Abstract
Targeted nucleases, primarily CRISPR-Cas-based systems, have revolutionized genome editing by enabling precise modification of target genes or transcripts. Many pre-clinical and clinical studies leverage this technology to develop treatments for human diseases; however, substantial off-target genotoxicity concerns delay its clinical translation. Despite the development of a wide array of tools, assays, and technologies aimed at identifying and quantifying off-target effects, the absence of standardized guidelines leads to inconsistent practices across studies. This review highlights the key challenges and potential solutions in ensuring the safety of gene editing studies for therapeutic applications, focusing on gRNA design, off-target sites prediction, and off-target activity measurement.
Keywords: MT: Clinical Applications, CRISPR-Cas9, off-target, safety, genome editing, gene therapy
Graphical abstract

Kalter and colleagues provide a comprehensive review of the key steps in designing a safe and precise guide RNA for CRISPR-Cas therapeutic applications, focusing on guide RNA design, the detection and quantification of off-target activity, the identification of structural variations, and the functional assessment of potential genotoxicity.
Introduction
The CRISPR-Cas toolkit
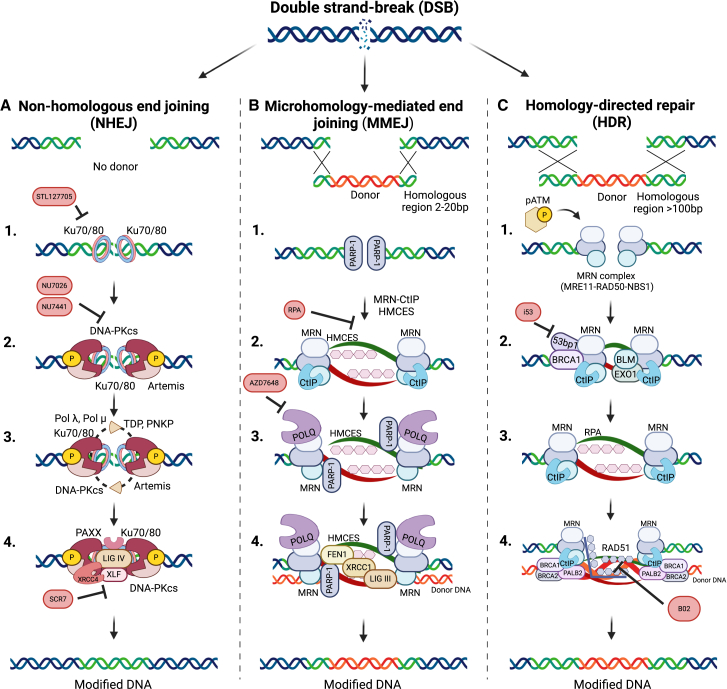
Clustered regularly interspaced short palindromic repeats (CRISPR)-Cas systems have been rapidly adopted in the genome-editing field, with promising applications in treating human diseases. Originally evolved as an adaptive immune mechanism in bacteria and archaea to combat bacteriophage infection, the CRISPR-Cas technology enables precise, base pair resolution modification at nearly any genomic locus (Figure 1).1,2 The CRISPR-Cas system includes two main components: an endonuclease from the Cas family and a guide RNA (gRNA). The diverse Cas family includes nucleases of varying sizes and cutting patterns; among them, Cas9 and Cas12a have already been tested in clinical trials.3 The gRNA includes a 20- to 24-nt spacer sequence that directs the endonuclease into a complementary sequence in the genome. Upon reaching the DNA target site, the Cas nuclease induces a double-strand break (DSB), subsequently repaired by the cell’s intrinsic repair mechanisms: predominantly non-homologous end joining (NHEJ) or homology-directed repair (HDR).4,5,6 NHEJ is a rapid, error-prone process that involves a simple ligation of the two ends of the break, often introducing short insertion or deletions (indels) at the DNA cut site, making it ideal for generating frameshift or nonsense mutations.7,8,9 A less common repair pathway is alternative NHEJ (alt-NHEJ), also referred to as microhomology-mediated end joining (MMEJ). This pathway requires 2–20 bp of microhomology at or near the break ends, which are subsequently repaired by the DNA polymerase POLQ, frequently resulting in large deletions at the cut site.10,11,12,13 In contrast, the HDR pathway leverages an exogenous template to accurately repair the DSB and mediates precise gene correction or the insertion of large transgenes (Figure 2).6,14,15,16 Both HDR-based and NHEJ-based genome-editing strategies have been utilized in many pre-clinical and clinical trials for the treatment of hereditary diseases17,18,19,20,21,22 and malignancies.23,24,25,26,27
Figure 1.
CRISPR-Cas DNA editors
General structure of Cas9 and Cas12a nucleases, BEs and PEs, either with or without serine integrase domain fusion. DD, deaminase domain; nCas, nicking Cas; PAM, protospacer adjacent motif; RT, reverse transcriptase; SI, serine integrase; UGI, uracyl-glycosilase inhibitor; Y, A-or-C bases. Created with BioRender.com.
Figure 2.
DSB repair pathways and small-molecule inhibitors
The repair of DSBs follows three major pathways: HDR, NHEJ, MMEJ. (A) In the HDR pathway, MRN and CtIP initiate DNA end resection; RAD51 then replaces RPA on single-stranded DNA, enabling homology-based strand invasion and template-directed repair. HDR inhibitors include AICAR and B02, targeting RAD52 and RAD51, respectively. (B) In the NHEJ repair pathway, Ku70/Ku80 binds to DNA ends, DNA-protein kinase catalytic subunit promotes end processing if needed, and XRCC4-DNA ligase IV complex seals the DNA break. (C) In the MMEJ pathway, PARP-1 competes with Ku70/80 for DSB binding, MRN/CtIP mediates limited resection, and POLQ aligns microhomologous sequences, facilitating error-prone synthesis and repair by the LIG3/XRCC1 complex. Created with BioRender.com.
The recent development of base editing (BE)28 and prime editing (PE)29 have expanded the CRISPR toolkit, enabling precise search and replace genome editing without the need for DSB induction. Base editors utilize Cas9 nickase (nCas9), which introduces a single-strand break, fused to a DNA deaminase domain. There are two main classes of BEs, distinguished by the type of deaminase used: adenine base editors, which facilitate the conversion of adenosine to guanine, and cytosine base editors (CBEs), which mediate cytidine to uracil conversion. CBEs typically also include a uracil DNA glycosylase inhibitor domain, which prevents base excision repair and enhances editing efficiency by stabilizing the uracil intermediate. BE allows for the precise correction of transition mutations through single base pair substitutions.30 However, BE can still result in unintended bystander deaminations when multiple editable bases fall within the deaminase activity window, potentially leading to additional single-nucleotide changes near the target site. In contrast, prime editors consist of a reverse transcriptase (RT) fused to nCas9, along with a prime-editing gRNA that directs the target site and encodes the intended genetic alteration. Unlike BEs, PEs do not rely on deaminase activity and therefore avoid bystander editing, while also supporting all 12 possible base-to-base swappings, as well as indels, offering greater flexibility compared to BE.31,32
A recent advancement in site-specific knockin strategies involves the use of integrases, particularly serine integrases. These enzymes mediate targeted insertion through a recombinase that contains attP attachment site and facilitates recombination at a ∼50-bp attB attachment site within the target genome. The programmable addition via site-specific targeting elements (PASTE) method further enhances this approach by incorporating PE to pre-generated att sites in the genome, facilitating the integration of large payloads (>100 kb) through integrase-mediated recombination. While more flexible and not restricted to a specific cell-cycle stage, integrase-based methods, unlike seamless HDR, leave residual “scars” in the genome after insertion.33
CRISPR-Cas off-target activity
While the CRISPR-Cas system is highly efficient and versatile, adverse editing events at unintended sites, known as off-target activity (OTA), remains a significant safety concern. OTA can occur when the CRISPR-Cas complex associates genomic loci with high sequence identity to the on-target sites, and is further complicated by the tolerance of DNA and RNA base bulges by CRISPR-Cas complexes (Figure 3A).34 CRISPR-Cas induced indels at OT sites can disrupt gene regulation and expression, potentially leading to various undesired effects, including oncogenic transformations.35,36 Additionally, substantial genotoxicity arises from larger structural variations (SVs) at both on-target and OTs, including translocations and large deletions, duplications, inversions, partial or full integration of HDR donors, and chromosomal loss (Figure 3B).37,38,39,40,41,42
Figure 3.
Mechanisms and consequences of OTA
(A) Schematic illustration of gRNA/DNA binding at an on-target site (left) and OT sites, which may involve mismatches, DNA bulges, or RNA bulges. Illustration is based on SpCas9. (B) DNA repair outcomes at on-target or OT sites and their potential consequences. Shown are insertion (INS), deletion (DEL), translocation (TRANS), inversion (INV), and large deletion (L DEL). Resulting effects include exonic frameshift mutations, disruption of intronic regulatory regions (e.g., non-coding RNAs [ncRNAs]), and gene fusions. Red lightning bolts indicate potential disruption of gene expression; the yellow caution sign denotes oncogenic potential. (C) Illustration of various strategies to mitigate OT effects, including optimized gRNA design, high-fidelity (HF) Cas variants, and alternative nucleases (nCas9, Cas12, BE, and PE). Created with BioRender.com.
In addition to OT mutations, the use of viral vectors for in vivo delivery of genome editing components or as HDR donor templates has been associated with unintended genomic integrations of vector or vector-derived fragments. For example, adeno-associated virus (AAV) HDR donors frequently integrate their inverted terminal repeats at both on-target and OT genomic sites.21,43 Furthermore, full-length or fragmented viral vector sequences have been observed to integrate at target genomic loci following in vivo delivery.39 In primary cells treated with viral HDR donors, concatemeric integrations of the full-length vector have specifically been reported at on-target sites.44 Integrations from non-viral double-stranded and single-stranded HDR DNA donors have also been documented, although typically at lower frequencies.45
The OTA of CRISPR-Cas systems are influenced by various factors, including the target site sequence, the delivery modality, unequal delivery of the Cas nuclease across treated cells, and the cell type (Figure 3C). A primary determinant is the gRNA sequence itself: gRNAs with unique target sequences, that is with minimal sequence identity to genomic regions other than the on-target, are expected to have a reduced number of potential OTs. Moreover, in most CRISPR-Cas systems, efficient DNA cleavage requires the presence of a protospacer adjacent motif (PAM), specifically 5′-NGG-3′, in the case of the prototypic Streptococcus pyogenes Cas9 (SpCas9). In nature, PAMs prevent self-targeting by ensuring that only foreign DNA is recognized and cleaved. In genome editing, this constraint limits the number of accessible genomic sites, but also inherently enhances specificity by reducing the likelihood of OTA. Engineered PAM-relaxed or PAM-less variants expand the range of targetable sites, although this increased flexibility may come at the cost of elevated OTA compared to the PAM-restricted wild-type enzyme.46,47 Chemical modifications on the gRNAs have also reduced OTA by altering the thermodynamic and kinetic properties of the gRNA-DNA heteroduplex formation and promoting increased dissociation rates at OT loci.48,49,50 Aside from the gRNA spacer itself, the context of the genomic locus, such as epigenetic features and the chromatin state, can influence OTA.51,52,53 Another key factor is the type of Cas protein utilized, with both natural and engineered Cas variants continuously being discovered and refined to offer enhanced specificity and efficiency.54,55 Recently, large language models have entered protein engineering, including CRISPR proteins.56 Optimized editing protocols including the delivery platform (see Cavazza et al.57 for a comprehensive review) and gRNA/Cas formulations have also been shown to affect OTA.58,59 Finally, BE and PE aim to minimize OT effects by circumventing the introduction of DSBs. For a comprehensive discussion of strategies to mitigate OTA, we refer readers to the paper by Wienert and Cromer.35 Despite these advancements, no current methods have fully eliminated OTA. Even at low frequencies, adverse mutations pose significant risks in therapeutic genome editing, where large numbers of cells are modified, and a single mutated cell could pose a substantial risk. The risk possesed by OTA depends largely on its genomic context. For example, OTAs in proto-oncogenes or coding regions are generally more concerning than OTs within intergenic regions or non-coding regions; however, these can promote the generation of SVs and cannot be overlooked. Therefore, a comprehensive OT analysis remains critical when developing CRISPR-based therapies for clinical use.
OTAs should be monitored carefully throughout the various stages of gRNA design, pre-clinical studies, and clinical studies (Figure 4). As a first step, gRNAs with minimal predicted OTA should be selected while considering genetic variations across populations. Once a gRNA or combination of gRNAs is chosen, potential OTs should be identified and assessed for actual damage under clinically relevant conditions. If adverse OT mutations are discovered, their functional consequences must be assessed thoroughly, with a particular focus on their potential to induce tumorigenicity. This review provides an overview of the tests and assays essential for evaluating the safety of genome-editing therapies for clinical applications and outlines the challenges and benefits of available tools and methods for each stage of the process, focusing on CRISPR-Cas9 applications. Of note, OTA can also be triggered by factors other than the gRNA. For instance, in the case of BE, nonspecific deamination of DNA or RNA can lead to unintended modifications. While this review focuses primarily on CRISPR-Cas9-based nucleases, we recommend further reading on other classes of genome editors.60,61
Figure 4.
Recommended workflow for CRISPR-based genome editing and genomic safety characterization
(A) Design phase: personalized gRNA design using in silico prediction tools (e.g., CRISPRme, Cas-OFFinder, COSMID, CRISPRon/off), selection of high-fidelity or engineered Cas variants, and optimization of delivery strategy (e.g., RNP, lipid nanoparticle, viral or non-viral vectors) for in vivo or ex vivo editing. (B) Preclinical OT nomination and validation: potential OTs are predicted (CRISPRme, Cas-OFFinder, CRISPRoff) and experimentally validated via orthogonal assays (e.g., CIRCLE-seq, Digenome-seq, GUIDE-seq, DISCOVER-seq). Quantification is performed by targeted deep sequencing (e.g., rhAmpSeq) and analyzed with tools like CRISPECTOR or CRISPResso2. (C) SVs and genomic rearrangement assessment: monitoring of large deletions, translocations, and other rearrangements using designated methods such as CAST-seq, PEM-seq, ddPCR, FISH, long-read sequencing. (D) Functional and safety validation: edited cells are evaluated via in vitro assays (cytotoxicity, tumorigenicity, differentiation, lineage tracking) and/or in vivo xenotransplantation into humanized mouse models to evaluate long-term safety, engraftment, and functional performance. (E) Regulatory and manufacturing considerations: all analyses are conducted under GMP conditions, using orthogonal methodologies aligned with FDA/EMA guidelines, with traceable data archiving for potential retrospective safety review. RNP, ribonucleoprotein; EMA, European Medicines Agency; FDA, US Food and Drug Administration. Created with BioRender.com.
In silico methods for gRNA design and selection
The concept of gRNA design
The first step in genome-editing experiments is to design an efficient and specific gRNA with maximal on-target efficiency and minimal OT potential. To do this, in silico methods are used to identify typically multiple or several on-target gRNA candidates that meet these on-target and OT criteria. When conducting knockout (KO) experiments of protein-coding genes, the strategy is to target the first couple of exons causing random edits leading to nonsense-mediated decay or dysfunctional proteins serving as KO. For non-coding RNAs, the strategy is typically to employ two gRNAs and remove the entire gene.62 Whereas KO studies allow for a pool of gRNAs to select from, specific edits such as a single genomic location often have limited gRNA possibilities—for example, by requiring the presence of a PAM or, in the case of BE, the availability of adenosine or cytosine bases. Hence, “gRNA design” is a matter of selecting gRNAs based on how well they meet the criteria. To meet the design criteria, computational prediction methods are applied. Computational methods for gRNA design generally fall into two main categories. The first category is sequence-only methods, which scan reference genomes to identify candidate gRNAs that meet specified requirements, such as target region or PAM sequence, and detect potential OTs with sequence similarity to the intended target. For the OT location detection, these methods employ sequence similarity to the gRNA. The second category is methods that score a potential on-target or OT site. This can be done by (1) hypothesis-driven algorithms, which apply established biological principles (e.g., mismatch position or kinetic modeling) to predict gRNA performance (see, for example, Eslami-Mossallam et al.63), and/or (2) machine learning (ML)-based algorithms, trained and tested on experimental datasets to predict cleavage efficiency at on-target and OT sites. ML methods are inherently limited by the quality and scope of their training data. The choice of computational method and training procedure may provide further limitations beyond those already present in the training data.
Datasets for building machine learning models
Various types of experimental datasets are available in the public domain, predominantly concerning SpCas9 cleavage results. These contain hundreds to tens of thousands of gRNAs for which their on-target efficiencies and/or corresponding OTAs have been measured by different experimental strategies either in vitro or in vivo. Early studies focused on generating loss-of-function data to train models using a functional readout from KO of coding genes (e.g., Doench et al.64). Most current datasets are indel based using high-throughput sequencing to measure the actual editing activity.65,66,67,68 The resulting reads are mapped to their respective genomic locations to call on-target and OT events, using various versions of mapping and sequence alignment algorithms. Hence, the mapping and sequence alignment tools must be carefully evaluated prior to selecting the data to build prediction models, as any errors introduced at the data-generation step would directly affect the prediction outcome.Additionally, ML methods trained on one dataset and evaluated on an external dataset are not expected to perform better than the correlation between them. Some of the datasets show moderate correlation (Spearman R ∼0.5–0.7),69 but not strong enough to ensure consistent performance across studies. Therefore, expectations for model performance on external datasets should be adjusted accordingly. Of note, each experimental dataset is generated in a specific cell type and may not be accurately transferred to others, as different cell types, editing modalities, and the active fractions of gene editing nucleases present in a given biological system can contribute to distinct editing profiles.65 Another challenge is that large-scale datasets, for example by Lazzarotto et al.,65 are far from covering the full mismatches space. Questions remain whether the observed gaps are due to limitations in data accuracy or underlying biological factors.
Prediction of on-target and OT activities
Several in silico methods have been made for both on-target and OT prediction using the above-mentioned datasets for training. The core concept is to assign scores to given on-target or OT sites that correlate with experimental activity data, such as log-transformed read counts from indel-based assays. Many different methods exist for predicting gRNA activity and the repair outcome of different Cas enzymes in different organisms, and while it is beyond the scope of this paper to provide a full overview, we emphasize some of the key methods below representing the main steps in the development of computational strategies.
For on-target efficiency prediction, a few nucleotides of its up- and downstream contexts are used as input to a computational method. For indel-based datasets, efficiency is measured as the fraction of reads carrying an edit. Input data can be featurized (for example, by counting specific dinucleotides and noting nucleotide positions), or it can be one-hot or sparsely/one-hot encoded, where each nucleotide in the target sequence is represented by a binary vector (one “1” and three “0s”). Whereas the former often is used for more “classical” ML and tree-based methods such as gradient boosting tree regression, the latter is most often used for machine/deep learning methods.
While the earlier methods for on-target efficiency prediction were based on functional readouts with the Azimuth package,64 a major advance for Cas9 gRNA on-target efficiency prediction was the DeepHF and DeepSpCas9 methods,70,71 which introduced large-scale, indel-based data followed by deep learning strategies for gRNA efficiency prediction. Whereas DeepHF's training data was biased toward highly efficient gRNAs, the DeepSpCas9 used a more balanced gRNA efficiency distribution, making it more compatible with new datasets. Xiang et al. subsequently integrated these data into CRISPRon, establishing a state-of-the-art model for Cas9 on-target efficiency prediction, validated on external, independent test sets.69 Notably, although these methods are based on deep learning, more classical ML methods, such as gradient-boosting regression trees, perform relatively close to the deep-learning based methods with the currently available data.69
Similar to on-target efficiency prediction, OT cleavage efficiency (activity) prediction is also a matter of scoring. However, before scoring potential OT regions, they need to be located. The simplest approach assumes no bulges in gRNA-DNA interactions, identifying OTs based solely on mismatches and enabling fast search with basic alignment algorithms. However, single-base “bulges” are often tolerated by Cas9 and allow OTA.34 Some tools that search for putative OTs do allow for bulges. The COSMID tool, for example, permits the identification of sites containing one base indel within the target sequence using a so-called tree-like data structure.72 Cas-OFFinder uses a more exhaustive search anchored in the PAMs and allows up to two bulges in gRNA or DNA.73
Some of the prediction tools do not provide a scoring, but merely locate potential OTs and the possible configurations of the mismatches and bulges within the spacer:DNA interaction. Scoring OTs solely on the number and position of mismatches and bulges is not advisable, as it has been demonstrated that single mismatches may reduce activity far more than multiple mismatches in some contexts.66 Furthermore, the distance of a gRNA:DNA mismatch from the PAM sequence impacts the level of OTA, with mismatches closer to the PAM generally having a more disruptive effect than distal mismatches.36,74,75 Mismatches within the PAM are also tolerated to some degree, with SpCas9 capable of recognizing the alternative PAM NAG.75 This has also been observed with other orthologs such as the Streptococcus thermophilus Cas9, which has a canonical PAM of NNAGAAT but can tolerate mismatches at positions 3, 4, 5, and 7.76 Therefore, alternative PAM sequences need to be considered when developing in silico prediction tools or when mapping reads from cell-based assays. Moreover, a recent in silico analysis supported by experimental results showed that, for SpCas9, the nucleotide context around the PAM strongly impacts gRNA efficiency, with Gs upstream of the PAM enhancing efficiency, whereas Gs downstream of the PAM tend to reduce activity.77 This positional effect partially explains why sequence context contributes to gRNA activity prediction. Furthermore, including the gRNA:DNA binding energy in the deep learning scheme for gRNA efficiency prediction was shown to improve the prediction accuracy,69 consistent with the observation of a sweet-spot binding energy interval in which the gRNAs have the highest efficiency.77 Additional parameters that have also been incorporated into scoring metrics include the nature of the mismatch,75 Cas9 binding data from chromatin immunoprecipitation,53,78 and DNA accessibility.79
Based on approaches that search for potential OT locations, a number of mainly ML algorithms have been developed.64,75,79,80,81,82,83,84,85,86 ML-based prediction tools generally outperform simpler baseline models in OT prediction. However, a binding-energy-based model, CRISPRoff,87 which approximates biophysical interactions within the Cas9-gRNA-DNA complex, surpassed top machine learning models of the time and continues to outperform them in some comparisons, as shown in recent benchmarks by Chen et al.88 Baseline models like CRISPRoff are simple and incorporate basic, interpretable properties, in contrast to ML methods, which despite advanced feature extraction, lack straightforward interpretability. Furthermore, mechanistic models like CRISPRoff are less dependent on organism-specific data, unlike ML models, which often rely on such data for training. The observation that simpler models, such as CRISPRoff, can sometimes outperform more sophisticated machine learning approaches is intriguing. We propose two explanations: (1) current training datasets may lack sufficient diversity for complex models to learn beyond what simpler rule-based algorithms already capture, and (2) the biological mechanisms driving OTA may be relatively simple and already well approximated by energy-based approaches. Without broader empirical data, it is difficult to determine which explanation is more accurate.
The recent CRISOT framework takes features of molecular dynamics into account in combination with a machine-learning framework.88 Whereas these methods mainly focus on mismatch patterns, only CRISPR-Net89 scores with bulges, using the locations and gRNA spacer:DNA interaction configurations.
Finally, many of the tools described above are available through web interfaces, including COSMID, CRISPOR, CHOP-CHOP, CRISPRon/off, PE-Designer, and PnB-Designer.72,90,91,92,93,94,95,96
Benchmarking in silico OT identification tools
Some review papers have attempted to provide a comprehensive overview of computational OT prediction, including reporting on the performance of a subset of available methods.97 However, these reviews face challenges in their benchmarks by blindly referencing performance reports from individual papers without ensuring that the methods are compared on identical datasets. Therefore, such comparisons are not informative in terms of deciding which methods to use. The overview of the different methods can, however, help users to obtain an overview of the different approaches.
When developing methods, especially ML-based methods, full evaluation of independent data is critical. Generally, a method's performance on external datasets will be limited by how closely the external data correlate with the training data (see above). This is also why benchmarking on external datasets often yields lower performance than evaluation on held-out data from the same experiment. Hence, a full evaluation of independent data is critical.98 For training, validation, and testing, this is more straightforward as all the OTs accompanying a gRNA can be used as independent held-out sets (assuming the gRNAs differ substantially). However, gRNAs vary widely in the number of associated OTs, and from a therapeutic perspective, there may be greater interest in algorithms that perform well on gRNAs with fewer OTs.
In addition to using publicly available datasets as external, independent OT references, Bao et al. generated a new dataset of validated OT sites from scratch.99 Evaluation of several computational methods revealed lower performance when tested on the novel set of validated OT sites from four gRNAs, compared to a dataset of published OT sites from 27 gRNAs that were part of the training data, demonstrating that reported model performance is likely overstated. Larger, unique datasets are required to confirm this observation, while other factors, such as batch effects of data generated in different labs, cannot be ruled out.99
In summary, computational pipelines for selecting optimal gRNAs have advanced significantly and continue to evolve with the integration of new data and models, serving as a valuable starting point for identifying candidate gRNAs. However, as evident from the evaluations of the computational methods mentioned above, in silico tools still exhibit high false positive rates and can predict thousands of OTs per gRNA, while the data typically suggest much fewer ones. This challenge is in part attributed to the limitations of the available data and in part to the OT location identification and scoring. Current experimental data used in these models do not fully account for three-dimensional genome structure or the molecular context near potential OTs, nor do they do so for on-target efficiency data. Furthermore, current computational tools for gRNA design are largely limited to well-characterized nucleases like SpCas9 and their standard PAMs, with limited applicability to novel or engineered CRISPR systems. These models typically assume a fixed gRNA scaffold and are trained on data from a narrow range of cell types (typically HEK293T or similar), reducing their generalizability. As a result, computational predictions should be considered a useful first filter, but empirical validation remains essential for selecting the most effective and specific gRNAs. Thus, a dataset with a higher number of potential false positives for subsequent experimental testing is preferable to one that might prematurely exclude true OTs. For the field to advance further with more accurate computational methods, we need even better data than those available today taking effectively as much of the cell state into account as possible.
Experimental assays for OT sites nomination
Cell-free and cell-based on-target nomination assays
Experimental assays for OT identification can be broadly classified as cell-free (in vitro) or cell-based assays (see Tables 1 and 2). Early cell-free methods made use of synthetic DNA libraries containing up to 1012 sequences covering all permutations of a target sequence with ≤8 mismatches.113 This was followed by several studies that used extracted genomic DNA as a substrate for Cas9 cleavage65,67,100,102,103 with different approaches to enrich for Cas9 cleaved products from a background of randomly sheared DNA, including biotinylated adaptor ligation,102 and circularization of DNA fragments.67 These cell-free studies allowed for a great degree of control of the reaction parameters as it is relatively straightforward to modify the enzyme (Cas9) and substrate (DNA) concentrations as well as the reaction time. Each of these parameters greatly influences how many potential OTs are identified, with increased time and Cas9 concentration resulting in more potential OTs being identified, although these additional sites generally have lower signals and fail to validate in follow-up targeted amplicon sequencing studies. The failure of these sites to validate could indicate that they are false positives or that they occur at a frequency below the limit of detection of high-throughput sequencing (∼0.1%).67 The in vitro assays are agnostic to cell-based factors or chromatin structure, which potentially allows for a broader scope of OT identification and more data for developing OT prediction models. The use of synthetic DNA libraries can also be used to identify variant-sensitive OTs facilitating the assessment of OTA in the context of global human genetic variation,104 but for clinical translation it may be more beneficial to assay therapeutic gRNAs in a relevant cell model with appropriate concentrations of the gene-editing components. For schematic overviews of key detection platforms and workflows, we refer readers to a review by Cavazza et al.,114 which provides clear illustrations of both in vitro and cell-based OTA detection strategies.
Table 1.
In vitro OT nomination assays
| Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Digenome-seq100 | in vitro Cas9 digestion, followed by next-generation sequencing (NGS) and computational analysis to detect indels | genome-wide; sensitive | requires high sequencing depth; high false positive rate; lacks the chromatin context |
| DIG-seq101 | modified version of Digenome-seq using chromatin DNA | genome-wide; sensitive; chromatin context | requires high sequencing depth |
| SITE-seq102 | in vitro Cas9 digestion; biotinylated adapters are then added to the cleaved ends; biotin selection and PCR amplify the target region for NGS | genome-wide; sensitive | requires high sequencing coverage; lacks the chromatin context |
| CIRCLE-seq67 | in vitro Cas9 digestion of circularized genomic DNAonly circular DNA containing nuclease cleavage sites is linearized and utilized for NGS library preparation | genome-wide; sensitive | requires large amounts of DNA; lacks the chromatin context |
| CHANGE-seq65 | a modified version of CIRCLE-seq that utilized enzymatic fragmentation instead of sonication | genome-wide; sensitive; lower amount of input DNA | expensive compared to CIRCLE-seq; lacks the chromatin context |
| RGEN-seq103 | in vitro Cas9 digestion followed by the ligation of the cut ends to a truncated NGS adapter that lacks the flow-cell binding site; a complementary sequencing adapter is then added in a subsequent step, allowing for the sequencing of only the cleaved DNA | genome-wide; sensitive; simplified workflow compared to other cell-free assays; amplification free | requires high sequencing depth; lacks the chromatin context |
| ONE-seq104 | in vitro Cas9 digestion in synthesized oligonucleotide libraries | enables testing thousands of sites in parallel; supports variant-aware analysis | limited to pre-defined regions; lacks the chromatin context |
Table 2.
In vivo OT nomination assays
| Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| BLESS105 | following CRISPR-Cas editing, cells are fixed, and nuclei are purified; DSB are captured using a biotinylated linker, allowing for their enrichment and subsequent sequencing | genome-wide; can be applied to in vivo-derived tissues | low sensitivity and high background; requires cell fixation; requires high cell input; centrifugation steps in the protocol shear the DNA and introduce background |
| BLISS106 | following CRISPR-Cas editing, cells are fixed onto a microscope slide; DSBs are ligated to an adapter containing T7 promoter and sequencing adapters; DNA is amplified using in vitro transcription, followed by NGS | genome-wide; higher sensitivity compared to BLESS | requires cell fixation; low sensitivity |
| IDLV integration107 | cells are edited in the presence of Integrative-Deficient Lentiviral Vectors (IDLV) vectors, which integrate the DSBs; linear-amplification mediated (LAM)-PCR is then performed using primers complementary to the IDLV sequences, followed by NGS | genome-wide; applicable to various cell types/nucleases | requires viral vector transduction; low integration bias |
| AAV integration39 | cells are edited in the presence of AAV vectors, which integrate into the DSBs; PCR amplification is then performed using primers complementary to the AAV sequences, followed by NGS | genome-wide; applicable to various cell types/nucleases | requires viral vector transduction; low integration bias |
| GUIDE-seq66 | cells are edited in the presence of a double-stranded oligonucleotide (dsODN), which integrates into the DNA breaks; PCR amplification is then performed using primers complementary to the DNA tag, followed by NGS | genome-wide; high sensitivity | some cells are sensitive to the dsODN (e.g., HSPCs); compatible only with nucleases that generate blunt ends at the DSBs, such as SpCas9 |
| OliTag-seq108 | optimized version of GUIDE-seq with a more stable tag and enhanced PCR amplification | genome-wide; high sensitivity | some cells are sensitive to the dsODN (e.g., HSPCs) |
| DISCOVER-seq109,110 | chromatin immunoprecipitation (ChIP-seq) targeting MRE11, a key protein involved in the DSB repair mechanism, followed by NGS | can be also applied in vivo; high specificity | lower resolution compared to other cell-based methods; requires cell optimization |
| DISCOVER-Seq+109 | optimized version of DISCOVER-seq using DNA-protein kinase catalytic inhibitor to achieve higher MRE11 levels | higher sensitivity compared to DISCOVER-seq | requires NHEJ inhibition, also in in vivo settings |
| INDUCE-seq111 | PCR-free, ligation-based method that labels DSBs in fixed nuclei, followed by direct sequencing to digitally quantify break sites | genome-wide; reduces amplification bias; high sensitivity and resolution | requires cell fixation; limited compatibility with certain cleavage profiles |
| SURRO-seq68 | transduction of synthesized lentiviral libraries containing custom OT sites and site-specific barcodes, followed by NGS | enables testing thousands of sites in parallel; supports variant-aware analysis | limited to predefined regions; requires viral vector transduction; background noise due to errors in the library synthesis |
| ABSOLVE-seq112 | transduction of synthesized lentiviral libraries containing custom OT sites and site-specific barcodes with unique molecular identifiers (UMI), followed by NGS | enables testing thousands of sites in parallel; supports variant-aware analysis; computational pipeline for noise filtering; contains UMI for improved quantification | limited to predefined regions; requires viral vector transduction; background noise due to errors in the library synthesis; complex computational pipeline required to handle the data |
Cell-based assays detect OTA in CRISPR-Cas-treated cells via the identification of DNA breaks,106,109,110,111 repair products such as indels or translocations,40,68,115,116 or the integration of exogenous DNA, such as virus vectors or short DNA tags, into the DNA break.39,66,107,108 The use of integrase deficient lentiviral vector capture into DNA breaks has been used to map nuclease OT sites in human cells but suffers from low sensitivity and can typically detect frequently cut OT sites with >1% indel formation.107,117 When short double-stranded DNA tags are used for DSB capture, the sensitivity of the approach is limited by the error rate of current sequencing technology (0.1%).66
Unlike methods that detect repair outcomes (indels or DNA integration), those that detect DNA breaks in cells are time sensitive as they cannot detect OTA once the DNA breaks have been repaired. This can lower their overall sensitivity; however, time-resolution studies using these approaches could provide invaluable data on Cas9 kinetics within human cells. The sensitivity of these methods that are reliant on detecting DNA breaks can be dramatically improved by inhibiting NHEJ DNA repair, which increases the availability of DNA DSB ends for detection.109
Other cell-based methods that rely on the detection of structural variants such as translocations induced by OTA are discussed further in the section “detection of adverse genomic rearrangements.”
In addition to the ex vivo experimental assays, animal models are sometimes used to evaluate editing specificity in vivo, particularly when assessing long-term effects or biodistribution.118 While these systems can provide important safety insights, it is important to acknowledge their limitations, as many human-specific DNA sequences are absent in these models, which may restrict the detection of clinically relevant OT effects. For a more comprehensive discussion of these considerations and the limitations of animal models in genome editing research, we refer readers to Soufizadeh et al.118 and Gopinath et al.119
Benchmarking experimental OT nomination assays
In studies that report cellular validation via deep sequencing of potential OTs, the false positive rates varied from 20% to 95%,99 indicating that either these experimental assays suffer from high false positive rates or that current sequencing technology is not sensitive enough to detect OTs with low editing rates.67 Improved sensitivity of deep sequencing for indel detection has been described using oversampling of input genomes, which in extreme cases comprise a single amplicon for an entire NextSeq run and improved sensitivity 100-fold.120
Many of these experimental tools have been developed to detect rare OT events and provide a worst-case scenario using DNA overexpression systems.59 How this compares to OT effects in primary cells of interest under Good Manufacturing Practice (GMP) conditions with CRISPR ribonucleoprotein complexes would also be useful for supporting the translation of CRISPR-based medicines to the clinic, and the differences in OT effects seen with different expression systems (DNA, mRNA, protein) could provide valuable information on the kinetics of OT editing.109,121
No formal benchmarking or direct comparison of the various experimental tools available using a set of standard gRNA sequences has been carried out, making it difficult for researchers to choose the most appropriate assay. These assays require specialized expertise in addition to significant investment and resources to establish in the lab, and a direct comparison using a set of validated gRNAs with known OT sites would not only allow for a fair and unbiased quantification of the true and false positive rates of each assay but also help researchers make an informed decision on which assays to use. Validation of OTA across different tools would also facilitate the creation of a database of validated OTs that could serve as a ground truth dataset to aid in the development of improved machine learning tools for OT identification. In the absence of a single gold standard assay, applying a combination of orthogonal approaches remains the most effective strategy.
Quantifying OTA
Since no single method can fully capture the OT landscape, regulatory bodies require the use of multiple orthogonal techniques from the wide array of in silico, cell-free, and cell-based approaches available (https://www.fda.gov/media/156894/download). Once a final list of potential OT sites is compiled, the presence of indels is quantified to address the level of activity of each nominated site. A state-of-the-art approach utilizes targeted amplification, such as the rhAmpSeq method,122 followed by deep sequencing, enabling the detection of even rare editing events. Ideally, this should be performed under conditions that simulate the clinical manufacturing process. The data are then analyzed in one of many available tools.42,123,124,125,126 CRISPResso2123 is widely used and offers various options, including specific analysis for different types of Cas enzymes and analysis of HDR donor integration rates. However, CRISPResso2 does not support direct comparison with untreated samples, resulting in high background control. CRISPECTOR42 uses a Bayesian classifier to analyze treated CRISPR-Cas9 samples against an untreated control sample, providing an accurate and statistical approach to assess editing levels. Furthermore, CRISPECTOR can detect translocations between the on-target and OT sites amplified.
Despite its sensitivity, the targeted PCR approach has several limitations, including susceptibility to PCR-induced bias, amplification challenges in certain genomic regions, and technical difficulties in scaling to panels exceeding 200 sites. An alternative solution is using hybridization-based technologies, such as Capture-seq,127 which leverages custom-designed probes to selectively enrich target regions of interest. Unlike PCR-based amplification methods, Capture-seq does not rely on primer binding or amplification efficiency, thereby reducing amplification bias and enabling more uniform coverage across OTs. This PCR-free approach is particularly valuable for accurate quantification of low-frequency editing events and has been applied in clinical studies such as Vertex’s exa-cel trial.128 Another approach for quantifying OT effects is utilizing RNA sequencing (RNA-seq) data. CRISPRroots129 integrates DNA:RNA binding properties, gene expression changes, and sequence variants to identify and prioritize potential OTs in Cas9 edited samples. However, its applicability is restricted to coding regions.
Personalized, variant-specific OT studies
The need for personalized genome editing
The human genome is diverse across populations and individuals, due to millions of single-nucleotide polymorphisms (SNPs), indels, and copy-number variants.130,131,132,133 Nevertheless, most computational pipelines for gRNA design, OT prediction, and OT analysis rely on a single consensus reference genome, typically GRCh37 (hg19) or GRCh38 (hg38). Genomic variants in CRISPR-Cas on-target or OT sequence can alter the binding efficiency of the nuclease and hence affect the efficiency or specificity of editing.134,135 Moreover, many of the CRISPR-Cas therapies are directed at diseases that are genetically enriched within specific populations, further underscoring the importance of considering genomic variations in therapeutic design. A recent study by Cancellieri et al. demonstrated that a Cas9/gRNA targeting the BCL11A enhancer used in Casgevy, the first approved CRISPR-based therapy, showed activity at an OT site specific to individuals of African ancestry as a result of a SNP creating a novel PAM site.136 In addition to affecting gRNA binding and OT profiles, SNPs can also impact the on-target integration efficiency of HDR-based strategies, as sequence mismatches within the homology arms have been shown to affect HDR efficacy.137 While viral HDR donors with ∼200- to 1,000-bp arms and single-stranded DNA donors with ∼30- to 100-bp arms generally perform well across individuals, in cases where high-frequency SNPs or deletions overlap the homology arms, personalized donor templates are required to ensure efficient and accurate editing.
The need for variant-specific OT assessment was acknowledged by regulators, who require researchers to address the potential impact of genetic heterogeneity on drug safety (https://www.fda.gov/media/171593/download).
Variant-aware OT prediction and measurement
In recent years, several large-scale projects have generated extensive sequencing data to provide a more accurate representation of human genome diversity. Databases such as the 1000 Genomes Project,138 gnomAD,139 and dbSNP140 offer comprehensive estimates of SNP distribution across diverse populations. Moreover, the recently developed pangenome reference sequence, which integrates assemblies from a genetically diverse cohort, enhances variant-aware OT site prediction.132 CRISPRme136 utilizes public databases for variant-aware OT prediction, accepting variant data from various databases and population-specific or individual VCF files. Individual information is particularly important when working with underrepresented populations in genomic studies or when developing therapies for founder mutations.
Variant-specific predictions often yield a large number of potential OTs, posing a challenge for confirmation through targeted sequencing approaches. A promising approach to address this challenge is the use of synthetic libraries that encompass all relevant variants, enabling high-throughput screening of multiple sites simultaneously. The in vitro ONE-seq104 assay, for example, leverages synthetic DNA fragments to estimate the CRISPR-Cas cleavage frequencies in different predicted OTs. While offering simplicity, in vitro assays lack the cellular context. In contrast, SURRO-seq68 and ABSOLVE-seq112 assays involve transducing cells with lentiviral libraries encoding various sites and measuring CRISPR-Cas cleavage frequencies in live cells. These methods have shown a strong correlation with editing rates in endogenous loci but suffer from a high signal-to-noise ratio and may be inaccurate, particularly for OT effects where editing occurs at levels below 1%.112
Following OT nomination, targeted deep sequencing in primary cells relevant to the specific therapeutic application remains the most accurate method for OT assessment, particularly when conducted under GMP-grade conditions. To account for genetic variation, sequencing data should be analyzed in the context of each sample’s unique genome sequence. CRISPResso2 supports allele-specific OT analysis for CRISPR-Cas experiments, including BE, PE, and HDR integration.123 However, it requires prior information about the sample’s genotype. The recently developed CRISPECTOR2.0141 software offers allele-specific quantification of CRISPR-Cas activity, contains a built-in allele-calling feature, and does not require prior genotype information.
The field of genome therapy is moving rapidly toward a more personalized approach, enhancing the safety and precision of treatments. The question remains whether allele-specific analysis should be population specific or individual specific. Population-specific approaches, which account for common variants within defined groups, offer a practical and cost-effective solution. They can be broadly applied, reduce resource demands, and improve accuracy over reference-only methods, although they may miss rare or private variants. In contrast, patient-specific analysis captures the full spectrum of individual variation, offering the highest precision, but at the cost of scalability, as it requires collecting a specimen from the specific patient or disease case to enable variant-aware OT prediction and editing strategy design. Such personalized analysis also poses regulatory challenges related to standardization and raises concerns about equitable access, especially in resource-limited settings. Ultimately, the choice between these approaches will depend on balancing precision with practical considerations of cost and feasibility.
Detection of adverse genomic rearrangements
CRISPR-Cas induces SVs
It is widely known that designer nucleases relying on DSBs often result in localized genomic sequence alterations, mainly indels derived from the NHEJ repair pathway. However, no matter how precise, a DSB is still a lesion that can trigger other DNA damage responses (DDRs), potentially resulting in more complex genomic resolutions, such as SVs.142,143,144,145,146,147,148,149,150 These aberrations consist of inter- and intra-chromosomal rearrangements, including inversions, large indels, and duplications.40,149,151,152 The biological consequences of these outcomes vary depending on their frequency and the extent of the affected region. For instance, large deletions can not only span several megabases but also result in chromosomal truncation or complete loss of a chromosome.38,40,142,143,152,153 Any large deletion can lead to complete gene loss if both homologous chromosomes are affected or to hemizygosity if only one is involved. The latter case presents various functional repercussions depending on gene dosage requirements, such as a neutral phenotypic effect, haploinsufficiency, loss of heterozygosity, or loss of imprinting.142
From a molecular standpoint, collateral editing at OTs is not different from that at the on-target site and can therefore induce the same type of SVs. However, simultaneous DSBs at different loci add the possibility of reciprocal translocations, not only between target and OTs but also between different OTs. In some cases, these rearrangements can generate other types of unbalanced translocations such as dicentric or acentric chromosomes (with the latter product generally being lost during subsequent mitoses) or more convoluted scenarios like chromothripsis.40,146,154 Unintentional targeting of a proto-oncogene or a tumor-suppressor gene is not the only risk potentially leading to carcinogenic development of the cell product. Even OT-driven reciprocal balanced translocations, without any loss of genetic material, could ultimately result in tumorigenesis, since, for example, a fusion of a regulatory element to a proto-oncogene has been reported to be a driver of malignant transformation.155,156,157,158,159 Until recently, SVs have often been overlooked, yet it is evident that their detection and characterization are critical for ensuring the safety and efficacy of gene-editing applications.
Detection of adverse SVs in genome-engineered cells
The identification and characterization of SVs derived from gene editing can be challenging, considering their diversity and sometimes low-frequency occurrence. Several methods allow their detection, which can be broadly categorized into biased and unbiased (or less biased, to be precise) assays (Table 3). Biased assays rely on previously known candidate sites. For instance, long-read sequencing can be used to outline the pattern of large deletions surrounding a region of interest.146,149,152,164 This approach can only be conducted within a pre-defined range, and the quantification of deletions should be carefully interpreted due to the preferential amplification of smaller over larger fragments. This problem could be overcome by using amplification-free capture methods, although this would require large amounts of input material. Conventional or digital droplet PCR (ddPCR) allows for straightforward detection of translocations involving the on-target and any suspected/predicted OTs by designing primers flanking the junctions.37,38,165,166 However, a concomitant sequence alteration preventing primer hybridization could yield a false negative result. Other techniques like fluorescence in situ hybridization (FISH), optical genome mapping (OGM), or comparative genomic hybridization (CGH) arrays offer additional probe-based detection methods for SVs, both in biased and unbiased contexts. The lower limit of detection of these methods, however, would not be enough to confidently discriminate against rare events from background signals.168,169,170,171
Table 3.
SV detection assays
| Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Long-read sequencing | |||
| targeted long-read sequencing151,152,160,161 | targeted PCR amplification followed by long-read sequencing using Oxford Nanopore Technologies (ONT) or Pacific Biosciences (PacBio) | capable of detecting large, complex, and repetitive SVs | limited to detection of known SVs; not suitable for genome-wide detection; PCR amplification bias |
| SMRT-OTS, NANO-OTS162 | amplification-free long-read sequencing for PacBio and ONT, respectively | amplification-free | higher error rate compared to short-read sequencing; high cost and longer run times; requires large amounts of DNA |
| Cas9 enrichment163 | amplification-free enrichment using CRISPR-Cas9 with gRNAs at both ends of the target region | amplification-free | limited to targeted regions; not suitable for genome-wide SV detection; requires prior knowledge of target loci |
| LongAmp-seq/long-range amplicon sequencing149,164 | optimization of long-range PCR and Illumina-based sequencing to quantify SVs | based on short-read sequencing; cost-effective | limited to predefined regions; amplification bias may miss some SVs |
| Probe-based methods | |||
| ddPCR37,38,165,166,167 | uses droplet microfluidics to partition samples into droplets, each containing a single DNA molecule labeled with fluorescent probes | absolute quantification of specific, known SVs | limited to predefined regions; amplification bias; may miss some SVs |
| FISH, FISH-IS168,169 | uses fluorescent probes that bind to specific DNA sequences to visualize SVs, typically in condensed chromosomes | direct visualization of chromosomal rearrangements; captures large genomic rearrangements | low resolution compared to sequencing; labor intensive; requires microscopy |
| OGM170,171 | OGM uses restriction enzymes to label DNA with unique fluorescent barcodes, which are then imaged and analyzed to create physical genome maps | detects a wider array of SVs and CNVs; amplification-free. | limited resolution for small SVs; lower throughput compared to sequencing-based methods; requires specialized equipment |
| array-CGH148 | genome-wide DNA copy-number analysis is performed based on the relative intensity of complementary fluorescent probes between sample and control DNA | can detect SVs that result in copy-number gain or loss | limited to detecting CNVs; unable to detect balanced SVs like inversions or translocations; low resolution; low sensitivity |
| Bait-prey assays | |||
| LAM-HTGTS, iHTGTS115,172,173 | LAM-PCR with biotinylated primer, located in proximity to a bait genomic region | genome-wide; inexpensive and scalable | detects only chromosomal rearrangements with the on-target (bait) site; requires large amounts of DNA; requires complex bioinformatics analysis |
| PEM-seq174 | primer extension with biotinylated primer, located in proximity to a bait genomic region | genome-wide; eliminates amplification noise | detects only chromosomal rearrangements with the on-target (bait) site; requires complex analysis; may miss small SVs or those in repetitive regions |
| UDiTaS116 | tagmentation with a custom Tn5 transposon, followed by an amplification using a bait primer | enzymatic fragmentation instead of sonication | limited to predefined loci; requires prior knowledge of target regions; not suitable for genome-wide analysis |
| CAST-seq40 | bait-prey strategy using decoy primers for an increased sensitivity | genome-wide; also detects on-target aberrations; higher sensitivity (0.01%) compared to other bait-prey assays | detects only chromosomal rearrangements with the on-target (bait) site; requires complex bioinformatics analysis |
When it comes to previously unidentified sites, HTGTS, UDiTaS, PEM-seq, and CAST-seq are methods capable of genome-wide detection of some species of SVs using an “anchor” or a “bait” genomic region.40,115,152,172,175 All these tools share a common principle based on using a primer or probe located near the DSB at the on-target site as bait to capture rearrangements with another locus. To improve sensitivity, CAST-seq uses decoy primers to inhibit the capture of unedited sequences, thereby increasing the detection limit of aberrant events to as low as 0.01%. In addition, the CAST-seq pipeline incorporates the assessment of on-target aberrations, providing a broader view of the genotoxicity landscape within the same protocol.152 The interrogation of on-target rearrangements is of particular importance, as these are often the most abundant and the most neglected SVs. However, studies have shown that the cell type, the genome editing platform, the locus, or the DNA repair modulators have an impact beyond OTA.37,40,148,151,152,176 For example, a recent study investigated the differential effects on specificity and SVs when comparing Cas9 nucleases to paired nCas9 in the context of promiscuous gRNAs.152 The results showed that the use of a dual nickase approach was valid to mitigate OT effects as previously reported,177 but it also altered the profile of on-target aberrations.152 This raises an important question: are large on-target aberrations inevitable or will we be able to mitigate their frequency and pattern?
While many OT detection assays were originally optimized for SpCas9, their suitability for other nucleases—particularly those with different DNA cleavage characteristics—remains uncertain. For example, blunt ends generated by Cas9 may be more prone to erroneous rejoining or chromosomal rearrangements than the staggered ends produced by enzymes like Cas12a.5 These differences in end structure not only influence the likelihood and nature of SV formation but may also affect how efficiently such events are captured by detection assays. Adapter ligation steps, in particular, may be less effective at capturing staggered ends, potentially leading to underrepresentation of SVs associated with type V nucleases. As the field expands to include a broader array of editors and platforms, there is a growing need to refine or redesign SV detection tools to account for cleavage-specific biases and ensure accurate characterization of genome integrity.
There remains an unmet need for technologies sensitive enough to trace other types of genomic aberrations, such as ultra-low-frequency chromosomal loss or those occurring in complex repetitive sequences. In most cases, SVs and small gene-disrupting edits are detrimental to cell viability, leading to the self-elimination of cells harboring such changes. However, the risk of such events causing malignant cell transformation or affecting tissue-relevant genes cannot be disregarded. We, therefore, call for genotoxicity testing for the detection of SVs to be extended beyond the current state of the art to allow a more comprehensive assessment of the potential spectrum of chromosomal aberrations. Even if we cannot yet predict how such rearrangements will affect patients, we need to have the relevant data today so that we can draw the necessary conclusions in the future if serious side effects occur.
Genomic stability assessment for genome-editing enhancers
Enhancing genome-editing precision and efficiency using small molecules
Gene-editing technologies, especially CRISPR-Cas9, have significantly advanced our ability to manipulate genomes with high precision. However, achieving efficient and precise editing remains a challenge, particularly due to OT effects and low rates of HDR, especially in post-mitotic and non-dividing cells. To address the limitations associated with HDR in these contexts, alternative strategies have been developed that harness NHEJ–mediated mechanisms, such as homology-independent targeted integration178 and obligate ligation-gated recombination.179 While these NHEJ-based methods are effective in achieving targeted insertion, they may not always result in the seamless integration of donor sequences as seen with HDR; instead, they enable efficient cassette incorporation at the desired locus, broadening the range of genomic modifications possible in HDR-refractory tissues.
In addition to these nuclease-based strategies, small molecules have emerged as powerful tools to enhance the efficiency and precision of gene editing.180 Recent advancements have improved gene editing outcomes, focusing on enhancing HDR, reducing OT effects, and ensuring genomic stability.181,182 Strategies to improve HDR can be grouped into four main categories: NHEJ inhibition, direct HDR enhancement, reduction of DNA damage sensing, and cell-cycle synchronization. These approaches are under active investigation and hold promise for future clinical applications.
Cell-cycle synchronization
Most cell-cycle regulatory compounds have shown proof-of-concept activity in immortalized cell lines, with minimal confirmation in target primary human cells such as hematopoietic stem and progenitor cells (HSPCs) or T cells.183 Consequently, very few genotoxic studies have been conducted on these compounds. Nocodazole and aphidicolin, which block cells in the M and S phases, respectively, were shown to be relatively cytotoxic to primary cells in vitro, limiting their investigative use despite a 10%–30% absolute increase in HDR in immortalized cell lines.183 Synchronization close to the G2 phase increases HDR, possibly due to chromatin accessibility, chromosome territories, and nuclear membrane structure instability.
P53 inhibition
Edited hematopoietic stem cells (HSCs) are significantly affected by culture procedures and the presence of DSBs and DNA donor templates. This is evidenced by increased p21 (G1 checkpoint regulator) expression driven by p53 activation and increased 53BP1 loci driven by the detection of open DNA ends.184 These factors trigger a signaling cascade that halts cell proliferation. Furthermore, long-term repopulating HSCs characterized by CD34+, CD45RA−, and CD90+ cells show a lower propensity for HDR outcomes. To address these bottlenecks, a medium containing SR-1, UM171, and transient expression of a p53 dominant-negative variant and the adenovirus 5 E4orf6/7 protein has been proposed. The latter upregulates the HDR apparatus in long-term HSCs, resulting in a 50% increase in editing efficiency in long-term human grafts. An additional promising molecule is GSE56, a dominant-negative mutant of the p53 protein. When co-electroporated with the CRISPR-Cas complex, GSE56 has been shown to enhance the engraftment of edited HSCs in vivo. These exceptional results were supplemented with genotoxic studies, revealing initial clinical translatability.185 However, it is imperative to note that p53 is a crucial tumor suppressor that plays a significant role in regulating the cell cycle, promoting DNA repair, and initiating apoptosis in response to DNA damage.186 Inhibition of p53 can have several consequences, particularly in the context of gene editing and HSCs.187 While there may be short-term benefits in gene editing, the long-term risks associated with genomic instability and potential transformation into cancerous cells must be considered carefully. The epigenetic modification induced by the E4orf6/7 protein, which dysregulates the activity of the cell-cycle controller E2F, could also be a similar factor of concern. However, the transient nature of these enhancers may bridge the natural cell response to DNA damage for the time needed to edit the cells in a controlled and still safe manner.
HDR direct enhancement
HDR is a repair pathway shown to be 10–30 times slower than NHEJ in primary cells and cell lines.167 To address this gap, a fusion protein bringing the Cas9 nuclease to the cleavage site with CtBP-interacting protein (CtIP) exonuclease and a dominant-negative variant of RNF168 (dnRNF168) has been proposed. This approach recruits HDR factors and inhibits NHEJ initiation.188 However, results in primary cells were not confirmed, and chromosomal aberration analysis with CAST-seq exposed an increased number of translocations at the on-target site with one OT site.188
NHEJ and alt-NHEJ inhibition
DSBs can be repaired by different pathways, depending on the targeted loci, cell-cycle state, culture, and cell type. Despite these variations, the NHEJ pathway remains the predominant repair mechanism.167 Its inhibition allows time for HDR to exert its activity and recruit the donor cassette at the edited site. Inhibition of DNA-PK, for example using NU7026 or NU7441, has been shown to suppress NHEJ while facilitating a higher frequency of HDR.178,189 STL127705, another inhibitor, targets the Ku70/80 complex.190 Inhibition of NHEJ using DNA-PK, coupled with the inhibition of the DNA polymerase theta (PolΘ), which mediated the alt-NHEJ pathway, has proven successful even in primary cells, boosting HDR up to 90% in iPSCs181 and HSPCs191 from 20% to 40% without drugs. The DNA-PK inhibitor AZD7648 outperformed M3814 in side-by-side studies, showing higher potency and less cytotoxicity. Additionally, the inhibition of 53BP1, a key player in the NHEJ pathway, has been shown to favor HDR, with up to 80% edited alleles utilizing the peptide i53. Deeper characterization showed that large deletions increased during these strategies but were normalized when utilizing the donor template,192 making NHEJ inhibition one of the most promising strategies. The inhibition of NHEJ, particularly when targeting canonical NHEJ exclusively, must be approached with caution as it can lead to larger genomic rearrangements via the alt-NHEJ and single-strand annealing pathways.5,193 If NHEJ inhibitors induce significant deletions, then PCR primers designed to detect NHEJ events may no longer anneal effectively. As demonstrated by Cullot et al.,193 such large deletions create a misleading impression of reduced NHEJ activity and an apparent increase in HDR events, when in reality, the result simply reflects the presence of large deletions at the target site.
Detecting genomic instability events induced by small molecules
The incredible efforts to improve HDR are finally yielding significant results in therapeutically relevant cells.181,182 These data are being deeply investigated for genomic instability. For example, DNA-PK inhibition with AZD7648 was shown to increase not only the frequency of large on-target deletions but also the number of induced translocations, both qualitatively and quantitatively.193 Recently, a relatively simple approach based on multiplexed digital PCR has been developed that can return a targeted qualification and quantification of editing outcomes.167 With this novel tool, it is possible to track the actual effects of NHEJ inhibition, revealing a bias in up to 90% of the alleles mainly due to the difficulty in detecting open ends with other techniques. This work could also demonstrate the “scarless” precise repair, along with insights into recurrent nuclease cutting and DNA repair rates. Furthermore, OTs were shown to have a greater chance of translocating with the on-target site, stressing the importance of accurate screening of designer editors. Despite these promising initial findings, comprehensive and unbiased characterization is still required to fully assess the benefits and potential drawbacks of these strategies and to guide the development of next-generation gene-editing tools. The use of these molecules for in vivo gene editing remains a matter of debate, even though some are already clinically approved as chemotherapeutic agents. This caution stems from the fact that many chemotherapies targeting DNA repair pathways are known to increase the risk of secondary malignancies, such as therapy-related myelodysplasia or acute myeloid leukemia. As a result, ex vivo gene therapy currently represents the most feasible therapeutic route. Nevertheless, the potential for in vivo applications cannot be ruled out, especially as advances in reducing genotoxicity or improving targeted delivery could enhance the safety and efficacy of these approaches for patients.
Functional consequences of genome-editing repair outcomes
Once an adverse CRISPR-Cas-induced mutation is identified in any of the described methods, it remains to evaluate its potential functional implications. In general, most OT mutations occur within intronic or non-coding regions and are unlikely to impact cellular function.194 Even in cases where mutations arise in exonic regions, loss-of-function variants are typically subjected to negative selection and are unlikely to propagate within the patient’s body. Of particular concern, however, are gain-of-function mutations, which, if advantageous, undergo clonal expansion, potentially contributing to tumorigenesis. When considering the mutation genotype, frameshift mutations present a heightened risk of disrupting normal gene function. The presence of large indels and genomic rearrangements is also concerning due to their potential to alter gene expression.
Linking a mutation to its biological effect remains an unresolved challenge. Specific assays can determine whether a specific function of edited cells was disrupted, for example, cytotoxicity, differentiation to subpopulation in progenitors, or infiltration to the target tissue. Computational strategies, such as deep learning algorithms can to some extent predict protein function based on a given mutation,195,196 but they are still limited, especially in non-coding regions. Most of the effort focuses on identifying the oncogenic potential of the engineered cells. State-of-the-art practices track clonal expansion in vitro to identify potential advantageous mutations and then sample modified cells over time and monitor specific indels through DNA sequencing or assessing gene expression via RNA-seq.37,197 Additionally, targeted amplification assays like real-time PCR or ddPCR can be employed to quantify specific indels or SVs over time. For a better resolution of linking mutation-phenotype, single-cell sequencing methods can be applied. Some studies incorporated single-cell RNA-seq to measure SVs over time and address the expression profile after editing.37,38 A later technology incorporates targeted single-cell DNA sequencing with immunostaining to achieve a one-to-one characterization of the mutations in an engineered sample to the observed phenotype.41
Another strategy for in vitro tumorigenicity assessment is using transformation assays, which measure the cell growth as a proxy for oncogenic potential. Soft agar culture assays are commonly used to assess tumorigenicity, wherein cells are seeded on an agar substrate and screened for proliferating clones based on colony size.198 Alternatively, the growth in low-attachment assay199,200 measures cell growth by quantifying the ATP production of cells seeded in low-attachment conditions. Both assays have been shown to identify adverse events in CRISPR-Cas-engineered cells with a detection limit of 1%–3%.201
The most widely used approach for measuring potential outcomes involves xenotransplantation of edited cells into immunodeficient mice, typically non-obese diabetic severe combined immunodeficiency- γ mice, for a period of weeks to months.51,202,203,204 This in vivo approach mimics the high cell dosage used in therapeutic products while preserving the “whole organism” context. Xenotransplantation enables the assessment of long-term engraftment, clonal dynamics, and lineage contribution of edited cells in a biologically relevant environment. Importantly, it provides a platform to monitor potential adverse effects, such as transformation or abnormal proliferation, over time, under selective pressures and physiological conditions that cannot be fully replicated in vitro. While xenotransplantation remains a powerful tool, it is limited by its extended duration and low throughput, and may lack clear physiological relevance to humans due to species-specific differences in DNA repair mechanisms, immune function, and cellular responses.205,206 Moreover, the relatively small number of engrafted cells and limited sampling sensitivity increases the likelihood of false negative results, making it difficult to detect rare but potentially deleterious mutations. These limitations underscore the need for complementary assays with higher sensitivity and resolution to ensure a more comprehensive evaluation of genome-editing outcomes.
In vivo editing presents unique challenges for functional outcome assessment, as the edited cells remain within the organism and cannot be readily monitored or recovered for direct analysis. While deep sequencing can detect specific mutations post-treatment, the limited availability of material often constrains sensitivity. Functional consequences may be assessed using the in vitro assays mentioned above; however, their predictive value for in vivo outcomes remains constrained. Surrogate approaches, such as editing primary human cells ex vivo followed by transplantation into immunodeficient mice, offer partial insights but lack full translational relevance. Although in vivo strategies can be evaluated in animal models, including mice and non-human primates, these systems do not fully recapitulate human-specific genomic or physiological contexts. Continued development of improved models and sensitive assays is therefore essential to accurately assess safety and efficacy in the in vivo setting.
Monitoring genotoxicity is also important for other factors aside from the CRISPR-Cas cleavage. Viral vector-based HDR templates, for example, are known to trigger the DDR pathway, which triggers p53 activation, and, consequently, apoptosis of modified cells.184,207 In vivo studies using recombinant AAV-mediated HDR CRISPR-Cas9 editing demonstrated a reduction in the HDR population over time.208 AAV toxicity can partly explain the pancytopenia observed in the first AAV-mediated HDR-based clinical trial aimed at correcting the HBB gene, which has since been halted.209
Overall, potential risks must be carefully evaluated, and any indications of possible genotoxicity should be flagged, thoroughly assessed, and, where possible, mitigated using the available technologies. While it may not be feasible to entirely eliminate adverse OT effects, it is crucial to monitor and report any potential events to ensure the safe implementation of gene therapy in clinical settings.
Conclusions
Gene therapy is a promising field with the potential to revolutionize the treatment of many medical conditions. As the risks associated with OTA are well recognized, ongoing advancements—including improved sequencing methods, computational algorithms, and enhanced gRNAs and Cas variants—continue to address these concerns.
Considering the over 100 active clinical trials utilizing genome-editing platforms, robust and comprehensive safety assessment frameworks are essential. Regulatory agencies recommend integrating multiple, orthogonal approaches to reduce bias and improve the reliability of OTA detection. These assessments must go beyond localized edits to include broader evaluations of genomic integrity, encompassing both on- and OT effects. However, in the absence of a universally mandated workflow, the ultimate pipeline remains at the discretion of the product developers, subject to eventual regulatory validation.
Current clinical strategies illustrate this variability. For instance, exa-cel (Casgevy)210 and trem-cel211 rely on in silico prediction combined with GUIDE-seq — their sole empirical assay — to nominate OTA sites. Although no OTA was detected at nominated sites, the impact on SVs remains unclear. In contrast, reni-cel (EDIT-301)212 and NTLA-2001213 extend their assessment by incorporating in vitro nomination assays (Digenome-seq and SITE-seq, respectively), with the latter additionally employing long-read sequencing strategies to detect SVs. These differences in genotoxicity surveys reflect how current frameworks allow for a case-by-case approach shaped by the specific therapeutic strategy and its risk-benefit profile. For example, Casgevy,210 designed to inactivate the BCL11A erythroid enhancer in autologous HSPCs for β-hemoglobinopathies, was approved despite criticism for relying on a non-representative reference genome. Its ex vivo setting, however, enables individualized post-editing OTA profiling before reinfusion into the patient, offering an additional layer of safety. Of note, OTA is often evaluated under a worst-case scenario, such as using high nuclease expression or cell-free in vitro assays. In practical settings, such as preclinical or clinical trials conducted under GMP conditions in cellular or tissue contexts, OTA is reported to be significantly lower.20,213,214 Nevertheless, caution should be taken when interpreting the absence of detectable OTA, as it may reflect limitations in assay sensitivity or the selection of inappropriate detection methods rather than an actual absence of OT effects.
With the progression of available tools and assays, safety assessments should be tailored for each CRISPR-based drug in an experimentally relevant setup, to select optimal gRNA candidates and thoroughly evaluate potential genotoxicity. Given the current limitations of each platform in detecting potential OTs, it is recommended that multiple orthogonal methods , including the in silico, cell-free, and cell-based approaches discussed in this paper, be employed to ensure a comprehensive OT assessment. Importantly, many established OT detection assays were developed for SpCas9 and may not be directly applicable to other CRISPR systems or to editors like BE and PE, which do not rely on DSBs. Therefore, adapting or developing new assays that align with the molecular mechanisms of these novel editors will be essential for accurately characterizing their specificity.
To support a more standardized and comprehensive safety evaluation strategy, we propose a five-step framework (Figure 4), encompassing (1) rational gRNA design and optimization, including computational selection, OT minimization, and pairing with engineered Cas variants and delivery strategies suited to the intended application; (2) OT site nomination and validation using in silico prediction and orthogonal experimental assays; (3) assessment of SVs and large-scale genomic rearrangements; (4) functional and long-term safety validation in relevant in vitro and in vivo models; and (5) consideration of regulatory and manufacturing factors, such as GMP compliance and traceability, which, while not the focus of this review, remain essential for clinical translation.
This roadmap is intended to guide developers in selecting the most appropriate tools, assays, and evaluation strategies at each stage of therapeutic development. However, the optimal configuration will inevitably vary depending on the biological system and delivery context. For example, editing HSCs, T cells, or hepatocytes presents distinct challenges in terms of delivery efficiency, genotoxic risk, and assay accessibility. Likewise, ex vivo approaches, such as those applied to HSPCs, offer opportunities for individualized, post-editing analysis before reinfusion, while in vivo therapies require robust preclinical models to assess biodistribution, immunogenicity, and long-term genotoxicity in situ. Therefore, safety assessments must be carefully contextualized to the specific cell type, editing modality, and therapeutic setting, while remaining aligned with rigorous scientific and regulatory standards. Although challenges remain in harmonizing specific standards, thresholds, detection limits, and protocols, the continuous innovation and collaborative efforts in the field offer a path toward achieving a high and consistent level of safety in gene therapy.
Acknowledgments
This publication is based upon work from COST Action Gene Editing for the treatment of Human Diseases, CA21113 (https://www.genehumdi.eu) supported by COST (European Cooperation in Science and Technology). J.G. was further supported by the Novo Nordisk Foundation (NNF21OC0068988) and the Independent Danish Research Foundation (9041-00317B). K.B. was further supported by Consejería de Universidad, Investigación e Innovación under Plan Andaluz de Investigación, Desarrollo e Innovación (PAIDI 2020) (ProyExcel_00875); by the Consejería de Salud y Consumo of the Junta de Andalucía (PI-0216-2024); and by Fundación Mutua Madrileña (FMM-AP16030-2024). K.B. also held a Nicolas Monardes contract from Consejería de Salud y Consumo, Junta de Andalucía (0006/2018). A.H. and T.C. were also supported by the European Union under the Horizon Europe grant (101057659 - EDITSCD). T.C. was further supported by the German Research Foundation (DFG, Project-ID 499552394 – SFB 1597). All figures in this paper were created with BioRender.com.
Author contributions
N.K.: writing – original draft, writing – review & editing, and visualization; A.S. and V.R.-D.: visualization; G.T., J.G., C.F.-G., K.B., and T.C.: writing – original draft and writing – review & editing; S.R.: writing – review & editing; C.L.: writing – original draft, writing – review & editing, and supervision; A.H.: writing – review & editing and supervision.
Declaration of interests
A.H. is the founder and chief scientific officer of CassidyBio. CassidyBio did not have input into the design, execution, interpretation, or publication of this work. T.C. and G.T. hold patents on CAST-seq. G.T. is current employees of AstraZeneca and may be AstraZeneca shareholders.
Declaration of generative AI in scientific writing
During the preparation of this work the authors used available AI tools (e.g., Copilot and ChatGPT) to enhance the quality of our writing. After using these tools, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Contributor Information
Giandomenico Turchiano, Email: g.turchiano@ucl.ac.uk.
Jan Gorodkin, Email: gorodkin@rth.dk.
Toni Cathomen, Email: toni.cathomen@uniklinik-freiburg.de.
Karim Benabdellah, Email: karim.benabdel@genyo.es.
Ciaran Lee, Email: ciaran.lee@ucc.ie.
Ayal Hendel, Email: ayal.hendel@biu.ac.il.
References
- 1.Doudna J.A., Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346 doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 2.Wang J.Y., Doudna J.A. CRISPR technology: A decade of genome editing is only the beginning. Science. 2023;379 doi: 10.1126/science.add8643. [DOI] [PubMed] [Google Scholar]
- 3.Porter S.N., Levine R.M., Pruett-Miller S.M. A Practical Guide to Genome Editing Using Targeted Nuclease Technologies. Compr. Physiol. 2019;9:665–714. doi: 10.1002/cphy.c180022. [DOI] [PubMed] [Google Scholar]
- 4.Chauhan V.P., Sharp P.A., Langer R. Altered DNA repair pathway engagement by engineered CRISPR-Cas9 nucleases. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2300605120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue C., Greene E.C. DNA Repair Pathway Choices in CRISPR-Cas9-Mediated Genome Editing. Trends Genet. 2021;37:639–656. doi: 10.1016/j.tig.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H., Ren S., Yu S., Pan H., Li T., Ge S., Zhang J., Xia N. Methods Favoring Homology-Directed Repair Choice in Response to CRISPR/Cas9 Induced-Double Strand Breaks. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21186461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickar-Oliver A., Gersbach C.A. The next generation of CRISPR-Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019;20:490–507. doi: 10.1038/s41580-019-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Men K., Duan X., He Z., Yang Y., Yao S., Wei Y. CRISPR/Cas9-mediated correction of human genetic disease. Sci. China Life Sci. 2017;60:447–457. doi: 10.1007/s11427-017-9032-4. [DOI] [PubMed] [Google Scholar]
- 9.Lieber M.R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seol J.H., Shim E.Y., Lee S.E. Microhomology-mediated end joining: Good, bad and ugly. Mutat. Res. 2018;809:81–87. doi: 10.1016/j.mrfmmm.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha S., Villarreal D., Shim E.Y., Lee S.E. Risky business: Microhomology-mediated end joining. Mutat. Res. 2016;788:17–24. doi: 10.1016/j.mrfmmm.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang H.H.Y., Pannunzio N.R., Adachi N., Lieber M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017;18:495–506. doi: 10.1038/nrm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain S.S., Majumdar R., Moore G.M., Narang H., Buechelmaier E.S., Bazil M.J., Ravindran P.T., Leeman J.E., Li Y., Jalan M., et al. Measuring nonhomologous end-joining, homologous recombination and alternative end-joining simultaneously at an endogenous locus in any transfectable human cell. Nucleic Acids Res. 2021;49 doi: 10.1093/nar/gkab262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun W., Liu H., Yin W., Qiao J., Zhao X., Liu Y. Strategies for Enhancing the Homology-Directed Repair Efficiency of CRISPR-Cas Systems. CRISPR J. 2022;5:7–18. doi: 10.1089/crispr.2021.0039. [DOI] [PubMed] [Google Scholar]
- 15.Rezazade Bazaz M., Dehghani H. From DNA break repair pathways to CRISPR/Cas-mediated gene knock-in methods. Life Sci. 2022;295 doi: 10.1016/j.lfs.2022.120409. [DOI] [PubMed] [Google Scholar]
- 16.Allen D., Kalter N., Rosenberg M., Hendel A. Homology-Directed-Repair-Based Genome Editing in HSPCs for the Treatment of Inborn Errors of Immunity and Blood Disorders. Pharmaceutics. 2023;15 doi: 10.3390/pharmaceutics15051329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dever D.P., Bak R.O., Reinisch A., Camarena J., Washington G., Nicolas C.E., Pavel-Dinu M., Saxena N., Wilkens A.B., Mantri S., et al. CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–389. doi: 10.1038/nature20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iancu O., Allen D., Knop O., Zehavi Y., Breier D., Arbiv A., Lev A., Lee Y.N., Beider K., Nagler A., et al. Multiplex HDR for disease and correction modeling of SCID by CRISPR genome editing in human HSPCs. Mol. Ther. Nucleic Acids. 2023;31:105–121. doi: 10.1016/j.omtn.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyu C., Shen J., Wang R., Gu H., Zhang J., Xue F., Liu X., Liu W., Fu R., Zhang L., et al. Targeted genome engineering in human induced pluripotent stem cells from patients with hemophilia B using the CRISPR-Cas9 system. Stem Cell Res. Ther. 2018;9:92. doi: 10.1186/s13287-018-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma A., Boelens J.J., Cancio M., Hankins J.S., Bhad P., Azizy M., Lewandowski A., Zhao X., Chitnis S., Peddinti R., et al. CRISPR-Cas9 Editing of the HBG1 and HBG2 Promoters to Treat Sickle Cell Disease. N. Engl. J. Med. 2023;389:820–832. doi: 10.1056/NEJMoa2215643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen D., Knop O., Itkowitz B., Kalter N., Rosenberg M., Iancu O., Beider K., Lee Y.N., Nagler A., Somech R., Hendel A. CRISPR-Cas9 engineering of the RAG2 locus via complete coding sequence replacement for therapeutic applications. Nat. Commun. 2023;14:6771. doi: 10.1038/s41467-023-42036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bengtsson N.E., Hall J.K., Odom G.L., Phelps M.P., Andrus C.R., Hawkins R.D., Hauschka S.D., Chamberlain J.R., Chamberlain J.S. Corrigendum: Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates pathophysiology in a mouse model for Duchenne muscular dystrophy. Nat. Commun. 2017;8 doi: 10.1038/ncomms16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z., Shi M., Ren Y., Xu H., Weng S., Ning W., Ge X., Liu L., Guo C., Duo M., et al. Recent advances and applications of CRISPR-Cas9 in cancer immunotherapy. Mol. Cancer. 2023;22:35. doi: 10.1186/s12943-023-01738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn N.F., Purdon T.J., van Leeuwen D.G., Lopez A.V., Curran K.J., Daniyan A.F., Brentjens R.J. CD40 Ligand-Modified Chimeric Antigen Receptor T Cells Enhance Antitumor Function by Eliciting an Endogenous Antitumor Response. Cancer Cell. 2019;35:473–488.e6. doi: 10.1016/j.ccell.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edri A., Ben-Haim N., Hailu A., Brycman N., Berhani-Zipori O., Rifman J., Cohen S., Yackoubov D., Rosenberg M., Simantov R., et al. Nicotinamide-Expanded Allogeneic Natural Killer Cells with CD38 Deletion, Expressing an Enhanced CD38 Chimeric Antigen Receptor, Target Multiple Myeloma Cells. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms242417231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legut M., Dolton G., Mian A.A., Ottmann O.G., Sewell A.K. CRISPR-mediated TCR replacement generates superior anticancer transgenic T cells. Blood. 2018;131:311–322. doi: 10.1182/blood-2017-05-787598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ottaviano G., Georgiadis C., Gkazi S.A., Syed F., Zhan H., Etuk A., Preece R., Chu J., Kubat A., Adams S., et al. Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for treatment of children with refractory B cell leukemia. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abq3010. [DOI] [PubMed] [Google Scholar]
- 28.Komor A.C., Kim Y.B., Packer M.S., Zuris J.A., Liu D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–424. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anzalone A.V., Randolph P.B., Davis J.R., Sousa A.A., Koblan L.W., Levy J.M., Chen P.J., Wilson C., Newby G.A., Raguram A., Liu D.R. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantor A., McClements M.E., MacLaren R.E. CRISPR-Cas9 DNA Base-Editing and Prime-Editing. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21176240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Z., Shang P., Mohanraju P., Geijsen N. Prime editing: advances and therapeutic applications. Trends Biotechnol. 2023;41:1000–1012. doi: 10.1016/j.tibtech.2023.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Lu C., Kuang J., Shao T., Xie S., Li M., Zhu L., Zhu L. Prime Editing: An All-Rounder for Genome Editing. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23179862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X., Du J., Yun S., Xue C., Yao Y., Rao S. Recent advances in CRISPR-Cas9-based genome insertion technologies. Mol. Ther. Nucleic Acids. 2024;35 doi: 10.1016/j.omtn.2024.102138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Y., Cradick T.J., Brown M.T., Deshmukh H., Ranjan P., Sarode N., Wile B.M., Vertino P.M., Stewart F.J., Bao G. CRISPR/Cas9 systems have off-target activity with insertions or deletions between target DNA and guide RNA sequences. Nucleic Acids Res. 2014;42:7473–7485. doi: 10.1093/nar/gku402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wienert B., Cromer M.K. CRISPR nuclease off-target activity and mitigation strategies. Front Genom. 2022;4 doi: 10.3389/fgeed.2022.1050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu Y., Foden J.A., Khayter C., Maeder M.L., Reyon D., Joung J.K., Sander J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuchida C.A., Brandes N., Bueno R., Trinidad M., Mazumder T., Yu B., Hwang B., Chang C., Liu J., Sun Y., et al. Mitigation of chromosome loss in clinical CRISPR-Cas9-engineered T cells. Cell. 2023;186:4567–4582.e20. doi: 10.1016/j.cell.2023.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nahmad A.D., Reuveni E., Goldschmidt E., Tenne T., Liberman M., Horovitz-Fried M., Khosravi R., Kobo H., Reinstein E., Madi A., et al. Frequent aneuploidy in primary human T cells after CRISPR-Cas9 cleavage. Nat. Biotechnol. 2022;40:1807–1813. doi: 10.1038/s41587-022-01377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanlon K.S., Kleinstiver B.P., Garcia S.P., Zaborowski M.P., Volak A., Spirig S.E., Muller A., Sousa A.A., Tsai S.Q., Bengtsson N.E., et al. High levels of AAV vector integration into CRISPR-induced DNA breaks. Nat. Commun. 2019;10:4439. doi: 10.1038/s41467-019-12449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turchiano G., Andrieux G., Klermund J., Blattner G., Pennucci V., El Gaz M., Monaco G., Poddar S., Mussolino C., Cornu T.I., et al. Quantitative evaluation of chromosomal rearrangements in gene-edited human stem cells by CAST-Seq. Cell Stem Cell. 2021;28:1136–1147.e5. doi: 10.1016/j.stem.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Moshref M., Lo J.H.H., McKay A., Camperi J., Schroer J., Ueno N., Wang S., Gulati S., Tarighat S., Durinck S., et al. Assessing a single-cell multi-omic analytic platform to characterize ex vivo-engineered T-cell therapy products. Front. Bioeng. Biotechnol. 2024;12 doi: 10.3389/fbioe.2024.1417070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amit I., Iancu O., Levy-Jurgenson A., Kurgan G., McNeill M.S., Rettig G.R., Allen D., Breier D., Ben Haim N., Wang Y., et al. CRISPECTOR provides accurate estimation of genome editing translocation and off-target activity from comparative NGS data. Nat. Commun. 2021;12:3042. doi: 10.1038/s41467-021-22417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breton C., Clark P.M., Wang L., Greig J.A., Wilson J.M. ITR-Seq, a next-generation sequencing assay, identifies genome-wide DNA editing sites in vivo following adeno-associated viral vector-mediated genome editing. BMC Genom. 2020;21:239. doi: 10.1186/s12864-020-6655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suchy F.P., Karigane D., Nakauchi Y., Higuchi M., Zhang J., Pekrun K., Hsu I., Fan A.C., Nishimura T., Charlesworth C.T., et al. Genome engineering with Cas9 and AAV repair templates generates frequent concatemeric insertions of viral vectors. Nat. Biotechnol. 2025;43:204–213. doi: 10.1038/s41587-024-02171-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bi C., Yuan B., Zhang Y., Wang M., Tian Y., Li M. Prevalent integration of genomic repetitive and regulatory elements and donor sequences at CRISPR-Cas9-induced breaks. Commun. Biol. 2025;8:94. doi: 10.1038/s42003-025-07539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walton R.T., Christie K.A., Whittaker M.N., Kleinstiver B.P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science. 2020;368:290–296. doi: 10.1126/science.aba8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L., Koseki S.R.T., Silverstein R.A., Amrani N., Peng C., Kramme C., Savic N., Pacesa M., Rodríguez T.C., Stan T., et al. PAM-flexible genome editing with an engineered chimeric Cas9. Nat. Commun. 2023;14:6175. doi: 10.1038/s41467-023-41829-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hendel A., Bak R.O., Clark J.T., Kennedy A.B., Ryan D.E., Roy S., Steinfeld I., Lunstad B.D., Kaiser R.J., Wilkens A.B., et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 2015;33:985–989. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen D., Rosenberg M., Hendel A. Using Synthetically Engineered Guide RNAs to Enhance CRISPR Genome Editing Systems in Mammalian Cells. Front Genome. 2020;2 doi: 10.3389/fgeed.2020.617910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryan D.E., Taussig D., Steinfeld I., Phadnis S.M., Lunstad B.D., Singh M., Vuong X., Okochi K.D., McCaffrey R., Olesiak M., et al. Improving CRISPR-Cas specificity with chemical modifications in single-guide RNAs. Nucleic Acids Res. 2018;46:792–803. doi: 10.1093/nar/gkx1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X., Scott D.A., Kriz A.J., Chiu A.C., Hsu P.D., Dadon D.B., Cheng A.W., Trevino A.E., Konermann S., Chen S., et al. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat. Biotechnol. 2014;32:670–676. doi: 10.1038/nbt.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen K.T., Fløe L., Petersen T.S., Huang J., Xu F., Bolund L., Luo Y., Lin L. Chromatin accessibility and guide sequence secondary structure affect CRISPR-Cas9 gene editing efficiency. FEBS Lett. 2017;591:1892–1901. doi: 10.1002/1873-3468.12707. [DOI] [PubMed] [Google Scholar]
- 53.Kuscu C., Arslan S., Singh R., Thorpe J., Adli M. Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat. Biotechnol. 2014;32:677–683. doi: 10.1038/nbt.2916. [DOI] [PubMed] [Google Scholar]
- 54.Vakulskas C.A., Dever D.P., Rettig G.R., Turk R., Jacobi A.M., Collingwood M.A., Bode N.M., McNeill M.S., Yan S., Camarena J., et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat. Med. 2018;24:1216–1224. doi: 10.1038/s41591-018-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park S.H., Lee C.M., Dever D.P., Davis T.H., Camarena J., Srifa W., Zhang Y., Paikari A., Chang A.K., Porteus M.H., et al. Highly efficient editing of the beta-globin gene in patient-derived hematopoietic stem and progenitor cells to treat sickle cell disease. Nucleic Acids Res. 2019;47:7955–7972. doi: 10.1093/nar/gkz475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qu Y., Huang K., Cousins H., Johnson W.A., Yin D., Shah M., Zhou D., Altman R., Wang M., Cong L. CRISPR-GPT: An LLM Agent for Automated Design of Gene-Editing Experiments. bioRxiv. 2024 doi: 10.1101/2024.04.25.591003. Preprint at. [DOI] [Google Scholar]
- 57.Cavazza A., Molina-Estévez F.J., Reyes Á.P., Ronco V., Naseem A., Malenšek Š., Pečan P., Santini A., Heredia P., Aguilar-González A., et al. Advanced delivery systems for gene editing: A comprehensive review from the GenE-HumDi COST Action Working Group. Mol. Ther. Nucleic Acids. 2025;36 doi: 10.1016/j.omtn.2025.102457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naeem M., Majeed S., Hoque M.Z., Ahmad I. Latest Developed Strategies to Minimize the Off-Target Effects in CRISPR-Cas-Mediated Genome Editing. Cells. 2020;9 doi: 10.3390/cells9071608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shapiro J., Iancu O., Jacobi A.M., McNeill M.S., Turk R., Rettig G.R., Amit I., Tovin-Recht A., Yakhini Z., Behlke M.A., Hendel A. Increasing CRISPR Efficiency and Measuring Its Specificity in HSPCs Using a Clinically Relevant System. Mol. Ther. Methods Clin. Dev. 2020;17:1097–1107. doi: 10.1016/j.omtm.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slesarenko Y.S., Lavrov A.V., Smirnikhina S.A. Off-target effects of base editors: what we know and how we can reduce it. Curr. Genet. 2022;68:39–48. doi: 10.1007/s00294-021-01211-1. [DOI] [PubMed] [Google Scholar]
- 61.Rao X., Zhao H., Shao C., Yi C. Characterizing off-target effects of genome editors. Curr. Opin. Biomed. Eng. 2023;28 doi: 10.1016/j.cobme.2023.100480. [DOI] [Google Scholar]
- 62.Arnan C., Ullrich S., Pulido-Quetglas C., Nurtdinov R., Esteban A., Blanco-Fernandez J., Aparicio-Prat E., Johnson R., Pérez-Lluch S., Guigó R. Paired guide RNA CRISPR-Cas9 screening for protein-coding genes and lncRNAs involved in transdifferentiation of human B-cells to macrophages. BMC Genom. 2022;23:402. doi: 10.1186/s12864-022-08612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eslami-Mossallam B., Klein M., Smagt C.V.D., Sanden K.V.D., Jones S.K., Jr., Hawkins J.A., Finkelstein I.J., Depken M. A kinetic model predicts SpCas9 activity, improves off-target classification, and reveals the physical basis of targeting fidelity. Nat. Commun. 2022;13:1367. doi: 10.1038/s41467-022-28994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doench J.G., Fusi N., Sullender M., Hegde M., Vaimberg E.W., Donovan K.F., Smith I., Tothova Z., Wilen C., Orchard R., et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 2016;34:184–191. doi: 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lazzarotto C.R., Malinin N.L., Li Y., Zhang R., Yang Y., Lee G., Cowley E., He Y., Lan X., Jividen K., et al. CHANGE-seq reveals genetic and epigenetic effects on CRISPR-Cas9 genome-wide activity. Nat. Biotechnol. 2020;38:1317–1327. doi: 10.1038/s41587-020-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsai S.Q., Zheng Z., Nguyen N.T., Liebers M., Topkar V.V., Thapar V., Wyvekens N., Khayter C., Iafrate A.J., Le L.P., et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 2015;33:187–197. doi: 10.1038/nbt.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai S.Q., Nguyen N.T., Malagon-Lopez J., Topkar V.V., Aryee M.J., Joung J.K. CIRCLE-seq: a highly sensitive in vitro screen for genome-wide CRISPR-Cas9 nuclease off-targets. Nat. Methods. 2017;14:607–614. doi: 10.1038/nmeth.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pan X., Qu K., Yuan H., Xiang X., Anthon C., Pashkova L., Liang X., Han P., Corsi G.I., Xu F., et al. Massively targeted evaluation of therapeutic CRISPR off-targets in cells. Nat. Commun. 2022;13:4049. doi: 10.1038/s41467-022-31543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiang X., Corsi G.I., Anthon C., Qu K., Pan X., Liang X., Han P., Dong Z., Liu L., Zhong J., et al. Enhancing CRISPR-Cas9 gRNA efficiency prediction by data integration and deep learning. Nat. Commun. 2021;12:3238. doi: 10.1038/s41467-021-23576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang D., Zhang C., Wang B., Li B., Wang Q., Liu D., Wang H., Zhou Y., Shi L., Lan F., Wang Y. Optimized CRISPR guide RNA design for two high-fidelity Cas9 variants by deep learning. Nat. Commun. 2019;10:4284. doi: 10.1038/s41467-019-12281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim H.K., Kim Y., Lee S., Min S., Bae J.Y., Choi J.W., Park J., Jung D., Yoon S., Kim H.H. SpCas9 activity prediction by DeepSpCas9, a deep learning-based model with high generalization performance. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aax9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cradick T.J., Qiu P., Lee C.M., Fine E.J., Bao G. COSMID: A Web-based Tool for Identifying and Validating CRISPR/Cas Off-target Sites. Mol. Ther. Nucleic Acids. 2014;3 doi: 10.1038/mtna.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bae S., Park J., Kim J.S. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics. 2014;30:1473–1475. doi: 10.1093/bioinformatics/btu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu P.D., Scott D.A., Weinstein J.A., Ran F.A., Konermann S., Agarwala V., Li Y., Fine E.J., Wu X., Shalem O., et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rock J.M., Hopkins F.F., Chavez A., Diallo M., Chase M.R., Gerrick E.R., Pritchard J.R., Church G.M., Rubin E.J., Sassetti C.M., et al. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat. Microbiol. 2017;2 doi: 10.1038/nmicrobiol.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corsi G.I., Qu K., Alkan F., Pan X., Luo Y., Gorodkin J. CRISPR/Cas9 gRNA activity depends on free energy changes and on the target PAM context. Nat. Commun. 2022;13:3006. doi: 10.1038/s41467-022-30515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Geen H., Henry I.M., Bhakta M.S., Meckler J.F., Segal D.J. A genome-wide analysis of Cas9 binding specificity using ChIP-seq and targeted sequence capture. Nucleic Acids Res. 2015;43:3389–3404. doi: 10.1093/nar/gkv137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh R., Kuscu C., Quinlan A., Qi Y., Adli M. Cas9-chromatin binding information enables more accurate CRISPR off-target prediction. Nucleic Acids Res. 2015;43 doi: 10.1093/nar/gkv575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stemmer M., Thumberger T., Del Sol Keyer M., Wittbrodt J., Mateo J.L. CCTop: An Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chuai G., Ma H., Yan J., Chen M., Hong N., Xue D., Zhou C., Zhu C., Chen K., Duan B., et al. DeepCRISPR: optimized CRISPR guide RNA design by deep learning. Genome Biol. 2018;19:80. doi: 10.1186/s13059-018-1459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin J., Wong K.C. Off-target predictions in CRISPR-Cas9 gene editing using deep learning. Bioinformatics. 2018;34:i656–i663. doi: 10.1093/bioinformatics/bty554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng H., Zheng Y., Zhao Z., Liu T., Li J. Recognition of CRISPR/Cas9 off-target sites through ensemble learning of uneven mismatch distributions. Bioinformatics. 2018;34:i757–i765. doi: 10.1093/bioinformatics/bty558. [DOI] [PubMed] [Google Scholar]
- 84.Abadi S., Yan W.X., Amar D., Mayrose I. A machine learning approach for predicting CRISPR-Cas9 cleavage efficiencies and patterns underlying its mechanism of action. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Listgarten J., Weinstein M., Kleinstiver B.P., Sousa A.A., Joung J.K., Crawford J., Gao K., Hoang L., Elibol M., Doench J.G., Fusi N. Prediction of off-target activities for the end-to-end design of CRISPR guide RNAs. Nat. Biomed. Eng. 2018;2:38–47. doi: 10.1038/s41551-017-0178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haeussler M., Schönig K., Eckert H., Eschstruth A., Mianné J., Renaud J.B., Schneider-Maunoury S., Shkumatava A., Teboul L., Kent J., et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016;17:148. doi: 10.1186/s13059-016-1012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alkan F., Wenzel A., Anthon C., Havgaard J.H., Gorodkin J. CRISPR-Cas9 off-targeting assessment with nucleic acid duplex energy parameters. Genome Biol. 2018;19:177. doi: 10.1186/s13059-018-1534-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Q., Chuai G., Zhang H., Tang J., Duan L., Guan H., Li W., Li W., Wen J., Zuo E., et al. Genome-wide CRISPR off-target prediction and optimization using RNA-DNA interaction fingerprints. Nat. Commun. 2023;14:7521. doi: 10.1038/s41467-023-42695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin J., Zhang Z., Zhang S., Chen J., Wong K.-C. CRISPR-Net: A Recurrent Convolutional Network Quantifies CRISPR Off-Target Activities with Mismatches and Indels. Adv. Sci. 2020;7 doi: 10.1002/advs.201903562. [DOI] [Google Scholar]
- 90.Concordet J.P., Haeussler M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018;46:W242–W245. doi: 10.1093/nar/gky354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Labun K., Montague T.G., Krause M., Torres Cleuren Y.N., Tjeldnes H., Valen E. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 2019;47:W171–W174. doi: 10.1093/nar/gkz365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anthon C., Corsi G.I., Gorodkin J. CRISPRon/off: CRISPR/Cas9 on- and off-target gRNA design. Bioinformatics. 2022;38:5437–5439. doi: 10.1093/bioinformatics/btac697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hwang G.H., Jeong Y.K., Habib O., Hong S.A., Lim K., Kim J.S., Bae S. PE-Designer and PE-Analyzer: web-based design and analysis tools for CRISPR prime editing. Nucleic Acids Res. 2021;49:W499–W504. doi: 10.1093/nar/gkab319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siegner S.M., Karasu M.E., Schröder M.S., Kontarakis Z., Corn J.E. PnB Designer: a web application to design prime and base editor guide RNAs for animals and plants. BMC Bioinf. 2021;22:101. doi: 10.1186/s12859-021-04034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cui Y., Xu J., Cheng M., Liao X., Peng S. Review of CRISPR/Cas9 sgRNA Design Tools. Interdiscip. Sci. 2018;10:455–465. doi: 10.1007/s12539-018-0298-z. [DOI] [PubMed] [Google Scholar]
- 96.Hwang G.H., Park J., Lim K., Kim S., Yu J., Yu E., Kim S.T., Eils R., Kim J.S., Bae S. Web-based design and analysis tools for CRISPR base editing. BMC Bioinf. 2018;19:542. doi: 10.1186/s12859-018-2585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu G., Zhang Y., Zhang T. Computational approaches for effective CRISPR guide RNA design and evaluation. Comput. Struct. Biotechnol. J. 2020;18:35–44. doi: 10.1016/j.csbj.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corsi G.I., Anthon C., Gorodkin J. Letter to the editor: Testing on external independent datasets is necessary to corroborate machine learning model improvement. Bioinformatics. 2023;39 doi: 10.1093/bioinformatics/btad327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bao X.R., Pan Y., Lee C.M., Davis T.H., Bao G. Tools for experimental and computational analyses of off-target editing by programmable nucleases. Nat. Protoc. 2021;16:10–26. doi: 10.1038/s41596-020-00431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim D., Bae S., Park J., Kim E., Kim S., Yu H.R., Hwang J., Kim J.I., Kim J.S. Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat. Methods. 2015;12:237–243. doi: 10.1038/nmeth.3284. [DOI] [PubMed] [Google Scholar]
- 101.Kim D., Kim J.S. DIG-seq: a genome-wide CRISPR off-target profiling method using chromatin DNA. Genome Res. 2018;28:1894–1900. doi: 10.1101/gr.236620.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cameron P., Fuller C.K., Donohoue P.D., Jones B.N., Thompson M.S., Carter M.M., Gradia S., Vidal B., Garner E., Slorach E.M., et al. Mapping the genomic landscape of CRISPR-Cas9 cleavage. Nat. Methods. 2017;14:600–606. doi: 10.1038/nmeth.4284. [DOI] [PubMed] [Google Scholar]
- 103.Kuzin A., Redler B., Onuska J., Slesarev A. RGEN-seq for highly sensitive amplification-free screen of off-target sites of gene editors. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-03160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Petri K., Kim D.Y., Sasaki K.E., Canver M.C., Wang X., Shah H., Lee H., Horng J.E., Clement K., Iyer S., et al. Global-scale CRISPR gene editor specificity profiling by ONE-seq identifies population-specific, variant off-target effects. bioRxiv. 2021 doi: 10.1101/2021.04.05.438458. Preprint at. [DOI] [Google Scholar]
- 105.Crosetto N., Mitra A., Silva M.J., Bienko M., Dojer N., Wang Q., Karaca E., Chiarle R., Skrzypczak M., Ginalski K., et al. Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat. Methods. 2013;10:361–365. doi: 10.1038/nmeth.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yan W.X., Mirzazadeh R., Garnerone S., Scott D., Schneider M.W., Kallas T., Custodio J., Wernersson E., Li Y., Gao L., et al. BLISS is a versatile and quantitative method for genome-wide profiling of DNA double-strand breaks. Nat. Commun. 2017;8 doi: 10.1038/ncomms15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang X., Wang Y., Wu X., Wang J., Wang Y., Qiu Z., Chang T., Huang H., Lin R.J., Yee J.K. Unbiased detection of off-target cleavage by CRISPR-Cas9 and TALENs using integrase-defective lentiviral vectors. Nat. Biotechnol. 2015;33:175–178. doi: 10.1038/nbt.3127. [DOI] [PubMed] [Google Scholar]
- 108.Yang Z.X., Deng D.H., Gao Z.Y., Zhang Z.K., Fu Y.W., Wen W., Zhang F., Li X., Li H.Y., Zhang J.P., Zhang X.B. OliTag-seq enhances in cellulo detection of CRISPR-Cas9 off-targets. Commun. Biol. 2024;7:696. doi: 10.1038/s42003-024-06360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zou R.S., Liu Y., Gaido O.E.R., Konig M.F., Mog B.J., Shen L.L., Aviles-Vazquez F., Marin-Gonzalez A., Ha T. Improving the sensitivity of in vivo CRISPR off-target detection with DISCOVER-Seq. Nat. Methods. 2023;20:706–713. doi: 10.1038/s41592-023-01840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wienert B., Wyman S.K., Richardson C.D., Yeh C.D., Akcakaya P., Porritt M.J., Morlock M., Vu J.T., Kazane K.R., Watry H.L., et al. Unbiased detection of CRISPR off-targets in vivo using DISCOVER-Seq. Science. 2019;364:286–289. doi: 10.1126/science.aav9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dobbs F.M., van Eijk P., Fellows M.D., Loiacono L., Nitsch R., Reed S.H. Precision digital mapping of endogenous and induced genomic DNA breaks by INDUCE-seq. Nat. Commun. 2022;13:3989. doi: 10.1038/s41467-022-31702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin J., Nguyen M.A., Lin L.Y., Zeng J., Verma A., Neri N.R., da Silva L.F., Mucci A., Wolfe S., Shaw K.L., et al. Scalable assessment of genome editing off-targets associated with genetic variants. bioRxiv. 2024 doi: 10.1101/2024.07.24.605019. Preprint at. [DOI] [Google Scholar]
- 113.Pattanayak V., Lin S., Guilinger J.P., Ma E., Doudna J.A., Liu D.R. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat. Biotechnol. 2013;31:839–843. doi: 10.1038/nbt.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cavazza A., Hendel A., Bak R.O., Rio P., Güell M., Lainšček D., Arechavala-Gomeza V., Peng L., Hapil F.Z., Harvey J., et al. Progress and harmonization of gene editing to treat human diseases: Proceeding of COST Action CA21113 GenE-HumDi. Mol. Ther. Nucleic Acids. 2023;34 doi: 10.1016/j.omtn.2023.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frock R.L., Hu J., Meyers R.M., Ho Y.J., Kii E., Alt F.W. Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. Nat. Biotechnol. 2015;33:179–186. doi: 10.1038/nbt.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giannoukos G., Ciulla D.M., Marco E., Abdulkerim H.S., Barrera L.A., Bothmer A., Dhanapal V., Gloskowski S.W., Jayaram H., Maeder M.L., et al. UDiTaS, a genome editing detection method for indels and genome rearrangements. BMC Genom. 2018;19:212. doi: 10.1186/s12864-018-4561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sander J.D., Ramirez C.L., Linder S.J., Pattanayak V., Shoresh N., Ku M., Foden J.A., Reyon D., Bernstein B.E., Liu D.R., Joung J.K. In silico abstraction of zinc finger nuclease cleavage profiles reveals an expanded landscape of off-target sites. Nucleic Acids Res. 2013;41 doi: 10.1093/nar/gkt716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Soufizadeh P., Mansouri V., Ahmadbeigi N. A review of animal models utilized in preclinical studies of approved gene therapy products: trends and insights. Lab. Anim. Res. 2024;40:17. doi: 10.1186/s42826-024-00195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gopinath C., Nathar T.J., Ghosh A., Hickstein D.D., Nelson E.J.R. Contemporary Animal Models For Human Gene Therapy Applications. Curr. Gene Ther. 2015;15:531–540. doi: 10.2174/1566523215666150929110424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller J.C., Patil D.P., Xia D.F., Paine C.B., Fauser F., Richards H.W., Shivak D.A., Bendaña Y.R., Hinkley S.J., Scarlott N.A., et al. Enhancing gene editing specificity by attenuating DNA cleavage kinetics. Nat. Biotechnol. 2019;37:945–952. doi: 10.1038/s41587-019-0186-z. [DOI] [PubMed] [Google Scholar]
- 121.Brinkman E.K., Chen T., de Haas M., Holland H.A., Akhtar W., van Steensel B. Kinetics and Fidelity of the Repair of Cas9-Induced Double-Strand DNA Breaks. Mol. Cell. 2018;70:801–813.e6. doi: 10.1016/j.molcel.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dobosy J.R., Rose S.D., Beltz K.R., Rupp S.M., Powers K.M., Behlke M.A., Walder J.A. RNase H-dependent PCR (rhPCR): improved specificity and single nucleotide polymorphism detection using blocked cleavable primers. BMC Biotechnol. 2011;11:80. doi: 10.1186/1472-6750-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clement K., Rees H., Canver M.C., Gehrke J.M., Farouni R., Hsu J.Y., Cole M.A., Liu D.R., Joung J.K., Bauer D.E., Pinello L. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 2019;37:224–226. doi: 10.1038/s41587-019-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Labun K., Guo X., Chavez A., Church G., Gagnon J.A., Valen E. Accurate analysis of genuine CRISPR editing events with ampliCan. Genome Res. 2019;29:843–847. doi: 10.1101/gr.244293.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanvicente-Garcia M., Garcia-Valiente A., Jouide S., Jaraba-Wallace J., Bautista E., Escobosa M., Sanchez-Mejias A., Guell M. CRISPR-Analytics (CRISPR-A): A platform for precise analytics and simulations for gene editing. PLoS Comput. Biol. 2023;19 doi: 10.1371/journal.pcbi.1011137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boel A., Steyaert W., De Rocker N., Menten B., Callewaert B., De Paepe A., Coucke P., Willaert A. BATCH-GE: Batch analysis of Next-Generation Sequencing data for genome editing assessment. Sci. Rep. 2016;6 doi: 10.1038/srep30330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chaudhari H.G., Penterman J., Whitton H.J., Spencer S.J., Flanagan N., Lei Zhang M.C., Huang E., Khedkar A.S., Toomey J.M., Shearer C.A., et al. Evaluation of Homology-Independent CRISPR-Cas9 Off-Target Assessment Methods. CRISPR J. 2020;3:440–453. doi: 10.1089/crispr.2020.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yen A., Zappala Z., Fine R.S., Majarian T.D., Sripakdeevong P., Altshuler D. Specificity of CRISPR-Cas9 Editing in Exagamglogene Autotemcel. N. Engl. J. Med. 2024;390:1723–1725. doi: 10.1056/NEJMc2313119. [DOI] [PubMed] [Google Scholar]
- 129.Corsi G.I., Gadekar V.P., Gorodkin J., Seemann S.E. CRISPRroots: on- and off-target assessment of RNA-seq data in CRISPR-Cas9 edited cells. Nucleic Acids Res. 2022;50:e20. doi: 10.1093/nar/gkab1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cargill M., Altshuler D., Ireland J., Sklar P., Ardlie K., Patil N., Shaw N., Lane C.R., Lim E.P., Kalyanaraman N., et al. Characterization of single-nucleotide polymorphisms in coding regions of human genes. Nat. Genet. 1999;22:231–238. doi: 10.1038/10290. [DOI] [PubMed] [Google Scholar]
- 131.Sachidanandam R., Weissman D., Schmidt S.C., Kakol J.M., Stein L.D., Marth G., Sherry S., Mullikin J.C., Mortimore B.J., Willey D.L., et al. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 2001;409:928–933. doi: 10.1038/35057149. [DOI] [PubMed] [Google Scholar]
- 132.Liao W.W., Asri M., Ebler J., Doerr D., Haukness M., Hickey G., Lu S., Lucas J.K., Monlong J., Abel H.J., et al. A draft human pangenome reference. Nature. 2023;617:312–324. doi: 10.1038/s41586-023-05896-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bergstrom A., McCarthy S.A., Hui R., Almarri M.A., Ayub Q., Danecek P., Chen Y., Felkel S., Hallast P., Kamm J., et al. Insights into human genetic variation and population history from 929 diverse genomes. Science. 2020;367 doi: 10.1126/science.aay5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lessard S., Francioli L., Alfoldi J., Tardif J.C., Ellinor P.T., MacArthur D.G., Lettre G., Orkin S.H., Canver M.C. Human genetic variation alters CRISPR-Cas9 on- and off-targeting specificity at therapeutically implicated loci. Proc. Natl. Acad. Sci. USA. 2017;114:E11257–E11266. doi: 10.1073/pnas.1714640114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scott D.A., Zhang F. Implications of human genetic variation in CRISPR-based therapeutic genome editing. Nat. Med. 2017;23:1095–1101. doi: 10.1038/nm.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cancellieri S., Zeng J., Lin L.Y., Tognon M., Nguyen M.A., Lin J., Bombieri N., Maitland S.A., Ciuculescu M.F., Katta V., et al. Human genetic diversity alters off-target outcomes of therapeutic gene editing. Nat. Genet. 2023;55:34–43. doi: 10.1038/s41588-022-01257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crane A.M., Kramer P., Bui J.H., Chung W.J., Li X.S., Gonzalez-Garay M.L., Hawkins F., Liao W., Mora D., Choi S., et al. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Rep. 2015;4:569–577. doi: 10.1016/j.stemcr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.1000 Genomes Project Consortium. Auton A., Brooks L.D., Durbin R.M., Garrison E.P., Kang H.M., Korbel J.O., Marchini J.L., McCarthy S., McVean G.A., Abecasis G.R. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gudmundsson S., Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., et al. Addendum: The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2021;597:E3–E4. doi: 10.1038/s41586-021-03758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smigielski E.M., Sirotkin K., Ward M., Sherry S.T. dbSNP: a database of single nucleotide polymorphisms. Nucleic Acids Res. 2000;28:352–355. doi: 10.1093/nar/28.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Assa G., Kalter N., Rosenberg M., Beck A., Markovich O., Gontmakher T., Hendel A., Yakhini Z. Quantifying allele-specific CRISPR editing activity with CRISPECTOR2.0. Nucleic Acids Res. 2024;52 doi: 10.1093/nar/gkae651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Alanis-Lobato G., Zohren J., McCarthy A., Fogarty N.M.E., Kubikova N., Hardman E., Greco M., Wells D., Turner J.M.A., Niakan K.K. Frequent loss of heterozygosity in CRISPR-Cas9-edited early human embryos. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2004832117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zuccaro M.V., Xu J., Mitchell C., Marin D., Zimmerman R., Rana B., Weinstein E., King R.T., Palmerola K.L., Smith M.E., et al. Allele-Specific Chromosome Removal after Cas9 Cleavage in Human Embryos. Cell. 2020;183:1650–1664.e15. doi: 10.1016/j.cell.2020.10.025. [DOI] [PubMed] [Google Scholar]
- 144.Boutin J., Rosier J., Cappellen D., Prat F., Toutain J., Pennamen P., Bouron J., Rooryck C., Merlio J.P., Lamrissi-Garcia I., et al. CRISPR-Cas9 globin editing can induce megabase-scale copy-neutral losses of heterozygosity in hematopoietic cells. Nat. Commun. 2021;12:4922. doi: 10.1038/s41467-021-25190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Simkin D., Papakis V., Bustos B.I., Ambrosi C.M., Ryan S.J., Baru V., Williams L.A., Dempsey G.T., McManus O.B., Landers J.E., et al. Homozygous might be hemizygous: CRISPR/Cas9 editing in iPSCs results in detrimental on-target defects that escape standard quality controls. Stem Cell Rep. 2022;17:993–1008. doi: 10.1016/j.stemcr.2022.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kosicki M., Tomberg K., Bradley A. Erratum: Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018;36:899. doi: 10.1038/nbt0918-899c. [DOI] [PubMed] [Google Scholar]
- 147.Adikusuma F., Piltz S., Corbett M.A., Turvey M., McColl S.R., Helbig K.J., Beard M.R., Hughes J., Pomerantz R.T., Thomas P.Q. Large deletions induced by Cas9 cleavage. Nature. 2018;560:E8–E9. doi: 10.1038/s41586-018-0380-z. [DOI] [PubMed] [Google Scholar]
- 148.Cullot G., Boutin J., Toutain J., Prat F., Pennamen P., Rooryck C., Teichmann M., Rousseau E., Lamrissi-Garcia I., Guyonnet-Duperat V., et al. CRISPR-Cas9 genome editing induces megabase-scale chromosomal truncations. Nat. Commun. 2019;10:1136. doi: 10.1038/s41467-019-09006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park S.H., Cao M., Pan Y., Davis T.H., Saxena L., Deshmukh H., Fu Y., Treangen T., Sheehan V.A., Bao G. Comprehensive analysis and accurate quantification of unintended large gene modifications induced by CRISPR-Cas9 gene editing. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abo7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mahmoud M., Gobet N., Cruz-Dávalos D.I., Mounier N., Dessimoz C., Sedlazeck F.J. Structural variant calling: the long and the short of it. Genome Biol. 2019;20:246. doi: 10.1186/s13059-019-1828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kosicki M., Allen F., Steward F., Tomberg K., Pan Y., Bradley A. Cas9-induced large deletions and small indels are controlled in a convergent fashion. Nat. Commun. 2022;13:3422. doi: 10.1038/s41467-022-30480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Klermund J., Rhiel M., Kocher T., Chmielewski K.O., Bischof J., Andrieux G., El Gaz M., Hainzl S., Boerries M., Cornu T.I., et al. On- and off-target effects of paired CRISPR-Cas nickase in primary human cells. Mol. Ther. 2024;32:1298–1310. doi: 10.1016/j.ymthe.2024.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schmidt J.K., Kim Y.H., Strelchenko N., Gierczic S.R., Pavelec D., Golos T.G., Slukvin I.I. Whole genome sequencing of CCR5 CRISPR-Cas9-edited Mauritian cynomolgus macaque blastomeres reveals large-scale deletions and off-target edits. Front Genome. 2022;4 doi: 10.3389/fgeed.2022.1031275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leibowitz M.L., Papathanasiou S., Doerfler P.A., Blaine L.J., Sun L., Yao Y., Zhang C.Z., Weiss M.J., Pellman D. Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing. Nat. Genet. 2021;53:895–905. doi: 10.1038/s41588-021-00838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Taniue K., Akimitsu N. Fusion Genes and RNAs in Cancer Development. Noncoding. RNA. 2021;7 doi: 10.3390/ncrna7010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gao Q., Liang W.W., Foltz S.M., Mutharasu G., Jayasinghe R.G., Cao S., Liao W.W., Reynolds S.M., Wyczalkowski M.A., Yao L., et al. Driver Fusions and Their Implications in the Development and Treatment of Human Cancers. Cell Rep. 2018;23:227–238.e3. doi: 10.1016/j.celrep.2018.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mertens F., Johansson B., Fioretos T., Mitelman F. The emerging complexity of gene fusions in cancer. Nat. Rev. Cancer. 2015;15:371–381. doi: 10.1038/nrc3947. [DOI] [PubMed] [Google Scholar]
- 158.Mitelman F., Johansson B., Mertens F. The impact of translocations and gene fusions on cancer causation. Nat. Rev. Cancer. 2007;7:233–245. doi: 10.1038/nrc2091. [DOI] [PubMed] [Google Scholar]
- 159.Drexler H.G., Gignac S.M., von Wasielewski R., Werner M., Dirks W.G. Pathobiology of NPM-ALK and variant fusion genes in anaplastic large cell lymphoma and other lymphomas. Leukemia. 2000;14:1533–1559. doi: 10.1038/sj.leu.2401878. [DOI] [PubMed] [Google Scholar]
- 160.Hendel A., Kildebeck E.J., Fine E.J., Clark J., Punjya N., Sebastiano V., Bao G., Porteus M.H. Quantifying genome-editing outcomes at endogenous loci with SMRT sequencing. Cell Rep. 2014;7:293–305. doi: 10.1016/j.celrep.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bi C., Wang L., Yuan B., Zhou X., Li Y., Wang S., Pang Y., Gao X., Huang Y., Li M. Long-read individual-molecule sequencing reveals CRISPR-induced genetic heterogeneity in human ESCs. Genome Biol. 2020;21:213. doi: 10.1186/s13059-020-02143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hoijer I., Johansson J., Gudmundsson S., Chin C.S., Bunikis I., Haggqvist S., Emmanouilidou A., Wilbe M., den Hoed M., Bondeson M.L., et al. Amplification-free long-read sequencing reveals unforeseen CRISPR-Cas9 off-target activity. Genome Biol. 2020;21:290. doi: 10.1186/s13059-020-02206-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gilpatrick T., Lee I., Graham J.E., Raimondeau E., Bowen R., Heron A., Downs B., Sukumar S., Sedlazeck F.J., Timp W. Targeted nanopore sequencing with Cas9-guided adapter ligation. Nat. Biotechnol. 2020;38:433–438. doi: 10.1038/s41587-020-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hwang G.-h., Lee S.-H., Oh M., Kim S., Habib O., Jang H.-K., Kim H.S., Kim C.H., Kim S., Bae S. Detailed mechanisms for unintended large DNA deletions with CRISPR, base editors, and prime editors. bioRxiv. 2024 doi: 10.1101/2024.01.04.574288. Preprint at. [DOI] [Google Scholar]
- 165.van Schendel R., Schimmel J., Tijsterman M. SIQ: easy quantitative measurement of mutation profiles in sequencing data. NAR Genom. Bioinform. 2022;4 doi: 10.1093/nargab/lqac063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Owens D.D.G., Caulder A., Frontera V., Harman J.R., Allan A.J., Bucakci A., Greder L., Codner G.F., Hublitz P., McHugh P.J., et al. Microhomologies are prevalent at Cas9-induced larger deletions. Nucleic Acids Res. 2019;47:7402–7417. doi: 10.1093/nar/gkz459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.White, N., Chalk, J., Hu, Y.-T., Pins, S., Antoniou, P., Wimberger, S., Svensson, S., Caetano-Silva, S., Mudde, A., Raj, R., et al. (2024). MEGA dPCR reveals chromosomal aberrations, NHEJ precise repair, recurrent nuclease cleavage and DSB half-life. 10.21203/rs.3.rs-4577114/v1. [DOI]
- 168.Maguire O., Wallace P.K., Minderman H. Fluorescent In Situ Hybridization in Suspension by Imaging Flow Cytometry. Methods Mol. Biol. 2016;1389:111–126. doi: 10.1007/978-1-4939-3302-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wiktor A.E., Van Dyke D.L., Stupca P.J., Ketterling R.P., Thorland E.C., Shearer B.M., Fink S.R., Stockero K.J., Majorowicz J.R., Dewald G.W. Preclinical validation of fluorescence in situ hybridization assays for clinical practice. Genet. Med. 2006;8:16–23. doi: 10.1097/01.gim.0000195645.00446.61. [DOI] [PubMed] [Google Scholar]
- 170.Sahajpal N.S., Mondal A.K., Tvrdik T., Hauenstein J., Shi H., Deeb K.K., Saxe D., Hastie A.R., Chaubey A., Savage N.M., et al. Clinical Validation and Diagnostic Utility of Optical Genome Mapping for Enhanced Cytogenomic Analysis of Hematological Neoplasms. J. Mol. Diagn. 2022;24:1279–1291. doi: 10.1016/j.jmoldx.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 171.Valkama A., Vorimo S., Kumpula T.A., Räsänen H., Savolainen E.R., Pylkäs K., Mantere T. Optical Genome Mapping as an Alternative to FISH-Based Cytogenetic Assessment in Chronic Lymphocytic Leukemia. Cancers (Basel) 2023;15 doi: 10.3390/cancers15041294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hu J., Meyers R.M., Dong J., Panchakshari R.A., Alt F.W., Frock R.L. Detecting DNA double-stranded breaks in mammalian genomes by linear amplification-mediated high-throughput genome-wide translocation sequencing. Nat. Protoc. 2016;11:853–871. doi: 10.1038/nprot.2016.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yin J., Liu M., Liu Y., Hu J. Improved HTGTS for CRISPR/Cas9 off-target detection. Bio. Protoc. 2019;9 doi: 10.21769/BioProtoc.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yin J., Liu M., Liu Y., Wu J., Gan T., Zhang W., Li Y., Zhou Y., Hu J. Optimizing genome editing strategy by primer-extension-mediated sequencing. Cell Discov. 2019;5:18. doi: 10.1038/s41421-019-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu Y., Yin J., Gan T., Liu M., Xin C., Zhang W., Hu J. PEM-seq comprehensively quantifies DNA repair outcomes during gene-editing and DSB repair. STAR Protoc. 2022;3 doi: 10.1016/j.xpro.2021.101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wen W., Quan Z.J., Li S.A., Yang Z.X., Fu Y.W., Zhang F., Li G.H., Zhao M., Yin M.D., Xu J., et al. Effective control of large deletions after double-strand breaks by homology-directed repair and dsODN insertion. Genome Biol. 2021;22:236. doi: 10.1186/s13059-021-02462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cho S.W., Kim S., Kim Y., Kweon J., Kim H.S., Bae S., Kim J.S. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014;24:132–141. doi: 10.1101/gr.162339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Suzuki K., Tsunekawa Y., Hernandez-Benitez R., Wu J., Zhu J., Kim E.J., Hatanaka F., Yamamoto M., Araoka T., Li Z., et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature. 2016;540:144–149. doi: 10.1038/nature20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maresca M., Lin V.G., Guo N., Yang Y. Obligate ligation-gated recombination (ObLiGaRe): custom-designed nuclease-mediated targeted integration through nonhomologous end joining. Genome Res. 2013;23:539–546. doi: 10.1101/gr.145441.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yeh C.D., Richardson C.D., Corn J.E. Advances in genome editing through control of DNA repair pathways. Nat. Cell Biol. 2019;21:1468–1478. doi: 10.1038/s41556-019-0425-z. [DOI] [PubMed] [Google Scholar]
- 181.Wimberger S., Akrap N., Firth M., Brengdahl J., Engberg S., Schwinn M.K., Slater M.R., Lundin A., Hsieh P.P., Li S., et al. Simultaneous inhibition of DNA-PK and Polϴ improves integration efficiency and precision of genome editing. Nat. Commun. 2023;14:4761. doi: 10.1038/s41467-023-40344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Selvaraj S., Feist W.N., Viel S., Vaidyanathan S., Dudek A.M., Gastou M., Rockwood S.J., Ekman F.K., Oseghale A.R., Xu L., et al. High-efficiency transgene integration by homology-directed repair in human primary cells using DNA-PKcs inhibition. Nat. Biotechnol. 2024;42:731–744. doi: 10.1038/s41587-023-01888-4. [DOI] [PubMed] [Google Scholar]
- 183.Lomova A., Clark D.N., Campo-Fernandez B., Flores-Bjurström C., Kaufman M.L., Fitz-Gibbon S., Wang X., Miyahira E.Y., Brown D., DeWitt M.A., et al. Improving Gene Editing Outcomes in Human Hematopoietic Stem and Progenitor Cells by Temporal Control of DNA Repair. Stem Cell. 2019;37:284–294. doi: 10.1002/stem.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schiroli G., Conti A., Ferrari S., Della Volpe L., Jacob A., Albano L., Beretta S., Calabria A., Vavassori V., Gasparini P., et al. Precise Gene Editing Preserves Hematopoietic Stem Cell Function following Transient p53-Mediated DNA Damage Response. Cell Stem Cell. 2019;24:551–565.e8. doi: 10.1016/j.stem.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ferrari S., Jacob A., Beretta S., Unali G., Albano L., Vavassori V., Cittaro D., Lazarevic D., Brombin C., Cugnata F., et al. Efficient gene editing of human long-term hematopoietic stem cells validated by clonal tracking. Nat. Biotechnol. 2020;38:1298–1308. doi: 10.1038/s41587-020-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kastenhuber E.R., Lowe S.W. Putting p53 in Context. Cell. 2017;170:1062–1078. doi: 10.1016/j.cell.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Haapaniemi E., Botla S., Persson J., Schmierer B., Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat. Med. 2018;24:927–930. doi: 10.1038/s41591-018-0049-z. [DOI] [PubMed] [Google Scholar]
- 188.Carusillo A., Haider S., Schäfer R., Rhiel M., Türk D., Chmielewski K.O., Klermund J., Mosti L., Andrieux G., Schäfer R., et al. A novel Cas9 fusion protein promotes targeted genome editing with reduced mutational burden in primary human cells. Nucleic Acids Res. 2023;51:4660–4673. doi: 10.1093/nar/gkad255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Robert F., Barbeau M., Éthier S., Dostie J., Pelletier J. Pharmacological inhibition of DNA-PK stimulates Cas9-mediated genome editing. Genome Med. 2015;7:93. doi: 10.1186/s13073-015-0215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Riesenberg S., Maricic T. Targeting repair pathways with small molecules increases precise genome editing in pluripotent stem cells. Nat. Commun. 2018;9:2164. doi: 10.1038/s41467-018-04609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cloarec-Ung F.M., Beaulieu J., Suthananthan A., Lehnertz B., Sauvageau G., Sheppard H.M., Knapp D.J.H.F. Near-perfect precise on-target editing of human hematopoietic stem and progenitor cells. eLife. 2024;12 doi: 10.7554/eLife.91288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.De Ravin S.S., Brault J., Meis R.J., Liu S., Li L., Pavel-Dinu M., Lazzarotto C.R., Liu T., Koontz S.M., Choi U., et al. Enhanced homology-directed repair for highly efficient gene editing in hematopoietic stem/progenitor cells. Blood. 2021;137:2598–2608. doi: 10.1182/blood.2020008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cullot G., Aird E.J., Schlapansky M.F., Yeh C.D., van de Venn L., Vykhlyantseva I., Kreutzer S., Mailänder D., Lewków B., Klermund J., et al. Genome editing with the HDR-enhancing DNA-PKcs inhibitor AZD7648 causes large-scale genomic alterations. Nat. Biotechnol. 2024 doi: 10.1038/s41587-024-02488-6. [DOI] [PubMed] [Google Scholar]
- 194.Smith R.H., Chen Y.C., Seifuddin F., Hupalo D., Alba C., Reger R., Tian X., Araki D., Dalgard C.L., Childs R.W., et al. Genome-Wide Analysis of Off-Target CRISPR/Cas9 Activity in Single-Cell-Derived Human Hematopoietic Stem and Progenitor Cell Clones. Genes. 2020;11 doi: 10.3390/genes11121501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Miosge L.A., Field M.A., Sontani Y., Cho V., Johnson S., Palkova A., Balakishnan B., Liang R., Zhang Y., Lyon S., et al. Comparison of predicted and actual consequences of missense mutations. Proc. Natl. Acad. Sci. USA. 2015;112:E5189–E5198. doi: 10.1073/pnas.1511585112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Livesey B.J., Marsh J.A. Using deep mutational scanning to benchmark variant effect predictors and identify disease mutations. Mol. Syst. Biol. 2020;16 doi: 10.15252/msb.20199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sharma R., Dever D.P., Lee C.M., Azizi A., Pan Y., Camarena J., Köhnke T., Bao G., Porteus M.H., Majeti R. The TRACE-Seq method tracks recombination alleles and identifies clonal reconstitution dynamics of gene targeted human hematopoietic stem cells. Nat. Commun. 2021;12:472. doi: 10.1038/s41467-020-20792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang Z. Assessing Tumorigenicity in Stem Cell-Derived Therapeutic Products: A Critical Step in Safeguarding Regenerative Medicine. Bioengineering. 2023;10 doi: 10.3390/bioengineering10070857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rotem A., Janzer A., Izar B., Ji Z., Doench J.G., Garraway L.A., Struhl K. Alternative to the soft-agar assay that permits high-throughput drug and genetic screens for cellular transformation. Proc. Natl. Acad. Sci. USA. 2015;112:5708–5713. doi: 10.1073/pnas.1505979112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Izar B., Rotem A. GILA, a Replacement for the Soft-Agar Assay that Permits High-Throughput Drug and Genetic Screens for Cellular Transformation. Curr. Protoc. Mol. Biol. 2016;116:28.8.1–28.8.12. doi: 10.1002/cpmb.26. [DOI] [PubMed] [Google Scholar]
- 201.Lemmens M., Fischer B., Zogg M., Rodrigues L., Kerr G., Del Rio-Espinola A., Schaeffer F., Maddalo D., Dubost V., Piaia A., et al. Evaluation of two in vitro assays for tumorigenicity assessment of CRISPR-Cas9 genome-edited cells. Mol. Ther. Methods Clin. Dev. 2021;23:241–253. doi: 10.1016/j.omtm.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Samuelson C., Radtke S., Zhu H., Llewellyn M., Fields E., Cook S., Huang M.L.W., Jerome K.R., Kiem H.P., Humbert O. Multiplex CRISPR/Cas9 genome editing in hematopoietic stem cells for fetal hemoglobin reinduction generates chromosomal translocations. Mol. Ther. Methods Clin. Dev. 2021;23:507–523. doi: 10.1016/j.omtm.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sato Y., Bando H., Di Piazza M., Gowing G., Herberts C., Jackman S., Leoni G., Libertini S., MacLachlan T., McBlane J.W., et al. Tumorigenicity assessment of cell therapy products: The need for global consensus and points to consider. Cytotherapy. 2019;21:1095–1111. doi: 10.1016/j.jcyt.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 204.Magis W., DeWitt M.A., Wyman S.K., Vu J.T., Heo S.J., Shao S.J., Hennig F., Romero Z.G., Campo-Fernandez B., Said S., et al. High-level correction of the sickle mutation is amplified in vivo during erythroid differentiation. iScience. 2022;25 doi: 10.1016/j.isci.2022.104374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shultz L.D., Keck J., Burzenski L., Jangalwe S., Vaidya S., Greiner D.L., Brehm M.A. Humanized mouse models of immunological diseases and precision medicine. Mamm. Genome. 2019;30:123–142. doi: 10.1007/s00335-019-09796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Radtke S., Humbert O., Kiem H.P. Mouse models in hematopoietic stem cell gene therapy and genome editing. Biochem. Pharmacol. 2020;174 doi: 10.1016/j.bcp.2019.113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Allen D., Weiss L.E., Saguy A., Rosenberg M., Iancu O., Matalon O., Lee C., Beider K., Nagler A., Shechtman Y., Hendel A. High-Throughput Imaging of CRISPR- and Recombinant Adeno-Associated Virus-Induced DNA Damage Response in Human Hematopoietic Stem and Progenitor Cells. CRISPR J. 2022;5:80–94. doi: 10.1089/crispr.2021.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pavel-Dinu M., Wiebking V., Dejene B.T., Srifa W., Mantri S., Nicolas C.E., Lee C., Bao G., Kildebeck E.J., Punjya N., et al. Gene correction for SCID-X1 in long-term hematopoietic stem cells. Nat. Commun. 2019;10:1634. doi: 10.1038/s41467-019-09614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ceglie G., Lecis M., Canciani G., Algeri M., Frati G. Genome editing for sickle cell disease: still time to correct? Front. Pediatr. 2023;11 doi: 10.3389/fped.2023.1249275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Frangoul H., Altshuler D., Cappellini M.D., Chen Y.S., Domm J., Eustace B.K., Foell J., de la Fuente J., Grupp S., Handgretinger R., et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and beta-Thalassemia. N. Engl. J. Med. 2021;384:252–260. doi: 10.1056/NEJMoa2031054. [DOI] [PubMed] [Google Scholar]
- 211.Lydeard J.R., Lin M.I., Ge H.G., Halfond A., Wang S., Jones M.B., Etchin J., Angelini G., Xavier-Ferrucio J., Lisle J., et al. Development of a gene edited next-generation hematopoietic cell transplant to enable acute myeloid leukemia treatment by solving off-tumor toxicity. Mol. Ther. Methods Clin. Dev. 2023;31 doi: 10.1016/j.omtm.2023.101135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.De Dreuzy E., Heath J., Zuris J.A., Sousa P., Viswanathan R., Scott S., Da Silva J., Ta T., Capehart S., Wang T., et al. EDIT-301: An Experimental Autologous Cell Therapy Comprising Cas12a-RNP Modified mPB-CD34+ Cells for the Potential Treatment of SCD. Blood. 2019;134:4636. doi: 10.1182/blood-2019-130256. [DOI] [Google Scholar]
- 213.Gillmore J.D., Gane E., Taubel J., Kao J., Fontana M., Maitland M.L., Seitzer J., O'Connell D., Walsh K.R., Wood K., et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021;385:493–502. doi: 10.1056/NEJMoa2107454. [DOI] [PubMed] [Google Scholar]
- 214.Akcakaya P., Bobbin M.L., Guo J.A., Malagon-Lopez J., Clement K., Garcia S.P., Fellows M.D., Porritt M.J., Firth M.A., Carreras A., et al. In vivo CRISPR editing with no detectable genome-wide off-target mutations. Nature. 2018;561:416–419. doi: 10.1038/s41586-018-0500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]