Abstract
The World Health Organization (WHO) has declared the mpox outbreak a public health emergency of international concern (PHEIC). Safe and efficient vaccines against the mpox virus (MPXV) are urgently needed to impede the surge in cases. Here, we report the results of a preclinical study employing different dosing strategies on a vaccine candidate named NTV, obtained via targeted gene deletion in the Tiantan strain vaccinia virus, resulting in a replication-deficient variant. Following optimisation of the NTV immunization dose and confirmation of its protective efficacy against MPXV in a mouse model, we demonstrate that a two-shot NTV regimen in macaques elicits significant neutralizing antibody and cellular immune responses, providing efficient protection against MPXV challenge. Notably, we find that a single NTV dose or long-term immunization in macaques offer effective protection against moderate or severe mpox disease by enhancing cellular immunity and rapidly evoking neutralizing antibodies. These results demonstrate the vaccine’s potential for emergency use and for long-lasting protection. Safety evaluations show no adverse effects in macaques receiving triple the standard dosage in three consecutive injections. These findings highlight the potential of the NTV vaccine candidate with key advantages, including robust immunogenicity, sustained protective efficacy, and safety in preclinical settings.
Subject terms: Pox virus, Viral infection, Preventive medicine, Live attenuated vaccines
Monkeypox or Mpox represents a worldwide health emergency, and efficient vaccines are urgently needed. Here authors present a modified vaccinia virus as vaccine candidate, generated from the Tiantan strain by targeted gene deletion to make it replication-deficient, which elicits robust immune response in both mouse and macaque models, with favourable safety and long-lasting protection profiles in the non-human primates.
Introduction
In August 2024, the World Health Organization (WHO) once again declared mpox a public health emergency of international concern (PHEIC) due to escalating incidence in Africa and a significant public health risk to other countries, higher fatality rate and probably more complex transmission route compared to the 2022-2023 mpox outbreak. The recent mpox outbreaks highlighted the urgent need for effective vaccines, particularly in Africa, where 107 mpox-related deaths were recorded in a week and vaccines have been largely unavailable1. Developing vaccines that are safe, effective, durable, and globally distributable is crucial for controlling the ongoing outbreak. To date, several vaccines have been approved for global use: ACAM2000 (a live replicating vaccinia virus vaccine, United States), MVA-BN (a non-replicating modified vaccinia virus vaccine, Denmark, marketed as JYNNEOS in the U.S.), LC16m8 (a third-generation, highly attenuated vaccinia virus vaccine, Japan)2–5. WHO recommends the MVA-BN and LC16m8 vaccines against mpox, with ACAM2000 as an alternative when other options are unavailable6. Although several countries have pledged to donate doses, access to current approved vaccines remains limited supply7.
Much of our understanding of poxvirus biology is derived from studies on the vaccinia virus (VACV), a large double-stranded DNA virus belonging to the orthopoxviruses family. The conservation of essential genes among orthopoxviruses has been known to provide cross-protection against VACV and mpox virus (MPXV) infection mediated by classical smallpox vaccine derived from live replicating vaccinia virus8,9. The Tiantan strain of vaccinia virus (Tiantan vaccinia virus, TTV), isolated and developed in China, played a pivotal role in the global eradication of smallpox, with 500 million people vaccinated. TTV is renowned for its strong immunogenicity and long-lasting immunity10. Our previous study showed that the Chinese population vaccinated with TTV before 1980 retains low levels of residual immunity against VACV and MPXV11. The non-replicating Tiantan vaccinia virus (NTV) was developed as a replication-deficient vaccine derived from TTV through targeted gene deletion12. There are certain differences in the terminal hairpin structure and ORF composition compared to MVA-BN. NTV shares 146 ORFs with MVA-BN, with 20 ORFs unique to NTV (mainly in region B) and 12 unique ORFs carried by MVA-BN (mainly in regions C-K)13. The proteins encoded by these differing ORFs are involved in virus host range, replication, transcription, translation, and virulence14. By screening in both primary chicken embryo and human cells, NTV cannot efficiently replicate in human cells, producing little to no progeny virus while propagate well in chicken embryo fibroblasts, enabling the production of high-titer virus and facilitating vaccine manufacturing as a reliable viral vector15–17. In mice and macaques, NTV has proven to be as effective as replication-competent TTV in stimulating neutralizing antibodies against MPXV and VACV11. More comprehensive data on NTV vaccines are still needed to evaluate their immune response, sustained immunity, protective efficacy against MPXV, and in vivo safety.
To explore the potential of the NTV vaccine candidate as a backup option to approved vaccines for mpox epidemics, we assess its immunogenicity, pre-clinical safety, protective efficacy under escalating-dose regimens, with a focus on extended long-lasting immunity and single-dose immunization in non-human primates. These promising findings support the development of NTV as a vaccine candidate, with a phase I clinical trial now underway.
Results
Immunogenicity and protective efficacy of NTV vaccine in mice
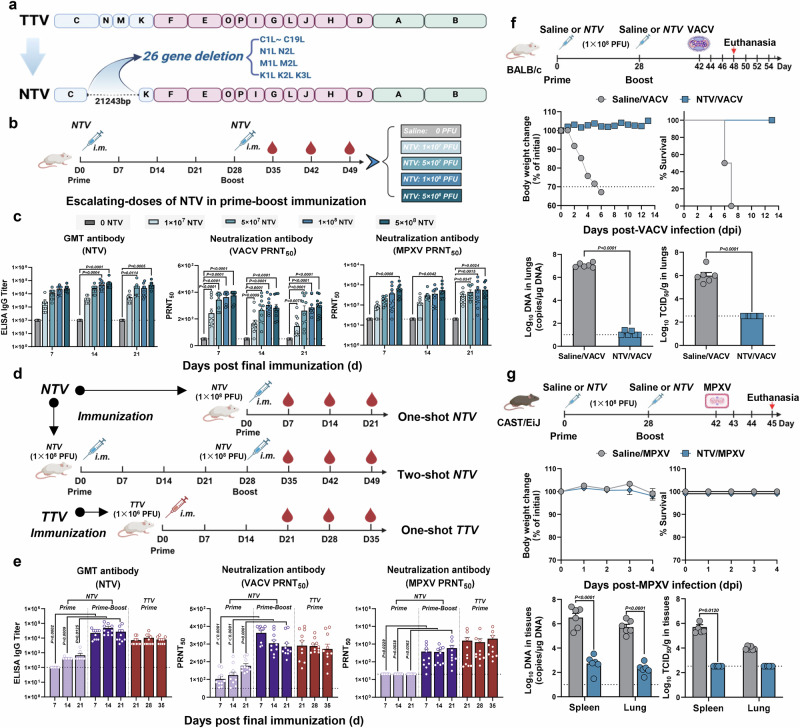
The NTV vaccine was derived from Dr. Tan’s Lab in National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention. Compared to the original strain (the TTV strain)17, the NTV genome lost 21,243 nucleosides between the C to K regions, resulting in the deletion of 26 genes associated with host range and virulence11,17,18, including C1L to C19L, N1L to N2L, M1L to M2L, and K1L to K3L (Fig. 1a). The NTV genome is 171,729 bp in length with a GC content of approximately 33% (National Genome Data Center in China, No: C_AA058750.1).
Fig. 1. The immunogenicity and protective efficacy of NTV vaccine in mice.
a Schematic diagram of TTV and NTV genome. The deleted genes in NTV genome compared with TTV genome are indicated. b Schematic illustrating experiment design in mice. Immunizations were performed with different doses of NTV or a mock saline treatment via intramuscular (i.m.) route on day 0 and 28 (n = 10/group). Created in BioRender. Yang, C. (2025) https://BioRender.com/qfm3k4e. c Humoral immune responses were assessed by binding antibody to NTV (geometric mean titers, GMT; left panel) via ELISA assay or by neutralizing antibodies to VACV (middle panel) and MPXV (right panel) using 50% plaque reduction neutralization tests (PRNT50) following NTV boost immunization (two-shot NTV immunization regimen). n = 10 biological replicates per group. d Schematic illustrating experiment design in mice. Immunizations with different shots of NTV or TTV via i.m. route (n = 10/group). Created in BioRender. Yang, C. (2025) https://BioRender.com/qfm3k4e. e Humoral immune responses were assessed by binding antibodies to NTV (GMT, left panel) via ELISA assay or by neutralizing antibodies to VACV (middle panel) and MPXV (right panel) via PRNT50 assay following NTV prime immunization (one-shot NTV immunization regimen), NTV boost immunization (two-shot NTV immunization regimen), and TTV immunization (one-shot NTV immunization regimen). n = 10 biological replicates per group. f, g Prime-boost regimen of NTV vaccine provides protection for mice against VACV and MPXV challenge. NTV (1 × 108 PFU) or saline were performed intramuscularly on day 0 and 28. Fourteen days after the NTV booster, BALB/c mice were challenged with 1 × 106 TCID50 of VACV WR via the intranasal (i.n.) route (f, n = 12/group), and CAST/EiJ mice were challenged with 1 × 106 TCID50 of MPXV clade IIb via the intraperitoneal (i.p.) route (g, n = 6/group). Created in BioRender. Yang, C. (2025) https://BioRender.com/h1at7w0. Weight loss and survival rate (n = 6/group), viral loads and titers in the lungs and spleen (n = 6/group) were detected after viral challenge. The dashed line indicates the limit of detection of the assay. Differences between the groups were evaluated using two-way ANOVA (c, g) or a two-sided unpaired Student’s t-tests (e, f). The data are presented as the means ± SEM. Source data are provided as a Source Data file.
Before moving to the non-human primate model, the immunogenicity and protective efficacy against viral challenge of NTV were initially evaluated in small animal models. For the immunogenicity study, BALB/c mice were immunized with NTV vaccine using escalating-doses (1 × 107 PFU, 5 × 107 PFU, 1 × 108 PFU and 5 × 108 PFU per dose) with prime-boost (two-shot) strategy. Blood samples were collected weekly (on days 7, 14, and 21 post-final dose for NTV and days 21, 28, and 35 post-prime for TTV) until the end of the study, and were analyzed for binding and neutralizing antibody against VACA and MPXV (Fig. 1b). Binding and neutralizing antibody levels induced by the 1 × 108 PFU dose of NTV were similar to those produced by the 5 × 108 PFU dose (Fig. 1c). Then, BALB/c mice were immunized with 1 × 108 PFU of NTV vaccine via either prime-only (one-shot) or prime-boost (two-shot) strategy. A group receiving a single dose of TTV (1 × 106 PFU/dose) was included as a comparison. Blood samples were collected at indicated days for analysis of binding and neutralizing antibody against VACA and MPXV (Fig. 1d). Moreover, antibody induction by NTV was observed following the prime dose and significantly increased after the booster. Importantly, comparable antibody levels were detected following the NTV booster and TTV immunization (Fig. 1e).
Next, NTV-specific cellular immune response was examined by enzyme-linked immunosorbent spot (ELISpot) assays on day 28 after one-shot immunization or on day 42 (day 14 after booster) following prime immunization (Supplementary Fig. 1a). Modest and comparable IFN-γ production was observed after either NTV and TTV priming, with a stronger T cell response detected after the NTV booster (Supplementary Fig. 1b). Similarly, binding and neutralizing antibody induction following the prime dose and a significant increase after the booster were confirmed in the rabbit model (Supplementary Fig. 1c–e).
To evaluate the protective efficacy of the two-shot NTV vaccine, we employed two small animal models: BALB/c mice challenged with the VACV WR strain (Fig. 1f) and CAST/EiJ mice challenged with the MPXV clade IIb strain (Fig. 1g). Protective efficacy was evaluated using two complementary endpoints: body weight loss and mortality, as well as tissue viral titers and viral loads. In the VACV challenge, NTV vaccination prevented body weight loss and mortality in BALB/c mice19 (Fig. 1f). In contrast, the MPXV challenge in CAST/EiJ mice showed no significant body weight loss or mortality in either vaccinated or control groups20 (Fig. 1g). Notably, pre-challenge vaccination significantly reduced tissue viral loads and viral titers in both models (Fig. 1f, g).
Potent humoral immune and T cell responses of NTV in rhesus macaques
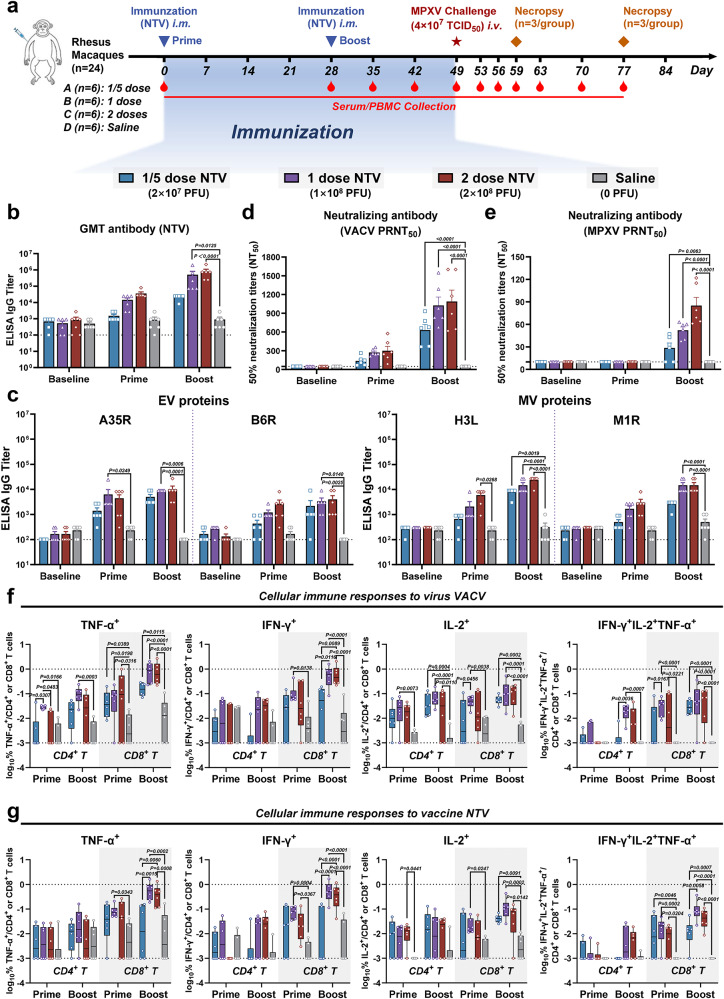
Next, we vaccinated twenty-four rhesus macaques, dividing them into four experimental groups (n = 6 per group). The groups received prime and boost immunizations with different doses of NTV (1/5 dose NTV: 2 × 107 PFU; 1 dose NTV: 1 × 108 PFU; 2 doses NTV: 2 × 108 PFU) or a mock saline treatment via intramuscular (i.m.) injection on days 0 and 28. Humoral and cellular immune responses were evaluated after immunization (Fig. 2a). We examined binding and neutralizing antibodies to VACV and MPXV at baseline, post-prime, and post-boost immunization in each macaque group. Comparably higher IgG titers were induced by the 1 dose and 2 doses vaccines compared to 0.2 doses group following the NTV boost (Fig. 2b). Similar trends were observed for MPXV antigen-specific IgG titers (Fig. 2c) targeting A35R and B6R (EV proteins) and H3L and M1R (MV proteins), as well as VACV- and MPXV-neutralizing antibodies (Fig. 2d, e), with significant increases detected after the booster immunization in all vaccinated macaques.
Fig. 2. Robust humoral and cellular immune responses in NTV-vaccinated rhesus macaques.
a Schematic diagram of two-dose NTV immunization regimen, sample collection, and challenge schedule in rhesus macaques. Created in BioRender. Yang, C. (2025) https://BioRender.com/pmjfete. Immunizations were performed with different doses of NTV (1/5 dose NTV: 2 × 107 PFU; 1 dose NTV: 1 × 108 PFU; 2 doses NTV: 2 × 108 PFU) or a mock saline treatment via intramuscular (i.m.) route on day 0 and 28, and challenge was performed with 4 × 107 TCID50 of MPXV via intravenous (i.v.) route on day 49 after initial immunization (n = 6/group). Specific IgG binding titers against NTV (b) or MPXV-specific antigen (c) (A35R, B6R (extracellular virion, EV proteins), H3L, M1R (mature virion, MV proteins)) at baseline (day 0), after NTV prime (day 28) and boost (day 42) immunization were determined by ELISA. n = 6 biological replicates per group. The PRNT50 was determined by neutralizing antibody assay based on VACV (d) or MPXV (e) at baseline (day 0), after NTV prime (day 28) and boost (day 42) immunization. The dashed line indicates the limit of detection of the assay. n = 6 biological replicates per group. CD4+ and CD8+ T cell responses to virus VACV (f) or vaccine NTV (g) by TNF-α, IFN-γ, and IL-2 intracellular cytokine staining (ICS) assays following NTV prime (day 28) and boost (day 42) immunization. The lower and upper limits of detection are indicated where applicable. n = 6 biological replicates per group. Responses depicted are % TNF-α, IFN-γ, IL-2 single-positive or TNF-α/IFN-γ/IL-2 triple-positive CD4+ or CD8+ T cells following VACV or NTV stimulation. Boxplots represent the median (central line), interquartile range (IQR, box boundaries), whiskers extending to the minimum to maximum values. Differences between the groups were evaluated using two-way ANOVA. The data are presented as the means ± SEM (b–e) or the min to max (f, g). Source data are provided as a Source Data file.
We next assessed the impact of different NTV doses on cellular immune responses by intracellular cytokine staining (ICS) assays. The proportions of TNF-α, IFN-γ, and IL-2 in CD4+ and CD8+ T cells from peripheral blood mononuclear cells (PBMCs) were measured using flow cytometry at baseline (pre-immunization), after the prime and post-boost immunizations with varying NTV doses (Supplementary Fig. 2a). CD8+ T cell responses to VACV and NTV were observed at the prime stage and increased after the booster. Moreover, CD8+ T cell function in 1 dose NTV group was comparable to that in the 2 doses group (Fig. 2f). CD4+ T cell responses showed similar trends but were generally lower than those of CD8+ T cell responses across most groups, except for CD4+ IL-2+ (Fig. 2g). Notably, besides the single-positive TNF-α, IFN-γ, and IL-2 T cell responses, polyfunctional T cells (double positive: IFN-γ+ IL-2+, IFN-γ+ TNF-α+, IL-2+ TNF-α+, and triple positive: IFN-γ+ IL-2+ TNF-α+) were also detected, indicating strong immune functionality, which may contribute to sufficient viral control in vivo (Fig. 2f, g, right panels and Supplementary Fig. 2b, c).
Protective efficacy of NTV prime-boost against MPXV challenge in rhesus macaques
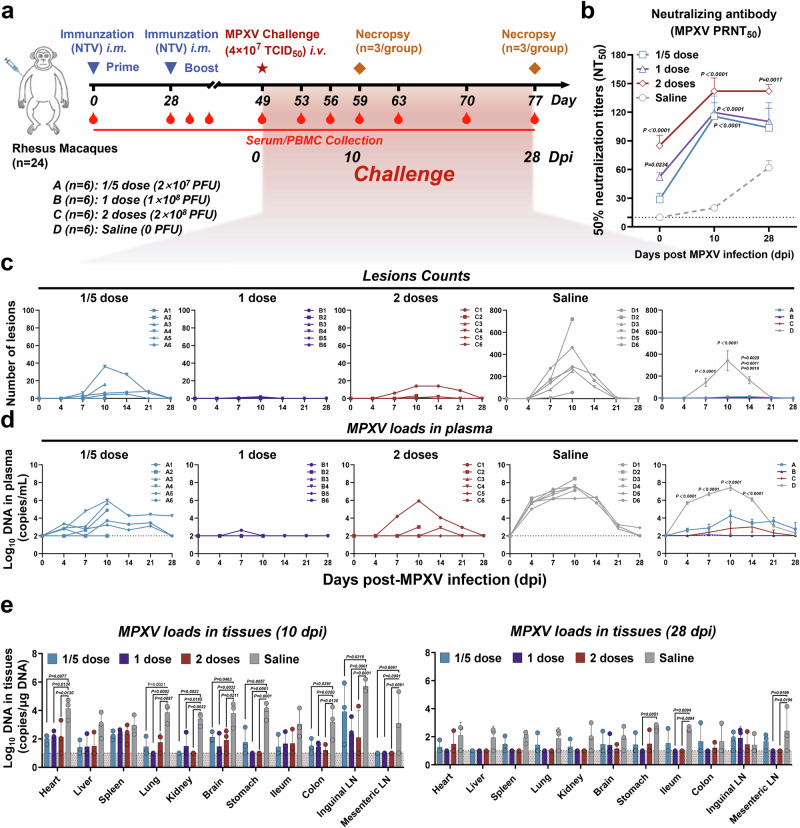
Twenty-one days after the NTV booster, all macaques were challenged with 4 × 107 TCID50 of MPXV clade IIb (MPXV-B.1-China-C-Tan-CQ01) via the intravenous (i.v.) route on day 49, and monitored for 10 days (with 3 macaques euthanized per group) and 28 days (with 3 remaining macaques euthanized per group) for neutralizing antibodies, and disease manifestation (such as weight loss, body temperature, and skin lesions), and viral loads in plasma and tissues (Fig. 3a). Despite initially slightly higher levels of neutralizing antibodies against MPXV in 1 dose and 2 doses groups, a faster and more robust increase was observed in all NTV-immunized groups when compared with the saline-treated control group (Fig. 3b). Clinical disease was evaluated by counting the number of skin lesions after MPXV challenge and classifying their severity based on the human grading system21. In the saline-treated group, animals developed a median of 142 lesions per animal by 7 days post-infection (dpi) (range 8-274), peaking at a median of 341 lesions (exceeding 250 as grade IV serious) by 10 dpi (range 57-719) over the entire body (limbs, face, perineal region, palms, and soles were included) (Fig. 3c and Supplementary Fig. 3a). In contrast, NTV-vaccinated animals exhibited significantly fewer skin lesions post-MPXV challenge, assessing their severity based on the human grading system. The 1/5 dose group showed a median of 11 lesions (range 0-36, grade I mild: 1-25), the 1 dose group had a median of 0.7 lesions (range 0-2, mild), and the 2 doses group displayed a median of 3.2 lesions (range 0-14, mild). These results indicate near-complete clinical protection in the 1 dose group, with all dose groups providing protection against moderate disease (26-100 lesions, Fig. 3c and Supplementary Fig. 3b). Additionally, no significant changes in body weight or body temperature were observed in any of the treatment groups throughout the MPXV IIb challenge observation period (Supplementary Fig. 3c, d). Next, we performed a pathological analysis of tissues during the acute phase of MPXV infection, examining samples from skin lesions, non-lesion skin, lung, spleen, liver, heart, brain, intestine, and lymph nodes collected from macaques autopsied at 10 dpi (Supplementary Fig. 4a). Our findings revealed no significant differences in the detected tissues, except for skin lesions, between the saline-treated infection model group and the two-shot NTV vaccine groups22 (Supplementary Fig. 4b).
Fig. 3. Prime-boost regimen of NTV vaccine protects rhesus macaques from MPXV challenge.
a Schematic illustrating experiment design in rhesus macaques. Created in BioRender. Yang, C. (2025) https://BioRender.com/pmjfete. b The PRNT50 was determined by neutralizing antibody assay based on MPXV on days 0, 10, and 28 post MPXV challenge in different doses NTV immunization groups. n = 6 biological replicates per group. c Poxvirus skin lesion counts were plotted and compared on days 0, 4, 7, 10, 14, 21, and 28 following MPXV challenge in different doses NTV immunization groups. n = 6 biological replicates per group. d Plasma log10 viral DNA copies/ml per animal were assessed on days 0, 4, 7, 10, 14, 21, and 28 following MPXV challenge in the different NTV dose immunization groups. n = 6 biological replicates per group. e Tissues samples (heart, liver, spleen, lung, kidney, brain, stomach, ileum, colon, inguinal lymph node (LN), mesenteric LN) log10 viral DNA copies/μg DNA collected from euthanized rhesus macaques on days 10 and 28 following MPXV challenge in the different NTV dose immunization group were assessed via quantitative PCR. n = 3 biological replicates per group. The dashed line indicates the limit of detection of the assay. Differences between the groups were evaluated using two-way ANOVA. The data are presented as the means ± SEM. Source data are provided as a Source Data file.
Plasma viral DNA in the NTV immunization groups was determined by quantitative reverse transcription polymerase chain reaction (qRT-PCR) assays following MPXV challenge. Viral loads in plasma were detectable at 4 dpi in the saline-treated group, with median DNA copies per milliliter on days 4, 7, 10 and 14 were 105.69, 106.70, 107.41, and 106.08, respectively (Fig. 3d). In comparison, the median DNA copies per milliliter was significantly lower in the 1/5 dose and 2 doses groups, at 104.25 and 102.82, respectively. Notably, no viral loads were detected in all monkeys’ plasma of the 1 dose group by 10 dpi (Fig. 3d). Then, we conducted correlation analyses between post-boost humoral/cellular immunity and disease severity parameters (skin lesions and plasma viral loads) at 10 dpi. Our data demonstrated a predominant inverse correlation between disease progression and cellular response, as well as neutralizing antibodies, especially CD8+ T-cell functionality encompassing both monofunctional and polyfunctional subsets (Supplementary Fig. 5a–c).
Three rhesus macaques from each group were sacrificed on either day 10 or day 28 post-MPXV infection, and viral loads in the heart, liver, spleen, lung, kidney, brain, stomach, ileum, colon, inguinal lymph node (LN), and mesenteric LN were assessed. At 10 dpi, the viral loads in most tissues of the saline-treated group were approximately 104 copies/μg DNA, with the highest levels in inguinal LN, reaching about 106 copies/μg DNA (Fig. 3e, left panel). In contrast, animals vaccinated with NTV showed lower viral loads compared to the saline-treated group (Fig. 3e, left panel). By 28 dpi, viral loads were either undetectable or present at detectable trace levels in tissues of all groups, consistent with the time to disappearance of skin lesions (Fig. 3e, right panel). Similarly, pathological analysis of tissues during the recovery phase (28 dpi) of MPXV infection showed no significant differences between the saline-treated infection model group and the two-shot NTV vaccine groups22 (Supplementary Fig. 6a, b). Taken together, all NTV immunization groups provided effective protection, and remarkably, the 1 dose NTV two-shot regimen provides near-complete protection against MPXV challenge in rhesus macaques.
Long-lasting protection of NTV prime-boost against MPXV challenge in rhesus macaques
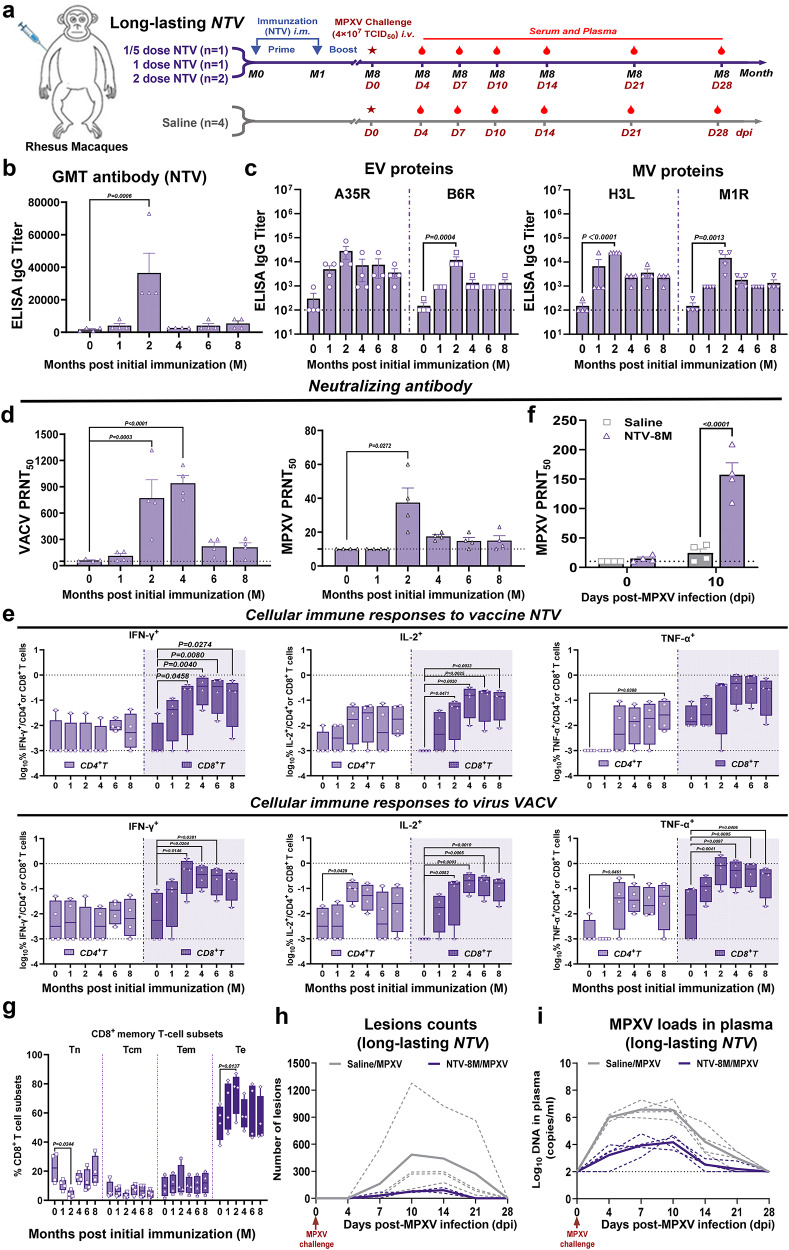
Long-lasting humoral and cellular memory immunity is critical for vaccinated individuals with protective immunity against mpox10,23. Next, using the above vaccinated macaques from different NTV dose groups (1/5 dose NTV, n = 1; 1 dose NTV, n = 1; 2 doses NTV, n = 2), we extended our experiment to evaluate the long-lasting protective efficacy of two-shot NTV immunization. MPXV challenge was performed eight months with robust CD8+ T cell response and relatively lower neutralizing antibody levels following the prime immunization, to assess the durability of NTV vaccine protection (Fig. 4a). We found that a significant increase in IgG titers against NTV at 2 months post initial immunization and rapidly decline after 4 months (Fig. 4b), but MPXV antigen-specific antibody titers peaked at 2 months post-initial immunization (1-month post-NTV boost) and remained the relative high levels through 8 months (Fig. 4c). VACV- and MPXV-neutralizing antibodies significantly increased at 2 months after primary immunization but decreased to lower levels by 6 and 4 months, respectively (Fig. 4d). Cellular immune responses to NTV or VACV were assessed by ICS assay at 0, 1, 2, 4, 6, and 8 months post-priming. CD8+ T-cell responses against NTV and VACV increased at 1 month post-priming and remained at relatively high levels at 8 months (Fig. 4e). CD4+ T cell responses were also present, though a bit lower than the CD8+ T cell responses. We observed significantly higher neutralizing antibody levels in the vaccinated group at day 10 post MPXV challenge (Fig. 4f). Moreover, we characterized the memory phenotype of T cells, revealing a significant decrease in naïve CD8+ T cells (Tn) frequencies accompanied by an expansion of terminally differentiated effector T cells (Te) at 2 months post initial immunization. While central memory (Tcm) and effector memory (Tem) subsets demonstrate no statistically significant changes, a trend toward Tem accumulation emerged over time (Fig. 4g and Supplementary Fig. 7a, b).
Fig. 4. Prime-boost regimen of NTV vaccine provides long-lasting protection for rhesus macaques against MPXV challenge.
a Schematic diagram of the long-lasting protective efficacy of NTV vaccine against MPXV challenge in rhesus macaques (n = 4/group). Created in BioRender. Yang, C. (2025) https://BioRender.com/pmjfete. Specific IgG binding titers against NTV (b) or MPXV-specific antigen (c) (A35R, B6R (extracellular virion, EV proteins), H3L, M1R (mature virion, MV proteins)) were determined by ELISA. n = 4 biological replicates per group. d The PRNT50 was determined by neutralizing antibody assay based on VACV (left panel) or MPXV (right panel). n = 4 biological replicates per group. e CD4+ and CD8+ T cell responses to vaccine NTV (upper panel) or virus VACV (lower panel) by TNF-α, IFN-γ, and IL-2 ICS assays. The lower and upper limits of detection are indicated where applicable. n = 4 biological replicates per group. Boxplots represent the median (central line), interquartile range (IQR, box boundaries), whiskers extending to the minimum to maximum values. f The PRNT50 was determined by neutralizing antibody assay post MPXV challenge. n = 4 biological replicates per group. g Proportion of CD8+ T cell subsets in PBMCs of rhesus macaques were analyzed via flow cytometry. The percentage levels of CD8+ T subsets (Tn: naïve T cells, Tcm: central memory T cells, Tem: effector memory T cells, Te: terminally differentiated effector T cells) were analyzed via flow cytometry. n = 4 biological replicates per group. Boxplots represent the median (central line), interquartile range (IQR, box boundaries), whiskers extending to the minimum to maximum values. Skin lesion counts (h), and plasma log10 viral DNA copies/ml (i) were assessed on indicated days following MPXV challenge in long-lasting NTV immunization (the purple lines) and saline treatment (the gray lines) groups. Solid lines indicate the median number of lesions (h) or viral loads (i) for each group, while dashed lines represent individual animal data. Differences between the groups were evaluated using one-way ANOVA (b–d) or two-way ANOVA (e–g). The data are presented as the means ± SEM (b–d, f), or the min to max (e, g). Source data are provided as a Source Data file.
Skin lesions were counted at 0, 4, 7, 10, 14, 21, and 28 days post MPXV challenge. The number of skin lesions in the NTV-vaccinated group was significantly lower than in the model control group, with a median of 76 lesions (26-100 as grade II moderate) compared to 432 lesions (exceeding 250 as grade IV serious) at 10 dpi, 8 months post-prime immunization (Fig. 4h). Consistent with the reduction in skin lesions, plasma viral loads were also significantly lower in the NTV-vaccinated group, with a median of 104.16 DNA copies per milliliter at 8 months post-challenge compared to 106.53 in the model control group (Fig. 4i). In summary, while neutralizing antibody levels in rhesus macaques decreased to low levels 8 months after NTV immunization, specific CD8+ T cell responses remained at a high level. Based on the human grading system, the median lesion count of 76 in the NTV-vaccinated group at 8 months post-infection indicates that vaccination provides long-lasting protection against severe mpox disease.
Protective efficacy of NTV prime-alone against MPXV challenge in rhesus macaques
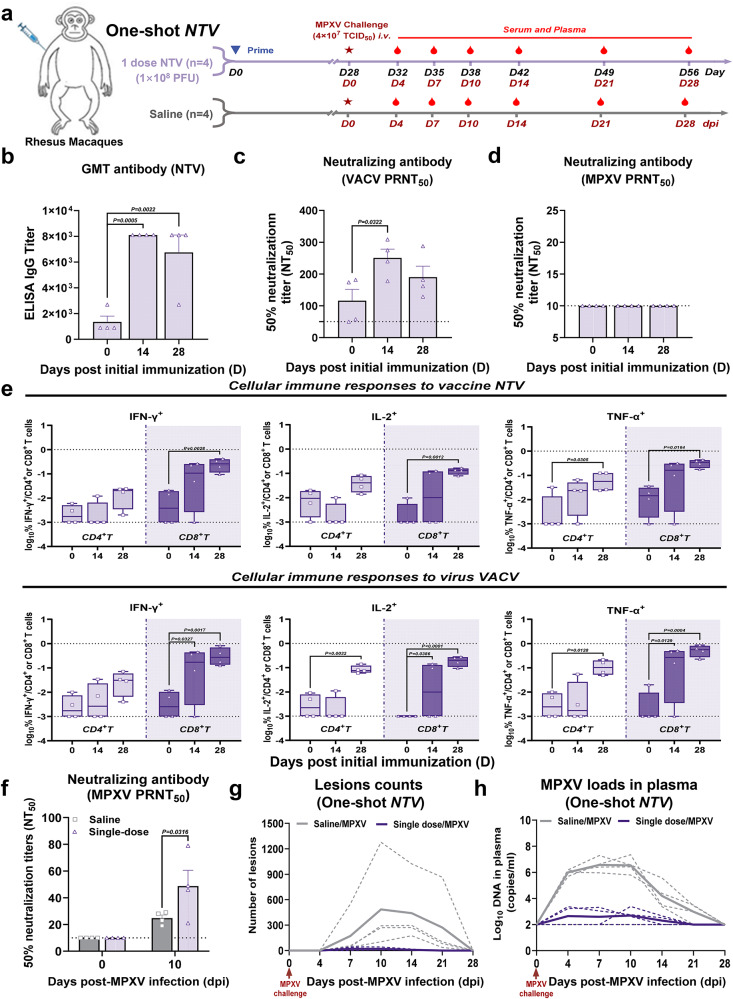
To assess the potential for urgent use of the NTV vaccine during an mpox outbreak, the protective efficacy of a single NTV immunization against MPXV was tested in rhesus macaques. Four animals received 1 dose of NTV, while another four were administered saline as a sham control group. All animals were challenged with 4×107 TCID50 of MPXV at 28 days post-immunization and monitored for another 28 days (Fig. 5a). The levels of binding and neutralizing antibodies in the vaccinated group were significantly increased at 14 and 28 days after immunization (Fig. 5b, c), although MPXV-specific neutralizing antibodies were not detected (Fig. 5d). Cellular immune responses to NTV or VACV were then assessed by ICS assay. IFN-γ-, IL-2-, and TNF-α-secreting CD8+ T cells in PBMC increased at 14 dpi and continue rising to a higher level by 28 dpi (Fig. 5e). In addition, IL-2-, and TNF-α-secreting CD4+ T cells also showed significant increases at 28 dpi (Fig. 5e).
Fig. 5. Single-dose regimen of NTV vaccine provides robust but incomplete protection for rhesus macaques against MPXV challenge.
a Schematic diagram of the single-dose immunization of NTV vaccine against MPXV in rhesus macaques. Created in BioRender. Yang, C. (2025) https://BioRender.com/pmjfete. Sample collection, and challenge schedule in rhesus macaques. Immunizations were performed intramuscularly (i.m.), and challenge was performed intravenously (i.v.) (n = 4/group). b Specific IgG binding titers against NTV on day 0, 14 and 28 post NTV immunization were determined by ELISA. n = 4 biological replicates per group. The PRNT50 was determined by neutralizing antibody assay based on VACV (c) or MPXV (d) on days 0, 14, and 28 post NTV immunization. n = 4 biological replicates per group. e CD4+ and CD8+ T cell responses to vaccine NTV (upper panel) or virus VACV (lower panel) by TNF-α, IFN-γ, and IL-2 ICS assays on days 0, 14, and 28 post NTV immunization. The lower and upper limits of detection are indicated where applicable. Responses depicted are % TNF-α, IFN-γ, IL-2 single-positive CD4+ or CD8+ T cells following VACV or NTV stimulation. The data are presented as the min to max. n = 4 biological replicates per group. f The PRNT50 was determined by neutralizing antibody assay based on MPXV on days 0, and 10 post MPXV challenge in single-dose NTV immunization and saline treatment groups. n = 4 biological replicates per group. g Poxvirus skin lesion counts were plotted and compared on days 0, 4, 7, 10, 14, 21, and 28 following MPXV challenge in single-dose NTV immunization and saline treatment groups. h Plasma log10 viral DNA copies/ml were assessed on days 0, 4, 7, 10, 14, 21, and 28 following MPXV challenge in single-dose NTV and saline immunization groups. The dashed line indicates the limit of detection of the assay. Differences between the groups were evaluated using one-way ANOVA (b–d) or two-way ANOVA (e, f). The data are presented as the means ± SEM (b–d, f) or the min to max (e). Source data are provided as a Source Data file.
A significantly higher level of neutralizing antibodies was observed in the NTV prime-alone group at day 10 post MPXV challenge compared to the model control group (Fig. 5f). Rhesus macaques immunized with a single-dose of NTV develop far fewer skin lesions at 7, 10, and 14 dpi compared to the saline-treated group (~20 lesions vs.~173 lesions, ~20 lesions vs.~485 lesions, ~9 lesions vs.~442 lesions) at 7, 10 and 14 days, respectively, indicating a well-controlled infection (Fig. 5g). Consistent with the reduction in skin lesions, viral loads in immunized macaques were also significantly lower than that in the saline group (Fig. 5h). Collectively, these results show that rhesus macaques immunized with a single dose of NTV provided incomplete protection against moderate mpox disease.
Preclinical safety evaluation of NTV escalating-dosing in cynomolgus macaques
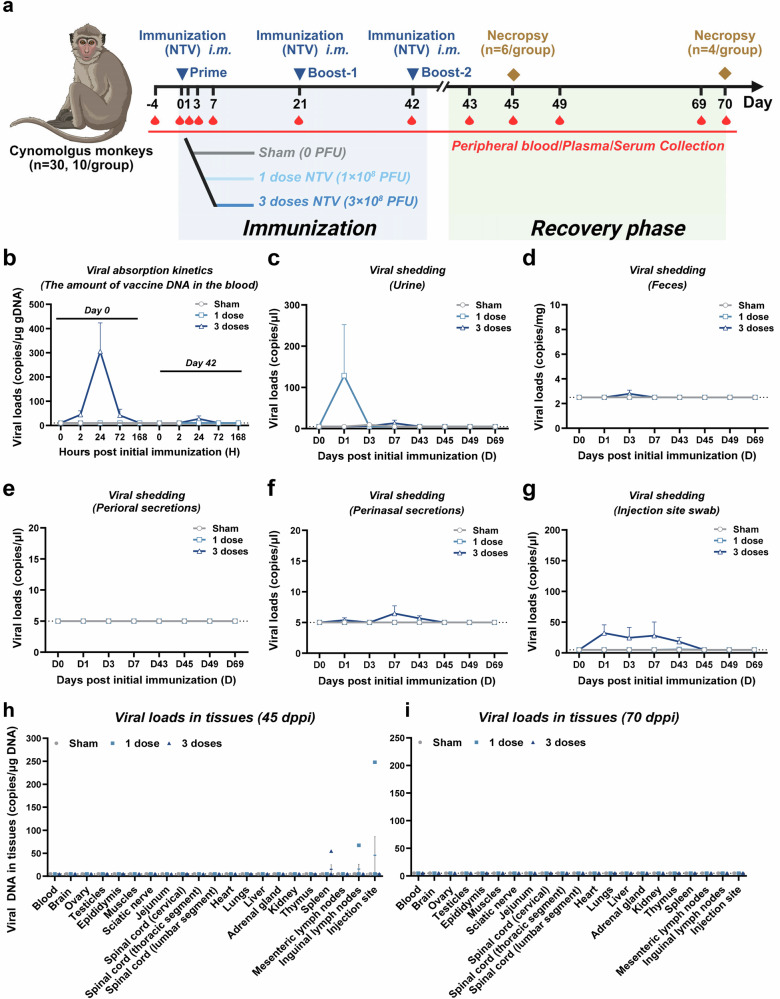
Further safety assessments of the NTV vaccine were conducted in thirty cynomolgus monkeys, that were divided into three groups. Two groups received three-shot intramuscular administrations of either a standard dose or triple the standard dose of NTV at 21-day intervals, while a control group was also included. To evaluate potential adverse effects, 6 monkeys were sacrificed on day 45 and 4 monkeys on day 70 following the initial immunization (Fig. 6a).
Fig. 6. Viral absorption kinetics, viral shedding, viral biodistribution of NTV vaccine in cynomolgus monkeys.
a Schematic diagram of immunization and sample collection, and challenge schedule in cynomolgus monkeys. Different doses of NTV were immunized via intramuscular (i.m.) route (n = 10/group). Created in BioRender. Yang, C. (2025) https://BioRender.com/ylcbdnw. b Viral absorption kinetics in cynomolgus monkeys immunized with different doses of NTV. Viral DNA loads in plasma of cynomolgus monkeys at 0 h, 2 h, 24 h, 72 h, and 168 h post prime (day 0), and boost-2 (day 42) immunization of different doses of NTV (saline, 1 dose and 3 doses). n = 10 biological replicates per group. Viral shedding in urine (c), feces (d), perioral secretions (e), perinasal secretions (f), and injection site swab (g) in cynomolgus monkeys immunized with different doses of NTV. Viral DNA loads in urine, feces, perioral secretions, perinasal secretions, and infection site swab in cynomolgus monkeys on days 0, 1, 3, 7, 43, 45, 49, 69 post prime immunization with different doses of NTV (saline, 1 dose and 3 doses). n = 10 biological replicates per group. Tissues viral DNA copies/μg DNA in cynomolgus monkeys euthanized at days 45 (h) and 70 (i) post prime immunization (dppi) of different doses of NTV (saline, 1 dose and 3 doses) were assessed. n = 5 biological replicates per group. The dashed line indicates the limit of detection of the assay. The data are presented as the means ± SEM. Source data are provided as a Source Data file.
During the study period, no abnormal reactions were observed at the local injection site in any of the animals. NTV vaccine DNA was detectable only in plasma from animals receiving the 3-dose regimen, peaking 24 hours after the initial immunization (10 out of 10 animals, ~300 copies/μg DNA) and rapidly declined. Following the second booster, only 2 animals showed detectable levels of 44 copies/μg DNA after 24 hours (Fig. 6b). Additionally, transient low-level viral shedding was detected in urine, feces, perinasal secretions, and at the injection site, but levels were below or near the detection limit after the second booster and completely undetectable in all tissues by 70 days post-immunization (Fig. 6c–i).
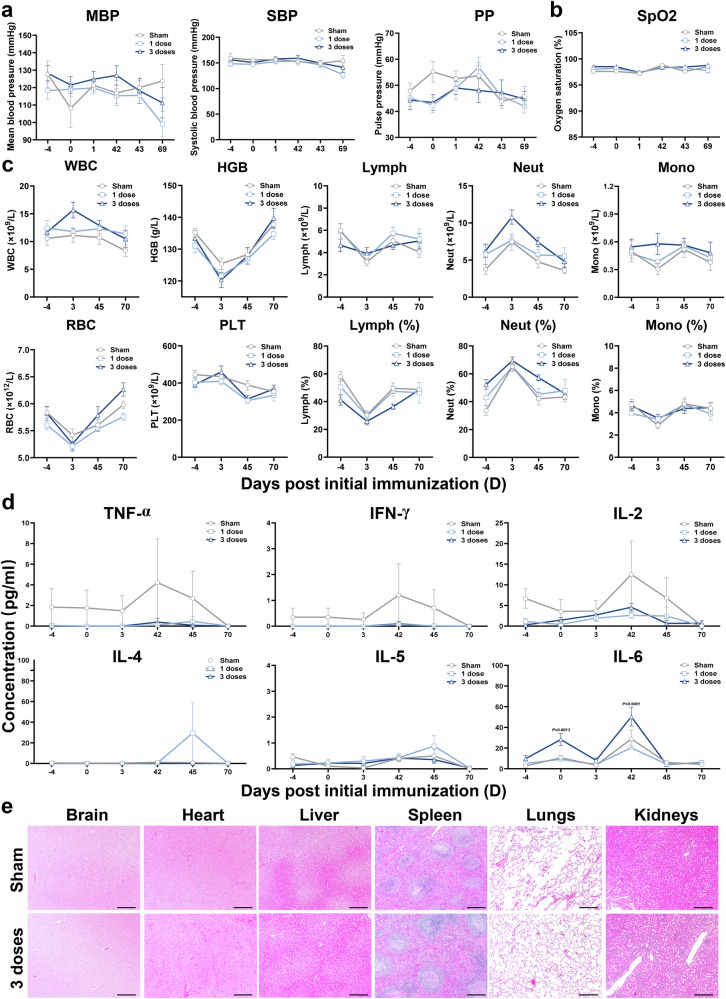
Furthermore, we did not detect significant abnormal changes in blood pressure, oxygen saturation, main blood hematology and clinical chemistry parameters during the observation period (Fig. 7a–c and Supplementary Fig. 8). In addition, we measured serial serum TNF-α, IFN-γ, IL-2, IL-4, IL-5 and IL-6 (Fig. 7d) as well as antinuclear antibodies (Supplementary Fig. 9) in different groups. Only IL-6 levels were elevated but were not considered related to vaccination, given a similar higher baseline level. Finally, we did not detect significant inflammatory abnormalities in brain, heart, liver, spleen, lung and kidney in all animals during the entire observation period (Fig. 7e).
Fig. 7. Blood pressure, oxygen saturation, hematology, cytokine expression, and histopathology of NTV vaccine in cynomolgus monkeys.
a Blood pressure in cynomolgus monkeys immunized with different doses of NTV. The mean blood pressure (MBP), systolic blood pressure (SBP), and pulse pressure (PP) in cynomolgus monkeys post prime immunization with different doses of NTV (saline, 1 dose and 3 doses) were measured by sphygmomanometer (BP-2010E). b Oxygen saturation in cynomolgus monkeys immunized with different doses of NTV. Oxygen saturation (SpO2) of cynomolgus monkeys post prime immunization with different doses of NTV (saline, 1 dose and 3 doses) were measured using pulse oximetry (CMC-60C). c Hematology in cynomolgus monkeys immunized with different doses of NTV. White blood cell count (WBC), red blood cell count (RBC), hemoglobin (HGB), blood platelet count (PLT), lymphocyte (Lymph), lymphocyte ratio (Lymph%, the percentage of lymphocyte count in the white blood cell count), neutrophil (Neut), neutrophil ratio (Neut%, the percentage of neutrophil count in the white blood cell count), monocyte (Mono), and monocyte ratio (Mono%, the percentage of monocyte count in the white blood cell count) in peripheral blood of cynomolgus monkeys post prime immunization with different doses of NTV (saline, 1 dose and 3 doses). d Secreted cytokine quantification in cynomolgus monkeys immunized with different doses of NTV were analyzed. TNF-α, IFN-γ, IL-2, IL-4, IL-5, and IL-6 in serum of cynomolgus monkeys post prime immunization with different doses of NTV (saline, 1 dose and 3 doses). n = 10 biological replicates per group (a–d). e Pathological analysis of cynomolgus monkeys with and without vaccination of 3 doses of NTV. Representative histopathology (H&E) of different tissues, brain, heart, liver, spleen, lungs, and kidneys of cynomolgus monkeys undergoing necropsy on day 45 immunization with saline or 3 doses of NTV. n = 5 biological replicates per group. Bar = 500 μm. Differences between the groups were evaluated using two-way ANOVA. The data are presented as the means ± SEM. Source data are provided as a Source Data file.
Discussion
Non-human primates, which develop mpox-like rashes, skin inflammation and prolonged viral loads after MPXV infection, serve as ideal animal models for studying mpox pathogenesis and immunity following infection and vaccine immunization24,25. Current vaccines aim to offer broad protection against MPXV and related orthopoxviruses, having demonstrated robust protection in non-human primate models19,25–28. Here, beyond demonstrating the robust immunogenicity and protective efficacy of the two-shot NTV vaccine against both VACV and MPXV challenges in small animal models, we conducted two efficacy and one safety studies using a total of 66 macaques to comprehensively evaluate the preclinical performance of the NTV vaccine candidate. We demonstrated that boosting with the NTV vaccine candidate significantly enhances cellular immune response and cross-reactive neutralizing antibodies, providing near-complete protection with 1 dose (1 × 108 PFU) in two-shot regimen against an intravenous MPXV clade IIb challenge in rhesus macaques. In addition, our data demonstrated a significant inverse relationship between T cell responses (CD4+ T and CD8+ T function after boost immunization) and disease severity (viral loads in plasma and skin lesions), which greatly strengthens the clinical relevance of our findings. Furthermore, there was a significant negative correlation between neutralizing antibodies and disease severity (viral loads in plasma). These studies suggest that humoral immunity and cellular immunity play important roles in MPXV challenge protection. Then we extended the observation both long-lasting immunities persisting for 8 months after priming and a single injection of NTV vaccine in rhesus macaques, conferred incomplete but long-lasting protection against severe mpox disease. Due to the limited sample size per dose, we prioritized evaluating shared immune response patterns and long-term protection across all animals rather than distinguishing dose-dependent effects. Future studies with larger cohorts are needed to systematically assess dose-dependent efficacy. These findings are consistent with previous evidence showing the MVA-BN vaccine’s ability to protect non-human primates from death, although sterile immunity was not achieved29,30. Moreover, considering the quick-access of vaccine for vulnerable populations based on the recent epidemic, we systematically evaluated the safety of the NTV vaccine in cynomolgus monkeys by recording various clinical observations, biological indicators, histopathology, as well as viral absorption kinetics, shedding and viral biodistribution at an extremely high dose than that for clinical use. Our results demonstrated no obvious side effects or immunopathological exacerbations, alleviating concerns about the high toxicity associated with TTV or similar second-generation live attenuated vaccines31,32.
Based on our dose-escalation findings, we identified correlates of protective efficacy for NTV boosting, which were associated with higher levels of neutralizing antibodies. Meanwhile, we observed that NTV boosting at low (1/5 dose), medium (1 dose), and high (2 doses) levels resulted in a rapid and substantial elevation in MPXV-neutralizing antibodies after challenge, along with a faster response compared to sham control macaques. A similar observation of a rapid residual neutralizing antibody response was made in challenge experiments following a single injection and long-lasting immunity. Our data confirmed the importance of both MPXV and VACV neutralizing antibody responses in macaques challenged with MPXV, which is consistent with previous reports33. The NTV vaccine in this study focused on quantifying neutralizing antibody titers and T-cell polyfunctionality as primary immunogenicity indicators, and these parameters were closely related to MPXV protection in nonhuman primate models24,25. Mucker et al found that in addition to the role of neutralizing antibodies, the Fab- and Fc-mediated functions of antibodies also play a key role in antagonizing orthopoxviruses by comparing the effectiveness of mRNA-1769, an mRNA vaccine, with MVA-BN vaccine in a non-human primate mpox infection model28. Based on these findings, we recognize that a comprehensive Fab- and Fc-mediated functional profiling of NTV may further elucidate the mechanisms of this vaccine. Moreover, previous studies demonstrated that vaccinia-neutralizing antibody levels induced by MVA-BN remained elevated sevenfold above baseline for six months post-vaccination, even with single-dose regimens. This persistent humoral response likely contributes to the mechanism by which non-replicating vaccine candidates mitigate disease severity34. Similar to MVA-BN, although NTV vaccine candidates cannot confer sterile immunity, their capacity to rapidly establish a residual antibody pool enables early viral neutralization, thereby curbing viremia and preventing severe pathological progression.
In real-world settings, a one-shot or two-shot MVA-BN vaccination in non-primed individuals generates relatively low levels of MPXV-neutralizing antibodies, but provides sufficient protection and significantly reduces the risk of MPXV infection, suggesting that other immune components may also contribute to protective efficacy6,35. In smallpox vaccine studies, long-lasting cross-protective cellular immunity against MPXV can persist for decades in individuals who received smallpox vaccination10,23. Also, additional studies have shown that long-lived VACV/MPXV-specific CD8+ T cells and robust effector memory T-cell responses play a critical role in providing antiviral immunity and mitigating the severity of mpox and other orthopoxvirus infection36–38. Thus, we investigated cellular immunity in addition to humoral response and found that boosting with NTV can elicit stronger VACV or vaccine-specific CD8+ T-cell responses, as indicated by elevated levels of IFN-γ, IL-2 and TNF-α, while significantly enhanced CD8+ T-cell responses were also detected following a single NTV injection. Moreover, both 1 dose and 2 doses of NTV could elicit robust CD8+ T cell response. Considering the potential for dose-dependent adverse effects and cost-effectiveness, 1 dose was selected as the optimal vaccine dose. This decision was also aligned with the principle of balancing immunogenicity, safety, and practical feasibility in vaccine development history. The dissociation observed between robust CD8+ T-cell persistence and suboptimal levels of neutralizing antibodies at 8 months after NTV vaccination raises critical questions about the immune correlates of protection against MPXV. While CD8+ T cells are known to mediate viral clearance through cytolytic mechanisms, their failure to fully prevent MPXV infection in this model may indicate the need for optimized booster vaccination to elicit durable and balanced T cell as well as humoral immunity. Further investigations are required to explore species-specific differences in immune response, lesion distribution, and pathogenesis. Although our study found that the proportion of Tn cells was significantly lower than baseline at 2 months post-immunization, and the proportion of Tcm cells were significantly upregulated. However, the identification of immunodominant cross-reactive epitopes through peptide library screening and MHC genotyping to unravel conserved T-cell immunity across orthopoxviruses remains to be investigated.
In summary, we developed a rhesus macaque model of mpox infection using MPXV clade IIb strain, isolated from the first imported case of mpox in men who have sex with men (MSM) in China in 2022. Moreover, we provided insights into mpox pathogenesis, long-lasting immunity, possibility of emergency use with single vaccine and preclinical safety. The current mpox epidemic is driven by close-contact transmission within the MSM population, approximately 40%-50% of MPXV-infected patients identified as HIV positive. HIV-infected individuals exhibit more severe infection consequences including severe skin lesions, higher rates of genital ulcers and bacterial superinfection, prolonged illness, and ultimately higher mortality especially in those with low CD4 T-cell counts. These indicate the urgent need for a vaccine tailored to immunocompromised individuals that can be rapidly produced and globally distributed. Compared to existing orthopoxvirus vaccines, the NTV vaccine candidate demonstrates advantages. While large-scale immunization with LC16m8 (a strain deficient in the immunogenic B5R extracellular enveloped protein) has shown improved safety and pediatric suitability, it remains contraindicated for immunocompromised individuals, including those with skin conditions or pregnancy39,40. In contrast, the replicative vaccine ACAM2000, though potentially more immunogenic, is prohibited in immunocompromised individuals such as pregnant women, people living with HIV and skin diseases, due to potential serious side effects41. Herein, NTV addresses these requirements through targeted deletion of 26 TTV genes linked to host range, immunosuppression, and virulence. This attenuation abolishes replication in human cells, substantially reducing virulence and enhancing safety without compromising immunogenicity. We demonstrated that NTV vaccine was safe in our monkey model, making NTV a promising candidate for immunocompromised populations and a valuable asset for controlling the ongoing mpox epidemic. Nevertheless, further studies are needed to validate its safety in immunocompromised individuals or animal models.
Collectively, these promising results suggest a clear path forward for the clinical development of the NTV vaccine candidate for human use, as it is currently advancing towards the pre-IND stage. Phases I clinical trials with NTV vaccine candidates are expected to begin this year (approval No. 2024LP02849).
Methods
Ethics statement
Animal studies were conducted following the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (Approval No. XJ24001, XJ23002, XJ23005, BSYYF20220903006). The animals were housed and cared for at the facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All procedures involving animals were conducted under anesthesia with 2% isoflurane (for mice) or 5 mg/kg zoletil 50 (for monkeys) prior to sample collection to ensure their well-being, and every effort was made to minimize any potential animal suffering.
Cells and viruses
Vero E6 (ATCC, CRL-1586) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO) supplemented with 10% or 2% heat-inactivated fetal bovine serum (FBS, GIBCO), 100 units/ml penicillin and 100 units/ml streptomycin, and the cells were grown at 37 °C under an atmosphere with 5% CO2.
Mpox virus Clade IIb (MPXV-B.1-China-C-Tan-CQ01) were isolated from first imported mpox case of mainland China in 202242. Vaccina virus Western Reverse strain (VACV WR) were kindly provided by Min Fang (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China). Viruses were cultivated in Vero cells and titrated via plaque assay as described previously42. All procedures were performed in a BSL-3 containment facility at the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences.
Mice study
Six- to eight-week-old BALB/c mice (female) were purchased from obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd., and four- to eight-week-old CAST/EiJ mice (6 males and 6 females) were obtained from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences. All mice were confirmed to be negative for MPXV and VACV prior to the experiments, and housed in small ventilated microisolator cages under pathogen-free conditions. The environment was temperature-controlled (21-23 °C) with a 12 h light and dark cycle, 50–60% relative humidity, and free access to food and water. The experiments involving the challenge of CAST/EiJ mice with MPXV clade IIb strain in this study were conducted in an animal biosafety level 3 (ABSL-3) containment facility at the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences.
The protective efficacy of NTV prime-boost immunization was evaluated against VACV challenge in BALB/c mice and MPXV challenge in CAST/EiJ mice. On day 0, mice received a prime immunization followed by a booster dose on day 28, administered intramuscularly, with either 1 dose NTV (1 × 108 PFU) or a sham saline treatment. Following 14 days after the boost immunization, the mice were inoculated with a 1 × 106 TCID50 dose of VACV WR strain via the intranasal route or a 1 × 106 TCID50 dose of MPXV clade IIb strain via the intraperitoneal route. Mice were monitored over the designated period for disease symptoms, including weight loss, survival, and viral loads and titers in tissues.
For the above mice immunization and infection, mice were anesthetized with 2% isoflurane. At the end of the experiments, mice were euthanized using 2% isoflurane, followed by cervical dislocation.
Rabbit study
Ninety- to one hundred-day-old rabbits (female) were obtained from Beijing Fuhao Experimental Animal Breeding Center. All rabbit were confirmed to be negative for MPXV and VACV prior to the experiments. To evaluate the humoral immune response of NTV in rabbits, twenty-four rabbits were randomly divided into four groups (n = 6/group), and inoculated with NTV (1 × 108 PFU) or TTV (1 × 106 PFU) via intramuscular route. Blood samples were collected on days 7, 14 and 21 after the final NTV immunization or on days 21, 28 and 35 after TTV immunization, and the levels of binding antibodies and neutralizing antibodies were measured.
Nonhuman primate studies
In this study, sixty-six nonhuman primates, including thirty-six rhesus macaques (18 males and 18 females, 3.0-5.0 years old) and thirty cynomolgus monkeys (15 males and 15 females, 3.1-3.6 years old), were confirmed to be negative for MPXV and VACV prior to the experiments. Basic information of nonhuman primates used in this study is presented in Supplementary Tables 1–3.
Study I, protective efficacy of NTV prime-boost immunization against MPXV challenge in rhesus macaques. Twenty-four rhesus macaques were divided into four groups (1/5 dose NTV: 2 × 107 PFU; 1 dose NTV: 1 × 108 PFU; 2 doses NTV: 2 × 108 PFU; sham control). On day 0, macaques received either a prime immunization followed by a boost on day 28 with different doses NTV or sham saline treatment via intramuscular route. After day 21 of boost immunization, twenty-four rhesus macaques were inoculated with 4 × 107 TCID50 dose of clade IIb MPXV (MPXV-B.1-China-C-Tan-CQ01) via the intravenous route and monitored for 10 days (3 euthanized in each group) and 28 days (3 euthanized in each group) for disease symptoms, including weight loss, body temperature, lesions, neutralizing antibody, plasma and tissues loads. Plasma viral loads and skin lesions reached their peak at 10 days post MPXV challenge and recovered by 28 days post-challenge in our previously completed study, which was consistent with previous reports19,24,25. Based on this, we selected 10 days and 28 days post-challenge as the experimental endpoints for our study.
Study II, protective efficacy of NTV single-dose immunization and long-lasting immunization against MPXV challenge in rhesus macaques. For long-lasting protection assay, four rhesus macaques were inoculated with different doses of NTV (1/5 dose NTV, n = 1; 1 dose NTV, n = 1; 2 doses NTV, n = 2) via i.m. route (collectively known as the long-lasting immunization group), 4 × 107 TCID50 of MPXV was administered at eight months post immunization, and the long-lasting protection of NTV vaccine was evaluated by observation of skin lesions and plasma viral loads. For single-dose protection assay, eight rhesus macaques were randomized into two groups of four and immunized with single-dose of NTV or administered a saline control (single-dose immunization group and control group). The animals were administered 4 × 107 TCID50 of MPXV at 28 days post immunization and monitored for another 28 days.
Study III, safety evaluation of NTV in cynomolgus macaques. Thirty cynomolgus monkeys (15/gender) were divided into three groups (5/gender/group) and intramuscularly immunized with saline control, 1 or 3 doses of NTV three times, with 21-day intervals between each vaccination. Three of the five animals of each sex in each group were dissected on day 45, and remaining 2/5 animals of each sex in each group were dissected on day 70. Collection of data on safety-related parameters and clinical observation, including viral absorption kinetics, viral shedding, viral biodistribution, blood pressure, oxygen saturation, hematology, cytokine expression, and histopathology were carried out during and after immunization.
For immunization, blood collection, and infection procedures, the macaques were anesthetized with Zoletil 50 (5 mg/kg). Euthanasia was conducted by bloodletting through the femoral artery under the condition of zoletil 50 anesthesia (5 mg/kg).
Measurement of skin lesions
Skin lesion assessments were performed with the experimental operator blinded to the rhesus macaque group assignments. Lesion counts were conducted by one individual and independently verified by a second observer. Rhesus macaques were anesthetized with Zoletil-50 (5 mg/kg), and evaluations were carried out in a biosafety cabinet. Lesions, including erythema, papules, nodules, blisters, and pustules, were quantified across the entire body, encompassing both haired and non-haired regions. In cases of confluent lesions, individual pock boundaries remained discernible, allowing accurate enumeration of each pock within the confluent areas without merging into a continuous mass. Disease severity was classified into four grades based on lesion counts, following established criteria21: mild (1-25), moderate (26-100), severe (101-250, grave), and serious (>250, plus grave).
Enzyme-linked immunosorbent assay (ELISA)
IgG antibody titers in the sera samples were determined by ELISA. Briefly, 96-well plates were coated with NTV (105 PFU/well) or 1 μg/ml of A35R (40886-V08H, Sino Biological), B6R (40902-V08H, Sino Biological), H3L (40893-V08H1, Sino Biological), M1R (40904-V07H, Sino Biological), respectively and incubated overnight at 4°C. After incubation, the plates were washed three times with 300 μl/well 1×PBST and blocked with 1% BSA (200 μl/well) for 2 h at 37°C. Then, the plates were washed three times with 300 μl/well 1×PBST. Next, 3-fold gradient dilutions of inactivated serum samples starting at 1: 100 was added to blocked 96-well plates (100 μl/well), and incubated for 1 h at 37 °C. After washed three times with 300 μl/well 1×PBST, the plates were treated with 100 μl horseradish peroxidase (HRP)-conjugated goat anti-monkey IgG, goat anti-rabbit IgG, or rabbit anti-mouse IgG (Abcam, 1: 5,000 dilution) for 1 h at 37 °C. The plates were washed three times with 300 μl/well 1×PBS-T and treated with 100 μl /well soluble TMB substrate (R&D, USA) for 15-30 min at room temperature in the dark. The reaction was terminated with stop solution (R&D, USA). Absorbance at 450/630 nm was recorded using SpectraMax iD5 multimode microplate reader (Molecular Devices, USA). The ELISA endpoint titers were defined as the highest reciprocal dilution of serum to given an absorbance greater than 2.1-fold of the background values.
Plaque reduction neutralization titer (PRNT) assay
The neutralizing antibodies titers in sera samples were determined by 50% plaque reduction neutralization test (PRNT50). Briefly, Vero E6 cells were seeded in 24-well plates and incubated overnight at 37 °C, 5% CO2 until the cells reached 90-100% confluency. On the day of cell seeding, serum samples were heat-inactivated at 56 °C for 30 min and used to generate a 2-fold dilution series in DMEM containing 2% FBS. The diluted serum samples were added to equal volume of VACV or MPXV (100 PFU/0.1 ml) in a 48-well plate and incubated for 1 h at 37 °C. Subsequently, 200 μl of each serum-virus mixture was added to Vero E6 cells and incubated for 1 h at 37 °C. One milliliter of 0.5% methylcellulose was added to each well and incubated at 37 °C for 2 days (VACV) or 3 days (MPXV) to plaque formation. Next, the plaques were fixed with 4% neutral buffered formaldehyde overnight and then stained with 0.4% crystal violet solution for the enumeration of the plaques. Neutralization end-point titers were calculated via point-to-point linear regression and based on the reciprocal dilution of the test serum that produced 50% plaque reduction compared to the virus only control via Reed-Muench method. All the work with infectious MPXV were performed in a BSL-3 containment facility.
ELISpot assay
Cellular immune responses in the NTV vaccinated mice were assessed using IFN-γ precoated ELISPOT kits (MabTech, 3321-2 A), according to the manufacturer’s protocol. Briefly, the plates were washed 4 times with PBS and blocked using RPMI 1640 containing 10% FBS (200 μl/well) and incubated for 30 min at room temperature. Immunized mouse splenocytes (200,000 cells/well) were stimulated with NTV (2 × 106 PFU/ml, 100 μl/well), 50 ng/ml phorbol myristate acetate (PMA) and 450 ng/ml ionomycin (Thermo Fisher) as positive control and RPMI 1640 media as negative control. Following incubation at 37 °C, 5% CO2 for 36 h, plates were washed with PBS 5 times and incubated with biotinylated anti-mouse IFN-γ antibody (0.1 μg/100 μl/well) for 2 h at room temperature. Wash the plates and incubate Streptavidin-ALP (1: 1000, 100 μl/well) for 1 h at room temperature. Finally, the plates were incubated with BCIP/NBT-plus substrate solution until spots exposed on the plates. Wash the plates with deionized water and dry in a dark place for 24 h. The spots were read by an automated Mabtech IRIS ELISPOT reader (Mabtech). The numbers of spot-forming cells (SFU) per 0.2 million cells were calculated by vSpot 7.0 software.
Flow cytometry
CD4+ and CD8+ T cell responses were quantitated by vaccine (NTV) or virus (VACV)-stimulated intracellular cytokine staining (ICS) assays. 1 × 106 peripheral blood mononuclear cells (PBMCs) were re-suspended in 100 μl of RPMI 1640 media supplemented with CD49d and CD28 monoclonal antibody (BD Biosciences, 1 μg/mL each). Each sample was assessed with mock (100 μl of R10; background control), vaccina (NTV) or virus (VACV) (1 × 107 PFU/ml, 100 μl/well), or 50 ng/ml PMA and 450 ng/ml ionomycin (Thermo Fisher) (100 μl; positive control) and incubated for 3 h at 37 °C with 5% CO2. Subsequently cells were incubated with 10 μl of BFA (sigma) in 100 μl of R10 for additional 15 h. After incubation, the cells were washed twice with wash buffer (PBS with 0.5% FBS). Cells were first labeled using Fixable Viability Dye (invitrogen) for 20 min at room temperature to exclude dead cells and then stained with surface markers anti-CD3-BV650 (563916, BD Bioscience, 1: 50 dilution), anti-CD4-BV510 (317444, Biolegend, 1: 50 dilution), anti-CD8-BUV737 (612754, BD Bioscience, 1: 50 dilution) for 30 min. Cells were then fixed and permeabilized with the Cytofix/Cytoperm kit (BD Bioscience) and stained with anti-IFN-γ-AF700 (557995, BD Bioscience, 1: 250 dilution), anti-IL-2-PE (559334, BD Bioscience, 1: 50 dilution), anti-TNF-α-FITC (554512, BD Bioscience, 1: 250 dilution) for 30 min. Finally, cells were resuspended in FACS buffer for acquisition and analysis using a BD LSR Fortessa flow cytometer and the data were analyzed by FlowJo software (Version 10.1).
For measurement of the proportion of memory T cell subsets during long-lasting immunity, 1 × 106 PBMCs were first labeled using Fixable Viability Dye (invitrogen) for 20 min at room temperature to exclude dead cells and then stained with surface markers anti-CD3-BV650 (563916, BD Bioscience, 1: 50 dilution), anti-CD4-BV510 (317444, Biolegend, 1: 50 dilution), anti-CD8-BUV737 (612754, BD Bioscience, 1: 50 dilution), anti-CD45RA-BV711 (740806, BD Bioscience, 1: 100 dilution), anti-CCR7-BV605 (353224, Biolegend, 1: 50 dilution) for 30 min. Cells were resuspended in FACS buffer for acquisition and analysis using a BD LSR Fortessa flow cytometer and the data were analyzed by FlowJo software (Version 10.1).
Quantification of viral DNA
Viral DNA from plasma and tissues were assessed by quantitative PCR (qPCR) assay following a previously described method43,44. Briefly, viral DNA was extracted and purified using a DNeasy Blood & Tissue Kit (QIAGEN). Quantitative PCR was carried out on an ABI 7500 Real-time PCR system (Applied Biosystems, Foster City, CA, USA) via TaqMan Gene Expression Master Mix (Applied Biosystems) using the following cycling protocol and primers: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The sequences of the primers and probes were targeted against the conserved region F3L as follows43: 5′- CTCATTGATTTTTCGCGGGATA-3′ (forward), 5′- GACGATACTCCTCCTCGTTGGT-3′ (reverse), and 5′-(FAM)- CATCAGAATCTGTAGGCCGT-(MGB)-3′ (probe). The detection limit for viral DNA in the plasma was 100 copies/ml, and that for viral DNA in tissues was 10 copies/μg of total DNA.
Viral absorption kinetics and viral shedding
Blood samples with EDTA anticoagulant were collected from cynomolgus monkeys infected with varying doses of NTV (normal saline, 1 and 3 doses) at 0, 2, 24, 72 and 168 h after the prime (day 0) and boost-2 (day 42) immunizations. The viral absorption kinetics were assessed by measuring the vaccine DNA in the blood. Total DNA was extracted, and quantitative PCR was carried out via TaqMan Gene Expression Master Mix (Applied Biosystems) with region of E9L as follow: 5′- TGGCAAACCGTAACATACCG -3′ (forward), 5′- AGGCCATCTATGATTCCATGC -3′ (reverse), and 5′-(FAM)- ACGCTTCGGCTAAGAGTTGCACATCCA -(TRAMA)-3′ (probe). The PCR amplification conditions were set at 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Data were acquired by three independent replicate experiments.
Viral shedding was assessed by analyzing viral DNA loads in urine, feces, perioral secretions, perinasal secretions, and infection site swab in cynomolgus monkeys on days 0, 1, 3, 7, 43, 45, 49, 69 after immunization with different doses of NTV (saline, 1 dose and 3 doses). The methods for total DNA extraction and quantitative PCR are consistent with those described above.
Cytokine analysis
Sera samples were collected from 30 cynomolgus macaques on days −4, 0, 3, 42, 45, and 70 following prime immunization with varying doses of NTV (saline, 1 dose, and 3 doses). The samples were isolated from peripheral blood collected from each group. Using a Cytometric Bead Array (CBA) kit (BD Bioscience), the expression levels of TNF-α, IFN-γ, IL-2, IL-4, IL-5, and IL-6 in the serum were analyzed by flow cytometry (Beckman DxFLEX Flow Cytometer).
Hematology, clinical chemistry and antinuclear antibody
Blood samples with EDTA anticoagulant were collected on days −4, 3, 45, and 70 post-immunizations from cynomolgus monkeys treated with different doses of NTV (saline, 1 dose, and 3 doses). Hematological analysis was conducted using an automated hematology analyzer. The parameters measured included white blood cell count (WBC), hemoglobin (HGB), red blood cell count (RBC), platelet count (PLT), lymphocyte count (Lymph), lymphocyte ratio (Lymph%, the percentage of lymphocyte count in the white blood cell count), neutrophil count (Neut), neutrophil ratio (Neut%, the percentage of neutrophil count in the white blood cell count), monocyte count (Mono), and monocyte ratio (Mono%, the percentage of monocyte count in the white blood cell count).
Blood samples were collected on days −4, 45, and 70 from cynomolgus monkeys treated with different doses of NTV (saline, 1 dose, and 3 doses) into tubes containing Gel & Clot Activator. After centrifugation, clinical chemistry parameters were measured using a TBA-120FR automatic biochemical analyzer (TOSHIBA). The parameters included aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), total protein (TP), albumin (ALB), total cholesterol (CHOL), blood urea nitrogen (BUN), creatinine (CREA), triglyceride (TGL), total bilirubin (TBI), and blood glucose (GLUC).
Blood samples were collected on days −4, 45, and 70 from cynomolgus monkeys treated with different doses of NTV (saline, 1 dose, and 3 doses) into tubes containing Gel & Clot Activator. After centrifugation, antinuclear antibody detection was performed using indirect immunofluorescence with an antinuclear antibody detection kit (Oumeng).
Histopathological analysis
Tissues from rhesus and cynomolgus monkeys, including skin lesions, non-lesioned skin, heart, liver, spleen, lung, kidney, brain, intestine, and lymph nodes, were collected at necropsy following the study period. Samples were fixed in 4% paraformaldehyde, processed into slides, and stained with hematoxylin and eosin (H&E). For skin sampling, lesions and adjacent non-lesioned skin were preferentially collected from the limbs. In animals with more than five lesions, five lesion sites and five corresponding non-lesioned areas were sampled; in those with fewer than five lesions, all lesions and five non-lesioned areas were analyzed. Slides were imaged using a 3D Histech Pannoramic MIDI microscope (Hungary) and examined with CaseViewer 2.4 software. Pathological evaluations were performed by a pathologist with over 10 years of histology experience and independently verified by a second pathologist.
Statistical analyses
Graphical representation and statistical analyses were performed using Prism 10.0 software (GraphPad software). All the data were presented as the means ± SEM. Differences between two independent samples were evaluated by two-tailed Student’s t-tests. Differences between multiple samples were analyzed by one-way or two-way analysis of variance (ANOVA). Exact P values are provided in figures. All experiments were conducted with at least 3 independent biological replicates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
This work was funded by the National Natural Science Foundation of China (82241068 to J.X., 82222041 to J.X.), the National Key Research and Development Project of China (2023YFC2309000 to J.X., 2022YFC2304100 to W.T., 2023YFC2606000 to Y.Z.), the Beijing Natural Science Foundation (Z220018 to J.X.) and Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2023-I2M-2-001 to J.X.). We thank Min Fang (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) for providing the VACV strain.
Author contributions
Conceptualization: J.X. and Y.Z.; Acquisition, analysis or interpretation of data: all authors. Animal care and procedures: Z.C., L.Z., J.M., Jingjing.Z., N.L., J.L., T.C., Y.H., D.Z., and Q.W.; Technical or material support: S.P., B.H., Junjie.Z., Z.Y., S.Q., Y.L., and Y.H.; Drafting of the manuscript: L.Z., D.L., and J.X.; writing—review & editing: L.Z., J.X., Y.Z., W.T., and D.L.; funding acquisition: J.X., Y.Z., and W.T.; resources: J.X., Y.Z., and W.T.; supervision: J.X. and Y.Z.
Peer review
Peer review information
Nature Communications thanks Rosamund Chapman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors affirm that all data supporting the findings in this study are accessible in the paper and Supplementary Information. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lin Zhu, Shuyuan Pan, Baoying Huang, Junjie Zhang.
Contributor Information
Dan Li, Email: lidan@chinaaids.cn.
Wenjie Tan, Email: tanwj@ivdc.chinacdc.cn.
Yuntao Zhang, Email: zhangyuntao@sinopharm.com.
Jing Xue, Email: xuejing@cnilas.org.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-62594-0.
References
- 1.Musambi, E. Mpox deaths rise by 107 in a week as Africa CDC calls the toll unacceptable. https://apnews.com/article/mpox-africa-cdc-outbreak-deaths-testing-vaccination-31e714fbc528feab3a58d0c36e2a847f (2024).
- 2.Grabenstein, J. D. & Hacker, A. Vaccines against mpox: MVA-BN and LC16m8. Expert Rev. Vaccines23, 796–811 (2024). [DOI] [PubMed] [Google Scholar]
- 3.WHO. Mpox global strategic preparedness and response plan. https://www.who.int/publications/m/item/mpox-global-strategic-preparedness-and-response-plan (2024). [PMC free article] [PubMed]
- 4.WHO. Strategic framework for enhancing prev ention and control of mpox-2024-2027. https://www.who.int/publications/i/item/9789240092907 (2024).
- 5.Organization, W. H. Smallpox and mpox (orthopoxviruses): WHO Position Paper. https://www.who.int/publications/i/item/who-wer-9934-429-456 (2024).
- 6.Wolff Sagy, Y. et al. Real-world effectiveness of a single dose of mpox vaccine in males. Nat. Med.29, 748–752 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinganda-Lusamaki, E. et al. Mpox is spreading-there are scientists’ key questions. Nature633, 16–17 (2024).39210015 [Google Scholar]
- 8.Moss, B. Understanding the biology of monkeypox virus to prevent future outbreaks. Nat. Microbiol.9, 1408–1416 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Gilchuk, I. et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell167, 684–694.e689 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, M. et al. Long-lasting humoral and cellular memory immunity to vaccinia virus Tiantan provides pre-existing immunity against mpox virus in Chinese population. Cell Rep.43, 113609 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Chu, Q. et al. Non-replicating vaccinia virus NTV as an effective next-generation smallpox and monkeypox vaccine: evidence from mouse and rhesus monkey models. Emerg. Microbes. Infec. 12, 2278900 (2023). [DOI] [PMC free article] [PubMed]
- 12.Qi, X. R. et al. The non-replicating recombinant vaccinia virus expressing six genes of HIV-1 can be passaged stably in CEF. Bing Du Xue Bao27, 135–143 (2011). [PubMed] [Google Scholar]
- 13.Zhang, W. C. et al. Accurate determination of the whole genome sequencing and open reading frames composition of non-replicating Tiantan strain of vaccinia virus based on novel long read sequencing platform. Chin. J. Microbiol. Immunol.4, 502–509 (2024). [Google Scholar]
- 14.Ruan, L., Lou, Y. & Lu, R. Non-replicating vaccinia virus TianTan strain. China Patent (2008).
- 15.Wen, B. et al. The novel replication-defective vaccinia virus (Tiantan strain)–based hepatitis C virus vaccine induces robust immunity in macaques. Mol. Ther.21, 1787–1795 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhan, Y. et al. Humoral and cellular immunity against both ZIKV and poxvirus is elicited by a two-dose regimen using DNA and non-replicating vaccinia virus-based vaccine candidates. Vaccine37, 2122–2130 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Zhao, Y. et al. Non-replicating vaccinia virus TianTan strain (NTV) translation arrest of viral late protein synthesis associated with anti-viral host factor SAMD9. Front. Cell. Infect. Mi. 10, 116 (2020). [DOI] [PMC free article] [PubMed]
- 18.Zhu, W. et al. The attenuation of vaccinia Tian Tan strain by the removal of the viral M1L-K2L genes. J. Virol. Methods144, 17–26 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuiani, A. et al. A multivalent mRNA monkeypox virus vaccine (BNT166) protects mice and macaques from orthopoxvirus disease. Cell187, 1363–1373.e12 (2024). [DOI] [PubMed]
- 20.Americo, J. L., Earl, P. L. & Moss, B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc. Natl. Acad. Sci. USA120, e2220415120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, X. & Lun, W. Skin manifestation of human monkeypox. J. Clin. Med.12, 914 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schafer, K. A. et al. Use of severity grades to characterize histopathologic changes. Toxicol. Pathol.46, 256–265 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Hammarlund, E. et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med.11, 1005–1011 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Aid, M. et al. Mpox infection protects against re-challenge in rhesus macaques. Cell186, 4652–4661.e4613 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob-Dolan, C. et al. Comparison of the immunogenicity and protective efficacy of ACAM2000, MVA, and vectored subunit vaccines for Mpox in rhesus macaques. Sci. Transl. Med.16, eadl4317 (2024). [DOI] [PubMed] [Google Scholar]
- 26.Buchman, G. W. et al. A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge. Vaccine28, 6627–6636 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatch, G. J. et al. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J. Virol.87, 7805–7815 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mucker, E. M. et al. Comparison of protection against mpox following mRNA or modified vaccinia Ankara vaccination in nonhuman primates. Cell187, 5540–5553.e10 (2024). [DOI] [PubMed]
- 29.Stittelaar, K. J. et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol.79, 7845–7851 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Earl, P. L. et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. USA105, 10889–10894 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCurdy, L. H., Larkin, B. D., Martin, J. E. & Graham, B. S. Modified vaccinia Ankara: potential as an alternative smallpox vaccine. Clin. Infect. Dis.38, 1749–1753 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Petersen, B. W., Harms, T. J., Reynolds, M. G. & Harrison, L. H. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupational exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. Morb. Mortal. Wkly Rep.65, 257–262 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Edghill-Smith, Y. et al. Smallpox vaccine–induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med.11, 740–747 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Ilchmann, H. et al. One- and two-dose vaccinations with modified vaccinia Ankara-Bavarian Nordic induce durable B-cell memory responses comparable to replicating smallpox vaccines. J. Infect. Dis.227, 1203–1213 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaeck, L. M. et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med.29, 270–278 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu, R. H., Fang, M., Klein-Szanto, A. & Sigal, L. J. Memory CD8+ T cells are gatekeepers of the lymph node draining the site of viral infection. Proc. Natl. Acad. Sci. USA104, 10992–10997 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickman, H. D. et al. Anatomically restricted synergistic antiviral activities of innate and adaptive immune cells in the skin. Cell Host Microbe13, 155–168 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adamo, S. et al. Memory profiles distinguish cross-reactive and virus-specific T cell immunity to mpox. Cell Host Microbe31, 928–936.e924 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy, J. S. et al. Safety and immunogenicity of LC16m8, an attenuated smallpox vaccine in vaccinia-naive adults. J. Infec. Dis.204, 1395–1402 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kidokoro, M., Tashiro, M. & Shida, H. Genetically stable and fully effective smallpox vaccine strain constructed from highly attenuated vaccinia LC16m8. Proc. Natl. Acad. Sci. USA102, 4152–4157 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen, B. W. et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Recomm. Rep.64, 1–26 (2015). [PubMed] [Google Scholar]
- 42.Huang, B. et al. Isolation and characterization of monkeypox virus from the first case of monkeypox—Chongqing Municipality, China, 2022. China CDC Wkly.4, 1019–1024 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei, Q. et al. The first strain of monkeypox isolated in the Chinese Mainland and preserved at the National Pathogen Resource Center of China. Infect. Med.1, 288–291 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao, H. et al. The first imported case of monkeypox in the Mainland of China—Chongqing Municipality, China, September 16, 2022. China CDC Wkly.4, 853–854 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors affirm that all data supporting the findings in this study are accessible in the paper and Supplementary Information. Source data are provided with this paper.