Abstract
Metabolic reprogramming is a key feature of clear cell renal cell carcinoma (ccRCC), and metabolic abnormality can lead to significant changes in gene expression, resulting in the immunosuppressive microenvironment. In this study, we used a combination of single-cell RNA sequencing and bulk RNA sequencing to investigate the relationships between ccRCC metabolic reprogramming and immune exhaustion. Metabolic subtypes of ccRCC patients were constructed using bulk RNA sequencing. Tumor cells of different metabolic subtypes were analyzed and extracted by the Scissor algorithm, using single-cell RNA sequencing. The molecular mechanisms of abnormal metabolic regulating tumor immunity were explored using cell–cell communication analysis. In addition, the correlations between relevant molecules and immune exhaustion signals were verified in ccRCC by immunohistochemistry. The molecular mechanisms of metabolic abnormalities leading to immune exhaustion were validated via Western blotting, ELISA, cell co-culture and immunotherapy models. ccRCC patients can be divided into MT1 and MT2 metabolic subtypes. The MT2 subtype has a poorer prognosis and lower response to immunotherapy. Abnormal metabolism of arachidonic acid is a prominent feature of the MT2 subtype, and activates the MDK signaling pathway. As a secreted protein, MDK can further recruit immunosuppressive cells, such as Treg, Tex, and TAM. Blocking the arachidonic acid COX metabolic pathway significantly reduces the expression and secretion levels of MDK, thereby reprogramming the tumor microenvironment to promote anti-tumor immunity. Abnormal metabolism of arachidonic acid plays an important role in promoting immune exhaustion by activating the MDK signaling pathway. MDK may serve as an important biomarker for predicting the immune therapy response in ccRCC. By reducing MDK secretion, targeting blockade of arachidonic acid metabolism may be an effective treatment strategy to enhance the efficacy of immunotherapy in ccRCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-025-01807-8.
Keywords: Clear cell renal cell carcinoma, Metabolic reprogramming, Arachidonic acid, MDK, Immunotherapy combined therapy
Introduction
Renal cell carcinoma (RCC) is considered one of the most lethal cancers of the urinary system, with approximately 430,000 new cases and 180,000 deaths worldwide in 2020 [1, 2]. Among the RCCs, clear cell renal cell carcinoma (ccRCC) is the most common subtype with the worst prognosis [3]. Surgical resection alone is often insufficient to control the disease progression and recurrence, and treatment decisions may involve targeted drug therapy, immunotherapy combined with targeted drug therapy, and dual immunotherapy combined treatment [3]. Although these therapies have greatly extended the overall survival of ccRCC patients, patients always suffer from adverse events such as drug resistance or distant metastasis during the treatment process [4]. Therefore, it is crucial to identify novel biomarkers and investigate the molecular mechanisms of ccRCC for more effective therapy strategies.
Metabolic reprogramming is a key feature of ccRCC, and the metabolic changes in tumor cells always lead to abnormalities in the tumor microenvironment (TME), thereby promoting tumor progression and immune exhaustion [4]. Accumulating evidence indicates that polyunsaturated fatty acids, such as arachidonic acid (AA), play a crucial regulatory role in cancer development [5–7]. Excessive intake of AA can lead to adverse effects in the human body, including cancer initiation and chronic inflammation [6, 8]. Long-term and sustained inflammation in TME is a hallmark of cancer progression, and is considered a major feature that leads to immune exhaustion [9, 10]. AA metabolites and related fatty acids (including prostaglandins, leukotrienes, lipoxins, and epoxyeicosanoic acids) exhibit strong abilities to regulate inflammation [6, 8]. Therefore, targeting the metabolism of polyunsaturated fatty acids such as AA in ccRCC may reduce the inflammation and prevent the exhaustion of tumor immune microenvironment and disease progression [11, 12].
Local inflammation in tumors can induce abnormal expression and dysfunction of various genes [13, 14]. Mounting evidence indicates that Midkine (MDK) plays a critical role in the inflammatory process [15], and its expression is highly regulated during inflammation [16]. MDK is a low molecular weight protein, and its recognition activity for glycosaminoglycans (GAGs) can significantly increase the affinity with receptor-like proteoglycans [17, 18]. In general, the interactions between MDK and receptors or receptor complexes enhance cancer cell growth, migration, metastasis, and immune exhaustion by activating downstream signaling cascades [18–20]. Research evidence shows that MDK can interact with immune cells, thereby regulating the role of immune cells in immunotherapy [16, 21]. In gastric cancer, liver cancer, and melanoma, data suggest that MDK inhibits the cytotoxicity of natural killer cells [15, 22], stimulates the accumulation of immunosuppressive bone marrow-derived suppressor cells in the TME [23], and finally leads to an immunosuppressive microenvironment [23, 24]. Therefore, the crosstalk between MDK and immune cells is worth of further exploration.
Recently, the application of single-cell RNA sequencing (scRNA-seq) technology in human cancers has revealed the important biological behaviors of immune cells in TME such as tumor infiltrating lymphocytes and myeloid cells [25]. scRNA-seq also greatly uncovers the reshaping of the immune microenvironment and the complex crosstalk between different cell types [26]. It is crucial to identify the cell subgroups that are associated with phenotypes such as disease stage, tumor metastasis, treatment response, and survival outcomes [27]. Therefore, using phenotype information from bulk RNA sequencing data to identify cell subpopulations which were highly correlated with disease phenotypes will help to improve the understanding of the disease [27].
In this study, by analyzing the data from scRNA-seq and bulk RNA-seq, we constructed the metabolic abnormal phenotype of ccRCC and identified tumor cells related to metabolic phenotype. Through further analysis of intercellular crosstalk, we found that the subtype of ccRCC tumor cells with high metabolism of AA contributed to an immune-suppressive microenvironment by secreting more MDK. In addition, blocking the arachidonic acid COX metabolic pathway significantly reduced the expression and secretion levels of MDK. Our study provides a novel insight into the immune microenvironment under different metabolic states of ccRCC, and uncovers the mechanisms which may be used to improve the therapeutic efficacy of combination immunotherapy for ccRCC patients.
Methods and materials
Data source
We searched for scRNA-seq and bulk RNA-seq data of ccRCC based on The Cancer Genome Atlas Program (TCGA, http://cancergenome.nih.gov/) and Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/). High-throughput transcriptome sequencing data (raw counts) of 522 ccRCC human samples with available clinical information from the TCGA-KIRC cohort were included and the raw counts were converted to FPKM for further analysis. Afterward, we downloaded Imvigor210 immunotherapy data from the GEO database for subsequent immunotherapy efficacy analysis. The scRNA-seq data came from the GSE156632 and GSE152938 datasets, with a total of 9 ccRCC samples and 6 normal samples.
Perform NMF clustering
We clustered TCGA-KIRC patients by nonnegative matrix factorization (NMF) using a published list of 2752 metabolic-related genes of all known human metabolic enzymes and transporters [28]. Before executing NMF, gene filtering was performed. First, candidate genes with low median absolute deviation (MAD) values (MAD ≤ 0.5) for all RCC patients were excluded. Afterward, the R package “survival” was used to perform Cox regression analysis to evaluate the association between all candidate genes and overall survival (OS). Finally, genes with high variability (MAD > 0.5) and significant prognostic value (P < 0.05) were used to perform NMF clustering. Subsequently, NMF clustering method was performed using the nmf R package on the TCGA-KIRC dataset [29].
Immune landscape analysis
We calculated the scores of immune landscape and metabolic pathway for each sample using ssGSEA. At the same time, the infiltration scores of each cell subset in single cells were calculated based on the marker genes in TCGA samples and the immune therapy cohort. The relative abundance of 22 immune cells in each sample was calculated based on the LM22 matrix using cibersort [30].
Single-cell data processing
Cells were filtered based on the following quality control metrics: (1) Total unique molecular identifiers (UMIs) per cell > 200 and < 5,000; (2) Total counts per cell < 30,000; (3) Percentage of mitochondrial < 30%. Only cells passing criteria were retained for downstream analysis. Finally, we obtained 68,024 cells for downstream analysis. Considering the heterogeneity among patients, we chose the batch effect correction algorithm Harmony for batch effect correction [31]. We use the functions NormalizeData and ScaleData in the Seurat package to normalize the expression matrix [32]. Then, we apply the FindVariable function to select the top 2000 variable genes for principal component analysis. We set PC to 30 and the resolution to 0.15 to use with the FindClusters function, resulting in the generation of 12 cell clusters. Through analysis of differentially expressed genes (DEGs) for each cluster and literature research, we ultimately determine 7 major cell types. DEGs are generated using the Seurat FindMarkers function.
Epithelial cell CNV assessment
We used InferCNV to infer CNVs in 6 RCC patients from single-cell datasets extracted from epithelial cells. As described in InferCNV, we used non-malignant cells, including immune cells, as a baseline to estimate the can of malignant cells [33]. Genes expressed in less than 20 cells were filtered out and classified according to their genomic position on each chromosome. The CNV heatmap of malignant cells in patients was visualized using the R package Pheatmap.
Scissor algorithm
We used Scissor algorithm to associate bulk sequencing data from TCGA-KIRC patients with single-cell data [34]. The metabolic subtyping of TCGA-KIRC was used as the phenotype in our Scissor (logistic regression) and the Scissor was run for each patient. We set the alpha parameter to 0.05 to select the malignant epithelial cells most related to the metabolic subtype.
Transcription factor activity inference
SCENIC is a tool used to assess transcription factor activity in scRNA-seq data, which can be used to construct gene regulatory networks and identify stable cell states [35]. We performed pySCENIC analysis on two representative metabolic subpopulations of cells. Only significantly upregulated or downregulated regulators were further analyzed.
Cellular communication analysis
We used CellChat to perform receptor-ligand pairing analysis between identified cell subpopulations and immune cells to identify intercellular communication [36]. We analyzed tumor cell-lymphoid cells and tumor cell-myeloid cells separately.
Cell culture and reagents
The human ccRCC cell lines 786-O,769-P and monocytic THP-1 cell line were purchased from ATCC and cultured in PRMI 1640(Gibco, C11875500BT) with 10% fetal bovine serum (Biological Industries, 04-001-1ACS). The mouse RCC cell line Renca were purchased from Procell Life Science&Technology (Wuhan, China) and cultured in a specific culture medium provided by Procell Life Science&Technology (CM-0568). All the above cells were maintained in a humidified incubator (5% CO2, 37 °C).
THP-1 monocytes were first differentiated into M0 macrophages by incubating with 150 nmol/L phorbol 12-myristate 13-acetate (PMA, S7791, Selleck) for 24 h. Recombinant human MDK (PeproTech 96-450-16-5) was purchased from Thermo Fisher Scientific Inc. (China).
Animal models
BALB/c mice (4–5 weeks old, 18–20 g) were purchased from BEIJING HFK BIOSCIENCE CO (Beijing, China). 5*105 RENCA cells were mixed with 30% Matrigel were injected into the left renal capsule of BALB/c mice. After 1 week of inoculation, the mice were then divided into three different treatment group. The first group was control group (PBS i.p.), the second group was treated with arachidonic acid (2 mg/kg i.p.) every three days, the third group was treated with celecoxib (5 mg/kg i.p.) every day. Anti-PD-1 (MedChemExpress, HY-P99144; 200 μg/mouse) was injected intraperitoneally into each mouse every 4 days. After 4 weeks of incubation, mice were sacrificed and tumors were removed and measured. Tumor volume was calculated with the following formula: volume = length * width * width/2.
Western blotting
Cells were seeded in 6-well plate and washed with cold PBS then lysed with NP-40 (20 mmol/L pH 7.5 Tris, 50 mmol/L NaCl, 10% glycerol, 1% NP-40) with 1% protease inhibitors (DINGGUO BIOLOGY). Protein concentration was determined with BCA assay (Beyotime, P0010S). Proteins were separated by SDS–PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, 1,620,177). The membranes were blocked with BSA (Beyotime, ST023) for 1 h and incubated overnight at 4 °C with primary antibodies. The membranes were washed three times with TBST buffer and then incubated with appropriate secondary antibodies for 1 h at room temperature the following day. The primary antibodies were as follows: Anti-Midkine (1:1000, Proteintech, 11009-1-AP), Anti-CD163(1:1000, Cohesion, CQA7212), Anti-CD206(1:1000, Cohesion, CPA1765), Anti-TIM3(1:1000, Cohesion, CQA1998).
Immunohistochemistry
All ccRCC tissue samples were embedded in paraffin and cut into 4-µm-thick slices. The sections were deparaffinized by xylene and rehydrated by ethanol then boiled with citrate antigen retrieval solution to retrieve antigens. The sections were blocked with serum for 1 h and then incubated overnight in 4 °C with primary antibodies against Anti-MK (1:100, Immunoway, YT5177), Anti-CD163(1:100, Cohesion,CQA7212), Anti-FOXP3 (1:100, Immunoway, YT5446) at 4 °C. After incubated with HRP-conjugated secondary antibody (MXB, KIT-9902) for 1 h, DAB (MXB, DAB-2031) was used for color development and then counterstained with hematoxylin. Sections were semi-quantitatively scored for the percentage of positive staining cells as we previously described: 0, negative; 1, < 10%; 2, 11–50%; and 3, > 50%. In addition, the staining intensity was scored as 0, no staining; 1, weak; 2, moderate; and 3, strong. Five random fields were analyzed under a light microscope. The samples were divided into two groups: 0–3, low expression; 4–6, high expression.
Enzyme-linked immunosorbent assay (ELISA)
The level of MDK in the supernatant of 786-O and 769-P cells was determined using human MDK ELISA kit (MM-0190H1) according to the manufacturer’s instructions. The microtiter plate was incubated for 30 min at 37 °C with dilute sample. HRP-Conjugate regent was added for 30 min at 37 °C and was stopped followed by measurement of absorbance at 450 nm. MDK concentrations in samples were calculated according to the standard sample OD value.
Results
Metabolic reprogramming reshapes the tumor immune microenvironment in ccRCC patients
By introducing 2752 metabolic genes, we used NMF clustering to divide ccRCC patients from TCGA database into two metabolic groups (MT1 and MT2) (Fig. 1B), and survival analysis indicated that the MT2 group had a significant worse overall survival (Fig. 1C). Then we tested and found that these two subgroups of patients showed different tumor metabolic states, indicating different differentiation trajectories of renal cancer cells in the process of metabolic reprogramming (Fig. 1D).
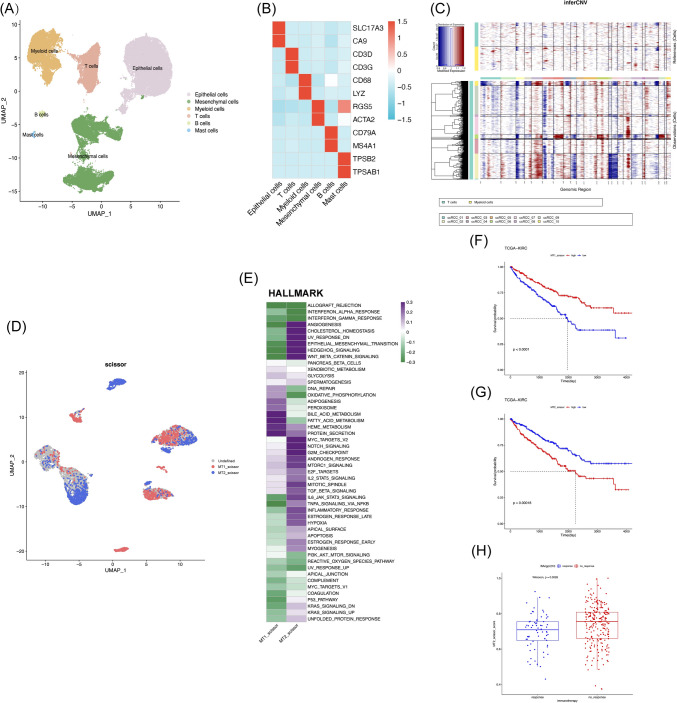
Fig. 1.
Metabolic reprogramming reshapes the tumor immune microenvironment in ccRCC patients. A The technical route of this article. B NMF algorithm recognizes two different metabolic subtypes (MT1, MT2) in TCGA-KIRC. C Kaplan–Meier curves for overall survival in MT1 and MT2 subtypes (log-rank test, p value < 0.0001). D MT1 and MT2 two metabolic groups have different metabolic landscapes. E The box plot shows the infiltration fraction of 22 different immune cells in two different subtypes of ccRCC patients. (ns, no significance, *P < 0.05, **P < 0.01, ***P < 0.001) in the TCGA-KIRC cohort. F GO_BP enrichment analysis of DEGs between MT1 and MT2. The red columns represent the pathway activated in MT1 patients, while the blue columns represent the pathway activated in MT2 patients
We used CIBERSORT to further estimate the immune infiltration of the MT1 and MT2 groups, and found significant differences in immune infiltration between the two groups. Among them, the Treg infiltration in the MT2 group was higher, whereas the M1-like macrophages decreased significantly (Fig. 1E). This suggested that the immune microenvironment in MT2 patients is suppressed, which may be due to the abnormal metabolism of tumor cells, resulting in the release of immune exhaustion signals.
To further investigate the heterogeneity between MT1 and MT2 subgroups, GO enrichment analysis was performed, and we found that in MT1 patients, the pathways were mainly enriched in respiratory and metabolic pathways such as electron transport chain and oxidative phosphorylation, while in MT2 patients, the pathways were mainly enriched in biological functions such as hypoxia response and angiogenesis (Fig. 1F). The changes in tumor function may further affect metabolism and the immune microenvironment.
scRNA-seq combined with bulk RNA-seq reveals representative cells of different metabolic subtypes
We collected the single-cell data from 9 tumor samples of ccRCC and 6 normal renal tissue samples for analysis. We divided all cells into 6 subgroups based on cell-specific markers, including epithelial cells (SLC17A3, CA9), mesenchymal cells (RGS5, ACTA2), myeloid cells (CD68, LYZ), T cells (CD3D, CD3G), B cells (CD79A, MS4A1), and mast cells (TPSB2, TPSAB1) (Fig. 2A, B).
Fig. 2.
scRNA-seq combined with bulk RNA-seq reveals representative cells of different metabolic subtypes. A UMAP plot of scRNA-seq dataset, with different colors representing different cell types. B Heatmaps display the expression level of representative marker genes in each cell type. Blue represents relatively low expression levels, while red represents high expression level. C The inferred CNV scores of each tumor sample’s epithelial cells, with red indicating high CNV levels and blue indicating low CNV levels. D The Scissor algorithm identifies two types of tumor cells that are most related to two metabolic subtypes (MT1 and MT2). The blue dot represents the representative cell of the MT1 type patient (MT1_scissor), and the red dot represents the representative cell of the MT2 type patient (MT2_scissor). E Activation of the HALLMARK pathway in MT1_scissor and MT2_scissor cells were assessed by GSVA. F High proportion of MT2_scissor cells in tumors will significantly reduce the overall survival rate of ccRCC patients (log-rank test, p value < 0.0001). G Low proportion of MT1_scissor cells in tumors will significantly reduce the overall survival rate of ccRCC patients (log-rank test, p value = 0.00018). H The proportion of the MT2_scissor cells in the immunotherapy response (response) and no-response groups (no_response) using the IMvigor210 cohort (Wilcoxon, p = 0.0026). The no-response group had a higher MT2_scissor proportion
To identify malignant tumor cells, we selected T cells and myeloid cells as references and inferred copy number variations (CNVs) in epithelial cells (Fig. 2C). We extracted malignant cells and mapped the phenotypic information of patients with either MT1 or MT2 subtype using the scissor algorithm. We selected representative cells MT1_scissor for patients with MT1 type, and MT2_scissor for patients with MT2 type (Fig. 2D).
By performing the analysis of tumor-related pathways, we found that MT1_scissor and MT2_scissor cells are distinctly different groups of cells. MT1_scissor cells activating multiple metabolic pathways, including oxidative phosphorylation and fatty acid metabolism. While in MT2_scissor cells, there is high activation in angiogenesis, epithelial-to-mesenchymal transition, and TGF-β pathway (Fig. 2E). And SCENIC transcription factor inference analysis revealed that the two types of cells have significant differences in transcriptional regulation, which also suggested that these two representative cells have different differentiation trajectories and biological functions in tumor progression (Fig. S1A). In addition, both cell groups have a significant impact on the prognosis of ccRCC, and the high percentage of MT2_scissor suggested a worse overall survival for ccRCC patients (Fig. 2F, G). We further verified the impact of two types of cells on the effectiveness of immunotherapy. Patients in the non-responsive immune group (NR) showed a higher percentage of MT2_scissor cells in their tumors (Fig. 2H). These results further confirmed our hypothesis that patients with MT2 subtype have exhausted immune microenvironment.
MT2_scissor cell remodels lymphocytes and promotes T cell exhaustion
As our data revealed significant immune microenvironment exhaustion and immune therapy failure in MT2 subtype patients, we next aimed to find out whether there were frequent cell-to-cell interactions between MT2_scissor cells and immune cells. To this end, we first clustered the lymphocyte cell types to identify different cellular subgroups within lymphocyte cells. We identified 7 lymphocyte subgroups based on cell-specific markers, including naive T cells (Tn, markers: SELL, TCF7, LEF1), T helper cells (Th, markers: IL7R, CCR7, CD40LG), cytotoxic T cells (Tc, markers: GZMH, GZMB, CD8A), exhausted T cells (Tex, markers: HAVCR2, TOX, LAG3), regulatory T cells (Tregs, markers: FOXP3, TIGIT, CTLA4), Natural killer T cells (NKT, markers: FCGR3A, KLRC1, FCER1G), and Natural killer cells (NK, Co-expression of NK cell and T cell markers) (Fig. 3A, B).
Fig. 3.
MT2_scissor cells promote T cell exhaustion through multiple mechanisms. A UMAP plots show clusters of T cells subtypes. Different colors indicate different subtypes of T cells. B Heatmap shows the expression levels of marker genes in each T cells subtypes. C Relative strength of outcoming signaling pathways. D Relative strength of incoming signaling pathways. E–G MT2_scissor specifically activates the MDK, CD79, and SPP1 signaling pathways during the interaction between the tumor cells and T cells subgroups. H Spearman correlation analysis between MT1_scissor cells and Treg and Tex in the TCGA-KIRC cohort. J Spearman correlation analysis between MT2_scissor cells and Treg and Tex in the TCGA-KIRC cohort
Next, we conducted cell interaction analysis and found that MT1_scissor and MT2_scissor exhibited strong heterogeneity during ligand secretion process (Fig. 3C). Additionally, a vary of signal reception intensities were observed among different lymphocyte subgroups (Fig. 3D). We further analyzed the contribution of ligands in the activation pathways, and found that MT2_scissor specifically activated the MK signaling pathway, CD70 signaling pathway and SPP1 signaling pathway, releasing corresponding ligands (MDK, CD70, SPP1) (Fig. 3E–G). The above pathways have been identified as key controllers to reduce toxic T cell infiltration and stimulate T cell exhaustion in some cancers [20, 37, 38] (Fig. 3E–G). Next, we investigated the correlations between two representative cell types and immune-suppressive T cells (Treg and Tex) in the TCGA-KIRC cohort. We found that compared to MT1_scissor (Treg: r = 0.008, p = 0.84; Tex: r = 0.095, p = 0.029) (Fig. 3H), MT2_scissor was notably correlated with the level of immune-suppressive T cells in ccRCC (Treg: r = 0.450, p < 0.001; Tex: r = 0.304, p < 0.001) (Fig. 3I).
Collectively, we concluded that MT2_scissor cells potentially reshape lymphocytes in the ccRCC microenvironment and inhibit immune therapy signals through regulating important immune inhibitory molecules such as MDK, CD70, and SPP1.
MT2_scissor cells recruit a variety of immunosuppressive tumor-associated macrophages
We then conducted another round of dimension reduction clustering in the myeloid cell population. Based on cell-specific markers, we identified nine cell subtypes, including two types of dendritic cells (CD1C + DC, CLEC9A + DC), six types of TAMs (C1QA + TAM, CCL3 + TAM, FOLR2 + TAM, IL1B + TAM), and cycling cells (Fig. 4A, B). We noticed a strong heterogeneity of tumor-associated macrophages (TAMs) in ccRCC patients, which is more complex than the previously defined M1-like (pro-inflammatory/anti-tumor) and M2-like (anti-inflammatory/pro-tumor) macrophage subgroups [39]. We evaluated 6 TAMs based on the relevant markers of M1-like and M2-like macrophages. Among them, CXCL10 + TAM tended to be M1-like macrophages, while the other 5 TAMs tended to be M2-like macrophages (Fig. 4C). This suggests that TAM plays a complex role in promoting tumor progression and regulating the immune microenvironment.
Fig. 4.
MT2_scissor cells recruit a variety of immunosuppressive tumor-associated macrophages (TAM). A UMAP plots show clusters of myeloid cells subtypes. Different colors indicate different subtypes of myeloid cells. B Heatmap shows the expression levels of marker genes in each myeloid cells subtypes. C GSVA scores of M1-like and M2-like macrophages markers for 6 types of TAMs. D Relative strength of outcoming signaling pathways. E Relative strength of incoming signaling pathways. F–H MT2_scissor specifically activates the MDK, COMPLEMENT, and PROS signaling pathways during the interaction between the tumor cells and myeloid cells subgroups. I Spearman correlation analysis between MT1_scissor cells and TAMs in the TCGA-KIRC cohort. J Spearman correlation analysis between MT2_scissor cells and TAMs in the TCGA-KIRC cohort
To further investigate the ways by which tumor cells recruit TAMs, how tumor cells recruit TAM, we conducted the analysis of cell communication. The results showed that there were differences in the release of ligand signals between MT1_scissor and MT2_scissor, and the response of TAMs was also different (Fig. 4D, E). MT2_scissor releases more MK signals, complement signals, and PROS signals, which are lacked in MT1_scissor (Fig. 4F–H). To detect the correlations between cells, we mapped the cell type information of scRNA-seq to RNA-seq data from TCGA-KIRC. We found a strong correlation between MT2_scissor and various immune-suppressive TAMs. These results provided solid evidence that MT2_scissor recruits more immunosuppressive TAMs and reconstructs the tumor microenvironment.
MDK significantly promotes the disease progression and immune exhaustion of ccRCC
Based on the above cell interaction analysis, we found that the MK signaling pathway plays an important role in promoting T cell exhaustion and recruiting immunosuppressive TAMs. MDK secreted by MT2_scissor is the most important ligand for activating this pathway (Fig. S1A, B).
We analyzed the role of MDK in ccRCC using the TCGA-KIRC database. We found that the expression of MDK was significantly increased in tumor tissues (Fig. 5A). Patients were stratified into high/low-MDK groups based on median expression. Survival analysis showed that patients with high MDK level had a significant poor overall survival rate (Fig. 5B). Furthermore, we found a notable positive correlation between MDK expression and the proportion of MT2_scissor tumor cells, while a significant negative correlation was observed with MT1_scissor (Fig. 5C, D). We also found that MDK has a strong correlation with the T stage (T1&T2 vs T3&T4), the N stage (N0 vs N1), the M stage (M0 vs M1), and the pathological grade (Stage I&Stage II vs Stage III& Stage IV) of cRCC, all of which indicated that high expression of MDK dramatically correlated with the clinical progression of ccRCC.
Fig. 5.
MDK significantly promotes the disease progression and immune exhaustion of ccRCC. A The expression level of MDK in tumor tissues in TCGA-KIRC dataset was significantly higher than that in normal tissues (***P < 0.001). B The expression level of MDK significantly affects the overall survival rate of ccRCC (log-rank test, P value < 0.0001). C The expression level of MDK is highly negatively correlated with the proportion of MT1_scissor cells in tumor tissue (r = − 0.348, p < 0.001). D The expression level of MDK is highly positively correlated with the proportion of MT2_scissor cells in tumor tissues (r = − 0.472, p < 0.001). E–H Correlation analysis of MDK expression levels with T stage (T1&T2 vs T3&T4), N stage (N0 vs N1), M stage (M0 vs M1), and pathological grading (stage I&II vs stage III&IV) in ccRCC. I The correlation between MDK and Treg, Tex cells in the TCGA-KIRC cohort. J The correlation between MDK and TAMs in the TCGA-KIRC cohort
Meanwhile, we found that MDK molecules have the same trend of immune cell recruitment as MT2_scissor. We found that MDK expression level was strong correlated with the level of Tregs, Tex cells and other TAMs. Concomitant increase of MDK with Treg/Tex/TAM abundance (Fig. 5I–J) suggests its potential role in immune cell recruitment, though mechanistic validation is warranted. As we have demonstrated that the proportion of MT2_scissor cells was possibly correlated the level of immune cells in ccRCC tumor tissues, we proposed that MT2_scissor remodels tumor immune microenvironment through regulating the MDK signaling pathway.
MDK is highly correlated with metabolic abnormalities and inflammatory responses in ccRCC
Our cell–cell communication analysis using CellChat pinpointed key pathways regulating T cell dysfunction (Tex and Treg) and M2 macrophage polarization. Critically, the MK signaling pathway exhibited uniquely elevated activity in M2_scissor cells, where it served as a master regulatory axis through the MDK-NCL ligand-receptor pair. This pathway orchestrates immunosuppressive networks encompassing Treg, Tex, and multiple TAM subtypes. While MDK is established as a potent immunomodulator mediating tumor immune evasion across malignancies, our study reveals a novel cellular source: metabolically reprogrammed M2_scissor cells significantly upregulate MDK expression, positioning them as pivotal architects of the immunosuppressive microenvironment.
To further validate the relationships between abnormal tumor metabolism and MDK expression, we analyzed the correlations between metabolic pathways and MDK expression level in the TCGA-KIRC database. The results showed clear correlations between a variety of metabolic pathways and high expression level of MDK (Fig. 6A). Among them, the pathways mostly interested us were the co-activation of AA metabolism and prostaglandin biosynthesis in renal cancer, which showed strong correlations with the expression of MDK (Fig. 6B, C). Previous studies have shown that these two metabolic pathways are a continuous process in the progression of renal cancer. AA is metabolized through the COX pathway to generate pro-inflammatory mediators such as prostaglandins, which in turn mediate tumor progression and immune exhaustion [11, 12].
Fig. 6.
MDK is highly correlated with metabolic abnormalities and inflammatory responses in ccRCC. A MDK expression level and metabolic pathways enrichment score heatmap in the TCGA-KIRC dataset. B There is a strong correlation between the expression level of MDK and arachidonic acid metabolism (r = 0.305, p < 0.001). C There is a strong correlation between the expression level of MDK and prostaglandin biosynthesis (r = 0.328, p < 0.001). D Correlation between MDK expression level and inflammatory factors (CSF1, IL2RA, IFNG, IL10, CXCL8, TNF). E The GSEA plot shows a strong between high expression of MDK and co-activation of cytokine interactions, arachidonic acid metabolism, and prostaglandin biosynthesis pathways in TCGA-KIRC
MDK is one of the growth factors that regulate inflammation [40], and research evidence shows that the expression of MDK is strongly induced during the inflammatory process [16]. Therefore, we examined the correlations between MDK and inflammation-related factors (CSF1, IL2RA, IFNG, IL10, CXCL8, TNF), and found that patients with high expression of MDK had significant high expression levels of these inflammatory factors (Fig. 6D). We also found a strong co-activation between the high expression of MDK and the activation of cytokine interaction, AA metabolism, and the prostaglandin synthesis pathway in renal cancer patients (Fig. 6E). The above evidence once again proved that there is a clear and close relationship between abnormal AA metabolism and immune microenvironment in kidney cancer. MDK significantly correlates with immunosuppressive microenvironment, suggesting its potential regulatory role.
AA metabolism promotes MDK expression and induces immune suppression
Based on our bioinformatic findings, we next experimentally tested MDK’s role in regulating the microenvironment using cell biology approaches. To examine the relationship between MDK and immune evasion in cancer, we analyzed several cases of ccRCC and divided them into MDK high expression (MDK-hi) and MDK low expression (MDK-lo) groups. Immunohistochemical results showed that FOXP3, a Treg cell marker, and CD163, an M2 macrophage marker, were both highly expressed in MDK high patients, indicating a strong correlation between levels of Treg cells, M2 macrophages, and MDK expression (Fig. 7A). Furthermore, we found a clear correlation between MDK and multiple T cell exhaustion signals (FOXP3, LAG3, PDCD1, TIGIT, CTLA4, BTLA) (Fig. S1C) and M2-like macrophage markers (CD163, FOLR2, MSR1, CCL3) (Fig. S1D). In addition, some studies have shown that the metabolism of AA can induce local inflammation by synthesizing downstream products such as prostaglandins through the COX pathway. MDK can be induced to express in inflammatory reactions [12, 16]. Therefore, we hypothesized that AA could induce the secretion of MDK, and the secretion of MDK may further inhibit anti-tumor immunity.
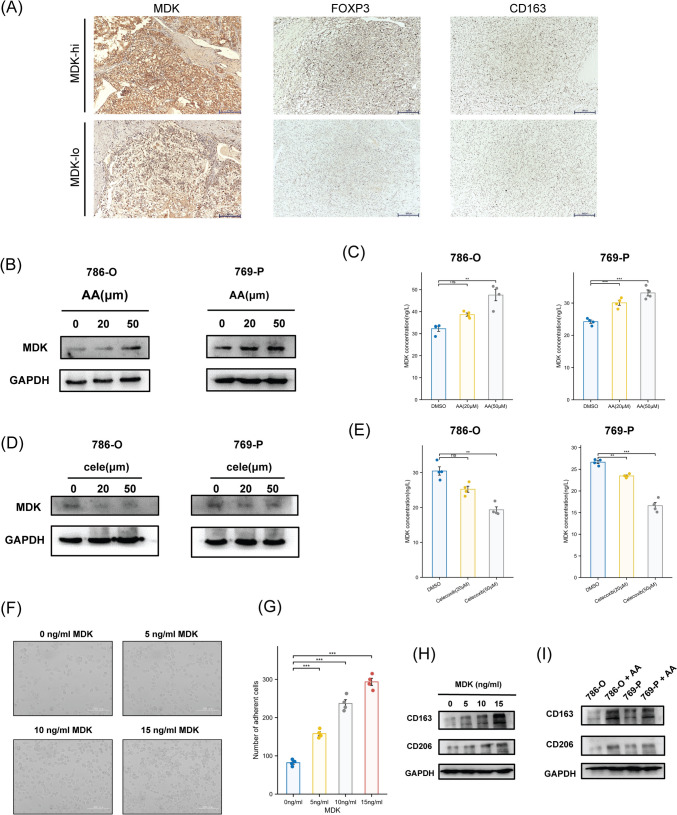
Fig. 7.
AA metabolism promotes MDK expression and induces immune suppression A Representative images of IHC staining with anti-MDK, anti-FOXP3 and anti-CD163. B The protein level of MDK of 786-O and 769-P cells were detected after treated with different concentrations of AA (0, 20, 50 µm). C The secretion level of MDK in media of 786-O and 769-P cells were detected by ELISA kit after treated with different concentrations of AA (0, 20, 50 µm). D The protein level of MDK of 786-O and 769-P cells were detected after treated with different concentrations of Celecoxib (0, 20, 50 µm). E The secretion level of MDK in media of 786-O and 769-P cells were detected by ELISA kit after treated with different concentrations of Celecoxib (0, 20, 50 µm). F–G 1 × 106 THP-1 human monocytes were seeded in 6-well plate and first treated with 150 nmol/L PMA for 24 h, and were then treated with different concentrations of MDK (0, 5, 10, 15 ng/ml) for 72 h. Number of adherent cells was statistically analyzed (Scale bar: 200 µm). H M2 macrophage markers (CD163, CD206) of THP-1 cells were detected after treated with different concentrations of MDK (0, 5, 10, 15 ng/ml). I M2 macrophage markers of THP-1 cells were detected after cocultured with 786-O and 769-P cells treated with AA
To authenticate this hypothesis, we first validated whether AA contributed to the upregulation of MDK. We treated ccRCC cell lines 786-O and 769-P with different concentrations of AA. The results from western blotting analysis showed that AA could promote the expression of MDK (Fig. 7B). Meanwhile, we found that there was a higher level of MDK in the ccRCC cell culture medium treated with AA, which suggested that AA can promote the secretion of MDK (Fig. 7C). Previous studies have shown that AA can catalyze the oxygen addition of COX-2 to produce prostaglandins and further induce inflammatory reactions [11, 12]. Celecoxib is a selective COX-2 inhibitor [41]. We then treated ccRCC cell lines with different concentrations of celecoxib and found that the expression of MDK showed a gradient decrease (Fig. 7D, E). This indicated that inhibiting the COX-2 pathway metabolism of AA may reduce the impact of MDK on the tumor microenvironment. To validate the AA/COX2-MDK, we conducted a combinatorial intervention assay in ccRCC cell lines (786-O and 769-P) comparing control (basal medium), AA monotherapy (50 μM), and AA + Celecoxib co-treatment (50 μM AA + 50 μM Celecoxib). Western blot and ELISA analyses demonstrated that AA alone significantly elevated intracellular MDK protein and secretion (*p < 0.001), whereas 50 μM Celecoxib abrogated AA-induced MDK upregulation, mechanistically establishing AA dependent MDK regulation (Fig. S1I–L).
To further verify whether MDK induces the differentiation of immunosuppressive macrophages (M2-like macrophages), human monocytic cell line THP-1 was treated with different concentrations of recombinant MDK protein and AA. Interestingly, treatment of recombinant MDK increased the number of adherent cells in THP-1, and cocultured with 786-O and 769-P treated with AA also induced THP-1 adhesion, while THP-1 treated with AA alone did not show obvious adhesion phenomenon (Fig. 7F, G; Fig. S1E, F). We also detected the expression of M2-like macrophage markers (CD163 and CD206) and found that both MDK (Fig. 7H) and cocultured with 786-O and 769-P with treatment of AA (Fig. 7I) stimulated the M2-like differentiation of macrophages. However, THP-1 treated with AA alone did not show obvious M2-like differentiation (Fig. S1G). These results demonstrated that tumor cells can induce MDK secretion through AA metabolism, and MDK promotes the M2-like transformation of macrophages.
Inhibition of the COX2 metabolism pathway of arachidonic acid reduce the secretion level of MDK and immune exhaustion in vivo.
To further validate the critical role of arachidonic acid (AA) metabolism in modulating MDK and impairing immunotherapy efficacy in renal cell carcinoma (RCC), we established an orthotopic murine renal tumor model using the Renca cell line and performed anti-PD-1 treatment (Fig. 8A). Compared with the control group (PD-1 mAb group), mice intraperitoneally injected with AA (PD-1 mAb + AA group) exhibited significantly increased tumor volumes, whereas intraperitoneal administration of the COX-2 inhibitor celecoxib suppressed tumor growth (PD-1 mAb + AA + Celecoxib group) (Fig. 8B–C).ELISA analysis of mouse serum revealed elevated MDK secretion levels in the AA-treated group, indicating that AA metabolism promotes MDK secretion in RCC (Fig. 8H). Furthermore, Western blotting analysis of orthotopic tumor tissues showed that mice in the AA group exhibited higher levels of MDK protein expression, alongside increased expression of the M2 macrophage marker CD206 and the exhaustion marker TIM3. In contrast, these markers were significantly downregulated in the celecoxib-treated group (Fig. 8G). Immunohistochemical analysis of the orthotopic RCC tissues further demonstrated that AA treatment enhanced the infiltration of M2 macrophages and Treg cells in tumor tissues, potentially contributing to RCC progression. Additionally, the AA-treated group showed more pulmonary metastatic nodules, while celecoxib effectively suppressed this effect (Fig. 8D–F).
Fig. 8.
A A sketch map of tumor formation in BALB/c mice. Renca cells were injected into the left renal cortex of mice, after 7 days, PBS, AA and Celecoxib was intraperitoneal injected in different groups. B–C Images of mice RCC tumors after orthotopic implantation for 6 weeks. D–F Representative images of IHC staining for FOXP3 and CD206 in mice RCC tumors and HE staining of mice lungs. G The protein expression of CD206, TIM3, MDK in mice RCC tumors. H Elisa experiment detecting the secretion levels of MDK in peripheral blood of mice
These findings confirm that inhibiting the COX-2 metabolic pathway of AA using celecoxib significantly reduces MDK secretion and effectively alleviates the immunosuppressive effects of MDK on the RCC immune microenvironment.
Discussion
Metabolic reprogramming in the TME is a key factor influencing tumor progression and anti-tumor immune responses. Disrupted metabolites and their regulatory pathways can significantly impact tumor progression and treatment responses. ccRCC is a tumor with a strong tumor metabolic heterogeneity [42, 43], and the disorder of various metabolites is an important factor for poor prognosis and treatment failure of ccRCC [44, 45]. Our research has revealed for the first time that abnormal metabolism of AA can significantly inhibit the immune microenvironment of ccRCC. In this study, we developed a new metabolic classification method for ccRCC and divided it into two categories based on distinct metabolic features. Subsequently, through a combination analysis of scRNA-seq and bulk RNA-seq, we confirmed that tumor cells play an important role in the metabolic heterogeneity of ccRCC, which significantly affects disease progression and immune response. Through cell interaction analysis, we found that the MDK pathway plays an important role in immune response, and that MDK is highly correlated with abnormal tumor metabolism, especially the AA metabolic pathway.
AA is a polyunsaturated omega-6 fatty acid that is present in phospholipids in cell membranes and is abundant in the brain, muscles, and liver [46]. It generates prostaglandins and thromboxanes through the cyclooxygenase (COX) pathway, and thereby participate in physiological processes such as inflammation and drug metabolism [47, 48]. Recent studies have shown that AA metabolism plays an important role in anti-tumor immunity. The combination of T cell-derived interferon (IFN γ) and AA can induce immunogenic tumor ferroptosis [49], and AA metabolism also affects macrophage polarization in mouse esophageal squamous cell carcinoma [50] and immune therapy in non-small cell lung cancer [51]. Our research also discovered a strong correlation between abnormal metabolism of AA and immune response in ccRCC. Through cell communication analysis, it is shown that MDK plays an important role in both abnormal metabolism of AA and immunosuppression. In our current study, we believe that the expression and secretion of MDK are regulated by AA metabolism. Inhibiting the COX2 pathway of AA metabolism can reduce the expression and secretion levels of MDK. However, the specific regulatory mechanism of AA metabolism abnormality and MDK remains unknown. Our study provides a reasonable explanation, which may be due to the dysregulation of continuous AA metabolism directly or indirectly promoting inflammatory reactions, further inducing the expression and secretion of MDK. At the same time, AA metabolism also produces other metabolites that can influence the tumor microenvironment, which requires further exploration.
MDK, a heparin-binding growth factor, is a soluble secreted protein that is associated with various physiological processes such as development, reproduction, and repair [52]. It plays a crucial role in the occurrence of malignant diseases including cancer [52]. MDK has been proven to promote tumor proliferation, invasion, and metastasis, thereby promoting tumor progression [53]. Through analysis of the TCGA database, we found that MDK is highly elevated in ccRCC tumor tissue, and high MDK level in ccRCC patients indicates a worse overall survival rate. MDK is also strongly correlated with the progression of ccRCC. As a secreted protein, MDK activates downstream signal transduction through receptor activation to generate biological responses [54, 55]. It participates in tumor inflammation and immune response [16, 55]. Through the analysis of scRNA-seq data, we further discovered that secreted MDK can induce and recruit Treg cells, M2-like macrophages, etc., which further exhausted the immune microenvironment of ccRCC. At last, analysis in our clinical renal tumor tissue samples showed that MDK expression is highly correlated with the infiltration level of Treg cells and M2-like macrophages. Cell experiments further confirmed that MDK can promote differentiation of THP-1 cells into M2-like macrophages.
In general, our research identifies that AA metabolism abnormalities induce MDK secretion, thereby affecting the immune microenvironment of ccRCC and suppressing the immune response of ccRCC. Our data reveal a novel network that influences the immune microenvironment, in which the metabolic differences between cells play an important role. MDK is a soluble cytokine with easy detectability in the peripheral circulatory system, making it an efficient biomarker for detection [56]. It is expected to become a novel biological marker for predicting the efficacy of immunotherapy for ccRCC [57, 58]. We also found that targeting the COX metabolic pathway that inhibits AA can reduce the secretion level of MDK, providing an effective combination immunotherapy strategy for treating ccRCC patients with high MDK expression, and implying the importance of diet management in ccRCC treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Jiaxi Yao: Formal analysis, Methodology, Validation, Writing—original draft, Writing—review & editing; Tong Xu: Conceptualization, Formal analysis, Methodology, Validation; Chenyuan Wang: Conceptualization, Formal analysis, Investigation, Methodology; Junfeng Xie: Investigation, Validation, Visualization; Qing Jiang: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaxi Yao and Tong Xu are contributed equally to this work.
References
- 1.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Barata PC, Rini BI. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin. 2017;67:507–24. 10.3322/caac.21411. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh JJ, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montecillo-Aguado M, Tirado-Rodriguez B, Huerta-Yepez S. The involvement of polyunsaturated fatty acids in apoptosis mechanisms and their implications in cancer. Int J Mol Sci. 2023. 10.3390/ijms241411691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz-Placín C, Castillejo-Rufo A, Estarás M, González A. Membrane lipid derivatives: roles of arachidonic acid and its metabolites in pancreatic physiology and pathophysiology. Molecules. 2023. 10.3390/molecules28114316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuller HM, et al. The cyclooxygenase inhibitor ibuprofen and the FLAP inhibitor MK886 inhibit pancreatic carcinogenesis induced in hamsters by transplacental exposure to ethanol and the tobacco carcinogen NNK. J Cancer Res Clin Oncol. 2002;128:525–32. 10.1007/s00432-002-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, et al. Regulation of inflammation in cancer by dietary eicosanoids. Pharmacol Ther. 2023;248: 108455. 10.1016/j.pharmthera.2023.108455. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Shadhu K, Xi C. Inflammation and pancreatic cancer: an updated review. Saudi J Gastroenterol. 2019;25:3–13. 10.4103/sjg.SJG_390_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fishbein A, Hammock BD, Serhan CN, Panigrahy D. Carcinogenesis: failure of resolution of inflammation? Pharmacol Ther. 2021;218: 107670. 10.1016/j.pharmthera.2020.107670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim W, Son B, Lee S, Do H, Youn B. Targeting the enzymes involved in arachidonic acid metabolism to improve radiotherapy. Cancer Metastasis Rev. 2018;37:213–25. 10.1007/s10555-018-9742-0. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, et al. Epigenetic regulation of chemokine (CC-motif) ligand 2 in inflammatory diseases. Cell Prolif. 2023;56: e13428. 10.1111/cpr.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falkowski L, Buddenkotte J, Datsi A. Epigenetics in T-cell driven inflammation and cancer. Semin Cell Dev Biol. 2023. 10.1016/j.semcdb.2023.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Saikia M, Cheung N, Singh AK, Kapoor V. Role of midkine in cancer drug resistance: regulators of its expression and its molecular targeting. Int J Mol Sci. 2023. 10.3390/ijms24108739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weckbach LT, Muramatsu T, Walzog B. Midkine in inflammation. ScientificWorldJournal. 2011;11:2491–505. 10.1100/2011/517152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204:127–43. 10.1016/s0304-3835(03)00450-6. [DOI] [PubMed] [Google Scholar]
- 18.Maeda N, et al. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J Biol Chem. 1999;274:12474–9. 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- 19.Muramatsu H, et al. LDL receptor-related protein as a component of the midkine receptor. Biochem Biophys Res Commun. 2000;270:936–41. 10.1006/bbrc.2000.2549. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, et al. Combined usage of MDK inhibitor augments interferon-γ anti-tumor activity in the SKOV3 human ovarian cancer cell line. Biomedicines. 2022. 10.3390/biomedicines11010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haffner-Luntzer M, et al. Midkine-deficiency delays chondrogenesis during the early phase of fracture healing in mice. PLoS ONE. 2014;9: e116282. 10.1371/journal.pone.0116282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao S, et al. Midkine upregulates MICA/B expression in human gastric cancer cells and decreases natural killer cell cytotoxicity. Cancer Immunol Immunother. 2012;61:1745–53. 10.1007/s00262-012-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding L, et al. Midkine inhibition enhances anti-PD-1 immunotherapy in sorafenib-treated hepatocellular carcinoma via preventing immunosuppressive MDSCs infiltration. Cell Death Discov. 2023;9: 92. 10.1038/s41420-023-01392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerezo-Wallis D, et al. Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state. Nat Med. 2020;26:1865–77. 10.1038/s41591-020-1073-3. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Liao K, Yang X, Wu C, Wu W. Using single-cell sequencing technology to detect circulating tumor cells in solid tumors. Mol Cancer. 2021;20: 104. 10.1186/s12943-021-01392-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei Y, et al. Applications of single-cell sequencing in cancer research: progress and perspectives. J Hematol Oncol. 2021;14:91. 10.1186/s13045-021-01105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun D, et al. Identifying phenotype-associated subpopulations by integrating bulk and single-cell sequencing data. Nat Biotechnol. 2022;40:527–38. 10.1038/s41587-021-01091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Possemato R, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–50. 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaujoux R, Seoighe C. A flexible R package for nonnegative matrix factorization. BMC Bioinformatics. 2010;11:367. 10.1186/1471-2105-11-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–59. 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korsunsky I, et al. Fast, sensitive and accurate integration of single-cell data with harmony. Nat Methods. 2019;16:1289–96. 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao Y, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573-3587.e3529. 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puram SV, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171:1611-1624.e1624. 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun D, et al. Identifying phenotype-associated subpopulations by integrating bulk and single-cell sequencing data. Nat Biotechnol. 2022;40:527–38. 10.1038/s41587-021-01091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aibar S, et al. SCENIC: single-cell regulatory network inference and clustering. Nat Methods. 2017;14:1083–6. 10.1038/nmeth.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin S, et al. Inference and analysis of cell-cell communication using Cell Chat. Nat Commun. 2021;12:1088. 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortiz-Cuaran S, et al. Epithelial-to-mesenchymal transition promotes immune escape by inducing CD70 in non-small cell lung cancer. Eur J Cancer. 2022;169:106–22. 10.1016/j.ejca.2022.03.038. [DOI] [PubMed] [Google Scholar]
- 38.Klement JD, et al. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J Clin Invest. 2018;128:5549–60. 10.1172/jci123360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M, et al. Metabolism, metabolites, and macrophages in cancer. J Hematol Oncol. 2023;16:80. 10.1186/s13045-023-01478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernández-Calle R, et al. Endogenous pleiotrophin and midkine regulate LPS-induced glial responses. Neurosci Lett. 2018;662:213–8. 10.1016/j.neulet.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 41.Stiller CO, Hjemdahl P. Lessons from 20 years with COX-2 inhibitors: importance of dose-response considerations and fair play in comparative trials. J Intern Med. 2022;292:557–74. 10.1111/joim.13505. [DOI] [PubMed] [Google Scholar]
- 42.Czyzyk-Krzeska MF, et al. Molecular and metabolic subtypes in sporadic and inherited clear cell renal cell carcinoma. Genes (Basel). 2021. 10.3390/genes12030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh JJ, et al. Overcome tumor heterogeneity-imposed therapeutic barriers through convergent genomic biomarker discovery: a braided cancer river model of kidney cancer. Semin Cell Dev Biol. 2017;64:98–106. 10.1016/j.semcdb.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai Y, et al. The tumour microenvironment and metabolism in renal cell carcinoma targeted or immune therapy. J Cell Physiol. 2021;236:1616–27. 10.1002/jcp.29969. [DOI] [PubMed] [Google Scholar]
- 45.Monjaras-Avila CU, et al. The tumor immune microenvironment in clear cell renal cell carcinoma. Int J Mol Sci. 2023. 10.3390/ijms24097946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahman M, Wright JT Jr, Douglas JG. The role of the cytochrome P450-dependent metabolites of arachidonic acid in blood pressure regulation and renal function: a review. Am J Hypertens. 1997;10:356–65. 10.1016/s0895-7061(96)00381-0. [DOI] [PubMed] [Google Scholar]
- 47.Borin TF, Angara K, Rashid MH, Achyut BR, Arbab AS. Arachidonic acid metabolite as a novel therapeutic target in breast cancer metastasis. Int J Mol Sci. 2017. 10.3390/ijms18122661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang T, et al. Arachidonic acid metabolism and kidney inflammation. Int J Mol Sci. 2019. 10.3390/ijms20153683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao P, et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022;40:365-378.e366. 10.1016/j.ccell.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu M, et al. Arachidonic acid metabolism controls macrophage alternative activation through regulating oxidative phosphorylation in PPARγ dependent manner. Front Immunol. 2021;12: 618501. 10.3389/fimmu.2021.618501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, et al. CYP4F2-catalyzed metabolism of arachidonic acid promotes stromal cell-mediated immunosuppression in non-small cell lung cancer. Cancer Res. 2022;82:4016–30. 10.1158/0008-5472.Can-21-4029. [DOI] [PubMed] [Google Scholar]
- 52.Muramatsu T. Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:410–25. 10.2183/pjab.86.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filippou PS, Karagiannis GS, Constantinidou A. Midkine (MDK) growth factor: a key player in cancer progression and a promising therapeutic target. Oncogene. 2020;39:2040–54. 10.1038/s41388-019-1124-8. [DOI] [PubMed] [Google Scholar]
- 54.Kerzerho J, et al. The angiogenic growth factor and biomarker midkine is a tumor-shared antigen. J Immunol. 2010;185:418–23. 10.4049/jimmunol.0901014. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer. J Hepatol. 2021;75:1128–41. 10.1016/j.jhep.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 56.Torres A, et al. CD44, TGM2 and EpCAM as novel plasma markers in endometrial cancer diagnosis. BMC Cancer. 2019;19:401. 10.1186/s12885-019-5556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jing X, Cui X, Liang H, Hao C, Han C. Diagnostic accuracy of ELISA for detecting serum midkine in cancer patients. PLoS ONE. 2017;12: e0180511. 10.1371/journal.pone.0180511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Song X, Shao Y, Wu C, Jiang J. Prognostic value of Midkine expression in patients with solid tumors: a systematic review and meta-analysis. Oncotarget. 2018;9:24821–9. 10.18632/oncotarget.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.