Abstract
Iodine deficiency with the resultant maternal hypothyroxinemia and the effects of endocrine disruptors can, individually or together, have a negative effect on embryonic and fetal brain development. This is the conclusion of a recent review by the authors which examined and critically discussed a total of 279 publications from the past 30 years on the effects of mild to moderate iodine deficiency, reduced maternal thyroxine levels, and the influence of endocrine disruptors on child brain development during pregnancy. Adequate iodine intake is important for all women of childbearing age to prevent negative psychological and social consequences for their children. An additional threat to the thyroid hormone system is the ubiquitous exposure to endocrine disruptors, which can increase the impact of maternal iodine deficiency on the neurocognitive development of their offspring. Ensuring an adequate iodine intake is therefore not only crucial for healthy fetal and neonatal development in general, but could also prevent the potential effects of endocrine disruptors. Due to the current deficient iodine status of women of childbearing age and of children and adolescents in Germany and most European countries, urgent measures are needed to improve the iodine intake of the population. Therefore, in the opinion of the AKJ, young women of childbearing age should be instructed to take iodine supplements continuously for at least 3 months before conception and during pregnancy. In addition, detailed strategies for detecting and reducing exposure to endocrine disruptors in accordance with the “precautionary principle” should be urgently developed.
Keywords: iodine deficiency, pregnancy, hypothyroxinemia, neurocognitive development, endocrine disruptors
Introduction
Thyroid hormones are especially important for embryonic/fetal and early postnatal neurocognitive development. Depending on the severity, duration and time of iodine deficiency in certain stages of life, iodine-deficiency disorders are associated with physical, neurological and mental deficiencies in humans. Severe iodine deficiency during pregnancy can have a number of negative impacts on the health of mother and child, including hypothyroidism, goiter, stillbirths, increased perinatal mortality, neurological damage and mental disability 1 2 .
In addition, exposure to endocrine-disrupting chemicals (EDCs) is increasing worldwide 3 4 5 . These endocrine disruptors are substances which are either present in nature or are produced artificially and released into the environment. The majority of EDCs specifically interfere with the thyroid metabolism and are therefore known as thyroid-disrupting chemicals (TDCs) 6 7 8 . The placenta is especially sensitive to EDCs because of its abundance of hormone receptors 9 . Exposure to these chemicals combined with an inadequate iodine intake can additionally harm the development, growth, differentiation and metabolic processes of the embryonic/fetal and neonatal brain 6 7 8 10 11 12 13 14 15 16 17 18 19 20 21 22 .
Both iodine deficiency and exposure to TDCs have a negative impact on general health and the socioeconomic system. The estimated annual cost of the seven EDC categories with the highest causation amounts to 33.1 billion Euros in Europe. The largest share of these costs relate to the loss of IQ and the increase in neurocognitive disorders 23 24 25 26 27 . In addition, a growing body of evidence suggests that exposure to TDCs, including through air pollution, not only affects brain function 13 28 29 30 31 but also has an impact on the outcomes of pregnancy and birth 32 33 34 35 36 .
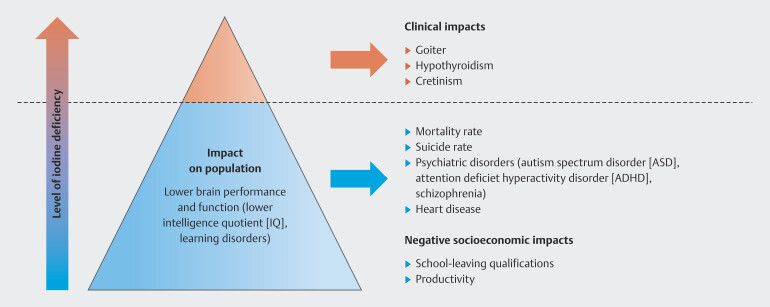
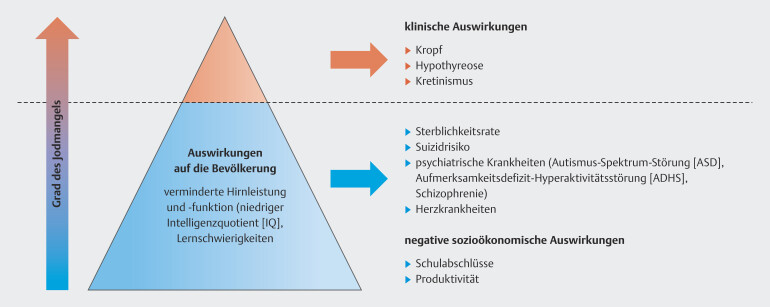
“Endemic goiter” has been synonymous with iodine deficiency for years and the aim has always been to prevent enlargement and overt dysfunction of the thyroid gland. However, there has been a paradigm shift in recent decades 37 , ever since the focus has moved to examining the consequences of mild to moderate iodine deficiency on the cognitive development of the embryo ( Fig. 1 ).
Fig. 1.
The paradigm shift relating to iodine deficiency (Fig. is based on data from 24 ).
Epidemiological and experimental studies on mild to moderate iodine deficiency carried out in the last two decades have shown that embryonic/fetal brain development can be affected not only in the infants of mothers with overt hypothyroidism but also those born to mothers with hypothyroxinemia in the early stages of pregnancy 38 39 40 41 42 . Low FT4, also known as hypothyroxinemia, is an indication of individual iodine deficiency. As FT4, but not FT3, is transported almost exclusively via the placenta in the first three months of pregnancy, slight changes in fetal brain development can be observed even if maternal thyroid hormone levels are low but still within reference ranges. The fetus is able to produce thyroid hormones from week 12–14 of gestation and is then dependent on iodine which is transported via the placenta, and no longer on maternal FT4 of which lower levels cross the placenta to reach the fetus from the 12th week of pregnancy.
Because of methodological issues with the definition, findings may not be homogeneous. Moreover, too little attention has been paid to isolated maternal hypothyroxinemia (IMH) because of some uncertainty regarding treatment. But IMH is clearly an indication of maternal iodine deficiency not reflected by elevated TSH levels, as the iodine-depleted thyroid gland reacts more sensitively to TSH 43 44 45 .
The pollution of our environment, with EDCs found in the air, the water, food, and sanitary products, is increasing worldwide and has reached potentially hazardous levels. Generally speaking, EDCs can affect the normal functioning of the endocrine system of humans and animals. They especially affect the thyroid hormone system, with negative impacts on fetal and neonatal brain development, growth, differentiation, and metabolic processes 6 7 .
The aim of a recently published review article was to highlight the importance of IMH caused by mild iodine deficiency and additional environmental factors such as EDCs and air pollution on the cognitive and psychosocial development of children and to identify measures for the prevention and treatment of IMH.
Method
The basis for compiling this opinion was a joint review article published in the international peer-reviewed journal Nutrients in 2023 2 . We also carried a search of the recent literature, focusing on relevant articles published between 2022 and September 2024 in PubMed, Medline, Cochrane, Web of Science, and Google Scholar using the search terms Iodine, Pregnancy, Thyroid Hormone, Thyroid Diseases, Endocrine Disruptors, Hypothyroxinemia, and Subclinical Hypothyroidism, which were searched for in combination using the operators AND and OR. The drafted key statements were voted on by the scientific advisory board of the Iodine Deficiency Working Group ( Arbeitskreis Jodmangel e. V. , AKJ).
Thyroid Function in Pregnancy
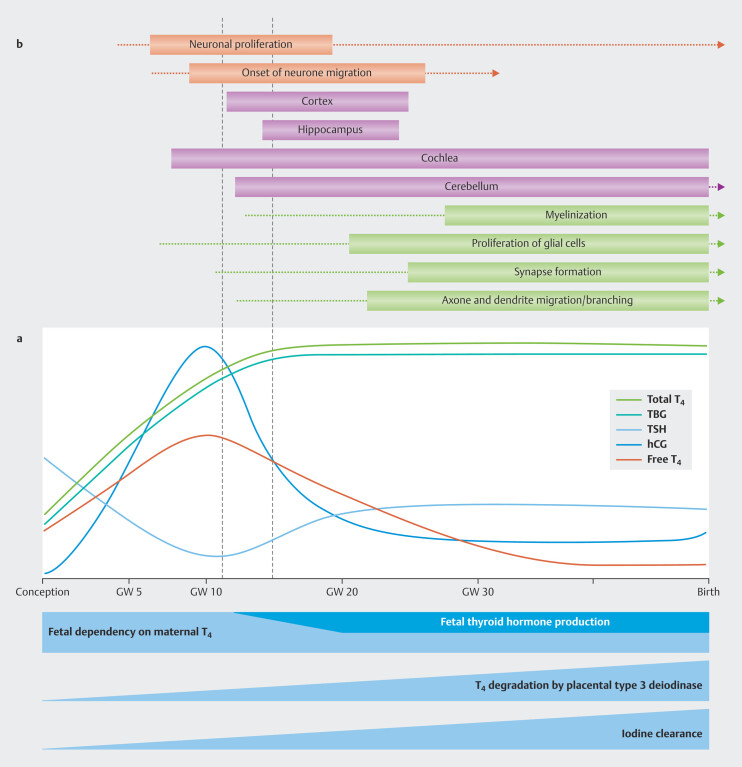
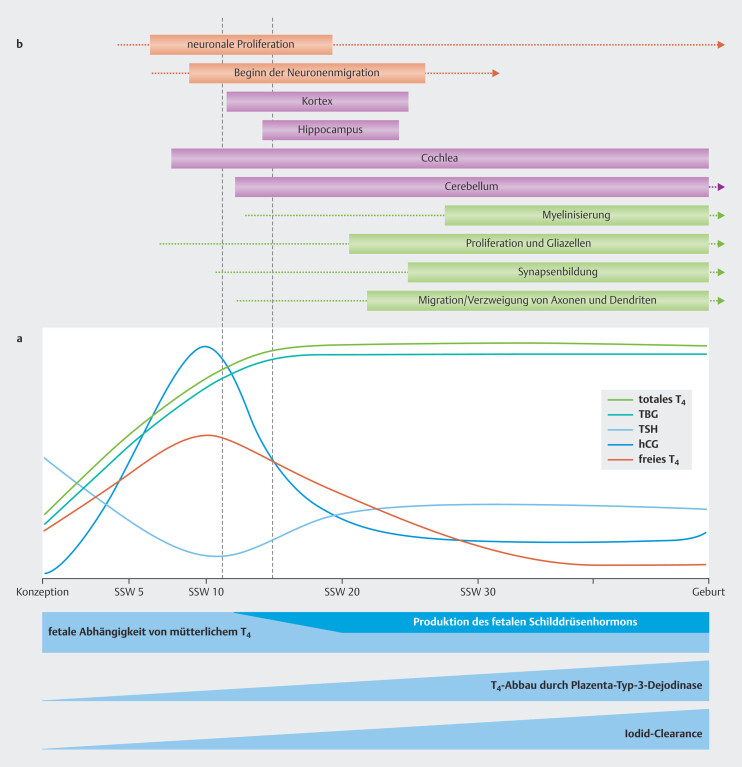
In pregnancy, the functions of the maternal thyroid are dynamically adapted to the thyroid hormone needs of the mother and embryo/fetus ( Fig. 2 a ). Pregnant women need about 50% more iodine because of their increased production of thyroid hormones, increased renal iodide clearance and the transplacental transfer of iodine to the fetus 46 47 . The average iodine supplementation recommendation during pregnancy is therefore 250 µg/day 48 .
Fig. 2.
Changes in thyroid physiology during pregnancy ( a ) and the relationship between thyroid hormone activity and brain development ( b ) (Fig. is based on data from 49 50 ). See text for further explanations (based on data from 2 ).
Median urine iodine concentrations (UIC) are used to assess iodine intake of the general population in the context of epidemiological studies. According to the criteria of the WHO, they should be over 100 µg/l, and over 150 µg/l during pregnancy and lactation 51 .
We know from recent epidemiological studies that the standard iodine intake is below the mean of what is required in about 30% of adults, 48% of women of childbearing age, and 44% of children and adolescents in Germany 52 53 54 . This is also the case in more than 70% (n = 21) of 29 European countries ( Table 1 ) 52 55 56 57 58 59 60 61 62 63 64 65 66 67 68 . A mean UIC figure of > 150 μg/l was only found in a few EU countries with mandatory universal salt iodization programs such as Bulgaria or Romania (see Table 1 ). Studies in other countries have shown that only mandatory universal salt iodization of more than 25 mg/kg can ensure sufficient iodine intake through nutrition across all sections of the population including pregnant women who have higher requirements 51 69 . Young women who are vegan or vegetarian and do not take iodine supplements are most at risk of low iodine status, iodine deficiency, and insufficient iodine intake.
Table 1 Iodine intake for the general population and for pregnant women in Europe (data from 2 ).
| Country | General population a | Pregnant women b | ||||||
| Median (UIC) (μg/l) |
Date of survey (N, S) |
Population | Iodine intake of the population | Median (UIC) (μg/l) |
Date of survey (N, S) |
Iodine intake | Legal status (year) e |
|
| Abbreviations: SAC = school-age children (normally aged 6–12 years); UIC = urine iodine concentration; USI = universal salt iodization; N = national representative data; S = only subnational data; Dates from a 68 , b 55 , c 70 , d 56 , e 62 , f 63 64 | ||||||||
| Austria | 111 | 2012 (N) |
SAC (7–14) |
adequate | 87 | 2009–2011 (S) |
inadequate | obligatory (1999) |
| Belgium | 113 | 2010/2011 (N) |
SAC (6–12) |
adequate | 124 | 2010 (N) |
inadequate | voluntary (2009) |
| Bulgaria | 182 | 2008 (N) |
SAC (7–11) |
adequate | 165 | 2003 (N) |
adequate | obligatory (2001) |
| Croatia | 248 | 2009 (N) |
SAC (7–11) |
adequate | 140 | 2009, 2015 (S) | inadequate | obligatory (1996) |
| Denmark | 145 | 2015 (S) |
SAC | adequate | 101 | 2012 (S) |
inadequate | obligatory (2000) f |
| Finland | 96 | 2017 (N) |
adults (25–74) |
inadequate | 115 | 2013–2017 (S) f |
inadequate | voluntary f |
| France | 136 | 2006–2007 (N) |
adults (18–74) |
adequate | 65 | 2006–2009 (S) |
inadequate | voluntary |
| Germany | 89 | 2014–2017 (N) |
SAC, adolescents (6–12) | inadequate | 54 | 2008–2011 (N) c |
inadequate | voluntary |
| Greece | 132 | 2018 (N) |
adults | adequate | 127 | 2008–2015 (S) |
inadequate | voluntary |
| Hungary | 228 | 2005 (S) |
SAC (10–14) |
adequate | 128 | 2018 (S) d |
inadequate | obligatory (2013) |
| Ireland | 111 | 2014–2015 (N) |
adolescent girls (14–15) | adequate | 107 | 2008–2010 (S) |
inadequate | voluntary |
| Italy | 118 | 2015–2019 (S) |
SAC | adequate | 72 | 2002–2013 (S) |
inadequate | obligatory (2005) |
| Netherlands | 130 | 2006 (S) |
adults (50–72) |
adequate | 223 | 2002–2006 (S) |
adequate | voluntary |
| Poland | 112 | 2009–2011 (S) |
SAC (6–12) |
adequate | 113 | 2007–2008 (S) |
inadequate | obligatory (2010) |
| Portugal | 106 | 2010 (N) |
SAC | adequate | 85 | 2005–2007 (N) |
inadequate | voluntary |
| Romania | 255 | 2015–16 (N) |
SAC (6–11) |
adequate | 206 | 2016 (S) |
adequate | obligatory (2009) |
| Spain | 173 | 2011–12 (N) |
SAC | adequate | 120 | 2002–2011 (S) |
inadequate | voluntary |
| Sweden | 125 | 2006–07 (N) |
SAC (6–12) |
adequate | 98 | 2006–2007; 2010–2012 (S) |
inadequate | voluntary (1936) f |
| Switzerland | 137 | 2015 (N) |
SAC (6–12) |
adequate | 136 | 2015 (N) |
inadequate | voluntary |
| United Kingdom | 166 | 2015–2016 (N) |
SAC, adolescents (4–18) | adequate | 99 | 2002–2011 (S) |
inadequate | no USI program |
As thyroxine-binding globulin (TBG) increases in pregnancy, determination of FT4 is imprecise as routine measurement of FT4 values is false resulting in figures that are either too low or too high because measurement methods depend on measuring TBG values.
In practice, this means that to ensure correct values, the mean normal FT4 range must be assumed to ensure that pregnant women have an adequate iodine intake. Additional supplementation with iodide tablets is necessary and is a useful preventive measure for all women wanting to have children 71 72 73 .
Impact of Mild Iodine Deficiency and Maternal Hypothyroxinemia on Prenatal Brain Development
A time frame (s. Fig. 2 b , between the two red dotted lines) has been identified in which a decrease in maternal thyroid hormones (FT4) has a particularly strong impact on neuronal proliferation and on the migration and development of the inner ear. Recognizing this early critical phase can have a direct clinical impact on the assessment of risk and the time frame for treatment options 74 75 . A lower fT4 transfer to the maternal placenta in this critical developmental stage probably has the greatest impact on the neurological development of the child 76 77 78 79 80 81 and also manifests in the form of permanent structural and functional anomalies 38 82 83 84 85 86 .
IMH ( Table 2 ) probably occurs much more often than subclinical hypothyroidism, 40 42 44 87 88 89 90 . IMH prevalence is assumed to be higher in countries with iodine deficiency 43 91 . Trimester-specific reference ranges for serum TSH and fT4 levels in an euthyroid pregnant population would have to be established as the gold standard for diagnosis 92 93 . Unfortunately, reference ranges are currently only available for TSH levels.
Table 2 Definition and prevalence of maternal thyroid disorders (data from 82 ).
|
Isolated maternal hypothyroxinemia (1.5–25%)
Serum fT4 concentration in the lower 5th or 10th percentile of the reference range with normal TSH concentrations |
| Overt hypothyroidism (0.3–0.5%) Elevated serum TSH levels together with decreased fT4 concentrations |
| Subclinical hypothyroidism (2–2.5%) Elevated serum TSH levels and normal fT4 concentrations |
| Autoimmune thyroid disease (10–20%) Presence of TPO and/or TG antibodies in serum with or without changes to TSH and fT4 concentrations |
In observational studies on the impairment of cognitive development and behavioral disorders in the context of mild iodine deficiency, maternal blood samples were usually taken between the 9th and the 13th week of gestation ( Table 3 ). The neurological examinations of the offspring were carried out between the ages of 6 months and 16 years 81 . The general study designs varied considerably. The differences relate to the criteria used to select mother-child pairs, the reference values and ranges used to determine the different levels of maternal hypothyroidism or hypothyroxinemia, and the different tests used to evaluate neurological development (s. Table 3 ).
Table 3 Observational studies on the negative impact on cognitive development and behavioral disorders in connection with mild iodine deficiency – characteristics of all studies included in the systematic evaluation (data from 94 ) (“sister articles” were combined).
| Author, Year [Reference] | Total number of tested participants | Country | Maternal thyroid disorder | Pregnancy week at TFT | Criteria for thyroid function disorder | Age of child at evaluation | Tests used to evaluate neurological development |
| Abbreviations: Co = continuous; HR = hypothyroxinemia; OH = overt hypothyroidism; SH = subclinical hypothyroidism; TFT = thyroid function test; TSH = thyroid-stimulating hormone; WISC = Wechsler Intelligence Scale for Children | |||||||
| Pop et al. 1999 95 | 220 | Netherlands | HR | 12 and 32 weeks | 10th percentile for fT4 (< 10.4 pmol/l) and 5th percentile for fT4 (< 9.8 pmol/l) | 10 months | Bayley Scales of Infant Development |
| Pop et al. 2003 96 | 125 | Netherlands | HR | 12, 24 and 32 weeks | fT4 < 10th percentile (12.10 pmol/l) | 1–2 years | Bayley Scales of Infant Development |
| Kasatkina et al. 2006 81 | 35 | Russia | HR | 1st and 3rd trimester | fT4 < 12.0 pmol/l | 6, 9 and 12 months | Gnome method, especially the Coefficient of Mental Development |
| Li et al. 2010 97 | 213 | China | SH and HR | 16 to 20 weeks | SH = TSH > 97.5 percentile (4.21 mU/l), HR = tT4 < 2.5 percentile (101.79 nmol/l) | 25–30 months | Bayley Scales of Infant Development |
| Henrichs et al. 2010 98 | 3659 | Netherlands | HR and Co TSH | 13,3 weeks | HR = fT4 10th percentile (< 11.76 pmol/l) and 5th percentile (< 10.96 pmol/l), Co TSH = TSH reference range 0.03–2.50 mU/l | 18 and 30 months | MacArthur-Bates Communication Development Inventories after 18 months, review of speech development after 30 months |
| Suárez-Rodríguez et al. 2012 80 | 70 | Spain | HR | 37 weeks | fT4 < 10th percentile (9.5 pmol/l) | 38 months and 5 years | McCarthy Scales of Children’s Abilities |
| Williams et al. 2012 99 | 166 | United Kingdom | SH and HR | + 1 hour after delivery | SH = TSH > 3.0 mU/l, HR = fT4 ≤ 10th percentile (11.6 pmol/l) or tT4 ≤ 10th percentile (108.4 nmol/l) |
5.5 years | McCarthy Scales of Children’s Abilities |
| Craig et al. 2012 100 | 196 | USA | HR | 2nd trimester | fT4 < 3rd percentile (11.84 pmol/l) | 2 years | Bayley Scale of Infant Development III |
| Ghassabian et al. 2014 79 /Korevaar et al. 2016 83 | 3737/5647 | Netherlands | HR and SH | 13.5/13.2 weeks | HR = fT4 < 5th percentile (10.99 pmol/l), SH = TSH > 2.50 mU/l |
6 years | Snijders-Oomen Non-verbal Intelligence Test, revision (mosaic patterns and categories) |
| Päkkilä et al. 2015 101 | 5295 | Finland | HR, SH and OH | Average 10.7 weeks | HR = fT4 < 11.4–11.09 pmol/l depending on the trimester, SH = TSH > 3.10–3.50 mU/l, depending on the trimester |
8 and 16 years | Severe and mild ADHD symptoms and normal behavior; teachers reported on the standard of the schoolwork of the child; self-report by the adolescent and WISC-reviewed |
| Grau et al. 2015 102 | 455 | Spain | HR | 1st and 2nd trimester | < 10th percentile (13.7–11.5 pmol/l depending on the trimester) | 1 and 6–8 years | Brunet-Lézine Scale and WISC-IV |
All studies, with the exception of the one by Grau et al. 102 which investigated the effects of low maternal fT4 levels at the end of the first trimester of pregnancy, report impairment of cognitive and motor development in exposed children 40 44 77 79 92 96 97 98 103 104 . The correlation gradually decreased with advancing pregnancy and disappeared by late pregnancy 42 101 105 .
Overall, none of the systematic reviews and meta-analyses showed clear threshold values for high TSH and/or low fT4 values in the serum of pregnant women which would clearly indicate an increased risk of neurological developmental disorders in their offspring. Such threshold values could not be determined because the epidemiological studies were not designed to show quantitative thresholds (s. Table 3 ).
Impact of Endocrine Disruptors (TDCs) on Thyroid Hormone System and the Role of Adequate Iodine Intake
TDCs do not just have a direct effect on pregnancy by acting as hormone agonists or antagonists but also have indirect effects by impairing maternal, placental, and fetal homeostasis. It is thought that the adverse health effects of TDCs including air pollution on offspring may be the result of two mechanisms: the first mechanism directly affects the placenta and therefore passes into the fetal circulation, and/or the second mechanism has an indirect impact through oxidative stress on the placenta which induces inflammation and epigenetic changes in the placenta and offspring 13 106 107 108 109 110 111 .
In view of the many different effects of all EDCs, such as low-dose effects, possible non-linear dose responses, cumulative effects which are often expected in cases of combined exposure, and cross-generational effects with different impacts during critical windows of exposure, it is currently unlikely that it is possible to define safe EDC contamination levels 26 84 112 113 114 115 .
Iodine deficiency is clearly able to promote adverse effects 116 . The urgency of the problem is due to the concurrence of the widely prevalent inadequate iodine intake and the continuously increasing exposure of humans to TDCs 6 32 117 118 119 . The studies on maternal hypothyroxinemia caused by mild to moderately severe iodine deficiency carried out to date have not taken additional prenatal exposure to TDCs into account (s. Table 4 , right-hand column).
Table 4 Potential thyroid-disrupting chemicals (TDCs) which target the signaling pathways of thyroid hormones (data from 2 ).
| Examples of chemicals | Target of TDC activities and outcomes | Changes in neurological development |
|
1
OCPs – are predominantly used in agriculture to protect crops, but they have been banned or their use has been greatly reduced in recent decades because of their environmental persistence and neurotoxicity.
2 PCBs – banned compounds used to produce electrical devices such as transformers and used in hydraulic fluids, heat transfer fluids, lubricants, and plasticizers. 3 Perchlorates, thiocyanate, and nitrate – exposure to these harmful substances occurs through foodstuffs or from other sources (e.g., thiocyanate in cigarette smoke or rocket fuels and perchlorate and nitrate in fertilizers). 4 Phthalates – are used to make plastics more flexible. They are also present in some food packaging, cosmetics, children’s toys, and medical devices. 5 Genistein – a substance which occurs naturally in plants with hormone-like activity found in soya products such as tofu or soya milk. 6 4NP – is used in the production of antioxidants, lubricant oil additives, detergents and washing-up liquids, emulsifiers, and solubilizers. 7 BP2 – is no longer approved for use as a UV filter in sun creams in the European Union. However, it is still contained in plastic materials and many cosmetics to prevent UV-related degradation. 8 Amitrole – is used as an herbicide. 9 PBDEs – are used in the production of flame retardants in household items such as upholstery foam and carpets. Although most PBDEs have been banned or are being gradually phased out, they persist in the environment. 10 Triclosan – may be present in some antimicrobial products and personal care products such as body washes. 11 Silymarin – a flavonoid compound which is a purified extract of the milk thistle plant. 12 Erythrosine, also known as Red Dye No. 3 – is an organo-iodine compound. It is a reddish-pink dye mainly used for food coloring. 13 Hydroxylated PBDEs (OH-BDEs) are abiotic and biotic transformation products of PBDEs which also occur naturally in marine systems. 14 Bisphenols, especially bisphenol A (BPA) – are used in the production of polycarbonate plastics and epoxy resins and are contained in many plastic products such as water bottles, food containers, CDs, DVDs, safety equipment, thermal paper, and medical devices. | ||
|
Organochlorine pesticides (OCPs)
1
Polychlorinated biphenyl compounds (PCB) 2 |
TSH-receptor signaling and reduced stimulation of thyroid follicular cells 120 | |
|
Perchlorate
3
Thiocyanate 3 Nitrate 3 Phthalates 4 |
Na+/I symporter (NIS) and inhibition of TH biosynthesis | |
| Propylthiouracil (PTU) Methimazole (MMI) Genistein 5 4-nonylphenol (NP) 6 Benzophenone-2 (BP2) 7 Herbicide (amitrole) 8 |
Inhibition of thyroid peroxidase (TPO) leads to lower TH synthesis and a subsequent reduction in circulating TH concentrations. | |
|
OH-PCBs
2
Polybrominated diphenyl ethers (PBDEs) 9 Phthalates 4 Genistein 5 |
TH distributor proteins : Displacement of T4 and T3 by the thyroid serum-binding protein transthyretin (TTR) and/or thyroid-binding globulin (TBG) disturbs TH homeostasis and decreases TH plasma levels. | |
|
Polychlorinated biphenyls (PCBs, OH-PCBs)
2
Triclosan 10 |
Upregulation of thyroid hormone catabolism through activation of key hepatic receptors leads to decrease of circulating TH levels 111 143 . | |
| Silymarin 11 | Disorders of cellular transmembrane transporters (MCT8, MCT10 and OATP1C1) inhibit T3 uptake. |
|
|
Erythrosine
12
6-n-propylthiouracil PCBs 2 |
Modification of deiodinase enzyme activities (DIO2, DIO3) through competitive inhibition of the enzyme or through interaction with its sulfhydryl cofactor. |
|
|
OH-PCBs
2
OH-BDEs 13 Bisphenols 14 |
Binding and transactivation of thyroid hormone receptor (TR) (TRα, TRβ) by some chemicals which bind TRs as antagonists and/or change the transcription; interactions with these TRs disrupt normal thyroid homeostasis which may possibly lead to anomalies in brain development 11 18 149 150 . | |
There are public health concerns about pregnant women with mild iodine deficiency who are exposed to perchlorate, thiocyanate, nitrate and other environmental “thyreostatic substances” 5 8 12 26 143 151 152 153 154 155 156 . A dose-effect model which investigated iodide and perchlorate exposure in foodstuffs showed that a low iodine intake of 75 μg/day and a daily perchlorate dose of 4.2 μg/kg would be sufficient to induce hypothyroxinemia, whereas a higher daily dose of perchlorate of about 34 µg/kg would be required if the iodine intake was sufficient (approx. 250 µg/day) 157 . Iodine deficiency can therefore worsen the effects of exposure to TDC, especially in pregnancy 5 8 12 17 18 26 .
Table 4 summarizes the well-characterized effects of TDCs on TH metabolism and the infant brain 116 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 144 145 146 147 148 149 150 158 . Air pollution is the main risk factor for the global disease burden, but the negative effects of exposure to airborne fine particulate matter measuring < 2.5 µm (PM 2.5 ) in pregnancy were previously not taken into account 159 160 161 . The available evidence suggests that intrauterine PM 2.5 exposure can change prenatal brain development through oxidative stress and systemic inflammation and lead to chronic neuroinflammation, microglial activation, and neuronal micturition disorders 28 162 163 . It was shown that exposure to fine particulate matter was associated with structural changes to the cerebral cortex of the child as well as impairment of core executive functions such as inhibitory control 164 165 166 167 .
Prevention and Treatment of IMH
As studies on the impact of IMH on the cognitive and motor development and the risk of neuropsychiatric disorders in children have shown a clear connection to early pregnancy, the key clinical question is whether these complications could be prevented at an early stage by iodine or levothyroxine substitution 39 43 89 . Treatment of IMH or subclinical hypothyroidism by administering levothyroxine in early pregnancy did not have any benefit on the neurological development of children based on evaluations when they were aged 6 and 9 years. However, levothyroxine supplementation was initiated, on average, in the 12th week of gestation, which is too late 168 169 . This is why the ATA guidelines do not recommend supplementation with levothyroxine 92 . However, based on new epidemiological data, the ETA guidelines suggest that levothyroxine supplementation should be carried out in the first trimester of pregnancy rather than during later stages of pregnancy 93 . The results of a recent study showed that early levothyroxine supplementation in women with TSH values of > 2.5 mU/l and fT4 < 7.5 pg/ml in or before the ninth week of gestation is safe and improves the course of pregnancy. Whether it also improves the neurological development of affected offspring has not yet been investigated. The data supports the recommendation to adopt threshold values for levothyroxine supplementation and start supplementation as early as possible, ideally before the end of the first trimester of pregnancy. TSH suppression must be avoided 170 .
A positive association has been demonstrated between maternal iodine intake starting even before conception and cognitive functions of her offspring at the age of 6–7 years 171 , but not if iodine substitution was only initiated in pregnancy 105 172 173 174 175 176 . Well designed, randomized controlled studies to study the neuropsychological development of children are currently in progress, which will investigate the impact of daily supplementation with 150–200 µg iodine in the period prior to preconception, during pregnancy and during lactation 177 178 179 180 .
The Krakow Declaration on Iodine, published by the Euthyroid Consortium and other organizations, raises important points on how iodine deficiency in Europe could be efficiently eliminated. The demands include
harmonizing universal salt iodization in all European countries,
carrying out regular monitoring and evaluation studies to continuously measure the benefit and potential damage of iodine enrichment programs, and
necessary social engagement to ensure that programs to prevent iodine deficiency disorders (IDD) are sustained 181 182 .
Conclusions for Clinical Practice
Iodine deficiency means that less FT4 and more FT3 is produced; rather than being elevated, TSH concentrations are decreased. Individual levels of iodine deficiency can be best determined based on hypothyroxinemia.
In clinical practice when dealing with women who want to have children this means that improving iodine intake should already start prior to conception. A low FT4 level is a useful supporting argument.
Some of the numerous endocrine-disrupting chemicals (EDCs) in the environment can negatively affect thyroid hormone metabolism and may even amplify the effects of iodine deficiency. These chemicals are also referred to as TDCs. As such TDCs may be below the detection limits in individuals, FT4 can serve as a marker for adequate iodine intake, especially in the first three months of pregnancy.
Of course, it is the responsibility of policy makers to persuade industry to reduce the prevalence of EDCs. But every one of us can also contribute to reducing the extent of EDCs released into the environment.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Interessenkonflikt Die Autorinnen/Autoren geben an, dass kein Interessenkonflikt besteht.
References/Literatur
- 1.Eastman CJ, Zimmermann MB. South Dartmouth (MA): MDText.com, Inc.; 2000. The iodine deficiency disorders. [Google Scholar]
- 2.Grossklaus R, Liesenkötter K-P, Doubek K et al. Iodine Deficiency, Maternal Hypothyroxinemia and Endocrine Disrupters Affecting Fetal Brain Development: A Scoping Review. Nutrients. 2023;15:2249. doi: 10.3390/nu15102249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice . Reducing Prenatal Exposure to Toxic Environmental Agents: ACOG Committee Opinion, Number 832. Obstet Gynecol. 2021;138:e40–e54. doi: 10.1097/AOG.0000000000004449. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz B, Terekeci H, Sandal S et al. Endocrine disrupting chemicals: exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev Endocr Metab Disord. 2020;21:127–147. doi: 10.1007/s11154-019-09521-z. [DOI] [PubMed] [Google Scholar]
- 5.Ghassabian A, Trasande L. Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect of Endocrine-Disrupting Chemicals on Child Neurodevelopment. Front Endocrinol (Lausanne) 2018;9:204. doi: 10.3389/fendo.2018.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Köhrle J, Frädrich C. Thyroid hormone system disrupting chemicals. Best Pract Res Clin Endocrinol Metab. 2021;35:101562. doi: 10.1016/j.beem.2021.101562. [DOI] [PubMed] [Google Scholar]
- 7.Demeneix BA. Evidence for Prenatal Exposure to Thyroid Disruptors and Adverse Effects on Brain Development. Eur Thyroid J. 2019;8:283–292. doi: 10.1159/000504668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mughal BB, Fini JB, Demeneix BA. Thyroid-disrupting chemicals and brain development: an update. Endocr Connect. 2018;7:R160–R186. doi: 10.1530/EC-18-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Y, Guo F, Liu K et al. The effect of endocrine-disrupting chemicals on placental development. Front Endocrinol (Lausanne) 2023;14:1.059854E6. doi: 10.3389/fendo.2023.1059854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gómez-Roig D, Pascal R, Cahuana MJ et al. Environmental Exposure during Pregnancy: Influence on Prenatal Development and Early Life: A Comprehensive Review. Fetal Diagn Ther. 2021;48:245–257. doi: 10.1159/000514884. [DOI] [PubMed] [Google Scholar]
- 11.Hamers T, Kortenkamp A, Scholze M et al. Transthyretin-Binding Activity of Complex Mixtures Representing the Composition of Thyroid-Hormone Disrupting Contaminants in House Dust and Human Serum. Environ Health Perspect. 2020;128:17015. doi: 10.1289/EHP5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisco G, De Tullio, AGiagulli VA et al. Interference on iodine uptake and human thyroid function by perchlorate-contaminated water and food. Nutrients. 2020;12:1669. doi: 10.3390/nu12061669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghassabian A, Pierotti L, Basterrechea M et al. Association of Exposure to Ambient Air Pollution With Thyroid Function During Pregnancy. JAMA Netw Open. 2019;2:e1912902. doi: 10.1001/jamanetworkopen.2019.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanphear BP. The impact of toxins on the developing brain. Annu Rev Public Health. 2015;36:211–230. doi: 10.1146/annurev-publhealth-031912-114413. [DOI] [PubMed] [Google Scholar]
- 15.Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355:240–248. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Jugan ML, Levi Y, Blondeau JP. Endocrine disruptors and thyroid hormone physiology. Biochem Pharmacol. 2010;79:939–947. doi: 10.1016/j.bcp.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Hartoft-Nielsen ML, Boas M, Bliddal S et al. Do Thyroid Disrupting Chemicals Influence Foetal Development during Pregnancy? J Thyroid Res. 2011;2011:342189. doi: 10.4061/2011/342189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noyes PD, Friedman KP, Browne P et al. Evaluating Chemicals for Thyroid Disruption: Opportunities and Challenges with in Vitro Testing and Adverse Outcome Pathway Approaches. Environ Health Perspect. 2019;127:95001. doi: 10.1289/EHP5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calsolaro V, Pasqualetti G, Niccolai F et al. Thyroid Disrupting Chemicals. Int J Mol Sci. 2017;18:2583. doi: 10.3390/ijms18122583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duntas LH, Stathatos N. Toxic chemicals and thyroid function: hard facts and lateral thinking. Rev Endocr Metab Disord. 2015;16:311–318. doi: 10.1007/s11154-016-9331-x. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert ME, Rovet J, Chen Z et al. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012;33:842–852. doi: 10.1016/j.neuro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Cediel-Ulloa A, Lupu DL, Johansson Y et al. Impact of endocrine disrupting chemicals on neurodevelopment: the need for better testing strategies for endocrine disruption-induced developmental neurotoxicity. Expert Rev Endocrinol Metab. 2022;17:131–141. doi: 10.1080/17446651.2022.2044788. [DOI] [PubMed] [Google Scholar]
- 23.Hetzel BS. The development of a global program for the elimination of brain damage due to iodine deficiency. Asia Pac J Clin Nutr. 2012;21:164–170. [PubMed] [Google Scholar]
- 24.Monahan M, Boelaert K, Jolly K et al. Costs and benefits of iodine supplementation for pregnant women in a mildly to moderately iodine-deficient population: a modelling analysis. Lancet Diabetes Endocrinol. 2015;3:715–722. doi: 10.1016/S2213-8587(15)00212-0. [DOI] [PubMed] [Google Scholar]
- 25.Großklaus R. Nutzen und Risiken der Jodprophylaxe. Einfluss von Jodsalz auf Schilddrüsenkrankheiten und die Gesundheit des Menschen. Präv Gesundheitsf. 2007;2:158–166. [Google Scholar]
- 26.Demeneix B, Slama R. Endocrine Disruptors: From Scientific Evidence to Human Health Protection. In Report Commissioned by the PETI Committee of the European Parliament; Policy Department for Citizen’s Rights and Constitutional Affairs. 2019. http://www.europarl.europa.eu/RegData/etudes/STUD/2019/608866/IPOL_STU(2019)608866_EN.pdf http://www.europarl.europa.eu/RegData/etudes/STUD/2019/608866/IPOL_STU(2019)608866_EN.pdf
- 27.Trasande L, Zoeller RT, Hass U et al. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis. Andrology. 2016;4:565–572. doi: 10.1111/andr.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa LG, Cole TB, Dao K et al. Developmental impact of air pollution on brain function. Neurochem Int. 2019;131:104580. doi: 10.1016/j.neuint.2019.104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivas I, Basagaña X, Cirach M et al. Association between Early Life Exposure to Air Pollution and Working Memory and Attention. Environ Health Perspect. 2019;127:57002. doi: 10.1289/EHP3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Angiulli A. Severe Urban Outdoor Air Pollution and Children’s Structural and Functional Brain Development, From Evidence to Precautionary Strategic Action. Front Public Health. 2018;6:95. doi: 10.3389/fpubh.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suades-González E, Gascon M, Guxens M et al. Air Pollution and Neuropsychological Development: A Review of the Latest Evidence. Endocrinology. 2015;156:3473–3482. doi: 10.1210/en.2015-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Padmanabhan V, Song W, Puttabyatappa M. Praegnatio Perturbatio-Impact of Endocrine-Disrupting Chemicals. Endocr Rev. 2021;42:295–353. doi: 10.1210/endrev/bnaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue K, Yan Q, Arah OA et al. Air Pollution and Adverse Pregnancy and Birth Outcomes: Mediation Analysis Using Metabolomic Profiles. Curr Environ Health Rep. 2020;7:231–242. doi: 10.1007/s40572-020-00284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pergialiotis V, Kotrogianni P, Christopoulos-Timogiannakis E et al. Bisphenol A and adverse pregnancy outcomes: a systematic review of the literature. J Matern Fetal Neonatal Med. 2018;31:3320–3327. doi: 10.1080/14767058.2017.1368076. [DOI] [PubMed] [Google Scholar]
- 35.Janssen BG, Saenen ND, Roels HA et al. Fetal Thyroid Function, Birth Weight, and in Utero Exposure to Fine Particle Air Pollution: A Birth Cohort Study. Environ Health Perspect. 2017;125:699–705. doi: 10.1289/EHP508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao X, Peng S, Xiang Y et al. Correlation between Prenatal Exposure to Polybrominated Diphenyl Ethers (PBDEs) and Infant Birth Outcomes: A Meta-Analysis and an Experimental Study. Int J Environ Res Public Health. 2017;14:268. doi: 10.3390/ijerph14030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, Eastman CJ. The changing epidemiology of iodine deficiency. Nat Rev Endocrinol. 2012;8:434–440. doi: 10.1038/nrendo.2012.43. [DOI] [PubMed] [Google Scholar]
- 38.Velasco I, Bath SC, Rayman MP. Iodine as Essential Nutrient during the First 1000 Days of Life. Nutrients. 2018;10:290. doi: 10.3390/nu10030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bath C. The effect of iodine deficiency during pregnancy on child development. Proc Nutr Soc. 2019;78:150–160. doi: 10.1017/S0029665118002835. [DOI] [PubMed] [Google Scholar]
- 40.Min H, Dong J, Wang Y et al. Maternal Hypothyroxinemia-Induced Neurodevelopmental Impairments in the Progeny. Mol Neurobiol. 2016;53:1613–1624. doi: 10.1007/s12035-015-9101-x. [DOI] [PubMed] [Google Scholar]
- 41.de Escobar GM, Obregón MJ, del Rey FE. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 2007;10:1554–1570. doi: 10.1017/S1368980007360928. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Xue F. The impact of gestational hypothyroxinemia on the cognitive and motor development of offspring. J Matern Fetal Neonatal Med. 2020;33:1940–1945. doi: 10.1080/14767058.2018.1529749. [DOI] [PubMed] [Google Scholar]
- 43.Dosiou C, Medici M. Isolated maternal hypothyroxinemia during pregnancy: knowns and unknowns. Eur J Endocrinol. 2017;176:R21–R38. doi: 10.1530/EJE-16-0354. [DOI] [PubMed] [Google Scholar]
- 44.Henrichs J, Ghassabian A, Peeters RP et al. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol (Oxf) 2013;79:152–162. doi: 10.1111/cen.12227. [DOI] [PubMed] [Google Scholar]
- 45.Moleti M, Trimarchi F, Vermiglio F. Doubts and Concerns about Isolated Maternal Hypothyroxinemia. J Thyroid Res. 2011;2011:463029. doi: 10.4061/2011/46302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glinoer D. The regulation of thyroid function during normal pregnancy: importance of the iodine nutrition status. Best Pract Res Clin Endocrinol Metab. 2004;18:133–152. doi: 10.1016/j.beem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Mégier C, Dumery G, Luton D. Iodine and Thyroid Maternal and Fetal Metabolism during Pregnancy. Metabolites. 2023;13:633. doi: 10.3390/metabo13050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.EFSA NDA Panel (EFSA Panel on Panel on Dietetic Products Nutrition and Allergies) . Scientific Opinion on Dietary Reference Values for iodine. EFSA Journal. 2014;12:3660. doi: 10.2903/j.efsa.2014.3660. [DOI] [Google Scholar]
- 49.Korevaar TIM, Medici M, Visser TJ et al. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol. 2017;13:610–622. doi: 10.1038/nrendo.2017.93. [DOI] [PubMed] [Google Scholar]
- 50.Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20:784–794. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization . 3. Geneva: World Health Organization; 2007. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. [Google Scholar]
- 52.The Iodine Global Network . Ottawa, Canada: IGN; 2020. Global scorecard of iodine nutrition in 2020 in the general population based on school-age children (SAC) [Google Scholar]
- 53.Hey I, Thamm M. Berlin: Robert Koch-Institut; 2019. Monitoring der Jod- und Natriumversorgung bei Kindern und Jugendlichen im Rahmen der Studie des Robert Koch-Instituts zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS Welle 2). Abschlussbericht. [Google Scholar]
- 54.Bundesinstitut für Risikobewertung (BfR) . Jodversorgung in Deutschland wieder rückläufig – Tipps für eine gute Jodversorgung. Fragen und Antworten zur Jodversorgung und zur Jodmangelvorsorge. FAQ des BfR vom 20. Februar 2020 (aktualisiert 9. Februar 2021) https://www.bfr.bund.de/cm/343/jodversorgung-in-deutschland-wieder-ruecklaeufig-tipps-fuer-eine-gute-jodversorgung.pdf https://www.bfr.bund.de/cm/343/jodversorgung-in-deutschland-wieder-ruecklaeufig-tipps-fuer-eine-gute-jodversorgung.pdf
- 55.The Iodine Global Network . Zurich, Switzerland: IGN; Global Scorecard of Iodine Nutrition in 2017 in the general population and in pregnant women (PW) [Google Scholar]
- 56.Katko M, Gazso AA, Hircsu I et al. Thyroglobulin level at week 16 of pregnancy is superior to urinary iodine concentration in revealing preconceptual and first trimester iodine supply. Matern Child Nutr. 2018;14:e12470. doi: 10.1111/mcn.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Candido AC, Morais NS, Dutra LV et al. Insufficient iodine intake in pregnant women in different regions of the world: a systematic review. Arch Endocrinol Metab. 2019;63:306–311. doi: 10.20945/2359-3997000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baldini E, Virili C, D’Armiento E et al. Iodine Status in Schoolchildren and Pregnant Women of Lazio, a Central Region of Italy. Nutrients. 2019;11:1647. doi: 10.3390/nu11071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medici M, Ghassabian A, Visser W et al. Women with high early pregnancy urinary iodine levels have an increased risk of hyperthyroid newborns: the population-based Generation R Study. Clin Endocrinol (Oxf) 2014;80:598–606. doi: 10.1111/cen.12321. [DOI] [PubMed] [Google Scholar]
- 60.Vandevijvere S, Amsalkhir S, Mourri AB et al. Iodine deficiency among Belgian pregnant women not fully corrected by iodine-containing multivitamins: a national cross-sectional survey. Br J Nutr. 2013;109:2276–2284. doi: 10.1017/S0007114512004473. [DOI] [PubMed] [Google Scholar]
- 61.Andersen SL, Sørensen LK, Krejbjerg A et al. Iodine deficiency in Danish pregnant women. Dan Med J. 2013;60:A4657. [PubMed] [Google Scholar]
- 62.Global Fortification Data Exchange . Global Fortification Coverage Data. 2021. https://www.gfdx.org https://www.gfdx.org
- 63.Nyström HF, Brantsæter AL, Erlund I et al. Iodine status in the Nordic countries – past and present. Food Nutr Res. 2016;60:31969. doi: 10.3402/fnr.v60.31969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miles EA, Vahlberg T, Calder PC et al. Iodine status in pregnant women and infants in Finland. Eur J Nutr. 2022;61:2919–2927. doi: 10.1007/s00394-022-02852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nazeri P, Mirmiran P, Shiva N et al. Iodine nutrition status in lactating mothers residing in countries with mandatory and voluntary iodine fortification programs: an updated systematic review. Thyroid. 2015;25:611–620. doi: 10.1089/thy.2014.0491. [DOI] [PubMed] [Google Scholar]
- 66.Manousou S, Andersson M, Eggertsen R et al. Iodine deficiency in pregnant women in Sweden: a national cross-sectional study. Eur J Nutr. 2020;59:2535–2545. doi: 10.1007/s00394-019-02102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindorfer H, Krebs M, Kautzky-Willer A et al. Iodine deficiency in pregnant women in Austria. Eur J Clin Nutr. 2015;69:349–354. doi: 10.1038/ejcn.2014.253. [DOI] [PubMed] [Google Scholar]
- 68.Johner SA, Thamm M, Schmitz R et al. Examination of iodine status in the German population: an example for methodological pitfalls of the current approach of iodine status assessment. Eur J Nutr. 2016;55:1275–1282. doi: 10.1007/s00394-015-0941-y. [DOI] [PubMed] [Google Scholar]
- 69.Dold S, Zimmermann MB, Jukic T et al. Universal salt iodization provides sufficient dietary iodine to achieve adequate iodine nutrition during the first 1000 days: A cross-sectional multicenter study. J Nutr. 2018;148:587–598. doi: 10.1093/jn/nxy015. [DOI] [PubMed] [Google Scholar]
- 70.Ittermann T, Albrecht D, Arohonka P et al. Standardized Map of Iodine Status in Europe. Thyroid. 2020;30:1346–1354. doi: 10.1089/thy.2019.0353. [DOI] [PubMed] [Google Scholar]
- 71.Eastman CJ, Ma G, Li M. Optimal Assessment and Quantification of Iodine Nutrition in Pregnancy and Lactation: Laboratory and Clinical Methods, Controversies and Future Directions. Nutrients. 2019;11:2378. doi: 10.3390/nu11102378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trumpff C, De Schepper J, Tafforeau J et al. Mild iodine deficiency in pregnancy in Europe and its consequences for cognitive and psychomotor development of children: a review. J Trace Elem Med Biol. 2013;27:174–183. doi: 10.1016/j.jtemb.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 73.Zimmermann M, Delange F. Iodine supplementation of pregnant women in Europe: a review and recommendations. Eur J Clin Nutr. 2004;58:979–984. doi: 10.1038/sj.ejcn.1601933. [DOI] [PubMed] [Google Scholar]
- 74.Chan S, Boelaert K. Optimal management of hypothyroidism, hypothyroxinemia and euthyroid TPO antibody positivity preconception and in pregnancy. Clin Endocrinol (Oxf) 2015;82:313–326. doi: 10.1111/cen.12605. [DOI] [PubMed] [Google Scholar]
- 75.Dhillon-Smith RK, Boelaert K. Preconception Counseling and Care for Pregnant Women with Thyroid Disease. Endocrinol Metab Clin North Am. 2022;51:417–436. doi: 10.1016/j.ecl.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 76.Miranda A, Sousa N. Maternal hormonal milieu influence on fetal brain development. Brain Behav. 2018;8:e00920. doi: 10.1002/brb3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costeira MJ, Oliveira P, Santos NC et al. Psychomotor development of children from an iodine-deficient region. J Pediatr. 2011;159:447–453. doi: 10.1016/j.jpeds.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 78.Kawahori K, Hashimoto K, Yuan X et al. Mild Maternal Hypothyroxinemia During Pregnancy Induces Persistent DNA Hypermethylation in the Hippocampal Brain-Derived Neurotrophic Factor Gene in Mouse Offspring. Thyroid. 2018;28:395–406. doi: 10.1089/thy.2017.0331. [DOI] [PubMed] [Google Scholar]
- 79.Ghassabian A, El Marroun H, Peeters RP et al. Downstream effects of maternal hypothyroxinemia in early pregnancy: nonverbal IQ and brain morphology in school-age children. J Clin Endocrinol Metab. 2014;99:2383–2390. doi: 10.1210/jc.2013-4281. [DOI] [PubMed] [Google Scholar]
- 80.Suárez-Rodríguez M, Azcona-San Julián C, Alzina de Aguilar V. Hypothyroxinemia during pregnancy: the effect on neurodevelopment in the child. Int J Dev Neurosci. 2012;30:435–438. doi: 10.1016/j.ijdevneu.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 81.Kasatkina EP, Samsonova LN, Ivakhnenko VN et al. Gestational hypothyroxinemia and cognitive function in offspring. Neurosci Behav Physiol. 2006;36:619–624. doi: 10.1007/s11055-006-0066-0. [DOI] [PubMed] [Google Scholar]
- 82.Moog NK, Entringer S, Heim C et al. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68–100. doi: 10.1016/j.neuroscience.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Korevaar TI, Muetzel R, Medici M et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 2016;4:35–43. doi: 10.1016/S2213-8587(15)00327-7. [DOI] [PubMed] [Google Scholar]
- 84.Préau L, Fini JB, Morvan-Dubois G et al. Thyroid hormone signaling during early neurogenesis and its significance as a vulnerable window for endocrine disruption. Biochim Biophys Acta. 2015;1849:112–121. doi: 10.1016/j.bbagrm.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 85.Abel MH, Korevaar TIM, Erlund I et al. Iodine Intake is Associated with Thyroid Function in Mild to Moderately Iodine Deficient Pregnant Women. Thyroid. 2018;28:1359–1371. doi: 10.1089/thy.2018.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alcaide Martin A, Mayerl S. Local Thyroid Hormone Action in Brain Development. Int J Mol Sci. 2023;24:12352. doi: 10.3390/ijms241512352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korevaar TI, Nieboer D, Bisschop PH et al. Risk factors and a clinical prediction model for low maternal thyroid function during early pregnancy: two population-based prospective cohort studies. Clin Endocrinol (Oxf) 2016;85:902–909. doi: 10.1111/cen.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berbel P, Mestre JL, Santamaría A et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid. 2009;19:511–519. doi: 10.1089/thy.2008.0341. [DOI] [PubMed] [Google Scholar]
- 89.Mohan V, Sinha RA, Pathak A et al. Maternal thyroid hormone deficiency affects the fetal neocorticogenesis by reducing the proliferating pool, rate of neurogenesis and indirect neurogenesis. Exp Neurol. 2012;237:477–488. doi: 10.1016/j.expneurol.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 90.Julvez J, Alvarez-Pedrerol M, Rebagliato M et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology. 2013;24:150–157. doi: 10.1097/EDE.0b013e318276ccd3. [DOI] [PubMed] [Google Scholar]
- 91.Elahi S, Nagra SA. Low maternal iodine intake and early pregnancy hypothyroxinemia: Possible repercussions for children. Indian J Endocrinol Metab. 2014;18:526–530. doi: 10.4103/2230-8210.137513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alexander EK, Pearce EN, Brent GA et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315–389. doi: 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- 93.Lazarus J, Brown RS, Daumerie C et al. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3:76–94. doi: 10.1159/000362597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thompson W, Russell G, Baragwanath G et al. Maternal thyroid hormone insufficiency during pregnancy and risk of neurodevelopmental disorders in offspring: A systematic review and meta-analysis. Clin Endocrinol (Oxf) 2018;88:575–584. doi: 10.1111/cen.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pop VJ, Kuijpens JL, van Baar AL et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–155. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 96.Pop VJ, Brouwers EP, Vader HL et al. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–288. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, Shan Z, Teng W et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf) 2010;72:825–829. doi: 10.1111/j.1365-2265.2009.03743.x. [DOI] [PubMed] [Google Scholar]
- 98.Henrichs J, Bongers-Schokking JJ, Schenk JJ et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab. 2010;95:4227–4234. doi: 10.1210/jc.2010-0415. [DOI] [PubMed] [Google Scholar]
- 99.Scottish Preterm Thyroid Group . Williams F, Watson J, Ogston S et al. Mild maternal thyroid dysfunction at delivery of infants born ≤ 34 weeks and neurodevelopmental outcome at 5.5 years. J Clin Endocrinol Metab. 2012;97:1977–1985. doi: 10.1210/jc.2011-2451. [DOI] [PubMed] [Google Scholar]
- 100.Craig WY, Allan WC, Kloza EM et al. Mid-gestational maternal free thyroxine concentration and offspring neurocognitive development at age two years. J Clin Endocrinol Metab. 2012;97:E22–E28. doi: 10.1210/jc.2011-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Päkkilä F, Männistö T, Hartikainen AL et al. Maternal and Child’s Thyroid Function and Child’s Intellect and Scholastic Performance. Thyroid. 2015;25:1363–1374. doi: 10.1089/thy.2015.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grau G, Aguayo A, Vela A et al. Normal intellectual development in children born from women with hypothyroxinemia during their pregnancy. J Trace Elem Med Biol. 2015;31:18–24. doi: 10.1016/j.jtemb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 103.Kampouri M, Margetaki K, Koutra K et al. Maternal mild thyroid dysfunction and offspring cognitive and motor development from infancy to childhood: the Rhea mother-child cohort study in Crete, Greece. J Epidemiol Community Health. 2021;75:29–35. doi: 10.1136/jech-2019-213309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andersen SL, Andersen S, Liew Z et al. Maternal Thyroid Function in Early Pregnancy and Neuropsychological Performance of the Child at 5 Years of Age. J Clin Endocrinol Metab. 2018;103:660–670. doi: 10.1210/jc.2017-02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chevrier J, Harley KG, Kogut K et al. Maternal Thyroid Function during the Second Half of Pregnancy and Child Neurodevelopment at 6, 12, 24, and 60 Months of Age. J Thyroid Res. 2011;2011:426427. doi: 10.4061/2011/426427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen H, Oliver BG, Pant A et al. Particulate Matter, an Intrauterine Toxin Affecting Foetal Development and Beyond. Antioxidants (Basel) 2021;10:732. doi: 10.3390/antiox10050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Johnson NM, Hoffmann AR, Behlen JC et al. Air pollution and Children’s health-a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ Health Prev Med. 2021;26:72. doi: 10.1186/s12199-021-00995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Costa LG, Cole TB, Dao K et al. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol Ther. 2020;210:107523. doi: 10.1016/j.pharmthera.2020.107523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ozaki K, Kato D, Ikegami A et al. Maternal immune activation induces sustained changes in fetal microglia motility. Sci Rep. 2020;10:21378. doi: 10.1038/s41598-020-78294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Street ME, Bernasconi S. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int J Mol Sci. 2020;21:1430. doi: 10.3390/ijms21041430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Genc S, Zadeoglulari Z, Fuss SH et al. The adverse effects of air pollution on the nervous system. J Toxicol. 2012;2012:782462. doi: 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bose S, Ross KR, Rosa MJ et al. Prenatal particulate air pollution exposure and sleep disruption in preschoolers: Windows of susceptibility. Environ Int. 2019;124:329–335. doi: 10.1016/j.envint.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vandenberg LN, Colborn T, Hayes TB et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dutta S, Haggerty DK, Rappolee DA et al. Phthalate Exposure and Long-Term Epigenomic Consequences: A Review. Front Genet. 2020;11:405. doi: 10.3389/fgene.2020.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xin F, Susiarjo M, Bartolomei MS. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin Cell Dev Biol. 2015;43:66–75. doi: 10.1016/j.semcdb.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Daniel S, Balalian AA, Insel BJ et al. Prenatal and early childhood exposure to phthalates and childhood behavior at age 7 years. Environ Int. 2020;143:105894. doi: 10.1016/j.envint.2020.105894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Neven KY, Cox B, Cosemans C et al. Lower iodine storage in the placenta is associated with gestational diabetes mellitus. BMC Med. 2021;19:47. doi: 10.1186/s12916-021-01919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Neven KY, Wang C, Janssen BG et al. Ambient air pollution exposure during the late gestational period is linked with lower placental iodine load in a Belgian birth cohort. Environ Int. 2021;147:106334. doi: 10.1016/j.envint.2020.106334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neven KY, Cox B, Vrijens K et al. Determinants of placental iodine concentrations in a mild-to-moderate iodine-deficient population: an ENVIRONAGE cohort study. J Transl Med. 2020;18:426. doi: 10.1186/s12967-020-02601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 121.Bouchard MF, Chevrier J, Harley KG et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eskenazi B, Kogut K, Huen K et al. Organophosphate pesticide exposure, PON1, and neurodevelopment in school-age children from the CHAMACOS study. Environ Res. 2014;134:149–157. doi: 10.1016/j.envres.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yamazaki K, Araki A, Nakajima S et al. Association between prenatal exposure to organochlorine pesticides and the mental and psychomotor development of infants at ages 6 and 18 months: The Hokkaido Study on Environment and Children’s Health. Neurotoxicology. 2018;69:201–208. doi: 10.1016/j.neuro.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 124.Wang S, Hu C, Lu A et al. Association between prenatal exposure to persistent organic pollutants and neurodevelopment in early life: A mother-child cohort (Shanghai, China) Ecotoxicol Environ Saf. 2021;208:111479. doi: 10.1016/j.ecoenv.2020.111479. [DOI] [PubMed] [Google Scholar]
- 125.Jeddy Z, Kordas K, Allen K et al. Prenatal exposure to organochlorine pesticides and early childhood communication development in British girls. Neurotoxicology. 2018;69:121–129. doi: 10.1016/j.neuro.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vermeir G, Covaci A, Van Larebeke N et al. Neurobehavioural and cognitive effects of prenatal exposure to organochlorine compounds in three year old children. BMC Pediatr. 2021;21:99. doi: 10.1186/s12887-021-02533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sagiv SK, Thurston SW, Bellinger DC et al. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol. 2010;171:593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taylor PN, Okosieme OE, Murphy R et al. Maternal perchlorate levels in women with borderline thyroid function during pregnancy and the cognitive development of their offspring: data from the Controlled Antenatal Thyroid Study. J Clin Endocrinol Metab. 2014;99:4291–4298. doi: 10.1210/jc.2014-1901. [DOI] [PubMed] [Google Scholar]
- 129.Moore BF, Shapiro AL, Wilkening G et al. Prenatal Exposure to Tobacco and Offspring Neurocognitive Development in the Healthy Start Study. J Pediatr. 2020;218:28–3400. doi: 10.1016/j.jpeds.2019.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Q, Chen XZ, Huang X et al. The association between prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts. Neurotoxicology. 2019;73:199–212. doi: 10.1016/j.neuro.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 131.Engel SM, Miodovnik A, Canfield RL et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118:565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van den Dries MA, Guxens M, Spaan S et al. Phthalate and Bisphenol Exposure during Pregnancy and Offspring Nonverbal IQ. Environ Health Perspect. 2020;128:77009. doi: 10.1289/EHP6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Olesen TS, Bleses D, Andersen HR et al. Prenatal phthalate exposure and language development in toddlers from the Odense Child Cohort. Neurotoxicol Teratol. 2018;65:34–41. doi: 10.1016/j.ntt.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 134.Ramhøj L, Frädrich C, Svingen T et al. Testing for heterotopia formation in rats after developmental exposure to selected in vitro inhibitors of thyroperoxidase. Environ Pollut. 2021;283:117135. doi: 10.1016/j.envpol.2021.117135. [DOI] [PubMed] [Google Scholar]
- 135.O’Shaughnessy KL, Kosian PA, Ford JL et al. Developmental Thyroid Hormone Insufficiency Induces a Cortical Brain Malformation and Learning Impairments: A Cross-Fostering Study. Toxicol Sci. 2018;163:101–115. doi: 10.1093/toxsci/kfy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Crofton KM, Gilbert M, Friedman KP . Paris: OECD Publishing; 2019. Adverse outcome pathway on inhibition of thyroperoxidase and subsequent adverse neurodevelopmental outcomes in mammals. [DOI] [Google Scholar]
- 137.Gibson EA, Siegel EL, Eniola F et al. Effects of Polybrominated Diphenyl Ethers on Child Cognitive, Behavioral, and Motor Development. Int J Environ Res Public Health. 2018;15:1636. doi: 10.3390/ijerph15081636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lam J, Lanphear BP, Bellinger D et al. Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis. Environ Health Perspect. 2017;125:86001. doi: 10.1289/EHP1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vuong AM, Yolton K, Xie C et al. Childhood polybrominated diphenyl ether (PBDE) exposure and neurobehavior in children at 8 years. Environ Res. 2017;158:677–684. doi: 10.1016/j.envres.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Herbstman JB, Mall JK. Developmental Exposure to Polybrominated Diphenyl Ethers and Neurodevelopment. Curr Environ Health Rep. 2014;1:101–112. doi: 10.1007/s40572-014-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Roze E, Meijer L, Bakker A et al. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y, Qian H. Phthalates and Their Impacts on Human Health. Healthcare (Basel) 2021;9:603. doi: 10.3390/healthcare9050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Charatcharoenwitthaya N, Ongphiphadhanakul B, Pearce EN et al. The association between perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant Thai women. J Clin Endocrinol Metab. 2014;99:2365–2371. doi: 10.1210/jc.2013-3986. [DOI] [PubMed] [Google Scholar]
- 144.Berghuis SA, Soechitram SD, Hitzert MM et al. Prenatal exposure to polychlorinated biphenyls and their hydroxylated metabolites is associated with motor development of three-month-old infants. Neurotoxicology. 2013;38:124–130. doi: 10.1016/j.neuro.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 145.Crofton KM, Zoeller RT. Mode of action: neurotoxicity induced by thyroid hormone disruption during development--hearing loss resulting from exposure to PHAHs. Crit Rev Toxicol. 2005;35:757–769. doi: 10.1080/10408440591007304. [DOI] [PubMed] [Google Scholar]
- 146.Paul KB, Hedge JM, Bansal R et al. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action. Toxicology. 2012;300:31–45. doi: 10.1016/j.tox.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang X, Ouyang F, Feng L et al. Maternal Urinary Triclosan Concentration in Relation to Maternal and Neonatal Thyroid Hormone Levels: A Prospective Study. Environ Health Perspect. 2017;125:67017. doi: 10.1289/EHP500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Johannes J, Jayarama-Naidu R, Meyer F et al. Silychristin, a Flavonolignan Derived From the Milk Thistle, Is a Potent Inhibitor of the Thyroid Hormone Transporter MCT8. Endocrinology. 2016;157:1694–1701. doi: 10.1210/en.2015-1933. [DOI] [PubMed] [Google Scholar]
- 149.Ruel MVM, Bos AF, Soechitram SD et al. Prenatal exposure to organohalogen compounds and Children’s mental and motor development at 18 and 30 months of age. Neurotoxicology. 2019;72:6–14. doi: 10.1016/j.neuro.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 150.Santoro A, Chianese R, Troisi J et al. Neuro-toxic and Reproductive Effects of BPA. Curr Neuropharmacol. 2019;17:1109–1132. doi: 10.2174/1570159X17666190726112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gaberšček S, Zaletel K. Epidemiological trends of iodine-related thyroid disorders: an example from Slovenia. Arh Hig Rada Toksikol. 2016;67:93–98. doi: 10.1515/aiht-2016-67-2725. [DOI] [PubMed] [Google Scholar]
- 152.Román GC. Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J Neurol Sci. 2007;262:15–26. doi: 10.1016/j.jns.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 153.Lewandowski TA, Peterson MK, Charnley G. Iodine supplementation and drinking-water perchlorate mitigation. Food Chem Toxicol. 2015;80:261–270. doi: 10.1016/j.fct.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 154.Leung AM, Pearce EN, Braverman LE. Environmental perchlorate exposure: potential adverse thyroid effects. Curr Opin Endocrinol Diabetes Obes. 2014;21:372–376. doi: 10.1097/MED.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ozpinar A, Kelestimur F, Songur Y et al. Iodine status in Turkish populations and exposure to iodide uptake inhibitors. PLoS One. 2014;9:e88206. doi: 10.1371/journal.pone.0088206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suh M, Abraham L, Hixon JG et al. The effects of perchlorate, nitrate, and thiocyanate on free thyroxine for potentially sensitive subpopulations of the 2001–2002 and 2007–2008 National Health and Nutrition Examination Surveys. J Expo Sci Environ Epidemiol. 2014;24:579–587. doi: 10.1038/jes.2013.67. [DOI] [PubMed] [Google Scholar]
- 157.Lumen A, Mattie DR, Fisher JW. Evaluation of perturbations in serum thyroid hormones during human pregnancy due to dietary iodide and perchlorate exposure using a biologically based dose-response model. Toxicol Sci. 2013;133:320–341. doi: 10.1093/toxsci/kft078. [DOI] [PubMed] [Google Scholar]
- 158.Kim MJ, Park YJ. Bisphenols and Thyroid Hormone. Endocrinol Metab (Seoul) 2019;34:340–348. doi: 10.3803/EnM.2019.34.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.World Health Organization . Geneva: World Health Organization; 2021. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide: executive summary. [PubMed] [Google Scholar]
- 160.World Health Organization . Geneva, Switzerland: World Health Organization; Sep 22, 2021. WHO Fact sheets Ambient (outdoor) air quality and health. [Google Scholar]
- 161.Burnett R, Chen H, Szyszkowicz M et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci U S A. 2018;115:9592–9597. doi: 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen H, Oliver BG, Pant A et al. Particulate matter, an intrauterine toxin affecting foetal development and beyond. Antioxidants (Basel) 2021;10:732. doi: 10.3390/antiox10050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Block ML, Elder A, Auten RL et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33:972–984. doi: 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Loftus CT, Ni Y, Szpiro AA et al. Exposure to ambient air pollution and early childhood behavior: A longitudinal cohort study. Environ Res. 2020;183:109075. doi: 10.1016/j.envres.2019.109075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peterson BS, Rauh VA, Bansal R et al. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry. 2015;72:531–540. doi: 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lubczyńska MJ, Muetzel RL, El Marroun H et al. Exposure to Air Pollution during Pregnancy and Childhood, and White Matter Microstructure in Preadolescents. Environ Health Perspect. 2020;128:27005. doi: 10.1289/EHP4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guxens M, Lubczyńska MJ, Muetzel RL et al. Air Pollution Exposure During Fetal Life, Brain Morphology, and Cognitive Function in School-Age Children. Biol Psychiatry. 2018;84:295–303. doi: 10.1016/j.biopsych.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 168.Lee SY, Pearce EN. Testing, Monitoring, and Treatment of Thyroid Dysfunction in Pregnancy. J Clin Endocrinol Metab. 2021;106:883–892. doi: 10.1210/clinem/dgaa945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Leung AM. No Benefit of Levothyroxine Among Pregnant Hypothyroid and/or Hypothyroxinemic Women on Offspring IQ at Age 9 years. Clin Thyroidol. 2018;30:100–103. [Google Scholar]
- 170.Runkle I, de Miguel MP, Barabash A et al. Early Levothyroxine Treatment for Subclinical Hypothyroidism or Hypothyroxinemia in Pregnancy: The St Carlos Gestational and Thyroid Protocol. Front Endocrinol (Lausanne) 2021;12:743057. doi: 10.3389/fendo.2021.743057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Robinson SM, Crozier SR, Miles EA et al. Preconception Maternal Iodine Status Is Positively Associated with IQ but Not with Measures of Executive Function in Childhood. J Nutr. 2018;148:959–966. doi: 10.1093/jn/nxy054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dineva M, Fishpool H, Rayman MP et al. Systematic review and meta-analysis of the effects of iodine supplementation on thyroid function and child neurodevelopment in mildly-to-moderately iodine-deficient pregnant women. Am J Clin Nutr. 2020;112:389–412. doi: 10.1093/ajcn/nqaa071. [DOI] [PubMed] [Google Scholar]
- 173.Machamba AAL, Azevedo FM, Fracalossi KO et al. Effect of iodine supplementation in pregnancy on neurocognitive development on offspring in iodine deficiency areas: a systematic review. Arch Endocrinol Metab. 2021;65:352–367. doi: 10.20945/2359-3997000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nazeri P, Shariat M, Azizi F. Effects of iodine supplementation during pregnancy on pregnant women and their offspring: a systematic review and meta-analysis of trials over the past 3 decades. Eur J Endocrinol. 2021;184:91–106. doi: 10.1530/EJE-20-0927. [DOI] [PubMed] [Google Scholar]
- 175.Harding KB, Peña-Rosas JP, Webster AC et al. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Syst Rev. 2017;3(03):CD011761. doi: 10.1002/14651858.CD011761.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Verhagen NJE, Gowachirapant S, Winichagoon P et al. Iodine Supplementation in Mildly Iodine-Deficient Pregnant Women Does Not Improve Maternal Thyroid Function or Child Development: A Secondary Analysis of a Randomized Controlled Trial. Front Endocrinol (Lausanne) 2020;11:572984. doi: 10.3389/fendo.2020.572984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Manousou S, Eggertsen R, Hulthén L et al. A randomized, double-blind study of iodine supplementation during pregnancy in Sweden: pilot evaluation of maternal iodine status and thyroid function. Eur J Nutr. 2021;60:3411–3422. doi: 10.1007/s00394-021-02515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lopes-Pereira M, Roque S, Costa P et al. Impact of iodine supplementation during preconception, pregnancy and lactation on maternal thyroid homeostasis and offspring psychomotor development: protocol of the IodineMinho prospective study. BMC Pregnancy Childbirth. 2020;20:693. doi: 10.1186/s12884-020-03376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bell MA, Ross AP, Goodman G. Assessing infant cognitive development after prenatal iodine supplementation. Am J Clin Nutr. 2016;104 3:928S–9234S. doi: 10.3945/ajcn.115.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Troendle JF. Statistical design considerations applicable to clinical trials of iodine supplementation in pregnant women who may be mildly iodine deficient. Am J Clin Nutr. 2016;104 3:924S–927S. doi: 10.3945/ajcn.115.110403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schaffner M, Mühlberger N, Conrads-Frank A et al. Benefits and Harms of a Prevention Program for Iodine Deficiency Disorders: Predictions of the Decision-Analytic EUthyroid Model. Thyroid. 2021;31:494–508. doi: 10.1089/thy.2020.0062. [DOI] [PubMed] [Google Scholar]
- 182.Volzke H, Erlund I, Grunert I et al. The Krakow Declaration on Iodine: Tasks and Responsibilities for Prevention Programs Targeting Iodine Deficiency Disorders. Eur Thyroid J. 2018;7:201–204. doi: 10.1159/000490143. [DOI] [PMC free article] [PubMed] [Google Scholar]