Abstract
The present study describes a rare case of plasmacytoid dendritic cell-associated acute myeloid leukemia (pDC-AML). A 70-year-old male patient was diagnosed with pDC-AML and underwent induction chemotherapy using the venetoclax + azacitidine (VA) regimen. After 3 weeks of treatment, bone marrow examination indicated a morphologic leukemia-free state (MLFS); however, the patient experienced persistent cytopenia, which was further complicated by severe pneumonia and gastrointestinal bleeding, both of which improved following treatment. After 3 weeks in MLFS, bone marrow morphology and minimal residual disease analysis revealed a relapse of leukemia. The patient subsequently underwent treatment with selinexor in conjunction with the VA regimen; however, due to severe thrombocytopenia, the family decided to discontinue further treatment. The patient subsequently succumbed shortly after discharge. pDC-AML is an extremely rare disease characterized by low complete remission rates and a poor prognosis. While the VA regimen demonstrates rapid efficacy and favorable safety in elderly patients, especially those unable to tolerate intensive chemotherapy, the risk of relapse remains substantial. CD123-targeted therapies may present potential new therapeutic options for this disease. Improving remission rates and extending survival in patients with pDC-AML remain pressing clinical challenges.
Keywords: acute myeloid leukemia, plasmacytoid dendritic cells, azacitidine, venetoclax, selinexor
Introduction
Acute myeloid leukemia (AML) is a clonal hematological malignancy marked by uncontrolled proliferation and impaired differentiation of myeloid precursors. The pathogenesis of AML involves diverse genetic mutations and dysregulated signaling pathways, such as FLT3-ITD and NPM1 mutations, which are frequently implicated in disease progression (1,2). According to GLOBOCAN 2022 data, ~119,000 new cases of AML are diagnosed globally each year, accounting for ~23% of all leukemia cases (3). In European Union countries, the incidence of AML is ~3.7/100,000 population, representing 25–30% of all leukemia cases (4). Data from the Surveillance, Epidemiology, and End Results database have indicated that the 5-year relative survival rate for patients with AML in the United States is 32.9%; however, this rate varies by age, reaching 69% in younger patients (<20 years) and reducing to 6.5% in older patients (≥65 years) (5). Standard treatment for AML typically involves a combination of chemotherapy and targeted agents. For high-risk patients, hematopoietic stem cell transplantation (HSCT) remains a key strategy to improve long-term survival outcomes (6,7). Furthermore, advances in genomic profiling have enabled more precise identification of molecular alterations, thus facilitating risk stratification and guiding personalized treatment approaches (8,9). Age is a critical prognostic factor in AML. Younger patients generally tolerate intensive chemotherapy better and have higher rates of complete remission. By contrast, older patients often have comorbidities and reduced treatment tolerance, requiring modified regimens such as low-intensity chemotherapy or hypomethylating agents (10,11). Among the various subtypes of AML, plasmacytoid dendritic cell-associated AML (pDC-AML) is extremely rare and exhibits distinct clinical and biological features. For example, RUNX1 mutations are the most common somatic alterations in pDC-AML, with a significantly higher incidence than in other AML subtypes (12). The pathogenesis of pDC-AML is complex, involving aberrant activation of multiple signaling pathways and inactivation of tumor suppressor genes. For example, dysregulation of inflammatory pathways and the JAK signaling pathway, as well as mutations in key genes such as NPM1, FLT3, IDH1/2 and TP53, serve important roles in the development and progression of pDC-AML (13–16). Although its incidence is low, pDC-AML is highly aggressive and poses a notable threat to patient health and survival (17).
Currently, treatment options for pDC-AML include conventional chemotherapy, targeted therapies, immunotherapies and HSCT. However, the classical ‘3+7’ regimen (cytarabine + anthracycline) exhibits limited efficacy in pDC-AML (18). With advancing insights into the molecular mechanisms of AML, targeted therapies and immunotherapies tailored to specific genetic mutations have shown promise. Nevertheless, even in cases achieving complete remission (CR), the risk of relapse remains high, and the overall survival (OS) of patients with pDC-AML is lower than that of patients with general AML. The prognosis is particularly poor in elderly individuals and those with adverse genetic features; notably, complex karyotypes and polyclonal genetic heterogeneity are considered important factors influencing the prognosis of patients with AML, with these effects being especially pronounced in the elderly population, thereby adding further complexity to clinical management (19–21).
As a distinct subtype of AML, pDC-AML presents unique challenges in treatment selection. The present study describes the case of an elderly patient with pDC-AML who was treated with the venetoclax + azacitidine (VA) regimen, and discusses the diagnostic and therapeutic characteristics in the context of a literature review.
Case report
In February 2024, a 70-year-old male patient was admitted to Jiaozuo People's Hospital (Jiaozuo, China) with complaints of ‘bilateral lower limb weakness for 2 weeks and fever for 1 day’. Physical examination upon admission revealed severe anemia, with markedly pale facial appearance. Enlarged, non-tender lymph nodes were palpable bilaterally in the cervical and axillary regions, with a maximum diameter of ~2×1 cm. A complete blood count indicated hematopoietic abnormalities: The white blood cell count was 16.98×109/l (normal range, 3.5–9.5×109/l), hemoglobin levels were 65 g/l (normal range, 115–150 g/l) and the platelet count was 5.00×109/l (normal range, 125–300×109/l).
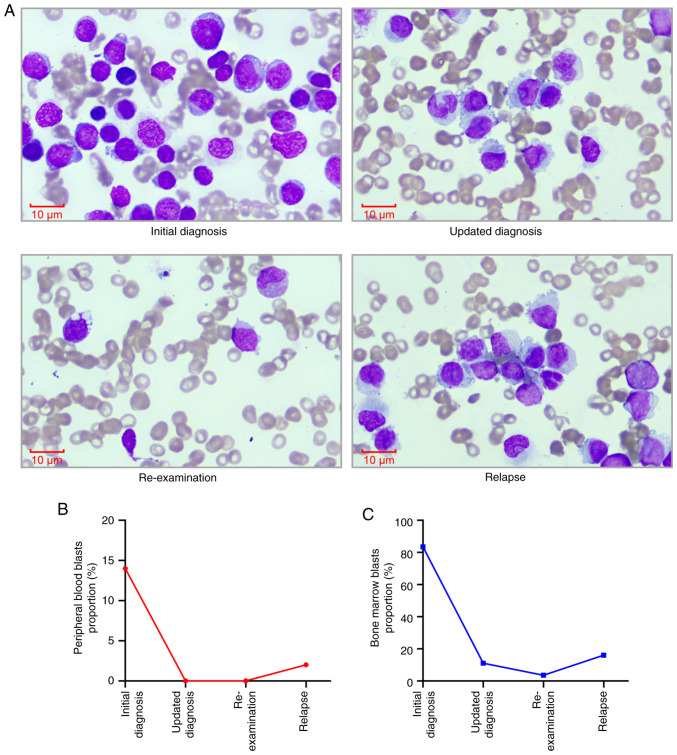
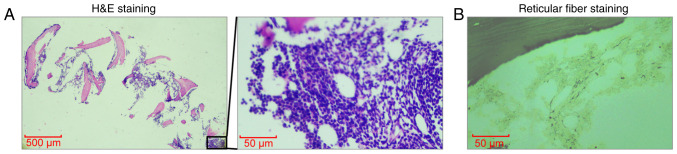
A peripheral blood smear revealed leukocytosis with a notable absence of granulocytes and ~14% blasts. Bone marrow cytology revealed markedly hypercellular marrow with notable reductions in granulocytic and erythroid lineages, and blasts accounting for 83.5% of nucleated cells (Fig. 1A). Karyotype analysis showed a normal 46, XY [20] profile. Bone marrow biopsy indicated active proliferation with diffuse infiltration by immature cells, sparse granulocytic and erythroid components, and mild reticulin fibrosis (Fig. 2A and B). For hematoxylin and eosin (H&E) staining, bone marrow tissue was fixed in 10% neutral buffered formalin at room temperature for 24 h, then dehydrated and embedded in paraffin. The embedded tissue was then sectioned into 4-µm slices, which were dewaxed with xylene, rehydrated through a graded alcohol series (100, 95, 90, 80 and 70%, 5 min each) and rinsed with distilled water. Subsequently, the sections were stained with hematoxylin for 4–8 min at room temperature, rinsed with tap water, then stained with eosin for 1–3 min at room temperature and rinsed again. The sections were successively immersed in 95% alcohol, anhydrous ethanol and xylene for 5 min each for dehydration. Finally, the samples were dried and mounted with neutral gum. The stained sections were examined under a light microscope (Olympus BX53; Olympus Corporation). For reticular fiber staining, the aforementioned paraffin-embedded bone marrow tissue sections were first treated with an oxidizing agent (potassium dichromate solution) for 10 min at room temperature. Then, the sections are treated with sodium metabisulfite for 5 min at room temperature and were immersed in a silver staining solution (Reticular Fiber Staining Kit; cat. no. HS2010; Celnovte Biotechnology Co., Ltd.) for ~15 min at room temperature, allowing silver ions to deposit on the reticular fibers and appear black. The development step was completed by treating the sections with formaldehyde solution for 2 min at room temperature, followed by fixation with sodium thiosulfate for 5 min at room temperature. Finally, the sections underwent dehydration and clearing sequentially, each step lasting ~5 min at room temperature, and were mounted with a coverslip. The stained sections were examined under a light microscope (Olympus BX53; Olympus Corporation).
Figure 1.
Morphological analysis and blast proportion changes in bone marrow cells before and after treatment. (A) Morphological examination of bone marrow cells conducted before and after treatment. Magnification, ×1,000; scale bar, 10 µm. (B) Proportion of blasts in peripheral blood before and after treatment. (C) Proportion of blasts in bone marrow before and after treatment.
Figure 2.
Histological examination of bone marrow via H&E and reticulin fiber staining. (A) H&E staining of bone marrow. Magnification, ×40 or ×400; scale bars, 500 or 50 µm. (B) Reticulin fiber staining of bone marrow. Magnification, ×40; scale bar, 50 µm. H&E, hematoxylin and eosin.
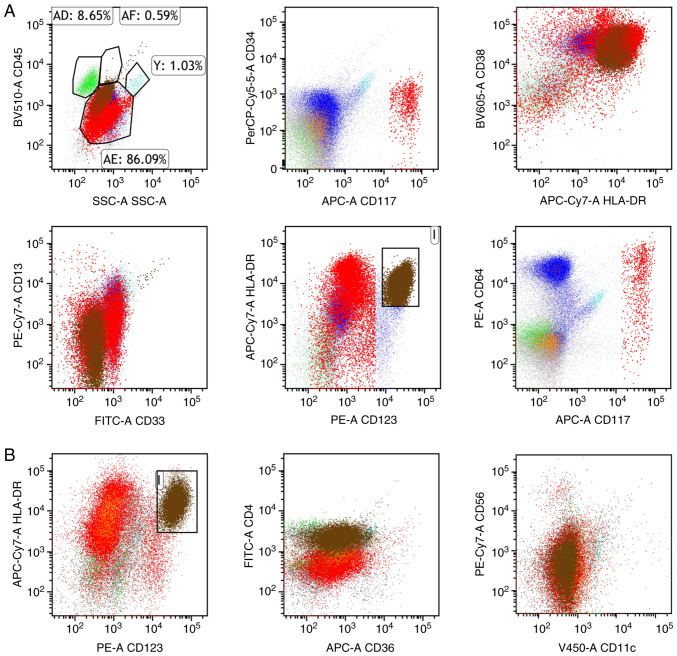
Flow cytometry identified aberrant leukemic immunophenotypes, with nucleated cells expressing HLA-DR, CD38, CD13, CD33 and CD64, along with partial expression of CD117 and TdT (Fig. 3A). There was no expression of cytoplasmic MPO (cMPO), CD34, CD123, CD10, CD19, CD22, CD20, cytoplasmic IgM (cIgM), CD15, CD14, CD64, CD56, CD7, CD2, CD3, CD4, CD8, CD5, CD11b, CD1a or cytoplasmic CD79a (cCD79a), suggesting atypical myeloid blasts. For flow cytometry, 100 µl EDTA-anticoagulated bone marrow samples were added to each tube and antibodies were added, including HLA-DR (cat. no. ab92511; Abcam), CD38 (cat. no. ab108403; Abcam), CD34 (cat. no. ab315802; Abcam), CD13 (cat. no. ab317440; Abcam), CD33 (cat. no. ab134115; Abcam), CD117 (cat. no. ab317843; Abcam), TdT (cat. no. ab183341; Abcam), cMPO (cat. no. ab208670; Abcam), CD123 (cat. no. ab280355; Abcam), CD10 (cat. no. ab227640; Abcam), CD19 (cat. no. ab320733; Abcam), CD22 (cat. no. ab254171; Abcam), CD20 (cat. no. ab219329; Abcam), cIgM (cat. no. ab212201; Abcam), CD15 (cat. no. ab241552; Abcam), CD14 (cat. no. ab314062; Abcam), CD64 (cat. no. ab109449; Abcam), CD56 (cat. no. ab220360; Abcam), CD7 (cat. no. ab109296; Abcam), CD2 (cat. no. ab314761; Abcam), CD3 (cat. no. ab243873; Abcam), CD4 (cat. no. ab213215; Abcam), CD8 (cat. no. ab237709; Abcam), CD5 (cat. no. ab300144; Abcam), CD11b (cat. no. ab224805; Abcam), CD1a (cat. no. ab313875; Abcam) and cCD79a (cat. no. ab133483; Abcam), all at a dilution of 1:1,000. The fluorescent conjugation kits including allophycocyanin (APC; cat. no. ab201807; Abcam), APC-Cy7 (cat. no. ab102859; Abcam), PerCP-Cy5.5 (cat. no. ab102911; Abcam), phycoerythrin (PE; cat. no. ab102918; Abcam), PE-Cy7 (cat. no. ab102903; Abcam), fluorescein isothiocyanate (cat. no. ab102884; Abcam) and Violet-450 (cat. no. ab312804; Abcam) were used to label the primary antibodies directly. Conjugation was performed by adding a modifier (1 mg/ml) to the primary antibody and incubating them for 3 h at room temperature, followed by the addition of a quencher and an additional 30-min incubation, in accordance with the manufacturer's instructions.
Figure 3.
Representative flow cytometry plots showing both the abnormal myeloid blast population and the pDCs. (A) Abnormal myeloid population (CD117part+, HLA-DR+, CD13+, CD33+, CD38+, CD64+, CD34−). The green population represents lymphocytes, brown indicates pDCs, red corresponds to myeloid blasts, blue denotes granulocytes, and orange represents monocytes. The brown cell populations in the boxes represent abnormal pDCs. (B) The brown cell populations abnormal pDCs (CD4+, CD123+, HLA-DR+, CD36−, CD56−, CD11c−). Boxed regions were used to highlight representative areas. pDCs, plasmacytoid dendritic cells.
After gentle mixing, the samples were incubated in the dark at room temperature for 20 min. Subsequently, 3% paraformaldehyde was added, and the samples were incubated again in the dark at room temperature for 10 min. Then, 1 ml purified water was added to the samples, which were shaken and incubated in the dark for another 10 min at room temperature. Subsequently, the samples were centrifuged at 160 × g for 5 min at room temperature and the supernatant was discarded. The pellet was resuspended in 2 ml PBS, centrifuged again at room temperature, and finally resuspended in 500 µl PBS. After mixing, the samples were analyzed using a flow cytometer (CytoFLEX; Beckman Coulter, Inc.) and data were processed with Kaluza 2.1.1 software (Beckman Coulter, Inc.).
Bone marrow cells from the patient were used for next-generation sequencing (NGS) for AML/myelodysplastic syndromes (MDS)/myeloproliferative neoplasms-related genes. For sequencing, the RNA was prepared using a commercial kit (cat. nos. DP431; Tiangen Biotech, Co., Ltd.). The purities and concentrations of RNA was confirmed by Nanodrop 2000 (Thermo Fisher Scientific, Inc.) and Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Inc.). The Qsep400 nucleic acid fragment analyzer (Hangzhou Houze Bio-Technology Co., Ltd.) was utilized to evaluate the integrity of RNA. The library was constructed using the KAPA EvoPlus Kit (cat. no. 9420053001; Kapa Biosystems, Roche Diagnostics), followed by 150 bp paired-end sequencing on the Illumina NextSeq 550 high-throughput sequencing platform (Illumina, Inc.). The sequencing was performed using the NextSeq 500/550 High Output Kit v2.5 (300 cycles; cat. no. 20024908; Illumina Inc.). The final library concentration was quantified using the Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Inc.), and the loading concentration was ~14 pM. The results identified multiple tier 1 mutations: ZRSR2 p.H191Y (96.60%), DNMT3A p.R882H (48.10%), RUNX1 p.I195fs (46.70%), ASXL1 p.G642fs (44.80%) and EZH2 p.L149R (44.80%). Additionally, multiple FLT3 mutations were detected, including p.N676K (17.30%), p.A680V (6.30%) and p.K663R (2.50%), as well as FLT3-TKD p.D835E (1.90%) and FLT3-ITD (0.16%). These genes are related with the pathogenesis and relapse of AML to some extent (12,22–36). Based on the morphological, immunological, cytogenetic and molecular features of the patient, they were diagnosed with AML.
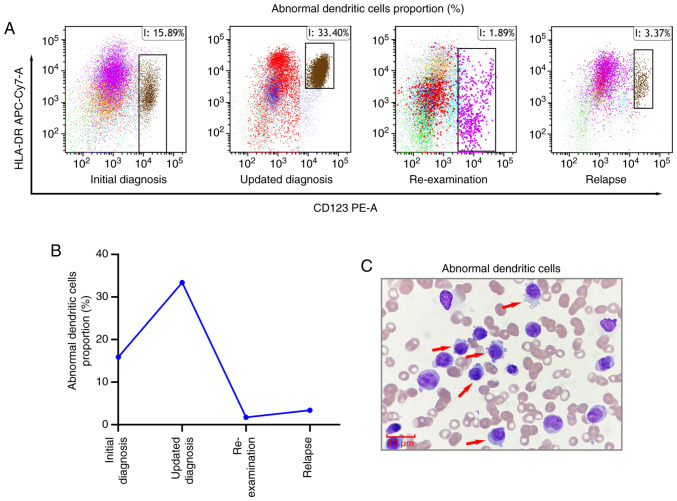
A total of 5 days after admission, considering that the patient was aged >60 years and had a history of recurrent pulmonary infections, they were deemed ‘unfit’ for intensive chemotherapy; therefore the patient commenced induction chemotherapy with the VA regimen (oral venetoclax: 100 mg on day 1, 200 mg on day 2, 400 mg on days 3–21; subcutaneous injection of azacitidine: 75 mg·m2·d−¹ for 7 days). After 2 weeks of treatment, as of early March 2024, follow-up assessments revealed the absence of leukemic cells in the peripheral blood smear (Fig. 1B). Bone marrow cytology indicated pancytopenia with a reduced blast percentage of ~11.0%, representing a significant decrease from the initial diagnosis (Fig. 1A and C). Minimal residual disease (MRD) analysis detected abnormal myeloid blasts (CD117part+, HLA-DR+, CD13+, CD33+, CD38+, CD64+, CD34−) accounting for 12.65% of nucleated cells (Fig. 3A). Furthermore, an abnormal dendritic cell population (CD4+, CD123+, HLA-DR+, CD36−, CD56−, CD11c−) was detected (Fig. 3B), comprising 33.40% of nucleated cells, which was a marked increase from baseline levels (Fig. 4A-C). However, the patient experienced persistent cytopenia, which was further complicated by severe pneumonia and gastrointestinal bleeding, both of which improved following treatment with broad-spectrum antibiotics (meropenem and vancomycin), hemostatic agents (tranexamic acid) and proton pump inhibitors (esomeprazole) as part of supportive care. Based on the bone marrow morphology and MRD findings, the diagnosis was updated to pDC-AML, and the patient continued venetoclax therapy without azacitidine.
Figure 4.
Analysis of abnormal dendritic cell proportions before and after treatment. (A) Flow cytometry analysis of abnormal plasmacytoid dendritic cells (CD4+, CD123+, HLA-DR+, CD36−, CD56− and CD11c−) in bone marrow before and after treatment. Only the CD123+/HLA-DR+ population is presented to highlight the key diagnostic features; other markers, such as CD4+, are not presented here. The cell populations in the boxes represent abnormal pDCs. (B) Abnormal pDCs proportions in bone marrow before and after treatment. (C) Morphological analysis of abnormal pDCs in bone marrow. Magnification, ×1,000; scale bar, 10 µm. pDCs, plasmacytoid dendritic cells.
Approximately 2 weeks later, re-examinations showed no blasts in the peripheral blood smear (Fig. 1B). Bone marrow cytology indicated a further reduction in blast percentage to 3.5% (Fig. 1A and C). Furthermore, MRD analysis revealed 1.89% abnormal dendritic cells, which was significantly reduced compared with previous measurement (Fig. 4A and B). These results suggested a morphologic leukemia-free state (MLFS).
Approximately 3 weeks later, the clinical condition of the patient deteriorated, with blasts reappearing in the peripheral blood smear (Fig. 1B). Bone marrow cytology indicated an increased blast percentage of 16% (Fig. 1A and C), and MRD analysis revealed 3.37% abnormal dendritic cells (Fig. 4A and B), confirming a relapse of leukemia. After 5 days, the patient underwent salvage therapy with a combination of selinexor and the VA regimen (selinexor: 40 mg orally twice weekly; oral venetoclax: 100 mg on day 1, 200 mg on day 2, 400 mg on days 3–21; subcutaneous injection of azacitidine: 75 mg·m2·d−¹ for 7 days). Unfortunately, the patient subsequently experienced severe myelosuppression, life-threatening pulmonary infections and gastrointestinal bleeding, and their condition continued to deteriorate. Consequently, the family decided to discontinue treatment, and the patient was discharged 2 days after the induction of salvage therapy. The patient subsequently succumbed 7 days later with an OS of 70 days.
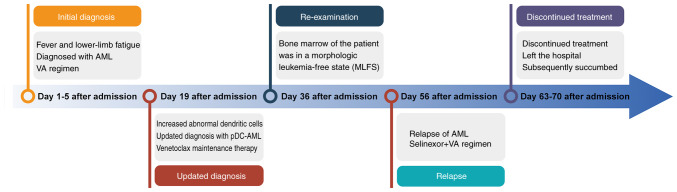
Fig. 5 provides a timeline summarizing the key events in the clinical course of the patient, including the initial presentation, diagnosis, treatments, disease progression and final outcome.
Figure 5.
Timeline of the clinical course. The timeline summarizes the key events in the patient's clinical course, including initial presentation, diagnosis, therapeutic interventions, disease progression and final outcome. AML, acute myeloid leukemia; pDC, plasmacytoid dendritic cell; VA, venetoclax + azacitidine.
Discussion
AML is an aggressive hematological malignancy predominantly affecting hematopoietic stem cells within the bone marrow; it is characterized by the clonal proliferation and differentiation arrest of myeloid blasts, leading to impaired normal hematopoiesis (37–39). pDCs are a crucial immune cell subset capable of rapidly producing interferon-γ (IFN-γ), which serve an essential role in bridging innate and adaptive immune responses. Upon pathogen recognition, pDCs secrete substantial amounts of type I IFNs (predominantly IFN-α and IFN-β), which are not only pivotal in antiviral defense but also exhibit significant antileukemic activity (40). In recent years, substantial progress has been made in the treatment of AML, particularly in the areas of targeted therapy, immunotherapy and epigenetic regulation. The BCL-2 inhibitor-based regimen, represented by venetoclax in combination with azacitidine, has become the first-line treatment option for elderly patients or those unfit for intensive chemotherapy (41). Moreover, novel targeted agents such as the FLT3 inhibitor gilteritinib and IDH1/2 inhibitor ivosidenib have markedly improved survival outcomes in patients with specific molecular subtypes (42–45). In the field of immunotherapy, CD3/CD123 bispecific antibodies and CD123-targeted chimeric antigen receptor-T cell therapies have shown promising therapeutic potential in relapsed or refractory AML, offering novel options for immune-based interventions (46–48). With regard to epigenetic therapies, oral azacitidine has demonstrated marked efficacy as a maintenance treatment in AML. A previous clinical study reported a 12-month relapse-free survival rate of 66.9% and an OS rate of 74.5% among treated patients (49). In addition, LSD1 inhibitors have shown considerable promise in cancer therapy. Dual inhibition of LSD1 and histone deacetylases has been shown to suppress tumor growth and to exert synergistic efficacy in multiple myeloma and other malignancies (50,51). Furthermore, MRD monitoring based on NGS and high-sensitivity flow cytometry, in combination with CRISPR-based functional screening, has provided new insights and strategies for individualized treatment and overcoming drug resistance (52–54). Future research will focus on optimizing combinatorial targeted and immune-based therapies, and developing MRD-guided, response-adaptive treatment strategies to further improve the prognosis of AML.
In patients with AML, pDCs display a notable reduction in type I IFN secretion, resulting in decreased production of these essential molecules. This deficiency compromises the resistance to viral infections, and diminishes the direct or indirect suppression of leukemic cells (55). Moreover, within the AML microenvironment, pDCs may undergo reprogramming, leading to the production of elevated levels of immunomodulatory cytokines such as interleukin-10; this cytokine suppresses T cells and other immune effector cells, thereby facilitating immune evasion and the survival of AML cells (56). Additionally, AML cells can secrete specific cytokines and chemokines to modulate pDCs, inducing the release of tumor-promoting factors, or interact with pDC receptors via ligand expression to evade immune surveillance, further promoting the survival and proliferation of AML cells (57).
The World Health Organization 5th edition classification of hematolymphoid tumors categorizes pDC-related neoplasms into two distinct categories: Blastic pDC neoplasm (BPDCN) and mature pDC proliferation (MPDCP) (58). The latter is exceedingly rare and is characterized by the presence of two clonal populations: pDCs and a myeloid neoplasm clone. The pDC population exhibits morphological similarities to normal pDCs and exhibits immunophenotypic characteristics akin to reactive pDCs, typically expressing CD68, CD123, CD303 and CD304, while lacking CD56 and exhibiting low Ki-67 expression (59–61). MPDCP frequently arises in MDS, chronic myelomonocytic leukemia and AML with monocytic differentiation (AML-M4/M5) (57). Clinically, it often presents with lymphadenopathy, erythema or papules. Despite reductions or resolution of pDC infiltration during remission, the overall prognosis remains poor (62). In healthy individuals, pDCs typically account for 0.2–0.8% of peripheral blood mononuclear cells and <0.4% of bone marrow nucleated cells; however, these values may vary with age, immune status or comorbidities (63). They characteristically express CD123, CD303 and CD304, without CD34 or CD117. By contrast, the abnormal pDC population in the present patient demonstrated a markedly increased proportion (15.89% at diagnosis, rising to 33.40% during chemotherapy) and aberrant immunophenotype (CD4+/CD123+/HLA-DR+, Fig. 3B), clearly distinguishing them from normal or reactive pDCs. This suggests that the quantitative criteria and qualitative markers of pDC may aid in the establishment of a diagnostic threshold for pDC-AML in the future.
In pDC-AML, leukemic stem cells (LSCs) exhibit aberrant differentiation capacity, and under specific stimuli or genetic alterations they may differentiate not only into myeloid blasts but also into pDCs, contributing to pDC proliferation (64). Thus, the relapse in the present case is likely driven by a common precursor: Either LSCs or even earlier abnormal hematopoietic progenitors, which give rise to both leukemic blasts and pDCs. Although the dominant population at relapse appears to be myeloid blasts (3.37%), the concurrent increase in pDCs (from 1.89 to 3.37%) suggests clonal evolution and co-proliferation, rather than an exclusive lineage-driven process. CD123 is expressed not only on pDCs but also on leukemic stem/progenitor cells in pDC-AML. Treatment with CD123-targeting agents such as tagraxofusp has resulted in reduction of both pDCs and CD34+ blasts, supporting the hypothesis that CD123-targeted therapy could still be a rational approach, even when relapse is primarily driven by myeloid blasts (57). Combination strategies, such as SL-401 with VA, may enhance efficacy. Moreover, since the leukemic blasts and pDCs in pDC-AML often share genetic mutations, therapies targeting shared molecular lesions could also represent viable alternatives (64).
Advances in diagnostic techniques have indicated that pDCs in AML may originate from malignant clones (65). A previous study reported that tumor-forming pDC infiltration was detected in 1.13% of AML cases between November 2013 and September 2016, while another analysis revealed significantly higher detection rates of tumor-forming pDC infiltration (38.03%) in AML-M4 and M5 subtypes. This suggests that pDCs in these subtypes may derive from leukemic blasts or stem cells (57). Furthermore, cross-lineage expression patterns and somatic mutations associated with poor prognosis, such as RUNX1, are more prevalent in pDC-AML compared with general AML cases; this underscores the impact of cellular origin or differentiation stage on biological behavior and clinical outcomes (17). In the present study, retrospective analysis of the initial diagnostic flow cytometry data (February 2024) revealed a minor population of CD4+/CD123+/HLA-DR+ cells (15.89% of nucleated cells). However, these cells were initially overlooked due to overlapping immunophenotypic features with myeloid blasts (partial CD34 expression) and the absence of a dedicated pDC-focused panel. This case highlights the diagnostic value of incorporating pDC-specific markers, such as CD303, CD304 and CD123 into standard AML immunophenotyping.
The present case involved an elderly patient who presented with clinical manifestations including fever, fatigue, palpitations, dyspnea and epistaxis. Initial diagnostic evaluations indicated anemia, severe thrombocytopenia and superficial lymphadenopathy. Bone marrow analysis revealed hyperplasia with a blast cell proportion of 83.5%, whereas NGS identified mutations in genes such as ZRSR2, DNMT3A, RUNX1, ASXL1, EZH2 and FLT3, all of which are associated with poor prognosis. Mutations in the ZRSR2 gene have been implicated in aberrant RNA splicing and impaired hematopoietic differentiation in AML (22). In myeloid malignancies, ZRSR2 mutations are frequently associated with dysfunctional pDCs, potentially disrupting their differentiation and activation (23). Moreover, emerging evidence has suggested that splicing defects driven by this mutation may promote abnormal expansion and impaired function of pDCs, ultimately influencing disease course and prognosis (12,60). DNMT3A mutations, among the most prevalent in AML, are thought to contribute to leukemogenesis via epigenetic dysregulation (24). In pDC-AML, DNMT3A mutations may drive malignant proliferation of pDCs by altering DNA methylation patterns, and disrupting gene expression programs involved in cell proliferation and differentiation (25,26). RUNX1 mutations enhance self-renewal of LSCs and block granulocytic differentiation, thus serving a central role in AML pathogenesis (27). In the context of pDC-AML, RUNX1 loss-of-function may further inhibit the terminal differentiation of pDCs, contributing to leukemic transformation (12). ASXL1 mutations are frequently observed in myeloid malignancies and are typically associated with poor prognosis (28,29). In AML, ASXL1 mutations often co-occur with mutations such as DNMT3A and FLT3-ITD, amplifying epigenetic dysregulation and accelerating disease progression (30,31). Aberrant expression of EZH2 in AML not only impairs cellular proliferation and differentiation but also contributes to therapy resistance and relapse, making it a promising therapeutic target (32). In pDC-AML, EZH2 may act synergistically with ASXL1 to maintain an undifferentiated pDC phenotype (33). The FLT3-ITD mutation is one of the most common kinase alterations in AML, found in ~30% of newly diagnosed cases (34). It frequently coexists with mutations such as RUNX1, and this combinatorial effect may result in greater tumor aggressiveness and drug resistance (35). Despite the development of multiple FLT3 inhibitors, resistance mechanisms often limit their clinical efficacy (34). Moreover, co-mutations involving FLT3-ITD, NPM1 and DNMT3A are associated with inferior overall and event-free survival outcomes (31). Understanding the interaction between FLT3-ITD and other cooperating mutations is therefore critical for improving risk stratification and therapeutic decision-making in AML (36). In the present study, abnormal pDCs, exhibiting a phenotype indicative of malignant infiltration, were detected in the bone marrow. The proportion of these cells increased to 33.40% during chemotherapy but subsequently decreased as treatment proved effective; however, upon disease progression, the pDC population expanded again, suggesting that these cells may originate from leukemic blasts or stem cells and that their infiltration could evolve alongside the progression of the myeloid malignancy. This finding highlights the potential role of pDCs in the pathogenesis of AML.
The standard first-line chemotherapy regimen for AML typically comprises the idarubicin + cytarabine regimen. Studies have suggested that AML cases with pDC infiltration may demonstrate resistance to a range of chemotherapeutic agents, including cytarabine, idarubicin, cladribine, homoharringtonine, fludarabine, decitabine, venetoclax, bortezomib, all-trans retinoic acid, vincristine, cyclophosphamide, methotrexate and methylprednisolone (57,66–69). Sensitivity to vinorelbine and carboplatin is moderate, whereas resistance to daunorubicin and sorafenib is evident. These patients typically present with elevated bone marrow blast counts and increased hemoglobin levels, necessitating a greater number of chemotherapy cycles to achieve CR. Moreover, these patients experience significantly reduced OS and progression-free survival (57).
BPDCN cells universally exhibit upregulation of CD123 and targeted therapies, such as the CD123-directed fusion protein SL-401, have shown promising efficacy in the treatment of BPDCN (70). In December 2018, the United States Food and Drug Administration approved tagraxofusp (SL-401) for BPDCN treatment. Considering the presence of abnormal CD123+ clones in pDC-AML, combining tagraxofusp with conventional chemotherapy could potentially enhance therapeutic efficacy (71,72). Current clinical trials are investigating the effectiveness of CD123-targeted therapies (73,74). Furthermore, a preclinical study using animal models of pDC-AML reported notable reductions in bone marrow cell populations, including CD34+ clones, following tagraxofusp treatment (12). Nonetheless, to the best of our knowledge, clinical data regarding the efficacy of tagraxofusp in patients with pDC-AML are currently unavailable (75).
In the present case, this patient was aged >60 years and had a history of recurrent pulmonary infections, and was thus deemed ‘unfit’ for intensive chemotherapy. In accordance with international and domestic guidelines, a treatment regimen comprising venetoclax (100 mg on day 1, 200 mg on day 2, 400 mg on days 3–21) combined with azacitidine (75 mg·m2·d−¹ for 7 days) was selected for induction chemotherapy. A total of 10 days following the initiation of treatment, a bone marrow biopsy revealed a MLFS; however, the patient subsequently experienced severe myelosuppression, life-threatening pulmonary infections and gastrointestinal bleeding. Despite supportive care and modifications to the treatment plan, leukemic blasts proliferated rapidly and the patient succumbed to disease progression shortly after being discharged, following the decision of the family to discontinue therapy. This case underscores the challenges and complexities involved in treating elderly patients with AML, particularly those with multisystem involvement and severe infections.
In summary, pDC-AML is a rare subtype of AML characterized by distinct clinical features and a poor prognosis. The development of individualized treatment strategies incorporating novel agents, such as the BCL-2 inhibitor venetoclax combined with demethylated drugs, is essential for improving patient outcomes. Furthermore, therapies targeting CD123 may offer additional therapeutic options; however, further clinical research and data are needed to validate their efficacy and establish optimal protocols.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The sequencing and raw data generated in the present study may be found in the BioProject database under accession number PRJNA1267814 or at the following URL: https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1267814.
Authors' contributions
ZP designed the project, provided the main conceptual ideas and wrote the manuscript. YZ and HX analyzed and interpreted the data, and wrote the manuscript. PP, ZZ, HW, JB, BZ, YZ, JG, ZF and ML contributed to data acquisition, as well as to data analysis and interpretation. QS designed the project, contributed the main conceptual ideas and the proof outline, actively participated in the entire writing process of the manuscript, and provided technical support. QS and ZP confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
As this case report includes clinical data and potentially identifiable information, approval was obtained from the Ethics Committee of Jiaozuo People's Hospital (Jiaozuo, China; approval no. 2024-078-k36) for its publication. This approval ensures that the patient's rights and privacy were adequately protected.
Patient consent for publication
As the patient succumbed to their condition, written informed consent for publication of the case report, including clinical details and images, was provided by the patient's family.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Martignoles JA, Delhommeau F, Hirsch P. Genetic hierarchy of acute myeloid leukemia: From clonal hematopoiesis to molecular residual disease. Int J Mol Sci. 2018;19:3850. doi: 10.3390/ijms19123850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye M, Zhang H, Yang H, Koche R, Staber PB, Cusan M, Levantini E, Welner RS, Bach CS, Zhang J, et al. Hematopoietic differentiation is required for initiation of acute myeloid leukemia. Cell Stem Cell. 2015;17:611–623. doi: 10.1016/j.stem.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 4.De Kouchkovsky I, Abdul-Hay M. ‘Acute myeloid leukemia: A comprehensive review and 2016 update’. Blood Cancer J. 2016;6:e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute, corp-author. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Leukemia-Acute Myeloid Leukemia (AML) https://seer.cancer.gov/statfacts/html/amyl.html [Google Scholar]
- 6.Forsberg M, Konopleva M. AML treatment: Conventional chemotherapy and emerging novel agents. Trends Pharmacol Sci. 2024;45:430–448. doi: 10.1016/j.tips.2024.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Huang BJ, Meyer LK, Alonzo TA, Wang YC, Lamble AJ, Ries RE, Wang W, Hirsch B, Raca G, Ma X, et al. Hematopoietic stem cell transplantation outcomes for high-risk AML: A report from the children's oncology group. J Clin Oncol. 2025;43:1961–1971. doi: 10.1200/JCO-24-01841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewald L, Dittmann J, Vogler M, Fulda S. Side-by-side comparison of BH3-mimetics identifies MCL-1 as a key therapeutic target in AML. Cell Death Dis. 2019;10:917. doi: 10.1038/s41419-019-2156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renard C, Corbel A, Paillard C, Pochon C, Schneider P, Simon N, Buchbinder N, Fahd M, Yakoub-Agha I, Calvo C. Preventive and therapeutic strategies for relapse after hematopoietic stem cell transplant for pediatric AML (SFGM-TC) Bull Cancer. 2025;112((1S)):S135–S145. doi: 10.1016/j.bulcan.2024.02.006. (In French) [DOI] [PubMed] [Google Scholar]
- 10.Tawfik B, Sliesoraitis S, Lyerly S, Klepin HD, Lawrence J, Isom S, Ellis LR, Manuel M, Dralle S, Berenzon D, et al. Efficacy of the hypomethylating agents as frontline, salvage, or consolidation therapy in adults with acute myeloid leukemia (AML) Ann Hematol. 2014;93:47–55. doi: 10.1007/s00277-013-1940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez HF. What is the optimal induction therapy for younger fit patients with AML? Curr Hematol Malig Rep. 2016;11:327–332. doi: 10.1007/s11899-016-0339-9. [DOI] [PubMed] [Google Scholar]
- 12.Xiao WB, Chan A, Waarts MR, Mishra T, Liu Y, Cai SF, Yao J, Gao Q, Bowman RL, Koche RP, et al. Plasmacytoid dendritic cell expansion defines a distinct subset of RUNX1-mutated acute myeloid leukemia. Blood. 2021;137:1377–1391. doi: 10.1182/blood.2020007897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nong T, Mehra S, Taylor J. Common driver mutations in AML: Biological impact, clinical considerations, and treatment strategies. Cells. 2024;13:1392. doi: 10.3390/cells13161392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naji NS, Sathish M, Karantanos T. Inflammation and Related signaling pathways in acute myeloid leukemia. Cancers (Basel) 2025;16:3974. doi: 10.3390/cancers16233974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HJ, Daver N, Kantarjian HM, Verstovsek S, Ravandi F. The role of JAK pathway dysregulation in the pathogenesis and treatment of acute myeloid leukemia. Clin Cancer Res. 2013;19:327–335. doi: 10.1158/1078-0432.CCR-12-2087. [DOI] [PubMed] [Google Scholar]
- 16.Li YF, Wan H, Jing Y. Molecular characterization and clinical treatment of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) patients with TP53 mutation. Clin Lymphoma Myeloma Leuk. 2021;21:841–851. doi: 10.1016/j.clml.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Feng Y, Deng X, Liu S, Qiang X, Gou Y, Li J, Yang W, Peng X, Zhang X. Tumor-forming plasmacytoid dendritic cells in acute myelocytic leukemia: A report of three cases and literature review. Int J Clin Exp Pathol. 2017;10:7285–7291. [PMC free article] [PubMed] [Google Scholar]
- 18.Tang K, Schuh AC, Yee KW. 3+7 combined chemotherapy for acute myeloid leukemia: Is it time to say goodbye? Curr Oncol Rep. 2021;23:120. doi: 10.1007/s11912-021-01108-9. [DOI] [PubMed] [Google Scholar]
- 19.Tawfik B, Pardee TS, Isom S, Sliesoraitis S, Winter A, Lawrence J, Powell BL, Klepin HD. Comorbidity, age, and mortality among adults treated intensively for acute myeloid leukemia (AML) J Geriatr Oncol. 2016;7:24–31. doi: 10.1016/j.jgo.2015.10.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdallah M, Xie Z, Ready A, Manogna D, Mendler JH, Loh KP. Management of acute myeloid leukemia (AML) in older patients. Curr Oncol Rep. 2020;22:103. doi: 10.1007/s11912-020-00964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medeiros BC, Othus M, Fang M, Appelbaum FR, Erba HP. Cytogenetic heterogeneity negatively impacts outcomes in patients with acute myeloid leukemia. Haematologica. 2015;100:331–335. doi: 10.3324/haematol.2014.117267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madan V, Cao Z, Teoh WW, Dakle P, Han L, Shyamsunder P, Jeitany M, Zhou S, Li J, Nordin HBM, et al. ZRSR1 Co-operates with ZRSR2 in regulating splicing of U12-type introns in murine hematopoietic cells. Haematologica. 2022;107:680–689. doi: 10.3324/haematol.2020.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Togami K, Chung SS, Madan V, Booth CAG, Kenyon CM, Cabal-Hierro L, Taylor J, Kim SS, Griffin GK, Ghandi M, et al. Sex-Biased ZRSR2 mutations in myeloid malignancies impair plasmacytoid dendritic cell activation and apoptosis. Cancer Discov. 2022;12:522–541. doi: 10.1158/2159-8290.CD-20-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang G, Cai X, Li D. Significance of targeting DNMT3A mutations in AML. Ann Hematol. 2025;104:1399–1414. doi: 10.1007/s00277-024-05885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai XY, Huang GQ, Zhou YM, Li DJ. Targeting calprotectin S100A8/A9 to overcome AML progression in DNMT3A-Mutant cells. Curr Med Sci. 2025;45:458–468. doi: 10.1007/s11596-025-00042-2. [DOI] [PubMed] [Google Scholar]
- 26.Palam LR, Ramdas B, Pickerell K, Pasupuleti SK, Kanumuri R, Cesarano A, Szymanski M, Selman B, Dave UP, Sandusky G, et al. Loss of Dnmt3a impairs hematopoietic homeostasis and myeloid cell skewing via the PI3Kinase pathway. JCI Insight. 2023;8:e163864. doi: 10.1172/jci.insight.163864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerritsen M, Yi G, Tijchon E, Kuster J, Schuringa JJ, Martens JHA, Vellenga E. RUNX1 mutations enhance self-renewal and block granulocytic differentiation in human in vitro models and primary AMLs. Blood Adv. 2019;3:320–332. doi: 10.1182/bloodadvances.2018024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang FC, Agosto-Peña J. Epigenetic regulation by ASXL1 in myeloid malignancies. Int J Hematol. 2023;117:791–806. doi: 10.1007/s12185-023-03586-y. [DOI] [PubMed] [Google Scholar]
- 29.Medina EA, Delma CR, Yang FC. ASXL1/2 mutations and myeloid malignancies. J Hematol Oncol. 2022;15:127. doi: 10.1186/s13045-022-01336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan W, Jia J, Wang J, Liu X, Yu W, Zhu X, Zhao T, Jiang Q, Ruan G, Zhao X, et al. Only FLT3-ITD co-mutation did not have a deleterious effect on acute myeloid leukemia patients with NPM1 mutation, but concomitant with DNMT3A co-mutation or a < 3log reduction of MRD2 predicted poor survival. Ann Hematol. 2024;103:4525–4535. doi: 10.1007/s00277-024-06001-6. [DOI] [PubMed] [Google Scholar]
- 31.Ebian HF, Elshorbagy S, Mohamed H, Embaby A, Khamis T, Sameh R, Sabbah NA, Hussein S. Clinical implication and prognostic significance of FLT3-ITD and ASXL1 mutations in Egyptian AML patients: A single-center study. Cancer Biomark. 2021;32:379–389. doi: 10.3233/CBM-210024. [DOI] [PubMed] [Google Scholar]
- 32.Fang J, Zhang J, Zhu L, Xin X, Hu H. The epigenetic role of EZH2 in acute myeloid leukemia. PeerJ. 2024;12:e18656. doi: 10.7717/peerj.18656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stomper J, Meier R, Ma T, Pfeifer D, Ihorst G, Blagitko-Dorfs N, Greve G, Zimmer D, Platzbecker U, Hagemeijer A, et al. Integrative study of EZH2 mutational status, copy number, protein expression and H3K27 trimethylation in AML/MDS patients. Clin Epigenetics. 2021;13:77. doi: 10.1186/s13148-021-01087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tecik M, Adan A. Therapeutic targeting of FLT3 in acute myeloid leukemia: Current status and novel approaches. Onco Targets Ther. 2022;15:1449–1478. doi: 10.2147/OTT.S384293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacharne S, Dovey OM, Cooper JL, Gu M, Friedrich MJ, Rajan SS, Barenboim M, Collord G, Vijayabaskar MS, Ponstingl H, et al. SETBP1 overexpression acts in the place of class-defining mutations to drive FLT3-ITD-mutant AML. Blood Adv. 2021;5:2412–2425. doi: 10.1182/bloodadvances.2020003443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li HD, Chen SS, Ding J, Zhang CL, Qiu HY, Xia XX, Yang J, Wang XR. Exploration of ETV6::ABL1-positive AML with concurrent NPM1 and FLT3-ITD mutations. Ann Hematol. 2024;103:4295–4304. doi: 10.1007/s00277-024-05917-3. [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell MR, Abboud CN, Altman J, Appelbaum FR, Coutre SE, Damon LE, Foran JM, Goorha S, Maness LJ, Marcucci G, et al. Acute myeloid leukemia. J Natl Compr Canc Netw. 2011;9:280–317. doi: 10.6004/jnccn.2011.0027. [DOI] [PubMed] [Google Scholar]
- 38.Cui P, Zhang Y, Cui M, Li Z, Ma G, Wang R, Wang N, Huang S, Gao J. Leukemia cells impair normal hematopoiesis and induce functionally loss of hematopoietic stem cells through immune cells and inflammation. Leukemia Res. 2018;65:49–54. doi: 10.1016/j.leukres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Miraki-Moud F, Anjos-Afonso F, Hodby KA, Griessinger E, Rosignoli G, Lillington D, Jia L, Davies JK, Cavenagh J, Smith M, et al. Acute myeloid leukemia does not deplete normal hematopoietic stem cells but induces cytopenias by impeding their differentiation. Proc Natl Acad Sci USA. 2013;110:13576–13581. doi: 10.1073/pnas.1301891110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Acker HH, Versteven M, Lichtenegger FS, Roex G, Campillo-Davo D, Lion E, Subklewe M, Van Tendeloo VF, Berneman ZN, Anguille S. Dendritic cell-based immunotherapy of acute myeloid leukemia. J Clin Med. 2019;8:579. doi: 10.3390/jcm8050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollyea DA, DiNardo CD, Arellano ML, Pigneux A, Fiedler W, Konopleva M, Rizzieri DA, Smith BD, Shinagawa A, Lemoli RM, et al. Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res. 2022;28:2753–2761. doi: 10.1158/1078-0432.CCR-21-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu R, Li L, Nguyen B, Seo J, Wu M, Seale T, Levis M, Duffield A, Hu Y, Small D. FLT3 tyrosine kinase inhibitors synergize with BCL-2 inhibition to eliminate FLT3/ITD acute leukemia cells through BIM activation. Signal Transduct Target Ther. 2021;6:186. doi: 10.1038/s41392-021-00578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao MY, Wang YF, Zhao Y, Ling LJ, He Y, Wen J, Zheng MY, Jiang HL, Xie CY. BCL-2 inhibitor synergizes with PI3Kδ inhibitor and overcomes FLT3 inhibitor resistance in acute myeloid leukaemia. Am J Cancer Res. 2022;12:3829–3842. [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J, Zhang P, Mao Y, Chen R, Cheng R, Li J, Sun H, Deng C, Zhong Z. CXCR4-mediated codelivery of FLT3 and BCL-2 inhibitors for enhanced targeted combination therapy of FLT3-ITD acute myeloid leukemia. Biomacromolecules. 2024;25:4569–4580. doi: 10.1021/acs.biomac.4c00561. [DOI] [PubMed] [Google Scholar]
- 45.Molenaar RJ, Radivoyevitch T, Nagata Y, Khurshed M, Przychodzen B, Makishima H, Xu M, Bleeker FE, Wilmink JW, Carraway HE, et al. IDH1/2 mutations sensitize acute myeloid leukemia to PARP inhibition and this is reversed by IDH1/2-mutant inhibitors. Clin Cancer Res. 2018;24:1705–1715. doi: 10.1158/1078-0432.CCR-17-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonnevaux H, Guerif S, Albrecht J, Jouannot E, De Gallier T, Beil C, Lange C, Leuschner WD, Schneider M, Lemoine C, et al. Pre-clinical development of a novel CD3-CD123 bispecific T-cell engager using cross-over dual-variable domain (CODV) format for acute myeloid leukemia (AML) treatment. Oncoimmunology. 2021;10:1945803. doi: 10.1080/2162402X.2021.1945803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watts J, Lin TL, Mims A, Patel P, Lee C, Shahidzadeh A, Shami P, Cull E, Cogle CR, Wang E, Uckun FM. Post-hoc analysis of pharmacodynamics and single-agent activity of CD3×CD123 bispecific antibody APVO436 in relapsed/refractory AML and MDS resistant to HMA or venetoclax plus HMA. Front Oncol. 2022;11:806243. doi: 10.3389/fonc.2021.806243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, Hoffman L, Aguilar B, Chang WC, Bretzlaff W, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122:3138–3148. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leber B, Ruiz MT, Elgendy H, Pettersson F, Prebet T, Vigil CE, Parikh RC, Korgaonkar S, Bello F, Davis KL, et al. Real-world treatment patterns and outcomes with oral azacitidine maintenance therapy in patients with acute myeloid leukemia. Cancer. 2025;131:e35845. doi: 10.1002/cncr.35845. [DOI] [PubMed] [Google Scholar]
- 50.Gajendran C, Tantry SJ, MNS Mohammed Z, Dewang P, Hallur M, Nair S, Vaithilingam K, Nagayya B, Rajagopal S, Sivanandhan D. Novel dual LSD1/HDAC6 inhibitor for the treatment of cancer. PLoS One. 2023;18:e0279063. doi: 10.1371/journal.pone.0279063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naveen Sadhu M, Sivanandhan D, Gajendran C, Tantry S, Dewang P, Murugan K, Chickamunivenkatappa S, Zainuddin M, Nair S, Vaithilingam K, Rajagopal S. Novel dual LSD1/HDAC6 inhibitors for the treatment of multiple myeloma. Bioorg Med Chem Lett. 2020;34:127763. doi: 10.1016/j.bmcl.2020.127763. [DOI] [PubMed] [Google Scholar]
- 52.Mukhopadhyay S, Huang HY, Lin Z, Ranieri M, Li S, Sahu S, Liu Y, Ban Y, Guidry K, Hu H, et al. Genome-Wide CRISPR screens identify multiple synthetic lethal targets that enhance KRASG12C inhibitor efficacy. Cancer Res. 2023;83:4095–4111. doi: 10.1158/0008-5472.CAN-23-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Y, Li H, Ju X, Gong B. Developing pioneering pharmacological strategies with CRISPR/Cas9 library screening to overcome cancer drug resistance. Biochim Biophys Acta Rev Cancer. 2024;1879:189212. doi: 10.1016/j.bbcan.2024.189212. [DOI] [PubMed] [Google Scholar]
- 54.Alvarez-Calderon F, Gregory MA, DeGregori J. Using functional genomics to overcome therapeutic resistance in hematological malignancies. Immunol Res. 2023;55:100–115. doi: 10.1007/s12026-012-8353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fatehchand K, Mehta P, Colvin CB, Buteyn NJ, Santhanam R, Merchand-Reyes G, Inshaar H, Shen B, Mo X, Mundy-Bosse B, et al. Activation of plasmacytoid dendritic cells promotes AML-cell fratricide. Oncotarget. 2021;12:878–890. doi: 10.18632/oncotarget.27949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayyadurai VAS, Deonikar P, Mclure KG, Sakamoto KM. Molecular systems architecture of interactome in the acute myeloid leukemia microenvironment. Cancers (Basel) 2022;14:756. doi: 10.3390/cancers14030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu L, Wang P, Zhang W, Li Q, Xiong J, Li J, Deng X, Liu Y, Yang C, Kong P, et al. Plasmacytoid dendritic cell infiltration in acute myeloid leukemia. Cancer Manag Res. 2020;12:11411–11419. doi: 10.2147/CMAR.S260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–1719. doi: 10.1038/s41375-022-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zalmaï L, Viailly PJ, Biichle S, Cheok M, Soret L, Angelot-Delettre F, Petrella T, Collonge-Rame MA, Seilles E, Geffroy S, et al. Plasmacytoid dendritic cells proliferation associated with acute myeloid leukemia: Phenotype profile and mutation landscape. Haematologica. 2020;106:3056–3066. doi: 10.3324/haematol.2020.253740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang W, Xu J, Khoury JD, Pemmaraju N, Fang H, Miranda RN, Yin CC, Hussein SE, Jia F, Tang Z, et al. Immunophenotypic and molecular features of acute myeloid leukemia with plasmacytoid dendritic cell differentiation are distinct from blastic plasmacytoid dendritic cell neoplasm. Cancers (Basel) 2022;14:3375. doi: 10.3390/cancers14143375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang Y, Wang Y, Chang Y, Yuan X, Hao L, Shi H, Lai Y, Huang X, Liu Y. Myeloid neoplasms with elevated plasmacytoid dendritic cell differentiation reflect the maturation process of dendritic cells. Cytometry A. 2020;97:61–69. doi: 10.1002/cyto.a.23953. [DOI] [PubMed] [Google Scholar]
- 62.Gong X, Li C, Wang Y, Rao Q, Mi Y, Wang M, Wei H, Wang J. Mature plasmacytoid dendritic cells associated with acute myeloid leukemia show similar genetic mutations and expression profiles to leukemia cells. Blood Sci. 2022;4:38–43. doi: 10.1097/BS9.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li G, Cheng L, Su L. Phenotypic and functional study of human plasmacytoid dendritic cells. Curr Protoc. 2021;1:e50. doi: 10.1002/cpz1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao W, Chan A, Waarts MR, Mishra T, Liu Y, Cai SF, Yao J, Gao Q, Bowman RL, Koche RP, et al. Plasmacytoid dendritic cell expansion defines a distinct subset of RUNX1-mutated acute myeloid leukemia. Blood. 2021;137:1377–1391. doi: 10.1182/blood.2020007897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lucas N, Duchmann M, Rameau P, Noël F, Michea P, Saada V, Kosmider O, Pierron G, Fernandez-Zapico ME, Howard MT, et al. Biology and prognostic impact of clonal plasmacytoid dendritic cells in chronic myelomonocytic leukemia. Leukemia. 2019;33:2466–2480. doi: 10.1038/s41375-019-0447-3. [DOI] [PubMed] [Google Scholar]
- 66.Klanova M, Lorkova L, Vit O, Maswabi B, Molinsky J, Pospisilova J, Vockova P, Mavis C, Lateckova L, Kulvait V, et al. Downregulation of deoxycytidine kinase in cytarabine-resistant mantle cell lymphoma cells confers cross-resistance to nucleoside analogs gemcitabine, fludarabine and cladribine, but not to other classes of anti-lymphoma agents. Mol Cancer. 2014;13:159. doi: 10.1186/1476-4598-13-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopez-Millan B, Diaz de la Guardia R, Roca-Ho H, Anguita E, Islam ABMMK, Romero-Moya D, Prieto C, Gutierrez-Agüera F, Bejarano-Garcia JA, Perez-Simon JA, et al. IMiDs mobilize acute myeloid leukemia blasts to peripheral blood through downregulation of CXCR4 but fail to potentiate AraC/Idarubicin activity in preclinical models of non del5q/5q-AML. Oncoimmunology. 2018;7:e1477460. doi: 10.1080/2162402X.2018.1477460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goulart H, Kantarjian H, Borthakur G, Daver N, DiNardo CD, Jabbour E, Pemmaraju N, Alvarado Y, Atluri H, Yilmaz M, et al. Cladribine, idarubicin, and cytarabine (CLIA) for patients with relapsed and/or refractory acute myeloid leukemia: A single-center, single-arm, phase 2 trial. Cancer. 2025;131:e35840. doi: 10.1002/cncr.35840. [DOI] [PubMed] [Google Scholar]
- 69.Sharon D, Cathelin S, Mirali S, Di Trani JM, Yanofsky DJ, Keon KA, Rubinstein JL, Schimmer AD, Ketela T, Chan SM. Inhibition of mitochondrial translation overcomes venetoclax resistance in AML through activation of the integrated stress response. Sci Transl Med. 2019;11:eaax2863. doi: 10.1126/scitranslmed.aax2863. [DOI] [PubMed] [Google Scholar]
- 70.Pemmaraju N, Deconinck E, Mehta P, Walker I, Herling M, Garnache-Ottou F, Gabarin N, Campbell CJV, Duell J, Moshe Y, et al. Recent advances in the biology and CD123-directed treatment of blastic plasmacytoid dendritic cell neoplasm. Clin Lymphoma Myeloma Leuk. 2024;24:e130–e137. doi: 10.1016/j.clml.2023.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Pammaraju N, Kantarjian H, Sweet K, Wang ES, Lane AA, Ali H, Stein AS, Yacoub A, Rizzieri D, Vasu S, et al. Poster: AML-397 Integrated Safety Analysis of Tagraxofusp, a CD123-Directed Targeted Therapy, in Patients With Hematologic Malignancies. Clin Lymphoma Myeloma Leuk. 2022;22:S246–S247. doi: 10.1016/S2152-2650(22)01287-3. [DOI] [Google Scholar]
- 72.DiPippo AJ, Wilson NR, Pemmaraju N. Targeting CD123 in BPDCN: An emerging field. Expert Rev Hematol. 2021;14:993–1004. doi: 10.1080/17474086.2021.1988848. [DOI] [PubMed] [Google Scholar]
- 73.Aldoss I, Clark M, Song JY, Pullarkat V. Targeting the alpha subunit of IL-3 receptor (CD123) in patients with acute leukemia. Hum Vaccin Immunother. 2020;16:2341–2348. doi: 10.1080/21645515.2020.1788299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lane AA. Targeting CD123 in AML. Clin Lymphoma Myeloma Leuk. 2020;20((Suppl 1)):S67–S68. doi: 10.1016/S2152-2650(20)30466-3. [DOI] [PubMed] [Google Scholar]
- 75.Roussel X, Garnache Ottou F, Renosi F. Plasmacytoid dendritic cells, a novel target in myeloid neoplasms. Cancers (Basel) 2022;14:3545. doi: 10.3390/cancers14143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequencing and raw data generated in the present study may be found in the BioProject database under accession number PRJNA1267814 or at the following URL: https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1267814.