Abstract
Hyperscanning has emerged as a prominent technique in social neuroscience, enabling the simultaneous recording of neural activity from multiple individuals engaged in interactive tasks. Despite its potential, functional magnetic resonance imaging (fMRI) hyperscanning remains underutilized due to technical and logistical challenges in synchronizing data across scanners. To address these barriers, we developed an internet-based fMRI hyperscanning platform in southern Taiwan, enabling synchronized acquisition between two sites located 305 km apart. The present study involving 33 dyads examined social and economic decision-making tasks under cooperative and competitive conditions, using a Sender–Receiver paradigm where monetary outcomes hinged on the Receiver’s choices and their alignment with the Sender’s suggestions. This design elucidated neural mechanisms underlying cooperation, competition, deception, and strategic interactions. The resulting dataset enables analyses of neural synchrony, temporal dynamics of functional connectivity, and condition-specific network interactions. By introducing a novel methodological framework, this work provides a valuable dataset to advance research on social decision-making and interactive neural processes.
Subject terms: Cooperation, Social behaviour
Background & Summary
Hyperscanning, a prominent method using one or more neuroimaging devices either synchronously or asynchronously, has enabled more refined social investigations1–3. Numerous studies have adopted various research paradigms, tools, and analytic methods to broaden and deepen the field. While EEG and fNIRS remain prevalent tools4,5, fMRI hyperscanning studies are relatively scarce. Since the first fMRI hyperscanning paper6 and subsequent publications over the past two decades7–9, hyperscanning has encountered several challenges10–14. To name a few, technical complexity and the need for collaboration among highly skilled teams have particularly deterred individual fMRI labs from pursuing this method. To bridge this gap, our team of fMRI practitioners in southern Taiwan created an internet-based hyperscanning method, drawing inspiration from earlier pioneering work15, which adopted multiple computers connected to a central server to synchronize onset triggers. Using Network Time Protocol (NTP) to synchronize acquisition timing across scanners located 305 km apart, we conducted hundreds of hyperscanning experiments. This dataset—the first openly available fMRI hyperscanning dataset acquired simultaneously from two geographically distant sites—represents the culmination of years of technical and experimental effort to support more accessible and ecologically valid social neuroscience research.
To further contribute to the literature, we recruited 33 dyads (with ages ranging from 20 to 30) to engage in two tasks involving social and economic decision-making while their brain activity was measured with fMRI. The study focused on cooperation and competition, with both conditions starting with the Sender suggesting a box and the Receiver making the final decision. In the cooperation condition, the dyad split a $200 reward if the Receiver chose the correct box. In the competition condition, the Receiver won the full reward if their choice matched the predetermined box; otherwise, the Sender won $150. These tasks were selected to examine how social context influences economic decision-making.
Although our previous results from this dataset were obtained using time-to-frequency-domain transformations (e.g., Fourier analysis) in event-related fMRI hyperscanning16, we believe there are several opportunities for reuse and extension. First, the temporal dynamics of brain activity and connectivity have not yet been explored, specifically how neural networks synchronize during various social interactions over different time scales. Additionally, our earlier focus was on the feedback stage, when the box was opened and the reward was revealed. Investigating dynamic changes in brain activity or interpersonal coherence is still needed. Third, interactions in the competition condition, where the Sender deceived the Receiver and the Receiver did not follow the Sender’s suggestions, remain of interest. Deception occurred in 45% of trials, and non-following occurred in 43% of trials. In “deceiving”, the Sender indicated the wrong box as more likely when they believed the left box had a higher probability. In “non-following”, the Receiver chose the left box despite the right box being suggested. Taken together, these examples highlight the significance of our data and its potential applications.
Methods
Portions of the Participants, Procedures, Experimental Task, and Neuroimaging Data Collection sections overlap with descriptions published in our previous work16. That prior work focused on reporting specific analyses based on preprocessed data (e.g., VTC and GLM files). In contrast, the present dataset describes and shares the complete raw dataset, including unprocessed functional and anatomical MRI data, behavioral responses, and participant demographics.
Participants
As described in our previous work16, we recruited thirty-three pairs of participants, aged 20 to 30 years (M = 22.74, SD = 2.03), from National Taiwan University (NTU) and National Cheng Kung University (NCKU), located in northern and southern Taiwan, respectively (305 km apart). All participants were native Taiwanese with normal or corrected-to-normal vision and no history of psychiatric or neurological disorders. Written informed consent was obtained from all participants, who followed the guidelines and regulations approved by the NCKU Governance Framework for Human Research Ethics (case number 106–254). For more details, visit https://rec.chass.ncku.edu.tw/en. Participants received NT $600 (~US $20) for their time in the experiment, along with a bonus based on the rewards earned from one randomly selected trial.
Procedures
The dyads learned the game rules during pre-experiment briefings and were informed of their roles as either Sender or Receiver, which was displayed at the start of each trial. Once the dyads were positioned in the scanner, practitioners at both sites initiated the experiment by counting down via an online communication platform. Scanner synchronization was verified through a Network Time Protocol (NTP) server-based system, ensuring timing discrepancies remained below 500 ms—well within the repetition time (TR) of 2 seconds.
Experimental task
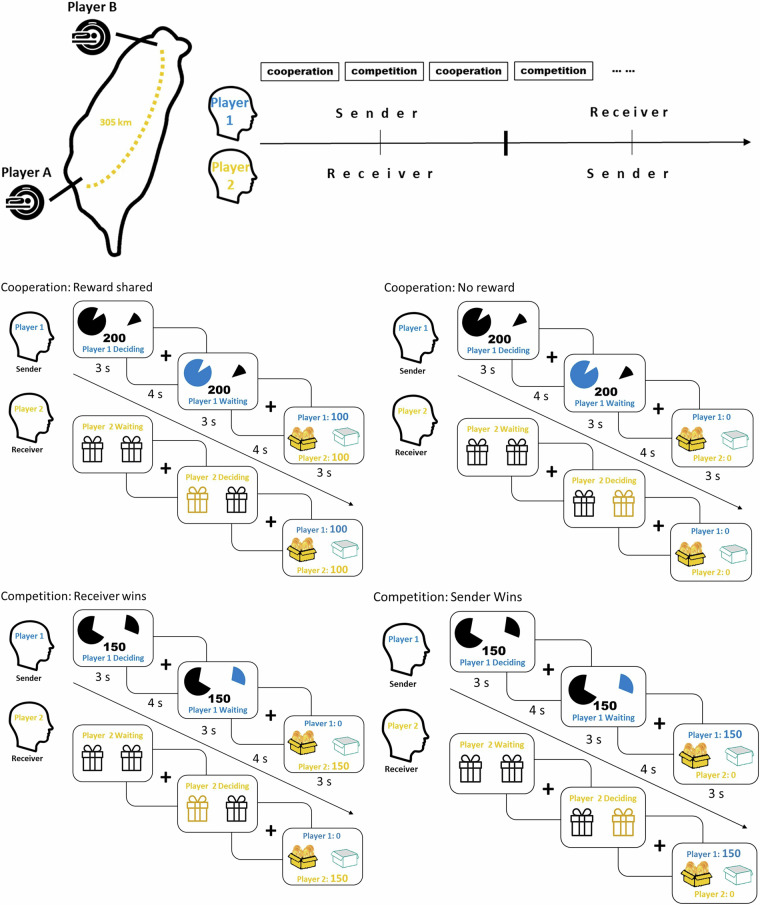
In the fMRI hyperscanning experiment called the “Opening Treasure Chest game”, both conditions started with the Sender recommending a box, followed by the Receiver making the final decision. This task resembled a “cheap talk” game17,18, where communication is free and uninformative unless the Sender and the Receiver share aligned interests.
Each trial lasted 17 seconds, divided into 3 seconds for the Sender’s decision, 4 seconds of fixation, 3 seconds for the Receiver’s decision, another 4 seconds of fixation, and 3 seconds for feedback. Inter-trial intervals ranged from 3 to 9 seconds. The conditions alternated between cooperation and competition, with roles of Sender and Receiver alternating every two rounds.
At the start of each trial, two boxes were presented, one containing a reward. The goal was to choose the correct box to either split NT $200 or take NT $150. The Sender, informed of the probabilities for each box (shown as pie charts), suggested a choice to the Receiver, who then made the final selection. In the cooperation condition, they split the $200 if successful. In the competition condition, the Receiver kept the chosen box, while the Sender received the unchosen one, with only one winning NT $150 (see Fig. 1).
Fig. 1.
The fMRI hyperscanning fMRI experiment involved both cooperation and competition, conducted simultaneously at two sites 305 km apart. The experiment consisted of 4 runs, each with 28 trials (14 cooperation and 14 competition). Player A and Player B alternated roles as Sender and Receiver. The Sender informed of the probability of the treasure box (NT$ 200 in the cooperation condition and NT$ 150 in the competition condition), suggested a box, while the Receiver made the final decision on which box to open.
To balance expected utility, the cooperation condition was set with a 75% success rate, giving each participant an expected utility of NT $75. The competition condition had a 50% success rate, resulting in the same expected utility. Participants were pseudo-randomly assigned roles as Player A or B, ensuring equal participation as both Sender and Receiver. The experiment lasted about 70 minutes per pair.
Neuroimaging data collection
Functional and anatomical MRI data were collected simultaneously using two different MRI scanners located 305 km apart. The first scanner, at the NCKU Mind Research and Imaging Center in Tainan (southern Taiwan), is a 3-Tesla General Electric Discovery MR750 (GE Medical Systems) with an 8-channel head coil. Functional scans were acquired using a T2* EPI sequence (TR = 2 s, TE = 33 ms, flip angle = 90°, 40 axial slices, voxel size = 3.5 × 3.5 × 3 mm³), and high-resolution T1-weighted structural scans were obtained using a 3D FSPGR sequence (TR = 7.65 ms, TE = 2.93 ms, inversion time = 450 ms, flip angle = 12°, 166 sagittal slices, voxel size = 0.875 × 0.875 × 1 mm³). The second scanner, located at NTU’s Imaging Center for the Body, Mind, and Culture Research in Taipei (northern Taiwan), is a 3-Tesla Siemens PRISMA with a 20-channel phase array coil. Functional scans were acquired using a T2*-weighted EPI sequence (TR = 2 s, TE = 24 ms, flip angle = 87°, 36 axial slices, voxel size = 3 × 3 × 3 mm³), and high-resolution T1-weighted structural scans were acquired using an MP-RAGE sequence (TR = 2.0 s, TE = 2.3 ms, inversion time = 900 ms, flip angle = 8°, 192 sagittal slices, voxel size = 0.938 × 0.938 × 0.94 mm³).
Neuroimaging data processing
DICOMs were first converted to NIFTI form using dcm2niix (citation) and organized into the Brain Imaging Data Structure using MRIcroGL (https://www.nitrc.org/projects/mricrogl).
Data Records
All data records adhere to the BIDS (Brain Imaging Data Structure) standard (version 1.0.0). Structural and functional neuroimaging data are publicly available as OpenNeuro Dataset ds004103. Each participant’s data is organized in participant-specific directories named < sub-XXA > for the NCKU site and < sub-XXB > for the NTU site. Each directory contains two subdirectories: ‘anat’ for structural data and ‘func’ for functional data. Task-relevant behavioral data are stored within the ‘func’ directory as *events.tsv files. Demographic information, including age and sex, is provided in the participants.tsv file. The complete dataset can be accessed openly via OpenNeuro at (https://openneuro.org/datasets/ds004103/versions/1.0.4.)19.
Technical Validation
Behavioral data
Across all 66 participants, senders adopted truth-telling strategies in the cooperation condition an average of 98.2% of the time (ranging from 88% to 100%). In the competition condition, this percentage dropped to 54.5% (ranging from 18% to 100%), while lying strategies were employed 45.5% of the time (ranging from 0% to 82.1%). Receivers’ ‘following’ rates (i.e., responding according to the sender’s suggestion) were 98.7% in the cooperation condition (SD = 2.4%, ranging from 89.3% to 100%). In the competition condition, the following rate was 57.9% (SD = 14.5%, ranging from 21.4% to 92.9%), and the ‘unfollowing’ rate was 42.1% (ranging from 7.1% to 78.6%). These findings align well with game theory predictions18. Overall, our task manipulations effectively elicited exclusive cooperation in the cooperation condition and a mix of truth-telling and lying, along with corresponding following and unfollowing behaviors, in the competition condition.
Structural neuroimaging data
In our previous work16, anatomical data were preprocessed using BrainVoyager QX1920. The pipeline included intensity inhomogeneity correction, manual AC-PC alignment, brain extraction, and transformation into Talairach space. Functional images were co-registered to anatomical scans, followed by spatial smoothing with a 6 mm FWHM Gaussian filter. These steps ensured standardized and analysis-ready anatomical data.
To calculate the T1-weighted Contrast-to-Noise Ratio (CNR), the process began with the acquisition of T1-weighted MRI images and their corresponding segmentation masks. For each subject, the T1-weighted image was adjusted using the scaling factors and additive offsets provided in the image metadata. Segmentation masks for gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) were then utilized. Gaussian smoothing was applied to these masks to refine them and reduce noise, ensuring that the masks more accurately represented the tissue regions.
In calculating CNR, the mean intensity values of GM and WM regions were extracted from the smoothed T1-weighted images. The standard deviation of the intensity values within the CSF mask, which is considered to represent the noise, was computed. CNR was then determined by calculating the absolute difference between the mean intensities of GM and WM, and normalizing this difference by the standard deviation of the CSF region. The resulting CNR value reflects the contrast between GM and WM tissues relative to the background noise, with higher values indicating better contrast and image quality. The process was repeated for each subject and dataset to obtain a mean CNR, providing a measure of contrast efficacy across the imaging data. At NCKU, the mean T1-weighted CNR was 1.14 ± 0.34, while at NTU, the mean T1-weighted CNR was 1.05 ± 0.06. The CNR of the two datasets (t(64) = 1.41, p = 0.163). These values suggest that the contrast between gray matter and white matter, normalized by the noise (standard deviation of the CSF), is relatively similar between the two datasets (Fig. 2).
Fig. 2.

The T1-weighted temporal contrast-to-noise ratio (CNR) is used to evaluate the quality of structural data across two scanner sites. Positive CNR values indicate that the activation signal is greater than the noise level. Error bars represent the 95% confidence intervals, providing a measure of the variability and reliability of the CNR estimates.
Functional neuroimaging data
In our previous work16, functional data were preprocessed using BrainVoyager QX20. Slice timing correction was applied via temporal interpolation to align slices within each volume. 3D motion correction realigned volumes using six motion parameters, which were also retained for use as nuisance regressors. Functional images were then co-registered to anatomical scans and transformed into Talairach space, producing Volume Time Course (VTC) files. Spatial smoothing (6 mm FWHM Gaussian kernel) enhanced signal-to-noise ratio, followed by high-pass filtering to remove low-frequency drift. Additional nuisance regressors (e.g., motion, white matter, CSF signals) were included to further denoise the data, ensuring high-quality inputs for subsequent analyses.
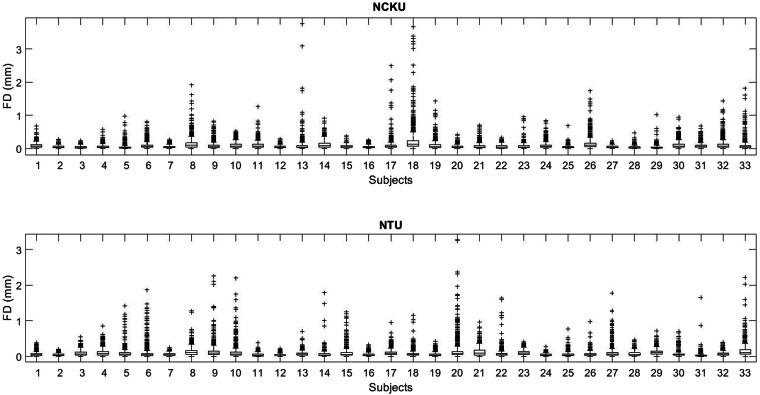
The degree of head motion was evaluated across all subjects, as excessive movement can compromise data quality and validity. We assessed motion using framewise displacement (FD), which quantifies volume-to-volume changes in head position by summing absolute differences in translational (x, y, z) and rotational movements, with rotational displacements converted to millimeters using a 50 mm head radius. Each point in Fig. 3 represents the FD of a single fMRI volume. Across all participants, average FD values were low, indicating minimal head motion. All fMRI acquisitions covered the whole brain, with 40 axial slices at the NCKU site (voxel size: 3.5 × 3.5 × 3 mm3) and 36 axial slices at the NTU site (voxel size: 3 × 3 × 3 mm3), ensuring consistent spatial coverage. The median FD was 0.051 mm for NCKU and 0.055 mm for NTU. The range of median FD across subjects was from 0.023 mm to 0.125 mm for NCKU and from 0.022 mm to 0.11 mm for NTU. Less than 5% of volumes were flagged as outliers, defined as having FD above the 95th percentile threshold.
Fig. 3.
Framewise displacement (FD) across all fMRI volumes and participants. Each point represents the FD of a single fMRI volume, reflecting volume-to-volume head motion. FD was computed by summing absolute translational and rotational displacements (the latter converted to millimeters using a 50 mm head radius). Median FD was 0.051 mm at NCKU and 0.055 mm at NTU, with low overall motion across participants. Less than 5% of volumes exceeded the 95th percentile FD threshold and were flagged as outliers.
ROI-based temporal signal-to-noise ratio (tSNR) and beta value comparisons
To ensure data quality and comparability across two different MRI scanners, a detailed analysis of the temporal signal-to-noise ratio (tSNR) and region-of-interest (ROI) beta values was conducted. The right temporoparietal junction (rTPJ) was selected as the ROI based on previous studies8,16. This ROI was defined through a meta-analysis of 128 studies using the keyword “TPJ”, which identified peak Montreal Neurological Institute (MNI) coordinates at [54, −55, 22] with a peak z-score of 8.82. This result corresponded to a false discovery rate (FDR) of 0.01 under the ‘association test’ criterion. The rTPJ is a well-established neural substrate involved in social cognition and theory of mind processing21–23.
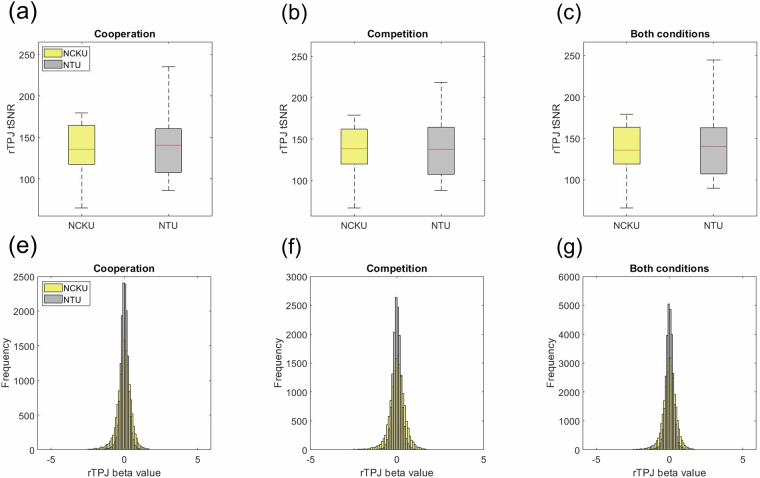
For each voxel within the rTPJ, the tSNR was calculated as the ratio of the mean signal to its standard deviation across each trial. To quantify regional signal quality, the median tSNR across all rTPJ voxels was computed for each trial. Subject-level tSNR values were then obtained by averaging these trial-level medians separately for cooperation and competition. This procedure was performed independently for each data acquisition site, yielding two distributions of subject-wise rTPJ tSNR values for statistical comparison. In the cooperation condition (Fig. 4a), the mean rTPJ tSNR was 136.38 ± 30.29 at NCKU and 142.25 ± 41.15 at NTU. A two-sample t-test revealed no significant difference between groups (t(64) = –0.661, p = 0.511, SD = 36.13). Similarly, for the competition condition (Fig. 4b), mean rTPJ tSNR values were 138.21 ± 29.67 at NCKU and 144.79 ± 43.99 at NTU, with no significant difference observed (t(64) = –0.713, p = 0.479, SD = 37.52). When data from both conditions were combined (Fig. 4c), the mean rTPJ tSNR was 137.29 ± 29.87 at NCKU and 143.52 ± 42.42 at NTU, again showing no significant difference (t(64) = –0.690, p = 0.492, SD = 36.69).
Fig. 4.
ROI-based comparison of temporal signal-to-noise ratio (tSNR) and beta values across two sites. (a–c) Boxplots of rTPJ tSNR values for NCKU and NTU participants under the cooperation, competition, and combined conditions. No significant differences were observed between sites in any condition. (d–f) Histograms of trial-level rTPJ beta values across the two sites under the same three conditions. Distributions show similar means and standard deviations, with overlapping 95% confidence intervals, confirming data consistency across MRI scanners (GE MR750 at NCKU; Siemens Prisma at NTU). These results support the comparability and homogeneity of data across acquisition sites.
Figure 4d–f illustrate the distributions of rTPJ beta values from participants scanned at NCKU and NTU. Beta values were extracted on a trial-by-trial basis and averaged across rTPJ voxels, resulting in 14,784 beta values per condition (33 participants × 8 FIR beta estimates × 14 trials × 4 runs). In the cooperation condition, the mean beta value was −0.023 ± 0.511 at NCKU and 0.007 ± 0.286 at NTU. The estimated distribution parameters were μ = −0.023 [95% CI: −0.032, −0.015] and σ = 0.511 [95% CI: 0.505, 0.516] for NCKU, and μ = 0.007 [95% CI: 0.003, 0.012] and σ = 0.286 [95% CI: 0.283, 0.289] for NTU. In the competition condition, the mean beta value was 0.011 ± 0.473 at NCKU and 0.001 ± 0.265 at NTU, with estimated parameters of μ = 0.011 [95% CI: 0.003, 0.019] and σ = 0.473 [95% CI: 0.467, 0.478] at NCKU, and μ = 0.001 [95% CI: −0.003, 0.005] and σ = 0.265 [95% CI: 0.262, 0.268] at NTU. When data from both conditions were combined, the mean beta value was −0.006 ± 0.492 at NCKU and 0.004 ± 0.276 at NTU, with distribution parameters of μ = −0.006 [95% CI: −0.012, −0.001] and σ = 0.492 [95% CI: 0.488, 0.496] for NCKU, and μ = 0.004 [95% CI: 0.001, 0.007] and σ = 0.276 [95% CI: 0.273, 0.278] for NTU. Overall, the similarity in the mean values, standard deviations, and 95% confidence intervals across both sites demonstrates a high level of data consistency between the two MRI scanner platforms. These findings support the reliability and homogeneity of the data, validating their suitability for pooled analysis across acquisition sites.
Acknowledgements
This work was supported by funding provided by Ministry of Science and Technology, Taiwan, to MHW (MOST-107-2420-H-006-007-/ MOST-108-2420-H-006-001-/ MOST-109-2420-H-006-002). We thank the NCKU Mind Research and Imaging Center (MRIC) at NCKU, and the imaging center for integrated Body, Mind, and Culture research at the NTU, for the generous support and equipment availability for this collaboration. Special thanks go to all the hyperscanning team for carrying out the experiment, as well as the preprocessing of fMRI data.
Author contributions
S.S.S. prepared and uploaded the data in B.I.D.S. format. C.C.K., D.Y.C. and M.H.W. planned the research. D.Y.C., J.T.C., and I.J.H. performed the experiments. L.S.W. and C.C.K. analyzed the data. L.S.W. and C.C.K. wrote the paper.
Code availability
Coherence analysis in our previous work16 related to this dataset is available in OSF at (https://osf.io/f75cp/)24.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Der-Yow Chen, Ming-Hung Weng, Chun-Chia Kung.
Contributor Information
Der-Yow Chen, Email: chendy@ncku.edu.tw.
Ming-Hung Weng, Email: mhweng@ncku.edu.tw.
Chun-Chia Kung, Email: cckung@kunglab-nckupsy.org.
References
- 1.Kelsen, B. A., Sumich, A., Kasabov, N., Liang, S. H. & Wang, G. Y. What has social neuroscience learned from hyperscanning studies of spoken communication? A systematic review. Neuroscience & Biobehavioral Reviews132, 1249–1262, 10.1016/j.neubiorev.2020.09.008 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Redcay, E. & Schilbach, L. Using second-person neuroscience to elucidate the mechanisms of social interaction. Nature Reviews Neuroscience20, 495–505, 10.1038/s41583-019-0179-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czeszumski, A. et al. Hyperscanning: a valid method to study neural inter-brain underpinnings of social interaction. Frontiers in Human Neuroscience14, 39, 10.3389/fnhum.2020.00039 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carollo, A. & Esposito, G. Hyperscanning literature after two decades of neuroscientific research: A scientometric review. Neuroscience551, 345–354, 10.1016/j.neuroscience.2024.05.045 (2024). [DOI] [PubMed] [Google Scholar]
- 5.Lu, W., Sun, Y., Gao, H. & Qiu, J. A review of multi-modal magnetic resonance imaging studies on perimenopausal brain: a hint towards neural heterogeneity. European Radiology33, 5282–5297, 10.1007/s00330-023-09549-5 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Montague, P. R. et al. Hyperscanning: Simultaneous fMRI during linked social interactions. NeuroImage16, 1159–1164, 10.1006/nimg.2002.1150 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Speer, S. P. & Boksem, M. A. Decoding fairness motivations from multivariate brain activity patterns. Social Cognitive and Affective Neuroscience14, 1197–1207, 10.1093/scan/nsz097 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang, L. S. et al. When “more for others, less for self” leads to co‐benefits: A tri‐MRI dyad‐hyperscanning study. Psychophysiology61, e14560, 10.1111/psyp.14560 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Hernandez‐Pena, L. et al. Neural correlates of static and dynamic social decision‐making in real‐time sibling interactions. Human Brain Mapping45, e26788, 10.1002/hbm.26788 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novembre, G. & Iannetti, G. D. Hyperscanning alone cannot prove causality. Multibrain stimulation can. Trends in Cognitive Sciences25, 96–99, 10.1016/j.tics.2020.11.003 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astolfi, L. et al. Raising the bar: Can dual scanning improve our understanding of joint action? NeuroImage216, 116813, 10.1016/j.neuroimage.2020.116813 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Misaki, M. et al. Beyond synchrony: the capacity of fMRI hyperscanning for the study of human social interaction. Social Cognitive and Affective Neuroscience16, 84–92, 10.1093/scan/nsaa143 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton, A. Fd. C. Hyperscanning: beyond the hype. Neuron109, 404–407, 10.1016/j.neuron.2020.11.008 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Tsoi, L., Burns, S. M., Falk, E. B. & Tamir, D. I. The promises and pitfalls of functional magnetic resonance imaging hyperscanning for social interaction research. Social and Personality Psychology Compass16, e12707, 10.1111/spc3.12707 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King-Casas, B. et al. Getting to know you: reputation and trust in a two-person economic exchange. Science308, 78–83, 10.1126/science.1108062 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Wang, L.-S. et al. Distinct cerebral coherence in task-based fMRI hyperscanning: cooperation versus competition. Cerebral Cortex33, 421–433, 10.1093/cercor/bhac075 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Spence, M. Job market signaling. The Quarterly Journal of Economics87, 355–374, 10.2307/1882010 (1973). [Google Scholar]
- 18.Crawford, V. P. & Sobel, J. Strategic information transmission. Econometrica: Journal of the Econometric Society, 1431–1451, 10.2307/1913390 (1982).
- 19.Shen, S.-S. et al. Collaborations and deceptions in strategic interactions revealed by hyperscanning fMRI. OpenNeuro [Dataset]. 10.18112/openneuro.ds004103.v1.0.4 (2025).
- 20.Goebel, R. BrainVoyager—past, present, future. NeuroImage62, 748–756, 10.1016/j.neuroimage.2012.01.083 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Koster-Hale, J. & Saxe, R. Theory of mind: A neural prediction problem. Neuron79, 836–848, 10.1016/j.neuron.2013.08.020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter, R. M. & Huettel, S. A. A nexus model of the temporal–parietal junction. Trends in Cognitive Sciences17, 328–336, 10.1016/j.tics.2013.05.007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igelström, K. M. & Graziano, M. S. The inferior parietal lobule and temporoparietal junction: a network perspective. Neuropsychologia105, 70–83, 10.1016/j.neuropsychologia.2017.01.001 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Wang, L.-S., Kung, C.-C., Weng, M.-H. & Chen, D.-Y. Distinct Cerebral Coherence in Task-Based fMRI Hyperscanning: Cooperation vs. Competition. OSF. November 16. 10.17605/OSF.IO/F75CP (2021). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Coherence analysis in our previous work16 related to this dataset is available in OSF at (https://osf.io/f75cp/)24.