Abstract
Background
Toxoplasmic encephalitis (TE) is one of the most common opportunistic central nervous system infections in patients with acquired immunodeficiency syndrome (AIDS) and a major cause of death. Magnetic resonance imaging (MRI) serves as a key adjunct in the clinical diagnosis of TE. This study aimed to identify the MRI characteristics of TE in patients with human immunodeficiency virus (HIV)/AIDS across different immune statuses.
Methods
A retrospective analysis was conducted on the clinical and imaging data of hospitalized patients with AIDS and concurrent TE treated at two centers between January 2019 and December 2023. Patients were divided into three groups based on CD4+ T-cell count levels (<50, 50–100, and >100 cells/µL). The differences in MRI imaging features between patients with varying immune statuses were analyzed.
Results
A total of 41 patients were included, 82.9% had multiple lesions on MRI, and 203 lesions larger than 2 mm were identified. The lesions predominantly presented with nodular and ring-enhancing patterns, mainly distributed at the gray–white matter junction and the thalamus–basal ganglia region. In the comparison of MRI features between the three groups, patients with >100 CD4+ T cells/µL had a higher prevalence of nodular lesions and eccentric target lesions (both P<0.05). For patients with <50 CD4+ T cells/µL, larger lesions (>2 cm) were more frequently observed on MRI (P<0.05). Additionally, the <50 CD4+ T cells/µL group showed significantly elevated cerebrospinal fluid protein levels as compared to the other two groups (P<0.05).
Conclusions
In patients with HIV/AIDS with secondary TE, MRI findings varied in terms of lesion size and enhancement patterns according to the immune status of the patient. These imaging characteristics provide valuable guidance for the clinical diagnosis and treatment of TE.
Keywords: Toxoplasmosis, brain, human immunodeficiency virus (HIV), acquired immunodeficiency syndrome (AIDS), magnetic resonance imaging (MRI)
Introduction
Toxoplasma gondii, a common opportunistic protozoan pathogen, can parasitize nucleated cells in humans and warm-blooded animals. In healthy individuals, Toxoplasma infections are typically self-limiting or asymptomatic. However, in immunocompromised patients, such as those with acquired immunodeficiency syndrome (AIDS), latent Toxoplasma infections may reactivate or reinfect, leading to severe symptoms or even death (1,2). Studies have shown (3-5) that Toxoplasma preferentially resides in brain tissues with abundant blood supply, and approximately 25% to 60% of patients coinfected with AIDS may develop toxoplasmic encephalitis (TE). Besides systemic symptoms such as fever and chills, the primary clinical manifestations of TE include headache, motor seizures, and limb dysfunction. However, these symptoms and signs lack specificity, making it difficult to distinguish TE from central nervous system tumors and other intracranial infections, such as tuberculosis or abscesses (6).
The gold standard for the clinical diagnosis of TE is brain biopsy, but its invasive nature limits widespread application. Although serological detection of Toxoplasma IgG and IgM antibodies serves as an important diagnostic criterion, the positivity rate is generally low. Imaging, as a crucial diagnostic adjunct, has the advantages of being painless, noninvasive, convenient, rapid, and accurate. On enhanced cranial magnetic resonance imaging (MRI), TE lesions typically present with a markedly enhanced ring-shaped outer edge, a weakly enhanced low-signal central area, and an eccentrically located focal high-signal center, known as the “eccentric target sign”. It appears to be the most characteristic finding, with a specificity of 95% and sensitivity of 25%, significantly enhancing the diagnostic efficiency for TE (7). However, this specific feature appears in fewer than 30% of cases, posing a diagnostic challenge. Based on the hypothesis that imaging features vary with immune status, clinical data and imaging characteristics of patients with AIDS and TE from two centers were analyzed according to CD4+ T-cell counts to determine the relationship between imaging features and different immune levels. The findings from this study may contribute to the stratified diagnosis of this condition. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2598/rc).
Methods
Study participants
This study collected clinical and imaging data of hospitalized patients with AIDS and concurrent TE treated at Beijing YouAn Hospital and The Sixth People’s Hospital of Zhengzhou between January 2019 and December 2023. AIDS was diagnosed according to the AIDS diagnosis and treatment guidelines (2021 edition) established by the AIDS Society of the Chinese Medical Association (8), with diagnosis confirmed in all patients via HIV immunoblotting. The diagnosis of TE was primarily based on central nervous system infection symptoms, cerebrospinal fluid (CSF) IgG and IgM tests after lumbar puncture, high-throughput sequencing of CSF, serum IgG and IgM tests, brain tissue pathology, or improvement in lesions and clinical symptoms following anti-Toxoplasma treatment.
Exclusion criteria included patients with CSF, serum findings, or trial treatment indicating the presence of other concurrent intracranial infections.
The 41 included patients were divided into the following three groups based on CD4+ T cell count: <50, 50–100, and >100 CD4+ T cells/µL.
Imaging protocol
At both sites, MRI scans were performed with an Achieva MR 1.5T scanner (Philips Healthcare, Best, the Netherlands) and a Tim-Trio MR 3.0T scanner (Siemens Healthineers, Erlangen, Germany) scanner. All patients underwent pretreatment cranial MRI plain and contrast-enhanced scans. The scanning range spanned from the skull base to the vertex. Prior to imaging, patients were thoroughly briefed and instructed to remain relaxed while lying comfortably in a supine position on the examination table. Foam padding was used on both sides of the head to minimize lateral movement. During the scan, patients were required to keep their eyes closed and remain awake.
The plain scan sequences included axial T1-weighted imaging [T1WI; repetition time (TR) =452 ms and echo time (TE) 2.46 ms], T2-weighted imaging (T2WI; TR =6,000 ms and TE =93 ms), fluid-attenuated inversion recovery (T2-FLAIR; TR =9,000 ms and TE =93 ms), and diffusion-weighted imaging (DWI; TR =4,200 ms, TE 93 ms, and b=0 and b=1,000 s/mm2), with apparent diffusion coefficient (ADC) maps being simultaneously generated. For contrast-enhanced imaging, T1WI (TR =471 ms and TE =2.46 ms) was performed in the axial, coronal, and sagittal planes, with a slice thickness of 4 mm and an interslice gap of 1 mm. The contrast agent used was gadopentetate dimeglumine (Gd-DTPA), administered at a dose of 0.1 mmol/kg and an injection rate of 2–3 mL/s via the median cubital vein.
All images were analyzed by two or more radiologists. In cases of disagreement, consensus was reached through further discussion. All intracranial lesions were observed, with only lesions larger than 2 mm being recorded to exclude partial volume effects.
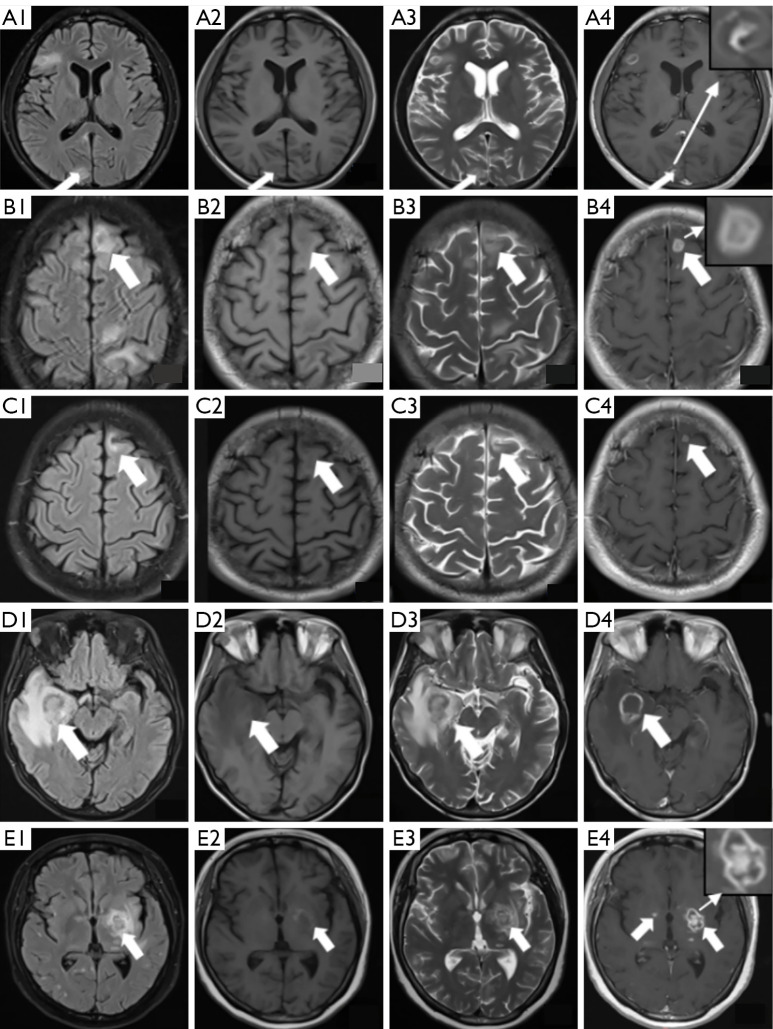
Image analysis included lesion count, distribution, size, and signal characteristics of the lesion center and edge on T1WI, T2WI, DWI, and ADC sequences, as well as enhancement patterns. Lesion distribution was described according to anatomical brain regions. Enhancement patterns were classified as punctate, nodular, ring, or target-like enhancement. Ring enhancement was further categorized into regular and irregular types. Target-like enhancement included an innermost enhanced core, a middle hypointense zone, and an outer hyperintense ring (as shown in Figure 1).
Figure 1.
Neuroimaging findings in four patients with AIDS with confirmed cerebral toxoplasmosis. (A1-A4) Case 1: a 34-year-old male with right-sided limb movement disorder (25 CD4+ T cells/µL). (A1) Axial FLAIR: slightly hyperintense lesion (15 mm × 12 mm) in the right occipital lobe (white arrow). (A2) Axial T1WI: patchy hypointense signal in the same region. (A3) Axial T2WI: hyperintense signal with perilesional edema (FLAIR hyperintensity, arrow). (A4) Postcontrast T1WI: target-like enhancement (central hypointensity + eccentric nodular enhancement, arrow). (B1-B4) Case 2: a 35-year-old male with chronic headache (88 CD4+ T cells/µL). (B1) Axial FLAIR: slightly hyperintense lesion (18 mm × 14 mm) in the left frontal lobe with mass effect (arrow). (B2) Axial T1WI: patchy hypointense signal in the same region. (B3) Axial T2WI: heterogeneous hyperintensity (arrow). (B4) Postcontrast T1WI: classic target enhancement (arrow). (C1-C4) Case 3: the same patient as in Case 2 after treatment. A 35-year-old male with chronic headache (88 CD4+ T cells/µL). (C1) Axial FLAIR: markedly reduced lesion (8 mm × 6 mm) in the left frontal lobe with resolution of perilesional edema (arrow). (C2) Axial T1WI: hypointense signal at the lesion site. (C3) Axial T2WI: residual hyperintensity within the lesion (arrow). (C4) Postcontrast T1WI: thin-walled peripheral enhancement (arrow), indicating treatment response. (D1-D4) Case 4: a 31-year-old male with left hemiparesis (1 CD4+ T cell/µL). (D1) Axial FLAIR: large hyperintense lesion (27.2 mm × 23.5 mm) in the right temporal lobe with surrounding edema and midline shift (arrow). (D2) Axial T1WI: hypointense signal in the same region. (D3) Axial T2WI: high signal intensity within the lesion (arrow). (D4) Postcontrast T1WI: irregular ring enhancement with a wall thickness of 2–4 mm (arrow). (E1-E4) Case 5: a 42-year-old female with speech dysfunction (132 CD4+ T cells/µL). (E1) Axial FLAIR: hyperintense lesion (10 mm × 8 mm) in the left basal ganglia with surrounding edema (arrow). (E2) Axial T1WI: hypointense lesion with subtle marginal hyperintensity, suggesting possible focal hemorrhage within the toxoplasmic lesion (arrow). (E3) Axial T2WI: high signal intensity in the lesion (arrow). (E4) Postcontrast T1WI: dual enhancement patterns—target-like enhancement on the left and punctate foci on the right (arrows). AIDS, acquired immunodeficiency syndrome; CD4+ T, CD4-positive T lymphocytes; FLAIR, fluid-attenuated inversion recovery; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging.
Statistical analysis
Data analysis was conducted with SPSS version 28.0 software (IBM Corp., Armonk, NY, USA). Categorical variables are presented as frequencies (%) and were compared between groups via the Fisher exact test or chi-squared test, as appropriate. Continuous variables were tested for normality with the Shapiro-Wilk test. Normally distributed data are expressed as the mean ± SD and were analyzed via one-way analysis of variance (ANOVA), with Student-Newman-Keuls (S-N-K) post hoc tests being applied if significant differences were detected. Nonnormally distributed data are presented as the median and IQR and were analyzed with the Kruskal-Wallis test for group comparisons and the Mann-Whitney test for pairwise comparisons. Effect sizes were quantified with the η² for ANOVA and nonparametric tests and with Cramer’s V for chi-squared tests. A two-tailed P value <0.05 indicated statistical significance.
Ethical considerations
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Ethical approval was obtained from both participating centers: the Medical Ethics Committee of Beijing YouAn Hospital (approval No. 2020047) and the Ethics Committee of The Sixth People’s Hospital of Zhengzhou (approval No. IEC-KY-2023-19). The requirement for individual informed consent was waived due to the retrospective and anonymized nature of the data.
Results
General characteristics of patients
A total of 41 patients with HIV/AIDS and concurrent TE meeting the inclusion criteria were enrolled during the study period, including 31 cases from Beijing YouAn Hospital and 10 cases from The Sixth People’s Hospital of Zhengzhou. Among these patients, 38 (92.7%) were male and 3 (7.3%) were female, with a mean age of 38±11.3 years (range, 19–65 years). The average duration of confirmed HIV infection was 2 months, with the longest diagnosis time being 20 years. Notably, in 6 cases, TE was the initial symptom that led to a diagnosis of HIV. The primary clinical manifestations were headache, dizziness, fever, and motor disorders, with the course of the disease ranging from 1 day to 1 year (mean 30 days). Among the patients, 68.3% (28/41) had other concurrent infections, with oral candidiasis, syphilis, Cytomegalovirus infection, and bacterial pneumonia being the most common, as shown in Table 1. Following treatment, 35 (85.4%) patients showed significant symptom improvement, 4 (9.8%) discharged themselves, and 2 (4.9%) died. The hospital stay ranged from 3 to 62 days (mean 29 days).
Table 1. The general condition of 41 patients with HIV/AIDS coinfected with TE.
| Variable | Values, n (%) |
|---|---|
| Symptom | |
| Headache | 28 (68.3) |
| Dizziness | 25 (61.0) |
| Fever | 19 (46.3) |
| Dyskinesia | 17 (41.5) |
| Twitch | 6 (14.6) |
| Abnormal mental behavior | 5 (12.2) |
| Speech disorder | 4 (9.8) |
| Nausea or vomiting | 3 (7.3) |
| Incontinence | 2 (4.9) |
| Coinfection | |
| Oral candidiasis | 15 (36.6) |
| Treponema pallidum | 13 (31.7) |
| Bacterial pneumonia | 9 (22.0) |
| CMV | 9 (22.0) |
| EBV | 8 (19.5) |
| Mycobacterium tuberculosis | 5 (12.2) |
| HBV | 2 (4.9) |
TE, toxoplasmic encephalitis; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HBV, hepatitis B virus.
Clinical and laboratory analysis
The HIV viral load in patients ranged from below the detection limit to 5,626,104 copies/mL, with detectable HIV virus in 35 cases (85.4%), and the average viral load was 37,680 copies/mL (328–244,972 copies/mL). The CD4+ T-cell count ranged from 1 to 294×106/L, with an average of 33×106/L. Only one patient had a CD4+ T-cell count above 200×106/L, while 24.4% (10/41) of patients had a count below 10×106/L. The CD4+:CD8+ ratio was below 0.5 for all patients, with an average ratio of 0.09 (IQR, 0.03–0.13).
The seropositivity rate of Toxoplasma IgG antibody in patients was 85.4% (35/41), while the positivity rate for IgM antibody was 19.5% (8/41). In CSF, the positivity rates for Toxoplasma IgG and IgM antibodies were 70.7% (29/41) and 12.2% (5/41), respectively. Additionally, seven patients were diagnosed with Toxoplasma infection via the high-throughput sequencing (next-generation sequencing) of CSF.
A comparison of laboratory data between the three groups revealed that only the difference in CSF protein content was statistically significant (P=0.015), as shown in Table 2.
Table 2. Demographic and clinical characteristics of patients with HIV/AIDS coinfected with TE under different immune statuses.
| Variable | <50 cells/µL (n=24) | 50–100 cells/µL (n=7) | >100 cells/µL (n=10) | F/H | P value | η²/V |
|---|---|---|---|---|---|---|
| Age (years) | 36.4±11.5 | 38.4±10.2 | 40.3±11.3 | 0.436 | 0.650 | 0.022 |
| Gender (male) | 23 (95.8) | 6 (85.7) | 8 (80.0) | 2.661 | 0.242 | 0.232 |
| Laboratory findings | ||||||
| Leukocyte count (×109/L) | 4.60±2.64 | 4.51±1.81 | 4.46±2.40 | 0.096 | 0.908 | 0.005 |
| Neutrophil ratio >0.7 | 12 (50.0) | 2 (28.6) | 2 (20.0) | 2.877 | 0.248 | 0.273 |
| CD8+ T-cell count | 5.047 | 0.041 | 0.414 | |||
| ≤200 cells/µL | 8 (33.3) | 0 | 0 | |||
| >200 cells/µL | 16 (66.7) | 7 (100.0) | 10 (100.0) | |||
| Cerebrospinal fluid | ||||||
| Leukocyte count (×106/L) | 6.6±3.0 | 5.6±1.8 | 6.7±4.7 | 1.345 | 0.528 | 0.069 |
| Protein (g/L) | 0.77±0.63 | 0.84±0.41 | 0.33±0.16 | 4.669 | 0.015 | 0.197 |
| Glucose (g/L) | 2.89±0.63 | 2.91±0.32 | 2.99±0.80 | 0.087 | 0.958 | 0.005 |
| Chloride (mmol/L) | 123.0±5.4 | 126.0±3.1 | 124.3±3.8 | 1.164 | 0.323 | 0.058 |
| Imaging findings | ||||||
| Number | ||||||
| Single (n=7) | 2 (8.3) | 2 (28.6) | 3 (30.0) | 3.473 | 0.144 | 0.217 |
| Multiple (n=34) | ||||||
| 2–5 (n=19) | 12 (50.0) | 2 (28.6) | 5 (50.0) | 1.597 | 0.519 | 0.162 |
| >5 (n=15) | 10 (41.7) | 3 (42.9) | 2 (20.0) | 1.578 | 0.598 | 0.196 |
| Distribution | ||||||
| Corticomedullary junction | 88 (69.3) | 30 (71.4) | 27 (79.4) | 1.346 | 0.510 | 0.081 |
| Thalamus-basal ganglia | 21 (16.5) | 10 (23.8) | 5 (14.7) | 1.401 | 0.496 | 0.083 |
| Cerebellum and brainstem | 18 (14.2) | 2 (4.8) | 2 (5.9) | 3.434 | 0.201 | 0.139 |
| Morphology | ||||||
| Ring-like | 65 (51.2) | 18 (42.9) | 13 (38.2) | 2.221 | 0.329 | 0.105 |
| Nodular | 41 (32.3) | 20 (47.6) | 18 (52.9) | 6.502 | 0.039 | 0.179 |
| Target sign | 4 (3.1) | 2 (4.8) | 5 (14.7) | 5.915 | 0.040 | 0.186 |
| Size (mm) | 9.8 (7.4–15.4) | 8.8 (6.7–10.9) | 8.7 (5.5–13.1) | 6.337 | 0.042 | 0.158 |
| Time of HIV diagnosis (days) | 30 (10.5–60) | 60 (20–240) | 60 (14–656.3) | 1.761 | 0.415 | 0.044 |
| Hospitalization time (days) | 30.8±13.8 | 33.6±19.3 | 27±7.4 | 0.506 | 0.607 | 0.026 |
| Outcome | 20 (83.3) | 6 (85.7) | 9 (90.0) | 0.267 | 0.875 | 0.078 |
Data are presented as the mean ± SD, n (%), or the median (IQR), as appropriate. AIDS, acquired immunodeficiency syndrome; CD8+ T-cell, CD8-positive T lymphocyte; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation; TE, toxoplasmic encephalitis.
Analysis of imaging characteristics
Lesion number and location
Among the 41 patients, 34 (82.9%) exhibited multiple lesions, while 7 had single lesions. The number of lesions per patient ranged from 1 to 12, and 203 lesions larger than 2 mm were detected, with sizes ranging from 2.7 to 48.6 mm. The majority of these lesions were ring-shaped (n=106, 52.2%) or nodular (n=80, 39.4%), while the remaining lesions displayed irregular shapes. The most common sites of lesions were the corticomedullary junction (n=144, 69.0%), followed by the thalamus-basal ganglia region (n=36, 19.3%), cerebellum (n=22, 10.8%), and brainstem (n=1, 0.5%). Edema was observed around all lesions, and gyrus-like edema was visible in the white matter surrounding lesions at the corticomedullary junction. Six patients showed lateral ventricle and brain parenchyma compression on the affected side, along with midline structural shifts (Figure 1).
Lesion morphology and signal changes
Plain scan
On MRI plain scans, lesions typically presented as hypointense on T1WI and hyperintense on T2WI and FLAIR. A target sign was observed in some lesions (n=11, 5.4%), characterized by a hypointense center surrounded by a ring of hyperintensity (Figure 1, A1-A4).
Contrast enhancement
The predominant enhancement patterns were ring enhancement (107, 52.7%) and nodular enhancement (80, 39.4%), with patchy (6, 3%), multiple punctate (3, 1.5%), spiral (3, 1.5%), and non-enhancing (4, 2.0%) also appearing. Among the ring-enhancing lesions, some exhibited a target sign (n=11, 5.4%) with a high-low-high intensity pattern from the outer to inner layers (Figure 1, A4,B4,E4).
Follow-up MRI
All patients received targeted anti-Toxoplasma therapy with pyrimethamine-sulfadiazine or combined clindamycin treatment after diagnosis. Among the 29 patients who underwent follow-up MRI, a marked reduction in the number of intracranial lesions, decreased perilesional edema, and alleviation of mass effect were observed (Figure 1, B1-C4). Specifically, in 6 cases, both the extent of enhancement and the number of enhancing lesions decreased on subsequent scans. Conversely, in another 6 cases, minimal or no improvement was observed.
Analysis of MRI characteristics in patients with different immune statuses
In the group with <50 CD4+ T cells/µL, 127 lesions were identified; in the 50–100 cells/µL group, 42 lesions were detected; and in the >100 cells/µL group, 34 lesions were identified. A significant difference was observed in the number of nodular-enhancing lesions across the groups (P=0.039). The eccentric target sign frequency also differed significantly, being higher in patients with ≥100 CD4+ T cells/µL (P=0.040) (Figure 1, D1-D4). There was a significant difference in lesion size between the three groups (P=0.042). Post hoc pairwise comparisons revealed that the group with <50 CD4+ T cells/µL had significantly larger lesion sizes as compared to the group with ≥100 CD4+ T cells/µL (P=0.037). No statistically significant differences in lesion size were observed in the other two group comparisons (P=0.053 and P=0.897, respectively). The comparisons of MRI characteristics are summarized in Table 2.
Discussion
TE is one of the most common opportunistic infections affecting the central nervous system in patients with AIDS and a leading cause of mortality in this population (4,9). TE primarily causes parenchymal damage and mass effects in the central nervous system and has nonspecific clinical manifestations. Diagnosis mainly relies on imaging studies, detection of specific antibodies in the blood or CSF, and genetic testing. However, specific antibody and genetic tests have a high rate of false negatives, making imaging an essential adjunct in diagnosing TE, with MRI showing greater sensitivity than computed tomography (10-13). Numerous studies have indicated (4,5,14) that the incidence of TE is closely related to the immune status of patients; nearly all cases occur in patients with CD4+ T-cell counts below 200 cells/µL, with incidence increasing exponentially as CD4+ T-cell counts decline. However, there is limited literature on the imaging characteristics of patients with AIDS with different immune statuses.
To further clarify the imaging characteristics in patients with varying degrees of immunosuppression, this study classified patients according to their CD4+ T-cell count at the time of TE infection. Patients were divided into three groups for imaging and laboratory comparisons. The results demonstrated a diversity of MRI enhancement patterns in patients with TE, including ring, nodular, spiral, miliary, patchy, and the characteristic eccentric target sign. Notably, multiple enhancement patterns frequently co-occurred. In patients with higher CD4+ T-cell counts (>100 cells/µL), nodular enhancement was more pronounced, and the eccentric target sign occurred more frequently. In contrast, a lower CD4+ T-cell count correlated with larger lesions and elevated CSF protein levels. These distinct MRI features, including lesion size, enhancement patterns, and CSF protein content, which vary depending on immune status, offer valuable insights for diagnosing TE.
In addition to specific antibody testing, routine and biochemical CSF analyses are also commonly used as adjunctive diagnostic methods for TE. Although TE primarily affects brain parenchyma and does not involve the meninges, routine and biochemical CSF analyses typically reveal normal or mild changes (15). However, this study found that 61% of patients had CSF protein levels exceeding 0.45 g/L, with CSF protein levels significantly increasing as CD4+ T-cell counts decreased. This may be associated with brain cell damage, necrosis, and hemorrhage during the progression of TE (16).
In terms of imaging characteristics, this study revealed significant differences across patients with TE of varying immune statuses. Studies (4,17,18) have shown that as Toxoplasma cysts rupture in infected areas of patients with TE, tachyzoites are repeatedly released, invading nearby brain cells, causing cellular damage, and forming localized necrotic foci, ultimately manifesting as one or more brain abscesses. This complex pathological process may involve liquefactive necrosis, gliosis, and granulation tissue, resulting in a range of different MRI presentations. This diversity in MRI enhancement patterns, with multiple enhancement types often coexisting, is also characteristic of TE (19). Among MRI enhancement patterns in patients with TE, nodular and ring-shaped lesions are most common, but the eccentric target sign is considered the most distinct (6,10,11).
The eccentric target sign is a typical MRI feature in patients with TE, and its formation mechanism is closely related to inflammatory responses and immune response. Pathologically, it consists of eccentric nodular enhancement around inflamed blood vessels located in the cerebral sulci. The central hypointense layer represents necrotic tissue, while the outer hyperintense layer indicates an inflammatory response. Numerous studies (6,10,20) have reported varying rates of target sign occurrence, but overall, its prevalence is relatively low; in our cohort, only 11 (26.8%) cases exhibited target-like changes similar to those reported in the literature. Moreover, nodular lesions and eccentric target signs were more common in patients with higher CD4+ T-cell counts (>100 cells/µL). This may be associated with immune responses in the progression of TE. Some studies (11,17,21) suggest that when CD4+ T-cell counts fall below 50 cells/µL, the appearance of nodular or ring-shaped enhancement becomes progressively less distinct as the cell count declines. Only when the CD4+ T-cell count reaches a certain threshold is an effective immune and inflammatory response triggered, leading to the gradual manifestation of ring enhancement. Additionally, one patient in this study initially showed no evident target sign but one appeared to develop on follow-up MRI 1 month after treatment, which may possibly be related to the gradual restoration of immune function following therapy.
Toxoplasma primarily forms cysts within the neuronal cells of the cerebral cortex, hippocampus, basal ganglia, and amygdala of infected individuals. As tachyzoites are repeatedly released from these cysts, patients with TE often develop multiple lesions in the brain parenchyma. The most common sites of infection are the frontal and parietal lobes, basal ganglia, and corticomedullary junction, although the cerebellum and brainstem may also be involved (21,22). Consistent with the literature, 82.9% of cases in our study exhibited multiple lesions, primarily distributed at the corticomedullary junction and thalamus-basal ganglia region. Additionally, we found that patients with <50 CD4+ T cells/µL exhibited significantly larger lesion sizes as compared to the other two patient groups. This may be related to the quantity of tachyzoite release. Bowen et al. (22) suggested that the conversion of Toxoplasma bradyzoites to tachyzoites largely depends on the patient’s immune status, with a higher conversion rate when CD4+ lymphocyte counts are <100 cells/µL. With the proliferation and release of tachyzoites, these parasites continuously invade adjacent cells, leading to local tissue cell necrosis and the formation of necrotic lesions. As this invasion process repeats, the affected lesion area progressively enlarges.
There were certain limitations to this study which should be addressed. First, the data in this study were collected retrospectively, introducing selection bias. Second, the relatively small sample size of patients with TE and the exclusive focus on their MRI characteristics limit the generalizability of the findings to larger populations or settings with constrained MRI availability. Third, the study did not comprehensively investigate the correlation between imaging characteristics and clinical manifestations. Future large-scale, multicenter studies are needed to validate these findings and further clarify this relationship.
Conclusions
MRI is an essential tool for the clinical diagnosis of TE. Patients with TE not only exhibit a variety of MRI enhancement patterns but also exhibit distinct characteristics in terms of lesion size and enhancement type depending on immune status. These findings provide valuable guidance for the diagnosis and treatment of TE and may aid clinicians in deepening their understanding of AIDS-associated TE and improve diagnostic accuracy.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Ethical approval was obtained from both participating centers: the Medical Ethics Committee of Beijing YouAn Hospital (approval No. 2020047) and the Ethics Committee of The Sixth People’s Hospital of Zhengzhou (approval No. IEC-KY-2023-19). The requirement for individual informed consent was waived due to the retrospective and anonymized nature of the data.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2598/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2598/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2598/dss
References
- 1.Mboera LEG, Kishamawe C, Kimario E, Rumisha SF. Mortality Patterns of Toxoplasmosis and Its Comorbidities in Tanzania: A 10-Year Retrospective Hospital-Based Survey. Front Public Health 2019;7:25. 10.3389/fpubh.2019.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewden C, Drabo YJ, Zannou DM, Maiga MY, Minta DK, Sow PS, Akakpo J, Dabis F, Eholié SP; IeDEA West Africa Collaboration. Disease patterns and causes of death of hospitalized HIV-positive adults in West Africa: a multicountry survey in the antiretroviral treatment era. J Int AIDS Soc 2014;17:18797. 10.7448/IAS.17.1.18797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang ZD, Wang SC, Liu HH, Ma HY, Li ZY, Wei F, Zhu XQ, Liu Q. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV 2017;4:e177-88. 10.1016/S2352-3018(17)30005-X [DOI] [PubMed] [Google Scholar]
- 4.Elsheikha HM, Marra CM, Zhu XQ. Epidemiology, Pathophysiology, Diagnosis, and Management of Cerebral Toxoplasmosis. Clin Microbiol Rev 2020;34:e00115-19. 10.1128/CMR.00115-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okome-Nkoumou M, Guiyedi V, Ondounda M, Efire N, Clevenbergh P, Dibo M, Dzeing-Ella A. Opportunistic diseases in HIV-infected patients in Gabon following the administration of highly active antiretroviral therapy: a retrospective study. Am J Trop Med Hyg 2014;90:211-5. 10.4269/ajtmh.12-0780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daher D, Shaghlil A, Sobh E, Hamie M, Hassan ME, Moumneh MB, Itani S, El Hajj R, Tawk L, El Sabban M, El Hajj H. Comprehensive Overview of Toxoplasma gondii-Induced and Associated Diseases. Pathogens 2021;10:1351. 10.3390/pathogens10111351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey RG, Geremia GK. CNS complications of AIDS: CT and MR findings. AJR Am J Roentgenol 1988;151:449-54. 10.2214/ajr.151.3.449 [DOI] [PubMed] [Google Scholar]
- 8.. Chinese guidelines for diagnosis and treatment of HIV/AIDS (2021 edition). Zhonghua Nei Ke Za Zhi 2021;60:1106-28. 10.3760/cma.j.cn112138-20211006-00676 [DOI] [PubMed] [Google Scholar]
- 9.Antinori A, Larussa D, Cingolani A, Lorenzini P, Bossolasco S, Finazzi MG, et al. Prevalence, associated factors, and prognostic determinants of AIDS-related toxoplasmic encephalitis in the era of advanced highly active antiretroviral therapy. Clin Infect Dis 2004;39:1681-91. 10.1086/424877 [DOI] [PubMed] [Google Scholar]
- 10.Masamed R, Meleis A, Lee EW, Hathout GM. Cerebral toxoplasmosis: case review and description of a new imaging sign. Clin Radiol 2009;64:560-3. 10.1016/j.crad.2008.09.016 [DOI] [PubMed] [Google Scholar]
- 11.Kumar GG, Mahadevan A, Guruprasad AS, Kovoor JM, Satishchandra P, Nath A, Ranga U, Shankar SK. Eccentric target sign in cerebral toxoplasmosis: neuropathological correlate to the imaging feature. J Magn Reson Imaging 2010;31:1469-72. 10.1002/jmri.22192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shyam babu C, Satishchandra P, Mahadevan A, Pillai Shibu V, Ravishankar S, Sidappa N, Udaykumar R, Ravi V, Shankar SK. Usefulness of stereotactic biopsy and neuroimaging in management of HIV-1 Clade C associated focal brain lesions with special focus on cerebral toxoplasmosis. Clin Neurol Neurosurg 2013;115:995-1002. 10.1016/j.clineuro.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang Y, Zhang X, Zhang L, Huang M, Tang C, Zhang L. Cerebral toxoplasmosis mimicking stroke in a woman living with undiagnosed HIV. J Infect Public Health 2024;17:102477. 10.1016/j.jiph.2024.102477 [DOI] [PubMed] [Google Scholar]
- 14.Martin-Iguacel R, Ahlström MG, Touma M, Engsig FN, Stærke NB, Stærkind M, Obel N, Rasmussen LD. Incidence, presentation and outcome of toxoplasmosis in HIV infected in the combination antiretroviral therapy era. J Infect 2017;75:263-73. 10.1016/j.jinf.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 15.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med 1992;327:1643-8. 10.1056/NEJM199212033272306 [DOI] [PubMed] [Google Scholar]
- 16.Lepennetier G, Hracsko Z, Unger M, Van Griensven M, Grummel V, Krumbholz M, Berthele A, Hemmer B, Kowarik MC. Cytokine and immune cell profiling in the cerebrospinal fluid of patients with neuro-inflammatory diseases. J Neuroinflammation 2019;16:219. 10.1186/s12974-019-1601-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farkash AE, Maccabee PJ, Sher JH, Landesman SH, Hotson G. CNS toxoplasmosis in acquired immune deficiency syndrome: a clinical-pathological-radiological review of 12 cases. J Neurol Neurosurg Psychiatry 1986;49:744-8. 10.1136/jnnp.49.7.744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidal JE. HIV-Related Cerebral Toxoplasmosis Revisited: Current Concepts and Controversies of an Old Disease. J Int Assoc Provid AIDS Care 2019;18:2325958219867315. 10.1177/2325958219867315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura S, Hara K, Kobayashi T, Sumitani R, Oura M, Maeda Y, Sogabe K, Yagi H, Takahashi M, Fujii S, Harada T, Bando Y, Abe M, Miki H. Toxoplasmic encephalitis with high (201)Tl uptake and retention mimicking malignant lymphoma in a patient with human immunodeficiency virus infection. Parasitol Int 2024;101:102895. 10.1016/j.parint.2024.102895 [DOI] [PubMed] [Google Scholar]
- 20.Zawadzki R, Modzelewski S, Naumowicz M, Matyja AM, Urbaniak AD, Zajkowska J, Kubas B. Evaluation of imaging methods in cerebral toxoplasmosis. Pol J Radiol 2023;88:e389-98. 10.5114/pjr.2023.130981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Offiah CE, Turnbull IW. The imaging appearances of intracranial CNS infections in adult HIV and AIDS patients. Clin Radiol 2006;61:393-401. 10.1016/j.crad.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 22.Bowen LN, Smith B, Reich D, Quezado M, Nath A. HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment. Nat Rev Neurol 2016;12:662-74. 10.1038/nrneurol.2016.149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as
Data Availability Statement
Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2598/dss