Abstract
Induced pluripotent stem cells (iPSCs) have substantial transformative potential in regenerative medicine, enabling tissue repair and restoration. However, their clinical application is limited by tumorigenic risks owing to their pluripotency. Biodistribution studies are crucial for elucidating the fate and tumorigenicity risk of iPSC-derived cell therapy products (CTPs). The lack of standardized biodistribution study protocols has led to inconsistent and unreliable study results, posing difficulties in the drug approval process. Therefore, we conducted a case study on the biodistribution of iPSC-derived pancreatic islet cells to develop a standardized biodistribution assessment protocol for iPSC-derived CTPs. We optimized a droplet digital polymerase chain reaction method targeting human-specific LINE1 sequences and validated its quantitativity for quantification across different tissues using a single calibration curve. We performed a long-term biodistribution study of iPSC-derived pancreatic islet cell in immunodeficient mice to assess biodistribution characteristics, which indicated that they remained localized at the transplantation site for one year, with no migration to other organs, suggesting long-term survival, minimal tumorigenicity risk, and advantages for clinical application. This study provides valuable insights into the standardization of biodistribution protocols for iPSC-derived CTPs.
Keywords: biodistribution, induced pluripotent stem cell, regenerative medicine, tumorigenicity, droplet digital PCR, cell therapy, pancreatic islet cell
Graphical abstract

A droplet digital PCR method was optimized, and its quantitativity was validated to evaluate the biodistribution characteristics of induced pluripotent stem cell-derived pancreatic islet cells. The study demonstrates their long-term survival, minimal tumorigenicity risk, and offers valuable insights into the standardization of biodistribution protocols of cell therapy products.
Introduction
Induced pluripotent stem cells (iPSCs) are cells that have been reprogrammed to regain pluripotency by introducing specific genes into somatic cells, enabling them to differentiate into various cell types.1 iPSCs hold substantial promise for the creation of genetic disease models, elucidation of disease mechanisms, and development of novel therapeutics.2,3 iPSCs have garnered considerable attention in regenerative medicine owing to their potential to provide innovative and fundamental treatment approaches to repair, reconstruct, and restore the function of diseased or damaged tissues or organs, offering curative solutions for diseases and injuries that are difficult to treat using conventional therapies.4 Thus, regenerative therapy using iPSC-derived cell therapy products (iCTPs) promises significant benefits for patients if the transplanted iCTPs persist for extended durations at the appropriate site without exhibiting abnormal biodistribution. However, iCTPs are generally considered to have a high tumorigenicity risk because residual undifferentiated iPSCs are naturally tumorigenic, forming teratomas. Moreover, the accumulation of genomic abnormalities during the establishment of an iPSC line and subsequent prolonged cell passage may induce cell transformations.5,6,7,8 Therefore, the effective evaluation and management of the tumorigenic risk of iCTPs is critical for their clinical application. A key method for assessing the tumorigenic risk of iCTPs is through biodistribution studies, which involve a detailed investigation of the distribution and behavior of transplanted iCTPs within the body.9 Because biodistribution studies can determine whether iCTPs persist at the transplanted site, migrate to unintended sites, and/or proliferate in ways that could induce tumor formation, they play a crucial role in ensuring that the use of iCTPs in medical applications is safe and effective.
The biodistribution of iCTPs has been evaluated using various methods, including imaging, flow cytometry, and quantitative polymerase chain reaction (qPCR).10,11,12,13,14 In particular, the Arthrobacter luteus element-targeting qPCR (Alu-qPCR) is frequently employed in preclinical biodistribution studies of human CTPs because of its enhanced sensitivity.15,16,17 The number of human cells in a volume of biological samples can be quantified using a calibration curve prepared by spiking standard cell lysates into a blank matrix18; however, qPCR methods are susceptible to matrix effects and/or variable DNA recovery rates across different matrices.19 Moreover, a further notable limitation of Alu-qPCR is nonspecific amplification in non-template control samples.16,18
We previously developed a droplet digital PCR method targeting human-specific LINE1 sequences (LINE1-ddPCR) as a quantification method for human CTPs in nonclinical biodistribution studies.19 This method minimizes the matrix effect and variability in DNA recovery by leveraging the endpoint assay feature of ddPCR and normalization through the addition of an external control (EC) gene (dog genomic DNA [gDNA]) to each sample, resulting in the accurate quantification of the number of human cells in different tissue samples using a single surrogate matrix calibration curve. However, the applicability of this method to quantify iCTPs in various biological samples from long-term biodistribution studies remains unclear. Moreover, the appropriate assay criteria for quantifying CTPs in preclinical biodistribution studies remain undetermined.
Biodistribution studies for iCTPs are limited, and no consensus on aspects of study design such as study duration, number of animals to use, sex, and tissue selection for quantification has been established.9 These considerations would affect the interpretation of the biodistribution characteristics of iCTPs. Generally, it is more comprehensive to evaluate cell distribution across all tissues, using a large number of animals over an extended period. However, from an animal welfare perspective, the number of animals used should be minimized. Furthermore, to expedite the development of innovative treatment options for patients, it is preferable to select relevant target tissues for bioanalysis.
Recently, the development of differentiation methods for human pluripotent stem cell-derived pancreatic islet cells (SC-islets) has advanced the treatment of type 1 diabetes.20,21,22,23,24,25,26 Correspondingly, recent clinical studies have demonstrated the feasibility of achieving glucose control through the intrahepatic transplantation of SC-islets in patients with type 1 diabetes27 and insulin-dependent type 2 diabetes,28 indicating that SC-islet transplantation could benefit patients. Although subcutaneously transplanted cells might have difficulty surviving for extended durations owing to reduced vascularization and hypoxia, allogeneic human iPSC-derived pancreatic islet cells (iPICs), a type of SC-islet, have been shown to improve diabetes in diabetic mice and pig models following subcutaneous transplantation with biodegradable scaffolds.29,30 This suggests that if cells that secrete insulin based on blood glucose levels survive at the transplant site, therapeutic efficacy can be achieved. Moreover, subcutaneously transplanted cells can be removed from the transplantation site in case of unexpected side effects, if the cells remain at the transplantation site without abnormal migration and tumorigenicity. Confirming the long-term persistence of iPICs at the transplantation site, without abnormal distribution and proliferation, is crucial for anticipating efficacy and safety.
In this study, we optimized the LINE1-ddPCR method, validated the quantitativity of the method as a bioanalytical assay for iCTPs in various matrices, and performed an in vivo biodistribution study in which iPICs were subcutaneously transplanted into immunodeficient mice. Additionally, we performed a long-term animal study of iPICs to standardize the fit-for-purpose biodistribution assessment for locally transplanted iCTPs.
Results
Assay optimization
Figure 1 shows a two-dimensional scatterplot showing the fluorescence intensity from the amplification of LINE1 (FAM) and EC (VIC) in the duplex assay. A clear separation of negative and positive droplets was observed in both the LINE1 and EC sequences. Although the fluorescence intensity of VIC did not differ between LINE1-positive and negative droplets, and that of FAM was slightly increased in EC-positive droplets.
Figure 1.
Simultaneous detection of LINE1 and EC sequences using a ddPCR assay
Blue, green, gray, and orange clusters represent LINE1-positive, EC-positive, double-negative, and double-positive droplets, respectively.
Assay validation
To confirm the quantitativity of this assay, quality control (QC) samples were prepared at three different concentrations of 3 (QC-L), 2 × 102 (QC-M), and 6 × 103 (QC-H) cells/20 mg tissue, 10 mg tissue, 0.2 mg tissue, or 50 μL blood on three separate days. The copy number of the LINE1 sequence was quantified using a single surrogate calibration curve with blank liver samples. The relative error (RE) and coefficient of variation (CV) values for the QC-H and QC-M samples ranged from −32.8% to 33.0% and 0.6% to 9.6%, respectively (Table 1). The RE and CV values for the QC-L samples ranged from −28.0% to 43.3% and 3.7% to 27.5%, respectively. These results met the required criteria: the RE and CV values were within 100.0% ± 35.0% for QC-H and QC-M, and within 100.0% ± 50.0% for QC-L. The regression analysis showed good linearity (r2 > 0.98) and similar calibration curve slopes within the range of 3–10,000 cells (slope = 0.000423–0.000527, within 1.24 times) across the three validation batches. Dilutions of QC samples were prepared by diluting the sample with a concentration of 5 × 104 cells/0.2 mg skin 10-fold (QC-HD1) and 100-fold (QC-HD2) with the control homogenate to confirm the accuracy and precision of the dilution procedure. The RE and CV values for QC-HD1 and QC-HD2 were within ±35% and below 35%, respectively (Table 2), indicating that the dynamic range could be expanded by 100-fold by diluting the samples. These results indicate an acceptable quantitativity for this assay method.
Table 1.
Validation of LINE1-ddPCR assay in NOG mouse samples
| Sample source | Nominal concentrationa | First assay |
Second assay |
Third assay |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean observed concentrationa | RE (%) | CV (%) | Mean observed concentrationa | RE (%) | CV (%) | Mean observed concentrationa | RE (%) | CV (%) | ||
| Liver | 3 | 3.15 | 5.0 | 11.8 | 3.26 | 8.7 | 9.0 | 3.09 | 3.0 | 14.9 |
| 200 | 198.00 | −1.0 | 1.7 | 197.00 | −1.5 | 3.0 | 204.00 | 2.0 | 3.7 | |
| 6,000 | 5,550.00 | −7.5 | 4.8 | 5,440.00 | −9.3 | 0.7 | 5,630.00 | −6.2 | 1.9 | |
| Kidney | 3 | 3.51 | 17.0 | 19.6 | 3.28 | 9.3 | 17.9 | 3.18 | 6.0 | 12.0 |
| 200 | 192.00 | −4.0 | 1.9 | 198.00 | −1.0 | 6.7 | 201.00 | 0.5 | 3.2 | |
| 6,000 | 5,640.00 | −6.0 | 3.2 | 5,610.00 | −6.5 | 4.6 | 5,590.00 | −6.8 | 1.4 | |
| Heart | 3 | 3.33 | 11.0 | 11.2 | 3.46 | 15.3 | 17.5 | 3.37 | 12.3 | 9.6 |
| 200 | 206.00 | 3.0 | 3.4 | 204.00 | 2.0 | 1.2 | 212.00 | 6.0 | 3.4 | |
| 6,000 | 5,890.00 | −1.8 | 2.9 | 5,650.00 | −5.8 | 0.8 | 5,880.00 | −2.0 | 1.2 | |
| Lung | 3 | 4.04 | 34.7 | 8.2 | 3.26 | 8.7 | 5.6 | 3.08 | 2.7 | 13.8 |
| 200 | 221.00 | 10.5 | 1.1 | 206.00 | 3.0 | 2.6 | 218.00 | 9.0 | 2.5 | |
| 6,000 | 7,080.00 | 18.0 | 9.6 | 6,190.00 | 3.2 | 2.4 | 6,310.00 | 5.2 | 1.7 | |
| Brain | 3 | 3.41 | 13.7 | 21.4 | 3.17 | 5.7 | 13.8 | 3.42 | 14.0 | 9.8 |
| 200 | 203.00 | 1.5 | 3.3 | 199.00 | −0.5 | 1.2 | 224.00 | 12.0 | 3.9 | |
| 6,000 | 6,090.00 | 1.5 | 4.9 | 5,700.00 | −5.0 | 4.9 | 5,940.00 | −1.0 | 2.7 | |
| Spleen | 3 | 3.83 | 27.7 | 8.3 | 3.38 | 12.7 | 5.7 | 3.28 | 9.3 | 17.2 |
| 200 | 216.00 | 8.0 | 1.8 | 197.00 | −1.5 | 4.8 | 220.00 | 10.0 | 2.9 | |
| 6,000 | 6,160.00 | 2.7 | 3.0 | 5,620.00 | −6.3 | 4.8 | 6,160.00 | 2.7 | 0.9 | |
| Testis | 3 | 4.12 | 37.3 | 11.1 | 2.92 | −2.7 | 10.0 | 4.14 | 38.0 | 5.7 |
| 200 | 225.00 | 12.5 | 3.3 | 212.00 | 6.0 | 3.6 | 232.00 | 16.0 | 1.4 | |
| 6,000 | 6,540.00 | 9.0 | 3.1 | 6,090.00 | 1.5 | 2.6 | 6,410.00 | 6.8 | 3.5 | |
| Ovary | 3 | 3.58 | 19.3 | 25.5 | 3.36 | 12.0 | 10.6 | 4.25 | 41.7 | 10.4 |
| 200 | 238.00 | 19.0 | 4.0 | 219.00 | 9.5 | 4.1 | 243.00 | 21.5 | 3.0 | |
| 6,000 | 6,670.00 | 11.2 | 4.9 | 5,950.00 | −0.8 | 5.2 | 6,710.00 | 11.8 | 2.0 | |
| Stomach | 3 | 3.91 | 30.3 | 8.2 | 3.88 | 29.3 | 9.0 | 3.41 | 13.7 | 7.6 |
| 200 | 234.00 | 17.0 | 1.6 | 220.00 | 10.0 | 9.0 | 244.00 | 22.0 | 0.6 | |
| 6,000 | 6,680.00 | 11.3 | 4.5 | 6,230.00 | 3.8 | 6.7 | 6,720.00 | 12.0 | 1.5 | |
| Small intestine | 3 | 3.92 | 30.7 | 13.8 | 4.18 | 39.3 | 27.5 | 4.30 | 43.3 | 13.6 |
| 200 | 236.00 | 18.0 | 4.7 | 234.00 | 17.0 | 3.8 | 266.00 | 33.0 | 3.6 | |
| 6,000 | 7,010.00 | 16.8 | 1.1 | 7,810.00 | 30.2 | 7.2 | 7,580.00 | 26.3 | 1.6 | |
| Large intestine | 3 | 2.87 | −4.3 | 20.6 | 3.88 | 29.3 | 12.2 | 3.06 | 2.0 | 12.3 |
| 200 | 166.00 | −17.0 | 6.0 | 220.00 | 10.0 | 6.0 | 186.00 | −7.0 | 3.6 | |
| 6,000 | 4,940.00 | −17.7 | 3.1 | 6,380.00 | 6.3 | 5.1 | 5,540.00 | −7.7 | 2.9 | |
| Skin | 3 | 2.54 | −15.3 | 5.4 | 3.08 | 2.7 | 20.9 | 2.52 | −16.0 | 9.1 |
| 200 | 163.00 | −18.5 | 4.1 | 180.00 | −10.0 | 5.7 | 153.00 | −23.5 | 4.3 | |
| 6,000 | 4,660.00 | −22.3 | 2.7 | 5,250.00 | −12.5 | 4.6 | 4,590.00 | −23.5 | 2.7 | |
| Blood | 3 | 3.42 | 14.0 | 3.7 | 2.84 | −5.3 | 26.7 | 2.16 | −28.0 | 8.0 |
| 200 | 181.00 | −9.5 | 2.7 | 191.00 | −4.5 | 8.4 | 135.00 | −32.5 | 2.3 | |
| 6,000 | 5,320.00 | −11.3 | 2.1 | 5,480.00 | −8.7 | 1.3 | 4,030.00 | −32.8 | 2.8 | |
| Calibration curves | ||||||||||
| Slope | 0.00042–0.00044 | 0.00044–0.00045 | 0.00051–0.00053 | |||||||
| R2 | 0.9831–0.9954 | 0.9905–0.996 | 0.995–0.9959 | |||||||
Abbreviations are as follows: CV, coefficient of variation; RE, relative error.
Liver, kidney, brain, heart, small intestine, large intestine, and testis: cells/20 mg tissue; spleen, lung, and ovary: cells/10 mg tissue; skin: cells/0.2 mg tissue; blood: cells/50 μL blood.
Table 2.
Accuracy and precision in the diluted QC samples
| Sample | Dilution factor | Nominal concentrationa | Mean observed concentrationa | RE (%) | CV (%) |
|---|---|---|---|---|---|
| QC-HD1 | 10 | 50,000 | 43,300.00 | −13.4 | 5.1 |
| QC-HD2 | 100 | 50,000 | 38,000.00 | −24.0 | 22.6 |
Abbreviations are as follows: CV, coefficient of variation; RE, relative error.
Cells/0.02 and 0.002 mg.
In vivo biodistribution study
iPICs were subcutaneously transplanted to 18 male and 18 female NOD.Cg-PrkdcscidIl2rgtm1Sug/ShiJic mice (NOG mice; In-Vivo Science Inc., Tokyo, Japan). Of these, 10 male and 10 female NOG mice underwent the same surgical procedure as the sham group to confirm the specificity of iPIC detection. Blood and tissue samples were obtained from three male and three female mice at 5, 13, 26, and 52 weeks after transplantation of iPICs for the treated group, and at 5 and 26 weeks for the sham group.
During the study period, five and three animals in the treatment and sham groups, respectively, were found dead or were euthanized. In these animals, decreased spontaneous respiration and locomotion were observed, and necropsy revealed an enlarged spleen.
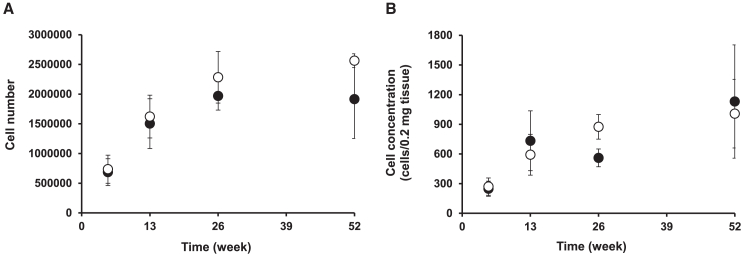
For quantification of the in vivo samples, QC samples were prepared to ensure assay quantitativity, and all QC samples met the criteria (Table S1). In the sham group, all tissue and blood samples were below the limit of quantification across all time points, indicating the selectivity of the LINE1-ddPCR method. The time profiles of cell concentrations and cell numbers at the transplantation site are shown in Figure 2. No clear differences were observed in the time profiles between cell concentration and cell number; however, the variability in the cell concentration was slightly greater than that in the cell number. The time-concentration profile fluctuated in males, whereas the cell number gradually increased severalfold over 26 weeks before reaching a steady state in both males and females. iPICs were not observed in the blood, liver, kidney, spleen, heart, lungs, brain, stomach, small intestine, large intestine, testis, uterus, or ovary at any given time point.
Figure 2.
Time profiles of cell numbers and cell concentrations of iPSCs at the transplantation site
(A) Each data point represents mean ± standard deviation of cell numbers (n = 3). (B) Each data point represents mean ± standard deviation of cell concentrations (n = 3). The open and closed circles indicate cell numbers in female and male, respectively.
Discussion
First, we optimized LINE1-ddPCR to quantify iPICs in the blood and various tissue samples using a single surrogate calibration curve. In a previous study, dog gDNA was used as an EC gene to normalize DNA recovery rates,19 whereas a synthetic plasmid containing canine MC1R genes was utilized in this study. Because gDNA is a biologically derived material, its use in biodistribution studies of iCTPs, which require the measurement of large quantities of samples from various matrices, raises ethical concerns regarding animal welfare. Synthetic plasmids are preferred to gDNA in terms of cost and quality. Furthermore, another advantage of synthetic plasmid DNA is its flexibility to adjust critical elements in PCR, such as minimizing cross-reactivity with genetic sequences present in target cells and host animals, as well as controlling the amplicon length. As shown in Figure 1, the simultaneous detection of LINE1 and canine MC1R inserted into the plasmid DNA was successfully demonstrated, similar to the case where gDNA was used as an EC gene.19 Because the positive and negative droplets could be clearly distinguished, this method could be used to quantify iPICs. However, there is scope for further improvement. FAM fluorescence intensity increased in the EC gene (VIC)-positive droplets, which was likely caused by fluorescence spillover from the VIC channel into the FAM channel. Accordingly, reducing the probe concentration for the EC gene or changing the fluorescent dye of the probe may help mitigate spillover.31
Second, we validated the quantitativity of the modified LINE1-ddPCR as a bioanalytical method to examine the large-scale biodistribution of iCTPs. As there are no established criteria for quantification in CTP biodistribution studies, in this study, we set the acceptance criteria for accuracy and precision based on our previous study18,19: ±35% RE and within 35% CV for QC-H and QC-M samples; ±50% RE and within 50% CV for QC-L samples. All QC samples met the criteria in three separate experiments, as shown in Table 1. Although these criteria are more stringent than recommendations from the Global CRO Council in Bioanalysis (GCC) and the proposal from the Multisite Evaluation Study on Analytical Methods for Non-clinical Safety Assessment of Human-Derived Regenerative Medical Products 2 (MEASURE2): ±50% RE and within 80% CV for the measured CAR-T in GCC recommendation, and ±50% RE and within 50% CV for QC samples in MEASURE2 proposal,32,33 they are considered more suitable for the quantitative evaluation of the biodistribution of iCTPs. This was achieved as a result of DNA correction using the EC gene and attenuation of matrix effects by ddPCR, enhancing the accuracy and precision of the method.32 Although further discussion is necessary to develop regulatory guidelines for assay validation, we believe that our results would provide valuable insights into establishing assay criteria for the quantification of CTPs.
Calibration curves were prepared for each plate during DNA extraction and ddPCR measurements. Because the difference in slope was within a maximum of 1.24-fold and the QC samples met the criteria regardless of which calibration curve was used for the calculation, a single calibration curve could be employed across the plates. Furthermore, the calibration curves were similar between runs, suggesting that the number of human cells in various biological samples can be calculated using a single calibration curve across runs. Thus, LINE1-ddPCR is advantageous in reducing the number of samples and costs and is applicable to long-term biodistribution studies of iCTPs, which require the quantification of a large number of samples with various matrices.
The bottleneck of LINE1-ddPCR is its narrow quantitative range of 3–10,000 cells (3,000-fold). This is critical because CTPs can exhibit significant proliferation in vivo beyond the dynamic range of ddPCR. Although qPCR—which offers a broader quantitative range despite the quantitative limitations caused by matrix effects—could be considered in the unlikely event that a sample exhibits significant proliferation of CTPs beyond the dynamic range of ddPCR, the LINE1-ddPCR assay demonstrated quantifiability even with a 100-fold dilution of the QC sample, effectively extending the quantitative range of the assay by 300,000-fold. This indicates that samples that exceeded the quantitative range of ddPCR could also be measured through dilution.
Third, we designed a study plan for assessing iPIC biodistribution. NOG mice were used because severely immunodeficient rodents, such as NOG mice, are recommended for nonclinical studies of CTPs to prevent immune rejection by the host due to xenogeneic transplantation.34 Generally, biodistribution studies of iCTPs should be conducted for as long as possible to assess the tumorigenic risk and cell fate. In this study, the duration was 52 weeks, considering the survival rate of the strain.35 Indeed, animal deaths were observed during the study period in both the treated and sham groups. Splenomegaly was observed in all these animals, suggesting that malignant lymphoma, which is frequently seen in this strain, was the cause of death,35 although a histopathological examination, not conducted in the present study, might be necessary for tumorigenicity evaluation. Because diabetes occurs in both males and females, both sexes were evaluated to assess the impact of sex differences on biodistribution and potential migration to reproductive organs. The minimal number of animals necessary to evaluate distribution variability was used, with three animals per time point per sex. Additional reserve animals were prepared for both the treated and sham groups to ensure an adequate number of animals at the 52-week time point, accounting for the possibility of natural death. As shown in Figure 2, the time profiles of the cell numbers at the transplantation site were successfully obtained with low variability. To observe the time-cell number profile and predict the cell fate in each tissue, four time points, spaced approximately in a geometric progression with a ratio of two, were evaluated over the course of one year. These sampling time points effectively captured the time profiles of cell concentration and cell numbers. Selecting the appropriate time points, considering the expected time profile of the cell concentration or cell numbers, is vital to effectively evaluate cell proliferation, decrease, and persistence. Similarly, evaluation of all tissues in the body allows for a comprehensive assessment of tumorigenic risk, with more tissue samples providing more extensive coverage. However, these evaluations require considerable time and effort. To expedite product delivery to patients and achieve disease eradication, it is preferable to select the necessary organs based on the objectives of the study. In this study, we evaluated tissues with abundant blood flow, those critical for maintaining life, and transplantation sites to assess the risk of cell leakage from the transplantation site, subsequent tumor formation, or adverse effects. To predict the biodistribution profile in humans, it is important that the route of administration (ROA) reflects the intended clinical ROA. While it is conceivable to elucidate the systemic distribution following intravenous administration to estimate the risk in cases where cells leak from the transplantation site and migrate into the systemic circulation, this approach may overestimate the risk. Additionally, the systemic distribution could vary depending on the number of migrated cells, and it is possible that cells leaking from the transplantation site could enter the arteries or lymphatic vessels instead of the veins. Therefore, the primary objective of this study was to determine whether cells could leak from the administration site; accordingly, only subcutaneous transplantation, as intended for clinical studies, was explored.
Finally, we studied the long-term biodistribution of iPICs in NOG mice. LINE1-ddPCR showed that the cells were detected only at the transplant site and not in other organs, indicating that iPICs survived at the transplantation site for up to one year without migrating to other organs, thereby demonstrating a low risk of leakage from the transplantation site. If Alu-qPCR had been used in this study, it is likely that noise would have been detected in non-template control samples.18 Moreover, the noise levels could vary between samples and fluctuate owing to matrix changes over the long-term study, making it difficult to conclusively determine that no migration occurred beyond the transplantation site, which is critical for evaluating the tumorigenicity of iCTPs and their applicability to clinical studies. The absence of noise when using LINE1-ddPCR provides a significant advantage over Alu-qPCR for application in long-term biodistribution studies.
At the transplant site, the number of cells per unit tissue weight (cell concentration) exhibited large variability compared with the number of cells per harvested sample. In addition, the time-concentration profile fluctuated slightly in males, whereas the cell number gradually increased severalfold over the course of 26 weeks before reaching a steady state in both males and females, with no evident sex differences. This suggests that the concentration of iPICs may vary depending on the ratio of iPICs to mouse-derived skin in the collected samples. In the present study, variation in the amount harvested at the transplantation site was minimal. Moreover, no clear differences in the time profiles of the cell numbers and concentration were observed, thus minimizing their impact on data interpretation. However, because cell concentration varies depending on the amount harvested, it could potentially lead to misinterpretations regarding cell number fluctuations, which could be critical in evaluating tumorigenic risk. Therefore, we recommend monitoring changes in the total number of cells per organ when evaluating the tumorigenicity risk of iCTPs with no clear proliferation, such as iPICs. However, when assessing products with expected proliferation, such as liver transplants, where the ratio of donor cells to host cells and organ weights can change significantly,36,37 careful considerations should be given to the units used for reporting. Furthermore, for pharmacokinetic analysis, concentration data are commonly used38,39,40; therefore, it is important to adopt the appropriate units, based on the purpose of the study.
At the transplantation site, the number of cells initially decreased post-transplantation, recovered to the original transplanted number (dosage) after six months, and remained constant for the following six months. Based on the observed rate of increase in cell numbers from week 5 to week 13, it is probable that the cell number prior to week 5 was even lower than that at week 5. However, because iPICs were not detected outside the transplantation site at any given time point, it is unlikely that the temporary decrease in cell number was due to migration and engraftment of iPICs to other tissues. The temporary decrease was believed to have occurred until sufficient angiogenesis was established at the transplant site, after which the cell numbers gradually recovered. Previous studies have reported that when neonatal porcine islets were subcutaneously transplanted into mice along with a fibrin scaffold, blood vessels were observed around the transplant site at 7 days post-transplantation, vascularization increased at days 14 and 21, and insulin-positive islets engrafted even at 22 weeks post-transplantation. In contrast, without the fibrin scaffold, a reduction in insulin-positive islets was observed at 3 weeks post-transplantation, with complete disappearance by 7 weeks.41 Thus, in subcutaneous transplantation, vascularization seems essential for the survival and therapeutic efficacy of transplanted cells.42 Although further studies are required to analyze the factors influencing the time-dependent changes in the number of iPICs after subcutaneous transplantation, the absence of cells outside the transplant site and the lack of proliferation beyond the transplanted cell count suggest that the risk of migration from the transplant site and tumorigenicity are negligible for iPICs. Furthermore, iPICs survived at the transplantation site for 52 weeks, suggesting that their therapeutic effects in clinical settings could persist for over a year. These characteristics are advantageous for patients, given that insulin pens conventionally used by patients require repeated injections. Therefore, these findings support the clinical application of iPICs.
This study had some limitations. Although this study demonstrated the applicability of LINE1-ddPCR in immunodeficient animals, it is necessary to evaluate their applicability to other animal species used to evaluate the efficacy of CTPs, such as dogs, pigs, and monkeys; we do believe that our method is applicable, based on the human specificity of LINE1 sequence.43,44,45,46 When using LINE1-ddPCR in animal species other than mice, it is necessary to design ECs that do not cross-react with the host’s gDNA. However, as synthetic plasmids are employed, this design is relatively straightforward. In biodistribution studies involving large animals, it will likely be a challenge to optimize homogenization methods due to the use of large tissue samples. Furthermore, the in vivo experiment was designed to show that the transplanted cells did not exhibit significant fluctuations in cell number and remained at the transplantation site without substantial proliferation; however, the same experimental design may not be applicable for products such as genetically engineered immune cells expressing chimeric antigen receptors that migrate to various tissues and demonstrate significant cellular dynamics. In the future, it is essential to accumulate further data on the in vivo distribution of various cellular products to advance the standardization of biodistribution studies and their analytical methods and to develop comprehensive guidelines.
Conclusion
In this study, we optimized LINE1-ddPCR, confirmed its quantitativity, developed a fit-for-purpose biodistribution study protocol, and conducted a long-term animal study of iPICs transplanted subcutaneously into immunodeficient mice to standardize the biodistribution assessment protocol for iCTPs. The present study successfully clarified the biodistribution profile of iPICs and justified their clinical use in terms of long-term persistence and low tumorigenicity risk, although the lack of histopathological examination should be noted. The key considerations for biodistribution studies derived from our study are summarized in Table 3. The insights gained from this study are crucial for standardizing biodistribution study protocols and advancing the research and development of iCTPs.
Table 3.
Key considerations for the biodistribution study of induced pluripotent stem cell-derived cell therapy products
| Considerations | |
|---|---|
| Bioanalytical assay method | unit notation: “cells/μL biofluid or mg tissue” is recommended |
| instrument: ddPCR is recommended to mitigate matrix effects | |
| standard sample: cell lysate is recommended | |
| primer/probe design: sensitivity, selectivity, and specificity should be considered | |
| PCR conditions: master mix, spillover, annealing temperature/duration, number of cycles, primer/probe concentrations, PCR input, replicate assays | |
| DNA extraction: use of an external control gene is proposed to normalize DNA variability | |
| calibration curve: a single surrogate calibration curve can be employed to minimize the number of animals used; linear regression: r2 > 0.98 | |
| QC samples: at least four samples at each concentration with each matrix, concentration, and dilution | |
| accuracy: within ±35% (50% for QC sample at lower concentrations) | |
| precision: <35% (50% for QC sample at lower concentrations) | |
| sample preparation: homogenization of the entire tissue is recommended to quantify CTPs heterogeneously distributed in organs | |
| In vivo biodistribution | duration: lifetime of the animal |
| species: immunodeficient animals | |
| sex: both male and female (depending on the clinical application) | |
| number of animals: at least three animals to evaluate individual variability; consider the natural mortality during the study duration | |
| tissue: tissues with abundant blood flow, those vital for maintaining life, and the transplantation site | |
| time points: multiple time points to capture cellular kinetics | |
| units: “cells/μL biofluid,” “cells/mg tissue,” or “cells/tissue” can be reported |
Abbreviations are as follows: CV, coefficient of variation; CTPs, cell therapy products; PCR, polymerase chain reaction; QC, quality control; RE, relative error.
Materials and methods
Materials
iPICs were prepared by Orizuru Therapeutics, Inc. (Kanagawa, Japan) as previously reported, with some modifications. The plasmid, including a portion of the canine MC1R sequence, was synthesized by Thermo Fisher Scientific (Waltham, MA, USA) as the EC for normalization of the DNA recovery rate. Primers and probes for the human LINE1 sequence and canine MC1R were obtained from Thermo Fisher Scientific.
ddPCR analysis
A QX ONE Droplet Digital PCR system (Bio-Rad Laboratories, Hercules, CA, USA) was used for the ddPCR assay. The primer/probe sets used in our previous study were used to detect LINE1 and EC.19 The ddPCR reaction was performed for 45 cycles (95°C for 0.5 min and 58°C for 1.5 min) after an initial denaturation at 95°C for 10 min. Following enzyme inactivation at 98°C for 10 min, the droplets were classified as positive or negative based on fluorescence intensity. Since no positive droplets were observed in our previous report when ddPCR Supermix for Probes was used for the ddPCR assay, ddPCR Multiplex Supermix (Bio-Rad Laboratories) was used as the master mix. The ddPCR reaction mixture contained 4× ddPCR Multiplex Supermix, primers (2,700 nmol/L for LINE1, 500 nmol/L for EC), probes (250 nmol/L), dithiothreitol (4 mmol/L), and restriction enzyme (ScaI, 10 U/μL; Takara Bio, Shiga, Japan), making up a final volume of 20 μL. All reactions were performed in triplicate. The copy numbers of the LINE1 and EC sequences were determined using QX ONE Software (ver. 1.2, Bio-Rad Laboratories).
Assay validation
Assay validation was conducted on three separate days. The cells were lysed at a concentration of 7,140 cells/μL with DNA/RNA Shield (Zymo Research, Irvine, CA, USA) combined with proteinase K (ProK) in a ratio of 9:1 at a temperature of 65°C. Subsequently, working cell lysates were prepared by serially diluting cell lysate with the DNA/RNA Shield/ProK mixture, achieving final concentrations ranging from 4.29 × 10−2 to 1.43 × 102 cells/μL. Mouse tissue and blood were obtained from male NOG mice. All experiments involving mice were approved by the Institutional Animal Care and Use Committee (IACUC) of the facility, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Control tissue samples were homogenized in DNA/RNA Shield to generate blank homogenates. To prepare calibration curve samples, working cell lysates were added to blank homogenates or blood samples. The concentrations ranged from 3, 10, 30, 102, 3 × 102, 103, 3 × 103, 6 × 103, to 104 cells per 20 mg tissue (for liver, kidney, heart, brain, small intestine, large intestine, and testis), 10 mg tissue (for spleen, lung, ovary, and stomach), 0.2 mg tissue (skin), or 50 μL of blood. QC samples were prepared at concentrations of 3 (QC-L), 2 × 102 (QC-M), and 6 × 103 (QC-H) cells/20 mg tissue, 10 mg tissue, 0.2 mg tissue, or 50 μL blood. A QC sample with a concentration of 5 × 104 cells/0.2 mg skin (QC-UH) was prepared. The diluted QC samples were prepared by diluting the QC-UH sample 10-fold (5 × 103 cells/0.2 mg tissue; QC-HD1) and 100-fold (5 × 102 cells/0.2 mg tissue; QC-HD2) with the control homogenate to confirm the accuracy and precision of the dilution procedure. The plasmid was added to all samples as an EC to normalize DNA variability among samples. DNA was extracted following the protocol described in our previous study, with minor modifications,19 using a MagMAX DNA Multi-Sample Ultra 2.0 Kit (Thermo Fisher Scientific). Briefly, DNA was extracted from 200 μL of tissue homogenate or 50 μL of blood using the KingFisher Apex system (MagMAX_Ultra2_200μL_Flex and MagMAX Ultra 2TissueV). The copy numbers of LINE1 and EC in the reaction mixture were determined as described in the “ddPCR analysis” section. The cell concentration in the QC samples was calculated using the equation Y = aX, where X is the nominal cell concentration, Y is the ratio of the LINE1/EC copy numbers in the reaction mixture, and a is the slope of the calibration curve. The assay criteria were based on our previous report,32 recommendations from the GCC, and a proposal from MEASURE2.33,34 The RE and CV value criteria were set within 100.0% ± 35.0% for QC-H, QC-M, QC-HD1, and QC-HD2 and within 100.0% ± 50.0% for QC-L.
Long-term biodistribution study
All procedures were approved by the IACUC of the contract research organization accredited by AAALAC. Seven-week-old male and female NOG mice were housed under a 12-h light-dark cycle with free access to water and a pelleted diet (CE-2; CLEA Japan, Inc., Tokyo, Japan). The room temperature was maintained at 23°C ± 3°C. The animals were divided into sham and treatment groups. A biodegradable implant containing 2.5 × 106 iPICs was transplanted into the subcutaneous space of treatment group animals. Blood and tissue samples were obtained from three male and three female animals at 5, 13, 26, and 52 weeks after transplantation. In the sham group, the same surgical procedure was performed, and blood and tissue samples were obtained at 5 and 26 weeks from three male and three female animals to confirm the specificity of iPIC detection. Samples were frozen immediately after weighing and stored at −70°C until DNA extraction. Because CTPs typically exhibit heterogeneous distribution within an organ, the entire organ was homogenized in DNA/RNA Shield to quantify the total CTPs in the organ. For the treatment group, the transplantation site (skin) was further diluted 10-fold with control skin homogenate. DNA extraction from samples and ddPCR assays were performed as described in the “assay validation” and “ddPCR analysis” sections. Sample analysis was conducted on two separate days: from 5, 13, and 26 weeks, and from 52 weeks.
Data availability
All datasets generated in this study will be made available upon request.
Acknowledgments
We thank Mr. Yukinori Yatsuda (Bio-Rad Laboratories K.K.) for helpful assistance with the ddPCR assay.
Author contributions
M.N., Y.M., H.U., T.W., H.H., and S.Y. contributed to the design of the experiments, interpretation of data, and writing of the manuscript; M.N. and S.Y. conducted the experiments.
Declaration of interests
H.U. is an employee of is Orizuru Therapeutics.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2025.101538.
Supplemental information
References
- 1.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Rowe R.G., Daley G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 2019;20:377–388. doi: 10.1038/s41576-019-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerneckis J., Cai H., Shi Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Signal Transduct. Target. Ther. 2024;9:112. doi: 10.1038/s41392-024-01809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chehelgerdi M., Behdarvand Dehkordi F., Chehelgerdi M., Kabiri H., Salehian-Dehkordi H., Abdolvand M., Salmanizadeh S., Rashidi M., Niazmand A., Ahmadi S., et al. Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy. Mol. Cancer. 2023;22:189. doi: 10.1186/s12943-023-01873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee A.S., Tang C., Rao M.S., Weissman I.L., Wu J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato Y., Bando H., Di Piazza M., Gowing G., Herberts C., Jackman S., Leoni G., Libertini S., MacLachlan T., McBlane J.W., et al. Tumorigenicity assessment of cell therapy products: The need for global consensus and points to consider. Cytotherapy. 2019;21:1095–1111. doi: 10.1016/j.jcyt.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Kuroda T., Yasuda S., Sato Y. Tumorigenicity studies for human pluripotent stem cell-derived products. Biol. Pharm. Bull. 2013;36:189–192. doi: 10.1248/bpb.b12-00970. [DOI] [PubMed] [Google Scholar]
- 8.Takei Y., Morioka M., Yamashita A., Kobayashi T., Shima N., Tsumaki N. Quality assessment tests for tumorigenicity of human iPS cell-derived cartilage. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-69641-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamiyama Y., Naritomi Y., Moriya Y., Yamamoto S., Kitahashi T., Maekawa T., Yahata M., Hanada T., Uchiyama A., Noumaru A., et al. Biodistribution studies for cell therapy products: Current status and issues. Regen. Ther. 2021;18:202–216. doi: 10.1016/j.reth.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michelotti F.C., Bowden G., Küppers A., Joosten L., Maczewsky J., Nischwitz V., Drews G., Maurer A., Gotthardt M., Schmid A.M., Pichler B.J. PET/MRI enables simultaneous in vivo quantification of beta-cell mass and function. Theranostics. 2020;10:398–410. doi: 10.7150/thno.33410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Himmelreich U., Dresselaers T. Cell labeling and tracking for experimental models using magnetic resonance imaging. Methods. 2009;48:112–124. doi: 10.1016/j.ymeth.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Nose N., Nogami S., Koshino K., Chen X., Werner R.A., Kashima S., Rowe S.P., Lapa C., Fukuchi K., Higuchi T. [18F]FDG-labelled stem cell PET imaging in different route of administrations and multiple animal species. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-90383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto S., Matsumoto S.I., Shimizu H., Hirabayashi H. Quantitative application of flow cytometry for the analysis of circulating human T cells: A preclinical pharmacokinetic study. Drug Metab. Pharmacokinet. 2020;35:207–213. doi: 10.1016/j.dmpk.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto S., Ding N., Matsumoto S.I., Hirabayashi H. Highly specific, quantitative polymerase chain reaction probe for the quantification of human cells in cynomolgus monkeys. Drug Metab. Pharmacokinet. 2021;36 doi: 10.1016/j.dmpk.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Walker J.A., Kilroy G.E., Xing J., Shewale J., Sinha S.K., Batzer M.A. Human DNA quantitation using Alu element-based polymerase chain reaction. Anal. Biochem. 2003;315:122–128. doi: 10.1016/s0003-2697(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 16.Funakoshi K., Bagheri M., Zhou M., Suzuki R., Abe H., Akashi H. Highly sensitive and specific Alu-based quantification of human cells among rodent cells. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bittorf P., Bergmann T., Merlin S., Olgasi C., Pullig O., Sanzenbacher R., Zierau M., Walles H., Follenzi A., Braspenning J. Regulatory-Compliant Validation of a Highly Sensitive qPCR for Biodistribution Assessment of Hemophilia A Patient Cells. Mol. Ther. Methods Clin. Dev. 2020;18:176–188. doi: 10.1016/j.omtm.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu H., Kuze Y., Higuchi T., Matsumoto S.I., Yamamoto S., Goto A., Moriya Y., Hirabayashi H. Development of a bioanalytical method for circulating human T cells in animals using Arthrobacter luteus-based quantitative polymerase chain reaction and its application in preclinical biodistribution studies. Regen. Ther. 2020;15:251–257. doi: 10.1016/j.reth.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama M., Yamamoto S., Hirabayashi H. Novel Cell Quantification Method Using a Single Surrogate Calibration Curve Across Various Biological Samples. AAPS J. 2023;25:26. doi: 10.1208/s12248-023-00791-9. [DOI] [PubMed] [Google Scholar]
- 20.Pagliuca F.W., Millman J.R., Gürtler M., Segel M., Van Dervort A., Ryu J.H., Peterson Q.P., Greiner D., Melton D.A. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A., O'Dwyer S., Quiskamp N., Mojibian M., Albrecht T., et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 22.Hogrebe N.J., Augsornworawat P., Maxwell K.G., Velazco-Cruz L., Millman J.R. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 2020;38:460–470. doi: 10.1038/s41587-020-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair G.G., Liu J.S., Russ H.A., Tran S., Saxton M.S., Chen R., Juang C., Li M.L., Nguyen V.Q., Giacometti S., et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived beta cells. Nat. Cell Biol. 2019;21:263–274. doi: 10.1038/s41556-018-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velazco-Cruz L., Song J., Maxwell K.G., Goedegebuure M.M., Augsornworawat P., Hogrebe N.J., Millman J.R. Acquisition of Dynamic Function in Human Stem Cell-Derived βCells. Stem Cell Rep. 2019;12:351–365. doi: 10.1016/j.stemcr.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bornstein S.R., Ludwig B., Steenblock C. Progress in islet transplantation is more important than ever. Nat. Rev. Endocrinol. 2022;18:389–390. doi: 10.1038/s41574-022-00689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q., Huang Y.X., Liu L., Zhao X.H., Sun Y., Mao X., Li S.W. Pancreatic islet transplantation: current advances and challenges. Front. Immunol. 2024;15 doi: 10.3389/fimmu.2024.1391504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witkowski P. Expanded FORWARD Trial Demonstrates Continued Potential for Stem Cell-Derived Islet Cell Therapy to Eliminate Need for Insulin for People with T1D. 2024. https://diabetes.org/newsroom/press-releases/expanded-forward-trial-demonstrates-continued-potential-stem-cell-derived
- 28.Wu J., Li T., Guo M., Ji J., Meng X., Fu T., Nie T., Wei T., Zhou Y., Dong W., et al. Treating a type 2 diabetic patient with impaired pancreatic islet function by personalized endoderm stem cell-derived islet tissue. Cell Discov. 2024;10:45. doi: 10.1038/s41421-024-00662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiyoshi H., Sakuma K., Tsubooka-Yamazoe N., Asano S., Mochida T., Yamaura J., Konagaya S., Fujii R., Matsumoto H., Ito R., Toyoda T. Characterization and reduction of non-endocrine cells accompanying islet-like endocrine cells differentiated from human iPSC. Sci. Rep. 2022;12:4740. doi: 10.1038/s41598-022-08753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamasaki M., Maki T., Mochida T., Hamada T., Watanabe-Matsumoto S., Konagaya S., Kaneko M., Ito R., Ueno H., Toyoda T. Xenogenic Engraftment of Human-Induced Pluripotent Stem Cell-Derived Pancreatic Islet Cells in an Immunosuppressive Diabetic Gottingen Mini-Pig Model. Cell Transplant. 2024;33 doi: 10.1177/09636897241288932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.dMIQE Group. Huggett J.F. The Digital MIQE Guidelines Update: Minimum Information for Publication of Quantitative Digital PCR Experiments for 2020. Clin. Chem. 2020;66:1012–1029. doi: 10.1093/clinchem/hvaa125. [DOI] [PubMed] [Google Scholar]
- 32.Wissel M., Poirier M., Satterwhite C., Lin J., Islam R., Zimmer J., Khadang A., Zemo J., Lester T., Fjording M., et al. Recommendations on qPCR/ddPCR assay validation by GCC. Bioanalysis. 2022;14:853–863. doi: 10.4155/bio-2022-0109. [DOI] [PubMed] [Google Scholar]
- 33.Fujita E., Yamamoto S., Hanada T., Jogasaki S., Koga Y., Yatsuda Y., Kakizaki Y., Jo Y., Asano Y., Yonezawa K., et al. Using qPCR and ddPCR to study biodistribution of cell therapy products: a multi-site evaluation. Cytotherapy. 2024;27:51–65. doi: 10.1016/j.jcyt.2024.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K., Ueyama Y., Koyanagi Y., Sugamura K., Tsuji K., et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita Y., Sato T., Noishiki K., Kobayashi K., Uchiyama A., Izumi H., Tamura T., Shikamura M., Oinishi Y., Saito M., Kawamata S. Data on long-term survival of the NOD/Shi-scid IL-2Rγnull (NOG) mouse in two facilities. J. Toxicol. Sci. 2021;46:453–469. doi: 10.2131/jts.46.453. [DOI] [PubMed] [Google Scholar]
- 36.Zhang R.R., Zheng Y.W., Taniguchi H. Generation of a Humanized Mouse Liver Using Human Hepatic Stem Cells. J. Vis. Exp. 2016;114 doi: 10.3791/54167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamaki Y., Shibata Y., Hayakawa M., Kato N., Machii A., Ikeda Y., Nanizawa E., Hayashi Y., Suemizu H., Ito H., Ishikawa T. Treatment with hepatocyte transplantation in a novel mouse model of persistent liver failure. Biochem. Biophys. Rep. 2022;32 doi: 10.1016/j.bbrep.2022.101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goto A., Yamamoto S., Igari T., Matsumoto S.I., Chisaki I., Iida K., Nakayama M., Oda A., Kakoi Y., Uchida A., et al. Quantitative Model Analysis and Simulation of Pharmacokinetics and Metastasis-Associated Lung Adenocarcinoma 1 RNA Knockdown Effect After Systemic Administration of Cholesterol-Conjugated DNA/RNA Heteroduplex Oligonucleotide Crossing Blood-Brain Barrier of Mice. J. Pharmacol. Exp. Ther. 2023;384:197–204. doi: 10.1124/jpet.122.001331. [DOI] [PubMed] [Google Scholar]
- 39.Ding N., Yamamoto S., Chisaki I., Nakayama M., Matsumoto S.I., Hirabayashi H. Utility of Gottingen minipigs for the prediction of human pharmacokinetic profiles after intravenous drug administration. Drug Metab. Pharmacokinet. 2021;41 doi: 10.1016/j.dmpk.2021.100408. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto S., Matsumoto S.I., Goto A., Ugajin M., Nakayama M., Moriya Y., Hirabayashi H. Quantitative PCR methodology with a volume-based unit for the sophisticated cellular kinetic evaluation of chimeric antigen receptor T cells. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-74927-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakama B.F., Seeberger K.L., Korbutt G.S. Fibrin supports subcutaneous neonatal porcine islet transplantation without the need for pre-vascularization. Xenotransplantation. 2020;27 doi: 10.1111/xen.12575. [DOI] [PubMed] [Google Scholar]
- 42.Zhou X., Xu Z., You Y., Yang W., Feng B., Yang Y., Li F., Chen J., Gao H. Subcutaneous device-free islet transplantation. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1287182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chong J.J.H., Yang X., Don C.W., Minami E., Liu Y.W., Weyers J.J., Mahoney W.M., Van Biber B., Cook S.M., Palpant N.J., et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poh K.K., Sperry E., Young R.G., Freyman T., Barringhaus K.G., Thompson C.A. Repeated direct endomyocardial transplantation of allogeneic mesenchymal stem cells: safety of a high dose, "off-the-shelf", cellular cardiomyoplasty strategy. Int. J. Cardiol. 2007;117:360–364. doi: 10.1016/j.ijcard.2006.04.092. [DOI] [PubMed] [Google Scholar]
- 45.Linke A., Müller P., Nurzynska D., Casarsa C., Torella D., Nascimbene A., Castaldo C., Cascapera S., Böhm M., Quaini F., et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc. Natl. Acad. Sci. USA. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perin E.C., Silva G.V., Assad J.A.R., Vela D., Buja L.M., Sousa A.L.S., Litovsky S., Lin J., Vaughn W.K., Coulter S., et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J. Mol. Cell. Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated in this study will be made available upon request.