Abstract
Background
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by endothelial dysfunction and inflammation. This study aimed to evaluate the effects of cilomilast (CIL), a phosphodiesterase-4 inhibitor, and tadalafil (TAD), a phosphodiesterase-5 inhibitor, on PAH induced by monocrotaline (MCT) in rats.
Methods
Forty Wistar albino rats were divided into five groups: control, MCT, MCT + CIL, MCT + TAD, and MCT + CIL + TAD. PAH was induced via MCT, and treatments were administered orally from days 21 to 35. Hemodynamic parameters, right ventricular pressure (RVP), echocardiographic findings, and histopathological lung and heart tissue changes were assessed. Nitric oxide (NO) levels in lung tissue were also measured.
Results
Tissue NO levels were significantly greater in the MCT + CIL + TAD group than in the MCT group (p = 0.01). The RVP was lower in the MCT + TAD and MCT + CIL + TAD groups than in the MCT group (p < 0.05) but not in the MCT + CIL group. Histopathologically, lung perivascular infiltration and pulmonary artery wall thickness were significantly reduced in the MCT + CIL + TAD group, indicating an anti-inflammatory effect. However, CIL alone did not significantly impact pulmonary artery thickening or RVP.
Conclusion
CIL alone had no significant effect on PAH progression, but its combination with TAD improved inflammation scores and NO levels. These findings suggest that targeting inflammation alongside vasodilation may offer therapeutic benefits in PAH. Further studies with different doses and PAH models are recommended.
Keywords: Cilomilast, Pulmonary arterial hypertension, Tadalafil, Monocrotaline, Rat
Background
The mechanisms leading to the onset and progression of pulmonary arterial hypertension (PAH) remain largely unclear. The complex interaction between dysfunction and inflammation of the pulmonary endothelial structure is a major factor in the development of this disease [1]. Pulmonary vascular remodeling in PAH cannot be explained only by medial hypertrophy and the accumulation of different vascular cells (pulmonary artery smooth muscle cells (PASMCs), endothelial cells, fibroblasts, myofibroblasts, and pericytes) in the pulmonary arterial wall. In these patients and animal models, varying degrees of perivascular inflammatory infiltrates, comprising T- and B-lymphocytes, macrophages, dendritic cells, and mast cells, have been demonstrated in pulmonary vascular lesions [1–4]. The impaired function of regulatory T cells, abnormalities involving T helper 17 cell immune polarization, and the presence of dendritic cells and other inflammatory cells in pulmonary vascular lesions are examples of conditions that suggest poor adaptation of the immune response [1, 2]. The correlations of the average perivascular inflammation score with the intima plus media and adventitia thickness and average pulmonary arterial pressure (PAP) have been demonstrated recently and support the importance of perivascular inflammation in the pulmonary vascular remodeling process [3–5].
In PAH, perivascular accumulation and intravascular infiltration of immune cells, as well as circulating levels of certain cytokines and chemokines, are abnormally elevated. These include interleukin (IL)-1, IL-6, IL-8, monocyte chemoattractant protein (MCP)-1, fractalkine, and tumor necrosis factor (TNF)-α [1]. Some of these cytokines and chemokines have been associated with poor clinical outcomes in PAH patients and have also been used as biomarkers for disease progression. Although the role of inflammation in the pathogenesis of PAH has been established, there is no high interest in studies in which cytokines and chemokines that play key roles in the pathogenesis of this disease are targeted.
Cilomilast (CIL) is a second-generation selective phosphodiesterase (PDE)-4 inhibitor with systemic action. It lacks a deep emetic effect while maintaining the therapeutic activity of first-generation PDE-4 inhibitors. The enzyme PDE-4, which is responsible for cyclic adenosine monophosphate (cAMP) degradation, is widely expressed in inflammatory and immune cells (eosinophils, neutrophils, monocytes, macrophages, T lymphocytes and B lymphocytes). It has long been recognized as a potential therapeutic target in various inflammatory conditions [6–8]. PDE-4 is an enzyme found extensively in lung tissue, and several studies have demonstrated that the inhibition of PDE-4 suppresses the release of various proinflammatory mediators, such as matrix metalloproteinase-9, leukotriene B4, neutrophil elastase, myeloperoxidase and reactive oxygen species (ROS), in both lung tissue and blood [9–12]. PDE-4 inhibitors also have corrective effects on proliferation and apoptosis [13, 14]. It has been reported that inhibition of PDE-4 can inhibit eosinophil infiltration into the lungs and reduce degranulation of granulocyte/macrophage colony-stimulating factor, TNF-α and platelet-activating factor [7, 11, 12, 15].
In this context, with respect to inflammation, which has the potential to be effective in the development or treatment of PAH, targeting and inhibiting a family of enzymes that can have a broad effect instead of individually targeting inflammatory cells, fibroblasts and smooth muscle cells that may play a role in the pathogenesis of this disease, in addition to more than one hundred mediators, including prostaglandins, leukotrienes, chemokines, cytokines, proteases and growth factors, can affect the treatment of the disease. No such effect has ever been attempted before as an adjuvant to vasodilator treatment already used in PAH, and it can be thought of as having the potential to make a difference. In this study, the use of CIL, a second-generation, selective PDE-4 inhibitor with systemic action as an adjuvant to the PDE-5-selective inhibitor tadalafil (TAD), which is a vasodilator agent that increases cyclic guanosine 3’,5’-monophosphate (cGMP) levels, was planned.
Methods
Animals and experimental design
Before initiating the study, approval was obtained from the Experimental Animal Ethics Board at İnönü University (2016 A-114). This study was funded by the Scientific Research Projects Fund of Inonu University (Project Number: TDK-2017-739). All of the animals used in the study were provided by the Experimental Animals Research Unit at Inonu University. Male Wistar albino rats were used in the study and housed under pathogen-free standard laboratory conditions (22 ± 2 °C, 60% humidity, 12 h light/12 h dark cycle) in polycarbonate-based cages and were fed 8 mm standard pellet feed. All the rats had the same characteristics. A total of 40 rats, 8 randomly selected from each group, were included in the study. The randomization was performed on Day 0 immediately after MCT administration, using a block-randomization protocol to evenly distribute baseline characteristics across treatment groups. MCT was used to construct the PAH model. MCT (Sigma‒Aldrich Co., St. Louis, MO, USA) was dissolved in 1 M HCl, and the pH was adjusted to 7.4 with 1 M NaOH [16–18]. The doses used in our study were selected based on prior publications demonstrating their pharmacological efficacy and tolerability in rodent models of PAH or inflammation: The CIL dose (3 mg/kg/day, oral) was based on studies showing anti-inflammatory efficacy in lung tissue without significant systemic toxicity [19]. The TAD dose (10 mg/kg/day, oral) was derived from studies that demonstrated hemodynamic improvement and pulmonary vascular remodeling attenuation in MCT-induced PAH [17].
In the control group, the vehicle solution was administered per orally (p.o.) once daily between days 21 and 35.
In the MCT group, the animals were sacrificed 35 days after subcutaneous (s.c.) administration of a single dose of 60 mg/kg MCT on the first day.
In the MCT + CIL group, following the s.c. administration of a single dose of 60 mg/kg MCT on the first day, 3 mg/kg CIL was administered p.o. once daily between days 21 and 35.
In the MCT + TAD group, following the s.c. administration of a single dose of 60 mg/kg MCT on the first day, 10 mg/kg TAD was administered p.o. once daily between days 21 and 35.
In the MCT + CIL + TAD group, following the s.c. administration of a single dose of 60 mg/kg MCT on the first day, 3 mg/kg CIL and 10 mg/kg TAD were administered p.o. once daily between days 21 and 35.
Echocardiography
The rats in each group underwent a cardiac transthoracic echocardiography (TTE) assessment on the day that the pressure recordings were invasively evaluated following the administration of the medication. TTE was performed by cardiovascular echocardiography standards under intraperitoneal ketamine/xylazine anaesthesia (ketamine hydrochloride, Parke-Davis Eczacıbası; 75 mg/kg and 5 mg/kg xylazine hydrochloride, Rompun®, Bayer Drug) via a Vivid S6 brand device and 10 S sector probe (GE Healthcare, United States) [20]. In brief, after the chests of the anaesthetized rats were shaved, 2D-MMode and Doppler measurements (Fig. 1) were performed while the rats were in the left decubitus position. Interventricular septum thickness (IVSD), posterior wall thickness (PWD), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular fractional shortening (LVFS), left ventricular ejection fraction (LVEF), left atrium and aortic root diameter, mitral valve E/A ratio, E-wave deceleration time (EDT), the ratio of the right ventricle (RV) to the left ventricle (LV), aortic and pulmonary valve velocities, pulmonary artery acceleration time (PAT), and heart rate (HR) were measured. Parasternal long-axis and apical four/five-chamber images were used for 2D and color flow imaging and spectral Doppler studies of the mitral valve and/or aortic outflow tract. LV, left atrium and aorta diameters were measured from the parasternal long axis. Pulmonary valve velocities with parasternal short-axis imaging were used to measure the PAT. All Doppler spectra were recorded for 5–10 cardiac cycles at a scanning rate of 100 mm/s. The color Doppler was preset at the Nyquist limit of 0.44 m/s. Estimated systolic PAP measurements by echocardiography were not performed because they did not produce practical and accurate results.
Fig. 1.
Parasternal long axis M Mode transechocardiographic view of the heart
Hemodynamic evaluation and surgical procedure
Before the procedure, 5 mg/kg 2% xylazine hydrochloride and 75 mg/kg ketamine hydrochloride were administered intraperitoneally to the rats for anaesthesia. For the recording of right ventricular systolic pressure (RVSP) and determination of systemic systolic, diastolic, and mean blood pressure (BP) and HR, the right jugular vein and one of the carotid arteries were generally cannulated individually. In cases of any problem, including bleeding or rupture during vascular access, the other vein was used as a backup. Changes in electrocardiography (ECG) signals were monitored via 3-lead ECG electrodes.
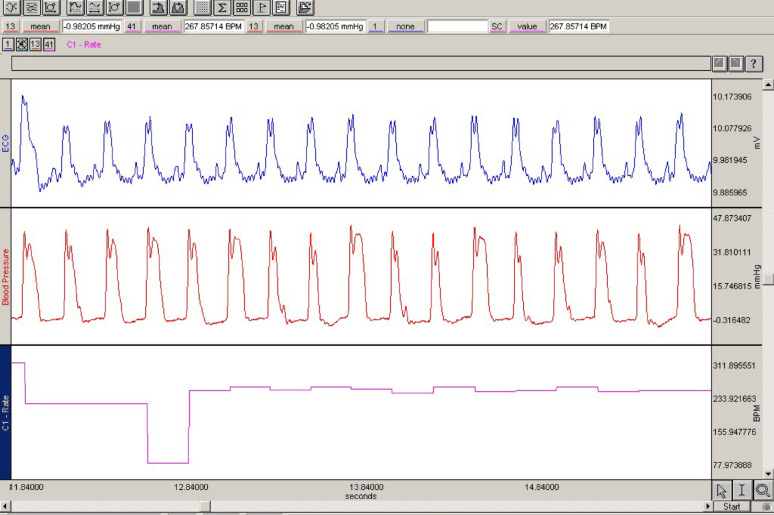
RVSP and systemic BP, HR, and ECG values were recorded for 3 min via the Biopac MP-100 Data Acquisition system computer recording program. To record the RVSP, the jugular vein was accessed, and a slightly curved catheter tip pressure transducer was inserted. The right low-pressure atrial waves were tracked on the pressure monitor, and the catheter was advanced 2–3 cm. Once the RV was reached, the pressure was recorded (Fig. 2). The RVSP was determined by averaging five consecutive heart cycles. The ECG was monitored through suitable simultaneous ECG electrodes. Systemic arterial BP was determined by carotid artery cannulation.
Fig. 2.
Right ventricular pressure and ECG recording findings
Following these procedures, the rats were euthanized with an overdose of ethyl-carbamate derivative (urethane® 3.6 g/kg, intraperitoneally). The anterior chest wall was cleaned with 10% povidone-iodine and sterile gauze, and the skin, subcutaneous, fascia, and thorax were exposed through a 3-cm median incision in the thorax. Lung and heart tissues harvested for biochemical and histopathological evaluation were divided into vertical and horizontal sections and stored at + 4 °C in vials containing 10% formaldehyde solution.
Histopathological analyses
At the end of the study, heart and lung tissues were fixed in 10% formaldehyde solution. Following tissue monitoring procedures, 4–5 μm thick sections were taken from the prepared paraffin blocks. To determine the general morphological structure, the tissue sections were stained via the hematoxylin and eosin (H&E) staining method. In our histopathological evaluation of lung and heart tissues using H&E staining, we primarily focused on mononuclear cell infiltration (e.g., lymphocytes and macrophages), which was the dominant feature observed in perivascular and interstitial areas.
Tissue sections were examined for inflammatory cell infiltration in 10 different areas at 20× magnification. Each area was scored according to the extent of histological changes: 0, normal; 1, less than 25%; 2, 25–50%; 3, or more than 50% change. Furthermore, the mean thickness of the 10 pulmonary arterioles with rounded lumens was measured at six different points and subsequently averaged. The method previously used for pulmonary arteriolar wall thickening and assessments was used [21].
Analyses were performed via the Leica Q Win Image Analysis System (Leica Micros Imaging Solutions Ltd., Cambridge, UK) with a Leica DFC-280 research microscope.
Biochemical analysis
A total of 0.1 g of lung tissue was weighed into plastic tubes, and 1 mL of PBS buffer (pH 7.4) was added. Samples of weighed tissue were homogenized on ice via a homogenizer until completely disintegrated. The resulting homogenates were then sonicated 4 times for 15 s on ice at 30-second intervals.
The activity of the catalase (CAT) enzyme was performed using the method recommended by Aebi [22]. The activity determination of the superoxide dismutase (SOD) enzyme was performed according to the method previously mentioned in the literature [23]. The amount of total glutathione (tGSH) was measured using the method reported by Akerboom et al. [24]. Malondialdehyde (MDA) measurement has been determined using the previously reported method in the literature [25].
Nitric oxide (NO) levels in tissue samples were measured via the Oxford Biomedical Research Colorimetric Nitric Oxide Test Kit. This kit provides a practical method to quantify NO2/NO3 (final products of in vitro NO production) in biological samples. The best index of total NO production is the sum of these two products. The results are expressed in mmol/mg protein. The protein concentrations of the tissue samples were measured via the Bradford method [26]. The absorption was measured at 595 nm with a UV‒VIS spectrophotometer. Bovine serum albumin was used as the protein standard.
To evaluate whether the combined use of MCT, TAD, and CIL has negative effects on the liver and kidney, routine kidney function tests and liver enzyme and serum lactate dehydrogenase (LDH) levels were also studied.
Statistics
The software used to perform the statistical analysis was IBM SPSS Statistics version 26. The normality assumption, which assumes that the data are normally distributed, was tested via the Shapiro‒Wilk test, which is a statistical test that compares observed data with a normal distribution. The Kruskal‒Wallis H test, which is a nonparametric test that compares three or more groups of data on the basis of their ranks, was used to compare the groups in terms of quantitative variables. The Mann‒Whitney U test, which is another nonparametric test that compares two groups of data on the basis of their ranks, was used for multiple comparisons, meaning that it was used to compare each pair of groups after finding a significant difference among the groups via the Kruskal‒Wallis H test. The Bonferroni correction, which is a method that adjusts the significance level to account for multiple testing and reduce the chance of false positives, was applied to the Mann‒Whitney U test. A value of p < 0.05 was considered statistically significant.
Results
Weight of rats
The weights of the rats in all the groups varied between 300 and 400 g. There was no difference in weight either before or after the intragroup study or at the beginning of the intergroup study.
Transthoracic echocardiogram results
The results from the transthoracic echocardiogram (TTE) performed just before the invasive hemodynamic assessment are detailed in Table 1. Accordingly, no difference was observed in the HR measured by TTE. IVSD, LVESD and PWD measurements of the left ventricle diameter were similar. LVEDD was significantly different, although not significantly different between the groups. LVEDD was greater in the MCT + TAD group than in the MCT + CIL group [7 (6–9) mm vs. 6 (5–7) mm]. As expected, there was no difference between the groups in terms of left ventricular systolic function parameters. While the aorta diameter was similar across all groups (the LA width was greater in the MCT + TAD group than in the control group ([5.0 (4.31–6.3) mm vs. 3.99 (3.14–4.93) mm).
Table 1.
Echocardiographic findings
| Parameter | Control (n = 8) | MCT (n = 8) | MCT + CIL (n = 8) | MCT + TAD (n = 8) | MCT + CIL + TAD (n = 8) | p value |
|---|---|---|---|---|---|---|
|
IVSD (mm) |
3 (2–3) |
3 (2–3) |
3 (2–3) |
3 (2–3) |
3 (2–3) |
0.869 |
|
LVEDD (mm) |
6 (6–7) |
7 (5–8) |
6 (5–7) |
7b (6–9) |
7 (5–8) |
0.027 |
|
LVESD (mm) |
4 (3–4) |
3 (2–4) |
3 (2–4) |
4 (3–5) |
4 (3–5) |
0.067 |
|
PWD (mm) |
2 (2–3) |
2 (2–3) |
2 (2–3) |
2 (2–3) |
2 (2–3) |
0.869 |
|
EF (%) |
81 (68–90) |
87 (78–93) |
87 (74–92) |
83 (77–91) |
85 (69–87) |
0.426 |
|
LA (mm) |
3.99 (3.14–4.93) |
4.61 (4.18–5.28) |
4.59 (3.93–5.61) |
5.00a (4.31–6.33) |
4.72 (3.95–7.04) |
0.015 |
|
Ao (mm) |
3.43 (2.84–3.85) |
3.47 (3.0-3.87) |
3.38 (3.03–4.13) |
3.55 (3.16–3.91) |
3.55 (3.36–4.15) |
0.438 |
|
E m/sn |
0.64 (0.62–0.83) |
0.65 (0.45–0.83) |
0.64 (0.54–0.81) |
0.66 (0.51-0.73) |
0.71 (0.58-0i73) |
0.927 |
|
A m/sn |
0.24 (0.15–0.35) |
0.35 (0.15-0.42) |
0.33a (0.2–0.66) |
0.25 (0.15–0.38) |
0.37a (0.20–0.48) |
0.040 |
| E/A ratio |
3.00 (2.13–4.13) |
2.05 (1.75-3.0) |
1.72a (1.22–3.18) |
2.60b (1.85–3.75) |
1.99 (1.52–3.18) |
0.013 |
|
EDT msn |
44 (37–55) |
48 (0–67) |
41 (18–59) |
48 (30–70) |
52 (33–78) |
0.229 |
|
PAT msn |
44 (33–55) |
33 (22–52) |
37 (30–59) |
37 (30–52) |
41 (26–48) |
0.097 |
|
LV/RV ratio |
1.30 (1.05–1.55) |
1.16 (1.1–1.35) |
1.33 (1.21.1.48) |
1.28 (1.0-1.27) |
1.27 (1.01–1.41) |
0.074 |
Data are median (min-max)
IVSD interventricular septal thickness, LVEDD left ventricle end diastolic diameter, LVESD left ventricle end systolic diameter, PWD posterior wall thickness, EF left ventricle ejection fraction, LA left atrial diameter, Ad aortic diameter, E left ventricle early mitral inflow velocity, A left ventricle late mitral inflow velocity, EDT E wave deceleration time, PAT pulmonary acceleration time
aStatistically different from the control group (p < 0.05);
bStatistically different group from MCT + CIL group (p < 0.05)
While the E-wave of mitral filling was similar between the groups, the A-wave was different between the MCT + CIL and MCT + CIL + TAD groups and the control group. The group with the lowest E/A ratio was MCT + CIL [(1.72 [1.22–3.18] vs. 3.0 [2.13–4.13] in the control group]). The E/A ratio was greater in the MCT + TAD group than in the MCT + CIL group [2.60 (1.85–3.75) vs. 1.72 (1.22–3.18)]. However, there was no difference in EDT.
There was no statistically significant difference between the groups in terms of the LV/RV ratio, which indicates the RV width, but the group with the lowest LV/RV ratio was the MCT group [1.30 (1.05–1.55) in the control group; 1.16 (1.1–1.35) in the MCT group]. While the flow velocity and PAT were found to be the lowest and close to the statistical significance limit in the MCT group, the values were close to each other in the other groups.
Hemodynamic parameters
Invasively measured HR, RV, and systemic arterial BP in all groups are shown in Table 2. Accordingly, the HR measured during TTE was similar across all groups. There was no difference between the groups in terms of systolic, diastolic, or mean BP. RVSP was significantly lower in the TAD and combined drug groups than in the MCT group ([31 (25–36) mmHg and 33 (26–42) mmHg vs. 43 (39–59)). The RVSP [38 (31–45) mmHg] in the MCT + CIL group remained as high as that in the MCT group.
Table 2.
Invasive hemodynamic results
| Parameter | Control (n = 8) | MCT (n = 8) | MCT + CIL (n = 8) | MCT + TAD (n = 8) | MCT + CIL + TAD (n = 8) | |
|---|---|---|---|---|---|---|
| HR (beat/min) | 299 (225–350) | 248 (226–413) | 295 (207–379) | 255 (190–365) | 285 (213–340) | |
| Systolic BP (mmHg) | 87 (80–112) | 94 (78–114) | 92 (53–128) | 107 (71–110) | 102 (75–119) | |
| Mean BP (mmHg) | 74 (64–91) | 70 (58–87) | 81 (45–114) | 77 (51–88) | 80 (67–96) | |
| Diastolic BP (mmHg) | 57 (48–80) | 50 (40–66) | 61 (35–111) | 54 (31–67) | 63 (40–75) | |
| RVSP (mmHg) | 30 (15–33)b | 43 (39–59)a | 38 (31–45)a | 31 (25–36)b | 33 (26–42)b | |
Data are written as Median (min-max)
BP Blood pressure, HR Heart rate, RVSP Right ventricle systolic pressure
aSignificantly different from the control group (p < 0.05)
bSignificantly different from the MCT group (p < 0.05)
Histopathological results
In the examination of lung tissue sections from the control group, bronchi, bronchioles, alveoli, and pulmonary interstitium structures were observed to be normal. On the other hand, significant mononuclear cell infiltration was observed around the pulmonary interstitium and pulmonary vessels in the sections from the MCT group. Pulmonary artery wall thickness was also significantly greater in the MCT group than in the control group. The infiltration observed in the MCT + CIL and MCT + TAD groups was similar to that in the MCT group, but in the MCT + CIL + TAD group, infiltration was significantly lower than that in the MCT group (Fig. 3; Table 3). The pulmonary artery wall thickness measured in the MCT + CIL group was similar to that in the MCT group and significantly lower in the MCT + TAD and MCT + CIL + TAD groups than in the MCT group (Fig. 4; Table 3).
Fig. 3.
Histological images of lung tissue from the control (A), MCT (B), MCT + CIL (C), MCT + TAD (D), and MCT + CIL + TAD (D) groups at 20X magnification. H&E-stained lung tissue sections of rats in each group (n = 8) were examined for mononuclear cell infiltration in 10 randomly selected areas. In the MCT group (B), perivascular infiltration (arrows) is noticeable. In MCT + CIL (C) and MCT + TAD groups (D), infiltration continues at a similar level to the MCT group. It is noteworthy that in the MCT + CIL + TAD group (E), infiltration decreased significantly
Table 3.
Heart and lung tissue infiltration scores and pulmonary arteriole wall thickness
| Parameters | Control (n = 8) |
MCT (n = 8) |
MCT + CIL (n = 8) |
MCT + TAD (n = 8) |
MCT + CIL + TAD (n = 8) |
P values |
|---|---|---|---|---|---|---|
| Infiltration on the heart | 0.0 ± 0.0 | 0.24 ± 0.43 | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0001 |
| Infiltration on the lung | 0.12 ± 0.33 | 1.20 ± 0.89a | 1.26 ± 1.04 | 1.07 ± 0.94 | 0.74 ± 0.76b | 0.0001 |
| Arteriolar wall thickness (µm) | 12.39 (5.11–27.54 | 13.75 (5.01–30.25)a | 13.68 (4.96–33.29) | 12.53 (4.13–29.97)b | 13.06 (4.48–30.90)b | 0.0001 |
Variables are summarized as ‘arithmetic mean ± standard deviation’ and ‘median (min-max)’. ap < 0.0001 vs. control group, bp < 0.05 vs. MCT group
Fig. 4.
Histological images of lung tissue from the control (A), MCT (B), MCT + CIL (C), MCT + TAD (D), and MCT + CIL + TAD (D) groups at 40X magnification. In H&E-stained lung tissue sections of rats in each group (n = 8), the tunica intima-media thickness of at least 10 pulmonary arteries was measured from 6 different points. Arrows indicate the pulmonary arteriole
The cardiac histologic evaluation of the heart in the control group revealed histologically normal sections. Although no significant histological changes were observed in the MCT group, small infiltration foci were noted in some sections of this group. This observed change was statistically significant between the control group and the MCT group. The cardiac tissue from the treated groups was found to have a normal histological appearance, similar to that of the control group (Fig. 5; Table 3).
Fig. 5.
Histological images of cardiac tissue from the control (A), MCT (B and C), MCT + CIL (D), MCT + TAD (E), and MCT + CIL + TAD (F) groups at 20X magnification. H&E-stained cardiac tissue sections of rats in each group (n = 8) were examined for mononuclear cell infiltration in 10 randomly selected areas. It is noteworthy that in the MCT group, all groups had a similar histological appearance, except for mild infiltration (arrows)
Biochemical parameters
The tests used for routine biochemical evaluation are shown in Table 4. There was no difference between the groups in terms of blood urea nitrogen (BUN), creatinine, aspartate transaminase (AST), or LDH levels.
Table 4.
Serum biochemical analysis
| Parameter | Control (n = 8) | MCT (n = 8) | MCT + CIL (n = 8) | MCT + TAD (n = 8) | MCT + CIL + TAD (n = 8) | p value |
|---|---|---|---|---|---|---|
| BUN (mg/dL) |
42 (33–49) |
37 (28–40) |
39 (28–43) |
37 (28–41) |
35 (28–48) |
0.1 |
| Creatinine (mg/dL) |
1.02 (0.58–1.30) |
0.73 (0.61–0.83) |
0.88 (0.72–1.08) |
0.88 (0.58–1.20) |
0.82 (0.57–1.14) |
0.101 |
| AST (IU/L) |
324 (230–392) |
214 (112–396) |
407 (229–596) |
292 (110–534) |
237 (104–519) |
0.121 |
| LDH (U/L) |
1563 (758–1816) |
1196 (565–2726) |
1065 (556–1988) |
1047.5 (420–1295) |
841 (337–1505) |
0.054 |
Data are written as Median (min-max)
BUN Blood urea nitrogen, AST aspartate aminotransferase, LDH lactate dehydrogenase
Each of the oxidative stress (OS) parameters examined from the lung tissue is shown below in relation to each of them. There was a significant difference between tissue CAT enzyme levels between the groups (Fig. 6). The order of CAT is located in the lowest MCT + CIL group [Control; 50.96 (33.68–67.3) U/mg protein, MCT; 70.14 (58.36–77.9) U/mg protein, MCT + CIL; 39.64 (32.24–51.14) U/mg protein, MCT + TAD; 67.19 (51.41–67.19) U/mg protein, MCT + CIL + TAD; 50.4 (30.49–61.91) U/mg protein]. There was a significant difference between the groups in terms of tissue SOD enzyme patterns (Fig. 6). Combined drug use group test was performed at the lowest value and was significantly higher than the other groups. [Control; 29.66 (24.65–42.67) U/mg protein, MCT; 35.32 (29.6-43.38) U/mg protein, MCT + CIL; 28.85 (26.14–34.83) U/mg protein, MCT + TAD; 31.22 (25.07–35.9) U/mg protein, MCT + CIL + TAD; 24.58 (22.39–33.83) U/mg protein]. In the evaluation of tissues in terms of tGSH, no significant difference was found between the groups [Figure 6, Control; 48.58 (34.48–50.91) nmol/mg protein, MCT; 50.32 (41.79–60.07) nmol/mg protein, MCT + CIL; 41.63 (30.95–47.97) nmol/mg protein, MCT + TAD; 47.99 (40.38–57.87) nmol/mg protein, MCT + CIL + TAD; 46.82 (42.1-59.58) nmol/mg protein, p = 0.074]. There was a significant difference between the groups in terms of tissue MDA levels (Fig. 6). The lowest MDA level was found in the group using combined drug. [Control; 27.4 (24.7–28.8) nmol/g wet tissue, MCT; 28.48 (27.7-28.48) nmol/g wet tissue, MCT + CIL; 27.75 (20.97–31.16) nmol/g wet tissue, MCT + TAD; 30.22 (26.27–34.69) nmol/g wet tissue, MCT + CIL + TAD; 16.99 (12.97–19.87) nmol/g wet tissue].
Fig. 6.
Oxidative stress parameters of lung tissue: A Catalase, B Superoxide dismutase, C Total glutathione, and D Malondialdehyde. Data were expressed as mean ± standard deviation (n = 8) * Significant difference between all groups (p < 0.001), significant difference between MCT + CIL and MCT and MCT + TAD (p < 0.05). **Significant difference between all groups (p = 0.013), significant difference between MCT + CIL + TAD and all groups (p < 0.05). *** No difference between groups (p = 0.074). *****Significant difference between all groups (p < 0.001), significant difference between MCT + CIL + TAD and all groups (p < 0.05)
There was a significant difference between the groups in terms of NO levels in lung tissues (Fig. 7). The NO level was lowest in the MCT group and highest in the control group [50.39 (34.54–69.42) nmol/g wet tissue vs. 113.209 (83.65–168.57) nmol/g wet tissue]. The tissue NO levels in the MCT + CIL group [61.22 (35.66–116.08) nmol/g wet tissue] were not statistically different from those in the MCT group [50.39 (34.54–69.42) nmol/g wet tissue]. However, in the combination group, the tissue NO level was significantly greater [84.16 (51.39–93.53) nmol/g wet tissue].
Fig. 7.
Nitric oxide levels from lung tissues. Data were presented as median (min-max) values (n = 8 rats per group)
Discussion
PAH is a rare but progressive and life-threatening condition characterized by elevated pulmonary arterial pressure and vascular resistance, ultimately leading to right heart failure and death. The estimated global prevalence of PAH is approximately 15–50 cases per million adults, with an incidence of 2–7 cases per million per year, varying by population and diagnostic criteria [1]. Despite advances in vasodilator therapy, PAH remains a disease with poor long-term outcomes, with a 5-year survival rate of approximately 60–70%. The progressive nature of pulmonary vascular remodeling and the limited reversibility of right ventricular dysfunction underscore the urgent need for novel or adjunctive treatment strategies that target not only hemodynamic burden but also inflammation and structural remodelling [27].
In PAH, abnormally elevated circulating levels of certain cytokines and chemokines, as well as increased perivascular accumulation and intravascular infiltration of immune cells, suggest that the complex interaction between pulmonary endothelial dysfunction and inflammation is a major factor in the development of this disease [28]. In this context, the addition of an agent with a proven anti-inflammatory effect in lung tissue to vasodilator therapy, which is frequently used in group 1 PAH, has never been tested before.
We acknowledge that the RVSP values in our model (ranging from 31 to 43 mmHg) are lower than those typically reported in studies showing severe PAH after MCT administration. This heterogeneity is described in the literature and may be ascribed to variances in sensitivity to MCT-induced PAH, which can greatly vary within Wistar albino rats. Although a consistent 60 mg/kg dose was used, potency can vary between lots [29]. We assessed RVSP on day 35, which may reflect an earlier or mid-phase of disease, whereas some studies measure RVSP later or use higher doses or adjuvant insults to amplify severity [30, 31]. Our findings likely represent a milder PAH phenotype. This may actually be advantageous for evaluating anti-inflammatory effects without the confounding impact of end-stage cardiac failure.
No significant effect of CIL alone or in combination was observed on the pressure-increasing effect of MCT on the basis of the measured RVSP. However, it should be noted that the numerical values measured in this study were not significantly different between the MCT group and the other groups, which may have limited the measurement of the effect. Nevertheless, the therapeutic potential of using TADs to reduce pulmonary pressure has been further confirmed.
In our study, evaluation of OS parameters revealed significant alterations in CAT, SOD, and MDA levels in the MCT group. Notably, MDA levels, as an indicator of lipid peroxidation, were markedly elevated in the MCT group and significantly reduced in the CIL + TAD combination group. These findings suggest that the combination therapy effectively alleviates OS. PDE-4 inhibitors not only exert anti-inflammatory effects by modulating PDE-4 enzyme activity in immune cells—particularly neutrophils and macrophages—but also reduce OS by suppressing the production of ROS. In this context, CIL has been shown in various preclinical studies to decrease the activity of ROS-producing enzymes such as myeloperoxidase and Β-Nicotinamide adenine dinucleotide phosphate hydrate oxidase. The consistency between these biochemical findings and the observed histological inflammation scores in our study supports the hypothesis that the antioxidant properties of CIL contribute meaningfully to the pathophysiology of PAH. On the other hand, no significant differences were observed in tGSH levels, suggesting that certain aspects of OS may be less responsive to the combination therapy.
In our study, when CIL alone was used in rats in which the PAH model was created with MCT, the tissue NO level was greater in the MCT group than in the control group, but the difference was not significant. However, the tissue NO level was significantly greater in the combined drug group. Regarding the synergistic increase in NO levels observed in the MCT + CIL + TAD group. TAD may preserve cGMP by inhibiting its degradation, enhancing NO signaling. CIL though not directly acting on the NO–cGMP axis, may reduce OS and inflammation, which are known to suppress endothelial nitric oxide synthase (eNOS) activity. Together, these agents may exert additive or synergistic effects, restoring eNOS function and NO bioavailability more effectively than either drug alone. As for cellular targets, PDE-4 is expressed in immune and inflammatory cells (macrophages, neutrophils, lymphocytes), and its inhibition may modulate cytokine release and leukocyte activity [6, 10, 32].
NO is produced primarily by the endothelium and is likely the most studied pulmonary vasodilator. The effect of NO on the vasculature is not limited to vasodilation. It inhibits vascular smooth muscle cell proliferation, DNA synthesis, and collagen production by activating cGMP [33, 34]. NO also inhibits the migration of vascular smooth muscle cells, independent of its effects on proliferation [35]. However, NO levels much higher than the vasodilatory concentration are required to inhibit proliferation, suggesting simultaneous activation of the cAMP kinase pathway by cGMP, which inhibits cell proliferation [36].
Disturbance of the NO pathway is a significant factor contributing to the pathobiology of PAH. The levels of the biochemical reaction products NO in exhaled air and NO in bronchoalveolar lavage (BAL) fluid are lower in the lungs of patients with PAH than in those of controls, and their levels are inversely proportional to the degree of PAH [37, 38]. Individuals with idiopathic PAH have significantly lower NO levels in exhaled breath than healthy nonsmoking controls and patients with associated PAH [39]. Early data revealed that eNOS expression, as measured by immunostaining, is decreased in the vascular endothelium of the pulmonary arteries of patients with PAH. This decrease has been reported to be inversely proportional to the severity of morphological arterial changes [40]. However, some investigators have shown increased or unchanged eNOS immunostaining in PAH [41]. Moreover, evidence of elevated eNOS III expression in plexiform lesions in PAH patients has also been suggested [42]. An unifying hypothesis developed to explain this situation is that there is a decrease in activity rather than in eNOS expression and that it effectively affects the level or activity of other NOS enzymes as well as the eNOS enzyme. In this context, it is highly probable that maintaining high endogenous NO levels in PAH, which directly contributes to pulmonary vascular remodelling, which is frequently observed in advanced stages of PAH, not only reduces PAP but also positively affects vascular remodelling, which is the major determinant of PAH.
In our study, the histopathological examination of the groups revealed that MCT, as expected, caused mononuclear cell infiltration around both the interstitium and the pulmonary vessel wall in the lung tissue and increased the thickness of the medium layer of the pulmonary small vessels. This finding is consistent with the changes observed in PAH, and both infiltration and wall thickness were reduced in the group in which CIL and TAD were combined. Inflammation continued in the monotherapy groups, whereas the increase in the thickness of the media layer in the MCT + TAD group decreased.
The increased activities of PDEs, which hydrolyse cAMP and cGMP, the second messengers induced by prostaglandin (PG)-I2 and NO in PAH, have long been established [43]. cAMP blocks cellular proliferation by targeting protein kinase A. It has been reported that it exerts this effect through the inhibition of cyclin-dependent kinase (CDK) 4, the activation of extracellular signal-regulated kinase (ERK)-11, and the upregulation of the CDK 2 inhibitor p27kipl [44]. The effect of cGMP on smooth muscle cell proliferation, which is not as strong as that of cAMP, has also been demonstrated.
The observation of perivascular inflammatory infiltrates involving mixed inflammatory cells before pulmonary vascular remodelling in experimental PAH indicates that incompatibility between the inflammatory and immune systems exists and contributes to remodelling in PAH. Consistent with this view, highly organized lymphocyte deposits may be found in the lungs of PAH patients [45]. Similarly, it has been reported that circulating inflammatory mediator levels follow a parallel course with a worse clinical outcome in PAH patients, and some changes may be observed in circulating cell subgroups [46].
Recent studies have provided evidence that pulmonary vascular cells are major sources of local resolvable signals that contribute to pulmonary vascular remodelling in PAH. PASMCs, endothelial cells, fibroblasts, and myofibroblasts from PAH patients exhibit distinct proinflammatory behaviors characterized by increased expression of various cytokines and chemokines and key inflammatory cell adhesion molecules, such as intercellular adhesion molecule (ICAM)-1 [1–3]. Local oversecretion of IL-1, IL6, leukotriene (LT)B4, macrophage migration inhibitory factor, leptin, and TNF-α and Forkhead Box O1 inactivation seem to play major roles in mediating structural and functional changes in the pulmonary vascular network in PAH [47, 48]. All these interaction channels between inflammation and PAH indicate that drugs that suppress inflammation may be effective in treating this disease.
In our study, there was no significant difference in mononuclear cell infiltration in the perivascular area or interstitium between the monotherapy groups, and inflammation was considerably suppressed in the group in which CIL and TAD were combined. In other words, the addition of CIL to the vasodilator TAD may have an anti-inflammatory effect, which suggests that different drug combinations with different activities may be more effective in treating PAH because of its complex pathophysiology rather than monotherapy or vasodilator therapy alone.
De Franceschi et al. studied the importance of the PDE-4 enzyme in sickle cell transgenic mice, in which PAH is a common cause of morbidity [49]. Although sickle cell anaemia (SCA) has been excluded from the WHO group 1 causes of PAH, they reported that exposing wild-type and transgenic sickle cell (Hbbs/Hbbs) mice to hypoxia (8% O2) for 7 days increased the number of neutrophils in both the BAL and peripheral circulation; increased the IL-1p, IL-10, IL-6 and TNF-α levels in BAL; and significantly increased endothelin-1, cyclo-oxygenase-2, angiotensin converting-enzyme and IL-1β gene activation. These authors reported that inflammation may play a key role in the pathogenesis of PAH in SCA. In the same study, prolonged hypoxia in the lungs of mice with SCA significantly increased the gene expression of PDE-1 in addition to PDE-4. When these mice were given the PDE-4 inhibitor rolipram, the upregulation of the PDE-4 and PDE-1 genes caused by hypoxia was prevented, and the development of PAH was inhibited, most likely through the modulation of both vascular tone and inflammatory factors. However, it should be noted that the pathogenesis of PAH is slightly different in the development of PAH in SCA, and microvascular occlusion caused by sickled erythrocytes and acute‒chronic lung injury caused by microinfarction are the major factors [50].
A study stressing the importance of PDE-4 inhibitors in PAH group 1 was previously conducted by Izikki et al. [51]. In that study, the efficacy of roflumilast, a PDE-4 inhibitor, in both MCT- and hypoxia-induced PAH was evaluated as monotherapy. They reported that roflumilast attenuated the development of PAH, RV hypertrophy, and distal vessel muscularization on day 21 when it was initiated immediately after MCT injection (60 mg × kg− 1 s.c.) and was used [(0.5–1.5 mg × kg(−1) day(−1)] until day 21. They reported that when roflumilast is used at high doses, it reduces the increases in IL-6 and MCP-1 mRNAs observed in lung tissue on day 21. In addition, it was strongly believed that PAH had reversed and that PAP and lung vascular structure were almost normal when roflumilast was used at high doses between 21 and 42 days after MCT. It was argued that this effect is also observed in the hypoxia model. Although more than a decade has passed since a study with such positive results, it is noteworthy that neither a preclinical nor a clinical study has been carried out in this field. In our study, we tested whether the addition of another PDE-4 inhibitor alone or in combination with an established treatment, the vasodilator TAD, affects PAH. Hence, our results revealed improved rates of inflammation and tissue NO in the combination group. Distal pulmonary artery muscularization was decreased in the combined and TAD groups, and no additional hemodynamic difference was detected in the combination group. CIL alone did not significantly affect either distal pulmonary artery muscularization or the PAP value. This may be due to the low dose of CIL we used or the poor group effect. However, while roflumilast is not a specific treatment for PAH, it is somewhat questionable whether it is used alone and is reported to be effective in PAH.
Growcot et al. studied proliferation, DNA synthesis, and apoptosis following the administration of drugs that affect platelet-derived growth factor and cAMP levels in culture medium to smooth muscle cells taken from the distal pulmonary arteries of patients with PAH [19]. They reported that the increase in intracellular cAMP after the administration of roflumilast and CIL was similar for both. This study reported that PDE-4 inhibition decreased both DNA synthesis and serum-stimulated PASMC proliferation and that dual therapy with iloprost and a PDE-4 inhibitor had a significantly greater antimitogenic effect. This study also reported that DNA synthesis induced by agonists was dose dependent for CIL, roflumilast, and rolipram. In the literature, there are common disorders, notably chronic obstructive pulmonary disease (COPD) and asthma, in which both molecules are strongly involved in the pathogenesis of inflammation [52]. The anti-inflammatory effects of CIL were evaluated in bronchial epithelial cells and sputum cells collected from smokers, COPD patients, and normal controls [32]. The TNF-α and IL-8 levels in bronchial epithelial and sputum cells from COPD patients were significantly greater than those in controls or smokers. CIL significantly reduced TNF-α secretion from bronchial epithelium and sputum cells and granulocyte/macrophage colony-stimulating factor secretion from sputum cells but did not affect IL-8 levels. CIL therapy also resulted in reductions in CD8+ and CD68+ cells in bronchial biopsies from COPD patients, which correlated with disease severity in COPD patients. Compared with those in the placebo group, significant reductions in subepithelial CD4+ cells (42%) and neutrophils (37%) were also reported. These results suggest that it has a potent anti-inflammatory effect [32, 53].
NO and other vasodilators use cGMP to regulate vascular tone [40, 54]. PDE-5 is highly specific to cGMP hydrolysis and is considered to be the PDE that provides the most active cGMP hydrolysis in smooth muscle cells under baseline conditions [55]. cGMP activates cGMP-dependent protein kinase (PK). Such an increase in cGMP causes a decrease in the intracellular Ca2+ concentration, leading to vasodilation [56]. These findings suggest that cAMP-dependent PK-A does not affect this activity. This finding was confirmed in PKG-1-deficient mice, in which cGMP-induced relaxation in aortic smooth muscle completely disappeared, and cAMP-induced relaxation was not affected [57]. However, the phosphorylation of the regulatory myosin-binding subunit of myosin phosphatase, inositol trisphosphate receptor-associated cGMP kinase substrate, and specific cAMP-induced PKA substrates, such as large calcium-activated K+ channels, is also a contributing factor to the reduction in intracellular calcium, resulting in weakening of muscle tone [58–61]. Therefore, both cGMP and cAMP modulate acute smooth muscle relaxation, mainly by reducing the level of intracellular calcium or activating the myosin phosphatase [55]. All of these effects may partly explain the vasodilatory effects of PDE-4 enzyme inhibitors in the various pH subgroups mentioned above [49, 51].
In our study, while the LV/RV ratio indicates RV width, which we can accept as a direct TTE parameter related to PAH, was the lowest but not statistically significant in the MCT group, the values were very close across all the treatment groups and the control group. In addition, the PAT value used in the TTE measurement of average PAP was also the lowest and close to the statistically significant limit in the MCT group, whereas it was found to be close to each other in the control and treatment groups. These results are inconsistent with the invasive hemodynamic and histological results. In their newly published study, Novelli et al. discussed the change in echocardiography parameters over time at a high frame rate after MCT using a 13 MHz linear probe in rats. In their study, the results for the RV started to be significant 3 weeks after MCT injection and reached their maximum value by week 5. In this study, parameters such as tricuspid annular plane systolic excursion (TAPSE) and RV fractional area change, which indicate RV systolic functions, were also used. In our study, echocardiography was performed on rats only at the end of the study, and TAPSE and RV fractional area changes were not considered because of the limitations of the imaging capability of the echocardiogram probe used, as well as the small numeric values found and their strong tendency to error [62]. We acknowledge that baseline hemodynamic confirmation (e.g., TTE on Day 21 before initiating treatment) was not performed, which limits our ability to confirm that PAH severity was equal among groups before therapy began. We agree that early echocardiographic or hemodynamic evaluation would have provided a more rigorous baseline control and will incorporate this approach in future studies.
Another significant result of our study is the variation in mitral filling wave patterns related to LV diastolic functions. Following the use of MCT, the early and late diastolic filling of the LV was affected, and a positive change was observed with the use of TAD compared with the other drug groups. In the histological examination, inflammatory cell infiltration was found in the heart tissues from the MCT group, which is likely to affect myocardial diastolic functions. Myocardial infiltration is one of the disadvantages of the MCT-PAH model, which was also reported by Gomez-Arroyo et al. [29]. In our study, myocardial infiltration improved in each of the treatment groups. In contrast to expectations, although the combination therapy group was thought to be the group that delivered the best results in terms of diastolic function, the group that received only TAD was more successful. Studies are available on the use of PDE-5 inhibitors in PAH development in heart failure patients with a preserved ejection fraction, which is very difficult to treat in adults. While some of these studies reported adverse outcomes, others reported improvements in both invasive hemodynamic parameters and clinical indicators [63, 64].
A limitation of this study is that while an anti-inflammatory agent was used, inflammatory markers such as IL-6 and TNF-α were not included in the study to demonstrate the mechanism of action. We have not performed ELISA assays for two key proinflammatory cytokines—TNF-α and IL-6—in lung tissue homogenates. However, due to tissue constraints and the study’s initial design, we were not able to perform immunohistochemistry for specific inflammatory cell markers (e.g., CD68 for macrophages, CD3 for T cells, etc.). Moreover, parameters such as echocardiographic TAPSE and RV fractional area changes are not used for the functional evaluation of the RV. In addition, direct PAP measurement was not included among the hemodynamic parameters evaluated; instead, RVSP was recorded, and parameters such as pulmonary vascular resistance and cardiac output were not measured. RV dysfunction in PAH involves not only hypertrophy but also structural and cellular alterations such as fibrosis, capillary rarefaction, and apoptosis. In the current study, our histological evaluation focused on overall inflammatory cell infiltration and medial hypertrophy, and we did not assess: fibrotic remodeling (e.g., via Masson’s trichrome or collagen-specific staining), capillary density (e.g., via CD31 or vWF immunostaining), and apoptosis (e.g., via TUNEL assay or caspase-3 expression). These are indeed important for a complete characterization of RV remodeling and function. Our current findings, while limited to hypertrophy and inflammation, provide a foundation for hypothesis generation regarding anti-remodeling effects of dual PDE inhibition, which will be tested in follow-up studies.
While we measured lung tissue NO levels as a readout of endothelial function, direct measurement of cGMP levels was not performed due to sample limitations and the study’s original scope. We now also explicitly state these as limitations of our study and suggest future investigations should include: cGMP quantification (e.g., via ELISA or immunohistochemistry), eNOS protein expression (e.g., Western blot or immunostaining) and immunophenotyping to identify the affected immune cell subpopulations.
Conclusion
In MCT-induced PAH, CIL alone had no significant effect on RVSP or distal pulmonary artery wall thickening, which indicates PAP. However, the combination of this PDE-4 inhibitor, which was developed as an anti-inflammatory agent, with TAD improves the inflammation and tissue NO levels that histologically accompany PAH or seem to be important in its pathogenesis. Different doses of this agent or other PAH models may be investigated in further studies.
Abbreviations
- PAH
Pulmonary arterial hypertension
- PASMCs
Pulmonary artery smooth muscle cells
- PAP
Pulmonary artery pressure
- MCP-1
Monocyte chemoattractant protein-1
- TNF-α
Tumor necrosis factor-alpha
- CIL
Cilomilast
- PDE-4
Phosphodiesterase-4
- cAMP
Cyclic adenosine monophosphate
- ROS
Reactive oxygen species
- TAD
Tadalafil
- cGMP
Cyclic guanosine monophosphate
- MCT
Monocrotaline
- p.o.
Per orally
- s.c.
Subcutaneous
- TTE
Transthoracic echocardiography
- IVSD
Interventricular septum thickness in diastole
- PWD
Posterior wall thickness in diastole
- LVEDD
Left ventricular end-diastolic diameter
- LVESD
Left ventricular end-systolic diameter
- LVFS
Left ventricular fractional shortening
- LVEF
Left ventricular ejection fraction
- EDT
E-wave deceleration time
- RV
Right ventricle
- LV
Left ventricle
- PAT
Pulmonary artery acceleration time
- HR
Heart rate
- ECG
Electrocardiography
- RVSP
Right ventricular systolic pressure
- BP
Blood pressure
- H&E
Hematoxylin and eosin
- PBS
Phosphate-buffered saline
- CAT
Catalase
- SOD
Superoxide dismutase
- tGSH
Total glutathione
- MDA
Malondialdehyde
- NO
Nitric oxide
- LDH
Lactate dehydrogenase
- BUN
Blood urea nitrogen
- AST
Aspartate transaminase
- OS
Oxidative stress
- eNOS
Endothelial nitric oxide synthase
- PG
Prostaglandin
- CDK
Cyclin-dependent kinase
- SCA
Sickle cell anaemia
- ICAM
Intercellular adhesion molecule
- LT
Leukotriene
- BAL
Bronchoalveolar lavage
- COPD
Chronic obstructive pulmonary disease
- PKG
Protein kinase
- TAPSE
Tricuspid annular plane systolic excursion
Author contributions
NE and AA are the coordinators of this study, and they planned the study protocol design. NE, HP and OO met the mandatory requirements for the study. OO and NE were responsible for drug administration and data collection. NE and OO performed the surgical procedures. Hemodynamic parameters and cardiac results, including ECG data, were evaluated by NE and ZU. NE performed the TTE, BA and AU conducted the biochemical analyses. Histopathological evaluations were carried out by NV and AY. ZK was responsible for the data, statistical analysis and interpretation of the results. NE and ZU were responsible for the design of the figures and tables. This manuscript was written by NE. The final manuscript was revised collaboratively by HP, ZU and AA.
Funding
This study was funded by the Scientific Research Projects Foundation of İnönü University (Project No: TDK-2017-739). We would like to thank the Scientific Research Project unit of İnönü University for their support.
Data availability
This study is a part of Necip Ermis’s doctoral thesis, which was finished in 2021 and was deposited in the Council of Higher Education Thesis Center (https://tez.yok.gov.tr/UlusalTezMerkezi/giris.jsp). The language of the main text is Turkish. Only the abstract is available in English.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Experimental Animal Ethics Board at İnönü University (2016 A-114).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53(1):1801887. [DOI] [PMC free article] [PubMed]
- 2.Hautefort A, Girerd B, Montani D, Cohen-Kaminsky S, Price L, Lambrecht BN, Humbert M, Perros F. T-helper 17 cell polarization in pulmonary arterial hypertension. Chest. 2015;147(6):1610–20. [DOI] [PubMed] [Google Scholar]
- 3.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(3):261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circul Res. 2014;115(1):165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, Patterson AJ, Agrawal R, Rabinovitch M, Ambler K, et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circul Res. 2011;109(8):867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro A, Jerez MJ, Gil C, Martinez A. Cyclic nucleotide phosphodiesterases and their role in Immunomodulatory responses: advances in the development of specific phosphodiesterase inhibitors. Med Res Rev. 2005;25(2):229–44. [DOI] [PubMed] [Google Scholar]
- 7.Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;9(9):Cd002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakkas LI, Mavropoulos A, Bogdanos DP. Phosphodiesterase 4 inhibitors in Immune-mediated diseases: mode of action, clinical applications, current and future perspectives. Curr Med Chem. 2017;24(28):3054–67. [DOI] [PubMed] [Google Scholar]
- 9.Grootendorst DC, Gauw SA, Verhoosel RM, Sterk PJ, Hospers JJ, Bredenbröker D, Bethke TD, Hiemstra PS, Rabe KF. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor Roflumilast in patients with COPD. Thorax. 2007;62(12):1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatzelmann A, Schudt C. Anti-inflammatory and Immunomodulatory potential of the novel PDE4 inhibitor Roflumilast in vitro. J Pharmacol Exp Ther. 2001;297(1):267–79. [PubMed] [Google Scholar]
- 11.Jones NA, Boswell-Smith V, Lever R, Page CP. The effect of selective phosphodiesterase isoenzyme Inhibition on neutrophil function in vitro. Pulm Pharmacol Ther. 2005;18(2):93–101. [DOI] [PubMed] [Google Scholar]
- 12.Kubo S, Kobayashi M, Iwata M, Takahashi K, Miyata K, Shimizu Y. Disease-modifying effect of ASP3258, a novel phosphodiesterase type 4 inhibitor, on subchronic cigarette smoke exposure-induced lung injury in guinea pigs. Eur J Pharmacol. 2011;659(1):79–84. [DOI] [PubMed] [Google Scholar]
- 13.Selige J, Hatzelmann A, Dunkern T. The differential impact of PDE4 subtypes in human lung fibroblasts on cytokine-induced proliferation and myofibroblast conversion. J Cell Physiol. 2011;226(8):1970–80. [DOI] [PubMed] [Google Scholar]
- 14.Park JW, Ryter SW, Kyung SY, Lee SP, Jeong SH. The phosphodiesterase 4 inhibitor rolipram protects against cigarette smoke extract-induced apoptosis in human lung fibroblasts. Eur J Pharmacol. 2013;706(1–3):76–83. [DOI] [PubMed] [Google Scholar]
- 15.Momose T, Okubo Y, Horie S, Suzuki J, Isobe M, Sekiguchi M. Effects of intracellular Cyclic AMP modulators on human eosinophil survival, degranulation and CD11b expression. Int Arch Allergy Immunol. 1998;117(2):138–45. [DOI] [PubMed] [Google Scholar]
- 16.McLean JH, Darby-King A, Harley CW. Potentiation and prolongation of long-term odor memory in neonate rats using a phosphodiesterase inhibitor. Neuroscience. 2005;135(2):329–34. [DOI] [PubMed] [Google Scholar]
- 17.Zhang WH, Liu CP, Zhang YJ, Ji YQ, Lu WX, Zeng Q. Additive effect of Tadalafil and Simvastatin on monocrotaline-induced pulmonary hypertension rats. Scandinavian Cardiovasc Journal: SCJ. 2012;46(6):374–80. [DOI] [PubMed] [Google Scholar]
- 18.Ma Z, Mao L, Rajagopal S. Hemodynamic characterization of rodent models of pulmonary arterial hypertension. J Vis Exp JoVE. 2016(110):53335. [DOI] [PMC free article] [PubMed]
- 19.Growcott EJ, Spink KG, Ren X, Afzal S, Banner KH, Wharton J. Phosphodiesterase type 4 expression and anti-proliferative effects in human pulmonary artery smooth muscle cells. Respir Res. 2006;7(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, Donal E, Sade LE, Ernande L, Garbi M, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2017;18(12):1301–10. [DOI] [PubMed] [Google Scholar]
- 21.Ayik S, Gunata M, Ozhan O, Yildiz A, Vardi N, Sonmez E, Ermis N, Ates N, Kilic E, Noma SAA, et al. Alamandin and especially melatonin attenuate pulmonary arterial hypertension induced by monocrotalin. Fundam Clin Pharmacol. 2024;38(6):1143–54. [DOI] [PubMed] [Google Scholar]
- 22.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis (second edition). Academic Press; 1974. pp. 673–684.
- 23.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244(22):6049–55. [PubMed] [Google Scholar]
- 24.Akerboom TP, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–82. [DOI] [PubMed] [Google Scholar]
- 25.Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966;16(2):359–64. [DOI] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 27.Mansoor M, Ibrahim AF. Emerging mechanistic insights and therapeutic strategies for pulmonary arterial hypertension: a focus on right ventricular dysfunction and novel treatment pathways. Biomedicines. 2025;13(3):600. [DOI] [PMC free article] [PubMed]
- 28.Mathew R. Inflammation and pulmonary hypertension. Cardiol Rev. 2010;18(2):67–72. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012;302(4):L363–369. [DOI] [PubMed] [Google Scholar]
- 30.Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, et al. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circul Res. 2004;94(3):385–93. [DOI] [PubMed] [Google Scholar]
- 31.Kuwabara Y, Tanaka-Ishikawa M, Abe K, Hirano M, Hirooka Y, Tsutsui H, Sunagawa K, Hirano K. Proteinase-activated receptor 1 antagonism ameliorates experimental pulmonary hypertension. Cardiovascular Res. 2019;115(8):1357–68. [DOI] [PubMed] [Google Scholar]
- 32.Profita M, Chiappara G, Mirabella F, Di Giorgi R, Chimenti L, Costanzo G, Riccobono L, Bellia V, Bousquet J, Vignola AM. Effect of cilomilast (Ariflo) on TNF-alpha, IL-8, and GM-CSF release by airway cells of patients with COPD. Thorax. 2003;58(7):573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klinger JR, Kadowitz PJ. The nitric oxide pathway in pulmonary vascular disease. Am J Cardiol. 2017;120(8s):S71–9. [DOI] [PubMed] [Google Scholar]
- 34.Nakaki T, Nakayama M, Kato R. Inhibition by nitric oxide and nitric oxide-producing vasodilators of DNA synthesis in vascular smooth muscle cells. Eur J Pharmacol. 1990;189(6):347–53. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circul Res. 1996;78(2):225–30. [DOI] [PubMed] [Google Scholar]
- 36.Suresh K, Shimoda LA. Lung circulation. Compr Physiol. 2016;6(2):897–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dweik RA. The lung in the balance: arginine, methylated arginines, and nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L15–17. [DOI] [PubMed] [Google Scholar]
- 38.Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med. 1998;158(3):917–23. [DOI] [PubMed] [Google Scholar]
- 39.Ozkan M, Dweik RA, Laskowski D, Arroliga AC, Erzurum SC. High levels of nitric oxide in individuals with pulmonary hypertension receiving Epoprostenol therapy. Lung. 2001;179(4):233–43. [DOI] [PubMed] [Google Scholar]
- 40.Lan NSH, Massam BD, Kulkarni SS, Lang CC. Pulmonary arterial hypertension: pathophysiology and treatment. Dis (Basel Switzerland). 2018;6(2):38. [DOI] [PMC free article] [PubMed]
- 41.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159(6):1925–32. [DOI] [PubMed] [Google Scholar]
- 42.Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, Yacoub MH, Polak JM. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Pathol. 1998;185(3):313–8. [DOI] [PubMed] [Google Scholar]
- 43.Maclean MR, Johnston ED, McCulloch KM, Pooley L, Houslay MD, Sweeney G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J Pharmacol Exp Ther. 1997;283(2):619–24. [PubMed] [Google Scholar]
- 44.Cornwell TL, Arnold E, Boerth NJ, Lincoln TM. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1994;267(5 Pt 1):C1405–1413. [DOI] [PubMed] [Google Scholar]
- 45.Marsh LM, Jandl K, Grünig G, Foris V, Bashir M, Ghanim B, Klepetko W, Olschewski H, Olschewski A, Kwapiszewska G. The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2018;51(1):1701214. [DOI] [PMC free article] [PubMed]
- 46.Aldabbous L, Abdul-Salam V, McKinnon T, Duluc L, Pepke-Zaba J, Southwood M, Ainscough AJ, Hadinnapola C, Wilkins MR, Toshner M, et al. Neutrophil extracellular traps promote angiogenesis: evidence from vascular pathology in pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2016;36(10):2078–87. [DOI] [PubMed] [Google Scholar]
- 47.Hurst LA, Dunmore BJ, Long L, Crosby A, Al-Lamki R, Deighton J, Southwood M, Yang X, Nikolic MZ, Herrera B, et al. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat Commun. 2017;8:14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Hiress M, Tu L, Ricard N, Phan C, Thuillet R, Fadel E, Dorfmüller P, Montani D, de Man F, Humbert M, et al. Proinflammatory signature of the dysfunctional endothelium in pulmonary hypertension. Role of the macrophage migration inhibitory Factor/CD74 complex. Am J Respir Crit Care Med. 2015;192(8):983–97. [DOI] [PubMed] [Google Scholar]
- 49.De Franceschi L, Platt OS, Malpeli G, Janin A, Scarpa A, Leboeuf C, Beuzard Y, Payen E, Brugnara C. Protective effects of phosphodiesterase-4 (PDE-4) Inhibition in the early phase of pulmonary arterial hypertension in Transgenic sickle cell mice. FASEB Journal: Official Publication Federation Am Soc Experimental Biology. 2008;22(6):1849–60. [DOI] [PubMed] [Google Scholar]
- 50.Hayes MM, Vedamurthy A, George G, Dweik R, Klings ES, Machado RF, Gladwin MT, Wilson KC, Thomson CC. Pulmonary hypertension in sickle cell disease. Annals Am Thorac Soc. 2014;11(9):1488–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Izikki M, Raffestin B, Klar J, Hatzelmann A, Marx D, Tenor H, Zadigue P, Adnot S, Eddahibi S. Effects of roflumilast, a phosphodiesterase-4 inhibitor, on hypoxia- and monocrotaline-induced pulmonary hypertension in rats. J Pharmacol Exp Ther. 2009;330(1):54–62. [DOI] [PubMed] [Google Scholar]
- 52.Shen LF, Lv XD, Chen WY, Yang Q, Fang ZX, Lu WF. Effect of Roflumilast on chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ir J Med Sci. 2018;187(3):731–8. [DOI] [PubMed] [Google Scholar]
- 53.Gamble E, Grootendorst DC, Brightling CE, Troy S, Qiu Y, Zhu J, Parker D, Matin D, Majumdar S, Vignola AM, et al. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(8):976–82. [DOI] [PubMed] [Google Scholar]
- 54.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91(16):7583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circul Res. 2003;93(4):280–91. [DOI] [PubMed] [Google Scholar]
- 56.Murad F. Cyclic Guanosine monophosphate as a mediator of vasodilation. J Clin Investig. 1986;78(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci. 2000;113(Pt 10):1671–6. [DOI] [PubMed] [Google Scholar]
- 58.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase ialpha. Sci (New York NY). 1999;286(5444):1583–7. [DOI] [PubMed] [Google Scholar]
- 59.Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, et al. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature. 2000;404(6774):197–201. [DOI] [PubMed] [Google Scholar]
- 60.Fukao M, Mason HS, Britton FC, Kenyon JL, Horowitz B, Keef KD. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at Serine 1072. J Biol Chem. 1999;274(16):10927–35. [DOI] [PubMed] [Google Scholar]
- 61.Schlossmann J, Feil R, Hofmann F. Signaling through NO and cGMP-dependent protein kinases. Ann Med. 2003;35(1):21–7. [DOI] [PubMed] [Google Scholar]
- 62.Novelli D, Fumagalli F, Staszewsky L, Ristagno G, Olivari D, Masson S, De Giorgio D, Ceriani S, Affatato R, De Logu F, et al. Monocrotaline-induced pulmonary arterial hypertension: Time-course of injury and comparative evaluation of Macitentan and Y-27632, a Rho kinase inhibitor. Eur J Pharmacol. 2019;865:172777. [DOI] [PubMed] [Google Scholar]
- 63.Kramer T, Dumitrescu D, Gerhardt F, Orlova K, Ten Freyhaus H, Hellmich M, Baldus S, Rosenkranz S. Therapeutic potential of phosphodiesterase type 5 inhibitors in heart failure with preserved ejection fraction and combined post- and pre-capillary pulmonary hypertension. Int J Cardiol. 2019;283:152–8. [DOI] [PubMed] [Google Scholar]
- 64.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, et al. Effect of phosphodiesterase-5 Inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study is a part of Necip Ermis’s doctoral thesis, which was finished in 2021 and was deposited in the Council of Higher Education Thesis Center (https://tez.yok.gov.tr/UlusalTezMerkezi/giris.jsp). The language of the main text is Turkish. Only the abstract is available in English.