Abstract
Efficient delivery of messenger RNA (mRNA) to the brain via systemic administration remains a challenge, primarily due to the blood-brain barrier. To address this challenge, we incorporated SR-57227, a ligand of serotonin [5-hydroxytryptamine type 3 (5-HT3)] receptor, into the design of ionizable lipids to develop lipid nanoparticles (LNPs) for systemic mRNA delivery to the brain. OS4T LNP was identified as an optimized formulation based on multiple assays. Following systemic administration, OS4T LNP achieved over a 50-fold increase in mRNA translation within brain tissues compared to US Food and Drug Administration–approved Onpattro LNPs (DLin-MC3-DMA). In an orthotopic glioblastoma (GBM) mouse model, engineered interleukin-12 mRNA–loaded OS4T LNPs significantly suppressed tumor growth and improved overall survival. This study demonstrates OS4T LNP as a promising platform for brain mRNA delivery and highlights its potential for treating GBM and other central nervous system disorders.
Peptide-modified lipid nanoparticles enable systemic IL-12 mRNA delivery for glioblastoma treatment.
INTRODUCTION
Brain mRNA delivery holds immense potential to revolutionize treatments for central nervous system (CNS) diseases, such as neurodegenerative diseases and brain tumors (1–3). Lipid nanoparticles (LNPs) are US Food and Drug Administration (FDA)–approved nonviral vectors for mRNA delivery, as they enable safe and efficient transport of mRNA across various biological barriers (4–6). Recent advancements in LNP technology have enabled targeted mRNA delivery to specific organs, such as lung, spleen, and bone marrow, following intravenous administration (7–10). However, delivering mRNA to the brain remains a substantial challenge due to the presence of the blood-brain barrier (BBB) (11, 12), which effectively prevents most macromolecules such as mRNA from entering the brain. To overcome this barrier, several strategies have been developed. For instance, nanoparticles are modified with antibodies or peptides targeting specific receptors on the BBB, including transferrin (13), low-density lipoprotein (14, 15), glucose transporter (16), and insulin receptors. In addition, external stimuli, such as magnetic fields, ultrasound, and glucose fasting, have been explored to enhance nanoparticle penetration into deep brain tissues (17–19). Previous studies have demonstrated that certain small molecules can cross the BBB via distinct mechanisms (20, 21). For example, SR-57227 is a potent serotonin [5-hydroxytryptamine type 3 (5-HT3)] receptor ligand with the ability to cross the BBB (22, 23). Inspired by these findings, we hypothesized that synthetic lipids derived from SR-57227 may improve the ability of LNPs to cross the BBB, offering potential as mRNA carriers for brain delivery via systemic administration. In this study, we designed and synthesized a series of SR-57227–derived lipids (SLs) incorporating three building blocks: SR-57227 head, amino linkers, and biodegradable lipid tails (Fig. 1A). These SLs effectively encapsulated mRNA to form SR-57227–derived LNPs (SLNPs; Fig. 1A). Through screening and optimization assays, we identified the optimal SLNP formulation, OS4 LNP, which showed significantly higher mRNA delivery efficiency to the brain following intravenous injection, compared to FDA-approved LNPs, including MC3 (Onpattro), SM-102 (Spikevax), and ALC-0315 (Comirnaty). To further improve the ability of OS4 LNP to cross the BBB, we conjugated cell-penetrating peptides (CPPs) onto the surface via postmodification. CPPs are a group of short peptides capable of traversing biological membranes, promoting the cellular uptake of otherwise membrane-impermeable cargoes (24–26). While studies have shown that certain CPPs can facilitate the transport of small drugs, biomacromolecules, and nanoparticles across the BBB (26), their potential to enhance the brain delivery of LNP-mRNA formulations via systemic administration remains relatively unexplored. Through the evaluation of 10 different CPPs, we identified Tat as the optimal candidate, which did not compromise LNP stability while enhancing their ability to penetrate the BBB. As a result, the formulation OS4T LNP, consisting of OS4 LNP conjugated with the Tat peptide, showed ~10-fold higher luciferase mRNA delivery efficiency in the brain compared to OS4 LNP alone. The brain delivery efficiency of OS4T LNPs was verified through the delivery of two additional mRNAs, green fluorescent protein (GFP) and Cre recombinase (Cre), resulting in high expression across four major brain cell types: neurons, astrocytes, microglia, and endothelial cells. Furthermore, OS4T LNPs loaded with engineered interleukin-12 (eIL-12) mRNA were demonstrated to treat an orthotopic glioblastoma (GBM) mouse model. Three doses of OS4T LNP–mRNA encoding eIL-12 significantly inhibited GBM growth and extended the median survival by over twofold to 37 days compared to the phosphate-buffered saline (PBS) group. The use of OS4T LNPs for efficient mRNA delivery to the brain provides insights into the design of biomaterials capable of crossing the BBB, potentially paving the way for more effective CNS-targeted therapies.
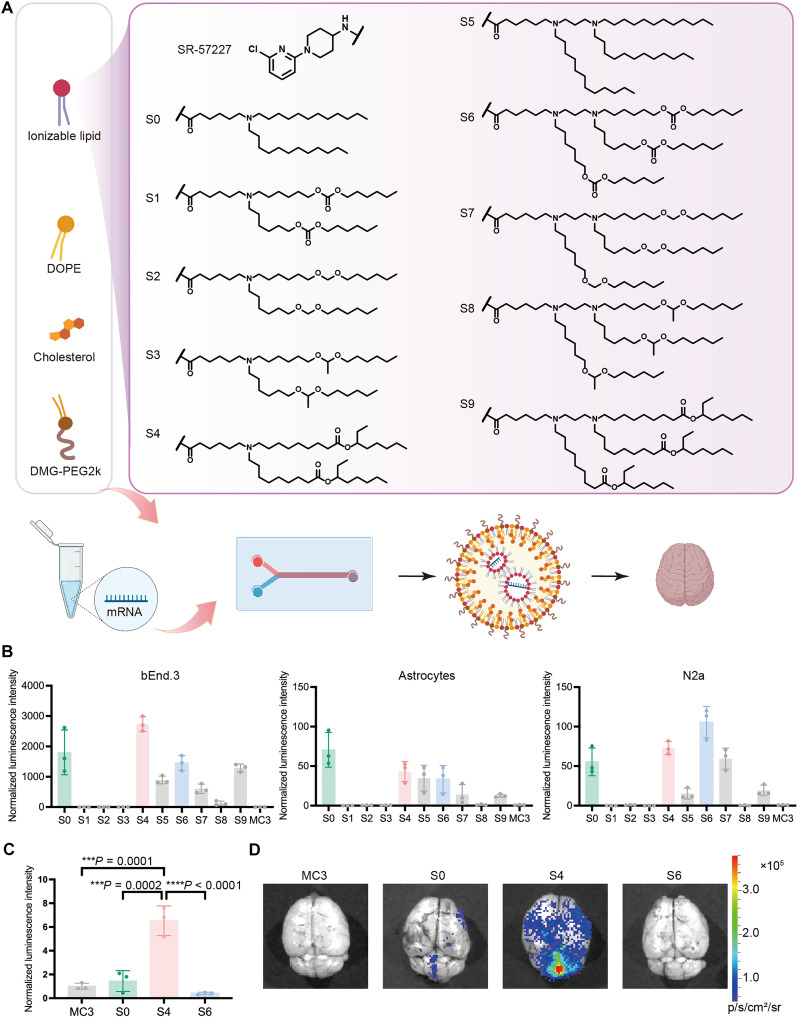
Fig. 1. Construction of SLNPs for brain delivery.
(A) The structure of SLs. (B) mRNA delivery efficiency in bEnd.3, astrocytes, and N2a cells. Data are presented as means ± SD. (C) Normalized intensity in the brain of mice treated with MC3, S0, S4, and S6 LNPs (n = 3 biological replicates). The intensity was normalized to MC3 LNP group. Data are presented as means ± SD. (D) Representative images of brain tissues. Statistical significance was analyzed by one-way analysis of variance (ANOVA) with the Tukey’s multiple comparisons test. ***P < 0.001; ****P < 0.0001. (A) was created in BioRender (X. Hou, 2025; https://BioRender.com/mjk1zye).
RESULTS
Synthesis, characterizations, and optimization of SLNPs
5-HT3 receptors are extensively distributed throughout the CNS (27, 28). Here, we used SR-57227, a potent 5-HT3 receptor ligand, to develop ionizable lipids for mRNA delivery to the brain. The series of SLs was designed with three structural modules: the SR-57227 core, two amino linkers, and five lipophilic tails (Fig. 1A). The lipids, S0, S1, S2, S3, S4, S5, S6, S7, S8, and S9, were synthesized and validated by 1H nuclear magnetic resonance and mass spectrometry (fig. S1). Next, each lipid was formulated with cholesterol (Chol), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (DMG-PEG2k), and firefly luciferase (FLuc) mRNA to generate SLNPs. The particle sizes of these SLNPs ranged from ~120 to 180 nm, with polydispersity indices (PDIs) below 0.3 (fig. S2). Most of these SLNPs exhibited negative charges, and their mRNA encapsulation efficiency ranged from 68.3 ± 9.1% to 83.2 ± 2.5% (fig. S3). To assess mRNA delivery efficiency, we incubated FLuc SLNPs with three representative types of mouse brain-associated cells: Neuro-2a (N2a), a neuroblastoma cell line; primary astrocytes; and bEnd.3, a brain endothelial cell line. As shown in Fig. 1B, S0, S4, and S6 LNPs exhibited the highest luminescence intensity across all three cell types, outperforming other SLNPs. This led to their selection for further in vivo validation of mRNA delivery efficiency in the brain. Six hours after intravenous injection of FLuc SLNPs (mRNA, 0.6 mg/kg), S4 LNP induced the highest luminescence intensity in brain tissues, which was 4.5- and 16.3-fold higher than S0 LNP and S6 LNP, respectively (Fig. 1C). These SLNPs showed similar distribution across other major organs, including the liver, lung, spleen, kidney, and heart (fig. S4). Consequently, S4 LNP was selected for further optimization to enhance mRNA delivery efficiency in the brain.
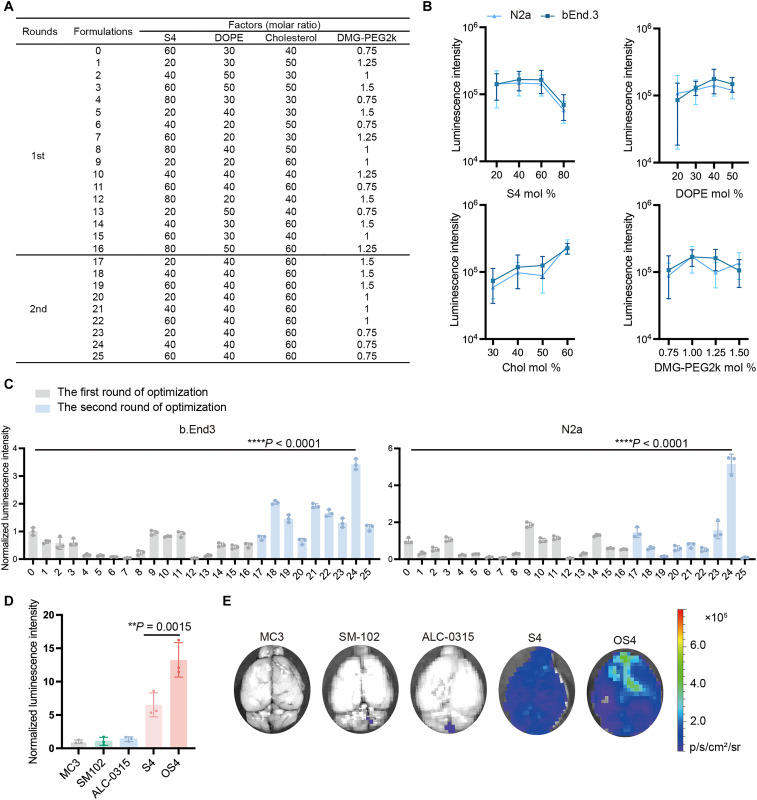
An orthogonal assay was used to refine the ratio of each lipid component (S4, DOPE, Chol, and DMG-PEG2k) in the S4 LNP formulation. In the first round, 16 formulations were prepared based on an L16 (4)4 orthogonal table (Fig. 2A), and their mRNA delivery efficiency was evaluated in N2a and bEnd.3 cell lines. Formulation 0 served as the baseline control, derived from initial evaluation before the optimization study. Each data point in Fig. 2B represents the average luminescence intensity of all LNP formulations sharing the same molar ratio for a specific lipid component (e.g., all formulations containing 20% S4). The trends of luminescence intensity for each lipid component were generally similar in both cell lines at different molar ratios. On the basis of these results, we selected the following ratios for further refinement: S4 mol % = 20, 40, and 60; DOPE mol % = 40; Chol mol % = 60; and PEG mol % = 0.75, 1.0, and 1.5. Thus, nine LNPs (formulations 17 to 25 in Fig. 2A) were prepared and tested in the second round. As exhibited in Fig. 2C, formulation 24 (S4/DOPE/Chol/DMG-PEG2k = 40:40:60:0.75) demonstrated superior mRNA delivery efficiency compared to other predicted formulations in both cell lines. Specifically, formulation 24 enhanced 3.4-fold (P < 0.001) and 5.2-fold (P < 0.001) mRNA delivery efficiency compared to the initial formulation 0 in bEnd.3 and N2a cell lines, respectively (Fig. 2C). Here, we named formulation 24, the optimized S4 LNP, as OS4 LNP. To validate the mRNA delivery efficiency in vivo, we intravenously treated mice with FLuc OS4 LNP (mRNA, 0.6 mg/kg) and other control LNPs including S4 LNP and FDA-approved formulations, MC3, SM-102, and ALC-0315. Six hours after administration, FLuc OS4 LNP resulted in about 13.3-, 12.5-, 9.6-, and 2-fold higher luminescence intensity in the brain than MC3, SM-102, ALC-0315, and S4 LNPs, respectively (Fig. 2, D and E).
Fig. 2. Optimization and evaluation of S4 LNP for brain delivery.
(A) The 16 (4)4 orthogonal table (first round) and predicted formulations (second round). (B) Effects of each lipid component at different molar ratios on mRNA delivery efficiency of S4 LNP (n = 4 biological replicates). Data are presented as means ± SEM. (C) Relative luminescence intensity in bEnd.3 and N2a cell lines. The intensity was normalized to formulation 0 group. Data are presented as means ± SD. (D) Normalized luminescence intensity in the brain of mice treated with MC3, SM-102, ALC-0315, S4, or OS4 LNPs (n = 3 biological replicates). The intensity was normalized to MC3 LNP group. Data are presented as means ± SD. (E) Representative images of brain tissues. Statistical significance was analyzed by one-way ANOVA with Tukey’s multiple comparisons test. **P < 0.01; ****P < 0.0001.
Enhancing brain delivery efficiency through CPP conjugation
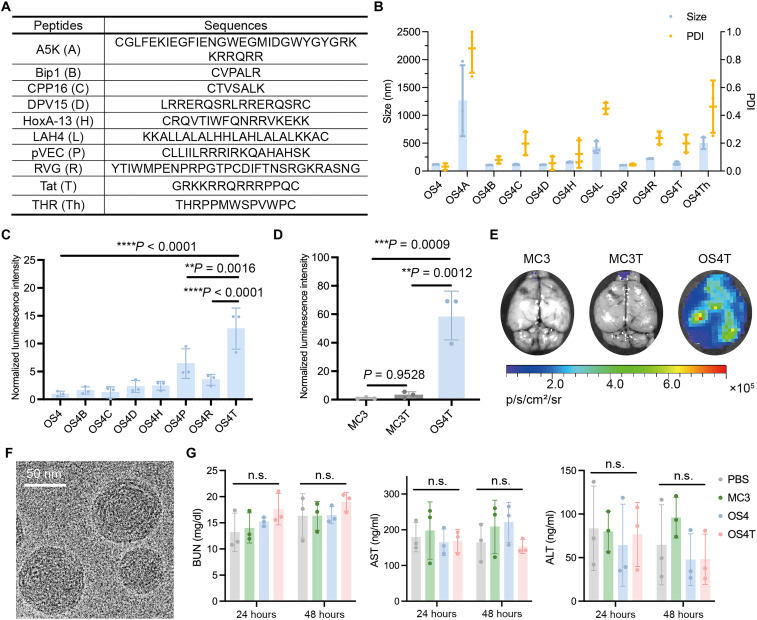
Although CPPs have been reported to facilitate the transport of various cargoes across the BBB (29, 30), few studies have specifically examined their impact on the BBB permeability of LNP-mRNA formulations following systemic administration (31). To further improve the brain delivery efficiency of OS4 LNP, we modified the LNP with a series of CPPs (Fig. 3A), originating from synthetic peptides and naturally occurring viral peptides (32–36). Each peptide was conjugated onto OS4 LNP through a Michael addition reaction. All CPP-conjugated OS4 LNPs exhibited positive charges (fig. S5). On the basis of the changes in size and PDI (Fig. 3B), conjugation of the A5K, THR, or LAH4 peptides caused aggregation of OS4 LNP, as the sizes of OS4A, OS4L, and OS4Th LNPs exceeded 450 nm (fig. S6). Therefore, the remaining seven CPP-conjugated OS4 LNPs were selected for in vivo evaluation of brain delivery efficiency. Although all these CPPs have been reported to possess BBB-penetrating capabilities, they exhibited varying potency in enhancing the brain delivery efficiency of OS4 LNP. As exhibited in Fig. 3C, vascular endothelial cadherin derived peptide (pVEC), rabies virus glycoprotein derived peptide (RVG), and transactivator of transcription peptide (Tat) conjugation significantly increased the luminescence intensity of OS4 LNP in the brain. Among them, Tat-conjugated OS4 LNP (OS4T) demonstrated the highest mRNA delivery efficiency to the brain, showing a 12.7-, 6.6-, and 2.0-fold increase compared to OS4, OS4R, and OS4P LNPs, respectively (Fig. 3C). Notably, OS4T LNP exhibited 59.1- and 17.2-fold greater brain delivery efficiency than MC3 LNP and Tat-conjugated MC3 (MC3T) LNP, respectively (Fig. 3, D and E). OS4T showed a spherical morphology under cryo–transmission electron microscopy (cryo-TEM) imaging (Fig. 3F). In addition, we observed that Tat-conjugated LNPs, OS4T and MC3T, induced higher luminescence signals in the lung compared to their unmodified counterparts, OS4 and MC3, respectively (fig. S7). This effect may be attributed to the increased positive charge, which has been reported to enhance LNP accumulation in the lung following systemic injection (7).
Fig. 3. The mRNA delivery efficiency and safety of CPP-conjugated OS4 LNPs.
(A) The list of different CPPs. (B) The size and PDI of CPP-conjugated OS4 LNPs. (C) Normalized luminescence intensity in the brain from the mice treated with CPP-conjugated OS4 LNPs (n = 3 biological replicates). The intensity was normalized to unmodified OS4 LNP group. (D) Quantification of luminescence intensity in the brain of mice treated with MC3, MC3T, or OS4T LNPs (n = 3 biological replicates). The intensity was normalized to MC3 LNP group. (E) Representative images of brain tissues. (F) Representative of cryo-TEM image of OS4T LNP. Scale bar, 50 nm. (G) The levels of blood urea nitrogen (BUN), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) in mice following intravenous administration of PBS, MC3, OS4, or OS4T LNPs (n = 3 biological replicates). Data are presented as means ± SD in (B), (C), (D), and (G). Statistical significance was analyzed by one-way ANOVA with Tukey’s multiple comparisons test. n.s., not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001.
To further understand OS4- and OS4T-mediated mRNA delivery, we performed cellular uptake and endosomal escape assays using LNPs encapsulating Alexa Fluor 647–labeled RNA. As shown in fig. S8A, MC3, OS4, and OS4T LNPs achieved comparable levels of cellular uptake. The in vitro biocompatibility of OS4 and OS4T LNPs was evaluated in N2a cells at the test dose (fig. S10), showing no significant cytotoxicity. To study the endosomal escape of these LNPs, we incubated N2a cells with calcein, a fluorescent dye that localizes within endosomes (37), and LNPs encapsulating Alexa Fluor 647–labeled RNA. Confocal imaging analysis revealed that green fluorescence appeared as punctate spots in the control group, which is consistent with calcein being trapped in endosomes. In the MC3 group, the prominent overlap of green and red fluorescence signals indicated limited endosomal escape, with LNPs largely retained within the endosomal compartments. In contrast, cells treated with OS4 and OS4T LNPs exhibited reduced colocalization with endosomes and a more diffused calcein signal (fig. S8, B and C), suggesting stronger endosomal escape. This observation aligns with the mRNA delivery efficiency results (Fig. 1B). To investigate the potential mechanisms of transport by OS4 and OS4T LNPs, we used a Transwell migration assay featuring bEnd.3 cells to model the BBB, following previously reported methods (fig. S9A) (21, 38). In this assay, bEnd.3 cells were pretreated with or without meta-chlorophenylpiperazine, a 5-HT receptor ligand, followed by incubation with FLuc OS4 or OS4T LNPs. The luminescence intensity of N2a cells in the lower chamber served as an indicator of the extent of LNP transport across the BBB. The transcytosis efficiency of OS4 and OS4T LNPs decreased in the presence of meta-chlorophenylpiperazine compared to the condition without the inhibitor (fig. S9B). None of these inhibitors used in this study had a notable impact on cell viability at the test dose (fig. S9C). This highlights the critical role of the BBB-crossing moiety, SR-57227, in the S4 lipid, indicating that both OS4 LNP and OS4T LNP can cross endothelial cells by 5-HT receptor–mediated transcytosis. To investigate the pathways, we pretreated bEnd.3 cells with 5-(N-ethyl-N-isopropyl)amiloride (EIPA; a macropinocytosis inhibitor), chlorpromazine (CPZ; a clathrin-mediated endocytosis inhibitor), or methyl-β-cyclodextrin (MβCD; a caveolae-mediated endocytosis inhibitor) before incubation with OS4 or OS4T LNPs in the Transwell migration assay. For OS4 LNP, transcytosis was reduced by over 90% with MβCD and more than 70% with EIPA, whereas CPZ did not significantly affect transcytosis (fig. S9B) compared to the condition without the inhibitor. These findings suggest that macropinocytosis- and caveolae-mediated pathways are critical for OS4 LNP to penetrate the BBB. For OS4T LNP, transcytosis was inhibited by ~75% with MβCD, 95% with EIPA, and 25% with CPZ, indicating that OS4T LNPs use multiple pathways to cross the BBB (fig. S9B). Notably, without any inhibitors, the luminescence intensity of N2a cells in the OS4T group was 1.4-fold higher than in the OS4 group (fig. S9D). This aligns with the observation that OS4T LNP demonstrated significantly greater mRNA delivery efficiency in the brain compared to OS4 LNP in vivo (Fig. 3C), highlighting the critical role of Tat in crossing the BBB. On the basis of these results, OS4T LNP was selected for the following studies.
To evaluate the in vivo biosafety profile, we intravenously administered PBS, MC3 LNP, OS4 LNP, or OS4T LNP (1.0 mg/kg) to mice. The blood levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and blood urea nitrogen (BUN) were measured at 24 and 48 hours. As shown in Fig. 3G, the levels of AST, ALT, and BUN in LNP-treated groups were comparable to those in the PBS-treated group. Furthermore, histopathological analysis of tissues, including the brain, heart, liver, lung, spleen, and kidney, at 48 hours postinjection revealed no notable pathological changes (fig. S11). These findings indicate the biocompatibility and safety of both OS4 and OS4T LNPs.
Efficient delivery of mRNA in different brain cells
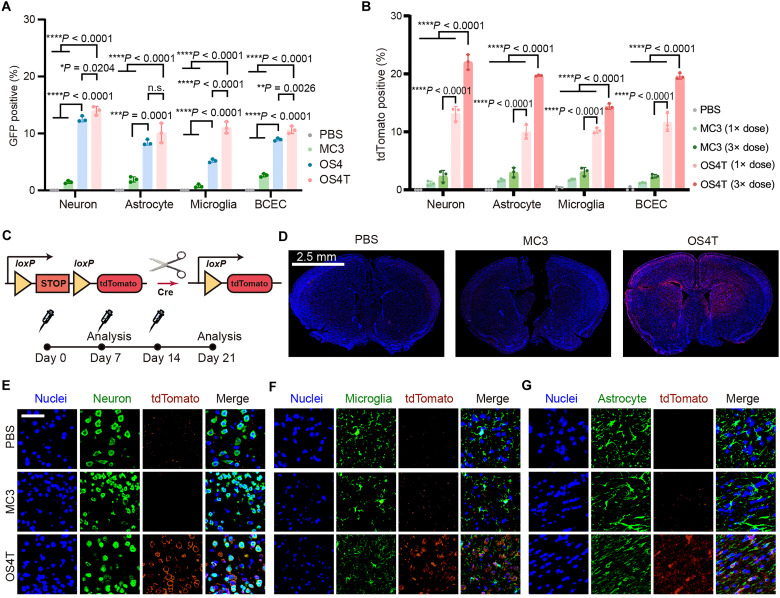
To assess the delivery efficiency and biodistribution of OS4T LNPs across different brain cell populations, we intravenously injected OS4T LNPs containing GFP mRNA into mice (mRNA, 1.0 mg/kg). At 12 hours postadministration, brain tissues were dissociated into single-cell suspensions, and the percentage of GFP+ cells was analyzed by flow cytometry following the gating strategy (fig. S12). Compared to MC3 LNP, OS4 LNP increased by about eight-, four-, six-, and threefold the mRNA delivery efficiency in neurons, astrocytes, microglia, and brain capillary endothelial cells (BCECs), respectively (Fig. 4A). After Tat conjugation, OS4T LNP displayed improved mRNA delivery efficiency in neurons, microglia, and BCECs compared with OS4 LNP.
Fig. 4. OST4 LNP enables effective mRNA delivery into different brain cells.
Flow cytometry analysis of GFP expression (A) or tdTomato expression (B) across different brain cells [neuronal nuclei (NeuN)+ for neuron, glial fibrillary acidic protein (GFAP)+ for astrocyte, CD11b+ for microglia, and CD31+ for BCEC]. Data are presented as means ± SD in (A) and (C) (n = 3 biological replicates). Statistical significance was analyzed by one-way ANOVA with Tukey’s multiple comparisons test. (C) Schematic illustrating the delivery of Cre mRNA to activate tdTomato expression, along with the administration regimen. (D) Representative immunofluorescence sections of brain tissues of Ai14 mice after three administrations with PBS, MC3, or OS4T LNPs (Cre mRNA, 1 mg/kg). Scale bar, 2.5 mm. The tdTomato expression in neurons (E), microglia (F), and astrocytes (G) after three administrations with PBS, MC3, or OS4T LNPs (Cre mRNA, 1 mg/kg). Scale bar, 50 μm. **P < 0.01; ***P < 0.001; ****P < 0.0001.
Next, we used Ai14 mice to validate mRNA delivery efficiency within the brain (Fig. 4C). Seven days after a single intravenous injection of Cre mRNA–loaded LNPs (1.0 mg/kg), tdTomato expression in different brain cell types was analyzed by flow cytometry. Compared to the MC3 group, which showed less than 2.0% tdTomato+ cells, the injection of OS4T LNP resulted in 13.1 ± 1.2% tdTomato+ neurons, 9.9 ± 1.2% tdTomato+ astrocytes, 10.2 ± 0.4% tdTomato+ microglia, and 11.6 ± 1.6% in tdTomato+ BCECs (Fig. 4B). We also studied whether multiple injections could enhance gene editing efficiency in Ai14 mice. Flow cytometry analysis revealed that triple injections of OS4T LNP resulted in a 1.6-, 2.0-, 1.5-, and 1.7-fold increase in tdTomato expression in neurons, astrocytes, BCECs, and microglia, respectively, compared to the single injection group (Fig. 4B). However, triple injections of MC3 LNP induced a limited increase of tdTomato signals across these cells (Fig. 4B). Immunofluorescence imaging further confirmed the enhanced mRNA delivery efficiency of OS4T LNP in the brain. In PBS and MC3 LNP groups, tdTomato signals in the brain were negligible on day 21 (Fig. 4D). In contrast, OS4T LNP containing Cre mRNA resulted in widespread tdTomato expression across most brain regions (Fig. 4D). Next, immunofluorescence staining on neurons (Fig. 4E), microglia (Fig. 4F), and astrocytes (Fig. 4G) was performed, revealing that tdTomato expression levels induced by OS4T LNP were higher compared to MC3 LNP.
Therapeutic efficacy of OS4T in an orthotopic mouse model of GBM
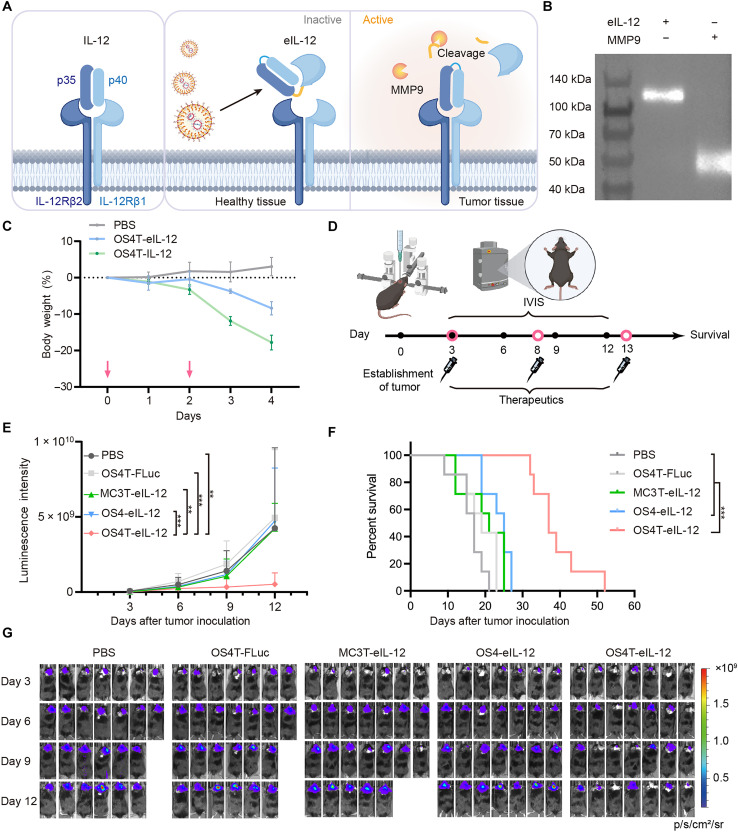
IL-12 is a promising antitumor cytokine due to its ability to activate multiple immune cell populations, including antigen-presenting cells (APCs) and T cells (39–41). Despite encouraging preclinical results, systemic administration of IL-12 has been associated with severe immune-related adverse events in clinical trials, leading to early termination of many studies (42, 43). One approach involves the intratumoral administration of IL-12, delivered either as a fusion with a tumor extracellular matrix–binding domain (44, 45) or through exosome (46) or LNP (47, 48) encapsulating mRNA encoding IL-12. While intratumoral injections have demonstrated improved tolerability, their repeated application in the treatment of brain cancers presents substantial clinical challenges. Recently, Hubbell and colleagues developed an eIL-12 by fusing it to the domain of its receptor (Fig. 5A), IL-12Rβ1(Q20–A261), through a matrix metalloproteinase (MMP)–sensitive linker (49). Given the high expression of MMP in tumor tissues, the linker is cleaved upon reaching the tumor site, locally releasing active IL-12. This design confines IL-12’s proinflammatory effects to the tumor site, reducing systemic side effects following intravenous administration. Thus, we developed an mRNA transcript encoding this eIL-12, containing sequences encoding IL-12, MMP-cleavable linkers, IL-12Rβ1(Q20–A261), and a FLAG tag (Fig. 5A). Next, we treated human embryonic kidney (HEK) 293 T cells (HEK293T) cells with OS4T LNP encapsulating eIL-12 mRNA, collected the supernatant, and performed Western blot analysis to detect the expressed protein. As shown in Fig. 5B, the molecular weight of intact eIL-12 matched its theoretical value. Furthermore, after incubation with MMP9, a molecular weight corresponding to the IL-12Rβ1(Q20–A261) segment was observed, confirming that cleavage had occurred. We then evaluated whether eIL-12 could mitigate systemic immune-related adverse events. For this study, we used healthy C3H/HeJ mice, which are a more representative model for human sensitivity to IL-12 compared to C57BL/6 mice (49, 50). The mice were treated with two doses of OS4T LNP encapsulating either IL-12 mRNA or eIL-12 mRNA, administered every other day, while monitoring body weight changes (Fig. 5C). Treatment with IL-12 mRNA resulted in ~20% body weight loss compared to PBS-treated controls, whereas eIL-12 mRNA induced less weight loss, demonstrating improved in vivo tolerability (Fig. 5C). Considering that LNPs may mediate delivery to off-target organs, such as the liver and spleen, following systemic administration, we evaluated interferon-γ (IFN-γ) levels, a key downstream cytokine indicative of IL-12 bioactivity, in the serum, liver, and spleen of mice treated with OS4T LNPs encapsulating either wild-type IL-12 or eIL-12 mRNA. As shown in fig. S13, mice treated with eIL-12 mRNA LNPs exhibited lower IFN-γ levels compared to those receiving wild-type IL-12 mRNA, suggesting that, although off-target delivery may occur, the eIL-12 construct helps to mitigate systemic immune-related adverse effects relative to the wild-type IL-12.
Fig. 5. Therapeutic efficacy of OS4T LNPs in an orthotopic mouse model of GBM.
(A) The eIL-12 expressed by OS4T LNPs can be activated by MMP9 within the tumor microenvironment. (B) Western blot analysis of the cleavage of eIL-12 by MMP9. (C) The body weight changes in C3H/HeJ mice (n = 5 biological replicates) following administration of OS4T loaded with IL-12 or eIL-12 mRNAs (mRNA, 1 mg/kg). The pink arrows indicate the time of administration. Data are presented as means ± SD. (D) Schematic of the treatment regimen in the CT-2A-Luc GBM model. (E) Luminescence intensity of orthotopic GBM tumor tissues in the mice intravenously treated with PBS, OS4T-FLuc mRNA, MC3T-eIL-12, OS4-eIL-12, or OS4T-eIL-12 LNPs, respectively (mRNA, 1 mg/kg). Data are presented as means ± SD. Statistical significance was analyzed by two-way ANOVA with Tukey’s multiple comparisons test. (F) Survival proportions over time (n = 7 biological replicates). Statistical significance was analyzed by Gehan-Breslow-Wilcoxon test. (G) Representative IVIS images of tumor-bearing mice. The missing image indicates that the mouse had already succumbed at this time. **P < 0.01; ***P < 0.001; ****P < 0.0001. (A) and (D) were created in BioRender (X. Hou, 2025; https://BioRender.com/mjk1zye).
To evaluate the therapeutic potential of OS4T LNP encapsulating eIL-12 mRNA (OS4T-eIL-12), we established a GBM mouse model using CT-2A-Luc cells, a high-grade glioma characterized by an immunosuppressive tumor microenvironment (51). Mice were intravenously injected with OS4T-eIL-12 (1 mg/kg) for three doses, administered every 5 days (Fig. 5D). Control groups included PBS, OS4T-FLuc mRNA, MC3T-eIL-12, and OS4-eIL-12 LNPs. Compared to control treatments, OS4T-eIL-12 LNP significantly inhibited tumor growth and extended survival (Fig. 5, E and F). The OS4T group exhibited an improvement in median survival, reaching 37 days, double that of the PBS group, which showed a median survival of 17 days (Fig. 5F). Moreover, no sustained body weight reduction was observed after the third treatment (fig. S14). These findings highlight the potential of systemic delivery of OS4T-eIL-12 LNP as a promising and tolerated strategy for effective GBM treatment.
To further investigate the immune response, we analyzed immune cell populations in tumor tissues (fig. S15). A single treatment with OS4T-eIL-12 led to the upregulation of M1 phenotype markers, including CD80, CD86, and inducible nitric oxide synthase (iNOS), in macrophages and microglia within brain tissues (fig. S16, A and B). The treatment also decreased the percentage of regulatory CD4+ T cells (fig. S17C) and activated CD8+ T cells in brain tissues, as indicated by an increased frequency of tumor necrosis factor–α (TNFα)+ and CD69+ CD8+ T cells (fig. S16D). A comparison of cytokine levels further demonstrates that OS4T-eIL-12 markedly enhances immune activation in the brain tissue of GBM-bearing mice (fig. S13D).
DISCUSSION
The application of LNP-mRNA in treatment and prophylaxis has gained unprecedented advancements, particularly following the rapid development of mRNA vaccines during the COVID-19 pandemic (52, 53). Although LNP-mRNA systems have emerged as a transformative platform for addressing conditions such as protein deficiencies, genetic mutations, or functional impairments (54–57), efficient mRNA delivery to the brain remains a formidable challenge due to the need to overcome several critical physiological barriers (58). The first is the restrictive nature of the BBB, a highly restrictive layer of endothelial cells connected by tight junctions. While some small molecules can traverse the BBB via passive diffusion, macromolecules such as LNPs rely on transcytosis, a process wherein they are encapsulated in vesicles on one side of the endothelial cell, transported across, and released on the opposite side (59). Beyond crossing the BBB, LNPs must navigate the interstitial space to reach target cell populations. Further challenges include achieving efficient cellular uptake and ensuring endosomal escape, particularly for mRNA, as successful protein translation occurs only when the mRNA is released into the cytoplasm. Overcoming these interconnected barriers is critical to achieving effective brain mRNA delivery.
Leveraging the capacity of certain small molecules to penetrate the BBB via high-affinity interactions with specific receptors or transporters, we propose a strategy for brain delivery by conjugating BBB-permeable molecules to ionizable lipids. This approach aims to enhance the ability of LNPs to effectively traverse the BBB. Our results demonstrated that the SLNP, OS4, achieved markedly improved delivery efficiency to the brain following systemic administration (Fig. 2, D and E). The chemical structure of S4 in OS4 LNPs features two branched ester chains (Fig. 1A), and this exhibited high brain delivery efficiency among all SLNPs tested (Fig. 1B). Previous studies have suggested that the head group of ionizable lipids can incorporate small-molecule ligands, thereby incorporating specific biological functions (10). As for the tail of ionizable lipid, the inclusion of biodegradable ester bonds has been reported to enhance delivery efficiency, potentially by accelerating intracellular release kinetics (60). Thus, the superior delivery efficiency of S4 is likely a result of the combined effects of both its head and tail structures.
To further improve brain delivery efficiency, we investigated the impact of various CPPs on the ability of OS4 LNP to cross the BBB. The CPP modification exhibited markedly different effects on LNP-mRNA formulations for brain delivery. Some CPPs, including A5K, LAH4, and THR peptides, caused LNP aggregation, while some others failed to noticeably improve the brain delivery efficiency of OS4 LNP. Among the tested CPPs, the Tat peptide emerged as the optimal choice, dramatically enhancing mRNA delivery to the brain without compromising the stability of OS4 LNP. Following intravenous administration, Tat-modified OS4 (OS4T) LNP achieved a 12.7-fold increase in brain mRNA delivery efficiency compared to unmodified OS4 LNP (Fig. 3D). All the CPPs converted the OS4 LNP surface charge from negative to positive; however, Tat modification did not result in the highest surface charge (fig. S5). Notably, although OS4B and OS4T exhibited similar zeta potentials (fig. S5), their brain delivery efficiencies differed to a large extent, with OS4T outperforming OS4B (Fig. 3C). This finding suggests that the improvement in delivery efficiency is not solely dependent on the surface charge of OS4 LNP. In addition, OS4 LNPs inherently exhibit high endosomal escape efficiency, and Tat modification did not further enhance the cellular uptake or endosomal escape of OS4 LNPs (fig. S8). However, the Transwell assay revealed that OS4T LNP exhibited a stronger BBB-crossing ability compared to unmodified OS4 LNP (fig. S9).
To investigate the effects of the small molecular ligand in S4 structure of ionizable lipids on brain delivery, we synthesized a control ionizable lipid, referred to as “amino lipid” (AL), which lacks the small molecule head group but contains the same tail group (fig. S17A). Using the same formulation as OS4 LNPs, we prepared AL LNPs, which had a larger average particle size compared to OS4 LNPs (fig. S17B). We then conjugated Tat to the AL LNPs; however, upon Tat conjugation, we observed aggregation and precipitation, with particle sizes exceeding 1 μm (fig. S17B). In addition, we compared OS4 LNPs and AL LNPs without Tat conjugation in an in vivo delivery study. As shown in fig. S17C, OS4 LNPs induced significantly higher luciferase intensity in the brain compared to AL LNPs, indicating that the small molecular head group plays a critical role in promoting brain delivery.
These findings suggest that the high brain delivery efficiency of OS4T is attributable to the combined contributions of both Tat and OS4 LNP. As a result, OS4T LNP can more effectively deliver mRNA into brain cells, including neurons, astrocytes, microglia, and BCECs compared to MC3 LNP following intravenous injection (Fig. 4, A and B). The therapeutic potential of OS4T LNP was further demonstrated in GBM mice by delivering eIL-12 mRNA. Consistent with its superior brain delivery efficiency, OS4T LNP encapsulating eIL-12 mRNA more effectively suppressed tumor growth and prolonged survival compared to treatments with MC3T or OS4 LNPs carrying eIL-12 mRNA (Fig. 5, E and F).
For future clinical translation of this system, there are several considerations. Although OS4T LNPs substantially enhanced delivery efficiency in the brain, a certain degree of off-target delivery remained unavoidable. This may lead to side effects, particularly in the context of IL-12–based immunotherapy. To address this, future studies may explore the incorporation of organ-specific microRNA binding sites into the untranslated regions of the mRNA to suppress undesired expression and enhance tissue specificity (61). In addition, while the findings indicate that OS4T LNP exhibit enhanced mRNA delivery efficiency across brain cell types, the distribution observed was broadly uniform, lacking specificity toward any particular cell type. Future efforts may focus on developing strategies for precise delivery to specific brain cell subtypes, which represent an important direction for advancing the field (62). In addition, we demonstrated that CPP conjugation enhances the brain delivery efficiency of LNPs. However, this study only selected 10 representative CPPs for evaluation. The diversity of CPPs can be further explored to identify superior candidates with BBB-crossing capacities. Collectively, this OS4T platform represents an important proof-of-concept study to advance LNP-mRNA delivery to the brain, which offers a possible avenue for treating brain disorders.
MATERIALS AND METHODS
Animal studies
The animal experiments conducted in this study adhered to the guidelines approved by the Icahn School of Medicine at Mount Sinai (IPROTO202200000134). All relevant ethical regulations were followed as applicable. Both male and female C57BL/6J (strain no.:000664), C3H/HeJ (strain no.:000659), and Ai14 (strain no.:007914) mice (6 to 10 weeks old), obtained from the Jackson Laboratory, were used for the experiments. Animals were randomly assigned to different groups.
Materials
All chemicals and solvents were purchased from Thermo Fisher Scientific unless otherwise listed. DLin-MC3-DMA (MC3), SM-102, ALC-0315, and ALC-0159 were purchased from MedKoo Biosciences (NC, USA). Chol, DOPE, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), DMG-PEG2k, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000 (DSPE-PEG2k-Mal) were purchased from Avanti Polar Lipids (AL, USA). Aldehydes were synthesized according to previously reported procedures (56). AL was synthesized according to previously reported procedures (63).
Characterization of LNPs and peptide conjugated LNPs
To prepare mRNA-loaded LNPs, we used a previously established method (63, 64). Initially, the organic solution was prepared by dissolving different lipids in ethanol at a defined molar ratio. Subsequently, the mRNA aqueous solution was mixed with the ethanol solution, maintaining a lipid-to-mRNA mass ratio of 10:1. For MC3 LNPs, a mixture of MC3 lipid, DSPC, Chol, and DMG-PEG2k is used in a molar ratio of 50/10/38.5/1.5. Similarly, SM-102 LNPs used SM-102, DSPC, Chol, and DMG-PEG2k in a molar ratio of 50/10/38.5/1.5. For ALC-0315 LNPs, the ethanol phase contained ALC-0315, DSPC, Chol, and ALC-0159 at a molar ratio of 45.8/10.4/42.2/1.6.
To conjugate peptides onto LNPs, we replaced the DMG-PEG2k by DSPE-PEG2k-Mal, enabling covalent attachment to the cysteine residue in peptides through a Michael addition reaction. After formulation, the LNPs were dialyzed in PBS before conjugation. Each candidate peptide contains a cysteine residue within its sequence. Peptides were then mixed with the LNPs in a 2:1 molar ratio (cysteine/Mal) to facilitate the Michael addition reaction, which was allowed to proceed for at least 1 hour at room temperature.
The hydrodynamic diameter and PDI of the different LNP formulations are measured using a Nano ZS Zetasizer (Malvern). The morphology of LNPs is then analyzed via cryo-TEM (Thermo Scientific Glacios), allowing for the detailed observation of particle structure and uniformity.
In vitro assays of LNPs loaded with FLuc-mRNA
The N2a cell line (CCL-131) and bEnd.3 cell line (CRL-2299) were obtained from the American Type Culture Collection (Manassas, VA, USA). Primary astrocytes were harvested from C57BL/6J (male/female) mice. Cells were seeded in 96-well plates at a concentration of 2 × 103 cells per well in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum and incubated at 37°C in a humidified atmosphere containing 5% CO2. After an overnight incubation, different LNPs were added to the wells. Following 18 hours of incubation, 50 μl of Bright-Glo luciferase substrate was added to each well. The plate was briefly mixed using a microplate shaker, and luminescence intensity was measured using a microplate reader.
Cellular uptake and endosome escape assays
N2a cells were seeded into 24-well plates at a concentration of 1 × 104 cells per well overnight. Subsequently, LNPs containing Alexa Fluor 647–tagged mRNA were added to each well. After 3 hours of incubation to allow uptake, the cells were washed with PBS, harvested, and analyzed using flow cytometry. For the endosome escape assay, N2a cells were seeded in each well of glass-bottom dishes containing DMEM (37°C with 5% CO2). After overnight incubation, calcein (Invitrogen) and LNPs loaded with Alexa Fluor 647–tagged mRNA were added to each well. After 2 hours of incubation to allow uptake, cells were washed with PBS and stained with Hoechst for 10 min to label the nucleus. Imaging was performed using a confocal laser scanning microscope (Leica DMi8).
In vivo assays of LNPs loaded with FLuc-mRNA
FLuc-mRNA loaded LNPs were intravenously injected into C57BL/6J mice (FLuc-mRNA, 0.6 mg/kg). After 6 hours, the mice administered an intraperitoneal injection of d-luciferin substrate. After 7 min, the mice were euthanized, and major organs were collected. Bioluminescence signals were quantified using regions of interest using the in vivo imaging system (IVIS; PerkinElmer).
BBB crossing mechanism study
The in vitro BBB Transwell model was established following previously described methods. Briefly, bEnd.3 cells were seeded into the upper chamber of a Transwell insert, while N2a cells were seeded in the lower chamber 1 day before treatment. Once a confluent endothelial monolayer was established, the upper chambers were pretreated with or without one of the following inhibitors: EIPA (10 μM), MβCD (4.5 mM), CPZ (5 μg/ml), or meta-chlorophenylpiperazine (10 μM). Following pretreatment, the upper chamber was incubated with OS4 or OS4T LNPs (loaded with FLuc-mRNA) for 18 hours. The N2a cells were then harvested using trypsin and treated with Bright-Glo luciferase substrate, and the luminescence intensity was measured using a microplate reader to assess mRNA delivery across the BBB model.
Cytotoxicity assay
N2a cells were seeded in 96-well plates at a density of 2 × 103 cells per well and incubated overnight. The following day, cells were treated with different LNP formulations at an mRNA dose of 0.5 μg/ml and incubated for 24 hours. After incubation, the culture medium was replaced with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-containing medium. Following an additional 4-hour incubation, the MTT-containing medium was removed, and the resulting formazan crystals were solubilized in 150 μl of dimethyl sulfoxide per well for 10 min at room temperature. Absorbance was measured at 490 nm using a microplate reader to determine relative cell viability. The cytotoxicity of the inhibitors used in the BBB Transwell model was evaluated using the same protocol.
In vivo biocompatibility evaluation
Mouse plasma and major organs were harvested 24 and 48 hours after intravenous administration of PBS, MC3 LNPs, OS4 LNPs, or OS4T LNPs (FLuc-mRNA, 1 mg/kg). Whole blood was collected in sodium citrate–coated tubes and centrifuged to isolate plasma. Levels of ALT, AST, and BUN were determined using an enzyme-linked immunosorbent assay (ELISA) kit. To assess potential systemic or local toxicity, we stained tissue sections with hematoxylin and eosin and scanned them under NanoZoomer S60 digital slide scanner (Hamamatsu Photonics K.K.).
Flow cytometry of brain cells
GFP mRNA– or Cre mRNA–loaded LNPs were prepared and administered intravenously to C57BL/6J or Ai14 mice, respectively. For GFP mRNA studies, brain tissues were harvested 12 hours postinjection, while for Cre mRNA studies, brain tissues were collected 5 days after the final dose. Following isolation, single-cell suspensions from the brain tissue were prepared (18, 65). Red blood cells were lysed using RBC lysis buffer, and myelin was removed using Myelin Removal Beads (MACS 130-096-433), following the manufacturer’s instructions. The purified single-cell suspensions were subsequently stained with specific markers for cell type identification: Neuronal nuclei (NeuN) (1:50 dilution, MACS 130-119-494) for neurons, glial fibrillary acidic protein (GFAP; 1:50, MACS 130-123-846) for astrocytes, CD11b (1:50, MACS 130-113-802) for microglia, and CD31 (1:50, MACS 130-111-541) for BCECs. For the GFP mRNA studies, rabbit polyclonal antibody to GFP (1:100, ab6556) and donkey anti-rabbit immunoglobulin G (IgG) H&L (1:100, ab150073) were used for GFP staining. Prior to immunofluorescence staining for NeuN and GFAP, the samples were incubated with a fixation-permeabilization buffer to enable intracellular staining. After the staining and washing, the cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) staining solution (Miltenyi Biotec) and analyzed using a flow cytometer.
Immunofluorescence staining of brain tissues
After the designated treatments, mice were anesthetized and perfused sequentially with PBS and 4% paraformaldehyde (PFA). The brains were harvested and fixed in 4% PFA, followed by preparation of paraffin section slides. Antigen retrieval was performed on the slides before staining. The sections were first incubated in blocking buffer for 1 hour and subsequently incubated overnight at 4°C with primary antibodies. After washing, the sections were incubated at room temperature for 2 hours with secondary antibodies. The following antibodies were used: NeuN antibody (1:50 dilution, Miltenyi Biotec, clone: REA1131) for neurons; GFAP antibody (1:300 dilution, Cell Signaling Technology, cat. no. 3655S) for astrocytes; anti-Iba1 antibody (1:5000 dilution, Abcam, ab283346) and goat anti-rat IgG H&L [fluorescein isothiocyanate (FITC)] (1:1000 dilution, Abcam, ab6840) for microglia; and tdTomato (E3G5L) rabbit monoclonal antibody (1:300 dilution, Cell Signaling Technology, cat. no. 20163) and goat anti-rabbit IgG H&L [Cyanine3 (Cy3)] (1:100 dilution, Abcam, ab6939) for tdTomato. The sections were mounted using a mounting medium containing DAPI to stain cell nuclei. Imaging was performed using a NanoZoomer S60 digital slide scanner (Hamamatsu Photonics K.K.).
eIL-12 mRNA
Although IL-12 exhibits potent anticancer activity, dose-limiting immune-related adverse events substantially hinder its clinical application, particularly for systemic administration. To minimize the systemic toxicity, we constructed eIL-12 mRNA sequence based on the previously reported fused IL-12 construct (49). The eIL-12 mRNA sequence was produced by incorporating IL-12p40, flexible linkers (such as glycine-glycine-glycine-serine), IL-12p35, a cleavable peptide sequence [(HPVGLLARVPLSLYSG)2(LSGRSDNH), specifically recognized by MMP9], IL-12Rβ1(Q20–A261), and Flag tag (DYKDDDDK). The complete sequence details are provided in table S1.
Cleavage assays of eIL-12 translated by mRNA
For in vitro detection of eIL-12, HEK293T cells were seeded into a six-well plate at a density of 1 × 105 cells per well. After 24 hours, the cells were treated with LNPs loaded with eIL-12 (mRNA at 1 μg/ml). These were incubated for an additional 24 hours; the proteins were then collected from the supernatant, and the cells were lysed to extract the proteins. Protein purification was performed using Anti-FLAG M2 Magnetic Beads according to the manufacturer’s protocol.
Recombinant mouse MMP9 protein was activated by p-aminophenylmercuric acetate (1 mM) before using. For cleavage assays, the purified proteins were incubated with activated MMP9 (5 μg/ml) for 30 min at 37°C. The samples were denatured and subjected to Western blot analysis. Protein separation was carried out on a Mini-PROTEAN TGX 4–15% precast gel (Bio-Rad), followed by transfer to a nitrocellulose membrane (Bio-Rad). IL-12 cleavage was detected via primary incubation with an anti-DDDDK tag (binds to FLAG tag sequence) antibody (1:1000 dilution, Abcam, ab1162) and secondary incubation with anti-rabbit IgG, horseradish peroxidase–linked antibody (1:1000 dilution, Cell Signaling Technology). Visualization was performed using a ChemiDoc MP Imaging System (Bio-Rad).
Body weight changes in C3H/HeJ mice
C3H/HeJ healthy mice (8 to 12 weeks old) were weighed on day 0. Mice were divided into three groups: PBS, OS4T-IL-12, and OS4T-eIL-12. Administrations were performed via tail vein on day 0 and day 2 (mRNA at 1.0 mg/kg). Body weight measurements were performed daily until day 4.
Treatment in an orthotopic GBM model
The orthotopic GBM model was established by implanting CT-2A-Luc cells into the brains of C57BL/6J mice. All mice received a painkiller (buprenorphine, 0.1 mg/kg) before undergoing deep anesthesia (induced with 4% isoflurane and maintained with 1.5% isoflurane). Before surgery, the skin was disinfected three times with povidone-iodine. Using a stereotaxic instrument, the coordinate origin was located, followed by a movement of 2 mm to the right and 1 mm upward. A needle was then slowly inserted to a depth of 3 mm into the brain and left in place for at least 60 s before beginning the injection. CT-2A-Luc cells (1 × 105 cells) were injected at a constant rate of 300 nl/min (2 μl per site). Upon completing the injection, the needle was left in the brain for at least 3 min before being withdrawn at a controlled rate of 0.75 mm/min. After surgery, the mice were closely monitored in a heating chamber set to 37°C until fully awake.
The tumor implantation was performed on day 0. The treatment began after 3 days of postimplantation. The mice were divided into five groups: PBS, OS4T-control mRNA, MC3T-eIL-12, OS4-eIL-12, and OS4T-eIL-12. Treatments were intravenously administered via tail vein on day 3, day 8, and day 13 (eIL-12 mRNA, 1.0 mg/kg). From day 3 to day 12, the mice were imaged by IVIS every 3 days to monitor the tumor growth. For the survival evaluation, mice were monitored for signs indicating an endpoint, including cranial protuberance, neurological deficits such as seizures or abnormal gait, and a body weight reduction exceeding 20%. Mice were euthanized either at the study’s conclusion or upon the first signs of reaching an end point.
Immune cell populations and cytokine levels within brain tissue were analyzed following treatment. GBM-bearing C57BL/6J mice (n = 5 per group) were intravenously injected with a single dose of PBS or OS4T-eIL-12 (1.0 mg/kg). Four days posttreatment, cytokine profiling and flow cytometry analysis were conducted on brain tissues. For IFN-γ, IL-6, and IL-12p70 cytokine measurements, brain tissue samples were analyzed by Eve Technologies. For flow cytometry, single-cell suspensions were prepared from brain tissues followed by myelin removal to enrich for immune cell populations before staining and analysis. Antibodies used in this study include CD45, APC (30-F11, Invitrogen, 17-0451-82); CD11b, Pacific Blue (M1/70.15, Invitrogen, RM2828); F4/80, PE-eFluor 610 (BM8, Invitrogen, 61-4801-82); Ly-6G/Ly-6C, Alexa Fluor 700 (RB6-8C5, Invitrogen, 56-5931-80); P2RY12-PE (S16007D, BioLegend, 848003); iNOS, PE-Cy7 (CXNFT, Invitrogen, 25-5920-80); CD80, FITC (B7-1, Invitrogen, 11-0801-81); CD86, FITC (B7-2, Invitrogen, 11-0862-81); CD206, Alexa Fluor 488 (MR6F3, Invitrogen, 53-2061-80); CD3e, PE (17A2, BioLegend, 100205); CD4, Pacific Blue (RM4-5, Invitrogen, MCD0428); CD25, Alexa Fluor 700 (PC61.5, Invitrogen, 56-0251-82); FOXP3, PE-eFluor 610 (FJK-16s, Invitrogen, 61-5773-80); CD69, FITC (H1.2F3, Invitrogen, 11-0691-82); CD8a, APC-eFluor 780 (53-6.7, Invitrogen, 47-0081-82); and TNFα, eFluor 450 (MP6-XT22, Invitrogen, 48732180). The cells were analyzed by an LSRFortessa flow cytometer (BD Biosciences) and FlowJo (version 10.8) using the following gating strategies (66): macrophages, CD45+CD11b+F4/80+Ly6C−Ly6G−P2ry12−; microglia, CD45+CD11b+F4/80+Ly6C−Ly6G−P2ry12+; regulatory T cells, CD45+CD3+CD4+CD25+Foxp3+; and CD8+ T cells, CD45+CD3+CD8+.
In vivo cytokine profiling after IL-12 mRNA LNP administration in healthy mice
C57BL/6J mice were intravenously injected with OS4T LNPs encapsulating either wild-type IL-12 mRNA, eIL-12 mRNA, or FLuc-mRNA as a negative control (mRNA, 1 mg/kg). A PBS group was also included (n = 3 per group). After 24 hours, serum, liver, and spleen tissues were collected, and levels of IFN-γ were measured by mouse IFN-γ ELISA kit (Invitrogen, cat. no. BMS606-2).
Statistical analysis
Data were statistically analyzed using GraphPad Prism. All relevant information on the statistical details of experiments is provided in figure legends. For in vitro studies, experiments were performed twice with similar results. Data are presented as means ± SD unless otherwise indicated. Sample sizes (n) for in vivo studies are also provided in the figure legends.
Acknowledgments
We thank the following core facilities at the Icahn School of Medicine: RNA NanoCore, Flow Cytometry Core, Preclinical Imaging Core, and Biorepository and Pathology Core Laboratory. We thank the Campus Microscopy and Imaging Facility at the Ohio State University.
Funding: This work was supported by the New York State Empire State Development Fund (135897) and the Icahn School of Medicine at Mount Sinai.
Author contributions: Conceptualization: D.C., X.H., C.W., S.W., and Y.D. Methodology: D.C., X.H., C.W., S.W., Z.L., M.T., K.G., H.L., Y.X., D.D.K., Y.Z., B.D., and Y.D. Validation: D.C., X.H., C.W., S.W., M.T., Z.L., H.L., Y.X., D.D.K., and Y.Z. Investigation: D.C., X.H., C.W., S.W., M.T., and K.G. Visualization: D.C., X.H., C.W., S.W., M.T., and Y.D. Formal analysis: D.C., X.H., C.W., S.W., and Y.D. Data curation: D.C., X.H., and Y.D. Software: X.H. Resources: D.C., X.H., K.G., and Y.D. Project administration: D.C., X.H., and Y.D. Funding acquisition: Y.D. Supervision: Y.D. Writing—original draft: D.C., X.H., C.W., S.W., and Y.D. Writing—review and editing: D.C., X.H., C.W., S.W., C.Y., and Y.D.
Competing interests: Y.D. is a scientific advisor for Arbor Biotechnologies. Y.D. is a cofounder and holds equity in Immunanoengineering Therapeutics. The other authors declare that they have no competing interests.
Data and materials availability: The lipids and LNPs can be provided by Y.D., pending scientific review and a completed material transfer agreement through Icahn School of Medicine at Mount Sinai. Requests for the lipids and LNPs should be submitted to yizhou.dong@mssm.edu. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Synthesis of ionizable lipids
Figs. S1 to S17
Table S1
REFERENCES AND NOTES
- 1.Barbier A. J., Jiang A. Y., Zhang P., Wooster R., Anderson D. G., The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Hajj K. A., Whitehead K. A., Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2, 17056 (2017). [Google Scholar]
- 3.Loughrey D., Dahlman J. E., Non-liver mRNA delivery. Acc. Chem. Res. 55, 13–23 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Hou X., Zaks T., Langer R., Dong Y., Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun B., Wu W., Narasipura E. A., Ma Y., Yu C., Fenton O. S., Song H., Engineering nanoparticle toolkits for mRNA delivery. Adv. Drug Deliv. Rev. 200, 115042 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Rampado R., Naidu G. S., Karpov O., Goldsmith M., Sharma P., Ezra A., Stotsky L., Breier D., Peer D., Lipid nanoparticles with fine-tuned composition show enhanced colon targeting as a platform for mRNA therapeutics. Adv. Sci. 12, e2408744 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Q., Wei T., Farbiak L., Johnson L. T., Dilliard S. A., Siegwart D. J., Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J., Eygeris Y., Ryals R. C., Jozić A., Sahay G., Strategies for non-viral vectors targeting organs beyond the liver. Nat. Nanotechnol. 19, 428–447 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Breda L., Papp T. E., Triebwasser M. P., Yadegari A., Fedorky M. T., Tanaka N., Abdulmalik O., Pavani G., Wang Y., Grupp S. A., Chou S. T., Ni H., Mui B. L., Tam Y. K., Weissman D., Rivella S., Parhiz H., In vivo hematopoietic stem cell modification by mRNA delivery. Science 381, 436–443 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma F., Yang L., Sun Z., Chen J., Rui X., Glass Z., Xu Q., Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci. Adv. 6, eabb4429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terstappen G. C., Meyer A. H., Bell R. D., Zhang W., Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 20, 362–383 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Gao J., Gunasekar S., Xia Z., Shalin K., Jiang C., Chen H., Lee D., Lee S., Pisal N. D., Luo J. N., Griciuc A., Karp J. M., Tanzi R., Joshi N., Gene therapy for CNS disorders: Modalities, delivery and translational challenges. Nat. Rev. Neurosci. 25, 553–572 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Barker S. J., Thayer M. B., Kim C., Tatarakis D., Simon M. J., Dial R., Nilewski L., Wells R. C., Zhou Y., Afetian M., Akkapeddi P., Chappell A., Chew K. S., Chow J., Clemens A., Discenza C. B., Dugas J. C., Dwyer C., Earr T., Ha C., Ho Y. S., Huynh D., Lozano E. I., Jayaraman S., Kwan W., Mahon C., Pizzo M., Robles-Colmenares Y., Roche E., Sanders L., Stergioulis A., Tong R., Tran H., Zuchero Y., Estrada A. A., Gadkar K., Koth C. M. M., Sanchez P. E., Thorne R. G., Watts R. J., Sandmann T., Kane L. A., Rigo F., Dennis M. S., Lewcock J. W., DeVos S. L., Targeting the transferrin receptor to transport antisense oligonucleotides across the mammalian blood-brain barrier. Sci. Transl. Med. 16, eadi2245 (2024). [DOI] [PubMed] [Google Scholar]
- 14.Dehouck B., Fenart L., Dehouck M. P., Pierce A., Torpier G., Cecchelli R., A new function for the LDL receptor: Transcytosis of LDL across the blood–brain barrier. J. Cell Biol. 138, 877–889 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J., Shangguan P., Lin M., Fu L., Liu Y., Han L., Chen S., Wang X., Lu M., Luo Z., Zhong Y., Shi B., Bai F., Dual-site Förster resonance energy transfer route of upconversion nanoparticles-based brain-targeted nanotheranostic boosts the near-infrared phototherapy of glioma. ACS Nano 17, 16840–16853 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Anraku Y., Kuwahara H., Fukusato Y., Mizoguchi A., Ishii T., Nitta K., Matsumoto Y., Toh K., Miyata K., Uchida S., Nishina K., Osada K., Itaka K., Nishiyama N., Mizusawa H., Yamasoba T., Yokota T., Kataoka K., Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat. Commun. 8, 1001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim T., Kim H. J., Choi W., Lee Y. M., Pyo J. H., Lee J., Kim J., Kim J., Kim J.-H., Kim C., Kim W. J., Deep brain stimulation by blood–brain-barrier-crossing piezoelectric nanoparticles generating current and nitric oxide under focused ultrasound. Nat. Biomed. Eng. 7, 149–163 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Wang X., Xie R., Burger J. C., Tong Y., Gong S., Overcoming the blood–brain barrier for gene therapy via systemic administration of GSH-responsive silica nanocapsules. Adv. Mater. 35, e2208018 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Meng Y., Reilly R. M., Pezo R. C., Trudeau M., Sahgal A., Singnurkar A., Perry J., Myrehaug S., Pople C. B., Davidson B., Llinas M., Hyen C., Huang Y., Hamani C., Suppiah S., Hynynen K., Lipsman N., MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci. Transl. Med. 13, eabj4011 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Cornelissen F. M. G., Markert G., Deutsch G., Antonara M., Faaij N., Bartelink I., Noske D., Vandertop W. P., Bender A., Westerman B. A., Explaining blood–brain barrier permeability of small molecules by integrated analysis of different transport mechanisms. J. Med. Chem. 66, 7253–7267 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Wang S., Xue Y., Zhong Y., Li H., Hou X., Kang D. D., Liu Z., Tian M., Wang L., Cao D., Yu Y., Liu J., Cheng X., Markovic T., Hashemi A., Kopell B. H., Charney A. W., Nestler E. J., Dong Y., Intravenous administration of blood-brain barrier-crossing conjugates facilitate biomacromolecule transport into central nervous system. Nat. Biotechnol., 10.1038/s41587-024-02487-7 (2024). [DOI] [PubMed] [Google Scholar]
- 22.Bachy A., Héaulme M., Giudice A., Michaud J.-C., Lefevre I. A., Souilhac J., Manara L., Emerit M. B., Gozlan H., Hamon M., Keane P. E., Soubrié P., Le Fur G., SR 57227A: a potent and selective agonist at central and peripheral 5-HT3 receptors in vitro and in vivo. Eur. J. Pharmacol. 237, 299–309 (1993). [DOI] [PubMed] [Google Scholar]
- 23.Nakamura Y., Kondo M., Koyama Y., Shimada S., SR 57227A is a partial agonist/partial antagonist of 5-HT3 receptor and inhibits subsequent 5-HT- or SR 57227A-induced 5-HT3 receptor current. Biochem. Biophys. Res. Commun. 508, 590–596 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Copolovici D. M., Langel K., Eriste E., Langel Ü., Cell-penetrating peptides: Design, synthesis, and applications. ACS Nano 8, 1972–1994 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Dougherty P. G., Sahni A., Pei D., Understanding cell penetration of cyclic peptides. Chem. Rev. 119, 10241–10287 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorko M., Jones S., Langel Ü., Cell-penetrating peptides in protein mimicry and cancer therapeutics. Adv. Drug Deliv. Rev. 180, 114044 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Derkach V., Surprenant A., North R., 5-HT3 receptors are membrane ion channels. Nature 339, 706–709 (1989). [DOI] [PubMed] [Google Scholar]
- 28.Sharp T., Barnes N. M., Central 5-HT receptors and their function; present and future. Neuropharmacology 177, 108155 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Xie J., Shen Z., Anraku Y., Kataoka K., Chen X., Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 224, 119491 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song N., Lu M., Liu J., Lin M., Shangguan P., Wang J., Shi B., Zhao J., A giant heterometallic polyoxometalate nanocluster for enhanced brain-targeted glioma therapy. Angew. Chem. Int. Ed. Engl. 63, e202319700 (2024). [DOI] [PubMed] [Google Scholar]
- 31.Han E. L., Tang S., Kim D., Murray A. M., Swingle K. L., Hamilton A. G., Mrksich K., Padilla M. S., Palanki R., Li J. J., Mitchell M. J., Peptide-functionalized lipid nanoparticles for targeted systemic mRNA delivery to the brain. Nano Lett. 25, 800–810 (2024). [DOI] [PubMed] [Google Scholar]
- 32.Zhang X., He T., Chai Z., Samulski R. J., Li C., Blood-brain barrier shuttle peptides enhance AAV transduction in the brain after systemic administration. Biomaterials 176, 71–83 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao Y., Wang J., Liu Y., Qu Y., Wang K., Zhang Y., Chang Y., Yang Z., Wan J., Liu J., Nakashima H., Lawler S. E., Chiocca E. A., Cho C. F., Bei F., Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood-brain barrier in rodents and primates. Nat. Biomed. Eng. 6, 1257–1271 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Cho C. F., Wolfe J. M., Fadzen C. M., Calligaris D., Hornburg K., Chiocca E. A., Agar N. Y. R., Pentelute B. L., Lawler S. E., Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 8, 15623 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foss D. V., Muldoon J. J., Nguyen D. N., Carr D., Sahu S. U., Hunsinger J. M., Wyman S. K., Krishnappa N., Mendonsa R., Schanzer E. V., Shy B. R., Vykunta V. S., Allain V., Li Z., Marson A., Eyquem J., Wilson R. C., Peptide-mediated delivery of CRISPR enzymes for the efficient editing of primary human lymphocytes. Nat. Biomed. Eng. 7, 647–660 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stalmans S., Bracke N., Wynendaele E., Gevaert B., Peremans K., Burvenich C., Polis I., De Spiegeleer B., Cell-penetrating peptides selectively cross the blood-brain barrier in vivo. PLOS ONE 10, e0139652 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel S., Kim J., Herrera M., Mukherjee A., Kabanov A. V., Sahay G., Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 144, 90–111 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barberio C., Withers A., Mishra Y., Couraud P. O., Romero I. A., Weksler B., Owens R. M., A human-derived neurovascular unit in vitro model to study the effects of cellular cross-talk and soluble factors on barrier integrity. Front. Cell. Neurosci. 16, 1065193 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiocca E. A., Yu J. S., Lukas R. V., Solomon I. H., Ligon K. L., Nakashima H., Triggs D. A., Reardon D. A., Wen P., Stopa B. M., Naik A., Rudnick J., Hu J. L., Kumthekar P., Yamini B., Buck J. Y., Demars N., Barrett J. A., Gelb A. B., Zhou J., Lebel F., Cooper L. J. N., Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Sci. Transl. Med. 11, eaaw5680 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinchieri G., Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Kang S., Mansurov A., Kurtanich T., Chun H. R., Slezak A. J., Volpatti L. R., Chang K., Wang T., Alpar A. T., Refvik K. C., Hansen O. I., Borjas G. J., Shim H.-N., Hultgren K. T., Gomes S., Solanki A., Ishihara J., Swartz M. A., Hubbell J. A., Engineered IL-7 synergizes with IL-12 immunotherapy to prevent T cell exhaustion and promote memory without exacerbating toxicity. Sci. Adv. 9, eadh9879 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atkins M. B., Robertson M. J., Gordon M., Lotze M. T., DeCoste M., DuBois J. S., Ritz J., Sandler A. B., Edington H. D., Garzone P. D., Mier J. W., Canning C. M., Battiato L., Tahara H., Sherman M. L., Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 3, 409–417 (1997). [PubMed] [Google Scholar]
- 43.Jia Z., Ragoonanan D., Mahadeo K. M., Gill J., Gorlick R., Shpal E., Li S., IL12 immune therapy clinical trial review: Novel strategies for avoiding CRS-associated cytokines. Front. Immunol. 13, 952231 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mansurov A., Ishihara J., Hosseinchi P., Potin L., Marchell T. M., Ishihara A., Williford J.-M., Alpar A. T., Raczy M. M., Gray L. T., Swartz M. A., Hubbell J. A., Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat. Biomed. Eng. 4, 531–543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Momin N., Mehta N. K., Bennett N. R., Ma L., Palmeri J. R., Chinn M. M., Lutz E. A., Kang B., Irvine D. J., Spranger S., Wittrup K. D., Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci. Transl. Med. 11, eaaw2614 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M., Hu S., Yan N., Popowski K. D., Cheng K., Inhalable extracellular vesicle delivery of IL-12 mRNA to treat lung cancer and promote systemic immunity. Nat. Nanotechnol. 19, 565–575 (2024). [DOI] [PubMed] [Google Scholar]
- 47.Li Y., Su Z., Zhao W., Zhang X., Momin N., Zhang C., Wittrup K. D., Dong Y., Irvine D. J., Weiss R., Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Cancer 1, 882–893 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu S., Xu Y., Solek N. C., Chen J., Gong F., Varley A. J., Golubovic A., Pan A., Dong S., Zheng G., Li B., Tumor-tailored ionizable lipid nanoparticles facilitate IL-12 circular RNA delivery for enhanced lung cancer immunotherapy. Adv. Mater. 36, e2400307 (2024). [DOI] [PubMed] [Google Scholar]
- 49.Mansurov A., Hosseinchi P., Chang K., Lauterbach A. L., Gray L. T., Alpar A. T., Budina E., Slezak A. J., Kang S., Cao S., Solanki A., Gomes S., Williford J.-M., Swartz M. A., Mendoza J. L., Ishihara J., Hubbell J. A., Masking the immunotoxicity of interleukin-12 by fusing it with a domain of its receptor via a tumour-protease-cleavable linker. Nat. Biomed. Eng. 6, 819–829 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leonard J. P., Sherman M. L., Fisher G. L., Buchanan L. J., Larsen G., Atkins M. B., Sosman J. A., Dutcher J. P., Vogelzang N. J., Ryan J. L., Effects of single-dose interleukin-12 exposure on interleukin-12–associated toxicity and interferon-γ production. Blood 90, 2541–2548 (1997). [PubMed] [Google Scholar]
- 51.Khalsa J. K., Cheng N., Keegan J., Chaudry A., Driver J., Bi W. L., Lederer J., Shah K., Immune phenotyping of diverse syngeneic murine brain tumors identifies immunologically distinct types. Nat. Commun. 11, 3912 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baden L. R., El Sahly H. M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S. A., Rouphael N., Creech C. B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B. S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polack F. P., Thomas S. J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J. L., Pérez Marc G., Moreira E. D., Zerbini C., Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kon E., Ad-El N., Hazan-Halevy I., Stotsky-Oterin L., Peer D., Targeting cancer with mRNA–lipid nanoparticles: key considerations and future prospects. Nat. Rev. Clin. Oncol. 20, 739–754 (2023). [DOI] [PubMed] [Google Scholar]
- 55.Liu C., Shi Q., Huang X., Koo S., Kong N., Tao W., mRNA-based cancer therapeutics. Nat. Rev. Cancer 23, 526–543 (2023). [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y., Hou X., Du S., Xue Y., Yan J., Kang D. D., Zhong Y., Wang C., Deng B., McComb D. W., Dong Y., Close the cancer–immunity cycle by integrating lipid nanoparticle–mRNA formulations and dendritic cell therapy. Nat. Nanotechnol. 18, 1364–1374 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pardi N., Krammer F., mRNA vaccines for infectious diseases — Advances, challenges and opportunities. Nat. Rev. Drug Discov. 23, 838–861 (2024). [DOI] [PubMed] [Google Scholar]
- 58.Ruan S., Zhou Y., Jiang X., Gao H., Rethinking CRITID procedure of brain targeting drug delivery: Circulation, blood brain barrier recognition, intracellular transport, diseased cell targeting, internalization, and drug release. Adv. Sci. 8, 2004025 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L., Wilhelm S., Exploiting endothelial transcytosis to reach into the brain. Nat. Mater. 22, 282–283 (2023). [DOI] [PubMed] [Google Scholar]
- 60.Mrksich K., Padilla M. S., Mitchell M. J., Breaking the final barrier: Evolution of cationic and ionizable lipid structure in lipid nanoparticles to escape the endosome. Adv. Drug Deliv. Rev. 214, 115446 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jain R., Frederick J. P., Huang E. Y., Burke K. E., Mauger D. M., Andrianova E. A., Farlow S. J., Siddiqui S., Pimentel J., Cheung-Ong K., McKinney K. M., Köhrer C., Moore M. J., Chakraborty T., MicroRNAs enable mRNA therapeutics to selectively program cancer cells to self-destruct. Nucleic Acid. Ther. 28, 285–296 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao J., Xia Z., Gunasekar S., Jiang C., Karp J. M., Joshi N., Precision drug delivery to the central nervous system using engineered nanoparticles. Nat. Rev. Mater. 9, 567–588 (2024). [Google Scholar]
- 63.Yan J., Zhang Y., Du S., Hou X., Li W., Zeng C., Zhang C., Cheng J., Deng B., McComb D. W., Zhao W., Xue Y., Kang D. D., Cheng X., Dong Y., Nanomaterials-mediated co-stimulation of toll-like receptors and CD40 for antitumor immunity. Adv. Mater. 34, 2207486 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X., Liu S., Sun Y., Yu X., Lee S. M., Cheng Q., Wei T., Gong J., Robinson J., Zhang D., Lian X., Basak P., Siegwart D. J., Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 18, 265–291 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ouellette J., Lacoste B., Isolation and functional characterization of primary endothelial cells from mouse cerebral cortex. STAR Protoc. 2, 101019 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeo A. T., Rawal S., Delcuze B., Christofides A., Atayde A., Strauss L., Balaj L., Rogers V. A., Uhlmann E. J., Varma H., Carter B. S., Boussiotis V. A., Charest A., Single-cell RNA sequencing reveals evolution of immune landscape during glioblastoma progression. Nat. Immunol. 23, 971–984 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthesis of ionizable lipids
Figs. S1 to S17
Table S1