Abstract
Background
Patients with obesity during childhood have an increased risk of fatal and non-fatal cardiovascular events during adulthood. The severity of obesity is commonly determined by BMI. However, children with relatively low BMI may have high cardiometabolic risk. Indeed, BMI-based obesity classifications might miss children at high cardiometabolic risk. Insulin has been suggested as a marker of cardiometabolic risk. In this study, we therefore estimated and compared cardiometabolic risk using either the BMI standard deviation score (BMI-SDS) or fasting insulin in an international cohort of children and adolescents with obesity and lean controls.
Methods
Study participants (712 with obesity and 99 lean controls), aged 3 to 18 years, were categorized according to their BMI-SDS as lean or obesity class I, II, or III, or by their fasting insulin quartiles as quartile 1, 2, 3, or 4 with the lean subjects in a separate control group. Prevalence of cardiometabolic risk factors was assessed in each group. Sensitivity and specificity analyses for cardiometabolic risk were conducted for both BMI-SDS and fasting insulin. Multiple regression, logistic regression, and receiver operating characteristic (ROC) analyses were performed between fasting insulin, BMI-SDS and cardiometabolic risk factors.
Results
An elevated prevalence of the cardiometabolic risk factors dyslipidemia, dysglycemia and hypertension was observed in both increasing BMI-SDS classes and increasing fasting insulin quartiles. Fasting insulin demonstrated higher areas under the curve (AUC) for detecting dyslipidemia, dysglycemia, and the combination of dyslipidemia, dysglycemia, and hypertension, compared to BMI-SDS. BMI-SDS demonstrated a higher AUC for detecting hypertension compared to fasting insulin. The same patterns were seen for the logistic regression. However, fasting insulin had an overall stronger association with the cardiometabolic risk factors studied compared to BMI-SDS.
Conclusions
In children and adolescents with obesity, fasting insulin provides complementary information to BMI-SDS in identifying those with elevated cardiometabolic risk factors. While neither marker alone offers strong predictive accuracy, incorporating fasting insulin into clinical assessment may help prioritize individuals who require more detailed evaluation. An elevated fasting insulin value may warrant further investigation among children and adolescents with obesity, independent of obesity class based on BMI-SDS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-025-02882-7.
Keyword: Obesity, Pediatric obesity, Hypertension, Cardiometabolic risk, Cardiovascular disease, Dysglycemia, Dyslipidemia, Insulin, Cardiovascular risk factors, BMI-SDS
Research insight
What is currently known about this topic?
BMI has several limitations and might underestimate cardiometabolic risk in children.
Insulin has been proposed as a biomarker to identify children at risk of cardiovascular disease.
What is the key research question?
Is fasting insulin a better predictor of cardiometabolic risk than BMI-SDS?
What is new?
Fasting insulin better predicted dyslipidemia and dysglycemia, in children with obesity.
Inflammation and hypertension were more strongly related to BMI-SDS.
Overall, fasting insulin better predicted cardiometabolic risk factors than BMI-SDS.
How might this study influence clinical practice?
Encourages incorporating fasting insulin into clinical practice when evaluating pediatric obesity.
Introduction
As the incidence of pediatric obesity increases, concerns about the cardiovascular health of patients with obesity during childhood grow. Despite data showing that children with obesity have high cardiovascular risk [1, 2], the disease was still severely undertreated in 2024 [3]. In adults, obesity is associated with major adverse cardiac events (MACE), which include death from cardiac causes, non-fatal myocardial infarction, and non-fatal stroke [4]. Obesity is also associated with other cardiovascular diseases such as heart failure with preserved ejection fraction (HFpEF), atrial fibrillation, hypertension, atherosclerosis, and coronary heart disease [5–8]. Compared to lean children, children and adolescents with obesity are five times more likely to have obesity as adults [9].
A high body-mass index (BMI) in childhood has been linked to an increased risk of future cardiovascular events. In a large prospective cohort study, pediatric BMI classified into four categories was associated with progressively higher hazard ratios (HR) for fatal and non-fatal cardiovascular events in adulthood [10]. Specifically, moving from one BMI class to the next corresponded to a 3.3-fold increase in HR for fatal cardiovascular events and a 3.9-fold increase for combined fatal and non-fatal events [10]. Furthermore, in the same study, individuals in the highest BMI class had an almost fivefold higher HR for cardiovascular events as adults [10]. Indeed, cardiovascular disease (CVD) is the leading cause of death in individuals with obesity [11, 12]. Moreover, cardiometabolic risk factors are present in children and adolescents with obesity, and the number of risk factors has been shown to increase with obesity class [2].
Yet, there are several limitations to using BMI at the individual level. For example, BMI does not account for the localization of excess fat in the body, such as the extent of ectopic fat in vital organs or abdominal fat [13, 14], or how that localization affects the cardiovascular risk [15]. Indeed, a high BMI does not necessarily correspond to a high cardiovascular risk in all individuals [15]. Also, a BMI-based classification of obesity has been shown to underestimate the prevalence of cardiometabolic risk during childhood [16]. Thus, in some countries, there has been a shift from BMI-based staging of obesity to the Edmonton Obesity Staging System (EOSS), which has a version adapted form for children, EOSS-P [17]. EOSS has been shown to better predict mortality in individuals living with obesity than BMI [18]. Nevertheless, EOSS-P is a tool that requires several pieces of information in order to stage the disease, which may not be easily available. While BMI remains the primary screening tool for obesity in the general pediatric population [19], it has limitations in identifying cardiometabolic risk severity among children who have obesity. More readily available biomarkers for risk assessment in pediatric obesity would enable better allocation of healthcare resources.
Insulin resistance has been proposed as a strong predictor of future cardiovascular events [20]. Even though fasting insulin is not considered a reliable marker of insulin sensitivity in children [21], elevated fasting insulin has been suggested as a possible marker for identifying children at high risk of developing CVD [22]. However, the relationship between fasting insulin and cardiometabolic risk in children and adolescents with obesity requires further investigation. Fasting insulin is not proposed as a diagnostic or stand-alone screening tool, but rather as a complementary biomarker to BMI-SDS that may help refine cardiometabolic risk stratification in children with obesity. The objectives of the present study were to investigate, firstly, the prevalence of cardiometabolic risk factors in children and adolescents with obesity depending on the class of obesity based on BMI-SDS. Secondly, the prevalence of cardiometabolic risk factors in children and adolescents with obesity based on quartiles of fasting insulin was investigated. Thirdly, multiple regression models were created to investigate the relationship between fasting insulin, BMI-SDS, and cardiometabolic risk factors. Lastly, we compared the sensitivity and specificity for predicting cardiometabolic risk factors, as determined by BMI-SDS or fasting insulin.
Methods
Study subjects
The study included children and adolescents with obesity from clinical obesity cohorts in Uppsala, Sweden, and Salzburg, Austria. Lean healthy controls were enrolled through a collaboration between local schools and the Universities, as well as through advertisements. The final study sample in this cross-sectional cohort study consisted of 811 individuals, of whom 712 had obesity and 99 did not. All data were collected at the first visit to the respective obesity clinic in Uppsala or Salzburg. The inclusion criteria were: under 18 years of age, age-adjusted BMI greater than 30, and acceptance of participation in the cohort. The exclusion criteria were: over 18 years of age, syndromic obesity, and age-adjusted BMI greater than 25 but less than 30, i.e., overweight.
Since the study sample consists of clinical data, some data points were missing. Therefore, the specific reported measurements have different sample sizes, which are presented in Supplementary Tables 1 and 2. To minimize the influence of acute infections, C-reactive protein (CRP) measurements above 10 mg/L were excluded, as previously discussed [23].
The study was performed in accordance with the Declaration of Helsinki, and approved by both the regional ethics committee in Uppsala (registration numbers 2010/036 and 2012/318) and Salzburg (registration number EK1544/2012). Written informed consent was obtained from adolescents 12 years or older and their legal guardians, or by the legal guardians in case of age under 12 years. Participation was voluntary, and participants could withdraw consent without providing a reason.
Subject stratification
Obesity was defined as BMI-SDS ≥ 2, as previously suggested [24]. Further sub-classification of obesity was based on the following categories: Class I (BMI-SDS ≥ 2, < 3), Class II (BMI-SDS ≥ 3, < 4), and Class III (BMI-SDS ≥ 4), corresponding to an adult BMI of above 30 but below 35 for Class I, above 35 but below 40 for Class II, and above 40 for Class III. The use of four classes of childhood obesity based on BMI-SDS has been investigated previously [25], but the differences between class III and IV were small; therefore we chose to use three classes of obesity, as has been done before in children [2], and is the classification used in adults [26]. Insulin quartiles were determined by calculating the quartiles of fasting insulin for all patients and then stratifying patients with obesity according to these quartiles. To facilitate comparison, we additionally performed a supplementary analysis using BMI-SDS quartiles, the results of which are presented in Supplementary Tables 4 and 5.
Subject characterization
All anthropometrical measurements were performed in accordance with clinical standards, as reported previously [27]. Body weight was measured to the nearest 0.1 kg using a calibrated electronic column scale, model 704 in Uppsala and model 801 in Salzburg (both: SECA, Hamburg, Germany). Height of the patient was determined to the nearest 0.1 cm with the patient standing barefoot using a stadiometer (Uppsala: Ulmer stadiometer, Busse, Elchingen, Germany; Salzburg: SECA, model 222 stadiometer, Hamburg, Germany). Height was measured twice and the mean value recorded. Waist circumference was determined horizontally using a flexible tape, anatomically midway between the arcus costalis inferior and the crista iliaca, having the patient standing with the feet together. Hip circumference was determined as the point of maximum girth of the buttocks. The sagittal abdominal diameter (SAD) was measured using an Abdometer (NovoNordisk, Bagsværd, Denmark) during a normal expiration, with the patient in the supine position with bent knees. Body mass index (BMI) was calculated as its definition, weight in kg divided by height in meters squared. Using Microsoft Excel (Microsoft Corporation, Redmond, WA) add-in LMS Growth, based on the WHO growth report v2.76, BMI-SDS was calculated.
Blood pressure was measured in mmHg after 5 min of rest, in either the left or right arm, while the patient was seated, using a standardized clinical sphygmomanometer with appropriate cuffs (Uppsala: CAS 740, CAS Medical Systems Inc., Branford, CT, USA; Salzburg: Carescape V100, Dinamap Technology/GE, Vienna, Austria).
Venous samples were drawn in the morning following an overnight fast. An oral glucose tolerance test (OGTT) was performed as previously described [28]. In short, a glucose dose of 1.75 g/kg body weight, up to a maximum of 75 g, was dissolved in water and consumed orally. Blood samples were collected via a venous catheter at fasting and at 120 min. All sampling was performed according to clinical routine.
Definitions of altered cardiometabolic risk factors were based on previous studies [2, 29] and were as follows: dyslipidemia was defined as total cholesterol levels > 5.18 mmol/L, or HDL cholesterol < 0.91 mmol/L, or LDL cholesterol > 3.37 mmol/L, or triglycerides > 1.70 mmol/L [25]. Non-HDL cholesterol was determined by subtracting HDL cholesterol from total cholesterol, and a value above 3.7 mmol/L was considered abnormal [29]. Both systolic and diastolic hypertension were defined as ≥ 95th percentile for age and sex, as previously described [25]. Dysglycemia, as used in the receiver operating characteristics (ROC) analysis, was defined as fasting glucose > 6.10 mmol/L, or 120-min glucose > 7.80 mmol/L, or HbA1c ≥ 42 mmol/mol, i.e. individuals with type 2 diabetes mellitus (T2DM) were also included.
Statistical analyses
Differences in proportions between groups were assessed using Fisher’s Exact Test for categorical variables where expected counts were small. For sex, a chi-square (χ2) test was performed. For other categorical comparisons, a chi-square test for equality of proportions with continuity correction was used, as the tables were expected to be 2 × 2. A one-way ANOVA followed by Tukey’s multiple comparison test was used for differences between continuous variables. Lean controls were excluded from the ANOVA. The Shapiro-Wilks tests and manual visualization of histograms were performed to assess normality of the variables. Multiple linear regression models were created to assess the associations between fasting insulin or BMI-SDS and the following variables: triglycerides, total-, LDL-, HDL-, and non-HDL cholesterol, HbA1c, as well as fasting glucose. This model was adjusted for age and sex. Another model, which adjusted for age, sex and height, was created to assess the association between fasting insulin or BMI-SDS with diastolic and/or systolic blood pressure. The following parameters were not normally distributed: age, height, fasting insulin, HDL-cholesterol, triglycerides, and fasting glucose. These were therefore logarithmically transformed prior to further analysis. Standardized β coefficients were calculated for the dependent and independent variables. Logistic regression analyses were performed in order to examine associations between BMI-SDS, fasting insulin and binary outcomes (dyslipidemia, dysglycemia, hypertension, and a combination of the three). ROC curve analyses were then performed using BMI-SDS and fasting insulin as continuous predictor variables to evaluate their ability to discriminate between individuals with and without dyslipidemia, dysglycemia, hypertension, or a combination of the three outcomes. The point with the highest combined specificity and sensitivity was determined from the ROC coordinates using the index described by Youden [30]. DeLong’s test was used to compare differences between two ROC areas under the curve (AUC). All statistical analyses were performed using RStudio v2024.09.01 (RStudio, PBC, Boston, MA) and GraphPad Prism v10.4.0 (GraphPad Software Inc., La Jolla, CA). P values below 0.05 were considered statistically significant.
Results
Patient characteristics
When children and adolescents were stratified according to BMI-SDS classes, 302 patients were classified as obesity class I, 368 as obesity class II, and 44 as obesity class III (Table 1). When children and adolescents with obesity were divided into quartiles according to fasting insulin levels (Table 2), 187 were classified as quartile 1 (Q1), 175 as quartile 2 (Q2), 176 as quartile 3 (Q3), and 174 as quartile 4 (Q4). The ranges of fasting insulin for each quartile were as follows: Q1: 0–13.89 mIU/L; Q2: 13.90–19.67 mIU/L; Q3: 19.68–30 mIU/L, and Q4: above 30 mIU/L. The material was not divided by the number of patients, but rather according to their insulin levels, which is why the quartiles vary in the number of patients.
Table 1.
Clinical characteristics stratified by BMI-SDS
| Parameter | Lean (n = 99) | Obesity class I (n = 302) | Obesity class II (n = 368) | Obesity class III (n = 44) | p-value |
|---|---|---|---|---|---|
| Age (years) | 13.6 (3.0) | 13.2 (2.6) | 13.1 (2.8) | 11.1 (4.9)a | < 0.0001 |
| Sex (% male) | 53.5 | 48.0 | 59.0 | 68.2 | n.s |
| Weight (kg) | 49.7 (15.1)a | 76.8 (18.2)a | 94.4 (26.2) | 99.1 (57.2) | < 0.0001 |
| Height (cm) | 160.0 (18.1) | 161.1 (12.8) | 162.5 (13.9) | 149.5 (23.7)a | < 0.0001 |
| BMI (kg/m2) | 18.8 (2.6)a | 29.1 (3.3)a | 34.9 (5.2)a | 39.9 (12.8)a | < 0.0001 |
| BMI-SDS | -0.2 (0.7)a | 2.6 (0.3)a | 3.3 (0.3)a | 4.4 (0.63)a | < 0.0001 |
| Systolic blood pressure (mmHg) | 108.5 (8.3)a | 117.2 (11.9)a | 123.1 (12.4) | 123.4 (16.9) | < 0.0001 |
| Diastolic blood pressure (mmHg) | 63.3 (8.3)a | 67.2 (9.6)a | 72.1 (9.6) | 73.9 (13.7) | < 0.0001 |
| LDL cholesterol (mmol/L) | 2.0 (0.5)a | 2.5 (0.8) | 2.6 (0.8) | 2.8 (0.7) | 0.01 |
| HDL cholesterol (mmol/L) | 1.5 (0.3)a | 1.2 (0.3) a | 1.1 (0.3) | 1.1 (0.2) | < 0.0001 |
| Triglycerides (mmol/L) | 0.7 (0.3)a | 1.2 (0.6) | 1.3 (0.8) | 1.3 (0.6) | 0.018 |
| Total cholesterol (mmol/L) | 3.9 (0.7)a | 4.2 (0.8) | 4.2 (0.8) | 4.4 (0.8) | n.s |
| Non-HDL cholesterol (mmol/L) | 2.4 (0.6)a | 3.0 (0.8) | 3.0 (0.9) | 3.3 (0.8) | 0.01 |
| Apolipoprotein A1 (g/L) | 1.4 (0.3)a | 1.3 (0.2) | 1.3 (0.2) | 1.2 (0.1) | 0.038 |
| Apolipoprotein B (g/L) | 0.6 (0.1)a | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | n.s |
| C-reactive protein (mg/L) | 0.7 (1.4)a | 2.3 (2.0)a | 3.5 (2.5) | 4.0 (3.0) | < 0.0001 |
| White blood cell count (109/L) | 5.5 (1.6)a | 6.9 (1.6)a | 7.4 (2.0) | 7.9 (2.0) | < 0.0001 |
| HbA1c (mmol/mol) | 34.2 (2.1) | 34.9 (3.9) | 35.5 (4.9) | 34.9 (3.3) | n.s |
| Fasting glucose (mmol/L) | 5.0 (0.6) | 5.2 (0.8) | 5.4 (1.0) | 5.4 (0.8) | 0.0013 |
| 120 min glucose (mmol/L) | 6.1 (1.1)a | 6.9 (1.8) | 7.4 (1.7) | 7.4 (1.7) | 0.003 |
| Fasting insulin (mIU/L) | 8.2 (4.0)a | 21.6 (24.4) | 27.2 (18.8) | 24.7 (24.0) | 0.004 |
| 120 min insulin (mIU/L) | 39.7 (26.1)a | 109.3 (94.4) | 157.4 (125.9) | 120.4 (97.4) | < 0.0001 |
| Waist circumference (cm) | 69.3 (8.3)a | 97.0 (10.2)a | 109.8 (14.1) | 112.0 (34.4) | < 0.0001 |
| Hip circumference (cm) | 83.9 (11.8)a | 103.0 (11.4) a | 112.3 (13.9) | 114.0 (28.5) | < 0.0001 |
| Waist/hip-ratio | 0.8 (0.1)a | 0.95 (0.1)a | 0.98 (0.1) | 1.0 (0.1) | < 0.0001 |
| Waist/height-ratio | 0.43 (0.03)a | 0.60 (0.05)a | 0.68 (0.05)a | 0.74 (0.15)a | < 0.0001 |
| Sagittal abdominal diameter (cm) | 16.7 (2.0)a | 22.2 (2.3)a | 24.9 (3.0)a | 26.5 (6.3)a | < 0.0001 |
| Creatinine (µmol/L) | 59.6 (15.7)a | 54.5 (11.5) | 54.1 (14.1) | 48.0 (15.9)a | < 0.01 |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 111.8 (20.2)a | 100.2 (17.0) | 96.8 (15.5) | 95.2 (20.0) | 0.014 |
All values are presented as mean ± standard deviation, if not otherwise stated. p-values from one-way ANOVA or χ2 test
asignificant different from all other groups in the post hoc analysis
Table 2.
Clinical characteristics as stratified by fasting insulin quartiles
| Parameter | Lean (n = 99) | Quartile 1 (n = 187) | Quartile 2 (n = 175) | Quartile 3 (n = 176) | Quartile 4 (n = 174) | p-value |
|---|---|---|---|---|---|---|
| Age (years) | 13.6 (3.0) | 11.7 (3.2)a | 13.1 (3.0) | 13.4 (2.5) | 13.9 (2.4) | < 0.0001 |
| Sex (% male) | 53.5 | 57.2 | 53.1 | 55.7 | 52.9 | n.s |
| Weight (kg) | 49.7 (15.1)a | 71.4 (24.0)a | 83.8 (25.3)a | 92.9 (27.2)a | 102.1 (25.3)a | < 0.0001 |
| Height (cm) | 160.0 (18.1) | 153.5 (16.0) a | 160.6 (14.5) | 164.2 (12.4) | 166.5 (11.1) | < 0.0001 |
| BMI (kg/m2) | 18.8 (2.6)a | 29.4 (5.1)a | 31.8 (5.4)a | 33.8 (6.6)a | 36.3 (6.0)a | < 0.0001 |
| BMI-SDS | -0.2 (0.7)a | 3.0 (0.6) | 3.0 (0.6) | 3.1 (0.5) | 3.3 (0.5) | < 0.0001 |
| Systolic blood pressure (mmHg) | 108.5 (8.3)a | 116.4 (11.5) | 118.4 (12.5) | 121.6 (11.9) | 126.1 (13.2)a | < 0.0001 |
| Diastolic blood pressure (mmHg) | 63.3 (8.3)a | 67.9 (10.1) | 68.5 (9.7) | 69.5 (9.8) | 74.5 (9.8)a | < 0.0001 |
| LDL cholesterol (mmol/L) | 2.0 (0.5)a | 2.4 (0.8) | 2.5 (0.8) | 2.5 (0.7) | 2.7 (0.7) | 0.016 |
| HDL cholesterol (mmol/L) | 1.5 (0.3)a | 1.3 (0.4) a | 1.2 (0.3) | 1.1 (0.2) | 1.1 (0.3) | < 0.0001 |
| Triglycerides (mmol/L) | 0.7 (0.3)a | 1.0 (0.6) a | 1.2 (0.6) | 1.3 (0.6) | 1.6 (0.9)a | < 0.0001 |
| Total cholesterol (mmol/L) | 3.9 (0.7) | 4.2 (0.8) | 4.2 (0.9) | 4.1 (0.8) | 4.3 (0.8) | 0.22 |
| Non-HDL cholesterol (mmol/L) | 2.4 (0.6)a | 2.9 (0.9) | 3.0 (0.9) | 3.0 (0.8) | 3.2 (0.8) | 0.0005 |
| Apolipoprotein A1 (g/L) | 1.4 (0.3)a | 1.3 (0.2) | 1.3 (0.2) | 1.2 (0.2) | 1.2 (0.2) | 0.0006 |
| Apolipoprotein B (g/L) | 0.6 (0.1)a | 0.7 (0.2) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | < 0.0001 |
| C-reactive protein (mg/L) | 0.7 (1.4)a | 2.6 (2.3) | 2.9 (2.2) | 3.0 (2.5) | 3.6 (2.7) | 0.002 |
| White blood cell count (109/L) | 5.5 (1.6)a | 6.7 (1.7) | 7.3 (1.7) | 7.1 (1.6) | 8.0 (2.0) a | < 0.0001 |
| HbA1c (mmol/mol) | 34.2 (2.1) | 34.3 (3.9) | 34.6 (2.6) | 34.8 (3.1) | 37.2 (6.5)a | < 0.0001 |
| Fasting glucose (mmol/L) | 5.0 (0.6) | 5.1 (1.0) | 5.2 (0.6) | 5.3 (0.7) | 5.7 (1.2)a | < 0.0001 |
| 120 min glucose (mmol/L) | 6.1 (1.1) | 6.6 (1.3) | 6.9 (1.3) | 7.1 (1.2) | 8.2 (2.5)a | < 0.0001 |
| Fasting insulin (mIU/L) | 8.2 (4.0) | 9.8 (3.4) | 17.1 (1.6) a | 24.5 (2.9) a | 48.6 (32.4) a | < 0.0001 |
| 120 min insulin (mIU/L) | 39.7 (26.1) | 63.1 (42.9) | 99.9 (66.8)a | 136.2 (72.0)a | 241.4 (152.4)a | < 0.0001 |
| Waist circumference (cm) | 69.3 (8.3)a | 95.4 (15.8)a | 102.1 (13.8)a | 107.8 (14.1)a | 114.2 (14.4) a | < 0.0001 |
| Hip circumference (cm) | 83.9 (11.8)a | 100.5 (14.4)a | 106.7 (14.9)a | 112.0 (13.7) | 115.8 (12.0) | < 0.0001 |
| Waist/hip-ratio | 0.8 (0.1)a | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) | 0.005 |
| Waist/height-ratio | 0.43 (0.03)a | 0.62 (0.08) | 0.64 (0.06) | 0.66 (0.07) | 0.69 (0.07)a | < 0.0001 |
| Sagittal abdominal diameter (cm) | 16.7 (2.0)a | 22.0 (2.9)a | 23.2 (2.8)a | 24.4 (3.2)a | 26.4 (3.1)a | < 0.0001 |
| Creatinine (µmol/L) | 59.6 (15.7) | 50.3 (12.0)a | 54.4 (12.5) | 54.8 (12.1) | 56.0 (15.5) | 0.0002 |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 111.8 (20.2)a | 103.2 (16.9)a | 97.9 (15.5) | 97.1 (16.3) | 94.1 (16.0) | < 0.0001 |
All values are presented as mean ± standard deviation, if not otherwise stated. p-values from one-way ANOVA or χ2 test
asignificant different from all other groups in the post hoc analysis
The patients in obesity class III were, on average, younger (11.1 ± 4.9 years of age), shorter (149.5 ± 23.7 cm of height), and had a higher BMI (39.9 ± 12.8 kg/m2) than the other patients and controls (Table 1). The patients in Q1 were on average younger (11.7 ± 3.2 years of age) than the other quartiles (Table 2). Patients in Q4 were on average heavier (102.1 ± 25.3 kg), with a higher BMI (36.3 ± 6.0 kg/m2), larger waist circumference (114.2 ± 14.4 cm), a greater sagittal abdominal diameter (26.4 ± 3.1 cm), but did not have a higher BMI-SDS (3.3 ± 0.5), than the other quartiles (Table 2).
Cardiometabolic risk factors
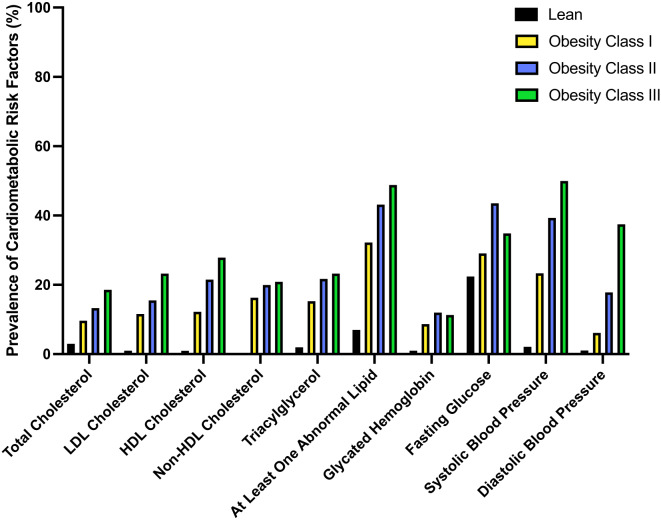
Patients stratified into obesity class II or III were more likely to have at least one abnormal blood lipid (43% and 49% of patients, respectively, p < 0.05 in both cases), compared to lean controls or patients considered to have obesity class I (7% and 32%, respectively), as illustrated in Fig. 1. Obesity class II differed in cardiometabolic risk factors from obesity class III only in the prevalence of diastolic hypertension (18% and 38%, respectively, Fig. 1). The patients in insulin Q4 were more likely to have at least one abnormal blood lipid (56% of patients), as well as more likely to have elevated triglycerides (32%), lowered HDL-cholesterol (30%), elevated HbA1c (21%), elevated fasting glucose (51%), systolic hypertension (46%), and diastolic hypertension (22%), compared to all other quartiles and the lean controls (Fig. 2). Indeed, the patients in insulin Q4 had, on average, a larger number of deviating cardiometabolic risk factors than did patients in other quartiles or lean controls (average 2.6 ± 1.9 risk factors; p < 0.0001 for all comparisons; Fig. 2). There were significantly more patients in insulin Q4 than in obesity class III (174 vs. 44, p = < 0.0001; data not shown).
Fig. 1.
Prevalence of cardiometabolic risk factors, stratified by obesity class based on BMI-SDS. Black bars = lean controls, yellow bars = obesity class I, blue bars = obesity class II, and green bars = obesity class III
Fig. 2.
Prevalence of cardiometabolic risk factors, stratified by obesity class based on quartiles of fasting insulin. Black bars = lean controls, yellow bars = quartile 1, blue bars = quartile 2, green bars = quartile 3, and red bars = quartile 4
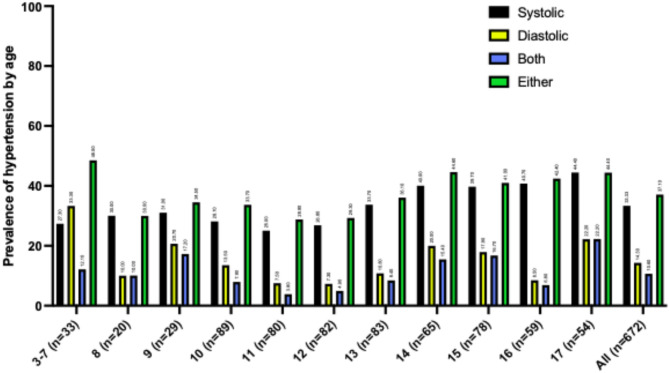
Blood pressure recordings were obtained from 672 children and adolescents with obesity aged 3 to 18 years (Fig. 3). The number of subjects in the 3–7 years old group was limited, so they were combined into one group (n = 33). For the groups aged 8–17 years, the number of subjects per age group ranged from 20 to 89. Fishers Exact test indicated a significant difference between groups (p < 0.0001). The prevalence of systolic hypertension across age groups increased from 27.3% in the 3–7-year group to 44.4% in the 17-year-old group. However, for diastolic hypertension, the prevalence decreasing slightly from 33.3% in 3–7-year-olds to 22.2% in 17-year-olds. Additionally, the combined prevalence of both systolic and diastolic hypertension with prevalence ranging from 12.1% in 3–7-year-olds to 22.2% in 17-year-olds. Finally, the prevalence of either systolic or diastolic hypertension showed prevalences slightly decreasing from 48.5% in 3–7-year-olds to 44.4% in 17-year-olds.
Fig. 3.
Prevalence of hypertension in children and adolescents with obesity in different age groups. Within each group, the data is divided as follows: black bars = prevalence of systolic hypertension, yellow bars = prevalence of diastolic hypertension, blue bars = prevalence of combined systolic and diastolic hypertension, and green bars = either systolic hypertension, diastolic hypertension, or a combination of both. Numbers above the bars indicate the percentage of prevalence
Multiple regression statistics for correlations between fasting insulin, BMI-SDS, and cardiometabolic risk markers are presented in Table 3. After adjusting for age and sex, both fasting insulin and BMI-SDS were significantly associated with triglycerides, LDL-cholesterol, HDL-cholesterol, and non-HDL cholesterol. Fasting insulin was significantly association with HbA1c and fasting glucose; BMI-SDS was not. BMI-SDS was significantly correlated with CRP, fasting insulin was not. The model adjusting for age, sex, and height found a significant relationship between fasting insulin and both systolic and diastolic blood pressure, as well as between BMI-SDS and both systolic and diastolic blood pressure.
Table 3.
Standardized β coefficients for the associations between fasting insulin, BMI-SDS, and markers of cardiometabolic risk in children and adolescents with obesity
| Biomarker | Predictor | Beta | p-value |
|---|---|---|---|
| Triglycerides | Fasting insulin | 0.43 | < 0.00001 |
| Triglycerides | BMI-SDS | 0.10 | 0.006 |
| Total cholesterol | Fasting insulin | 0.07 | 0.25 |
| Total cholesterol | BMI-SDS | 0.02 | 0.62 |
| LDL cholesterol | Fasting insulin | 0.14 | 0.025 |
| LDL cholesterol | BMI-SDS | 0.09 | 0.024 |
| HDL cholesterol | Fasting insulin | − 0.31 | < 0.00001 |
| HDL cholesterol | BMI-SDS | -0.22 | < 0.00001 |
| Non-HDL cholesterol | Fasting insulin | 0.17 | 0.006 |
| Non-HDL cholesterol | BMI-SDS | 0.10 | 0.012 |
| HbA1c | Fasting insulin | 0.31 | < 0.00001 |
| HbA1c | BMI-SDS | − 0.03 | 0.44 |
| Fasting glucose | Fasting insulin | 0.30 | < 0.00001 |
| Fasting glucose | BMI-SDS | 0.07 | 0.053 |
| Systolic blood pressure | Fasting insulin | 2.36 | 0.0008 |
| Systolic blood pressure | BMI-SDS | 3.44 | < 0.00001 |
| Diastolic blood pressure | Fasting insulin | 2.1 | 0.0006 |
| Diastolic blood pressure | BMI-SDS | 2.97 | < 0.00001 |
| C-reactive protein | Fasting insulin | 0.03 | 0.43 |
| C-reactive protein | BMI-SDS | 0.38 | < 0.00001 |
Risk prediction
The logistic regression models are presented in Table 4. While each unit increase in BMI-SDS (i.e., increasing one class of obesity), significantly increased the odds of hypertension by 267% (p < 0.0001) and the odds of having a combination of hypertension, dyslipidemia and dysglycemia by 224% (p = 0.04), isolated dysglycemia or dyslipidemia was not significantly predicted by increasing 1 unit of BMI-SDS. Each unit increase in fasting insulin increased the risk of dyslipidemia by 3.4% (p < 0.0001), dysglycemia by 3.9% (p < 0.0001), and the combination of hypertension, dyslipidemia and dysglycemia by 2% (p = 0.0005). One unit increase in fasting insulin did not significantly increase the odds ratio of hypertension.
Table 4.
Logistic regression models for the associations between fasting insulin, BMI-SDS, and dyslipidemia, impaired glucose tolerance and hypertension in children and adolescents with obesity
| Model | Variable | Coefficient | Odds ratio | p-value |
|---|---|---|---|---|
| Dyslipidemia | Sex | − 0.2135 | 0.808 | 0.20864 |
| BMI-SDS | 0.3302 | 1.391 | 0.08523 | |
| Fasting insulin | 0.0339 | 1.034 | < 0.00001 | |
| Age | 0.0281 | 1.029 | 0.38358 | |
| Dysglycemia | Sex | − 0.5007 | 0.6061 | 0.00605 |
| BMI-SDS | 0.1889 | 1.208 | 0.35628 | |
| Fasting insulin | 0.0385 | 1.039 | < 0.00001 | |
| Age | 0.0872 | 1.091 | 0.01295 | |
| Hypertension | Sex | 0.0523 | 1.054 | 0.77742 |
| BMI-SDS | 0.9833 | 2.673 | < 0.00001 | |
| Fasting insulin | 0.0065 | 1.007 | 0.10539 | |
| Age | 0.0219 | 1.022 | 0.67418 | |
| Height | 0.0031 | 1.003 | 0.77747 | |
| All three | Sex | − 0.7260 | 0.48 | 0.044 |
| BMI-SDS | 0.8080 | 2.24 | 0.0392 | |
| Fasting insulin | 0.0203 | 1.02 | 0.0005 | |
| Age | 0.2347 | 1.26 | 0.00067 |
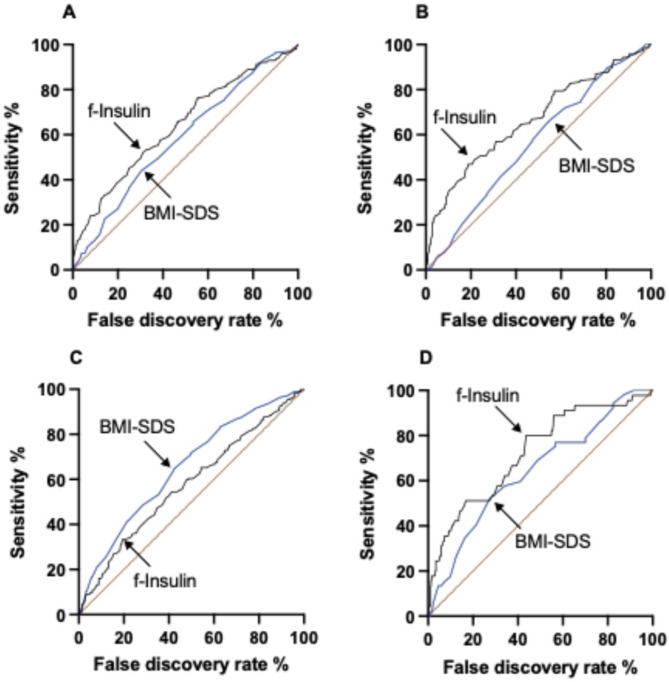
When investigating dyslipidemia, the ROC AUC of fasting insulin was larger (0.64 vs. 0.58) than that of BMI-SDS (Fig. 4A, p = 0.002). The Youden cutoffs with the highest combined sensitivity and specificity, the AUC, as well as the sensitivity and specificity of each analysis is presented in Table 5. For predicting dysglycemia, fasting insulin had a higher ROC AUC (0.67 vs. 0.56) than BMI-SDS (Fig. 4B, p = 0.003). Fasting insulin had a smaller ROC AUC (0.58 vs. 0.65) for predicting hypertension, than BMI-SDS (Fig. 4C p = 0.012). For predicting the combination of hypertension, dyslipidemia and dysglycemia, fasting insulin had a higher ROC AUC (0.72 vs. 0.64) than BMI-SDS (Fig. 4D, p < 0.0001). When looking at the material only in the group with class I obesity (supplementary Table 2), the ROC AUC was greater for BMI-SDS than for fasting insulin with regards to hypertension (0.60 vs. 0.57, p < 0.0001), but smaller for dysglycemia (0.54 vs. 0.67, p < 0.0001) as well as dyslipidemia (0.56 vs. 0.60, p < 0.0001). No lean controls were included in any of the ROC analyses.
Fig. 4.
Receiver operating characteristic curves for cutoffs of fasting plasma insulin (black line) and BMI-SDS (blue line) as predictors of A dyslipidemia, B dysglycemia, C hypertension, and D a combination of the three in obese adolescents. Arrows indicate optimal cutoffs as defined by Youden’s index
Table 5.
Receiver operating characteristics of fasting insulin and BMI-SDS in predicting dyslipidemia, dysglycemia, hypertension, and the combined outcome of hypertension, dyslipidemia, and dysglycemia
| Outcome | Test | AUC | Youden cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Dyslipidemia | Fasting Insulin | 0.64 | 23.58 mIU/L | 52.2% (46.0–58.2%) | 68.8% (64.1–73.2%) |
| BMI-SDS | 0.58 | 3.25 units | 44.0% (38.3–49.9%) | 69.5% (65.0–73.7%) | |
| Dysglycemia | Fasting Insulin | 0.67 | 27.89 mIU/L | 46.9% (40.2–53.7%) | 81.3% (77.4–84.6%) |
| BMI-SDS | 0.56 | 2.93 units | 66.1% (59.7–72.0%) | 45.4% (41.0–49.9%) | |
| Hypertension | Fasting Insulin | 0.58 | 30.05 mIU/L | 33.5% (27.6–39.9%) | 80.5% (76.4–84.1%) |
| BMI-SDS | 0.65 | 3.01 units | 64.5% (58.5–70.2%) | 57.8% (53.1–62.4%) | |
| All three | Fasting Insulin | 0.72 | 20.92 mIU/L | 80.0% (66.2–89.1%) | 56.4% (52.4–60.3%) |
| BMI-SDS | 0.64 | 3.37 units | 51.9% (38.7–64.9%) | 72.7% (69.1–75.9%) |
Sensitivity and specificity is presented with its 95% confidence interval in parenthesis
AUC, Area under the curve
Discussion
Cardiometabolic risk factors such as hyperinsulinemia, dyslipidemia, dysglycemia and hypertension are seen in children and adolescents with obesity [10, 25]. While there is variability in the development of cardiometabolic complications in these patients [10], there is a clear association between the number of cardiometabolic risk factors in childhood and the presence of atherosclerosis in the coronary arteries in young adulthood [31]. In order to guide treatment efforts and minimize future complications, tools that can identify individuals at high risk early on are needed. Commonly, BMI-based measures are used to identify such subjects. However, BMI-SDS has limitations in children and adolescents with severe obesity and may fail to identify those at elevated cardiovascular risk [16, 32]. Indeed, it has previously been proposed that a patient's individual cardiometabolic risk, rather than BMI alone, should guide treatment decisions such as bariatric surgery and/or pharmacotherapy [25].
In the present study, we tested whether BMI-SDS or hyperinsulinemia better predicted dysglycemia, dyslipidemia, and hypertension in children and adolescents with obesity. Whereas younger individuals at risk might benefit from staging the disease according to BMI-SDS, a greater number of individuals appear to be stratified as high-risk if staging the disease based on fasting insulin. We emphasize that fasting insulin is not intended to function as an independent diagnostic or screening tool, but rather as an adjunct to BMI measures that may enhance cardiometabolic risk stratification in pediatric obesity. Indeed, our study shows that obesity class III is associated with dyslipidemia in children as young as 11 years old. It is well-known that obesity is associated with increased cardiometabolic risk, and our findings are in line with previous studies in pediatric obesity [2, 25]. However, the associations between cardiometabolic risk factors and fasting insulin as a tool to help guide the staging of cardiometabolic risk remain less explored.
Hyperinsulinemia has long been proposed as an independent risk factor for ischemic heart disease [33]. Notably, fasting insulin has previously been included in cardiac risk scores [34]. Several prospective and cross-sectional studies in adults suggest that insulin resistance and hyperinsulinemia are major cardiometabolic risk factors, independent of other risk factors, even in individuals without diabetes mellitus [35]. Indeed, prediabetic individuals with insulin resistance show a worse cardiometabolic profile than prediabetic individuals without insulin resistance [36]. In the San Antonio Heart Study, fasting hyperinsulinemia was independently associated with elevated cardiovascular risk factors in adults without diabetes [37]. However, other studies in adults have not found an association between fasting insulin and CVD [38, 39]. Nevertheless, in the Department of Veterans Affairs High-Density Lipoprotein Intervention Trial, nondiabetic adult patients in the highest quartile of fasting insulin had a higher incidence of MACEs than those in the other quartiles of fasting insulin, which was not seen for quartiles of fasting glucose [40]. A study in children with overweight found an increase in cardiovascular risk factors with increasing quartiles of both fasting and 2-h insulin, although no comparison with BMI-SDS was made [41]. In line with this, the results from the present study show that, when stratifying children and adolescents with obesity by fasting insulin rather than BMI-SDS, a larger number of individuals with multiple cardiovascular risk factors are identified, which lends support to the role of fasting insulin as a potential biomarker for disease severity in childhood obesity. Therefore, longitudinal studies exploring fasting insulin as a predictor of future morbidity and mortality would be of interest. We recognize that fasting insulin is not considered a definitive marker of insulin resistance in children due to physiological factors and the absence of standardized clinical thresholds [21]. However, fasting insulin remains a clinically practical measurement, due to its simplicity. Our findings suggest that fasting insulin complements BMI by improving risk stratification in children and adolescents with obesity. While fasting insulin cannot replace direct measures of insulin sensitivity, its inclusion in clinical assessments may enhance identification of individuals at elevated cardiometabolic risk. In addition, puberty strongly influences insulin levels, via the physiological insulin resistance induced during this time period. The study presented here did not aim to investigate the biological nature of the association, but rather the usefulness of fasting insulin as a biomarker for disease severity. The results presented here should be further investigated different age groups, Tanner stages and sexes due to the highly likely impact of puberty on the insulin levels.
Two-hour glucose has been proposed to be better than fasting glucose at identifying patients who are at high cardiovascular risk [35]. In the present study, fasting insulin Q4 had significantly higher 2-h glucose than the other quartiles, while BMI-SDS class III did not differ from class I or II in 2-h glucose. This suggests that hyperinsulinemia at fasting might better than BMI-SDS identify individuals with obesity at high risk of future cardiac events than does BMI-SDS.
Elevated triglycerides are associated with obesity during childhood [42], and have been coupled with incident CVD [38, 39]. Indeed, both elevated triglycerides and total cholesterol during childhood increase the risk of both fatal and non-fatal cardiovascular events in adulthood [10]. In the present study, severity of obesity classified by BMI-SDS failed to distinguish elevated triglycerides in plasma between groups, whereas individuals in Q4 of fasting insulin had markedly higher triglycerides than those in the other quartiles of fasting insulin. Indeed, multiple regression analysis found that fasting insulin is more strongly associated with elevated triglycerides than BMI-SDS. High levels of insulin stimulate lipogenesis, when glucose and fatty acids are converted into triglycerides in the liver and adipose tissue [43], and inhibit lipolysis in adipose tissue [44], which provides a plausible mechanistic explanation for this observation.
Triglycerides are incorporated into VLDL-cholesterol molecules [45] and further processed into LDL-cholesterol [45]. LDL particles, for which LDL-cholesterol can be measured as a proxy, contribute to the atherosclerotic process of the vessel wall [46]. In this study, BMI-SDS and fasting insulin both positively correlated with LDL-cholesterol. However, in multiple regression analysis LDL-cholesterol was better associated with fasting insulin than with BMI-SDS. Elevated levels of LDL-cholesterol during childhood would increase both the rate of plaque progression and the future size of the plaque burden [46]. Indeed, children with obesity have been shown to have a 3.34 HR for a fatal cardiovascular event during adulthood, and these events occurred at an average age of 47 years [10], which is considerably earlier than the average fatal cardiovascular event in the general population [47]. Autopsies performed on teenagers and young adults, who died from non-cardiac causes, revealed early accumulation of atherosclerotic plaques in arteries, and that the size and complexity of these plaques seemed to increase with age [48]. Thus, it appears that LDL-trapping in the arterial wall begins already early in life, and that the atherosclerotic plaques progressively enlarge long before MACEs begin to occur [46]. Taken together, there is a possible mechanistic link between elevated insulin, dyslipidemia, and the pathophysiology of the atherosclerotic process. In fact, each unit increase in insulin significantly increased the likelihood of identifying a patient with dyslipidemia, while one unit of increase in BMI-SDS did not. Notably, insulin shows large biological variation in our study (standard deviation of 21 mIU/L). Hence, a 1 mIU/L increase in insulin is relatively small, whereas an increase in 1 BMI-SDS is equivalent to moving up to the next obesity class.
Dysglycemia, manifested as impaired glucose tolerance and T2DM, occurs in children and adolescents with obesity [49, 50]. Patients diagnosed with T2DM live with an elevated risk of cardiovascular events [51], and the risk for cardiovascular events occurs at lower LDL-cholesterol levels than for individuals without T2DM [52]. Indeed, a large population study of individuals without symptoms of angina pectoris, which investigated the atherosclerotic burden in the coronary and carotid arteries using computed tomography and ultrasound, showed increased atherosclerotic burden with increasing degrees of glycemic intolerance [53]. In the present study, fasting insulin was better at predicting dysglycemia and more strongly associated with fasting glucose as well as HbA1c, than was BMI-SDS. As with dyslipidemia, one unit increase in fasting insulin had a significant effect on the odds ratio of having dysglycemia, while one unit increase in BMI-SDS did not.
Hypertension is an important factor in the development of cardiovascular disease. Systolic hypertension increases both the transcytosis of LDL into the arterial wall [46] and arterial injury [54]. Hypertension during adolescence increases the risk of renal disease and CVD, as well as all-cause mortality [55, 56]. Indeed, a longitudinal study in 1,897,048 adolescents aged 16–19 showed that a diastolic blood pressure above 80 mmHg was associated with an increased risk of stroke before the age of 52 [57]. In addition to hypertension [58, 59], obesity is emerging as an independent risk factor for HFpEF [59, 60]. The findings from this and other studies [25], demonstrating that children and adolescents with obesity develop hypertension at remarkably young ages, are alarming. Since exposure time to both obesity and hypertension increases the risk of developing HFpEF, early diagnosis and intensification of treatment during youth are warranted [15]. The link between obesity in childhood and hypertension is well known and related to a higher BMI [25, 61] and our results show that BMI-SDS is better than fasting insulin at predicting hypertension.
Obesity class III is associated with many diseases. A recent study of 270,657 adults showed that the associations between class III and comorbidities are strongest with obstructive sleep apnea, T2DM, and MASLD, but weaker for atherosclerotic CVD [62]. This aligns with our findings that obesity class based on BMI might not be optimal for identifying patients at high cardiometabolic risk.
Obesity treatments, such as promoting health behavior and lifestyle treatment, incretin therapy, and bariatric surgery, improves the lipid profile in children and adolescents [63–65], as well as in adults [51]. Indeed, successful treatment of pediatric obesity lowers the risks of both morbidity and mortality in the early years of adulthood [66]. These observations underscore the need for intense and early treatment of pediatric obesity, and the allocation of resources for early prevention. Since reducing the cumulative exposure to LDL-cholesterol slows atherosclerotic progression, and this process begins early in life, it would be valuable to explore the potential benefits of lowering LDL-cholesterol earlier than current clinical guidelines recommend. Obesity class I in children is commonly identified in primary health care [17]. When performing the ROC analyses in this subgroup, fasting insulin better predicted cardiometabolic health than BMI-SDS did. This emphasizes the usefulness of fasting insulin determining which patients in primary care should be referred for further evaluation.
This study has several strengths; it includes a relatively large sample of children and adolescents with obesity from two clinics, as well as age-matched lean controls. The material includes a broad set of cardiometabolic risk factors, which provides a comprehensive assessment of cardiometabolic health. However, the study is limited by its cross-sectional design, which prevents the investigation of causal links in the data. Additionally, CVD outcomes are not available. Also, there are missing data in the material, which can introduce bias. A key limitation of our study is the potential confounding effect of puberty on fasting insulin levels, as insulin resistance naturally increases during puberty in both children with obesity and normal weight. While we adjusted for age and sex in our analyses, and for height when assessing blood pressure, these adjustments may not fully capture the complex, nonlinear physiological changes associated with pubertal stages. Unfortunately, Tanner staging data were not available in this study, preventing more precise adjustment for pubertal status. Future longitudinal studies aimed at validating the predictive utility of fasting insulin over time are warranted. These studies should incorporate standardized pubertal staging to account for physiological insulin resistance during adolescence, in order to better delineate the impact of puberty on fasting insulin levels and improve cardiometabolic risk stratification in pediatric obesity. Furthermore, the relatively small number of participants in the lean control group and in Class III obesity may limit the statistical power to detect differences within these subgroups. In addition, the study is conducted on a highly selected group of patients from tertiary care, and which limits the generalizability of the findings. Importantly, fasting insulin is not meant to replace direct measurement of lipids, blood pressure, or glucose, but rather to serve as a complementary marker to BMI-based measures. Similar to BMI-SDS, which is not interpreted in isolation, fasting insulin may add clinical value by identifying early metabolic alterations, such as compensatory hyperinsulinemia, that can precede overt cardiometabolic disease. It may help identify children with obesity who are at higher risk, capturing early signs that BMI alone might miss, and thus help prioritize which individuals should undergo more comprehensive risk evaluation or follow-up. Moreover, BMI-SDS is an index rather than a direct measure of adiposity and may not fully capture individual differences in fat mass or distribution. Similarly, fasting insulin is an indirect marker of insulin resistance and subject to biological and analytical variability. Future studies using direct assessments of adiposity and insulin sensitivity may better delineate their relationships with cardiometabolic risk. Fasting insulin assays are subject to considerable inter-laboratory variability, which limits the generalizability of specific cut-offs and reduces clinical applicability [67]. This limitation underscores the need for harmonization across insulin assays if fasting insulin is to be widely used in clinical practice. Until such standardization is achieved, specific cut-off values should be interpreted with caution and always in the context of the assay used.
Although fasting insulin and BMI-SDS showed statistically significant associations with various cardiometabolic risk factors, the ROC AUC values were modest. This indicates that neither marker alone provides strong discriminatory power to reliably classify individuals at high cardiometabolic risk. Therefore, these markers should not be interpreted as definitive diagnostic tools but rather as part of a multifactorial risk assessment strategy that incorporates additional clinical and biochemical indicators. The cost of analyzing fasting insulin must be weighed against established markers such as lipids, blood pressure, and fasting glucose. Our findings suggest that fasting insulin may offer complementary information, particularly for identifying higher-risk individuals among children with obesity. Further studies are needed to assess the cost-effectiveness and clinical utility of incorporating fasting insulin into routine evaluation protocols.
In conclusion, obesity presenting in childhood or adolescence is associated with high cardiometabolic risk. Although obesity class based on BMI-SDS is of great value, it fails to identify many individuals with a significant cardiometabolic burden. Our findings do not challenge the role of BMI as the essential initial screening measure for obesity in children. Rather, our findings show that fasting insulin could complement BMI-SDS by identifying those within the obesity population who are at elevated cardiometabolic risk and may benefit from targeted intervention. We propose that measuring fasting insulin in children and adolescents with obesity could be incorporated into clinical routine in primary health care. Our results indicate that an elevated fasting insulin value may warrant further investigation among children and adolescents with obesity, independent of obesity class based on BMI-SDS. However, the findings need to be validated longitudinally in a larger cohort.
Supplementary Information
Acknowledgements
We are grateful to all the children, adolescents, and their families who participated in this study. The authors would also like to extend their gratitude to the collaborators, nurses, and research assistants who made this project possible. The authors are especially grateful for the contributions of Malte Lidström, RN, and Marie Dahlbom, RN to many parts of this project.
Author contributions
RS, SEA, AF, DW and PB supervised, designed, and led the study, while all authors contributed. RS, SEA and PB wrote the first draft. RS, SYC and HM conducted the statistical analyses. All authors critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of the work. The corresponding author, DW, the first author RS, and the last author PB, are the manuscripts guarantors and confirm that all listed authors meet authorship criteria. They also attest that no individuals meeting the criteria have been omitted and take responsibility for the overall content.
Funding
Support from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement number 279153 (Beta- JUDO), the Regional Research Council in Uppsala-Örebro, Sweden, the Swedish Diabetes Foundation, the Swedish Society for Diabetology, the Gillbergska Foundation, the Swedish Innovation Agency (Project number 2020-02417), the Swedish Foundation for Strategic Research (grant number CMP22-0014), Uppsala University (grant ECHO zones), and the Swedish Research Council (2016-01040) was received for the study.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Uppsala University.
Declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki and approved by both the regional ethics committee in Uppsala (registration numbers 2010/036 and 2012/318) and in Salzburg (registration number EK1544/2012). Written informed consent was obtained from adolescents and their legal guardians 12 years or older, or by the legal guardians in case of age under 12 years. Participation was voluntary, and withdrawal of consent without any reason needed was possible throughout the entirety of the study.
Consent for publication
All participants and their legal guardians provided consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: Figures 1 and 2 have been corrected in PDF version.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/26/2025
The original online version of this article was revised: Figures 1 and 2 have been corrected in PDF version.
Change history
11/1/2025
A Correction to this paper has been published: 10.1186/s12933-025-02975-3
References
- 1.Baker JL, Olsen LW, Sørensen TIA. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357(23):2329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med. 2015;373(14):1307–17. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez PJ, Do D, Gratzl S, Cartwright BMG, Stucky NL, R DW. Treatment of obesity in US children and adolescents before and after the AAP guidelines [Internet]. medRxiv; 2024 [cited 2024 Nov 20]. p. 2024.10.25.24316147. Available from: https://www.medrxiv.org/content/10.1101/2024.10.25.24316147v1
- 4.Deanfield J, Verma S, Scirica BM, Kahn SE, Emerson SS, Ryan D, et al. Semaglutide and cardiovascular outcomes in patients with obesity and prevalent heart failure: a prespecified analysis of the SELECT trial. The Lancet. 2024;404(10454):773–86. [DOI] [PubMed] [Google Scholar]
- 5.Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389(12):1069–84. [DOI] [PubMed] [Google Scholar]
- 6.Foy AJ, Mandrola J, Liu G, Naccarelli GV. Relation of obesity to new-onset atrial fibrillation and atrial flutter in adults. Am J Cardiol. 2018;121(9):1072–5. [DOI] [PubMed] [Google Scholar]
- 7.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension. Circ Res. 2015;116(6):991–1006. [DOI] [PMC free article] [PubMed]
- 8.Henning RJ. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: a review of the pathophysiology and treatment of obesity. Am J Cardiovas Dis. 2021;11(4):504. [PMC free article] [PubMed] [Google Scholar]
- 9.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016;17(2):95–107. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs DR, Woo JG, Sinaiko AR, Daniels SR, Ikonen J, Juonala M, et al. Childhood cardiovascular risk factors and adult cardiovascular events. N Engl J Med. 2022;386(20):1877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. [DOI] [PMC free article] [PubMed]
- 13.Nuttall FQ. Body mass index: obesity, BMI, and health: a critical review. Nutr Today. 2015;50(3):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risérus U, de Faire U, Berglund L, Hellénius ML. Sagittal abdominal diameter as a screening tool in clinical research: cutoffs for cardiometabolic risk. j Obes. 2010;2010: 757939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borlaug BA, Jensen MD, Kitzman DW, Lam CSP, Obokata M, Rider OJ. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiological targets. Cardiovasc Res. 2022;118(18):3434–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zapata JK, Azcona-Sanjulian MC, Catalán V, Ramírez B, Silva C, Rodríguez A, et al. BMI-based obesity classification misses children and adolescents with raised cardiometabolic risk due to increased adiposity. Cardiovasc Diabetol. 2023;22(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Wolf A, Nauwynck E, Vanbesien J, Staels W, De Schepper J, Gies I. Optimizing childhood obesity management: the role of Edmonton obesity staging system in personalized care pathways. Life. 2024;14(3):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ. 2011;183(14):E1059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman DS, Zemel BS, Dietz WH, Daymont C. Screening accuracy of bmi for adiposity among 8- to 19-year-olds. Pediatrics. 2024;154(1): e2024065960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Diabetes Care. 2009;32(2):361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy-Marchal C, Arslanian S, Cutfield W, Sinaiko A, Druet C, Marcovecchio ML, et al. Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab. 2010;95(12):5189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hrafnkelsson H, Magnusson KT, Sigurdsson EL, Johannsson E. Association of BMI and fasting insulin with cardiovascular disease risk factors in seven-year-old Icelandic children. Scand J Prim Health Care. 2009;27(3):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giollabhui NM, Ellman LM, Coe CL, Byrne ML, Abramson LY, Alloy LB. To exclude or not to exclude: considerations and recommendations for C-Reactive Protein values higher than 10 mg/L. Brain Behav Immun. 2020;31(87):898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284–94. [DOI] [PubMed] [Google Scholar]
- 25.Reinehr T, Tittel SR, Holle R, Wiegand S, Gellhaus I, Hebebrand J, et al. Comparison of cardiovascular risk factors between children and adolescents with classes III and IV obesity: findings from the APV cohort. Int J Obes. 2021;45(5):1061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253. [PubMed]
- 27.Aydin BK, Stenlid R, Ciba I, Cerenius SY, Dahlbom M, Bergsten P, et al. High levels of FSH before puberty are associated with increased risk of metabolic syndrome during pubertal transition. Pediatr Obes. 2022;17(8): e12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenlid R, Manell H, Halldin M, Kullberg J, Ahlström H, Manukyan L, et al. High DPP-4 concentrations in adolescents are associated with low intact GLP-1. J Clin Endocrinol Metab. 2018;103(8):2958–66. [DOI] [PubMed] [Google Scholar]
- 29.Schipper HS, de Ferranti S. Cardiovascular risk assessment and management for pediatricians. Pediatrics. 2022;150(6): e2022057957. [DOI] [PubMed] [Google Scholar]
- 30.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5. [DOI] [PubMed] [Google Scholar]
- 31.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338(23):1650–6. [DOI] [PubMed] [Google Scholar]
- 32.Júlíusson PB, Roelants M, Benestad B, Lekhal S, Danielsen Y, Hjelmesaeth J, et al. Severe obesity is a limitation for the use of body mass index standard deviation scores in children and adolescents. Acta Paediatr. 2018;107(2):307–14. [DOI] [PubMed] [Google Scholar]
- 33.Després JP, Lamarche B, Mauriège P, Cantin B, Dagenais GR, Moorjani S, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334(15):952–8. [DOI] [PubMed] [Google Scholar]
- 34.Öhrvall M, Berglund L, Vessby B. Sagittal abdominal diameter compared with other anthropometric measurements in relation to cardiovascular risk. Int J Obes. 2000;24(4):497–501. [DOI] [PubMed] [Google Scholar]
- 35.Adeva-Andany MM, Martínez-Rodríguez J, González-Lucán M, Fernández-Fernández C, Castro-Quintela E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab Syndr. 2019;13(2):1449–55. [DOI] [PubMed] [Google Scholar]
- 36.Haffner SM, Mykkänen L, Festa A, Burke JP, Stern MP. Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation. 2000;101(9):975–80. [DOI] [PubMed] [Google Scholar]
- 37.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263(21):2893–8. [DOI] [PubMed]
- 38.Welin L, Eriksson H, Larsson B, Ohlson LO, Svärdsudd K, Tibblin G. Hyperinsulinaemia is not a major coronary risk factor in elderly men. The study of men born in 1913. Diabetologia. 1992;35(8):766–70. [DOI] [PubMed]
- 39.Yarnell JW, Sweetnam PM, Marks V, Teale JD, Bolton CH. Insulin in ischaemic heart disease: are associations explained by triglyceride concentrations? The Caerphilly prospective study. Br Heart J. 1994;71(3):293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubins HB, Robins SJ, Collins D, Nelson DB, Elam MB, Schaefer EJ, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the department of veterans affairs high-density lipoprotein intervention trial (VA-HIT). Arch Intern Med. 2002;162(22):2597–604. [DOI] [PubMed] [Google Scholar]
- 41.Libman IM, Barinas-Mitchell E, Bartucci A, Chaves-Gnecco D, Robertson R, Arslanian S. Fasting and 2-Hour Plasma Glucose and Insulin: relationship with risk factors for cardiovascular disease in overweight nondiabetic children. Diabetes Care. 2010;33(12):2674–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tersander B, Olsson R, Aydin BK, Stenlid R, Ciba I, Manell H. Obesity-related subclinical hypothyroidism in childhood: elevated triglycerides but not basal metabolic rate. Pediatr Res. 2024. [DOI] [PMC free article] [PubMed]
- 43.Najjar SM, Yang Y, Fernström MA, Lee SJ, DeAngelis AM, Rjaily GAA, et al. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab. 2005;2(1):43–53. [DOI] [PubMed] [Google Scholar]
- 44.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003;31(Pt 6):1120–4. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Fang Z, Luo Q, Wang X, Warda M, Das A, et al. Unlocking the mysteries of VLDL: exploring its production, intracellular trafficking, and metabolism as therapeutic targets. Lipids Health Dis. 2024;23(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ference BA, Braunwald E, Catapano AL. The LDL cumulative exposure hypothesis: evidence and practical applications. Nat Rev Cardiol. 2024;21(10):701–16. [DOI] [PubMed] [Google Scholar]
- 47.Woodruff RC, Tong X, Khan SS, Shah NS, Jackson SL, Loustalot F, et al. Trends in cardiovascular disease mortality rates and excess deaths, 2010–2022. Am J Prev Med. 2024;66(4):582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNamara JJ, Molot MA, Stremple JF, Cutting RT. Coronary artery disease in combat casualties in Vietnam. JAMA. 1971;216(7):1185–7. [PubMed] [Google Scholar]
- 49.Manell H, Staaf J, Manukyan L, Kristinsson H, Cen J, Stenlid R, et al. Altered plasma levels of glucagon, GLP-1 and glicentin during OGTT in adolescents with obesity and type 2 diabetes. J Clin Endocrinol Metab. 2016;101(3):1181–9. [DOI] [PubMed] [Google Scholar]
- 50.Stenlid R, Cerenius SY, Wen Q, Aydin BK, Manell H, Chowdhury A, et al. Adolescents with obesity treated with exenatide maintain endogenous GLP-1, reduce DPP-4, and improve glycemic control. Front Endocrinol (Lausanne). 2023;14:1293093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20(10):685–95. [DOI] [PubMed] [Google Scholar]
- 52.Taskinen MR, Borén J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239(2):483–95. [DOI] [PubMed] [Google Scholar]
- 53.Östgren CJ, Otten J, Festin K, Angerås O, Bergström G, Cederlund K, et al. Prevalence of atherosclerosis in individuals with prediabetes and diabetes compared to normoglycaemic individuals-a Swedish population-based study. Cardiovasc Diabetol. 2023;22(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim HL. Arterial stiffness and hypertension. Clin Hypertens. 2023;1(29):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sundström J, Neovius M, Tynelius P, Rasmussen F. Association of blood pressure in late adolescence with subsequent mortality: cohort study of Swedish male conscripts. BMJ. 2011;22(342): d643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leiba A, Fishman B, Twig G, Gilad D, Derazne E, Shamiss A, et al. Association of adolescent hypertension with future end-stage renal disease. JAMA Intern Med. 2019;179(4):517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fishman B, Vinograd A, Tsur AM, Bardugo A, Bendor CD, Libruder C, et al. Adolescent blood pressure and early age stroke. NEJM Evid. 2024;3(12):EVIDoa2400193. [DOI] [PubMed] [Google Scholar]
- 58.Kasiakogias A, Rosei EA, Camafort M, Ehret G, Faconti L, Ferreira JP, et al. Hypertension and heart failure with preserved ejection fraction: position paper by the European society of hypertension. J Hypertens. 2021;39(8):1522–45. [DOI] [PubMed] [Google Scholar]
- 59.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34(19):1424–31. [DOI] [PubMed] [Google Scholar]
- 60.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69(9):1129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szczudlik E, Stępniewska A, Bik-Multanowski M, Brandt-Heunemann S, Flehmig B, Małecka-Tendera E, et al. The age of the obesity onset is a very important factor for the development of metabolic complications and cardiovascular risk in children and adolescents with severe obesity. Eur J Pediatr. 2024;183(9):3833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao Z, Tchang BG, Albert M, Blumenthal RS, Nasir K, Blaha MJ. Associations between Class I, II, or III obesity and health outcomes. NEJM Evid. 2025;4(4):EVIDoa2400229. [DOI] [PubMed] [Google Scholar]
- 63.Pratt RE, Kavey RE, Quinzi D. Combined dyslipidemia in obese children: response to a focused lifestyle approach. J Clin Lipidol. 2014;8(2):181–6. [DOI] [PubMed] [Google Scholar]
- 64.Price PH, Kaizer AM, Daniels SM, Jenkins TM, Inge TH, Eckel RH. Physical activity improves lipid and weight loss outcomes post-metabolic bariatric surgery in adolescents with severe obesity. Obesity (Silver Spring). 2019;27(6):989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK, et al. Once-weekly semaglutide in adolescents with obesity. New Engl J Med. 2022;387(24):2245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Putri RR, Danielsson P, Ekström N, Ericsson Å, Lindberg L, Marcus C, et al. Effect of pediatric obesity treatment on long-term health. JAMA Pediatr. 2025;179(3):302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Werner M, Tönjes A, Stumvoll M, Thiery J, Kratzsch J. Assay-dependent variability of serum insulin levels during oral glucose tolerance test: influence on reference intervals for insulin and on cut-off values for insulin sensitivity indices. Clin Chem Lab Med. 2008;46(2):240–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Uppsala University.