiotechnol.22, 175 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zuris, J. A. et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol.33, 73–80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu, F. et al. Cryo-shocked tumor cells deliver CRISPR–Cas9 for lung cancer regression by synthetic lethality. Sci. Adv.10, eadk8264 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brocken, D. J., Tark-Dame, M. & Dame, R. T. dCas9: a versatile tool for epigenome editing. Curr. Issues Mol. Biol.26, 15–32 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Wang, C. et al. dCas9-based gene editing for cleavage-free genomic knock-in of long sequences. Nat. Cell Biol.24, 268–278 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu, S. et al. CRISPR–dCas9 mediated cytosine deaminase base editing in Bacillus subtilis. ACS Synth. Biol.9, 1781–1789 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Tasan, I. et al. CRISPR/Cas9-mediated knock-in of an optimized TetO repeat for live cell imaging of endogenous loci. Nucleic Acids Res.46, e100 (2018). [DOI] [PMC free article] [PubMed]