Abstract
Optimal intraoperative fluid management is essential to improve surgical outcomes and reduce complications. The Pleth Variability Index (PVI), a dynamic and non-invasive indicator of fluid responsiveness, has been proposed as a tool for goal-directed fluid management. This systematic review and meta-analysis aimed to evaluate the effectiveness of PVI-guided fluid therapy compared to conventional fluid management (CFM) in non-cardiac surgeries. A comprehensive search of PubMed, Embase, and Cochrane databases up to January 2024 identified eligible studies. Primary outcomes included total intraoperative fluid volume and crystalloid administration. Secondary outcomes included hemodynamic parameters, renal function markers, acid-base balance, and hospital length of stay (LOS). Random-effects models were applied, and subgroup and sensitivity analyses were performed. Nine studies comprising 1,105 patients were included. Compared to conventional fluid management, PVI-guided therapy significantly reduced total fluid volume (mean difference [MD] − 761.23 mL; 95% CI − 1267.42 to − 255.03) and crystalloid administration (MD − 655.05 mL; 95% CI − 1096.48 to − 213.62), without significant differences in colloid use, urine output, norepinephrine requirement, arterial pressure, acid-base balance, or LOS. Subgroup analysis of abdominal surgeries confirmed the observed reduction in fluid volumes. PVI-guided fluid management allows for a more restrictive and individualized approach without compromising hemodynamic or metabolic stability. While the heterogeneity across studies limits generalizability, these findings support the clinical value of PVI as a non-invasive tool for perioperative fluid optimization, especially in settings where invasive monitoring is not feasible. Further trials are needed to evaluate its impact on long-term outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10877-025-01334-7.
Keywords: Pleth Variability Index (PVI), Goal-directed fluid therapy, Perioperative fluid management, Systematic review, Meta-analysis
Introduction
Modern anesthesia extends beyond inducing unconsciousness; It incorporate precision medicine strategies to optimize perioperative hemodynamics and improve patient outcomes. Among these, Precise intraoperative fluid management plays a pivotal role, as both hypovolemia and hypervolemia can contribute to significant complications during the perioperative period [1, 2, 3, 4, 5], increasing morbidity and mortality [6], hospital stays [7, 8], and healthcare costs [9]. Achieving an optimal fluid balance is therefore a cornerstone of perioperative care, particularly in high-risk surgical populations.
Conventional fluid management (CFM) relies on static parameters such as heart rate, blood pressure, and urine output. However, these measures have limited predictive value for fluid responsiveness, leading to inaccurate intravascular volume assessment and suboptimal fluid administration. To address this challenge, dynamic parameters such as Stroke Volume Variation (SVV), Pulse Pressure Variation (PPV), and the Pleth Variability Index (PVI) have been introduced to guide individualized fluid therapy strategies [10]. PVI, derived from the respiratory variations in the pulse oximeter waveform, offers a non-invasive and continuous assessment of fluid responsiveness, making it an attractive alternative to more invasive monitoring methods [11].
Although prior studies have explored PVI’s predictive accuracy, the evidence regarding its effectiveness in guiding intraoperative fluid therapy remains fragmented. Some reports suggest that PVI-guided goal-directed fluid management (GDFM) can reduce fluid administration while maintaining hemodynamic stability, but findings have been inconsistent across different surgical settings [12, 13, 14]., Furthermore, questions remain regarding the impact of PVI-guided therapy on postoperative recovery and whether it should complement or replace existing hemodynamic monitoring techniques [15].
This systematic review and meta-analysis aim to consolidate current evidence on the efficacy of PVI-guided fluid management in non-cardiac surgeries. Specifically, we assess whether PVI-based GDFM optimizes intraoperative fluid administration without compromising hemodynamic stability, acid-base balance, or postoperative outcomes. By addressing the limitations of previous studies, we seek to clarify PVI’s role in perioperative medicine and identify areas for future research.
Methods
Study design and registration
This systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16] and the Cochrane Collaboration Handbook for Systematic Review of Interventions [17]. The study protocol was registered in the International Prospective Register of Systematic Reviews under the registration number CRD42025649617.
Eligibility criteria
Studies were included if they met the following criteria: 1- randomized controlled trials (RCTs), 2- comparing PVI-guided goal-directed fluid management (GDFM) to CFM, 3- conducted in patients undergoing non-cardiac surgeries, and 4- reporting at least one relevant perioperative outcome.
We excluded studies that 1- involved duplicate or overlapping populations, 2- focused on pediatric or obstetric patients, or 3- did not specifically use PVI as a fluid responsiveness parameter. PVI-GDFM was defined as intraoperative fluid administration based on dynamic PVI monitoring, whereas CFM relied on conventional static parameters such as heart rate, blood pressure, and urine output.
Search strategy and data extraction
A systematic search was conducted in PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception to January 2024 using the following search terms: “Pleth Variability Index” OR “PVI” OR “Pulse Oximeter Variability” OR “Plethysmographic Variability” OR “Non-Invasive Monitoring”. The complete search strategy is detailed in Supplementary Methods in Supplementary Data.
Additionally, reference lists of all included studies, as well as previous systematic reviews and meta-analyses, were manually screened to identify any additional relevant studies. Two independent reviewers (V.F. and R.C.) extracted data using standardized spreadsheets. Extracted data included study characteristics, number and demographics of participants in each group, type of surgical procedure, intraoperative fluid management strategy, and reported clinical outcomes.
Outcomes
This meta-analysis primarily outcome was intraoperative fluid balance, with a specific focus on total fluid, crystalloid and colloids infusion. Secondary outcomes included norepinephrine requirement, mean arterial pressure (MAP), urine output, 24-hour creatinine, arterial pH, bicarbonate, lactate levels, blood loss, and hospital length of stay (LOS).
Risk of bias assessment
Two authors (V.F. and B.W.) independently assessed the risk of bias. Disagreements were resolved with a third author. The Cochrane Risk of Bias-2 (RoB-2) tool was used to evaluate the risk of bias in randomized trials. RoB-2 evaluates five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result [18].
Publication bias assessment
Publication bias was evaluated through visual inspection of funnel plots and analysis of control lines. Due to the limited number of included studies, no formal quantitative assessment of small-study effects or publication bias was conducted.
Sensitivity analyses
To explore potential sources of variability and address heterogeneity, we conducted subgroup analyses, categorizing studies based on key characteristics such as abdominal surgery. Additionally, we performed sensitivity analyses to assess the robustness of our findings. Specifically, we applied a leave-one-out (LOO) approach [19] for total fluid infused and crystalloids infused, sequentially removing each study one at a time and reanalyzing the data. This method allowed us to evaluate the influence of individual studies on the pooled results and ensure the stability of our findings.
Statistical analysis
We pooled risk ratios (RR) and mean differences (MD) with 95% confidence intervals (CI) for categorical and continuous outcomes, respectively. To account for heterogeneity in methodology and demographics across studies, we applied a random-effects model [20]. We assessed heterogeneity with I² statistics and Cochrane Q test; p-values < 0.10 and I² >25% were considered significant for heterogeneity. Statistical analyses were performed using R statistical software, version 4.2.3 (R Foundation for Statistical Computing) [21].
Results
Study selection and characteristics
The initial database search conducted on November 8, 2024, identified 4,433 records. After removing duplicates and applying the eligibility criteria, 23 studies were selected for full-text review (Fig. 1). Of these, 9 studies met the inclusion criteria and were included in this systematic review and meta-analysis [15, 22, 23, 24, 25, 26, 27, 28, 29]. The primary reasons for exclusion were: (1) Studies not specifically evaluating PVI- GDFM; (2) Not meet the predefined methodological criteria.
Fig. 1.
PRISMA flow diagram detailing the study selection process, including identification, screening, and inclusion of studies in the meta-analysis
A total of 1,105 patients were included across the selected studies, with 551 patients receiving PVI-GDFM and 554 receiving CFM. The overall mean patient age was 54.4 years, with similar baseline across studies (Table 1). The surgical procedures included abdominal, orthopedic, gynecological, and major general surgeries, and enrollment periods spanning studies published between 2010 and 2023 were generally comparable, as summarized in (Table 1).
Table 1.
Baseline characteristics of the included studies
| Study | Patients (n) | Age (M ± SD) PVI/CFM | Sex % Fem | BMI (M ± SD) PVI/CFM | ASA (I/II/III) PVI/CFM | Type of surgery | PVI Threshold for Intervention |
|---|---|---|---|---|---|---|---|
| Abdelhamid 2023 | 66 | 40.4 ± 10.9 / 41.9 ± 9.8 | 42.4% | 27.4 ± 3.5 / 26.4 ± 2.5 | 29/4/0 / 27/6/0 | Elective lumbar spine procedures under general anesthesia in the prone position | > 13% for > 5 min |
| Cesur 2019 | 70 | 58.7 ± 14.4 / 62.3 ± 10.5 | 41.4% | 25.5 ± 4.9 / 26.8 ± 4.2 | 22/13/0 / 19/16/0 | Elective colorectal surgery | > 13% for > 5 min |
| Demirel 2017 | 60 | 36.3 ± 10.8 / 40.1 ± 11.9 | 61.7% | 43.1 ± 3.3 / 43.6 ± 4.5 | 0/15/15 / 0/12/18 | Laparoscopic Roux-en-Y Gastric Bypass Surgery in Morbidly Obese Patients | > 14% for 5 min |
| Fischer 2020 | 438 | 65.0 ± 10.0 / 66.0 ± 10.0 | 36.8% | 29.0 ± 6.0 / 30.0 ± 6.0 | 36/149/28 / 30/152/39 | Adult patients having elective orthopedic surgery | > 13% |
| Forget 2010 | 82 | 59.0 ± 14.0 / 61.0 ± 12.0 | 39.0% | NA | 0/22/19 / 0/22/19 | Major abdominal surgery under general anesthesia | > 13% for ≥ 5 min |
| Hokenek 2022 | 78 | 48.7 ± 5.4 / 48.7 ± 6.1 | NA | 30.9 ± 5.6 / 30.0 ± 4.7 | All patients ASA I/II/III | Elective total abdominal hysterectomy and bilateral salpingo-oophorectomy under general anesthesia | > 13% (when MAP ≤ 65 mmHg and/or > 20% decrease in MAP) |
| Wang 2023 | 211 | 70.7 ± 5.4 / 70.7 ± 5.2 | 31.3% | 22.4 ± 3.0 / 22.9 ± 3.2 | 12/80/15 / 7/79/18 | Major gastrointestinal surgical in elderly patients | > 13% (when MAP ≥ 65 mmHg) for 5 min |
| Yilmaz 2022 | 64 | 53.0 ± 6.0 / 51.0 ± 8.0 | NA | 31.5 ± 5.4 / 31.9 ± 5.8 | 3/21/5 / 2/29/3 | Elective laparoscopic total hysterectomy | > 13% |
| Yu 2014 | 30 | 58.0 ± 5.0 / 59.0 ± 6.0 | 43.3% | NA | All patients ASA I/II | Elective abdominal surgery under combined general and epidural anesthesia | > 13% over 5 min |
n, number of patients; PVI, pulse variability index; M, mean; SD, standard deviation; CFM, conventional fluid management; ASA, American Society of Anesthesiologists; BMI, body mass index (kg/m2); MAP, mean arterial pressure; min, minutes; Fem, female
Primary outcomes: fluid administration
Total fluid infused
The pooled analysis indicated that the PVI-guided group received significantly less total fluid than the standard group (MD − 761.23 mL; 95% CI − 1267.42 to − 255.03; p < 0.01; I² = 95%, Fig. 2a). Notably, individual studies consistently showed a trend toward reduced fluid administration in the PVI group, with effect sizes ranging from − 1063.00 mL to − 335.20 mL.
Fig. 2.
Meta-analysis comparing total fluid infused (a), total crystalloids infused (b), and total colloids infused (c) between the Pleth Variability Index (PVI) group and the standard management group. Mean differences (MD) and 95% confidence intervals (CI) were analyzed using a random-effects model
Crystalloids and colloids infused
Our results showed a significant reduction in the volume of crystalloids infused in the PVI-guided group compared with the standard group (MD − 655.05 mL; 95% CI − 1096.48 to − 213.62; p < 0.01; I² = 97%, Fig. 2b). In contrast, there were no significant differences between groups in colloid volume (MD 185.24 mL; 95% CI − 76.14 to 446.63; p = 0.16; I² = 97%, Fig. 3c).
Fig. 3.
Meta-analysis evaluating the impact of the Pleth Variability Index (PVI) versus standard management on norepinephrine requirement (a), mean arterial blood pressure (b), and urine output (c). Results are presented as risk ratios (RR) or mean differences, with 95% confidence intervals (CI), using a random-effects model
Secondary Outcomes.
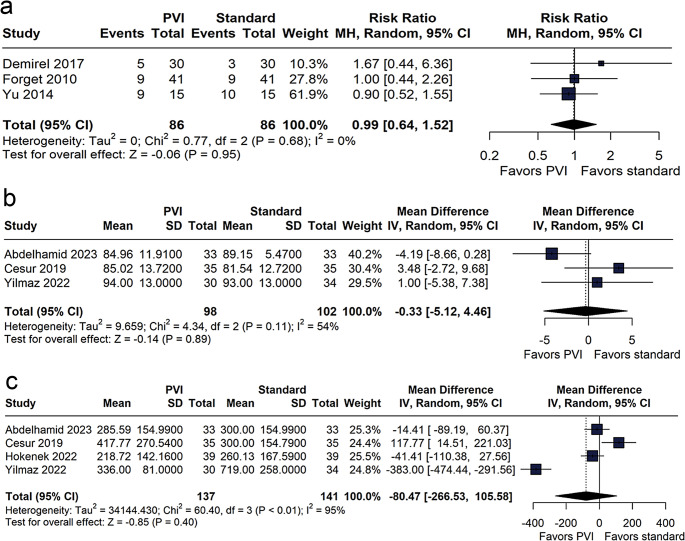
Norepinephrine requirement, MAP, and urine output
The proportion of patients requiring noradrenaline was comparable between the PVI-guided and standard care groups (RR 0.99; 95% CI 0.64 to 1.52; p = 0.95; I² = 0%, Fig. 3a). Likewise, no significant difference in MAP was observed between the groups (MD − 0.33 mmHg; 95% CI − 5.12 to 4.46; p = 0.89; I² = 54%, Fig. 3b). Analysis of urine output indicated no statistically significant difference between the groups (MD − 80.47 mL; 95% CI − 266.53 to 105.58; p = 0.40; I² = 95%, Fig. 3c).
Arterial pH, Bicarbonate, and Lactate.
pH levels were comparable between groups (MD 0.00; 95% CI − 0.02 to 0.03; p = 0.83; I² = 73%, Fig. 4a). Bicarbonate levels differed by MD − 0.48 mmol/L (95% CI − 1.15 to 0.19; p = 0.16; I² = 29%, Fig. 4b), and intraoperative blood lactate levels were similarly matched (MD − 0.03 mmol/L; 95% CI − 0.40 to 0.34; p = 0.87; I² = 86%). One hour postoperatively, lactate levels showed no significant difference (MD 0.02 mmol/L; 95% CI − 0.14 to 0.18; p = 0.79; I² = 73%, Fig. 4c). Overall, no significant differences in acid-base parameters were observed between the groups.
Fig. 4.
Meta-analysis comparing the effects of using the Pleth Variability Index (PVI) versus standard management on acid-base parameters (a), serum bicarbonate (b), and postoperative blood lactate (c). Mean differences and 95% confidence intervals (CI) were analyzed using a random-effects model
Intraoperative blood loss, LOS, and postoperative creatinine
There was no statistical difference between groups regarding (MD − 5.41 mL; 95% CI − 43.09 to 32.28; p = 0.78; I² = 12%, Fig. 5a). The LOS was virtually the same (MD − 0.01 days; 95% CI − 0.24 to 0.21; p = 0.90; I² = 0%, Fig. 5b), and 24-hour postoperative creatinine levels also showed no significant difference (MD − 0.02 mg/dL; 95% CI − 0.07 to 0.02; p = 0.25; I² = 0%, Fig. 5c).
Fig. 5.
Meta-analysis evaluating blood loss (a), length of hospital stay (LOS) (b), and postoperative creatinine levels (c) in patients managed with the Pleth Variability Index (PVI) versus standard management. Results are presented as mean differences (MD) with 95% confidence intervals (CI) using a random-effects model
Subgroup analysis.
Abdominal surgeries.
In the subgroup analysis of abdominal surgeries, PVI-guided fluid management resulted in a significant reduction in crystalloid volume compared with conventional fluid management (MD − 655.05 mL; 95% CI − 1096.48 to − 213.62; p < 0.01; I² = 97.3%, Fig. 6a). A parallel reduction in total fluid volume was also observed (MD − 761.16 mL; 95% CI − 1267.34 to − 254.99; p < 0.01; I² = 94.9%, Fig. 6b), although high heterogeneity was noted in both outcomes, likely reflecting differences in surgical complexity and fluid management protocols. Sensitivity analyses confirmed these findings, suggesting that the reduction in total fluid volume was primarily driven by decreased crystalloid administration.
Fig. 6.
Subgroup analysis comparing total fluid infused (a) and total crystalloids infused (b) in abdominal surgeries between the intervention and control groups. Mean differences (MD) and 95% confidence intervals (CI) were analyzed using a random-effects model
Sensitivity analyses
The leave-one-out sensitivity analysis confirmed the robustness of the PVI-guided intervention, as excluding any single study did not alter the direction or significance of the effect estimates. However, persistent high heterogeneity suggests that other factors (e.g., methodological differences or population characteristics) may contribute to the variability observed. The results for the sensitivity analysis are presented in Supplementary Figs. 1, 2.
Risk of bias assessment
Among the nine included studies, the majority were classified as having either a low risk of bias or some concerns. Specifically, eight out of nine studies were categorized as having an overall low risk of bias or some concerns in specific domains. Only one study was identified as having a high risk of bias [7] primarily due to concerns in the domain related to deviations from intended interventions (Supplementary Fig. 3).
The assessment showed that most studies had a low risk in key domains such as deviations from interventions, missing outcome data, and measurement of outcomes. However, concerns were noted in the randomization process for multiple studies, indicating potential limitations in allocation concealment or blinding methods.
There was no clear evidence of publication bias based on the visual inspection of the funnel plot for key outcomes (Supplementary Figs. 4 and 5). However, the small number of studies included in each individual analysis limits the ability to formally assess publication bias quantitatively. Some concerns in the risk of bias assessment.
Discussion
This systematic review and meta-analysis of nine randomized controlled trials involving 1,105 surgical patients evaluated the effectiveness of PVI-guided goal-directed fluid management (PVI-GDFM) compared to conventional fluid management (CFM). Our findings demonstrate that PVI-GDFM significantly reduces total fluid and crystalloid administration without compromising hemodynamic stability, renal function, acid-base balance, or postoperative outcomes. These results support PVI as a viable, non-invasive tool for intraoperative fluid optimization, particularly in settings where invasive hemodynamic monitoring is impractical.
Among the primary outcomes, PVI-GDFM was associated with a significant reduction in total fluid volume (− 761.23 mL) and crystalloid administration (− 655.05 mL) compared to CFM. Despite this more restrictive approach, patients in the PVI group maintained stable hemodynamic parameters, including mean arterial pressure (MAP) and vasopressor requirements, with no significant differences relative to the control group. Renal function, assessed by intraoperative urine output and postoperative creatinine levels, was similarly preserved. Additionally, metabolic markers (arterial pH, bicarbonate levels, and blood lactate concentrations demonstrated no disruption in acid-base balance). Collectively, these findings suggest that reduced fluid administration under PVI guidance does not compromise tissue perfusion or organ function.
Subgroup analysis focusing on abdominal surgeries reinforced the clinical utility of PVI-guided fluid management in surgical contexts associated with substantial fluid shifts and increased risk of fluid overload. These procedures are often characterized by third-space losses, intra-abdominal pressure changes due to pneumoperitoneum, and surgical trauma, which make fluid balance particularly challenging. In this subgroup, PVI-guided therapy led to a more pronounced reduction in total fluid and crystalloid administration compared to conventional strategies, without inducing hemodynamic instability. Excessive fluid in these settings has been linked to impaired tissue healing, delayed gastrointestinal recovery, and cardiopulmonary complications [7, 8].
Our sensitivity analyses, including leave-one-out procedures, confirmed the robustness of these results and demonstrated that no single study disproportionately influenced the pooled estimates. Notably, Yu et al. [22] reported that PVI-guided therapy under combined general and epidural anesthesia significantly reduced fluid volumes and lactate levels, further supporting its effect on tissue perfusion.
While these results are promising, it is important to interpret them within the framework of modern perioperative care. In the context of enhanced recovery after surgery (ERAS) protocols, major improvements in clinical outcomes are increasingly driven by multimodal strategies rather than single interventions. Therefore, expecting a solitary change in fluid therapy to significantly affect outcomes such as mortality or hospital length of stay may be unrealistic. Instead, fluid optimization using tools like PVI should be viewed as a component that contributes incremental but meaningful gains in recovery when embedded in a comprehensive protocol. Although this analysis did not show a reduction in major postoperative complications, the ability to reduce intraoperative fluid volumes safely may still offer clinical value. PVI-GDFM can serve as a supportive, non-invasive tool for fluid optimization within multimodal perioperative care pathways, where cumulative effects, not individual interventions, drive outcome improvements.
We chose to conduct a subgroup analysis on abdominal surgeries due to their physiologic complexity and the availability of methodologically homogeneous data within this category. Other subtypes, such as orthopedic and gynecologic surgeries, were underrepresented and presented greater variability in design and endpoints, limiting the feasibility of separate subgroup analyses.
Although not part of our pooled analysis, kidney transplant recipients illustrate a compelling clinical scenario where non-invasive monitoring may be particularly beneficial. These patients often have fragile vascular access due to long-term dialysis or vascular disease, making the avoidance of unnecessary arterial or central venous cannulation crucial. In this context, continuous assessment of fluid responsiveness through PVI could provide valuable clinical information with minimal procedural risk. The transplant setting exemplifies a broader principle: in patients where invasive monitoring is contraindicated or impractical, PVI represents a practical alternative worthy of prospective investigation.
Looking forward, further research should prioritize high-quality, adequately powered randomized controlled trials that examine the long-term and patient-centered effects of PVI-guided fluid therapy. Standardization of PVI thresholds and fluid intervention protocols is also needed to reduce inter-study variability and enhance reproducibility. Comparative studies combining PVI with other dynamic monitors such as SVV or esophageal Doppler could clarify the best strategies for individualized perioperative care. Moreover, implementation studies assessing integration of PVI within ERAS protocols may provide insights into its cost-effectiveness and clinical scalability.
Despite the promising findings of this meta-analysis, several limitations warrant consideration. The high degree of heterogeneity in fluid volume outcomes likely reflects differences in surgical procedures, populations, and institutional practices. The absence of standardized PVI intervention thresholds across studies may have contributed to inconsistent effects. Nevertheless, subgroup and sensitivity analyses reinforced the direction and consistency of the findings. Additionally, the limited focus on long-term outcomes across the included trials restricts conclusions about broader postoperative benefits. We also acknowledge variability in CFM definitions; however, strict eligibility criteria and systematic risk-of-bias assessments helped ensure methodological rigor.
Conclusion
This meta-analysis suggests that PVI-guided fluid management reduces intraoperative fluid administration without compromising physiological stability. These findings support the use of PVI as a non-invasive tool for individualized fluid optimization, particularly in abdominal surgeries and in patients where invasive monitoring is unfeasible. The heterogeneity observed highlights the need for further research to refine its application and define its role in contemporary perioperative medicine.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
V.F. conceived the study and led the development of the research question and design. V.F. and B.W. conducted the systematic search. B.W., M.I., J.B., M.S., and R.C. assisted with data extraction. V.F. and B.W. performed the statistical analysis. V.F. drafted the manuscript with advisory input and critical revisions from C.D. and M.A.L. All authors reviewed and approved the final version of the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cai J, Tang M, Wu H, Yuan J, Liang H, Wu X, Xing S, Yang X, Duan XD. Association of intraoperative hypotension and severe postoperative complications during non-cardiac surgery in adult patients: A systematic review and meta-analysis. Heliyon. 2023;9:e15997. 10.1016/j.heliyon.2023.e15997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory A, Stapelfeldt WH, Khanna AK, Smischney NJ, Boero IJ, Chen Q, Stevens M, Shaw AD. Intraoperative hypotension is associated with adverse clinical outcomes after noncardiac surgery. Anesth Analg. 2021;132:1654–65. 10.1213/ANE.0000000000005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, Cywinski J, Thabane L, Sessler DI. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–15. 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 4.Silva PL, Cruz FF, Fujisaki LC, Oliveira GP, Samary CS, Ornellas DS, Maron-Gutierrez T, Rocha NN, Goldenberg R, Garcia CS, Morales MM, Capelozzi VL, Gama de Abreu M, Pelosi P, Rocco PR. Hypervolemia induces and potentiates lung damage after recruitment maneuver in a model of sepsis-induced acute lung injury. Crit Care. 2010;14:R114. 10.1186/cc9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care. 2014;4:21. 10.1186/s13613-014-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An R, Pang QY, Liu HL. Association of intra-operative hypotension with acute kidney injury, myocardial injury and mortality in non-cardiac surgery: A meta-analysis. Int J Clin Pract. 2019;73:e13394. 10.1111/ijcp.13394. [DOI] [PubMed] [Google Scholar]
- 7.Shin CH, Long DR, McLean D, Grabitz SD, Ladha K, Timm FP, Thevathasan T, Pieretti A, Ferrone C, Hoeft A, Scheeren TWL, Thompson BT, Kurth T, Eikermann M. Effects of intraoperative fluid management on postoperative outcomes: A hospital registry study. Ann Surg. 2018;267:1084–92. 10.1097/SLA.0000000000002220. [DOI] [PubMed] [Google Scholar]
- 8.Miller TE, Mythen M, Shaw AD, Hwang S, Shenoy AV, Bershad M, Hunley C. Association between perioperative fluid management and patient outcomes: a multicentre retrospective study. Br J Anaesth. 2021;126:720–9. 10.1016/j.bja.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Oh TK, Hwang JW, Jeon YT, Do SH. Perioperative cumulative fluid balance and its association with an increase in costs after major surgery. J Clin Med. 2018;7. 10.3390/jcm7090227. [DOI] [PMC free article] [PubMed]
- 10.Bednarczyk JM, Fridfinnson JA, Kumar A, Blanchard L, Rabbani R, Bell D, Funk D, Turgeon AF, Abou-Setta AM, Zarychanski R. Incorporating dynamic assessment of fluid responsiveness into goal-directed therapy: A systematic review and meta-analysis. Crit Care Med. 2017;45:1538–45. 10.1097/CCM.0000000000002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelsattar Alamir MM, Abd EFE, Seidy MI, Sayed W, Abdel Monem M, Mohammed S. Plethysmography variation index versus pulse pressure variation as an indicator of fluid responsiveness in colorectal surgeries during immediate postoperative period. Egypt J Anaesth. 2024;40:119–26. 10.1080/11101849.2024.2312340. [Google Scholar]
- 12.Chaves RCF, Barbas CSV, Queiroz VNF, Serpa Neto A, Deliberato RO, Pereira AJ, Timenetsky KT, Silva Junior JM, Takaoka F, de Backer D, Celi LA, Correa TD. Assessment of fluid responsiveness using pulse pressure variation, stroke volume variation, plethysmographic variability index, central venous pressure, and inferior Vena Cava variation in patients undergoing mechanical ventilation: a systematic review and meta-analysis. Crit Care. 2024;28:289. 10.1186/s13054-024-05078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin JY, Ho KM. Use of plethysmographic variability index derived from the Massimo((R)) pulse oximeter to predict fluid or preload responsiveness: a systematic review and meta-analysis. Anaesthesia. 2012;67:777–83.10.1111/j.1365-2044.2012.07117.x. [DOI] [PubMed]
- 14.Sandroni C, Cavallaro F, Marano C, Falcone C, De Santis P, Antonelli M. Accuracy of plethysmographic indices as predictors of fluid responsiveness in mechanically ventilated adults: a systematic review and meta-analysis. Intensive Care Med. 2012;38:1429–37. 10.1007/s00134-012-2621-1. [DOI] [PubMed] [Google Scholar]
- 15.Cesur S, Cardakozu T, Kus A, Turkyilmaz N, Yavuz O. Comparison of conventional fluid management with PVI-based goal-directed fluid management in elective colorectal surgery. J Clin Monit Comput. 2019;33:249–57. 10.1007/s10877-018-0163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142. 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernan MA, Hopewell S, Hrobjartsson A, Junqueira DR, Juni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 19.Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010. 1:112– 25.10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003. 327:557–60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. 10.1016/0197-2456(86)90046-2. [DOI] [PubMed]
- 22.Yu Y, Dong J, Xu Z, Shen H, Zheng J. Pleth variability index-directed fluid management in abdominal surgery under combined general and epidural anesthesia. J Clin Monit Comput. 2015;29:47–52. 10.1007/s10877-014-9567-5. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz G, Akca A, Kiyak H, Can E, Aydin A, Salihoglu Z. Pleth variability index-based goal-directed fluid management in patients undergoing elective gynecologic surgery. Sisli Etfal Hastan Tip Bul. 2022;56:220–6. 10.14744/SEMB.2021.81073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Zhang Y, Zheng J, Dong X, Wu C, Guo Z, Wu X. Intraoperative pleth variability index-based fluid management therapy and Gastrointestinal surgical outcomes in elderly patients: a randomised controlled trial. Perioper Med (Lond). 2023;12:16. 10.1186/s13741-023-00308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hokenek UD, Gurler HK, Saracoglu A, Kale A, Saracoglu KT. Pleth variability index guided volume optimisation in major gynaecologic surgery. J Coll Physicians Surg Pak. 2022;32:980–6. 10.29271/jcpsp.2022.08.980. [DOI] [PubMed] [Google Scholar]
- 26.Forget P, Lois F, de Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg. 2010;111:910–4. 10.1213/ANE.0b013e3181eb624f. [DOI] [PubMed] [Google Scholar]
- 27.Fischer MO, Lemoine S, Tavernier B, Bouchakour CE, Colas V, Houard M, Greub W, Daccache G, Hulet C, Compere V, Taing D, Lorne E, Parienti JJ, Hanouz JL. Optimization using the Pleth Variability Index Trial G (2020) Individualized fluid management using the pleth variability index: a randomized clinical trial. Anesthesiology 133:31–40. 10.1097/ALN.0000000000003260 [DOI] [PubMed]
- 28.Demirel I, Bolat E, Altun AY, Ozdemir M, Bestas A. Efficacy of Goal-Directed fluid therapy via pleth variability index during laparoscopic Roux-en-Y gastric bypass surgery in morbidly obese patients. Obes Surg. 2018;28:358–63. 10.1007/s11695-017-2840-1. [DOI] [PubMed] [Google Scholar]
- 29.Abdelhamid B, Matta M, Rady A, Adel G, Gamal M. Conventional fluid management versus plethysmographic variability index-based goal directed fluid management in patients undergoing spine surgery in the prone position - a randomised control trial. Anaesthesiol Intensive Ther. 2023;55:186–95. 10.5114/ait.2023.130792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.