Abstract
Background and Objective
Chromogranin A (CgA) is extensively recognized as a biomarker in neuroendocrine neoplasms (NENs) due to its secretion alongside peptide hormones and biogenic amines in neuroendocrine cells. Despite its widespread clinical use, the reliability of CgA as a diagnostic and prognostic tool remains controversial because of its variable expression in various diseases and the influence of factors such as medication and disease characteristics. This review critically examines the role of CgA beyond neuroendocrine contexts, particularly in gastrointestinal conditions where increased levels may mislead clinical diagnostics.
Methods
This review was conducted by performing a search on the PubMed database regarding CgA and both pathological and non-pathological conditions, excluding NENs.
Key Content and Findings
Conditions such as chronic atrophic gastritis (CAG), proton pump inhibitor usage, and inflammatory bowel diseases (IBDs), among others, can lead to elevated CgA levels, often without any malignant association. Studies reviewed underscore the necessity for cautious interpretation of elevated CgA levels to avoid misdiagnosis and unnecessary anxiety in patients. The review further discusses the implications of non-neuroendocrine diseases contributing to elevated CgA levels, emphasizing the need for improved specificity in testing and a greater awareness among clinicians about the factors influencing CgA levels.
Conclusions
This comprehensive understanding assists in better managing patient outcomes through more accurate diagnosis and appropriate therapeutic interventions.
Keywords: Neuroendocrine tumors, tumor marker, accuracy, chromogranin A (CgA), diagnosis
Introduction
Neuroendocrine neoplasms (NENs) are malignancies arising from the neuroendocrine system’s secretory cells, typically exhibiting slow growth and synthesizing diverse peptide hormones and biogenic amines. Gastroenteropancreatic (GEP) NENs encompass tumors located in the gastrointestinal system and pancreatic NENs, which may exhibit hormonal activity or inactivity based on their origin (1). Chromogranin A (CgA) is a key biomarker in neuroendocrine and endocrine cells, noted for its concomitant release with peptide hormones and biogenic amines (2).
Granins, such as CgA, are essential elements of the secretory mechanism in neuroendocrine cells and are important for several processes, including granulogenesis, protein sorting within secretory pathways, and the maturation and condensation of secretory granules (3) CgA is ubiquitous in all neuronal types and is crucial for producing dense-core granules throughout the diffuse neuroendocrine system. Elevated CgA levels may result from NENs and other illnesses unrelated to neuroendocrine tumors, including benign and malignant diseases (4).
NENs are classified according to their functional status, with 70% deemed non-functional and 30% functional. Among functional pancreatic NENs, insulinomas and gastrinomas are the most common types (5). Conversely, carcinoid syndrome is a disorder usually arising from functional NENs located in the jejunum-ileum, although it might also be present in lung primary NENs (6).
CgA levels are associated with tumor differentiation and disease progression but are inadequate for detecting localized non-functioning tumors due to little production. CgA is frequently utilized for monitoring NENs; nevertheless, its efficacy in assessing disease progression or forecasting therapeutic outcomes is variable and lacks robust data. Detecting elevated CgA levels in individuals lacking a NEN diagnosis may erroneously suggest the necessity of initiating a diagnostic process to identify a neuroendocrine tumor.
This review aims to equip healthcare providers, particularly within the gastroenterology field, with the knowledge to manage elevated CgA levels calmly and without hastily categorizing individuals as cancer suspects when they do not have a NEN diagnosed. It highlights that such elevations are often linked to gastroenterological conditions, underscoring the importance of careful and informed patient handling in these scenarios. We present this article in accordance with the Narrative Review reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-113/rc).
Methods
The literature search was conducted using the PubMed database, limiting the results to articles published in English without any time constraints. The search focused on the keyword chromogranin A and its association with diseases or physiological conditions. This review was not conducted using the methodological approach of a systematic review but rather that of a narrative review.
The research was carried out independently by two authors (F.P., E.R.), and the search results were manually compared. In case of discrepancies, a third author (D.C.) was involved to determine whether the article was relevant to the research objectives.
The details of the search strategy for this narrative review are provided in Table 1.
Table 1. Search strategy summary.
| Items | Specification |
|---|---|
| Dates of search | June 2024 to August 2024 |
| Database searched | PubMed |
| Search terms used | “Chromogranin A” AND “Proton Pump Inhibitors” OR “Chronic Atrophic Gastritis” OR “Helicobacter Pylori” OR “Gastrointestinal diseases” OR “Inflammatory Bowel Disease” OR “Renal Dysfunction” OR “Heart failure” OR “Pregnancy” OR “Endocrine Disorders” OR “Neurological Diseases” OR “Drugs” OR “Tumors” |
| Timeframe | From inception to August 2024 |
| Inclusion criteria and exclusion criteria | Inclusion criteria: all study types, published in English |
| Exclusion criteria: articles related to neuroendocrine tumors | |
| Selection process | The literature search was conducted by F.P. and E.R.. In case of discrepancies, a third author (D.C.) was involved |
Gastrointestinal causes of increased CgA
CgA and chronic atrophic gastritis (CAG)
Gastric mucosa atrophy occurring in CAG involves the thinning or loss of the stomach’s original glands, often replaced by altered gland types or fibrosis. When this occurs in the stomach’s corpus area, the resulting decrease in parietal cells compromises the production of gastric acid and intrinsic factor. This impairment can lead to the poor absorption of iron and vitamin B12, causing anemia. Additionally, the alteration in the stomach’s internal environment increases the risk of developing gastric cancer. Persistent stimulation of gastrin-producing cells in the antrum can promote hyperplasia of endocrine-like cells, potentially leading to type 1 gastric NEN and elevation of plasma CgA levels (7,8).
Peracchi et al. (9) evaluated plasma CgA levels as a potential marker for detecting gastric NENs in patients with autoimmune CAG. Plasma CgA levels were assessed using an enzyme-linked immunosorbent assay (ELISA) kit among three groups: 45 healthy volunteers, 9 patients with type 1 gastric NENs, and 43 CAG patients, some exhibiting enterochromaffin-like (ECL) cell hyperplasia/dysplasia.
Results highlighted that CgA levels were significantly elevated in CAG patients compared to healthy controls, with even higher levels in those with gastric NENs and ECL cell hyperplasia/dysplasia. In CAG patients without NEN, CgA levels positively correlated with the presence and severity of ECL cell lesions. Despite these correlations, the study found a considerable variation in CgA levels among CAG patients based on their ECL cell status, underscoring a complex interaction between CgA expression and ECL cell activity.
The sensitivity of the CgA assay for identifying patients with type 1 gastric NENs was 100%, indicating that all patients with gastric NENs had elevated CgA levels. However, the specificity was notably low at 23%, suggesting that elevated CgA levels were also common in many CAG patients without NENs. This low specificity limits the clinical utility of CgA as a standalone diagnostic tool for identifying gastric NENs in CAG patients.
The study by Campana et al. (10) explored the pattern of CgA plasma levels across a broad patient cohort, including 238 individuals with NEN, 42 patients with CAG both with and without ECL cell hyperplasia, and 48 healthy participants. Diagnostic assessments included routine biochemical check-ups, imaging techniques, endoscopy, and histological evaluations.
Key findings from the research revealed that CgA plasma levels were significantly higher in NEN patients compared to those with CAG or healthy participants (P<0.001). More specifically, CgA levels were elevated in NEN patients exhibiting diffuse disease over those with localized or hepatic involvement (P<0.001). Additionally, patients with Zollinger-Ellison syndrome displayed notably higher CgA levels than those with other types of NENs (P<0.001).
The study established a CgA cut-off range of 18 to 19 U/L between healthy participants and NEN patients, achieving an 85.3% sensitivity and a 95.8% specificity. For distinguishing all non-neoplastic participants (healthy participants, CAG patients, and disease-free patients) from those with NENs, the optimal cut-off range was identified as 31 to 32 U/L, resulting in a 75.3% sensitivity and an 84.2% specificity. To maximize specificity at 95%, a higher cut-off range of 84 to 87 U/L was necessary, though this reduced the sensitivity to 55%.
The study’s conclusion underscores the effectiveness of using CgA plasma levels as a diagnostic tool for NENs, emphasizing the need for specific cut-off values to differentiate NENs from non-neoplastic conditions that may also elevate CgA levels.
The study by Sanduleanu et al. (11) reported that, in patients with CAG, especially those who are Helicobacter pylori (H. pylori)-positive and undergoing PPI therapy, there’s a clear correlation between elevated serum CgA and the severity of gastritis features. For instance, the study found that the prevalence of body gland atrophy was significantly higher in H. pylori-positive patients on PPIs compared to H. pylori-negative patients—53.5% versus 2.7%, respectively. Additionally, ECL cell hyperplasia was notably more prevalent in the H. pylori-positive, PPI-treated group (41.9%) compared to H. pylori-negative subjects (5.4%). These statistics highlight the interaction between H. pylori infection, acid-suppressive therapy, and increased serum CgA levels, reflecting both glandular atrophy and ECL cell proliferation.
Based on this evidence, it is clear that elevated CgA levels are more frequently linked to benign conditions such as CAG rather than NENs. This underscores the non-specific nature of CgA increases in gastric conditions, often appearing in various non-malignant disorders. Thus, an elevated CgA typically suggests benign gastric issues like CAG over NENs, highlighting the importance of careful interpretation and further diagnostic measures, such as endoscopy, to accurately determine the underlying condition. Although CgA levels rise with the histological severity of ECL cell lesions in CAG, its specificity for diagnosing gastric NENs is limited.
CgA and proton pump inhibitors
The effect of gastric acid suppression on plasma CgA levels was reported more than 25 years ago. The case-control study by Sanduleanu et al. (12) investigated gastrin and CgA serum levels in dyspeptic patients undergoing medium to long-term gastric acid suppressive therapy. Key findings of that study include patients on PPIs show significantly higher levels of these markers compared to those on H2-receptor antagonists or untreated controls. Elevated serum gastrin and CgA correlated with the degree and duration of acid inhibition, highlighting a potential risk for gastric ECL cell hyperfunction or proliferative changes.
It has been reported that CgA levels may rise even after a short period of PPI therapy; the study by Mosli et al. (13) explored how quickly CgA levels increased upon initiating PPI therapy and the duration required after stopping PPI for CgA levels to normalize. The experiment involved 17 healthy subjects who were administered lansoprazole 30 mg daily for 7 days, with CgA levels measured before, during, and after discontinuation of PPI. Results indicate a significant increase in CgA during PPI use across all assays used, with levels beginning to normalize within a week of discontinuing PPI. However, a two-week cessation was recommended for CgA levels to return to baseline fully.
This finding was further confirmed by other reports, strongly supporting the evidence of a direct influence of PPIs on plasma CgA levels (14-16).
Based on this evidence, it is clear that the use of PPI for conditions entirely different from the presence of a NEN, such as dyspepsia or gastroesophageal reflux, induces an early and significant increase in plasma CgA levels.
It has been shown that Vasostatin-1 (VS-1), a fragment derived from CgA, has a higher specificity compared to total CgA in diagnosing NENs, as conditions like proton-pump inhibitor use do not elevate it (17). Based on this, VS-1 could be a more reliable marker for diagnosing and monitoring NENs, potentially offering a more standardized and accurate approach for clinical assessments; however, VS-1 is still not commonly used in clinical practice.
CgA and H. pylori infection
Elevated CgA levels are observed in patients with H. pylori infection, aligning with increases seen in CAG cases, both with and without ECL cell hyperplasia. For patients with H. pylori, increased CgA levels are often seen due to the infection’s effect on gastric mucosa, leading to an environment conducive to neuroendocrine changes and potentially higher CgA secretion (10).
In patients with H. pylori infection treated with PPIs, the CgA levels are considerably elevated. For example, the study by Sanduleanu et al. reported that, in H. pylori-positive patients on PPIs, the median CgA level was observed at a higher range (going up to 26.1 nmol·L−1) compared to H. pylori-negative individuals, where the median CgA level was around 3.7 nmol·L−1. This notable increase indicates a significant enhancement of gastric ECL cell activity in response to the dual impact of H. pylori infection and prolonged acid suppression therapy (11).
This confirms that in H. pylori infection, regardless of CAG presence, alterations in ECL cells, or potential NEN presence, CgA levels can be altered.
CgA and inflammatory bowel disease (IBD)
In patients with IBD, CgA elevation is driven by inflammation-induced activation of neuroendocrine cells in the gastrointestinal tract. These cells, normally responsible for secreting various neuroendocrine markers, including CgA, become hyperactive during inflammatory states, thus increasing CgA output. CgA plays a crucial role in modulating inflammation itself; it inhibits the activation of endothelial cells, which are central to vascular responses in inflammation, and reduces blood vessel permeability that inflammatory cytokines like tumor necrosis factor (TNF)-α can exacerbate. This modulation helps mitigate vascular complications associated with inflammation. Elevated CgA levels—observed in about 30% to 50% of IBD patients with active disease—also potentially serve as a feedback mechanism to control excessive inflammation, thereby protecting the gut mucosa from further damage and marking its utility as a biomarker for monitoring disease activity (18).
The study by Sciola et al. (19) focused on evaluating plasma CgA levels in 119 patients with IBD, including those diagnosed with ulcerative colitis and Crohn’s disease, and compared these levels to those of 85 healthy controls. The results revealed that IBD patients had significantly higher mean CgA levels compared to controls, specifically 20.4 versus 11.3 U/L. Elevated CgA levels were detected in 55% of patients with active IBD and 24% in remission. Furthermore, CgA levels correlated with the activity of the disease and were linked with serum TNF-α levels, suggesting a connection between CgA and inflammation. Based on these findings, CgA has previously been proposed as a potentially reliable marker of disease activity in IBD patients (20), although it has not been incorporated into clinical practice for evaluating these patients.
An increase in neuroendocrine cells was also observed in pouch mucosa of patients with ulcerative colitis undergoing proctocolectomy (21). The study by Giuffrida et al. identified a marked increase in CgA and serotonin-positive cells in the pouch mucosa of patients with ulcerative colitis. Specifically, the number of CgA-positive cells per 100 crypt cells was significantly higher in the pouch mucosa (median 13.0%, 25th–75th percentile: 11.0–15.7%) compared to control ileum (median 6.6%, 25th–75th percentile: 5.1–8.8%). Similarly, serotonin-positive cells were significantly more abundant in the pouch mucosa compared to controls, underscoring the pronounced neuroendocrine changes associated with inflammatory bowel conditions.
CgA and other gastrointestinal diseases
Elevated CgA levels have also been reported in different liver diseases. Specifically, CgA above the upper reference limit was observed in 32% of patients with chronic hepatitis and in 50% of patients with cirrhosis (22). The median CgA levels were 15.3 U/L for chronic hepatitis and 26.4 U/L for cirrhosis, indicating a notable increase in the progression of liver disease severity. This elevation in CgA levels reflects the potential involvement of neuroendocrine components in the pathology of chronic liver conditions.
It has also been reported that, in the setting of non-alcoholic fatty pancreas dyspeptic patients, CgA levels in the duodenal mucosa were significantly elevated in the pancreatic fibrotic group compared to the non-fibrotic group, with a notable increase observed in those with pancreatic fibrosis (P=0.001) (23).
CgA levels have been studied even in subjects with irritable bowel syndrome (24), with one study funding transiently elevated CgA levels in diarrhoea-predominant IBS (25).
Finally, serum CgA levels were reported to be elevated in patients with refractory celiac disease (26), reflecting an increase in serotonin-producing neuroendocrine cells within the duodenal mucosa. This elevation of CgA underscores its potential role in contributing to the persistent inflammatory response characteristic of refractory celiac disease.
Non-gastrointestinal causes of increased CgA
CgA and renal dysfunction
The study by Hsiao et al. (27) highlighted that CgA levels correlate significantly with the severity of renal impairment, as indicated by serum creatinine levels. Specifically, as renal function deteriorates, CgA concentrations increase correspondingly. For instance, in patients with mid-range renal disease (serum creatinine between 2 and 7.5 mg/dL) and those with end-stage renal disease (serum creatinine greater than 7.5 mg/dL), there is a notable escalation in plasma CgA levels. This correlation underscores the kidney’s role in clearing CgA, with impaired renal function leading to its accumulation in the plasma. Thus, when assessing CgA as a marker for neuroendocrine activity or disease, it is crucial to consider the patient’s renal status and creatinine levels to avoid diagnostic inaccuracies.
Elevated CgA levels have also been observed in diabetic patients with early renal damage, even if with normal creatinine values. In the study by Yu et al. (28), the serum CgA levels in diabetic patients were investigated to evaluate its utility in diagnosing early diabetic nephropathy. It categorized 219 type 2 diabetes mellitus patients into groups based on urine albumin to creatinine ratios: normoalbuminuria, microalbuminuria, and macroalbuminuria, reflecting the progression of diabetic nephropathy. The findings revealed that serum CgA levels correlate with urine albumin to creatinine ratios and increase progressively with the severity of diabetic nephropathy. Elevated serum CgA levels were noted even before significant elevations in creatinine, suggesting that CgA could serve as an early biomarker for diabetic nephropathy, independent of creatinine levels, which traditionally indicate later stages of renal impairment.
It has been demonstrated that serum concentrations of CgA correlate strongly with the estimated glomerular filtration rate in patients with chronic kidney disease, demonstrating an inverse relationship where CgA levels increase as estimated glomerular filtration rate (eGFR) decreases (29). This correlation suggests that CgA levels are influenced by renal function, necessitating GFR-dependent reference limits for accurate interpretation in clinical assessments of chronic kidney disease patients.
CgA and heart failure
In heart diseases, CgA levels are elevated, reflecting changes in sympathetic tone and activity of the adrenomedullary system. Increased plasma CgA is associated with coronary artery disease, heart failure, and hypertension, linking it to cardiovascular mortality and adverse cardiac events (30). Alterations in CgA are linked to its role in neuroendocrine modulation of cardiovascular function. The increase in CgA levels in heart conditions such as heart failure is primarily due to its hyperglycosylation in the myocardium, which impairs the conversion of CgA to its active peptide fragments. This disrupted processing impacts the myocardial and coronary function, exacerbating heart failure progression (31). In heart diseases, alterations in CgA levels are notably linked to cardiac dysfunction and the overall stress response from the sympathetic nervous system. Goetze et al. (32) showed that patients with heart failure and higher CgA levels had a significantly increased risk of mortality. Specifically, the hazard ratio for cardiovascular mortality was 5.35 when CgA levels were above the median, with a 95% confidence interval of 1.74–16.43, highlighting its potential utility as a prognostic marker in cardiovascular conditions.
CgA and pregnancy
During pregnancy, the placenta, an endocrine organ with a crucial role in the hormonal and physiologic changes aimed at maintaining gestation and fetal growth, produces a variety of hormones and molecules, including CgA. As outlined in a notable review by Bralewska et al., CgA levels increase with the course of gestation, possibly indicating a potential role of CgA in the preparation of maternal tissue for delivery (33). Although its influence in the gestational period has not yet been well established, it’s important to highlight that an elevation of levels of CgA is expected in pregnant women.
CgA and hyperthyroidism/hyperparathyroidism
An elevation of the CgA levels has also been observed in endocrine disorders, such as hyperthyroidism and hyperparathyroidism. A study by Al-Shoumer et al. found that untreated hyperthyroid patients exhibit significant elevation in CgA levels, with a positive correlation with the severity of the condition (34). The exact mechanism of the elevation in CgA levels is not well known. Still, it is thought to result from the interaction of thyroid hormones with the sympathetic nervous system, generating a sympathovagal imbalance.
CgA and neurological disease
CgA is present in both the peripheral and the central nervous system. Thus, it has been studied as a potential marker of various neurological diseases, such as Alzheimer’s disease, Amyotrophic Lateral Sclerosis, and Parkinson’s disease, since CgA levels may be altered in serum and cerebrospinal fluid. However, studies concerning serum CgA in Parkinson’s disease produced contradictory results, with some showing a positive correlation between the serum levels of CgA and the severity of Parkinson’s disease (35) and others showing lower levels of CgA (36).
Moreover, several studies have shown a positive correlation between anxiety/depression and the level of CgA. A study by Li et al., including 263 healthy workers divided into two groups (anxiety/no-anxiety groups or depression/no-depression groups), showed that the anxiety and depression group had significantly higher plasma CgA levels than that in the no-anxiety group (P<0.001) (37). This outlines the possible complex relationship between CgA levels and some neurological diseases.
CgA and other drugs
Apart from PPI, the relationship between CgA levels and other drugs has been poorly investigated, especially in specific settings rather than in large studies.
Steroid treatment or glucocorticoid excess, which can lead to up-regulation of CgA mRNA, may increase the concentration of CgA by about two times (38).
As far as antidepressants are concerned, a case report identified increased CgA levels with carcinoid syndrome-like symptoms in a patient treated with duloxetine (39). Serotonin reuptake inhibitor treatment may also increase the secretion of hormones and neuropeptides, including CgA, determining false positive levels. However, to our knowledge, a clear association between this class of drugs and CgA levels has not been described in the literature and needs to be further evaluated.
CgA and non-neuroendocrine tumors
As reported by the study of Uhlig et al. (40), CgA is elevated in various non-NENs, as seen in 6.3% of 9,237 analyzed tumors. Besides basal cell carcinomas of the skin, where 50% were positive for CgA, and adrenocortical carcinomas, with a positivity of 91.7%, other notable expressions were observed in adenocarcinomas from different origins. For example, adenocarcinomas from the female genital tract showed an 18.9% positivity rate for neuroendocrine differentiation, while those from the pancreatic-/hepato-/biliary tract and prostate had positivity rates of 15.8% and 14.9%, respectively. Despite the detectable expression of CgA across these tumor types, the study concluded that elevated CgA levels were not correlated with aggressive tumor features or poor outcomes, suggesting its limited clinical significance in the prognostic assessment of these cancers.
Other studies have reported that CgA may be elevated in non-NENs (41-43), including breast cancer (47.2% positivity), lung cancer (52.4%), and gastrointestinal cancer (35.7%). Additionally, other cancers like gynecological (58.3%), genitourinary (66.6%), hematological (100%), and head and neck cancers (75%) also showed significant elevations in CgA levels. This broad expression across various tumor types underscores the potential of CgA as a marker for neuroendocrine differentiation in non-neuroendocrine tumors, although its clinical significance in these contexts remains to be fully understood. Therefore, when considering the role of CgA as a specific marker for NENs, it is important to keep in mind that numerous other non-NENs can express elevated levels of CgA. In this context, the marker must be evaluated with great caution in the attempt to attribute a neuroendocrine nature to a neoplasm for which a histological confirmation is not yet available.
Tips for proper interpretation of CgA in clinical practice
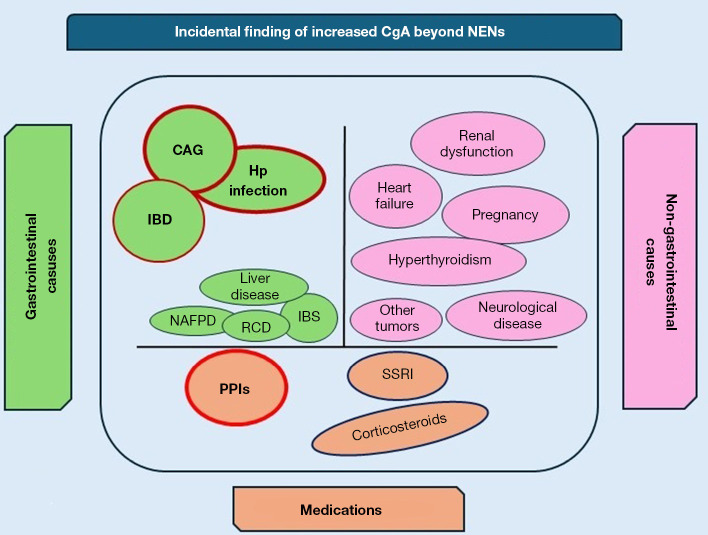
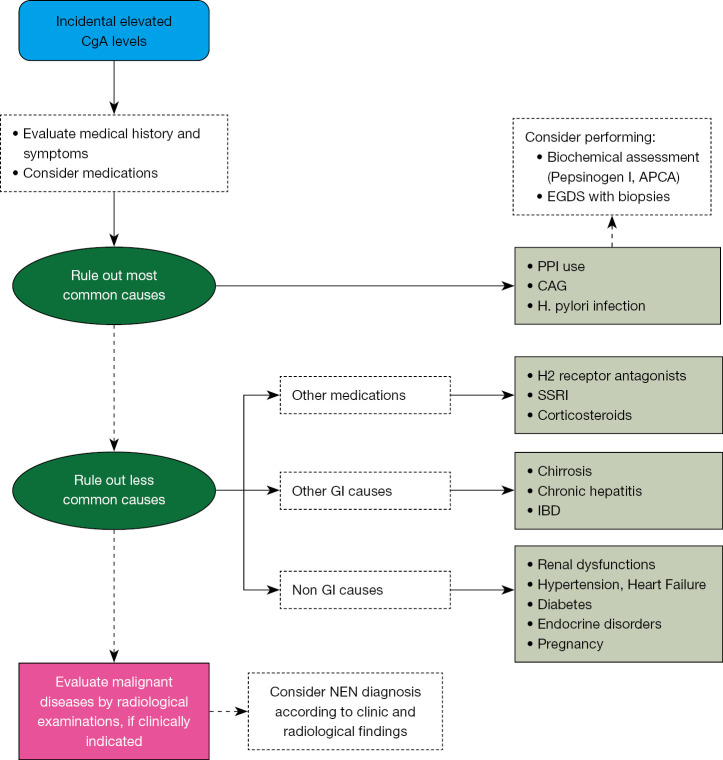
Despite CgA being proposed for several years as the most accurate tumor marker for the diagnosis, prognostic assessment, and monitoring of disease progression during medical therapy in patients with NENs, its role has progressively diminished over recent years due to increased awareness of the vast number of conditions other than NENs that can cause a false positive result (44) (Figure 1). Like most so-called tumor markers, CgA should never be used as a screening method or in any other clinical context where a NEN diagnosis is not already established, as the risk of a false positive is extremely high (Table 2). This could trigger an incorrect diagnostic path, causing anxiety in patients who might fear they have a NEN, and leading to the unnecessary use of instrumental and economic resources aimed at ruling out a diagnostic suspicion based solely on CgA. When faced with an incidental finding of elevated CgA levels, a thorough medical history, including an assessment of the medications the patient is taking, is often sufficient to justify the detected values. As a second step, assessing the presence of an underlying gastric pathology, particularly CAG, is advisable by measuring parietal cell antibodies, pepsinogen I, and anti-H. pylori antibodies, and considering the performance of a gastroscopy with chromoendoscopy and multiple biopsies, which is currently considered the diagnostic standard for identifying patients with CAG (45) (Figure 2). Recent guidelines from ENETS do not include CgA in the diagnostic pathway for NENs but limit its use to monitoring the disease when the diagnosis is already confirmed (46-48). From a purely probabilistic perspective, it is crucial to note that the overall prevalence of NENs is less than 30 per 100,000 individuals (49). In contrast, benign conditions unrelated to NEN diagnosis, which can also lead to elevated levels of CgA, are significantly more common. For instance, CAG has a prevalence that varies greatly—up to 27%—depending on the study methodology used (7). This condition is quite prevalent in the adult population. H. pylori infections affect approximately 20–60% of individuals (50), and the use of PPIs is estimated to range between 14% and 25% of the adult population in some European countries (51,52). IBDs show a prevalence of about 0.3% (53). These conditions, all of which are more common than NENs, can lead to an increase in CgA levels (Table 3).
Figure 1.
Incidental finding of increased CgA beyond NENs. CAG, chronic atrophic gastritis; CgA, chromogranin A; Hp, Helicobacter pylori; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; NAFPD, non-alcoholic fatty pancreas disease; NEN, neuroendocrine neoplasm; PPIs, proton pump inhibitors; RCD, refractory celiac disease; SSRI, selective serotonin reuptake inhibitor.
Table 2. Do’s & Don’ts when interpreting chromogranin A level.
| Do’s |
| Interpret with context: always consider patient history, especially medication use and gastrointestinal conditions, before diagnosing based on elevated chromogranin A levels |
| Evaluate gastric conditions first: when chromogranin A is elevated, first evaluate for benign gastrointestinal conditions, such as chronic atrophic gastritis or the use of proton pump inhibitors |
| Utilize multidisciplinary approaches: engage with other specialties to improve diagnostic accuracy, avoiding unnecessary neuroendocrine neoplasm assessments |
| Don’ts |
| Avoid using chromogranin A as a screening tool for NENs: elevated chromogranin A alone is insufficient for diagnosing NENs due to false positives from non-malignant conditions |
| Don’t overlook renal function impact: elevated chromogranin A can result from renal impairment; assess kidney health to avoid misleading conclusions |
NEN, neuroendocrine neoplasm.
Figure 2.
Proposed algorithm for the interpretation of incidental elevated CgA levels. APCA, anti-parietal cells antibodies; CAG, chronic atrophic gastritis; CgA, chromogranin A; EGDS, esophagogastroduodenoscopy; GI, gastrointestinal; IBD, inflammatory bowel disease; NEN, neuroendocrine neoplasm; PPI, proton pump inhibitor; SSRI, selective serotonin reuptake inhibitors.
Table 3. Causes of CgA elevation.
| Conditions | Clinical scenario |
|---|---|
| Non-oncological conditions | |
| Medications | PPI† (14–25%); H2RAs; SSRI; corticosteroids |
| GI disease | CAG† (27%); Hp infection† (20–60%); IBD† (0.3%); IBS; chronic hepatitis; cirrhosis |
| Non-GI disease | Renal dysfunction; CV diseases; endocrine diseases |
| Oncological conditions | |
| GI neoplasia | GI-NET; tumors of the pancreatic-/hepato-/biliary tract |
| Non-GI neoplasia | Basal cell carcinoma; adrenocortical carcinoma; prostatic cancer; breast cancer; lung cancer; genitourinary cancer; hematological cancer; head and neck cancers |
†, most common causes. In brackets, the estimated prevalence of conditions in the general population. CAG, chronic atrophic gastritis; CV, cardiovascular; CgA, chromogranin A; GI, gastrointestinal; H2RAs, H2 receptor antagonists; Hp, Helicobacter pylori; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; NET, neuroendocrine tumor; PPI, proton pump inhibitor; SSRI, selective serotonin reuptake inhibitor.
Conclusions
Numerous frequent clinical conditions, completely devoid of pathological significance or otherwise attributable to benign conditions that have nothing to do with a NEN, can significantly increase CgA levels. Among these, the most frequent causes are the use of PPIs, CAG, and IBDs. For this reason, the gastroenterological community should be adequately informed about the possibility that an incidental finding of increased CgA may be related to one of these conditions, contributing to the multidisciplinary management of NENs, now considered a quality criterion in providing care to these patients (54), to avoid initiating specific diagnostic pathways for suspected NEN that could lead to unjustified anxiety in patients, and unnecessarily consume health and economic resources.
More innovative approaches through the use of new biomarkers, primarily the NETest (55,56), a multi-analyte blood biomarker, will likely be the future in the landscape of tumor markers in NENs.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-113/rc
Funding: Manuscript published thanks to the Sapienza University Grant Atenero 2023 RM123188F2A0536B.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-113/coif). The authors have no conflicts of interest to declare.
References
- 1.Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin 2018;68:471-87. 10.3322/caac.21493 [DOI] [PubMed] [Google Scholar]
- 2.Bevere M, Masetto F, Carazzolo ME, et al. An Overview of Circulating Biomarkers in Neuroendocrine Neoplasms: A Clinical Guide. Diagnostics (Basel) 2023;13:2820. 10.3390/diagnostics13172820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modlin IM, Gustafsson BI, Moss SF, et al. Chromogranin A--biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol 2010;17:2427-43. 10.1245/s10434-010-1006-3 [DOI] [PubMed] [Google Scholar]
- 4.Gorr SU, Jain RK, Kuehn U, et al. Comparative sorting of neuroendocrine secretory proteins: a search for common ground in a mosaic of sorting models and mechanisms. Mol Cell Endocrinol 2001;172:1-6. 10.1016/S0303-7207(00)00342-7 [DOI] [PubMed] [Google Scholar]
- 5.Magi L, Marasco M, Rinzivillo M, et al. Management of Functional Pancreatic Neuroendocrine Neoplasms. Curr Treat Options Oncol 2023;24:725-41. 10.1007/s11864-023-01085-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grozinsky-Glasberg S, Davar J, Hofland J, et al. European Neuroendocrine Tumor Society (ENETS) 2022 Guidance Paper for Carcinoid Syndrome and Carcinoid Heart Disease. J Neuroendocrinol 2022;34:e13146. 10.1111/jne.13146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahner E, Zagari RM, Zullo A, et al. Chronic atrophic gastritis: Natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig Liver Dis 2019;51:1621-32. 10.1016/j.dld.2019.09.016 [DOI] [PubMed] [Google Scholar]
- 8.Lamberti G, Panzuto F, Pavel M, et al. Gastric neuroendocrine neoplasms. Nat Rev Dis Primers 2024;10:25. 10.1038/s41572-024-00508-y [DOI] [PubMed] [Google Scholar]
- 9.Peracchi M, Gebbia C, Basilisco G, et al. Plasma chromogranin A in patients with autoimmune chronic atrophic gastritis, enterochromaffin-like cell lesions and gastric carcinoids. Eur J Endocrinol 2005;152:443-8. 10.1530/eje.1.01862 [DOI] [PubMed] [Google Scholar]
- 10.Campana D, Nori F, Piscitelli L, et al. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol 2007;25:1967-73. 10.1200/JCO.2006.10.1535 [DOI] [PubMed] [Google Scholar]
- 11.Sanduleanu S, De Bruïne A, Stridsberg M, et al. Serum chromogranin A as a screening test for gastric enterochromaffin-like cell hyperplasia during acid-suppressive therapy. Eur J Clin Invest 2001;31:802-11. 10.1046/j.1365-2362.2001.00890.x [DOI] [PubMed] [Google Scholar]
- 12.Sanduleanu S, Stridsberg M, Jonkers D, et al. Serum gastrin and chromogranin A during medium- and long-term acid suppressive therapy: a case-control study. Aliment Pharmacol Ther 1999;13:145-53. 10.1046/j.1365-2036.1999.00466.x [DOI] [PubMed] [Google Scholar]
- 13.Mosli HH, Dennis A, Kocha W, et al. Effect of short-term proton pump inhibitor treatment and its discontinuation on chromogranin A in healthy subjects. J Clin Endocrinol Metab 2012;97:E1731-5. 10.1210/jc.2012-1548 [DOI] [PubMed] [Google Scholar]
- 14.Raines D, Chester M, Diebold AE, et al. A prospective evaluation of the effect of chronic proton pump inhibitor use on plasma biomarker levels in humans. Pancreas 2012;41:508-11. 10.1097/MPA.0b013e318243a0b6 [DOI] [PubMed] [Google Scholar]
- 15.Pregun I, Herszényi L, Juhász M, et al. Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion 2011;84:22-8. 10.1159/000321535 [DOI] [PubMed] [Google Scholar]
- 16.Giusti M, Sidoti M, Augeri C, et al. Effect of short-term treatment with low dosages of the proton-pump inhibitor omeprazole on serum chromogranin A levels in man. Eur J Endocrinol 2004;150:299-303. 10.1530/eje.0.1500299 [DOI] [PubMed] [Google Scholar]
- 17.Corsello A, Di Filippo L, Massironi S, et al. Vasostatin-1: A novel circulating biomarker for ileal and pancreatic neuroendocrine neoplasms. PLoS One 2018;13:e0196858. 10.1371/journal.pone.0196858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massironi S, Zilli A, Cavalcoli F, et al. Chromogranin A and other enteroendocrine markers in inflammatory bowel disease. Neuropeptides 2016;58:127-34. 10.1016/j.npep.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 19.Sciola V, Massironi S, Conte D, et al. Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm Bowel Dis 2009;15:867-71. 10.1002/ibd.20851 [DOI] [PubMed] [Google Scholar]
- 20.Zissimopoulos A, Vradelis S, Konialis M, et al. Chromogranin A as a biomarker of disease activity and biologic therapy in inflammatory bowel disease: a prospective observational study. Scand J Gastroenterol 2014;49:942-9. 10.3109/00365521.2014.920910 [DOI] [PubMed] [Google Scholar]
- 21.Giuffrida P, Vanoli A, Biletta E, et al. Increase in chromogranin A- and serotonin-positive cells in pouch mucosa of patients with ulcerative colitis undergoing proctocolectomy. Dig Liver Dis 2018;50:1205-13. 10.1016/j.dld.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 22.Massironi S, Fraquelli M, Paggi S, et al. Chromogranin A levels in chronic liver disease and hepatocellular carcinoma. Dig Liver Dis 2009;41:31-5. 10.1016/j.dld.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 23.Huang CT, Liang YJ. Comparison of Duodenal Mucosal Chromogranin-A Expression in Non-Alcoholic Fatty Pancreas Dyspeptic Patients with and without Endosonography-Diagnosed Early Chronic Pancreatitis: A Case Series Study. Case Rep Gastroenterol 2019;13:102-12. 10.1159/000497777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin A as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol 2010;45:1435-9. 10.3109/00365521.2010.503965 [DOI] [PubMed] [Google Scholar]
- 25.Sidhu R, McAlindon ME, Leeds JS, et al. The role of serum chromogranin A in diarrhoea predominant irritable bowel syndrome. J Gastrointestin Liver Dis 2009;18:23-6. [PubMed] [Google Scholar]
- 26.Di Sabatino A, Giuffrida P, Vanoli A, et al. Increase in neuroendocrine cells in the duodenal mucosa of patients with refractory celiac disease. Am J Gastroenterol 2014;109:258-69. 10.1038/ajg.2013.426 [DOI] [PubMed] [Google Scholar]
- 27.Hsiao RJ, Mezger MS, O'Connor DT. Chromogranin A in uremia: progressive retention of immunoreactive fragments. Kidney Int 1990;37:955-64. 10.1038/ki.1990.71 [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Wang H, Su X, et al. Serum chromogranin A correlated with albuminuria in diabetic patients and is associated with early diabetic nephropathy. BMC Nephrol 2022;23:41. 10.1186/s12882-022-02667-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikkelsen G, Åsberg A, Hultström ME, et al. Reference limits for chromogranin A, CYFRA 21-1, CA 125, CA 19-9 and carcinoembryonic antigen in patients with chronic kidney disease. Int J Biol Markers 2017;32:e461-6. 10.5301/ijbm.5000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe T. The Emerging Roles of Chromogranins and Derived Polypeptides in Atherosclerosis, Diabetes, and Coronary Heart Disease. Int J Mol Sci 2021;22:6118. 10.3390/ijms22116118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ottesen AH, Christensen G, Omland T, et al. Glycosylated Chromogranin A: Potential Role in the Pathogenesis of Heart Failure. Curr Heart Fail Rep 2017;14:478-88. 10.1007/s11897-017-0360-x [DOI] [PubMed] [Google Scholar]
- 32.Goetze JP, Hilsted LM, Rehfeld JF, et al. Plasma chromogranin A is a marker of death in elderly patients presenting with symptoms of heart failure. Endocr Connect 2014;3:47-56. 10.1530/EC-14-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bralewska M, Pietrucha T, Sakowicz A., Chromogranin A: An Endocrine Factor of Pregnancy. Int J Mol Sci 2023;24:4986. 10.3390/ijms24054986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Shoumer KA, Vasanthy BA. Serum chromogranin A concentration in hyperthyroidism before and after medical treatment. J Clin Endocrinol Metab 2009;94:2321-4. 10.1210/jc.2008-2231 [DOI] [PubMed] [Google Scholar]
- 35.Xu DJ, Wei LY, Li HF, et al. Serum levels of chromogranins and secretogranins correlate with the progress and severity of Parkinson's disease. Kaohsiung J Med Sci 2019;35:146-50. 10.1002/kjm2.12026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gmitterova K, Varges D, Schmitz M, et al. Chromogranin A Analysis in the Differential Diagnosis Across Lewy Body Disorders. J Alzheimers Dis 2020;73:1355-61. 10.3233/JAD-191153 [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Song Y, Dang W, et al. The associations between anxiety/depression and plasma chromogranin A among healthy workers: Results from EHOP study. J Occup Health 2020;62:e12113. 10.1002/1348-9585.12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rozansky DJ, Wu H, Tang K, et al. Glucocorticoid activation of chromogranin A gene expression. Identification and characterization of a novel glucocorticoid response element. J Clin Invest 1994;94:2357-68. 10.1172/JCI117601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karger S, Wiesner T, Kersting A, et al. Increased chromogranin a and carcinoid syndrome-like symptoms in a patient treated with duloxetine. Endocr Pract 2014;20:e215-8. 10.4158/EP14162.CR [DOI] [PubMed] [Google Scholar]
- 40.Uhlig R, Dum D, Gorbokon N, et al. Synaptophysin and chromogranin A expression analysis in human tumors. Mol Cell Endocrinol 2022;555:111726. 10.1016/j.mce.2022.111726 [DOI] [PubMed] [Google Scholar]
- 41.Tropea F, Baldari S, Restifo G, et al. Evaluation of chromogranin A expression in patients with non-neuroendocrine tumours. Clin Drug Investig 2006;26:715-22. 10.2165/00044011-200626120-00005 [DOI] [PubMed] [Google Scholar]
- 42.Leone N, Pellicano R, Brunello F, et al. Elevated serum chromogranin A in patients with hepatocellular carcinoma. Clin Exp Med 2002;2:119-23. 10.1007/s102380200016 [DOI] [PubMed] [Google Scholar]
- 43.Panzuto F, Severi C, Cannizzaro R, et al. Utility of combined use of plasma levels of chromogranin A and pancreatic polypeptide in the diagnosis of gastrointestinal and pancreatic endocrine tumors. J Endocrinol Invest 2004;27:6-11. 10.1007/BF03350903 [DOI] [PubMed] [Google Scholar]
- 44.Mariën L, Islam O, Chhajlani S, et al. The Quest for Circulating Biomarkers in Neuroendocrine Neoplasms: a Clinical Perspective. Curr Treat Options Oncol 2023;24:1833-51. 10.1007/s11864-023-01147-3 [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez-Carrasco M, Esposito G, Libânio D, et al. Image-enhanced endoscopy for gastric preneoplastic conditions and neoplastic lesions: a systematic review and meta-analysis. Endoscopy 2020;52:1048-65. 10.1055/a-1205-0570 [DOI] [PubMed] [Google Scholar]
- 46.Lamarca A, Bartsch DK, Caplin M, et al. European Neuroendocrine Tumor Society (ENETS) 2024 guidance paper for the management of well-differentiated small intestine neuroendocrine tumours. J Neuroendocrinol 2024;36:e13423. 10.1111/jne.13423 [DOI] [PubMed] [Google Scholar]
- 47.Panzuto F, Ramage J, Pritchard DM, et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for gastroduodenal neuroendocrine tumours (NETs) G1-G3. J Neuroendocrinol 2023;35:e13306. 10.1111/jne.13306 [DOI] [PubMed] [Google Scholar]
- 48.Kos-Kudła B, Castaño JP, Denecke T, et al. European Neuroendocrine Tumour Society (ENETS) 2023 guidance paper for nonfunctioning pancreatic neuroendocrine tumours. J Neuroendocrinol 2023;35:e13343. 10.1111/jne.13343 [DOI] [PubMed] [Google Scholar]
- 49.Fraenkel M, Kim M, Faggiano A, et al. Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer 2014;21:R153-63. 10.1530/ERC-13-0125 [DOI] [PubMed] [Google Scholar]
- 50.Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420-9. 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 51.Lassalle M, Le Tri T, Bardou M, et al. Use of proton pump inhibitors in adults in France: a nationwide drug utilization study. Eur J Clin Pharmacol 2020;76:449-57. 10.1007/s00228-019-02810-1 [DOI] [PubMed] [Google Scholar]
- 52.Abrahami D, McDonald EG, Schnitzer M, et al. Trends in acid suppressant drug prescriptions in primary care in the UK: a population-based cross-sectional study. BMJ Open 2020;10:e041529. 10.1136/bmjopen-2020-041529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769-78. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 54.Magi L, Mazzuca F, Rinzivillo M, et al. Multidisciplinary Management of Neuroendocrine Neoplasia: A Real-World Experience from a Referral Center. J Clin Med 2019;8:910. 10.3390/jcm8060910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Öberg K, Califano A, Strosberg JR, et al. A meta-analysis of the accuracy of a neuroendocrine tumor mRNA genomic biomarker (NETest) in blood. Ann Oncol 2020;31:202-12. 10.1016/j.annonc.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 56.Gertner J, Tsoli M, Hayes AR, et al. The Clinical Utility of the NETest in Patients with Small Intestinal Neuroendocrine Neoplasms (Si-NENs): A "Real-Life" Study. Cancers (Basel) 2024;16:2506. 10.3390/cancers16142506 [DOI] [PMC free article] [PubMed] [Google Scholar]