Abstract
Equilibrative nucleoside transporters (ENTs) facilitate the equilibrative movement of nucleosides and nucleobases across cell membranes in a sodium-independent manner. ENT1 (SLC29A1) and ENT2 (SLC29A2) also transport nucleoside analogs and can affect the pharmacokinetics and pharmacodynamics of drugs used in cancer, viral infections, and inflammatory disorders. ENT1 and ENT2 may be differentiated functionally by their sensitivity to inhibition by nitrobenzylthioinosine (NBMPR), and we used this difference in NBMPR sensitivity to create a HeLa-based ENT2 inhibition assay. We then screened a library of 1600 diverse compounds composed of drugs and natural products for inhibition against ENT1 and ENT2, selecting a subset of compounds for side-by-side comparison of dose-response studies. We used these screening data to build machine learning models for ENT1 and ENT2 inhibition, employing dataset balancing and conformal prediction to adjust for the asymmetrical nature of the data. A random forest model predicted a prospective test set of 44 additional molecules (from the MedChem Express Drug Repurposing Library [2700 compounds]) as potential ENT1 inhibitors with 59% accuracy. This resulted in the identification of the Food and Drug Administration-approved drugs isradipine, avanafil, and istradefylline as inhibitors of ENT1. These new experimental and computational methods and models for these clinically relevant transporters can be used to evaluate drug-transporter interactions early in drug discovery, before testing in vivo.
Significance Statement
Recent regulatory guidance have suggest the inclusion of the equilibrative nucleoside transporters (eg, ENT1 and ENT2) as transporters with emerging clinical relevance for in vitro and in vivo assessment. We have screened over 1600 diverse molecules, allowing us to build machine learning models that in turn were further used to make predictions to validate the models. Our combined experimental and machine learning approach resulted in the identification of multiple Food and Drug Administration-approved medications as inhibitors of ENT1 or ENT2.
Key words: Compound library, Conformal prediction, Drug screening, Equilibrative nucleoside transporters, Machine learning models, Random forest model
Graphical abstract
1. Introduction
Equilibrative nucleoside transporters (ENTs) comprise the SLC29 family of transporters, within the solute carrier (SLC) superfamily, facilitating the equilibrative movement of nucleosides and nucleobases across cell membranes in a sodium-independent manner.1 The cellular uptake and extracellular release of these building blocks are essential for various physiological processes, including DNA and RNA synthesis, energy metabolism, and extracellular signaling.2 ENT1 (SLC29A1) and ENT2 (SLC29A2) are the most extensively studied and characterized of the 4 isoforms, and unlike ENT3 and ENT4, are ubiquitously expressed across diverse cell types.3 They are present at important biological interfaces, such as the blood-testes4 and blood-retinal barriers,5 and are also suspected to transport nucleoside analogs.3 Understandably, ENT1 and ENT2 are therefore known to have an effect on the pharmacokinetics and pharmacodynamics of many drugs used in the treatment of cancer (cladribine and clofarabine), viral infections (ribavirin and zalcitabine), and inflammatory disorders (azacitidine and decitabine).6,7 Other drug classes, like benzodiazepines, tyrosine-kinase inhibitors, and alcohol (ethanol), have been shown to inhibit ENT1 and ENT2 function, indicating a wealth of potential for drug-drug interactions (DDIs) involving these transporters.8
Predicting drug interactions with these transporters could be useful during drug discovery, either to capitalize on the ENT1-ENT2 transepithelial transport pathway or to avoid harmful DDI caused by the inhibition of these transporters. Computational models of drug-transporter interactions have used techniques like machine learning and pharmacophore modeling to identify potential substrates/inhibitors of transporters, or to identify structure-activity relationships for transporter-substrate selectivity.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 We have previously developed Bayesian machine learning models to characterize drug interaction with ENT1 and ENT2, using both internally15 and externally generated inhibition and substrate data.19 However, these models were limited by both the size and chemical diversity of the datasets used to build them. Prediction of chemical classes outside of nucleoside analogs necessitates the generation of a more structurally diverse ENT1 and ENT2 inhibition data.
ENT1 and ENT2 can be differentiated functionally by their sensitivity to inhibition by nitrobenzylthioinosine (NBMPR). ENT1 transport is blocked by nanomolar concentrations of NBMPR, whereas ENT2-mediated nucleoside transport requires micromolar concentrations to achieve similar levels of inhibition.20 In the current study, we used this difference in NBMPR sensitivity to design a screen for ENT2 transport without the need for an ENT1 knockout cell line. Our HeLa ENT2-Flpn cell assay measures the transport of [3H]uridine in the presence of 100 nM NBMPR, allowing for the distinction between ENT1- and ENT2-mediated transport. We used this cell line in a side-by-side screen of ENT1 and ENT2 inhibition for a library of 1600 diverse compounds composed of drugs and natural products, followed by a comparison of dose-response studies for 14 pharmaceutically relevant compounds. We then leveraged this novel screening data to build machine learning models for ENT1 and ENT2 inhibition. Using the combined strategies of careful dataset curation and conformal prediction, we created a random forest (RF) model that predicted a prospective test set of ENT1 inhibitors with 59% accuracy. We demonstrated that this model performed with better precision and accuracy on a prospective set than a balanced model for the same target, despite better cross-validation statistics on the balanced model. These models will allow us to identify effective inhibitors of ENTs with the potential of some clinically relevant compounds and the related risk for unwanted DDI.
2. Materials and methods
[3H]Uridine (specific activity 38 Ci/mmol) was purchased from Moravek. NBMPR was purchased from Tocris. HeLa S2 cells were purchased from American Type Culture Collection (Catalog number CCL-2.2) and propagated in Ham’s F-12 media (Sigma-Aldrich) supplemented with 2.5 g/L sodium bicarbonate, 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin.
2.1. Generation of ENT2 FlpIn cell lines
Zeocin-resistant ENT1 KO HeLa FlpIn cells15 were seeded at 750,000 cells/well in duplicate in a 6-well plate 1 day prior to transfection. The following day, cells were transfected with 2.5 μg of human ENT2 packaged into pcDNA5/FRT vector, together with the Flp recombinase pOG44 (Invitrogen) in a ratio of 1:9 μg. Plasmids were diluted in 250 μL of Opti-MEM (Gibco), 5 μL of P3000, and 7.5 μL of lipofectamine was added and mixed via pipetting, then left to incubate at room temperature for 15 minutes. The transfection mix was added in a dropwise fashion to target cells in 4 mL of Ham’s F12K Media. Twenty-four hours after transfection, cells were selected in media containing hygromycin (700 μg/mL). Resistant cells were grown up to confluency and maintained in Ham’s F12K Media containing hygromycin (100 μg/mL).
2.2. Cell culture
HeLa S2 cells and HeLa S3 ENT2 FlpIn cells were cultured in Ham’s F12K Media supplemented with 2.5 g/L sodium bicarbonate, 10% v/v FBS, 1% v/v penicillin, and streptomycin HeLa S3 ENT2 FlpIn medium that was supplemented with hygromycin for selective pressure. Cells were housed in an incubator kept at 37 °C and 5% CO2. When 80%–90% confluent, cells were subcultured 1:6 every 3–4 days (following recommendations by the American Type Culture Collection protocol) and maintained in a humidified 5% CO2 incubator at 37 °C.
2.3. Transport experiments with radiolabeled compounds
Transport experiments were performed as previously described with minor modifications.16,21 Briefly, HeLa cells (300,000 cells/mL) were plated into corning poly-d-lysine-covered MicroWell 96-well optical bottom plates (ThermoFisher Scientific) at 200 μL/well and grown to confluence. Cell culture media was aspirated, and cells were washed 1 time with 300 μL of room temperature Waymouth’s buffer (WB; 2.5 mM CaCl 2H2O, 28 mM D-glucose, 13 mM HEPES, 135 mM NaCl, 1.2 mM MgCl, 0.8 mM MgSO4 7H2O, pH 7.4), using a Biotek 405 LS Microplate Washer (BioTek). Cells were incubated with 50 mL of transport buffer dispensed with an Integra VIAFLO 96-well multichannel pipette (Integra Lifesciences), containing WB, approximately 20 nM [3H]uridine, and additional compounds as noted in the figure legends. After selected time intervals, transport was halted by washing 2 times with 300 mL of ice-cold WB. Following the washes, 200 mL of MicroScint-20 scintillation cocktail (Perkin Elmer) was added per well, and the plates were sealed with microplate film (USA Scientific) and incubated at room temperature for 2 hours before measurements were taken. Total accumulated radioactivity was determined by using a Wallac 1450 MicroBeta TriLux liquid scintillation counter (Perkin Elmer).
2.4. Drug screening
Compounds from the Spectrum collection (MicroSource Discovery Systems, Inc), provided by the University of Arizona High Throughput Screening and Assay Development Core at the Arizona Center for Drug Discovery, distributed in 80 wells of 96-well plates (1 mM per well in 100% DMSO), were screened for their inhibitory effectiveness against transport of uridine into HeLa cells expressing either ENT1 or ENT2; a total of 1600 compounds (1598 for ENT2) were assessed. Each compound was diluted to a concentration of 20 μM, pH 7.4, to a final concentration of 2% DMSO using a VIAFLO multichannel electronic pipet (Integra Biosciences). The following compounds were purchased from Sigma and Tocris (ketoconazole, sinensetin, medrysone, myricetin, benzbromarone, canagliflozin, gefitinib, quercetin, kanamycin, domperidone, naringin, methapyrilene, flumazenil, and losartan) for dose-response testing.
2.5. Data analysis
Results are presented as means ± SD. Statistical analyses were performed using ANOVA with Tukey’s post-test, with P < .05 considered statistically significant (Prism, 8 GraphPad Software Inc).
2.6. Machine learning
Machine learning models were created as described in detail previously by Martinez-Guerrero et al.22 Briefly, ENT1 and ENT2 datasets from the library screen were subjected to cleaning by removing compounds with high SDs (≥10%) between replicates in the corresponding assay, as well as any compounds that lacked discrete, organic SMILES strings. SMILES strings were canonicalized according to RDKit23 parameters, salts and solvents were removed, and duplicate measurements of the same molecule were averaged. Each dataset was binarized so that the active class was composed of molecules that inhibited the transport of [3H] uridine by at least 50%, relative to control, and a 20% stratified test set was generated for each dataset using the Python scikit-learn library. Balanced datasets with a 1:1 ratio of active: inactive compounds were generated using the RandomUnderSampler from the imbalanced-learn library in Python.
Machine learning models were initially generated using either extended-connectivity fingerprints (ECFPs) or functional-class fingerprints (FCFPs)24 (diameter = 6 atoms, size = 1024 bits). AdaBoost Classifier, Bernoulli Naïve Bayes, deep learning (DL), k-nearest neighbors, logistic regression, RF, support vector classification (SVC), and XGBoost models were generated using the Assay Central software and evaluated using 5-fold cross-validation statistics (single-fold 20% leave-out set for DL models), as well as performance on the stratified test set. Conformal predictions were calculated using a method combining class-conditional conformal predictors25 for a prediction coverage level alpha (α) such that a compound (Ytest) in a test set has a 1-α probability of being included in the prediction set (C(Xtest)) if the class label (y) is true.
For cross-conformal predictors,26 the dataset is divided into N folds, and a model trained with N-1 folds is calibrated on the remaining fold (n), where is the quantile of the adjusted (1-α) scores in the training set. This is repeated N times, using a different fold as a calibration set each time, and the union of all predictions for the N sets is used to calibrate the conformal predictor.
Predictions were performed on a cleaned, digital version of the Drug Repurposing Compound Library HY-L035 (MedChem Express) and limited to unique compounds that were available as 10 mM in DMSO and were not present in the ENT1/ENT2 screen. DataWarrior (openmolecules.org) was used to identify compounds with a flavonoid substructure, and to select 80 diverse compounds from the model predictions.
2.7. Chemical descriptor comparison
Chemical descriptors for compounds in the ENT1 and ENT2 screens were calculated using the cxcalc command line tool from Chemaxon, and two-tailed Mann-Whitney test was performed in GraphPad Prism 10.
3. Results
3.1. Functional assessment of ENT1 and ENT2 cell lines
The relative expression of ENT1 and ENT2 can be markedly different between lines of HeLa cells, as measured by the relative levels of “NBPMR-sensitive” transport of nucleosides.27, 28, 29 To evaluate the functional activity of ENT1 and ENT2 in 2 different cell lines, we measured the accumulation of the labeled substrate [3H]uridine in the presence of different concentrations of NBMPR over the course of 2 minutes. The HeLa S2 cell line appeared to functionally express only ENT1, as the uptake of [3H]uridine was reduced 65%–75% in the presence of 100 nM NBMPR (Fig. 1A), a reduction that was statistically unchanged in the presence of either 100 μM NBMPR or 5 mM unlabeled uridine (P = .2). To characterize ENT2 function, we inserted an ENT2 overexpression cassette into an ENT1 HeLa S3 knockout cell line,15 then performed the same NBMPR challenge. In this HeLa FlpIn ENT2 cell line, only ∼55% of total [3H]uridine uptake was blocked in the presence of 100 nM NBMPR, indicating a certain amount of residual ENT1 activity (Fig. 1B). However, total uptake was reduced by ∼80% in the presence of 100 μM NBMPR or 5 mM uridine, indicating that a portion of total [3H]uridine uptake in this cell was controlled by ENT2. We therefore measured the ENT2 activity in this cell line as the difference in transport blocked by 100 nM NBMPR and that blocked by 100 μM NBMPR.
Fig. 1.
Characterization of hENT1 and hENT2 activity by the inhibition by 100 nM and 100 μM of NBMPR of [3H]uridine uptake into wild-type (WT) HeLa and HeLa FlpIn cell lines overexpressing hENT2.
3.2. Characterization of ENTs transport activity
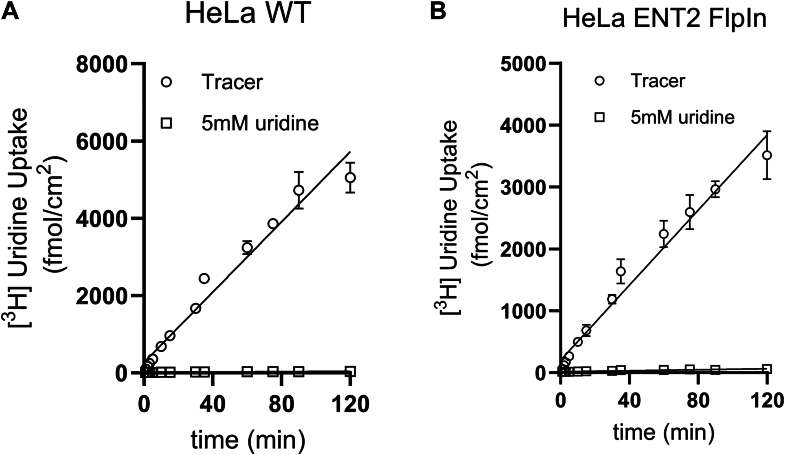
ENT activity was assessed using uridine as a model transported substrate. We measured the accumulation of [3H]uridine over the course of 120 minutes in both cell lines (Fig. 2). A saturating concentration of unlabeled uridine (5 mM) was used to establish the fraction of total [3H]uridine accumulation that was carrier mediated. Although the total amount of [3H]uridine accumulated in the ENT1 cell line (Fig. 2A) was greater than that accumulated in the ENT2 cell line (Fig. 2B), both reactions failed to reach steady-state within this time frame. The failure to approach a steady-state accumulation of uridine over this extended incubation indicates that accumulated uridine was quickly metabolized to a form(s) that was effectively trapped within the cells for each line.
Fig. 2.
Time course of uridine into wild-type (WT) HeLa cells and equilibrative nucleoside transporter 2 (hENT2) overexpressing HeLa cells. Time course of [3H]uridine (∼10 nM) open squares represent transport measured in the presence of 5 mM unlabeled uridine. Each point is the mean of 4 replicate measures of uptake determined in a single representative experiment.
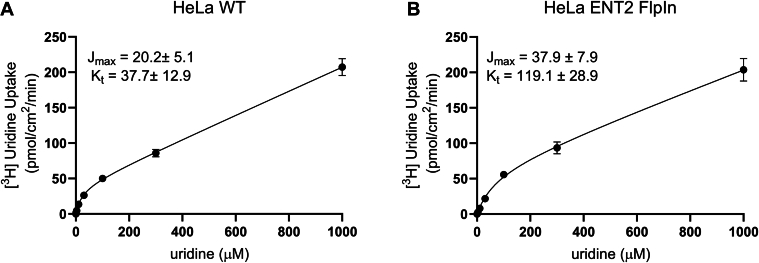
To determine uridine transport kinetics in each cell line, we evaluated [3H]uridine uptake after 5 minutes, which provided estimates of the initial rate of uptake. The rate of transport mediated by both transporters was a saturable function of increasing uridine concentration and was adequately described by the Michaelis-Menten equation (plus a first-order component that reflected the “nonblockable” fraction of total uptake that reflected the combination of diffusion, binding, and incomplete elimination of labeled substrate during the rinse process). The Jmax values for ENT1 and ENT2 were 20.2 ± 5.1 pmol·cm−2·min−1 and 37.9 ± 7.9 pmol·cm−2·min−1, respectively (Fig. 3, A and B). The Kt for ENT1 was 3 times lower than that for ENT2 (37.7 ± 12.9 vs 119.1 ± 28.9 μM) (Fig. 3, A and B), which is in agreement with previously reported kinetic parameters.15
Fig. 3.
Kinetics of radiolabeled uridine uptake at pH 7.4. Wild-type (WT) HeLa cells and HeLa cells overexpressing hENT1 were exposed for 5 minutes to WB (pH 7.4) containing: ∼10 nM [3H]uridine and increasing concentrations of unlabeled uridine. Each open circle is the mean (±SD) of total uptakes measured in 3 separate experiments, each determined in 4 replicate wells. Jmax, maximal rate of transport, Kt, Michaelis constant.
3.3. Inhibition of transport activity
We determined the effect of ∼1600 structurally distinct compounds from the Spectrum collection on ENT1 and ENT2-mediated uridine transport. Figure 4 shows the effect of a 20 μM concentration of each compound on mediated transport of ∼15 nM [3H]uridine (total uptake corrected for 5 mM unlabeled uridine block). Data are presented in rank order of increasing inhibitory potency; 185 (11.6%) and 91 (5.7%) of these compounds inhibited uridine transport by at least 50% for ENT1 and ENT2, respectively, whereas 406 (25.4%) and 412 (25.6%) compounds inhibited transport by at least 20% for each. Seventy of these compounds fell under the 50% threshold for both ENT1 and ENT2 (Supplemental Table 1), whereas the nonoverlapping inhibitors for each transporter showed no correlation of activities for the other enzyme (Supplemental Fig. 1; Supplemental Table 1).
Fig. 4.
(A) Inhibitory effect of 1600 test compounds from the Spectrum collection on the hENT1 and hENT2-mediated transport of 10 nM labeled uridine. The 5-minute accumulation of substrate was measured in the presence of a 20 μM concentration of each test agent. The scatter dot represents each individual value (expressed relative to uptake measured in the absence of inhibitor, ie, “control”) determined in 2–3 separate experiments, each measured in triplicate. The dots are arranged from no inhibition (left side) to complete inhibition (right side). The gray area represents the “inactive” inhibitors (>20% control, to the left). The horizontal blue striped area indicates above 20% inhibition; The horizontal red striped area indicates above 50% inhibition.
3.4. IC50 values for inhibition of [3H]uridine uptake produced by selected compounds
Fourteen compounds were selected for IC50 evaluation of [3H]uridine uptake, representing different levels of single-point ENT1 and ENT2 inhibition (Fig. 5; Table 1). A 3-fold dilution series was performed for each compound, and the IC50 value was determined using the following equation:
where J∗ is the rate of transport of the substrate (uridine) from a concentration of the labeled substrate equal to [S∗] and Japp is a constant related to the Jmax for uridine times the ratio of relative affinity of the transporter for inhibitor and substrate; [I] is the concentration of unlabeled inhibitor; and Dns is a rate constant that describes the nonsaturable component of labeled substrate accumulation. The use of IC50 here instead of Ki reflects the fact that the kinetic mechanism of inhibition is not determined by this assay.
Fig. 5.
Inhibition by ketoconazole (A), sinensetin (B), medrysone (C), myrcetin (D), benzbromarone (E), canagliflozin (F), gefitinib (G), quercetin (H), kanamycin (I), domperidone (J), naringin (K), methapyrilene (L), flumazenil (M), and losartan (N) uptake into WT Hela cells (teal squares) or HeLa cells overexpressing hENT2 (purple circles). Five-minute uptakes of ∼10 nM [3H]uridine was measured in the presence of increasing concentrations of each inhibitor. Each point is the mean (±SD) of results determined in 2–3 separate experiments. The IC50 values are listed in Table 1.
Table 1.
Inhibitory activity of select compounds against ENT1 and ENT2
| ENT1 |
ENT2 |
|||
|---|---|---|---|---|
| % Inhibition (20 μM) | IC50 (μM) ± SD | % Inhibition (20 μM) | IC50 (μM) ± SD | |
| Ketoconazole | 98.4 | 7.2 ± 1.2 | 89.4 | 22.8 ± 0.7 |
| Sinensetin | 95.9 | 1.25 ± 0.3 | 82.5 | 22.2 ± 2.2 |
| Medrysone | 95.1 | 4.44 ± 0.9 | 56.3 | 209.6 ± 89.2 |
| Myricetin | 82.4 | 47.4 ± 1.7 | 51.2 | 28.2 ± 1.6 |
| Benzbromarone | 79.6 | 29.75 ± 2.1 | 25.4 | 262 ± 107.6 |
| Canagliflozin | 74.5 | 23.99 ± 5.1 | 56.5 | 22.34 ± 2.4 |
| Gefitinib | 63.45 | 3.6 ± 0.3 | 54.6 | 3.07 ± 0.01 |
| Quercetin | 53.07 | 20.6 ± 6.8 | 84.3 | 116.9 ± 24.6 |
| Kanamycin | 43.7 | – | 11.7 | ≥5000 |
| Domperidone | 24.41 | 5.42 ± 0.2 | 64.3 | 0.16 ± 0.04 |
| Naringin | 24.4 | 101 ± 8.9 | 8.5 | 266.1 ± 81.5 |
| Methapyrilene | 23.6 | ≥1500 | 7.6 | 418.7 ± 128.1 |
| Flumazenil | 20 | 121 ± 0.7 | 85.11 | 3.82 ± 0.3 |
| Losartan | 0 | ≥5000 | 33.03 | 172.2 ± 60.2 |
3.5. Generation of machine learning models for ENT1 and ENT2 inhibition
We used the in vitro screening data from the ENT1 and ENT2 assays to create machine learning models to predict inhibitory activity against both transporters individually. The screening data were “cleaned” as described by Martinez-Guerrero et al,22 and an activity threshold of 50% was set to divide the compounds into active and inactive classes (Table 2). The cleaned datasets were markedly imbalanced, with only 15.2% of compounds classified as active for ENT1, and 6.6% of compounds active for ENT2. A 20% stratified test set was removed from each dataset, leaving the remaining 80% for heavily imbalanced model training sets. We used these training sets to compare models using 8 different machine learning algorithms (AdaBoost Classifier, Bernoulli Naïve Bayes, DL, k-nearest neighbors, logistic regression, RF, SVC, XGBoost) and 2 different molecular fingerprints (ECFP, FCFP) per transporter (Supplemental Table 2). Although the cross-validation performance for each algorithm was comparable for both fingerprint types (Supplemental Table 2), the FCFP outperformed the ECFP on the stratified test set for most of the algorithms, specifically with regards to precision (Supplemental Table 3).
Table 2.
Composition of ENT1 and ENT2 activity datasets from transporter screening data
| ENT1 |
||||
|---|---|---|---|---|
| Number of Compounds |
% Active | |||
| Active | Inactive | Total | ||
| Full screen | 184 | 1416 | 1600 | 11.5 |
| Below 10% SD | 152 | 863 | 1015 | 15 |
| Post-clean | 145 | 812 | 957 | 15.2 |
| Stratified test set | 29 | 163 | 192 | 15.1 |
| Imbalanced training set | 116 | 649 | 765 | 15.2 |
| Balanced training set | 116 | 116 | 232 | 50 |
| ENT2 | ||||
| Number of Compounds |
% Active |
|||
| Active |
Inactive |
Total |
||
| Full screen | 93 | 1505 | 1598 | 5.9 |
| Below 10% SD | 86 | 1286 | 1372 | 6.3 |
| Post-clean | 85 | 1213 | 1298 | 6.5 |
| Stratified test set | 17 | 243 | 260 | 6.5 |
| Imbalanced training set | 68 | 970 | 1038 | 6.6 |
| Balanced training set | 68 | 68 | 136 | 50 |
Precision measures the ratio of true positive (tp) predictions over the sum of tp and false positive (fp) predictions for the set (Pre = tp/(tp + fp)), and a large negative class can skew other metrics, such as accuracy, when predictions are randomly sorted into the larger class. This is particularly important in the case of our screening data, where the active class is not only much smaller than the inactive class, but is also chemically diverse. Supplemental Fig. 2 shows the comparison in chemical space of the active versus inactive compounds for ENT1 (a) and ENT2 (b), generated using Molecular ACCess System keys as molecular fingerprints. Except for a few small clusters of compounds, there is little structural similarity among active inhibitors from either the ENT1 or ENT2 screens Supplemental Fig. 2; neither do the active inhibitors from these screens occupy distinct regions of chemical space from the inactive compounds, because the inhibitors occupy the same regions of chemical space as the noninhibitors (Supplemental Fig. 2). Because precision measures the ratio of true positives in the prediction set, regardless of the total number of predictions, we decided to use exclusively FCFPs going forward.
Despite the slight advantage FCFPs had over ECFPs with these datasets, the cross-validation statistics and test-set predictions showed that these models still struggled to identify active compounds (Supplemental Tables 2 and 3). We thought this was likely due to the extreme imbalance between the active and inactive classes and attempted to mitigate this by performing a data-balancing technique called random undersampling. Random undersampling balances a dataset by removing random compounds from the larger class until the 2 classes are equal in size. Other data-balancing techniques like weighted learning or the synthetic minority oversampling technique (SMOTE)30 result in less loss of data from the majority class. However, our attempts at adding weights to the samples in these datasets did not result in a noticeable increase in model performance (data not shown), and we did not want to introduce synthetic data to these datasets when the structures of the few active compounds were so structurally diverse. For each dataset, we randomly undersampled the majority class a total of 10 times, then built classification models for each training set and compared the cross-validation statistics (Supplemental Table 4). Overall, the models for each of the balanced training sets displayed better cross-validation statistics than their imbalanced counterparts. However, we selected only one balanced dataset each for ENT1 and ENT2 for the final models, based on general cross-validation performance (bolded in S4).
Finally, despite the lack of clustering of active compounds in chemical space (Supplemental Fig. 2), there are several fundamental chemical properties that do differentiate the ENT1 and ENT2 inhibitors from the noninhibitors, including molecular weight, solubility (LogP, LogD), number of hydrogen donors, and number of rings (aromatic and nonaromatic) (Supplemental Table 5); all these properties have long been considered important for the pharmacokinetic profile of a drug.31,32
3.6. Conformal predictions for model validation
This focus on true positive predictions informed the decision to use conformal predictions with these models. Typically, classification models return predictions for compound activity in the form of probability-like scores for the positive class (active) class, and these scores are binarized on an internal threshold to return an “active” or “inactive” prediction. In this case, a prediction is returned for every compound in the prediction set, and there is no measure of model confidence associated with the predictions. Conformal predictions allow for a measure of confidence in model predictions by setting a user-defined acceptable false positive rate based on a calibration set.33 The result of this technique is that the model will only return predictions for compounds if they fall within a certain measurement of uncertainty, or alpha (α), such that a compound has a 1-α probability of being returned in a prediction set if the prediction is true. For example, an alpha score of 0.2 represents an acceptable error rate of 20% in the predictions returned. To define this error rate, the conformal predictor must be trained on a calibration set with rank-ordered class probabilities. This requires a continuous distribution of probability scores for each class that is broad enough to be meaningful and reflect some measure of model confidence. Of the machine learning algorithms that we have described thus far, RF and SVC best satisfy these requirements (Supplemental Fig. 3). Because RF performed better than SVC on the imbalanced ENT2 datasets (Supplemental Tables 2 and 3), we selected this algorithm to move forward with our conformal predictions. We varied the conformal prediction alpha score from 0.01 to 0.3, reflecting an acceptable error rate for the conformal predictions from 1% to 30%, and then used the imbalanced and balanced RF models for ENT1 and ENT2 to predict activity on the stratified test sets for each paired target. We then determined the optimal alpha score for each target based on the precision scores of the prediction sets. For ENT1, the highest precision scores for both the imbalanced and balanced models were seen at alpha = 0.03 (Fig. 6A; Supplemental Table 6), whereas the highest precision scores for both ENT2 models were seen at alpha = 0.01 (Fig. 6B; Supplemental Table 6). However, by definition, the smaller the chosen alpha threshold for acceptable error in the prediction set, the smaller the ultimate size of the prediction set. At the chosen alpha thresholds, the ENT1 imbalanced and balanced models returned predictions of “active” for 15 and 22 compounds, respectively (Fig. 6C; Supplemental Table 6). The ENT2 models, however, returned active predictions for far fewer compounds at the chosen alpha; the ENT2 imbalanced model only returned active predictions for 2 compounds at alpha = 0.01 (Fig. 6D), and the ENT2 balanced model returned active predictions for only 9 compounds. Although larger values of alpha necessarily correspond to a larger number of active predictions by the ENT2 models, such that the number of conformal predictions at alpha = 0.03 was comparable to those seen for the ENT1 models, the precision score for the ENT2 balanced model drops precipitously at this value, reflecting that the increased number of active predictions does not correlate with an increased rate of true active predictions at this value.
Fig. 6.
Comparison of model performance using conformal predictions on a stratified test set. (A) Precision scores for conformal predictions of the ENT1 full and ENT1 balanced models against the ENT1 stratified test set for the given values of alpha. (B) Precision scores for conformal predictions of the ENT2 full and ENT2 balanced models against the ENT2 stratified test set for the given values of alpha. (C) Number of compounds scored active by the ENT1 full and ENT1 balanced models at the given values of alpha. (D) Number of compounds scored active by the ENT2 full and ENT2 balanced models at the given values of alpha. Dotted lines represent the selected alpha value for the given target.
The ENT1 and ENT2 imbalanced and balanced models were then used to predict activity for compounds in the MedChem Express Drug Repurposing Library, using the alpha values determined by the stratified test set predictions. Because the ENT1 models predicted 5 times as many active inhibitors from this library as the corresponding full and balanced ENT2 models (Supplemental Table 7), and because there was a large overlap in both the ENT1 and ENT2 predictions (Supplemental Table 7) as well as ENT1 and ENT2 inhibitors in the initial screen (Supplemental Table 1), we decided to pursue only the predictions from the ENT1 models. Of 2703 unique molecules from the Drug Repurposing Library, only 122 compounds were predicted active by one, or both, of the ENT1 inhibition models at alpha = 0.03 (Supplemental Table 7). Eighteen of these compounds were flavonoids, which were set aside for future evaluation along with the remaining flavonoids in the MedChemExpress library, because this class of compounds was already well represented in the compounds we chose to follow up with from the initial screen (Table 1). A chemically diverse subset of 80 compounds from the remaining 104 predictions were submitted for testing in the ENT1 inhibition assay. Using the same threshold of 50% inhibition at 20 μM for active ENT1 inhibitors, the imbalanced and balanced models each correctly predicted 26 inhibitors from the Drug Repurposing Library (Table 3), though only 13 of these correct predictions were shared between the models (Table 4). The smaller number of false positives predicted by the ENT1 imbalanced model in the prediction set results in a higher precision score than the balanced model (0.59 imbalanced, 0.47 balanced). Additionally, because the machine learning models did not predict any inactive compounds at this alpha score, the precision scores directly reflect the accuracy of these prediction sets (59% imbalanced, 47% balanced) (Table 4). Of the 39 active ENT1 inhibitors revealed by these predictions between the 2 models, 11 are Food and Drug Administration-approved drugs that are currently prescribed, and another 7 have made it as far as stage III clinical trials (Table 4).
Table 3.
Results of predictions from ENT1 inhibition models
| Imbalanced Model | Balanced Model | |
|---|---|---|
| Total predicted inhibitors | 44 | 55 |
| True positive inhibitors | 26 | 26 |
| False positive inhibitors | 18 | 29 |
| Precision score (accuracy) | 0.59 (59%) | 0.47 (47%) |
Table 4.
Inhibitory activity and drug status for correctly predicted inhibitors of ENT1
| Compound | Predictive Model | ENT1 % Inhibition (±SD) |
Maximum Clinical Trail Stage |
|---|---|---|---|
| PF-06372865 | Balanced | 100.45 (0.02) | II |
| Avanafil | Imbalanced | 100.12 (0.01) | FDA-approved |
| Sabizabulin | Both | 99.63 (0.32) | III |
| Ensifentrine | Imbalanced | 98.22 (0.74) | III |
| Radequinil | Both | 98.17 (0.19) | II |
| CFTR corrector 2 | Balanced | 97.98 (1.08) | I |
| BNC105 | Both | 97.87 (1.06) | II |
| Istradefylline | Balanced | 96.11 (1.73) | FDA-approved |
| Asundexian | Balanced | 95.39 (0.23) | II |
| Isradipine | Imbalanced | 92.67 (1.54) | FDA-approved |
| Everolimus | Imbalanced | 91.97 (0.56) | FDA-approved |
| Inobrodib | Imbalanced | 91.38 (1.78) | I |
| Apabetalone | Both | 88.08 (1.91) | III |
| Orelabrutinib | Balanced | 87.51 (0.53) | III |
| Farudodstat | Imbalanced | 86.78 (3.73) | II |
| Nomegestrol acetate | Both | 82.86 (2.17) | III |
| Lotamilast | Both | 81.26 (0.90) | II |
| Mirodenafil (dihydrochloride) | Both | 79.35 (2.82) | III |
| Odevixibat | Imbalanced | 78.33 (0.88) | FDA-approved |
| Alpha-Estradiol | Imbalanced | 77.22 (2.19) | FDA-approved |
| Nicardipine (hydrochloride) | Balanced | 76.78 (5.34) | FDA-approved |
| Azemiglitazone | Balanced | 74.95 (4.00) | II |
| (R)-Lansoprazole | Both | 73.57 (1.24) | FDA-approved |
| C188-9 | Imbalanced | 72.99 (5.06) | II |
| Nifedipine | Both | 72.56 (4.24) | FDA-approved |
| Bicyclol | Balanced | 72.28 (2.62) | III |
| Phenoxodiol | Imbalanced | 69.32 (2.33) | II |
| GLPG3970 | Balanced | 68.08 (8.81) | II |
| Silybin A | Balanced | 67.52 (0.48) | III |
| Arctigenin | Both | 67.51 (0.83) | – |
| NRC-2694 | Imbalanced | 67.27 (11.1) | – |
| Merestinib dihydrochloride | Imbalanced | 58.49 (4.13) | II |
| Cimicoxib | Balanced | 57.32 (0.24) | II |
| Galunisertib | Balanced | 56.16 (4.26) | II |
| FT011 | Both | 54.94 (2.47) | II |
| Pterostilbene | Both | 54.71 (4.96) | II |
| Alpha-Asarone | Balanced | 52.42 (3.92) | – |
| Omeprazole (sodium) | Both | 51.32 (3.77) | FDA-approved |
| Vandetanib | Imbalanced | 50.28 (4.75) | FDA-approved |
FDA, Food and Drug Administration.
4. Discussion
The International Transporter Consortium white paper from 2013 highlighted the importance of ENTs related to drug delivery for nucleoside drugs,34 and more recent regulatory guidance recommendations have suggested the inclusion of the ENT transporters as additional membrane transporters with emerging clinical relevance for assessment.3 Unfortunately, compared with other drug transporters (eg, organic cation transporters, organic anion-transporting polypeptides, multidrug and toxin extrusions, and breast cancer resistance protein), the selectivity of ENTs has received comparatively little attention,15,19,35 which also impacts the ability to predict these DDIs. The objective of this study was to screen a relatively large database of diverse molecular structures, including drugs and natural compounds, to study their interaction with ENT1 and ENT2 to better clarify the impact of these transporters on the excretion of clinically relevant compounds and enable structure-activity relationship modeling and machine learning. Mirroring our previous efforts,19 there were far more “active” inhibitors of ENT1 (184, ∼12%) that were identified compared with ENT2 (93, ∼6%).
Interestingly, we found that a significant component of the active inhibitors (26% for ENT1 and 33% for ENT2) were food components and natural products. For example, for ENT1, 20 μM isocotoin (a natural product with antiviral activity) inhibited ∼65% of uridine uptake with a predicted IC50 of 12 μM. Epicatechin gallate (a flavan-3-ol, a type of flavonoid present in green tea) inhibited ∼85% of uridine uptake with a predicted IC50 of 4 μM. The interaction of natural products and drugs is a common hidden problem encountered in clinical practice.36 Food components such as flavonoids can alter the pharmacokinetics of other drugs causing unwanted drug-nutrient/food interactions.37,38 An example of such an interaction is the immunosuppressant tacrolimus that, when used after organ transplant, has been reported to have an interaction with flavonoids (eg, sinensetin and nobiletin) in the clementine fruit.37 We found that tacrolimus, as well as sinensetin and dimethyl-nobiletin, are potent inhibitors of ENT1 with 20 μM of each producing inhibition of transport activity (∼80%, Supplemental Table 1) and a more moderate inhibition of ENT2 (35%, Supplemental Table 1). This highlights the potential of ENT1 to be involved not only in DDI but also drug-food interactions that could be clinically relevant. Inhibition of these transporters, especially in the intestine, kidney, and or the liver, can lead to altered bioavailability and, thus, an increased risk for potentially adverse effects. Although the capacity of ENTs to interact with diverse classes of compounds, like flavonoids and cannabinoids, has received less attention than nucleoside analogs,3,39,40 the apparent breadth of their substrate selectivity highlights the potential clinical importance of ENTs as a pathway for the disposition of several drug classes, including bioactive compounds from food, and environmental toxins. Because of this, we set aside all the flavonoid compounds from our compound prediction library (MedChemExpress Drug Repurposing Library) for future inhibitor and/or substrate analysis.
Our ENT1 and ENT2 screening data were used to build machine learning models to predict inhibition by compounds of diverse chemical classes with different algorithms. As is common with such compound screens, the active class of compounds represented a small percentage of the overall dataset. We employed several modeling techniques to address this issue, including random undersampling of the majority class and the use of conformal predictions to instill a measurable level of confidence in the model outputs. We also strategically prioritized precision in both the cross-validation and test set statistics to maximize the proportion of true positive inhibitors identified by our models. Our ENT2 models did not perform to the same level as our ENT1 models, likely due to the smaller starting percentage of active compounds. We selected one balanced ENT1 RF model and compared the performance on a prospective test set with that of the imbalanced ENT1 RF model. Our results showed that the imbalanced ENT1 model predicted activity of a prospective test set with 59% accuracy, compared with 47% seen with the balanced model. These results suggest that when the dataset being modeled and the compounds being predicted upon are both structurally diverse compound libraries, the inclusion of more chemical structures in the training set may be more beneficial to future predictions than the increased cross-validation performance of a balanced dataset. However, seeing as our smaller, balanced model identified just as many true positives as the larger imbalanced model, and that half of these predictions were unique to either model, it is not unreasonable to infer that the balanced model identified some chemical characteristics as important that were not identified by the larger model.
A benefit of RF models is the ability to perform feature importance analysis to identify which features in the dataset were the most informative for training the model, which can then be leveraged to guide synthesis in the future design of new inhibitors. Because our models were trained using FCFPs folded into 1024 bits, each “feature” in this analysis is represented by one bit. Supplemental Fig. 4A shows how the most important feature, or bit, of the ENT1 imbalanced model represents a repeated chemical motif found in the training set. This is consistent with FCFPs describing the “pharmacophore role” a substructure plays in a molecule, rather than explicit atom identities like ECFPs.24 However, when using feature importance analysis to inform chemical synthesis, it is always important to remember that these models are trained on bits and not substructures. Therefore, it is important to identify when different motifs are assigned the same bit because they perform the same functional role in the molecule (Supplemental Fig. 4A), and when there is true “bit collision,” which arises when 2 different molecular features generate the same identifier (Supplemental Fig. 4B).
It is worth noting that our results were restricted to establishing the inhibition of ENT1 and ENT2 transport activity. We did not identify the mechanism of this inhibition (eg, competitive vs noncompetitive), nor did our results distinguish between compounds that may also serve as substrates for these transporters versus those that simply blocked activity. This will be the topic of future work. Although such information would likely assist in the development of a more robust predictive modeling approach, the current results are expected to aid in the planning and interpretation of drug discovery associated with this physiologically and pharmacologically important family of transporters. Although we have used several machine learning algorithms and approaches here to model ENT inhibition, there are more recent techniques such as few shot learning and large language modeling that we have successfully employed for other target classes41, 42, 43 that have yet to be applied to transporter datasets,44 and which could be leveraged in the future to create even more powerful models to understand the interactions between these increasingly important transporters and the types of small molecules that humans encounter daily.
Between our 2 ENT1 inhibition models, we identified 39 compounds that inhibit ENT1 activity at 20 μM, 11 of which are Food and Drug Administration-approved drugs. Although 20 μM is a rather high concentration, it is not outside clinical relevance for a single-point inhibition screen. Several of the drugs characterized here have clinical concentrations in the low micromolar range, including avanafil, canagliflozin, and ketoconazole (Fig. 5; Supplemental Table 8). For ketoconazole, which we describe in this study with an IC50 of 7.2 μM against ENT1 (Table 1), there are documented human plasma levels of 3500 and 6000 ng/mL, corresponding to 6.6 and 11.3 μM (Supplemental Table 8). The approaches we describe here could be used to elucidate the roles of these transporters in drug disposition and DDI.
Conflict of interest
SE is the founder and owner of Collaborations Pharmaceuticals, Inc. PAV, JSH, RR, and TRL are employees. All other authors have no conflicts of interest.
Acknowledgments
SE kindly acknowledges Dr Pat Walters for suggesting we assess different descriptors for the t-distributed Stochastic Neighbor Embedding visualization.
Financial support
This work was supported by the National Institutes of Health National Institute of General Medical Sciences (Grant 1R42GM131433) [to S.E. and N.J.C].
Data availability
The authors declare that all the data supporting the findings of this study are available within the paper and its Supplemental Data.
CRediT authorship contribution statement
Lucy J. Martinez-Guerrero: Formal analyses, Investigation, Methodology, Writing – original draft. Patricia A. Vignaux: Formal analyses, Data curation, Writing – original draft. Dominique O. Farrera: Methodology. Joshua S. Harris: Data curation. Renuka Raman: Writing - Review & Editing. Thomas R. Lane: Supervision, Project administration, Data curation. Stephen H. Wright: Conceptualization, Supervision, Writing - Review & Editing. Sean Ekins: Conceptualization, Resources, Supervision, Writing - Review & Editing. Nathan J. Cherrington: Conceptualization, Resources, Supervision, Writing - Review & Editing.
Footnotes
L.J.M.-G. and P.A.V. contributed equally to this work.
This article has supplemental material available at jpet.aspetjournals.org.
Supplemental material
References
- 1.Boswell-Casteel R.C., Hays F.A. Equilibrative nucleoside transporters—a review. Nucleosides Nucleotides Nucleic Acids. 2017;36(1):7–30. doi: 10.1080/15257770.2016.1210805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naes S.M., Ab-Rahim S., Mazlan M., Abdul Rahman A. Equilibrative nucleoside transporter 2: properties and physiological roles. Biomed Res Int. 2020;2020 doi: 10.1155/2020/5197626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hau R.K., Wright S.H., Cherrington N.J. Addressing the clinical importance of equilibrative nucleoside transporters in drug discovery and development. Clin Pharmacol Ther. 2023;114(4):780–794. doi: 10.1002/cpt.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hau R.K., Wright S.H., Cherrington N.J. Drug transporters at the human blood-testis barrier. Drug Metab Dispos. 2023;51(5):560–571. doi: 10.1124/dmd.122.001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L., Liu X. Roles of drug transporters in blood-retinal barrier. Adv Exp Med Biol. 2019;1141:467–504. doi: 10.1007/978-981-13-7647-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson J.M., Lamont I.L. Nucleoside analogues as antibacterial agents. Review. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter C.J., Mekkawy A.H., Morris D.L. Role of human nucleoside transporters in pancreatic cancer and chemoresistance. World J Gastroenterol. 2021;27(40):6844–6860. doi: 10.3748/wjg.v27.i40.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermann R., Krajcsi P., Fluck M., et al. Cladribine as a potential object of nucleoside transporter-based drug interactions. Clin Pharmacokinet. 2022;61(2):167–187. doi: 10.1007/s40262-021-01089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astorga B., Ekins S., Morales M., Wright S.H. Molecular determinants of ligand selectivity for the human multidrug and toxin extrusion proteins, MATE1 and MATE2-K. J Pharmacol Exp Ther. 2012;341(3):743–755. doi: 10.1124/jpet.112.191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekins S., Polli J.E., Swaan P.W., Wright S.H. Computational modeling to accelerate the identification of substrates and inhibitors for transporters that affect drug disposition. Clin Pharmacol Ther. 2012;92(5):661–665. doi: 10.1038/clpt.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain S., Ecker G.F. In Silico Approaches to predict drug-transporter interaction profiles: data mining, model generation, and link to cholestasis. Methods Mol Biol. 2019;1981:383–396. doi: 10.1007/978-1-4939-9420-5_26. [DOI] [PubMed] [Google Scholar]
- 12.Zdrazil B., Hellsberg E., Viereck M., Ecker G.F. From linked open data to molecular interaction: studying selectivity trends for ligands of the human serotonin and dopamine transporter. MedChemComm. 2016;7(9):1819–1831. doi: 10.1039/c6md00207b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin L., Yee S.W., Kim R.B., Giacomini K.M. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov. 2015;14(8):543–560. doi: 10.1038/nrd4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang C., Swaan P.W. Computational approaches to modeling drug transporters. Eur J Pharm Sci. 2006;27(5):411–424. doi: 10.1016/j.ejps.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Miller S.R., Zhang X., Hau R.K., et al. Predicting drug interactions with human equilibrative nuceloside transporters 1 and 2 using functional knockout cell lines and bayesian modeling. Mol Pharmacol. 2021;99(2):147–162. doi: 10.1124/molpharm.120.000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandoval P.J., Zorn K.M., Clark A.M., Ekins S., Wright S.H. Assessment of substrate-dependent ligand interactions at the organic cation transporter OCT2 using six model substrates. Mol Pharmacol. 2018;94(3):1057–1068. doi: 10.1124/mol.117.111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane T.R., Urbina F., Zhang X., et al. Machine learning models identify new inhibitors for human OATP1B1. Mol Pharm. 2022;19(11):4320–4332. doi: 10.1021/acs.molpharmaceut.2c00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekins S., Clark A.M., Wright S.H. Making transporter models for drug–drug interaction prediction mobile. Drug Metab Dispos. 2015;43(10):1642–1645. doi: 10.1124/dmd.115.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller S.R., Lane T.R., Zorn K.M., Ekins S., Wright S.H., Cherrington N.J. Multiple computational approaches for predicting drug interactions with human equilibrative nucleoside transporter 1. Drug Metab Dispos. 2021;49(7):479–489. doi: 10.1124/dmd.121.000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangravite L.M., Badagnani I., Giacomini K.M. Nucleoside transporters in the disposition and targeting of nucleoside analogs in the kidney. Eur J Pharmacol. 2003;479(1-3):269–281. doi: 10.1016/j.ejphar.2003.08.076. [DOI] [PubMed] [Google Scholar]
- 21.Severance A.C., Sandoval P.J., Wright S.H. Correlation between apparent substrate affinity and OCT2 transporter turnover. J Pharmacol Exp Ther. 2017;362(3):405–412. doi: 10.1124/jpet.117.242552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Guerrero L., Vignaux P.A., Harris J.S., et al. Computational approaches for predicting drug interactions with human organic anion transporter 4 (OAT4) Mol Pharm. 2025;22(4):1847–1858. doi: 10.1021/acs.molpharmaceut.4c00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RDKit: Open-source cheminformatics. https://www.rdkit.org
- 24.Rogers D., Hahn M. Extended-connectivity fingerprints. J Chem Inf Model. 2010;50(5):742–754. doi: 10.1021/ci100050t. [DOI] [PubMed] [Google Scholar]
- 25.Ding T., Angelopoulos A., Bates S., Jordan M., Tibshirani R.J. In: Advances in Neural Information Processing Systems 36. Dix J., editor. Springer; 2024. Class-conditional conformal prediction with many classes. [Google Scholar]
- 26.Vovk V. Cross-conformal predictors. Ann Math Artif Intell. 2015;74(1-2):9–28. doi: 10.1007/s10472-013-9368-4. [DOI] [Google Scholar]
- 27.Lauzon G.J., Paterson A.R.P. Binding of the nucleoside transport inhibitor nitrobenzylthioinosine to HeLa cells. Mol Pharmacol. 1977;13(5):883–891. doi: 10.1016/S0026-895X(25)12451-6. [DOI] [PubMed] [Google Scholar]
- 28.Dahlig-Harley E., Eilam Y., Paterson A.R., Cass C.E. Binding of nitrobenzylthioinosine to high-affinity sites on the nucleoside-transport mechanism of HeLa cells. Biochem J. 1981;200(2):295–305. doi: 10.1042/bj2000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller S.R., Hau R.K., Jilek J.L., Morales M.N., Wright S.H., Cherrington N.J. Nucleoside reverse transcriptase inhibitor interaction with human equilibrative nucleoside transporters 1 and 2. Drug Metab Dispos. 2020;48(7):603–612. doi: 10.1124/dmd.120.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chawla N.V., Bowyer K.W., Hall L.O., Kegelmeyer W.P. SMOTE: synthetic minority over-sampling technique. J Artif Intell Res. 2002;16:321–357. doi: 10.1613/jair.953. [DOI] [Google Scholar]
- 31.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 32.Shearer J., Castro J.L., Lawson A.D.G., MacCoss M., Taylor R.D. Rings in clinical trials and drugs: present and future. J Med Chem. 2022;65(13):8699–8712. doi: 10.1021/acs.jmedchem.2c00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shafer G., Vovk V. A tutorial on conformal prediction. J Mach Learn Res. 2008;9(3):371–421. [Google Scholar]
- 34.Hillgren K.M., Keppler D., Zur A.A., et al. Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94(1):52–63. doi: 10.1038/clpt.2013.74. [DOI] [PubMed] [Google Scholar]
- 35.Rehan S., Shahid S., Salminen T.A., Jaakola V.P., Paavilainen V.O. Current progress on equilibrative nucleoside transporter function and inhibitor design. SLAS Discov. 2019;24(10):953–968. doi: 10.1177/2472555219870123. [DOI] [PubMed] [Google Scholar]
- 36.Joshi R., Medhi B. Natural product and drugs interactions, its clinical implication in drug therapy management. Saudi Med J. 2008;29(3):333–339. [PubMed] [Google Scholar]
- 37.Weiss J., Gattuso G., Barreca D., Haefeli W.E. Nobiletin, sinensetin, and tangeretin are the main perpetrators in clementines provoking food-drug interactions in vitro. Food Chem. 2020;319 doi: 10.1016/j.foodchem.2020.126578. [DOI] [PubMed] [Google Scholar]
- 38.Bushra R., Aslam N., Khan A.Y. Food-drug interactions. Oman Med J. 2011;26(2):77–83. doi: 10.5001/omj.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pimple S.R. University of Tennessee Health Science Center; 2008. Flavonoids and Related Compounds as Nucleoside Transporter Inhibitors. [DOI] [Google Scholar]
- 40.Carrier E.J., Auchampach J.A., Hillard C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A. 2006;103(20):7895–7900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baskin I.I., Winkler D., Tetko I.V. A renaissance of neural networks in drug discovery. Expert Opin Drug Discov. 2016;11(8):785–795. doi: 10.1080/17460441.2016.1201262. [DOI] [PubMed] [Google Scholar]
- 42.Ekins S. The next era: Deep learning in pharmaceutical research. Pharm Res. 2016;33(11):2594–2603. doi: 10.1007/s11095-016-2029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korotcov A., Tkachenko V., Russo D.P., Ekins S. Comparison of deep learning with multiple machine learning methods and metrics using diverse drug discovery datasets. Mol Pharm. 2017;14(12):4462–4475. doi: 10.1021/acs.molpharmaceut.7b00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder S.H., Vignaux P.A., Ozalp M.K., et al. The Goldilocks paradigm: comparing classical machine learning, large language models, and few-shot learning for drug discovery applications. Commun Chem. 2024;7(1):134. doi: 10.1038/s42004-024-01220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this study are available within the paper and its Supplemental Data.