Abstract
Objective
To evaluate the immunogenicity of adjuvanted recombinant zoster vaccine (RZV) in patients with rheumatoid arthritis receiving upadacitinib 15 mg once daily (QD) with background methotrexate.
Methods
Eligible patients in SELECT-COMPARE (NCT02629159) receiving upadacitinib 15 mg QD and background methotrexate received RZV at weeks 0 and 12. Antibody titres were collected at weeks 0, 4, 16 and 60 (prevaccination, 4 weeks after first dose, and 4 and 48 weeks after second dose). The primary endpoint was the proportion of patients achieving a satisfactory humoral response to RZV at week 16 (≥ 4-fold increase in prevaccination anti-glycoprotein E (gE) antibody titres). Cell-mediated immune (CMI) response to RZV (≥ 2-fold increase in prevaccination gE-specific CD4+ [2+] T-cell frequency) was assessed at each time point in a subcohort of 38 patients. Safety was assessed for 30 days after each vaccination.
Results
Overall, 93 patients received both RZV doses (78.5% female; mean age, 62.4 years). At baseline, 49.5% used concomitant corticosteroids (median daily dose, 5.0 mg). Satisfactory humoral responses to RZV were observed in 87.8% (95% CI 81.0 to 94.5) of patients at week 16. Age and concomitant corticosteroid use did not affect RZV antibody response. Over 60% of patients achieved a CMI response to RZV at all time points. No serious adverse events were reported. One patient developed herpes zoster 4 months after the second RZV dose.
Conclusions
Most patients receiving upadacitinib 15 mg QD with background methotrexate achieved satisfactory humoral and CMI responses 4 weeks after the second RZV vaccination (week 16).
Keywords: Antirheumatic Agents; Vaccination; Arthritis, Rheumatoid
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
In this study, most patients vaccinated with RZV while using upadacitinib 15 mg once daily and background methotrexate achieved satisfactory humoral and cell-mediated immune responses to RZV at weeks 4, 16 and 60. Factors such as age, RA severity, and the use or dose of concomitant corticosteroids did not affect humoral response to RZV.
RZV was well tolerated, and no serious adverse events were reported within 30 days following vaccination.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The results of this study suggest that patients with RA receiving upadacitinib and methotrexate concurrently can achieve satisfactory immune responses to RZV. This study was not designed to specifically assess the efficacy of RZV in reducing the rate of HZ infection among patients with RA treated with upadacitinib.
Introduction
Individuals with rheumatoid arthritis (RA) are approximately twice as likely to develop infections compared with those without RA.1 This increased susceptibility can be attributed to various factors, such as immune dysfunction due to RA, comorbid conditions and the use of immunosuppressive therapies like corticosteroids (CSs) and disease-modifying anti-rheumatic drugs (DMARDs),2 which can include conventional synthetic DMARDs (csDMARDs), biologic DMARDs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) such as Janus kinase (JAK) inhibitors. Of particular concern is the heightened risk of developing herpes zoster (HZ), an opportunistic infection resulting from the reactivation of the dormant varicella-zoster virus infection. Studies have shown that patients with RA have a two- to almost threefold higher risk of developing HZ compared with the general population.3,6 Moreover, recent research has highlighted that the use of JAK inhibitors for treating moderately to severely active RA is associated with an elevated incidence rate of HZ, with rates of 2.8 per 100 patient-years reported for tofacitinib and baricitinib7 and 3.0 to 5.3 per 100 patient-years for upadacitinib. 8To provide optimal patient care and minimise the risk of infections in RA, it is important to prioritise infection prevention, with vaccination serving as a valuable strategy to stimulate and enhance protective immunity. Recognising the increased risk of vaccine-preventable infections, the American College of Rheumatology9 and the European League Against Rheumatism10 recommend vaccination for immunosuppressed adults with RA. The US Centers for Disease Control and Prevention emphasise the importance of using the non-live adjuvanted recombinant zoster vaccine (RZV; Shingrix GlaxoSmithKline;11 approved 2017) for preventing HZ in adults aged 50 years or older.12
Furthermore, results from clinical trials13,17 and real-world evidence studies18 19 have shown the efficacy and safety of RZV in healthy and immunocompromised adults; however, the biological effects of RZV in patients with RA receiving JAK inhibitors, particularly those receiving upadacitinib with background methotrexate, have not been well studied. To gain a better understanding of vaccination response and optimise vaccination strategies for patients with RA, short- and long-term data on the immunogenicity of RZV are needed. Therefore, the objective of this analysis was to evaluate the immunogenicity of RZV through week 60 in patients with RA who were receiving upadacitinib 15 mg once daily (QD) and background methotrexate.
Methods
Study design and patient selection
Patients enrolled in the ongoing phase 3 open-label extension trial SELECT-COMPARE (NCT02629159) were invited to participate in this vaccine substudy. The SELECT-COMPARE trial included patients with moderately to severely active RA who had an inadequate response to methotrexate.20 In the substudy, patients aged 50 years or older were required to be receiving stable doses of upadacitinib 15 mg QD and background methotrexate (with or without other csDMARDs) for at least 8 weeks before the first RZV vaccination through at least 4 weeks after the second vaccination. Throughout this period, any background anti-rheumatic medications, including CSs, had to remain unchanged. Patients using CSs were required to maintain a consistent daily dose of less than 10 mg of prednisone or equivalent CS therapy for a minimum of 4 weeks following the second vaccination. Moreover, patients had to meet the prescribing specifications outlined in the local label requirements to be eligible for RZV administration. Patients were allowed to initiate or change their dose of oral or parenteral CSs or csDMARDs starting 4 weeks after the second vaccination. Patients were excluded from the substudy if they had previously received RZV or the live-attenuated HZ vaccine (Zostavax, Merck & Co., Inc)21 within 8 weeks before the substudy baseline visit. Patients who had been taking a daily dose of prednisone or equivalent CS therapy exceeding 10 mg per day within 8 weeks before and at the baseline visit or had received any CS injection within 8 weeks before the baseline visit were also not eligible. Additionally, patients who had received any vaccine within 4 weeks before the baseline visit and/or expected to receive any vaccination during the first 16 weeks of the substudy were not included. Other exclusion criteria were a history of severe allergic reaction to any component of RZV, acute illness at the time of vaccination that could potentially alter immune response and a history of and/or presence of symptoms related to varicella or HZ infection, including post-herpetic neuralgia, within 6 months before the baseline visit.
The vaccine substudy was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The substudy was approved by an investigational review board or ethics committee at each study site. All participating patients provided written informed consent.
Treatments and dosing
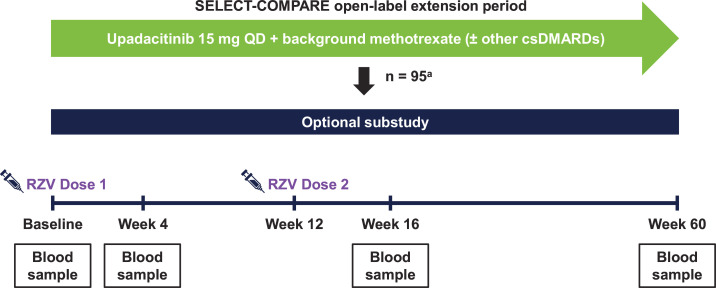
Eligible patients who were receiving upadacitinib 15 mg QD plus stable methotrexate (with or without other csDMARDs) were given two separate, single-dose 0.5 mL intramuscular injections of RZV (Shingrix, GlaxoSmithKline Biologicals, Belgium).11 The RZV injections were administered at baseline and week 12, following the instructions provided on the local label prescribing information. Each RZV dose consisted of 50 µg of recombinant varicella-zoster virus glycoprotein E (gE) antigen combined with a liposomal-based AS01B adjuvant system.11 Blood samples for measuring antibody titres were collected at four substudy visits: prevaccination (week 0), 4 weeks after the first dose (week 4), 4 weeks after the second dose (week 16) and 48 weeks after the second dose (week 60) (figure 1).
Figure 1. Vaccine substudy design. aNumber of patients who received ≥1 RZV dose. csDMARDs, conventional synthetic disease-modifying anti-rheumatic drugs; QD, once daily; RZV, adjuvanted recombinant zoster vaccine.
Immunogenicity and safety measures
The primary endpoint was to assess the proportion of patients who achieved a satisfactory humoral response to RZV 4 weeks after receiving the second dose (week 16). Consistent with previous studies,16 22 a satisfactory humoral response was defined as a ≥4-fold increase in the concentration of anti-gE antibody titres compared with the prevaccination concentration (week 0), as measured using an ELISA. Secondary endpoints included assessing the proportion of patients who achieved a satisfactory humoral response to RZV 4 weeks after the first dose (week 4) and 48 weeks after the second dose (week 60); and the geometric mean fold rise (GMFR) of anti-gE antibody levels at weeks 4, 16 and 60 compared with pre-vaccination levels was evaluated.
An exploratory analysis was conducted in a subcohort of 38 patients to assess cell-mediated immunogenicity. The exploratory endpoint was to evaluate the frequency of gE-specific CD4+ [2+] T cells expressing ≥2 activation markers (interferon-gamma, interleukin-2, tumour necrosis factor-alpha, cluster of differentiation 40 ligand) at weeks 4, 16 and 60 using intracellular cytokine staining and flow cytometry. A cell-mediated immune response to RZV was defined as a ≥2-fold increase in the frequencies of gE-specific CD4+ [2+] T cells compared with the prevaccination frequencies.16 The GMFR in gE-specific CD4+ [2+] T-cell levels at weeks 4, 16 and 60 compared with prevaccination levels was also assessed.
Safety was evaluated by monitoring and analysing adverse events (AEs), serious AEs that occurred within 30 days after each vaccination and AEs that led to study discontinuation. AEs were categorised by system organ class and preferred term according to the Medical Dictionary for Regulatory Activities V.26.0. The severity of AEs and their relationship to upadacitinib and RZV were assessed by the investigator.
Statistical analysis
A total sample size of approximately 85 patients was planned for the substudy, based on an assumed humoral response rate of 70%13 and accounting for a 20% dropout rate. The planned sample size provided a margin of error (half width of CI) of approximately 11% when using a two-sided 95% CI for the response rate. However, as a substudy of the SELECT-COMPARE trial, these analyses were not powered to conduct inferential statistics, and the results were descriptive only.
Baseline patient characteristics were summarised for the substudy. Immunogenicity measures were evaluated for all patients who received both vaccine doses. The proportion of patients who achieved a satisfactory humoral response to RZV was reported as counts and percentages with 95% CI calculated using logarithmically transformed assay results and then back-transformed to the original scale.
Subgroup analyses were performed at weeks 16 and 60 to examine the impact of age, RA disease severity as categorised by the Clinical Disease Activity Index (CDAI) and the use and dose of concomitant CSs on humoral response to RZV. Additionally, the GMFR in anti-gE antibody levels and their corresponding 95% CIs were summarised. In the exploratory analysis, the counts and the proportion of patients who achieved a cell-mediated immune response to RZV were presented, along with the GMFR in the frequencies of gE-specific CD4+ [2+] T cells and their respective 95% CIs. The GMFR was calculated by logarithmically transforming the assay results and then back-transforming them to the original scale. For non-positive frequency values of gE-specific CD4+ [2+] T cells, a technical cut-off was assigned to the original values to calculate the fold rise. Immunogenicity assessments were conducted using available blood samples, and missing values were not imputed. No adjustments for multiplicity were made in this substudy.
Safety analyses were conducted on all patients who received at least 1 dose of RZV in the substudy. The number and proportion of patients who experienced AEs within 30 days postvaccination were presented overall and by baseline CS use. In addition, HZ exposure-adjusted event rates (EAERs) were calculated as the number of events per 100 patient-years. HZ events were assessed for substudy patients and those in the main SELECT-COMPARE study who did not participate in the substudy and did not receive RZV or live-attenuated HZ vaccine. For substudy patients, HZ events occurring from the date of the first RZV dose to the last substudy visit for each patient who received upadacitinib were reported. In the main SELECT-COMPARE study, the observation period for HZ events started from the first upadacitinib dose date to the last-patient-last-visit date of the substudy. Missing safety data were not imputed.
Results
Baseline patient characteristics
Among the 95 patients who received ≥1 RZV dose, 93 (97.9%) received both doses, while 2 (2.1%) received one dose. A total of 86 (90.5%) patients completed the vaccine substudy through week 60 (online supplemental figure 1). During the substudy, 5 patients discontinued upadacitinib for various reasons, including lost to follow-up (n = 2), AEs (n = 1), withdrawal of consent (n = 1) and COVID-19 infection (n = 1). Among the patients who received both RZV doses, most were female (78.5%) and White (79.6%), with a mean (SD) age of 62.4 (7.5) years at baseline (table 1). Patients had been experiencing symptoms of RA for a median (range) duration of 11.7 (4.9–41.6) years and were exposed to upadacitinib for a median (range) of 3.9 (2.9–5.8) years. Overall, patients exhibited low disease activity based on a mean (SD) CDAI score of 5.3 (6.3) and Disease Activity Score 28-joint count C-reactive protein of 2.9 (1.2). Two patients (2.1%) included in the analysis did not have a record of methotrexate use at baseline but were later determined to have not met the enrolment criteria of receiving concomitant methotrexate at baseline. Because the analyses were conducted using an intent-to-treat approach, these patients remained in the analyses. One-half (49.5%) of the patients used oral CSs, and the median (range) daily dose was 5.0 mg (2.5 mg–10.0 mg). Most patients provided evaluable blood samples during the substudy visits at week 4 (n = 90/93, 96.8%), week 16 (n = 90/93, 96.8%) and week 60 (n = 84/93, 90.3%).
Table 1. Baseline demographics and clinical characteristics.
| Characteristic | UPA 15 mg QD (n=93)* |
|---|---|
| Female, n (%) | 73 (78.5) |
| Age, years, mean (SD) | 62.4 (7.5) |
| Body mass index, kg/m2, mean (SD) | 29.9 (6.1) |
| Race, n (%) | |
| Black or African American | 14 (15.1) |
| White | 74 (79.6) |
| Multiple | 5 (5.4) |
| Geographic Region, n (%) | |
| North America | 35 (37.6) |
| South or Central America | 37 (39.8) |
| Other† | 21 (22.6) |
| Duration of RA since initial symptoms, years, median (range) | 11.7 (4.9–41.6) |
| Duration of RA since initial diagnosis, years, median (range) | 10.2 (4.2–34.1) |
| Duration of upadacitinib treatment, years, median (range) | 3.9 (2.9–5.8) |
| Concomitant methotrexate use, n (%) | 91 (97.8)‡ |
| Methotrexate dose, mg, median (range) | 15.0 (7.5–25.0) |
| Concomitant oral corticosteroid use, n (%) | 46 (49.5) |
| Oral corticosteroid dose, mg, median (range) | 5.0 (2.5–10.0) |
| CDAI, mean (SD) | 5.3 (6.3) |
| CDAI score ≤ 2.8, n (%) | 43 (46.2) |
| CDAI score >2.8 to ≤10, n (%) | 36 (38.7) |
| CDAI score >10, n (%) | 14 (15.1) |
| DAS28-CRP, mean (SD) | 2.9 (1.2) |
Baseline demographic and clinical characteristics were assessed during the substudy baseline visit.
Number of patients who received both RZV doses.
Patients were all from South Africa.
Two patients took methotrexate for less than 42 days in the 8-week period before baseline and did not meet study entry criteria but were entered into the study.
CDAI, Clinical Disease Activity Index; DAS28-CRP, Disease Activity Score 28-joint count C-reactive protein; QD, once daily; RA, rheumatoid arthritis; RZV, adjuvanted recombinant zoster vaccine; UPA, upadacitinib.
Vaccine immunogenicity
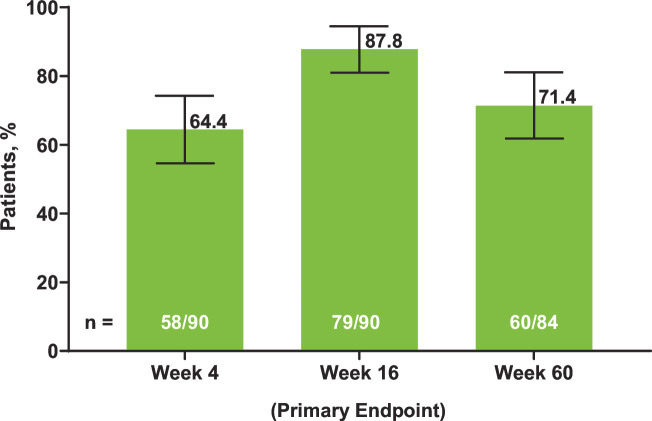
Satisfactory humoral response to RZV (≥4-fold increase in prevaccination gE antibody titres) was achieved in 64.4% (n = 58/90 (95% CI 54.6% to 74.3%)) of patients at week 4, which increased to 87.8% (n = 79/90 (95% CI 81.0% to 94.5%)) at week 16 (primary endpoint) and 71.4% (n = 60/84 (95% CI 61.8% to 81.1%)) at week 60 (figure 2). Individual variation in gE antibody titres by visit is shown in box plots in online supplemental figure 2.
Figure 2. Proportion of patients achieving satisfactory humoral responses to RZV at weeks 4, 16 and 60. Error bars indicate 95% CI. Satisfactory humoral response was defined as a ≥4-fold increase in the concentration of anti-glycoprotein E antibody titres compared with the prevaccination concentration (week 0). The number of patients was based on the availability of blood samples collected at baseline and the substudy visits. RZV, adjuvanted recombinant zoster vaccine.
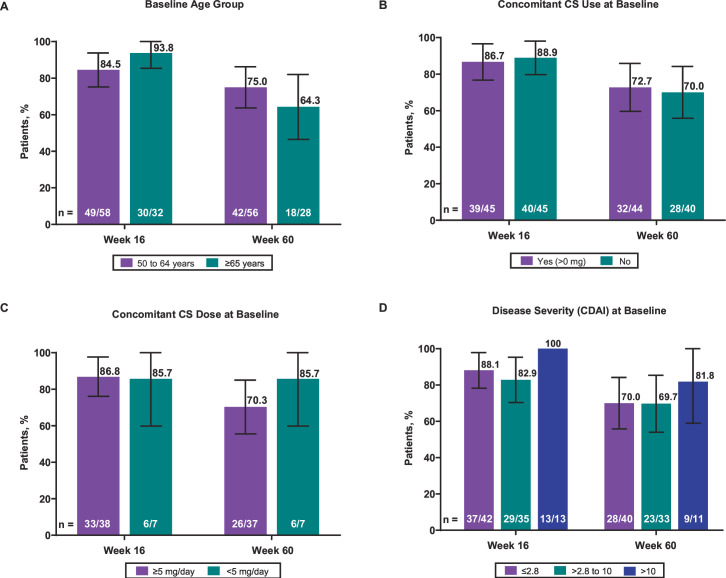
The rates of humoral response were comparable between patients aged 50 to 64 years and those aged 65 years and older at week 16 (84.5%, n = 49/58 (95% CI 75.2% to 93.8%) vs 93.8%, n=30/32 (95% CI 85.4% to 100%), respectively) and week 60 (75.0%, n = 42/56 (95% CI 63.7% to 86.3%) vs 64.3%, n = 18/28 (95% CI 46.5% to 82.0%), respectively) (figure 3A). Similarly, rates of humoral response at weeks 16 and 60 were comparable between patients with and without baseline concomitant CS use at week 16 (86.7%, n = 39/45 (95% CI 76.7% to 96.6%) vs 88.9%, n=40/45 (95% CI 79.7% to 98.1%), respectively) and week 60 (72.7%, n = 32/44 (95% CI 59.6% to 85.9%) vs 70.0%, n = 28/40 (95% CI 55.8% to 84.2%), respectively) (figure 3B). Due to the overlapping 95% CIs, a definitive conclusion could not be made about the numeric differences observed in humoral responses based on patients stratified by baseline concomitant CS dose (week 16, ≥5 mg/day 86.8%, n = 33/38 (95% CI 76.1% to 97.6%) vs <5 mg/day, 85.7%, n = 6/7 (95% CI 59.8% to 100%); week 60, ≥5 mg/day, 70.3%, n = 26/37 (95% CI 55.5% to 85.0%) vs <5 mg/day, 85.7%, n = 6/7 (95% CI 59.0% to 100%)) (figure 3C) or baseline RA disease severity (week 16, CDAI of ≤2.8, 88.1%, n = 37/42 (95% CI 78.3% to 97.9%) vs CDAI of >2.8 to 10, 82.9%, n = 29/35 (95% CI 70.4% to 95.3%) vs CDAI of >10, 100%, n = 13/13 (95% CI 100% to 100%); week 60, CDAI of ≤2.8, 70.0%, n = 28/40 (95% CI 55.8% to 84.2%) vs CDAI of >2.8 to 10, 69.7%, n = 23/33 (95% CI 54.0% to 85.4%) vs CDAI of >10, 81.8%, n = 9/11 (95% CI 59.0% to 100%)) (figure 3D).
Figure 3. (A) The effect of age, (B) baseline concomitant CS use, (C) baseline concomitant CS dose and (D) baseline RA disease severity on humoral responses to RZV at weeks 16 and 60. Error bars indicate 95% CI. Satisfactory humoral response was defined as a ≥4-fold increase in the concentration of anti-glycoprotein E antibodies compared with the prevaccination concentration (week 0). The number of patients was based on the availability of blood samples collected at baseline and the substudy visits. Disease severity was classified by the CDAI. CS, corticosteroid; CDAI, Clinical Disease Activity Index; RA, rheumatoid arthritis; RZV, adjuvanted recombinant zoster vaccine.
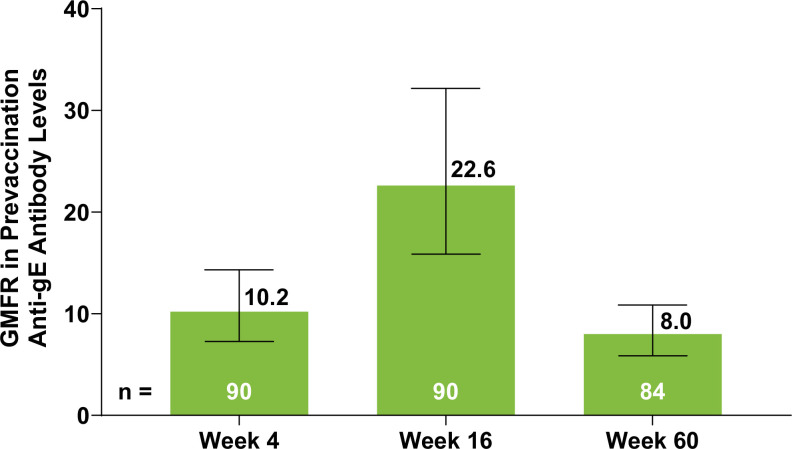
Additionally, the geometric mean anti-gE antibody levels demonstrated an increase at week 4 (10.2, n=90 (95% CI 7.3 to 14.3)) and week 16 (22.6, n = 90 (95% CI 15.9 to 32.2)) compared with prevaccination levels, followed by a decrease at week 60 (8.0, n=84 (95% CI 5.9 to 10.9)) (figure 4).
Figure 4. GMFR in prevaccination anti-gE antibody levels at weeks 4, 16 and 60 after RZV vaccination. Error bars indicate 95% CI. The number of patients was based on the availability of blood samples collected at baseline and the substudy visits. gE, glycoprotein E; GMFR, geometric mean fold rise; RZV, adjuvanted recombinant zoster vaccine.
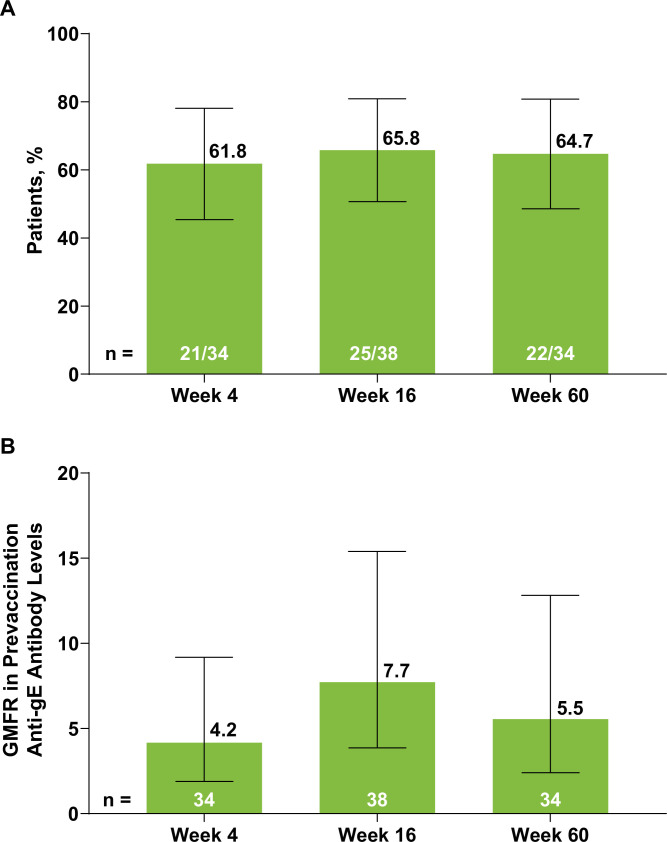
In the exploratory analysis conducted on a subcohort of 38 patients, over 60% of patients achieved a cell-mediated immune response to RZV at all time points (figure 5A). A similar pattern was noted in the GMFR for the frequency of gE-specific CD4+ [2+] T cells, with levels peaking at week 16 (7.7, n = 38 (95% CI 3.9 to 15.4)) and subsequently decreasing by week 60 (5.5, n = 34 (95% CI 2.4 to 12.8)) (figure 5B). At week 60, approximately half of patients (52.9%, n=18/34) had achieved both humoral response and cell-mediated immune response, with some patients exhibiting only a humoral response (23.5%, n=8/34) or cell-mediated response (11.8%, n=4/34) or neither immune response (11.8%, n=4/34) (online supplemental table 1).
Figure 5. (A) Cell-mediated immune responses to RZV and (B) GMFR in prevaccination gE-specific CD4+ [2+] T-cell levels at weeks 4, 16 and 60. Error bars indicate 95% CI. Responders for cell-mediated immune response (figure 5A) were patients with a ≥2-fold increase in the frequencies of gE-specific CD4+ [2+] T cells compared with the prevaccination frequencies. A technical cut-off was assigned to non-positive frequency values of gE-specific CD4+ [2+] T cells to calculate the fold rise. The number of patients was based on the availability of blood samples collected at baseline and the substudy visits. gE, glycoprotein E; GMFR, geometric mean fold rise; RZV, adjuvanted recombinant zoster vaccine.
Safety
During the 30 days following vaccination, a total of 37 patients (38.9%) experienced an AE, with the majority being mild or moderate (98.9%) in severity (table 2). One patient was reported to have severe hypersensitivity. The most commonly reported AEs were injection site pain (4.2%) followed by increased blood creatine phosphokinase (3.2%), COVID-19 infection (3.2%), headache (3.2%), hypertension (3.2%) and urinary tract infection (3.2%). As assessed by the investigator, 11 patients had AEs that were considered possibly related to upadacitinib, and 16 patients had AEs that were considered possibly related to RZV within 30 days following vaccination.
Table 2. Safety through 30 days after each vaccination with RZV.
| Event, n (%) | UPA 15 mg QD (n = 95)* |
|---|---|
| Any AE | 37 (38.9) |
| AEs occurring in >1 patient | |
| Injection site pain | 4 (4.2) |
| Increased blood creatine phosphokinase | 3 (3.2) |
| COVID-19 | 3 (3.2) |
| Headache | 3 (3.2) |
| Hypertension | 3 (3.2) |
| Urinary tract infection | 3 (3.2) |
| Back pain | 2 (2.1) |
| Allergic dermatitis | 2 (2.1) |
| Injection site erythema | 2 (2.1) |
| Lower respiratory tract infection | 2 (2.1) |
| Myalgia | 2 (2.1) |
| Nausea | 2 (2.1) |
| Rheumatoid arthritis | 2 (2.1) |
| Severe AE† | 1 (1.1) |
| Serious AE | 0 |
| AE with reasonable possibility of being related to UPA‡ | 11 (11.6) |
| AE with reasonable possibility of being related to RZV§ | 16 (16.8) |
| AE leading to discontinuation of UPA | 0 |
| Death¶ | 0 |
Number of patients who received ≥1 RZV dose.
Hypersensitivity.
AEs were assessed by the investigator, and patients could have had more than one AE. Three patients had increased blood creatine phosphokinase, and one patient each had hiatus hernia, nausea, injection site pain, lower respiratory tract infection, urinary tract infection, decreased haemoglobin, dyslipidaemia, myalgia, rheumatoid arthritis (worsening) and headache.
AEs were assessed by the investigator, and patients could have had more than one AE. Four patients had injection site pain; three patients had headache; and two patients each had nausea, injection site erythema and myalgia. One patient each had application site pain, asthenia, fatigue, injection site reaction, pain, vaccination site erythema, vaccination site pain, vaccination site reaction, vaccination site swelling, hypersensitivity, urinary tract infection, arthralgia, rheumatoid arthritis, lethargy, allergic dermatitis, erythema and hypertension.
One death resulting from respiratory failure due to COVID-19 pneumonia was observed in a 68-year-old male patient 239 days after receiving the second RZV dose.
AE, adverse event; QD, once daily; RZV, adjuvanted recombinant zoster vaccine; UPA, upadacitinib.
During the substudy, one patient (EAER=0.9 per 100 patient-years) experienced a moderate case of HZ that was unilateral and involved multiple dermatomes 120 days after receiving the second RZV dose, which led to the interruption of upadacitinib treatment. In the main SELECT-COMPARE study among patients treated with upadacitinib 15 mg QD who did not receive RZV, a total of 132 events (2.8 events/100 patient-years of HZ) were observed.
No serious AEs, AEs leading to discontinuation of upadacitinib or deaths were reported within 30 days after either RZV dose. A 68-year-old male patient died due to respiratory failure caused by COVID-19 pneumonia, which occurred 239 days after receiving the second RZV dose and was not considered related to study medication.
Overall, safety outcomes were generally comparable between patients who did and did not use concomitant CSs at baseline (online supplemental table 2).
Discussion
We evaluated the short-term and long-term immunogenicity of RZV in patients with RA who were receiving upadacitinib 15 mg QD and concomitant methotrexate. Our results suggest that most patients achieved a satisfactory humoral response to RZV (≥4-fold increase in prevaccination gE antibody titres) at week 16, even when administered alongside methotrexate. Further, the majority of patients had satisfactory cell-mediated immune responses at week 16 in our exploratory substudy. As expected from previous studies,16 humoral and cell-mediated immune responses were reduced after the 44-week follow-up period, but most patients maintained satisfactory responses throughout that time point and in the long-term follow-up study.23 Further, we observed the vaccine to be safe in this population, with few individuals having systemic AEs and none having a serious AE in the 30 days postvaccination. Our immunogenicity findings suggest that this vaccine should be used in patients receiving upadacitinib and concomitant methotrexate. Further studies evaluating efficacy should be conducted.
Although the majority of patients mounted satisfactory responses in this study, the magnitude of humoral and cell-mediated immune response was lower than that observed in prior studies of non-immunosuppressed patients. For example, in the pivotal phase 3 clinical trial of RZV conducted in healthy individuals, a humoral response rate of 97.8% and a GMFR in anti-gE antibody levels of 39.1-fold over baseline were observed 1 month after the second dose of RZV, compared with a GMFR of 22 in our study.16 Further, the cell-mediated responses in that phase 3 trial were also several fold higher than what we observed in our study. Given our patients’ underlying disease and use of immunosuppressive therapy, we anticipated a diminished magnitude in humoral and cellular response compared with that of a healthy population.
To date, there is limited RZV data in populations with immune-mediated inflammatory diseases. Several small clinical trials have been conducted in other immunosuppressed settings; for example, reduced response to RZV was observed in patients who had undergone autologous haematopoietic stem cell transplantation.24 In patients with RA, a recent non-randomised clinical trial, which measured humoral response using a methodology similar to our study, found that 80.5% of patients achieved a ≥4-fold increase in anti-gE antibody levels 6 weeks after receiving their second RZV dose (approximately 3.5 months since the first dose); however, later time points were not measured.17 Additionally, a prospective study examining the immunogenicity of RZV in 53 RA patients used a comparable definition of humoral response and found that 80.0% of those treated with csDMARDs, 60.9% with bDMARDs and 50.0% with tsDMARDs achieved a satisfactory humoral response 4 weeks after the second RZV dose, but the differences between treatment groups were not significant.25 Several other studies have assessed the immunogenicity of RZV in RA populations by measuring anti-varicella-zoster virus immunoglobulin G antibody levels, although results have been variable and limited by small sample sizes.18 25 26
In our analysis, factors such as age and baseline use of concomitant CSs did not appear to affect RZV antibody responses. These findings are generally consistent with a study that evaluated the pneumococcal 13-valent conjugate vaccine in patients with RA who were also receiving concomitant upadacitinib and methotrexate.27 That study found that concomitant CS use did not affect antibody responses, but older age was associated with a decreased humoral response to pneumococcal 13-valent conjugate vaccine.27
In addition to the humoral response, there was a cell-mediated immune response to RZV in a subcohort of 38 patients, which provides additional indirect insights into the vaccine’s efficacy and potential in HZ prevention.16 25 28 Cell-mediated immunity is an important immune effector for controlling HZ infection; specifically, T-cell immunity appears to be more important than humoral immunity for HZ control, especially as age-related susceptibility to the disease correlates with a decline in specific T-cell immunity but not specific antibody titre.16 29 Our findings are similar to those observed in immune-compromised patients and in patients receiving immunosuppressants or immune-modifying drugs.30
In this substudy through week 60 (107.7 patient-years of upadacitinib exposure), there was one (EAER of 0.9 per 100 patient-years) moderate case of breakthrough HZ infection, which was unilateral and affected multiple dermatomes, and occurred 4 months after the second RZV dose. We have reported the rate of HZ events through week 60, which is longer than in typical vaccination clinical trials. Notably, in the main SELECT-COMPARE study among patients treated with upadacitinib 15 mg QD who did not receive RZV, a total of 132 events (2.8 events per 100 patient-years) of HZ were observed (unpublished data). The limitation of such a comparison is much higher upadacitinib exposure among all unvaccinated patients of the SELECT-COMPARE study (reaching 4743.1 patient-years by the time of the vaccination sub-study analysis). Furthermore, the ongoing SELECT-COMPARE long-term extension study is following patients for up to 5 years. Thus, updated reporting for this substudy may be possible in the future. Overall, the RZV vaccination was well tolerated, with no reports of serious AEs. Safety outcomes were generally similar between patients who used CSs at baseline and those who did not. Notably, the AE rates were considerably lower in our study compared with other studies involving immunocompetent and immunocompromised adults,17 18 25 31 32 which may be attributed to the use of JAK inhibitors. Injection site pain was the most common AE, occurring in 4.2% of patients in our study, whereas other studies have reported more than 40% of patients experiencing an injection site reaction or pain,17 18 25 with 68.9% of patients aged over 70 years reporting injection site pain after HZ subunit vaccine in the ZOE-70 trial.15
Our substudy was limited in several ways. Our sample size was relatively small, and most patients (~80%) were White, which potentially limits generalisability to other racial and ethnic groups. The substudy was a single-arm open-label trial, so it lacked a placebo control group with which to compare potential treatment effects on RZV responses in the absence of JAK inhibitor or methotrexate exposure. In addition, inferential statistical tests were not conducted, and the data reported were descriptive only. A further consideration is that approximately 50% of patients were receiving concomitant CSs (mean dose, 5.8 mg/day) at the substudy baseline visit (first vaccination visit), which may not be representative of the broader population with RA treated with upadacitinib or other JAK inhibitors. For example, in the recent UPHOLD real-world study that enrolled 1701 patients (mean age, 56.9 years) with moderate-to-severe RA initiating upadacitinib with or without csDMARDs, 43.2% of patients were receiving concomitant CSs (mean dose, 8 mg/day) at baseline.33 Thus, although our results should be interpreted with appropriate caution, it is reassuring that we observed adequate humoral and cell-mediated immune responses to RZV in most patients despite the slightly higher rate of concomitant CS use than that reported in the large, real-world population in the UPHOLD study. In our small substudy, heterogeneity was observed in baseline characteristics and immunogenicity responses, as would be expected. Despite the limitations, this substudy provides indirect insights into the potential effectiveness of RZV, with respect to the average level of humoral and cell-mediated response in the assessed population. These findings can be confirmed in future randomised controlled trials that enrol relatively larger cohorts that can directly control for potential confounders, such as differences in baseline disease severity, immune status and treatment duration. Future studies should also investigate whether temporary suspension of upadacitinib or methotrexate use before vaccination could improve the immune response to RZV, especially in older adults who are more likely to have less vigorous immune response to vaccination.
In summary, our study found that most patients achieved satisfactory humoral and cell-mediated immune responses to RZV at weeks 4, 16 and 60. Age, RA disease severity and the use or dose of concomitant CSs did not affect humoral response to RZV. Additionally, RZV was well tolerated, and no serious AEs were reported within 30 days after vaccination. These findings support the potential of RZV as an effective long-term preventive strategy against HZ infections in RA patients who are at higher risk and receiving multiple immunosuppressive treatments. Further studies evaluating RZV efficacy within RA should be conducted.
Supplementary material
Acknowledgements
AbbVie and the authors thank the patients, study sites and investigators who participated in the study. Medical writing support was provided by Julia Zolotarjova, MSc, MWC, of AbbVie. Editorial support was provided by Angela T Hadsell, BA, of AbbVie, and Abegale Templar, PhD, of JB Ashtin and funded by AbbVie. These data were previously presented at the 2024 EULAR Annual Congress of Rheumatology in Vienna, Austria.
Footnotes
Funding: AbbVie Inc. (North Chicago, IL, USA) funded this study and participated in the study design, research, data collection, analysis, interpretation of data, and the review and approval of the publication. All authors had access to relevant data and participated in the drafting, review and approval of this publication. No honouraria or payments were made for authorship.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involved human participants and was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The substudy was approved by an investigational review board or ethics committee at each study site. Participants gave informed consent to participate in the study before taking part.
Data availability free text: AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymised, individual and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent and scientific research and will be provided following review and approval of a research proposal, Statistical Analysis Plan and execution of a Data Sharing Agreement. Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select 'Home'.
Data availability statement
Data are available upon reasonable request.
References
- 1.Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 2.Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford) 2013;52:53–61. doi: 10.1093/rheumatology/kes305. [DOI] [PubMed] [Google Scholar]
- 3.Dlamini ST, Htet KM, Theint ECC, et al. Herpes Zoster Risk in Patients with Rheumatoid Arthritis and Its Association with Medications Used. Int J Environ Res Public Health. 2023;20:2123. doi: 10.3390/ijerph20032123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smitten AL, Choi HK, Hochberg MC, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57:1431–8. doi: 10.1002/art.23112. [DOI] [PubMed] [Google Scholar]
- 5.Veetil BMA, Myasoedova E, Matteson EL, et al. Incidence and time trends of herpes zoster in rheumatoid arthritis: a population-based cohort study. Arthritis Care Res (Hoboken) 2013;65:854–61. doi: 10.1002/acr.21928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yun H, Yang S, Chen L, et al. Risk of Herpes Zoster in Autoimmune and Inflammatory Diseases: Implications for Vaccination. Arthritis Rheumatol . 2016;68:2328–37. doi: 10.1002/art.39670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opdam MAA, Broeder N den, van den Bemt BJF, et al. Higher infection risk for JAK inhibitors tofacitinib and baricitinib compared to subcutaneous biological DMARDs. Clin Rheumatol. 2024;43:2133–8. doi: 10.1007/s10067-024-06980-x. [DOI] [PubMed] [Google Scholar]
- 8.Winthrop KL, Nash P, Yamaoka K, et al. Incidence and risk factors for herpes zoster in patients with rheumatoid arthritis receiving upadacitinib: a pooled analysis of six phase III clinical trials. Ann Rheum Dis. 2022;81:206–13. doi: 10.1136/annrheumdis-2021-220822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bass AR, Chakravarty E, Akl EA, et al. 2022 American College of Rheumatology Guideline for Vaccinations in Patients With Rheumatic and Musculoskeletal Diseases. Arthritis Care & Research. 2023;75:449–64. doi: 10.1002/acr.25045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furer V, Rondaan C, Heijstek MW, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79:39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 11.GlaxoSmithKline SHINGRIX® (Zoster vaccine recombinant, adjuvanted). Prescribing information. [-Oct-2024]. https://www.fda.gov/media/108597/download?attachment Available. Accessed.
- 12.Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103–8. doi: 10.15585/mmwr.mm6703a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vink P, Ramon Torrell JM, Sanchez Fructuoso A, et al. Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Chronically Immunosuppressed Adults Following Renal Transplant: A Phase 3, Randomized Clinical Trial. Clin Infect Dis. 2020;70:181–90. doi: 10.1093/cid/ciz177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–96. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med. 2016;375:1019–32. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham AL, Heineman TC, Lal H, et al. Immune Responses to a Recombinant Glycoprotein E Herpes Zoster Vaccine in Adults Aged 50 Years or Older. J Infect Dis. 2018;217:1750–60. doi: 10.1093/infdis/jiy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Källmark H, Bergström T, Nagel J, et al. Serologic immunogenicity and safety of herpes zoster subunit vaccine in patients with rheumatoid arthritis receiving Janus kinase inhibitors. Rheumatology (Oxford) 2024;63:2024–33. doi: 10.1093/rheumatology/kead552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esteban-Vazquez A, Steiner M, Castañeda E, et al. The Real-World Study of Immunogenicity and Safety of the Adjuvant Recombinant Vaccine against Varicella Zoster Virus in Patients with Immune-Mediated Inflammatory Diseases Treated with Janus Kinase Inhibitors. Vaccines (Basel) 2023;11:1610. doi: 10.3390/vaccines11101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh R, Singer D, Chmielewski-Yee E, et al. Effectiveness and safety of recombinant zoster vaccine: A review of real-world evidence. Hum Vaccin Immunother. 2023;19:2263979. doi: 10.1080/21645515.2023.2263979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischmann R, Pangan AL, Song I-H, et al. Upadacitinib Versus Placebo or Adalimumab in Patients With Rheumatoid Arthritis and an Inadequate Response to Methotrexate: Results of a Phase III, Double-Blind, Randomized Controlled Trial. Arthritis Rheumatol . 2019;71:1788–800. doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 21.Merck & Co Inc ZOSTAVAX® (Zoster vaccine live). Prescribing information. [-Oct-2024]. https://www.fda.gov/media/82524/download Available. Accessed.
- 22.Racine É, Gilca V, Amini R, et al. A systematic literature review of the recombinant subunit herpes zoster vaccine use in immunocompromised 18–49 year old patients. Vaccine (Auckl) 2020;38:6205–14. doi: 10.1016/j.vaccine.2020.07.049. [DOI] [PubMed] [Google Scholar]
- 23.Boutry C, Hastie A, Diez-Domingo J, et al. The Adjuvanted Recombinant Zoster Vaccine Confers Long-Term Protection Against Herpes Zoster: Interim Results of an Extension Study of the Pivotal Phase 3 Clinical Trials ZOE-50 and ZOE-70. Clin Infect Dis. 2022;74:1459–67. doi: 10.1093/cid/ciab629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastidas A, de la Serna J, El Idrissi M, et al. Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster After Autologous Stem Cell Transplantation: A Randomized Clinical Trial. JAMA. 2019;322:123–33. doi: 10.1001/jama.2019.9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima S, Iwamoto T, Kobayashi Y, et al. Immunogenicity and influence on disease activity of recombinant zoster vaccine in patients with rheumatoid arthritis treated with DMARDs. RMD Open. 2024;10:e003902. doi: 10.1136/rmdopen-2023-003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venerito V, Stefanizzi P, Cantarini L, et al. Immunogenicity and Safety of Adjuvanted Recombinant Zoster Vaccine in Rheumatoid Arthritis Patients on Anti-Cellular Biologic Agents or JAK Inhibitors: A Prospective Observational Study. Int J Mol Sci. 2023;24:6967. doi: 10.3390/ijms24086967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winthrop K, Vargas JI, Drescher E, et al. Evaluation of response to 13-valent conjugated pneumococcal vaccination in patients with rheumatoid arthritis receiving upadacitinib: results from a phase 2 open-label extension study. RMD Open. 2022;8:e002110. doi: 10.1136/rmdopen-2021-002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laing KJ, Ford ES, Johnson MJ, et al. Recruitment of naive CD4+ T cells by the recombinant zoster vaccine correlates with persistent immunity. J Clin Invest. 2023;133:e172634. doi: 10.1172/JCI172634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341–57. doi: 10.1007/82_2010_31. [DOI] [PubMed] [Google Scholar]
- 30.Dagnew AF, Vink P, Drame M, et al. Immune responses to the adjuvanted recombinant zoster vaccine in immunocompromised adults: a comprehensive overview. Hum Vaccin Immunother. 2021;17:4132–43. doi: 10.1080/21645515.2021.1930846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dagnew AF, Rausch D, Hervé C, et al. Efficacy and serious adverse events profile of the adjuvanted recombinant zoster vaccine in adults with pre-existing potential immune-mediated diseases: a pooled post hoc analysis on two parallel randomized trials. Rheumatology (Oxford) 2021;60:1226–33. doi: 10.1093/rheumatology/keaa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mwakingwe-Omari A, Lecrenier N, Naficy A, et al. Recombinant zoster vaccine in immunocompetent and immunocompromised adults: A review of clinical studies. Hum Vaccin Immunother. 2023;19:2278362. doi: 10.1080/21645515.2023.2278362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Östör A, Feist E, Sidiropoulos P, et al. Achievement of treatment targets and maintenance of response with upadacitinib in patients with moderate-to-severe rheumatoid arthritis in real-world practice: 1-year outcomes from the UPHOLD observational study. Arthritis Res Ther . 2025;27:84. doi: 10.1186/s13075-025-03528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.