Abstract
Decreased masticatory function, which is termed ‘masticatory dysfunction’, has long been considered a sign and symptom of underlying oral diseases. Masticatory function usually improves when the underlying diseases are treated. However, in older patients with systemic diseases, persistent masticatory dysfunction may occur even after individuals receive dental treatment. In this narrative review, the authors review of the current research on masticatory dysfunction associated with systemic factors, and proposed the concept of Dysmasema - a persistent condition of masticatory dysfunction refractory to dental treatment and associated with aging and systemic factors. The rationale for the need for the new concept (i.e., Dysmasema) and the elements needed to identify Dysmasema – namely, low masticatory performance, subjective eating difficulty, and poor nutritional status – are addressed. This new proposal, which is based on clinical, epidemiological, and pathological perspectives, contributes to the precise diagnosis and treatment of older adults by linking masticatory dysfunction with systemic factors.
Key words: Mastication, Masticatory performance, Eating difficulty, Geriatrics, Oral health
Background
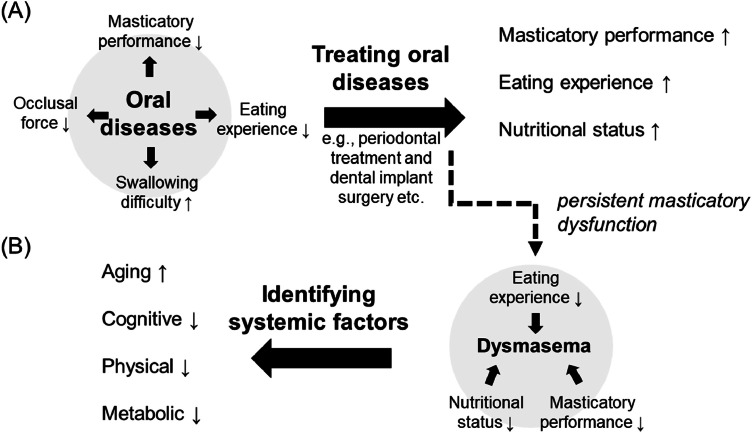
The terms ‘masticatory deficit’, ‘hypofunction of mastication’, or ‘masticatory disorder’ have been widely used in the dental literature to describe declines in masticatory function as evaluated by subjective eating experience or standard tests (Table 1). In literature, the concept of masticatory dysfunction has been used as an umbrella term for a broad spectrum of problems related to mastication attributed to multiple factors.1,2 Masticatory dysfunction is associated with oral diseases and poor oral health1 and multiple age-related systemic disorders, such as frailty.3 For decades, masticatory dysfunction has been considered a sign and symptom of underlying oral diseases. For example, complete edentulism leads to severe problems in chewing, occlusion, and swallowing4 as well as the risk of malnutrition and sarcopenia.5,6 Dental treatment, such as installing dentures, helps to improve masticatory function.7 Therefore, the major goal of dental treatment is to identify underlying oral diseases leading to masticatory dysfunction. When the underlying oral diseases, such as periodontitis or toothache, are managed, the patient’s masticatory function is expected to improve, compared to the condition when the diseases are not treated (Figure 1A).
Table 1.
Terms describing the problems related to decreased masticatory function.
| Term | Definition |
|---|---|
| Masticatory dysfunction | ‘a reduction in normal masticatory function due to structural factors such as tooth loss, or functional factors such as weaker bite force’1 |
| Masticatory performance | ‘a measure of the comminution of food attainable under standardized testing conditions’8 |
| Masticatory efficiency | ‘the effort required to achieve a standard degree of comminution of food’8 |
| Masticatory ability | (masticatory function) ‘… the ability of an individual to masticate solid food’9 |
| Eating difficulties | ‘any activity and emotional requirement and relations, which alone or in combination interfere with the process of preparing food, transferring food into the mouth, chewing and swallowing’10 |
| Masticatory disorder | A construct evaluated by the questionnaire ‘screening for masticatory disorders in older adults’, consisting of 2 factors: masticatory function and masticatory perception11 |
| Masticatory muscle disorders | A set of diagnoses defined related to temporomandibular disorders, including muscle pain and movement disorders12 |
Fig. 1.
Associations between oral diseases, masticatory dysfunction, and Dysmasema. (A) Oral diseases, such as periodontitis or dental caries, which may lead to tooth loss, are associated with multiple forms of oral dysfunction, including lower masticatory performance and eating difficulties. Dental treatment that focuses on restoring deficits, such as periodontal treatment or dental implant surgery, improves masticatory dysfunction and nutritional status. (B) In contrast to the condition in which masticatory function is significantly improved by dental treatment (i.e., [A]), Dysmasema refers to the condition in which masticatory dysfunction is persistent, even when dental treatment is completed. Persistent masticatory dysfunction can be evaluated by lower masticatory performance, experience of eating difficulties, and worse nutritional status. Notably, in older adults with systemic diseases, systemic factors (e.g., sarcopenia or cognitive impairment) may have a greater impact on masticatory function. Therefore, identifying the underlying systemic factors may play a critical role in improving masticatory function.
In older adults, the idea that ‘masticatory function will improve as long as oral diseases are treated’ becomes more complicated owing to the complex associations among mastication, aging, and systemic factors (e.g., frailty and cognitive impairment). For example, installing prostheses and treating orofacial pain have a significant effect on improving masticatory performance.13,14 However, in patients with dementia or frailty, the treatment was less effective because of the decline in general mental and physical capacity.15, 16, 17, 18 As discussed in this article, patients with systemic diseases may suffer persistent eating difficulty or show lower masticatory performance after dental treatment, compared to the individuals without the systemic diseases.15, 16, 17, 18, 19 Furthermore, recent advancements in dental medicine, neuroscience, and geriatric medicine have highlighted that persistent masticatory dysfunction may be a problem related to multiple factors, such as sarcopenia or neurodegeneration,20,21 eventually leading to poor nutritional status.
Therefore, in this narrative review, we suggest a new focus on persistent masticatory dysfunction related to aging and systemic factors. Based on current evidence from clinical, geriatric, and neuroscience research,15, 16, 17, 18, 19, 20, 21, 22, 23 we propose the term Dysmasema to describe persistent masticatory dysfunction in older adults, and we discuss its defining elements and practical implications.
Dysmasema: Persistent decline in masticatory function related to aging and systemic factors
In this article, we propose the concept of Dysmasema (in Greek, δυσ for ‘bad’ and μάσημα for ‘chewing’), which refers to persistent masticatory dysfunction refractory to dental treatment and is associated with aging and systemic factors. We related this concept specifically to aging and cause (a) masticatory dysfunction is a common symptom in older individuals,22,24, 25, 26 and (b) systemic conditions, such as neurodegenerative disorders, are highly associated with aging. Researchers have concentrated on the association between oral function and oral healthcare,4,22,27 and dental treatment is considered a necessary element for systemic health. While most masticatory dysfunction caused by oral diseases (e.g., periodontitis and tooth loss) can be improved by dental treatment,13, 14, 28 i.e., being reactive to the treatment effect, we consider Dysmasema as the condition when masticatory dysfunction persists even patients have received dental treatment.
In this article, we first explained the difference between Dysmasema, which is not resolved by dental treatment and persists, and the masticatory dysfunction which is improved by dental treatment (Section 3). Next, we explain the rationale why Dysmasema should be treated as a standalone clinical condition due to its clinical significance in dental treatment, epidemiological connections with aging, and potential pathological mechanisms in neuroscience (Section 4). The section is followed by the proposed elements for assessing Dysmasema in clinical practice (Section 5), and the difference between Dysmasema and other forms of masticatory dysfunction is discussed (Section 6).
Reactive masticatory dysfunction vs. Dysmasema
In the literature, masticatory dysfunction and related terms (Table 1) are used in different conditions: (1) to describe someone with subjective difficulty in eating (e.g., ‘chewing difficulty’)27; (2) to describe a low masticatory ability,9 such as a lower masticatory performance or masticatory efficiency, as assessed by standard tests8; or (3) to imply a functional problem due to anatomical deficits (e.g., tooth loss).29 While the terms are used in various conditions, there is 1 aspect that remains consistent in all the literature: these terms describe either a sign (hypofunction) or a symptom, e.g., increased eating difficulty10 secondary to a condition about oral diseases of poor oral health. We prefer to name masticatory dysfunction as a sign/symptom secondary to oral diseases ‘reactive masticatory dysfunction’, which responds to functional/anatomical deficits in orofacial structure (e.g., tooth loss). As shown in previous studies, masticatory dysfunction in response to deficits (e.g., tooth loss) can be significantly improved by treating oral diseases, including installing dental prostheses to replace missing teeth7 and receiving periodontal treatment,28 orthodontic treatment,30 and implant-supported overdenture.13 The findings from a systematic review revealed that, in patients with complete edentulism, wearing a denture was associated with improved nutritional status, eating-related quality of life (ERQoL), and eating function compared with those who did not wear a denture.7 In general, the findings suggest substantial improvement in masticatory function after dental treatment (Table 2).
Table 2.
Differences between reactive masticatory dysfunction and Dysmasema.
| Reactive masticatory dysfunction | Dysmasema | |
|---|---|---|
| Reversibility | Masticatory function is improved with dental treatment focusing on oral diseases | Masticatory function is not significantly improved with dental treatment focusing on oral diseases |
| Chronicity | Acute (immediately after tooth loss) or chronic (periodontitis) | Chronic; usually a long-lasting condition after multiple dental treatments |
| Association with oral conditions | The degree of dysfunction is proportional to anatomical (tooth loss) or functional (hyposalivation) factors | The degree of dysfunction may not be proportional to deficits in oral conditions |
| Association with systemic factors | Patients maintain physical and mental fitness | Systemic factors, including physical (e.g., sarcopenia) and mental (e.g., dementia) disorders, play a key role |
| Neurological mechanism | Focusing on the neuromuscular system at the peripheral (e.g., masticatory muscles) | Focusing on systemic deficits in the neuromuscular system |
However, even if installing a denture leads to a significant improvement in masticatory function (as shown in the case of reactive masticatory dysfunction), this does not mean that masticatory function is fully restored (as in the dentate condition). Compared with dentate individuals, edentulous patients who wear a complete denture still have decreased nutritional status, ERQoL, and eating ability.7 Longitudinal research also revealed that patients wearing a denture also reported lower health-related quality of life, compared with dentate subjects.31 Furthermore, in the domain of ERQoL, edentulous patients perceive difficulty in eating hard foods.32 These findings highlight the importance of modifying eating habits in elderly patients. In addition to dental treatment, dietary intervention customized to patients’ personal eating habits contributes to improving their nutritional status.33,34
The associations between the effects of dental treatment and functional recovery should be further examined in terms of aging and age-related systemic diseases. Under the global challenge of population aging, mastication plays a pivotal role not only in oral health but also in nutritional intake and systemic health in elderly people.22,35,36 Accumulating evidence has suggested that, in contrast to older adults with reactive masticatory dysfunction, older adults with systemic diseases, including neurodegenerative disorders,16,18,19 frailty,15,17 and diabetes,23 have long-lasting declines in masticatory function even after receiving effective dental treatment (Table 3). For example, patients with dementia show weaker improvements in masticatory performance and chewing parameters after receiving removable dentures than healthy controls.16, 37 Patients with Parkinson’s disease show lower masticatory performance than healthy controls 2 months after receiving dentures.19 Notably, treatment effectiveness is associated with the quality of dental care. Older patients with systemic diseases also show deficits in their medical behavior. For example, patients with dementia have more difficulty communicating with and deciding on a treatment plan,38 and patients with frailty are more likely to discontinue dental treatment.39 In other words, systemic factors may lead to suboptimal conditions for treatment. The persistent functional decline associated with these factors suggests a clinical difference from reactive masticatory dysfunction (Table 2).
Table 3.
Selected evidence of persistent masticatory dysfunction associated with systemic factors.
| Source | Systemic factors | Dental treatment | Findings |
|---|---|---|---|
| Campos et al.16 | Alzheimer’s disease | Removable denture, 2-month F/U | ‘After removable prosthesis insertion, elders with AD continued to show lower masticatory efficiency values than controls….’16 |
| Jockusch et al.18 | Dementia | Removable denture | In patients with moderate dementia, increased chewing problems were found, even the patients remained using a removable denture18 |
| Ribeiro et al.19 | Parkinson's disease | Removable denture, 2-month F/U | (After receiving dental treatment and a new denture,) ‘The PD group showed a decreased range of jaw motion, longer duration and slower velocity of the masticatory cycle (P < 0.05), higher X50 value, and lower maximum bite force (P < 0.05)’19 |
| Alchalabi et al.15 | Frailty | Complete denture | ‘Frail individuals with CD reported chewing difficulties 7.8 times more often than nonfrail individuals without CD.‘15 |
| Castrejon-Perez et al.17 | Frailty | Complete denture, 4-year f/U | ‘Self-reported use of nonfunctional complete dentures, dissatisfaction with dentures and a reduced ACHF with complete dentures were associated with a higher FI both cross-sectionally and prospectively’17 |
| Roumanas et al.23 | Diabetes | Conventional and implant-supported overdentures, 6-month F/U | ‘After 7 months of adaptation to new dentures, patients consumed fewer difficult-to-chew foods than with their original dentures’23 |
Note: The included studies are systematically searched via PubMed with the combination of the following keywords: (cardiovascul*[tiab] OR obesity[tiab] OR arthri*[tiab] OR diabet*[tiab] OR frail*[tiab] OR dement*[tiab]) AND ("dental treatment"[tiab] OR prosthes*[tiab] OR denture*[tiab]) AND (masticat*[tiab] OR chew*[tiab] OR eating[tiab]). Only the studies published before 2024.8.1 are selected.
Rationale for Dysmasema as a new concept
We here proposed Dysmasema, defined as persistent decline in masticatory function related to aging and systemic factors, as a standalone concept for decreased masticatory function rather than merely a sign/symptom of oral disease. First, from a clinical perspective, treating oral diseases is important to improve masticatory dysfunction (Figure 1A). However, for older patients with systemic diseases (Figure 1B), a focus on potential systemic factors may play a critical role in managing masticatory dysfunction since their oral function is closely associated with general physical and cognitive factors.20,21 In other words, the evaluation of these systemic factors plays a dominant role in developing a dental treatment plan. Second, epidemiologically, Dysmasema has been widely observed in patients with age-related diseases (Table 3). The focus on Dysmasema corroborates previous investigations of the associations between oral and geriatric health in general.22 Third, Dysmasema may be related to specific pathological mechanisms, as demonstrated by recent findings from neuroscience and geriatrics. Mastication is conceived as a neuromuscular process but is not confined to the peripheral neuromuscular structure. While deficits in teeth or the masticatory muscles may cause masticatory dysfunction,11,12 emerging evidence has suggested that systemic factors related to neuromuscular function may play a key role in Dysmasema. For example, masseter muscle volume is associated with frailty and cognitive impairment and is further associated with poor nutritional status,40,41 and patients with neurodegenerative disorders have difficulty chewing. Sarcopenia is considered a major factor for masticatory dysfunction.20 Neuroscience research has revealed that changes in mastication are associated with neuroplastic effects on the brain.42,43 Animal studies revealed that changes in dental occlusion or tooth loss induced changes in sensorimotor cortices in the brain, i.e., an effect of dental factors on cortical plasticity.42,43 Human subjects, nevertheless, showed a functional adaptation to these changes. For example, patients who received a new denture showed different brain activation before and after the treatment, with a gradual change in masticatory function.44 Based on the evidence, we conceived that most older individuals can adapt themselves to the dental changes with no long-lasting masticatory dysfunction. However, patients who have deficits in this neuroplasticity may progress into Dysmasema because their brain functions are less able to cope with the dental changes. In addition, cognitive and emotional processing, such as the perception of taste/smell and food texture, plays a key role in mastication.45 Taste and smell are further associated with systemic diseases and the effect of long-term medication.46 Individuals with such dysfunction usually adopt various adaptive strategies to improve their performance, such as changing from the edentulous side to the dentate side to chew or spend more time chewing.47,48 Notably, individuals may still experience difficulty eating a balanced diet and develop a maladaptive strategy to cope with their oral condition, such as avoiding the consumption of some hard foods, which further worsens their nutritional status.47 Adaptive behavior is closely associated with mental function49; therefore, Dysmasema, especially in patients with neurodegenerative disorders, may represent an inability to learn and maintain adaptive behavior for eating. These findings collectively reveal the complexity of the mechanisms underlying Dysmasema.
Elements to identify Dysmasema
We propose 3 elements to identify Dysmasema (Table 4). First, Dysmasema is associated with persistent declined masticatory function, evidenced by a lower masticatory performance from objective assessments. Second, Dysmasema is associated with a persistent subjective difficulty in eating, evidenced by self-reported experience of eating daily meals. Third, individuals with Dysmasema show a worse nutritional status, evidenced by the questionnaires on nutrient intake and the related body metrics, such as the limb circumference that reflects the condition of sarcopenia (Table 4).
Table 4.
Elements for the clinical diagnosis of Dysmasema.
| Elements | Procedure | Assessments | Remark |
|---|---|---|---|
| Low masticatory performance | Using assessments of masticatory performance under standardized conditions and testing food, including assessments of crushing and mixing abilities | Various standard assessments have been developed and show good reliability and validity, including the assessment of cutting/crunching ability (e.g., assessing the size of food particles) and food mixing ability (e.g., assessing the degree of color mixing of gums) during mastication.50 | No consensus was formed for the cutoff point of assessments of masticatory performance. |
| Eating difficulty | Using questionnaires of eating experience, including subjective difficulty in eating and adaptive strategies to improve eating | There have been standard questionnaires for the general difficulty in chewing and swallowing food, such as the Screening for Masticatory Disorders in Older Adults (SMDOA),11 and for intaking specific foods, e.g., the Chewing Function Questionnaire.51 Notably, culture/region-specific questionnaires are designed for food intake experience.52,53 | Questions should focus on foods in patients’ daily meals. |
| Poor nutritional status | Using objective assessments for measuring body indices | Validated assessments, such as Mini Nutrition Assessment54 or the Subjective Global Assessment,55 should be used. The assessment of body metrics, such as calf circumference (CC) and mid-upper arm circumference (MUAC), also provide useful indices for a detrimental nutritional status. | The body metrics CC and MUAC have been used to screen sarcopenia in older individuals.56,57 |
We consider the use of objective assessments the pivotal role in assessing Dysmasema because these tests can be conducted in a standard condition. Their effectiveness in tracing the changes in masticatory function has been evidenced by clinical research, e.g., the improved masticatory function after treatment of temporomandibular disorders (TMD).14,58 Therefore, these assessments are useful for identifying persistent masticatory dysfunction in longitudinal follow-ups. Various standard assessments have been developed and show good reliability and validity, including the assessment of cutting/crunching ability (e.g., assessing the size of food particles) and food mixing ability (e.g., assessing the degree of color mixing of gums) during mastication.50 In contrast to lower masticatory performance, ‘eating difficulty’ is considered a judgment by one’s personal experience. We consider both the objective element, i.e., a sign of lower masticatory function, and the subjective element, i.e., feelings of difficulty in eating, to be necessary elements for identifying Dysmasema. There is a weak correlation between the performance of objective tests and subjective eating experience,59,60 suggesting the necessity of evaluating both elements independently. There have been standard questionnaires for the general difficulty in chewing and swallowing food, such as the Screening for Masticatory Disorders in Older Adults (SMDOA)11 and for taking specific foods, e.g., the Chewing Function Questionnaire.51 Notably, eating difficulty should be evaluated with a patient-centered approach. The intake of food types in daily meals is highly culturally and regionally specific to patients. Culture/region-specific questionnaires should be used for food intake experience.52,53
Critically, lower masticatory performance and eating difficulty are associated with nutritional status.36 For example, patients with complete edentulism or insufficient functional units have a greater risk of malnutrition than do individuals with functional dentition.6 Validated assessments of nutritional status, including the Mini Nutrition Assessment (MNA),54 the Subjective Global Assessment (SGA),55 and patient-generated SGA, are critical elements in assessing Dysmasema. We believe that the use of body metrics, such as calf circumference (CC) and mid-upper arm circumference (MUAC), which reflect the status of sarcopenia,56,57 also provide useful indices for a detrimental nutritional status.
Comparison between Dysmasema and other existing concepts of masticatory dysfunction
It is noteworthy that lower masticatory performance, eating difficulty, and poor nutritional status, should be considered as the core elements for Dysmasema, rather than a rigid set of clinical criteria for differential diagnosis – which requires further consensus of experts. Still, these 3 elements help us to differentiate between Dysmasema and other related conditions. First, lower masticatory performance is considered as an oral ‘hypofunction’.26 Hypofunction refers to a lower functional performance, quantified by standard tests, below a cutoff point. However, Dysmasema differs from hypofunction because patients need to present not only hypofunction from standard assessments but also difficulty in eating daily meals.
Second, we consider the dysfunction caused by TMDs and Dysmasema to be different. First, in patients with TMDs, masticatory performance can mostly be improved after regular treatment, as evidenced by previous research.14,58 Therefore, we consider the dysfunction a reactive masticatory dysfunction. However, Dysmasema, according to our definition, is closely associated with aging-related systemic factors. In patients with myofascial pain and masticatory disorders, if the diseases are associated with systemic factors, such as chronic distress,61 the condition is considered Dysmasema.
Third, there have been many concepts regarding declined oral health or oral function associated with systemic health, such as oral hypofunction,26 oral frailty,24,25 or chronic oral disorders in older individuals.22 Dysmasema differs from these concepts by additionally focusing on poor nutritional status, especially the use of questionnaires and body metrics, including the MUAC and CC, to assess malnutrition.56,57
Future directions
Our proposal of Dysmasema aims to highlight new directions for research on mastication instead of raising a new banner to revolutionize current clinical diagnosis and treatment methods. The precision of scientific definition and practicability in clinical management can be limited because there is insufficient clinical research focusing on Dysmasema. We hope this proposal would evoke more discussion between inter-disciplinary experts in the near future so that its diagnosis criteria and guidelines of clinical management can be formed by consensus. Before reaching a consensus, we expect further clarification on the basis of research evidence for the following aspects:
-
1.
The chronicity and time course of Dysmasema need further clarification. There have been few long-term (>5 years) studies on the persistence of masticatory dysfunction in patients receiving dental treatment, especially for very old patients (>80 years) or those with systemic diseases. Longitudinal research on the long-term effects of dental treatment is needed to clarify the clinical profile of patients with Dysmasema. Furthermore, since these patients usually have their signs/symptoms controlled by medication, the effects of long-term medication on oral function should not be underestimated. In general, more observational research is needed to differentiate patients with reactive vs. persistent masticatory dysfunction.
-
2.
Dysmasema is associated with specific neurological mechanisms. Recently, chronic pain has been proposed as a unique disease because of its specific neurological mechanism.62 Research has suggested that changes in masticatory function are associated with neuroplastic effects on the brain.42,43 The hypothesis that Dysmasema may be related to alterations in the central nervous system has remained unexplored.
-
3.
A practical and valid index for quantifying Dysmasema requires more research: while standard tests of masticatory performance and questionnaires of eating difficulty have been widely used,50 the cutoff point to define normal vs. hypomastication function has remained unclear. Establishing a practical and valid index combining both objective and subjective assessments would be the foundation for a precise diagnosis of Dysmasema.
-
4.
A guideline of clinical management on Dysmasema should be based on more discussion and consensus from experts after the issues above-stated are clarified. Nevertheless, we would like to suggest the importance of ‘stop and watch’ when dentists identify a potential case of Dysmasema. For example, patients with cognitive impairment are likely to show poor oral health and masticatory dysfunction and also have difficulty verbally communicating how painful they are when chewing with a denture.63 In this case, we believe that dentists should first consult experts from geriatrics or neurology for cross-disciplinary opinions, rather than ‘pushing on’ finishing the denture, which not just provides limited benefits but also causes long-lasting health conditions, such as malnutrition, as the concept of Dysmasema suggests.
Contribution
C-S Lin initiated the concept and drafted the manuscript; C-S Lin and T-C Chen discussed the content, finalized the conclusions, and prepared the final manuscript for submission.
Declaration of competing interest
The authors report no competing interests.
References
- 1.Lahoud T., Yu A.Y., King S. Masticatory dysfunction in older adults: A scoping review. J Oral Rehabil. 2023;50(8):724–737. doi: 10.1111/joor.13493. [DOI] [PubMed] [Google Scholar]
- 2.Bousiou A., Konstantopoulou K., Polychronopoulou A., Halazonetis D.J., Schimmel M., Kossioni AE. Sociomedical and oral factors affecting masticatory performance in an older population. Clin Oral Investig. 2022;26(4):3477–3486. doi: 10.1007/s00784-021-04316-6. [DOI] [PubMed] [Google Scholar]
- 3.Kojima G., Taniguchi Y., Iwasaki M., Aoyama R., Urano T. Associations between self-reported masticatory dysfunction and frailty: a systematic review and meta-analysis. PLoS One. 2022;17(9) doi: 10.1371/journal.pone.0273812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dibello V., Lobbezoo F., Lozupone M., et al. Oral frailty indicators to target major adverse health-related outcomes in older age: a systematic review. Geroscience. 2023;45(2):663–706. doi: 10.1007/s11357-022-00663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moynihan P.J., Teo JL. Exploring oral function, protein intake, and risk of sarcopenia: a scoping review. JDR Clin Trans Res. 2024;9(1):4–20. doi: 10.1177/23800844231157259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zelig R., Goldstein S., Touger-Decker R., et al. Tooth Loss and Nutritional Status in Older Adults: a systematic review and meta-analysis. JDR Clin Trans Res. 2022;7(1):4–15. doi: 10.1177/2380084420981016. [DOI] [PubMed] [Google Scholar]
- 7.Moynihan P., Varghese R. Impact of wearing dentures on dietary intake, nutritional status, and eating: a systematic review. JDR Clin Trans Res. 2022;7(4):334–351. doi: 10.1177/23800844211026608. [DOI] [PubMed] [Google Scholar]
- 8.The Academy of Prosthodontics The glossary of prosthodontic terms 2023: tenth edition. J Prosthet Dent. 2023;130(4 Suppl 1):e7–e126. doi: 10.1016/j.prosdent.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Elgestad Stjernfeldt P., Faxen-Irving G., Wardh I. Masticatory ability in older individuals: a qualitative interview study. Gerodontology. 2021;38(2):199–208. doi: 10.1111/ger.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klinke M.E., Wilson M.E., Hafsteinsdottir T.B., Jonsdottir H. Recognizing new perspectives in eating difficulties following stroke: a concept analysis. Disabil Rehabil. 2013;35(17):1491–1500. doi: 10.3109/09638288.2012.736012. [DOI] [PubMed] [Google Scholar]
- 11.Cavalcanti R.V.A., Magalhaes Junior H.V., Pernambuco L.A., Lima K.C. Screening for masticatory disorders in older adults (SMDOA): An epidemiological tool. J Prosthodont Res. 2020;64(3):243–249. doi: 10.1016/j.jpor.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman E., Ohrbach R., Truelove E., et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for Clinical and research applications: recommendations of the international RDC/TMD consortium network* and orofacial pain special interest groupdagger. J Oral Facial Pain Headache. 2014;28(1):6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boven G.C., Raghoebar G.M., Vissink A., Meijer HJ. Improving masticatory performance, bite force, nutritional state and patient's satisfaction with implant overdentures: a systematic review of the literature. J Oral Rehabil. 2015;42(3):220–233. doi: 10.1111/joor.12241. [DOI] [PubMed] [Google Scholar]
- 14.Pereira L.J., Steenks M.H., de Wijer A., Speksnijder C.M., van der Bilt A. Masticatory function in subacute TMD patients before and after treatment. J Oral Rehabil. 2009;36(6):391–402. doi: 10.1111/j.1365-2842.2008.01920.x. [DOI] [PubMed] [Google Scholar]
- 15.Alchalabi L., Schaffner E., Imboden M., Difloe-Geisert J.C., Zitzmann N.U. Probst-Hensch N. Removable dental prostheses and difficulties with chewing among frail individuals: Results from the Swiss SAPALDIA cohort. Swiss Dent J. 2024;134(2):53–71. doi: 10.61872/sdj-2024-02-04. [DOI] [PubMed] [Google Scholar]
- 16.Campos C.H., Ribeiro G.R. Rodrigues Garcia RCM. Mastication and oral health-related quality of life in removable denture wearers with Alzheimer disease. J Prosthet Dent. 2018;119(5):764–768. doi: 10.1016/j.prosdent.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Castrejon-Perez R.C., Borges-Yanez S.A., Ramirez-Aldana R., Nasu I., Saito Y. Complete dentures associated with frailty among edentulous older Japanese people: A prospective analysis. Commun Dent Oral Epidemiol. 2024;52(4):572–580. doi: 10.1111/cdoe.12956. [DOI] [PubMed] [Google Scholar]
- 18.Jockusch J., Riese F., Theill N., Sobotta B.A.J. Nitschke I. Aspects of oral health and dementia among Swiss nursing home residents. Z Gerontol Geriatr. 2021;54(5):500–506. doi: 10.1007/s00391-020-01739-w. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro G.R., Campos C.H., Rodrigues Garcia R.C.M. Parkinson's disease impairs masticatory function. Clin Oral Investig. 2017;21(4):1149–1156. doi: 10.1007/s00784-016-1879-z. [DOI] [PubMed] [Google Scholar]
- 20.Kugimiya Y., Iwasaki M., Ohara Y., et al. Association between sarcopenia and oral functions in community-dwelling older adults: a cross-sectional study. J Cachexia Sarcopenia Muscle. 2023;14(1):429–438. doi: 10.1002/jcsm.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin C.S., Lin H.H., Wang S.J., Fuh JL. Association between regional brain volume and masticatory performance differed in cognitively impaired and non-impaired older people. Exp Gerontol. 2020;137 doi: 10.1016/j.exger.2020.110942. [DOI] [PubMed] [Google Scholar]
- 22.MacEntee M.I., Brondani M., Avivi-Arber L., Bartlett D., Donnelly L., Duyck J., et al. Clinical oral disorders in adults screening protocol (CODA-SP) from the 2019 Vancouver IADR Consensus Symposium. Gerodontology. 2021;38(1):5–16. doi: 10.1111/ger.12496. [DOI] [PubMed] [Google Scholar]
- 23.Roumanas E.D., Garrett N.R., Hamada M.O., Kapur KK. Comparisons of chewing difficulty of consumed foods with mandibular conventional dentures and implant-supported overdentures in diabetic denture wearers. Int J Prosthodont. 2003;16(6):609–615. [PubMed] [Google Scholar]
- 24.Tanaka T., Hirano H., Ikebe K., et al. Consensus statement on “oral frailty” from the japan geriatrics society, the Japanese society of gerodontology, and the Japanese association on sarcopenia and frailty. Geriatr Gerontol Int. 2024;24(11):1111–1119. doi: 10.1111/ggi.14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parisius K.G.H., Verhoeff M.C., Lobbezoo F., et al. Towards an operational definition of oral frailty: A e-Delphi study. Arch Gerontol Geriatr. 2024;117 doi: 10.1016/j.archger.2023.105181. [DOI] [PubMed] [Google Scholar]
- 26.Minakuchi S., Tsuga K., Ikebe K., et al. Oral hypofunction in the older population: Position paper of the Japanese Society of Gerodontology in 2016. Gerodontology. 2018;35(4):317–324. doi: 10.1111/ger.12347. [DOI] [PubMed] [Google Scholar]
- 27.Woo J., Tong C., Yu R. Chewing Difficulty Should be Included as a Geriatric Syndrome. Nutrients. 2018;10(12):1–12. doi: 10.3390/nu10121997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira L.J., Gazolla C.M., Magalhaes I.B., et al. Influence of periodontal treatment on objective measurement of masticatory performance. J Oral Sci. 2012;54(2):151–157. doi: 10.2334/josnusd.54.151. [DOI] [PubMed] [Google Scholar]
- 29.Alvarenga M.O.P., Ferreira R.O., Magno M.B., Fagundes N.C.F., Maia L.C., Lima RR. Masticatory dysfunction by extensive tooth loss as a risk factor for cognitive deficit: a systematic review and meta-analysis. Front Physiol. 2019;10:832. doi: 10.3389/fphys.2019.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanon G., Contardo L., Reda B. The impact of orthodontic treatment on masticatory performance: a literature review. Cureus. 2022;14(10) doi: 10.7759/cureus.30453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen P.F., McMillan AS. A longitudinal study of quality of life outcomes in older adults requesting implant prostheses and complete removable dentures. Clin Oral Implants Res. 2003;14(2):173–179. doi: 10.1034/j.1600-0501.2003.140206.x. [DOI] [PubMed] [Google Scholar]
- 32.Al-Sultani H.F., Field J.C., Thomason J.M., Moynihan PJ. The impact of replacement conventional dentures on eating experience. JDR Clin Trans Res. 2019;4(1):29–40. doi: 10.1177/2380084418803091. [DOI] [PubMed] [Google Scholar]
- 33.McCrum L.A., Watson S., McGowan L., et al. Development and feasibility of a tailored habit-based dietary intervention coupled with natural tooth replacement on the nutritional status of older patients. Pilot Feasibility Stud. 2020;6:120. doi: 10.1186/s40814-020-00654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson S., McCrum L.A., McGuinness B., et al. Establishing healthy eating ‘habits’: a pilot randomised controlled trial of a habit-based dietary intervention following oral rehabilitation for older adults. Nutrients. 2023;15(3):1–21. doi: 10.3390/nu15030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peyron M.A., Woda A., Bourdiol P., Hennequin M. Age-related changes in mastication. J Oral Rehabil. 2017;44(4):299–312. doi: 10.1111/joor.12478. [DOI] [PubMed] [Google Scholar]
- 36.Tada A., Miura H. Systematic review of the association of mastication with food and nutrient intake in the independent elderly. Arch Gerontol Geriatr. 2014;59(3):497–505. doi: 10.1016/j.archger.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Campos C.H., Ribeiro G.R., Stella F., Rodrigues Garcia R.C. Mandibular movements and bite force in Alzheimer's disease before and after new denture insertion. J Oral Rehabil. 2017;44(3):178–186. doi: 10.1111/joor.12476. [DOI] [PubMed] [Google Scholar]
- 38.Geddis-Regan A., Kerr K., Curl C. The impact of dementia on oral health and dental care, part 2: approaching and planning treatment. Prim Dent J. 2020;9(2):31–37. doi: 10.1177/2050168420923862. [DOI] [PubMed] [Google Scholar]
- 39.Niesten D., van Mourik K., van der Sanden W. The impact of frailty on oral care behavior of older people: a qualitative study. BMC Oral Health. 2013;13:61. doi: 10.1186/1472-6831-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin C.S., Liu L.K., Lee W.J., et al. Low masseter muscle mass is associated with frailty in community-dwelling older adults: I-Lan Longitudinal Aging Study. Exp Gerontol. 2022;163 doi: 10.1016/j.exger.2022.111777. [DOI] [PubMed] [Google Scholar]
- 41.Lin C.S., Liu L.K., Chen L.K., Fuh JL. Association between masseter muscle volume, nutritional status, and cognitive status in older people. Arch Gerontol Geriatr. 2023;113 doi: 10.1016/j.archger.2023.105038. [DOI] [PubMed] [Google Scholar]
- 42.Avivi-Arber L., Lee J.C., Sessle BJ. Dental occlusal changes induce motor cortex neuroplasticity. J Dent Res. 2015;94(12):1757–1764. doi: 10.1177/0022034515602478. [DOI] [PubMed] [Google Scholar]
- 43.Avivi-Arber L., Seltzer Z., Friedel M., et al. Widespread volumetric brain changes following tooth loss in female mice. Front Neuroanat. 2016;10:121. doi: 10.3389/fnana.2016.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luraschi J., Korgaonkar M.S., Whittle T., Schimmel M., Muller F., Klineberg I. Neuroplasticity in the adaptation to prosthodontic treatment. J Orofac Pain. 2013;27(3):206–216. doi: 10.11607/jop.1097. [DOI] [PubMed] [Google Scholar]
- 45.Le Reverend B., Saucy F., Moser M., Loret C. Adaptation of mastication mechanics and eating behaviour to small differences in food texture. Physiol Behav. 2016;165:136–145. doi: 10.1016/j.physbeh.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Schiffman S.S., Zervakis J. Taste and smell perception in the elderly: effect of medications and disease. Adv Food Nutr Res. 2002;44:247–346. doi: 10.1016/s1043-4526(02)44006-5. [DOI] [PubMed] [Google Scholar]
- 47.Zelig R., Jones V.M., Touger-Decker R., et al. The eating experience: adaptive and maladaptive strategies of older adults with tooth loss. JDR Clin Trans Res. 2019;4(3):217–228. doi: 10.1177/2380084419827532. [DOI] [PubMed] [Google Scholar]
- 48.Woda A., Foster K., Mishellany A., Peyron MA. Adaptation of healthy mastication to factors pertaining to the individual or to the food. Physiol Behav. 2006;89(1):28–35. doi: 10.1016/j.physbeh.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Frisancho AR. The study of human adaptation. Human Evolution Biol. 2010:17–28. [Google Scholar]
- 50.Goncalves T., Schimmel M., van der Bilt A., et al. Consensus on the terminologies and methodologies for masticatory assessment. J Oral Rehabil. 2021;48(6):745–761. doi: 10.1111/joor.13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Persic S., Palac A., Bunjevac T., Celebic A. Development of a new chewing function questionnaire for assessment of a self-perceived chewing function. Community Dent Oral Epidemiol. 2013;41(6):565–573. doi: 10.1111/cdoe.12048. [DOI] [PubMed] [Google Scholar]
- 52.Hsu K.J., Lee H.E., Lan S.J., Huang S.T., Chen C.M., Yen Y.Y. Evaluation of a self-assessed screening test for masticatory ability of Taiwanese older adults. Gerodontology. 2012;29(2):e1113–e1120. doi: 10.1111/j.1741-2358.2012.00632.x. [DOI] [PubMed] [Google Scholar]
- 53.Koshino H., Hirai T., Toyoshita Y., et al. Development of new food intake questionnaire method for evaluating the ability of mastication in complete denture wearers. Prosthodontic Res Pract. 2008;7(1):12–18. [Google Scholar]
- 54.Bauer J.M., Kaiser M.J., Anthony P., Guigoz Y., Sieber CC. The Mini Nutritional Assessment–its history, today's practice, and future perspectives. Nutr Clin Pract. 2008;23(4):388–396. doi: 10.1177/0884533608321132. [DOI] [PubMed] [Google Scholar]
- 55.Makhija S., Baker J. The Subjective Global Assessment: a review of its use in clinical practice. Nutr Clin Pract. 2008;23(4):405–409. doi: 10.1177/0884533608321214. [DOI] [PubMed] [Google Scholar]
- 56.Hu F.J., Liu H., Liu X.L., et al. Mid-upper arm circumference as an alternative screening instrument to appendicular skeletal muscle mass index for diagnosing sarcopenia. Clin Interv Aging. 2021;16:1095–1104. doi: 10.2147/CIA.S311081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawakami R., Murakami H., Sanada K., et al. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr Gerontol Int. 2015;15(8):969–976. doi: 10.1111/ggi.12377. [DOI] [PubMed] [Google Scholar]
- 58.Kumbuloglu O., Saracoglu A., Bingol P., Hatipoglu A., Ozcan M. Clinical study on the comparison of masticatory efficiency and jaw movement before and after temporomandibular disorder treatment. Cranio. 2013;31(3):190–201. doi: 10.1179/crn.2013.030. [DOI] [PubMed] [Google Scholar]
- 59.Kim H.E., Lee H. Factors affecting subjective and objective masticatory function in older adults: Importance of an integrated approach. J Dent. 2021;113 doi: 10.1016/j.jdent.2021.103787. [DOI] [PubMed] [Google Scholar]
- 60.Cusson V., Caron C., Gaudreau P., Morais J.A., Shatenstein B., Payette H. Assessing older adults' masticatory efficiency. J Am Geriatr Soc. 2015;63(6):1192–1196. doi: 10.1111/jgs.13443. [DOI] [PubMed] [Google Scholar]
- 61.Schmitter M., Keller L., Giannakopoulos N., Rammelsberg P. Chronic stress in myofascial pain patients. Clin Oral Investig. 2010;14(5):593–597. doi: 10.1007/s00784-009-0330-0. [DOI] [PubMed] [Google Scholar]
- 62.Treede R.D., Rief W., Barke A., et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11) Pain. 2019;160(1):19–27. doi: 10.1097/j.pain.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 63.Lobbezoo F., Delwel S., Weijenberg R.A.F., Scherder EJA. Orofacial pain and mastication in dementia. Curr Alzheimer Res. 2017;14(5):506–511. doi: 10.2174/1567205013666160602233535. [DOI] [PubMed] [Google Scholar]