ABSTRACT
Background and Aims
Reproductive history, including age at first childbirth, may be associated with female dementia prevalence and sex disparities. This study investigates the relationship between reproductive timing and dementia outcomes, emphasizing socioeconomic and cultural influences, particularly in low‐ and middle‐income countries (LMICs) where disparities in healthcare and education may exacerbate risks.
Methods
Country‐level ecological analysis was conducted using data from 204 countries. Variables were sourced from international databases, including the Institute for Health Metrics and Evaluation (IHME), the United Nations, and the World Bank. The primary outcomes were female dementia prevalence and sex disparity in dementia prevalence. Predictor variables included age at first childbirth, ageing in females, economic affluence, genetic predisposition, and urban living. Statistical methods included scatterplots, correlation analyses, partial correlations, stepwise multiple regression, and principal component analysis.
Results
Later age at first childbirth was strongly associated with increased female dementia prevalence (R² = 0.7314, n = 126) and greater sex disparity in dementia outcomes (R² = 0.6362, n = 126). Subgroup analyses revealed stronger associations in LMICs than in high‐income countries. Economic affluence was positively associated with both later age at first childbirth and higher female dementia prevalence. Other variables, such as ageing and genetic predisposition, also showed independent associations.
Conclusion
Delayed age at first childbirth is significantly associated with higher female dementia prevalence and sex disparities, particularly in LMICs. These findings highlight the need for individual‐level, longitudinal studies to explore potential causal pathways and to inform sex‐sensitive public health strategies for dementia prevention across diverse global contexts.
Keywords: age at first childbirth, female dementia prevalence, public health strategies, reproductive history, sex disparities
Summary
This study examines the relationship between age at first childbirth and female dementia prevalence, highlighting that delayed childbirth is associated with increased dementia risk and greater sex disparities in dementia prevalence.
- These findings highlight the need for further investigation through individual‐level, longitudinal studies to explore potential sex‐specific determinants of dementia risk, particularly in low‐ and middle‐income countries.
- Quick Points
-
◦Delayed age at first childbirth is significantly associated with higher female dementia prevalence and greater sex disparities in dementia outcomes, highlighting the importance of reproductive history in cognitive health.
-
◦The study highlights the amplified risks in low‐ and middle‐income countries, where limited healthcare access and educational disparities exacerbate the relationship between delayed childbirth and dementia.
-
◦Findings contribute to the existing literature by emphasizing the interplay of biological, socioeconomic, and cultural factors in shaping dementia risk among women.
-
◦This study highlights the need for individual‐level investigations to explore how sex specific factors such as maternal health, stress exposure, and cognitive stimulation may be associated with dementia risk across diverse global populations.
-
◦These results support the integration of reproductive health into dementia prevention frameworks, with implications for reducing the global burden of dementia, especially among women in resource‐limited settings.
-
◦
1. Introduction
Dementia remains one of the most pressing global health challenges, with profound social and economic consequences [1]. It is a leading cause of disability and dependency among older adults, disproportionately affecting women, who consistently exhibit higher prevalence rates compared to men [2, 3]. This pronounced sex disparity in dementia prevalence is observed across diverse demographic and socioeconomic contexts, underscoring the complex interplay of biological, environmental, and sociocultural factors influencing dementia risk [4]. The influence of reproductive history [5], particularly age at first childbirth, on dementia outcomes remains underexplored [6], despite extensive research on established risk factors such as ageing, genetic predisposition, and lifestyle factors [4, 7].
Reproductive history, specifically the age at first childbirth, is a key aspect of women's health, encompassing biological, psychological, and social dimensions that can significantly influence long‐term health trajectories [8]. The timing of first childbirth reflects broader demographic transitions, characterized by delayed reproduction due to urbanization, increased educational attainment, and economic affluence [9]. While these shifts often bring improvements in women's opportunities and overall health, they may simultaneously introduce new risks for chronic diseases [10], including dementia. The biological mechanisms underlying these associations may include cumulative estrogen exposure [11, 12], which plays a protective role in brain health by promoting neuronal growth and reducing inflammation [13]. Delayed childbirth, however, may alter these hormonal trajectories and potentially influence susceptibility to neurodegenerative diseases [5, 14].
Societal changes associated with delayed childbirth, such as increased stress from career and family responsibilities [15, 16], sedentary lifestyles [17], and greater reliance on processed foods [18, 19], may further exacerbate dementia risk. These factors are particularly relevant in low‐ and middle‐income countries (LMICs), where healthcare access and educational disparities often magnify the impact of reproductive and lifestyle factors on health outcomes [20]. Despite its relevance, the relationship between age at first childbirth and female dementia prevalence, as well as the extent of sex disparities in dementia prevalence, has been inadequately explored in existing research [21].
This study investigates the role of age at first childbirth in predicting female dementia prevalence and sex disparities in dementia outcomes, using data from 204 countries. The analysis incorporates comprehensive data sets from internationally recognized sources, examining key sociodemographic and biological variables, including ageing in females, economic affluence, genetic predisposition, and urban living. By integrating these predictors, the study seeks to elucidate the independent and confounded associations of reproductive history and other factors with dementia outcomes.
The decision to examine age at first childbirth as an independent variable stems from its potential to capture broader fertility trends and societal transitions, while also reflecting women's long‐term health trajectories [22]. Delayed childbirth often accompanies improvements in healthcare access and educational attainment, which can reduce certain health risks [23]. However, postponing childbirth may also alter hormonal exposure and influence lifestyle factors that are linked to elevated dementia risk, such as stress, physical inactivity, and diet [5, 14]. This study quantifies the association between age at first childbirth and dementia outcomes to address key gaps in understanding the determinants of sex disparities in dementia prevalence. In doing so, it offers empirical evidence to support future research focused on gender‐specific factors in cognitive health. It is hypothesized that a higher mean age at first childbirth is significantly associated with increased female dementia prevalence and wider sex disparities in dementia across countries.
2. Materials and Methods
2.1. Data Sources
This study employs a cross‐sectional ecological design at the country level, with all six variables sourced from data published by the United Nations and its affiliated agencies, previous publication and Institute for Health Metrics and Evaluation (IHME). A comprehensive list of countries was compiled from the United Nations World Fertility Data, comprising 204 reporting units with available total fertility rate data [24]. Not all variables were available for every country, but over 116 reporting units were included. Each country was treated as an independent unit of study. The data sourcing organizations use terms such as “location”, “population”, and “country” interchangeably to denote a single reporting entity rather than a sovereign state. For data analysis, each country was treated as an independent unit of study.
2.1.1. Female Dementia Prevalence Rate
This is the dependent variable, defined as the number of diagnosed dementia cases per 100,000 females in 2021. Data were obtained from the Global Burden of Disease Study 2019 (GBD 2019), published by the IHME [2, 25]. It captures the national‐level burden of dementia among women, who consistently exhibit a higher prevalence of dementia than men.
2.1.2. Sex Disparity in Dementia Prevalence
This derived variable represents the difference between female and male dementia prevalence rates, calculated as: Sex disparity = Female dementia prevalence rate − Male dementia prevalence rate. Using data from the GBD 2019 data set by IHME, this variable quantifies the extent to which dementia is more prevalent in females than in males across countries.
2.1.3. Age at First Childbirth
The independent variable is the mean age of women at the birth of their first child, based on the most recent available data. It was obtained from the World Fertility Report 2012, published by the United Nations Department of Economic and Social Affairs, Population Division [26]. This measure represents a weighted average of maternal age at first birth, calculated using age‐specific fertility rates, and serves as a proxy for national reproductive timing.
2.1.4. Economic Affluence
Economic affluence was measured by Gross Domestic Product (GDP) per capita adjusted for Purchasing Power Parity (PPP) in 2018 international US dollars. Data were retrieved from the World Bank [27]. This indicator accounts for differences in the cost of goods, services, and living standards across countries, and is considered a key socioeconomic confounder linked to dementia outcomes [28].
2.1.5. Ageing in Females
Ageing in females was measured using female life expectancy at birth in 2018, with data sourced from the World Bank [27]. This demographic indicator reflects the ageing structure of the female population and serves as a proxy for population‐level ageing, a dominant risk factor for dementia.
2.1.6. Genetic Predisposition
Genetic predisposition was indexed using the Biological State Index (Ibs), which estimates the accumulation of deleterious genes within a population as a result of relaxed natural selection. Data were derived from a 2018 peer‐reviewed publication by You & Henneberg [29]. This index reflects population‐level genetic vulnerability to non‐communicable diseases, including dementia [30].
2.1.7. Urban Living
Urban living was represented by the percentage of the population residing in urban areas in 2018, with data sourced from the World Bank [31]. This indicator captures demographic and lifestyle transitions, including healthcare access and environmental exposures that are relevant to dementia risk [32, 33].
2.2. Regression Diagnostic Analysis
Multicollinearity is a common issue that can impact the quality of data in regression model analysis. It occurs when independent and confounding variables are highly correlated, potentially compromising the reliability of regression results. To address this, multicollinearity was assessed by calculating the correlation among six variables (age at first childbirth, ageing in females, economic affluence, genetic predisposition, urban living, and dementia prevalence in females) using a multiple linear regression model. The analysis was repeated by substituting female dementia prevalence with an alternative dependent variable, sex disparity in dementia prevalence. Multicollinearity was ruled out, as tolerance values across all variables ranged from 0.165 to 0.439, while variance inflation factor (VIF) values ranged from 2.276 to 6.049 in both diagnostic analyses, ensuring that tolerance values were ≥ 0.10 and VIF values were ≤ 10. These results confirm that the model is robust and not adversely affected by multicollinearity [34].
2.3. Data Analysis
To investigate the predictive influence of age at first childbirth on female dementia prevalence and sex disparity in dementia prevalence at the population level, the analysis followed a structured six‐step approach [35, 36]:
1. Scatter plots: Scatter plots were generated to visually examine the relationship between age at first childbirth and on female dementia prevalence and sex disparity in dementia prevalence, respectively. These plots provided insights into data distribution and helped assess data quality.
2. Bivariate correlations: Pearson's r and nonparametric rho correlations were calculated to evaluate the strength and direction of relationships between age at first childbirth and on female dementia prevalence, and sex disparity in dementia prevalence respectively. These analyses accounted for the potential impacts of non‐normal data distributions on correlation coefficients.
3. Partial correlation analysis: Partial correlations using Pearson's method were conducted to explore the independent predictive power of age at first childbirth on female dementia prevalence and sex disparity in dementia prevalence, respectively. By alternately treating age at first childbirth as both a predictor and control variable, the analysis assessed how age at first childbirth influenced female dementia prevalence and sex disparity in dementia prevalence, respectively, and its role in explaining confounding factors' predictive effects.
4. Multiple linear regression: Standard multiple linear regression (stepwise method) was used to model the relationship between predictors and female dementia prevalence and sex disparity in dementia prevalence, respectively. Two models were applied: one including age at first childbirth as a predictor and another excluding it, to evaluate how age at first childbirth influenced the effects of ageing, economic affluence, genetic predisposition, and urban living on female dementia prevalence and sex disparity in dementia prevalence, respectively.
5. Factor analysis (Principal Component Analysis (PCA)): PCA was conducted to explore the interrelationships among predictors and assess the contribution of age at first childbirth to the shared variance within the principal component. As a complement to traditional multicollinearity diagnostics (tolerance and VIF), PCA was selected for its robustness in capturing the role of birth rate within the broader predictive construct.
6. Grouped analysis by population characteristics: The analysis included subgroup comparisons to explore the correlation between age at first childbirth and female dementia prevalence and sex disparity in dementia prevalence, respectively, across diverse global contexts. Countries were classified based on World Bank income categories (low‐, middle‐, and high‐income), United Nations and WHO regions, and additional criteria such as cultural, geographic, or socioeconomic factors. Specific groupings included the Asia Cooperation Dialogue, Asia‐Pacific Economic Cooperation, the Arab World, the European Economic Area, English‐speaking nations, Latin America, the OECD, and others. This grouping allowed for comparative analyses of how demographic and socioeconomic factors influenced female dementia prevalence and sex disparity in dementia prevalence, respectively.
Given the WHO's report that over 55 million people globally are living with dementia, with the majority in low‐ and middle‐income countries, a Fisher r‐to‐z transformation was performed. This comparison assessed age at first childbirth's predictive role on female dementia prevalence and sex disparity in dementia prevalence respectively across income‐level classifications (e.g., LMIC vs. high‐income countries) and development classifications (e.g., United Nations‐developing vs. developed countries).
The statistical methods included scatter plots, Pearson's correlation, Spearman's rho, partial correlation, multiple linear regression (Enter and Stepwise methods), and PCA. Scatter plots were created using Microsoft Excel 2016, while all other analyses were conducted in SPSS (version 29). Significance levels were set at p < 0.05, p < 0.01, and p < 0.001, with regression criteria defined as a probability of F to enter ≤ 0.05 and to remove ≥ 0.10.
3. Results
The relationship between age at first childbirth and female dementia prevalence, as well as sex disparity in dementia prevalence, was analysed using quadratic regression models. A strong association was observed between later age at first childbirth and increased female dementia prevalence, as depicted by the equation y = 5.6901x2 − 82.525x − 332.72 (R 2 = 0.7314, n = 126). This suggests a significant curvilinear trend, with dementia prevalence rising sharply at higher ages of first childbirth.
Similarly, sex disparity in dementia prevalence demonstrated a moderate quadratic relationship with age at first childbirth (y = 2.193x2− 16.36x − 412.9, R 2 = 0.6362, n = 126). While the trend was less pronounced compared to female dementia prevalence, the results indicate that disparity also increases with delayed childbirth, particularly beyond 25 years.
These findings highlight a critical link between reproductive history and later‐life dementia risk, emphasizing the importance of investigating underlying biological and sociocultural mechanisms (Figure 1).
Figure 1.
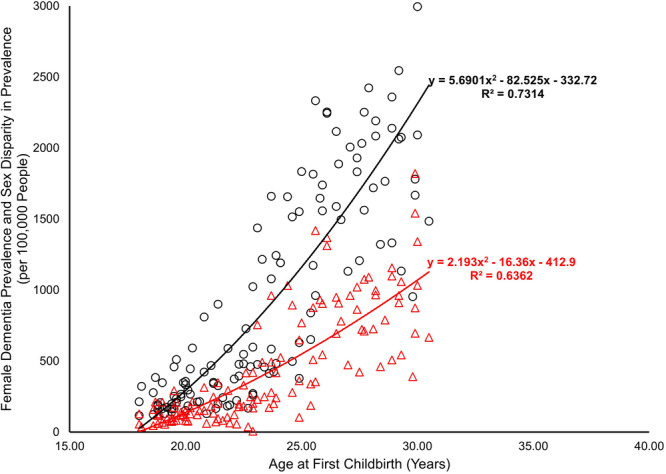
Predictive power of age at first childbirth on female dementia prevalence and sex disparity in prevalence (female minus male) data source and definition: The mean age at first childbirth, representing age at first childbirth, was obtained from the World Fertility Report 2012 by the United Nations. Dementia prevalence data, including female prevalence and sex disparity (calculated as the difference between female and male prevalence rates), were sourced from the Institute for Health Metrics and Evaluation, University of Washington, with rates reported as the number of diagnosed cases per 100,000 individuals diagnosed in 2021.
The scatterplots confirmed the strong and significant relationship between age at first childbirth, and female dementia prevalence and sex disparity in dementia prevalence. This relationship was further confirmed through nonparametric and Pearson r analyses using raw data.
Bivariate and partial correlation analyses were conducted to examine the factors associated with female dementia prevalence and sex disparity in dementia prevalence. Age at first birth demonstrated the strongest positive correlation with both outcomes (r = 0.852, ρ = 0.868, p < 0.001, n = 126 for female dementia prevalence; r = 0.795, ρ = 0.825, p< 0.001, n = 126 for sex disparity) (Table 1). This association remained significant in partial correlation (r = 0.714 and 0.688 for female dementia prevalence and sex disparity respectively, p < 0.001, n = 126 respectively) (Table 1), indicating that delayed childbirth is a key factor influencing dementia outcomes.
Table 1.
Bivariate and partial correlation analysis of factors associated with female dementia prevalence and sex disparity in dementia prevalence.
| Independent/control variable | Variable mean | Dependent variable: Female dementia prevalence (mean = 851.12) | Independent/control variable | Dependent variable: Sex disparity in dementia prevalence (Mean = 380.31) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson's r (95% CIs) | Nonparametric ρ (95% CIs) | Partial correlation (95% CIs) | Partial correlation (95% CIs) | Pearson's r (95% CIs) | Nonparametric ρ (95% CIs) | Partial correlation (95% CIs) | Partial correlation (95% CIs) | |||
| Mean age at 1st birth | 23.61 | 0.852*** | 0.868*** | 0.714*** | — | Mean age at 1st birth | 0.795*** | 0.825*** | 0.688*** | — |
| Ageing, female | 72.21 | 0.830*** | 0.842*** | — | 0.608*** | Ageing, female | 0.830*** | 0.842*** | — | 0.708*** |
| Genetic predisposition | 0.93 | 0.596*** | 0.697*** | — | 0.373*** | Genetic predisposition | 0.596*** | 0.697*** | — | 0.761*** |
| Economic affluence | 2710.46 | 0.805*** | 0.834*** | — | 0.731*** | Economic affluence | 0.805*** | 0.834*** | — | 0.456*** |
| Urban living | 60.40 | 0.598*** | 0.580*** | — | 0.436*** | Urban living | 0.598*** | 0.580*** | — | 0.505*** |
Note: Data source and definition: The mean age at first childbirth, representing age at first childbirth, was obtained from the World Fertility Report 2012 by the United Nations. Dementia prevalence data, including female prevalence and sex disparity (calculated as the difference between female and male prevalence rates), were sourced from the Institute for Health Metrics and Evaluation, University of Washington, with rates reported as the number of diagnosed cases per 100,000 individuals diagnosed in 2021. Economic affluence was represented by per capita GDP PPP, measured as the purchasing power parity value of all final goods and services produced within a territory in 2018, sourced from the World Bank. Genetic predisposition was indexed using the Biological State Index (Ibs), reflecting dementia‐related genetic background accumulation due to reduced natural selection, as detailed in a previous publication (You and Henneberg, 2022). Aging in females was represented by life expectancy at birth, sourced from the World Bank 2018. Urban living was indexed by urbanization, measured as the percentage of the population living in urban areas in 2018, also sourced from the World Bank.
p < 0.001. Number of country range, 116–126.
Other variables, including ageing in females (r = 0.830, ρ = 0.842, p< 0.001, n = 126) and economic affluence (r = 0.805, ρ = 0.834, p< 0.001, n = 126), also exhibited strong positive correlations with female dementia prevalence, with independent effects confirmed in partial analyses (r = 0.608 and r = 0.731, respectively, n = 126) (Table 1). For sex disparity, these variables similarly showed strong bivariate correlations, with partial correlations of r = 0.708 for ageing in females and r = 0.456 for economic affluence (Table 1).
Moderate associations were observed for genetic predisposition (r = 0.596, ρ = 0.697, p < 0.001, n = 126) and urban living (r = 0.598, ρ = 0.580, p< 0.001, n = 126) across both outcomes, with partial correlations indicating stronger effects of genetic predisposition on sex disparity (r = 0.761, n = 126) compared to female dementia prevalence (r = 0.373, n = 126) (Table 1).
Stepwise multiple linear regression identified distinct independent predictors for female dementia prevalence and sex disparity in dementia prevalence.
For female dementia prevalence, ageing in females emerged as the sole significant predictor (R 2 = 0.459, β = 0.703, p< 0.001, n = 122), explaining 45.9% of the variance (Table 2). Other factors, including age at first childbirth, economic affluence, genetic predisposition, and urban living, were not significant contributors.
Table 2.
Independent predictors of female dementia prevalence and sex disparity in dementia prevalence identified through stepwise multiple linear regression.
| Predictor | Dependent variable: Female dementia prevalence, all countries, n = 204 | ||||||
|---|---|---|---|---|---|---|---|
| R square | Beta (95% CIs) | Sig. | Predictor | R square | Beta (95% CIs) | Sig. | |
| Age at first childbirth | Not incorporated | Age at first childbirth | 0.728 | 0.684 | 0.000 | ||
| Ageing, female | 0.459 | 0.703 | 0.000 | Ageing, female | 0.739 | 0.206 | 0.016 |
| Economic affluence | Not insignificant | Economic affluence | Not insignificant | ||||
| Genetic predisposition | Not insignificant | Genetic predisposition | Not insignificant | ||||
| Urban living | Not insignificant | Urban living | Not insignificant | ||||
| Age at first childbirth | Not incorporated | Age at first childbirth | 0.635 | 0.797 | 0.000 | ||
| Ageing, female | 0.372 | 0.613 | 0.000 | Ageing, female | Not significant | ||
| Economic affluence | Not significant | Economic affluence | Not significant | ||||
| Genetic predisposition | Not significant | Genetic predisposition | Not significant | ||||
| Urban living | Not significant | Urban living | Not significant | ||||
Note: Data source and definition: The mean age at first childbirth, representing age at first childbirth, was obtained from the World Fertility Report 2012 by the United Nations. Dementia prevalence data, including female prevalence and sex disparity (calculated as the difference between female and male prevalence rates), were sourced from the Institute for Health Metrics and Evaluation, University of Washington, with rates reported as the number of diagnosed cases per 100,000 individuals diagnosed in 2021. Economic affluence was represented by per capita GDP PPP, measured as the purchasing power parity value of all final goods and services produced within a territory in 2018, sourced from the World Bank. Genetic predisposition was indexed using the Biological State Index (Ibs), reflecting dementia‐related genetic background accumulation due to reduced natural selection, as detailed in a previous publication (You and Henneberg, 2022). Aging in females was represented by life expectancy at birth, sourced from the World Bank 2018. Urban living was indexed by urbanization, measured as the percentage of the population living in urban areas in 2018, also sourced from the World Bank.
Number of country range, 116–126.
For sex disparity in dementia prevalence, age at first childbearing was the dominant predictor (R 2 = 0.728, β = 0.684, p< 0.001, n = 122), accounting for 72.8% of the variance. Ageing in females also contributed significantly (R 2 increased to 0.739, β = 0.206, p< 0.050, n = 122) (Table 2), though its impact was less pronounced. Other variables, including economic affluence, genetic predisposition, and urban living, were not significant.
Factor analysis (principal component) complemented stepwise regression by revealing age at first childbirth as a single dominant component, accounting for 75.225% of the total variance and indicating a shared underlying mechanism among the predictors of female dementia prevalence and sex disparity. The Kaiser–Meyer–Olkin measure of sampling adequacy (KMO = 0.827) and Bartlett's Test of Sphericity (χ 2 = 471.651, p < 0.001) confirmed the suitability of the data for factor analysis (Table 3). Female ageing and age at first childbirth emerged as the most significant contributors, with high loadings (0.945 and 0.897, respectively) (Table 3), emphasizing their central role in dementia outcomes. Other factors, including economic affluence, genetic predisposition, and urban living, also loaded strongly, highlighting their interconnected influence.
Table 3.
Factor analysis summary (principal component) highlighting age at first childbirth as a significant predictor for female dementia prevalence and sex disparity in dementia prevalence.
| Measure | Results |
|---|---|
| KMO and Bartlett's test | |
| Kaiser–Meyer–Olkin (KMO) | 0.827 |
| Bartlett's test of sphericity | χ² = 471.651, df = 10, p < 0.001 |
| Component | Initial Eigenvalues |
| 1 | 3.761 |
| 2 | 0.544 |
| 3 | 0.406 |
| 4 | 0.168 |
| 5 | 0.120 |
| Communalities | Initial |
| Mean age at 1st childbirth | 1.000 |
| Economic affluence | 1.000 |
| Genetic predisposition | 1.000 |
| Female aging | 1.000 |
| Urban living | 1.000 |
| Component matrix (component 1) | |
| Age at first childbirth | 0.897 |
| Economic affluence | 0.882 |
| Genetic predisposition | 0.781 |
| Female aging | 0.945 |
| Urban living | 0.822 |
| Total variance explained by component 1 | 75.225% out of 100% |
Note: Data source and definition: The mean age at first childbirth, representing age at first childbirth, was obtained from the World Fertility Report 2012 by the United Nations. Dementia prevalence data, including female prevalence and sex disparity (calculated as the difference between female and male prevalence rates), were sourced from the Institute for Health Metrics and Evaluation, University of Washington, with rates reported as the number of diagnosed cases per 100,000 individuals diagnosed in 2021. Economic affluence was represented by per capita GDP PPP, measured as the purchasing power parity value of all final goods and services produced within a territory in 2018, sourced from the World Bank. Genetic predisposition was indexed using the Biological State Index (Ibs), reflecting dementia‐related genetic background accumulation due to reduced natural selection, as detailed in a previous publication (You and Henneberg, 2022). Aging in females was represented by life expectancy at birth, sourced from the World Bank 2018. Urban living was indexed by urbanization, measured as the percentage of the population living in urban areas in 2018, also sourced from the World Bank.
***p < 0.001. Number of country range, 116–126.
The analyses in Table 4 revealed significant associations between the mean age at first childbirth and both female dementia prevalence and sex disparity in dementia prevalence across various country groupings. Globally, the correlations were strong and highly significant for female dementia prevalence (r = 0.852, p < 0.001) and sex disparity (r = 0.795, p < 0.001) (Table 4), indicating that a higher age at first childbirth is associated with higher dementia prevalence and greater sex disparity. Stratification by income levels showed that LMICs exhibited stronger correlations (r = 0.717, p < 0.001 for female prevalence; r = 0.651, p < 0.001 for sex disparity) compared to high‐income countries (r = 0.488, p < 0.001 and r = 0.363, p < 0.050, respectively) (Table 4). Fisher r‐to‐z comparisons confirmed these differences, with LMICs demonstrating significantly stronger associations (p < 0.050). United Nations classifications underscored notable disparities: the correlation between age at first childbirth and dementia outcomes was significantly stronger in developing countries (r = 0.852, p < 0.001) than in developed ones (r = 0.496, p < 0.001), with Fisher r‐to‐z tests confirming a statistically significant difference (p < 0.001). Additionally, regional comparisons revealed varying strengths of association. The Americas, Western Pacific, and Europe showed the strongest links between delayed childbirth and dementia indicators, whereas weaker or nonsignificant associations were observed in Africa and the Eastern Mediterranean.
Table 4.
Age at first childbirth predicting female dementia prevalence rate and sex disparity in dementia prevalence in various country groupings.
| Country groupings | Female dementia prevalence | Sex disparity in dementia prevalence | Sample size | ||
|---|---|---|---|---|---|
| Pearson's r | Nonparametric rho | Pearson's r | Nonparametric rho | n | |
| Worldwide | 0.852*** | 0.868*** | 0.795*** | 0.825*** | 126 |
| World Bank income classifications | |||||
| High income | 0.488*** | 0.266 | 0.363* | 0.220 | 41 |
| Upper middle income | 0.660*** | 0.675*** | 0.580*** | 0.628*** | 28 |
| Low middle income | 0.613*** | 0.569*** | 0.551*** | 0.468** | 37 |
| Low income | 0.403 | 0.693*** | 0.266 | 0.351 | 19 |
| Low‐ and middle‐income countries (LMIC) | 0.717*** | 0.741*** | 0.651*** | 0.643*** | 84 |
| Fisher r‐to‐z transformation (LMIC vs. high‐income) |
z = 1.87, p < 0.050 |
z = 3.46, p < 0.001 |
z = 2.02, p < 0.050 |
z = 2.74, p < 0.010 |
|
| United Nations (common practice) | |||||
| Developed | 0.496*** | 0.433** | 0.327*** | 0.308* | 45 |
| Developing | 0.852*** | 0.868*** | 0.795*** | 0.825*** | 126 |
| Fisher r‐to‐z transformation (Developing vs. developed) |
z = 4.03, p < 0.001 |
z = 4.82, p < 0.001 |
z = 4.17, p < 0.001 |
z = 4.78, p < 0.001 |
|
| WHO regions | |||||
| Africa | 0.371* | 0.369* | 0.287 | 0.244 | 36 |
| Americas | 0.847*** | 0.538* | 0.861*** | 0.500* | 15 |
| Eastern Mediterranean | 0.647 | 0.600 | 0.043 | 0.200 | 6 |
| Europe | 0.619*** | 0.578*** | 0.436** | 0.445** | 48 |
| South‐East Asia | 0.663 | 0.762* | 0.776* | 0.810* | 8 |
| Western Pacific | 0764** | 0.809*** | 0.701* | 0.718** | 12 |
| Countries grouped with various factors | |||||
| Asia Cooperation Dialogue | 0.715*** | 0.696*** | 0.700*** | 0.724*** | 18 |
| Asia‐Pacific Economic Cooperation | 0.661** | 0.700** | 0.608* | 0.675** | 14 |
| Arab world | 0.653 | 0.700 | 0.016 | 0.300 | 5 |
| European Economic Area | 0.253 | 0.191 | 0.011 | 0.076 | 29 |
| European Union | 0.224 | 0.182 | −0.060 | 0.050 | 27 |
| English as official language | 0.854*** | 0.809*** | 0.7835*** | 0.769*** | 30 |
| Latin America and Caribbean | 0.394 | 0.289 | 0.429 | 0.237 | 13 |
| Organisation for Economic Co‐operation and Development | 0.434** | 0.283 | 0.310 | 0.222 | 35 |
| Southern African Development Community | 0.762** | 0.591* | 0.747** | 0.495 | 13 |
| Shanghai Cooperation Organization | 0.598* | 0.765*** | 0.568* | 0.730*** | 17 |
Note: Data source and definition: The mean age at first childbirth, representing age at first childbearing, was obtained from the World Fertility Report 2012 by the United Nations. Dementia prevalence data, including female prevalence and sex disparity (calculated as the difference between female and male prevalence rates), were sourced from the Institute for Health Metrics and Evaluation, University of Washington, with rates reported as the number of new cases per 100,000 individuals diagnosed in 2021.
p < 0.05, **p < 0.01, ***p < 0.001. Number of country range, 116–126.
4. Discussion
This study highlights the critical influence of age at first childbirth on female dementia outcomes and sex disparities in dementia prevalence, based on data from 204 countries. As a country‐level ecological analysis, the findings reflect population‐level associations and should not be interpreted as causal at the individual level. Future research using individual‐level, longitudinal data is essential to confirm these patterns, clarify causal pathways, and inform targeted prevention strategies.
4.1. Biological Implications of Delayed Childbirth
The observed relationship between age at first childbirth and dementia prevalence may be attributed to biological mechanisms, including cumulative estrogen exposure [5, 14]. Estrogen plays a protective role in brain health by promoting neuronal growth and reducing inflammation [11], potentially mitigating neurodegenerative processes [12]. Delayed childbirth may disrupt these hormonal trajectories, leading to extended periods of low estrogen levels, which could increase susceptibility to cognitive decline [13]. Additionally, delayed childbearing is often linked to prolonged exposure to environmental and occupational stressors [37], further compounding dementia risk over time [38]. These findings align with previous studies suggesting that reproductive timing significantly impacts overall health and underscore the importance of considering reproductive factors in dementia research [39].
While age at first childbirth has been proposed as a potential risk factor for dementia [30], this study focuses specifically on female dementia prevalence. This approach aligns with earlier research that explores the relationship between female reproductive behaviour and dementia risk [40], taking into account the unique biological, psychological, and social factors influencing women's health outcomes.
Late childbirth may also imply a lower fertility rate, as most women cease childbearing at around the age of 50 [41, 42]. From Darwin's evolutionary perspective, higher total fertility rates reflect reduced reliance on birth control, thereby allowing for greater biological variation in fertility [43, 44]. Even a small degree of this variation creates opportunities for natural selection to operate [43].
We acknowledge that the strong associations observed in this ecological study should not be interpreted as diminishing the importance of other well‐established dementia risk factors, such as those identified in the 2024 Lancet Commission report by Livingston et al. [1]. Rather, reproductive timing may serve as a population‐level proxy for a broad constellation of social, behavioural, and environmental exposures that also influence dementia risk. The factors influencing the decision to delay childbirth may overlap with those that increase vulnerability to dementia. Additionally, the experience of parenting itself may introduce exposures that can affect cognitive health over time. These exposures may include both risk‐enhancing factors, such as stress, financial strain, and sleep deprivation, and potentially protective factors, such as increased physical activity, social engagement, emotional connection, and intellectual stimulation. The timing and duration of these exposures may play an important role in shaping long‐term dementia outcomes. These considerations further support the need for individual‐level, longitudinal research to clarify causal pathways and mechanisms. Sociocultural and economic shifts influencing reproductive patterns and health. Delayed childbirth is often linked to urbanization, higher education, and improved healthcare access, which generally enhance health outcomes. Urbanization improves medical services, infrastructure, and preventive care, while education increases health awareness, promotes healthy behaviours, and empowers informed decision‐making.
4.2. Sociocultural and Economic Contexts
Sociocultural and economic shifts influencing reproductive patterns and health [41]. Delayed childbirth is often linked to urbanization, higher education, and improved healthcare access, which generally enhance health outcomes [45, 46]. Urbanization improves medical services, infrastructure, and preventive care, while education increases health awareness, promotes healthy behaviours, and empowers informed decision‐making [47].
However, these societal advancements can also introduce unintended risks for dementia. Urbanized lifestyles are often characterized by sedentary behaviour, with reduced physical activity contributing to cardiovascular and metabolic health challenges that are known risk factors for cognitive decline [48]. Furthermore, dietary changes, including increased consumption of processed and calorie‐dense foods, can exacerbate these metabolic issues, potentially elevating the risk of neurodegenerative conditions [49, 50]. Elevated stress levels from juggling career demands and family responsibilities in urban settings can further compound these risks by negatively affecting mental and physical health over time [51].
These findings highlight the complex interplay between societal progress and health risks, emphasizing the need for holistic public health strategies that mitigate the unintended consequences of modern lifestyles while promoting the benefits of education and healthcare access [52].
4.3. Family Support and Healthy Lifestyle
A later age at first childbirth often correlates with lower total fertility rates, as most women experience menopause, marking the end of natural fertility, between 45 and 55 years of age. Total fertility rate, a common measure of family size, suggests that delayed childbirth typically results in smaller family sizes and, consequently, smaller family networks [42, 53].
Family support and interactions within larger family networks have been shown to contribute to improved health outcomes by fostering greater life satisfaction and encouraging healthy behaviours [29]. Within these networks, family members are more likely to remind or recommend necessary medical examinations and promote healthier lifestyles. This supportive dynamic positively influences psychological well‐being, which is associated with practicing healthy habits and understanding the benefits of regular medical examinations. Bai et al. found that individuals with positive psychological well‐being are more inclined to adopt healthy lifestyles and reduce their risk of chronic conditions, such as cancer [29, 54, 55]. Similar mechanisms may apply to dementia risk reduction, where family interactions and support act as protective factors against cognitive decline [30].
4.4. Differential Impact of Age at First Childbirth in High‐Income and Low‐ and Middle‐Income Countries
Subgroup analyses reveal significant associations between age at first childbirth and dementia prevalence, with patterns differing between low/middle‐income and high‐income countries. In low‐ and middle‐income countries, where childbearing typically begins earlier, the timing of first birth appears to be more strongly associated with dementia outcomes. This disparity likely stems from differences in healthcare access, educational attainment, and socioeconomic resources [56].
Limited healthcare infrastructure and inadequate resources in low‐ and middle‐income countries can amplify the impact of reproductive timing on long‐term health outcomes [57]. The combination of earlier childbearing and restricted healthcare access creates unique vulnerabilities affecting dementia risk. By contrast, in high‐income countries, better healthcare access and health literacy appear to moderate the impact of childbirth timing on cognitive health [58].
These findings underscore the relationship between socioeconomic factors and reproductive history in determining dementia risk. Effective public health strategies should address these disparities through improved healthcare access and education, with interventions tailored to local contexts and typical patterns of reproductive timing [59].
Comparative studies focusing on high‐ and low‐income settings are particularly needed to unravel the contextual influences on reproductive timing and dementia risk [60]. Qualitative research exploring women's lived experiences and decision‐making processes around childbirth could provide valuable insights into the sociocultural dimensions of reproductive health [61]. Additionally, investigating the role of male reproductive history and its potential contributions to sex disparities in dementia outcomes could offer a broader perspective on the interplay between sex and cognitive health [62].
4.5. Sex Disparities in Dementia Prevalence
The study's findings regarding sex disparities in dementia prevalence are particularly notable. Women consistently exhibit higher dementia prevalence rates than men [2, 3], a disparity exacerbated by delayed childbirth. This could be partially attributed to women's longer life expectancy. Even after controlling for ageing in females, the age at first childbirth remained moderately and significantly correlated with sex disparities. Residual factors may contribute to an increased cumulative risk of developing dementia.
Additionally, hormonal differences, social roles, and caregiving responsibilities may contribute to heightened vulnerability among women [63, 64]. The results of this study emphasize the necessity of addressing sex‐specific risk factors in dementia prevention strategies. While ageing remains a dominant predictor of dementia prevalence, reproductive history emerges as a key factor influencing the magnitude of sex disparities, highlighting the associations between reproductive history and dementia outcomes.
5. Limitations and Strengths
This study stands out for its comprehensive data set, rigorous methodologies, and global perspective, offering valuable insights into the relationship between reproductive history and dementia outcomes. However, it is limited by ecological bias or the “ecological fallacy”, where population‐level findings may not directly apply to individuals. Furthermore, the analyses were not adjusted for many established individual‐level dementia risk factors, including cardiovascular conditions, depression, and physical inactivity, due to data limitations inherent to ecological designs. Additionally, the dementia prevalence rates used may underrepresent the true burden of disease, as they are based on diagnosed cases and may not capture undiagnosed or unreported individuals, especially in countries with limited healthcare access. Broad indicators like GDP and urbanization provide context but do not capture individual experiences. The cross‐sectional design also limits the ability to explore the longitudinal relationship between age at first childbirth and dementia risk. Longitudinal studies with individual‐level data are necessary to establish causal pathways and clarify the biological, psychological, and social determinants of dementia. Nonetheless, the consistent and significant correlations observed in this study lend credibility to the association and underscore the importance of cohort studies with larger samples and extended follow‐ups.
Several potential confounders, including healthcare access, cultural attitudes, nutrition, and environmental factors like UV exposure and temperature, could not be analysed due to data limitations. While residual confounding remains, the study's robust analytical framework enhances reliability. Future research should examine how factors like early‐life nutrition, maternal healthcare access, and cardiovascular health interact with reproductive history to influence dementia risk.
The ecological approach allowed extensive data inclusion, avoiding some biases of individual‐level studies, and GDP PPP served as a proxy for key factors like age at first childbirth, healthcare, and education. However, collecting all related data as separate confounders was not feasible.
While limited by cross‐sectional design and ecological bias, this study's strengths in methodology and scope underscore significant trends between reproductive history and dementia outcomes. These findings inform future research and public health strategies that address socioeconomic, cultural, and biological factors.
6. Public Health and Policy Implications
The findings highlight crucial implications for public health policy and dementia prevention. Recognizing the role of reproductive history in dementia risk emphasizes the need for sex‐specific strategies. Future individual‐level research is needed to determine whether delayed childbirth is associated with dementia risk, which could eventually inform health promotion and educational efforts. Addressing socioeconomic disparities requires enhancing healthcare infrastructure, ensuring maternal care access, and raising awareness about dementia risks, especially in low‐ and middle‐income countries.
Integrating reproductive health into dementia prevention frameworks is essential. Reproductive health programs can include cognitive health education to emphasize the long‐term effects of reproductive timing. Sex‐sensitive healthcare policies prioritizing women's health across the lifespan can further reduce disparities. Collaboration among governments, healthcare providers, and community stakeholders is critical for creating culturally relevant interventions tailored to diverse populations.
7. Conclusion
Delayed childbirth may be a risk factor for female dementia development and may exacerbate sex disparities in dementia prevalence. By examining reproductive history in individual‐level studies, future research may clarify its relevance to dementia prevention efforts. The findings of these studies provide a basis for targeted interventions and policies. Addressing biological, sociocultural, and economic factors is essential to reducing dementia's global burden, particularly among women. This study highlights the need for sex‐sensitive strategies and actionable solutions tailored to diverse global contexts, advancing both scientific understanding and effective public health approaches.
Author Contributions
W.Y. conceptualized the research idea, developed the research hypothesis and study design, and independently conducted data collection and analysis. W.Y. also interpreted the statistical relationships, contextualized the findings, and drafted the manuscript. The final manuscript was solely reviewed, edited, and approved by W.Y. The author, Dr. Wenpeng You, had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics Statement
The data used in this study are secondary and publicly accessible, sourced from previous publications and global repositories maintained by the World Health Organization and the World Bank. As these data are aggregated at the population level and contain no identifiable information about individuals or communities, ethical approval and informed consent were not required. This study was classified as involving only negligible risk and using non‐identifiable human data. Accordingly, it was exempted from ethical review by the Office of Research Ethics, Compliance, and Integrity (ORECI) at the University of Adelaide under Ethics Approval Numbers 36289 and 35404.
Consent
The authors have nothing to report.
Conflicts of Interest
The author declares no conflicts of interest.
Transparency Statement
The author, Wenpeng You, affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Acknowledgments
Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
Data Availability Statement
The author confirms that all data used in this study were obtained from publicly accessible data sets provided by recognized international organizations, including the Institute for Health Metrics and Evaluation (IHME), the United Nations Population Division, and the World Bank. The sources and details of these data sets are fully described in Section Results, 2. No restrictions apply to accessing these data.
References
- 1. Livingston G., Huntley J., Sommerlad A., et al., “Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission,” Lancet 396, no. 10248 (2020): 413–446, 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. IHME , Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results (Institute for Health Metrics and Evaluation (IHME), 2020), http://ghdx.healthdata.org/gbd-results-tool. [Google Scholar]
- 3. Nebel R. A., Aggarwal N. T., Barnes L. L., et al., “Understanding the Impact of Sex and Gender in Alzheimer's Disease: A Call to Action,” Alzheimer's & Dementia: Journal of the Alzheimer's Association 14, no. 9 (2018): 1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.“Global Status Report on the Public Health Response to Dementia: Executive Summary,” WHO, published 2021, https://iris.who.int/bitstream/handle/10665/344707/9789240034624-eng.pdf?utm_source=chatgpt.com.
- 5. Gong J., Harris K., Peters S. A. E., and Woodward M., “Reproductive Factors and the Risk of Incident Dementia: A Cohort Study of UK Biobank Participants,” PLoS Medicine 19, no. 4 (2022): e1003955, 10.1371/journal.pmed.1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.“Pregnancy and Reproductive History May Impact Dementia Risk Plus, the Move to Re‐Think the Impact of Hormone Therapy on Cognition 2018,” AAIC, published December 30, 2024, https://aaic.alz.org/releases_2018/AAIC18-Mon-women-dementia-risk.asp.
- 7. You W., Henneberg R., and Henneberg M., “Healthcare Services Relaxing Natural Selection May Contribute to Increase of Dementia Incidence,” Scientific Reports 12, no. 1 (2022): 8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pirkle C. M., de Albuquerque Sousa A. C. P., Alvarado B., and Zunzunegui M. V., “Early Maternal Age at First Birth Is Associated With Chronic Diseases and Poor Physical Performance in Older Age: Cross‐Sectional Analysis From the International Mobility in Aging Study,” BMC Public Health 14 (2014): 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Molina‐García L., Hidalgo‐Ruiz M., Cocera‐Ruíz E. M., Conde‐Puertas E., Delgado‐Rodríguez M., and Martínez‐Galiano J. M., “The Delay of Motherhood: Reasons, Determinants, Time Used to Achieve Pregnancy, and Maternal Anxiety Level,” PLoS One 14, no. 12 (2019): e0227063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nea F. M., Kearney J., Livingstone M. B. E., Pourshahidi L. K., and Corish C. A., “Dietary and Lifestyle Habits and the Associated Health Risks in Shift Workers,” Nutrition Research Reviews 28, no. 2 (2015): 143–166. [DOI] [PubMed] [Google Scholar]
- 11. Thakkar R. D., Wang R., Sareddy G. R., Vadlamudi R. K., and Brann D. W., “Estrogen Neuroprotection and Anti‐inflammation Actions in the Hippocampus,” in Estrogens and Memory: Basic Research and Clinical Implications (2020), 401. [Google Scholar]
- 12. Wise P. M., Dubal D. B., Wilson M. E., Rau S. W., and Böttner M., “Minireview: Neuroprotective Effects of Estrogen—New Insights Into Mechanisms of Action,” Endocrinology 142, no. 3 (2001): 969–973. [DOI] [PubMed] [Google Scholar]
- 13. Villa A., Vegeto E., Poletti A., and Maggi A., “Estrogens, Neuroinflammation, and Neurodegeneration,” Endocrine Reviews 37, no. 4 (2016): 372–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jett S., Malviya N., Schelbaum E., et al., “Endogenous and Exogenous Estrogen Exposures: How Women's Reproductive Health Can Drive Brain Aging and Inform Alzheimer's Prevention,” Frontiers in Aging Neuroscience 14 (2022): 831807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Testa A., Mijares L., and Jackson D. B., “The Impact of Prior Incarceration on Cognitive Trajectories Among Older Adults: Evidence From the Health and Retirement Study,” Journals of Gerontology, Series B: Psychological Sciences and Social Sciences 80, no. 2 (2024): gbae194, 10.1093/geronb/gbae194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quashie N. T., Saenz J. L., Sheehan C., Lopez A., “Gendered Marital Power, Depression, and Cognition Among Older Adults in Mexico,” Journals of Gerontology, Series B: Psychological Sciences and Social Sciences 80, no. 5 (2024): gbae193, 10.1093/geronb/gbae193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raichlen D. A., Aslan D. H., Sayre M. K., et al., “Sedentary Behavior and Incident Dementia Among Older Adults,” Journal of the American Medical Association 330, no. 10 (2023): 934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.“Cutting Back on Ultra‐Processed Foods Linked With Lower Dementia Risk,” Harvard, published 2022, https://www.health.harvard.edu/mind-and-mood/cutting-back-on-ultra-processed-foods-linked-with-lower-dementia-risk?utm_source=chatgpt.com.
- 19. Lane M. M., Gamage E., Du S., et al., “Ultra‐Processed Food Exposure and Adverse Health Outcomes: Umbrella Review of Epidemiological Meta‐Analyses,” BMJ 384 (2024): e077310, 10.1136/bmj-2023-077310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mielke M. M., Aggarwal N. T., Vila‐Castelar C., et al., “Consideration of Sex and Gender in Alzheimer's Disease and Related Disorders From a Global Perspective,” Alzheimer's & Dementia: Journal of the Alzheimer's Association 18, no. 12 (2022): 2707–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi L. C. and Ehrlich J. R., “Closing the Data Gaps on Trends in Dementia and Related Care in Low‐And Middle‐Income Countries,” Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 79 (2024): S5–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh M., Shekhar C., and Gupta J., “Transition in the Ages at Key Reproductive Events and Its Determinants in India: Evidence From NFHS 1992–93 to 2019–21,” BMC Women's Health 23, no. 1 (2023): 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.“Society at a Glance 2024: OECD Social Indicators,” OECD, published 2024, https://www.oecd.org/en/publications/2024/06/society-at-a-glance-2024_08001b73.html#report.
- 24.“World Fertility Data 2008,” The United Nations, published 2012, http://www.un.org.
- 25.“Global Burden of Disease Study 2019 (GBD 2019) Covariates 1980–2019,” IHME, published March 12, 2023, https://ghdx.healthdata.org/record/global-burden-disease-study-2019-gbd-2019-covariates-1980-2019.
- 26.“Table A.6. Mean Age at First Birth (United Nations, Department of Economic and Social Affairs, Population),” United Nations, published 2013, https://www.un.org/en/development/desa/population/publications/dataset/fertility/wfr2012/Data/Data_Sources/TABLE%20A.6.%20Mean%20age%20at%20first%20birth.xlsx.
- 27.“Life Expectancy at Birth, Total (Years),” The World Bank, published April 5, 2022, https://data.worldbank.org/indicator/SP.DYN.LE00.IN.
- 28. Deckers K., Cadar D., van Boxtel M. P. J., Verhey F. R. J., Steptoe A., and Köhler S., “Modifiable Risk Factors Explain Socioeconomic Inequalities in Dementia Risk: Evidence From a Population‐Based Prospective Cohort Study,” Journal of Alzheimer's Disease 71, no. 2 (2019): 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. You W., Rühli F. J., Henneberg R. J., and Henneberg M., “Greater Family Size Is Associated With Less Cancer Risk: An Ecological Analysis of 178 Countries,” BMC Cancer 18, no. 1 (2018): 924, 10.1186/s12885-018-4837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. You W. and Henneberg M., “Large Household Reduces Dementia Mortality: A Cross‐Sectional Data Analysis of 183 Populations,” PLoS One 17, no. 3 (2022): e0263309, 10.1371/journal.pone.0263309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.“Urban Population (% of Total) 2014,” World Bank, published 2022, http://data.worldbank.org.
- 32. Ravindranath V. and Sundarakumar J. S., “Changing Demography and the Challenge of Dementia in India,” Nature Reviews Neurology 17, no. 12 (2021): 747–758, 10.1038/s41582-021-00565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tian W., Cao K., Kwan M. P., Chiu M., and Chen H., “How Does Urbanization Affect the Cognitive Function Among Older Adults: A Geospatial Analysis in China,” Health & Place 88 (2024): 103259. [DOI] [PubMed] [Google Scholar]
- 34. O'Brien R. M., “A Caution Regarding Rules of Thumb for Variance Inflation Factors,” Quality & Quantity 41, no. 5 (2007): 673–690, 10.1007/s11135-006-9018-6. [DOI] [Google Scholar]
- 35. You W., Feng S., and Donnelly F., “Total Meat (Flesh) Supply May be a Significant Risk Factor for Cardiovascular Diseases Worldwide,” Food Science & Nutrition 11, no. 6 (2023): 3203–3212, 10.1002/fsn3.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. You W., Cusack L., and Donnelly F., “A Lack of Nurse Autonomy Impacts Population Health When Compared to Physician Care: An Ecological Study,” Scientific Reports 13, no. 1 (2023): 12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luo J., Beam C. R., and Gatz M., “Is Stress an Overlooked Risk Factor for Dementia? A Systematic Review From a Lifespan Developmental Perspective,” Prevention Science 24, no. 5 (2023): 936–949. [DOI] [PubMed] [Google Scholar]
- 38. García‐Moreno J. A., Cañadas‐Pérez F., García‐García J., and Roldan‐Tapia M. D., “Cognitive Reserve and Anxiety Interactions Play a Fundamental Role in the Response to the Stress,” Frontiers in Psychology 12 (2021): 673596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alonzo A. A., “Long‐Term Health Consequences of Delayed Childbirth: NHANES III,” Women's Health Issues 12, no. 1 (2002): 37–45. [DOI] [PubMed] [Google Scholar]
- 40. Wolfova K., Hubbard R. A., Kearns P. B., et al., “Number of Children and Risk of Dementia: A Cohort Study,” Journal of Epidemiology and Community Health 79 (2025): 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Balasch J. and Gratacós E., “Delayed Childbearing: Effects on Fertility and the Outcome of Pregnancy,” Current Opinion in Obstetrics & Gynecology 24, no. 3 (2012): 187–193. [DOI] [PubMed] [Google Scholar]
- 42. Schmidt L., Sobotka T., Bentzen J. G., and Nyboe Andersen A., “Demographic and Medical Consequences of the Postponement of Parenthood,” Human Reproduction Update 18, no. 1 (2012): 29–43. [DOI] [PubMed] [Google Scholar]
- 43. Henneberg M., “Natural Selection Through Differential Fertility in Human Populations: Quantitative Evaluation of Selection Intensity,” Przeglad Antropologiczny 46 (1980): 21–60. [Google Scholar]
- 44. Darwin C., The Descent of Man, and Selection in Relation to Sex. New ed. (John Murray, 1901). [Google Scholar]
- 45. Zabak S., Varma A., Bansod S., and Pohane M. R., “Exploring the Complex Landscape of Delayed Childbearing: Factors, History, and Long‐Term Implications,” Cureus 15, no. 9 (2023): e46291, 10.7759/cureus.46291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li H., Nawsherwan, Fan C., Mubarik S., Nabi G., and Ping Y. X., “The Trend in Delayed Childbearing and Its Potential Consequences on Pregnancy Outcomes: A Single Center 9‐Years Retrospective Cohort Study in Hubei, China,” BMC Pregnancy and Childbirth 22, no. 1 (2022): 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tripathi S. and Maiti M., “Does Urbanization Improve Health Outcomes: A Cross Country Level Analysis,” Asia‐Pacific Journal of Regional Science 7, no. 1 (2023): 277–316. [Google Scholar]
- 48. Owen N., Salmon J., Koohsari M. J., Turrell G., and Giles‐Corti B., “Sedentary Behaviour and Health: Mapping Environmental and Social Contexts to Underpin Chronic Disease Prevention,” British Journal of Sports Medicine 48, no. 3 (2014): 174–177. [DOI] [PubMed] [Google Scholar]
- 49. Gomes Gonçalves N., Vidal Ferreira N., Khandpur N., et al., “Association Between Consumption of Ultraprocessed Foods and Cognitive Decline,” JAMA Neurology 80, no. 2 (2023): 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Claudino P. A., Bueno N. B., Piloneto S., et al., “Consumption of Ultra‐Processed Foods and Risk for Alzheimer's Disease: A Systematic Review,” Frontiers in Nutrition 10 (2024): 1288749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Neto M., Chambel M. J., and Carvalho V. S., “Work–Family Life Conflict and Mental Well‐Being,” Occupational Medicine 68, no. 6 (2018): 364–369. [DOI] [PubMed] [Google Scholar]
- 52. Faghy M. A., Whitsel L., Arena R., Smith A., and Ashton R. E. M., “A United Approach to Promoting Healthy Living Behaviours and Associated Health Outcomes: A Global Call for Policymakers and Decisionmakers,” Journal of Public Health Policy 44, no. 2 (2023): 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prince C., Sharp G. C., Howe L. D., Fraser A., and Richmond R. C., “The Relationships Between Women's Reproductive Factors: A Mendelian Randomisation Analysis,” BMC Medicine 20, no. 1 (2022): 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. You W., Symonds I., and Henneberg M., “Low Fertility may be a Significant Determinant of Ovarian Cancer Worldwide: An Ecological Analysis of Cross‐Sectional Data From 182 Countries,” Journal of Ovarian Research 11, no. 1 (2018): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bai A., Li H., Huang Y., et al., “A Survey of Overall Life Satisfaction and Its Association With Breast Diseases in Chinese Women,” Cancer Medicine 5, no. 1 (2016): 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mattap S. M., Mohan D., McGrattan A. M., et al., “The Economic Burden of Dementia in Low‐and Middle‐Income Countries (LMICs): a Systematic Review,” BMJ Global Health 7, no. 4 (2022): e007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meherali S., Rehmani M., Ali S., and Lassi Z. S., “Interventions and Strategies to Improve Sexual and Reproductive Health Outcomes Among Adolescents Living in Low‐and Middle‐Income Countries: A Systematic Review and Meta‐Analysis,” Adolescents 1, no. 3 (2021): 363–390. [Google Scholar]
- 58. Rostamzadeh A., Stapels J., Genske A., et al., “Health Literacy in Individuals at Risk for Alzheimer's Dementia: A Systematic Review,” Journal of Prevention of Alzheimer's Disease 7 (2020): 47–55. [DOI] [PubMed] [Google Scholar]
- 59. Tavananezhad N., Bolbanabad A. M., Ghelichkhani F., Effati‐Daryani F., and Mirghafourvand M., “The Relationship Between Health Literacy and Empowerment in Pregnant Women: A Cross‐Sectional Study,” BMC Pregnancy and Childbirth 22, no. 1 (2022): 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zubiagirre U., Ibarrondo O., Larrañaga I., Soto‐Gordoa M., Mar‐Barrutia L., and Mar J., “Comorbidity and Household Income as Mediators of Gender Inequalities in Dementia Risk: A Real‐World Data Population Study,” BMC Geriatrics 24, no. 1 (2024): 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yuill C., McCourt C., Cheyne H., and Leister N., “Women's Experiences of Decision‐Making and Informed Choice About Pregnancy and Birth Care: A Systematic Review and Meta‐Synthesis of Qualitative Research,” BMC Pregnancy and Childbirth 20 (2020): 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Han S.‐L., Liu D. C., Tan C. C., Tan L., and Xu W., “Male‐and Female‐Specific Reproductive Risk Factors Across the Lifespan for Dementia or Cognitive Decline: A Systematic Review and Meta‐Analysis,” BMC Medicine 21, no. 1 (2023): 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pinquart M. and Sorensen S., “Gender Differences in Caregiver Stressors, Social Resources, and Health: An Updated Meta‐Analysis,” Journals of Gerontology Series B: Psychological Sciences and Social Sciences 61, no. 1 (2006): P33–P45. [DOI] [PubMed] [Google Scholar]
- 64. Zygouri I., Cowdell F., Ploumis A., Gouva M., and Mantzoukas S., “Gendered Experiences of Providing Informal Care for Older People: A Systematic Review and Thematic Synthesis,” BMC Health Services Research 21 (2021): 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The author confirms that all data used in this study were obtained from publicly accessible data sets provided by recognized international organizations, including the Institute for Health Metrics and Evaluation (IHME), the United Nations Population Division, and the World Bank. The sources and details of these data sets are fully described in Section Results, 2. No restrictions apply to accessing these data.


