Abstract
Drug therapy, including chemotherapy and immunotherapy, remains a cornerstone of cancer treatment; however, significant toxic side effects are often unavoidable, inhibiting tumor growth while causing damage to multiple organ systems. Polymeric nanomedicines have shown substantial promise in addressing the limitations of small-molecule drugs, such as poor solubility, rapid clearance, low tumor retention, and adverse effects, thereby enhancing the therapeutic index. Despite these advances, clinical outcomes indicate that the overall survival rates of cancer patients post-treatment are often not significantly higher than those achieved with standard small-molecule drugs. This is largely due to the inadequate tumor targeting and limited tumor penetration of polymeric drugs despite their drug release and targeting capabilities. While actively tumor-targeted and selectively activated drug strategies can potentially improve drug targeting, traditional approaches have yielded unsatisfactory results due to insufficient differences in targets, such as markers and stimuli, between tumor and normal tissues. Recent innovations focus on utilizing drug or external stimuli, such as light, radiation, and ultrasound, to amplify tumor-associated markers or stimuli, enabling more precise tumor targeting and selective drug activation. Based on these innovations, actively targeted or selectively activated polymeric nanomedicines can further enhance drug accumulation within tumors and improve therapeutic outcomes. Moreover, the integration of actively tumor-targeting and tumor-selectively activated strategies represents a significant advancement, which achieves simultaneously enhanced drug accumulation and selective activation within the tumors. This review highlights the significant potential, challenges, and advanced strategies of polymeric nanomedicines in targeted tumor therapy, emphasizing the need for ongoing research to optimize their effectiveness and ultimately improve patient outcomes, paving the way for more effective and less toxic cancer treatment options.
Keywords: Polymeric nanomedicines, Drug delivery, Tumor targeting, Selective activation, Controlled release
1. Introduction
Drug therapy is integral to cancer treatment [1,2], targeting and eliminating cancer cells, reducing tumor size, and preventing disease progression [3]. Chemotherapy, which uses cytotoxic drugs to kill rapidly dividing cancer cells, remains the primary systemic treatment and constitutes approximately 70% of cancer drug therapies [4]. Immunotherapy, a groundbreaking approach, has revolutionized cancer treatment by leveraging the body's immune system to recognize and attack cancer cells [5] and offers a more personalized therapeutic option [6,7]. Despite significant improvements in cancer survival rates due to drug therapy, substantial challenges persist particularly severe side effects. Chemotherapy can induce systemic toxicity, while immunotherapy may trigger cytokine storms, leading to further organ dysfunction [8,9]. Thus, enhancing the quality of life for cancer patients remains a critical objective [10,11].
Nanomedicines are pharmaceutical agents that utilize nanoscale materials, such as polymers, liposomes, protein, and inorganic compounds, typically ranging from 1 to 200 nanometers, to deliver therapeutic small-molecule agents [[12], [13], [14]]. Nanomedicines have made significant progress in overcoming the limitations of small-molecule drugs, such as poor solubility, rapid clearance, low tumor retention, and adverse effects, thereby significantly increasing the therapeutic index [15]. However, the overall survival of cancer patients post-nanomedicine treatment is not significantly enhanced compared to standard small-molecule drug treatment. Thus, ongoing research continues to refine these therapies [16] to control the targeting and release of nanomedicines, further enhancing efficacy, reducing associated risks, and remodeling the tumor microenvironment [17].
While only a limited number of nanomedicines have been approved for clinical use, polymeric nanomedicines constitute a significant portion due to their biocompatibility, biodegradability, stability, design versatility, and multifunctionality [[18], [19], [20]]. Table 1 shows examples of polymeric nanomedicines approved for cancer therapy in the clinic and representative polymeric nanomedicines that have been or are being used in clinical trials. Polymeric nanoparticles have emerged as a highly promising platform for targeted and controlled drug delivery [21,22]. Strategies such as active tumor-targeted delivery [23], tumor-selective drug activation [[24], [25], [26]], and multiple tumor-targeted delivery approaches have been developed to overcome the associated challenges in low drug delivery efficacy [27]. Active tumor-targeting nanomedicines are engineered with targeting moieties that specifically recognize and bind to tumor-associated markers, allowing for selective accumulation in tumors and the delivery of therapeutic agents while minimizing damage to healthy tissues. Tumor-selective activation [28], another promising strategy, employs stimuli-responsive materials that release their drug cargo in response to specific tumor microenvironmental cues (e.g., low pH, redox, hypoxia, and enzymes [29]) or external stimuli (e.g., radiotherapy, phototherapy, ultrasound (US), and irradiation). This approach further enhances drug selectivity and minimizes off-target effects. A more advanced approach, the multiple tumor-targeted strategy, combines active targeting with selective activation to enhance the accumulation and release of drugs within tumors. The goal of these strategies is to enhance the precision of drug delivery to tumor sites [30], maximizing therapeutic efficacy while minimizing adverse effects on healthy tissue. Thus, the success of these approaches critically depends on targets differentially expressed between tumor cells and normal tissue [31,32]. Previous review articles have summarized past and partial progress; however, this review will discuss key advancements and the current progress of the three previously mentioned polymeric nanomedicine strategies, with a highlight on their design and effects tailored to specific tumor markers [21,22,[27], [28], [29],[33], [34], [35]].
Table 1.
Examples of polymeric nanomedicines approved for cancer therapy in the clinic [36].
| Name | Therapeutic | Formulation | Indications | Year | Country/ Region |
|---|---|---|---|---|---|
| Oncaspar | Asparaginase | Polymeric conjugate | Acute lymphoblastic leukemia | 1994 | United States (US)/European Union (EU) |
| SMANCS | Neocarzinostatin | Polymeric conjugate | Hepatocellular carcinoma | 1994 | Japan |
| Eligard | Leuprolide acetate | Polymeric matrix | Advanced prostate cancer | 2002 | US |
| Genexol PM | PTX | Polymeric micelles | Breast cancer and non-small cell lung cancer (NSCLC) | 2006 | South Korea |
| Nanoxel | PTX | Polymeric nanoparticles | Breast cancer, pancreatic cancer, and NSCLC | 2008 | India |
| Docetaxel PM | Docetaxel | Polymeric micelles | Breast cancer, gastric cancer, NSCLC, ovarian cancer, prostate cancer, and squamous cell cancer | 2013 | South Korea |
| Apealea | PTX | Polymeric micelles | Ovarian cancer, primary peritoneal cancer, and fallopian tube cancer | 2018 | US/EU |
2. Actively tumor-targeted delivery strategy
Actively tumor-targeted delivery provides drug foundational specificity, which leverages interactions between the targeting moiety on polymeric nanomedicine and overexpressed altered markers in tumors due to inherent tumor biology, drug intervention, or external treatments. These markers may be found on the surface of tumor cells, within the cells, near the tumor vasculature, or on intratumoral immune cells. The targeted moieties of polymeric nanomedicine typically include proteins [37], peptides [38], antibodies [39], antibody fragments [[40], [41], [42]], sugars [43], biologically active ligands, aptamers, and small molecules, which bind to tumor-associated markers through bio-orthogonal reactions [23,[44], [45], [46]]. The following sections present representative studies (Table 2).
Table 2.
Overview of key studies on actively tumor-targeted delivery strategies.
| Polymeric nanomedicines | Loaded drugs | Targeting moiety & tumor-associated markers | Mechanism of action | Ref. |
|---|---|---|---|---|
| Polycaprolactone-block-poly(glutamic acid-stat-alendronic acid) vesicles (PCL67-b-P[Glu6-stat-(Glu-ADA)16]) | Doxorubicin (DOX) | Alendronic acid (ADA) & hydroxyapatite (HA) in bone tissue | 1. Bone targeting: ADA chelates divalent Ca²⁺ ions in HA, enabling specific bone tumor accumulation. 2. SPECT/CT imaging: 99mTc chelated with ADA for real-time tumor imaging. 3. pH-sensitive drug release: Acidic tumor microenvironment promotes DOX release due to weakened electrostatic interactions. | [50] |
| Poly(mannose acrylamide-co-1-(azidomethyl)−4-vinylbenzene)-bufalin conjugate (P2) | Bufalin (Buf) (Cytotoxic drug) | Mannose & mannose receptors on HepG2 cells | 1. Targeting: Mannose binds to mannose receptors on HepG2 cells for selective liver cancer targeting. 2. Drug release: Ester bonds hydrolyzed by esterase release Buf for anticancer effects. | [51] |
| Poly[(fluorene vinylene)-co-isoluminol]-poly(ethylene glycol)-folic acid nanoparticles (PFV-L@PEG-FA-NPs) | PFV-luminol (chemiluminescent agent) | Folic acid & folate receptors (FR) on tumor cells | 1. Targeting: Folic acid binds to folate receptors for selective tumor targeting. 2. Imaging: Chemiluminescence triggered by high ROS levels enables tumor imaging with high sensitivity and resolution. 3. PDT: ROS generation for photodynamic therapy without external light sources. | [52] |
| Poly(N-acryloyl glycinamide)-co-(3-O-sulfated galactose methacrylate) (PNAGA-co-SO4–3-GalMA) | SO4–3 galactose (3-O-sulfated galactose) | Mannose & CD206 on macrophages | 1. Targeting: SO4–3-Gal specifically binds to the cysteine-rich (CR) domain of CD206 on tumor-associated macrophages (TAMs). 2. Biological effect: Binding forms stable intracellular complexes, blocking CD206 recycling and endocytosis. This modulates TAMs' immunosuppressive functions, promoting immune activation. | [53] |
| Generation 7 Poly(amidoamine) (PAMAM) dendrimer-aPD-L1 conjugates (G7-aPD-L1) | Anti-PD-L1 antibody (aPD-L1) | Anti-PD-L1 monoclonal antibody (aPDL1) & PD-L1 on tumor cells | 1. Targeting: aPD-L1 antibodies conjugated to dendrimers bind specifically to PD-L1 proteins overexpressed on tumor cells. 2. Biological effect: Multivalent binding enhances PD-L1 blockade, restoring T-cell activity, increasing cytokine production, and reducing tumor chemoresistance. | [54] |
| Poly(L-glutamic acid)-Fc binding peptide conjugate (PGLU-Fc-III-4C) | Anti-PDL1 antibody (αPDL1) Anti-CD3 antibody (αCD3e)Anti-4–1BB antibody (α4–1BB) | Anti-PD-L1 monoclonal antibody (aPDL1) & PD-L1 on tumor cells | 1. Targeting: PGLU-Fc-III-4C binds Fc regions of αPDL1, αCD3e, and α4–1BB antibodies, enabling simultaneous targeting of PDL1 on tumor cells and CD3e/4–1BB on CD8+ T cells. 2. Biological effect: Bridges tumor cells and CD8+ T cells, enhancing T cell activation and proliferation while promoting tumor cell killing with reduced systemic toxicity. | [56] |
| Trastuzumab-poly(L-glutamic acid)-DM1 Conjugate (T-NPLG-DM1) | Maytansine (DM1) | Anti-HER2 monoclonal antibody (aHER2) & HER2 on SKBR-3 tumor cells | 1. Targeting: Trastuzumab specifically binds to HER2 receptors overexpressed on SKBR-3 tumor cells, while the Fc-III-4C peptide ensures high-affinity binding to the Fc region of trastuzumab. 2. Biological effect: Delivers DM1 to tumor cells, inhibiting tubulin polymerization and inducing apoptosis, with improved tumor suppression rates with DAR increases. | [57] |
| Anti-PD-L1-poly(L-glutamic acid)-SN38 conjugate (aPDL1-NPLG-SN38) | SN38 (cytotoxic drug) | Anti-PD-L1 monoclonal antibody (aPDL1) & PD-L1 on MC38 tumor cells | 1. Targeting: Anti-PD-L1 monoclonal antibody (aPDL1) binds specifically to PD-L1 overexpressed on MC38 tumor cells, enabling precise tumor targeting. 2. Biological effect: Ester bonds cleaved by esterase release SN38, which inhibits topoisomerase I, leading to DNA damage and apoptosis. | [58] |
| Anti-HER2-poly(L-glutamic acid)-MMAE conjugate (aHER2-P-MMAE) | MMAE (microtubule inhibitor) | Anti-HER2 monoclonal antibody (aHER2) & HER2 on SKOV-3 ovarian tumor cells | 1. Targeting: aHER2 binds to HER2 overexpressed on SKOV-3 cells for tumor-specific drug delivery. 2. Biological effect: MMAE released by enzymatic cleavage disrupts microtubule assembly, inducing tumor cell apoptosis. | [59] |
| Fourth-generation poly(amidoamine) dendrimers conjugated with doxorubicin, polyethylene glycol, transferrin, and tamoxifen (G4-DOX-PEG-Tf-TAM) | DOX & tamoxifen (TAM) | Tf & transferrin receptors (TfRs) on brain capillary endothelium and glioma cells | 1. Targeting: Tf binds to TfR on BBB and glioma cells, promoting drug carrier transport and tumor cell uptake. 2. Biological effect: TAM inhibits MDR proteins to reduce drug efflux; DOX is released in acidic lysosomal environments, inducing glioma cell apoptosis. | [60] |
| Poly(amidoamine)-polyethylene glycol-wheat germ agglutinin-transferrin dendrimer (PAMAM-PEG-WGA-Tf) (fourth-generation PAMAM dendrimer) | DOX (cytotoxic drug) | Wheat germ agglutinin (WGA) & sugar moieties on brain endothelial cells. Tf & TfRs on brain capillary endothelial cells and glioma cells | 1. Targeting: WGA binds to sugar moieties on brain endothelial cells (adsorptive endocytosis); Tf binds to TfRs on BBB and glioma cells (receptor-mediated transcytosis). 2. Biological effect: DOX is released intracellularly, inducing glioma cell apoptosis. | [61] |
| Paclitaxel-loaded hyperbranched poly[(amine-ester)-co-(D,L-lactide)]/1,2-dipalmitoyl-sn‑glycero-3-phosphoethanolamine copolymer conjugated with cyclic RGD peptide and transferrin (HPAE-co-PLA/DPPE-RGD/Tf) | Paclitaxel (PTX) (cytotoxic drug) | cRGDfK peptide (RGD) & integrin αvβ3 on angiogenic tumor endothelium. Tf & TfRs on tumor cells | 1. Targeting: RGD binds to integrin αvβ3 on tumor vasculature, enhancing migration and accumulation; Tf binds to TfRs on tumor cells, improving cellular uptake via receptor-mediated endocytosis. 2. Biological effect: PTX is encapsulated in the hydrophobic core of the polymer and released intracellularly, disrupting microtubule dynamics and inducing apoptosis in tumor cells. | [62] |
| Docetaxel-loaded poly(lactic-co-glycolic acid) nanoparticles conjugated with anti-EGFR and anti-PD-L1 antibodies (DRT-DTX-PLGA) | Docetaxel (DTX) (cytotoxic drug) | Anti-EGFR antibody (panitumumab, Pmab) & EGFR receptors on A549 cells. Anti-PD-L1 antibody & PD-L1 receptors on U87-MG cells | 1. Targeting: Anti-EGFR antibody binds to EGFR receptors overexpressed on A549 cells; Anti-PD-L1 antibody binds to PD-L1 receptors overexpressed on U87-MG cells, enabling dual-receptor-mediated endocytosis. 2. Biological effect: DTX is released intracellularly, disrupting microtubule dynamics and inducing apoptosis in cancer cells. | [63] |
| Poly(D,L-lactide-co-glycolic acid) nanoparticles coated with chimeric antigen receptor T-cell membranes (CAR-T-MNPs) | Cisplatin (Cis-Pt) | CAR-T-cell membranes & HER2 on tumor cells | 1. Targeting: CAR-T-cell membranes actively target HER2+ cancer cells, enhancing nanoparticle uptake. 2. Biological effect: CisPt induces DNA damage by forming Pt-DNA adducts, leading to apoptosis in HER2+ tumor cells. | [67] |
| Cancer cell membrane-coated conjugated oligomer nanoparticles (C-BTA NPs) | BTA (photothermal agent) | Tumor cell membrane & tumor microenvironment | 1. Targeting: Tumor cell membrane coating enables homologous targeting and immune evasion, enhancing nanoparticle accumulation at tumor sites. 2. Biological effect: BTA absorbs NIR-II light, converting it to heat for photothermal therapy (PTT), leading to tumor cell ablation. | [68] |
| A15 peptide-decorated poly(L-glutamic acid)-cisplatin conjugates (A15-PGA-CisPt) | CisPt (cytotoxic drug) | A15 peptide & fibrin clots in coagulation sites induced by DMXAA in tumors | 1. Targeting: A15 peptide binds to fibrin clots via FXIIIa-catalyzed transglutamination in DMXAA-induced coagulation sites.2. Biological effect: Cisplatin is released through chloride ion exchange and polymer degradation, inducing tumor cell apoptosis. | [70] |
| FXIIIa substrate peptide-decorated BLZ945 nanoparticles (A15-BLZ-NPs) (BLZ945-PLA: BLZ945-poly(D,L-lactide); MAL-PEG5k-PLA5k: maleimide-poly(ethylene glycol)-poly(D,L-lactide)) | BLZ945 (CSF-1R inhibitor) | A15 peptide & fibrin clots in coagulation sites induced by CA4-NPs | 1. Targeting: A15 peptide binds to fibrin clots via FXIIIa-catalyzed crosslinking in CA4-NP-induced coagulation sites. 2. Biological effect: BLZ945 inhibits M2-type macrophages, reduces immunosuppression, and promotes CD8+T cell infiltration. | [71] |
| Coagulation targeting peptide-decorated poly(L-glutamic acid)-graft-maleimide poly(ethylene glycol)/combretastatin A4 conjugate (A15-PLG-CA4) | Combretastatin A4 (CA4, vascular-disrupting agent) | A15 peptide (GNQEQVSPLTLLKXC) & fibrin clots in tumor blood vessels | 1. Targeting: A15 peptide binds to fibrin clots via FXIIIa-catalyzed transglutamination in tumor blood vessels. 2. Biological effect: CA4 disrupts tumor vasculature, induces intratumoral hemorrhage, and amplifies FXIIIa activity for enhanced tumor targeting. | [72] |
| Des-Arg9-kallidin-modified poly(γ-glutamic acid)-based nanoparticles loaded with 5-ALA, magnetic upconverting nanoparticles, and anti-PDL1 antibody (d-K @ γ-PGA @ 5-ALA @ MUCNP @ aPDL1) | 5-ALA (photosensitizer), aPDL1 (immune checkpoint inhibitor) | d-K (des-Arg9-kallidin): a B1R-specific kinin ligand & B1R receptors overexpressed on GBM cells and blood-tumor barrier (BTB) | 1. Targeting: D-K binds specifically to B1R (only upregulated in the GBM microenvironment), enhancing nanoparticle accumulation and transiently increasing BTB permeability. 2. Biological effect: 5-ALA generates PpIX for ROS-mediated photodynamic therapy (PDT), while aPDL1 blocks PD-L1 to boost antitumor immune response. | [73] |
| Anti-PDL1 antibody-poly(L-glutamic acid)-grafted-R848 nanoparticles (aPDL1-PLG/R848 NPs) | R848 (TLR7/8 agonist) | aPDL1 antibody & PD-L1 overexpressed on tumor cells | 1. Targeting: aPDL1 specifically binds to PD-L1 on tumor cells, enhancing nanoparticle accumulation and tumor-specific delivery. 2. Biological effect: R848 activates TLR7/8 to induce a Th1-type immune response and reprogram the immunosuppressive tumor microenvironment. | [74] |
2.1. Inherent tumor-associated marker-targeted delivery
Tumor-associated markers are biological molecules that are either overexpressed or specifically expressed in tumor cells or within the tumor microenvironment. These markers can be employed for drug delivery, such as human epidermal growth factor receptor 2 (HER2)/neu, programmed death-ligand 1 (PDL1), CD19/CD20, integrin, transferrin receptor, glutathione (GSH) transporters [47,48]. This section covers a range of actively tumor-targeted strategies, highlighting delivery methods for diverse tumor types or immune cells with challenges, the delivery of immunotherapeutic drugs, and antibody-mediated targeting by polymeric nanomedicine employing a single targeting moiety. It also addresses polymeric nanomedicines specifically engineered for active tumor targeting that incorporate dual targeting moieties.
Recent research highlights the potential of nanoformulations in targeting different tumor types and immune cells. A study on membrane-crosslinked polymersomes decorated with the surface brain tumor-targeting peptide ligand angiopep-2 demonstrated that varying their elasticity significantly influenced drug delivery efficacy [49]. The rigid polymersomes exhibited superior uptake in brain tumor cells and effective penetration of the blood-brain barrier (BBB), enhancing their accumulation and penetration in glioblastoma models despite having shorter circulation times than their softer counterparts. Additionally, an alendronic acid-based bone-targeting polymer vesicle was developed for the simultaneous imaging and treatment of malignant bone tumors [50]. Single photon emission computed tomography/computed tomography (SPECT/CT) was used to track drug distribution and showed that this vesicle successfully reduced tumor sizes in rabbit models from over 2.0 cm³ to < 0.6 cm³, showcasing its dual diagnostic and therapeutic capabilities. A water-soluble mannose-modified polymer-drug conjugate enhanced bufalin's solubility and targeting for liver cancer treatment [51], while chemiluminescent polymeric PFV-L@PEG-FA-NPs nanoparticles constructed by the self-assembly of polyfluorene vinylene (PFV)-luminol and the amphiphilic copolymer DSPE-PEG/DSPE-PEG-FA provided advanced imaging and tumor-targeted photodynamic therapy capabilities [52]. By employing SO4–3-Gal glycopolymers targeting its cysteine-rich lectin domain, researchers revealed a previously unrecognized mechanism of CD206 inhibition [53]. The formation of stable intracellular complexes blocked receptor recycling to the cell surface, thus inhibiting its function. Both in vitro and in vivo findings highlight the glycopolymers’ potential as modulators of CD206, paving the way for innovative immunotherapies.
Considering the successful and prospective application of immunotherapy in clinics, researchers have exerted more effort toward immune-related delivery in various cancers in recent years. Multivalent poly(amidoamine) dendrimers have been employed to prepare dendrimer-immune checkpoint inhibitor conjugates (G7-aPD-L1), which substantially mediate multivalent interactions and increase the binding avidity of immune checkpoint inhibitors, thereby improving the antagonist effect [54]. A mannan-decorated pathogen-like polymeric nanoparticle was utilized as a protein vaccine carrier to elicit robust anticancer immunity, with mannan as the shell, polylactic acid-polyethylenimine (PLA-PEI)-assembled nanoparticles as the core, and protein antigens and the Toll-like receptor 9 (TLR9) agonist CpG absorbed onto the PLA-PEI core via electrostatic interactions [43]. Most studies on active targeting are still in the basic study stage.
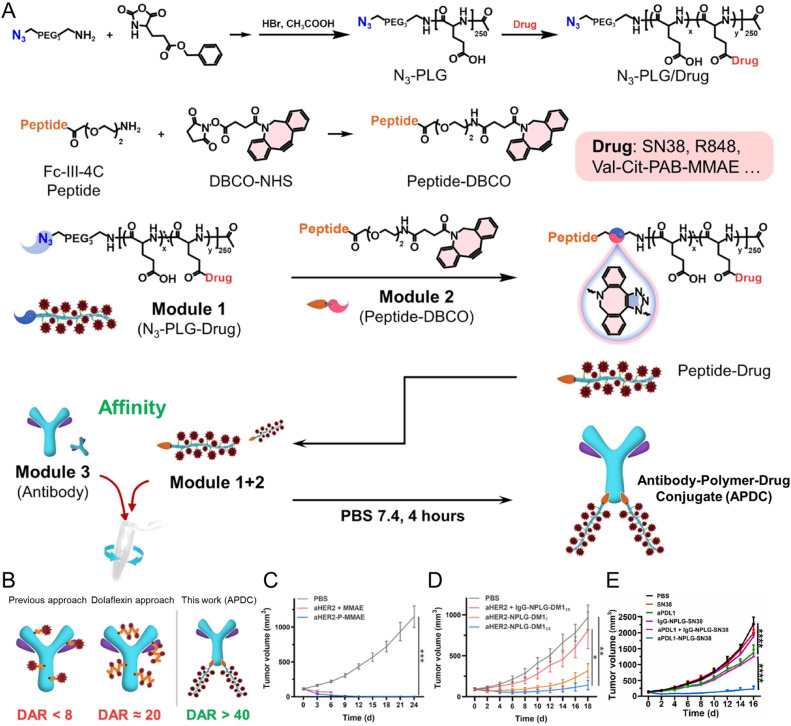
The clinical success of antibody-drug conjugates is largely attributed to the tumor-targeting ability of antibodies [55]. Thus, nanomedicine with antibodies as a targeting moiety becomes promising for tumor targeting and translation. Building on this success, recent innovations in nanomedicine have focused on antibody-polymer conjugates, which offer even greater versatility and efficacy. These conjugates leverage antibodies’ targeting capabilities and the tunable properties of polymers to enhance drug delivery to tumor sites. Notably, Xue et al. developed a platform for constructing multispecific antibodies (MsAbs) using poly(L-glutamic acid)-multiple Fc binding peptides (PGLU-Fc-III-4C), enabling simple assembly in aqueous solutions without the need for purification [56]. This approach allows for the generation of tri-specific antibodies (TsAbs), which can effectively engage T cells and stimulate antitumor immune responses, as demonstrated in murine models. Compared to traditional free monoclonal antibodies (mAbs), these MsAbs exhibited superior tumor drug distribution and reduced systemic toxicity (Fig. 1). Liu and Zhang et al. further advanced the field by incorporating click chemistry and Fc binding peptides, to increase the conjugation ratio of drug on antibodies [[57], [58], [59]]. Their method allowed for the creation of antibody-polymer-drug conjugates with ultra-high drug-to-antibody ratios, demonstrating significant tumor suppression in multiple cancer models (Fig. 2). These conjugates targeted antigens such as PDL1 and HER2 and improved tumor biodistribution by 2- to 4-fold, showcasing their potential for enhanced therapeutic outcomes. These developments in Fc-binding peptide-based antibody-polymer conjugates represent a promising frontier in cancer therapy, offering improved specificity, higher drug payloads, and reduced side effects compared to conventional approaches.
Fig. 1.
An Fc binding peptide-based multi-specific antibody (MsAb) and antibody-drug conjugate building platform. (A-C) An Fc binding peptide-based platform for MsAb. (A) Synthesis approach of PGLU-Fc-III-4C. (B) Fc binding peptide-based platform for the generation of multispecific antibodies. (C) The underlying mechanisms, tumor growth curve and mice body weight change in the tumor inhibition experiment of PDL1/CD3e/4–1BB TsAb treated MC38 mice. Reproduced under terms of the CC-BY license. [56] Copyright 2023, American Society for Clinical Investigation.
Fig. 2.
Different antibody-polymer-drug conjugates (APDCs) were prepared using polymer linkers based on a “Lego-like” assembly.(A-B) Schematic illustration of APDC. (C-E) The tumor growth curves in the tumor inhibition experiments of (C) aHER2-P-MMAE treated SKOV-3 tumor bearing mice (Reproduced with permission. [57] Copyright 2023, Wiley-VCH Verlag GmbH & Co.), (D) aHER-2-NPLG-DM1 treated SKOV-3 tumor bearing mice (Reproduced with permission. [58] Copyright 2024, Elsevier Ltd.), and (E) aPDL1-NPLG-SN38 treated MC38 bearing mice (Reproduced with permission. [59] Copyright 2023, Elsevier Ltd.).
Dual-active targeting polymeric nanomedicines have also been investigated. One example is a dual-targeting drug carrier (PAMAM-PEG-WGA-Tf), synthesized using a polyethylene glycol (PEG)ylated fourth-generation (G4.0) PAMAM dendrimer with transferrin (Tf) and wheat germ agglutinin (WGA) on its surface, and doxorubicin (DOX) encapsulated in its core. This carrier was evaluated for its ability to penetrate the BBB and target tumors [60,61]. Another approach involved the synthesis of a hyperbranched amphiphilic copolymer, poly[(amine-ester)-co-(D,L-lactide)]/1,2-dipalmitoyl-sn‑glycero-3-phosphoethanolamine copolymer (HPAE-co-PLA/DPPE), functionalized with a cyclic RGD peptide (cRGDfK) and Tf to prepare paclitaxel-loaded nanoparticles for dual-targeting chemotherapy [62]. Additionally, dual-receptor specific poly(lactic-co-glycolic acid) (PLGA) nanoparticles, targeting both the epidermal growth factor receptor and PD-L1, were developed for the enhanced delivery of docetaxel in cancer therapy [63]. These studies offer new strategies for targeted drug delivery.
Cell membrane-coated polymeric nanodrugs have gained significant attention for their potential targeting ability in tumor therapy. They are fabricated by coating the surface of polymeric nanodrugs with membranes derived from specific cell types, leveraging surface receptors, adhesion molecules, and immune evasion characteristics to extend biocompatibility and specifically target cancer cells [[64], [65], [66]]. One notable example is anti-HER2 CAR-T cell membrane-coated PLGA nanoparticles (CAR-T-MNPs) [67], which demonstrate exceptional targeting capabilities for HER2-positive tumors. Anti-HER2 CAR genes were introduced into Jurkat T cells through genetic transfection, and their membranes were subsequently extracted and coated onto PLGA nanoparticles, resulting in a biomimetic drug carrier system. In vivo studies demonstrated that CAR-T-MNPs effectively prolonged nanoparticle circulation time and significantly increased tumor accumulation in HER2-positive lung cancer mouse models, achieving a five-fold enhancement in tumor accumulation compared to uncoated PLGA nanoparticles. Moreover, CAR-T-MNPs effectively reduced non-specific toxicity, such as damage to kidney and lung tissues. These findings indicate that by targeting HER2-expressing tumors, CAR-T-MNPs not only enhance drug delivery efficiency but also significantly improve therapeutic outcomes. Similarly, tumor cell membrane-coated nanoparticles incorporating conjugated oligomers with strong near-infrared II (NIR-II) absorption (C-BTA NPs) have been developed to enhance NIR-II photothermal therapy (PTT) and multimodal imaging [68]. These nanoparticles exhibited superior tumor homing capabilities, high photothermal conversion efficiency (60%), and effective tumor ablation under NIR-II laser irradiation, with no significant toxicity to major organs. These advancements underline the potential of CMNPs to enhance tumor-specific drug delivery by integrating the stability and multifunctionality of polymeric cores with the homotypic targeting and immune evasion properties of cell membranes. Tumor-targeting bacteria and their membrane vesicles are also employed for tumor-targeting delivery [69]. These approaches offer a promising direction for advancing cancer-targeted therapies and personalized medicine.
2.2. Drug-amplified tumor-associated marker targeted delivery
Although tumor-associated markers are inherently overexpressed in tumors compared to normal tissues, a greater difference in expression is always advantageous. However, the targeting efficiency of traditional tumor-targeting nanotherapeutics is often limited by insufficient in vivo accumulation at the target site. Drug-amplified or -induced tumor coagulation targeting strategies have been developed to address this limitation. Song et al. synthesized coagulation-targeted cisplatin-loaded nanoparticles (A15-PGA-CisPt) with a substrate for activated blood coagulation factor XIII on the surface. In a tumor vessel coagulation environment induced by a vascular-disrupting agent (DMXAA), the nanoparticles selectively accumulated in solid tumors via transglutamination. The combination of DMXAA and A15-PGA-CisPt increased therapeutic platinum delivery to the tumor by 7.5-fold, reducing tumor burden with minimal systemic toxicity compared to free cisplatin [70]. Wei et al. designed FXIIIa substrate peptide A15-modified BLZ945 nanoparticles (A15-BLZ-NPs), with BLZ945-poly(D,L-lactide) (BLZ945-PLA) encapsulated in PEG-poly(D,L-lactide) (PEG5k-PLA5k), and A15 conjugated to the PEG segment. These nanoparticles crosslinked with fibrin by elevated FXIIIa and specifically targeted intratumoral coagulation sites induced by polymeric vascular-disrupting agents, such as CA4-NPs. In vivo studies demonstrated that CA4-NPs enhanced BLZ945 distribution in tumors, achieving a 3.75-fold increase in BLZ945 content in the CA4-NP + A15-BLZ-NP group compared to A15-BLZ-NP monotherapy [71].
Wang et al. developed a self-amplifying tumor coagulation-targeting strategy based on a chain reaction mechanism to further improve targeting efficacy [72]. A coagulation-targeting peptide (GNQEQVSPLTLLKXC, termed A15)-decorated poly(L-glutamic acid)-graft-maleimide poly(ethylene glycol)/combretastatin A4 conjugate (A15-PLG-CA4), was synthesized to create a self-amplifying nanotherapeutic platform capable of homing to tumors. Upon administration to tumor-bearing mice, A15-PLG-CA4 initiated a chain reaction consisting of intratumoral hemorrhage, coagulation factor XIIIa (FXIIIa) target amplification, blood clot binding, and CA4 release in tumors. This chain reaction increased the concentration of accessible targets (FXIIIa), enhancing therapeutic efficacy. Tumor inhibition studies in C26 tumor models compared the efficacy of A15-PLG-CA4 and A15-PLG-CA4/BLZ945 with the nontargeting peptide conjugates A15’-PLG-CA4 and A15’-PLG-CA4/BLZ945. At day 8 post-administration, the A15-PLG-CA4 group achieved an 86.2% tumor suppression rate, significantly higher than the 63.1% in the A15’-PLG-CA4 group. The A15-PLG-CA4/BLZ945 combination showed the highest tumor suppression rate (87%) by day 9, outperforming A15’-PLG-CA4/BLZ945. Mechanistically, FXIIIa activity in C26 tumors treated with A15-PLG-CA4 was increased 4.1-fold at 8 h compared to baseline, and the total CA4 concentration at 24 h was 2.9 times higher than in controls. These findings underscore the enhanced antitumor effects of A15-PLG-CA4 in large C26 tumors (about 500 mm³), demonstrating its advanced tumor-targeting capacity and potential for improving targeted drug delivery (Fig. 3). Thus, the self-amplifying tumor-targeting approach represents a significant advancement in the field, providing novel insight into enhancing the precision and efficacy of targeted drug delivery.
Fig. 3.
Self-amplifying nanotherapeutic drugs targeting tumors via a chain reaction mechanism. (A) Synthesis and preparation of the A15-PLG-CA4 drug delivery platform. (B) Schematic representation of the self-amplifying tumor coagulation targeting mechanism of A15-PLG-CA4. (C-D) Tumor growth curves in C26 tumor-bearing mice treated with A15-PLG-CA4 (C) and A15-PLG-CA4/BLZ945 (D) in tumor inhibition experiments. (E) Biodistribution of the drug in mice following A15-PLG-CA4 treatment. Reproduced with permission. [72] Copyright 2020, Wiley-VCH Verlag GmbH & Co.
2.3. External approach-amplified tumor-associated marker targeted delivery
External techniques, including surgical resection, radiotherapy, phototherapy, and US, can amplify certain tumor-associated markers, highlighting the importance of spatial control and creating new opportunities for targeted drug delivery. This review emphasizes several tumor-targeting strategies that leverage these methods. Biodegradable polymeric nanoparticles have been developed to locally induce immune responses within the central nervous system. These nanoparticles encapsulate an anti-PDL1 antibody (aPDL1), upconverting nanoparticles, and the photosensitizer 5-ALA, while being conjugated to a B1R kinin ligand on their surface to facilitate transport across the blood-tumor barrier [73]. Additionally, Cui et al. designed tumor-targeting nano-adjuvants (aPDL1-PLG/R848 NPs) composed of aPDL1, an Fc-III-4C peptide linker (Fc-linker), and poly(L-glutamic acid)-grafted-R848. Under US irradiation, antigen-presenting cells are activated by the dual stimulation of in situ antigens and immune adjuvants. US further increased the expression of tumoral PDL1 and enhanced the biodistribution of aPDL1-PLG/R848 NPs by up to four-fold [74], surpassing the delivery efficacy of inherent PDL1 tumor targeting.
3. Tumor-selective activation strategy
The tumor-associated stimuli-activated prodrug design represents a promising strategy for enhancing the selectivity and efficacy of antitumor drugs [75]. A typical prodrug comprises a masking moiety and a parent drug, which are covalently linked by a cleavable linker. Ideally, the active component of the parent drug remains inhibited until it reaches the tumor site [76]. Consequently, the linker must be cleaved by tumor-associated stimuli, such as hypoxia, enzymes, reactive oxygen species (ROS), reducing agents, and low pH in the tumor and its microenvironment [77,78]. The following section discusses various designs of stimuli-activatable polymeric nanomedicines (Table 3).
Table 3.
Overview of polymeric nanomedicines designed for tumor-selective activation.
| Polymeric nanomedicines | Loaded drugs | Selective cleavable linker & tumor-associated markers | Mechanism of action | Ref. |
|---|---|---|---|---|
| Methoxy poly(ethylene glycol)-b-poly[(N-2-hydroxyethyl)-aspartamide]-g-thioketal-R848 (mPEG-b-PHEA-g-TK-R848) | R848 (TLR7/8 agonist) | Thioketal (TK) linker & ROS in the tumor | 1. Responsive release: ROS in the tumor microenvironment cleaves the TK linker, selectively releasing active R848. 2. Biological effects: R848 activates the TLR7/8 pathway, enhances dendritic cell antigen presentation, and activates CD8+ T cells to promote antitumor immunity. | [79] |
| IMDQ-Me combined with Poly(2-propionic-3-methylmaleic anhydride)-based methacrylamide polymer (p(PMMA-MA)) | IMDQ-Me (TLR7/8 agonist) | 2-propionic-3-methylmaleic anhydride linker & pH in endolysosomal compartments (tumor cells) | 1. Responsive release: Acidic pH in endolysosomes triggers the cleavage of the 2-propionic-3-methylmaleic anhydride linker, releasing IMDQ-Me. 2. Biological effects: IMDQ-Me activates TLR7/8, promoting dendritic cell maturation and immune activation. | [80] |
| Poly(ethylene glycol)-block-poly(diisopropylaminoethyl methacrylate)-block-poly(dopamine) & Poly(ethylene glycol)-block-poly(thioketal doxorubicin) (PEG-PDPA-PDA & PEG-PtkDOX) | β-lapachone (Lap), doxorubicin (DOX) | Thioketal (TK) linker & ROS in tumor cells; pH-responsive polymer & acidic tumor intracellular environment | 1. Responsive release: Acidic pH induces disassembly of PEG-PDPA-PDA, releasing Lap, which generates H2O2 via NQO1 overexpression in tumor cells. H2O2 is converted to hydroxyl radicals through the Fenton reaction, triggering ROS-induced cleavage of the TK linker and releasing DOX. 2. Biological effects: Cascade ROS generation and DOX release enhance chemodynamic and chemotherapy combination therapy, inducing oxidative damage and tumor cell death. | [81] |
| Poly(ethylene glycol)-tocopheryl succinate-block-paclitaxel-single thioether-oleic acid nanoparticles (PEG-TPGS2k-b-PTX-S-OA NPs) | Paclitaxel (PTX) | Single thioether linker & ROS/GSH (tumor intracellular environment) | 1. Responsive release: ROS oxidizes the thioether to sulfone, followed by GSH-triggered thiolysis, leading to the hydrolysis of ester bonds and release of PTX. 2. Biological effects: Rapid and selective PTX release, enhanced tumor apoptosis, and significant tumor growth inhibition. | [83] |
| Hydroxy poly(ethylene glycol)-b-poly[(N-2-aminoethyl)-aspartamide]-g-diselenide-doxorubicin (HO-Se-Se-PEG-P(AEMA-SS-DOX)) | DOX | Diselenide bond & ROS (tumor microenvironment); disulfide bond & GSH (tumor cells) | 1. Responsive release: High extracellular ROS cleaves the diselenide bond, releasing the PD-L1 antagonist D-peptide (DPPA-1) for immune checkpoint blockade and consumes ROS to enhance T-cell activity. Intracellularly, high GSH levels cleave the disulfide bond, releasing DOX for immunogenic cell death (ICD) and generating H2O2 to synergize ferroptosis. 2. Biological effects: Enhances ICD, promotes cytotoxic T-cell proliferation, inhibits tumor growth and metastasis, and prolongs survival. | [84] |
| Tirapazamine (TPZ) combined with Poly(L-glutamic acid)-graft‑methoxy poly(ethylene glycol)/combretastatin A4 nanoparticles (PLG-g-mPEG/CA4-NPs) | Combretastatin A4 (CA4) (vascular-disrupting agent) | Nitro group & hypoxia (tumor microenvironment) | 1. Responsive release: CA4-NPs selectively disrupt tumor blood vessels, inducing vascular shutdown and creating profound hypoxia in the tumor microenvironment. 2. Biological effects: Elevated hypoxia enhances the activation of hypoxia-activated prodrug (HAP) tirapazamine (TPZ) to its cytotoxic form, leading to tumor-specific chemotherapy, significant tumor shrinkage, and metastasis inhibition. | [85] |
| Hypoxia-sensitive imiquimod (hs-IMQ) combined with Poly(L-glutamic acid)-graft‑methoxy poly(ethylene glycol)/combretastatin A4 (PLG-g-mPEG/CA4, referred to as CA4-NPs) | Combretastatin A4 (CA4) (vascular-disrupting agent) & imiquimod (IMQ) (TLR7/8 agonist) | Nitroreductase (NTR) & tumor hypoxia (tumor microenvironment) | 1. Responsive release: CA4-NPs release CA4 selectively in tumor regions with physiological pH (7.4). Hypoxia-induced NTR catalyzes the activation of hs-IMQ, converting it into active IMQ, specifically in hypoxic tumor regions. 2. Biological effects: CA4 disrupts tumor vasculature, induces hypoxia, and promotes the infiltration of immature plasmacytoid dendritic cells (pDCs). Active IMQ converts immature pDCs into their activated form, promoting IFN-α secretion, priming natural killer (NK) cells, and activating cytotoxic T lymphocytes (CTLs) to enhance antitumor immunity. | [86] |
| Poly(L-glutamic acid)-graft‑methoxy poly(ethylene glycol)/combretastatin A4 combined with azide-masked resiquimod (PLG-g-mPEG/CA4 + R848-N3) | Combretastatin A4 (CA4) (vascular-disrupting agent) and R848-N3 (TLR7/8 agonist) | Azide group & CYP 450 in hypoxic microenvironment | 1. Responsive release: CA4-NPs exacerbate tumor hypoxia by disrupting tumor blood vessels, while CYP450 enzymes in the hypoxic tumor microenvironment reduce R848-N3 to active R848. 2. Biological effects: R848 activates the TLR7/8 pathway, enhances dendritic cell activation, polarizes macrophages to the M1 phenotype, and promotes CD8+ T cell-mediated antitumor immunity. | [87] |
| Poly(L-glutamic acid)-g‑methoxy poly(ethylene glycol)/combretastatin A4/BLZ945 nanoparticles combined with ethyl-(E)−6-diazo-2-((4-((4-(dimethylamino)phenyl)diazenyl)benzyl)oxy)carbonyl)amino)−5-oxohexanoate (PLG-g-mPEG/CA4/BLZ945 + Azo-DON) | Azo-DON (glutamine metabolism inhibitor) and combretastatin A4 (CA4) (vascular-disrupting agent) | Azo linker & azo-reductase in hypoxic microenvironment | 1. Responsive release: Azo-DON is reduced to active DON by azo-reductase (AZOR) in hypoxic tumor regions, while CBP increases tumor hypoxia by disrupting tumor vasculature. 2. Biological effects: DON inhibits glutamine metabolism, inducing tumor cell starvation and death while enhancing T cell cytotoxicity and reducing immunosuppression. | [88] |
| Poly(L-glutamic acid)-graft‑methoxy poly(ethylene glycol)/combretastatin A4 nanoparticles (PLG-g-mPEG/CA4, referred to as CA4-NPs) | HDON (glutamine metabolism inhibitor) and combretastatin A4 (CA4) (vascular-disrupting agent) | Nitrobenzyl linker & NTR in hypoxic microenvironments | 1. Responsive release: HDON is selectively reduced to active DON by NTR in hypoxic tumor regions, while CA4-NPs enhance tumor hypoxia by disrupting vasculature.2. Biological effects: DON blocks glutamine metabolism, inducing tumor cell starvation and apoptosis. CA4 further enhances hypoxia, while DON maintains T cell activity and reduces immunosuppressive MDSCs. | [89] |
| Poly(L-glutamic acid)−3-(3-(5-hydroxypentyl)ureido)benzo[e][1,2,4]triazine 1,4-dioxide nanoparticles (PLG-TPZH—NPs) | TPZH (DNA-damaging agent) | Ester bond linker & esterase | 1. Responsive release: Esterase in the tumor microenvironment cleaves the ester bond linker, sustainably releasing TPZH. 2. Biological effects: TPZH is activated in hypoxic conditions, generating cytotoxic benzotriazinyl radicals, which cause DNA strand breaks and apoptosis in tumor cells. | [90] |
| Poly(L-glutamic acid)-grafted Azo-plinabulin nanoparticles (PLG-AzoP-NPs) | Amino derivative of plinabulin (AmP) (vascular-disrupting agent) | Azo linker & hypoxic microenvironment | 1. Responsive release: AzoP-NPs are activated by reductases such as nitroreductase in hypoxic tumor environments, reducing azo bonds to amino groups and releasing the active drug AmP. 2. Biological effects: AmP disrupts tumor vasculature, amplifies tumor hypoxia, and induces tumor cell death by nutrient and oxygen deprivation. | [91] |
| Methoxy poly(ethylene glycol)-b-poly(D,L-lactide) co-loaded with plinabulin stearic mono-chloromethyl (PSM) and hexyl chain-containing tirapazamine derivative (TPZHex) (mPEG-b-PDLLA/PT-NPs) | PSM (vascular-disrupting agent), TPZHex (DNA-damaging agent) | Ester bond & esterase; acetoxymethyl ester linker & hypoxic microenvironment | 1. Responsive release: Esterase in the tumor microenvironment hydrolyzes PSM, releasing plinabulin to disrupt tumor vasculature and amplify hypoxia; TPZHex is activated in hypoxia, releasing free radicals that damage tumor cell DNA. 2. Biological effects: PSM disrupts tumor blood vessels, causing nutrient and oxygen deprivation; TPZHex kills tumor cells by inducing DNA damage under hypoxic conditions. | [92] |
| Maleimide-modified poly(ethylene glycol)-b-poly(D,L-lactide) nanoparticles loaded with azide-masked resiquimod (MAL-PEG-PDLLA/MAL-NPs) | R848-N3 (TLR7/8 agonist) | Azide linker & radiation-induced reduction | 1. Responsive release: Radiation reduces the azide linker, selectively releasing active R848 at the tumor site. 2. Biological effects: R848 activates dendritic cells (DCs), enhances antigen presentation, stimulates cytotoxic T lymphocytes (CTLs), and reverses tumor immunosuppression. | [93] |
| Methoxy poly(ethylene glycol)-b-poly(D,L-lactide) nanoparticles loaded with hypoxia-activated 6-diazo-5-oxo-L-norleucine (HDON—NPs) | HDON (glutamine metabolism inhibitor) | Nitro linker & hypoxia-induced NTR in tumors | 1. Responsive release: Hypoxia-induced NTR reduces HDON to DON, selectively blocking glutamine metabolism in tumor cells. 2. Biological effects: DON inhibits tumor glutamine metabolism, induces starvation therapy, and activates CD4+ and CD8+ T cells to enhance antitumor immunity. | [94] |
| Semiconducting polymeric nanoagonist conjugated with a stimulator of interferon genes (STING) agonist via singlet oxygen-cleavable diphenoxyethene linker (SPNM) | MSA-2 (STING pathway agonist) | Diphenoxyethene linker & sono-irradiation-induced singlet oxygen | 1. Responsive release: Sono-irradiation generates ¹O₂, cleaving the diphenoxyethene linker to release MSA-2 at the tumor site. 2. Biological effects: MSA-2 activates the STING pathway, induces interferon-β (IFN-β) secretion, promotes dendritic cell (DC) maturation, and enhances CD8+ T cell infiltration for systemic antitumor immunity. | [95] |
| Methoxy poly(ethylene glycol)-block-poly(L-glutamic acid) grafted with riboflavin and cisplatin prodrug (mPEG-b-PLG-g-Rf/cisPt(IV)) | Cisplatin prodrug (Pt(IV), DNA-damaging agent) | Leaving group of Pt(IV) & ultrasound (US)-induced •O₂⁻ | 1. Responsive release: US irradiation generates •O₂⁻, which reduces Pt(IV) to active Pt(II) at the tumor site. 2. Biological effects: Pt(II) binds to DNA, causing DNA crosslinking and double-strand breaks, leading to tumor cell apoptosis. | [96] |
3.1. Inherent tumor-associated stimuli-responsive drug activation
A recent study by Ren et al. presented an approach to enhance the efficacy of TLR7/8 agonists by developing ROS-responsive nanoparticles designated as R848 NPs (Fig. 4A, B) [79]. These nanoparticles, constructed from methoxy poly(ethylene glycol)-b-poly-[(N-2-hydroxyethyl)-aspartamide]-g-thioketal-R848, leverage the unique tumor microenvironment to enable the selective release of the active R848 in response to endogenous tumor ROS levels. This chemical modification of R848 to specifically block the C-4 amino group is a strategic innovation aimed at mitigating the severe systemic cytokine storms typically associated with TLR agonists, thus enhancing the safety profile of this therapeutic approach. The study integrated US as a dual-modality to induce immune cell death and promote the intratumoral accumulation of R848 NPs. This combined treatment, denoted R848 NPs + US, demonstrated significant advantages in the immunological landscape of the tumor microenvironment. By enhancing antigen presentation by dendritic cells (DCs), facilitating the distribution of active R848, and modulating systemic inflammatory responses, this strategy effectively upregulated the expression of costimulatory molecules on DCs. The culmination of these effects was the robust activation of CD8+ T cell immunity, which further synergized αPDL1 therapy, leading to a notable suppression of CT26 tumors (with a suppression rate of 99.0% and a Q value of 1.68). This multi-faceted and safe approach not only promoted immediate antitumor responses but also elicited memory immunity, which is critical for preventing tumor rechallenge. By intelligently engineering nanoparticles to respond to the tumor microenvironment and combining them with US to enhance their therapeutic effects, these studies pave the way for future research aimed at improving the precision and efficacy of TLR7/8 agonists.
Fig. 4.
Tumor selective activation strategies. (A) Schematic illustration of ROS-responsive TLR7/8 nanoagonists R848 NPs. (B) Treatment schedule, tumor growth curves, and representative tumor images at the end of the in vivo antitumor experiment on BALB/c mice bearing CT26 tumors. Reproduced with permission. [79] Copyright 2024, Elsevier B.V. (C) Redox-sensitive drug release mechanism of PTX-S-OA triggered by GSH/ROS. Reproduced under terms of the CC-BY license. [81] Copyright 2016, American Society for Clinical Investigation. (D) Schematic illustration of the cascade of ROS generation and drug release. Reproduced with permission. [83] Copyright 2019, Wiley-VCH Verlag GmbH & Co.
In another promising approach, reactive polymers that conjugate primary and secondary amines have been designed to facilitate drug release under acidic conditions [80]. Utilizing methacrylamide-based monomers with pendant 2-propionic-3-methylmaleic anhydride groups, researchers have created polymers that allow for the stable conjugation of amines while resisting radical polymerization conditions. These anhydride-functionalized methacrylates are synthesized using reversible addition-fragmentation chain transfer polymerization, leading to hydrophilic polymers suitable for targeted drug delivery. The acidic environment of tumors triggers the release of therapeutics, with primary amines forming irreversible imide bonds, while secondary amines enable quantitative release. In vitro studies indicated that these pH-responsive polymers effectively released immune-stimulatory drugs under endosomal conditions, demonstrating high cellular uptake and low toxicity. Drug conjugates modified with secondary amines exhibit improved receptor activity and enhanced immune cell maturation compared to those with primary amines, highlighting the importance of pH-sensitive mechanisms in therapeutic optimization. Overall, 2-propionic-3-methylmaleic anhydride-based polymers provide a versatile platform for the pH-triggered delivery of various drugs.
Recent advances in dual and multiple stimuli-responsive polymeric nanomedicines also demonstrate significant potential for enhancing therapeutic efficacy in cancer treatment [[81], [82], [83], [84]]. A redox dual-responsive prodrug nanosystem self-assembled from hydrophobic small-molecule paclitaxel (PTX) and oleic acid conjugates (OA) has been reported [83] (Fig. 4C). This system features thioether-linked conjugates (PTX-S-OA) and dithioether-inserted conjugates (PTX-2S-OA), designed to exploit the redox heterogeneity of tumors. PTX-S-OA demonstrated superior redox sensitivity compared to PTX-2S-OA, facilitating a more rapid and selective release of free PTX from the prodrug nanoassemblies upon redox stimuli. With a drug-loading capacity of 57.4%, the PEGylated PTX-S-OA nanoassemblies exhibited potent antitumor activity in human epidermoid carcinoma xenograft models. This innovative prodrug nanosystem effectively addresses the limitations associated with conventional hydrophobic prodrugs. Furthermore, a promising approach involves the utilization of ROS not only as therapeutic agents in chemodynamic therapy but also as stimuli for activating antitumor drug release. One such innovation is a pH/ROS dual-responsive nanomedicine that combines β-lapachone (Lap) with a pH-responsive polymer and a ROS-responsive polyprodrug (Fig. 4D) [81]. In an acidic intracellular environment, this nanomedicine triggers pH-mediated disassembly, leading to the release of Lap. Once released, Lap efficiently generates hydrogen peroxide, which is subsequently converted into highly toxic hydroxyl radicals via the Fenton reaction. This cascade reaction not only amplifies the therapeutic effect but also promotes the ROS-induced cleavage of the thioketal linker, resulting in the release of DOX from the polyprodrug. In vivo studies have shown that this coordinated generation of ROS and antitumor drug release effectively inhibited tumor growth, highlighting the potential of cascade reaction designs in enhancing antitumor efficacy.
3.2. Drug-amplified tumor-associated stimuli-responsive drug activation
Tumor-associated stimuli are inherently high in tumors compared to normal tissues. However, the efficiency of traditional tumor-selective activated nanotherapeutics is often limited by insufficient in vivo alterations at the tumor site. Thus, drug-amplified tumor-associated stimuli-responsive drug activation has been developed to address this limitation.
A cooperative strategy that utilizes a combretastatin A4 nanodrug (CA4-NPs) in conjunction with a matrix metalloproteinase 9 (MMP9)-activated DOX prodrug (MMP9-DOX-NPs) was developed by Jiang et al. Combretastatin A4 is a well-known vascular-disrupting agent that selectively disrupts immature tumor blood vessels, exacerbating the hypoxic tumor state. CA4-NPs treatment increased MMP9 expression in the tumors by 5.6-fold, and enhanced the tumor-selective release of MMP9-DOX-NPs by 3.7-fold in an orthotopic mouse mammary adenocarcinoma 4T1 tumor model. The sequential delivery of CA4-NPs and MMP9-DOX-NPs demonstrated enhanced antitumor efficacy with reduced systemic toxicity compared to non-cooperative controls. Yang, Shen, Sun, and Xu reported a sequential delivery system composed of a polymeric vascular-disrupting agent containing combretastatin A4 and hypoxia-activated prodrugs (HAPs). In this system, combretastatin A4 amplified hypoxic signaling within the tumor microenvironment, followed by the selective activation of HAPs via hypoxia-induced nitroreductase, azoreductase, or cytochrome P450 (CYP450) overactivity. These HAPs included tirapazamine (TPZ) [85], nitrobenzyl-based imiquimod (hs-IMQ) [86], R848-N3 [87], Azo-based hypoxic-activated 6-diazo-5-oxo-l-norleucine (Azo-DON) [88], nitrobenzyl-based hypoxia-activated 6-diazo-5-oxo-l-norleucine (HDON) [89], and urea-containing TPZ derivatives (TPZH—NPs) [90]. The tumor biodistribution and activity of these hypoxia-activated prodrugs were significantly enhanced by combining them with polymeric vascular-disrupting agents, achieving increases of up to 3.7- to 6.3-fold compared to single HAP treatments.
Building upon the amplified hypoxia signal induced by vascular-disrupting agents (VDAs), Xu and Lv et al. further developed a self-activating drug delivery system. Plinabulin, a potent VDA that has progressed to phase III clinical trials, has the potential to enhance tumor vessel blockade; however, its rigid structure poses significant challenges in physical encapsulation and chemical conjugation. Thus, an amino derivative of plinabulin (AmP) was grafted onto poly(L-glutamic acid) using a hypoxia-responsive Azo linker to address this, which resulted in the formulation of AzoP-NPs (Fig. 5A-B) [91]. This system selectively activated AmP under intrinsic tumor hypoxia, thereby disrupting tumor vessels and further amplifying hypoxia, ultimately inhibiting tumor growth. In a 4T1 tumor model, AzoP-NPs exhibited selective biodistribution within the tumor, with a free AmP concentration that was 18.6-fold higher at 24 h post-treatment than after direct AmP administration and significantly greater than in other tissues. Additionally, methoxy poly(ethylene glycol)-b-poly(D,L-lactide) (mPEG-b-PDLLA) was further prepared to co-deliver plinabulin and TPZ to the tumor site, concurrently disrupting blood vessels and eliminating tumor cells, thereby addressing both the symptoms and root causes of tumor progression (Fig. 5C) [92]. Plinabulin was converted into an esterase-responsive prodrug (PSM), while TPZ was synthesized into a hexyl chain-containing derivative (TPZHex) for effective co-delivery. Both PSM and TPZHex were co-encapsulated with mPEG-b-PDLLA, forming nanodrugs (PT-NPs). Upon reaching the tumor site, PT-NPs responded to the overexpression of esterase, releasing plinabulin to disrupt blood vessels and induce nutritional and oxygen deficiencies. TPZHex was activated in response to increased hypoxia, leading to tumor cell death. PT-NPs demonstrated enhanced therapeutic efficacy in the treatment of 4T1 tumors, achieving a tumor suppression rate of 92.9% and a cure rate of 20%. These studies represent a more advanced strategy for drug delivery.
Fig. 5.
Self-activated drug deliveries. (A) Synthesis and preparation of the AzoP-NPs. (B) Schematic image for release of AmP in hypoxic tumor microenvironment. Reproduced under terms of the CC-BY license. [91] Copyright 2022, Elsevier B.V. (C) Chemical structure and benefits of esterase- and hypoxia-responsive prodrugs PSM, TPZHex, and PT-NPs. Reproduced under terms of the CC-BY license. [92] Copyright 2024, Elsevier B.V.
3.3. External approach-amplified tumor-associated stimuli-responsive drug activation
Recent advances in the field of drug delivery have highlighted the potential of utilizing exogenous approaches to amplify tumor-associated stimuli and further enhance the selectivity and efficacy of therapeutics, particularly in the context of cancer treatment. Strategies, such as the use of maleimide-modified resiquimod prodrug nanoparticles (MAL-NPs) combined with radiotherapy (RT) and anti-PD1 therapy, demonstrate another dimension of this approach [93]. RT amplified ROS levels in tumors and promoted the selective activation of the resiquimod prodrug. MAL-NPs enhanced immune checkpoint inhibitor therapy by promoting antigen endocytosis in dendritic cells while being selectively activated within the tumor microenvironment. This dual mechanism not only amplified the therapeutic effect but also elicited a systemic immune response, effectively addressing the challenges associated with conventional immunotherapy. Additionally, the integration of US to increase hypoxia and activate hypoxia-activated 6-diazo-5-oxo-L-norleucine prodrug nanoparticles (HDON—NPs) illustrated the utility of physical stimuli in augmenting therapeutic outcomes [94]. This synergistic approach significantly enhanced tumor suppression rates while simultaneously modulating the tumor microenvironment. Furthermore, the development of semiconducting polymeric nanoagonists (SPNM) for sono-activated immunotherapy showed the versatility of using external stimuli to achieve spatiotemporal control of drug delivery and immune activation [95]. By generating ROS and selectively releasing immunotherapeutic agents, SPNM facilitated a robust antitumor immune response.
Another study illustrated the efficacy of US in converting Pt(IV) prodrugs to their active Pt(II) forms in an aqueous environment [96]. Notably, the incorporation of riboflavin (Rf), a well-known sonosensitizer, significantly enhanced the reduction rate of the prodrug by facilitating the generation of superoxide anion radicals (•O2−). This synergistic effect underscores the potential of Rf in optimizing US-mediated prodrug activation. By covalently co-loading Rf and tetravalent cisplatin [cisPt(IV)] prodrug into a poly(L-glutamic acid) matrix, the researchers successfully created a US catalysis-activated nanoprodrug [P-Rf/cisPt(IV)], specifically designed for antitumor therapy. Notably, even at low-intensity US irradiation (1.5 W/cm²), the P-Rf/cisPt(IV) was effectively converted to cisPt(II) in vivo, leading to significant antitumor effects (Fig. 6). This highlights the potential of US-activated nanoprodrugs for enhancing drug efficacy and minimizing the systemic toxicity typically associated with traditional chemotherapy.
Fig. 6.
Ultrasound irradiation-induced superoxide anion radical mediates the reduction of tetravalent platinum prodrug for anti-tumor therapy. (A) Structure and ultrasound induced cisplatin release of polymeric tetravalent platinum P-Rf/cisPt(IV). (B) In vitro evidences of ultrasound induced cisplatin release of polymeric tetravalent platinum P-Rf/cisPt(IV). (C) Mechnism evaluation for ultrasound induced cisplatin release of polymeric tetravalent platinum P-Rf/cisPt(IV). (D) In vivo evidences of ultrasound induced cisplatin release,biodistribution, and tumor inhibition of polymeric tetravalent platinum P-Rf/cisPt(IV). [96] COPYRIGHT & PERMISSIONS © 2024 Chinese Chemical Society.
In summary, the strategic amplification of tumor-associated stimuli for selective drug activation by external approaches represents a promising direction in cancer therapy, enhancing therapeutic efficacy while minimizing off-target effects. The innovative designs of these activatable systems offer more effective and precise cancer treatments, potentially revolutionizing cancer treatment modalities.
4. Multiple tumor-targeted delivery
Multiple tumor-targeted delivery integrates active tumor-targeted drug delivery and tumor-selective drug activation, fully endowing polymeric nanomedicines with the ability to be actively enriched and selectively activated in tumors. The approach holds significant potential to enhance tumor-targeted drug therapy. Currently, relatively few designs exist in this area; however, the following section will highlight some examples (Table 4).
Table 4.
Key examples of multiple tumor-targeted polymeric nanomedicines.
| Polymeric nanomedicines | Loaded drugs | Targeting moiety & tumor-associated markers | Selective cleavable linker & tumor-associated markers | Mechanism of action | Ref. |
|---|---|---|---|---|---|
| Cyclic RGD-modified dextran-hydrazone-doxorubicin/dihydralazine self-assembled polymeric nanoparticles (cRGD-Dex-DOX/HDZ NPs) | Doxorubicin (DOX) (DNA intercalator); dihydralazine (HDZ) (vasodilator) | cRGD & Integrin αvβ3 in tumor vessel and tumor cells | Hydrazone group (pH-sensitive linker) & acidic tumor microenvironment | 1. Active targeting via cRGD-integrin αvβ3 interaction. 2. The hydrazone bond is cleaved in acidic tumor microenvironments, releasing DOX and HDZ. 3. HDZ dilates tumor blood vessels, reducing hypoxia and ECM stiffness, which enhances nanoparticle accumulation and penetration. DOX intercalates into DNA, inducing cytotoxicity, while improved vascularity promotes T-cell infiltration for enhanced antitumor immunity. | [97] |
| c(RGDfK)-modified poly(lactic-co-glycolic acid) nanoparticles encapsulating RA-S-S-Cy and catalase (c(RGDfK)-PLGA-RA-S-S-Cy NPs) | RA-S-S-Cy (cyclopeptide RA-V + Cy5.5 dye); catalase | c(RGDfK) & integrin αvβ3 in tumor vessel and tumor cells | Disulfide bond & GSH (tumor cells); catalase & H2O2 (tumor cells) | 1. c(RGDfK) binds to αvβ3 integrin, enhancing tumor-cell-specific uptake. 2. The disulfide bond in RA-S-S-Cy is cleaved by intracellular GSH, releasing RA-V and activating Cy5.5 fluorescence. Catalase reacts with H2O2 to generate O2, alleviating tumor hypoxia and enhancing drug release. 3. RA-V induces apoptosis and inhibits tumor growth, while O2 generation improves the tumor microenvironment and enhances chemotherapeutic efficacy. | [98] |
| CD163 monoclonal antibody-modified Azide-poly(ethylene glycol)-block-poly(2-(4-formylbenzoyloxy)ethyl methacrylate)-doxorubicin nanoparticles (mAb-CD163-PDNPs) | DOX | CD163 monoclonal antibody (mAb-CD163) & CD163 on M2-type tumor-associated macrophages (TAMs) | Schiff base bond (-CH N-) & acidic in tumor microenvironments | 1.CD163 monoclonal antibody targeted to M2-type macrophagesin the tumors. 2. The Schiff base bond is hydrolyzed in acidic tumor microenvironments, releasing DOX from the polymeric backbone. 3. Depletes M2-type macrophages in TAMs, reduces immunosuppression, enhances drug retention at the tumor site, and inhibits tumor cell proliferation. | [99] |
| Doxorubicin and CdSeTe quantum dots-loaded poly(2-(hexamethyleneimino)ethyl methacrylate)-b-poly(ethylene glycol)-b-poly(diethylenetriaminepentaacetic acid methacrylate)-b-poly(1-vinylimidazole)-b-poly(4-vinylphenylboronic acid) (DOX@CdSeTe@PC7A-PEG-DTPA-VI-PBA) | DOX, CdSeTe quantum dots (QDs) | Phenylboronic acid (PBA) & sialic acid overexpressed on tumor cell surfaces | Protonation of 2-(hexamethyleneimino)ethyl methacrylate (C7A) & acidic tumor microenvironment | 1. PBA specifically binds to sialic acid on tumor cells for active targeting. 2. Protonation of C7A under weakly acidic conditions triggers micelle rupture and DOX release. 3. DOX inhibits tumor proliferation; CdSeTe QDs produce ROS under light irradiation for enhanced tumor cell apoptosis. | [100] |
| Hydroxybenzyl alcohol-ursodeoxycholic acid polymeric prodrug nanoparticles (pHU), surface-coated with taurodeoxycholic acid (TUDCA), referred to as t-pHUDs | DOX (DNA intercalator); hydroxybenzyl alcohol (HBA) (antiangiogenic agent); ursodeoxycholic acid (UDCA) (cytoprotective agent) | Taurodeoxycholic acid (TUDCA) & P-selectin overexpressed on tumor cells | Peroxalate linker & H2O2 (tumor microenvironment) | 1. TUDCA binds P-selectin on tumor cells, enhancing tumor localization. 2. Peroxalate linker reacts with H2O2, releasing DOX, HBA, and UDCA. 3. DOX intercalates into DNA, inducing tumor cell death. HBA suppresses VEGF expression, reducing angiogenesis. UDCA mitigates DOX-induced cardiotoxicity and hepatotoxicity. | [101] |
| Fibrin-targeting peptide-modified poly(L-glutamic acid)-g-poly(ethylene glycol)-dibenzocyclooctyne/TPZP nanoparticles) (FT11-TPZP-NPs) | TPZP (a urea-containing tirapazamine derivative) | FT11 peptide; & fibrin in blood clots within tumors | Ester bond & esterase in tumor cells; azo bond & azoreductase elevated in hypoxic microenvironments | 1. FT11 peptide binds to fibrin, allowing for targeted accumulation in tumors. 2. CA4-NPs induce coagulation and exacerbate hypoxia, enhancing TPZP activation. 3. TPZP is released and activated in hypoxic tumor environments, leading to increased cytotoxicity. 4. This strategy improves tumor targeting and reduces systemic toxicity, achieving high tumor inhibition rates. | [102] |
| Poly(L-glutamic acid)-graft‑methoxy poly(ethylene glycol)/combretastatin A4 nanoparticles (CA4-NPs); apcitide peptide-decorated poly(L-glutamic acid)-graft-IMDQ-N3 (apcitide-PLG-IMDQ-N3) | Combretastatin A4 (CA4); imidazoquinoline derivative (IMDQ-N3) | Apcitide peptide & GPIIb-IIIa receptor on activated platelets | Azido group (-N3) & CYP450 in hypoxic microenvironment | 1. CA4-NPs disrupt tumor vasculature, causing hemorrhage and hypoxia, enhancing 2. Apcitide-PLG-IMDQ-N3 targeting activated platelets in coagulated tumors. 3. IMDQ-N3 is selectively reduced to an amino group under hypoxic conditions via CYP enzymes, stimulating TLR7/8, enhancing DC activation, M1 macrophage polarization, and CD8+ T cell priming, inducing robust antitumor immunity. | [103] |
The first example focuses on a dihydralazine (HDZ)-loaded nanoparticle drug delivery system containing a pH-responsive, cyclic RGD peptide-modified prodrug based on DOX (cRGD-Dex-DOX) (Fig. 7A) [97]. In acidic tumor cell microenvironments, the hydrazone group in the polymeric prodrug can be cleaved, leading to the release of DOX. This formulation is engineered to enhance antitumor efficacy by exploiting the acidic microenvironment commonly found in tumors, thereby facilitating targeted drug release at the site of action.
Fig. 7.
Multiple tumor-targeted delivery strategies. (A) Schematic illustration of pH-responsive cRGD-Dex-DOX/HDZ nanoparticles. Reproduced under terms of the CC-BY license. [97] Copyright 2023, Wiley-VCH Verlag GmbH & Co. (B) Schematic illustration of the RA-S-S-Cy@PLGA NPs structure. Reproduced under terms of the CC-BY license. [98] Copyright 2019, Ivyspring International. (C) Schematic illustration of synthesis and self-assembly of mAb-CD163-PDNPs nanoparticles modified by CD163 monoclonal antibody. (D) Tumor volume, changes in mouse body weight during mAb-CD163-PDNPs treatment and representative picture of tumor after treatment on day 14. Reproduced under terms of the CC-BY license. [99] Copyright 2023, MDPI. Co. (E) Chemical structure of TPZP and schematic diagram of CA4-NPs, FT11-TPZP-NPs combined therapy. Reproduced under terms of the CC-BY license. [102] Copyright 2024, China Science Publishing & Media Ltd,.
Another notable case involves a c(RGDfK)-targeted and ROS/GSH dual-activatable theranostic nanosystem (RA-S-S-Cy@PLGA NPs) designed for selective therapy against hypoxic tumors with in situ fluorescence chemotherapeutic drug tracking (Fig. 7B) [98]. The theranostic agent (RA-S-S-Cy) is composed of a disulfide bond cleavable by high intracellular GSH levels, a NIR fluorophore for tracking, and the natural cyclopeptide RA-V as the active anticancer drug. Upon GSH-induced cleavage, the system releases RA-V and triggers an increase in NIR fluorescence, enabling simultaneous drug release and tumor imaging. RA-S-S-Cy is encapsulated within c(RGDfK)-targeted PLGA nanoparticles, along with catalase for oxygen generation, to enhance tumor targeting and redox dual-responsiveness. The results showed improved therapeutic efficacy both in vitro and in vivo, as well as successful in situ monitoring of drug release via NIR fluorescence. This nanosystem offers a promising approach for precise, hypoxia-targeted cancer therapy and provides valuable insight into drug-release mechanisms.
Yang et al. developed CD163 monoclonal antibody-modified Schiff-base bond-conjugated DOX polymeric prodrug nanoparticles (mAb-CD163-PDNPs), specifically designed to enhance antitumor effects by selectively targeting and depleting M2-type tumor-associated macrophages (TAMs) (Fig. 7C-D) [99]. The study demonstrated that these nanoparticles achieved a tumor inhibition rate of 81%, highlighting their potential to effectively deplete pro-tumor macrophages while simultaneously delivering the chemotherapeutic agent DOX. The mAb-CD163-PDNP formulation involves a sophisticated approach where DOX is covalently bonded to an amphiphilic polymer via a Schiff base reaction, enabling self-assembly into nanoparticles in aqueous solutions. The nanoparticles are further modified through a “click” reaction, facilitating the attachment of the CD163 monoclonal antibody to ensure specific targeting to TAMs. In vitro studies revealed that these prodrug nanoparticles responded to an acidic tumor microenvironment, leading to targeted drug release and enhanced cellular uptake and offering an avenue for the development of targeted immunotherapies in malignant tumors. Overall, mAb-CD163-PDNPs represent significant progress in the quest for effective cancer treatments that leverage the unique characteristics of the tumor microenvironment.
A fourth approach explores a tumor-targeting and imaging micelle system, which was developed for phenylboronic acid-targeted and pH-triggered anticancer polymeric drug and combined photodynamic therapy (PDT) [100]. Charge-switchable polymer nano micelles composed of poly(2-(hexamethyleneimino) ethyl methacrylate)-b-poly(ethylene glycol) monomethyl ether methacrylate)-b-poly(diethylenetriaminepentaacetic acid methacrylate)-b-poly(1-vinyl imidazole)-b-poly(4-vinyl phenylboronic acid) (PC7A-PEG-DTPA-VI-PBA) were designed to release DOX more efficiently in acidic environments (pH 5.0) compared to a neutral pH (7.4), thus improving targeted drug delivery to tumor sites. The imidazole groups in the micelles coordinate CdSeTe quantum dots to enable PDT, while phenylboronic acid enhances tumor targeting. Additionally, DTPA chelates 99mTc for SPECT imaging, facilitating precise tumor localization. In vitro experiments revealed that DOX-loaded micelles were not cytotoxic to normal cells but significantly inhibited the proliferation of B16F10 melanoma cells and promoted apoptosis. In vivo studies using C57BL tumor-bearing mice demonstrated efficient tumor growth inhibition, highlighting excellent pH responsiveness, biocompatibility, and potential of the micelles as a multifunctional drug delivery system for cancer therapy.
Another study introduced polymeric prodrug nanoparticles (t-pHUDs) for targeted cancer therapy, combining drug delivery and therapeutic action [101]. The nanoparticles were surface-decorated with TUDCA for p-selectin-mediated cancer cell targeting, which enhanced tumor accumulation. DOX release was triggered by H2O2, a characteristic of the tumor microenvironment, ensuring selective drug release and minimizing off-target toxicity. In animal models, t-pHUDs exhibited superior anticancer efficacy compared to free DOX or pHU nanoparticles while significantly reducing cardiotoxicity and hepatotoxicity. This approach shows great promise for improving the therapeutic index and minimizing side effects, with strong translational potential for cancer treatment.
Another novel approach was used to enhance the efficacy and selectivity of hypoxia-activated prodrugs (HAPs) by utilizing a urea-containing derivative of tirapazamine (TPZP) combined with a fibrin-targeted nanodrug delivery system (Fig. 7E) [102]. The urea modification of TPZ significantly improved its hypoxic cytotoxicity, with TPZP exhibiting 20-fold higher cytotoxicity compared to TPZ while maintaining hypoxia selectivity. TPZP was formulated into fibrin-targeting nanoparticles (FT11-TPZP-NPs) to further optimize delivery and reduce off-target effects. The inclusion of CA4-NPs, a polymeric vascular-disrupting agent, exacerbated tumor hypoxia and increased fibrin levels in tumors, creating a highly favorable microenvironment for the targeted accumulation and activation of FT11-TPZP-NPs. This strategy ensures that the drug is selectively activated in hypoxic tumors, minimizing systemic toxicity while maximizing therapeutic efficacy. In preclinical CT26 tumor models, a single-dose treatment of FT11-TPZP-NPs combined with CA4-NPs achieved a tumor inhibition rate of 98.1%, with the complete elimination of tumors in four out of six cases. The efficiency of this active targeting and selective activation mechanism demonstrates the potential for greatly improving the therapeutic outcomes of HAPs in cancer therapy.
Finally, Xu et al. introduced an innovative drug delivery strategy that combines active targeting and selective activation, which significantly enhanced antitumor efficacy while reducing systemic toxicity of the immune agonist [103]. The designed imidazoquinoline (IMDQ) nano-missile (apcitide-PLG-IMDQ-N3) integrated a tumor-targeting mechanism with a “safety-catch” activation system. Poly(L-glutamic acid)-graft‑methoxy poly(ethylene glycol)/combretastatin A4 (termed CA4-NPs), a polymeric vascular-disrupting agent acting as a “radar detector”, remodels the tumor microenvironment by amplifying coagulation and hypoxia. Subsequently, apcitide-PLG-IMDQ-N3 targets the GPIIb-IIIa on activated platelets in these coagulated regions through surface apcitide and selectively activates IMDQ in hypoxic conditions by changing the -N3 group of IMDQ-N3 to an -NH2 group. The results demonstrated that an intravenous injection of apcitide-PLG-IMDQ-N3 combined with CA4-NPs led to a 59.7-fold increase in activated IMDQ at tumor sites compared to the IMDQ-N3 prodrug. The biodistribution results confirmed the high tumor uptake of activated IMDQ (22.66%ID/g). This approach not only minimized systemic toxicity but also effectively primed tumor-specific CD8+ T cells and enhanced their immunological memory. The treatment successfully eradicated large 4T1 breast cancer and CT26 colon cancer tumors (about 500 mm³) in mice while also providing protection against tumor rechallenge (Fig. 8). By employing a highly selective activation mechanism in conjunction with tumor-specific targeting, this “safety-catch”-equipped IMDQ nano-missile represents a promising avenue for clinical applications, offering both precision and amplified immunogenic effects for tumor immunotherapy.
Fig. 8.
Tumor microenvironment remodeling-mediated sequential drug delivery potentiates treatment efficacy. (A) Synthesis of IMDQ-N3, PLG-IMDQ-N3 and apcitide-PLG-IMDQ-N3. (B) Schematic illustration for CA4-NPs induced coagulation cascade, active recognition and binding of apcitide on apcitide-PLG-IMDQ-N3 and active GPIIa-IIIb in active platelet, and activation of apcitide-PLG-IMDQ-N3 under hypoxia. (C) Schematic of the sequential drug delivery strategy with tumor microenvironment remodeling. (D) Biodistribution of active IMDQ of tumor-bearing mice post different treatment. (E) Tumor growth curves of mice post treatment in the tumor inhibition experiment and re-challenge experiment. Reproduced with permission. [103] Copyright 2024, Wiley-VCH Verlag GmbH & Co.
These studies provide a framework for enhancing the precision and potency of drug treatments to significantly advance the development of more effective anticancer strategies.
5. Conclusion and perspective
In summary, this review on tumor-targeted delivery and the release of polymeric nanomedicine highlights the significant potential, challenges, and advanced strategies of using polymeric nanomedicine in targeted tumor therapy. There are three primary strategies: active tumor-targeted delivery, tumor-selective drug activation, and multiple tumor-targeted delivery (Fig. 9). The inherent tumor-associated markers or stimuli are not sufficiently different between tumor and normal tissues. Thus, drug or external approaches (light, radiation, and US) are first utilized in advanced strategies to amplify tumor-associated markers or stimuli. Then, active tumor-targeted or tumor selectively activated polymeric nanomedicine is given to achieve better drug accumulation or selective activation, providing foundational specificity or refining spatial control. Furthermore, multiple tumor-targeted polymeric nanomedicines synchronizing active tumor-targeting or tumor-selectively activated ability could achieve first tumor accumulation and then selective activation of the drug after drug or external approaches (such as light, radiation, and US) to amplify tumor-associated markers or stimuli. Future efforts should focus on exploring external approaches, e.g., light, radiation, and US-based chemical cleavable prodrugs and tumor-associated markers, optimizing and simplifying polymeric nanomedicine design, and improving active targeting and selective activation mechanisms to achieve enhanced tumor-targeted delivery, release, and therapeutic efficacy.
Fig. 9.
Schematic illustration of advanced tumor-targeted delivery and release strategies of polymeric nanomedicines, including actively tumor-targeted delivery, tumor selective drug activation and multiple tumor-targeted delivery.
Immunotherapy represents a revolutionary cancer treatment approach, leveraging the immune system to recognize and attack tumor cells. The effective delivery of immunotherapeutic agents is essential to address challenges such as off-target effects, immune-related adverse events, and enhancing therapeutic efficacy [104,105]. Immunotherapeutic delivery systems have become a prominent focus in the field of drug delivery research. Furthermore, designing combination therapies is urgently needed to enhance tumor-targeting efficiency and achieve synergistic effects in cancer treatment.
The development of theranostic nanoparticles has introduced a promising imaging technology for monitoring tumor-targeting efficiency. This approach enables the simultaneous co-delivery of molecular imaging modalities and therapeutic drugs to the tumor site [106]. Molecular imaging provides non-invasive and real-time visualization techniques, offering detailed insight into molecular and biochemical processes. The commonly used molecular imaging modalities include magnetic resonance imaging, optical imaging, US imaging, computed tomography, and positron emission tomography/SPECT [107]. For example, fluorescence imaging and photoacoustic imaging, which are based on NIR optical imaging, have demonstrated significant potential in this field [108,109]. We anticipate that further explorations will yield breakthroughs in cancer therapy, which will ultimately lead to better quality of life and extend the survival of tumor patients.
Declaration of competing interest
The authors declare that they have no conflicts of interest in this work.
Acknowledgments
This work was financially supported by the National Key R&D Program of China (2022YFE0110200), the National Natural Science Foundation of China (52273157, 52073279, 52025035), the Department of Science and Technology of Jilin Province (20240305041YY), and the Youth Innovation Promotion Association of Chinese Academy of Sciences (2022224).
Biographies
Na Shen is an associated professor at the Key Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Science. Her research interests include controlled targeting and release, and host response of polymeric nanomedicines in cancer treatment.
Hai Sun is a professor at the Key Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Science. His research focuses on the industrialization of medical-grade polylactic acid and the development of high-performance absorbable medical devices.
Zhaohui Tang is a professor at the Key Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Science. His research interests include biodegradable polymeric drug carrier, nanomedecine, tumor targeting drug delivery and host immune response in cancer treatment.
Contributor Information
Hai Sun, Email: sunhai@ciac.ac.cn.
Zhaohui Tang, Email: ztang@ciac.ac.cn.
References
- 1.Anand U., Dey A., Chandel A.K.S., et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023;10(4):1367–1401. doi: 10.1016/j.gendis.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debela D.T., Muzazu S.G., Heraro K.D., et al. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021;9 doi: 10.1177/20503121211034366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu B., Zhou H., Tan L., et al. Exploring treatment options in cancer: Tumor treatment strategies. Sign. Transd. Targ. Ther. 2024;9(1):175. doi: 10.1038/s41392-024-01856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savage P., Mahmoud S. Development and economic trends in cancer therapeutic drugs: A 5-year update 2010-2014. Br. J. Cancer. 2015;112(6):1037–1041. doi: 10.1038/bjc.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D-R, Wu X.-L., Sun Y.-L. Therapeutic targets and biomarkers of tumor immunotherapy: Response versus non-response. Sign. Transd. Targ. Ther. 2022;7(1):331. doi: 10.1038/s41392-022-01136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blass E., Ott P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021;18(4):215–229. doi: 10.1038/s41571-020-00460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin U., Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359(6382):1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 8.Wang F., Yang S., Palmer N., et al. Real-world data analyses unveiled the immune-related adverse effects of immune checkpoint inhibitors across cancer types. NPJ Precis. Oncol. 2021;5(1):82. doi: 10.1038/s41698-021-00223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schagen S.B., Tsvetkov A.S., Compter A., et al. Cognitive adverse effects of chemotherapy and immunotherapy: Are interventions within reach? Nat. Rev. Neurol. 2022;18(3):173–185. doi: 10.1038/s41582-021-00617-2. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A., Jasrotia S., Kumar A. Effects of chemotherapy on the immune system: Implications for cancer treatment and patient outcomes. Naunyn Schmiedebergs Arch. Pharmacol. 2024;397(5):2551–2566. doi: 10.1007/s00210-023-02781-2. [DOI] [PubMed] [Google Scholar]
- 11.Su J.-X., Li S.-J., Zhou X.-F., et al. Chemotherapy-induced metastasis: Molecular mechanisms and clinical therapies. Acta Pharmacol. Sin. 2023;44(9):1725–1736. doi: 10.1038/s41401-023-01093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J., Kantoff P.W., Wooster R., et al. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Canc. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luobin L., Wanxin H., Yingxin G., et al. Nanomedicine-induced programmed cell death in cancer therapy: Mechanisms and perspectives. Cell Death Discov. 2024;10(1):386. doi: 10.1038/s41420-024-02121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B., Hu S., Teng Y., et al. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Sign. Transduct. Target. Ther. 2024;9(1):200. doi: 10.1038/s41392-024-01889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell M.J., Billingsley M.M., Haley R.M., et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stater E.P., Sonay A.Y., Hart C., et al. The ancillary effects of nanoparticles and their implications for nanomedicine. Nat. Nanotechnol. 2021;16(11):1180–1194. doi: 10.1038/s41565-021-01017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y., Hu P., Shi J. Nanomedicine remodels tumor microenvironment for solid tumor immunotherapy. J. Am. Chem. Soc. 2024;146(15):10217–10233. doi: 10.1021/jacs.3c14005. [DOI] [PubMed] [Google Scholar]
- 18.Elmowafy E.M., Tiboni M., Soliman M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharmaceut. Investigat. 2019;49(4):347–380. [Google Scholar]
- 19.Xu W., Ling P., Zhang T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv. 2013;2013(1) doi: 10.1155/2013/340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng C., Wu J., Cheng R., et al. Functional polypeptide and hybrid materials: Precision synthesis via α-amino acid n-carboxyanhydride polymerization and emerging biomedical applications. Prog. Polym. Sci. 2014;39(2):330–364. [Google Scholar]
- 21.El-Say K.M., HS El-Sawy. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017;528(1–2):675–691. doi: 10.1016/j.ijpharm.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 22.Tang Z., He C., Tian H., et al. Polymeric nanostructured materials for biomedical applications. Prog. Polym. Sci. 2016:6086–6128. [Google Scholar]
- 23.Rosenblum D., Joshi N., Tao W., et al. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018;9(1):1410. doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao Y., Li H., Ge X., et al. Tumor-selective activation of toll-like receptor 7/8 agonist nano-immunomodulator generates safe anti-tumor immune responses upon systemic administration. Angewandte Chemie Int. Edition. 2022;61(52) doi: 10.1002/anie.202214992. [DOI] [PubMed] [Google Scholar]
- 25.Y. Zhao, Y.-Q. Xie, S. Van Herck, et al., Switchable immune modulator for tumor-specific activation of anticancer immunity, Sci. Adv. 7 (37) eabg7291. [DOI] [PMC free article] [PubMed]
- 26.Xia H., Qin M., Wang Z., et al. A ph-/enzyme-responsive nanoparticle selectively targets endosomal toll-like receptors to potentiate robust cancer vaccination. Nano Lett. 2022;22(7):2978–2987. doi: 10.1021/acs.nanolett.2c00185. [DOI] [PubMed] [Google Scholar]
- 27.Kamaly N., Xiao Z., Valencia P.M., et al. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012;41(7):2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin X., Li Y. Strategies to design and synthesize polymer-based stimuli-responsive drug-delivery nanosystems. Chembiochem. 2020;21(9):1236–1253. doi: 10.1002/cbic.201900550. [DOI] [PubMed] [Google Scholar]
- 29.Hu J., Zhang G., Liu S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev. 2012;41(18):5933–5949. doi: 10.1039/c2cs35103j. [DOI] [PubMed] [Google Scholar]
- 30.Manzari M.T., Shamay Y., Kiguchi H., et al. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mat. 2021;6(4):351–370. doi: 10.1038/s41578-020-00269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Tanani M., Rabbani S.A., Babiker R., et al. Unraveling the tumor microenvironment: Insights into cancer metastasis and therapeutic strategies. Cancer Lett. 2024 doi: 10.1016/j.canlet.2024.216894. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Z., Ukidve A., Kim J., et al. Targeting strategies for tissue-specific drug delivery. Cell. 2020;181(1):151–167. doi: 10.1016/j.cell.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Nicolas J., Mura S., Brambilla D., et al. Couvreur P, design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013;42(3):1147–1235. doi: 10.1039/c2cs35265f. [DOI] [PubMed] [Google Scholar]
- 34.Delplace V., Couvreur P., Nicolas J. Recent trends in the design of anticancer polymer prodrug nanocarriers. Polym. Chem. 2014;5(5):1529–1544. [Google Scholar]
- 35.Arranja A.G., Pathak V., Lammers T., et al. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol. Res. 2017:11587–11595. doi: 10.1016/j.phrs.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Q., Xiang J., Qiu N., et al. Tumor abnormality-oriented nanomedicine design. Chem. Rev. 2023;123(18):10920–10989. doi: 10.1021/acs.chemrev.3c00062. [DOI] [PubMed] [Google Scholar]
- 37.Salvati A., Pitek A.S., Monopoli M.P., et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013;8(2):137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 38.Tian T., Zhang H.-X., He C.-P., et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018:150137–150149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Escareno N., Hassan N., Kogan M.J., et al. Microfluidics-assisted conjugation of chitosan-coated polymeric. J. Colloid. Interface Sci. 2021:591440–591450. doi: 10.1016/j.jcis.2021.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Pastorino F., Brignole C., Marimpietri D., et al. Doxorubicin-loaded fab′ fragments of anti-disialoganglioside immunoliposomes selectively inhibit the growth and dissemination of human neuroblastoma in nude mice. Cancer Res. 2003;63(1):86–92. [PubMed] [Google Scholar]
- 41.Lewis S.M., Wu X., Pustilnik A., et al. Generation of bispecific igg antibodies by structure-based design of an orthogonal fab interface. Nat. Biotechnol. 2014;32(2):191. doi: 10.1038/nbt.2797. [DOI] [PubMed] [Google Scholar]
- 42.Cheng W.W.K., Allen T.M. Targeted delivery of anti-cd19 liposomal doxorubicin in b-cell lymphoma: A comparison of whole monoclonal antibody, fab' fragments and single chain fv. J. Controll. Rel. 2008;126(1):50–58. doi: 10.1016/j.jconrel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y., Ma S., Zhao J., et al. Mannan-decorated pathogen-like polymeric nanoparticles as nanovaccine carriers for eliciting superior anticancer immunity. Biomaterials. 2022;284:121489. doi: 10.1016/j.biomaterials.2022.121489. [DOI] [PubMed] [Google Scholar]
- 44.Qiao J., Tian F., Deng Y., et al. Bio-orthogonal click-targeting nanocomposites for chemo-photothermal synergistic therapy in breast cancer. Theranostics. 2020;10(12):5305–5321. doi: 10.7150/thno.42445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo Z., Hu D., Gao D., et al. High-specificity in vivo tumor imaging using bioorthogonal nir-iib nanoparticles. Adv. Mater. 2021;33(49) doi: 10.1002/adma.202102950. [DOI] [PubMed] [Google Scholar]
- 46.Meng D., Pan H., He W., et al. In situ activated nk cell as bio-orthogonal targeted live-cell nanocarrier augmented solid tumor immunotherapy. Adv. Funct. Mater. 2022;32(29) [Google Scholar]
- 47.Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C. 2016;60:569–578. doi: 10.1016/j.msec.2015.11.067. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen T.T.K., Woo S.M., Seo S.U., et al. Enhanced anticancer efficacy of trail-conjugated and odanacatib-loaded plga nanoparticles in trail resistant cancer. Biomaterials. 2025;312 doi: 10.1016/j.biomaterials.2024.122733. [DOI] [PubMed] [Google Scholar]
- 49.Zheng M., Du Q., Wang X., et al. Tuning the elasticity of polymersomes for brain tumor targeting. Adv. Sci. 2021;8(20) doi: 10.1002/advs.202102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou X., Yan N., Cornel E.J., et al. Bone-targeting polymer vesicles for simultaneous imaging and effective malignant bone tumor treatment. Biomaterials. 2021 doi: 10.1016/j.biomaterials.2020.120345. [DOI] [PubMed] [Google Scholar]
- 51.Xu J., Lin S., Hu H., et al. Tumor-targeting polymer–drug conjugate for liver cancer treatment in vitro. Polymers (Basel) 2022;14(21):4515. doi: 10.3390/polym14214515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L., Zhang X., Ren Y., et al. Chemiluminescent conjugated polymer nanoparticles for deep-tissue inflammation imaging and photodynamic therapy of cancer. J. Am. Chem. Soc. 2024;146(9):5927–5939. doi: 10.1021/jacs.3c12132. [DOI] [PubMed] [Google Scholar]
- 53.Mastrotto F., Pirazzini M., Negro S., et al. Sulfation at glycopolymer side chains switches activity at the macrophage mannose receptor (cd206) in vitro and in vivo. J. Am. Chem. Soc. 2022;144(50):23134–23147. doi: 10.1021/jacs.2c10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bu J., Nair A., Iida M., et al. An avidity-based pd-l1 antagonist using nanoparticle-antibody conjugates for enhanced immunotherapy. Nano Lett. 2020;20(7):4901–4909. doi: 10.1021/acs.nanolett.0c00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dumontet C., Reichert J.M.M., Senter P.D.D., et al. Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023;22(8):641–661. doi: 10.1038/s41573-023-00709-2. [DOI] [PubMed] [Google Scholar]
- 56.Xue F., Yao H., Cui L., et al. An fc binding peptide-based facile and versatile build platform for multispecific antibodies. Nano Lett. 2023;23(10):4191–4200. doi: 10.1021/acs.nanolett.3c00071. [DOI] [PubMed] [Google Scholar]
- 57.Zhang H., Zhang Z., Wang X., et al. Preparation of trastuzumab-dm1 conjugate with a high drug-to-antibody ratio for breast cancer therapy. Nano Today. 2024;54:102134. [Google Scholar]
- 58.Zhang H., Sun J., Zhang Y., et al. Preparation of an ultrahigh-dar pdl1 monoclonal antibody-polymeric-sn38 conjugate for precise colon cancer therapy. Biomaterials. 2023;301:122285. doi: 10.1016/j.biomaterials.2023.122285. [DOI] [PubMed] [Google Scholar]
- 59.Liu Z., Zhang H., Sun J., et al. Organic-solvent-free “lego-like” modular preparation of fab-nondestructive antibody-drug conjugates with ultrahigh drug-to-antibody ratio. Adv. Mater. 2023;35(46) doi: 10.1002/adma.202300377. [DOI] [PubMed] [Google Scholar]
- 60.Li Y., He H., Jia X., et al. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials. 2012;33(15):3899–3908. doi: 10.1016/j.biomaterials.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 61.He H., Li Y., Jia X.-R., et al. Pegylated poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials. 2011;32(2):478–487. doi: 10.1016/j.biomaterials.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Xu Q., Liu Y., Su S., et al. Anti-tumor activity of paclitaxel through dual-targeting carrier of cyclic rgd and transferrin conjugated hyperbranched copolymer nanoparticles. Biomaterials. 2012;33(5):1627–1639. doi: 10.1016/j.biomaterials.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 63.Emami F., Duwa R., Banstola A., et al. Dual receptor specific nanoparticles targeting egfr and pd-l1 for enhanced delivery of docetaxel in cancer therapy. Biomed. Pharmacother. 2023;165 doi: 10.1016/j.biopha.2023.115023. [DOI] [PubMed] [Google Scholar]
- 64.Xu C.H., Ye P.J., Zhou Y.C., et al. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 2020;15:1051–1114. doi: 10.1016/j.actbio.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 65.Huang Y., Gao X., Chen J. Leukocyte-derived biomimetic nanoparticulate drug delivery systems for cancer therapy. Acta Pharmaceut. Sinica B. 2018;8(1):4–13. doi: 10.1016/j.apsb.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc. Res. 1996;32(4):733–742. [PubMed] [Google Scholar]
- 67.Yaman S., Ramachandramoorthy H., Iyer P., et al. Targeted chemotherapy via her2-based chimeric antigen receptor (car) engineered t-cell membrane coated polymeric nanoparticles. Bioactive Mater. 2024;34:422–435. doi: 10.1016/j.bioactmat.2023.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y., Luo Y., Gao Y., et al. Carrier-free biomimetic organic nanoparticles with super-high drug loading for targeted nir-ii excitable triple-modal bioimaging and phototheranostics. Small. 2024;20 doi: 10.1002/smll.202406003. [DOI] [PubMed] [Google Scholar]
- 69.Ijaz M., Hasan I., Chaudhry T.H., et al. Bacterial derivatives mediated drug delivery in cancer therapy: A new generation strategy. J. Nanobiotechnol. 2024;22(1):510. doi: 10.1186/s12951-024-02786-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song W., Tang Z., Zhang D., et al. A cooperative polymeric platform for tumor-targeted drug delivery. Chem. Sci. 2016;7(1):728–736. doi: 10.1039/c5sc01698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei Q., Shen N., Yu H., et al. Fxiiia substrate peptide decorated blz945 nanoparticles for specifically remodeling tumor immunity. Biomater. Sci. 2020;8(20):5666–5676. doi: 10.1039/d0bm00713g. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Shen N., Wang Y., et al. Self-amplifying nanotherapeutic drugs homing to tumors in a manner of chain reaction. Adv. Mater. 2021;33(7) doi: 10.1002/adma.202002094. [DOI] [PubMed] [Google Scholar]
- 73.Zhang M., Jiang X., Zhang Q., et al. Biodegradable polymeric nanoparticles containing an immune checkpoint inhibitor (apdl1) to locally induce immune responses in the central nervous system. Adv. Funct. Mater. 2021;31(38) [Google Scholar]
- 74.Cui L., Yao H., Xue F., et al. A tumor-targeting nano-adjuvant for in situ vaccine based on ultrasound therapy. Aggregate. 2024;5(3):e504. [Google Scholar]
- 75.Fralish Z., Chen A., Khan S., et al. The landscape of small-molecule prodrugs. Nat. Rev. Drug Discov. 2024;23(5):365–380. doi: 10.1038/s41573-024-00914-7. [DOI] [PubMed] [Google Scholar]
- 76.Prange C.J., Hu X., Tang L. Smart chemistry for traceless release of anticancer therapeutics. Biomaterials. 2023;303 doi: 10.1016/j.biomaterials.2023.122353. [DOI] [PubMed] [Google Scholar]
- 77.Khan R.U., Shao J., Liao J.-Y., et al. Ph-triggered cancer-targeting polymers: From extracellular accumulation to intracellular release. Nano Res. 2023;16(4):5155–5168. doi: 10.1007/s12274-022-5252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su Y., Jin G., Zhou H., et al. Development of stimuli responsive polymeric nanomedicines modulating tumor microenvironment for improved cancer therapy. Med. Rev. 2023;3(1):4–30. doi: 10.1515/mr-2022-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren X., Yao H., Sun J., et al. Ros-responsive tlr7/8 nanoagonist and ultrasound modulate dendritic cells antigen presentation. Nano Today. 2024;57:102402. [Google Scholar]
- 80.Heck A.G., Stickdorn J., Rosenberger L.J., et al. Polymerizable 2-propionic-3-methylmaleic anhydrides as a macromolecular carrier platform for ph-responsive immunodrug delivery. J. Am. Chem. Soc. 2023;145(50):27424–27436. doi: 10.1021/jacs.3c08511. [DOI] [PubMed] [Google Scholar]
- 81.Wang S., Yu G., Wang Z., et al. Enhanced antitumor efficacy by a cascade of reactive oxygen species generation and drug release. Angewandte Chemie-Int. Edition. 2019;58(41):14758–14763. doi: 10.1002/anie.201908997. [DOI] [PubMed] [Google Scholar]
- 82.Fu X., Hosta-Rigau L., Chandrawati R., et al. Multi-stimuli-responsive polymer particles, films, and hydrogels for drug delivery. Chem. 2018;4(9):2084–2107. [Google Scholar]
- 83.Luo C., Sun J., Liu D., et al. Self-assembled redox dual-responsive prodrug-nanosystem formed by single thioether-bridged paclitaxel-fatty acid conjugate for cancer chemotherapy. Nano Lett. 2016;16(9):5401–5408. doi: 10.1021/acs.nanolett.6b01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y., Cao Y., Ma K., et al. A triple-responsive polymeric prodrug nanoplatform with extracellular ros consumption and intracellular h2o2 self-generation for imaging-guided tumor chemo-ferroptosis-immunotherapy. Adv. Healthc. Mater. 2024;13(16) doi: 10.1002/adhm.202303568. [DOI] [PubMed] [Google Scholar]
- 85.Yang S., Tang Z., Hu C., et al. Selectively potentiating hypoxia levels by combretastatin a4 nanomedicine: Toward highly enhanced hypoxia-activated prodrug tirapazamine therapy for metastatic tumors. Adv. Mater. 2019;31(11) doi: 10.1002/adma.201805955. [DOI] [PubMed] [Google Scholar]
- 86.Shen N., Wu J., Yang C., et al. Combretastatin a4 nanoparticles combined with hypoxia-sensitive imiquimod: A new paradigm for the modulation of host immunological responses during cancer treatment. Nano Lett. 2019;19(11):8021–8031. doi: 10.1021/acs.nanolett.9b03214. [DOI] [PubMed] [Google Scholar]
- 87.Sun J., Liu Z., Yao H., et al. Azide-masked resiquimod activated by hypoxia for selective tumor therapy. Adv. Mater. 2023;35(29) doi: 10.1002/adma.202207733. [DOI] [PubMed] [Google Scholar]
- 88.Xu H., Zheng M., Yang C., et al. Azo-based hypoxic-activated 6-diazo-5-oxo-L-norleucine (don) prodrug combined with vascular disrupting agent nanoparticles for tumor-selective glutamine metabolism blockade. Chem. Engineer. J. 2024;481:148281. [Google Scholar]
- 89.Zheng M., Xu H., Huang Y., et al. Hypoxia-activated glutamine antagonist prodrug combined with combretastatin a4 nanoparticles for tumor-selective metabolic blockade. J. Controll. Rel. 2024;365:480–490. doi: 10.1016/j.jconrel.2023.11.054. [DOI] [PubMed] [Google Scholar]
- 90.Xu Y., Lv J., Kong C., et al. A novel hypoxia-activated polymeric tirapazamine derivative for enhanced antitumor therapy. J. Polymer Sci. 2023;61(12):1111–1119. [Google Scholar]
- 91.Xu Y., Lv J., Shen N., et al. A self-activating nanoized vascular disrupting agent for selective anti-tumor therapy. Biomaterials. 2022:121736. doi: 10.1016/j.biomaterials.2022.121736. [DOI] [PubMed] [Google Scholar]
- 92.Lv J., Xu Y., Liu Y., et al. Co-delivery of plinabulin and tirapazamine boosts anti-tumor efficacy by simultaneously destroying tumor blood vessels and killing tumor cells. Biomaterials. 2024;309:122586. doi: 10.1016/j.biomaterials.2024.122586. [DOI] [PubMed] [Google Scholar]
- 93.Sun J., Yao H., Ren X., et al. Radiation-activated resiquimod prodrug nanomaterials for enhancing immune checkpoint inhibitor therapy. Nano Lett. 2024;24(9):2921–2930. doi: 10.1021/acs.nanolett.4c00114. [DOI] [PubMed] [Google Scholar]
- 94.Zheng M., Liu Z., Xu H., et al. Synergistic enhancement of ultrasound therapy for tumors using hypoxia-activated 6-diazo-5-oxo-L-norleucine (don) prodrug nanoparticles. Nano Res. 2024;17(7):6323–6331. [Google Scholar]
- 95.Jiang J., Zhang M., Lyu T., et al. Sono-driven sting activation using semiconducting polymeric nanoagonists for precision sono-immunotherapy of head and neck squamous cell carcinoma. Adv. Mater. 2023;35(30) doi: 10.1002/adma.202300854. [DOI] [PubMed] [Google Scholar]
- 96.Kong C., Li Y., Xu Y., et al. Ultrasound irradiation-induced superoxide anion radical mediates the reduction of tetravalent platinum prodrug for anti-tumor therapy. CCS Chem. 2024:1–29. [Google Scholar]
- 97.Zhang L., Huang J., Buratto D., et al. A ph-responsive nanoparticle delivery system containing dihydralazine and doxorubicin-based prodrug for enhancing antitumor efficacy. Aggregate. 2024;5(1):e434. [Google Scholar]
- 98.Chen H., Li F., Yao Y., et al. Redox dual-responsive and o2‑evolving theranostic nanosystem for highly selective chemotherapy against hypoxic tumors. Theranostics. 2019;9(1):90–103. doi: 10.7150/thno.30259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang Z., Li H., Zhang W., et al. Cd163 monoclonal antibody modified polymer prodrug nanoparticles for targeting tumor-associated macrophages (tams) to enhance anti-tumor effects. Pharmaceutics. 2023;15(4):1241. doi: 10.3390/pharmaceutics15041241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qi Q., Zeng X., Peng L., et al. Tumor-targeting and imaging micelles for ph-triggered anticancer drug release and combined photodynamic therapy. J. Biomater. Sci., Polymer Edition. 2020;31(11):1385–1404. doi: 10.1080/09205063.2020.1760698. [DOI] [PubMed] [Google Scholar]
- 101.Kim N., Kwon S., Kwon G., et al. Tumor-targeted and stimulus-responsive polymeric prodrug nanoparticles to enhance the anticancer therapeutic efficacy of doxorubicin. J. Controll. Rel. 2024;369:369351–369362. doi: 10.1016/j.jconrel.2024.03.046. [DOI] [PubMed] [Google Scholar]
- 102.Xu Y., Lv J., Kong C., et al. Introducing urea into tirapazamine derivatives to enhance anticancer therapy. Natl. Sci. Rev. 2024;11(4):nwae038. doi: 10.1093/nsr/nwae038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu Y., Lv J., Liu F., et al. Tumor microenvironment remodeling-mediated sequential drug delivery potentiates treatment efficacy. Adv. Mater. 2024;36(23):2312493. doi: 10.1002/adma.202312493. [DOI] [PubMed] [Google Scholar]
- 104.Riley R.S., June C.H., Langer R., et al. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18(3):175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pérez-Herrero E., Lanier O.L., Krishnan N., et al. Drug delivery methods for cancer immunotherapy. Drug Deliv. Transl. Res. 2024;14(1):30–61. doi: 10.1007/s13346-023-01405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hosseini S.M., Mohammadnejad J., Salamat S., et al. Theranostic polymeric nanoparticles as a new approach in cancer therapy and diagnosis: A review. Mater. Today Chem. 2023;29 [Google Scholar]
- 107.Shahbazi-Gahrouei D., Moradi Khaniabadi P., Moradi Khaniabadi B., et al. Medical imaging modalities using nanoprobes for cancer diagnosis: A literature review on recent findings. J. Res. Med. Sci. 2019;24:38. doi: 10.4103/jrms.JRMS_437_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L., Liu Y., Huang H., et al. Multifunctional nanotheranostics for near infrared optical imaging-guided treatment of brain tumors. Adv. Drug Deliv. Rev. 2022;190 doi: 10.1016/j.addr.2022.114536. [DOI] [PubMed] [Google Scholar]
- 109.Roy S., Bag N., Bardhan S., et al. Recent progress in nir-ii fluorescence imaging-guided drug delivery for cancer theranostics. Adv. Drug Deliv. Rev. 2023;197 doi: 10.1016/j.addr.2023.114821. [DOI] [PubMed] [Google Scholar]