Abstract
Diffuse leptomeningeal glioneuronal tumors (DLGNTs) are rare, and optimal treatment remains undefined. We aim to comprehensively characterize their clinical and molecular features, offering granular insights into presentations and therapies to elucidate prognostic factors and therapeutic targets. Histologic, molecular, and clinical data of 30 patients with DLGNT were analyzed. Median age at diagnosis was 7.5 years (range: 0.9–20 years). Disease was localized at diagnosis in 16 patients (53.3%), predominantly in the spinal cord (14/16, 87.5%). KIAA1549::BRAF fusion occurred in 27 (96.4%) of 28 patients. DNA methylation profiling of 23 tumors classified 4 (17.4%) as DLGNT MC-1, 3 (13.0%) as DLGNT MC-2, and 16 (69.6%) as DLGNT, but not to a specific subclass. Median follow-up was 57.5 months. Most patients (90.0%) received adjuvant therapy. Chemotherapy was the first-line adjuvant therapy in 19 patients (70.4%); targeted therapy in 5 patients (18.5%), and radiotherapy in 2 patients (7.4%). Median progression-free survival (PFS) after first chemotherapy, targeted therapy, or radiotherapy was 44 (1–77) months, 18 (4–39) months, and 16.5 (9–23) months, respectively. Five-year PFS was 15.9% ± 8.0, and 5 year overall survival (OS) was 83.3% ± 8.8. Patients older than 9 years at diagnosis (p = 0.002) and those with MC-2 (p = 0.04) had worse 5 year OS. Multi-omic analysis revealed simultaneous activation of multiple signaling pathways, which may serve as potential therapeutic targets. DLGNT remains challenging to treat, with poor outcomes across modalities. Further investigation of treatment, including targeted therapies addressing activated pathways, is needed to improve patient survival.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00401-025-02924-0.
Keywords: Diffuse leptomeningeal glioneuronal tumor, DLGNT, Patient outcomes, Treatment responses, Activated signaling pathways
Introduction
Diffuse leptomeningeal glioneuronal tumors (DLGNT) are rare neoplasms of the CNS that were first included as a provisional entity in the 2016 WHO classification [1]. DLGNT preferentially occurs in children and is characterized by diffuse leptomeningeal dissemination. DLGNT frequently harbors KIAA1549::BRAF fusions and chromosome arm 1p deletion [2]. DNA methylation profiling has identified two DLGNT methylation subclasses, MC-1 and MC-2 [3]. The 2021 WHO classification of CNS tumors does not assign a definitive grade to DLGNT due to limited outcome data. Importantly, prognostic factors and optimal treatment strategies for DLGNT remain poorly understood. Methylation class, 1q status, Ki-67 labeling, and age have previously been reported as prognostic factors, with worse outcomes seen with MC-2, 1q gain, age >9, and Ki-67 of ≥4% in one study and >7% in another [3–5]. However, the reported cohorts lack detailed clinical annotations, and thus, optimal therapeutic approaches are largely unknown.
Detailed descriptions of treatment and outcome data are essential to understanding the responses of DLGNT to current treatment modalities and identifying risk factors associated with survival outcomes. Additionally, understanding the activated signaling pathways in DLGNT may shed light on potential novel therapeutic strategies. In this study, we retrospectively analyzed a multi-institutional cohort of patients with DLGNT, providing detailed clinical and molecular characterization. The DNA methylome and transcriptome of DLGNT were analyzed to infer activated signaling pathways in the tumors. The findings were validated using deep proteomics and phosphoproteomics analysis.
Methods
Study population
Thirty patients with a diagnosis of DLGNT reviewed at St. Jude Children’s Research Hospital (St. Jude) were identified between January 2009 and May 2025. Comprehensive treatment and histopathologic data were collected. Twelve patients were treated at St. Jude, 16 at other institutions in the United States, one in Brazil, and one in Lebanon. Institutional review board approval was obtained at St. Jude and outside institutions based on local guidelines.
Histopathologic and molecular studies
All tumor samples were centrally reviewed by a board-certified neuropathologist at St. Jude (JC). The tumors were classified as DLGNT based on the essential diagnostic criteria listed in the CNS WHO 2021. The Ki-67 labeling index was quantified via masked assessment without knowledge of the clinical outcome data or molecular status of the samples to ensure unbiased evaluation.
Genome-wide DNA methylation profiling and data analysis
Genomic DNA was prepared using a QIAamp DNA FFPE Tissue Kit (Qiagen) for genome-wide methylation profiling on an Illumina Infinium MethylationEPIC platform, as previously described [6–11]. Normal tissues from donors without brain tumors were obtained from the normal frontal cortex (n = 5), cerebellum (n = 4), pons (n = 5), and spinal cord (n = 3) for comparison. Differential methylation analysis was performed using ChAMP 2.22.0 in R 4.1.0 with default parameters [12]. All differentially methylated regions were queried against MSigDB using the champ. GSEA function with default parameters, as previously described [6]. Tumor methylation profiles were classified by the Molecular Neuropathology brain tumor classifier (www.molecularneuropathology.org) v12.8. A matching score higher than 0.9 was used as the threshold for classifying molecular tumor type and subclass.
Transcriptome sequencing (RNA-seq) and data analysis
Total RNA was isolated from archived formalin-fixed paraffin-embedded (FFPE) sections using a PureLinkTM FFPE RNA Isolation Kit (Thermo Fisher Scientific), as previously described [6–11]. Purified RNA was quantified on a Qubit 3 Fluorometer and subjected to RNA sequencing following the Illumina TruSeq Stranded Total RNA protocol (500 ng RNA minimum). All sequencing data were generated after 100 cycles of paired-end runs on an Illumina HiSeq 2500 or HiSeq 4000. The RNA-seq data were aligned to the human reference genome (build GRCh38). Differential gene expression was analyzed using the edgeR (v4.2.2) and limma (v3.60.6) packages in R (v4.4.0) with the default parameters, as previously described [6, 13, 14]. Only annotated protein-coding genes were considered in the analysis. The differentially expressed genes were queried against MSigDB using qusage (Quantitative Set Analysis for Gene Expression, v.2.38.0) to identify enriched signaling pathways in DLGNT [15].
Deep-proteome and phosphoproteome profiling and data analysis
Total proteins were extracted from FFPE sections of tumor, frontal cortex, cerebellum, pons, and spinal cord samples by following a previously established protocol with some modifications [16]. Briefly, the tissue pellets were incubated in the nuclease digestion solution (1% SDS and 100U of Benzonase [Merck] in 0.2 M HEPES, pH 8) at 37°C for one hour. Afterward, an equal volume of 20% SDS was added, and the mixture was heated at 95°C for 45 min and then treated with 10X Tris(2-carboxyethyl)phosphine hydrochloride and chloroacetamide solution (100 mM TCEP and 400 mM CAA) at 37°C for 30 min. The lysates were then subjected to paramagnetic beads cleanup: a 1:1 mixture of Sera-Mag Speed Beads (GE Life Sciences, CAT# 45152105050250 and CAT# 65152105050250) was added, yielding a concentration of 1 μg protein: 10 μg beads in 50% ethanol (v/v). The proteins were incubated with the beads at room temperature for 10 min. After the beads had settled on a magnetic rack, the supernatant was removed, and the beads were rinsed with 80% ethanol three times. Proteins were digested overnight at 37°C with the digestion solution [50 mM HEPES pH 8.0 + trypsin/lysC mix (1 μg trypsin/lysC:50 μg protein)]. Digested peptides were labeled by 18-plex TMTpro reagents (Thermo Fisher Scientific). At least 1 mg protein per sample was used for whole-proteome and phosphoproteome analyses. The TMT-labeled peptides were equally pooled, desalted on Sep Pak C18 cartridge (Waters), and fractionated via offline basic pH reverse-phase liquid chromatography (LC). Two offline LC runs were performed: 5% of the sample for whole-proteome (3 h gradient, 80 concatenated fractions) analysis and 95% for phosphoproteome (∼1 h gradient, 40 fractions) analysis on an XBridge C18 column (3.5 μm particle size, 4.6 mm × 25 cm, Waters; buffer A: 10 mM ammonium formate, pH 8.0; buffer B: 95% acetonitrile, 10 mM ammonium formate, pH 8.0). Whole-proteome fractions were analyzed by acidic pH reverse-phase liquid chromatography-tandem mass spectrometry (LC-MS/MS), and phosphoproteome fractions underwent phosphopeptide enrichment by TiO2 beads (GL Sciences) as previously reported, followed by acidic pH reverse-phase LC-MS/MS analysis [17].
Database search was performed using the published JUMP software suite, a tag-based hybrid search engine [18, 19]. The protein database was generated by combining downloaded Swiss-Prot, TrEMBL (Released 2023-01-20), and UCSC databases and removing redundancy, followed by concatenation with a decoy database. Oxidation (M) was included in the search as a variable modification. TMTpro tags on Lysine residues and peptide N-termini and carbamidomethyl cysteines were set as fixed modifications. The default settings were used for other parameters. Phosphorylation (STY) was included as an additional variable modification for phosphoproteome analysis. Peptide/protein quantification was performed using the JUMP software suite [18, 19]. Differential protein expression was analyzed using the edgeR and limma packages in R with the default parameters [13, 14].
Statistical analysis
Descriptive statistics were used to analyze clinical data. The date of diagnosis was defined as the date of initial tissue acquisition, either from the first biopsy or surgical resection. Localized disease was defined as tumor confined to a single, defined area without evidence of leptomeningeal spread or multiple areas of tumor. Disease progression was determined based on radiologic evidence of tumor growth, clinical deterioration, or the need for intervention. Survival outcomes were assessed using the Kaplan–Meier method to estimate progression-free survival (PFS) and overall survival (OS). Follow-up duration was measured from the date of diagnosis to the date of death, occurrence of an event, or last follow-up. OS was defined as the time from diagnosis to either death from any cause or the last follow-up, whichever occurred first. PFS was defined as the time from diagnosis to the first documented disease progression, death from any cause, or last follow-up, whichever occurred first. Five-year PFS and OS were used to estimate outcomes based on clinical and molecular features. Two-year PFS or median PFS were reported when describing therapeutic approaches, as most patients had a short progression-free interval. The comparisons between patient subgroups were conducted via the log-rank test. Response to therapy was evaluated using the Response Assessment in Pediatric Neuro-Oncology (RAPNO) criteria [20].
The moderated t-statistics implemented by the limma package in R (v4.4.0) was used for significance analysis. Benjamini and Hochberg’s method was used to adjust and control the false-discovery rate (FDR). For gene set enrichment analysis (GSEA), the nominal p-value estimated the statistical significance of the enrichment score for a single gene set, and the FDR was adjusted for gene set size and multiple hypotheses testing. A p-value of less than 0.05 was considered statistically significant for all analyses.
Results
Clinical and molecular characteristics
The median age of the study’s 30 patients at diagnosis was 7.5 years (range: 0.9–20 years). There was a male predominance, with 22 (73.3%) males (Fig. 1). The disease was localized in 16 patients (53.3%) at the time of diagnosis; the remainder had disseminated disease at presentation. Fourteen (87.5%) of the 16 patients with localized disease had tumors in the spinal cord; the other two had tumors in the brain. All DLGNT tumors had a loss of chromosome arm 1p. KIAA1549::BRAF fusions were present in 27 (96.4%) of 28 tumors with enough material for testing, and one tumor had an NTRK2 gene fusion. No additional mutations were identified in the tumors. Fifteen (50.0%) tumors had 1q gain. Ki-67 labeling at the time of initial tissue diagnosis ranged from 1 to 10%, with 10 (33.3%) tumors having Ki-67 labeling of at least 4%. DNA methylome analysis was performed for 23 tumors (76.6%). Of these, 4 (17.4%) matched DLGNT MC-1, 3 (13.0%) matched DLGNT MC-2, and 16 (69.6%) matched DLGNT but not a specific subclass. Additional clinical and molecular data are summarized in Fig. 1 and Supplemental Table 1.
Fig. 1.
Genetic alterations and clinical characteristics of the study cohort. Patient/tumors are arranged in columns, with categories labeled along the rows. DLGNT = diffuse leptomeningeal glioneuronal tumor; MC-1 = DLGNT Methylation Class-1; MC-2 = DLGNT Methylation Class-2; CSI = craniospinal irradiation
Diagnostic and treatment approaches
Biopsy was the most common neurosurgical intervention at the time of diagnosis (14/30, 46.7%), followed by subtotal resection (STR) (8/30, 26.6%) and gross or near-total resection (GTR/NTR) (8/30, 26.6%) (Fig. 1). Of those who had a biopsy as initial intervention, 12/14 (85.7%) had disseminated disease on imaging. Of those who had a resection (STR/GTR/NTR), 14/16 (87/5%) had localized disease on imaging. All biopsies were immediately followed by adjuvant therapy, while only 25% (2/8) of GTR/NTRs and 50% (4/8) STRs were immediately followed by adjuvant therapy, with the remainder being observed with surveillance imaging. In total, 27 patients (90.0%) received adjuvant therapy, either at the time of initial diagnosis or at the time of progression. Chemotherapy was used as first-line adjuvant therapy in 19 patients (70.4%), while targeted therapy and radiotherapy were used as first-line treatments in 5 patients (18.5%) and 2 patients (7.4%), respectively.
A swimmer plot and table with treatment details are provided in Fig. 2 and Supplemental Table 1. Twenty-three (76.7%) patients received at least one regimen of cytotoxic chemotherapy during the course of disease, with 36 regimens (median, 1; mean, 1.5) used among them. Carboplatin combined with either vincristine or vinblastine was the most common first-line chemotherapy regimen (11/23, 47.8%), followed by temozolomide monotherapy (6/23, 26.1%). Three patients (13.0%) received carboplatin, vincristine, and temozolomide as their first-line chemotherapy. Among the 13 subsequent chemotherapy regimens used in six patients, vinblastine monotherapy was the most common (5/13, 38.7%), followed by thioguanine, procarbazine, lomustine, and vincristine (TPCV) (2/13, 15.4%), and temozolomide combined with irinotecan (2/13, 15.4%). Across all 36 chemotherapy regimens, progression occurred after 24 of the regimens (66.7%).
Fig. 2.
Swimmer plot of patients with DLGNT. Events and interventions are described in the figure key. DLGNT = diffuse leptomeningeal glioneuronal tumor; STR = subtotal resection; NTR = near-total resection; GTR = gross total resection
Fifteen (50.0%) patients received at least one regimen of targeted therapy. Of the 29 targeted therapy regimens used in these 13 patients, trametinib monotherapy was the most common (16/29, 55.2%), followed by tovorafenib (6/29, 20.7%), and mirdametinib (3/29, 10.3%). One patient received two concomitant agents (tovorafenib and pimasertib) as the seventh line of therapy. Three patients (10.3%) had a radiologic partial response. Progression occurred after 21 (72.4%) of these 29 regimens.
Twelve patients (40.0%) received radiotherapy, with nine patients (75.0%) receiving craniospinal irradiation (CSI) and 3 (25.0%) receiving focal radiotherapy. Two patients received radiotherapy as their first adjuvant therapy (one CSI, one focal). The remaining ten patients received a median of two prior systemic therapies before radiation. Progression occurred in eight patients (66.7%) after receiving radiotherapy.
Patient outcomes
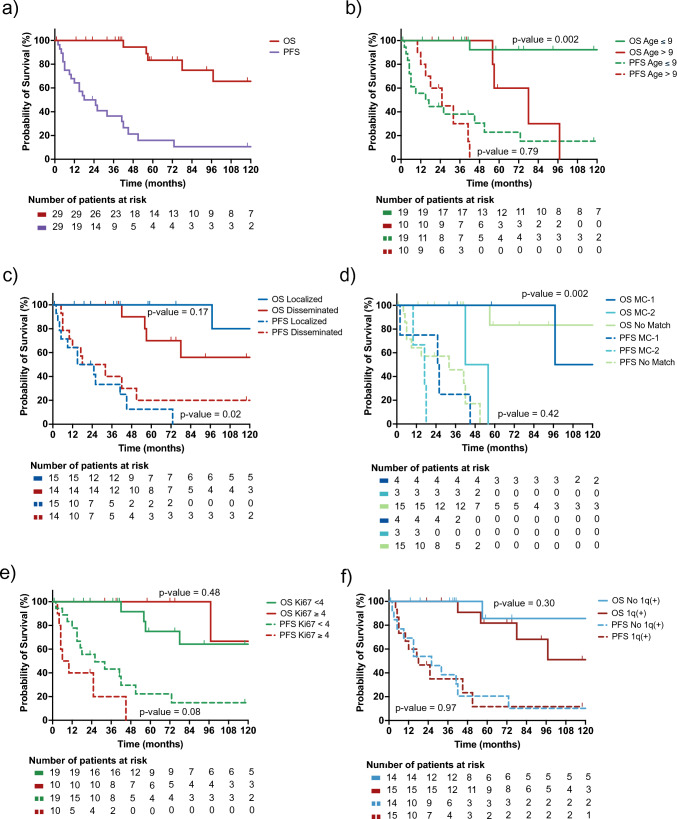
The median follow-up time was 57.5 months (1–179 months). Five patients (16.7%) died, all from tumor progression. The 5 year PFS was 15.9% ± 8.0, with a median time to progression of 21.5 months (2–73 months); the 5 year OS was 83.3% ± 8.8 (Fig. 3a). The 5 year OS was lower in patients older than 9 years at diagnosis than in those younger than 9 years at diagnosis (60.0% ± 21.9 vs. 92.3% ± 7.4, p = 0.002). However, there was no difference in 5 year PFS when stratified by age (Fig. 3b). There was no difference between the 5 year OS of those with localized or disseminated disease at diagnosis, but the 5 year PFS of patients with localized disease was lower than that of patients with disseminated disease (0.0% ± 11.0 vs. 34.3% ± 14.8, p = 0.02) (Fig. 3c). Those with tumors that methylated to MC-2 had worse 5year OS compared to MC-1 (0.0% ±35.4% vs. 100 ±0.0%, p = 0.04) or those who matched to DLGNT but not a specific subclass (83.3 ±15.2, p = 0.004). There was no difference in PFS between groups in this cohort (Figure 3d). When stratified by Ki-67 labeling or by the tumor’s 1q status, neither 5 year OS nor 5 year PFS differed between patients (Fig. 3e and f).
Fig. 3.
Kaplan–Meier curves of cohort outcomes. a PFS and OS of the whole cohort. b PFS and OS based on age, with 9 years as a threshold. c PFS and OS based on disease spread at diagnosis, localized vs. disseminated. d PFS and OS based on methylation class status. e PFS and OS based on Ki-67, with 4% as a threshold. f PFS and OS based on 1q status. P-value calculated by performing log-rank testing. DLGNT = diffuse leptomeningeal glioneuronal tumor; MC-1 = DLGNT Methylation Class-1; MC-2 = DLGNT Methylation Class-2; No match = matched to DLGNT, but not to a DLGNT subclass
There was no significant difference in PFS by surgical intervention (p = 0.052). Median PFS after STR was 9.5 months (range: 3–45 months), 26 months after GTR/NTR (range: 6–41 months), and 32 months after biopsy (range: 2–73 months) (Fig. 4a). Median PFS was 44 months (range: 1–77 months) after the patient’s first chemotherapy regimen and 11 months (range: 4-39 months) after the first targeted therapy regimen (Fig. 4b). Median PFS after radiotherapy was 16.5 months (range: 9–23 months). The 2 year and median PFS of the therapeutic modalities did not differ (p = 0.23), nor did those of patients treated with CSI and those who received focal radiotherapy (Fig. 4c). Figure 4d describes the PFS after the different lines of adjuvant therapy (first to seventh line), with subsequent treatments having worse median PFS (p = 0.002).
Fig. 4.
Kaplan–Meier curves of treatment outcomes. a PFS after surgery. b PFS after the use of the different treatment modalities. c PFS after radiotherapy, focal vs craniospinal irradiation (CSI). d PFS after different lines of therapy, from the first line to the seventh line. P-value calculated by performing log-rank testing
Simultaneous activation of multiple signaling pathways in DLGNTs
Genome-wide DNA methylome profiling of 24 tumors and 17 normal tissues was performed to identify differentially methylated regions (DMRs) and genes, as well as their associated activated signaling and metabolic pathways in DLGNT (Fig. 5a). Differential methylation analysis revealed 1874 differentially methylated genes within the DMRs, which were further investigated by global gene set enrichment analysis (GSEA) to determine their biological functions. The 19 most overrepresented GSEA terms indicated significant enrichment of pathways involved in apoptosis, cell-cycle control, T-cell receptor signaling, the MYC network, and MAPK, WNT, and p53 signaling (Fig. 5b).
Fig. 5.
Study design (a) and gene set enrichment analysis (b) comparing genes in the differentially methylated regions in DLGNT vs. normal CNS tissue
Twelve tumors and 17 normal tissues with sufficient material were also subjected to RNA sequencing and transcriptome analysis to confirm the DNA methylome findings. Differential expression analysis and GSEA of the transcriptome data revealed the upregulation of genes in MAPK, PI3K/AKT/mTOR, WNT, and p53 signaling in DLGNT, supporting the findings of our DNA methylome analysis (Fig. 6). The results also showed transcriptional activation of MYC targets, cell-cycle checkpoint activation, and increased apoptosis in DLGNT, consistent with the findings of differential methylation analysis. Orthogonal validation by deep proteomics and phosphoproteomics revealed similar findings, supporting the simultaneous activation of multiple signaling pathways in DLGNT at the DNA methylome, transcriptome, proteome, and phosphoproteome levels (Suppl Figure 1 and Suppl Figure 2).
Fig. 6.
Transcriptome analysis showing activation of multiple pathways in DLGNT
Discussion
This study presents the largest molecularly characterized and clinically annotated cohort of DLGNT patients, providing detailed treatment and outcome data. DLGNTs showed a male predominance in our cohort, which is consistent with prior reports [2–4, 21]. Nearly two-thirds of the tumors, while showing methylome profiles matching DLGNT, could not be classified into a specific DLGNT methylation subclass. No CNS WHO grade is assigned to DLGNT, but according to the 2021 WHO Classification of CNS tumors, most DLGNTs are low-grade tumors with a clinical course most aligned to WHO grade 2 entities [22]. Although DLGNT is histologically low-grade, our data support that DLGNT demonstrates high progression rates, comparable to those typically associated with CNS WHO grade 2 or even grade 3 tumors. For example, IDH-mutant gliomas are classified as CNS WHO grade 2 or 3 tumors and have a 5 year PFS of approximately 30–40% [23–25], and the 5 year PFS was about 18% in our cohort.
Historically, DLGNT has been associated with widespread leptomeningeal involvement. However, our cohort demonstrates that, contrary to common belief, it is common to have localized disease at diagnosis, especially in the spinal cord. Given these findings, it is essential to consider DLGNT in the differential diagnosis of localized spinal tumors. Furthermore, radiologic surveillance of the neural axis should be considered in patients with localized DLGNT, given the likelihood of eventual leptomeningeal spread. As approximately half of the tumors did not exhibit leptomeningeal dissemination at presentation, reconsidering the term ‘diffuse leptomeningeal glioneuronal tumor’ may be warranted to accurately reflect the disease spectrum.
In our cohort, age at diagnosis and MC-2 are factors associated with inferior outcomes, whereas Ki-67 labeling index and 1q status did not demonstrate prognostic value. Previous studies have suggested that methylation class, 1q status, Ki-67, and age are prognostic in DLGNT [3–5]. These discrepancies could be multifactorial, ranging from biases in cohort characteristics to differences in analysis and therapeutic approaches.
BRAF alterations are frequently considered a tumor-agnostic drug target, with most patients having a clinical benefit from targeted therapy [26]. However, in this study, despite KIAA1549::BRAF gene fusions being present in more than 95% of tumors, the disease was poorly controlled with single-agent targeted therapy against BRAF or MEK-1/2, with infrequent radiologic response and short time to progression. This was similar in the only patient who received a combination of targeted agents, who was heavily pretreated. Importantly, our multi-omics analysis, incorporating DNA methylome, transcriptome, proteome, and phosphoproteome data, revealed the simultaneous activation of multiple signaling pathways in DLGNT. Contrary to the perception that pediatric low-grade gliomas are single-pathway diseases, our data suggest that inhibiting multiple pathways might be required for disease control in patients with DLGNT. Clinical trials are necessary to validate this approach prospectively. Furthermore, the results of our multi-omics analysis should be considered when enrolling patients with DLGNT in active clinical trials using MAP kinase inhibitors and when analyzing the subsequent trial results.
Our study found that PFS was shorter in patients with localized disease compared to disseminated disease. Additionally, the extent of resection did not significantly affect PFS, although there was a trend towards a longer time to progression in patients who underwent biopsy compared to those who received a STR or GTR/NTR. Treatment patterns likely explain these findings. Patients with disseminated disease were more likely to undergo biopsy followed immediately by adjuvant therapy. In contrast, those with localized disease typically underwent some degree of resection followed by surveillance and did not receive systemic therapy until the time of progression. Gross total resection is the standard of care for pediatric low-grade gliomas when it can be safely achieved, offering a 5 year PFS rate exceeding 90% without adjuvant therapy [27]. Our data, however, indicate that patients who underwent GTR or NTR did not have improved PFS compared to those who had a STR. Furthermore, a substantial proportion experienced disease progression following GTR. Together, these findings raise important questions regarding the appropriateness of close surveillance in patients with DLGNT after resection and suggest that adjuvant therapy may be beneficial regardless of the extent of resection.
Disease control with cytotoxic chemotherapy and radiotherapy was of short duration for patients with DLGNT. Most patients who received radiotherapy experienced progression, all within 2 years of radiation. This differs from the greater than 70% 5 year event-free survival seen in patients with metastatic pediatric-grade gliomas after CSI radiation [28]. Patients in our cohort received radiation at varying times during their disease course, and both focal and CSI radiation were used. Given the tendency for localized disease to eventually disseminate as well as the inferior PFS with subsequent progressions, treating with CSI earlier in the disease course may provide better disease control. Ultimately, better treatments for DLGNT are clearly and urgently needed.
Our study does have limitations. First, although this is a sizeable cohort considering the rarity of DLGNT and published data, a larger one could allow for more robust statistical analyses and uncover additional details about factors associated with disease progression. Second, although the dataset has granular details on the treatments used, different chemotherapy regimens were used, and the timing of radiotherapy varied. Although broad conclusions can be drawn from these data, prospectively collected data with consistent treatment would be needed to provide more robust conclusions on treatment approaches and define the optimal treatment for DLGNT.
In summary, our study reveals that DLGNT exhibits diverse clinical and molecular characteristics, yet it consistently yields poor outcomes, regardless of these features, and remains poor across all treatment modalities. Our data show that DLGNT has clinical courses similar to those of CNS WHO grade 2 or grade 3 tumors, providing evidence that may support an approach to treatment more typical for pediatric high-grade gliomas. Due to DLGNT’s rarity, establishing optimal therapeutic strategies will require the prospective collection of outcomes after more homogeneous treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Emily Walker for technical assistance with MethylationEPIC array and Sai Prabhas Konduru for technical assistance with RNA-seq. We thank Cherise Guess for providing scientific editing of this manuscript.
Author contribution
Conceived and designed the study: DCM and JC. Performed experiments and data analysis: MKM, XL, KY, AAH, HT, LVF, and JP. Provided clinical information: NL, JB, JDB, DRB, FC, SLC, MCD, JBF, LH, JKK, TL, ML, AM, BCM, DN, BP, JS, IS, SS, RT, IQ, and GWR. Created the figures and drafted the manuscript: MM, XL, DCM, and JC. All authors have read and approved the final version.
Funding
DCM and JC receive support from the St. Jude Comprehensive Cancer Center (NCI grant P30CA021765) and American Lebanese Syrian Associated Charities. JC receives support from the NCI Program Project P01CA096832 and the V Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
Data will be provided upon reasonable request to the corresponding authors.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Margit K. Mikkelsen and Xiaoyu Li are Co-first authors.
Contributor Information
Daniel C. Moreira, Email: daniel.moreira@stjude.org
Jason Chiang, Email: jason.chiang@stjude.org.
References
- 1.Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820 [DOI] [PubMed] [Google Scholar]
- 2.Bajin IY, Levine A, Dewan MC et al (2024) Understanding diffuse leptomeningeal glioneuronal tumors. Childs Nerv Syst 40(8):2359–2366 [DOI] [PubMed] [Google Scholar]
- 3.Deng MY, Sill M, Chiang J et al (2018) Molecularly defined diffuse leptomeningeal glioneuronal tumor (DLGNT) comprises two subgroups with distinct clinical and genetic features. Acta Neuropathol 136:239–253 [DOI] [PubMed] [Google Scholar]
- 4.Wiśniewski K, Brandel MG, Gonda DD, Crawford JR, Levy ML (2022) Prognostic factors in diffuse leptomeningeal glioneuronal tumor (DLGNT): a systematic review. Childs Nerv Syst 38(9):1663–1673 [DOI] [PubMed] [Google Scholar]
- 5.Chiang J, Moreira DC, Li X, Furtado LV (2022) Prognostic significance of chromosome arm 1q gain and methylation class in molecularly defined diffuse leptomeningeal glioneuronal tumor. Acta Neuropathol 144(6):1185–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Moreira DC, Bag AK, Qaddoumi I, Acharya S, Chiang J (2023) The clinical and molecular characteristics of progressive hypothalamic/optic pathway pilocytic astrocytoma. Neuro-oncol 25(4):750–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang J, Bagchi A, Li X et al (2024) High-grade glioma in infants and young children is histologically, molecularly, and clinically diverse: results from the SJYC07 trial and institutional experience. Neuro-oncol 26(1):178–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang J, Diaz AK, Makepeace L et al (2020) Clinical, imaging, and molecular analysis of pediatric pontine tumors lacking characteristic imaging features of DIPG. Acta Neuropathol Commun 8:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang J, Li X, Jin H, Wu G, Lin T, Ellison DW (2022) The molecular characteristics of low-grade and high-grade areas in desmoplastic infantile astrocytoma/ganglioglioma. Neuropathol Appl Neurobiol 48(4):e12801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang JC, Harreld JH, Tanaka R et al (2019) Septal dysembryoplastic neuroepithelial tumor: a comprehensive clinical, imaging, histopathologic, and molecular analysis. Neuro-oncol 21(6):800–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He C, Xu K, Zhu X et al (2021) Patient-derived models recapitulate heterogeneity of molecular signatures and drug response in pediatric high-grade glioma. Nat Commun 12(1):4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris TJ, Butcher LM, Feber A et al (2014) ChAMP: 450k chip analysis methylation pipeline. Bioinformatics 30(3):428–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy DJ, Chen Y, Smyth GK (2012) Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40(10):4288–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smyth GK (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3(1):1 [DOI] [PubMed] [Google Scholar]
- 15.Yaari G, Bolen CR, Thakar J, Kleinstein SH (2013) Quantitative set analysis for gene expression: a method to quantify gene set differential expression including gene-gene correlations. Nucl Acids Res 41(18):e170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes CS, McConechy MK, Cochrane DR et al (2016) Quantitative profiling of single formalin fixed tumour sections: proteomics for translational research. Sci Rep 6(1):34949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai B, Wang X, Li Y et al (2020) Deep multilayer brain proteomics identifies molecular networks in Alzheimer’s disease progression. Neuron 105(6):975–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Li Y, Wu Z, Wang H, Tan H, Peng J (2014) Jump: a tag-based database search tool for peptide identification with high sensitivity and accuracy. Mol Cell Proteom 13(12):3663–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu M, Cho J-H, Kodali K et al (2017) Extensive peptide fractionation and y 1 ion-based interference detection method for enabling accurate quantification by isobaric labeling and mass spectrometry. Anal Chem 89(5):2956–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fangusaro J, Witt O, Driever PH et al (2020) Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 21(6):e305–e316 [DOI] [PubMed] [Google Scholar]
- 21.Lu VM, Di L, Gernsback J et al (2022) Contemporary outcomes of diffuse leptomeningeal glioneuronal tumor in pediatric patients: a case series and literature review. Clin Neurol Neurosurg 218:107265 [DOI] [PubMed] [Google Scholar]
- 22.WHO Classification of Tumours Editorial Board (2021) WHO classification of tumours series: central nervous system tumours, 5th edn, vol 6. International Agency for Research on Cancer, Lyon, France
- 23.Miller JJ, Loebel F, Juratli TA et al (2019) Accelerated progression of IDH mutant glioma after first recurrence. Neuro Oncol 21(5):669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen E, Hamisch C, Ruess D et al (2019) Observation after surgery for low grade glioma: long-term outcome in the light of the 2016 WHO classification. J Neuro-Oncol 145:501–507 [DOI] [PubMed] [Google Scholar]
- 25.Wijnenga MM, French PJ, Dubbink HJ et al (2018) The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro-oncol 20(1):103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanrahan AJ, Chen Z, Rosen N, Solit DB (2024) BRAF—a tumour-agnostic drug target with lineage-specific dependencies. Nat Rev Clin Oncol 21(3):224–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wisoff JH, Sanford RA, Heier LA et al (2011) Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery 68(6):1548–1555 [DOI] [PubMed] [Google Scholar]
- 28.Tsang DS, Murphy ES, Ezell SE, Lucas JT Jr, Tinkle C, Merchant TE (2017) Craniospinal irradiation for treatment of metastatic pediatric low-grade glioma. J Neurooncol 134(2):317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be provided upon reasonable request to the corresponding authors.