Abstract
This study compared the lateral approach laparoscopic spleen-preserving distal pancreatectomy (LA LSPDP) with the conventional approach to LSPDP (CA LSPDP) for benign to borderline malignant tumors in the pancreatic tail. A multicenter retrospective cohort of patients undergoing LA LSPDP or CA LSPDP for pancreatic tail tumors with a tumor center located beyond the left lateral border of the aorta was analyzed. A 1:1 propensity score matching (PSM) yielded 56 patients per group. A total of 172 patients were planned for LSPDP. After PSM, the tumor sizes were comparable (3.1 cm vs 3.3 cm, p = 0.549). However, resected specimens were longer in the CA LSPDP group (8.4 cm vs. 7.7 cm, p < 0.001). Rates of conversion to open surgery, the use of Warshaw’s technique, and the need for combined splenectomy were not significantly different between the two groups. However, the LA LSPDP group had a shorter operation time (127.1 min vs. 161.1 min, p = 0.002) and less blood loss (106.2 cc vs. 291.4 cc, p = 0.001). The postoperative complication rates were similar (35.7% vs. 27.3%, p = 0.339). LA LSPDP is a safe and effective technique that reduces operative time and blood loss in pancreatic tail tumors; however, larger prospective studies are needed to confirm this finding.
Keywords: Laparoscopy, Distal pancreatectomy, Splenic vessel preservation, Left lateral approach, Pancreatic neoplasms
Subject terms: Pancreatic cancer, Pancreatitis
Introduction
Laparoscopic distal pancreatectomy (LDP) is a well-validated surgical procedure for removing tumors from the body or tail of the pancreas. LDP can usually be performed with splenectomy. Since the inception of LDP, inquiries have been made concerning the fundamental role of the spleen in the immune system. Therefore, surgeons have increasingly adopted laparoscopic spleen-preserving distal pancreatectomy (LSPDP) for benign and borderline tumors of the distal pancreas to minimize unnecessary splenectomy. This has resulted in LSPDP becoming a prevalent procedure1,2.
From the body to the tail of the pancreas, complex short branches of the splenic vein are embedded in the sulcus on the posterior side of the pancreas. The splenic hilum is closely abutted at the end of the pancreatic tail. Traditional LSPDP starts with medial dissection of the upper and lower borders of the pancreas. Dissection is made medial-to-lateral towards the splenic hilum3–5. Especially for pancreatic tail tumors located laterally to the left border of the aorta, it is challenging to dissect short branches in the splenic vein toward the end of the tail owing to the anatomical features of the pancreas. In addition, the camera scope is too far to reach the end of the pancreas tail. The operator does not accurately recognize the complex vascular structure, which can increase the risk of intraoperative bleeding.
Unlike the conventional approach to LSPDP (CA LSPDP), the lateral approach (LA LSPDP) places the patient in the right lateral decubitus position. It is commonly used in laparoscopic left adrenalectomy and laparoscopic splenectomy4. In this approach, the surgeon first encounters the splenic hilum around the pancreatic tail, enabling easier exposure and accurate dissection between the complex splenic vein branches and pancreatic tail tumor. Several authors have reported that the lateral approach is a feasible method for LSPDP of pancreatic tail tumors, with potential benefits in terms of surgical outcomes and spleen preservation6–8.
To date, the literature on the lateral approach for LSPDP is limited to technical reports, a small number of case series from a single center, and several retrospective studies. This study aimed to compare the postoperative outcomes of patients who underwent either LA LSPDP or CA LSPDP at four large-volume centers, particularly for pancreatic lesions located in the tail.
Materials and methods
Study design and patient selection
Between November 2012 and October 2023, patients with planned LSPDP at the Department of Hepatobiliary and Pancreatic Surgery, Seoul St. Mary’s Hospital, Incheon St. Mary’s Hospital, Bucheon St. Mary’s Hospital, and St. Vincent’s Hospital were enrolled in this multicenter retrospective cohort study. LSPDP using a conventional approach was initiated at our hospital in 2010. The lateral LSPDP approach was first introduced in 2015. All consecutive spleen-preserving distal pancreatectomies were performed by eight surgeons (T. Y., Y. C., C. H., G. H., D. D., Y. G., T. H., and S. E.), each possessing advanced expertise in the field of pancreas surgery and practicing at individual high-volume centers. We included patients who were confirmed to have a benign or borderline malignant tumor in the pancreatic tail, with the tumor center located beyond the left lateral border of the aorta, based on preoperative radiologic studies. Patients were excluded from this study if any of the following criteria were met: the maximum tumor length exceeded 8 cm, suggesting that open surgery would be preferred over minimally invasive approaches, the need for multi-visceral resection beyond the pancreas and spleen, the tumor’s center was located medial to the left lateral border of the aorta, follow-up data were unavailable, or concurrent procedures were required.
In all the LSPDP cases, preservation of the splenic artery and vein was the original surgical strategy. Nevertheless, LDP using the Warshaw technique or even splenectomy has been performed in specific conditions where dissection of vascular structures is difficult or the tumor has severe adhesion to splenic vessels, resulting in substantial intraoperative bleeding. This study was reviewed and approved by the Institutional Review Board of the main research institution, The Catholic University of Korea Hospital (KC22RISI0484). All methods were performed in accordance with the relevant guidelines and regulations. Using 1:1 propensity score matching based on patient age and American Society of Anesthesiologists (ASA) score, 56 patients were selected from each of LA LSPDP and CA LSPDP groups for comparative analysis.
Data collection
Demographic data, perioperative outcomes, and complication rates were prospectively recorded in the database and retrospectively analyzed. Demographic data included age, sex, body mass index (BMI), Charlson Comorbidity Index (CCI), ASA classification, comorbidities, previous abdominal surgery history, surgical methods, tumor location, tumor size, specimen length, and clinicopathological diagnosis. Perioperative outcomes included the following: whole operative time (WOT), estimated blood loss (EBL), conversion to Warshaw technique, conversion to combined splenectomy, or open surgery, clinically relevant postoperative pancreatic fistula (CR-POPF), days to drain removal, postoperative complications, incidence of reoperation, readmission and mortality within 90 days, and length of hospital stay (LOS).
Definition
Pancreatic tail tumors were defined as those with centers located away from the left border of the aorta. Tumor location was determined by the distance between the lateral aortic margin and the center of the tumor. Postoperative complications were classified according to the Clavien-Dindo classification9. Pancreatic fistula was graded according to the International Study Group of Pancreatic Fistula (ISGPF) definition and classified as biochemical leak (previously ISGPF grade A) or CR POPF (ISGPF grades B and C)10. Pathologists collected pathological specimens with the final pathological diagnosis and maximum tumor diameter.
Treatment and follow-up
All patients underwent the same clinical protocol during hospitalization, regardless of the surgical approach. The patient was given parenteral nutrition on the first day after the operation, when vital signs were stabilized. Octreotide was not routinely used unless there was a clear evidence of POPF. On the fifth day after surgery, the patient was evaluated using abdominal computed tomography (CT) to confirm that there was no pancreatic leakage or other intra-abdominal complications. The oral diet was then resumed. The patient maintained drains for at least five days after the surgery. If the amount of drainage was less than 100 ml per day without evidence of bleeding or pancreatic leakage, the drain was removed. Patients on a tolerable diet without evidence of infection were discharged at 7–8 days after the operation.
Surgical procedure
Lateral approach for LSPDP
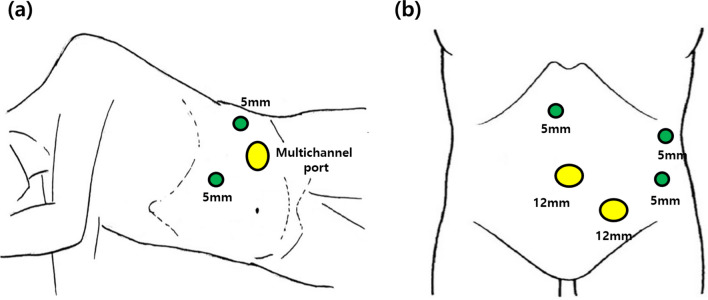
The patient was placed in the right lateral decubitus position under general anesthesia. A 3 cm transverse skin incision was made on the mid-clavicular line in the left mid-abdominal quadrant and extended down to the layer of the abdominal fascia. We inserted a multichannel trocar (Glove Port, Nelis, Seoul, Korea), consisting of four ports and two rings. This trocar provided access to a 10 mm 30-degree laparoscope, 12 mm endovascular stapler (endoscopic linear stapler), 5 mm laparoscopic instrument, and two rings with gas insulation and exsufflation gates. Two additional 5-mm trocars were placed in the subxiphoid and left axillary line and used as access points for a 5-mm laparoscopic grasper and dissector (Fig. 1a). Surgery was initiated with dissection of the splenocolic ligament to divide the transverse colon. A lateral incision was then extended from the lateral side to the medial side, without injuring the gastroepiploic or short gastric vessels. A lesser sac was also identified. The end of the pancreatic tail was divided from the splenic hilum using a vascular sealing device (LigaSure V; Tyco Healthcare, Tokyo, Japan). Subsequently, the pancreatic tail was lifted anteromedially. The dissection was continued along the superior border of the pancreatic parenchyma with ligation of the small branches of the splenic vessels (Fig. 2). Finally, pancreas transection was performed using an endoscopic linear stapler (Signia™ Stapling System with a 60 mm purple reload, Medtronic Japan, Tokyo) to allow an adequate margin (> 1 cm) from the mass. We occasionally performed reinforcement sutures when the pancreatic stump was crushed during stapling and leakage of pancreatic juice was a concern. After transection, the pancreatic stump was covered with a polyglycolic acid mesh sheet (Neoveil®). A closed suction drain was placed on the stump of the pancreas through a subxiphoid 5-mm port site, and the specimen was bagged. The specimen was extracted through the incision site of a multichannel trocar. If the tumor size was > 3 cm, the incision was expanded as much as possible.
Fig. 1.
Trocar placement for (a) the lateral approach in laparoscopic spleen-preserving distal pancreatectomy and (b) the conventional approach in laparoscopic spleen-preserving distal pancreatectomy.
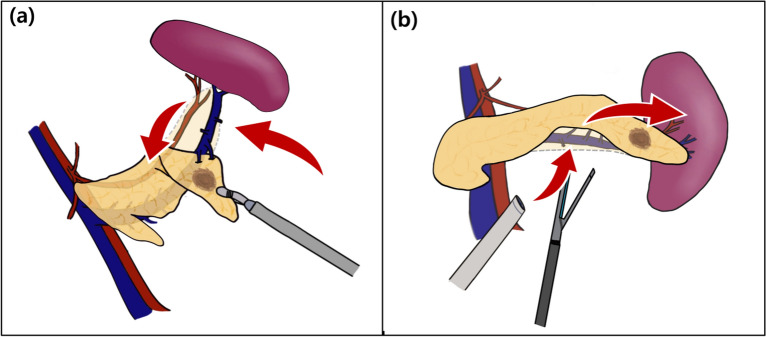
Fig. 2.
Schematic layout of laparoscopic spleen-preserving distal pancreatectomy, depicting the (a) lateral and (b) conventional approaches.
Conventional approach for LSPDP
The patient was placed in a supine position with legs spread apart and tilted to the right reverse Trendelenburg position, typically using five trocars (Fig. 1b). The dissection was performed in a medial-to-lateral manner. The extent of resection was similar for the two types of surgical approaches. Briefly, after entering the lesser sac, dissection was performed along the lower border of the pancreas within the avascular plane and then along the posterior aspect of the pancreas. After the distal part of the pancreas was dissected to mobilize as much as possible, the pancreatic parenchyma was transected at a distance of ≥ 1 cm from the tumor using a linear stapler. The pancreas was then dissected toward the lateral end while isolating the splenic blood vessels. Finally, depending on the length of the specimen, if necessary, the incision was expanded. The specimens were extracted from an umbilical trocar site.
Statistical analyses
Continuous variables are expressed as mean ± standard deviation, and categorical variables are presented as numbers (%). Statistical analyses were performed using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables. The propensity score was calculated using a logistic regression model that included preoperative variables considered to be directly associated with postoperative outcomes. The lateral approach LDP group and the control group underwent 1:1 nearest-available matching of the logit of the propensity score with a caliper width of 0.20 of the standard deviation of the score. All statistical analyses were conducted using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). All comparisons were two-sided, and a P-value < 0.05 was considered to be significant.
Results
Patient characteristics
During the study period, 172 patients underwent LSPDP for benign or borderline malignant tumors located in the pancreatic tail. Among these patients, CA LSPDP was planned in 116 patients and LA LSPDP was attempted in the remaining 56 patients. Patients who underwent LA LSPDP were younger, had lower CCI and ASA scores, and had a lower incidence of diabetes mellitus. After 1:1 propensity score matching, 56 patients in the LA LSPDP group and 56 patients in the CA LSPDP group were finally included. The baseline characteristics of the patients in the two groups were well matched. The most common diagnoses were NET (26.8%), benign cysts or intrapancreatic accessory spleens (19.6%), and SPN (17.9%) in the LA LSPDP group. The most common diagnoses in the CA LSPDP group were SCN (23.2%), IPMN (19.6%), and NET (16.1%). Tumor location (4.3 cm vs. 4.8 cm, p = 0.252) and Tumor size (3.1 cm vs. 3.3 cm, p = 0.549) was comparable between the two groups. However, the resected specimen was longer in the CA LSPDP group (8.4 cm vs. 7.7 cm, p < 0.001). The patient demographics before and after propensity score matching are shown in Table 1.
Table 1.
Demographic and clinical characteristics of patients in LA LSPDP and CA LSPDP groups.
| Characteristics | Before propensity score matching | After propensity score matching | ||||
|---|---|---|---|---|---|---|
| LA LSPDP (N = 56) |
CA LSPDP (N = 116) |
p value | LA LSPDP (N = 56) |
CA LSPDP (N = 56) |
p value | |
| Age, yrs | 51.9 ± 18.19 | 59.7 ± 15.91 | 0.004 | 51.9 ± 18.19 | 53.8 ± 17.72 | 0.575 |
| Sex, N (%) | 0.611 | 0.43 | ||||
| Male | 18 (32.1) | 43 (37.1) | 18 (32.1) | 22 (39.3) | ||
| Female | 38 (67.9) | 73 (62.9) | 38 (67.9) | 34 (60.7) | ||
| BMI, kg/m2 | 25 ± 4.04 | 24.8 ± 3.33 | 0.721 | 25 ± 4.04 | 25.1 ± 3.38 | 0.966 |
| Charlson Comorbidity index | 1.5 ± 1.66 | 2.4 ± 1.84 | 0.005 | 1.5 ± 1.66 | 1.8 ± 1.66 | 0.461 |
| ASA score, N (%) | 0.011 | 0.324 | ||||
| 1 | 18 (32.1) | 19 (16.4) | 18 (32.1) | 13 (23.2) | ||
| 2 | 37 (66.1) | 90 (77.6) | 37 (66.1) | 42 (75) | ||
| 3 | 1 (1.8) | 7 (6) | 1 (1.8) | 1 (1.8) | ||
| Comorbidity, N (%) | ||||||
| Hypertension | 16 (28.6) | 51 (44) | 0.052 | 16 (28.6) | 20 (35.7) | 0.418 |
| Diabetes mellitus | 8 (14.3) | 33 (28.4) | 0.041 | 8 (14.3) | 13 (23.2) | 0.226 |
| Cardiac disease | 5 (8.9) | 13 (11.2) | 0.647 | 5 (8.9) | 4 (7.1) | 1.000 |
| COPD | 0 | 3 (2.6) | 0.552 | 0 | 0 | |
| Previous history of operation | 17 (30.4) | 31 (26.7) | 0.619 | 17 (30.4) | 12 (41.4) | 0.281 |
| Pathologys, N (%) | 0.393 | 1.000 | ||||
| IPMN | 8 (14.3) | 38 (32.8) | 8 (14.3) | 11 (19.6) | ||
| NET | 15 (26.8) | 14 (12.1) | 15 (26.8) | 9 (16.1) | ||
| SPN | 10 (17.9) | 6 (5.2) | 10 (17.9) | 4 (7.1) | ||
| SCN | 7 (12.5) | 21 (18.1) | 7 (12.5) | 13 (23.2) | ||
| MCN | 2 (3.6) | 15 (12.9) | 2 (3.6) | 9 (16.1) | ||
| Pancreatitis | 0 | 6 (5.2) | 0 | 1 (1.8) | ||
| Pseudocyst | 3 (5.4) | 3 (2.6) | 3 (5.4) | 2 (3.6) | ||
| Others | 11 (19.6) | 13 (11.2) | 11 (19.6) | 7 (12.5) | ||
| Tumor location, cm* | 4.3 ± 1.34 | 5 ± 1.27 | 0.078 | 4.3 ± 1.34 | 4.8 ± 1.22 | 0.252 |
| Tumor size, cm | 3.1 ± 1.95 | 3.5 ± 2.14 | 0.233 | 3.1 ± 1.95 | 3.3 ± 2.16 | 0.549 |
| Specimen length, cm | 7.7 ± 1.69 | 9.4 ± 2.55 | < 0.001 | 7.7 ± 1.69 | 8.4 ± 2.45 | < 0.001 |
LA LSPDP lateral approach laparoscopic spleen-preserving distal pancreatectomy; CA LSPDP conventional approach to laparoscopic spleen-preserving distal pancreatectomy; BMI body mass index; ASA American Society of Anesthesiologists; COPD chronic obstructive pulmonary disease; IPMN intraductal papillary mucinous neoplasm; NET neuroendocrine tumor; SPN solid pseudopapillary neoplasm; SCN serous cystic neoplasm; MCN mucinous cyst neoplasm.
*Tumor location was defined as the distance from the lateral border of the aorta to the center of the tumor.
Surgical outcomes
One case (1.8%) of open conversion occurred in the CA LSPDP group. In cases of intraoperative bleeding or inadequate dissection of the splenic vessels, conversion to Warshaw’s technique is necessary. Splenectomy is required in more severe cases. Our study found that 10 (27.8%) patients in the LA LSPDP group and 14 (38.9%) patients in the CA LSPDP group underwent conversion to Warshaw’s technique, with no significant difference (p = 0.317) between the two groups. Additionally, splenectomy was performed in one (1.8%) patient in the LA LSPDP group and three (5.4%) patients in the CA LSPDP group, showing no significant difference (p = 0.618) between the two groups. The LA LSPDP group demonstrated significantly shorter WOT (127.1 min vs. 161.1 min, p = 0.002) and lower EBL (106.2 cc vs. 291.4 cc, p = 0.001) than the CA LSPDP group. Although the transfusion rate was lower in the LA LSPDP group, the difference was not statistically significant (0% vs. 8.9%, p = 0.057). There were no significant differences in CR-POPF (5.4% vs. 10.7%, p = 0.489), postoperative complications (35.7% vs. 27.3%, p = 0.339), or length of hospital stay (7.8 days vs. 7.9 days, p = 0.752). Table 2 summarizes perioperative outcomes after propensity score matching.
Table 2.
Comparative analysis of surgical outcomes between LA LSPDP and CA LSPDP groups.
| LA LSPDP (N = 56) | CA LSPDP (N = 56) | P-value | |
|---|---|---|---|
| Conversion to open surgery, N(%) | 0 | 1 (1.8) | 1.000 |
| Conversion to Warshaw’s technique, N(%) | 10 (27.8) | 14 (38.9) | 0.317 |
| Conversion to combined splenectomy, N (%) | 1 (1.8) | 3 (5.4) | 0.618 |
| Whole operation time, min | 127.1 ± 65.43 | 161.1 ± 47.04 | 0.002 |
| Estimated blood loss, cc | 106.2 ± 134.41 | 291.4 ± 355.74 | 0.001 |
| Transfusion, N(%) | 0 | 5 (8.9) | 0.057 |
| CR-POPF | 3 (5.4) | 6 (10.7) | 0.489 |
| B | 3 (5.4) | 6 (10.7) | |
| C | 0 | 0 | |
| Days until drain removal | 7.3 ± 5.02 | 11.1 ± 14.19 | 0.071 |
| Postoperative complication, N (%) | |||
| Atelectasis | 16 (28.6) | 14 (25) | 0.67 |
| Pneumonia | 0 | 2 (3.6) | 0.495 |
| PPH | 0 | 0 | |
| DGE | 0 | 0 | |
| Wound infection | 2 (3.6) | 2 (3.6) | 1.000 |
| Clavien-Dindo classification, N (%) | 0.564 | ||
| Minor | 17 (30.4) | 13 (23.2) | |
| Major | 3 (5.4) | 7 (12.5) | |
| Reoperation | 0 | 0 | |
| Re-admission, N(%) | 4 (7.1) | 5 (8.9) | 1.000 |
| Morbidity, N(%) | 20 (35.7) | 15 (27.3) | 0.339 |
| Mortality (< 90 days), N(%) | 0 | 1 (1.8) | 0.495 |
| Length of hospital stay, day | 7.8 ± 1.67 | 7.9 ± 2.44 | 0.752 |
LA LSPDP lateral approach laparoscopic spleen-preserving distal pancreatectomy; CA LSPDP conventional approach to laparoscopic spleen-preserving distal pancreatectomy; WT Warshaw’s technique; CR-POPF clinically relevant postoperative pancreatic fistula; PPH Post- pancreatectomy haemorrhage; DGE delayed gastric emptying.
Surgical outcomes in obese patients
Among the patients included in the study, we performed a subgroup analysis for 55 obese individuals, defined as those with a BMI of 25 or higher according to the World Health Organization (WHO) criteria for Asian populations. Twenty-six patients in the LA LSPDP group and 29 patients in the CA LSPDP group were analyzed. None of the patients in either group required conversion to open surgery during the procedure. Five (19.2%) patients in the LA LSPDP group and nine (31%) patients in the CA LSPDP group underwent conversion to Warshaw’s technique. The difference between the two groups was not statistically significant (p = 0.316). However, the CA LSPDP group had a significantly higher rate of combined splenectomy (10 (34.5%) patients in the CA LSPDP group vs. 3 (11.5%) patients in the LA LSPDP group, p = 0.046). Obese patients in the LA LSPDP group had a significantly shorter WOT (133.6 min vs. 166.8 min, p = 0.038) and less intraoperative blood loss (137.9 cc vs. 388.6 cc, p = 0.008) than those in the CA LSPDP group (Table 3).
Table 3.
Subgroup analysis of perioperative outcomes between LA LSPDP and CA LSPDP groups stratified by obesity.
| LA LSPDP (N = 26) | CA LSPDP (N = 29) | P-value | |
|---|---|---|---|
| Conversion to open surgery, N (%) | 0 | 0 | |
| Conversion to Warshaw’s technique, N (%) | 5 (19.2) | 9 (31.0) | 0.316 |
| Conversion to distal pancreatectomy, N (%) | 3 (11.5) | 10 (34.5) | 0.046 |
| Whole operation time, min | 133.6 ± 63.38 | 166.8 ± 51.87 | 0.038 |
| Estimated blood loss, cc | 137.9 ± 179.92 | 388.6 ± 444.85 | 0.008 |
| Transfusion, N (%) | 0 | 3 (10.3) | 0.238 |
| CR-POPF | 2 (7.7) | 4 (13.8) | 0.672 |
| Days until drain removal | 8 ± 5.15 | 12.4 ± 15.81 | 0.177 |
| Postoperative complication, N (%) | |||
| Atelectasis | 13 (50) | 7 (24.1) | 0.047 |
| Pneumonia | 0 | 1 (3.4) | 1.000 |
| PPH | 0 | 0 | |
| DGE | 0 | 0 | |
| Wound infection | 0 | 2 (6.9) | 0.492 |
| Clavien-Dindo classification, N (%) | 0.819 | ||
| Minor | 11 (42.3) | 8 (27.6) | |
| Major | 2 (7.7) | 5 (17.2) | |
| Reoperation | 0 | 0 | |
| Re-admission, N(%) | 1 (3.8) | 3 (10.3) | 0.613 |
| Morbidity, N(%) | 13 (50) | 8 (28.6) | 0.107 |
| Mortality (< 90 days), N (%) | 0 | 1 (3.6) | 1.000 |
| Length of hospital stay, day | 7.8 ± 1.37 | 8 ± 2.41 | 0.66 |
LA LSPDP lateral approach laparoscopic spleen-preserving distal pancreatectomy; CA LSPDP conventional approach to laparoscopic spleen-preserving distal pancreatectomy; WT Warshaw’s technique; CR-POPF clinically relevant postoperative pancreatic fistula; PPH Post- pancreatectomy haemorrhage; DGE delayed gastric emptying.
Discussion
In this study, we presented the postoperative outcomes of patients who underwent LA LSPDP and investigated the benefits of this lateral approach compared with the conventional medial-to-lateral approach in the supine position. Examining improvements in surgical techniques may not always result in differences in postoperative morbidities. However, other results, such as operation time and blood loss, could better reflect small but significant differences in terms of technical ease. Easier techniques of LSPDP could be accepted by more surgeons and extend its applicability to more pancreatic lesions, thereby extending the benefits to more patients. Our results showed that the LA LSPDP group had a shorter WOT and relatively less EBL compared to the CA LSPDP group. No significant differences in postoperative complications, including CR-POPF, were observed between the two groups. Therefore, LA LSPDP can serve as a safe and adaptable method for select patients with benign or borderline malignant pancreatic tumors located lateral to the left border of the aorta.
Considering the high life expectancy of patients with benign or borderline malignant lesions of the distal pancreas, they should be informed about the risk of developing long-term sequelae such as new-onset diabetes mellitus (NODM) following distal pancreatectomy (DP). Therefore, surgeons should pay careful attention to the extent of pancreatic resection. Systematic reviews and meta-analyses have reported that the average incidence of NODM after DP ranges from 14 to 29%11,12. Patients who undergo DP have a higher likelihood of developing NODM than those who undergo pancreaticoduodenectomy. This is closely related to the asymmetric distribution of β-islet cells, whose density gradually increases from the head of the pancreas to the body and tail13,14. Several studies have reported that the length of the resected pancreas, age, sex, BMI, surgical blood loss, and splenectomy are independent risk factors for the development of NODM after DP15–17. The pathological report of our study showed no significant difference in tumor size between the two groups. However, the specimen length was notably shorter in the LA LSPDP group than in the CA LSPDP group (7.7 cm vs. 8.4 cm, p < 0.001). This suggests that LA LSPDP could prevent unnecessary resection of the pancreatic parenchyma, offering an advantage of preserving the function of the remnant pancreas. However, our study did not examine the outcomes of the remaining pancreatic functions, indicating the need for further investigation. In addition, during CA LSPDP, more dissection near the remnant pancreas is typically performed, resulting in a higher incidence of crushing injury or capsule tearing of the pancreatic parenchyma, which could lead to a postoperative pancreatic fistula. LA LSPDP allows pancreatic transection while preserving the capsule of the pancreas on the remnant side. The lateral approach ensures that only a portion of the pancreas requiring removal is dissected, thereby minimizing unnecessary contact with the remnant pancreatic tissue. In addition, the incidence of CR-POPF was notably higher in the CA LSPDP group (10.7%) than in the LA LSPDP group (5.4%), although this difference was not statistically significant (p = 0.489). Similarly, our study found a higher rate of biochemical pancreatic fistula in the CA LSPDP group (30.4%) than in the LA LSPDP group (19.6%), although the difference was not statistically significant (p = 0.19). This suggests that the drainage of amylase-rich fluid is more effectively reduced in LA LSPDP.
Separation of the splenic vein from the pancreatic parenchyma is the most critical point during LSPDP, with preservation of the splenic vessels. There are many short branches from the splenic vein to the pancreatic parenchyma, making them a major site of intraoperative bleeding. In particular, towards the tail of the pancreas, the splenic vein is embedded in the pancreatic parenchyma, making it more difficult to isolate. In CA LSPDP, the operation proceeds from the medial to the lateral direction, starting with pancreatic resection. The surgeon mainly spends considerable time on the initial concentration in the dissection of the pancreatic parenchyma by separating the splenic artery and vein from the pancreatic parenchyma. As it progresses to the pancreatic tail, the operator enters the complex anatomical structure of the splenic vessels and gradually loses concentration, which can ultimately increase the risk of bleeding. In LA LSPDP, the procedure begins with separation of splenic vessels from the pancreatic tail, which is the most complex and challenging process. This allows the operator to focus intensely on safely managing the separation of the splenic vessels from the pancreatic parenchyma, thus addressing the highest risk of bleeding first.
Surgery is more complex and challenging in obese patients because of the copious amount of visceral fat in their abdominal cavity. Many studies have reported that obesity is a potential risk factor for increased postoperative complications such as blood loss, operation time, and wound infection rate18,19. In the lateral approach technique, excessive visceral fat is dropped by gravity in the right decubitus position, making it easier to expose the pancreas and the spleen. We conducted a sub-analysis of obese patients in both groups. The LA LSPDP group had a shorter operative time, less bleeding, and a lower rate of conversion to DP than the CA LSPDP group. These results demonstrate that the lateral approach can be safely applied in obese patients. This approach can facilitate the resection of the renocolic ligament, effectively displacing the transverse colon towards the caudal side of the patient, thereby providing a more spacious surgical field. Furthermore, in patients with a history of upper abdominal surgery, accessing the pancreas and identifying anatomical structures can be challenging due to intra-abdominal adhesions in the supine position. Even after gastric surgery or pancreaticoduodenectomy, accessing the pancreas, which is situated posterior to the gastrojejunal anastomosis, can be exceedingly challenging in the supine position because of limited surgical view. However, the lateral position of the patient offers the advantage of a relatively adhesion-free operative field without impeding access to the body or tail of the pancreas behind the gastroenteric anastomosis.
The current study had several limitations that should be considered when interpreting the results. First, while PSM reduced bias secondary to confounding, it could not fully adjust for all potential differences in the LA LSPDP group versus the CA LSPDP group. Second, the application of LA LSPDP becomes increasingly challenging as the tumor location moves more medially from the pancreatic tail. Currently, there are no precise guidelines or evidence to define how medially a tumor can be located in the pancreatic tail for LA LSPDP. Moreover, the decision to apply this technique varies among surgeons based on their individual preferences and experiences. Further studies should be conducted in the future. Finally, we analyzed the early postoperative outcomes between the two groups. However, a larger cohort study is needed to determine the long-term effects of LA LSPDP.
In conclusion, the lateral approach for pancreatic tail tumors can save operation time and result in less blood loss than the conventional approach. In particular, it is a safe and easily accessible method for preserving splenic vessels even in morbidly obese patients. However, further large-scale studies are needed to validate our findings.
Acknowledgements
No additional investigators were included in the study.
Author contributions
Study conception and design: SEP and THH; Acquisition of data: SEP, TYL, YCY, CHS, GHN, YKW, and DDY; Statistical analysis: SEP; Analysis and interpretation of data: SEP and THH; Drafting of manuscript: SEP; Critical revision: SEP and THH; Guarantor of article: THH. All authors have approved the final version of the manuscript, including a list of authors.
Funding
The authors have received no specific funding for this study.
Data availability
Research data supporting the findings of this study are available from the corresponding author upon reasonable request. These data are not publicly available because of privacy or ethical restrictions.
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved and carefully monitored by the Institutional Review Board (KC22RISI0484). Written informed consent was obtained from all participants. The authors are accountable for all aspects of this work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Warshaw, A. L. Distal pancreatectomy with preservation of the spleen. J. Hepatobiliary Pancreat. Sci.17(6), 808–812. 10.1007/s00534-009-0226-z (2010). [DOI] [PubMed] [Google Scholar]
- 2.Warshaw, A. L. Conservation of the spleen with distal pancreatectomy. Arch. Surg.123(5), 550–553. 10.1001/archsurg.1988.01400290032004 (1988). [DOI] [PubMed] [Google Scholar]
- 3.Fanget, F., Thievenaz, R. & Lifante, J. C. Laparoscopic resection of a benign cystic pedicled pancreatic lesion in right lateral position (with video). J. Visc. Surg.155(4), 333–334. 10.1016/j.jviscsurg.2018.06.004 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Strickland, M. et al. Lateral approach in laparoscopic distal pancreatectomy is safe and potentially beneficial compared to the traditional medial approach. Surg. Endosc.29(9), 2825–2831. 10.1007/s00464-014-3997-5 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Kooby, D. A. et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann. Surg.248(3), 438–446. 10.1097/SLA.0b013e318185a990 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Abraham, D., Thomas, J. K., Joseph, P. & Paul, M. J. Lateral laparoscopic approach to pancreatic tail insulinomas. World J. Endocrine Surg.4(1), 3–7. 10.5005/jp-journals-10002-1082 (2012). [Google Scholar]
- 7.Honore, C., Honore, P. & Meurisse, M. Laparoscopic spleen-preserving distal pancreatectomy: description of an original posterior approach. J. Laparoendosc. Adv. Surg. Tech. A17(5), 686–689. 10.1089/lap.2006.0222 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Nakamura, M. et al. Lateral approach for laparoscopic splenic vessel-preserving distal pancreatectomy. Surgery150(2), 326–331. 10.1016/j.surg.2011.05.014 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Clavien, P. A. et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann. Surg.250(2), 187–196. 10.1097/SLA.0b013e3181b13ca2 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Bassi, C. et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery161(3), 584–591. 10.1016/j.surg.2016.11.014 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Chen, Z. et al. A novel clinical model for risk prediction and stratification of new-onset diabetes mellitus after distal pancreatectomy. Hepatobiliary Surg. Nutr.12(6), 868–881. 10.21037/hbsn-22-382 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu, J., Sun, R., Han, X. & Liu, Z. New-onset diabetes mellitus after distal pancreatectomy: a systematic review and meta-analysis. J. Laparoendosc. Adv. Surg. Tech. A30(11), 1215–1222. 10.1089/lap.2020.0090 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Wang, X. et al. Quantitative analysis of pancreatic polypeptide cell distribution in the human pancreas. PLoS ONE8(1), e55501. 10.1371/journal.pone.0055501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, X. et al. Regional differences in islet distribution in the human pancreas–preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS ONE8(6), e67454. 10.1371/journal.pone.0067454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang, J. S. et al. Endocrine function impairment after distal pancreatectomy: incidence and related factors. World J. Surg.40(2), 440–446. 10.1007/s00268-015-3228-9 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Dai, M. et al. Risk factors for new-onset diabetes mellitus after distal pancreatectomy. BMJ Open Diabetes Res. Care8(2), e001778. 10.1136/bmjdrc-2020-001778 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imamura, S. et al. High incidence of diabetes mellitus after distal pancreatectomy and its predictors: a long-term follow-up study. J. Clin. Endocrinol. Metab.109(3), 619–630. 10.1210/clinem/dgad634 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kodera, Y. et al. Obesity and outcome of distal gastrectomy with D2 lymphadenectomy for carcinoma. Hepatogastroenterology51(58), 1225–1228 (2004). [PubMed] [Google Scholar]
- 19.House, M. G. et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J. Gastrointest. Surg.12(2), 270–278. 10.1007/s11605-007-0421-7 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data supporting the findings of this study are available from the corresponding author upon reasonable request. These data are not publicly available because of privacy or ethical restrictions.