Abstract
Metaplasia is an adaptative response to injury and inflammation and can be a precursor to dysplasia and cancer. Metaplasia in the esophagus, termed Barrett’s esophagus, is the replacement of the stratified squamous epithelium by glandular tissue comprising gastric and/or intestinal cell lineages. Metaplasia in the stomach can be divided further into pyloric metaplasia, in which corpus glands become more antral-like, or gastric intestinal metaplasia (GIM), in which gastric cells are replaced by intestinal cell lineages, with the latter subdivided into complete and incomplete. The routine diagnosis of metaplasia and dysplasia is performed by examining hematoxylin and eosin-stained sections and mucin immunohistochemistry. However, these methods fail to capture the cellular diversity across glands and the molecular changes in cells that can predict possible progression to dysplasia or cancer. The use of immunohistochemistry- or immunofluorescence-based biomarkers can improve our understanding of gland phenotypes and aid the differentiation of metaplastic and dysplastic transitions. Here, we provide an overview of the pathophysiology of metaplasia in the esophagus and stomach and detail the current understanding of biomarker expression across metaplastic transitions. We suggest a cohort of biomarkers that can differentiate between metaplastic phenotypes in the esophagus (gastric-type and intestinal-type) and the stomach (pyloric metaplasia, incomplete GIM, and complete GIM) that might be used in research and clinical settings. Importantly, we detail the status of dysplasia biomarkers in both the esophagus and stomach, which may have clinical relevance in stratification of high-risk patients.
Keywords: Cell State, Dysplasia, Esophagus, Intestinal, Metaplasia, Pyloric, Stomach
Metaplasia is a form of progenitor cell plasticity in which normal cell lineages are replaced by a different cell type, either from the same tissue, as observed in pancreatic acinar-to-ductal metaplasia, or from a different organ, as observed in esophageal metaplasia.1 Metaplasia is an adaptative response to injury and inflammation, and may be a precursor for dysplasia and cancer.1 Esophageal metaplasia, termed Barrett’s esophagus (BE), is characterized by the replacement of a stratified squamous epithelium in the distal esophagus near the gastroesophageal junction (GEJ) by a columnar epithelium, which contains gastric and/or intestinal cell lineages2 (Figure 1A). The presence of intestinal metaplasia (IM) has been associated with the progression of BE to esophageal adenocarcinoma (EAC) in a subset of cases.3 Similarly, the presence of gastric IM (GIM) is associated with increased risk of gastric cancer. The clinical diagnosis of BE in the United States is currently contingent upon the presence of IM. Importantly, professional guidelines differ around the world, with the presence of metaplastic columnar epithelium sufficient to diagnose BE in the United Kingdom,4 and thus, there remain discrepancies in the clinical definition of BE.3 For this overview, we will use the United Kingdom definition of BE, as it is the most inclusive and subclassifies metaplastic glands into either gastric or intestinal type.
Figure 1.
Overview of metaplastic phenotypes in the esophagus and stomach. (A) Metaplastic glands in the esophagus can resemble gastric glands (gastric metaplasia) or intestinal glands (IM). Gastric metaplastic glands lack goblet cells, have CDX2-negative foveolar cells, and may contain parietal and chief cells. Intestinal metaplastic glands contain goblet cells and CDX2 positive foveolar cells, lack parietal and chief cells, and may contain Paneth cells in the deep glands. Expression of MUC5AC, CDH17, and TFF3 can distinguish gastric-type and intestinal-type glands in metaplasia of the esophagus. Abnormal p53 expression (increase or absence) is commonly observed in dysplasia. P16 and Cyclin D1 expression is frequently increased in dysplastic glands. (B) PM is characterized by foveolar hyperplasia in the upper gland and SPEM cells in the base of the gland. Incomplete GIM glands also contain SPEM cells at their base along with goblet cells throughout the gland. Complete GIM glands resemble a small intestinal gland with abundant well-defined goblet cells, absorptive enterocytes, and lack of SPEM cells. Complete GIM glands may contain Paneth cells. Pyloric metaplasia glands can be identified by AQP5 and CD44v9 staining of SPEM cells but negative for intestinal cell markers (CDH17, TFF3, CD10, and DEFA5). Incomplete GIM stains positive for SPEM cell markers (AQP5, CD44v9) and intestinal cell markers (CDH17, TFF3) but negative for CD10 and DEFA5. Complete GIM has no SPEM cell marker expression and expresses CDH17, TFF3 and CD10. DEFA5 is only present in complete GIM glands but not all complete GIM glands will be positive for DEFA5. TROP2 and CEACAM5 expression is increased in dysplasia. SOX2 expression is reduced in dysplasia. Colored text denotes expression; red = positive, blue = negative.
Metaplasia in the stomach can be divided into 2 major subtypes, namely pyloric metaplasia (PM) and IM (Figure 1B). In gastric PM, the corpus glands of the stomach become more antral-like with loss of parietal and chief cells and expansion of foveolar and deep antral gland-like mucous cells, termed spasmolytic-polypeptide-expressing metaplasia (SPEM) cells.5,6 GIM can be subclassified as complete or incomplete. Complete GIM harbors entire intestinal glands containing a crypt to villus structure resembling the small intestine.5 By contrast, incomplete GIM is a disorganized gland containing both intestinal and gastric cell lineages and is associated with a higher risk of dysplastic transition and progression to cancer.5,7 Importantly, a mixture of gland phenotypes and cell lineages is observed in both BE and gastric metaplasia.
Tissue biopsies allow the diagnosis and classification of metaplasia. Much of the classification of metaplasia has been performed through examination of hematoxylin and eosin (H&E)-stained sections and mucin histochemistry. Although these methods are extremely useful in defining patterns of metaplasia, they cannot elucidate the molecular changes within cells that might predict progression to dysplasia and cancer. To gain this level of detail that can be both quantifiable and predictable, we must employ a cohort of immunohistochemistry (IHC)- or immunofluorescence (IF)-based biomarkers that can define these transitions towards carcinogenesis. Herein, we examine the present knowledge to establish biomarker-based criteria for metaplasia and cell state transitions markers in BE and the stomach.
Columnar Metaplasia in the Esophagus
Pathophysiology of Metaplasia in the Esophagus
Esophageal columnar metaplasia is an adaptive response to chronic injury caused by gastroesophageal reflux disease (GERD).1 In patients with GERD, a region of acid exposure extends beyond the GEJ, with studies estimating that the distal esophagus could be exposed to acid for up to 10% of the day.8 Repeated acid exposure and bile damage to the normal stratified squamous epithelium triggers chronic inflammation and induces adaptive repair through metaplasia.8 These metaplastic mucous-secreting columnar cells, represented by either the gastric or the intestinal lineage, are more resilient to acid and bile exposure compared with the normal squamous epithelium.8,9 Multiple cell types have been proposed to be the cell of origin for metaplasia, including basal cells of the esophageal squamous epithelium, esophageal submucosal glands, and cells from the gastric cardia.10, 11, 12, 13, 14, 15 Substantial evidence from single-cell transcriptomics, lineage tracing, mouse models and esophagectomy patient specimens demonstrate that metaplastic lesions in the esophagus can originate from cells in the gastric cardia and that BE cells can progress into esophageal adenocarcinoma.9,13,16, 17, 18, 19 However, evidence from porcine and human studies, including clonality studies, injury repair models, and esophagectomy tissue pathology, supports esophageal submucosal glands as a source of progenitor cells during epithelial injury repair and as a potential origin for BE.11,12,20, 21, 22 Single-cell transcriptomics have identified expression of SPEM markers, similar to those observed in metaplasia of the stomach, in gastric type metaplastic glands in the esophagus,19 which has led to the suggestion that a SPEM-like transition may be a precursor for BE.23
Early studies described heterogeneity in the esophageal metaplastic glands with 3 different types of metaplastic glands identified: atrophic gastric-fundic epithelium, junctional-type epithelium with cardia mucus glands, and specialized intestinal mucosa with mucous glands and goblet cells.2,24 This gland heterogeneity has now been subdivided further into 5 different gland phenotypes: atrophic corpus, oxyntocardiac, cardiac, specialized intestinal, and mature intestinal.25 Broadly speaking, the first 3 gland types contain gastric cell lineages and can be described as gastric metaplasia, whereas the latter 2, which exhibit intestinal lineages including goblet cells, connote IM26 (Figure 1A). Cardiac type and specialized intestinal glands are by far the most common phenotypes.25 Interestingly, these glands have different spatial and temporal distribution. Intestinalized glands are frequently found proximally and are commonly identified at the transition between the squamous epithelium and columnar metaplasia, whereas gastric-like glands, specifically cardiac glands, are frequently observed near the GEJ.27 The density of goblet cells follows this spatial pattern, with high numbers of goblet cells observed in proximal glands and with lower numbers in the distal glands near the GEJ.27 This spatial distribution of goblet cells correlates with an esophageal luminal pH gradient that is lowest in the distal segment of BE (pH 2.2) and increases in the proximal segment of BE (pH 4.4).28 Bile acid solubility is pH-dependent, and duodenal bile acids, which are more soluble in the proximal intermediate pH range, are known to induce goblet cell differentiation and may contribute to these spatial differences in goblet cell density.29 Temporally, the initial metaplastic gland is likely a cardia-like gland that can then evolve to a more differentiated gastric gland or an intestinal gland.29 Longitudinal studies of patients who have had a gastroesophagectomy reveal a cardiac-like mucosa in the distal esophagus within a few months which, in some patients, develops into an intestinalized epithelium after several years.17,30 Indeed, clonal tracing of glands has demonstrated that gastric and intestinal metaplastic glands can originate from a shared progenitor.25,31
Interestingly, gland heterogeneity has been associated with BE progression to dysplasia. Areas of nondysplastic BE that are adjacent to dysplasia exhibit higher gland diversity compared with other areas of nondysplastic BE.25 Yet, the use of gland diversity as a biomarker is challenging, as pathologists rely upon small biopsy samples for diagnosis, which may not fully reflect gland heterogeneity. The discrepancy over whether intestinal cells must be present to diagnosis BE, as in the United States, or if the presence of columnar metaplasia is sufficient for diagnosis, as in the United Kingdom, is still a topic of debate.3,4,32 A large-scale longitudinal study of BE progression in the United Kingdom showed a significantly lower risk of progression in patients with gastric metaplasia alone compared with those patients with intestinal cells.33 Gastric metaplasia exhibits lower mutational burden than intestinalized glands.19,33 Therefore, it has been proposed that identifying patients with IM is most relevant for surveillance.34 The cytosponge-trefoil factor 3 (TFF3) is an IHC biomarker-based nonendoscopic test that detects intestinalized TFF3+ metaplastic cells and has shown an improved detection of BE in clinical trials.35 Importantly, clonal tracing of EAC lesions has identified that EAC can originate from nongoblet cells expressing gastric-like metaplastic glands.36 Thus identifying only goblet cell-positive metaplasia may miss some at-risk patients.36 Combining the identification of dysplastic cells on cytosponge samples, identified by p53 IHC and glandular atypia, with patient demographics has shown promise in the stratification of high-risk patients.37 Similarly, the TissueCypher BE test (TSP-9) combines protein expression, using an IF biomarker panel of 9 proteins (p53, p16, AMACR, HER2, CK20, CD68, COX-2, HIF-1α, CD45RO), and features of tissue architecture to stratify BE patients into low, intermediate, or high risk of progression.38,39 The TSP-9 test has exhibited encouraging results in clinical trials and its use has been endorsed by an American Gastroenterological Association clinical practice guideline.39,40
Biomarkers of Columnar Metaplasia in the Esophagus
BE diagnosis in the United States requires the presence of goblet cells, which can be observed using routine H&E staining. Goblet cells are columnar cells with a large mucin-filled globule in the cytoplasm and a small triangular nucleus in the base.41 Additional staining with Alcian Blue (pH 2.5) or Periodic Acid Schiff (PAS) can be used to identify goblet cells in humans, which will appear bright blue or dark purple, respectively.41 In the context of BE, Alcian blue staining reflects expression of mucin 2 (MUC2), whereas PAS reflects mucin 5 subtype AC (MUC5AC). Subtle changes in the appearance of nuclei, such as darker and enlarged nuclei, can indicate low-grade dysplasia (LGD).41 However, LGD is difficult to diagnose reliably on H&E and has poor interobserver agreement. High-grade dysplasia (HGD) exhibits more obvious abnormalities, including prominent nucleoli, loss of nuclear polarity, increased mitosis, and glandular cribriforming.41 For an extensive characterization of metaplastic gland phenotypes and transitions in the esophagus, staining for protein biomarkers is required.
We have compiled an extensive list of proteins previously tested in human esophageal tissues to identify metaplasia and/or dysplasia (Table 1). We have attempted to identify markers that can distinguish between gastric-type and intestinal-type metaplasia in the esophagus. However, most reports have only validated markers in IM, and thus, specific markers of gastric-type glands are limited.26 Importantly, intestinal-type glands in the esophagus are comprised of a mixture of gastric and intestinal cell markers, similar to the incomplete GIM phenotype.42 For example, intestinal metaplastic glands often express both gastric mucins (MUC5AC, mucin 6 [MUC6]) and intestinal mucins (MUC2).43 Trefoil factor proteins are coexpressed with these mucins; trefoil factor 1 (TFF1)/MUC5AC, trefoil factor 2 (TFF2)/MUC6, and TFF3/MUC2.43 Caudal type homeobox 2 (CDX2) and cadherin-17 (CDH17) are robust markers of intestinal-type metaplasia, and their lack of expression has also been observed in gastric-type metaplasia.44 We can surmise that several markers used in the stomach may have utility in identifying gastric-type metaplasia in the esophagus. For example, claudin 18 (CLDN18) is a specific marker of gastric lineages and, when used in combination with the intestinal marker CDH17, can distinguish between gastric and intestinal cell lineages in pediatric patients with gut mispatterning.45 Similarly, we would predict gastric-type metaplasia would lack expression of other common intestinal cell type markers, such is villin or TFF3.
Table 1.
Biomarker Expression of Metaplasia in the Esophagus
| Marker | Esophageal metaplasia |
|||||
|---|---|---|---|---|---|---|
| Gastric-type metaplasia |
Intestinal-type metaplasia |
Dysplasia |
||||
| Lower gland | Upper gland | Lower gland | Upper gland | Lower gland | Upper gland | |
| Aurora Kinase A | Negative46 | Negative37,46 | Positive37,46 | Positive37,46 | ||
| β-catenin | – | Membrane positivity47 | Cytoplasmic and nuclear positivity with/without loss of membrane positivity47 | |||
| CD44H (and V6) | – | Weak positive48 | Negative48 | Positive48 | Positive48 | |
| CDH17 | Negative44 | Positive44 | Positive44 | |||
| CDX1 | – | Positive49 | Positive49 | – | ||
| CDX2 | Negative or focal positivity44,50, 51, 52, 53 | Positive44,50, 51, 52, 53, 54, 55 | Positive44,54, 55, 56 | |||
| c-Myc | – | Weak positive37,55 | Positive37,55 | |||
| Cyclin A | – | Positive57 | Negative57 | Positive57 | Positive57 | |
| Cyclin D1 | – | Negative or focal positivity47 | Positive47 | Positive47 | ||
| Cytokeratin 7 | – | Positive58, 59, 60 | Positive58, 59, 60 | – | ||
| Cytokeratin 20 | – | Negative58, 59, 60 | Positive58, 59, 60 | – | ||
| Das-1 | – | Positive58,60,61 | – | |||
| EGFR | – | Negative or weak positive62 | Positive62 | |||
| FABP1 | Negative44 | Negative44 | Positive44 | Positive44 | Positive44 | |
| FXR | – | Positive63 | Negative63 | |||
| GATA4 | – | Positive64 | – | |||
| GATA6 | – | – | Positive65 | Positive65 | ||
| HepPar-1 | Negative44,53 | Positive44,53 | Positive44 | |||
| HIF-2α | Negative66 | Negative66 | Weak positive66 | |||
| Jagged | – | Weak positive55 | Positive55 | |||
| MCM2 | – | Negative or focal positivity47 | Negative47 | Positive47 | Positive47 | |
| MUC2 | Negative52 | Negative52 | Negative42,43,47,52,60 | Positive42,43,47,52,60 | Positive43,47,56 | Positive43,47,56 |
| MUC5AC | Negative25 | Positive25 | Negative42,43,60 | Positive42,43,60 | Negative43,56 | Positive43,56 |
| MUC6 | – | Positive42,43 | Negative42,43 | Positive42,43 | Negative42,43 | |
| P120-catenin | – | Membrane positivity55 | Cytoplasmic positivity55 | |||
| P16 | – | Negative or weak positive47 | Negative47 | Cytoplasmic and nuclear positivity47 | ||
| P504S | – | Negative or weak positive67 | Positive67 | |||
| p53 | – | Negative or weak positive37,47,56,68,69 | Positive37,47,56,68,69 | |||
| SCD | – | Weak positive70 | Positive70 | |||
| SHH | – | Focal positivity47 | – | |||
| SOX2 | – | Positive71 | Reduced expression71 | |||
| SOX9 | Weak focal positivity51 | Positive51 | Positive51 | – | ||
| TFF1 | – | Negative43 | Positive43 | Negative43 | Positive43 | |
| TFF2 | – | Positive42,43 | Negative42,43 | Positive43 | Negative43 | |
| TFF3 | – | Positive43,47,72,73 | Positive43,47,72,73 | Positive42,43,47 | Positive42,43,47 | |
| TROP2 | – | Weak positive74 | Positive75 | |||
| Villin | Negative or scattered positive cells53 | Positive53,76 | Positive53,76 | Positive56 | Positive56 | |
NOTE: Expression of proteins, using IHC or IF, in human esophageal tissues displaying gastric-type metaplasia, intestinal-type metaplasia and dysplasia. Where possible, the localization of marker expression has been denoted as either lower and/or upper gland. Merged wells indicate that the localization details of the marker were not present in the reported literature. Wells are color-coded as follows: blue = negative; red = positive; yellow = weak expression or changed cellular localization.
IF, immunofluorescence; IHC, immunohistochemistry.
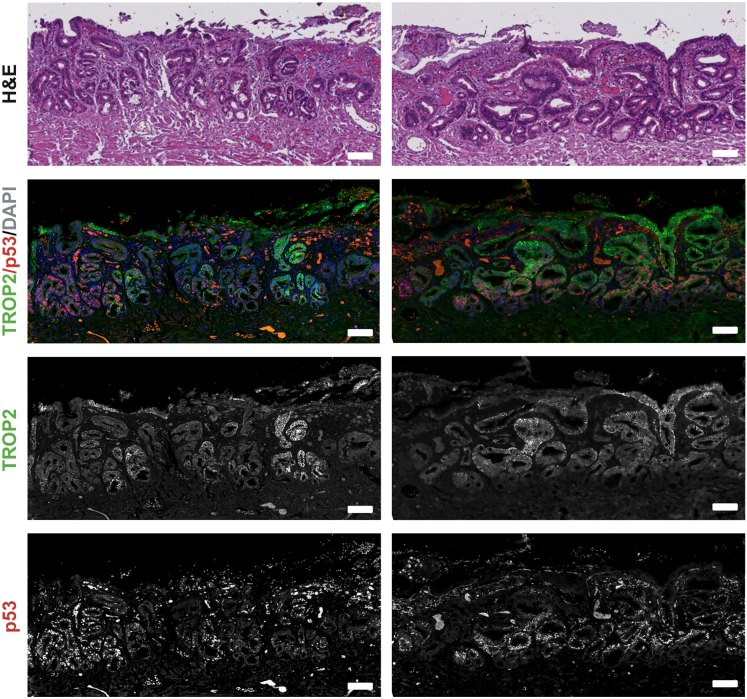
The presence of dysplasia, especially HGD, increases the risk of progression to EAC. Therefore, a sensitive and specific method to identify dysplasia is essential to stratify the most at-risk patients for additional screening and to advance molecular and cell biology studies of EAC initiation. P53 expression has been repeatedly shown to increase in dysplasia and correlates with the acquisition of TP53 mutations, which cause stabilization of the p53 protein. This occurs early in the metaplasia-dysplasia-cancer cascade and is associated with progression.47, 77, 78 likely due to differentiation arrest of gastric cardia stem cells.75 However, a subset of high-grade dysplastic lesions loses p53 protein expression, and thus, careful evaluation for abnormal staining patterns including absence of p53 expression is crucial.47,77,68 Markers associated with cell cycle regulation and proliferation (Cyclin D1, minichromosome maintenance proteins 2–7 [MCM2], Aurora kinase A) are all increased in dysplasia.47,46 Several proteins have altered subcellular localization. For example, β-catenin is predominantly located at the membrane in metaplasia with increased nuclear staining occurring in dysplasia, indicative of increased Wnt signaling.47 Overall, these markers associated with increase cell proliferation have shown promise for detecting dysplasia, but validation in follow-up studies is required to assess the consistency and reliability of these markers.47 Interestingly, recent publications indicate that dysplastic markers validated in the stomach, such as trophoblast cell surface antigen 2 (TROP2), may also have specificity in the esophagus.75 Indeed, we have promising results indicating coexpression of TROP2 and p53 in areas of dysplastic BE (Figure 2). Importantly, TROP2 is expressed in normal squamous esophageal epithelium, but TROP2 levels are reduced in nondysplastic columnar metaplasia before increasing in dysplasia.74
Figure 2.
Dual expression of p53 and TROP2 in high-grade dysplastic BE. Representative images of high-grade dysplastic BE tissue stained with either H&E or costained with anti-TROP2 and anti-p53 antibodies by immunofluorescence. TROP2 is often coexpressed with p53, which is increased in dysplastic tissues. Scale bar = 100 μm.
A Biomarker Panel to Distinguish Columnar Metaplasia and Dysplasia of the Esophagus
To differentiate metaplastic and dysplastic transitions reliably, we suggest a panel of reliable protein markers that have been validated in human esophageal tissues (Figure 1A). Intestinal-type glands can be confidently identified by the presence of goblet cells and the intestinal markers CDH17 or TFF3.44,47,50 Specific gastric-type gland markers have not been validated, but the lack of CDH17 or TFF3 expression combined with the presence of MUC5AC,25 which is also expressed in intestinal-type glands, can identify gastric-type glands. Abnormal p53 expression is a dominant marker of dysplasia and predictive of progression.37,47,77,68 Importantly, abnormal expression can be scored based on the percentage of p53+ nuclei per gland or the total absence of p53 staining in all epithelial cells within a gland, reflecting the presence of a stabilizing p53 mutation or p53 loss, respectively.77 In addition to p53, we suggest using a second biomarker such as P16 or Cyclin D1, which are both increased in dysplasia.47 Distinguishing metaplastic and dysplastic transitions in the esophagus is critical for basic research as we elucidate the mechanisms of BE and EAC initiation and progression.
Clinically, there are several noninvasive biomarker-based screening tests, including the Cytosponge-TFF3 and TSP-9 test, which are currently being used for early detection, surveillance, and risk stratification of patients with BE.35,39,40 There is an ongoing debate regarding the appropriate surveillance intervals for patients with BE depending upon histology and risk factors. Longitudinal studies have found that intestinal-type BE glands give rise to dysplasia and EAC, and thus, are the most relevant for patient diagnosis.33 However, given that studies have found that gastric-type glands are a precursor to intestinal-type glands,30 it may be that these patients with gastric-type only are an early cohort and still warrant surveillance, but just at less frequent intervals. Additionally, recent results suggest that gland diversity in BE is associated with dysplasia.25 These studies highlight the importance of differentiating the types of metaplastic glands in BE for risk stratification. There is a lack of research comparing marker expression between gastric and intestinal-type glands in BE, but established gastric metaplasia markers could potentially be used to help differentiate BE gland phenotypes. Future studies should address this gap to improve our understanding of disease progression.
Metaplasia in the Stomach
Pathophysiology of Metaplasia in the Stomach
The pathway to gastric carcinogenesis in both humans and mice is perhaps the best and most studied example of a metaplasia to dysplasia cascade.79 The cascade begins with gastric epithelial damage and loss of chief cells and parietal cells, most often in the setting of chronic Helicobacter pylori (H. pylori) infection.5,80 This initial damage leads to the development of pyloric metaplasia in the corpus of the stomach. The pyloric metaplastic gland consists of SPEM cell lineages in the deep glands, with more luminal foveolar lineages towards the surface81 (Figure 1B). This corpus gland metaplasia phenotype resembles the lineage characteristics of the antral glands without expression of gastrin.
Pyloric metaplasia is considered a reparative lineage that can appear in individual damaged corpus glands. Chronic injury and inflammation foster the development of intestinal lineages. This pathway of intestinalization was first noted as precancerous by Pelayo Correa and others in the field.82,83 Two types of IM are observed in patients in both the corpus and antrum. Complete IM glands contain well-formed mature goblet cells and mature absorptive type intestinal enterocytes.84 Additionally, these glands often demonstrate the presence of Paneth cells at their bases.84 Similar to fully mature goblet-cell columnar metaplasia in BE, these alterations in the lining of the stomach may be considered a successful adaptation to chronic mucosal injury. However, their precancerous nature seems less apparent.
Incomplete IM represents a gland phenotype with a mixture of gastric and intestinal lineages.84 SPEM cell lineages are retained at the base of glands deep to a group of proliferative cells that are surmounted towards the lumen by immature intestinalized lineages and immature goblet cells.5,85 This gland structure carries the highest risk for development of gastric cancer.7 Metaplasia in individual gland units can develop somewhat autonomously, replacing normal secretory lineages. The metaplastic mucosa often comprises an admixture of complete and incomplete IM glands. Thus, a gland with dangerous implications may exist within a greater field of benign compete IM. Therefore, it is crucial to distinguish with high certainty the markers for incomplete IM separately from complete IM and determine which precancerous metaplasia units or glands can progress to dysplasia and cancer.
As with esophageal IM, the cell(s) of origin for gastric metaplasia may not be restricted to one cell type or lineage. Mouse models have been informative. In the corpus, it has been reported that SPEM cell lineages can arise from transdifferentiation of chief cells in mucous metaplasia.86,87 Others have reported that gastric metaplasia/SPEM following inflammation and injury to the corpus arises from isthmal stem/progenitor cells.88 It is conceivable, albeit not established, that the cell of origin may change temporally or switch during a particular phase of injury/repair.
Biomarkers of Metaplasia in the Stomach
Parietal and chief cell morphology is distinctive in H&E staining. Parietal cells are large oval-triangular shaped cells with a central nucleus and a pink staining granular cytoplasm. Chief cells are found in the base of glands and have a distinct blue staining base of cytoplasm with a granular cytoplasm. PM is characterized by the presence of mucus-secreting antral gland cells in the lower gland and loss of both parietal and chief cells and can be diagnosed by H&E staining.89 Complete and incomplete GIM is also identified by H&E staining. Complete GIM resembles small intestinal crypts with pink enterocytes forming a brush border, abundant well-formed goblet cells, and Paneth cells with eosinophilic granules.90 Incomplete GIM is a less differentiated phenotype and does not have any brush border cells or Paneth cells.84 Incomplete GIM glands contain a mixture of goblet cells with heterogenous morphologies containing variable and irregular sized mucin droplets in the cytoplasm.84,90
GIM was originally classified into 3 subtypes (types 1–3) based on the type of mucins secreted.84,90 PAS stains neutral mucins magenta, Alcian blue (pH 2.5) stains acid mucins blue, and high iron diamine (HID) stains sulfomucins brown.84,90 Neutral mucins are normally expressed in the stomach and acid mucins are found throughout the small intestine and colon, whereas sulfomucins are restricted to the colon.84,90 Complete GIM, previously known as type 1, resembles a small intestinal crypt, and as such, stains positive for acid mucins in goblet cells and negative for mucins in columnar cells.84,90 Incomplete GIM, which includes type 2 and 3, has a mixture of gastric and intestinal lineages and thus, has variable staining of all mucins with acid mucins in goblet cells and acid/neutral mucins in columnar cells.84,90 The presence of sulfomucins in columnar cells distinguishes type 2 and 3, with type 3 positive for sulfomucins.84,90 Although HID staining can be quite informative, the reagents are toxic, thus the staining is rarely performed.
We have compiled an extensive list of proteins that have been tested in human gastric metaplasia and dysplasia (Table 2). We have attempted to identify and nominate biomarkers that can distinguish between PM, GIM (complete and incomplete), and dysplasia (Figure 1B; Figure 3). As previously noted, GIM has historically been classified into 3 subtypes. For reports using this classification system, we have reclassified type 1 as complete and both type 2 and 3 as incomplete. Unfortunately, some very early reports do not differentiate between complete and incomplete GIM, and these are noted in Table 2. PM and incomplete GIM share SPEM cell lineages at the base of glands, and thus, both stain positively for cell markers such as aquaporin-5 (AQP5), CD44 variant 9 (CD44v9), griffonia simplicifolia lectin II (GSII), MUC6, and TFF2.5 These markers are expressed in normal deep antral glands, and 3 of them (GSII, MUC6, and TFF2) are also expressed in the mucous neck cells of the corpus, but for the purposes of this review, we will designate these 5 markers as SPEM cell markers.
Table 2.
Biomarker Expression of Metaplasia in the Stomach
| Protein | Gastric metaplasia |
|||||||
|---|---|---|---|---|---|---|---|---|
| PM |
Incomplete IM |
Complete IM |
Dysplasia |
|||||
| Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | |
| ANPEP | – | Positive91 | – | – | ||||
| AQP5 | Positive85 | Negative85 | Positive85 | Negative85 | – | Negative85 | - | |
| CD10 | – | Negative85,92 | Negative85,92 | Positive85,92 | – | |||
| CD133 | – | Negative93,94 | Negative93,94 | Positive (50% dysplastic samples)93 | ||||
| CD44 | – | Positive94 | Positive94 | Strongly positive94 | ||||
| CD44v9 | Positive85 | Negative85 | Positive85,95 | Negative85,95 | Positive85 | Negative85 | Negative95 | |
| CDH17 | Negative96 | Positive (entire gland)96,97 | – | |||||
| CDX1 | – | Positive49,98,99 | Positive49,98,99 | Positive49,98,99 | Positive49,98,99 | Positive99 | Positive99 | |
| CDX2 | Negative100 | Positive98,101,102 | Positive98,101,102 | Strongly positive98,101, 102, 103 | Strongly positive98,101, 102, 103 | Positive98,101,102 | Positive98,101,102 | |
| CEACAM5 | – | Positive98 | Positive98 | Negative98 | Weakly positive98 | Positive98 | Positive98 | |
| Clusterin | Positive104,105 | Negative104,105 | Positive104,105 | Negative104,105 | Negative104 | – | ||
| Das1 | – | Positive (30% of samples)92 | Negative92 | Negative92 | – | |||
| DEFA5/HD5 | Negative85,96 | Negative85,106 | Positive85,96,106 | Negative85,96,106 | – | |||
| DMBT1 | Negative105 | Variable105 | Positive (base of gland)105 | – | ||||
| FABP1 | Negative96 | Positive (entire gland)96 | – | |||||
| GSII | Positive85 | Negative85 | Positive85 | Negative85 | Negative85 | – | ||
| LTF | Positive105 | Negative105 | Positive105 | Negative105 | Negative105 | – | ||
| MUC1 | Negative107 | Positive107 | Positive107 | Positive107 | Negative107 | – | ||
| MUC2 | – | Positive101,107 | Positive101,103,107 | Positive101 | Positive101 | |||
| MUC5AC | Negative96 | Positive96 | Positive96,101,107, 108, 109 | Positive96,101,107, 108, 109 | Few scattered positive cells96,101,103,107, 108, 109 | Variable101 | ||
| MUC6 | Positive108 | Negative108 | Positive101,107,108 | Negative101,107,108 | Negative101,107,108 | Variable101 | ||
| MUC13 | Negative96 | Positive (upper gland)96 | – | |||||
| PDX1 | Variable108,110 | Strongly positive108 | – | |||||
| SCD1 | – | Positive (upper gland)70 | Positive70 | |||||
| SOX2 | – | Positive98 | Negative98 | Negative98,103 | Reduced expression98 | Negative98 | ||
| SOX9 | – | Positive (lower gland)111 | – | |||||
| TFF1 | Negative112 | Positive112 | Negative109,113 | Positive109,113 | Negative109,113 | Positive109,113 | – | |
| TFF2 | Positive89,100,105 | Negative100,105 | Positive113 | Negative113 | Negative113 | Positive (50% of samples)114 | ||
| TFF3 | Negative85 | Positive85,108,113 | Positive85,108,113 | Positive85,95,108,113 | Positive85,95,108,113 | Positive95 | Positive95 | |
| TROP2 | Negative95 | Positive85,95 | Negative85 | Positive95 | ||||
| UEA1 | Negative85 | Positive85 | Negative85 | Negative85 | – | |||
| Villin | – | Positive97,102,103 | – | |||||
| WFDC2/HE4 | Positive115 | Negative115 | Positive (base of gland)115 | – | ||||
NOTE: Expression of proteins, using IHC or IF, in human gastric tissues displaying PM, gastric IM (incomplete and complete) and dysplasia. Merged wells across subtypes indicate that there was no differentiation of subtype in the reported literature (eg, reports on GIM staining that have not divided into incomplete and complete). Where possible, the localization of marker expression has been denoted as either lower and/or upper gland. Merged wells indicate that the localization details of the marker were not present in the reported literature. Wells are color coded as follows: blue = negative; red = positive; yellow = weak or variable expression.
GIM, gastric intestinal metaplasia; IF, immunofluorescence; IHC, immunohistochemistry; IM, intestinal metaplasia; PM, pyloric metaplasia.
Figure 3.
Unique staining patterns of gastric metaplasia subtypes and dysplasia. Schematics and representative images denote the different metaplastic phenotypes in the stomach. Normal corpus mucosa contains H/K ATPase-positive parietal cells and UEAI-positive foveolar cells. PM and incomplete IM both have CD44v9-positive SPEM cells at the base of their glands, but incomplete IM glands also express TROP2 and basolateral CEACAM5. In dysplasia, CEACAM5 expression is increased and more diffuse. Complete IM is negative for CD44v9, TROP2, and CEACAM5. Complete IM contains TFF3-positive goblet cells and CD10-positive brush border. Scale bar = 100 μm.
Although PM and incomplete GIM are similar in expressing SPEM cell markers at the base, the top of these glands differs significantly. PM is characterized by foveolar cell hyperplasia and a lack of goblet cells. The upper PM gland can be distinguished by MUC5AC/ulex europaeus agglutinin-1 (UEA1)-positive foveolar cells and the absence of intestinal markers such as CDH17, CDX2, TFF3, and Villin.85,96, 97, 100 In contrast, complete GIM stains positive for these intestinal markers, but lacks expression of SPEM cell markers. Expression of CD10, a brush border marker, or defensin alpha 5 (DEFA5), a Paneth cell marker, confirms assignment of glands as complete GIM85,92 (Figure 3). Incomplete intestinal metaplasia characteristically expresses SPEM markers in gland base cells and basolateral TROP2 in more luminal cell lineages,95,98 whereas complete GIM glands do not express TROP2 (Figure 3).
Distinguishing incomplete GIM from dysplasia is the most difficult transition state to discern, but several more specific markers have been proposed recently that might help define progression to dysplasia. Both carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) and TROP2 are expressed in incomplete GIM, but their expression increases further in dysplasia.98 Combining CEACAM5 and TROP2 staining quantification increases the ability to differentiate dysplasia from incomplete GIM, especially when loss of SPEM markers such as CD44v9 or AQP5 are noted98 (Figure 3). Additionally, SRY-box transcription factor 2 (SOX2) expression is reduced in dysplasia compared to incomplete GIM, and thus the combination of 5 markers might further increase sensitivity for dysplasia detection.98
A Biomarker Panel to Distinguish Metaplasia and Dysplasia of the Stomach
To differentiate gastric metaplasia subtypes, we propose a limited panel of biomarkers (Figure 1B). PM can be distinguished from GIM by the presence of SPEM cell markers, such as AQP5 and CD44v985 in the bases of glands with a lack of expression for intestinal markers, such as CDH17 and TFF385,96,100 (Figure 3). Incomplete GIM expresses both SPEM cell markers and intestinal markers.85 Complete GIM does not express any SPEM cell markers and expresses intestinal markers, namely CDH17 and TFF3.95,96,98 Complete GIM can also be distinguished by the presence of CD10,85 a brush border marker, and DEFA5,85 a Paneth cell marker. Importantly, CD10 and DEFA5 are specific for complete GIM, but the lack of staining may not exclude complete GIM, as CD10 staining can be inconsistent and Paneth cell abundance is variable. Therefore, it is important to consider a few different biomarkers plus the histological appearance when distinguishing different metaplastic glands. We suggest that costaining of TROP2 and CEACAM5 can highlight incomplete GIM and transition to dysplasia. Although both markers are expressed in nondysplastic incomplete GIM, their expression is increased in dysplasia, and CEACAM5 staining often colocalizes with TROP2 on the basolateral membranes of dysplastic cells.98 SOX2, CD44v9, and AQP5 expression is lost in dysplasia and can be used as further markers for confirmation.98 As with all IHC or IF staining, there are several technical variables that may influence staining, such as tissue fixation duration, antigen retrieval, and antibody. Therefore, we strongly recommend the use of positive and negative controls when establishing these marker panels.
Correlation of Human Metaplastic Biomarkers in Mouse Models
Several key anatomical differences exist between the mouse and human esophagus and GEJ. The mouse esophagus has a keratinized stratified squamous epithelium and lacks submucosal glands, and the forestomach is lined with a squamous epithelium as opposed to glandular tissue that is present in the distal stomach.116 Overexpression of interleukin 1β (IL-1β) in the esophagus and forestomach in a transgenic mouse model induces metaplasia and dysplasia at the squamocolumnar junction, which mimics human BE.13 These metaplastic glands do not have classical goblet cells but do contain mucus-secreting columnar cells and express MUC5AC, TFF2, and MUC2.13,117 Exposure to bile acids accelerates the progression of metaplasia and eventual dysplasia.13 However, L2-IL-1β mice can develop both oral and esophageal squamous epithelial dysplasia and squamous cell carcinoma rather than just EAC.118 Despite this limitation, the L2-IL-1β mouse is the predominant mouse model used in BE research, and the effects of high-fat diet, overexpression of CDX2, and hypergastrinemia on columnar metaplasia progression have all been studied using this model system.116
The heterogeneity of gland phenotypes observed in human esophageal metaplasia is not observed in the L2-IL-1β mouse. Although some of the key human markers have been validated in these mice, a large proportion have not.116 Dysplastic lesions have been identified based on H&E histology rather than markers, and thus, we do not know if the expression patterns of human dysplastic markers will be recapitulated in these models. P53 is the most robust human marker for dysplastic BE. Introduction of a TP53 mutation in the cardia progenitor cells of the L2-IL-1β mouse accelerates columnar metaplasia progression and increases dysplasia.75 As expected, these lesions stain positively for p53. These dysplastic lesions also stain positively for TROP2, a robust marker of gastric dysplasia, in both humans and mice, and TROP2 is emerging as a potential marker of esophageal intestinal dysplasia in humans.75 Interestingly, CD44v9 expression was detected in the metaplastic glands, but not the dysplastic glands of these mice.75 Initial evidence suggests these markers may show specificity for metaplastic and dysplastic glands in human BE, although further studies are required.75
In the past 20 years, several mouse models have recapitulated the induction of metaplasia in the stomach. Most of these models demonstrate the induction of PM following loss of parietal cells either through Helicobacter sp. infection119,120 or drug-induced loss of parietal cells.121, 122, 123 In these mouse models, SPEM cells at the base of PM express AQP5 and CD44v9, mirroring patterns observed in the human stomach. More recently, several genetic models of targeted inducible expression of Kras(G12D) expression have led to models that induce a spectrum of metaplastic lesions from PM to incomplete IM and dysplasia.70, 124, 125 Again, in these models, SPEM lineages in PM as well as in incomplete GIM express CD44v9 and AQP5. Additionally, IM lineages express TFF3, and incomplete IM lesions express TROP2 and CLDN4 as in humans. Dysplastic lesions in mice express TROP2 in addition to CEACAM5 and SCD1, similar to findings in humans. Together, these results suggest that there are similarities in biomarker expression along the metaplasia to dysplasia sequence in both humans and mice.
It should be noted that certain biomarkers are present only in humans or mice. Gastric intrinsic factor (GIF), which is a strong marker for chief cells in mice, is expressed only in parietal cells in humans.126 Similarly, gastrokine 3 (GKN3) expression marks mucous neck cells and SPEM lineages in mice, but GKN3 expression has not been confirmed in humans.127,128 Although Alcian blue staining of goblet cells in intestinal metaplasia is often used in humans, Alcian blue staining is less reliable in mice, where strong Alcian blue staining is often seen in nongoblet cell morphology lineages including deep antral gland cells and some SPEM lineages.129 These differences likely accrue from specific differences in terminal glycosylation patterns.
Conclusions
The metaplasia-dysplasia cascade, a precancerous process, is observed across several cancers. Metaplasia of the esophagus and stomach in humans shares several characteristics, including the presence of chronic inflammation, the presence of both gastric and intestinal cell lineages, gland diversity and heterogeneity, and some overlapping diagnostic protocols. Identifying metaplastic and dysplastic transitions is critical for the study of the pathophysiology of metaplasia and in cancer initiation. The strategic interpretation of immunostaining markers can distinguish different metaplastic gland lineages from each other as well as from dysplasia (Figure 1). These markers provide more specific, definitive, and quantifiable indicators of gland pathophysiology, and we acknowledge the importance of multi-omics in this pursuit but emphasize the value of these markers for a more targeted approach. Importantly, these patterns of biomarker expression are observed in some mouse models of esophageal metaplasia, gastric atrophic gastritis, gastric metaplasia, and dysplasia. Previous interpretation of intramucosal pathology has been dominated by evaluation of H&E staining, which can often be controversial.130 The use of IHC biomarkers therefore allows specific correlation between mouse pathology with human pathology. These biomarkers also provide critical criteria for analyzing studies in vitro as well as for animal studies that seek to alter the progression of metaplasia to dysplasia. Utilization of these biomarkers can thereby provide quantitative assessment of changes in protein expression characteristic of changes in cellular behaviors during the metaplastic and dysplastic transitions.
The presence of dysplasia is a major risk factor for cancer initiation in both the esophagus and stomach. The identification of high-risk patients is crucial, and there are several promising dysplasia markers that should be considered during diagnosis. Abnormal p53 expression, such as elevated expression and total absence of expression, is the most widely validated BE dysplasia marker. Other BE dysplasia markers require further validation across different cohorts to confirm sensitivity and specificity. Many of these biomarkers illustrate changes in localization or expression. Similarly, TROP2 and CEACAM5 expression is increased in dysplastic lesions in the stomach, but some expression can also be detected in the nondysplastic incomplete GIM glands. These differences in expression may be obvious when metaplasia and dysplasia are present in the same slide, but more difficult to interpret if only one is present. We strongly recommend the use of positive and negative controls for all protein staining to aid interpretation. We anticipate that the gastric dysplastic markers will have validity as esophageal dysplasia markers too. Indeed, a recent publication showed TROP2 staining in a patient with dysplastic BE,75 and further studies should seek to validate the sensitivity of these markers in a larger cohort of patients with BE. The use of dysplastic markers to aid risk stratification of patients should be pursued to assist with both identifying high-risk patients for early intervention and reducing endoscopic screening in patients with lower risk.
Acknowledgments
The authors are grateful to all members of the National Institutes of Health Program on the Origins of Gastroesophageal Cancer Consortium for their advice and support.
NCI POGEC Consortium includes Julian A Abrams (Columbia University), Michael Quante (Universitätsklinikum Freiburg), Harris H Wang (Columbia University), Jianwen Que (Columbia University), Marcia Cruz-Correa (University of Puerto Rico), Anil K Rustgi (Columbia University), James M Wells (Cincinnati Children's Hospital Medical Center), Silas Maniatis (New York Genome Center), Karol Nowicki-Osuch (German Cancer Research Center), Sandra Ryeom (Columbia University), Jaffer A Ajani (MD Anderson Cancer Center), Wa Xian (University of Houston), Frank McKeon (University of Houston), Eunyoung Choi (Vanderbilt University), James R Goldenring (Vanderbilt University), Jeanine Genkinger (Columbia University), Jianhua Hu (Columbia University), Chin Hur (Columbia University), Nicholas Tatonetti (Columbia University), Timothy C Wang (Columbia University), Michael Zody (New York Genome Center), Joel T Gabre (Columbia University), David A Katzka (Columbia University), and Maria Gonzalez-Pons (University of Puerto Rico)
CRediT Authorship Contributions
Karen J. Dunbar (Investigation, Writing - Original Draft, Writing - Review & Edit, Visualization, Conceptualization, Project Administration)
Su-Hyung Lee (Investigation, Project Administration)
Yookyung Won (Investigation)
Emily M. Esquea (Investigation)
Jeong Yun Yang (Writing - Review & Editing)
Daniel O. Kechele (Writing - Review & Editing)
Sandra Ryeom (Writing - Review & Editing, Funding aquisition, Conceptualization)
Julian A. Abrams (Writing - Review & Editing, Funding aquisition, Conceptualization)
Frank D. McKeon (Writing - Review & Editing, Funding aquisition, Conceptualization)
James M. Wells (Writing - Review & Editing, Funding aquisition, Conceptualization)
Micheal Quante (Writing - Review & Editing, Funding aquisition, Conceptualization
Eunyoung Choi (Writing - Review & Editing, Funding aquisition, Conceptualization)
Jianwen Que (Writing - Review & Editing, Funding aquisition, Conceptualization)
Timothy C. Wang (Writing - Review & Editing, Funding aquisition, Conceptualization)
Anil K Rustgi (Writing - Review & Editing, Funding aquisition, Conceptualization)
James R. Goldenring (Writing - Original Draft, Writing - Review & Editing, Funding aquisition, Conceptualization)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was funded by National Institutes of Health (NIH)/National Cancer Institute (NCI) grants R01CA272903 (Karen J. Dunbar, Emily M. Esquea, Anil K. Rustgi, James M. Wells), R01CA272898 (Julian A. Abrams, Micheal Quante), R01CA272891 (Sandra Ryeom), R01CA272901 (Timothy C. Wang, Jianwen Que), R01CA272906 (Frank D. McKeon), R01CA272687 (Eunyoung Choi, James R. Goldenring), U24CA272897 (NCI POGEC consortium), P30CA013696 (Anil K. Rustgi), and the American Gastroenterology Association Research Scholar Award AGA2024-13-03 (Karen J. Dunbar).
Contributor Information
Karen J. Dunbar, Email: kd2840@cumc.columbia.edu.
James R. Goldenring, Email: jim.goldenring@vumc.org.
References
- 1.Giroux V., Rustgi A.K. Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer. 2017;17:594–604. doi: 10.1038/nrc.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paull A., Trier J.S., Dalton M.D., et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med. 1976;295:476–480. doi: 10.1056/NEJM197608262950904. [DOI] [PubMed] [Google Scholar]
- 3.Rajendra S. Diagnosis and management of Barrett’s esophagus: an updated ACG Guideline. Am J Gastroenterol. 2022;117:1880. doi: 10.14309/ajg.0000000000001896. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald R.C., di Pietro M., Ragunath K., et al. British Society of Gastroenterology British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 5.Goldenring J., Mills J. Cellular plasticity, reprogramming, and regeneration: metaplasia in the stomach and beyond. Gastroenterology. 2022;162:415–430. doi: 10.1053/j.gastro.2021.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt P., Lee J., Joshi V., et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 7.González C.A., Sanz-Anquela J.M., Companioni O., et al. Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: results of the Spanish follow-up multicenter study. J Gastroenterol Hepatol. 2016;31:953–958. doi: 10.1111/jgh.13249. [DOI] [PubMed] [Google Scholar]
- 8.Souza R., Spechler S. Mechanisms and pathophysiology of Barrett oesophagus. Nat Rev Gastroenterol Hepatol. 2022;19:605–620. doi: 10.1038/s41575-022-00622-w. [DOI] [PubMed] [Google Scholar]
- 9.McDonald S.A., Lavery D., Wright N.A., Jansen M. Barrett oesophagus: lessons on its origins from the lesion itself. Nat Rev Gastroenterol Hepatol. 2015;12:50–60. doi: 10.1038/nrgastro.2014.181. [DOI] [PubMed] [Google Scholar]
- 10.Minacapelli C.D., Bajpai M., Geng X., et al. Barrett’s metaplasia develops from cellular reprograming of esophageal squamous epithelium due to gastroesophageal reflux. Am J Physiol Gastrointest Liver Physiol. 2017;312:G615–G622. doi: 10.1152/ajpgi.00268.2016. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson A.M., Graham T.A., Simpson A., et al. Barrett’s metaplasia glands are clonal, contain multiple stem cells and share a common squamous progenitor. Gut. 2012;61:1380–1389. doi: 10.1136/gutjnl-2011-301174. [DOI] [PubMed] [Google Scholar]
- 12.Que J., Garman K.S., Souza R.F., Spechler S.J. Pathogenesis and cells of origin of Barrett’s esophagus. Gastroenterology. 2019;157:349–364.e1. doi: 10.1053/j.gastro.2019.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quante M., Bhagat G., Abrams J., et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012;21:36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y., Urbanska A.M., Hayakawa Y., et al. Gastrin stimulates a cholecystokinin-2-receptor-expressing cardia progenitor cell and promotes progression of Barrett’s-like esophagus. Oncotarget. 2017;8:203–214. doi: 10.18632/oncotarget.10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang M., Li H., Zhang Y., et al. Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature. 2017;550:529–533. doi: 10.1038/nature24269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowicki-Osuch K., Zhuang L., Jammula S., et al. Molecular phenotyping reveals the identity of Barrett’s esophagus and its malignant transition. Science. 2021;373:760–767. doi: 10.1126/science.abd1449. [DOI] [PubMed] [Google Scholar]
- 17.Leodolter A., Nocon M., Vieth M., et al. Progression of specialized intestinal metaplasia at the cardia to macroscopically evident Barrett’s esophagus: an entity of concern in the ProGERD study. Scand J Gastroenterol. 2012;47:1429–1435. doi: 10.3109/00365521.2012.733952. [DOI] [PubMed] [Google Scholar]
- 18.Dunn L.J., Shenfine J., Griffin S.M. Columnar metaplasia in the esophageal remnant after esophagectomy: a systematic review. Dis Esophagus. 2015;28:32–41. doi: 10.1111/dote.12129. [DOI] [PubMed] [Google Scholar]
- 19.Nowicki-Osuch K., Zhuang L., Cheung T.S., et al. Single-cell RNA sequencing unifies developmental programs of esophageal and gastric intestinal metaplasia. Cancer Discov. 2023;13:1346–1363. doi: 10.1158/2159-8290.CD-22-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leedham S.J., Preston S.L., McDonald S.A., et al. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut. 2008;57:1041–1048. doi: 10.1136/gut.2007.143339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krüger L., Gonzalez L.M., Pridgen T.A., et al. Ductular and proliferative response of esophageal submucosal glands in a porcine model of esophageal injury and repair. Am J Physiol Gastrointest Liver Physiol. 2017;313:G180–G191. doi: 10.1152/ajpgi.00036.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Furstenberg R.J., Li J., Stolarchuk C., et al. Porcine esophageal submucosal gland culture model shows capacity for proliferation and differentiation. Cell Mol Gastroenterol Hepatol. 2017;4:385–404. doi: 10.1016/j.jcmgh.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin R.U., Mills J.C. Are gastric and esophageal metaplasia relatives? The case for Barrett’s stemming from SPEM. Dig Dis Sci. 2018;63:2028–2041. doi: 10.1007/s10620-018-5150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bozymski E.M., Herlihy K.J., Orlando R.C. Barrett’s esophagus. Ann Intern Med. 1982;97:103–107. doi: 10.7326/0003-4819-97-1-103. [DOI] [PubMed] [Google Scholar]
- 25.Evans J., Carlotti E., Lin M., et al. Clonal transitions and phenotypic evolution in Barrett’s esophagus. Gastroenterology. 2022;162:1197–1209.e13. doi: 10.1053/j.gastro.2021.12.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald S.A., Graham T.A., Lavery D.L., et al. The Barrett’s gland in phenotype space. Cell Mol Gastroenterol Hepatol. 2015;1:41–54. doi: 10.1016/j.jcmgh.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandrasoma P., Der R., Dalton P., et al. Distribution and significance of epithelial types in columnar-lined esophagus. Am J Surg Pathol. 2001;25:1188–1193. doi: 10.1097/00000478-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Theodorou D., Ayazi S., DeMeester S.R., et al. Intraluminal pH and goblet cell density in Barrett’s esophagus. J Gastrointest Surg. 2012;16:469–474. doi: 10.1007/s11605-011-1776-3. [DOI] [PubMed] [Google Scholar]
- 29.Biswas S., Quante M., Leedham S., Jansen M. The metaplastic mosaic of Barrett’s oesophagus. Virchows Arch. 2018;472:43–54. doi: 10.1007/s00428-018-2317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton S.R., Yardley J.H. Regnerative of cardiac type mucosa and acquisition of Barrett mucosa after esophagogastrostomy. Gastroenterology. 1977;72(4 Pt 1):669–675. [PubMed] [Google Scholar]
- 31.Lavery D., Martinez P., Gay L., et al. Evolution of oesophageal adenocarcinoma from metaplastic columnar epithelium without goblet cells in Barrett’s oesophagus. Gut. 2016;65:907–913. doi: 10.1136/gutjnl-2015-310748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghaus S., Neumann H., Muhammad H., et al. Diagnosis and surveillance of Barrett’s esophagus: addressing the transatlantic divide. Dig Dis Sci. 2016;61:2185–2193. doi: 10.1007/s10620-016-4138-x. [DOI] [PubMed] [Google Scholar]
- 33.Black E., Ococks E., Devonshire G., et al. Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) Consortium. Understanding the malignant potential of gastric metaplasia of the oesophagus and its relevance to Barrett’s oesophagus surveillance: individual-level data analysis. Gut. 2024;73:729–740. doi: 10.1136/gutjnl-2023-330721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubenstein J.H., Sawas T., Wani S., et al. AGA Clinical Practice Guideline on endoscopic eradication therapy of Barrett’s esophagus and related neoplasia. Gastroenterology. 2024;166:1020–1055. doi: 10.1053/j.gastro.2024.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzgerald R.C., di Pietro M., O’Donovan M., et al. BEST3 Trial team. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396:333–344. doi: 10.1016/S0140-6736(20)31099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Killcoyne S., Fitzgerald R.C. Evolution and progression of Barrett’s oesophagus to oesophageal cancer. Nat Rev Cancer. 2021;21:731–741. doi: 10.1038/s41568-021-00400-x. [DOI] [PubMed] [Google Scholar]
- 37.Ross-Innes C., Chettouh H., Achilleos A., et al. BEST2 study group. Risk stratification of Barrett’s oesophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol. 2017;2:23–31. doi: 10.1016/S2468-1253(16)30118-2. [DOI] [PubMed] [Google Scholar]
- 38.Critchley-Thorne R.J., Duits L.C., Prichard J.W., et al. A tissue systems pathology assay for high-risk Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2016;25:958–968. doi: 10.1158/1055-9965.EPI-15-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khoshiwal A.M., Frei N.F., Pouw R.E., et al. TissueCypher SURF LGD Study Pathologists Consortium The tissue systems pathology test outperforms pathology review in risk stratifying patients with low-grade dysplasia. Gastroenterology. 2023;165:1168–1179.e6. doi: 10.1053/j.gastro.2023.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Muthusamy V.R., Wani S., Gyawali C.P., Komanduri S., CGIT Barrett’s Esophagus Consensus Conference Participants AGA Clinical Practice Update on new technology and innovation for surveillance and screening in Barrett’s esophagus: expert review. Clin Gastroenterol Hepatol. 2022;20:2696–2706.e1. doi: 10.1016/j.cgh.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin F., Hernandez Gonzalo D., Lai J., Liu X. Histopathology of Barrett’s esophagus and early-stage esophageal adenocarcinoma: an updated review. Gastrointest Disord. 2019;1:147–163. [Google Scholar]
- 42.Lavery D., Nicholson A., Poulsom R., et al. The stem cell organisation, and the proliferative and gene expression profile of Barrett’s epithelium, replicates pyloric-type gastric glands. Gut. 2014;63:1854–1863. doi: 10.1136/gutjnl-2013-306508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warson C., van de Bovenkamp J., Korteland-Van Male A., et al. Barrett’s esophagus is characterized by expression of gastric-type mucins (MUC5AC, MUC6) and TFF peptides (TFF1 and TFF2), but the risk of carcinoma development may be indicated by the intestinal-type mucin, MUC2. Hum Pathol. 2002;33:660–668. doi: 10.1053/hupa.2002.124907. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava S., Kern F., Sharma N., et al. FABP1 and Hepar expression levels in Barrett’s esophagus and associated neoplasia in an Asian population. Dig Liver Dis. 2017;49:1104–1109. doi: 10.1016/j.dld.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez J.G., Rankin S., Paul E., et al. RFX6 regulates human intestinal patterning and function upstream of PDX1. Development. 2024;151 doi: 10.1242/dev.204379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rugge M., Fassan M., Zaninotto G., et al. Aurora kinase A in Barrett’s carcinogenesis. Hum Pathol. 2010;41:1380–1386. doi: 10.1016/j.humpath.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Choi Y., Bedford A., Pollack S. The aberrant expression of biomarkers and risk prediction for neoplastic changes in Barrett’s esophagus-dysplasia. Cancers (Basel) 2024;16:2386. doi: 10.3390/cancers16132386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lagorce-Pages C., Paraf F., Dubois S., et al. Expression of CD44 in premalignant and malignant Barrett’s oesophagus. Histopathology. 1998;32:7–14. doi: 10.1046/j.1365-2559.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- 49.Silberg D., Furth E., Taylor J., et al. CDX1 protein expression in normal, metaplastic, and neoplastic human alimentary tract epithelium. Gastroenterology. 1997;113:478–486. doi: 10.1053/gast.1997.v113.pm9247467. [DOI] [PubMed] [Google Scholar]
- 50.Groisman G., Amar M., Meir A. Expression of the intestinal marker Cdx2 in the columnar-lined esophagus with and without intestinal (Barrett’s) metaplasia. Mod Pathol. 2004;17:1282–1288. doi: 10.1038/modpathol.3800182. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X., Westerhoff M., Hart J. Expression of SOX9 and CDX2 in nongoblet columnar-lined esophagus predicts the detection of Barrett’s esophagus during follow-up. Mod Pathol. 2015;28:654–661. doi: 10.1038/modpathol.2014.157. [DOI] [PubMed] [Google Scholar]
- 52.Steininger H., Pfofe D., Müller H., et al. Expression of CDX2 and MUC2 in Barrett’s mucosa. Pathol Res Pract. 2005;201:573–577. doi: 10.1016/j.prp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Shi X., Bhagwandeen B., Leong A. CDX2 and villin are useful markers of intestinal metaplasia in the diagnosis of Barrett esophagus. Am J Clin Pathol. 2008;129:571–577. doi: 10.1309/UWK3NAHV31GFHM3J. [DOI] [PubMed] [Google Scholar]
- 54.Phillips R.W., Frierson H.F., Moskaluk C.A. Cdx2 as a marker of epithelial intestinal differentiation in the esophagus. Am J Surg Pathol. 2003;27:1442–1447. doi: 10.1097/00000478-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Karamchandani D., Lehman H., Ohanessian S., et al. Increasing diagnostic accuracy to grade dysplasia in Barrett’s esophagus using an immunohistochemical panel for CDX2, p120ctn, c-Myc and Jagged1. Diagn Pathol. 2016;11:23. doi: 10.1186/s13000-016-0473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown I., Whiteman D., Lauwers G. Foveolar type dysplasia in Barrett esophagus. Mod Pathol. 2010;23:834–843. doi: 10.1038/modpathol.2010.59. [DOI] [PubMed] [Google Scholar]
- 57.Lao-Sirieix P., Lovat L., Fitzgerald R. Cyclin A immunocytology as a risk stratification tool for Barrett’s esophagus surveillance. Clin Cancer Res. 2007;13:659–665. doi: 10.1158/1078-0432.CCR-06-1385. [DOI] [PubMed] [Google Scholar]
- 58.DeMeester S., Wickramasinghe K., Lord R., et al. Cytokeratin and DAS-1 immunostaining reveal similarities among cardiac mucosa, CIM, and Barrett’s esophagus. Am J Gastroenterol. 2002;97:2514–2523. doi: 10.1111/j.1572-0241.2002.06033.x. [DOI] [PubMed] [Google Scholar]
- 59.Ormsby A., Goldblum J., Rice T., et al. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288–294. doi: 10.1016/s0046-8177(99)90007-2. [DOI] [PubMed] [Google Scholar]
- 60.Piazuelo M., Haque S., Delgado A., et al. Phenotypic differences between esophageal and gastric intestinal metaplasia. Mod Pathol. 2004;17:62–74. doi: 10.1038/sj.modpathol.3800016. [DOI] [PubMed] [Google Scholar]
- 61.Glickman J., Wang H., Das K., et al. Phenotype of Barrett’s esophagus and intestinal metaplasia of the distal esophagus and gastroesophageal junction - an immunohistochemical study of cytokeratins 7 and 20, Das-1 and 45MI. Am J Surg Pathol. 2001;25:87–94. doi: 10.1097/00000478-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Cronin J., McAdam E., Danikas A., et al. Epidermal growth factor receptor (EGFR) is overexpressed in high-grade dysplasia and adenocarcinoma of the esophagus and may represent a biomarker of histological progression in Barrett’s esophagus (BE) Am J Gastroenterol. 2011;106:46–56. doi: 10.1038/ajg.2010.433. [DOI] [PubMed] [Google Scholar]
- 63.De Gottardi A., Dumonceau J., Bruttin F., et al. Expression of the bile acid receptor FXR in Barrett’s esophagus and enhancement of apoptosis by guggulsterone in vitro. Mol Cancer. 2006;5:48. doi: 10.1186/1476-4598-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stavniichuk R., DeLaForest A., Thompson C., et al. GATA4 blocks squamous epithelial cell gene expression in human esophageal squamous cells. Sci Rep. 2021;11:3206. doi: 10.1038/s41598-021-82557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alvarez H., Opalinska J., Zhou L., et al. Widespread hypomethylation occurs early and synergizes with gene amplification during esophageal carcinogenesis. PLoS Genetics. 2011;7 doi: 10.1371/journal.pgen.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griffiths E., Pritchard S., McGrath S., et al. Increasing expression of hypoxia-inducible proteins in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Br J Cancer. 2007;96:1377–1383. doi: 10.1038/sj.bjc.6603744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmad J., Arthur K., Maxwell P., et al. A cross sectional study of p504s, CD133, and Twist expression in the esophageal metaplasia dysplasia adenocarcinoma sequence. Dis Esophagus. 2015;28:276–282. doi: 10.1111/dote.12181. [DOI] [PubMed] [Google Scholar]
- 68.Kastelein F., Biermann K., Steyerberg E., et al. ProBar-study group Aberrant p53 protein expression is associated with an increased risk of neoplastic progression in patients with Barrett’s oesophagus. Gut. 2013;62:1676–1683. doi: 10.1136/gutjnl-2012-303594. [DOI] [PubMed] [Google Scholar]
- 69.Helminen O., Melkko J., Saarnio J., et al. Predictive value of p53, Ki67 and TLR5 in neoplastic progression of Barrett’s esophagus: a matched case-control study. Virchows Arch. 2022;481:467–476. doi: 10.1007/s00428-022-03340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Won Y., Jang B., Lee S., et al. Oncogenic fatty acid metabolism rewires energy supply chain in gastric carcinogenesis. Gastroenterology. 2024;166:772–786.e14. doi: 10.1053/j.gastro.2024.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Olphen S., Biermann K., Spaander M., et al. SOX2 as a novel marker to predict neoplastic progression in Barrett’s esophagus. Am J Gastroenterology. 2015;110:1420–1428. doi: 10.1038/ajg.2015.260. [DOI] [PubMed] [Google Scholar]
- 72.Ross-Innes C., Debiram-Beecham I., O’Donovan M., et al. BEST2 Study Group Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lao-Sirieix P., Boussioutas A., Kadri S., et al. Non-endoscopic screening biomarkers for Barrett’s oesophagus: from microarray analysis to the clinic. Gut. 2009;58:1451–1459. doi: 10.1136/gut.2009.180281. [DOI] [PubMed] [Google Scholar]
- 74.Hoppe S., Meder L., Gebauer F., et al. Trophoblast cell surface antigen 2 (TROP2) as a predictive bio-marker for the therapeutic efficacy of sacituzumab govitecan in adenocarcinoma of the esophagus. Cancers (Basel) 2022;14:4789. doi: 10.3390/cancers14194789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lian G., Malagola E., Wei C., et al. p53 mutation biases squamocolumnar junction progenitor cells towards dysplasia rather than metaplasia in Barrett’s oesophagus. Gut. 2025;74:182–196. doi: 10.1136/gutjnl-2024-332095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacLennan A., Orringer M., Beer D. Identification of intestinal-type Barrett’s metaplasia by using the intestine-specific protein villin and esophageal brush cytology. Mol Carcinog. 1999;24:137–143. doi: 10.1002/(sici)1098-2744(199902)24:2<137::aid-mc8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 77.Redston M., Noffsinger A., Kim A., et al. Abnormal TP53 predicts risk of progression in patients with Barrett’s esophagus regardless of a diagnosis of dysplasia. Gastroenterology. 2022;162:468–481. doi: 10.1053/j.gastro.2021.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stachler M.D., Camarda N.D., Deitrick C., et al. Detection of mutations in Barrett’s esophagus before progression to high-grade dysplasia or adenocarcinoma. Gastroenterology. 2018;155:156–167. doi: 10.1053/j.gastro.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Correa P., Piazuelo M. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hayakawa Y., Nakagawa H., Rustgi A.K., et al. Stem cells and origins of cancer in the upper gastrointestinal tract. Cell Stem Cell. 2021;28:1343–1361. doi: 10.1016/j.stem.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldenring J. Pyloric metaplasia, pseudopyloric metaplasia, ulcer-associated cell lineage and spasmolytic polypeptide-expressing metaplasia: reparative lineages in the gastrointestinal mucosa. J Pathol. 2018;245:132–137. doi: 10.1002/path.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Correa P. A human-model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 83.Dixon M.F., Genta R.M., Yardley J.H., Correa P. Classification and grading of gastritis - the updated Sydney System. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Shah S.C., Gawron A.J., Mustafa R.A., Piazuelo M.B. Histologic subtyping of gastric intestinal metaplasia: overview and considerations for clinical practice. Gastroenterology. 2020;158:745–750. doi: 10.1053/j.gastro.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee S., Jang B., Min J., et al. Up-regulation of aquaporin 5 defines spasmolytic polypeptide-expressing metaplasia and progression to incomplete intestinal metaplasia. Cell Mol Gastroenterol Hepatol. 2022;13:199–217. doi: 10.1016/j.jcmgh.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nam K.T., Lee H.-J., Sousa J.F., et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037.e9. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caldwell B., Meyer A.R., Weis J.A., et al. Chief cell plasticity is the origin of metaplasia following acute injury in the stomach mucosa. Gut. 2022;71:1068–1077. doi: 10.1136/gutjnl-2021-325310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hata M., Kinoshita H., Hayakawa Y., et al. GPR30-expressing gastric chief cells do not dedifferentiate but are eliminated via PDK-dependent cell competition during development of metaplasia. Gastroenterology. 2020;158:1650–1666.e15. doi: 10.1053/j.gastro.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rugge M., Sacchi D., Genta R., et al. Histological assessment of gastric pseudopyloric metaplasia: Intra- and inter-observer consistency. Dig Liver Dis. 2021;53:61–65. doi: 10.1016/j.dld.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 90.Correa P., Piazuelo M., Wilson K. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493–498. doi: 10.1038/ajg.2009.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoft S.G., Brennan M., Carrero J.A., et al. Unveiling cancer-related metaplastic cells in both Helicobacter pylori infection and autoimmune gastritis. Gastroenterology. 2025;168:53–67. doi: 10.1053/j.gastro.2024.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koulis A., Di Costanzo N., Mitchell C., et al. CD10 and Das1: a biomarker study using immunohistochemistry to subtype gastric intestinal metaplasia. BMC Gastroenterol. 2022;22:197. doi: 10.1186/s12876-022-02268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Min J., Zhang C., Bliton R., et al. Dysplastic stem cell plasticity functions as a driving force for neoplastic transformation of precancerous gastric mucosa. Gastroenterology. 2022;163:875–890. doi: 10.1053/j.gastro.2022.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang T., Ong C., Shi J., et al. Sequential expression of putative stem cell markers in gastric carcinogenesis. Br J Cancer. 2011;105:658–665. doi: 10.1038/bjc.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Riera K., Jang B., Min J., et al. Trop2 is upregulated in the transition to dysplasia in the metaplastic gastric mucosa. J Pathol. 2020;251:336–347. doi: 10.1002/path.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee H., Nam K., Park H., et al. Gene expression profiling of metaplastic lineages identifies CDH17 as a prognostic marker in early stage gastric cancer. Gastroenterology. 2010;139:213–215.e3. doi: 10.1053/j.gastro.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grötzinger C., Kneifel J., Patschan D., et al. LI-cadherin: a marker of gastric metaplasia and neoplasia. Gut. 2001;49:73–81. doi: 10.1136/gut.49.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jang B., Lee S., Dovirak I., et al. CEACAM5 and TROP2 define metaplastic and dysplastic transitions in human antral gastric precancerous lesions and tumors. Gastric Cancer. 2024;27:263–274. doi: 10.1007/s10120-023-01458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma L., Cao Y., Hu J., Chu M. Casein kinase 2 interacting protein 1 positively regulates caudal-related homeobox 1 in intestinal-type gastric cancer. Chin Med J (English) 2020;133:154–164. doi: 10.1097/CM9.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lennerz J., Kim S., Oates E., et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Q., Teh M., Ito K., et al. CDX2 expression is progressively decreased in human gastric intestinal metaplasia, dysplasia and cancer. Mod Pathol. 2007;20:1286–1297. doi: 10.1038/modpathol.3800968. [DOI] [PubMed] [Google Scholar]
- 102.Xiao Z., Ru Y., Sun J., et al. Expression of CDX2 and villin in gastric cardiac intestinal metaplasia and the relation with gastric cardiac carcinogenesis. Asian Pac J Cancer Prev. 2012;13:247–250. doi: 10.7314/apjcp.2012.13.1.247. [DOI] [PubMed] [Google Scholar]
- 103.Tsukamoto T., Inada K., Tanaka H., et al. Down-regulation of a gastric transcription factor, Sox2, and ectopic expression of intestinal homeobox genes, Cdx1 and Cdx2: inverse correlation during progression from. J Cancer Res Clin Oncol. 2004;130:135–145. doi: 10.1007/s00432-003-0519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weis V., Sousa J., LaFleur B., et al. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut. 2013;62:1270–1279. doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sousa J., Ham A., Whitwell C., et al. Proteomic profiling of paraffin-embedded samples identifies metaplasia-specific and early-stage gastric cancer biomarkers. Am J Pathol. 2012;181:1560–1572. doi: 10.1016/j.ajpath.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen B., Porter E., Reynoso E., et al. Human defensin 5 expression in intestinal metaplasia of the upper gastrointestinal tract. J Clin Pathol. 2005;58:687–694. doi: 10.1136/jcp.2004.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reis C., David L., Correa P., et al. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999;59:1003–1007. [PubMed] [Google Scholar]
- 108.Can N., Puyan F., Altaner S., et al. Mucins, trefoil factors and pancreatic duodenal homeobox 1 expression in spasmolytic polypeptide expressing metaplasia and intestinal metaplasia adjacent to gastric carcinomas. Arch Med Sci. 2020;16:1402–1410. doi: 10.5114/aoms.2013.36923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song J.Y., Kim B.W., Lee A.W., et al. Expression of MUC5AC and trefoil peptide 1 (TFF1) in the subtypes of intestinal metaplasia. Clin Endosc. 2012;45:151–154. doi: 10.5946/ce.2012.45.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leys C., Nomura S., Rudzinski E., et al. Expression of Pdx-1 in human gastric metaplasia and gastric adenocarcinoma. Hum Pathol. 2006;37:1162–1168. doi: 10.1016/j.humpath.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 111.Kimura M., Mutoh H., Sugano K. SOX9 is expressed in normal stomach, intestinal metaplasia, and gastric carcinoma in humans. J Gastroenterol. 2011;46:1292–1299. doi: 10.1007/s00535-011-0443-5. [DOI] [PubMed] [Google Scholar]
- 112.Newton J., Allen A., Westley B., May F. The human trefoil peptide, TFF1, is present in different molecular forms that are intimately associated with mucus in normal stomach. Gut. 2000;46:312–320. doi: 10.1136/gut.46.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim B., Kim K., Lee B., et al. Expression of trefoil peptides in the subtypes of intestinal metaplasia. Peptides. 2004;25:779–783. doi: 10.1016/j.peptides.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 114.Hu G., Yu B., Dong W., et al. Expression of TFF2 and Helicobacter pylori infection in carcinogenesis of gastric mucosa. World J Gastroenterol. 2003;9:910–914. doi: 10.3748/wjg.v9.i5.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nozaki K., Ogawa M., Williams J., et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–522. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martinez-Uribe O., Becker T.C., Garman K.S. Promises and limitations of current models for understanding Barrett’s esophagus and esophageal adenocarcinoma. Cell Mol Gastroenterol Hepatol. 2024;17:1025–1038. doi: 10.1016/j.jcmgh.2024.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kong J., Sai H., Crissey M.A., et al. Immature myeloid progenitors promote disease progression in a mouse model of Barrett’s-like metaplasia. Oncotarget. 2015;6:32980–33005. doi: 10.18632/oncotarget.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muthupalani S., Annamalai D., Feng Y., et al. IL-1β transgenic mouse model of inflammation driven esophageal and oral squamous cell carcinoma. Sci Rep. 2023;13 doi: 10.1038/s41598-023-39907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang T., Goldenring J., Dangler C., et al. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 120.Fox J., Li X., Cahill R., et al. Hypertrophic gastropathy in Helicobacter felis - infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996;110:155–166. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 121.Nomura S., Yamaguchi H., Ogawa M., et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G362–G375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 122.Nam K., Lee H., Sousa J., et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037.39. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huh W., Khurana S., Geahlen J., et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24.e7. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Choi E., Hendley A., Bailey J., et al. Expression of activated Ras in gastric chief cells of mice leads to the full spectrum of metaplastic lineage transitions. Gastroenterology. 2016;150:918–930.e13. doi: 10.1053/j.gastro.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim H., Jang B., Zhang C., et al. Targeting stem cells and dysplastic features with dual MEK/ERK and STAT3 suppression in gastric carcinogenesis. Gastroenterology. 2024;166:117–131. doi: 10.1053/j.gastro.2023.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee E., Seetharam B., Alpers D., Deschryverkecskemeti K. Immunohistochemical survey of cobalamin-binding proteins. Gastroenterology. 1989;97:1171–1180. doi: 10.1016/0016-5085(89)91687-9. [DOI] [PubMed] [Google Scholar]
- 127.Geahlen J., Lapid C., Thorell K., et al. Evolution of the human gastrokine locus and confounding factors regarding the pseudogenicity of GKN3. Physiolog Genomics. 2013;45:667–683. doi: 10.1152/physiolgenomics.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Menheniott T., Peterson A., O’Connor L., et al. A novel gastrokine, Gkn3, marks gastric atrophy and shows evidence of adaptive gene loss in humans. Gastroenterology. 2010;138:1823–1835. doi: 10.1053/j.gastro.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 129.Goldenring J., Nomura S. Differentiation of the gastric mucosa III. Animal models of oxyntic atrophy and metaplasia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G999–G1004. doi: 10.1152/ajpgi.00187.2006. [DOI] [PubMed] [Google Scholar]
- 130.Rogers A.B. Histologic scoring of gastritis and gastric cancer in mouse models. Methods Mol Biol. 2012;921:189–203. doi: 10.1007/978-1-62703-005-2_22. [DOI] [PubMed] [Google Scholar]