Abstract
Filarial infections trigger a complex immune response characterized by the production of different antibodies, particularly immunoglobulin G (IgG) and immunoglobulin M (IgM). These immunoglobulins play a key role in diagnosing the disease, with IgM typically indicating recent infection and IgG reflecting past or ongoing exposure. Assessing their presence provides valuable insight into an individual's immune response and infection history. This study examined the levels of IgG and IgM in people living with lymphedema in the Kamwenge district, Western Uganda, to better understand their immunological status in relation to filarial infection.
This cross-sectional study, conducted in the Kamwenge district, aimed to assess the presence of anti-filarial antibodies among lymphedema patients. A total of 154 participants, predominantly female (71.4%), with a mean age of 54.7 years, were selected through simple random sampling. Serological testing using the Abbexa Filariasis IgG/IgM Rapid Test revealed that 10.4% tested positive for IgG, and 1.9% for IgM antibodies. We enrolled a total of 154 participants, the majority of whom were female 110 (71.4%) while 44 (28.6%) were male. The participants had a mean age of 54.7 years, with a standard deviation of 15.6 years. Overall, 10.4% (n=16) tested positive for filarial antibodies. Specifically, 10.4% (n=16) were positive for filarial IgG, while 1.9% (n=3) tested positive for IgM antibodies. The serological findings demonstrated a low prevalence of recent filarial infections, with a higher occurrence of past or chronic exposure among participants. This suggests that while active transmission may be limited, lymphatic filariasis remains an ongoing public health concern in the Kamwenge district. These results emphasize the need for continued surveillance, early detection, and targeted interventions to effectively manage and mitigate the burden of filarial-related lymphedema in the region.
Keywords: filarial igg and igm antibodies, kamwenge uganda, lymphatic filariasis, lymphedema, podoconiosis
Introduction
Lower limb lymphedema, commonly known as "elephantiasis," is a chronic condition caused by dysfunction of the lymphatic system, resulting in the accumulation of localized fluid [1,2]. It is characterized by the accumulation of lymphatic fluid in the interstitial tissues due to a malfunctioning lymphatic system [3,4]. Despite its debilitating nature, lymphedema remains insufficiently addressed in scientific research, medical education, and clinical practice. The build-up of protein-rich fluid in the interstitial tissues due to inadequate lymphatic drainage can lead to complications such as fibrosis, fat deposition, and various skin abnormalities, including papillomas, dermal thickening, and acanthosis [4]. Lymphedema is generally categorized as either primary or secondary, based on its origin. While secondary lymphedema results from external damage or disease affecting the lymphatic system, primary lymphedema arises from inherent structural or functional lymphatic abnormalities [5]. Around 70% of primary lymphedema cases are idiopathic with no currently known genetic cause [5]. However, the remaining 30% are linked to genetic mutations, with approximately 20 genes implicated in lymphatic dysfunction [6]. This condition is also a feature of certain genetic syndromes, including Turner syndrome, Noonan syndrome, tuberous sclerosis, and capillary malformation-arteriovenous malformation syndrome [7]. Currently, there is no definitive cure for primary or secondary lymphedema. Treatment primarily aims to manage symptoms and prevent complications [7,8]. Importantly, the approach to prevention and treatment depends on identifying the specific underlying cause.
The immune response to filarial infection is complex and involves the production of various immunoglobulins, notably immunoglobulin G (IgG) and immunoglobulin M (IgM) [9,10]. The detection of these antibodies serves as a crucial diagnostic tool for identifying active or past infections and understanding the immune status of individuals affected by filariasis [11]. IgM antibodies typically indicate a recent infection, while IgG antibodies suggest either chronic infection or past exposure to the filarial antigens. The presence of these antibodies can also provide insights into the underlying pathophysiological mechanisms contributing to lymphedema [12,13].
The diagnosis of lymphedema caused by filarial typically involves clinical assessment and laboratory tests. Traditionally, the diagnosis has relied on the identification of the adult worms or their microfilariae in blood samples [11,14]. Most filarial species exhibit nocturnal periodicity, meaning their microfilariae (larval stage) are primarily found in the peripheral blood during specific hours, often at night (10 PM to 2 AM) [15,16]. This necessitates inconvenient blood collection times, which can be challenging for both patients and healthcare workers in the Kamwenge district. Serological rapid tests to detect IgG and IgM antibodies against filarial antigens are reliable diagnostic alternatives; however, they have not yet been conducted among patients in the Kamwenge district. These serological tests can provide valuable information about the immune status of individuals and facilitate the identification of those at risk of developing lymphedema. Therefore, this study aimed to detect filarial IgG and IgM antibodies among individuals with lymphedema in the Kamwenge district, Western Uganda.
Materials and methods
Study design, population, and setting
This was a cross-sectional sero-survey. Participants with lymphedema were selected through simple random sampling and included individuals who had lived in the Kamwenge district for more than three months.
Inclusion and exclusion criteria
Participants were included in the study if they exhibited signs of lymphedema, regardless of age, and were either receiving care at Rukunyu Hospital or had been invited from the Kamwenge district community. Eligibility required informed consent from adult participants or emancipated minors, while for children above seven years, both caregiver consent and the child’s assent were mandatory. The study excluded individuals who showed no signs or symptoms of lymphedema, those currently undergoing treatment, and those who did not provide informed consent.
Sample size calculation
The sample size for this study was determined using the Kish and Leslie formula (1965), which is suitable for estimating proportions in cross-sectional studies.
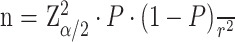
Here, Z is the standard normal deviate corresponding to a 95% confidence level (Z=1.96); P is the estimated prevalence of lymphedema in the Kamwenge district, which was 34.7% (P=0.347), based on a recent study [4]; and r is the desired margin of error, set at 8% (r=0.08). Substituting these values into the Kish and Leslie formula:
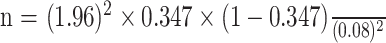
n = 136 participants
To account for potential non-response or incomplete data, we included an additional 10%, resulting in a final minimum sample size of 150 lymphedema patients to be recruited from Rukunyu Hospital.
Laboratory procedures
Sample Collection and Processing
A vein was selected and punctured using a sterile vacutainer needle and holder, and blood was collected into an ethylenediaminetetraacetic acid (EDTA) vacutainer tube. Following standard venepuncture procedures, approximately 2 mL of venous blood was drawn from each participant into a clean, labelled collection tube. The median cubital vein was identified, and the surrounding skin was disinfected by wiping with cotton wool soaked in 70% alcohol and allowing it to air dry. The collected blood samples were then transported to the laboratory, where an immunochromatographic test (ICT) was performed to detect the presence of Filarial IgG and IgM antibodies.
Circulating Filarial IgG and IgM Testing
Two millilitres of blood were collected into EDTA vacutainer tubes, transported to the laboratory, centrifuged, aliquoted, and analyzed for filarial IgG and IgM antibodies using the immunochromatographic test (ICT) with the Abbexa Filariasis IgG/IgM rapid serological test. This assay offers excellent diagnostic accuracy, with a sensitivity of 92.3% for IgG and 98.5% for IgM, and a specificity of 100% for both IgG and IgM, indicating a high level of reliability in detecting both positive and negative cases.
Data management and statistical analysis
Data were double-entered by the principal investigator into Microsoft Excel (Redmond, WA: Microsoft Corp.), which was exported to Stata software version 17 (College Station, TX: Stata Corp LLC) for cleaning and statistical analysis. Age was tested for normality using the Shapiro-Wilk normality test and was normally distributed (p=0.08797). Age was summarized using mean±standard deviation. The mean age in participants with filarial IgG positivity was compared with the mean age in participants without filarial IgG positivity using the Student's t-test. Categorical variables were summarized using frequencies and proportions and compared using Fisher's exact test. A p-value of less than 0.05 was considered statistically significant.
Results
Out of 154 participants, the majority, 110 (71.4%), were female, while 44 (28.6%) were male. The mean age of the participants was 54.7 (±15.6) years, one (0.6%) were below 10 years of age, six (3.9%) were between 11 and 24 years of age, 45 (29.2%) were between 25 and 49 years of age, and 102 (66.2%) were above 50 years of age. Overall, 10.4% (n=16) tested positive for filarial antibodies. Specifically, 10.4% (n=16) were positive for filarial IgG, while 1.9% (n=3) tested positive for IgM antibodies.
Seventy-nine (51.3%) participants had not attained the primary level of education, 68 (44.2%) had attained the primary level, while eight (7.9%) participants had attained the secondary level of education. Forty-four (29.1%) of the participants were single, while 71 (47.0%) were married, 26 (17.2%) separated or divorced, three (2.0%) cohabiting, and seven (4.6%) widowed.
A total of 132 (85.7%) participants were Bakiga; three (1.9%) were Banyankole; and 19 (12.3%) belonged to other tribes. A total of 114 (75.0%) participants were peasants or farmers engaged in agriculture and grazing, while 137 (91.3%) resided in rural settings. A total of 149 (96.8%) participants had all lower limbs affected, and 16 (11.3%) had discharging wounds (Table 1 and Figure 1).
Table 1. Sociodemographic and behavioral characteristics of study participants.
*For some variables (marital status, religion, occupation, residence type, and discharging wounds), the total (n) varies due to missing values.
| Variables | Total* (n=154) | Filarial IgG positivity | p-Value | ||
| Yes, n=16 (10.4%) | No, n=138 (89.6%) | ||||
| Gender | Male | 44 (28.6%) | 4 (25.0%) | 40 (29.0%) | 0.74 |
| Female | 110 (71.4%) | 12 (75.0%) | 98 (71.0%) | ||
| Age (years), mean±SD | 54.70±15.6 | 55.13±15.21 | 54.66±15.69 | 0.91 | |
| Age groups | ≤10 | 1 (0.6%) | 0 (0.0%) | 1 (0.7%) | 1.00 |
| 11-24 | 6 (3.9%) | 0 (0.0%) | 6 (4.3%) | ||
| 25-49 | 45 (29.2%) | 5 (31.3%) | 40 (29.0) | ||
| ≥50 | 102 (66.2%) | 11 (68.8%) | 91 (65.9%) | ||
| Education | None | 79 (51.3%) | 10 (62.5%) | 69 (50.0%) | 0.49 |
| Primary | 68 (44.2%) | 6 (37.5%) | 62 (44.9%) | ||
| Secondary | 7 (4.5%) | 0 (0.0%) | 7 (5.1%) | ||
| Marital status | Single | 44 (29.1%) | 4 (25.0%) | 40 (29.6%) | 0.75 |
| Married | 71 (47.0%) | 7 (43.8%) | 64 (47.4%) | ||
| Separated/divorced | 26 (17.2%) | 3 (18.8%) | 23 (17.0%) | ||
| Cohabiting | 3 (2.0%) | 1 (6.3%) | 2 (1.5%) | ||
| Widowed | 7 (4.6%) | 1 (6.3%) | 6 (4.4%) | ||
| Tribe | Munyankole | 3 (1.9%) | 1 (6.3%) | 2 (1.4%) | 0.42 |
| Mukiga | 132 (85.7%) | 13 (81.3%) | 119 (86.2%) | ||
| Others | 19 (12.3%) | 2 (12.5%) | 17 (12.3%) | ||
| Religion | Catholic | 64 (42.1%) | 8 (53.3%) | 56 (40.9%) | 0.57 |
| Protestant | 66 (43.4%) | 7 (46.7%) | 59 (43.1%) | ||
| Moslem | 1 (0.7%) | 0 (0.0%) | 1 (0.7%) | ||
| Pentecostal | 11 (7.2%) | 0 (0.0%) | 11 (8.0%) | ||
| Others | 10 (6.6%) | 0 (0.0%) | 10 (7.3%) | ||
| Occupation | Housewife | 9 (5.9%) | 1 (6.7%) | 8 (5.8%) | 0.97 |
| Business | 8 (5.3%) | 1 (6.7%) | 7 (5.1%) | ||
| Peasant farmer/grazing | 114 (75.0%) | 12 (80.0%) | 102 (74.5%) | ||
| Student | 2 (1.3%) | 0 (0.0%) | 2 (1.5%) | ||
| Civil servant | 1 (0.7%) | 0 (0.0%) | 1 (0.7%) | ||
| Others | 18 (11.8%) | 1 (6.7%) | 17 (12.4%) | ||
| Residence type | Rural | 137 (91.3%) | 14 (93.3%) | 123 (91.1%) | 0.77 |
| Semi-urban/trading centre | 13 (8.7%) | 1 (6.7%) | 12 (8.9%) | ||
| Limbs affected | Lower limbs | 149 (96.8%) | 16 (100.0%) | 133 (96.4%) | 0.74 |
| Upper limbs | 2 (1.3%) | 0 (0.0%) | 2 (1.4%) | ||
| All limbs | 3 (1.9%) | 0 (0.0%) | 3 (2.2%) | ||
| Discharging wounds | Yes | 16 (11.3%) | 0 (0.0%) | 16 (12.7%) | 0.14 |
| No | 125 (88.7%) | 15 (100.0%) | 110 (87.3%) | ||
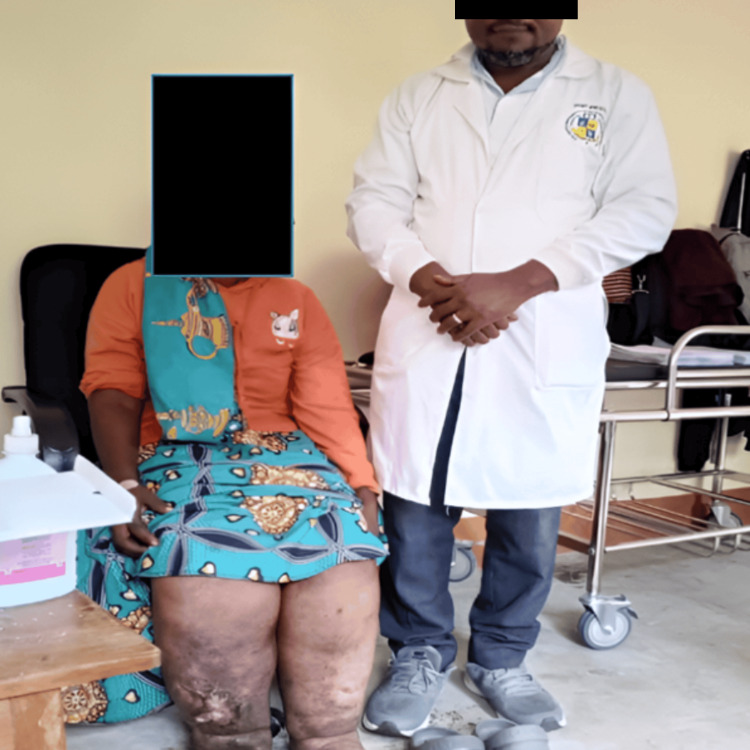
Figure 1. The principal investigator with one of the participants who had lymphedema, an ulcerative wound on her right leg, and cellulitis on both legs.
Discussion
The study investigated cases of lymphedema in the Kamwenge district, Western Uganda, focusing on both filarial-related and non-filarial forms of the condition. Serological testing revealed that 10.4% (n=16) of participants were positive for filarial antibodies, all of whom had IgG, indicating past or chronic exposure. In contrast, only 1.9% (n=3) tested positive for IgM antibodies, suggesting a low rate of recent infections. The positivity rate in this study is consistent with findings from previous research, likely due to the use of similar laboratory methods and study designs [17]. However, it appears lower than rates reported in some other communities, which may reflect the impact of ongoing control measures such as Mass Drug Administration (MDA) with ivermectin and albendazole, as well as the widespread use of insecticide-treated mosquito nets. These interventions are known to reduce transmission in endemic areas [17,18].
Conversely, the majority of participants tested negative for filarial antibodies, suggesting the potential presence of non-filarial elephantiasis, specifically podoconiosis. This aligns with earlier studies conducted in Busiriba sub-county, the Kamwenge district, which confirmed the existence of podoconiosis in the region [4,19].
The identification of confirmed cases of filarial elephantiasis in the Kamwenge district indicates that the burden of elephantiasis in the region extends beyond podoconiosis and is further complicated by the presence of lymphatic filariasis [4]. Prolonged exposure of bare feet to irritant volcanic soils, especially without protective footwear, remains a significant risk factor for podoconiosis [19,20]. These findings underscore the need for further research into the genetic and chemical contributors to podoconiosis. To address both forms of elephantiasis effectively, key recommendations include promoting health education on the transmission of filarial worms, encouraging early diagnosis, ensuring proper foot hygiene, and advocating for the consistent use of protective footwear. Public awareness campaigns and community education on prevention and control strategies for both filarial and non-filarial elephantiasis are also essential in mitigating the health burden in the region.
Limitations
Rapid diagnostic tests are limited by their inability to quantify antibody levels, differentiate between filarial species, and their susceptibility to false-positive or false-negative results. Although the presence of IgG and IgM suggests past exposure or recent infection, it does not confirm active disease. Accurate determination of current infection and differentiation from previous exposure requires antigen-based tests, such as circulating filarial antigen (CFA) assays. The study’s cross-sectional design restricts the ability to determine causality between filarial infection and lymphedema, as it captures data at only one point in time without reflecting disease progression.
Conclusions
Serological testing revealed that a small proportion of participants were positive for filarial antibodies, with all positive cases exhibiting IgG, indicative of past or chronic exposure. In contrast, only a few individuals tested positive for IgM, suggesting recent infection. These results point to a low level of ongoing transmission but a notable history of exposure to lymphatic filariasis within the Kamwenge district. This highlights the continued public health relevance of the disease and underscores the need for sustained surveillance, early case detection, and targeted public health strategies to effectively manage filarial-associated lymphedema. Additionally, the findings support the utility of antibody seroprevalence testing as a valuable surveillance tool, particularly in regions nearing the elimination of lymphatic filariasis. Nonetheless, integrating antibody-based surveillance into national control programs requires further investigation to define thresholds that distinguish between active transmission and elimination status. Longitudinal studies tracking antibody levels before and after multiple rounds of mass drug administration (MDA) are essential for understanding the relationship between antibody and antigen responses and for determining whether antibody testing can reliably indicate infection and transmission dynamics.
Acknowledgments
The authors would like to extend their sincere appreciation to Dr. William Mucunguzi, the District Health Officer, and Dr. Ivan Mujuni, Medical Director at Rukunyu Hospital, for their invaluable support throughout the study. They are also grateful to Mr. Beyanga Evarist, Mr. Asiimwe Kalemire Gerevasio, Mr. Atayebwa Solomon Raul Muyambi, and the other Village Health Team (VHT) members for their coordination and assistance during data collection. Additionally, the authors acknowledge the staff of the Rukunyu Hospital Laboratory Department for their support during both sample collection and analysis.
Disclosures
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Mbarara University of Science and Technology Research Ethics Committee issued approval #MUST-2024-1711.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Vicent Mwesigye, Joanita Berytah Tebulwa, Benson Musinguzi, Bosco Bekita Agaba, Francis Bajunirwe, Joel Bazira, Edgar Mulogo, Itabangi Herbert, Frederick Byarugaba
Acquisition, analysis, or interpretation of data: Vicent Mwesigye, Charlse Nkubi Bagenda, Edgar Mulogo, Itabangi Herbert, Frederick Byarugaba
Drafting of the manuscript: Vicent Mwesigye, Joanita Berytah Tebulwa, Benson Musinguzi, Bosco Bekita Agaba, Charlse Nkubi Bagenda, Francis Bajunirwe, Joel Bazira, Edgar Mulogo, Itabangi Herbert, Frederick Byarugaba
Critical review of the manuscript for important intellectual content: Vicent Mwesigye, Edgar Mulogo, Itabangi Herbert, Frederick Byarugaba
Supervision: Edgar Mulogo, Itabangi Herbert, Frederick Byarugaba
References
- 1.Global preventive and management strategies and their effectiveness in patients with secondary lymphedema: a scoping review. Mwesigye V, Atwine D, Munguciada EF, et al. Cureus. 2025;17 doi: 10.7759/cureus.83627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elia R, Maruccia M. Pearls and Pitfalls in Skin Ulcer Management. Cham, Switzerland: Springer; 2024. Lymphedema and wound care; pp. 649–660. [Google Scholar]
- 3.Lymphedema: pathophysiology and clinical manifestations. Grada AA, Phillips TJ. J Am Acad Dermatol. 2017;77:1009–1020. doi: 10.1016/j.jaad.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Sero-antigen prevalence of lymphatic filariasis and risk factors of podoconiosis in Busiriba sub-county, Kamwenge district, Southwestern Uganda, August-September 2018. Mwesigye V, Musinguzi B, Okongo B, Mucunguzi W, Kakaire MN, Migisha R. BMC Res Notes. 2024;17 doi: 10.1186/s13104-024-06801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Current concepts in the management of primary lymphedema. Senger JB, Kadle RL, Skoracki RJ. Medicina (Kaunas) 2023;59 doi: 10.3390/medicina59050894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biology of lymphedema. Brix B, Sery O, Onorato A, Ure C, Roessler A, Goswami N. Biology (Basel) 2021;10 doi: 10.3390/biology10040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlögel MJ, Brouillard P, Boon LM, Vikkula M. Lymphedema: Presentation, Diagnosis, and Treatment. Cham, Switzerland: Springer; 2015. Genetic causes of lymphedema; pp. 19–31. [Google Scholar]
- 8.Current understanding of pathological mechanisms of lymphedema. Sung C, Wang S, Hsu J, Yu R, Wong AK. Adv Wound Care (New Rochelle) 2022;11:361–373. doi: 10.1089/wound.2021.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Induction of immunoglobulin G4 in human filariasis: an indicator of immunoregulation. Adjobimey T, Hoerauf A. Ann Trop Med Parasitol. 2010;104:455–464. doi: 10.1179/136485910X12786389891407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Immunity in filarial infections: lessons from animal models and human studies. Kwarteng A, Ahuno ST. Scand J Immunol. 2017;85:251–257. doi: 10.1111/sji.12533. [DOI] [PubMed] [Google Scholar]
- 11.Mackenzie CD, Souza A, Geary TG. Human and Animal Filariases. Hoboken, NJ: John Wiley & Sons; 2022. Diagnosis and assessment of human filarial infections: current status and challenges; pp. 97–124. [Google Scholar]
- 12.IgG antibody subclasses in human filariasis. Differential subclass recognition of parasite antigens correlates with different clinical manifestations of infection. Hussain R, Grögl M, Ottesen EA. J Immunol. 1987;139:2794–2798. [PubMed] [Google Scholar]
- 13.Unraveling the dynamics of human filarial infections: immunological responses, host manifestations, and pathogen biology. Rajamanickam A, Babu S. Pathogens. 2025;14 doi: 10.3390/pathogens14030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ElShewy K. Medical Parasitology: A Body System Approach. Cham, Switzerland: Springer; 2024. Blood and lymphatic parasites; pp. 165–189. [Google Scholar]
- 15.da Rocha EM, Fontes G, Ehrenberg JP. Arthropod Borne Diseases. Cham, Switzerland: Springer; 2016. Lymphatic filariasis; pp. 369–381. [Google Scholar]
- 16.The 24-hour periodicity of microfilariae: biological mechanisms responsible for its production and control. Hawking F. Proc R Soc Lond B. 1967;169:59–76. [Google Scholar]
- 17.Anti-filarial antibodies are sensitive indicators of lymphatic filariasis transmission and enable identification of high-risk populations and hotspots. Lawford H, Mayfield H, Sam FA, et al. Int J Infect Dis. 2024;147 doi: 10.1016/j.ijid.2024.107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geneva, Switzerland: World Health Organization; 2023. Public health pesticide management: a course module. [Google Scholar]
- 19.Risk factors for podoconiosis: Kamwenge district, Western Uganda, September 2015. Kihembo C, Masiira B, Lali WZ, et al. Am J Trop Med Hyg. 2017;96:1490–1496. doi: 10.4269/ajtmh.16-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.With bare feet in the soil: podoconiosis, a neglected cause of tropical lymphoedema. Chandler DJ, Grijsen ML, Fuller LC. Dermatology. 2021;237:236–247. doi: 10.1159/000506045. [DOI] [PubMed] [Google Scholar]