Abstract
Aging is a slow, progressive, and inevitable process that affects multiple organs and tissues, including the cardiovascular system. The most frequent cardiac and vascular alterations that are observed in older adults (especially patients aged ≥80 years) are diastolic and systolic dysfunction, progressive stiffening of the vascular wall and endothelial impairment usually driven by an excess of extracellular matrix (ECM) and profibrotic substances, reduced levels of matrix metalloproteinases (MMPs), or by amyloid and calcium deposits in myocardium and valves (especially in aortic valves). Moreover, deformation of the heart structure and shape, or increased adipose tissue and muscle atrophy, or altered ion homeostasis, chronotropic disability, reduced heart rate, and impaired atrial sinus node (SN) activity are other common findings. Interestingly, aging is often associated with oxidative stress, alterations in the mitochondrial structure and function, and a low-grade proinflammatory state, characterized by high concentrations of cytokines and inflammatory cells, without evidence of infectious pathogens, in a condition known as ‘inflammaging’. Aging is a well-recognized independent risk factor for cardiovascular disease and easily leads to high mortality, morbidity, and reduced quality of life. Recently, several efforts have been made to mitigate and delay these alterations, aiming to maintain overall health and longevity. The primary purpose of this review was to provide an accurate description of the underlying mechanisms while also exploring new therapeutic proposals for oxidative stress and inflammaging. Moreover, combining serum biomarkers with appropriate imaging tests can be an effective strategy to stratify and direct the most suitable treatment.
Keywords: cardiovascular aging, cardiac amyloidosis, heart failure, ejection fraction, oxidative stress
1. Introduction
Cardiovascular aging is a progressive and inevitable biological process that profoundly affects both the structure and function of the heart and vascular system. As the global population ages, age-related cardiovascular conditions—such as heart failure, aortic stenosis, and vascular stiffening—are becoming increasingly prevalent and represent a major burden on healthcare systems worldwide.
This review article explores the complex pathophysiological mechanisms underlying cardiovascular aging, including myocardial remodeling, extracellular matrix (ECM) accumulation, altered matrix metalloproteinase (MMP) activity, endothelial dysfunction, and vascular wall stiffening. A central focus is placed on the role of oxidative stress and chronic low-grade inflammation—commonly referred to as “inflammaging”—as key drivers of these degenerative processes. These molecular pathways contribute to reduced myocardial contractility, impaired vascular compliance, and increased susceptibility to atherosclerosis and arrhythmias.
We also highlight the impact of mitochondrial dysfunction, cellular senescence, and maladaptive neurohormonal activation on cardiovascular health in older adults. Special attention is given to emerging diagnostic tools, including circulating and urinary biomarkers, which may allow for early identification and risk stratification of patients with age-related cardiovascular changes. The potential clinical relevance of molecules such as B-type natriuretic peptide (BNP), interleukin (IL)-6, high-sensitivity cardiac troponin T (hs-cTnT), and fibroblast growth factor 21 (FGF21) is discussed, alongside non-invasive imaging techniques. In addition to pathophysiological insights, the review outlines a range of preventive and therapeutic strategies aimed at delaying or mitigating cardiovascular aging. These include lifestyle modifications—such as regular physical exercise and dietary interventions—as well as pharmacological approaches targeting inflammation, oxidative stress, and MMP regulation. Ultimately, the review provides a thorough and up-to-date synthesis of current knowledge in the field of cardiovascular aging, with the goal of informing future research and improving care for an increasingly elderly population.
2. Age-Related Changes
Aging is a slow, progressive, and inevitable process that involves several organs and tissues, including the cardiovascular system, and is commonly considered an independent risk factor for cardiac and vascular diseases [1, 2, 3].
By 2030, an increase in population growth and ageing (approximately 20% of the entire population will be aged 65 years or older) will promote additional rises in cardiovascular diseases (CVDs) of at least 40% in all deaths. Thus, this scenario is associated with increased therapeutic costs and a negative economic impact on hospitalizations and the entire health system [4].
A constant and gradual decline in various physiological processes is a natural result of aging, and it is generally defined as a complex process that begins in the fourth decade of life, resulting from a combination of social, biological, and psychological factors [5].
Alterations in metabolism and organ and tissue functions can be used to characterize human aging. In older adults, the size of the trachea, bronchi, and number of alveoli are reduced, along with a decrease in vital capacity (VC) and total pulmonary capacity (CPT), and lung compliance. These alterations easily lead to an increase in dead space and reduced blood oxygenation. Moreover, the function and motility of respiratory cilia are impaired, thus resulting in higher susceptibility to infections [6].
The gastrointestinal system is affected by aging, with reduced gut and stomach motility, a reduction in teeth, saliva, peristalsis, and pancreatic function, as well as dysfunctional regeneration activity by the liver.
A decrease in strength and muscle mass, as well as bone density, leads to higher susceptibility to osteoporosis and fractures, which are common changes in the musculoskeletal system [7]. Moreover, the genitourinary system is easily impaired through reduced kidney filtration function and loss of sphincter activity [8]. Meanwhile, the tegumentary system and sensory organs are involved in alterations such as skin atrophy, dryness of the mucous membranes, reduced vision, and reduced hearing ability. The ability of the skin to respond to damage or inflammation is reduced, as are nerve fibers and sweat glands [9]. In addition, psychosocial maladaptation to the environment, with a sense of loneliness, a hostile attitude, and the need to depend on a caregiver, are all significant consequences of aging. Furthermore, as it is well known, aging involves various alterations in the nervous system ranging from neuronal atrophy or cell death, reduced memory, increased white matter [10], which can lead to the onset of neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and frontotemporal lobar dementia (FLTD) [11].
3. Age-Related Changes in the Cardiovascular System
3.1 Role of MMPs and ECM
The most frequent cardiac and vascular alterations observed in older individuals (especially patients aged 80 years) are diastolic and systolic dysfunction of the heart, progressive stiffening of the vascular wall, and endothelial dysfunction [12, 13].
Several authors have described how various cardiac and vascular diseases are linked to an altered balance between MMPs, ECM, and tissue inhibitors of metalloproteinases (TIMPs) [14, 15].
MMPs belong to a large family of zinc-dependent endopeptidases responsible for demolishing protein substrates of the ECM, such as collagen and elastin. They are commonly made up of a catalytic sequence, a hinge region, and a hemopexin sequence and are classified according to their substrates (gelatinase, collagenase, etc.). Commonly, these proteinases are secreted in an inactive form by vascular smooth muscle cells, fibroblasts, and leukocytes [16].
A disequilibrium between MMPs and TIMPs has been described by various authors in patients with arterial hypertension, in the formation of arterial plaques, in the remodeling of varicose veins and aortic aneurysms [17] and some exogen MMP inhibitors (MMPi) showed promising results in the management of vascular diseases [17, 18, 19].
Specifically, MMPs play a key role in arterial remodeling through various mechanisms, including endothelial inflammation, degradation of elastin fibers, arterial wall calcification and fibrosis, as well as alterations in the adventitial wall. Endothelial inflammation appears to be associated with cell necrosis and apoptosis (resulting from p53 and caspase activation), thrombosis, platelet aggregation, reduced nitric oxide production (involving heat shock protein 90 catabolism), and alterations in vasodilation. Moreover, MMPs can directly destroy elastin fibers through the phosphorylation of proteins involved in elastogenesis, such as ERK-1/2, or through direct digestion. In addition, calcification and fibrosis of the arterial wall are due, respectively, to both increased cakpain-1, which can physiologically reduce the activity of calcification inhibitors, and to direct activation of tissue growth factors (TGFs), such as TGF-beta1. Interestingly, the destruction of elastin fibrils leads to the release of a TGF-binding protein, thereby enhancing its activation, collagen production, and fibronectin. Even the thickening of the adventitial wall is attributed to the actions of MMPs, TGF, and inflammatory cells, resulting in a negative feedback loop [20].
The ECM can be viewed as a robust, three-dimensional macromolecular mechanical support present in all tissues and organs, composed of various interconnecting fibers that reinforce the cytoskeletal architecture. In addition to being a valid anchor structure, the ECM modifies and adapts in response to different mechanical stresses, thereby strengthening the rigidity of the structure to which it is attached. In physiological conditions, ECM is a solid protein structure composed of collagens, proteoglycans/glycosaminoglycans, fibronectin, elastin, and other glycoproteins, as well as laminin, which aligns and supports myocytes. MMPs or various other proteases are responsible for the constant remodeling of this structure and are important in preventing overproduction [21]. Conversely, some substances, such as transforming growth factor- (TGF-), can increase the levels of ECM and reduce MMPs [22, 23].
3.2 Vascular Aging
Vascular involvement in older adults can be easily summarized by two key factors: wall stiffening and endothelial dysfunction [24]. A reduction in elastic fibers, accompanied by the deposition of collagen and calcium, is a relatively common finding in the arterial vessels of older people [25].
Specifically, a high elastolytic activity is documented in the walls of older individuals and frail subjects [26]. A high activity of proteases with elastolytic properties, such as MMP-1, -2, -9, -13, and -14, or cathepsins has also been observed [20, 27, 28, 29].
Several studies have reported an imbalance between MMPs/TIMPs and/or MMPs/ECM in older individuals, and among these alterations, MMP-2 has been shown to be strictly related to cardiovascular disease [26, 27].
Interestingly, these peptidases are, in turn, upregulated by oxidative stress, growth factors, or inflammatory stimuli [30]. A close connection between elastin degradation products and the activation of wall repair mechanisms has also been described. Amorphous elastin degradation products can act as an inflammatory chemotactic stimulus for cells, such as leukocytes, smooth muscle cells, and fibroblasts, or even exhibit angiogenic activity. Thus, continuous exposure to elastases during aging can lead to a chronic inflammatory state, known as ‘inflammaging’, and, again, a negative feedback loop [31].
The progressive reduction in wall elasticity and increased stiffening lead to elevated blood pressure, morbidity, and mortality [32, 33]. Meanwhile, other alterations have also been described, including reduced sensitivity to vasoconstrictors and vasodilators, as well as a marked reduction in neoangiogenesis. As mentioned above, the following endothelial dysfunctions have also been studied: impaired wall integrity and abnormal responses to damage, leading to a greater susceptibility to atherogenesis [34]. Coronary arteries are also involved in these alterations.
3.3 The Aging Heart
In older adults, myocytes are constantly solicited by oxidative stress, resulting in reduced contractile tissue, an increase in fibroblast cells, compensatory hypertrophy of remaining tissue, decreased elasticity, and diastolic dysfunction [1, 35, 36]. The response of cardiomyocytes to apoptosis is also reduced, resulting in impaired cell renewal [37].
Structural and functional alterations of the ECM are commonly found in the hearts of older adults. Moreover, any alteration of the levels of ECM, MMPs, and profibrotic substances in older individuals leads to an excess of ECM and stiffness of vessel walls, myocardium, and reduced contractility [22, 38, 39]. In addition, several studies have documented the importance of deformation of heart structure and shape (ventricular septum), or amyloid and calcium deposits in myocardium and valves (especially the aortic valve), and muscle atrophy with increased adipose tissue [40].
A certain degree of chronotropic disability, characterized by reduced heart rate and atrial sinus node (SN) malfunction, especially in individuals with coexisting diabetes [41], is a common finding in older adults. This is primarily related to the loss of cells, accompanied by fibrotic alterations in the sinoatrial (SA) node and conduction elements [42]. These elements cause common conditions, such as arrhythmias or a diminished autonomic drive [43].
Other functional disorders have been recognized, including reduced myocardial utilization of calcium (Ca2+), a reduction in the endothelial nitric oxide (NO) concentration, and dysfunction of adrenergic capacity with reduced sympathetic tone at both cardiac and vascular sites [1, 44].
Moreover, the aging heart can be indirectly affected by the vascular system: a ventricular afterload and decreased output are relatively common findings [45] as a direct consequence of pulse pressure widening, arterial wall stiffness, and systolic arterial hypertension [46]. The most common pathways involved are hyperglycemia and insulin resistance, collagen damage by oxidative stress, and fibrogenesis resulting from alterations in the renin–angiotensin–aldosterone system (RAAS) [47, 48].
4. Cellular Aging, Inflammatory Responses, and Oxidative Stress
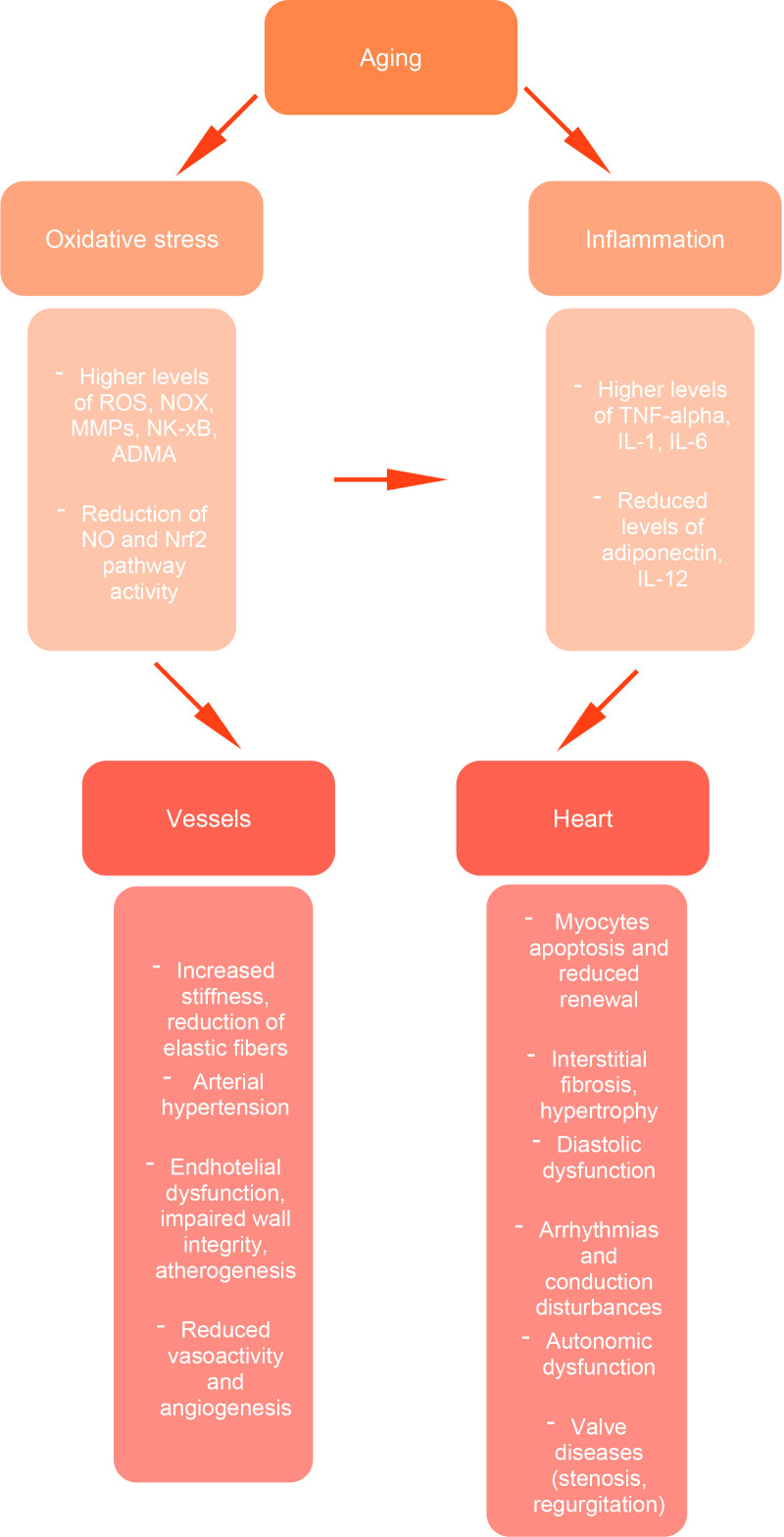
Further, cellular senescence and aging are frequently associated with a low-grade pro-inflammatory state in the absence of evident infectious pathogens, called ‘inflammaging’, which is characterized by high concentrations of TNF-, macrophages, monocytes, and lymphocytes [49, 50, 51, 52, 53]. Fig. 1 shows the complex interplay between oxidative stress, chronic inflammation, and their downstream effects on the vascular and cardiac systems, highlighting key molecular pathways and structural changes that drive age-related cardiovascular dysfunction.
Fig. 1.
Cardiovascular aging: pathogenetic mechanisms. Aging promotes oxidative stress and chronic inflammation, leading to increased levels of reactive oxygen species (ROS), matrix metalloproteinases (MMPs), and proinflammatory cytokines (TNF-, IL-1, IL-6), along with a reduction in protective pathways, such as nitric oxide (NO) signaling and Nrf2 activity. These alterations contribute to structural and functional damage at both the vascular level (increased stiffness, endothelial dysfunction, impaired angiogenesis) and the cardiac level (myocyte apoptosis, interstitial fibrosis, diastolic dysfunction, arrhythmias, autonomic dysregulation, and valve diseases). Together, these processes underlie the age-related decline in cardiovascular function. NOX, NADPH oxidase; MMPs, matrix metalloproteinases; ADMA, asymmetric dimethylarginine; IL, interleukin.
The etiology and physiopathological mechanisms remain unknown. According to the antagonistic pleiotropy theory of aging, an inflammatory state in adolescence and adulthood could have positive and beneficial effects, because it would have a contrasting action against pathogens; however, this inflammatory state can then also cause damage if it is perpetrated chronically in older adults [50, 51], being associated strongly with a worse prognosis [54], high mortality and morbidity [51], and includes cardiac alterations, such as myocardial hypertrophy, interstitial fibrosis and diastolic dysfunction [55]. Mitochondria are the primary structures that modulate oxidative stress, and therefore, cellular aging is indirectly affected. Meanwhile, mitochondria normally respond through various adaptive control mechanisms that are activated in response to perceived oxidative stress. These include the unfolded protein response specific to mitochondria (UPRmt), the formation of mitochondrial-derived vesicles (MDVs), and mitophagy, which removes damaged or dysfunctional mitochondrial components or entire mitochondria. In cases of irreversible damage, cells may also activate programmed cell death pathways, such as apoptosis or necrosis [2].
Under normal conditions, oxidative stress and reactive oxygen species (ROS) usually enhance the activation of the antioxidant response, which includes the activation of signaling pathways such as NF-B, the mitogen-activated protein kinase (MAPK), and Keap1–Nrf2–antioxidant pathway, a nuclear factor whose activation normally induces transcription of antioxidant genes [48]. All these mechanisms trigger a series of beneficial adaptive responses, such as cell growth, autophagy, and inflammation.
However, increased oxidative stress and an impaired cellular response to ROS are commonly observed in older adults. This increase is associated with elevated serum levels of ROS, resulting in DNA damage, alterations in various mitochondrial proteins, and further amplification of ROS production, establishing a self-perpetuating vicious cycle [1].
In detail, the most common alterations are reduced mitochondrial function and high levels of NADPH oxidase (NOX); both of which activate an increase in ROS, MMPs, and NK-xBA (ADMA), and a reduction in NO and the Nrf2 pathway [48].
Additionally, protein carbonylation and relative formation of ketones and aldehydes are other irreversible consequences of oxidative stress in older adults [56]. Interestingly, high levels of carbonylated proteins are found in patients with PD [57], AD [58], cardiac amyloidosis (CA), especially the transthyretin form, thus suggesting a strong link between amyloid fibrils, oxidative stress, and age-related disease [59, 60, 61].
Post-transcriptional changes in chaperones and proteins can damage transthyretin, thus resulting in their extracellular deposition, such as in the myocardium, with a direct toxic activity in a vicious circle [62, 63].
In older people, mitochondria appear swollen with few mitochondrial crests and are characterized by impaired ATP metabolism and accumulation of intralysosomal lipofuscin. Moreover, altered and swollen mitochondria tend to become larger and less capable of performing phagocytosis. Similar alterations also occur in smooth muscle cells with additional anatomical and functional repercussions on the arterial vascular walls [62, 63, 64].
Interestingly, the activity of peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1), which is involved in mitochondrial homeostasis and the equilibrium between biogenesis and degradation, appears to be reduced in older adults, resulting in impaired heart function and activity [65].
In addition, not only systemic and local age-related changes, but also comorbidities commonly seen in frail individuals, such as diabetes mellitus, obesity, dyslipidemia, and hypertension, can have a direct impact on the heart [66].
In a prospective study conducted by Vasan et al. [66] with a mean follow-up of 5 years, low serum levels of insulin-like growth factor-1 (IGF-1) were strongly related to cachexia, ventricular dysfunction, and worsening of heart failure in older subjects.
Recently, growing evidence has emerged regarding the role of microRNAs as regulators in the pathogenesis and development of CVDs, as well as their involvement in senescence pathways and comorbidities [67, 68, 69].
5. Potential Research Opportunities Using Novel Biomarkers for the Early Diagnosis of Cardiovascular System Aging
As previously mentioned, aging is strongly related to CVDs and is a well-recognized independent risk factor [1, 2, 3, 4, 5]. Older individuals have higher serum levels of BNP, hs-cTnT, C-reactive protein (CRP), and IL-6 compared to younger subjects [46, 47, 48, 49, 50]. Secretory molecules easily detectable in biological fluids such as urine or blood could be commonly used in clinical routine as biomarkers for the early diagnosis of cardiovascular disease and as a prevention strategy, especially in vulnerable populations, such as older individuals [70].
Higher values of serum BNP are related to a reduced left atrial (LA) reservoir and conduit strain rate, as measured by speckle-tracking echocardiography, and may correlate with heart failure in older adults [50].
Several authors have proposed urine as a simple and cost-effective biological fluid for diagnostic purposes, as it can be easily collected even in outpatient settings [70]. Interestingly, high BNP values in the urine are strongly related to CVDs [71].
As in the heart, biomarkers have also proven useful in vascular ageing. Indeed, elevated levels of circulating fibroblast growth factor 21 (cFGF21) are more frequently observed in older adults [72]. According to a systematic review and meta-analysis by Zhang et al. [73], these levels are strongly associated with an increased risk of CVDs.
Circulating levels of CRP, IL-6, IL-1, or oxidized low-density lipoprotein (ox-LDL) are increased in vascular ageing and are closely related to inflammaging and oxidative stress [74, 75].
In addition, several authors have found an interesting relationship between brachial–ankle pulse wave velocity (PWV), blood levels of fibulin-1, and vascular age, suggesting a possible role for this biomarker in arterial stiffness and cardiovascular burden [76, 77].
Vascular healing is also compromised in the aging process: bone marrow-derived endothelial progenitor cells (EPCs), which normally contribute to vascular wall repair and neoangiogenesis, exhibit both reduced numbers and impaired function in older adults [78, 79, 80].
6. Intervention Measures
Cardiovascular aging can be slowed by general preventive strategies, such as an adequate dietary regimen, regulation of caloric and sodium intake, as well as specific nutrients. In addition, both regular physical exercise and the cessation of alcohol and smoking abuse, or the reduction of psychosocial stress, can permit the delay of cardiovascular ageing [1, 2].
Chen et al. [81] summarized the positive effects of regular physical activity on various signaling pathways in the cardiovascular system. El Assar et al. [48] highlighted the benefits of inflammation (reduced levels of TNF-, IL-6, and higher levels of IL-10 and adiponectin), on oxidative stress (decreasing levels of ROS and higher levels of Nrf2), and various effects on mitochondrial activity (especially an increase in PGC-1 activity).
In addition, a physiological hypertrophic response of myocardial tissue, which would be less vulnerable to ischemic insult and cardiac remodeling, was observed after physical exercise [81]. Moreover, constant and regular aerobic activity can have a cardioprotective effect through the regulation of RNA and various signaling cascades [81].
Physical activity upregulates the sympathetic tone and downregulates the parasympathetic tone, resulting in increased heart rate, blood flow to the heart, and improved contraction and systolic activity through the Frank–Starling mechanism [82, 83].
Specifically, moderate physical exercise has proven effective in reducing glucose and insulin levels, blood pressure, and body mass index (BMI) [84, 85], regardless of age group [86].
A reduction in myocardial oxidative stress driven by reduced ROS and higher antioxidants levels, together with augmented cardiac, neoangiogenesis and lymphangiogenesis activities derived from vascular growth factors and endothelial vasodilatation, in addition to metabolic changes, such as glucose utilization or adenosine triphosphate (ATP) production, are all positive benefits on the cardiovascular system induced by active exercise, and are also observed in the oldest population [87, 88].
In addition to general measures, other authors have emphasized the importance of exogenous agents that can modulate the activity of MMPs. Additionally, biological and endogenous inhibitors, such as TIMPs and MMPs, can be downregulated by different exogenous agents (biological or pharmacological), including statins, captopril, sulfonamides, or cilostazol [89, 90].
Thus, MMP expression and its function are regulated by numerous factors, including biological effectors, endogenous inhibitors, epigenetic regulators, miRNAs, and pharmacological agents. Recently, several authors have utilized pharmacological modulators of MMPs to prevent or modify the course of CVDs [30, 89].
7. Conclusions and Future Directions
Early identification of cardiac and vascular ageing biomarkers, along with targeted treatment of inflammatory pathways and modulation of key signaling molecules, or early management of comorbidities, may offer significant benefits in older populations. These strategies could not only aid in cardiovascular prevention but also help slow the progressive and continuous deterioration of the systems involved in the ageing process.
Inflammation and oxidative stress appear to share common molecular pathways, and several promising pharmacological treatments with both antioxidant and anti-inflammatory properties may represent reasonable therapeutic options in older adults. In addition, modulation of MMP inhibitory activity could have beneficial effects in reducing and preventing CVDs, even in older populations.
Nonetheless, general lifestyle interventions, such as regular physical activity, a balanced diet, and caloric restriction, can significantly support cardiovascular health, particularly in older adults. A deeper understanding of the underlying signaling pathways may aid in identifying specific therapeutic targets in the near future; however, further studies are required to validate these approaches.
Acknowledgment
Not applicable.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
MT, RP, LM, EP, FT, CT have been involved in the draft preparation, made contributions to conception, and revised it critically. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Not applicable.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Tana M, Piccinini R, Moffa L, Tana C. Heart Failure with Preserved Ejection Fraction and Cardiac Amyloidosis in the Aging Heart. International Journal of Molecular Sciences . 2024;25:11519. doi: 10.3390/ijms252111519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Triposkiadis F, Xanthopoulos A, Butler J. Cardiovascular Aging and Heart Failure: JACC Review Topic of the Week. Journal of the American College of Cardiology . 2019;74:804–813. doi: 10.1016/j.jacc.2019.06.053. [DOI] [PubMed] [Google Scholar]
- [3].Wei JY. Age and the cardiovascular system. The New England Journal of Medicine . 1992;327:1735–1739. doi: 10.1056/NEJM199212103272408. [DOI] [PubMed] [Google Scholar]
- [4].Joseph P, Lanas F, Roth G, Lopez-Jaramillo P, Lonn E, Miller V, et al. Cardiovascular disease in the Americas: the epidemiology of cardiovascular disease and its risk factors. Lancet Regional Health. Americas . 2025;42:100960. doi: 10.1016/j.lana.2024.100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li Q, Xiao N, Zhang H, Liang G, Lin Y, Qian Z, et al. Systemic aging and aging-related diseases. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology . 2025;39:e70430. doi: 10.1096/fj.202402479RRR. [DOI] [PubMed] [Google Scholar]
- [6].Cho SJ, Stout-Delgado HW. Aging and Lung Disease. Annual Review of Physiology . 2020;82:433–459. doi: 10.1146/annurev-physiol-021119-034610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ticinesi A, Lauretani F, Milani C, Nouvenne A, Tana C, Del Rio D, et al. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis. Nutrients . 2017;9:1303. doi: 10.3390/nu9121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fang Y, Gong AY, Haller ST, Dworkin LD, Liu Z, Gong R. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Research Reviews . 2020;63:101151. doi: 10.1016/j.arr.2020.101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hussein RS, Bin Dayel S, Abahussein O, El-Sherbiny AA. Influences on Skin and Intrinsic Aging: Biological, Environmental, and Therapeutic Insights. Journal of Cosmetic Dermatology . 2025;24:e16688. doi: 10.1111/jocd.16688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Azam S, Haque ME, Balakrishnan R, Kim IS, Choi DK. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Frontiers in Cell and Developmental Biology . 2021;9:683459. doi: 10.3389/fcell.2021.683459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nature Reviews. Neurology . 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- [12].Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003; 107: 714–720. https://doi.org/10.1161/01.cir.0000048123.22359.a0. Erratum in Circulation . 2020;141:e809. doi: 10.1161/CIR.0000000000000778. [DOI] [PubMed] [Google Scholar]
- [13].Tana M, Tana C, Rossi D, Mantini C, Gallina S, Ricci F, et al. Thromboembolic and bleeding risk in cardiac amyloidosis. Journal of Thrombosis and Haemostasis: JTH . 2024;22:2381–2392. doi: 10.1016/j.jtha.2024.05.018. [DOI] [PubMed] [Google Scholar]
- [14].Siefert SA, Sarkar R. Matrix metalloproteinases in vascular physiology and disease. Vascular . 2012;20:210–216. doi: 10.1258/vasc.2011.201202. [DOI] [PubMed] [Google Scholar]
- [15].Liu J, Khalil RA. Matrix Metalloproteinase Inhibitors as Investigational and Therapeutic Tools in Unrestrained Tissue Remodeling and Pathological Disorders. Progress in Molecular Biology and Translational Science . 2017;148:355–420. doi: 10.1016/bs.pmbts.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang X, Khalil RA. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Advances in Pharmacology (San Diego, Calif.) . 2018;81:241–330. doi: 10.1016/bs.apha.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochemical Pharmacology . 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. Experientia Supplementum (2012) . 2012;103:209–279. doi: 10.1007/978-3-0348-0364-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tanase DM, Valasciuc E, Anton IB, Gosav EM, Dima N, Cucu AI, et al. Matrix Metalloproteinases: Pathophysiologic Implications and Potential Therapeutic Targets in Cardiovascular Disease. Biomolecules . 2025;15:598. doi: 10.3390/biom15040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang M, Kim SH, Monticone RE, Lakatta EG. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension (Dallas, Tex.: 1979) . 2015;65:698–703. doi: 10.1161/HYPERTENSIONAHA.114.03618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Knox A. Arterial Aging, Metalloproteinase Regulation, and the Potential of Resistance Exercise. Current Cardiology Reviews . 2018;14:227–232. doi: 10.2174/1573403X14666180801153801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovascular Research . 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Advanced Drug Delivery Reviews . 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- [24].Tan D, Yang X, Yang J, Fan G, Xiong G. PCSK9 in Vascular Aging and Age-Related Diseases. Aging and Disease . 2025 doi: 10.14336/AD.2024.1713. (online ahead of print) [DOI] [PubMed] [Google Scholar]
- [25].Laurent S, Boutouyrie P, Benetos A. Pathophysiology of hypertension in the elderly. The American Journal of Geriatric Cardiology . 2002;11:34–39. doi: 10.1111/j.1076-7460.2002.00857.x. [DOI] [PubMed] [Google Scholar]
- [26].Azevedo A, Prado AF, Feldman S, de Figueiredo FAT, Dos Santos MCG, Issa JPM. MMPs are Involved in Osteoporosis and are Correlated with Cardiovascular Diseases. Current Pharmaceutical Design . 2018;24:1801–1810. doi: 10.2174/1381612824666180604112925. [DOI] [PubMed] [Google Scholar]
- [27].Paiva KBS, Granjeiro JM. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Progress in Molecular Biology and Translational Science . 2017;148:203–303. doi: 10.1016/bs.pmbts.2017.05.001. [DOI] [PubMed] [Google Scholar]
- [28].Freitas-Rodríguez S, Folgueras AR, López-Otín C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochimica et Biophysica Acta. Molecular Cell Research . 2017;1864:2015–2025. doi: 10.1016/j.bbamcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- [29].Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. Journal of Hypertension . 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- [30].Amin M, Pushpakumar S, Muradashvili N, Kundu S, Tyagi SC, Sen U. Regulation and involvement of matrix metalloproteinases in vascular diseases. Frontiers in Bioscience (Landmark Edition) . 2016;21:89–118. doi: 10.2741/4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Antonicelli F, Bellon G, Debelle L, Hornebeck W. Elastin-elastases and inflamm-aging. Current Topics in Developmental Biology . 2007;79:99–155. doi: 10.1016/S0070-2153(06)79005-6. [DOI] [PubMed] [Google Scholar]
- [32].Cremer A, Lainé M, Papaioannou G, Yeim S, Gosse P. Increased arterial stiffness is an independent predictor of atrial fibrillation in hypertensive patients. Journal of Hypertension . 2015;33:2150–2155. doi: 10.1097/HJH.0000000000000652. [DOI] [PubMed] [Google Scholar]
- [33].Störk S, van den Beld AW, von Schacky C, Angermann CE, Lamberts SWJ, Grobbee DE, et al. Carotid artery plaque burden, stiffness, and mortality risk in elderly men: a prospective, population-based cohort study. Circulation . 2004;110:344–348. doi: 10.1161/01.CIR.0000134966.10793.C9. [DOI] [PubMed] [Google Scholar]
- [34].North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circulation Research . 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tana M, Tana C, Guglielmi MD, Stefanelli A, Mantini C, Porreca E. Current Perspectives on Atrial Amyloidosis: A Narrative Review. Reviews in Cardiovascular Medicine . 2024;25:73. doi: 10.31083/j.rcm2502073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tana M, Tana C, Palmiero G, Mantini C, Coppola MG, Limongelli G, et al. Imaging findings of right cardiac amyloidosis: impact on prognosis and clinical course. Journal of Ultrasound . 2023;26:605–614. doi: 10.1007/s40477-023-00789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Papp Z, Czuriga D, Balogh L, Balogh Á, Borbély A. How cardiomyocytes make the heart old. Current Pharmaceutical Biotechnology . 2012;13:2515–2521. [PubMed] [Google Scholar]
- [38].Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. Journal of Molecular and Cellular Cardiology . 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. Journal of Molecular and Cellular Cardiology . 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Waller BF. The old-age heart: normal aging changes which can produce or mimic cardiac disease. Clinical Cardiology . 1988;11:513–517. doi: 10.1002/clc.4960110802. [DOI] [PubMed] [Google Scholar]
- [41].Sugita Y, Ito K, Yoshioka Y, Sakai S. Association of complication of type 2 diabetes mellitus with hemodynamics and exercise capacity in patients with heart failure with preserved ejection fraction: a case-control study in individuals aged 65-80 years. Cardiovascular Diabetology . 2023;22:97. doi: 10.1186/s12933-023-01835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Murray K, Wahid M, Alagiakrishnan K, Senaratne J. Clinical electrophysiology of the aging heart. Expert Review of Cardiovascular Therapy . 2022;20:123–139. doi: 10.1080/14779072.2022.2045196. [DOI] [PubMed] [Google Scholar]
- [43].Fumagalli S, Potpara TS, Bjerregaard Larsen T, Haugaa KH, Dobreanu D, Proclemer A, et al. Frailty syndrome: an emerging clinical problem in the everyday management of clinical arrhythmias. The results of the European Heart Rhythm Association survey. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology . 2017;19:1896–1902. doi: 10.1093/europace/eux288. [DOI] [PubMed] [Google Scholar]
- [44].Ferrari AU. Modifications of the cardiovascular system with aging. The American Journal of Geriatric Cardiology . 2002;11:30–33. doi: 10.1111/1467-8446.00044-i1. [DOI] [PubMed] [Google Scholar]
- [45].Florea VG. Classifying systolic and diastolic heart failure. JAMA . 2007;297:1058–1059. doi: 10.1001/jama.297.10.1058-b. author reply 1059. [DOI] [PubMed] [Google Scholar]
- [46].Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arteriosclerosis, Thrombosis, and Vascular Biology . 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- [47].Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circulation Research . 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- [48].El Assar M, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radical Biology & Medicine . 2013;65:380–401. doi: 10.1016/j.freeradbiomed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- [49].Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, et al. Source of Chronic Inflammation in Aging. Frontiers in Cardiovascular Medicine . 2018;5:12. doi: 10.3389/fcvm.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences . 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- [51].Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and ‘Garb-aging’. Trends in Endocrinology and Metabolism: TEM . 2017;28:199–212. doi: 10.1016/j.tem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- [52].Andrade B, Jara-Gutiérrez C, Paz-Araos M, Vázquez MC, Díaz P, Murgas P. The Relationship between Reactive Oxygen Species and the cGAS/STING Signaling Pathway in the Inflammaging Process. International Journal of Molecular Sciences . 2022;23:15182. doi: 10.3390/ijms232315182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lewis ED, Wu D, Meydani SN. Age-associated alterations in immune function and inflammation. Progress in Neuro-psychopharmacology & Biological Psychiatry . 2022;118:110576. doi: 10.1016/j.pnpbp.2022.110576. [DOI] [PubMed] [Google Scholar]
- [54].Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews. Cardiology . 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tomomatsu T. Aged and the cardiovascular system. Nihon Ishikai zasshi. Journal of the Japan Medical Association . 1971;65:775–779. [PubMed] [Google Scholar]
- [56].Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clinica Chimica Acta; International Journal of Clinical Chemistry . 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- [57].Silvestro S, Raffaele I, Mazzon E. Modulating Stress Proteins in Response to Therapeutic Interventions for Parkinson’s Disease. International Journal of Molecular Sciences . 2023;24:16233. doi: 10.3390/ijms242216233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu G, Yang C, Wang X, Chen X, Wang Y, Le W. Oxygen metabolism abnormality and Alzheimer’s disease: An update. Redox Biology . 2023;68:102955. doi: 10.1016/j.redox.2023.102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ando Y, Nyhlin N, Suhr O, Holmgren G, Uchida K, el Sahly M, et al. Oxidative stress is found in amyloid deposits in systemic amyloidosis. Biochemical and Biophysical Research Communications . 1997;232:497–502. doi: 10.1006/bbrc.1996.5997. [DOI] [PubMed] [Google Scholar]
- [60].Fiore M, Cambieri C, Libonati L, Moret F, D’Andrea E, Di Certo MG, et al. Oxidative Stress in Transthyretin-Mediated Amyloidosis: An Exploratory Study. Antioxidants (Basel, Switzerland) . 2024;13:998. doi: 10.3390/antiox13080998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Choi DH, Cristóvão AC, Guhathakurta S, Lee J, Joh TH, Beal MF, et al. NADPH oxidase 1-mediated oxidative stress leads to dopamine neuron death in Parkinson’s disease. Antioxidants & Redox Signaling . 2012;16:1033–1045. doi: 10.1089/ars.2011.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Terman A, Brunk UT. Myocyte aging and mitochondrial turnover. Experimental Gerontology . 2004;39:701–705. doi: 10.1016/j.exger.2004.01.005. [DOI] [PubMed] [Google Scholar]
- [63].Terman A, Brunk UT. The aging myocardium: roles of mitochondrial damage and lysosomal degradation. Heart, Lung & Circulation . 2005;14:107–114. doi: 10.1016/j.hlc.2004.12.023. [DOI] [PubMed] [Google Scholar]
- [64].Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology . 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- [65].Zhu X, Shen W, Yao K, Wang H, Liu B, Li T, et al. Fine-Tuning of PGC1α Expression Regulates Cardiac Function and Longevity. Circulation Research . 2019;125:707–719. doi: 10.1161/CIRCRESAHA.119.315529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Vasan RS, Sullivan LM, D’Agostino RB, Roubenoff R, Harris T, Sawyer DB, et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Annals of Internal Medicine . 2003;139:642–648. doi: 10.7326/0003-4819-139-8-200310210-00007. [DOI] [PubMed] [Google Scholar]
- [67].Quiat D, Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. The Journal of Clinical Investigation . 2013;123:11–18. doi: 10.1172/JCI62876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tana C, Giamberardino MA, Cipollone F. microRNA profiling in atherosclerosis, diabetes, and migraine. Annals of Medicine . 2017;49:93–105. doi: 10.1080/07853890.2016.1226515. [DOI] [PubMed] [Google Scholar]
- [69].Hemmeryckx B, Hohensinner P, Swinnen M, Heggermont W, Wojta J, Lijnen HR. Antioxidant Treatment Improves Cardiac Dysfunction in a Murine Model of Premature Aging. Journal of Cardiovascular Pharmacology . 2016;68:374–382. doi: 10.1097/FJC.0000000000000423. [DOI] [PubMed] [Google Scholar]
- [70].Aging Biomarker Consortium, Bao H, Cao J, Chen M, Chen M, Chen W, et al. Biomarkers of aging. Science China Life Sciences . 2023;66:893–1066. doi: 10.1007/s11427-023-2305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Campbell RT, Jasilek A, Mischak H, Nkuipou-Kenfack E, Latosinska A, Welsh PI, et al. The novel urinary proteomic classifier HF1 has similar diagnostic and prognostic utility to BNP in heart failure. ESC Heart Failure . 2020;7:1595–1604. doi: 10.1002/ehf2.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yang N, Zhang Y, Huang Y, Yan J, Qian Z, Li H, et al. FGF21 at physiological concentrations regulates vascular endothelial cell function through multiple pathways. Biochimica et Biophysica Acta. Molecular Basis of Disease . 2022;1868:166558. doi: 10.1016/j.bbadis.2022.166558. [DOI] [PubMed] [Google Scholar]
- [73].Zhang Y, Yan J, Yang N, Qian Z, Nie H, Yang Z, et al. High-Level Serum Fibroblast Growth Factor 21 Concentration Is Closely Associated With an Increased Risk of Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Frontiers in Cardiovascular Medicine . 2021;8:705273. doi: 10.3389/fcvm.2021.705273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gopcevic KR, Gkaliagkousi E, Nemcsik J, Acet Ö, Bernal-Lopez MR, Bruno RM, et al. Pathophysiology of Circulating Biomarkers and Relationship With Vascular Aging: A Review of the Literature From VascAgeNet Group on Circulating Biomarkers, European Cooperation in Science and Technology Action 18216. Frontiers in Physiology . 2021;12:789690. doi: 10.3389/fphys.2021.789690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gkaliagkousi E, Lazaridis A, Dogan S, Fraenkel E, Tuna BG, Mozos I, et al. Theories and Molecular Basis of Vascular Aging: A Review of the Literature from VascAgeNet Group on Pathophysiological Mechanisms of Vascular Aging. International Journal of Molecular Sciences . 2022;23:8672. doi: 10.3390/ijms23158672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sang Y, Mao KM, Huang Y, Wu XF, Wang XF, Ruan L, et al. Relationship between the Plasma Fibulin-1 Levels, Pulse Wave Velocity, and Vascular Age in Asymptomatic Hyperuricemia. Current Medical Science . 2021;41:94–99. doi: 10.1007/s11596-021-2324-3. [DOI] [PubMed] [Google Scholar]
- [77].Luo M, Yan D, Liang X, Huang Y, Luo P, Yang Z, et al. Association Between Plasma Fibulin-1 and Brachial-Ankle Pulse Wave Velocity in Arterial Stiffness. Frontiers in Cardiovascular Medicine . 2022;9:837490. doi: 10.3389/fcvm.2022.837490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Balistreri CR, Pisano C, Bertoldo F, Massoud R, Dolci S, Ruvolo G. Red Blood Cell Distribution Width, Vascular Aging Biomarkers, and Endothelial Progenitor Cells for Predicting Vascular Aging and Diagnosing/Prognosing Age-Related Degenerative Arterial Diseases. Rejuvenation Research . 2019;22:399–408. doi: 10.1089/rej.2018.2144. [DOI] [PubMed] [Google Scholar]
- [79].Buffa S, Borzì D, Chiarelli R, Crapanzano F, Lena AM, Nania M, et al. Biomarkers for vascular ageing in aorta tissues and blood samples. Experimental Gerontology . 2019;128:110741. doi: 10.1016/j.exger.2019.110741. [DOI] [PubMed] [Google Scholar]
- [80].Rodríguez-Carrio J, Alperi-López M, López P, Alonso-Castro S, Carro-Esteban SR, Ballina-García FJ, et al. Red cell distribution width is associated with endothelial progenitor cell depletion and vascular-related mediators in rheumatoid arthritis. Atherosclerosis . 2015;240:131–136. doi: 10.1016/j.atherosclerosis.2015.03.009. [DOI] [PubMed] [Google Scholar]
- [81].Chen H, Chen C, Spanos M, Li G, Lu R, Bei Y, et al. Exercise training maintains cardiovascular health: signaling pathways involved and potential therapeutics. Signal Transduction and Targeted Therapy . 2022;7:306. doi: 10.1038/s41392-022-01153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Stamford BA. Exercise and the elderly. Exercise and Sport Sciences Reviews . 1988;16:341–379. [PubMed] [Google Scholar]
- [83].Howden EJ, Sarma S, Lawley JS, Opondo M, Cornwell W, Stoller D, et al. Reversing the Cardiac Effects of Sedentary Aging in Middle Age-A Randomized Controlled Trial: Implications For Heart Failure Prevention. Circulation . 2018;137:1549–1560. doi: 10.1161/CIRCULATIONAHA.117.030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet (London, England) . 2017;390:2643–2654. doi: 10.1016/S0140-6736(17)31634-3. [DOI] [PubMed] [Google Scholar]
- [85].Oja P, Kelly P, Murtagh EM, Murphy MH, Foster C, Titze S. Effects of frequency, intensity, duration and volume of walking interventions on CVD risk factors: a systematic review and meta-regression analysis of randomised controlled trials among inactive healthy adults. British Journal of Sports Medicine . 2018;52:769–775. doi: 10.1136/bjsports-2017-098558. [DOI] [PubMed] [Google Scholar]
- [86].Murtagh EM, Nichols L, Mohammed MA, Holder R, Nevill AM, Murphy MH. The effect of walking on risk factors for cardiovascular disease: an updated systematic review and meta-analysis of randomised control trials. Preventive Medicine . 2015;72:34–43. doi: 10.1016/j.ypmed.2014.12.041. [DOI] [PubMed] [Google Scholar]
- [87].Jenkins GP, Evenson KR, Herring AH, Hales D, Stevens J. Cardiometabolic Correlates of Physical Activity and Sedentary Patterns in U.S. Youth. Medicine and Science in Sports and Exercise . 2017;49:1826–1833. doi: 10.1249/MSS.0000000000001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].El Assar M, Álvarez-Bustos A, Sosa P, Angulo J, Rodríguez-Mañas L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. International Journal of Molecular Sciences . 2022;23:8713. doi: 10.3390/ijms23158713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Cui N, Hu M, Khalil RA. Biochemical and Biological Attributes of Matrix Metalloproteinases. Progress in Molecular Biology and Translational Science . 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Simões G, Pereira T, Caseiro A. Matrix metaloproteinases in vascular pathology. Microvascular Research . 2022;143:104398. doi: 10.1016/j.mvr.2022.104398. [DOI] [PubMed] [Google Scholar]