Abstract
Bone defects are a common pathology in bone tissue diseases, and existing therapeutic interventions have significant limitations, highlighting the need for innovative strategies and advanced biomaterials. DNA, traditionally recognized as a prominent genetic material, also possesses exceptional properties as a biological material, making it an ideal nanoscale building block for creating various DNA-based biomaterials, such as DNA framework materials and DNA hydrogels. DNA-based biomaterials offer notable advantages, including structural versatility, biocompatibility, and, crucially, programmability, which position them as promising candidates for bone tissue engineering. This review explores recent advancements in the use of DNA-based biomaterials for bionic mineralization and drug delivery systems, as well as their future potential in this field.
Keywords: DNA nanotechnology, DNA framework materials, DNA hydrogels, Bone tissue engineering, Programmable biomaterials
1. Introduction
Bone defects represent a prevalent clinical pathology within the domain of bone tissue, arising from diverse factors such as severe trauma, tumor resection in bone cancer, congenital diseases, and debridement of infected bone tissue [[1], [2], [3]]. While bone possesses inherent regenerative and remodeling capacities, these mechanisms have limitations [4,5]. When defects surpass a critical threshold, self-repair becomes inadequate, leading to incomplete regeneration or nonunion fractures that necessitate surgical interventions like bone grafting. However, both autologous and allogeneic bone grafts have limitations [[6], [7], [8], [9]]. As a result, there is a growing need for safer and more stable treatment options to overcome the challenges of bone grafting, making this a critical area of research.
Tissue engineering is a bioengineering approach focused on restoring, reconstructing, or replacing damaged or lost tissues and organs. It has achieved significant advancements across various fields [[10], [11], [12], [13]]. Notably, bone tissue engineering holds substantial promise and provides potential alternatives for addressing bone defects [[14], [15], [16]]. Biocompatibility, mechanical stability, and biodegradability are fundamental prerequisites for materials employed in bone tissue engineering [17,18]. To achieve optimal treatment outcomes, replicating the intricate, microscopically porous architecture of native bone is essential, including the appropriate porosity, pore sizes, and robust osteoinductive properties [[19], [20], [21], [22]]. However, available osteogenic materials currently fail to fulfill all these requirements simultaneously, and the osteogenic potential remains limited, underscoring the urgent need for novel biomaterials.
DNA nanotechnology is a rapidly advancing field that exploits the potential of synthetic DNA strands to fold into designed shapes and structures, forming diverse 2D and 3D fine materials and showing extraordinary potential in biomimetic patterns of complex buildings [[23], [24], [25]]. DNA-based biomaterials are known for their exceptional programmability, enabling precise control over their shapes, configurations, sizes, surface chemistries, biological functions, and molecular organization [[26], [27], [28], [29]]. This unique attribute opens up novel avenues in diverse biomedical applications, including but not limited to biosensing, bioimaging, disease diagnosis, drug delivery, and tissue engineering [[30], [31], [32], [33]]. For instance, Cui et al. covalently coupled cysteine protease inhibitors to DNA nanostructures, enabling selective modulation of lysosomal protease activity in tumor-associated macrophages, thereby enhancing antigen presentation and inhibiting tumor growth [34]. Furthermore, highly programmable DNA sequences simplify loading specific therapeutic oligonucleotides and drugs into the delivery system. Immunostimulatory DNA sequences, like unmethylated cytosine-phosphate-guanine motifs, can be loaded into DNA hydrogels for biomedical applications [35,36].
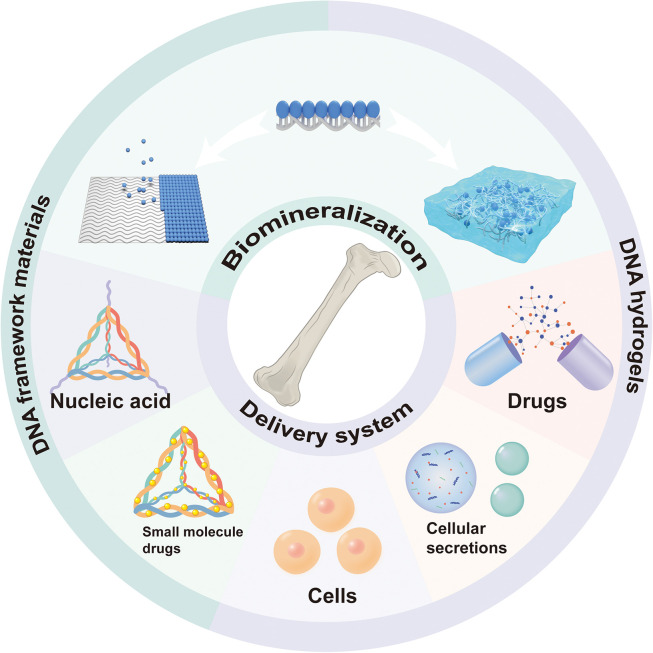
DNA nanotechnology allows for the construction of DNA framework materials and DNA hydrogels, the two main types of DNA-based biomaterials used in bone tissue engineering. Nanomaterials typically display size and morphology distributions within a specific range, challenging precise control. In contrast, DNA nanotechnology allows for the assembly of biomaterials with precise structures, uniform molecular weights, and controllable morphologies and sizes, making them promising materials for bone tissue engineering. DNA framework materials are engineered to provide a scaffold for other functional components, such as molecules, nanoparticles, and living cells, to be assembled into desired configurations [37]. In contrast, DNA hydrogels manifest as 3D networks that consist of DNA molecules as the component, demonstrating a remarkable capacity for water absorption and swelling. These two classes of DNA-based biomaterials exhibit properties that differ from other nanomaterials, mainly due to the programmability of DNA. Their distinctive features include precise size and shape control, high-precision synthesis, tunable physicochemical properties, multifunctionality, responsiveness to specific stimuli, robust biocompatibility, and inherent stability. This impressive spectrum of qualities has propelled programmable DNA-based biomaterials into the forefront of diverse biomedical fields.
The programmability of DNA biomaterials has made a significant impact on bone tissue engineering (Fig. 1). This unique feature allows scientists to create precisely controlled bionic mineralized templates and delivery systems, enabling the controlled release of therapeutic substances. These advancements contribute significantly to the development of more efficient biomaterials for therapeutic applications in bone tissue engineering. Despite the growing interest in DNA biomaterials, a comprehensive review of their role in bone tissue engineering remains lacking [[38], [39], [40], [41], [42]]. Thus, the primary goal of this review is to provide a clear and comprehensive overview of the advancements of DNA biomaterials for bone tissue engineering (Fig. 2). We will begin by exploring the use of DNA biomaterials as bionic mineralized templates. Next, we will examine their application in constructing delivery systems, including DNA frameworks and hydrogels, for bone tissue engineering. Finally, we will discuss the challenges and opportunities ahead, outlining future directions for the sustained development and broader application of DNA biomaterials in bone tissue engineering.
Fig. 1.
A timeline of DNA-based biomaterials relevant to bone tissue engineering. DNA-based biomaterials are used in bone tissue engineering mainly as bionic mineralization templates and delivery systems. Although not a comprehensive account, discoveries and major developments are listed. QCM, quartz crystal microbalance; CaP, calcium phosphate; HAP, hydroxyapatite; SBF, simulated body fluid; TDF, tetrahedral DNA framework; ADSCs, adipose-derived stem cells; nSi, silicate nanodisks; Dex, dexamethasone; pAsp, polyaspartic acid; dsDNA, double-stranded DNA; BMSCs, bone marrow mesenchymal stem cells. TLR4, toll-like receptor 4; VEGF, vascular endothelial growth factor.
Fig. 2.
Schematic representation of DNA-based biomaterials for bone tissue engineering.
2. DNA biomaterials as biomimetic mineralized templates
Biomimetic mineralization plays a crucial role in bone tissue engineering, as it aims to replicate the natural process of mineral deposition in bones. Biomineralization is the natural process that forms hard tissues such as teeth and bones in the human body and plays a crucial role in providing the necessary structural and functional properties of these tissues [43]. Simulating biomineralization offers valuable insights into the hierarchical structure of naturally mineralized tissues and has the potential to advance tissue regeneration strategies [[44], [45], [46], [47]]. The simulation of calcium phosphate (CaP) mineralization is crucial, as it represents a major inorganic component of hard tissues and holds great significance in the field of biomedicine [[48], [49], [50]].
DNA has emerged as an ideal template for biomimetic CaP mineralization due to its high negative charge density, programmable structure, and optimal inter-base pair spacing conducive to mineralization [51,52]. The inter-base pair spacing in DNA aligns with the typical crystallographic spacing observed in CaP minerals along the c-axis [53]. By utilizing DNA as a template, researchers can gain valuable insights into the intricate processes involved in biomineralization and potentially develop innovative approaches for tissue regeneration in biomedicine [54,55]. Furthermore, DNA exhibits potential osteogenic-inducing properties. Studies have shown that DNA can exert osteogenic induction [56], and the release of extracellular phosphate and adenine through DNA degradation promotes osteogenic differentiation [[57], [58], [59]]. In the field of bone tissue engineering, two main types of DNA materials related to CaP biomineralization have been investigated: DNA framework materials and DNA hydrogels. Considering the significant advantages offered by DNA materials in bone tissue engineering and the critical role of CaP biomineralization, this section will focus on these two types of materials.
2.1. Biomimetic mineralization of DNA framework materials
The programmability of DNA enables precise control over the arrangement and spatial organization of binding sites, allowing for the incorporation of bioactive molecules, growth factors, or drugs into the framework. It enables the replication of hierarchical structures found in natural bone tissues, which is essential for achieving enhanced mechanical properties and functionality. Moreover, DNA framework materials exhibit inherent biocompatibility, ensuring compatibility with living systems and minimizing adverse reactions. It is of utmost importance in bone tissue engineering, as the materials must support cell attachment, proliferation, and differentiation. Additionally, the stable and durable properties of DNA framework materials ensure the preservation of the desired structural characteristics throughout the mineralization process and in subsequent applications. This stability is crucial for preserving the integrity of the biomimetic mineralized scaffold and its long-term performance in bone regeneration.
The use of DNA framework materials as templates for biomimetic mineralization enables the incorporation of biologically relevant components, such as calcium phosphate (CaP), which is a major inorganic component of bone tissues. CaP mineralization is essential for imparting the desired mechanical strength and bioactivity to the engineered bone constructs. Based on the CaP mineralization properties of DNA molecules, DNA framework materials can be utilized as templates for CaP mineralization [[60], [61], [62], [63]]. Table 1 summarizes current research using DNA framework materials for CaP biomimetic mineralization. Liu et al. demonstrated the broad applicability of DNA templates in CaP mineralization by successfully forming various 2D and 3D DNA nanostructures for mineralization (Fig. 3A, B) [60]. The results demonstrated the successful formation of a CaP mineralized layer on all prescribed DNA templates after incubation (Fig. 3B). Specifically, the rectangular DNA templates exhibited a mineralized layer with an approximate thickness of 2.5 nm. Moreover, the CaP shell formed on the DNA templates offered the additional advantage of protecting nucleic acids within the complexes in the intracellular environment (Fig. 3C, D). This protective function provides a unique and robust mechanism for intelligent drug delivery, characterized by the ability to precisely control geometric features and maintain long-term in vivo bioactivities.
Table 1.
Comparative study of DNA framework materials as templates for calcium phosphate mineralization.
| Type of DNA framework material | Surface modifier | Mineralization condition | Incubation time | Before mineralization | After mineralization | Ref. | |
|---|---|---|---|---|---|---|---|
| 1D | Three strands double helix | Polyaspartic acid | 1.67 Ca/P mole ratio | 3 h | 1–2 nm (height) | 12.5–63.3 nm (height) | [61] |
| 2D | Triangular DNA origami | N/A | pH 9.0 | 24 h | 1.9 nm (height) | 4.4 nm (height) | [60] |
| Rectangular DNA template | N/A | pH 9.0 | 24 h | 2.0 nm (height) | 4.5 nm (height) | [60] | |
| N/A | 1.33 Ca/P mole ratio, pH 6.5 | 8 h | 2.01 nm (height) | 2.65 nm (height) | [62] | ||
| SSEE peptide | 1.67 Ca/P mole ratio, pH 8.0 | 20–30 min | 2.5 nm (height) | height increase of over 30 nm | [63] | ||
| 3D | TDF | N/A | pH 9.0 | 3 h | 6.8 nm (edge length) | 27.7 nm (diameter) | [60] |
| 12.6 nm (edge length) | 36.6 nm (diameter) | ||||||
| DNA origami rod | N/A | 1.33 Ca/P mole ratio, pH 6.5 | 8 h | 2.01 nm (height) | 5.75 nm (height) | [62] | |
| DNA origami tube | N/A | 1.33 Ca/P mole ratio, pH 6.6 | 8 h | 3.53 nm (height) | 5.20 nm (height) | [62] | |
| DNA nanotube | SSEE peptide | 1.67 Ca/P mole ratio, pH 8.0 | 20–30 min | 6.8 nm (width) | 111.0 nm (height) | [63] | |
Fig. 3.
CaP mineralization of DNA framework materials. (A) Schematic of CaP mineralization of DNA framework material templates with tetrahedral DNA as a detailed example. (B) TEM, FESEM, and AFM images of DNA templates after mineralization, using TDF as an example. Scale bar, 100 nm on the left and 30 nm on the right. (C) Schematic of immunostimulatory oligonucleotides (CpG) delivered using TDF and combined with the mineralization process, and AFM images of TDF-CpG (left) and mineralized TDF-CpG (TDF-CpG-CaP) (right). Solid circles showed TDF-CpGs with typical hollow structures; dashed circles showed collapsed TDF-CpGs. Upon mineralization, the hollow structures of TDF were filled in, but the tail-like structures of CpG remained. Scale bars: 100 nm; AFM height scale bar, 4 nm. (D) Different samples of stimulated macrophage RAW-264.7 expression level of tumor necrosis factor-α. TDF-CpG-CaP showed better immunostimulatory activity. (A-D) Adapted with permission [60]. Copyright 2019, CELL PRESS. (E-G) Biomimetic mineralization of DNA templates using particle attachment crystallization strategy. (E) Schematic of particle attachment crystallization strategy for biomimetic mineralization of DNA templates. (F) Rectangular DNA origami’s (I-origami) size schematic (top), AFM images after mineralization (m-I-origami) (middle), and height statistics (bottom). (G) Schematic of streptavidin pre-immobilized on I-origami that remained bound to biotinylated transferrin after mineralization. Adapted with permission [62]. Copyright 2020, AMER CHEMICAL SOC.
Wu et al. employed a particle attachment crystallization strategy in their study, which enabled a precise, controllable, and slow mineralization process, thereby preserving the intricate structural features encoded by the DNA template [62]. The approach involved saturating the DNA nanostructure with Mg2+, leading to the preferential formation of Posner’s clusters in the bulk solution through the interaction of Ca2+ and PO43−. Subsequently, the exchange of Ca2+ and Mg2+ resulted in the deposition of these clusters onto the DNA template (Fig. 3E). This technique yielded mineralized materials closely matching the DNA template size and retained immobilized functional components, such as streptavidin, for further modifications (Fig. 3F, G). Wu’s approach effectively addresses the challenge of losing DNA template addressability, a significant obstacle encountered in DNA nanostructure mineralization, and holds considerable implications for the precise control of DNA template mineralization.
Further control over the mineralization process can be achieved by modifying additional components present on the DNA template. Polyaspartic acid (pAsp) is a peptide mineral inducer capable of serving as a precursor for CaP crystallization [[64], [65], [66], [67]]. Kim et al. achieved guidance and control of mineralization by covalently attaching pAsp to a three-stranded double-helical DNA structure, resulting in a linear distribution of mineralization along the DNA under specific mineralization conditions (Fig. 4A, B) [61]. However, the mineralized deposits produced in their study are amorphous calcium phosphates, which serve as a precursor that can transform into hydroxyapatite (HAP) under certain conditions. This highly organized HAP nanocrystal represents one of the primary components of bone and is essential for the mechanical properties and physiological functions of mineralized tissues in humans [[68], [69], [70], [71]]. To promote mineralization and regulate HAP formation, Danesi et al. combined DNA framework materials with a mineral-promoting peptide (Fig. 4C, D) [63], which is an extracellular matrix protein found in teeth and bones [72,73]. They designed a functionalized DNA rectangular origami capable of rapid mineralization when subjected to mineralization conditions (Fig. 4D). By increasing the concentration of Ca2+ and extending the incubation period to 24 h, the generated deposits exhibited the characteristic features of HAP (Fig. 4E).
Fig. 4.
CaP mimetic mineralization of surface-modified DNA templates. (A-B) Mineralization of polyaspartate-modified DNA templates. (A) Schematic of the constructed polyaspartate covalently linked three-stranded double helix DNA structure (3sDH-pAsp). (B) AFM images of 3sDH-pAsp incubated under mineralization conditions for 30 min and 3 h Adapted with permission [61]. Copyright 2019, ROYAL SOC CHEMISTRY. (C-E) Mineralization of SSEE peptide-modified DNA templates. (C) AFM images of SSEE peptide-functionalized DNA nanotubes after 20–30 min incubation under mineralized conditions. (D) AFM images of SSEE peptide-functionalized DNA rectangular origami after 20–30 min incubation under mineralized conditions. (E) Raman spectral characterization of HAP (control) and SSEE-functionalized DNA rectangular origami incubated under mineralized conditions for 24 h Confirmation of HAP by the appearance of a weak peak at 960 cm-1. Adapted with permission [63]. Copyright 2021, AMER CHEMICAL SOC.
DNA framework materials serve as versatile, programmable, and biocompatible templates for biomimetic mineralization. Their ability to precisely control mineralization processes, support functional modifications, and maintain stability makes them ideal candidates for developing durable biomimetic scaffolds. The use of DNA as a template for biomimetic mineralization has the potential to enhance the regenerative capacity and functional outcomes of engineered bone constructs, ultimately advancing bone tissue engineering.
2.2. Biomimetic mineralization of DNA hydrogels
Hydrogels have found extensive utility in tissue regeneration engineering. However, in the case of bone, a highly mineralized tissue, conventional hydrogels exhibit significant limitations due to their lack of mineralization capacity. In contrast, mineralized hydrogels more closely resemble the chemical composition and structure of natural bone. These hydrogels exhibit bioactive properties that support cell adhesion, proliferation, and differentiation while offering enhanced mechanical robustness [74]. Furthermore, mineralized hydrogels can act as reservoirs of osteogenic raw materials, progressively releasing inorganic minerals during their in vivo degradation, thereby inciting the stimulation of fresh bone regeneration [75]. Hydrogels embrace two principal mineralization strategies: embedding inorganic phase particles within the hydrogel and autonomous biomimetic mineralization of the hydrogel in a mineralization solution [76]. Xu et al. engineered mineralized hydrogels endowed with elevated strength and toughness, wherein hydroxyapatite (HAP) crystals formed within the hydrogel promoted the attachment, proliferation, and osteogenic differentiation of C2C12 cells in vitro [77].
In bone tissue engineering, DNA hydrogels effectively mimic the natural tissue environment, providing an ideal matrix for cellular growth and tissue regeneration. Additionally, the inherent biocompatibility and biodegradability of DNA render DNA hydrogels a secure and biodegradable scaffold, well-suited for filling bone defect sites and recruiting bioactive constituents to amplify intrinsic bone regeneration mechanisms. As elucidated in the previous section, DNA templates hold the ability to guide the biomimetic mineralization process. Similarly, DNA hydrogels, predominantly composed of DNA, are equally amenable to biomimetic mineralization. The combination of DNA hydrogel properties with biomimetic mineralization strategies offers more possibilities for bone tissue engineering.
Athanasiadou et al. employed a simple mineralization method to prepare CaP-mineralized DNA hydrogels (Fig. 5A, B) [78]. The hydrogels were immersed in a mineralization solution (calcium chloride solution and disodium phosphate solution) for 16 h, and the formation of HAP was confirmed by X-ray diffraction and other analyses. As a biodegradable scaffold, these DNA hydrogels exhibited excellent interaction with osteoblasts, maintaining cellular activity and promoting new bone regeneration in rats. Wu et al. developed DNA hydrogels with an autonomous mineralization system and integrated G4-Hemin DNAzyme functionality using the rolling circle amplification (RCA) technique [79]. In this autonomous mineralization system, pyrophosphatase (PPase) promoted the hydrolysis of the RCA byproduct pyrophosphate (PPi) to form orthophosphate (Pi), which subsequently reacted with calcium ions to form calcium orthophosphate (CaPi) (Fig. 5C). The CaPi species were later deposited within the amplified DNA, resulting in the formation of a mineralized DNA hydrogel (Fig. 5D). The mineralization conferred exceptional biostability to the hydrogel, rendering it resistant to degradation by nucleases and reducing the degradation rate by approximately 75% compared to conventional DNA hydrogels (Fig. 5E). The noteworthy feature of this mineralization strategy lies in its autonomous nature. Unlike passive mineralization methods that involve prolonged immersion in concentrated salt solutions, often leading to the formation of a mineral crust, the proposed approach offers controlled mineralization kinetics and uniform mineral distribution. In addition, this mineralized DNA hydrogel incorporates the catalytic ability of DNAzyme to improve the osteogenic microenvironment by restoring mitochondrial homeostasis, facilitating high-quality bone defect repair (Fig. 5F, G).
Fig. 5.
CaP mineralization of DNA hydrogels. (A-B) DNA hydrogels were mineralized by soaking in the mineralization solution for 16 h. (A) Schematic of the self-assembled DNA hydrogel. (B) SEM images of DNA hydrogels after 16 h of mineralization. Adapted with permission [78]. Copyright 2023, NATL ACAD SCIENCES. (c-g) Autonomous mineralized DNA hydrogel with improved osteogenic microenvironment for bone repair. (C) Schematic of mineralized DNAzyme hydrogel preparation. Autonomous mineralization used byproducts of rolling circle amplification. (D) SEM images of non-mineralized (left) and mineralized (right) DNA hydrogels. (E) The stability of MDH was verified by PAGE electrophoresis and grayscale analysis. (F) DCFH fluorescence assay for reactive oxygen species content in BMSCs under different treatments. The greater intensity of green fluorescence indicated higher intracellular reactive oxygen species content. (G) Micro-CT images after implantation of different hydrogels in rat bone defects. Adapted with permission [79]. Copyright 2023, WILEY-V C H VERLAG GMBH. NH, normal DNA Hydrogel, without mineralization reaction and DNA-Hemin; MH, mineralized DNA Hydrogel, without DNA-Hemin; MDH, mineralized DNAzyme hydrogel; N-BMSC, BMSC in the normal state; Os-BMSC, BMSC in the oxidative stress state.
3. DNA biomaterials as delivery systems
Delivery systems play a critical role in the treatment of various diseases, as they enhance the uptake of therapeutic components, reducing cytotoxicity and improving therapeutic efficiency [80]. In bone tissue engineering, effective bone regeneration requires the targeted delivery of appropriate drugs, cytokines, and therapeutic nucleic acids to the affected site. Therefore, the development of an ideal delivery system for bone tissue engineering is essential to enhance therapeutic efficacy.
Delivery systems commonly used in bone tissue engineering include polymer-based scaffolds, hydrogels, calcium phosphate nanoparticles, liposomes, microspheres and microcapsules [[81], [82], [83]]. These materials rely on overall physicochemical properties, which makes precise control challenging. DNA offers distinct advantages in providing precise control for drug delivery. DNA can be designed into specific structures based on sequence programmability and can be easily endowed with specific functions, enabling intelligent responsiveness and precise recognition of sequences or molecules for controlled or targeted release [84,85]. Meanwhile, DNA materials can be of low immunogenicity and biodegradability, avoiding issues such as poor degradation or toxic accumulation. Herein, we mainly introduce research using DNA framework materials and DNA hydrogels as delivery systems for bone tissue engineering. These systems exhibit great potential for the precise delivery of therapeutic agents to specific target regions, making them highly attractive in regenerative medicine.
3.1. DNA framework materials as delivery systems
In drug delivery, DNA framework materials offer a versatile platform for the encapsulation and controlled release of therapeutic agents. Their programmable design enables the creation of compartments or channels within the framework, allowing drugs to be loaded and released in a controlled manner [86,87]. This precise control over drug release kinetics can enhance therapeutic efficacy, reduce side effects, and improve patient outcomes.
Tetrahedral DNA framework (TDF) is an attractive delivery material in bone tissue engineering. It is a framework DNA nanostructure formed through the self-assembly of four single-stranded DNA and offers several advantages, including high yield, stability, biocompatibility, programmability, and cell-penetrating properties [42,88,89]. Numerous studies have demonstrated that TDFs stimulate osteogenic differentiation, stem cell proliferation and vascular regeneration, and possess anti-inflammatory and antioxidant properties [[90], [91], [92], [93], [94], [95]]. These benefits arise from TDF’s ability to activate signaling pathways like Notch and Wnt/β-catenin, thereby promoting cell proliferation and osteogenic differentiation [90,91]. Besides, TDF exhibits anti-inflammatory and antioxidative effects by inhibiting phosphorylation of MAPK subfamilies, modulating oxidative stress and inflammation in macrophages [[93], [94], [95]]. These characteristics make TDF highly promising for application in bone tissue engineering. Notably, TDF’s ability to enhance cellular endocytosis is a prominent advantage, facilitating cellular entry through a caveolin-mediated pathway without transfection agents [96,97]. As a result, TDF is considered an ideal carrier material for effective application in bone tissue engineering [[98], [99], [100]]. Various methods, such as direct ligation, sticky-end hybridization, embedding, and encapsulation, are commonly employed to utilize TDF as carriers for delivering functional molecules [101]. In the subsequent sections, we will delve into the application of TDF as a carrier material in bone tissue engineering.
3.1.1. Delivery of nucleic acid drugs
MicroRNA (miRNA) and small interfering RNA (siRNA) are nucleic acid drugs that play crucial roles in establishing complex regulatory networks to control biochemical reactions within cells. However, their applications are limited by instability, susceptibility to enzymatic degradation, and insufficient membrane permeability [102,103]. TDF-based delivery systems have emerged as promising approaches to address these challenges and improve cellular uptake efficiency.
In a study conducted by Li et al., MiR335–5p was specifically incorporated into TDFs to form complexes (Fig. 6A) [104]. MiR335–5p, known for its ability to activate the Wnt pathway and promote osteogenic differentiation and new bone formation [[105], [106], [107]], exhibited promising results in bone regeneration. The miRNA and TDF complex significantly increased the expression of alkaline phosphatase and the deposition of calcium nodules in bone marrow mesenchymal stem cells while reducing adipogenic differentiation (Fig. 6B). However, it was observed that TDFs remained bound to miRNA after cellular delivery, potentially limiting downstream biochemical activity.
Fig. 6.
TDF as a delivery system in bone tissue engineering. (A-D) TDF delivery of microRNA. (A) Schematic of MiR@TDF complex formation by TDF loading MiR335–5p. (B) MiR@TDF promoted osteogenic differentiation of BMSCs. Alkaline phosphatase assay (top), alizarin red staining of calcium nodules (middle), Oil red O staining for lipid droplets (bottom). Scale bar = 20 μm. Adapted with permission [104]. Copyright 2021, Wiley-VCH. (C) Schematic of the process of TDF loading MiR2861 through sticky ends. (D) Schematic of enzymatic cleavage to release MiR2861 in the extracellular environment. Adapted with permission [108]. Copyright 2021, WILEY-V C H VERLAG. (E-G) TDF delivery of small molecule drugs applied to bone tissue engineering. (E) Clindamycin (CLI) was loaded onto TDF through electrostatic adsorption. (F) Confocal microscopy images of BMSCs uptake of TDF, TDF-CLI, and ss-DNA. Cy5 labeled TDF, TDF-CLI, and ss-DNA. (G) Bacterial colony counting data of different samples treated for 24 h at 0.25 minimum inhibitory concentration. Adapted with permission [113]. Copyright 2022, ELSEVIER SCIENCE SA.
Another approach introduced bio-switchable nanocomposites using RNase H-responsive sequences to attach sticky-end TDFs (stTDFs) to a model miRNA (MiR-2861), resulting in stTDF-MiR (Fig. 6C) [108]. This system efficiently released MiR-2861 upon cellular entry, subsequently downregulating histone deacetylase 5, upregulating Runx2 protein, and promoting osteogenesis in bone defect areas (Fig. 6D). RNase H facilitated miRNA release by selectively cleaving RNA in DNA/RNA hybrids, optimizing therapeutic efficacy [109]. This study demonstrates a targeted approach for enhancing miRNA function within cells, offering valuable insight into gene-based regenerative therapies.
The TDF-based delivery system leverages the programmability of DNA to achieve controlled release of miRNAs, enhancing delivery accuracy and therapeutic efficiency. This approach provides a reliable platform for a broader range of tissue engineering applications.
3.1.2. Delivery of small molecule drugs
Despite exhibiting promising efficacy, small molecule drugs frequently encounter limitations such as insufficient cellular uptake, toxicity to non-targeted cells, and susceptibility to drug resistance [[110], [111], [112]]. Achieving desired effects may require high doses or repeated administration, leading to serious side effects and poor patient compliance. It is crucial to utilize a suitable drug delivery system to overcome these challenges, improve drug solubility and stability, and reduce toxicity and resistance. TDF-based drug delivery systems have the potential to address these challenges by enhancing drug uptake, enabling targeted delivery, and promoting improved drug accumulation.
TDF-based systems can be constructed as platforms for drug delivery by utilizing the programmability of DNA and the versatility of binding. Through electrostatic interactions, hydrogen bonding, and other interactions, TDFs can be loaded with a range of therapeutic agents, including anti-infectives and anti-inflammatories. Li et al. loaded the anti-infective drug clindamycin (CLI) onto TDF by electrostatic attraction (Fig. 6E) and utilized the carrier properties of TDF to improve the cell penetration and antimicrobial effect of the drug (Fig. 6F, G) to effectively treat infected bone defects [113]. In addition, flavonoids can inhibit inflammation and prevent bone destruction in bone tissue engineering through hydrogen bonding with guanine and cytosine residues inserted into DNA [[114], [115], [116], [117]]. This unique versatility allows TDF-based systems to deliver various therapeutic agents, highlighting their potential across different medical fields.
TDF demonstrates remarkable potential as a carrier in bone tissue engineering (as summarized in Table 2) [[118], [119], [120]]. It facilitates the delivery of therapeutic molecules, including nucleic acids and small molecule drugs, thereby fulfilling the requirements for effective bone regeneration therapy by leveraging its exceptional cellular permeability and tissue penetration abilities. Future research may focus on improving TDF stability and loading efficiency, as well as exploring site-specific functionalization to optimize therapeutic outcomes.
Table 2.
Summary of tetrahedral DNA as delivery systems in bone tissue engineering. TLR4, Toll-like receptor 4.
| Compound loaded |
Mechanism of loading | Mechanisms of treatment | Ref. | |
|---|---|---|---|---|
| Type | Name | |||
| Nucleic acid drugs | MiR335–5p | direct connection | inhibition of Dickkopf-1 expression and upregulation of the Wnt pathway | [104] |
| MiR-2861 | sticky-end hybridization | inhibition of histone deacetylase expression and up-regulation of Runx2 protein expression | [108] | |
| miR-21–5p | sticky-end hybridization | induction of osteogenesis and angiogenesis in the aging microenvironment. | [118] | |
| miR205 | direct connection | activation of TGF-β/BMP signaling pathway and enhancement of both osteogenic and angiogenic functions. | [119] | |
| TLR4-siRNA | sticky-end hybridization | downregulation of TLR4 expression and regulation of macrophage mitochondrial homeostasis | [120] | |
| Small molecule drug | clindamycin | electrostatic adsorption | TDF enhances the antibacterial effect of clindamycin | [113] |
3.2. DNA hydrogels as delivery systems
Hydrogel is a widely used material in bone tissue engineering, serving as a cell carrier that provides anchoring sites for cell adhesion and growth. Additionally, it serves as a reservoir for bioactive compounds and drugs, permitting their sustained and controlled release, thereby orchestrating the augmentation of bone regeneration. Compared to natural and synthetic polymer-based hydrogels, DNA hydrogels stand out for their ability to self-assemble via rationally designed DNA modules under mild conditions, offering distinct advantages. This process occurs under mild conditions, rendering them a favorable choice. Importantly, DNA hydrogels exhibit an inherent capacity for customizable cargo-triggered or sustained release properties, functioning as adept delivery vehicles. Furthermore, DNA’s programmability gives DNA hydrogels enhanced responsiveness to physiological cues, extending beyond conventional abiotic stimuli like pH, light, and temperature [[121], [122], [123]]. These hydrogels can also respond to a range of physiological signals, including nucleic acids, enzymes, and ATP [[124], [125], [126]]. This expanded responsiveness renders DNA hydrogels a highly sought-after delivery medium in bone tissue engineering.
3.2.1. Delivery of cells
Bone marrow mesenchymal stem cells (BMSCs) are widely recognized as optimal seed cells for bone tissue regeneration due to their remarkable proliferative capacity, potential for multi-lineage differentiation, low immunogenicity, and direct involvement in bone tissue regeneration [[127], [128], [129]]. However, BMSCs are limited by their low abundance in stem cell therapy, and the high shear force generated during direct injection of BMSCs can severely damage the cells or even cause cell death [130]. DNA hydrogels have emerged as promising carriers for BMSCs, effectively enriching and delivering these cells. The porous three-dimensional structure, high water content, and low cytotoxicity of DNA hydrogels can mimic the natural extracellular matrix, create a suitable microenvironment for cells, and promote cells’ proliferation, adhesion, and differentiation while maintaining a high degree of cellular activity [[131], [132], [133], [134], [135]]. Furthermore, DNA hydrogels mediate signaling and material exchange between nascent and existing tissues, which is crucial for promoting bone regeneration. Additionally, the programmability of DNA hydrogels enables tunable physicochemical properties, specific capture, and conditionally triggered release of cells. These unique properties position DNA hydrogels as an ideal carrier for delivering BMSCs, ensuring their optimal growth and differentiation for successful bone tissue regeneration.
Overcoming the challenge of the low abundance of BMSCs in bone marrow is crucial for stem cell-based therapies. To address this, Yao et al. developed an innovative method utilizing double-rolling circle amplification (double-RCA) to construct a DNA network structure capable of efficiently capturing BMSCs (Fig. 7A) [136]. This technique involved the generation of two elongated DNA strands through double-RCA, with one strand containing an aptamer sequence for specific cell anchoring. The aptamer exhibits a high affinity for the ALPL protein, which is overexpressed on the membrane surface of BMSCs, ensuring specific anchoring and facilitating the enrichment of BMSCs. The two DNA strands formed a network structure by physical cross-linking, enabling cellular encapsulation that maintains a high level of BMSC activity and the ability to harvest cells by nuclease degradation of the hydrogel under mild conditions (Fig. 7B-D). This approach offers a promising solution for BMSC extraction, advancing stem cell-based therapies.
Fig. 7.
DNA hydrogels as cell carriers in bone tissue engineering. (A-D) Specific capture and release of BMSCs from DNA hydrogels generated by double-rolling circle amplification. (A) Schematic of the formation of double-rolling circle amplified DNA network structure, specific capture of BMSCs and nuclease-triggered release. The Apt19S aptamer has a high affinity for ALPL proteins on the membranes of BMSCs and ensures cell-specific anchoring. (B) Microscopic images of nuclease-triggered released BMSCs (left) and after 24 h of culture (right). Live and dead cells were stained with calcein-AM (green) and propidium iodide (PI, red), respectively. (C) Fluorescence microscopy images of cell mixtures of BMSCs and SMCs before (left) and after cell capture (right). BMSCs were stained with CM-Dil (red), and SMCs were stained with CM-DiO (green). SMCs, smooth muscle cells. (D) Statistics of the capture frequency of BMSCs in (C). This method was able to pick out BMSCs at a frequency of up to 81%. Adapted with permission [136]. Copyright 2020, AMER CHEMICAL SOC. (E-G) DNA hydrogels that provided efficient protection for BMSCs. (E) Schematic of the preparation of DNA hydrogels loaded with BMSCs. (F) Viability of BMSCs stained with calcein-AM (green, live) and PI (red, dead) after bidirectional rubbing in conventional liquid media or DNA hydrogels. (G) Percentage survival of BMSCs after bidirectional rubbing. Adapted with permission [137]. Copyright 2021, WILEY-V C H VERLAG GMBH.
Ensuring efficient delivery and long-term survival of cells is crucial for enhancing the effectiveness of stem cell-based therapies. However, high shear forces during cell injection can damage cells and hinder therapeutic outcomes. Yan et al. demonstrated that DNA hydrogels loaded with BMSCs could protect cells from high shear forces during injection (Fig. 7E) [137]. Through the supramolecular dynamic interactions of DNA (where DNA double strands unwind under deformation and reform when stress is relieved), the hydrogel exhibits shear-thinning properties, minimizing shear damage and enhancing cell protection during delivery (Fig. 7F, G). Although this approach may not fully meet the mechanical demands for treating large bone defects, the study’s exploration of achieving exceptional cyto-protection using DNA hydrogels introduces novel perspectives for bone defect treatments. These insights have significant implications for preserving seed cell viability thus enhancing therapeutic efficiency.
3.2.2. Delivery of cellular secretions
Cellular secretions play a critical role in treating bone tissue defects, containing various substances with therapeutic potential [[138], [139], [140], [141]]. Among these substances, interleukin 10 (IL-10) is a cytokine that has bone regenerative effects by promoting mesenchymal stem cell adhesion, migration, and osteogenic differentiation, while also providing anti-inflammatory effects [142]. However, the short half-life of IL-10 and the need for multiple doses present challenges, highlighting the importance of achieving sustained cytokine delivery [143]. To address this challenge, Li et al. developed a strategy for sustained IL-10 delivery by incorporating it into a physically cross-linked DNA hydrogel [144]. This approach could extend to other growth factors in bone repair, such as platelet-derived growth factor, fibroblast growth factor, and vascular endothelial growth factor, offering a promising solution to facilitate successful bone regeneration.
Vasculature is crucial in bone repair, and vascular endothelial growth factor (VEGF) supports vascularized bone regeneration [145]. However, its application is hindered by its rapid degradation and deactivation in vivo. The earlier discussed technique involving the direct physical encapsulation of cellular growth factors within DNA hydrogels emerges as a strategy capable of preserving the functionality of these factors, ensuring sustained release. The inclusion of nanocomponents, such as black phosphorus nanosheets (BPNSs), further enhances the hydrogel’s release capacity. Notably, black phosphorus nanosheets (BPNSs) stand out due to their noteworthy biomineralization properties and osteoinductive potential [[146], [147], [148]]. These nanosheets create dynamic networks, effectively extending VEGF release and enhancing both scaffold mechanics (Fig. 8A-C) [149]. These approaches show promise in overcoming cytokine delivery challenges, promoting effective bone regeneration, and preventing complications from inadequate vascularization at bone repair sites.
Fig. 8.
Sustained release of VEGF from BPNS-DNA hydrogel to promote vascularized bone regeneration. (A) Schematic of incorporation of BPNSs loaded with VEGF into DNA hydrogel combined with 3D printed polycaprolactone scaffold to promote vascularized bone regeneration. (B) Release profiles of VEGF in two types of DNA hydrogel scaffolds. (C) Immunohistochemical staining images of OCN and CD31 after 4 weeks of repairing cranial defects in rats with different samples, the yellow-brown area is the positive expression area of OCN and CD31. Adapted with permission [149]. Copyright 2022, KEAI PUBLISHING LTD.
Exosomes are extracellular vesicles containing proteins, lipids, and nucleic acids that mediate intercellular interactions and are essential for bone cell communication. They play crucial roles in various stages of bone defect healing, such as regulating the secretion of inflammatory factors, promoting angiogenesis, and promoting cell differentiation [150]. As promising materials for tissue regeneration, exosomes offer advantages such as low immunogenicity and high stability, positioning them as potential alternatives to stem cell therapy [151,152]. However, the rapid clearance of exosomes from the bloodstream following injection poses a limitation to their administration [153]. Thus, developing suitable delivery systems is essential for achieving controlled and sustained exosome release for effective bone regeneration. DNA hydrogels, with their excellent editability and biocompatibility, offer numerous possibilities for exosome delivery.
For bone defects in patients with diabetes mellitus (DM), attaining optimal therapeutic outcomes can be challenging due to metabolic imbalances and the overexpression of pro-inflammatory markers such as matrix metalloproteinases (MMPs), which impede bone healing [[154], [155], [156]]. To address this, Jing et al. developed a PEG/DNA hybrid hydrogel system responsive to MMP-9, a pathological marker (Fig. 9A, B) [157]. These hydrogels, loaded with exosomes from stem cells of the apical papilla (SCAP-Exo), show superior angiogenic and osteogenic properties, effectively promoting vascularized bone regeneration (Fig. 9C, D). Leveraging the programmability of DNA, researchers designed DNA linkers containing MMP-9 responsive sequences. MMP-9 exhibits a high affinity for this DNA sequence. In the absence of activation, the DNA linkers help maintain the structural stability of the hydrogel. However, in the pathological regions of DM rats, MMP-9 binds to the DNA, causing the loss of the linker’s functionality. This results in the collapse of the hydrogel network and the subsequent release of the encapsulated exosomes, promoting bone regeneration.
Fig. 9.
DNA hydrogels for controlled release of exosomes are applied in bone tissue engineering. (A-D) Exosomes were directly physically encapsulated in a dynamic bio-responsive PEG/DNA hybrid hydrogel for vascularized bone regeneration in diabetes mellitus (DM) rats. (A) Schematic of the synthesis and behavior of SCAP-Exo-loaded PEG/ DNA hybrid hydrogel in response to MMP-9 stimulation. (B) Exosome release profile of this hydrogel in PBS and MMP-9 solution. (C) Immunofluorescence staining images of CD31 (angiogenic marker, red) after bone defects in DM rats with different sample treatments. Scale bar = 100 μm. (D) Micro-CT images of bone defect areas in DM rats with different sample treatments. Adapted with permission [157]. Copyright 2022, AMER CHEMICAL SOC. (E) Schematic of sustained or light-controlled release of exosomes achieved by tethering exosomes in hydrogel. Exosomes were functionalized by ATRP initiator and cholesterol-modified DNA double strands (Chol-dsDNA-iBBr), and the polymer chains were grafted directly from the exosomes to form exosome-tethered hydrogels. Modification of the DNA strand with a photocleavable group, p-nitrophenyl, enabled the photocontrolled release of exosomes. Adapted with permission [158]. Copyright 2022, AMER CHEMICAL SOC.
Yereni et al.’s study cleverly leveraged the functionalization and programmability of DNA by designing a cholesterol-modified DNA strand (Fig. 9E) [158]. The cholesterol moiety binds to the lipid membrane of exosomes via non-covalent interactions, anchoring the exosomes, while the other end of the DNA strand is modified with a 5′-α-bromoisobutyrate group, enabling covalent bonding with the hydrogel backbone to secure the exosomes within the hydrogel for sustained release. Additionally, a p-nitrophenyl group was incorporated as a photocleavable linker, enabling light-triggered exosome release. This approach significantly extended the duration of exosome release, achieving a sustained release over one month, surpassing the release duration obtained by physical encapsulation with DNA hydrogels alone. It offers a promising strategy to enhance the therapeutic efficacy of exosomes in bone tissue engineering.
3.2.3. Delivery of small molecule drugs
In bone tissue engineering, the softness and tunable fluidic properties of DNA hydrogels enable them to be injected into bone defect sites via syringes, fulfilling the need for filling irregular bone defects. Additionally, the tunability of the crosslink density of the DNA crosslinked network allows it to control the sustained release of the carried drug to meet the long-term properties required for bone repair.
Dexamethasone (Dex) is a classical small molecule osteogenic model drug that stimulates stem cell proliferation, promotes osteogenic differentiation, induces macrophage M2 polarization, and facilitates tissue regeneration through cytokine secretion [[159], [160], [161]]. The bioavailability and therapeutic effectiveness of Dex can be improved by DNA hydrogel delivery systems that enable sustained release over an extended period [162,163].
Ren et al. demonstrated the sustained release capability of DNA hydrogels for Dex by encapsulating it into a poly (lactic-co-glycolic acid) polymer and combining it with DNA to form a composite DNA hydrogel [164]. This delivery approach extended the complete release duration from 60 h to 7 days. Basu et al. developed another injectable DNA hydrogel loaded with Dex, featuring two cross-linking modes: double-linked DNA cross-linking and electrostatic interaction with silicate nanodiscs (nSi) (Fig. 10A) [165]. The incorporation of nSi enhanced the mechanical elasticity of the hydrogel and enabled a tunable rate of Dex release (Fig.10B, C). Through the action of Dex, the hydrogel upregulated osteogenic markers in cells and demonstrated favorable bone regeneration properties.
Fig. 10.
Sustained release of Dex from nSi-DNA hydrogels. (a) Schematic of composite DNA hydrogels prepared by a two-step gel method. In the first step, double-stranded DNA was denatured and cooled to form a pre-gel, and A-type network points were formed by complementary base pairing between DNA strands; in the second step, silicate nanodisks (nSi) were introduced to form B-type network points through electrostatic interactions between nSi and DNA strands. (b) SEM images of DNA hydrogel. The addition of nSi reduced the pore size of the hydrogel. Scale bar = 100 μm. (c) Release of Dex from DNA hydrogels with different nSi contents. Adapted with permission [165]. Copyright 2018, AMER CHEMICAL SOC.
While nSi enhances mechanical properties, its cost and potential cytotoxicity limit its application [166,167]. In contrast, chitosan (CS), a natural polymer, exhibits better biocompatibility and is more cost-effective than nSi [168,169]. Chen et al. developed injectable DNA-CS hybrid hydrogels with adjustable porosity and rheology by utilizing the positively charged amino groups of CS to electrostatically interact with DNA strands [170]. These hybrid hydrogels, when loaded with Dex, induced macrophage M2 polarization and promoted osteogenic differentiation, showcasing their potential in bone tissue engineering.
The studies discussed above highlight the significant potential of DNA hydrogels in bone tissue engineering (Table 3). These hydrogels can serve as carriers for cells, providing a suitable environment for cell survival, proliferation, and differentiation. Furthermore, they can be employed as delivery systems for cell secretions and drugs, enabling sustained release to enhance bioavailability and therapeutic efficacy. The programmability of DNA enables the incorporation of motifs that respond to external triggers, allowing for the creation of intelligent hydrogel systems with enhanced responsiveness to stimuli, targeted delivery, and controlled release. Overall, these findings underscore the versatility and promise of DNA hydrogels as a platform for bone tissue engineering, offering opportunities for improved drug delivery, enhanced responsiveness to external stimuli, and targeted release, thus paving the way for more effective treatments in the future.
Table 3.
Summary of DNA hydrogels as delivery systems in bone tissue engineering.
| Materials loaded |
Nano-complexes | Mechanism of loading | Types of hydrogels | Preparation methods | Characteristics | Ref. | |
|---|---|---|---|---|---|---|---|
| Type | Name | ||||||
| Cells | bone marrow mesenchymal stem cells | N/A | cell and aptamer specific binding | pure DNA hydrogel | rolling circle amplification; physical crosslinking | controlled capture and release of cells | [136] |
| N/A | physical encapsulation | pure DNA hydrogel | sticky end hybridization; physical crosslinking | excellent shear resistant delivery system | [137] | ||
| Cellular secretions | interleukin-10 | N/A | physical encapsulation | pure DNA hydrogel | sticky end hybridization; physical crosslinking | continuous delivery of cytokines | [144] |
| vascular endothelial growth factor | black phosphorus nanosheets | physical encapsulation | nanocomposite hydrogel | physical crosslinking | [149] | ||
| exosomes | N/A | physical encapsulation | hybridized DNA hydrogel | chemical crosslinking | controlled release of exosomes | [157] | |
| N/A | exosomes were tethered to the hydrogel by noncovalent interactions | hybridized DNA hydrogel | chemical crosslinking | further prolonged the release time of exosomes | [158] | ||
| M2 macrophage-derived extracellular vesicles | silver nanoclusters | physical encapsulation | nanocomposite hydrogel | sticky end hybridization; physical crosslinking | sustained release M2 macrophage-derived extracellular vesicles | [168] | |
| Drugs | dexamethasone | Silicate nanodiscs | physical encapsulation | nanocomposite hydrogel | physical crosslinking | sustained release drugs | [165] |
| Chitosan | physical encapsulation | nanocomposite hydrogel | physical crosslinking | [170] | |||
4. Conclusions and perspectives
DNA-based biomaterials have emerged as a promising platform for advancing bone tissue engineering due to their inherent programmability, biocompatibility, stability, and versatility. DNA offers high precision and controllability, enabling the precise construction of DNA biomaterials in various shapes and sizes through DNA nanotechnology. This technology allows for functionalization via sequence design, chemical modification, and binding with functional molecules. DNA can be engineered to bind specific target genes or form secondary structures to interact with target proteins, enabling highly specific targeting. In addition, as a natural molecule, DNA can be degraded in the body by nuclease enzymes, avoiding problems such as toxicity buildup often associated with polymers or inorganic materials. However, DNA-based materials also face several challenges, such as high production costs, difficulties in large-scale manufacturing, and susceptibility to nuclease degradation. Researchers are actively addressing these issues. For instance, enzymatic synthesis of DNA avoids the toxic reagents required in traditional chemical synthesis, shortens synthesis time, and is better suitable for large-scale production [171]. Moreover, DNA nanomaterials can be assembled in ways that reduce nuclease exposure, thereby improving stability [172]. Exogenous implants often pose immunogenicity challenges, which can significantly reduce treatment efficacy. However, DNA materials, as natural cellular components, exhibit excellent biocompatibility. Further efforts to minimize DNA materials’ immunogenicity have been made; for example, Liu et al. developed l-DNA hydrogels from the d-DNA mirror isomer l-DNA, showing high stability and reduced pro-inflammatory response, thus offering a platform with high biostability and low immunogenicity for biomedical applications [173].
Extensive investigation has been dedicated to exploiting DNA’s potential as a mineralization template. Mineralized DNA framework materials demonstrate enhanced stability and mechanical properties, making them effective for drug delivery. Furthermore, adjusting the mineralization mode allows precise control of mineralization kinetics, maintains the integrity of the DNA template, and activates post-mineralization cascades. Among 3D DNA-based framework materials, TDF stands out as an exceptional vessel for transporting therapeutic agents relevant to bone tissue engineering, primarily due to its remarkable cell-penetrating capacity.
DNA hydrogels serve dual roles, functioning as cell-loaded scaffolds and delivery systems for therapeutics. Their stimulus-responsive properties enable intelligent therapeutic release. Mineralization integration enhances their stability and osteogenic activity, positioning them as strong candidates for high-quality bone regeneration. Despite their many advantages, the applications of DNA hydrogels may be limited by their inherent mechanical weaknesses. Typically, DNA hydrogels are weakly bonded structures, mainly formed by hydrogen bonds, and lack the necessary rigidity to withstand the pressures and prolonged loading in bone tissue engineering. However, strategies like increased cross-linking, incorporation of nano-components, polymer hybridization, 3D printing, and mineralization can help address these limitations [174,175]. Recently, Lachance-Brais et al. achieved a storage modulus of 105 Pa in DNA hydrogels by integrating polyadenine with cyanuric acid, surpassing previous records for unmodified DNA hydrogels [176]. In addition, DNA hydrogels are susceptible to nuclease degradation in vivo, and their degradation rate often exceeds the rate of bone regeneration. This mismatch can lead to problems such as insufficient mechanical support and difficulties in effectively integrating with newborn bone. However, by optimizing DNA network design and implementing post-modification techniques, the degradation rates can be better controlled, improving the hydrogels’ performance in bone tissue engineering. These advancements offer promising alternatives to conventional methods, addressing key challenges and enhancing the potential of DNA hydrogels in this field.
To further enhance the efficiency and viability of DNA-based biomaterials in bone tissue engineering, several challenges must be addressed. First, replicating the complex, multi-layered structure of natural bone could facilitate the creation of filler materials that closely match natural bone composition. Second, developing lower-cost DNA-based biomaterials would improve accessibility and affordability. Third, integrating vascular and neural regeneration with bone repair could result in more comprehensive bone regeneration outcomes. Fourth, creating stimulus-responsive materials would increase the precision and control of therapeutic molecule delivery. Fifth, achieving a refined equilibrium between mechanical robustness and biodegradability remains a vital pursuit. Additionally, curtailing treatment duration for larger bone defects, enhancing antimicrobial or anticancer attributes for addressing infections or tumors, and further harnessing the programmable potential of DNA-based biomaterials warrant in-depth exploration and research.
Beyond bone tissue engineering, DNA materials have recently shown strong performance in cell and organoid cultures. As extracellular matrices with tunable stress relaxation, cross-linking thermodynamics, kinetics, and degradability, DNA materials better mimic the tissue microenvironment and support a wide variety of cells and organoids [177]. Moreover, DNA materials can serve as nano-microspheres with cellular-level stiffness, providing biochemical cues within organoids to guide their development [178]. These emerging applications highlight the unique properties of DNA as a biomaterial, suggesting broader biomedical potential.
Overall, DNA-based biomaterials hold significant potential in bone tissue engineering and open doors to innovative therapeutic methodologies. Their programmability, biocompatibility, stability, and versatility make them a powerful tool in biomedicine. By mimicking extracellular matrices and leveraging their customizable characteristics, DNA-based biomaterials hold substantial promise for application in tissue engineering. Their role in drug delivery systems also opens new possibilities for targeted and controlled therapeutic release. With ongoing research, the impact of DNA biomaterials on medical applications is expected to grow, potentially revolutionizing strategies in bone tissue engineering.
Declaration of competing interest
The authors declare that they have no conflicts of interest in this work.
Acknowledgments
This work was supported by the National Key R&D Program of China (2022YFA1603603, 2023YFB3810202 and 2022YFB3808200), National Natural Science Foundation of China (32271385, 22074046 and 22377076), Basic and Applied Basic Research Foundation of Guangdong Province (2022A1515010887 and 2022A1515011839), and Shanghai Pujiang Program (22PJ1408500, to Huan Zhang).
Biographies
Xiaoyue Xu is a graduate student at the School of Biomedical Sciences and Engineering, South China University of Technology. Her research primarily focuses on developing and applying hydrogels for bone tissue engineering. Specifically, she investigates how hydrogels can be used as programmable biomaterials to influence cell interactions. By leveraging the unique properties of hydrogels, she aims to advance the understanding of cellular behavior and improve strategies for tissue regeneration and repair.
Honglu Zhang(BRID: 07717.00.28229) is an associate professor at School of Sensing Science and Engineering, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University. His research interests mainly focus on engineering nucleic acids nanomaterials for applications in biomedicine and plant engineering, as well as integration of biotechnology and information technology (BT&IT) for data sensing, manipulation, and storage.
Kunyu Zhang(BRID: 08167.00.80072) is an associate professor at the School of Biomedical Sciences and Engineering, South China University of Technology. He is enthusiastic about the design of functional hydrogels to reproduce the biochemical and biophysical signals existing in the natural cellular microenvironment and is particularly interested in their unique ability to regulate cellular behaviors and promote tissue regeneration.
Huan Zhang(BRID: 05139.00.86598) is an associate professor at School of Agriculture and Biology, Shanghai Jiao Tong University. Her research focuses on the design of nucleic acids nanostructures (e.g. framework nucleic acids) as programmable materials and their application in biology, including plant engineering, agriculture and biomedicine. She has published over forty papers in academic journals, including Nat. Nanotech., PNAS, Nat. Protoc., Nat. Commun.
Contributor Information
Honglu Zhang, Email: z.hl@sjtu.edu.cn.
Kunyu Zhang, Email: kyuzhang@scut.edu.cn.
Huan Zhang, Email: zhang_huan@sjtu.edu.cn.
References
- 1.Nauth A., Schemitsch E., Norris B., et al. Critical-size bone defects: Is there a consensus for diagnosis and treatment? J. Orthop. Trauma. 2018;32:S7–S11. doi: 10.1097/BOT.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 2.Panetta N.J., Gupta D.M., Slater B.J., et al. Tissue engineering in cleft palate and other congenital malformations. Pediatr. Res. 2008;63:545–551. doi: 10.1203/PDR.0b013e31816a743e. [DOI] [PubMed] [Google Scholar]
- 3.Keating J.F., Simpson A.H.R.W., Robinson C.M. The management of fractures with bone loss. J. Bone Joint Surg. Br. 2005;87:142–150. doi: 10.1302/0301-620x.87b2.15874. [DOI] [PubMed] [Google Scholar]
- 4.Rioja A.Y., Daley E.L.H., Habif J.C., et al. Distributed vasculogenesis from modular agarose-hydroxyapatite-fibrinogen microbeads. Acta Biomater. 2017;55:144–152. doi: 10.1016/j.actbio.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henkel J., Woodruff M.A., Epari D.R., et al. Bone regeneration based on tissue engineering conceptions — A 21st century perspective. Bone Res. 2013;1:216–248. doi: 10.4248/BR201303002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koons G.L., Diba M., Mikos A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020;5:584–603. [Google Scholar]
- 7.Ehrler D.M., Vaccaro A.R. The use of allograft bone in lumbar spine surgery. Clin. Orthop. 2000;371:38–45. doi: 10.1097/00003086-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Oryan A., Alidadi S., Moshiri A., et al. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. 2014;9:18. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betz R.R. Limitations of autograft and allograft: New synthetic solutions. Orthopedics. 2002;25:S561–S570. doi: 10.3928/0147-7447-20020502-04. [DOI] [PubMed] [Google Scholar]
- 10.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:874–880. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 11.Miotto M., Gouveia R.M., Ionescu A.M., et al. 4D corneal tissue engineering: Achieving time-dependent tissue self-curvature through localized control of cell actuators. Adv. Funct. Mater. 2019;29 [Google Scholar]
- 12.Kargozar S., Baino F., Hamzehlou S., et al. Bioactive glasses: Sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018;36:430–444. doi: 10.1016/j.tibtech.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Ritzau-Reid K.I., Spicer C.D., Gelmi A., et al. An electroactive oligo-EDOT platform for neural tissue engineering. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.202003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal R., García A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015;94:53–62. doi: 10.1016/j.addr.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Eyisoylu H., Qin X., et al. 3D bioprinting of graphene oxide-incorporated cell-laden bone mimicking scaffolds for promoting scaffold fidelity, osteogenic differentiation and mineralization. Acta Biomater. 2021;121:637–652. doi: 10.1016/j.actbio.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J., Jia J., Kim J.P., et al. Ionic colloidal molding as a biomimetic scaffolding strategy for uniform bone tissue regeneration. Adv. Mater. 2017;29 doi: 10.1002/adma.201605546. [DOI] [PubMed] [Google Scholar]
- 17.Takizawa T., Nakayama N., Haniu H., et al. Titanium fiber plates for bone tissue repair. Adv. Mater. 2018;30 doi: 10.1002/adma.201703608. [DOI] [PubMed] [Google Scholar]
- 18.Feng P., Wu P., Gao C., et al. A multimaterial scaffold with tunable properties: Toward bone tissue repair. Adv. Sci. 2018;5 doi: 10.1002/advs.201700817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., Zhu L., Wang Z., et al. Development of a centrally vascularized tissue engineering bone graft with the unique core-shell composite structure for large femoral bone defect treatment. Biomaterials. 2018;175:44–60. doi: 10.1016/j.biomaterials.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Won J., Lee Y., Park J., et al. Hierarchical microchanneled scaffolds modulate multiple tissue-regenerative processes of immune-responses, angiogenesis, and stem cell homing. Biomaterials. 2020;227 doi: 10.1016/j.biomaterials.2019.119548. [DOI] [PubMed] [Google Scholar]
- 21.Hollister S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 22.Swanson W.B., Omi M., Zhang Z., et al. Macropore design of tissue engineering scaffolds regulates mesenchymal stem cell differentiation fate. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ong L.L., Hanikel N., Yaghi O.K., et al. Programmable self-assembly of three-dimensional nanostructures from 10,000 unique components. Nature. 2017;552:72–77. doi: 10.1038/nature24648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong F., Zhang F., Liu Y., et al. DNA origami: Scaffolds for creating higher order structures. Chem. Rev. 2017;117:12584–12640. doi: 10.1021/acs.chemrev.6b00825. [DOI] [PubMed] [Google Scholar]
- 25.Chen J., Seeman N.C. Synthesis from DNA of a molecule with the connectivity of a cube. Nature. 1991;350:631–633. doi: 10.1038/350631a0. [DOI] [PubMed] [Google Scholar]
- 26.Chakraborty A., Ravi S.P., Shamiya Y., et al. Harnessing the physicochemical properties of DNA as a multifunctional biomaterial for biomedical and other applications. Chem. Soc. Rev. 2021;50:7779–7819. doi: 10.1039/d0cs01387k. [DOI] [PubMed] [Google Scholar]
- 27.Seeman N.C., Sleiman H.F. DNA nanotechnology. Nat. Rev. Mater. 2018;3:17068. [Google Scholar]
- 28.Greschner A.A., Ropagnol X., Kort M., et al. Room-temperature and selective triggering of supramolecular DNA assembly/disassembly by nonionizing radiation. J. Am. Chem. Soc. 2019;141:3456–3469. doi: 10.1021/jacs.8b10355. [DOI] [PubMed] [Google Scholar]
- 29.Edwardson T.G.W., Carneiro K.M.M., McLaughlin C.K., et al. Site-specific positioning of dendritic alkyl chains on DNA cages enables their geometry-dependent self-assembly. Nat. Chem. 2013;5:868–875. doi: 10.1038/nchem.1745. [DOI] [PubMed] [Google Scholar]
- 30.Li P., Fu L., Liao Z., et al. Chitosan hydrogel/3D-printed poly(ε-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121131. [DOI] [PubMed] [Google Scholar]
- 31.Xavier M., Kyriazi M.-E., Lanham S., et al. Enrichment of skeletal stem cells from human bone marrow using spherical nucleic acids. ACS Nano. 2021;15:6909–6916. doi: 10.1021/acsnano.0c10683. [DOI] [PubMed] [Google Scholar]
- 32.Toda M., Ohno J., Shinozaki Y., et al. Osteogenic potential for replacing cells in rat cranial defects implanted with a DNA/protamine complex paste. Bone. 2014;67:237–245. doi: 10.1016/j.bone.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Diaz A.Patino, Bracaglia S., Ranallo S., et al. Programmable cell-free transcriptional switches for antibody detection. J. Am. Chem. Soc. 2022;144:5820–5826. doi: 10.1021/jacs.1c11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui C., Chakraborty K., Tang X.A., et al. A lysosome-targeted DNA nanodevice selectively targets macrophages to attenuate tumours. Nat. Nanotechnol. 2021;16:1394–1402. doi: 10.1038/s41565-021-00988-z. [DOI] [PubMed] [Google Scholar]
- 35.Tanifuji T., Nishimura M., Kusamori K., et al. Intradermal delivery of Cryj1 loaded in CpG DNA hydrogel for inhibiting allergic reactions in mice. J. Controlled Release. 2023;354:429–438. doi: 10.1016/j.jconrel.2023.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Wei H., Zhao Z., Wang Y., et al. One-step self-assembly of multifunctional DNA nanohydrogels: An enhanced and harmless strategy for guiding combined antitumor therapy. ACS Appl. Mater. Interfaces. 2019;11:46479–46489. doi: 10.1021/acsami.9b15874. [DOI] [PubMed] [Google Scholar]
- 37.Ge Z., Gu H., Li Q., et al. Concept and development of framework nucleic acids. J. Am. Chem. Soc. 2018;140:17808–17819. doi: 10.1021/jacs.8b10529. [DOI] [PubMed] [Google Scholar]
- 38.Sidharthan D.S., Abhinandan R., Balagangadharan K., et al. Advancements in nucleic acids-based techniques for bone regeneration. Biotechnol. J. 2022;17 doi: 10.1002/biot.202100570. [DOI] [PubMed] [Google Scholar]
- 39.Laird N.Z., Acri T.M., Tingle K., et al. Gene- and RNAi-activated scaffolds for bone tissue engineering: Current progress and future directions. Adv. Drug Deliv. Rev. 2021;174:613–627. doi: 10.1016/j.addr.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S., Wu X., Wang X., et al. Hydrogels for bone organoid construction: From a materiobiological perspective. J. Mater. Sci. Technol. 2023;136:21–31. [Google Scholar]
- 41.Storrie H., Mooney D. Sustained delivery of plasmid DNA from polymeric scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2006;58:500–514. doi: 10.1016/j.addr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Zhang T., Tian T., Lin Y., et al. Functionalizing framework nucleic-acid-based nanostructures for biomedical application. Adv. Mater. 2022;34 doi: 10.1002/adma.202107820. [DOI] [PubMed] [Google Scholar]
- 43.Tang S., Ke X., Wang H., et al. Biomineralization-inspired intermediate precursor for the controllable gelation of polyphenol–macromolecule hydrogels. ACS Appl. Mater. Interfaces. 2022;14:44890–44901. doi: 10.1021/acsami.2c15068. [DOI] [PubMed] [Google Scholar]
- 44.Khalifehzadeh R., Arami H. DNA-templated strontium-doped calcium phosphate nanoparticles for gene delivery in bone cells. ACS Biomater. Sci. Eng. 2019;5:3201–3211. doi: 10.1021/acsbiomaterials.8b01587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramaniam S., Fang Y.-H., Sivasubramanian S., et al. Hydroxyapatite-calcium sulfate-hyaluronic acid composite encapsulated with collagenase as bone substitute for alveolar bone regeneration. Biomaterials. 2016;74:99–108. doi: 10.1016/j.biomaterials.2015.09.044. [DOI] [PubMed] [Google Scholar]
- 46.Son J., Kim J., Lee K., et al. DNA aptamer immobilized hydroxyapatite for enhancing angiogenesis and bone regeneration. Acta Biomater. 2019;99:469–478. doi: 10.1016/j.actbio.2019.08.047. [DOI] [PubMed] [Google Scholar]
- 47.James B.D., Guerin P., Iverson Z., et al. Mineralized DNA-collagen complex-based biomaterials for bone tissue engineering. Int. J. Biol. Macromol. 2020;161:1127–1139. doi: 10.1016/j.ijbiomac.2020.06.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dorozhkin S. Calcium orthophosphates in nature, biology and medicine. Materials. 2009;2:399–498. [Google Scholar]
- 49.Wang X., Yu Y., Ji L., et al. Calcium phosphate-based materials regulate osteoclast-mediated osseointegration. Bioact. Mater. 2021;6:4517–4530. doi: 10.1016/j.bioactmat.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu X., Hu L., Wang G., et al. DNA-mediated biomineralization of calcium-deficient hydroxyapatite for bone tissue engineering. New J. Chem. 2020;44:4755–4761. [Google Scholar]
- 51.Lee J.W., Lee B., Park C.H., et al. Monolithic DNApatite: An elastic apatite with sub-nanometer scale organo–inorganic structures. Adv. Mater. 2024;34 doi: 10.1002/adma.202406179. [DOI] [PubMed] [Google Scholar]
- 52.Patoine K., Ta K., Gilbert A., et al. Equilibrium interactions of biomimetic DNA aptamers produce intrafibrillar calcium phosphate mineralization of collagen. Acta Biomater. 2024;179:234–242. doi: 10.1016/j.actbio.2024.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Uskoković V., Uskoković D.P. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. B Appl. Biomater. 2011;96B:152–191. doi: 10.1002/jbm.b.31746. [DOI] [PubMed] [Google Scholar]
- 54.Ngourn S.C., Butts H.A., Petty A.R., et al. Quartz crystal microbalance analysis of DNA-templated calcium phosphate mineralization. Langmuir. 2012;28:12151–12158. doi: 10.1021/la300949y. [DOI] [PubMed] [Google Scholar]
- 55.Takeshita T., Matsuura Y., Arakawa S., et al. Biomineralization of hydroxyapatite on DNA molecules in SBF: Morphological features and computer simulation. Langmuir. 2013;29:11975–11981. doi: 10.1021/la402589j. [DOI] [PubMed] [Google Scholar]
- 56.Baig M.M.F.A., Dissanayaka W.L., Zhang C. 2D DNA nanoporous scaffold promotes osteogenic differentiation of pre-osteoblasts. Int. J. Biol. Macromol. 2021;188:657–669. doi: 10.1016/j.ijbiomac.2021.07.198. [DOI] [PubMed] [Google Scholar]
- 57.Chai Y.C., Carlier A., Bolander J., et al. Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater. 2012;8:3876–3887. doi: 10.1016/j.actbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Shih Y.-R.V., Hwang Y., Phadke A., et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci. 2014;111:990–995. doi: 10.1073/pnas.1321717111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y.-P., Chu Y.-L., Tsuang Y.-H., et al. Anti-inflammatory effects of adenine enhance osteogenesis in the osteoblast-like MG-63 cells. Life. 2020;10:116. doi: 10.3390/life10070116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X., Jing X., Liu P., et al. DNA framework-encoded mineralization of calcium phosphate. Chem. 2020;6:472–485. [Google Scholar]
- 61.Kim F., Chen T., Burgess T., et al. Functionalized DNA nanostructures as scaffolds for guided mineralization. Chem. Sci. 2019;10:10537–10542. doi: 10.1039/c9sc02811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu S., Zhang M., Song J., et al. Fine Customization of calcium phosphate nanostructures with site-specific modification by DNA templated mineralization. ACS Nano. 2021;15:1555–1565. doi: 10.1021/acsnano.0c08998. [DOI] [PubMed] [Google Scholar]
- 63.Danesi A.L., Athanasiadou D., Aderinto A.O., et al. Peptide-decorated DNA nanostructures promote site-specific hydroxyapatite growth. ACS Appl. Mater. Interfaces. 2022;14:1692–1698. doi: 10.1021/acsami.1c19271. [DOI] [PubMed] [Google Scholar]
- 64.Gower L.B., Odom D.J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth. 2000;210:719–734. [Google Scholar]
- 65.Wang Y.-W., Christenson H.K., Meldrum F.C. Confinement increases the lifetimes of hydroxyapatite precursors. Chem. Mater. 2014;26:5830–5838. [Google Scholar]
- 66.de Wildt B.W.M., van der Meijden R., Bartels P.A.A., et al. Bioinspired silk fibroin mineralization for advanced in vitro bone remodeling models. Adv. Funct. Mater. 2022;32 [Google Scholar]
- 67.Quan B.D., Wojtas M., Sone E.D. Polyaminoacids in biomimetic collagen mineralization: Roles of isomerization and disorder in polyaspartic and polyglutamic acids. Biomacromolecules. 2021;22:2996–3004. doi: 10.1021/acs.biomac.1c00402. [DOI] [PubMed] [Google Scholar]
- 68.Olszta M.J., Cheng X., Jee S.S., et al. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007;58:77–116. [Google Scholar]
- 69.Lotsari A., Rajasekharan A.K., Halvarsson M., et al. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018;9:4170. doi: 10.1038/s41467-018-06570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Onuma K., Saito M.M., Yamakoshi Y., et al. Coherent surface structure induces unique epitaxial overgrowth of metastable octacalcium phosphate on stable hydroxyapatite at critical fluoride concentration. Acta Biomater. 2021;125:333–344. doi: 10.1016/j.actbio.2021.02.024. [DOI] [PubMed] [Google Scholar]
- 71.Du L., Bian S., Gou B., et al. Structure of clusters and formation of amorphous calcium phosphate and hydroxyapatite: From the perspective of coordination chemistry. Cryst. Growth Des. 2013;13:3103–3109. [Google Scholar]
- 72.Abbarin N., San Miguel S., Holcroft J., et al. The enamel protein amelotin is a promoter of hydroxyapatite mineralization. J. Bone Miner. Res. 2015;30:775–785. doi: 10.1002/jbmr.2411. [DOI] [PubMed] [Google Scholar]
- 73.Fisher L.W., Torchia D.A., Fohr B., et al. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem. Biophys. Res. Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- 74.Douglas T.E.L., Skwarczynska A., Modrzejewska Z., et al. Acceleration of gelation and promotion of mineralization of chitosan hydrogels by alkaline phosphatase. Int. J. Biol. Macromol. 2013;56:122–132. doi: 10.1016/j.ijbiomac.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Green D.W., Ben-Nissan B., Yoon K.S., et al. Natural and synthetic coral biomineralization for human bone revitalization. Trends Biotechnol. 2017;35:43–54. doi: 10.1016/j.tibtech.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Gkioni K., Leeuwenburgh S.C.G., Douglas T.E.L., et al. Mineralization of Hydrogels for Bone Regeneration. Tissue Eng. Part B Rev. 16. 2010:577–585. doi: 10.1089/ten.TEB.2010.0462. [DOI] [PubMed] [Google Scholar]
- 77.Xu B., Zheng P., Gao F., et al. A mineralized high strength and tough hydrogel for skull bone regeneration. Adv. Funct. Mater. 2017;27 [Google Scholar]
- 78.Athanasiadou D., Meshry N., Monteiro N.G., et al. DNA hydrogels for bone regeneration. Proc. Natl. Acad. Sci. U. S. A. 2023;120 doi: 10.1073/pnas.2220565120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu H., Huang S., Zhu Y., et al. Self-mineralizing Dnazyme hydrogel as a multifaceted bone microenvironment amendment for promoting osteogenesis in osteoporosis. Adv. Funct. Mater. 2023;33:2212669. [Google Scholar]
- 80.Li J., Zeng H., Li L., et al. Advanced generation therapeutics: Biomimetic nanodelivery system for tumor immunotherapy. ACS Nano. 2023;17:24593–24618. doi: 10.1021/acsnano.3c10212. [DOI] [PubMed] [Google Scholar]
- 81.Bose S., Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012;8:1401–1421. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levingstone T.J., Herbaj S., Dunne N.J. Calcium Phosphate Nanoparticles for Therapeutic Applications in Bone Regeneration. Nanomaterials (Basel) 2019;9:1570. doi: 10.3390/nano9111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu M., Zeng X., Ma C., et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5:17014. doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y., Xu H., Jiang L., et al. Sulfonium-driven neoantigen-released DNA nanodevice as a precise vaccine for tumor immunotherapy and prevention. ACS Nano. 2022;16:19509–19522. doi: 10.1021/acsnano.2c09708. [DOI] [PubMed] [Google Scholar]
- 85.Yu L., Xu Y., Al-Amin M., et al. Cytodirect: A nucleic acid nanodevice for specific and efficient delivery of functional payloads to the cytoplasm. J. Am. Chem. Soc. 2023;145:27336–27347. doi: 10.1021/jacs.3c07491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y., Cai Z., Ma W., et al. A DNA tetrahedron-based ferroptosis-suppressing nanoparticle: Superior delivery of curcumin and alleviation of diabetic osteoporosis. Bone Res. 2024;12:14. doi: 10.1038/s41413-024-00319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han Y., Wu Y., Wang F., et al. Heterogeneous DNA hydrogel loaded with Apt02 modified tetrahedral framework nucleic acid accelerated critical-size bone defect repair. Bioact. Mater. 2024;35:1–16. doi: 10.1016/j.bioactmat.2024.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goodman R.P., Berry R.M., Turberfield A.J. The single-step synthesis of a DNA tetrahedron. Chem. Commun. 2004;12:1372–1373. doi: 10.1039/b402293a. [DOI] [PubMed] [Google Scholar]
- 89.He Y., Ye T., Su M., et al. Hierarchical self-assembly of DNA into symmetric supramolecular polyhedral. Nature. 2008;452:198–201. doi: 10.1038/nature06597. [DOI] [PubMed] [Google Scholar]
- 90.Shao X., Lin S., Peng Q., et al. Effect of tetrahedral DNA nanostructures on osteogenic differentiation of mesenchymal stem cells via activation of the Wnt/β-catenin signaling pathway. Nanomedicine Nanotechnol. Biol. Med. 2017;13:1809–1819. doi: 10.1016/j.nano.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 91.Zhou M., Liu N., Shi S., et al. Effect of tetrahedral DNA nanostructures on proliferation and osteo/odontogenic differentiation of dental pulp stem cells via activation of the notch signaling pathway. Nanomedicine Nanotechnol. Biol. Med. 2018;14:1227–1236. doi: 10.1016/j.nano.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 92.Shi S., Tian T., Li Y., et al. Tetrahedral framework nucleic acid inhibits chondrocyte apoptosis and oxidative stress through activation of autophagy. ACS Appl. Mater. Interfaces. 2020;12:56782–56791. doi: 10.1021/acsami.0c17307. [DOI] [PubMed] [Google Scholar]
- 93.Zhou M., Gao S., Zhang X., et al. The protective effect of tetrahedral framework nucleic acids on periodontium under inflammatory conditions. Bioact. Mater. 2021;6:1676–1688. doi: 10.1016/j.bioactmat.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Q., Lin S., Shi S., et al. Anti-inflammatory and antioxidative effects of tetrahedral DNA nanostructures via the modulation of macrophage responses. ACS Appl. Mater. Interfaces. 2018;10:3421–3430. doi: 10.1021/acsami.7b17928. [DOI] [PubMed] [Google Scholar]
- 95.Zhao D., Cui W., Liu M., et al. Tetrahedral framework nucleic acid promotes the treatment of bisphosphonate-related osteonecrosis of the jaws by promoting angiogenesis and M2 polarization. ACS Appl. Mater. Interfaces. 2020;12:44508–44522. doi: 10.1021/acsami.0c13839. [DOI] [PubMed] [Google Scholar]
- 96.Liang L., Li J., Li Q., et al. Single-particle tracking and modulation of cell entry pathways of a tetrahedral DNA nanostructure in live cells. Angew. Chem. Int. Ed. 2014;53:7745–7750. doi: 10.1002/anie.201403236. [DOI] [PubMed] [Google Scholar]
- 97.Wiraja C., Zhu Y., Lio D.C.S., et al. Framework nucleic acids as programmable carrier for transdermal drug delivery. Nat. Commun. 2019;10:1147. doi: 10.1038/s41467-019-09029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu Q., Li H., Wang L., et al. DNA nanotechnology-enabled drug delivery systems. Chem. Rev. 2019;119:6459–6506. doi: 10.1021/acs.chemrev.7b00663. [DOI] [PubMed] [Google Scholar]
- 99.Zhang T., Tian T., Zhou R., et al. Design, fabrication and applications of tetrahedral DNA nanostructure-based multifunctional complexes in drug delivery and biomedical treatment. Nat. Protoc. 2020;15:2728–2757. doi: 10.1038/s41596-020-0355-z. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y., Ma W., Zhu Y., et al. Inhibiting methicillin-resistant staphylococcus aureus by tetrahedral DNA nanostructure-enabled antisense peptide nucleic acid delivery. Nano Lett. 2018;18:5652–5659. doi: 10.1021/acs.nanolett.8b02166. [DOI] [PubMed] [Google Scholar]
- 101.Lin Y., Li Q., Wang L., et al. Advances in regenerative medicine applications of tetrahedral framework nucleic acid-based nanomaterials: An expert consensus recommendation. Int. J. Oral Sci. 2022;14:51. doi: 10.1038/s41368-022-00199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun Y., Ye X., Cai M., et al. Osteoblast-targeting-peptide modified nanoparticle for siRNA/microRNA delivery. ACS Nano. 2016;10:5759–5768. doi: 10.1021/acsnano.5b07828. [DOI] [PubMed] [Google Scholar]
- 103.Zhao Y., Zhang T., Shen X., et al. Tumor necrosis factor alpha delivers exogenous inflammation-related microRNAs to recipient cells with functional targeting capabilities. Mol. Ther. 2022;30:3052–3065. doi: 10.1016/j.ymthe.2022.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li D., Yang Z., Luo Y., et al. Delivery of miR335-5p-pendant tetrahedron DNA nanostructures using an injectable heparin lithium hydrogel for challenging bone defects in steroid-associated osteonecrosis. Adv. Healthc. Mater. 2022;11 doi: 10.1002/adhm.202101412. [DOI] [PubMed] [Google Scholar]
- 105.Zhang L., Tang Y., Zhu X., et al. Overexpression of MiR-335-5p promotes bone formation and regeneration in mice. J. Bone Miner. Res. 2017;32:2466–2475. doi: 10.1002/jbmr.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang J., Tu Q., Bonewald L.F., et al. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J. Bone Miner. Res. 2011;26:1953–1963. doi: 10.1002/jbmr.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sui L., Wang M., Han Q., et al. A novel lipidoid-microRNA formulation promotes calvarial bone regeneration. Biomaterials. 2018;177:88–97. doi: 10.1016/j.biomaterials.2018.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li S., Liu Y., Tian T., et al. Bioswitchable delivery of microRNA by framework nucleic acids: Application to bone regeneration. Small. 2021;17 doi: 10.1002/smll.202104359. [DOI] [PubMed] [Google Scholar]
- 109.Xue H., Ding F., Zhang J., et al. DNA tetrahedron-based nanogels for siRNA delivery and gene silencing. Chem. Commun. 2019;55:4222–4225. doi: 10.1039/c9cc00175a. [DOI] [PubMed] [Google Scholar]
- 110.Yadav P., Ambudkar S.V., Prasad N.Rajendra. Emerging nanotechnology-based therapeutics to combat multidrug-resistant cancer. J. Nanobiotechnol. 2022;20:423. doi: 10.1186/s12951-022-01626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chehelgerdi M., Chehelgerdi M., Allela O.Q.B., et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer. 2023;22:169. doi: 10.1186/s12943-023-01865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun G., Rong D., Li Z., et al. Role of small molecule targeted compounds in cancer: Progress, opportunities, and challenges. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.694363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li J., Lai Y., Li M., et al. Repair of infected bone defect with clindamycin-tetrahedral DNA nanostructure complex-loaded 3D bioprinted hybrid scaffold. Chem. Eng. J. 2022;435 [Google Scholar]
- 114.Khan N.M., Haseeb A., Ansari M.Y., et al. A wogonin-rich-fraction of scutellaria baicalensis root extract exerts chondroprotective effects by suppressing IL-1β-induced activation of AP-1 in human. OA chondrocytes. Sci. Rep. 2017;7:43789. doi: 10.1038/srep43789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chi Y.S., Lim H., Park H., et al. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. Biochem. Pharmacol. 2003;66:1271–1278. doi: 10.1016/s0006-2952(03)00463-5. [DOI] [PubMed] [Google Scholar]
- 116.Shi S., Chen Y., Tian T., et al. Effects of tetrahedral framework nucleic acid/wogonin complexes on osteoarthritis. Bone Res. 2020;8:6. doi: 10.1038/s41413-019-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khan N.M., Ahmad I., Ansari M.Y., et al. Wogonin, a natural flavonoid, intercalates with genomic DNA and exhibits protective effects in IL-1β stimulated osteoarthritis chondrocytes. Chem. Biol. Interact. 2017;274:13–23. doi: 10.1016/j.cbi.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qi L., Hong S., Zhao T., et al. DNA tetrahedron delivering miR-21-5p promotes senescent bone defects repair through synergistic regulation of osteogenesis and angiogenesis. Adv. Healthc. Mater. 2024;13 doi: 10.1002/adhm.202401275. [DOI] [PubMed] [Google Scholar]
- 119.Jiang W., Hong S., Liu K., et al. A tetrahedral DNA nanostructure-mediated miRNA inhibitor delivery system: Type H vessel-related bone healing during distraction osteogenesis. Chem. Eng. J. 2024;496 [Google Scholar]
- 120.Zhang T., Ma H., Zhang X., et al. Functionalized DNA nanomaterials targeting toll-like receptor 4 prevent bisphosphonate-related osteonecrosis of the jaw via regulating mitochondrial homeostasis in macrophages. Adv. Funct. Mater. 2023;33 [Google Scholar]
- 121.Hu Y., Gao S., Lu H., et al. Acid-resistant and physiological pH-responsive DNA hydrogel composed of A-Motif and i-Motif toward oral insulin delivery. J. Am. Chem. Soc. 2022;144:5461–5470. doi: 10.1021/jacs.1c13426. [DOI] [PubMed] [Google Scholar]
- 122.Liu X., Zhang J., Fadeev M., et al. Chemical and photochemical DNA "gears" reversibly control stiffness, shape-memory, self-healing and controlled release properties of polyacrylamide hydrogels. Chem. Sci. 2019;10:1008–1016. doi: 10.1039/c8sc04292f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Borum R.M., Moore C., Mantri Y., et al. Supramolecular loading of DNA hydrogels with dye–drug conjugates for real-time photoacoustic monitoring of chemotherapy. Adv. Sci. 2022;10 doi: 10.1002/advs.202204330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Du X., Bi Y., He P., et al. Hierarchically structured DNA-based hydrogels exhibiting enhanced enzyme-responsive and mechanical properties. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 125.Fischer A., Zhang P., Ouyang Y., et al. DNA-tetrahedra corona-modified hydrogel microcapsules: "Smart" ATP- or microRNA-responsive drug carriers. Small. 2022;18 doi: 10.1002/smll.202204108. [DOI] [PubMed] [Google Scholar]
- 126.Yue L., Wang S., Wulf V., et al. Stiffness-switchable DNA-based constitutional dynamic network hydrogels for self-healing and matrix-guided controlled chemical processes. Nat. Commun. 2019;10:4774. doi: 10.1038/s41467-019-12697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Annamalai R.T., Hong X., Schott N.G., et al. Injectable osteogenic microtissues containing mesenchymal stromal cells conformally fill and repair critical-size defects. Biomaterials. 2019;208:32–44. doi: 10.1016/j.biomaterials.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li W., Miao W., Liu Y., et al. Bioprinted constructs that mimic the ossification center microenvironment for targeted innervation in bone regeneration. Adv. Funct. Mater. 2022;32 [Google Scholar]
- 129.Yan Y., Cheng B., Chen K., et al. Silk fibroin hydrogels: Enhanced osteogenesis of bone marrow-derived mesenchymal stem cells by a functionalized silk fibroin hydrogel for bone defect repair. Adv. Healthc. Mater. 2019;8 doi: 10.1002/adhm.201801043. [DOI] [PubMed] [Google Scholar]
- 130.Amer M.H., White L.J., Shakesheff K.M., et al. The effect of injection using narrow-bore needles on mammalian cells: Administration and formulation considerations for cell therapies. J. Pharm. Pharmacol. 2015;67:640–650. doi: 10.1111/jphp.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gačanin J., Synatschke C.V., Weil T. Biomedical applications of DNA-based hydrogels. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 132.Jin J., Xing Y., Xi Y., et al. A triggered DNA hydrogel cover to envelop and release single cells. Adv. Mater. 2013;25:4714–4717. doi: 10.1002/adma.201301175. [DOI] [PubMed] [Google Scholar]
- 133.Zhou B., Yang B., Liu Q., et al. Effects of univariate stiffness and degradation of DNA hydrogels on the transcriptomics of neural progenitor cells. J. Am. Chem. Soc. 2023;145:8954–8964. doi: 10.1021/jacs.2c13373. [DOI] [PubMed] [Google Scholar]
- 134.Yuan T., Shao Y., Zhou X., et al. Highly permeable DNA supramolecular hydrogel Promotes neurogenesis and functional recovery after completely transected spinal cord injury. Adv. Mater. 2021;33 doi: 10.1002/adma.202102428. [DOI] [PubMed] [Google Scholar]
- 135.Ge Z., Li W., Zhao R., et al. Programmable DNA hydrogel provides suitable microenvironment for enhancing TSPCS therapy in healing of tendinopathy. Small. 2023;19 doi: 10.1002/smll.202207231. [DOI] [PubMed] [Google Scholar]
- 136.Yao C., Tang H., Wu W., et al. Double rolling circle amplification generates physically cross-linked DNA network for stem cell fishing. J. Am. Chem. Soc. 2020;142:3422–3429. doi: 10.1021/jacs.9b11001. [DOI] [PubMed] [Google Scholar]
- 137.Yan X., Yang B., Chen Y., et al. Anti-friction MSCs delivery system improves the therapy for severe osteoarthritis. Adv. Mater. 2021;33 doi: 10.1002/adma.202104758. [DOI] [PubMed] [Google Scholar]
- 138.Tan J., Zhang M., Hai Z., et al. Sustained release of two bioactive factors from supramolecular hydrogel promotes periodontal bone regeneration. ACS Nano. 2019;13:5616–5622. doi: 10.1021/acsnano.9b00788. [DOI] [PubMed] [Google Scholar]
- 139.Wu M., Chen G., Li Y. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Papageorgiou P., Vallmajo-Martin Q., Kisielow M., et al. Expanded skeletal stem and progenitor cells promote and participate in induced bone regeneration at subcritical BMP-2 dose. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119278. [DOI] [PubMed] [Google Scholar]
- 141.Grunewald M., Kumar S., Sharife H., et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. 2021;373:eabc8479. doi: 10.1126/science.abc8479. [DOI] [PubMed] [Google Scholar]
- 142.Moore K.W., de Waal Malefyt R., Coffman R.L., et al. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 143.Kordulewska N.K., Topa J., Tańska M., et al. Stability of interleukin-1β, -4, -6, -8, -10, -13, interferon-γ and tumor necrosis factor-α in human sera after repetitive freeze-thaw cycles and long storage. J. Pharm. Biomed. Anal. 2021;196 doi: 10.1016/j.jpba.2021.113900. [DOI] [PubMed] [Google Scholar]
- 144.Li W., Wang C., Wang Z., et al. Physically cross-linked DNA hydrogel-based sustained cytokine delivery for in situ diabetic alveolar bone rebuilding. ACS Appl. Mater. Interfaces. 2022;14:25173–25182. doi: 10.1021/acsami.2c04769. [DOI] [PubMed] [Google Scholar]
- 145.Li G., Gao F., Yang D., et al. ECM-mimicking composite hydrogel for accelerated vascularized bone regeneration. Bioact. Mater. 2024;42:241–256. doi: 10.1016/j.bioactmat.2024.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qing Y., Li R., Li S., et al. Advanced black phosphorus nanomaterials for bone regeneration. Int. J. Nanomedicine. 2020;15:2045–2058. doi: 10.2147/IJN.S246336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang Z., Zhao J., Tang W., et al. Multifunctional nanoengineered hydrogels consisting of black phosphorus nanosheets upregulate bone formation. Small. 2019;15 doi: 10.1002/smll.201901560. [DOI] [PubMed] [Google Scholar]
- 148.Yang B., Yin J., Chen Y., et al. 2D-black-phosphorus-reinforced 3D-printed scaffolds: A stepwise countermeasure for osteosarcoma. Adv. Mater. 2018;30 doi: 10.1002/adma.201705611. [DOI] [PubMed] [Google Scholar]
- 149.Miao Y., Chen Y., Luo J., et al. Black phosphorus nanosheets-enabled DNA hydrogel integrating 3D-printed scaffold for promoting vascularized bone regeneration. Bioact. Mater. 2023;21:97–109. doi: 10.1016/j.bioactmat.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gao M., Gao W., Papadimitriou J.M., et al. Exosomes-the enigmatic regulators of bone homeostasis. Bone Res. 2018;6:36. doi: 10.1038/s41413-018-0039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin Z., Xiong Y., Meng W., et al. Exosomal PD-L1 induces osteogenic differentiation and promotes fracture healing by acting as an immunosuppressant. Bioact. Mater. 2022;13:300–311. doi: 10.1016/j.bioactmat.2021.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kang Y., Xu C., Meng L., et al. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact. Mater. 2022;18:26–41. doi: 10.1016/j.bioactmat.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xing Y., Yerneni S.S., Wang W., et al. Engineering pro-angiogenic biomaterials via chemoselective extracellular vesicle immobilization. Biomaterials. 2022;281 doi: 10.1016/j.biomaterials.2021.121357. [DOI] [PubMed] [Google Scholar]
- 154.Navaratna D., Fan X., Leung W., et al. Cerebrovascular degradation of TRKB by MMP9 in the diabetic brain. J. Clin. Invest. 2013;123:3373–3377. doi: 10.1172/JCI65767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chang M., Nguyen T.T. Strategy for treatment of infected diabetic foot ulcers. Acc. Chem. Res. 2021;54:1080–1093. doi: 10.1021/acs.accounts.0c00864. [DOI] [PubMed] [Google Scholar]
- 156.Zhou W., Duan Z., Zhao J., et al. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact. Mater. 2022;17:1–17. doi: 10.1016/j.bioactmat.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jing X., Wang S., Tang H., et al. Dynamically bioresponsive DNA hydrogel incorporated with dual-functional stem cells from apical papilla-derived exosomes promotes diabetic bone regeneration. ACS Appl. Mater. Interfaces. 2022;14:16082–16099. doi: 10.1021/acsami.2c02278. [DOI] [PubMed] [Google Scholar]
- 158.Yerneni S.S., Lathwal S., Cuthbert J., et al. Controlled release of exosomes using atom transfer radical polymerization-based hydrogels. Biomacromolecules. 2022;23:1713–1722. doi: 10.1021/acs.biomac.1c01636. [DOI] [PubMed] [Google Scholar]
- 159.Yuasa M., Yamada T., Taniyama T., et al. Dexamethasone enhances osteogenic differentiation of bone marrow- and muscle-derived stromal cells and augments ectopic bone formation induced by bone morphogenetic protein-2. PLOS ONE. 2015;10 doi: 10.1371/journal.pone.0116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bhunia S., Jaiswal M.K., Singh K.A., et al. 2D covalent organic framework direct osteogenic differentiation of stem cells. Adv. Healthc. Mater. 2022;11 doi: 10.1002/adhm.202101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Feng Q., Xu M., Yu Y.Y., et al. High-dose dexamethasone or all-trans-retinoic acid restores the balance of macrophages towards M2 in immune thrombocytopenia. J. Thromb. Haemost. 2017;15:1845–1858. doi: 10.1111/jth.13767. [DOI] [PubMed] [Google Scholar]
- 162.Wang Q., Jiang J., Chen W., et al. Targeted delivery of low-dose dexamethasone using PCL-PEG micelles for effective treatment of rheumatoid arthritis. J. Controlled Release. 2016;230:64–72. doi: 10.1016/j.jconrel.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 163.Park J.H., Jiang Y., Zhou J., et al. Genetically engineered cell membrane-coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs. Sci. Adv. 2021;7:eabf7820. doi: 10.1126/sciadv.abf7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ren N., Sun R., Xia K., et al. DNA-Based hybrid hydrogels sustain water-insoluble ophthalmic therapeutic delivery against allergic conjunctivitis. ACS Appl. Mater. Interfaces. 2019;11:26704–26710. doi: 10.1021/acsami.9b08652. [DOI] [PubMed] [Google Scholar]
- 165.Basu S., Pacelli S., Feng Y., et al. Harnessing the noncovalent interactions of DNA backbone with 2D silicate nanodisks to fabricate injectable therapeutic hydrogels. ACS Nano. 2018;12:9866–9880. doi: 10.1021/acsnano.8b02434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Song Y., Li X., Wang L., et al. Nanomaterials in humans: Identification, characteristics, and potential damage. Toxicol. Pathol. 2011;39:841–849. doi: 10.1177/0192623311413787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu L., Zhang M., Zhang Q., et al. Graphene nanosheets damage the lysosomal and mitochondrial membranes and induce the apoptosis of RBL-2H3 cells. Sci. Total Environ. 2020;734 doi: 10.1016/j.scitotenv.2020.139229. [DOI] [PubMed] [Google Scholar]
- 168.Agudelo D., Kreplak L., Tajmir-Riahi H.A., et al. Microscopic and spectroscopic analysis of chitosan-DNA conjugates. Carbohydr. Polym. 2016;137:207–213. doi: 10.1016/j.carbpol.2015.09.080. [DOI] [PubMed] [Google Scholar]
- 169.Casettari L., Vllasaliu D., Castagnino E., et al. PEGylated chitosan derivatives: Synthesis, characterizations and pharmaceutical applications. Prog. Polym. Sci. 2012;37:659–685. [Google Scholar]
- 170.Chen F., He Y., Li Z., et al. A novel tunable, highly biocompatible and injectable DNA-chitosan hybrid hydrogel fabricated by electrostatic interaction between chitosan and DNA backbone. Int. J. Pharm. 2021;606 doi: 10.1016/j.ijpharm.2021.120938. [DOI] [PubMed] [Google Scholar]
- 171.Seydel C. DNA writing technologies moving toward synthetic genomes. Nat. Biotechnol. 2023;41:1504–1509. doi: 10.1038/s41587-023-02006-0. [DOI] [PubMed] [Google Scholar]
- 172.Bila H., Kurisinkal E.E., Bastings M.M.C. Engineering a stable future for DNA-origami as a biomaterial. Biomater. Sci. 2019;7:532–541. doi: 10.1039/c8bm01249k. [DOI] [PubMed] [Google Scholar]
- 173.Yang B., Zhou B., Li C., et al. Biostable l-DNA Hydrogel with Improved Stability for Biomedical Applications. Angew Chem. Int. Ed Engl. 2022;61 doi: 10.1002/anie.202202520. [DOI] [PubMed] [Google Scholar]
- 174.Le J., Osmanovic D., Klocke M.A., et al. Fueling DNA self-assembly via gel-released regulators. ACS Nano. 2022;16:16372–16384. doi: 10.1021/acsnano.2c05595. [DOI] [PubMed] [Google Scholar]
- 175.Chaudhuri O., Gu L., Klumpers D., et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15:326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lachance-Brais C., Rammal M., Asohan J., et al. Small molecule-templated DNA hydrogel with record stiffness integrates and releases DNA nanostructures and gene silencing nucleic acids. Adv. Sci. 2023;10 doi: 10.1002/advs.202205713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Peng Y., Hsiao S., Gupta K., et al. Dynamic matrices with DNA-encoded viscoelasticity for cell and organoid culture. Nat. Nanotechnol. 2023;18:1463–1473. doi: 10.1038/s41565-023-01483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Afting C., Walther T., Drozdowski O.M., et al. DNA microbeads for spatio-temporally controlled morphogen release within organoids. Nat. Nanotechnol. 2024;19:1849–1857. doi: 10.1038/s41565-024-01779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]