Summary
Background
Proteomic biomarkers for Parkinson’s disease (PD) are critical for identifying new targets for disease-modifying therapies and expanding our understanding of disease pathophysiology.
Methods
Deep proteome analysis of a cerebrospinal fluid (CSF) cohort (40 PD, 40 controls) coupled with previous data from the substantia nigra (SN) proteome was used to discover low abundance biomarkers involved in PD pathogenesis. We validated our findings using parallel reaction monitoring mass spectrometry with an independent cohort of CSF samples (80 PD, 80 controls). We further evaluated our biomarkers with a separate cohort of 80 individuals with Dementia with Lewy Bodies (DLB). We then correlated our candidate biomarkers with motor and cognitive performance.
Findings
We identified 3683 unique proteins, 1425 of them quantified across all 80 discovery samples and 505 that separated PD from controls. Using a stepwise criterion and integrating with 1140 differentially expressed proteins in SN, we identified 34 candidate biomarker proteins. The validation study resulted in 8 proteins (VSTM2A, VGF, SCG2, PI16, OMD, FAM3C, EPHA4, and CCK) with expression patterns and effect sizes like the discovery set. When controlling for age and gender, CCK and OMD maintained their significance and two additional proteins trended toward significance (VGF and PI16, p = 0.057). PI16 and OMD were upregulated in PD, while the others were downregulated. Our investigation is the first to our knowledge to identify PI16 as a possible biomarker and to identify CCK in the CSF of individuals with PD. Combining 4 of the proteins had modest ability to separate PD from controls. CCK and VGF significantly predicted MoCA total scores amongst the DLB group.
Interpretation
These candidate biomarkers add to our understanding of PD pathophysiology and the relationship between PD and DLB. They provide further research directions toward disease-modifying therapies.
Funding
National Institute of Neurological Disorders and Stroke.
Keywords: Parkinson’s disease, Biomarkers, Proteomics, Cerebrospinal fluids, Mass spectrometry
Research in context.
Evidence before this study
We searched PubMed with the search terms “mass spectrometry,” “Parkinson disease,” “cerebrospinal fluid,” and “substantia nigra” for articles published on or before May 27, 2025, in any field. We found a total of 7 articles. No studies combined substantia nigra and cerebrospinal fluid proteomes to identify potential biomarkers. We identified numerous investigations that evaluated CSF and SN proteomes separately. We also performed separate searches of each of our eight identified proteins in PubMed. We did not identify any studies that found VSTM2A and PI16 to be associated with PD, nor did we find any studies with CCK in the CSF. VSTM2A has been found to be associated with Alzheimer’s disease and there is a theoretical relationship between blood-brain barrier permeability and PI16.
Added value of this study
Identification of novel proteins that relate to PD pathophysiology is critical to advancing the field toward disease-modifying therapies. With our unique approach, we were able to identify novel proteins that may be related to PD pathophysiology. These proteins represent potential therapeutic targets, thus opening the door to further investigation.
Implications of all the available evidence
Our investigation is the first to identify two potentially critical biomarkers of PD pathophysiology. We further confirmed the importance of additional proteins, especially the granin family, as being important in PD pathophysiology. The relationship of our potential biomarkers to cognition, and previous research regarding their association with Alzheimer's disease (AD), highlights the importance of co-pathology in the development of PD-related cognitive changes.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease following Alzheimer’s disease (AD).1 Although the cause and mechanisms are still being elucidated, many proteins involved in PD have been identified.2 Discovering additional proteins that are potential biomarkers for PD is critical for improving diagnosis and identifying new targets for disease-modifying therapy. Mass spectrometry-based proteomics is widely used in the unbiased discovery of PD biomarkers, but the sensitivity and specificity of the identified candidate biomarkers precludes clinical utility. Since most regulatory proteins in cells are of low abundance, reliable protein biomarkers in cerebrospinal fluid (CSF) may also be scarce. To date, CSF proteome analysis depth has not been adequate to detect such low abundance proteins. Attempts to increase the proteome depth by depleting abundant CSF proteins can create biases by removing low abundance proteins, which may be strong PD biomarker candidates. A further confounding factor is the significant clinical heterogeneity in PD, and by extension the variability in CSF protein makeup and concentrations. Larger cohorts with standardized processing methods are essential for discovery of reliable PD biomarkers.
The aim of this investigation was to use an unbiased mass-spectrometry approach to identify CSF-based biomarkers for PD while addressing the limitations of previous investigations. For in-depth proteome analysis, we utilized a state-of-the-art mass spectrometer with pre-fractionation of peptides before liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis (Fig. 1) and incorporated data from substantia nigra (SN) samples for the selection of candidate biomarkers. The SN data sought to ensure that the CSF-identified biomarkers are based within the known pathophysiology of PD. The top 34 candidate biomarkers were then analysed in our validation cohort, identifying 8 proteins as potential diagnostic and progression markers of PD and further supporting the inflammatory and insulin-resistance related hypothesis of PD pathophysiology. We also present data on marker concentration in a separate Dementia with Lewy Bodies (DLB) cohort, suggesting that these markers may also be relevant to synuclein-associated cognitive change.
Fig. 1.
Data generation and analysis workflow. CSF and SN samples were processed, fractionated, and run through LC-MS/MS, resulting in potential peptide biomarkers (A). The peptides were then compared between individuals with PD and controls in the CSF and SN, resulting in potential biomarkers that were likely associated with PD pathophysiology (B). The peptides of interest were then validated in a different cohort of individuals with PD and controls using PRM assays (C) followed by statistical analyses to identify key diagnostic markers (D). Created with BioRender.com.
Methods
Study design and cohorts
Ethics
Individuals from both the discovery and validation cohort signed consent forms at their respective institutions. All individuals agreed to have their samples and data shared with qualified investigators.
Discovery
The discovery and selection of candidate biomarkers was based on tandem mass tag-mass spectrometry (TMT-MS) proteomic profiling from a CSF specimen set of 40 PDs and 40 age, gender, and education level matched controls, a subset of the Johns Hopkins (JH) site of the NINDS PDBP,3 chosen based on individuals and visits with the most available CSF. Participants from the JH PDBP were enrolled between 2012 and 2018 and underwent clinical assessments and annual lumbar punctures. Inclusion criteria for PD participants included a UK Brain Bank diagnosis of PD,4 modified to allow first-degree relatives with parkinsonism, with symptoms bothersome enough to warrant symptomatic therapy. Controls could not have a first-degree relative with parkinsonism or essential tremor and a screening Montreal Cognitive Assessment in the normal range (i.e., a score of 26 or greater) CSF was processed according to PDBP standardized operating procedures.5 Findings were further corroborated by cross-comparison with previously reported TMT-MS data from an SN specimen set of 15 PDs and 15 HCs.6
Validation
The validation study included 80 subjects with PD and 80 demographically matched controls. We also analysed 80 CSF samples from individuals with DLB. All validation and DLB CSF were from the BioSEND repository.5 CSF from PD and control subjects were from 5 sites, while the DLB samples were from 14 different sites (Supplementary Table S1). Consequently, we avoided direct comparison of biomarker values between PD or controls and DLB, as the results could be confounded with site-specific differences. All CSF samples were processed per NINDS standardized procedures and sent to BioSEND.
Prior to MS analysis, CSF samples used for validation underwent a single freeze/thaw cycle at BioSEND to allow for aliquoting into 200 μL and then refrozen. This single thaw/freeze cycle is the only known processing difference between discovery and validation samples.
Sample/specimen processing and data generation
Acquisition of untargeted MS data for biomarker discovery
The sample preparation and LC-MS/MS analysis were conducted as described previously with minor modifications.7 Briefly, CSF proteins were lysed in 4 M urea and 50 mM triethylammonium bicarbonate (TEAB), then reduced with 10 mM dithiothreitol for 1 h at room temperature (RT) and alkylated with 30 mM iodoacetamide for 30 min at RT in the dark. The proteins were digested with Lys-C and trypsin and then labelled with 11-plex TMT. The peptides were pooled and pre-fractionated by basic pH reversed-phase liquid chromatography into 24 fractions. The peptide samples were analysed on an Orbitrap Fusion Lumos Tribrid mass spectrometer coupled with an Ultimate 3000 RSLCnano liquid chromatography system (Thermo Scientific). The tandem mass spectrometry data were searched against the human UniProt database (May 2018 release, containing protein entries with common contaminants) using the SEQUEST search algorithm embedded in the Thermo Proteome Discoverer platform (version 2.2, Thermo Fisher Scientific) for protein identification and quantitation.
Acquisition of targeted proteomics data for biomarker validation
The sample preparation and parallel reaction monitoring (PRM)-MS analysis were conducted as described previously with minor modifications.7 Briefly, isotopically labelled peptides (SpikeTides L, JPT Peptide Technologies GmbH, Berlin, Germany) were used for PRM-MS analysis. The CSF proteins were digested using LysC and trypsin. The PRM-MS analysis was conducted on an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific) coupled with Ultimate 3000 RSLCnano liquid chromatography system (Thermo Fisher Scientific) over 60 min.
Previously generated untargeted MS data from SN specimens to support candidate biomarker selection
The descriptions of sample selection criteria, preparation methods, and data analysis have been reported previously.6 Briefly, 15 PD and 15 HC samples were sonicated in 8 M urea/50 mM TEAB, reduced by 10 mM Tris (2-carboxyethyl) phosphine hydrochloride, alkylated by 40 mM chloroacetamide, digested with LysC and trypsin, labelled with three batches of 11-plex TMT, prefractionated by basic pH reversed-phase liquid chromatography, and analysed by LC-MS/MS.
Statistical bioinformatics approaches and considerations
Data generation, preprocessing and analytical quality assessment
The CSF samples (both discovery and validation sets) were randomized and blinded. After the mass spectrometry data were acquired, they were unblinded. PRM-MS analysis was conducted in duplicates, and the means were used in downstream data analysis. To assess PRM-MS assay quality for each candidate biomarker, we estimated the median absolute differences between duplicates divided by the means of the duplicates of all samples to approximate a coefficient of variation (CV). Additionally, we estimated the ratio of mean biomarker standard deviation (SD) among all samples over the mean absolute differences between duplicates, defining this as the signal-to-noise ratio which served as a measurement of the biological information contents relative to analytical variation in data.
Analysis for biomarker discovery using untargeted MS data
Untargeted MS profile data from the CSF and SN specimen sets were independently subjected to univariate differential analyses for discovery, with results cross-compared for corroboration. To identify candidate biomarkers with stable performance among samples within and between sets and/or supported by existing knowledge of protein co-regulatory patterns using criteria: 1) up-regulated in PD patients with high consistency (small SD in bootstrap runs) in either CSF or SN sets; or 2) slightly greater variability in bootstrap analysis but consistent up- or down-regulation cross both CSF and SN sets; and 3) highly connected nodes in differential dependency network analysis.8 Fisher’s exact test for cell-type enrichment analysis was performed by comparing DISCO’s brain cell-type marker gene list (https://www.immunesinglecell.org/) with differentially expressed proteins corresponding to SN data which filtered by adjusted p-value ≤ 0.05 by the Benjamini-Hochberg adjustment method.
Analysis for biomarker validation using targeted data
A primary objective of the validation study was to identify candidate biomarker from discovery that displays the same significant dysregulation patterns. We plotted the individual samples in a biplot with selected biomarkers overlaid on a 2D principal component analysis plot to illustrate the relative relationship and strength of individual biomarkers.
Multivariate predictive models
The PRM targeted data of biomarkers that survived validation were further evaluated as a multivariate predictive model through cross-validation using 60/40 split for their ability in combination to detect PD from controls. The area-under-curves (AUCs) from receiver-operating-characteristic (ROC) analysis estimated. The objective was not to derive a final model for future use but to assess whether the biomarkers are complementary with respect to PD detection.
Role of the funders
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the report.
Results
Sample sets and data generation workflow
The overall data generation and analysis workflow and sample sets involved are illustrated in Fig. 1 for discovery (A & B) using untargeted TMT LC-MS/MS analysis and validation (C & D) using PRM- MS assays.
Study cohort demographic/clinicopathological characterization
Discovery cohort
The demographic and clinical characteristics of individuals in our discovery cohort are listed in Table 1. Individuals with PD and controls were well matched in age, education, and race (p-values all > 0.05). The control population was approximately 52% female while the PD population was approximately 28% female (p = 0.02). Expected differences in clinical characteristics included significantly greater motor, depression, and anxiety symptomatology amongst individuals with PD compared to controls. While our PD cohort had significantly lower Montreal Cognitive Assessment (MoCA) scores (p = 0.05), the mean MoCA scores of both groups were within normal range (≥26).
Table 1.
Discovery cohort demographic and clinical characteristics.
 |
Control and PD participants were well matched on age and education level. There were more female control participants. There were expected clinical assessment differences between the two groups.
MDS-UPDRS: Movement Disorder Society Unified Parkinson’s Disease Rating Scale; LEDD: Levodopa equivalent daily dosing; MoCA: Montreal cognitive assessment.
Validation cohort
The demographic and clinicopathological characteristics of the validation PD, control, and DLB cohorts are listed in Table 2. PD and control groups were well-matched regarding age, gender, education, and race. Expected differences in clinical characteristics, including motor, depression, and anxiety symptomatology, were observed (all p values < 0.05). PD individuals had slightly higher MoCA scores (p = 0.47), though not clinically different (mean of 26.1 versus 25.9). While not statistically compared, DLB individuals were demographically and clinically different from those with PD and controls with regards to age, gender, disease duration, motor symptomatology, cognition, depression, and anxiety.
Table 2.
Validation cohort demographic and clinical characteristic.
 |
MS data generation
Quantitative proteome analysis of CSF samples
For the analysis of 80 CSF samples (40 PD/40 controls) using the 11-plex TMT method, we conducted 8 batches of TMT experiments. The 11th channel of each batch was used for a common reference pool prepared by pooling 80 CSF samples. In total, 7,168,678 MS/MS spectra were acquired, with 878,596 MS/MS spectra assigned to peptides, identifying 42,165 peptides and 3683 proteins (Supplementary Table S2 and Supplementary Data S1). This number of identified proteins reaches ∼76% of ∼4400 proteins annotated in the CSF Proteome Resource as of July 2024—indicating an in-depth CSF proteome analysis.9
Identification of candidate biomarkers
CSF discovery sample set
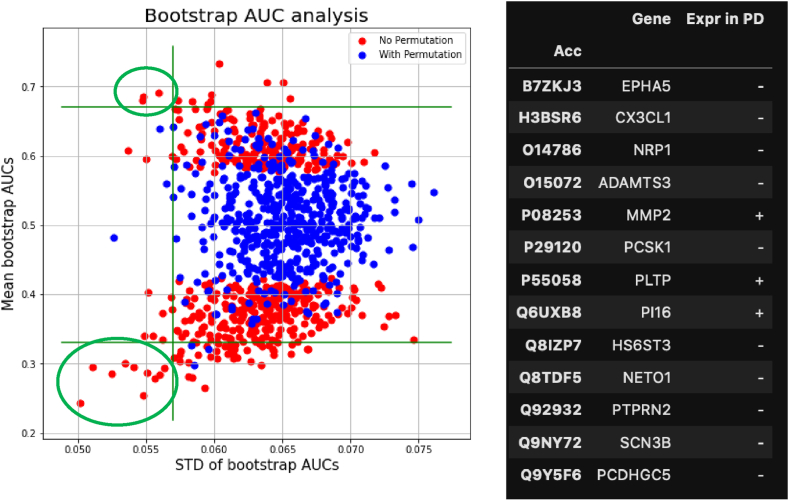
Amongst the 3683 unique proteins, 1452 of them were detected across all 80 samples and pre-tested for their ability to differentiate between PD and control samples using Wilcoxon rank sum test at a p-value cutoff of 0.1. The 505 proteins that passed this selection were analysed in bootstrap ROC analysis to identify candidate biomarkers with statistically stable performance. To balance between number of selections for downstream validation and control for false discovery rate, the criteria that mean AUC > 0.67 (or < 0.23 for down-regulated proteins) and SD AUC < 0.057 were used, resulting in 13 candidate biomarkers that were then put with our SN discovery set (top left and bottom left, circled by green lines) (Fig. 2).
Fig. 2.
Bootstrap ROC analysis to select candidate biomarkers with stable performance against variation in samples. Red dots in the plot are the mean and SD of AUCs from repeated bootstrap samples of the original samples (n = 508). Blue dots are the mean and SD of AUCs from repeated bootstrap samples of label permutated original samples. Green circles are 13 candidate biomarkers that resulted from this analysis.
Integration of CSF and SN discovery set to determine the final discovery set
In the previous publication, the SN analysis revealed 1140 proteins differentially expressed between individuals with PD and controls.6 We extended these results by integrating the CSF data with re-analysis (Supplementary Table S2 and Supplementary Data S2) of the previously published SN data.6 We went through the following steps:
-
1.
List of candidates that were differentiating in both CSF and SN set: BST1, SUSD5, DDAH1, LGALS1, CHGA, EPHA4, NTRK2, NPDC1, LUZP2, HS6ST3, FAM3C, RDX, MDK, THY1, ALDH1A1, SCG2, ADGRB1, PNOC.
-
2.
Highly connected nodes from Differential Dependency Network (DDN) analysis of SN set: RAB8B, DLD, GSPT1, ANXA7, SLC19A1
-
3.
Increased in PDs in CSF set with extremely small variation in bootstrap analysis: MMP2, PLTP, PI16
-
4.
Increased in PDs in SN set with extremely small variation in bootstrap analysis: PCNP, SORT1, TPD52L2, LUC7L2, MT1F, CD63, MCEE, NXT1
These 34 proteins completed our discovery set of biomarkers (Table 3). We then sought to develop a PRM targeted assay for these 34 proteins and validate them.
Table 3.
Up/down expression patterns in CSF and SN of the combined biomarkers.
| Protein ID | PD expr in CSF | p-value in CSF | PD expr in SN | p-values in SN |
|---|---|---|---|---|
| BST1 | + | 0.0028 | + | 0.0033 |
| SUSD5 | – | 0.0048 | + | 0.0190 |
| DDAH1 | – | 0.0033 | + | 0.0211 |
| LGALS1 | + | 0.0022 | + | 0.0027 |
| CHGA | – | 0.0024 | + | 0.0001 |
| EPHA4 | – | 0.0061 | + | 0.0051 |
| NTRK2 | – | 0.0049 | + | 0.0155 |
| NPDC1 | – | 0.0044 | – | 0.1404 |
| LUZP2 | – | 0.0003 | – | 0.0677 |
| HS6ST3 | – | 0.0007 | – | 0.0677 |
| FAM3C | – | 0.0020 | + | 0.0057 |
| RDX | + | 0.0056 | + | 0.0008 |
| MDK | + | 0.0043 | + | 0.0445 |
| THY1 | – | 0.0023 | – | 0.1598 |
| ALDH1A1 | + | 0.0098 | – | 0.0003 |
| SCG2 | – | 0.0091 | + | 0.0211 |
| ADGRB1 | – | 0.0028 | + | 0.0733 |
| PNOC | – | 0.0082 | + | 0.0090 |
| RAB8B | + | 0.00001 | ||
| DLD | – | 0.0475 | + | 0.0002 |
| GSPT1 | + | 0.0002 | ||
| ANXA7 | + | 0.0001 | ||
| SLC19A1 | + | 0.0001 | ||
| MMP2 | + | 0.0029 | ||
| PLTP | + | 0.0021 | – | 0.3779 |
| PI16 | + | 0.0012 | + | 0.1227 |
| PCNP | + | 0.0000 | ||
| SORT1 | + | 0.3308 | + | 0.0000 |
| TPD52L2 | + | 0.0000 | ||
| LUC7L2 | + | 0.0000 | ||
| MT1F | + | 0.0000 | ||
| CD63 | + | 0.0000 | ||
| MCEE | + | 0.0000 | ||
| NXT1 | + | 0.0000 |
p-values from the Mann–Whitney U test (equivalent to testing whether ROC/AUC > 0.5). Not adjusted for multiple testing as these candidates were selected using procedures that take into consideration of multiple testing.
Validation study
Comparison of expression patterns of candidate biomarkers between discovery and validation
Among the 34 selected PD candidate biomarker proteins, 20 proteins were not detectable by PRM-MS. Thus, we added 24 more proteins based on the differential expression levels in PD CSF and 5 more proteins based on the previous publication.10 Among the 29 additional proteins, 18 proteins were detectable by PRM-MS. Therefore, we analysed PRM-MS for 14 proteins initially selected and 18 proteins added later using 80 PD and 80 HC CSF samples (Supplementary Table S3). Among the selected candidate biomarkers, the PRM-MS assay for SCN3B failed to produce meaningful data. For the remaining biomarkers, the PRM assays demonstrated a high analytical precision, with most candidates having CV < 5% and all but 2 of them < 10%. The mean ratio of biological versus analytical SD was 13.7, indicating a high information content.
We then evaluated which of the candidate proteins had similar expression patterns and effect size in the discovery and validation analysis (Supplementary Table S4). This process identified 8 proteins as candidate biomarkers for PD diagnosis (Table 4). Controlling for age and gender, two proteins (CCK and OMD) maintained their significance and two additional proteins trended toward significance (VGF and PI16, both with p = 0.057). PI16 and OMD were upregulated in PD while the others were downregulated relative to controls. We observed significant differences in biomarker expression between male and female subjects. The up/down-regulation expression patterns between PD and controls were retained in male or female. However, the significances were noticeably less in female, potentially due to the smaller number of female subjects in the study (Supplementary Table S5).
Table 4.
Biomarkers with consistent up/down expression trends between PD and controls in the validation set as in the original discovery study at significance < 0.1 (Wilcoxon Rank Sum test).
 |
Among them, PI16 and OMD were up-regulated in PD, while the others were down-regulated in PD, all relative to controls. Adjustment for age, or age and gender were done by including them in logistic regression as covariates.
Principal component analysis (PCA) and exploratory analysis of candidate biomarkers and clinical measurements
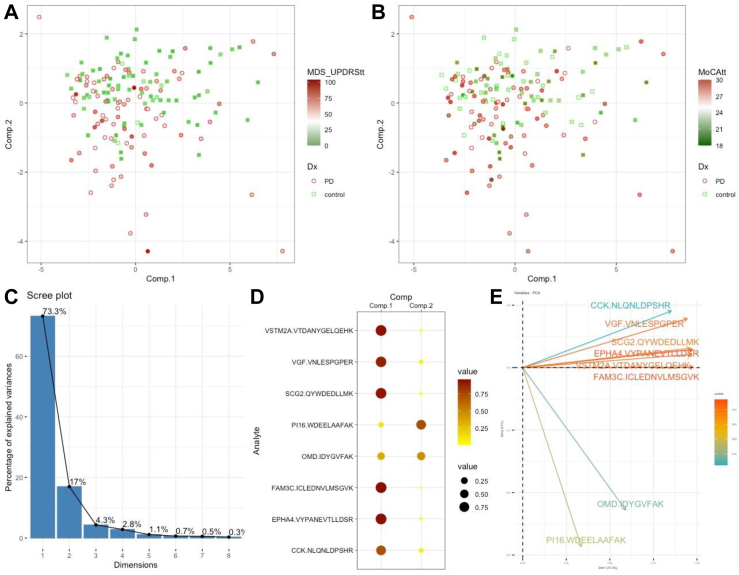
PCA analysis revealed two primary components: component 1 with 6 biomarkers downregulated in PD and component 2 with 2 biomarkers upregulated. Together, components 1 and 2 account for 90.1% of variance (Fig. 3C and D), indicating two components can explain most variance. Although there was a significant overlap between the PD and NMD groups, the samples with high MDS-UPDRS Part III score were better separated on component 2, while they were not separated by MoCA total score (Fig. 3A and B).
Fig. 3.
Principal component analysis using validated biomarkers. A. Distribution of PD (n = 80) and control (n = 80) subjects with subjects shaded by MDS-UPDRS total score. B. Distribution of PD and control subjects with subjects shaded by MoCA total score. C–E. PCA components 1 and 2 account for 90.1% of data variances. PI16 and OMD contributed mostly to component 2 while the other 6 biomarkers contributed in a similar fashion to component 1.
We focused on PI16, OMD, CCK, and VGF based on both their individual discriminatory power (Table 4) and non-redundant complementary values (Fig. 3E) to derive a multivariate index assay model to segregate PD from controls. The individual proteins and multivariate model did not correlate with motor symptom severity, though there was an overall positive correlation (Fig. 4). PI16 and OMD were negatively correlated with MoCA total score while CCK and VGF were positively correlated, but none of these relationships were statistically significant. The combination model had a modest negative correlation but failed to show a significant relationship between the biomarkers and cognitive symptomatology. However, the multivariate model, derived with 60/40 split of training and testing, was statistically significant in differentiating PD from controls (p = 0.0065, Fig. 5) and achieved a ROC/AUC of 0.7 (plot omitted) in testing, indicating a modest ability to separate PD from controls.
Fig. 4.
Pearson correlations between individual proteins listed on the y-axis and motor symptomatology (top row, A–E) and cognitive function (bottom row, F–J). The y-axis labelled “MDL” is a composite of the 4 other peptides listed. Only the relationship between CCK and VGF and cognition in DLB was significant (n = 80 PD, 80 DLB).
Fig. 5.
Data from controls (n = 80) and PD (n = 80) were split 60/40 for training and testing using four proteins PI16, OMD, CCK, VGF with the output of the model indicative of the risk of PD. The boxplot shows the distribution of the model output in the training samples (left) and the testing samples (right). The AUC from ROC analysis is 0.7.
DLB peptide analysis
Although we could not directly compare our biomarkers’ performance between individuals with PD and DLB, we evaluated how each marker relates to clinical performance measures in DLB. Within the DLB group, markers were either unrelated or negatively correlated with motor symptomatology and none of the relationships were significant. CCK and VGF significantly predicted MoCA total score. The multivariate model derived using only PD/control data inversely correlated with MoCA total score (Fig. 4).
Discussion
We conducted PD biomarker discovery and validation using MS-based proteomics on CSF combining with SN data and identified 8 potential PD diagnostic markers, 4 of which may relate to cognition in DLB. We reasoned that if the biomarkers we discovered in the CSF were bona fide PD biomarkers, we could highly likely observe the changes in SN as well. However, we still included candidate biomarkers discovered only in CSF since the SN brain samples are at the terminal stage of PD, in which many dopaminergic neuronal cells died, and thereby, the increased proteins in SN could be predominantly represented by non-dopaminergic neuronal cells. As expected, our analysis of the increased proteins in the PD group of SN with q-value < 0.05 exhibited the enrichment for non-neuronal cells such as oligodendrocytes, astrocytes, microglia, and endothelial cells (Supplementary Fig. S1A). In contrast, our analysis of the decreased proteins in the PD group of SN with q-value < 0.05 exhibited the enrichment for neuronal cells (Supplementary Fig. S1B). Interestingly, the major proteins changed in SN of PD in our previous study were mitoribosome proteins, but only two mitoribosome proteins (RACK1 and RPS3) were detected in the CSF of our discovery experiment. Moreover, none of the proteins exhibited statistically significant changes in CSF of PD patients. This discrepancy could be explained by the fact that CSF was collected at baseline, while SN was obtained from the terminal stage of PD progression, or the mitoribosome changes secreted from SN were diluted or cancelled out by the mitoribosome proteins secreted from other brain regions.
Our results align with previous studies that identified OMD,11 VGF,11 SCG2,12 EPHA4,13 as potential CSF biomarkers for PD versus controls. Among the other 4 proteins, CCK has been previously identified in SN14 and FAM3C has been found to separate DLB from controls15; our work reinforces their roles as a potential synuclein-based markers. The remaining two proteins, PI16 and VSTM2A are novel as PD biomarkers and more research is needed regarding their potential role in PD pathophysiology. Our study is the first to our knowledge to associate CCK, VGF, and a composite score of PI16, OMD, CCK and VGF with worsening cognitive symptoms in DLB.
VGF and SCG2 were identified by us and others as CSF biomarkers for PD and DLB.16 These proteins are part of the granin family, which function in the regulated secretory pathway responsible for delivery of peptides and neurotransmitters, including dopamine.12 VGF is part of the nigrostriatal circuits that are downregulated in PD. VGF levels were previously identified as reduced in early PD but normalized as the disease progresses.17 Our cross-sectional findings reinforced this with a slight, but not significant, VGF increase related to motor symptomatology. SCG2 was previously noted to be reduced in the CSF of PD compared to controls.
EPHA4, a receptor involved in inflammatory responses,18 was also identified by us and others as possible CSF biomarkers for PD.10 The inflammatory response relationship is intriguing because it adds to the evidence of the link between neuroinflammatory pathways and PD pathogenesis.
FAM3C was previously identified as separating individuals with atypical parkinsonism from controls.19 It is an exosomal protein and a possible biomarker for oncologic diseases including non-small cell lung cancer.20 While its relationship to PD has not been elucidated, it is related to gluconeogenesis by suppressing hepatic gluconeogenic gene expression independent of insulin21. More research is needed to better understand this protein’s role in impaired glucose metabolism observed in PD.
While our investigation is the first to our knowledge to identify CCK in the CSF of individuals with PD, it was previously found to be reduced in the SN, but not caudate or putamen.14 This follows other findings that CCK and dopamine are both found in dopaminergic neurons and that CCK modulates dopamine release,22 with recent interest in CCK as a therapy target in both Alzheimer's disease (AD) and PD.22
The remaining two proteins—VSTM2A and PI16—have not previously been associated with PD or PD pathophysiology to our knowledge. The downregulation of VSTM2A aligns with emerging evidence linking VSTM2A to neurodegenerative disorders, including AD, where altered VSTM2A levels have also been observed.23 VSTM2A acts as an innate antagonist of programmed death ligand 1 (PD-L1), modulating immune responses by blocking the PD-1/PD-L1 interaction and promoting T cell activation.24 In the context of PD, reduced VSTM2A expression could lead to enhanced PD-L1/PD-1 signalling, potentially suppressing neuroprotective immune surveillance and contributing to chronic neuroinflammation—a hallmark of PD pathology.24 Similarly, studies in AD have noted dysregulation of neuronal and synaptic proteins, including VSTM2A, suggesting a broader role in maintaining neuronal health and immune balance during neurodegeneration.23,25 The consistent downregulation of VSTM2A in both PD and AD raises the possibility that loss of its immunomodulatory function may be a shared mechanism contributing to disease progression in these disorders. Further research is warranted to clarify whether restoring VSTM2A levels could offer neuroprotective benefits in PD and related neurodegenerative diseases.23,24 PI16 is expressed in the fibroblasts in the epi- and perineum of the peripheral nervous system and the meninges of the central nervous26 system and may increase neuropathic pain by raising the permeability of the blood nerve barrier, resulting in increased immune cell filtration.26 Alterations in the blood brain barrier have been documented as part of the pathophysiology of PD and may contribute to the neuroinflammation observed in the disease. One hypothesis then is that PI16 may increase the blood-brain barrier permeability observed in PD, but this is conjecture based on the role of PI16 elsewhere.
Six of the proteins that we identified have been noted as being related to AD, highlighting the overlap in pathology and pathophysiology between AD and PD. CCK, SCG2, and VGF are possible CSF biomarkers for AD versus controls.13 FAM3C serves as an endogenous suppressor of amyloid-beta production in AD27 and alterations in FAM3C expression may be increase amyloid-beta burden. EPHA4 serves as an amyloid-beta oligomer (ABO) receptor and, when aberrantly activated by the ABO, leads to dendritic spine elimination in the neurons, possibly explaining the synaptic spine alterations observed in AD.28 VSTM2A is differentially expressed gene in AD compared to controls as discussed above.25 These AD-associated proteins identified as PD biomarkers align with the canonical AD biomarkers, tau, phospho-tau, amyloid-beta 42, which predict cognitive decline in PD.29 Further, there is co-pathology observed in autopsy cases with about 30–40% of PD autopsies also demonstrating significant AD-type co-pathology.30 These biomarkers in PD CSF may represent tau-based processes in our PD participants. However, tau and synuclein interact with each other31 and some of our biomarker proteins may represent common pathophysiology between PD and AD.
The significance of CCK, VGF, and our 4 primary proteins with MoCA scores in DLB reinforces the relationship between cognition and our protein biomarkers. These proteins may contribute to or be part of the development of cortical synuclein, tau, or both. More research is needed to clarify this relationship.
The gender differences observed between the different peptide concentrations could be explained by a combination of hormonal, genetic, and neurobiological factors. Oestrogen may play a protective role by influencing dopamine regulation and secretory pathways, wherease sex-specific patterns in neuroinflammation and genetic risk factors could further modulate peptide expression. For example, SCG2 is upregulated in women with PD and linked to neuroprotection and secretory processes.32 A detailed discussion of sex differences in each peptide is beyond the scope of this article but clearly more research is needed. These findings highlight the importance of considering sex differences in biomarker studies and therapeutic strategies for PD.
Although we could not detect 20 out of 34 candidate PD biomarker proteins discovered in the discovery phage, it does not necessarily mean they are less important than the ones detected by PRM-MS. ADGRB1 decreased in dopaminergic neurons of the PD mouse model, which is intoxicated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). AMP-activated protein kinase (AMPK) is predicted to be involved in the downregulation of ADGRB1 in the mouse model.33 Mice lacking full-length ADGRB1 exhibit social deficits, increased seizure susceptibility, and altered brain development, implicating it in neurodevelopmental and neuropsychiatric disorders.34 ANXA7 can induce neuronal apoptosis by affecting glutamate release in rats with subarachnoid haemorrhage (SAH), and downregulation of ANXA7 attenuates early brain injury, suggesting a role in neuronal cell death relevant to neurodegeneration.35 BST1 is known to be associated with the susceptibility of PD36 and is also known to be associated with REM sleep disorder.37 CD63 is a well-established marker for exosomes and is involved in lysosomal pathways.38,39 Considering the emerging role of the lysosome in PD, CD63 could be deeply involved in the PD pathogenesis process.40 NTRK2 is a neurotrophic receptor tyrosine kinase and is a receptor for brain-derived neurotrophic factor (BDNF).41,42 Since BDNF is known to be reduced in the brains of PD patients, NTRK2 could be another promising biomarker for PD.42,43 Little is known about the relevance of the remaining potential biomarker proteins (DDAH1, DLD, GSPT1, LUC7L2, LUZP2, MCEE, MDK, MT1F, NXT1, PCNP, PNOC, RAB8B, RDX, SLC19A1, and TPD52L2) to neurodegenerative diseases. Further study is required to investigate the relevance of these proteins to PD.
This study utilized CSF from two cohorts to identify potential PD biomarkers. CSF limits the sensitivity of MS-based proteomics compared with antibody-based assays because of the high dynamic range of protein abundances.11 By design our biomarkers reflect both the CSF and tissue. While we included DLB findings to demonstrate the relationship between these proteins and neurodegeneration in synucleinopathies, further research is needed to validate these peptides in DLB populations. In addition, both PD-based populations in this research had mid-stage disease; the impact of medication and treatment on our findings cannot be assessed and further research is needed in drug-naive PD populations.
Although our study yielded promising results, several limitations should be acknowledged. We conducted the discovery experiment by prefractionating peptides into 24 fractions based on their hydrophobicity before conducting the LC-MS/MS analysis. On the other hand, we conducted the PRM-MS analysis without prefractionation of the peptides due to the nature of the PRM-MS analysis, increasing peptide complexity. This was the main reason why we could not detect 20/34 proteins in the PRM-MS analysis. One possible way to overcome this limitation is to conduct PRM-MS after immunoprecipitating the target proteins. However, this will need a larger volume of CSFs and optimization of the experiment. Furthermore, this can also cause sample-to-sample variation due to immunoprecipitation bias. Alternatively, enzyme-linked immunosorbent assay (ELISA) or multiplexed antibody-based approaches could be applied.
In conclusion, we applied MS-based proteomics to analyze CSF from PD patients and used autopsy tissue to further ensure the relationship between our findings and PD pathophysiology. We observed interesting biomarker candidates in PD, including two novel biomarkers and a composite of 4 markers that separate PD from controls. Our findings strengthen previous biomarker candidates and identify potential future pharmaceutical targets for PD disease-modifying medications.
Contributors
Z.Z., T.M.D., C.H.N., and L.S.R. designed research; S.O., J.J., Y.J., L.S.R. and C.B. conducted the experiment; S.O., Z.Z., C.B., J.H.K. and C.H.N. performed data analysis. S.O, J.J., Y.J., A.Y.P., T.M.D., C.H.N., J.H.K. and L.S.R. wrote the manuscript; T.M.D., C.H.N., and L.S.R. supervised research. All authors read and approved the final version of the manuscript.
Data sharing statement
Clinical data for the discovery and validation cohort are all available on the NINDS Data Management Resource. Individual Global Unique Identifiers (GUIDS) used for the discovery and validation cohorts will be made available upon request from other researchers. Study protocol and sample processing information are currently available through the DMR and BioSEND repository.
The discovery dataset is accessible on the ProteomeXchange (PX) consortium through the PRIDE repository (PXD055996), under the project titled ‘Discovery and validation of biomarkers for Parkinson’s disease from human cerebrospinal fluid using mass spectrometry-based proteomics analysis’ reviewer_pxd055996@ebi.ac.uk. The validation dataset is available on the PeptideAtlas SRM Experiment Library (PASSEL) with the Dataset Identifier ‘PASS05882’.
Declaration of interests
We have no competing interests to report.
Acknowledgements
This work was supported by an NIH grant (U01NS097049 to T.M.D. and L.S.R.). We acknowledge the NIH High-End Instrumentation grant (S10OD021844, to T.M.D.) for the support of the Orbitrap Fusion Lumos Tribrid mass spectrometer. Data and biospecimens used in preparation of this manuscript were obtained from the Parkinson’s Disease Biomarkers Program (PDBP) Consortium, supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health. Investigators include: Roger Albin, Roy Alcalay, Alberto Ascherio, Thomas Beach, Sarah Berman, Bradley Boeve, F. DuBois Bowman, Shu Chen, Alice Chen-Plotkin, William Dauer, Ted Dawson, Paula Desplats, Richard Dewey, Ray Dorsey, Jori Fleisher, Kirk Frey, Douglas Galasko, James Galvin, Dwight German, Steven Gunzler, Lawrence Honig, Xuemei Huang, David Irwin, Kejal Kantarci, Anumantha Kanthasamy, Daniel Kaufer, Qingzhong Kong, James Leverenz, Allan Levey, Carol Lippa, Irene Litvan, Oscar Lopez, Jian Ma, Lara Mangravite, Karen Marder, Nandakumar Narayanan, Laurie Orzelius, Vladislav Petyuk, Judith Potashkin, Liana Rosenthal, Rachel Saunders-Pullman, Clemens Scherzer, Michael Schwarzschild, Nicholas Seyfried, Tanya Simuni, Andrew Singleton, David Standaert, Debby Tsuang, David Vaillancourt, Jerrold Vitek, David Walt, Andrew West, Cyrus Zabetian, and Jing Zhang. The PDBP Investigators have not participated in reviewing the data analysis or content of the manuscript. Samples from the NINDS BioSEND, which receives government support under a cooperative agreement grant (U24 NS095871) awarded by the National Institute of Neurological Disorders and Stroke (NINDS), were used in this study. We thank contributors who collected samples used in this study, as well as patients and their families, whose help and participation made this work possible.
ChatGPT-4o and ChatGPT3.5 were both used to reduce the word count after the document was fully written. The software was told to reduce each paragraph by anywhere from 5 to more than 20 words without changing content or meaning. Separate paragraphs were then pasted into the text box and the AI output was reviewed line by line, with many but not all the recommended changes incorporated after confirming that the content and meaning had indeed not changed. Perplexity and Perplexity Pro were used to help summarize available data regarding the different peptides identified and to ensure that our research on each peptide was complete. The author(s) have reviewed and confirmed the validity of the text and take(s) full responsibility for the content of the publication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2025.105844.
Contributor Information
Zhen Zhang, Email: zzhang7@jhmi.edu.
Ted M. Dawson, Email: tdawson@jhmi.edu.
Chan Hyun Na, Email: chanhyun@jhmi.edu.
Liana S. Rosenthal, Email: Liana.Rosenthal@jhmi.edu.
Appendix A. Supplementary data
References
- 1.Mhyre T.R., Boyd J.T., Hamill R.W., Maguire-Zeiss K.A. Parkinson’s disease. Subcell Biochem. 2012;65:389–455. doi: 10.1007/978-94-007-5416-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirooznia S.K., Rosenthal L.S., Dawson V.L., Dawson T.M. Parkinson disease: translating insights from molecular mechanisms to neuroprotection. Pharmacol Rev. 2021;73(4):33–97. doi: 10.1124/pharmrev.120.000189. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal L.S., Drake D., Alcalay R.N., et al. The NINDS Parkinson’s disease biomarkers program. Mov Disord. 2016;31(6):915–923. doi: 10.1002/mds.26438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biospecimen collection, processing, and shipment manual Parkinson’s disease biomarker Program (PDBP) study. 2020. https://biosend.org/docs/biospecimens/pd/BioSEND%20Manual%20of%20Procedures%20PDBP%208.24.2020%20with%20appendix.pdf [cited 2024 June 10]; Available from: [Google Scholar]
- 6.Jang Y., Pletnikova O., Troncoso J.C., et al. Mass spectrometry-based proteomics analysis of human substantia nigra from Parkinson’s disease patients identifies multiple pathways potentially involved in the disease. Mol Cell Proteomics. 2023;22(1) doi: 10.1016/j.mcpro.2022.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh S., Jang Y., Na C.H. Discovery of biomarkers for amyotrophic lateral sclerosis from human cerebrospinal fluid using mass-spectrometry-based proteomics. Biomedicines. 2023;11(5) doi: 10.3390/biomedicines11051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B., Li H., Riggins R.B., et al. Differential dependency network analysis to identify condition-specific topological changes in biological networks. Bioinformatics. 2009;25(4):526–532. doi: 10.1093/bioinformatics/btn660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guldbrandsen A., Farag Y., Kroksveen A.C., et al. CSF-PR 2.0: an interactive literature guide to quantitative cerebrospinal fluid mass spectrometry data from neurodegenerative disorders. Mol Cell Proteomics. 2017;16(2):300–309. doi: 10.1074/mcp.O116.064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi M., Movius J., Dator R., et al. Cerebrospinal fluid peptides as potential Parkinson disease biomarkers: a staged pipeline for discovery and validation. Mol Cell Proteomics. 2015;14(3):544–555. doi: 10.1074/mcp.M114.040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karayel O., Virreira Winter S., Padmanabhan S., et al. Proteome profiling of cerebrospinal fluid reveals biomarker candidates for Parkinson’s disease. Cell Rep Med. 2022;3(6) doi: 10.1016/j.xcrm.2022.100661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rotunno M.S., Lane M., Zhang W., et al. Cerebrospinal fluid proteomics implicates the granin family in Parkinson’s disease. Sci Rep. 2020;10(1):2479. doi: 10.1038/s41598-020-59414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastos P., Ferreira R., Manadas B., Moreira P.I., Vitorino R. Insights into the human brain proteome: disclosing the biological meaning of protein networks in cerebrospinal fluid. Crit Rev Clin Lab Sci. 2017;54(3):185–204. doi: 10.1080/10408363.2017.1299682. [DOI] [PubMed] [Google Scholar]
- 14.Studler J.M., Javoy-Agid F., Cesselin F., Legrand J.C., Agid Y. CCK-8-Immunoreactivity distribution in human brain: selective decrease in the substantia nigra from parkinsonian patients. Brain Res. 1982;243(1):176–179. doi: 10.1016/0006-8993(82)91135-0. [DOI] [PubMed] [Google Scholar]
- 15.Tsamourgelis A., Swann P., Chouliaras L., O'Brien J.T. From protein biomarkers to proteomics in dementia with Lewy Bodies. Ageing Res Rev. 2023;83 doi: 10.1016/j.arr.2022.101771. [DOI] [PubMed] [Google Scholar]
- 16.van Steenoven I., Koel-Simmelink M.J.A., Vergouw L.J.M., et al. Identification of novel cerebrospinal fluid biomarker candidates for dementia with Lewy bodies: a proteomic approach. Mol Neurodegener. 2020;15(1):36. doi: 10.1186/s13024-020-00388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocco C., Corda G., Lisci C., et al. VGF peptides as novel biomarkers in Parkinson’s disease. Cell Tissue Res. 2020;379(1):93–107. doi: 10.1007/s00441-019-03128-1. [DOI] [PubMed] [Google Scholar]
- 18.Soliman E., Leonard J., Basso E.K.G., et al. Efferocytosis is restricted by axon guidance molecule EphA4 via ERK/Stat6/MERTK signaling following brain injury. J Neuroinflamm. 2023;20(1):256. doi: 10.1186/s12974-023-02940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magdalinou N.K., Noyce A.J., Pinto R., et al. Identification of candidate cerebrospinal fluid biomarkers in parkinsonism using quantitative proteomics. Parkinsonism Relat Disord. 2017;37:65–71. doi: 10.1016/j.parkreldis.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Wang L.-Z., Soo R.A., Thuya W.L., et al. Exosomal protein FAM3C as a potential novel biomarker for non-small cell lung cancer. J Clin Oncol. 2014;32(15_suppl) in ASCO annual meeting. [Google Scholar]
- 21.Chen Z., Wang J., Yang W., et al. FAM3C activates HSF1 to suppress hepatic gluconeogenesis and attenuate hyperglycemia of type 1 diabetic mice. Oncotarget. 2017;8(62):106038–106049. doi: 10.18632/oncotarget.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reich N., Holscher C. Cholecystokinin (CCK): a neuromodulator with therapeutic potential in Alzheimer’s and Parkinson’s disease. Front Neuroendocrinol. 2024;73 doi: 10.1016/j.yfrne.2024.101122. [DOI] [PubMed] [Google Scholar]
- 23.Modeste E.S., Ping L., Watson C.M., et al. Quantitative proteomics of cerebrospinal fluid from African Americans and Caucasians reveals shared and divergent changes in Alzheimer’s disease. Mol Neurodegener. 2023;18(1):48. doi: 10.1186/s13024-023-00638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Y., Liu J.J., Zhou Y., et al. VSTM2A reverses immunosuppression in colorectal cancer by antagonizing the PD-L1/PD-1 interaction. Mol Ther. 2024;32(11):4045–4057. doi: 10.1016/j.ymthe.2024.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arzouni N., Matloff W., Zhao L., Ning K., Toga A.W. Identification of dysregulated genes for late-onset alzheimer’s disease using gene expression data in brain. J Alzheimers Dis Parkinsonism. 2020;10(6) [PMC free article] [PubMed] [Google Scholar]
- 26.Singhmar P., Trinh R.T.P., Ma J., et al. The fibroblast-derived protein PI16 controls neuropathic pain. Proc Natl Acad Sci USA. 2020;117(10):5463–5471. doi: 10.1073/pnas.1913444117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe N., Nakano M., Mitsuishi Y., et al. Transcriptional downregulation of FAM3C/ILEI in the Alzheimer’s brain. Hum Mol Genet. 2021;31(1):122–132. doi: 10.1093/hmg/ddab226. [DOI] [PubMed] [Google Scholar]
- 28.Vargas L.M., Cerpa W., Muñoz F.J., Zanlungo S., Alvarez A.R. Amyloid-beta oligomers synaptotoxicity: the emerging role of EphA4/c-Abl signaling in Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt A):1148–1159. doi: 10.1016/j.bbadis.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Liu C., Cholerton B., Shi M., et al. CSF tau and tau/Abeta42 predict cognitive decline in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(3):271–276. doi: 10.1016/j.parkreldis.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irwin D.J., Hurtig H.I. The contribution of tau, amyloid-beta and alpha-synuclein pathology to dementia in Lewy body disorders. J Alzheimers Dis Parkinsonism. 2018;8(4) doi: 10.4172/2161-0460.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan X., Uronen R.L., Huttunen H.J. The interaction of alpha-synuclein and Tau: a molecular conspiracy in neurodegeneration? Semin Cell Dev Biol. 2020;99:55–64. doi: 10.1016/j.semcdb.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Cerdan A., Andreu Z., Hidalgo M.R., et al. Unveiling sex-based differences in Parkinson’s disease: a comprehensive meta-analysis of transcriptomic studies. Biol Sex Differ. 2022;13(1):68. doi: 10.1186/s13293-022-00477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi J.S., Bae W.Y., Nam S., Jeong J.W. New targets for Parkinson’s disease: adhesion G protein-coupled receptor B1 is downregulated by AMP-activated protein kinase activation. OMICS. 2018;22(7):493–501. doi: 10.1089/omi.2018.0047. [DOI] [PubMed] [Google Scholar]
- 34.Shiu F.H., Wong J.C., Yamamoto T., et al. Mice lacking full length Adgrb1 (Bai1) exhibit social deficits, increased seizure susceptibility, and altered brain development. Exp Neurol. 2022;351 doi: 10.1016/j.expneurol.2022.113994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Q.S., Wang W.X., Lin Y.X., et al. Annexin A7 induction of neuronal apoptosis via effect on glutamate release in a rat model of subarachnoid hemorrhage. J Neurosurg. 2020;132(3):777–787. doi: 10.3171/2018.9.JNS182003. [DOI] [PubMed] [Google Scholar]
- 36.Li J., Luo J., Liu L., Fu H., Tang L. The association between CD157/BST1 polymorphisms and the susceptibility of Parkinson’s disease: a meta-analysis. Neuropsychiatr Dis Treat. 2019;15:1089–1102. doi: 10.2147/NDT.S190935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mufti K., Yu E., Rudakou U., et al. Novel associations of BST1 and LAMP3 with REM sleep behavior disorder. Neurology. 2021;96(10):e1402–e1412. doi: 10.1212/WNL.0000000000011464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshimura A., Adachi N., Matsuno H., et al. The Sox2 promoter-driven CD63-GFP transgenic rat model allows tracking of neural stem cell-derived extracellular vesicles. Dis Model Mech. 2018;11(1) doi: 10.1242/dmm.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Acunzo P., Hargash T., Pawlik M., Goulbourne C.N., Pérez-González R., Levy E. Enhanced generation of intraluminal vesicles in neuronal late endosomes in the brain of a Down syndrome mouse model with endosomal dysfunction. Dev Neurobiol. 2019;79(7):656–663. doi: 10.1002/dneu.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navarro-Romero A., Montpeyo M., Martinez-Vicente M. The emerging role of the lysosome in Parkinson’s disease. Cells. 2020;9(11) doi: 10.3390/cells9112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z., Simmons M.S., Perry R.T., Wiener H.W., Harrell L.E., Go R.C.P. Genetic association of neurotrophic tyrosine kinase receptor type 2 (NTRK2) with Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147(3):363–369. doi: 10.1002/ajmg.b.30607. [DOI] [PubMed] [Google Scholar]
- 42.Ibrahim A.M., Chauhan L., Bhardwaj A., et al. Brain-derived neurotropic factor in neurodegenerative disorders. Biomedicines. 2022;10(5) doi: 10.3390/biomedicines10051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tessarollo L., Yanpallewar S. TrkB truncated isoform receptors as transducers and determinants of BDNF functions. Front Neurosci. 2022;16 doi: 10.3389/fnins.2022.847572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.