Abstract
Background
The triglyceride-glucose index (TyG) has emerged as a reliable proxy for insulin resistance and metabolic dysfunction, showing associations with various health outcomes. While the relationship between metabolic health and respiratory function has been established, the association between TyG and lung function remains unclear, particularly in Asian populations. Therefore, we investigated whether TyG is associated with decreased lung function in a large sample of healthy Koreans.
Methods
We analyzed data from 89,809 healthy Korean adults (46,739 men, mean age: 38.5 years) who underwent health examinations in 2019, stratifying participants into quartiles based on their TyG index. Lung function impairment was defined using the lower limit of normal (LLN) derived from spirometric values at the fifth percentile of our population. We calculated adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for lung function impairment, using the lowest TyG quartile as the reference group.
Results
Mean TyG index was 8.34 ± 0.57. Subjects in the highest TyG quartile exhibited the lowest predicted forced expiratory volume in one second (FEV1%) and forced vital capacity (FVC%) after adjusting for covariates (P < 0.001). Also, FEV1(L) /FVC(L) ratio significantly differ among the four quartiles (P < 0.001). Compared to the lowest quartile (Q1), the aORs with 95% CI for FEV1% below the LLN across increasing quartiles (Q2 to Q4) were 1.150 (1.002–1.320), 1.272 (1.103–1.466), and 1.535 (1.310–1.799), respectively. For FVC% below the LLN, aORs were 1.233 (1.065–1.428), 1.334 (1.159–1.536), and 1.745 (1.506–2.021), respectively. Both trends were statistically significant (all P for trend < 0.001). In contrast, the aORs for FEV1/FVC below the LLN showed no significant differences among groups (P for trend = 0.186).
Conclusions
We found a significant association between higher TyG index values and decreased lung function in a large sample of healthy Koreans. Longitudinal studies are needed to establish causality and explore the long-term implications of this relationship on respiratory health.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-025-03869-6.
Keywords: Triglyceride-glucose index, Insulin resistance, Spirometry, Lung function, Healthy population
Introduction
Chronic lung diseases (CRDs) are leading causes of morbidity and mortality, and the incidence of these is increasing globally [1]. Additionally, most CRDs are irreversible. Thus, it is imperative to identify risk factors for their development to ensure timely interventions and an improved prognosis. As lung dysfunction often precedes overt CRDs [2], early identification and treatment of related modifiable factors is important to prevent various CRDs and their complications. Interestingly, metabolic syndrome (MS) can cause lung dysfunction and CRD progression [3–5]. Of the components of MS, insulin resistance (IR) is a critical mediator of the link between lung dysfunction and MS [6]. Furthermore, lung dysfunction predicts the development of IR [7], and vice versa [8]. Taken together, early detection of IR could contribute to preventive strategies for CRDs.
However, measurement of IR is challenging outside of research settings [9]. The hyperinsulinemic-euglycemic clamp test is the gold standard method for evaluating IR, but it is impractical as it is expensive and laborious [9]. Laboratory surrogates, such as the homeostatic model assessment of insulin resistance (HOMA-IR), require knowledge of the fasting insulin level and is relatively costly [9]. These methods are therefore unsuitable for large-scale screening of the general population. In contrast to these, the triglyceride-glucose index (TyG), based on a spot fasting glucose (FBS) and triglyceride (TG) measurement, is ordered routinely in clinical and screening settings, is a simple and inexpensive surrogate marker of IR, and has been used for large-scale screening of healthy subjects [10]. Furthermore, both glucotoxicity [11] and lipotoxicity [12] as assessed by TyG are among the classic risk markers of lung dysfunction. This has led to speculation that TyG could be an efficient surrogate marker for early identification of IR and thereby facilitate the identification of healthy people at high risk for lung dysfunction.
Till now, most studies on TyG have been performed in the context of cardiovascular-metabolic diseases (CVMDs) [13–17]. In contrast, only three studies to date [18–20] have attempted to evaluate the relationship between TyG and lung function in western populations, including in subjects with overt preexisting CVMDs and CRDs, which are related to impaired lung function [3, 4, 7, 21, 22], regardless of IR. A recent Chinese study was conducted on heathy subjects, but the sample size was relatively small [23]. Consequently, the effects of TyG on lung function remain largely uncharacterized, especially in Asians. Furthermore, the association between TyG and lung function in asymptomatic healthy subjects is unknown. Therefore, our aim in this study was to explore the relationship between TyG and lung function in a large group of healthy Koreans.
Methods
Study design and population
This cross-sectional study utilized data from the Kangbuk Samsung Health Study (KSHS) database, in which South Koreans aged 18 years or older have undergone a comprehensive annual or biennial health examination at the Total Healthcare Center of Kangbuk Samsung Hospital, Republic of Korea, since January 1, 2002. These screenings are mandated by South Korean law, specifically the Industrial Safety and Health Law, which requires employers to provide free health check-ups for their employees. Most examinees were employees and family members of various companies or local governmental organizations. The remaining participants voluntarily registered for screening examinations.
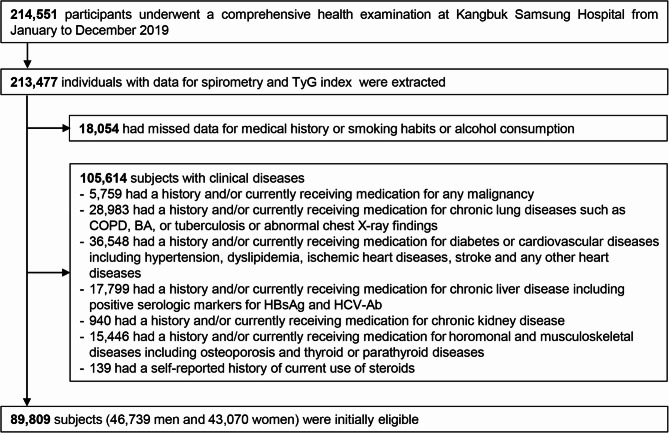
This study began with data from 214,551 subjects who underwent a comprehensive health examination in 2019. Inclusion criteria were participants aged 18 years or older with recorded spirometry and TyG values (n = 213,477). Among this cohort, we excluded subjects with missing medical history data and/or data about smoking habits or alcohol consumption (n = 18,054). We further excluded participants with either a self-reported history and/or those patients currently receiving treatment for any medical conditions (n = 105,614). However, detailed comorbidities were unavailable, because the medical history questionnaire only required yes/no responses. As some individuals had more than one exclusion criterion, 89,809 participants were finally included in the analysis (Fig. 1). We also compared participants with missing and non-missing data across key variables and found no significant differences, supporting the MCAR assumption (P = 0.0827).
Fig. 1.
Flow chart of the study participants. BA, bronchial asthma; COPD, chronic obstructive pulmonary disease; HBsAg, hepatitis B virus surface antigen; HCV-Ab, hepatitis C virus antibody; TyG, triglyceride-glucose index
The study was reviewed and approved by the Institutional Review Board of Kangbuk Samsung Hospital (KBSMC 2024-01-038). Given that the study involved the analysis of de-identified data obtained during routine health screening examination, the requirement for informed consent was waived by the IRB. This study also complied with the 1975 Declaration of Helsinki and its later amendments and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Data collection, anthropometric measurements, and laboratory tests
The comprehensive health-screening program collected socio-demographic data, anthropometric measurements, behavioral factors, and laboratory data. Information on demographics, smoking and drinking habits, exercise frequency, medical history and medication use were obtained by standardized, self-administered questionnaires. Subjects were classified as nonsmokers, ex-smokers or current smokers based on their self-reported smoking habits. Average alcohol consumption was calculated based on the frequency and amount of alcohol consumed per drinking day and then categorized as none, non-heavy (< 20 g ethanol/day), or heavy (≥ 20 g ethanol/day) [24]. Weekly frequency of moderate physical activity (defined as more than 30 min of activity per day inducing slight breathlessness) was also assessed, and regular exercise was defined as exercising ≥ 3 times/week [24].
Physical characteristics and serum biochemical parameters were measured by trained nurses as reported previously [6, 24]. Height and weight were determined with individuals shoeless and wearing a lightweight hospital gown, using automated instruments (InBody 3.0 and Inbody 720, Biospace Co., Seoul, Republic of Korea). Waist circumference (WC) was measured at a level midline between the lower rib margin and iliac crest. Body mass index (BMI) was calculated by dividing weight (kg) by the square of height (m2). Obesity was defined as a body mass index (BMI) ≥ 25 kg/m2 [25]. Blood pressure (BP) was measured with a standard sphygmomanometer after at least 5 min of seated rest. Measurements were performed twice at 5-minute intervals and averaged for analysis. Mean BP (MBP) was calculated as diastolic BP + 0.33 × (systolic BP − diastolic BP).
Blood sample was taken after at least a 10-h fast. Methods for measuring serum levels of liver enzymes, creatinine, lipid profiles, FBS, and glycated hemoglobin (HbA1c) have been described previously [6, 24]. Total coefficients of variation for these measurements were < 5% for both the normal range control and high-level quality control materials during the study period, respectively. TG was determined by an enzymatic colorimetric assay using a Cobas 8000 c702 chemistry analyzer (Roche Diagnostics, Tokyo, Japan). FBS was measured using the hexokinase method on a Cobas Integra 800 apparatus (Roche Diagnostics). Total coefficients of variation for TG and FBS were 0.94–1.36% and 0.65–1.63% for the low-level quality control materials and 0.90–1.16% and 0.55–1.08% for the high-level quality control materials, respectively. TyG was calculated as the natural logarithm of [TG (mg/dl) × FBS (mg/dl)/2] [10]. Metabolic syndrome (MS) was defined using the National Cholesterol Education Program Adult Treatment Panel III (NECP-ATPIII) [26]. The Laboratory Medicine Department at Kangbuk Samsung Hospital, where all laboratory analyses were conducted, is accredited and participates annually in inspections and surveys by the Korean Association of Quality Assurance for Clinical Laboratories.
Lung function measurements
Spirometry was conducted in accordance with the guidelines provided by the American Thoracic Society/European Respiratory Society guidelines [22], using the Vmax22 system (Sensor-Medics, Yorba Linda, CA, USA). The methods for spirometry tests were reported previously [6, 27]. Predicted values for forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were calculated using equations for a representative Korean population sample [28]. Regarding the predicted FEV1% (FEV1%) and FVC% (FVC%), we divided the measured value (L) by the predicted value (L) and converted the quotient into a percentage. Ratio of FEV1 to FVC (FEV1/FVC) was calculated using the actual measurements.
Spirometric values at the fifth percentile in our population were considered the lower limit of normal (LLN) range, as in a previous study [29]. Impaired lung function was defined as FEV1% or FVC% less than the LLN, and obstructive lung function (OLF) was defined as FEV1/FVC < LLN [22].
Statistical analyses
Demographic and clinical characteristics of the study subjects were compared among groups by gender and quartiles of TyG. Data are expressed as means ± standard deviations or medians and interquartile ranges for continuous variables and as numbers (%) for categorical variables. The normality of continuous variables was checked with the Kolmogorov-Smirnov test. Differences in characteristics stratified by TyG quartiles were examined by Chi-Square test or Fisher’s exact test for categorical variables, and one-way analysis of variance or the Kruskal Wallis test for continuous variables. Analysis of covariance (ANCOVA) was performed to test the significance of differences in mean values of lung function parameters among study groups after adjusting for age, sex, BMI, waist circumference, and continuous variables with P < 0.05 in univariate analyses. Post hoc analysis was performed using the Bonferroni correction to compare mean spirometric values between study groups. We also conducted multiple linear regression analyses treating both TyG and lung function parameters as continuous variables. To control for potential confounding factors, we adjusted for age, sex, BMI, WC, smoking (pack-years), alcohol consumption (g/day), moderate physical activity frequency (times/week), MBP, HbA1c, lipid profiles, liver enzymes, and creatinine.
To analyze the significance of differences among groups by quartiles of TyG, all covariates were transformed into categorical variables: high or low and with or without. Differences among four groups were tested using the chi square test or Fisher’s exact test. Multivariate analysis using binary logistic regression was performed to estimate odds ratios (ORs) with 95% confidence intervals (CI) for lung dysfunction in quartiles 2–4 of TyG, with the lowest (1st) quartile as the reference group. We fitted three models to progressively adjust for potential confounders: model 1 was adjusted for age, sex, smoking, alcohol habits and regular exercise; model 2 was adjusted as for model 1 plus for metabolic components including BMI, waist circumference, MBP, HbA1c, total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol and the presence of MS; model 3 was adjusted as in model 2 plus for variables with P < 0.050 in univariate analyses. In the subgroup analysis, categorical and nominalized continuous variables were used. The modulatory effect was confirmed by adding the interaction term of variables defining the subgroup to the multivariate regression analysis. Because FVC (L) and FEV1 (L) were strongly correlated (r = 0.943, P < 0.001), these parameters were assessed separately to avoid confounding effects. All tests were two-sided, and P values < 0.050 were considered statistically significant. Data were analyzed using IBM SPSS Statistics 24.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics of the study population
The characteristics of the 89,809 eligible subjects (52.0% male, 38.5 ± 7.3 years) are summarized according to gender in Table 1. Mean BMI and TyG were 23.2 ± 3.3 kg/m2. The prevalence of MS was higher in men (26.3%) than women (9.4%). The mean TyG level was 8.59 in men and 8.14 in women. Men were more likely to have smoked, and to drink alcohol, and had higher blood pressure and worse levels of metabolic markers, compared with women. Regarding spirometric parameters, values of FEV1, FEV1%, FVC, and FVC% was higher and FEV1/FVC ratio was lower in men than women (P < 0.001).
Table 1.
Baseline characteristics of study participants by gender
| Characteristics | Overall Subjects (n = 89,809) |
Male (n = 46,739) |
Female (n = 43,070) |
p value | ||
|---|---|---|---|---|---|---|
| Age (years) | 38.5 ± 7.3 | 39.1 ± 7.3 | 37.9 ± 7.4 | < 0.001 | ||
| Waist circumference (cm) (n = 89,804) | 80.2 ± 9.5 | 85.4 ± 7.7 | 74.6 ± 7.9 | < 0.001 | ||
| BMI (kg/m2) | 23.2 ± 3.3 | 24.6 ± 2.9 | 21.8 ± 3.0 | < 0.001 | ||
| Smoking (pack-years) | 0.0 (0.0–10.0) | 7.0 (0.0–18.0) | 0.0 (0.0–0.0) | < 0.001 | ||
| Smoking habits | ||||||
| Non smoker | 55,460 (61.8) | 16,277 (34.8) | 39,183 (91.0) | < 0.001 | ||
| Former smoker | 20,574 (22.9) | 17,560 (37.6) | 3014 (7.0) | < 0.001 | ||
| Current smoker | 13,775 (15.3) | 12,902 (27.6) | 873 (2.0) | < 0.001 | ||
| Alcohol consumption (g/day) | 6.0 (2.0–14.0) | 10.0 (4.0–25.0) | 3.0 (1.0–6.0) | < 0.001 | ||
| Alcohol intake | ||||||
| Non | 7197 (8.0) | 1730 (3.7) | 5467 (12.7) | < 0.001 | ||
| moderate (≤ 20 g) | 66,183 (73.7) | 31,464 (67.3) | 34,719 (80.6) | < 0.001 | ||
| heavy (> 20) | 16,429 (18.3) | 13,545 (29.0) | 2884 (6.7) | < 0.001 | ||
|
Moderate physical activity frequency (times/week) (n = 89,544) |
0.0 (0.0–1.0) | 0.0 (0.0–2.0) | 0.0 (0.0–1.0) | < 0.001 | ||
| Total bilirubin (mg/dL) (n = 89,794) | 0.79 ± 0.36 | 0.90 ± 0.37 | 0.67 ± 0.30 | < 0.001 | ||
| ALT (U/L) (n = 89,648) | 17 (12–25) | 22 (17–32) | 13 (10–17) | < 0.001 | ||
| Serum creatinine (mg/dL) | 0.80 ± 0.17 | 0.92 ± 0.12 | 0.66 ± 0.10 | < 0.001 | ||
| Total cholesterol (mg/dL) | 192 ± 31 | 197 ± 31 | 187 ± 30 | < 0.001 | ||
| Triglyceridemia (mg/dL) | 89 (64–130) | 111 (79–160) | 73 (56–97) | < 0.001 | ||
| HDL cholesterol (mg/dL) (n = 89,807) | 62 ± 17 | 55 ± 14 | 70 ± 16 | < 0.001 | ||
| LDL cholesterol (mg/dL) (n = 89,806) | 123 ± 30 | 132 ± 29 | 114 ± 29 | < 0.001 | ||
| Fasting glucose (mg/dl) | 93 ± 10.7 | 96 ± 11 | 91 ± 9 | < 0.001 | ||
| HbA1c (%) (n = 89,805) | 5.4 ± 0.4 | 5.5 ± 0.4 | 5.4 ± 0.3 | |||
| Systolic BP (mmHg) | 108 ± 12 | 114 ± 11 | 102 ± 10 | < 0.001 | ||
| Diastolic BP (mmHg) | 70 ± 9 | 74 ± 9 | 66 ± 8 | < 0.001 | ||
| MBP | 83 ± 10 | 87 ± 9 | 78 ± 8 | < 0.001 | ||
| Metabolic syndrome | 16,369 (18.2) | 12,300 (26.3) | 4069 (9.4) | < 0.001 | ||
| TyG index | 8.34 ± 0.57 | 8.59 ± 0.57 | 8.14 ± 0.47 | < 0.001 | ||
| Measured FEV1 (L) | 3.30 ± 0.68 | 3.79 ± 0.51 | 2.76 ± 0.37 | < 0.001 | ||
| FEV1% | 98.00 ± 10.71 | 99.09 ± 10.96 | 97.01 ± 10.37 | < 0.001 | ||
| Measured FVC (L) | 4.00 ± 0.87 | 4.66 ± 0.61 | 3.28 ± 0.42 | < 0.001 | ||
| FVC% | 98.31 ± 10.69 | 99.70 ± 10.96 | 97.03 ± 10.09 | < 0.001 | ||
| FEV1(L)/FVC(L) ratio | 0.829 ± 0.059 | 0.814 ± 0.056 | 0.845 ± 0.058 | < 0.001 | ||
Data are presented as means ± standard deviations, medians (interquartile range), or number of subjects with percentages in parenthesis. We recorded subject numbers with available clinical parameters. Unless otherwise indicated, the available subject number was 89,809. Detailed documentation on missing values for some variables is unavailable, given that our study utilized pre-existing health check-up data. However, the MCAR test became non-significant (p = 0.0827), indicating that the data were missing completely at random
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; BP, blood pressure; FEV1%, percent predicted forced expiratory volume in 1 s; FVC%, percent predicted forced vital capacity; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MBP, mean blood pressure; MCAR, Missing Completely at Random; TyG, triglyceride glucose index. MBP = diastolic BP (average systolic BP-average diastolic BP)/3
Lung function according to quartile of TyG
Table 2 displays the comparison of lung function parameters between study groups after adjusting for multiple covariates (age, sex, BMI, waist circumference, smoking (pack-years), alcohol consumption (g/day), moderate physical activity frequency (times/ week), MBP, HbA1c, lipid profiles, liver enzymes, and creatinine). The highest quartile group had the lowest FEV1 (L), FEV1%, FVC (L), FVC% and FEV1 (L) / FVC (L) ratio values (P < 0.001). All these spirometric values were significantly different among groups (P < 0.001). (Fig. 2). In addition, the fully adjusted multivariate linear regression models for the associations were presented in Supplementary Table 1. TyG was significantly inversely correlated with lung function values including FEV1 (L), FEV1%, FVC (L), FVC% and FEV1 (L) / FVC (L). Specifically, per one unit increased TyG was associated with decline in FEV1 (L) (β: − 48 ml, 95% CI: − 56 ml to– 39ml, P < 0.001), FEV1% (β: − 0.928, 95% CI: − 1.151 to − 0.705, P < 0.001), FVC (L) (β: − 31 ml, 95% CI: − 41 ml to − 0.20ml, P < 0.001 ), FVC% ( β: − 0.230, 95% CI: − 0.450 to − 0.011, P = 0.040) and FEV1(L)/FVC(L) ratio (β: − 0.006, 95% CI: − 0.007 to − 0.004, P < 0.001 ).
Table 2.
Adjusted mean values of lung function parameters according to quartile of TyG
| Category | p value by ANCOVA | Adjusted p valuea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (≤ 7.97) (n = 22,572) |
Q2 (7.98–8.33) (n = 22,784) |
Q3 (8.34–8.73) (n = 21,874) |
Q4 (≥ 8.74) (n = 22,579) |
Q1 vs. Q2 | Q1 vs. Q3 | Q1 vs. Q4 | Q2 vs. Q3 | Q2 vs. Q4 | Q3 vs. Q4 | ||
| FEV1 (L) | 3.333 ± 0.003 | 3.322 ± 0.002 | 3.313 ± 0.002 | 3.296 ± 0.002 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.002 | < 0.001 | 0.002 |
| FEV1% | 98.465 ± 0.079 | 98.149 ± 0.069 | 97.790 ± 0.075 | 97.360 ± 0.114 | < 0.001 | 0.007 | < 0.001 | < 0.001 | 0.003 | < 0.001 | 0.004 |
| FVC (L) | 4.060 ± 0.004 | 4.033 ± 0.003 | 4.010 ± 0.002 | 3.981 ± 0.003 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| FVC% | 98.963 ± 0.029 | 98.640 ± 0.019 | 98.387 ± 0.018 | 98.313 ± 0.020 | < 0.001 | 0.020 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| FEV1(L)/FVC(L) ratio | 0.834 ± 0.001 | 0.828 ± 0.001 | 0.826 ± 0.001 | 0.825 ± 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.009 | 0.003 | 0.785 |
Data are presented as adjusted means ± standard errors. The multivariable model was adjusted for age, sex, body mass index, waist circumference, and continuous variables with p < 0.05 in univariate analyses, comprising smoking (pack-years), alcohol consumption (g/day), moderate physical activity frequency (times/week), mean blood pressure, hemoglobin A1c, lipid profiles, liver enzymes, and creatinine
Abbreviations: FEV1%, percent predicted forced expiratory volume in 1 s; FVC%, percent predicted forced vital capacity; TyG, triglyceride glucose index
aAdjusted p values using Bonferroni correction
Fig. 2.
Adjusted mean values of spirometric values (standard errors) according to quartile of TyG. (A) FEV1 (L); (B) predicted FEV1 (%); (C) FVC (L); (D) predicted FVC (%); (E) FEV1/FVC. Q1 is the lowest quartile. The multivariable model was adjusted for age, sex, body mass index and continuous variables with p < 0.05 in univariate analyses. FEV1%, percent predicted forced expiratory volume in 1 s; FVC%, percent predicted forced vital capacity
Comparison of clinical and laboratory parameters among groups stratified by quartile of TyG
A comparison of clinical and laboratory characteristics among the four groups divided by TyG quartile is presented in Table 3. Subjects in higher TyG quartiles were older, more obese and more likely to be men, to have smoked, and to drink alcohol (all P < 0.001). Furthermore, the prevalence of unfavorable cardio-metabolic parameters such as lipid profiles, FBS, HbA1c and MBP increased with increasing quartile of TyG (P < 0.001), whereas total bilirubin decreased. Similarly, MS was more prevalent in the higher TyG groups (p < 0.001). Additionally, there were statistically significant differences in liver enzyme and creatinine levels among groups. With respect to lung function parameters, the prevalence of impaired lung function increased significantly across quartiles (from Q2 to Q4) (P < 0.001) compared with the lowest quartile group (Q1) (Table 3).
Table 3.
Comparisons of demographic and clinical parameters between four groups stratified by quartile of TyG index
| All subjects (N = 89,809) |
Q1 (≤ 7.97) (N = 22,572) |
Q2 (7.98–8.33) (N = 22,784) |
Q3 (8.34–8.73) (N = 21,874) |
Q4 (≥ 8.74) (N = 22,579) |
P value | |
|---|---|---|---|---|---|---|
| Age (≥ 39 years) | 39,361 (43.8) | 7,498 (33.2) | 9,349 (41.0) | 10,387 (47.5) | 12,127 (53.7) | < 0.001 |
| Sex (male) | 46,739 (52.0) | 6,141 (27.2) | 9,314 (40.9) | 13,122 (60.0) | 18,162 (80.4) | < 0.001 |
| High waist circumference (N = 89,804)a | 19,967 (22.2) | 2,098 (9.3) | 3,538 (15.5) | 5,450 (24.9) | 8,881 (39.3) | < 0.001 |
| BMI (≥ 25 kg/m2 = obesity) | 23,981 (26.7) | 1,998 (8.9) | 3,937 (17.3) | 6,464 (29.6) | 11,582 (51.3) | < 0.001 |
| Current smokers | 13,775 (15.3) | 1,427 (6.3) | 2,365 (10.4) | 3,638 (16.6) | 6,345 (28.1) | < 0.001 |
| Heavy alcohol intake (> 20 g/day) | 16,429 (18.3) | 2,399 (10.6) | 3,143 (13.8) | 4,227 (19.3) | 6,660 (29.5) | < 0.001 |
| Regular exercise (≥ 3 times/week) (N = 89,544) | 11,805 (13.2) | 3,497 (15.5) | 3,056 (13.5) | 2,786 (12.8) | 2,466 (11.0) | < 0.001 |
| Elevated bilirubin (> 1.9 mg/dL) (N = 89,794) | 1,192 (1.3) | 370 (1.6) | 301 (1.3) | 267 (1.2) | 254 (1.1) | < 0.001 |
| Elevated ALT (> 40 U/L) (N = 89,648) | 7,434 (8.3) | 491 (2.2) | 801 (3.5) | 1,699 (7.8) | 4,443 (19.7) | < 0.001 |
| Elevated serum creatinine (> 1.2 mg/dL) | 209 (0.2) | 24 (0.1) | 43 (0.2) | 61 (0.3) | 81 (0.4) | < 0.001 |
| Hypercholesterolemia (≥ 220 mg/dL) | 15,296 (17.0) | 1,626 (7.2) | 2,611 (11.5) | 4,003 (18.3) | 7,056 (31.3) | < 0.001 |
| Hypertriglyceridemia (≥ 150 mg/dL) | 16,348 (18.2) | 0 (0.0) | 0 (0.0) | 32 (0.1) | 16,316 (72.3) | < 0.001 |
| Low HDL cholesterolb (n = 89,807) | 20,039 (22.3) | 792 (3.5) | 2,150 (9.4) | 4,973 (22.7) | 12,124 (53.7) | < 0.001 |
| Hyper LDL cholesterol (≥ 159 mg/dL)(N = 89,806) | 10,434 (11.6) | 680 (3.0) | 1,476 (6.5) | 3,035 (13.9) | 5,243 (23.2) | < 0.001 |
| Hyperglycemia after fasting (≥ 100 mg/dl) | 14,297 (15.9) | 640 (2.8) | 1,965 (8.6) | 3,735 (17.1) | 7,957 (35.2) | < 0.001 |
| Metabolic syndrome | 16,369 (18.2) | 210 (0.9) | 821 (3.6) | 2,275 (10.4) | 13,063 (57.9) | < 0.001 |
| HbA1c ≥ 6.5 (N = 89,805) | 568 (0.6) | 3 (0.0) | 14 (0.1) | 52 (0.2) | 499 (2.2) | < 0.001 |
| MBP ≥ 83 | 40,252 (44.8) | 5,421 (24.0) | 8,067 (35.4) | 10,872 (49.7) | 15,892 (70.4) | < 0.001 |
| FVC% < LLNc | 3,095 (3.4) | 655 (2.9) | 706 (3.1) | 809 (3.7) | 925 (4.1) | < 0.001 |
| FEV1% < LLNc | 3,564 (4.0) | 712 (3.2) | 799 (3.5) | 872 (4.0) | 1,181 (5.2) | < 0.001 |
| FEV1(L)/FVC(L) ratio < LLNc | 1,499 (1.7) | 224 (1.0) | 325 (1.4) | 412 (1.9) | 538 (2.4) | < 0.001 |
Data are presented as the number of subjects with percentages in parentheses. We recorded subject numbers with available clinical parameters. Unless otherwise indicated, the available subject number was 89,809. Detailed documentation on missing values for some variables is unavailable, given that our study utilized pre-existing health check-up data. However, the MCAR test became non-significant (p = 0.0827), indicating that the data were missing completely at random
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; BP, blood pressure; FEV1%, percent predicted forced expiratory volume in 1 s; FVC%, percent predicted forced vital capacity; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MBP, mean blood pressure; MCAR, Missing Completely at Random; TyG, triglyceride glucose index. MBP = diastolic BP (average systolic BP-average diastolic BP
aHigh waist circumference was defined as ≥ 90 cm in males and ≥ 85 cm in females
bLow HDL was defined as < 40 mg/dL in male and < 50 mg/d/L in female
cThe value of LLN was 81%, 82% and 73% for FEV1%, FVC% and FEV1(L)/FVC(L) ratio, respectively
Odds ratios (ORs) for lung dysfunction according to quartile of TyG
Multiple regression analysis was performed to assess the effects of TyG on lung dysfunction. According to the fully adjusted logistic regression analysis (model 3), the risk of having lung dysfunction was higher for subjects with a higher TyG, using the lowest quartile of TyG as a reference (Table 4).
Table 4.
Multiple logistic regression analysis of impaired lung function by quartile of TyG
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | p for trend | OR (95% CI) | p value | p for trend | OR (95% CI) | p value | p for trend | |
| FEV1%< LLN | < 0.001 | < 0.001 | < 0.001 | ||||||
| Q1 (0-0.5233) [reference] | 1 | 1 | 1 | ||||||
| Q2 (0.5234–0.6612) | 1.160 (1.015–1.331) | 0.035 | 1.152 (1.004–1.323) | 0.044 | 1.150 (1.002–1.320) | 0.048 | |||
| Q3 (0.6613–0.8319) | 1.315 (1.143–1.514) | < 0.001 | 1.277 (1.109–1.471) | < 0.001 | 1.272 (1.103–1.466) | < 0.001 | |||
| Q4 (≥ 0.8320) | 1.697 (1.461–1.972) | < 0.001 | 1.546 (1.321–1.806) | < 0.001 | 1.535 (1.310–1.799) | < 0.001 | |||
| FVC%< LLN | < 0.001 | < 0.001 | < 0.001 | ||||||
| Q1 (0-0.5233) [reference] | 1 | 1 | 1 | ||||||
| Q2 (0.5234–0.6612) | 1.259 (1.111–1.428) | < 0.001 | 1.239 (1.093–1.405) | < 0.001 | 1.233 (1.065–1.428) | 0.005 | |||
| Q3 (0.6613–0.8319) | 1.456 (1.293–1.640) | < 0.001 | 1.401 (1.242–1.580) | < 0.001 | 1.334 (1.159–1.536) | < 0.001 | |||
| Q4 (≥ 0.8320) | 1.945 (1.730–2.186) | < 0.001 | 1.784 (1.577–2.019) | < 0.001 | 1.745 (1.506–2.021) | < 0.001 | |||
| FEV1(L)/FVC(L) ratio < LLN | 0.563 | 0.381 | 0.186 | ||||||
| Q1 (0-0.5233) [reference] | 1 | 1 | 1 | ||||||
| Q2 (0.5234–0.6612) | 0.888 (0.744–1.060) | 0.187 | 0.884 (0.741–1.055) | 0.173 | 0.879 (0.717–1.078) | 0.215 | |||
| Q3 (0.6613–0.8319) | 0.886 (0.744–1.055) | 0.173 | 0.875 (0.773–1.044) | 0.137 | 0.873 (0.712–1.070) | 0.191 | |||
| Q4 (≥ 0.8320) | 0.922 (0.773–1.090) | 0.366 | 0.901 (0.747–1.087) | 0.278 | 0.847 (0.679–1.057) | 0.141 | |||
Model 1 was adjusted for age, sex, smoking status, heavy alcohol intake and regular exercise. Model 2 was adjusted as in model 1 plus metabolic components including BMI, waist circumference, MBP, HbA1c, total cholesterol, high-density lipoprotein cholesterol, low density lipoprotein cholesterol, and the presence of metabolic syndrome. Model 3 was adjusted as in model 2 plus variables with a p value < 0.05 in the univariate analyses. LLN was 81%, 82% and 73% for FEV1%, FVC % and FEV1(L)/FVC(L), respectively
Abbreviations: BMI, body mass index; FEV1%, percent predicted forced expiratory volume in 1 s; FVC%, percent predicted forced vital capacity; HbA1c, hemoglobin A1c; LLN, lower limit of normal; MBP, mean blood pressure; TyG, triglyceride glucose index. MBP = diastolic BP(average systolic BP-average diastolic BP)/3
Specifically, the aORs (95% CIs) for having FEV1% < LLN across increasing quartiles (from Q2 to Q4) were 1.150 (1.002–1.320), 1.272 (1.103–1.466), and 1.535 (1.310–1.799), respectively (P for trend < 0.001). Similar results were observed for FVC% < LLN (P for trend < 0.001). However, the difference in aORs for OLF among groups were consistently not significant (P for trend = 0.186). (Fig. 3).
Fig. 3.
Multivariable-adjusted odds ratios for lung dysfunction according to quartile of lung TyG. aOR for impaired lung function (FEV1% < LLN (A) and FVC% < LLN (B)) increased with increasing quartile (Q2-4) of TyG. However, difference in aORs for FEV1(L)/FVC(L) < LLN among groups were consistently not significant. Reference values were set as the lowest quartile (Q1) of TyG. Models were adjusted for potential covariates and metabolic laboratory markers (age, sex, smoking status, heavy alcohol intake, regular exercise, metabolic components and variables with a p value < 0.05 in univariate analyses). LLN was 81%, 82%, and 73% for FEV1%, FVC % and FEV1(L)/FVC(L), respectively. FEV1%, percent predicted forced expiratory volume in 1 s; FVC%, percent predicted forced vital capacity; LLN, lower limit of normal
In subgroup analyses, as part of a sensitivity analysis, the association between TyG and decreased lung function was more pronounced in males than females. However, no statistically significant interaction was observed for other covariates. (Supplementary Table 2)
Discussion
Our findings underscore the relationship between metabolic health and lung function in a healthy Korean population. The inverse association between TyG index and both FEV1 and FVC suggests that metabolic dysfunction may play a crucial role in lung health, even in individuals without overt clinical diseases. This relationship highlights the potential utility of the TyG index as an early predictor of lung dysfunction. By extending these observations to a Korean population, our study broadens the applicability of previous research predominantly conducted in Western cohorts. These results emphasize the importance of considering metabolic parameters in assessing and maintaining impaired lung health across diverse populations [3, 5].
To date, only four studies have evaluated the relationship between TyG and lung function [18–20, 23]. Our findings are in accordance with the results of previous studies with the exception of Wu et al.’s study where TyG was not associated with lung function after additional adjustment for HOMA-IR or metabolic syndrome [18]. However, careful consideration of the demographics of the study subjects is required when assessing the relationship between TyG and lung function. Compared to ours and the other two studies [19, 23], Wu et al.’ s study [18] included much more subjects with CVMDs and CRDs, both of which affect lung function [3, 4, 7, 21, 22], and IR [5, 13–17, 30]. IR is more common in patients with CVMDs and CRDs than healthy subjects. Furthermore, these patients are more prone to lung dysfunction. Therefore, there is a good chance that the association between TyG and lung function may have been distorted in Wu et al.’s study [18]. Additionally, the association between TyG and lung function in this previous study was likely attenuated by additional adjustments for markers of metabolic dysfunction. It should also be noted that the median age of enrolled subjects in previous studies (more than 56.9 years of age) [18, 19] was much higher than that of our study (38.5 years ) and Yang et al.’s study (45 years) [23]. IR is associated with aging, and aging in turn causes premature airway closure, which can result in airway injury [31]. The aging process may potentiate the metabolic hazard of IR in lung function deterioration. This could have consequently affected the study findings. Taken together, it is necessary to explore the relationship between TyG and lung function in younger healthy population like ours and Yang and colleagues’ [23]. However, Yang et al.’s study [23] including previous studies [18–20], potentially overlooked important biological differences of gender. In contrast, our study identified a more pronounced association in males, suggesting a possible effect modification by sex. This sex-based difference may be explained by sex hormone and differences in fat distribution. Given that the median age of our study population was 38.5 years, a substantial proportion of the female participants were likely premenopausal. Estrogen is known to enhance insulin sensitivity and modulate lipid metabolism, potentially offering a protective effect against TyG-associated metabolic impairment of lung function [32]. Compared to females, males may be more susceptible to TyG-related metabolic stress due to higher visceral fat accumulation and lower baseline insulin sensitivity [33]. These findings may reflect sex differences in metabolic regulation and respiratory outcomes, particularly in a relatively young population. However, further studies that include postmenopausal women and longitudinal designs may further clarify the modifying effects of sex hormones. Additionally, our study was designed to evaluate the relation between TyG and lung function in a large sample of Asian subjects (n = 89,809), compared to Yang et al.’s study (n = 696) [23]. Previous studies in Western subjects [18–20] cannot necessarily be extrapolated to other populations. Of note, research exploring novel risk-prediction variables in diverse global populations is important. Thus, our results are more relevant to the general population than those of the previous studies [18–20, 23], and extend the utility of TyG to the Oriental population.
Interestingly, previous studies [18, 20] found no association between TyG and OLF when analyzed as a continuous [20] or dichotomizing measure [18]. In contrast, we observed an interesting discrepancy regarding the association between TyG and the FEV1/FVC ratio depending on the analytic method and variable treatment. When TyG was categorized into quartiles and analyzed via logistic regression with FEV1/FVC < LLN as the outcome, no statistically significant association was observed. However, when TyG was treated as a continuous variable in ANCOVA and linear regression models, a significant inverse association with FEV1/FVC was detected. This difference may stem from methodological factors. Logistic regression with dichotomized outcomes such as FEV1/FVC < LLN may reduce statistical power and potentially mask weaker associations, particularly when the prevalence of the event is relatively low as like our study population (1.7%). In contrast, treating both TyG and lung function parameters as continuous variables in ANCOVA or linear models retains more variability and may detect subtler associations. This approach aligns with previous research suggesting that continuous variable modeling can uncover associations that categorical analyses might overlook [34]. Furthermore, we propose some plausible explanations for why lung physiology is less sensitive to early OLF changes resulting from metabolic insults, compared to FEV1 or FVC. First, TyG was negatively associated with both FEV1 and FVC, resulting in a null net effect on the ratio of these two measures. Second, OLF might be more closely associated with systemic inflammation [35] than IR [36], although IR is also associated with chronic low-grade inflammation [37]. However, it is unclear how much of the IR-related effects occur independently of systemic inflammation and vice versa, because IR is associated with chronic low-grade inflammation [37]. Third, functional debility of the airways might have gone undetected on screening spirometry especially in our healthy subjects, because OLF predominantly reflects obstruction of large airways [38]. Therefore, careful consideration is required when assessing OLF based on screening spirometry, especially in healthy young and middle-aged subjects.
Pathophysiologically, the current results are consistent with recent evidences indicating that IR negatively impacts lung function [6, 8, 39]. IR can contribute to chronic low-grade inflammation, oxidative stress, and cellular dysfunction in non-adipose tissues, including the lungs [3, 5, 37]. These pathophysiological changes can ultimately lead to a decline in lung function. Although the exact mechanisms by which TyG, a surrogate marker of IR, affects lung function remain unclear, the underlying mechanism can be explained by the crucial roles of glucotoxicity and lipotoxicity associated with the modulation of IR [10, 40] and lung function impairment [11, 12]. Glucotoxicity, due to persistent hyperglycemia, results in the formation of advanced glycation end-products (AGEs), which bind to RAGE (receptors for AGEs) expressed in alveolar and endothelial cells. This interaction triggers reactive oxygen species (ROS) production and further activates nuclear factor κB (NF-κB), contributing to chronic inflammation and structural remodeling of the lung parenchyma [41]. In addition, lipotoxicity arises from excessive free fatty acid (FFA) accumulation in pulmonary tissues, particularly type II alveolar epithelial cells and alveolar macrophages. This leads to mitochondrial dysfunction, surfactant synthesis disruption, activation of inflammatory signaling pathways and adhesion of inflammatory cells to the pulmonary capillary endothelium [3]. Finally, both glucotoxicity and lipotoxicity can cause airway damage and a decrease in lung function. Considering the above mechanisms, decreased lung function could be viewed as the pulmonary manifestation of IR. Therefore, TyG can serve as an indicator of impaired lung function.
We demonstrated a significant association between IR as assessed using TyG and lung dysfunction in this study. Our findings have several clinical implications. Lung dysfunction is a maker of CRDs and CVMDs [7, 21, 22], which are potentially preventable diseases with significant health and economic impacts worldwide [1, 42]. Therefore, this study is clinically important in that early detection and modulation of IR, especially in the healthy population, can reduce mortality risk related to respiratory and other non-respiratory complications. Furthermore, TyG can easily be used to screen for IR, whether in an apparently healthy population or patients with comorbidities, which is important given the increasing public health impact of decreased lung function [7, 21, 22] and the increasing prevalence of obesity and metabolic syndrome-related IR [26]. However, we found no association between TyG and OLF. Thus, the association between TyG and lung function is non-specific and could reflect reduced effort, restrictive impairment, or be an early consequence of small airway disease [22]. However, our finding is less likely related to reduced effort given that our participants were generally highly educated and asymptomatic. Furthermore, ANCOVA analysis treating FEV1/FVC ratio as continuous variables showed statistical significance, in contrast to logistic regression with dichotomized outcomes (OLF). Given that dichotomizing outcomes may lead to reduce statistical power and obscure subtle differences that could be detected with continuous measurement [34], our results suggest possible subclinical airflow limitation, supporting the small airway remodeling mechanism as described by McDonough et al. [43]. Therefore, our findings have important clinical implications given the clinical significance of restrictive ventilatory impairment [7, 44] and small airway disease [43]. Major strengths of our study are its large sample size and focus on healthy subjects without overt disease. Another strength is that we adjusted various confounders that may have impacted the results. This gave us increased precision and sufficient statistical power and makes our findings more relevant to healthy individuals. However, TyG thresholds vary significantly depending on ethnicity, study populations and outcome conditions and currently lacks universally accepted thresholds [45]. Thus, further studies are needed to determine standardized and population-specific cut-off values that can enhance clinical utility and interpretability of the TyG in assessing lung health, given the absence of established reference cut-off values for the TyG in relation to lung function outcomes.
Our study also has several limitations. First, the cross sectional nature of this study precludes determination of a causal relationship between TyG and lung dysfunction. Hence, further longitudinal follow-up studies are needed to validate our findings. Second, medical histories were self-reported, which may have resulted in reporting bias. This might have affected outcomes, as subclinical diseases can contribute to lung dysfunction, especially in individuals with IR [3, 4, 7, 13–17, 21, 22, 30]. Due to the cross sectional nature of current study, the results may also have been skewed by unadjusted confounders, such as dietary factors, passive smoking, occupational exposures and other clinical characteristics of the study population that can affect TyG and lung function [20, 46], despite controlling for several confounders. More studies with a prospective design are therefore needed. Third, we cannot exclude the possibility of intra-individual changes in both lung function and TyG. This may have biased the results. To address this issue, dynamic monitoring of TyG during the follow-up period is required.
Further large, population-based cohort study could be also necessary to confirm and further clarify our findings, ensuring robust estimates and generalizability, because a large cohort study, using the large sample size and population-level analyses may mitigate this variability.
Fourth, our results were obtained from middle-aged healthy Korean adults, which limits the generalizability of the findings. Finally, our LLN values were classified as the bottom 5% of the study population because those for the Korean population have not been determined. Therefore, our LLN values might be different from those defined as the 5th percentile of a normal healthy Korean population [22]. Our findings should therefore be validated in future studies using LLN values from a normal healthy Korean population.
In conclusion, we found a strong link between the TyG index, a practical and cost-effective marker for IR, and lung function. This study supports and extends previous findings that IR confers higher risk for lung dysfunction [6–8, 39]. Thus, clinicians need to monitor individuals with high TyG to prevent various lung dysfunction-driven comorbidities. However, longitudinal and prospective interventional studies are needed to confirm our findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- aOR
adjusted odds ratios
- BMI
Body mass index
- CIs
Confidence intervals
- CRDs
Chronic lung diseases
- CVMDs
Cardiovascular-metabolic diseases
- FBS
Fasting glucose
- FEV1%
Predicted forced expiratory volume in one second
- FVC%
Predicted forced vital capacity
- HbA1c
Glycated hemoglobin
- HDL cholesterol
High-density lipoprotein cholesterol
- HOMA-IR
Homeostatic model assessment of insulin resistance
- IR
Insulin resistance
- LDL cholesterol
Low density lipoprotein cholesterol
- LLN
Lower limit of normal
- MBP
Mean blood pressure
- MS
Metabolic syndrome
- OLF
Obstructive lung function
- TC
Total cholesterol
- TG
Triglyceride
- TyG
Triglyceride-glucose index
- WC
Waist circumference
Author contributions
Conceptualization: Jae-Uk Song and Jonghoo LeeData curation and Formal analysis: Jae-Uk Song and Sungwon LimFunding acquisition: Jae-Uk SongInvestigation: Jae-Uk Song and Hyun-il GilMethodology: Min-Jung Kwon, Soo-Youn Ham, Hyun-il Gil, and Jae-Uk SongProject administration: Jae-Uk Song and Jonghoo LeeResources: Jae-Uk SongSoftware: Jae-Uk Song and Hyun-il GilSupervision: Jae-Uk Song and Jonghoo Lee Validation: Min-Jung Kwon, Jae-Uk Song, and Jonghoo LeeVisualization: Hyun-il Gil, Sungwon Lim, and Soo-Youn Ham Writing - original draft: Hyun-il Gil, Sungwon Lim, Jae-Uk Song, and Jonghoo Lee Writing - review & editing: Hyun-il Gil, Sungwon Lim, Min-Jung Kwon, Soo-Youn Ham, Jonghoo Lee, and Jae-Uk Song.
Funding
This work was supported by Medical Research Funds from Kangbuk Samsung Hospital.
Data availability
Data availability: The data are not publicly available outside of the hospital because of Institutional Review Board restrictions (the data were not collected in a way that can be distributed widely). However, the analytical methods are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Kangbuk Samsung Hospital (KBSMC 2024-01-038), which waived the requirement for informed consent owing to the use of de-identified data for analysis purposes. This study complied with the 1975 Declaration of Helsinki and its later amendments and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hyun-Il Gil and Sungwon Lim contributed equally to this work.
Contributor Information
Jonghoo Lee, Email: lovlet@jejunu.ac.kr.
Jae-Uk Song, Email: khfever76@gmail.com.
References
- 1.Collaborators GBDCRD. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med. 2020;8:585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colak Y, Afzal S, Nordestgaard BG, Lange P, Vestbo J. Importance of early COPD in young adults for development of clinical COPD: findings from the Copenhagen general population study. Am J Respir Crit Care Med. 2021;203:1245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baffi CW, Wood L, Winnica D, Strollo PJ Jr., Gladwin MT, Que LG, et al. Metabolic syndrome and the lung. Chest. 2016;149:1525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang NN, Wang ZH, Li SH, Ge YY, Liu X, Sui DX. Pulmonary function in metabolic syndrome: A Meta-Analysis. Metab Syndr Relat Disord. 2022;20:606–17. [DOI] [PubMed] [Google Scholar]
- 5.Papaioannou O, Karampitsakos T, Barbayianni I, Chrysikos S, Xylourgidis N, Tzilas V, et al. Metabolic disorders in chronic lung diseases. Front Med (Lausanne). 2017;4:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Park HK, Kwon MJ, Ham SY, Gil HI, Lim SY, et al. The impact of insulin resistance on the association between metabolic syndrome and lung function: the Kangbuk Samsung health study. Diabetol Metab Syndr. 2023;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engstrom G, Hedblad B, Nilsson P, Wollmer P, Berglund G, Janzon L. Lung function, insulin resistance and incidence of cardiovascular disease: a longitudinal cohort study. J Intern Med. 2003;253:574–81. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Kim HS, Min HK, Lee SW. Association between insulin resistance and lung function trajectory over 4 years in South korea: community-based prospective cohort. BMC Pulm Med. 2021;21:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh B, Saxena A. Surrogate markers of insulin resistance: A review. World J Diabetes. 2010;1:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. [DOI] [PubMed] [Google Scholar]
- 11.McKeever TM, Weston PJ, Hubbard R, Fogarty A. Lung function and glucose metabolism: an analysis of data from the third National health and nutrition examination survey. Am J Epidemiol. 2005;161:546–56. [DOI] [PubMed] [Google Scholar]
- 12.Cirillo DJ, Agrawal Y, Cassano PA. Lipids and pulmonary function in the third National health and nutrition examination survey. Am J Epidemiol. 2002;155:842–8. [DOI] [PubMed] [Google Scholar]
- 13.Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pranata R, Huang I, Irvan, Lim MA, Vania R. The association between triglyceride-glucose index and the incidence of type 2 diabetes mellitus-a systematic review and dose-response meta-analysis of cohort studies. Endocrine. 2021;74:254–62. [DOI] [PubMed] [Google Scholar]
- 15.Feng X, Yao Y, Wu L, Cheng C, Tang Q, Xu S. Triglyceride-Glucose index and the risk of stroke: A systematic review and Dose-Response Meta-Analysis. Horm Metab Res. 2022;54:175–86. [DOI] [PubMed] [Google Scholar]
- 16.Nabipoorashrafi SA, Seyedi SA, Rabizadeh S, Ebrahimi M, Ranjbar SA, Reyhan SK, et al. The accuracy of triglyceride-glucose (TyG) index for the screening of metabolic syndrome in adults: A systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2022;32:2677–88. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Yang W, Jiang X. Association between Triglyceride-Glucose index and hypertension: A Meta-Analysis. Front Cardiovasc Med. 2021;8:644035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu TD, Fawzy A, Brigham E, McCormack MC, Rosas I, Villareal DT, et al. Association of Triglyceride-Glucose index and lung health: A Population-Based study. Chest. 2021;160:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Lu Z, Wang X, Zhang J, Zhang D, Li S. The chain mediating role of C-reactive protein and triglyceride-glucose index between lung function and cognitive function in a systemic low-grade inflammation state. J Psychiatr Res. 2022;155:380–6. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Liu W, Zhu X, Xu M, Lin B, Bai Y. Associations of dietary inflammation index and composite dietary antioxidant index with preserved ratio impaired spirometry in US adults and the mediating roles of triglyceride-glucose index: NHANES 2007–2012. Redox Biol. 2024;76:103334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–9. [DOI] [PubMed] [Google Scholar]
- 22.Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022;60:2101499. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Wang S, Jia B, Chen S. Association between Triglyceride-Glucose index and lung function parameters in the general population undergoing health examinations. Diabetes Metab Syndr Obes. 2024;17:4031–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang Y, Kim BK, Yun KE, Cho J, Zhang Y, Rampal S, et al. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63:2679–86. [DOI] [PubMed] [Google Scholar]
- 25.Western Pacific Region, World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment Sydney. Health Communications Australia; 2000.
- 26.Jung CH, Lee WJ, Song KH. Metabolically healthy obesity: a friend or foe? Korean J Intern Med. 2017;32:611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Park HK, Kwon MJ, Ham SY, Gil HI, Lim SY, et al. Increased Apolipoprotein b/apolipoprotein A-I ratio is associated with decline in lung function in healthy individuals: the Kangbuk Samsung health study. J Korean Med Sci. 2024;39:e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi HS, Park YB, Yoon HK, Lim SY, Kim TH, Park JH, et al. Validation of previous spirometric reference equations and new equations. J Korean Med Sci. 2019;34:e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong Y, Ra SW, Shim TS, Lim CM, Koh Y, Lee SD, et al. Poor interpretation of pulmonary function tests in patients with concomitant decreases in FEV1 and FVC. Respirology. 2008;13:569–74. [DOI] [PubMed] [Google Scholar]
- 30.Sauerwein HP, Schols AM. Glucose metabolism in chronic lung disease. Clin Nutr. 2002;21:367–71. [DOI] [PubMed] [Google Scholar]
- 31.Lalley PM. The aging respiratory system–pulmonary structure, function and neural control. Respir Physiol Neurobiol. 2013;187:199–210. [DOI] [PubMed] [Google Scholar]
- 32.Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of Pear shape. Biol Sex Differ. 2012;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo B, Lee SH, Kim SY, Leem AY, Chung KS, Kim EY, et al. Relationship between airway obstruction and C-reactive protein levels in a community-based population of Korea. Int J Tuberc Lung Dis. 2019;23:1228–34. [DOI] [PubMed] [Google Scholar]
- 36.Fimognari FL, Pasqualetti P, Moro L, Franco A, Piccirillo G, Pastorelli R, et al. The association between metabolic syndrome and restrictive ventilatory dysfunction in older persons. J Gerontol Biol Sci Med Sci. 2007;62:760–5. [DOI] [PubMed] [Google Scholar]
- 37.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuomi T, Heliovaara M, Palosuo T, Aho K. Smoking, lung function, and rheumatoid factors. Ann Rheum Dis. 1990;49:753–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters MC, Schiebler ML, Cardet JC, Johansson MW, Sorkness R, DeBoer MD, et al. The impact of insulin resistance on loss of lung function and response to treatment in asthma. Am J Respir Crit Care Med. 2022;206:1096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu L, Ma L, Nicholson LF, Black PN. Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respir Med. 2011;105:329–36. [DOI] [PubMed] [Google Scholar]
- 42.Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: A compass for future health. J Am Coll Cardiol. 2022;80:2361–71. [DOI] [PubMed] [Google Scholar]
- 43.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the united states: data from the first National health and nutrition examination survey follow up study. Thorax. 2003;58:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurniawan LB. Triglyceride-Glucose index as A biomarker of insulin resistance, diabetes mellitus, metabolic syndrome, and cardiovascular disease: A review. EJIFCC. 2024;35:44–51. [PMC free article] [PubMed] [Google Scholar]
- 46.Parro J, Aceituno P, Droppelmann A, Mesias S, Munoz C, Marchetti N, et al. Secondhand tobacco smoke exposure and pulmonary function: a cross-sectional study among non-smoking employees of bar and restaurants in santiago, Chile. BMJ Open. 2017;7:e017811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability: The data are not publicly available outside of the hospital because of Institutional Review Board restrictions (the data were not collected in a way that can be distributed widely). However, the analytical methods are available from the corresponding author upon request.