Abstract
Background
Circulating miRNAs (cmiRNAs) are emerging as valuable non-invasive biomarkers for monitoring disease progression and therapeutic response in cancer. Their stability in biological fluids, tissue-specific expression, and functional roles in tumor biology make them particularly suitable for liquid biopsy approaches. However, challenges related to quantification accuracy and assay standardization have limited their clinical translation. Digital PCR (dPCR) offers a highly sensitive and reproducible solution for absolute quantification of low-abundance transcripts, addressing many of these limitations.
Methods
We developed and analytically validated the first duplex dPCR assay for the simultaneous detection of miR-4488 and miR-579-3p in serum samples from patients with BRAF-mutant metastatic melanoma receiving MAPK inhibitor therapy. These two cmiRNAs were previously identified by our group as biomarkers predictive of treatment response. Using fluorescently labelled probes, both targets were co-amplified in a single reaction. The assay was tested for analytical performance, including comparison with singleplex formats and quantitative Real-Time PCR (qRT-PCR). We then applied the duplex assay to assess the prognostic potential of the expression ratio between the two miRNAs, termed miRatio, at baseline and over treatment timepoints.
Results
The duplex assay maintained analytical performance comparable to singleplex reactions while reducing sample and reagent use. Compared to qRT-PCR, dPCR showed superior sensitivity, particularly for detecting low-abundance miRNAs like miR-4488. miRatio effectively predicts disease outcome when measured at baseline prior to MAPKi therapy and exhibits dynamic changes during treatment, supporting its potential as a longitudinal biomarker. ROC analysis demonstrated strong prognostic value, with improved accuracy over previous qRT-PCR-based evaluations.
Conclusions
This study highlights duplex dPCR as a robust, sensitive, and scalable technology for circulating miRNA quantification in liquid biopsy applications. By enabling absolute and simultaneous detection of miR-4488 and miR-579-3p, the assay provides a technically advanced platform for real-time monitoring in metastatic melanoma. While miRatio remains a promising biomarker, the key innovation of this work is the development of a duplex assay suitable for clinical implementation in precision oncology.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-025-06941-1.
Keywords: Digital PCR, Duplex assay, Liquid biopsy, Circulating MiRNAs, Metastatic melanoma
Introduction
In recent years, liquid biopsy has gained increasing relevance in oncology as a minimally invasive approach for the molecular profiling and longitudinal monitoring of tumors [1]. By analyzing tumor-derived components released into biological fluids such as blood, serum, plasma, urine, or cerebrospinal fluid, liquid biopsy provides real-time insights into tumor dynamics while overcoming key limitations of traditional tissue biopsies, including invasiveness, sampling bias, and poor representation of spatial and temporal heterogeneity [2, 3]. Several classes of circulating biomarkers have been explored in this context, including circulating tumor cells (CTCs) [4], circulating tumor DNA (ctDNA) [5], extracellular vesicles (EVs) [6], and tumor-educated platelets (TEPs) [7]. Each of these analytes captures distinct aspects of tumor biology, disease progression, and immune modulation. Among them, circulating miRNAs (cmiRNAs) have emerged as particularly attractive biomarkers due to their remarkable stability in biofluids, their active secretion by tumor cells, and their involvement in critical cancer-related processes such as proliferation, immune escape, and therapeutic resistance [8–10]. However, despite growing evidence supporting their clinical utility, the implementation of cmiRNAs in routine practice is still hindered by technical challenges, including variability in RNA extraction efficiency, low abundance in serum or plasma, and the lack of standardized normalization strategies in qRT-PCR-based workflows [11]. Digital PCR (dPCR) has recently emerged as a powerful alternative for miRNA quantification in liquid biopsy settings. By partitioning the sample into thousands of nanoliter-scale reactions and enabling end-point detection, dPCR allows absolute quantification of nucleic acids without the need for standard curves or reference genes. This approach significantly improves analytical sensitivity, precision, and reproducibility, especially in low-input and inhibitor-rich samples such as serum [12, 13]. Moreover, dPCR supports multiplexing, enabling the simultaneous detection of multiple targets in a single reaction, an essential feature for the efficient evaluation of complex molecular signatures in limited clinical specimens [14]. Metastatic melanoma remains one of the most aggressive and treatment-resistant malignancies, with poor survival outcomes once distant dissemination occurs [15]. Despite major advances in targeted therapies (BRAF and MEK inhibitors) and immune checkpoint inhibitors (ICIs), most patients eventually experience disease progression due to intrinsic or acquired resistance mechanisms [16, 17]. There is thus a critical need for robust, non-invasive biomarkers capable of predicting therapeutic response and guiding treatment decisions in real time. Our group has previously identified two cmiRNAs, miR-4488, an oncogenic miRNA, and miR-579-3p, a tumor-suppressive miRNA, that are differentially expressed in relation to response to BRAF/MEK inhibitors in metastatic melanoma patients. Importantly, we demonstrated that the expression ratio between these two miRNAs, termed miRatio, provides superior predictive accuracy compared to the individual miRNAs alone, likely reflecting the dynamic balance between oncogenic and tumor-suppressive regulatory networks [18].
In the present study, we report the development and technical validation of the first duplex dPCR assay specifically designed for the simultaneous quantification of two cancer-related cmiRNAs in serum. This duplex assay allows absolute and parallel measurement of miR-4488 and miR-579-3p in a single reaction, providing a precise and reproducible readout of the miRatio biomarkers. To our knowledge, this is the first application of a duplex dPCR strategy to the analysis of a composite miRNA signature in oncology, representing a methodological advancement with potential for clinical translation. Our aim is to evaluate the utility of this assay as a prognostic tool for early detection of therapeutic resistance in metastatic melanoma, with broader implications for personalized medicine in solid tumors.
Material and methods
Experimental design
This retrospective study involved a cohort of 60 patients (30 males and 30 females) diagnosed with BRAF-mutated metastatic melanoma. The general characteristics of the 60 BRAF-mutated melanoma patients are summarized in Table 1. All patients received the first line targeted therapy with MAPK pathway inhibitors between April 2013 and February 2020. Treatment regimens included either BRAF inhibitors (vemurafenib or dabrafenib) as monotherapy or in combination with MEK inhibitors (cobimetinib or trametinib), according to current clinical guidelines and physician discretion. Peripheral blood samples were collected from each patient either prior to and/or during the initiation of MAPK-targeted therapy. Approximately 1 mL of serum was isolated and stored under controlled conditions in the institutional biobanks of the National Cancer Institute IRCCS "G. Pascale Foundation" (Naples, Italy) and the IRCCS Regina Elena National Cancer Institute (Rome, Italy), in order to preserve sample integrity. The detailed clinical information on patients’ cohorts are available in our previous work [18]. Additionally, serum samples from 12 healthy donors (average age 54 years, range 49–59; 6 males and 6 females), stored under controlled conditions in the institutional biobanks of the IRCCS Regina Elena National Cancer Institute (Rome, Italy), were included in the study. These samples were used both to optimize experimental conditions, minimizing the use of valuable patient-derived material, and to enable comparative analysis between healthy donors and melanoma patients. The human melanoma cell line A375, kindly provided by Dr. Paolo A. Ascierto from the National Cancer Institute of Naples, “Fondazione G. Pascale” (Naples, Italy), harbors the BRAF-V600E mutation and was originally derived from a metastatic lesion in a 54-year-old female patient diagnosed with malignant melanoma. Cells were cultured in RPMI medium supplemented with 10% fetal bovine serum (FBS).
Table 1.
The table summarizes general characteristics of the 60 BRAF-mutated melanoma patients
| Characteristic | N | (%) | |
|---|---|---|---|
| Patients | 60 | 100% | |
| BRAF variant | V600E | 58 | 96,70% |
| V600K | 2 | 3,30% | |
| Gender | Male | 30 | 50% |
| Female | 30 | 50% | |
| Stage | IV | 55 | 91,70% |
| IIIB | 3 | 5% | |
| IIIC | 2 | 3,30% | |
| Age | Mean (min–max) | 51,4 (20–78) | – |
Based on our previous study [18] and according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1), patients were stratified into two groups based on clinical outcome. The first group, referred to as Disease Control (DC), included patients who achieved complete response (CR), partial response (PR), or stable disease (SD) as their best objective response. The second group comprised patients who developed Progressive Disease (PD) within six months from the start of MAPK-targeted therapy. For each patient, approximately three serum samples were collected at different treatment time points:
Baseline (T0): Corresponding to the pre-treatment time point, samples were available for 48 patients (15 PD and 33 DC).
Time Point 1 (T1): Representing the first available sample after the initiation of therapy, this time point was available for 50 patients (16 PD and 34 DC). The average collection time was 1.5 months after T0 (standard deviation: 1.08 months).
Progression/Response (T2): Samples at this time point were available for 40 patients (18 PD and 22 DC). For PD, T2 corresponded to disease progression (defined as a sample collected immediately before or within one month of documented progression). For DC, T2 corresponded to a period of documented response, the average time from T0 to T2 was 7.0
Due to constraints in sample availability, not all patients contributed samples at every time point.
RNA extraction, reverse transcription and evaluation of cmiRNAs by real-time PCR
Total RNA, including miRNAs, was extracted from 200 μL of serum using the miRNeasy Mini Kit (Qiagen, 217204), according to the manufacturer’s instructions. Due to the typically low RNA concentrations in serum, often below the detection thresholds of both the NanoDrop spectrophotometer and the Qubit fluorometer, a fixed input volume of 2 μL of total RNA was used for each reverse transcription reaction, as recommended by the manufacturer. For cellular samples, total RNA was extracted using the phenol–chloroform method (ThermoFisher Scientific), following the manufacturer’s protocol and as previously reported [19]. RNA concentration was quantified using a NanoDrop One C spectrophotometer (ThermoFisher Scientific). For each reverse transcription reaction, 10 ng of total RNA was used as input. All RNA samples were eluted in 20 μL of nuclease-free water, aliquoted, and stored at − 80 °C until further use.
Reverse transcription was performed using the TaqMan™ Advanced miRNA cDNA Synthesis Kit (ThermoFisher Scientific, A28007), which enables universal reverse transcription of all miRNAs and includes a preamplification step designed to enrich target sequences prior to detection. This protocol ensures both optimal reverse transcription efficiency and consistent cDNA quality for downstream applications. The expression levels of cmiRNAs were analyzed using TaqMan MicroRNA Assay probes and quantified by qRT-PCR on a QuantStudio 6 Flex Real-Time PCR System (Thermo Fisher Scientific). Each reaction was performed in a final volume of 10 μL, containing 5 μL of 2 × TaqMan Fast Advanced Master Mix (ThermoFisher Scientific, 4444557), 0.5 μL of 20 × TaqMan® MicroRNA Assay probe (Thermo Fisher Scientific), 1 μL of cDNA template, and 3.5 μL of nuclease-free water. A total of 9 μL from each prepared reaction mix was loaded into the wells. The thermal cycling conditions were as follows: initial enzyme activation at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 10 s and annealing/extension at 60 °C for 20 s. The relative expression levels were calculated and reported as 2−Ct values.
Digital PCR Analyses
Samples were analyzed using the QuantStudio™ Absolute Q Digital PCR chip/plate-based system (ThermoFisher Scientific). Reactions were prepared in a final volume of 10 μL, comprising 2 μL of 5 × Master Mix (ThermoFisher Scientific, A52490), 0.5 μL of 20 × TaqMan® probe (ThermoFisher Scientific), 1 μL of cDNA template, and 6.5 μL of nuclease-free water. A volume of 9 μL from each reaction mix was loaded onto MAP16 plates (ThermoFisher Scientific, A52865), followed by the addition of 15 μL of Isolation Buffer (ThermoFisher Scientific, A52730) into each well containing the PCR mixture. For the duplex assay, a commercial probe specific for miR-4488 labeled with FAM (ThermoFisher Scientific, A25576) and a custom probe specific for miR-579-3p labeled with VIC (ThermoFisher Scientific, CCU001S) were used. All dPCR steps, including sample partitioning, thermal cycling, and data acquisition, were performed on the Applied Biosystems QuantStudio Absolute Q Digital PCR System. Thermal cycling conditions were as follows: a pre-heating step at 96.0 °C for 10 min, followed by 40 cycles of denaturation at 96 °C for 5 s and annealing/extension at 60 °C for 15 s. Fluorescence threshold values for FAM and VIC channels were automatically determined using ThermoFisher's analysis software, manually reviewed, and then applied to the corresponding cDNA samples. dPCR operates by partitioning each PCR reaction into thousands of individual reactions, where the target is randomly distributed across all partitions. After amplification, the presence of the target is detected by fluorescence in positive partitions. Thanks to partitioning, dPCR is less affected by PCR inhibitors, as they are diluted across thousands of individual micro-reactions, minimizing their impact on each amplification event. This allows for accurate quantification even in complex matrices such as serum.
Statistical analysis
Receiver Operating Characteristic (ROC) curve analysis was performed to evaluate the discriminatory power of the miRatio, defined as the ratio between miR-4488 and miR-579-3p, in distinguishing between DC and PD, as well as between healthy donors and melanoma patients. The optimal cut-off value for sensitivity and specificity was determined based on the highest Youden’s Index. Statistical analyses were performed using the Mann–Whitney U test for comparisons between independent groups (e.g., T0: DC vs PD), and the Wilcoxon matched-pairs signed-rank test for comparisons between paired measures obtained by different methodological approaches(e.g., singleplex vs duplex). Progression-free survival (PFS) curves were generated using the Kaplan–Meier method and potential differences between subgroups were evaluated using the log-rank test. For these analyses, patient groups were stratified according to miRatio categories, defined by the optimal cut-off derived from the ROC curve. Univariate and multivariate Cox proportional hazards regression models were conducted to estimate crude and adjusted hazard ratios (HR) along with their relative 95% confidence intervals (95% CIs). Correlation between the two formats was assessed using the Pearson’s correlation coefficient. A p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using GraphPad Prism software, version 8.0.2.
Results
Comparing the sensitivity of dPCR vs qRT-PCR in the detection of miR-4488 and miR-579-3p in serum samples from healthy donors and melanoma patients
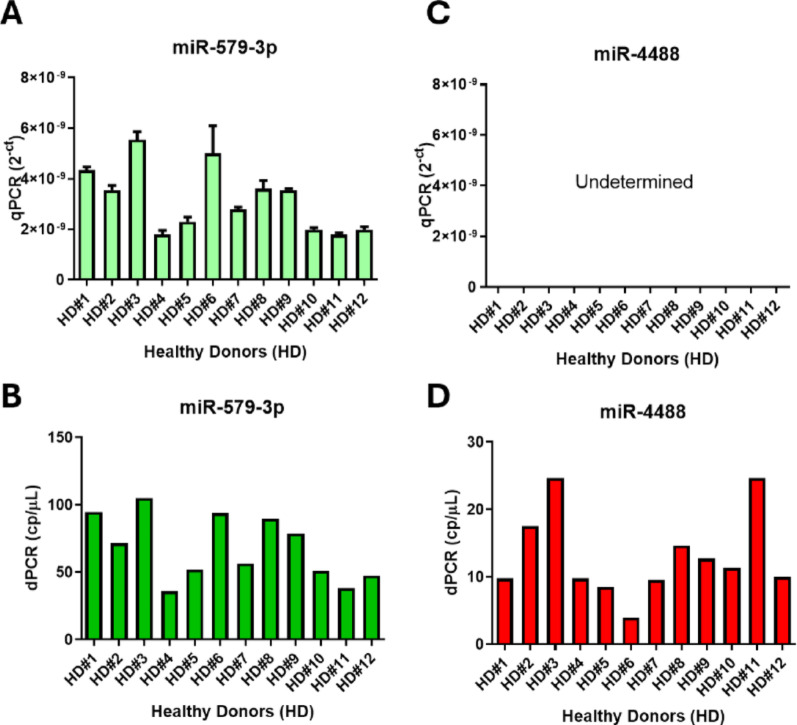
Given the intrinsic complexity of serum as a biological matrix, because of low RNA content and presence of potential PCR inhibitors, we first evaluated the performance of qRT-PCR, the current gold standard in liquid biopsy-based diagnostics, in comparison to dPCR for the detection of miR-4488 and miR-579-3p. To this end, we analyzed serum samples from healthy donors (n = 12) using both methodologies. Both techniques successfully detected miR-579-3p, with comparable relative expression levels (Fig. 1 left, panels A-B). In contrast, miR-4488 was consistently undetectable by qRT-PCR, whereas dPCR reliably quantified its expression across all samples (Fig. 1 right, panels C–D).
Fig. 1.
Comparison of circulating miR-4488 and miR-579-3p levels in healthy donors’ serum using qRT-PCR and dPCR. Quantification of miR-579-3p (panels A and B) and miR-4488 (panels C and D) in serum samples from healthy donors was performed using qRT-PCR (A, C) and dPCR (B, D). qRT-PCR results are shown as relative expression values (2−Ct), while dPCR provides absolute quantification, expressed as copies per microliter
This discrepancy is in line with the low basal expression of miR-4488 in healthy individuals, as it is recognized as an oncogenic miRNA (oncomiR) upregulated only in malignant conditions [18, 19]. These findings highlight the superior analytical sensitivity of dPCR, particularly for low-abundance circulating targets. Quantitative profiling confirmed that miR-579-3p was more abundant than miR-4488 across both detection platforms. Specifically, dPCR measured average concentrations of 70.18 copies/µL for miR-579-3p and 12.51 copies/µL for miR-4488. While qRT-PCR successfully detected miR-579-3p in all samples, miR-4488 levels remained below its detection threshold. This further underscores the advantage of dPCR in capturing low-copy-number miRNAs in complex biological matrices such as serum.
To determine whether the limited detection observed in serum was due to technical issues or to the biological nature of the sample, we analyzed RNA extracted from a melanoma cell line previously used in our studies [19, 20] as a positive control. In this context, both qRT-PCR and dPCR successfully detected and quantified miR-4488 and miR-579-3p, showing consistent and linear results across serial dilutions (Supplementary Fig. 1). These findings confirm that both assays perform reliably under optimal RNA conditions.
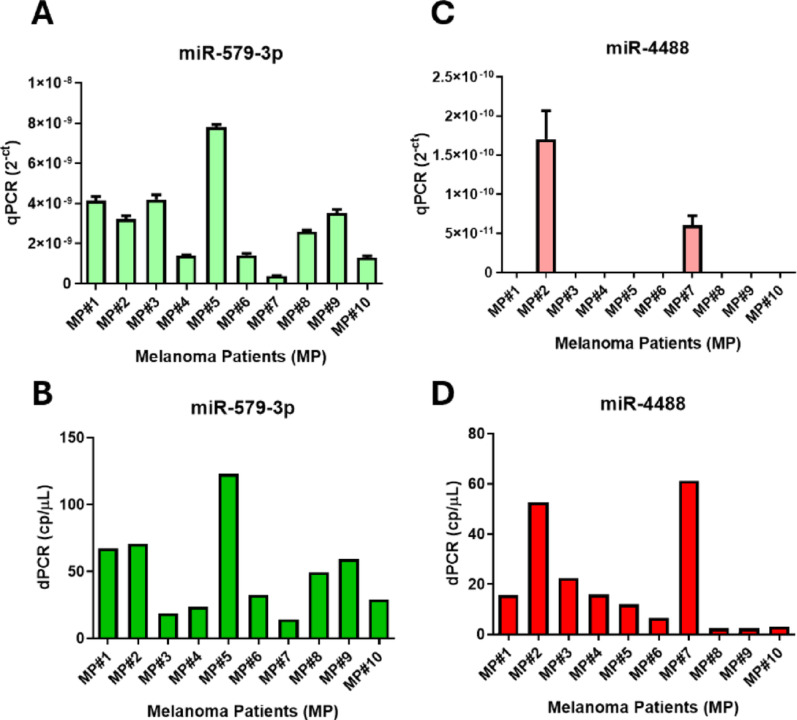
After testing the assay in healthy donors, we next evaluated its performance in a pathological context by analyzing baseline serum samples from metastatic melanoma patients (n = 10). As observed in healthy samples, miR-579-3p was robustly detected in all patients by both qRT-PCR and dPCR (Fig. 2 left, panels A-B). In contrast, miR-4488 was detected by qRT-PCR in only two cases, while dPCR successfully quantified it in all samples (Fig. 2 right, panels C-D). This is in line with our previous findings, as most patients in this cohort belong to DC group, typically showing low baseline levels of miR-4488 and higher levels of miR-579-3p [18]. This setup revealed that qRT-PCR could detect miR-4488 only when expression exceeded ~ 40 copies/µL by dPCR, whereas dPCR reliably quantified the target even below this threshold, confirming its superior sensitivity and ability to detect low-abundance transcripts at levels of just a few copies per microliter. Overall, these results support the preferred use of dPCR for its greater sensitivity in the detection of cmiRNAs.
Fig. 2.
Comparison of circulating miR-4488 and miR-579-3p levels in melanoma patients’ serum using qRT-PCR and dPCR. Quantification of miR-579-3p (panels A and B) and miR-4488 (panels C and D) in serum samples from melanoma patients using qRT-PCR (A, C) and dPCR (B, D). qRT-PCR results are expressed as relative levels (2−Ct), while dPCR provides absolute quantification in copies per microliter
Development and validation of a dPCR Assay for the simultaneous detection of miR-4488 and miR-579-3p and quantification of their expression ratio (miRatio)
Given the superior sensitivity of dPCR in detecting low-abundance cmiRNAs, we selected this technological solution for further studies. An additional advantage of dPCR is the ability to develop multiplex assays, allowing the simultaneous quantification of more than one miRNA at a time in a single reaction tube, an important feature when working with limited patient-derived material. To enable this, we used differently labeled probes: a FAM-labeled probe for miR-4488 and a VIC-labeled probe for miR-579-3p, as detailed in the Materials and Methods section.
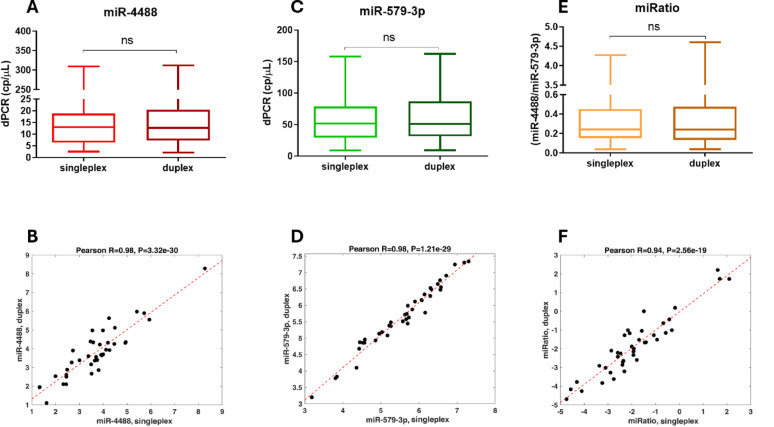
To proceed to the initial set up the duplex assay, we analyzed 40 serum samples, first quantifying each miRNA individually (singleplex), then together in the multiplex format (duplex). Quantification was highly consistent across methods, with Pearson’s correlation coefficient equal to r = 0.98 for both miRNAs, and no significant differences between singleplex and duplex results (Fig. 3 left, panels A–B, C–D). We then calculated the ratio between miR-4488 and miR-579-3p (miRatio), previously identified as a more robust prognostic biomarker than each individual miRNA [18]. miRatio values from the duplex format strongly correlated with singleplex-derived values (r = 0.94; Fig. 3 right, panels E–F), confirming the reliability of the assay.
Fig. 3.
Comparison of singleplex and duplex dPCR assays for circulating miR-4488 and miR-579-3p quantification. Expression levels of miR-4488 (A) and miR-579-3p (C) measured by singleplex and duplex dPCR across 40 serum samples. Correlation between the two formats is shown for miR-4488 (B) and miR-579-3p (D) using Pearson’s correlation coefficient. Panel E compares miRatio (miR-4488/miR-579-3p) values from both approaches, with corresponding correlation in (F). All expression levels are reported as copies per microliter. Statistical comparisons were performed using the Wilcoxon matched-pairs signed-rank test; ns = not significant
Based on these findings, we selected the duplex assay for all subsequent analyses because it offers analytical precision, reduced sample and reagent requirements, and lower technical variability. Moreover, focusing on miRatio aligns with our data and prior evidence suggesting that combined biomarker signatures outperform single-analyte approaches in predicting therapy response [18].
Once the technical validation of the duplex assay was established, we decided to assess its prognostic and predictive potential in the clinical setting. This will be the focus of the next section.
Assessing the prognostic role of miR-4488/miR-579-3p ratio in melanoma patients by multiplex dPCR
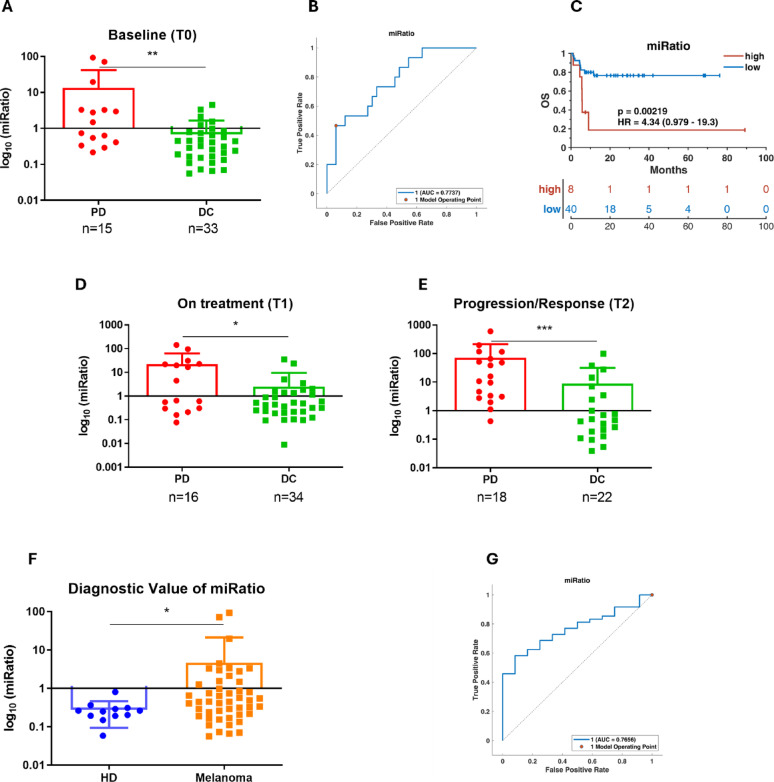
To investigate the prognostic value of the miRatio as measured by duplex dPCR in predicting treatment response in metastatic melanoma, we analyzed a cohort of 60 patients harboring BRAF mutations, all treated with BRAF and/or MEK inhibitors. Serum samples were collected at three key time points: 1) prior to therapy initiation (T0, baseline), 2) approximately 1,5 months after starting therapy (T1, on treatment), and 3) at either the time of disease progression or during a sustained clinical response (T2, relapse/response). In particular for PD, T2 was defined as the time of disease progression, for DC, T2 corresponded to a period of sustained clinical benefit, occurring on average 7.0 months after T0. Due to constraints in sample availability, not all patients contributed samples at every time point; a detailed breakdown is provided in the Materials and Methods section. At baseline, we analysed serum from 48 patients (15 PD and 33 DC) to assess whether the miRatio, quantified by dPCR, could discriminate between groups with different clinical outcomes. As expected from our previous work [18], miRatio levels were significantly higher in PD (median 13.364) than in DC (median 0.682), suggesting a strong association between high miRatio values and poor prognosis (Fig. 4, panel A). ROC curve analysis confirmed the predictive power of the miRatio, with an area under the curve (AUC) of 0.777, supporting its potential utility as a biomarker (Fig. 4, panel B). Most importantly, this AUC value exceeded what obtained in our previous qRT-PCR-based study (AUC 0.702), highlighting the improved overall accuracy and specificity achieved with dPCR in quantifying low-abundance circulating transcripts. Kaplan–Meier analysis further supported the prognostic relevance of the miRatio, revealing a significant difference in progression-free survival (p = 0.00219) and a hazard ratio (HR) of 4.34 (95% CI: 0.979–19.3) (Fig. 4, panel C). Building on these encouraging results, we extended the analysis to include longitudinal samples representing, to our knowledge, the first investigation of miRatio dynamics by dPCR during the course of targeted therapy. At T1, we evaluated serum from 50 patients (16 PD and 34 DC) to determine whether the prognostic signal of the miRatio persisted after therapy initiation. Consistently, mean miRatio values remained significantly elevated in PD (22.211) compared to DC (2.470), indicating that miRatio levels may reflect persistent oncogenic signaling and early signs of therapeutic failure (Fig. 4, panel D).
Fig. 4.
Prognostic and diagnostic value of miRatio in melanoma patients. Panel A shows baseline serum miRatio levels in DC and PD to targeted therapy, with significantly higher values observed in PD. Panel B presents the ROC curve evaluating the predictive performance of baseline miRatio in classifying treatment response. Panel C displays Kaplan–Meier survival curves stratifying patients by high or low baseline miRatio, demonstrating its prognostic relevance. KM curve generated using the 2.79 threshold calculated by ROC curve in panel (B). Panels D and E show longitudinal changes in miRatio levels in melanoma patients: panel D displays values at the first on-treatment timepoint (T1), while panel (E) reports levels at later timepoints corresponding to disease progression or sustained response (T2). Panel F compares miRatio values between healthy donors and melanoma patients, revealing significant upregulation in the latter group. Panel G presents the ROC curve assessing the diagnostic accuracy of miRatio in distinguishing melanoma patients from healthy controls. Statistical significance was evaluated using the Mann–Whitney U test. *p < 0.05; **p < 0.01; ***p < 0.001
To further explore the association between miRatio and disease trajectory, we analyzed samples collected at progression in PD (n = 18) and during prolonged response in DC (n = 22). Results revealed a sharp increase in miRatio at the time of progression (mean 71.302), while levels remained stably low in DC (mean 8.904) (Fig. 4, panels E). These findings support the utility of miRatio as a dynamic biomarker capable of capturing real-time changes in tumor behavior and onset of resistance. dPCR plots from representative DC and PD patients are shown in Supplementary Fig. 2, illustrating the distinct expression profiles of miR-4488 and miR-579-3p and supporting the utility of miRatio as a predictive biomarker.
Finally, we assessed the diagnostic value per se of the miRatio by comparing baseline serum samples from BRAF mutated melanoma patients and healthy donors. Melanoma patients exhibited significantly higher miRatio values (mean 4.645) compared to controls (mean 0.213), and ROC analysis yielded an AUC of 0.765 (Fig. 4, panels F-G). This suggests that the miRatio may also serve as a diagnostic indicator, with potential applications in early disease detection or risk stratification.
Discussion
This study describes the development and initial clinical validation of the first duplex assay based on dPCR for the simultaneous quantification of miR-4488 and miR-579-3p in serum samples from patients with BRAF mutated metastatic melanoma. dPCR enables absolute quantification with superior sensitivity and reproducibility compared to conventional qRT-PCR, making it particularly well-suited for liquid biopsy applications, where circulating biomarkers are typically found at low concentrations and sample volume is limited. By enabling the concurrent detection of two cmiRNAs within a single reaction, this duplex assay not only optimizes biospecimen usage but also offers a broader molecular insight into disease progression and therapeutic response.
This work builds upon our previous findings, where miR-4488 and miR-579-3p were identified as circulating biomarkers associated with response to BRAF/MEK-targeted therapy. That study introduced the concept of miRatio, the expression ratio between miR-4488 and miR-579-3p, which outperformed individual miRNAs in predicting PFS [18]. Importantly, we also found that miRatio was a superior predictor of PFS compared to serum lactate dehydrogenase (LDH) levels in melanoma patients. This approach leverages upon the biological complementarity of the two molecules: miR-4488 has been associated with tumor aggressiveness and poor prognosis [19], while miR-579-3p exhibits tumor-suppressive activity through the inhibition of oncogenic pathways such as BRAF and MDM2 [20]. Their integration into a single metric improves both biological interpretation and clinical applicability, offering a dynamic representation of the balance between oncogenic and oncosuppressive signaling.
In the present study, we re-evaluated miRatio using dPCR to take advantage of its enhanced analytical accuracy. This not only confirmed its prognostic relevance in a more robust technological setting and enabled, for the first time, a longitudinal assessment of circulating levels across multiple treatment timepoints, but also revealed improved predictive performance, with ROC analysis yielding an AUC of 0.777, surpassing the 0.702 obtained in our previous qRT-PCR-based study, thus highlighting the superior sensitivity and specificity of dPCR in detecting low-abundance circulating transcripts. The AUC of 0.77 could be further improved by integrating miRatio with additional clinical or molecular parameters, an approach that could be evaluated in future prospective clinical trials. Of note, the improvement of AUC measuring miRatio over time revealed dynamic fluctuations that suggest its potential role as a real-time indicator of disease trajectory and therapeutic efficacy, rather than a purely static biomarker. It should be noted that, in the present study, miRatio was measured at the first available time point after therapy initiation, which occurred, on average, 1.5 months after treatment started. However, it remains possible that miRatio could detect early signs of therapeutic failure within just a few days or weeks from treatment initiation. This hypothesis warrants evaluation in future prospective studies.
The duplex nature of the assay represents a notable technical advancement. By simultaneously quantifying two miRNAs in a single well, this platform reduces sample consumption and processing time, minimizes variability, and increases assay throughput, all features that facilitate clinical implementation. To the best of our knowledge, this is the first dPCR-based duplex assay with demonstrated prognostic value in an oncological setting. Although previous studies have explored multiplexed dPCR approaches, such as in cellular RNA from blood mononuclear cells, our work uniquely targets cmiRNAs in serum, offering a minimally invasive and clinically applicable tool for patient monitoring [14].
These findings also support the growing interest in composite biomarkers. Given the biological complexity of cancer, single miRNA markers often lack sufficient predictive accuracy. Several studies have proposed individual miRNAs as potential biomarkers in melanoma. For instance, miR-21 has been suggested as a diagnostic marker, being consistently overexpressed in melanoma tissues and detectable in the bloodstream, where it correlates with tumor aggressiveness [21]. As a prognostic marker, miR-182 has been linked to increased metastatic potential and poor survival outcomes [22]. Regarding therapeutic response, miR-34a has emerged as a promising candidate, with elevated levels associated with drug resistance and immune evasion mechanisms, suggesting its role in predicting treatment efficacy [23].
However, the performance of individual markers is frequently inconsistent across studies and patient cohorts. In this context, ratio-based or multi-marker models have gained traction for their ability to integrate complex molecular signals. Notable examples include the MELmiR-7 panel, a seven-miRNA signature associated with disease stage and survival [24], and the MEL38 signature, a 38-miRNA panel validated for diagnostic and prognostic purposes across melanoma subtypes [25]. Other signatures such as MEL12, integrating 12 miRNAs, have been linked to patient outcome and clinical progression [26].
Moreover, ratio-based models have shown particular promise. Notably, the miR-1246/miR-485-3p ratio proposed by Levati et al. demonstrated strong prognostic value in plasma samples from metastatic melanoma patients treated with BRAF/MEK inhibitors [27]. Similarly, our results reinforce the utility of composite metrics, demonstrating that dynamic biomarkers like miRatio can better capture the interplay between tumor progression and therapeutic response than single miRNA measurements. Notably, the miRNA signatures reported in these studies were identified using qRT-PCR [24, 27] or Nanostring-based platforms [25, 26], which differ from our approach based on duplex dPCR. The technical advantages of dPCR in miRNA quantification have been described earlier in the manuscript, underscoring its relevance and superior performance in the context of liquid biopsy applications.
The potential of miRNA profiles in cancer management extends beyond melanoma, with numerous clinical trials exploring miRNAs as minimally invasive biomarkers for early detection, diagnosis, and therapy response across various malignancies. Ongoing studies are assessing the diagnostic value of circulating or tissue miRNA signatures in cancers such as lymphoma, leukemia, lung, and thyroid cancer (ClinicalTrials.gov). Additionally, innovative approaches aim to apply miRNA classifiers for cancer screening through non-invasive samples such as stool, e.g., in an ongoing trial investigating novel miRNA markers for colorectal cancer detection (NCT05346757), or to determine the tumor origin in cancers of unknown primary (CUP), as exemplified by the miRview® mets assay [28]. For example, miRNA panels are being explored for detecting prostate cancer and predicting therapeutic responses to androgen deprivation or chemotherapy (NCT04662996, NCT02366494) (ClinicalTrials.gov). Other trials are investigating exosomal miRNA panels to predict resistance or sensitivity to standard regimens in breast, colon, and non-small-cell lung cancers (NCT02466113). Another example is the 24-microRNA signature classifier (MSC), which has shown strong potential as a non-invasive tool to enhance lung cancer screening. Integrated with low-dose CT (LDCT), it improved diagnostic accuracy and reduced false positives [29]. More recently, the MSC was validated in the large prospective BioMILD study (NCT02247453), confirming its ability to stratify risk among individuals with indeterminate nodules and demonstrating high negative predictive value, thus supporting its clinical utility in refining screening and follow-up strategies [30]. To date, only one miRNA-based diagnostic signature (ThyraMIR v2, combined with the ThyGeNEXT oncogene panel) has been approved for clinical use to stratify thyroid nodules [31].
Despite promising advances, the translational journey of cmiRNAs remains challenged by limited consistency across studies. Variability in pre-analytical and analytical conditions, such as sample type (serum, plasma, exosomes), extraction protocols, normalization strategies, detection methods (qRT-PCR, microarrays, sequencing, or dPCR), and cohort heterogeneity, has contributed to discrepancies in reported results and hindered broader clinical application [32, 33]. To address these limitations, the National Cancer Institute (NCI) has developed evidence-based biospecimen practices (BEBP) specifically for cmiRNA research. These guidelines offer standardized protocols for blood collection, processing, storage, and RNA extraction to support reproducibility and facilitate inter-laboratory comparisons, with flexibility to accommodate both exploratory and clinical settings [34].
Importantly, the BEBP guidelines were published in 2024, after the completion of sample collection and processing for the present study. Therefore, full adherence to these procedures was not feasible. Nevertheless, the recognition of these standardized protocols highlights the importance of future studies adopting harmonized workflows to ensure maximal reproducibility and clinical translatability.
As noted in recent reports, these challenges are not unique to cmiRNAs but also affect other circulating biomarkers such as cell-free DNA and proteins [35]. Advancing miRNA biomarker research will require large, well-annotated cohorts, rigorous pre-analytical standardization, and the adoption of robust analytical platforms. In this context dPCR stands out as a powerful tool, as it enables absolute quantification without the need for standard curves or endogenous reference genes. This eliminates one of the main sources of technical variability in qRT-PCR-based workflows, where normalization strategies can introduce bias, especially in the context of heterogeneous clinical samples. By bypassing the requirement for housekeeping miRNAs or spike-in controls, dPCR reduces inter-group variability and enhances data comparability across laboratories. In line with this, an increasing number of studies have embraced dPCR for the analysis of cmiRNAs, further validating its reliability and applicability in the context of liquid biopsy [12, 36].
An important consideration in interpreting the results of this study is the detection of both miR-4488 and miR-579-3p in the serum of healthy donors, albeit at lower concentrations compared to melanoma patients. This finding raises fundamental questions about the cellular origin of these cmiRNAs and the mechanisms regulating their release into the bloodstream. It remains unclear whether the observed cmiRNA levels predominantly reflect tumor-derived material, immune system activity, or contributions from other normal tissues [37]. Several evidence suggests that several miRNAs previously proposed as cancer biomarkers can also be actively secreted by blood cells, platelets, and other stromal components, thereby complicating their attribution to tumor-specific processes. Consequently, it is plausible that fluctuations in the bloodstream levels of these miRNAs may mirror dynamic changes in blood cell populations or systemic physiological responses rather than tumor burden alone [38, 39]. A deeper understanding of the cellular source and release mechanisms, whether through extracellular vesicles, protein complexes, or passive leakage, will be essential to refine the specificity and clinical interpretability of cmiRNA-based biomarkers. In this context it is important to mention that miR-4488 is reported to be expressed by normal melanocytes and is upregulated in melanocyte-derived exosomes following ultraviolet B (UVB) irradiation [40]. Given the well-established role of UVB in melanocyte transformation and melanoma development, one could speculate that miR-4488 is expressed at low levels in normal skin cells, becomes upregulated in melanoma cells, and reaches even higher levels in therapy-resistant tumors. Interestingly, in our previous work, we observed an opposite pattern for miR-579-3p, which is highly expressed in melanocytes but progressively downregulated in melanoma and even more so in drug-resistant cells [20]. These findings suggest that the dynamic balance between these two miRNAs during melanoma progression and therapy resistance could be reflected in their circulating levels, potentially offering insight into their roles as complementary biomarkers.
Lastly, although our findings are encouraging, they are based on a relatively small cohort. However, a notable strength of the study is the multicenter origin of the patient samples, which were collected from two independent institutions at different time points. Despite these variations, the duplex assay showed consistent performance, supporting the robustness and reproducibility of miRatio across distinct clinical settings. Still, further validation in larger, prospectively collected cohorts is essential to confirm its generalizability and clinical utility. Evaluating its applicability across diverse melanoma subtypes, treatment regimens, and disease stages will also provide deeper insights into its role as a predictive biomarker in precision oncology.
Taken together, this work underscores the versatility of miRatio as a non-invasive biomarker with potential applications in diagnosis, prognosis, and longitudinal monitoring of metastatic melanoma. The integration of solid dPCR with duplex quantification offers a technically robust and clinically relevant tool for liquid biopsy-based stratification. These results contribute to the ongoing efforts toward the clinical translation of cmiRNA-based biomarkers and support future multi-omic approaches aimed at personalizing cancer management.
Conclusions
We developed and validated the first duplex dPCR assay for the simultaneous quantification of miR-4488 and miR-579-3p in serum from metastatic melanoma patients. This method enables precise, reproducible, and high-throughput detection of both cmiRNAs in a single reaction, addressing the limitations of singleplex assays and enhancing the analytical power of liquid biopsy approaches. Applying this duplex assay, we confirmed the clinical utility of the miRatio (miR-4488/miR-579-3p), a previously proposed ratiometric biomarker, as a circulating indicator of therapeutic response to MAPK-targeted therapies. Although both miRNAs are detectable in healthy donors, their relative modulation in melanoma patients underscores the value of ratio-based metrics in capturing tumor-related molecular dynamics. The cellular origin of these cmiRNAs remains to be elucidated, an essential step toward fully understanding their biological role. This work lays the foundation for future prospective studies and supports the integration of dPCR-based miRNA signatures into precision oncology workflows for improved therapeutic stratification and non-invasive disease monitoring.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Bioresource: Biobank IRCCS Regina Elena National Cancer Institute (BBIRE), Italy (code BBMRI IT_1504858990324590).
Abbreviations
- CTCs
Circulating tumor Cells
- ctDNA
Circulating tumor DNA
- EVs
Extracellular vesicles
- TEPs
Tumor-educated platelets
- miRNAs
MicroRNAs
- qRT-PCR
Quantitative real time polymerase chain reaction
- dPCR
Digital polymerase chain reaction
- ICIs
Immune checkpoint inhibitors
- MAPK
Mitogen-activated protein kinase
- IRCCS
Istituto di Ricovero e Cura a Carattere Scientifico
- RPMI
Roswell Park Memorial Institute (medium)
- FBS
Fetal bovine serum
- cmiRNAs
Circulating microRNAs
- Ct
Threshold cycle
- FAM
Fluorescein amidite (fluorescent dye)
- VIC
Victoria (fluorescent dye from ThermoFisher)
- ROC
Receiver operating characteristic
- DC
Disease control
- CR
Complete response
- PR
Partial response
- SD
Stable disease
- PD
Progressive disease
- PFS
Progression-free survival
Author contributions
Alex De Gregorio materially executed all dPCR and qRT-PCR analyses on serum samples with the support of Valentina De Pascale. Andrea Sacconi and Irene Terrenato have performed statistical analysis to plot ROC and Kaplan-Meier curves. Chiara Mandoj, Giovanni Cigliana, Teresa Meinardi, Francesca Guerrresi, and Gabriele Madonna recruited serum samples of melanoma patients from Istituto Nazionale Tumori IRCCS “Regina Elena” of Rome and Istituto Nazionale Tumori IRCCS “G. Pascale” of Naples. Fabio Valenti and Mariaelena Capone annotated in the dedicated databases and provided the clinical information from patients analysed in this study. Michelangelo Russillo, Virginia Ferraresi, and Paolo Antonio Ascierto are the medical oncologist who followed and monitored melanoma patients. Maurizio Fanciulli and Giovanni Blandino provide support for the study and revised the manuscript. Alex De Gregorio, Gennaro Ciliberto, and Luigi Fattore wrote and edited the manuscript. Rita Mancini provide support and revised the manuscript. Gennaro Ciliberto and Luigi Fattore coordinated the study.
Funding
This study was supported by Italian Association for Cancer Research (AIRC) grant IG24451 to R. Mancini and by Ministry of Enterprises and Made in Italy grants BIOMIND and SPID to R. Mancini and G. Ciliberto. The study was also supported by MUR-PNRR M4C2I1.3 PE6 project PE00000019 Heal Italia (CUP H83C22000550006) to G. Ciliberto, by Italian Minister of Health through PNC Project HUB – Advanced Diagnostics (HLS-DA) PNC-E3-2022-23683266 G. Blandino and partially supported by Italian Minister of Health through Ricerca Corrente 2022–2024 Linea 2/1 to P.A. Ascierto. We thank the Lazio Innova grant A0613-2023-078139 to L. Fattore.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The datasets used in this study are openly available at 10.5281/zenodo.15533924.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committees of both participating institutions: the IRCCS “G. Pascale Foundation” (Approval Numbers DSC/1504, June 11, 2014 and DSC/2893, April 11, 2015) and the IRCCS Regina Elena National Cancer Institute-IFO (Approval Number 8393, July 23, 2017), in accordance with the Declaration of Helsinki. Inclusion criteria specified patients aged ≥ 18 years with histologically confirmed, locally advanced or metastatic melanoma (Stage IIIB-IIIC or IV according to the American Joint Committee on Cancer, 7th Edition), eligible for MAPK-targeted therapy, and capable of providing informed consent. Samples from healthy donors were collected with the approval of the IRCCS Regina Elena National Cancer Institute (Rome, Italy) institutional ethics committee (Approval Number 7182, May 24, 2019). Written informed consent was obtained from all participants before inclusion in the study.
Consent for publication
All the authors agreed for publication of this research.
Competing interests
All the authors declare no conflict of interest with the exceptions of P.A.A. P.A.A. has/had a consultant/advisory role for Bristol MyersSquibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, AstraZeneca, Sun Pharma, Sanofi, Idera, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, Oncosec, Nouscom, Lunaphore, Seagen, iTeos, Medicenna, Bio-Al Health, ValoTX, Replimmune. He also received research funding from Bristol Myers Squibb, Roche-Genentech, Pfizer, Sanofi. Travel support by Pfizer. The funders of this study had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alix-Panabières C, Pantel K. Advances in liquid biopsy: from exploration to practical application. Cancer Cell. 2025;43(2):161–5. [DOI] [PubMed] [Google Scholar]
- 2.Ma L, Guo H, Zhao Y, Liu Z, Wang C, Bu J, et al. Liquid biopsy in cancer current: status, challenges and future prospects. Signal Transduct Target Ther. 2024;9(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monette A, Aguilar-Mahecha A, Altinmakas E, Angelos MG, Assad N, Batist G, et al. The society for immunotherapy of cancer perspective on liquid biopsy and radiomics based technologies for immuno-oncology biomarker discovery and application. Clin Cancer Res. 2025. 10.1158/1078-0432.CCR-24-3791. [DOI] [PubMed] [Google Scholar]
- 4.Allen TA. The role of circulating tumor cells as a liquid biopsy for cancer: advances, biology, technical challenges, and clinical relevance. Cancers (Basel). 2024. 10.3390/cancers16071377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartolomucci A, Nobrega M, Ferrier T, Dickinson K, Kaorey N, Nadeau A, et al. Circulating tumor DNA to monitor treatment response in solid tumors and advance precision oncology. NPJ Precis Oncol. 2025;9(1):1–19. 10.1038/s41698-025-00876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Q-F, Li W-J, Hu K-S, Gao J, Zhai W-L, Yang J-H, et al. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer. 2022;21(1): 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Zhang H, Li H, Xiong J, Wang J, Huang Y. Application of tumor-educated platelets as new fluid biopsy markers in various tumors. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2023;25(1):114–25. [DOI] [PubMed] [Google Scholar]
- 8.Shi W, Wartmann T, Accuffi S, Al-Madhi S, Perrakis A, Kahlert C, et al. Integrating a microRNA signature as a liquid biopsy-based tool for the early diagnosis and prediction of potential therapeutic targets in pancreatic cancer. Br J Cancer. 2024;130(1):125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim SY, Boyd SC, Diefenbach RJ, Rizos H. Circulating MicroRNAs: functional biomarkers for melanoma prognosis and treatment. Mol Cancer. 2025;24(1): 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakrabortty A, Patton DJ, Smith BF, Agarwal P. miRNAs: potential as biomarkers and therapeutic targets for cancer. Genes (Basel). 2023;14(7):1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takizawa S, Matsuzaki J, Ochiya T. Circulating micrornas: challenges with their use as liquid biopsy biomarkers. Cancer Biomark. 2022;35(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirillo PDR, Margiotti K, Mesoraca A, Giorlandino C. Quantification of circulating microRNAs by droplet digital PCR for cancer detection. BMC Res Notes. 2020;13(1):1–6. 10.1186/s13104-020-05190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coccaro N, Tota G, Anelli L, Zagaria A, Specchia G, Albano F. Digital PCR: a reliable tool for analyzing and monitoring hematologic malignancies. Int J Mol Sci. 2020. 10.3390/ijms21093141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busato F, Ursuegui S, Deleuze JF, Tost J. Multiplex digital PCR for the simultaneous quantification of a miRNA panel. Anal Chim Acta. 2025;1335:343440. 10.1016/j.aca.2024.343440. [DOI] [PubMed] [Google Scholar]
- 15.Saginala K, Barsouk A, Aluru JS, Rawla P, Barsouk A. Epidemiology of melanoma. Med Sci. 2021. 10.3390/medsci9040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ascierto PA, Ferrucci PF, Fisher R, Del Vecchio M, Atkinson V, Schmidt H, et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med. 2019;25(6):941–6. [DOI] [PubMed] [Google Scholar]
- 17.Lei Q, Wang D, Sun K, Wang L, Zhang Y. Resistance mechanisms of anti-PD1/PDL1 therapy in solid tumors. Front Cell Dev Biol. 2020;8:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruggiero CF, Fattore L, Terrenato I, Sperati F, Salvati V, Madonna G, et al. Identification of a miRNA-based non-invasive predictive biomarker of response to target therapy in BRAF-mutant melanoma. Theranostics. 2022;12(17):7420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castaldo V, Minopoli M, Di Modugno F, Sacconi A, Liguoro D, Frigerio R, et al. Upregulated expression of miR-4443 and miR-4488 in drug resistant melanomas promotes migratory and invasive phenotypes through downregulation of intermediate filament nestin. J Exp Clin Cancer Res. 2023;42(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fattore L, Mancini R, Acunzo M, Romano G, Laganà A, Pisanu ME, et al. MiR-579-3p controls melanoma progression and resistance to target therapy. Proc Natl Acad Sci U S A. 2016;113(34):E5005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melnik BC. MiR-21: an environmental driver of malignant melanoma? J Transl Med. 2015;13: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sameti P, Tohidast M, Amini M, Bahojb Mahdavi SZ, Najafi S, Mokhtarzadeh A. The emerging role of MicroRNA-182 in tumorigenesis; a promising therapeutic target. Cancer Cell Int. 2023;23(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gajos-Michniewicz A, Czyz M. Role of miRNAs in melanoma metastasis. Cancers (Basel). 2019. 10.3390/cancers11030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark MS, Klein K, Weide B, Haydu LE, Pflugfelder A, Tang YH, et al. The prognostic and predictive value of melanoma-related micrornas using tissue and serum: a microRNA expression analysis. EBioMedicine. 2015;2(7):671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Laar R, Lincoln M, Van Laar B. Development and validation of a plasma-based melanoma biomarker suitable for clinical use. Br J Cancer. 2018;118(6):857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Laar R, Latif B, King S, Love C, Taubenheim N, Kalansooriya E, et al. Validation of a microRNA liquid biopsy assay for diagnosis and risk stratification of invasive cutaneous melanoma. Br J Dermatol. 2023;189(3):292–301. [DOI] [PubMed] [Google Scholar]
- 27.Levati L, Bassi C, Mastroeni S, Lupini L, Antonini Cappellini GC, Bonmassar L, et al. Circulating miR-1246 and miR-485-3p as promising biomarkers of clinical response and outcome in melanoma patients treated with targeted therapy. Cancers (Basel). 2022. 10.3390/cancers14153706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quang MT, Nguyen MN. The potential of microRNAs in cancer diagnostic and therapeutic strategies: a narrative review. J Basic Appl Zool. 2024. 10.1186/s41936-024-00360-2. [Google Scholar]
- 29.Sozzi G, Boeri M, Rossi M, Verri C, Suatoni P, Bravi F, et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(8):768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boeri M, Sabia F, Ledda RE, Balbi M, Suatoni P, Segale M, et al. Blood microRNA testing in participants with suspicious low-dose CT findings: follow-up of the BioMILD lung cancer screening trial. Lancet Reg Health. 2024;46: 101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma T, Marshall C, Dantey KE, Thompson DV, Banizs A, Finkelstein SD, et al. The role of the ThyGeNEXT oncogene panel used in combination with the expanded miRNA panel ThyraMIRv2 in indeterminate thyroid nodules: a large, blinded, real-world, observational study. Cancer Cytopathol. 2024;132(9):556–63. [DOI] [PubMed] [Google Scholar]
- 32.Zendjabil M. Preanalytical, analytical and postanalytical considerations in circulating microRNAs measurement. Biochem medica. 2024;34(2):20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babayan A, Neumann MHD, Herdean A, Shaffer JM, Janning M, Kobus F, et al. Multicenter evaluation of independent high-throughput and RT-qPCR technologies for the development of analytical workflows for circulating miRNA analysis. Cancers (Basel). 2020. 10.3390/cancers12051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greytak SR, Engel KB, Hoon DSB, Elias KM, Lockwood CM, Guan P, et al. Evidence-based procedures to improve the reliability of circulating miRNA biomarker assays. Clin Chem Lab Med. 2024;62(1):60–6. [DOI] [PubMed] [Google Scholar]
- 35.Felekkis K, Papaneophytou C. The circulating biomarkers league: combining miRNAs with cell-free DNAs and proteins. Int J Mol Sci. 2024. 10.3390/ijms25063403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gattuso G, Longo F, Spoto G, Ricci D, Lavoro A, Candido S, et al. Diagnostic and prognostic significance of a four-miRNA signature in colorectal cancer. Int J Mol Sci. 2025;26(3):1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allegra A, Alonci A, Campo S, Penna G, Petrungaro A, Gerace D, et al. Circulating microRNAs: new biomarkers in diagnosis, prognosis and treatment of cancer (review). Int J Oncol. 2012;41(6):1897–912. [DOI] [PubMed] [Google Scholar]
- 38.Duttagupta R, Jiang R, Gollub J, Getts RC, Jones KW. Impact of cellular miRNAs on circulating miRNA biomarker signatures. PLoS ONE. 2011;6(6): e20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, et al. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res (Phila). 2012;5(3):492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Z, Sun J, Shao J, Xu J. Ultraviolet B irradiation enhances the secretion of exosomes by human primary melanocytes and changes their exosomal miRNA profile. PLoS ONE. 2020;15(8): e0237023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The datasets used in this study are openly available at 10.5281/zenodo.15533924.