Abstract
Background
Variations in DNA methylation within the DNA damage response (DDR) mechanism could have significant implications for glioma prognosis and immune responses. This study aimed to explore the global DNA methylation landscape of DDR genes in gliomas and identify key epigenetically regulated genes influencing glioma biology and immunity.
Methods
This study incorporated a range of public and local glioma datasets. Multiple clinical, bioinformatic, and in vitro experimental analyses were conducted to explore clinical and biological aspects.
Results
Global DNA methylation variations in DDR genes correlated with distinct glioma prognoses, with five CpGs identified as potent predictors. Hierarchical clustering and a risk-score model based on these CpGs unveiled immune-related prognostic subgroups in glioblastomas (GBMs) and lower-grade gliomas (LGGs). NSUN5, epigenetically regulated by one of these CpGs, highlighted the biological significance of the DDR CpG panel. In vitro, NSUN5 displayed tumor-suppressor-like activities in GBM cells, but clinically, it was an unfavorable prognostic marker. Depletion of NSUN5 shifted cytosolic DNA sensing from a STING-dependent (cGAS-STING) pathway to a STING-independent (DNA-PK-HSPA8) pathway, leading to a delayed but more robust type I interferon (IFN) response in GBM cells and enhancing microglial M1 polarization and chemotaxis. This may partially account for the functional discrepancy of NSUN5 observed between experimental and clinical contexts.
Conclusion
This study highlights the complex interplay between DNA methylation, the DDR mechanism, cytosolic DNA sensing, and glioma immunity. These findings may inspire novel strategies for DNA sensing-based immunotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-025-06360-2.
Keywords: Glioma, DNA damage response, DNA methylation, Cytosolic DNA sensing, NSUN5
Introduction
Glioblastomas (GBMs) represent the most prevalent and aggressive subtype of gliomas [1]. The current therapeutic approach primarily relies on genotoxic treatments, encompassing radiotherapy (RT) and temozolomide (TMZ). Exposure to these exogenous genotoxic agents induces DNA double-strand breaks (DSBs) and disrupts genomic integrity, which are particularly detrimental to cancer [2–5]. Additionally, endogenous factors, such as aldehydes and reactive oxygen species, constantly threaten tumor DNA, resulting in replication-associated DSBs and chromosomal mis-segregation [5]. To counteract these insults, tumor cells employ robust DNA damage response (DDR) mechanisms, consisting of a complex network of sensors, mediators, and effectors [2–5]. Impairments within the DDR system have profound impacts on GBM prognosis and treatment efficacy [2, 6]. In 2020, Olivieri et al. [3]. conducted 31 CRISPR-Cas9 screens targeting 27 genotoxic agents in human cells, identifying 842 core genes crucial for the response to DNA damage. In this study, these core genes will serve as proxies for the intricate human DDR system.
DNA methylation plays a critical role in regulating gene expression [7] and is frequently altered in GBMs, leading to changes in the expression of DDR genes [8]. While some studies have examined the impact of abnormal DNA methylation of individual DDR genes in GBM biology and immune response [8–10], comprehensive evidence at the genome-wide level is lacking. Analyzing global DNA methylation patterns of DDR genes could provide valuable insights for personalized medicine in GBMs.
In our study, while exploring the associations between global DDR methylation alterations and GBM immunity, we identified a novel DDR gene, NSUN5. NSUN5, a 5-methylcytosine RNA methyltransferase, plays a pivotal role in maintaining ribosomal function and protein synthesis [11]. Previous research [11] has demonstrated that epigenetic loss of NSUN5 in gliomas results in an unmethylated status at the C3782 position of 28 S rRNA, leading to a general depletion of protein synthesis and the activation of an adaptive translational program to ensure cellular survival under stressful conditions. Through bioinformatic and integrative omics-data analysis, we have, for the first time, linked NSUN5 with cytosolic DNA sensing pathways. These pathways, including both STING-dependent and STING-independent signaling, are recognized as crucial interfaces linking DDR to tumor immunity [12–14]. Elucidating the specific regulatory role of NSUN5 on cytosolic DNA sensing pathways may provide deeper insights into how DDR methylation abnormalities contribute to GBM prognosis and immunity.
Collectively, this study first describes the close associations between global DNA methylation variations in DDR genes and glioma prognosis and immunity. Second, we utilized a selective panel of five CpGs to delineate novel glioma subtypes that are biologically and clinically relevant. Subsequently, we validated a risk-score signature incorporating these CpGs for predicting the prognosis of the most aggressive non-G-CIMP GBM subtype. Our biological studies focused on the RNA methyltransferase NSUN5, which is regulated by one of the identified CpGs. In vitro experiments revealed dual roles of NSUN5 in GBMs; specifically, depletion of NSUN5 redirects cytosolic DNA sensing from a STING-dependent (cGAS-STING) pathway to a STING-independent (DNA-PK-HSPA8) pathway, promoting a robust type I interferon (IFN) response in GBM cells and enhancing microglial M1 polarization and chemotaxis. Conversely, in an immune-free environment, NSUN5 deficiency exhibited tumor-promoting functions in tumor cells. Ultimately, this study aims to emphasize the intricate interplay among DNA methylation, the DDR machinery, cytosolic DNA sensing, and tumor immunity, thereby inspiring the development of novel strategies for DNA-sensing-based immunotherapy.
Materials and methods
Datasets of gliomas and cell lines
DNA methylation datasets and corresponding clinical information were obtained for GBMs, lower-grade gliomas (LGG), non-tumor brain tissues (NTB), and glioma cell lines. These datasets were sourced from The Cancer Genome Atlas (TCGA) [15, 16], the Gene Expression Omnibus (GEO) [17–22], and the Chinese Glioma Genome Atlas (CGGA) [23]. Additional datasets were provided by the authors [7, 24]. Whenever available, paired transcriptome datasets for these samples were also downloaded. Other transcriptome datasets for gliomas and NTBs were obtained from additional sources [25–27]. Detailed information regarding sample sources, clinical and molecular data, detection techniques, and data processing methodology are provided in the Supplementary Methods.
Hierarchical cluster analysis (HCA) and risk-score modeling
Initial CpG selection was conducted as previously described [7]. Batch effects were adjusted using M-value transformation and the Empirical Bayes method [28, 29]. In this study, the human DDR system was characterized by leveraging a set of 842 core genes whose loss results in either sensitivity or resistance to DNA-damaging agents [3]. These genes were identified in a comprehensive genome-scale analysis employing 31 CRISPR-Cas9 screens conducted in human cells against 27 distinct genotoxic agents [3]. By intersecting these 842 DDR genes with the initially screened CpGs, 1095 CpGs located within the genomic regions of 635 DDR genes were identified. Subsequently, 190 CpGs with high variability in DNA methylation (standard deviation [SD] of β value ≥ 0.1 among GBM samples) were selected for HCA, which was performed using the Euclidean distance and complete linkage method. Furthermore, a risk-score signature was developed using a discovery-validation approach (Supplementary Figure S1). Briefly, two temozolomide (TMZ)-treated cohorts (TCGA-GBM and RAUH) were used as discovery cohorts. A univariate Cox regression model was employed to correlate the 190 highly variable CpGs with overall survival (OS) in the discovery cohorts (Supplementary Figure S1). A panel of five CpGs was then considered for multivariate Cox regression analysis. After adjusting for age, MGMT methylation status, and treatment, all five CpGs were identified as potent and independent prognostic markers (Supplementary Figure S1). The risk-score signature was formulated as previously reported [7], with the cutoff for low- and high-risk groups set at the median risk-score value in the discovery cohorts.
Formalin-fixed paraffin-embedded (FFPE) samples
Surgical samples of 86 primary gliomas (WHO grade 2 to 4) were collected from the Department of Neurosurgery, Xijing Hospital, between 2012 and 2019. Additionally, 10 brain tissues from traumatic brain injury cases were collected as NTB controls. These samples were stored as FFPE blocks. Written informed consent was obtained from all participants, and the study was approved by the Institutional Review Board. Patient information, as well as clinical and molecular data, are provided in the Supplementary Methods.
Pyrosequencing
Total DNA was extracted using the EZ DNA Methylation-Gold™ Kit (Beijing Tianmo). Pyrosequencing was performed on the PyroMark Q96 ID platform (Qiagen). Primer sequences are detailed in the Supplementary Methods.
Immunohistochemistry (IHC) and Immunofluorescence (IF)
FFPE samples were used for IHC with the primary antibody NSUN5 (Abcam, ab121633), as previously described [30]. Immunoreactivity was scored on a scale of 0 to 431. In vitro GBM cells and FFPE samples were also used for IF with multiple primary antibodies, followed by Alexa Fluor® secondary antibodies, as detailed in the Supplementary Methods.
In vitro functional assays
Cell lines including GBM (A172, U251, and DBTRG-05MG) and microglia (HMC3), were cultured as described in the Supplementary Methods. Drugs used in this study included temozolomide (TMZ), 5-Aza-2′-deoxycytidine (5-AzadC), STING inhibitor (H-151), and DNA-PK inhibitor (Nu7441). Small interfering RNAs (siRNAs) and lentiviral vectors were used to generate in vitro knockdown and overexpression of NSUN5 in GBM cells. Total RNA, DNA, and proteins were extracted as previously described [7]. Various functional assays, including quantitative real-time PCR (qRT-PCR), RNA sequencing (RNA-Seq), western blotting, cell viability and TMZ cytotoxicity assay, cell cycle and apoptosis assays, cell migration and invasion assays, protein stability assays, enzyme-linked immunosorbent assay (ELISA), and co-culture experiments are described in the Supplementary Methods.
Bioinformatic and statistical analyses
Bioinformatic analyses, including single-cell RNA-Seq data analysis, gene set enrichment analysis (GSEA) [31], gene set variation analysis (GSVA) [32], Tumor Immune Dysfunction and Exclusion (TIDE) [33], and CIBERSORT [34], are detailed in the Supplementary Methods. Frequency data were analyzed using Fisher’s exact test or chi-square test. Continuous data were analyzed using Student’s t-test or Mann-Whitney test. Survival data were estimated using the Kaplan-Meier Method and compared using the log-rank test. The correlation between DNA methylation and gene expression was evaluated using Pearson correlation analysis. Prognostic correlations and independence were assessed using univariate and multivariate Cox regression models. Meta-analysis was conducted using the inverse-variance approach with either fixed- or random-effect models based on the heterogeneity test, considering P < 0.1 or I²>50% as statistically significant. For prognostic classification, cut-offs were defined as median values or calculated using the maxstat R package [35]. All statistical calculations were performed using SPSS and R software, with two-sided P values ≤ 0.05 considered statistically significant.
Results
Global and selective DNA methylation variations in DDR genes were linked to distinct prognostic subtypes across all diffuse gliomas
We first conducted HCA based on global DNA methylation variations in the core DDR genes (190 highly variable CpGs). The HCA consistently resulted in distinct tumor clusters associated with different OS among GBMs and other grade gliomas (Supplementary Figure S2 and Table S1). The findings suggest that global DNA methylation alterations in DDR genes play a role in glioma prognosis.
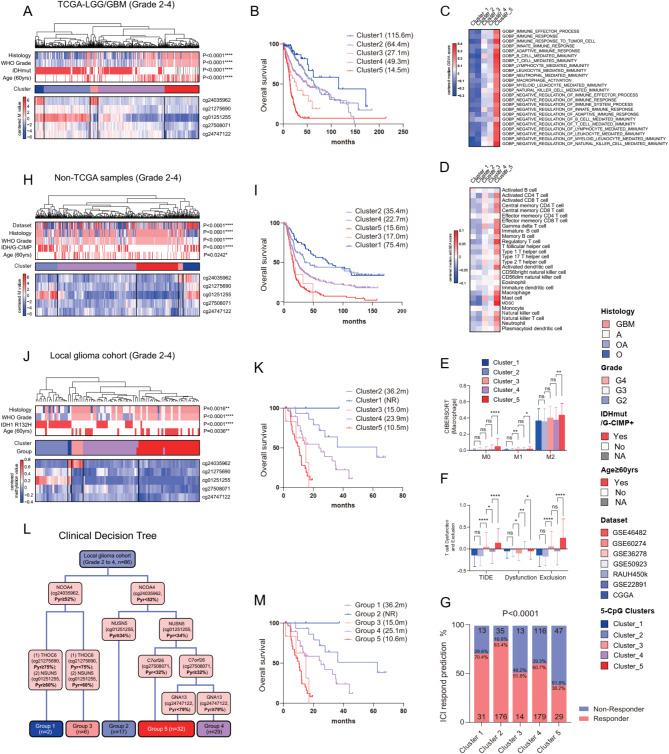
Next, we employed a meticulous multi-step selection approach to identify a panel of five CpGs within five DDR-relevant genes (Supplementary Figure S1). Each CpG was independently associated with OS of GBMs (Supplementary Figure S1). HCA using this 5-CpG panel clustered TCGA glioma samples into five distinct clusters, each characterized by unique 5-CpG methylation patterns and prognostic outcomes (Fig. 1A-B). Bioinformatic analysis revealed associations between these tumor clusters and unique immune transcriptional profiles (Fig. 1C-D). Additionally, these clusters demonstrated differential levels of TIIC infiltration, distinct T cell functions, and varied responses to immune checkpoint blockade (ICB) therapy (Fig. E-G).
Fig. 1.
Hierarchical clustering analysis (HCA) on the selective 5-CpG methylation defined distinct prognostic and immune-relevant glioma subtypes; the heatmaps of glioma clusters defined by HCA on the 5-CpG methylation (Illumina DNA methylation chips) in (A) TCGA and (H) non-TCGA samples; the heatmaps of glioma clusters defined by HCA on 5-CpG methylation (pyrosequencing data) in (J) local glioma cohort; each row represents a CpG and each column represents a sample grouped by HCA; clinical and molecular features are indicated for each sample; survival curves of each glioma clusters in (B) TCGA, (I) non-TCGA and (K) local glioma cohort; (L) a clinical decision tree using the 5-CpG pyrosequencing data and (M) survival curves of each group; (C) the heatmaps of median enrichment scores of immune-relevant gene sets defined by GSVA and (D) median enrichment scores of the abundance of TIICs defined by ssGSEA; (E) macrophage subtypes, (F) T cell dysfunction and exclusion scores and (G) the distribution of predicted responders to ICI therapy among the glioma clusters in TCGA-LGG/GBM
The 5-CpG-based HCA classification was validated in non-TCGA diffuse gliomas (Fig. 2H, I) and FFPE samples using pyrosequencing (Fig. 2J, K). A clinical decision tree was developed to facilitate straightforward clinical application (Fig. 2L, M). The 5-CpG-based glioma classification was independent of known prognostic factors (Supplementary Figure S3). Overall, our findings demonstrate the utility of the 5-CpG methylation panel in delineating novel, clinically and biologically distinct glioma subtypes, each characterized by unique prognostic outcomes and local immune statuses.
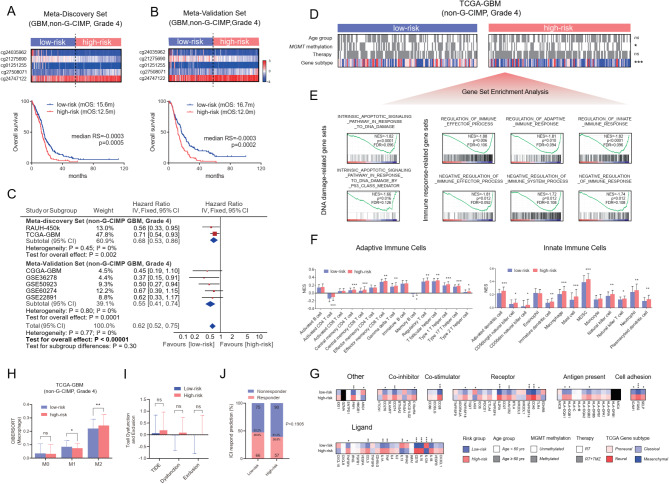
Fig. 2.
Identification and validation of a prognostically and biologically relevant risk-score signature based on the five-CpG methylation in non-G-CIMP GBMs; Survival curves of low-risk and high-risk groups defined by the DDR risk-score signature in (A) meta-discovery cohort of non-G-CIMP GBMs (TCGA-GBM and RAUH-450k collectively), (B) meta-validation cohort of non-G-CIMP GBMs (GSE22891, CGGA-GBM, GSE50923, GSE6074 and GSE36278 collectively); the cutoff was pre-defined as median risk score value (-0.0003) in the meta-discovery cohort; the heatmaps of the five-CpG methylation are shown respectively; each row represents a CpG and each column represents a sample ordered by its risk score; survival curves in each cohort are shown in Supplementary Figure S3; (C) meta-analysis of the 5-CpG risk-score signature in each cohort; (D) the correlation of the risk groups with clinical and molecular features in TCGA-GBM; (E) representative GSEA enrichment plots of the significantly enriched gene sets in high-risk tumors from TCGA-GBM; (F) the abundance of adaptive and innate immune infiltrating cells between low-risk and high-risk tumors from TCGA-GBM; (G) the expression levels of immune checkpoint molecules, (H) macrophage subtypes, (I) T cell dysfunction and exclusion scores and (J) the distribution of predicted responders to ICI therapy between low-risk and high-risk groups from TCGA-GBM; statistical significance at the level of *<0.05, ** <0.01, *** <0.001 and **** <0.0001
Construction and validation of the five DDR CpG-based risk-score signature for non-G-CIMP GBMs
Given the limited representation of GBMs by the G-CIMP subtype, which features unique DNA methylation profiles, favorable prognosis, and exclusive IDH mutations [7], this subtype was excluded from the development of the 5-CpG risk-score signature. The risk score was calculated as follows: risk score = (-1.099 × β value of cg24035962) + (-2.368 × β value of cg21275690) + (-1.104 × β value of cg01251255) + (1.217 × β value of cg27508071) + (1.101 × β value of cg24747122). Patients were categorized into low- and high-risk groups based on a predefined cutoff (Fig. 2A, B). The prognostic value of the 5-CpG signature was validated in multiple independent cohorts of non-G-CIMP GBMs and other diffuse gliomas, demonstrating its robustness, consistency, and independence (Fig. 2A-C, Supplementary Figure S4-7 and Table S2).
Bioinformatic analysis further revealed associations between the 5-CpG-based risk subgroups and TCGA gene expression subtypes, with enrichment of high-risk tumors in the mesenchymal subtype (Fig. 2D and Supplementary Figure S8). GSEA uncovered distinct transcriptional profiles between risk subgroups, notably enriching DDR- and immunity-related gene sets in high-risk tumors (Fig. 2E and Supplementary Figure S8 and Table S3-6). Additionally, high-risk tumors showed increased TIIC infiltration and elevated expression of immune checkpoint molecules (Fig. 2F, G and Supplementary Figure S8). Overall, these findings underscore the potential of the 5-CpG signature as a robust prognostic tool and highlight its biological relevance to DDR- and immunity-related profiles in non-G-CIMP GBMs.
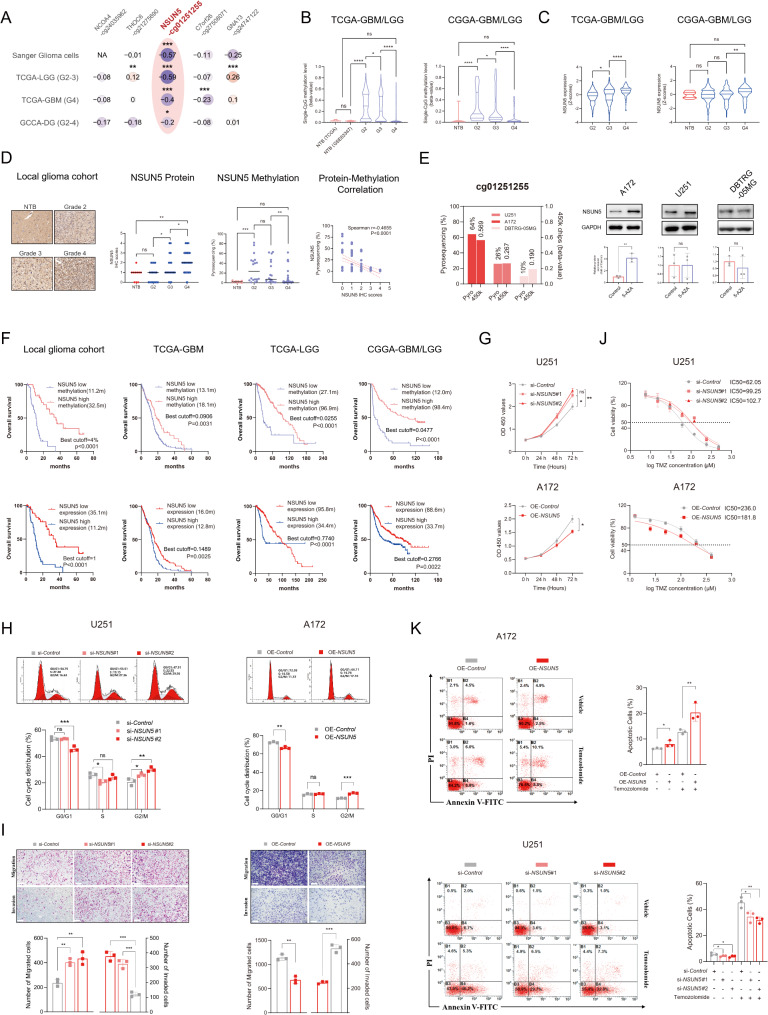
NSUN5, regulated by DNA methylation, demonstrated anti-tumor activities in GBM cell lines in vitro but served as an unfavorable prognostic biomarker in clinical glioma samples
Among the five CpGs, cg01251255 exhibited a significant negative correlation with NSUN5 gene expression (Fig. 3A), prompting further investigation of this CpG-gene pair. NSUN5 was hypermethylated in LGGs but remained unaltered in GBMs compared to NTBs (Fig. 3B). NSUN5 expression appeared to increase with tumor grade, though variations were observed across different datasets (Fig. 3C, D and Supplementary Figure S9). The CpG-gene correlation was validated in FFPE glioma samples (Fig. 3D). Demethylation treatment upregulated NSUN5 in high-methylation cell lines (A172), but not in low-methylation ones (U251 and DBTRG-05MG) (Fig. 3E), indicating that DNA methylation is a primary regulator of NSUN5 in gliomas.
Fig. 3.
NSUN5, regulated by DNA methylation, demonstrated anti-tumor activities in GBM cell lines in vitro but served as an unfavorable prognostic biomarker in clinical glioma samples; (A) Pearson correlation analysis between CpG methylation and gene expression for the 5 identified CpGs in different datasets; (B) CpG methylation (cg01251255) and (C) NSUN5 expression between gliomas of all grades and NTB from different datasets; (D) IHC staining of NSUN5 protein and pyrosequencing data in local glioma cohort, and their correlation; scale bar = 50 μm; (E) CpG methylation and protein levels of NSUN5 in different GBM cell lines with demethylation treatment of 5-Aza-dC (10 nM). (F) Survival curves between gliomas with low vs. high NSUN5 methylation (expression) in local glioma cohort and public datasets; best cutoffs are calculated for each cohort by the maximal ranked log-rank statistic; (G) CCK-8 proliferation assay in U251 and A172; (H) Cell cycle assay by flow cytometry in U251 and A172; (I) Migration and invasion assay in U251 and A172; scale bar = 200 μm; (J) TMZ cytotoxicity assay in U251 and A172; (K) Apoptosis assay by flow cytometry in U251 and A172
In clinical glioma samples, higher NSUN5 expression (or low NSUN5 methylation) was associated with shorter OS, suggesting NSUN5 as an unfavorable prognostic biomarker in clinical settings (Fig. 3F and Supplementary Figure S9). However, in vitro studies showed tumor-suppressor roles of NSUN5 in GBM cells, including inhibition of cell proliferation, disruption of the cell cycle, promotion of apoptosis, and reduction of migration and invasion (Fig. 3G-I and Supplementary Figure S10). Additionally, NSUN5 increased TMZ sensitivity in GBM cells (Fig. 3J-K). These in vitro findings contrasted with observations in clinical glioma samples.
NSUN5 depletion facilitated a shift from STING-dependent to STING-independent cytosolic DNA sensing in GBM cells through differential modulation of translation efficiency
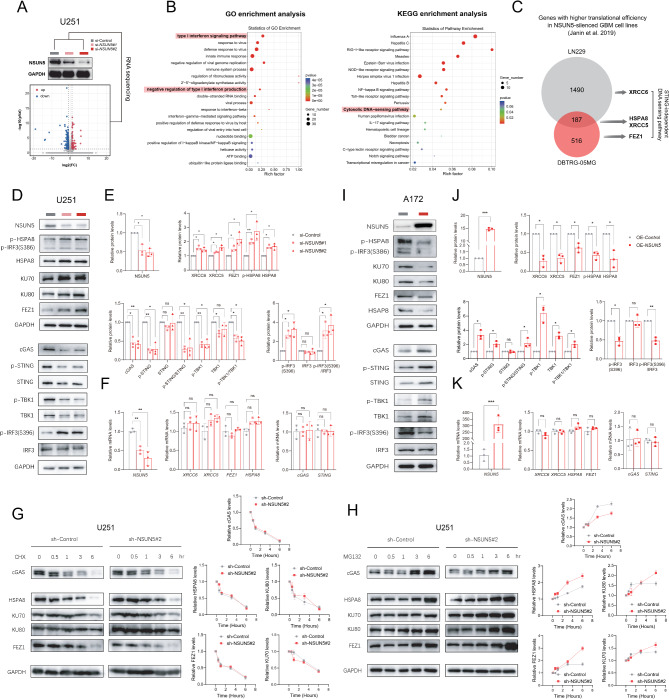
To elucidate the functional paradox of NSUN5, we conducted RNA-Seq analysis on U251 cells following NSUN5 knockdown, revealing 219 differentially expressed genes (DEGs) enriched in immune-related annotations, particularly the cytosolic DNA-sensing pathway and downstream type I IFN signaling (Fig. 4A, B; Supplementary Table S7). NSUN5 is an RNA methyltransferase responsible for 5-methylcytosine modification in rRNA. NSUN5 deficiency leads to hypomethylation at the C3782 position of human 28 S rRNA, inducing structural alterations in the 28 S ribosomal subunit [11]. In glioma cells, NSUN5 loss disrupts normal protein synthesis but selectively enhances translation of stress-responsive genes [11]. Re-examination of integrated Ribo-Seq and RNA-Seq data provided by Janin et al. [11]. suggested molecular clues underlying this paradox (Fig. 4C). Specifically, four components of a novel DNA-PK-mediated, STING-independent DNA-sensing pathway (HSPA8, XRCC5, XRCC6, FEZ1) [36, 37] showed enhanced translational efficiency following NSUN5 knockdown in GBM cells (Fig. 4C). Our experiments validated the elevated protein levels and activity of these four components, with no significant changes in mRNA levels (Fig. 4D-F). Treatment with MG132, a proteasome inhibitor, revealed increased protein synthesis of the four components after NSUN5 knockdown (Fig. 4G), while treatment with cycloheximide (CHX) showed no discernible differences in their protein degradation rates (Fig. 4H). Conversely, NSUN5 knockdown decreased cGAS protein levels without altering mRNA, reducing STING and TBK1 activity in GBM cells (Fig. 4D-F). MG132 and CHX treatments indicated that cGAS protein synthesis, but not degradation, was inhibited after NSUN5 knockdown (Fig. 4G-H). NSUN5 overexpression suppressed the DNA-PK-HSPA8 signal and enhanced the cGAS-STING signal in A172 cells without significant transcriptional changes (Fig. 4I-K). Overall, these findings suggest that NSUN5 loss triggers a switch from cGAS-STING-mediated to DNA-PK-mediated, STING-independent DNA sensing in GBM cells, potentially via differential regulation of translation efficiency.
Fig. 4.
NSUN5 favored switch of cytosolic DNA-sensing modes via differential regulation of translation efficiency in GBM cell lines; (A) Volcano plots of differentially expressed genes (DEGs) from RNA sequencing on U251 cells after transfection of si-Control and si-NSUN5#2; NSUN5 knockdown was confirmed by Western blot; (B) GO and KEGG analysis of enriched pathways using DEGs; (C) Venn diagram of genes with higher translational efficiency in NSUN5-silenced GBM cell lines reported by Janin et al. [15]; (D-E) Western blot images of proteins involved in cGAS-mediated STING-dependent signaling or DNA-PK-HSAP8-mediated STING-independent signaling, and corresponding statistical results in U251; (F) qRT-PCR data in U251; (I-J) Western blot images and corresponding statistical results in A172; (K) qRT-PCR data in A172; (G) Western blot images of CHX treatment experiments for protein degradation; (H) Western blot images of MG132 treatment experiments for protein synthesis
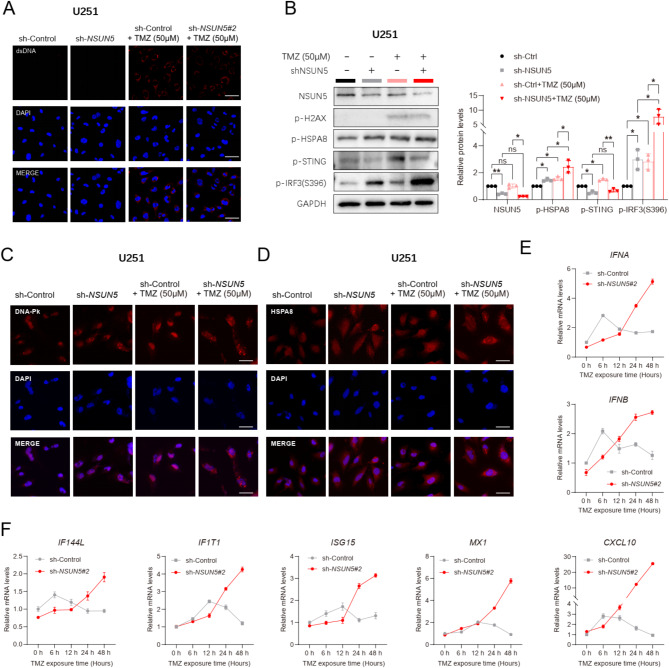
Upon exposure to TMZ, NSUN5 loss amplified cytosolic DNA-PK-HSPA8 signaling and elicited a delayed yet more robust type I IFN response in GBM cells
The DNA-damaging agent TMZ was employed to induce DNA damage (marked by p-H2AX) and augment cytosolic double-stranded (ds) DNA accumulation in U251 cells (Fig. 5A-B). In NSUN5-silenced U251 cells, TMZ enhanced the DNA-PK-HSAP8-IRF3 signaling cascade (Fig. 5B). Additionally, NSUN5 knockdown, in synergy with TMZ, increased the cytosolic localization of DNA-PK and HSAP8, potentially augmenting their contributions to cytosolic DNA sensing (Fig. 5C-D). NSUN5 deficiency led to a modest reduction in baseline expressions of type I IFN genes (IFNA, IFNB) and interferon-stimulated genes (ISGs; CXCL10, MX1, IF144L, IF1T1, ISG15) in GBM cells (U251, DBTRG-05MG; Fig. 5E-F and Supplementary Figure S11). However, upon TMZ exposure, NSUN5-silenced GBM cells exhibited a delayed (peaking at 24–48 h) but more robust type I IFN response compared to controls, which peaked at 6 h (Fig. 5E-F and Supplementary Figure S11). Conversely, NSUN5 overexpression reversed these effects in A172 cells (Supplementary Figure S12). Collectively, these findings suggest that NSUN5 loss activates a DNA-PK-mediated, STING-independent DNA sensing pathway, eliciting a delayed yet potent type I IFN response in GBM cells treated with TMZ.
Fig. 5.
Loss of NSUN5, in cooperation with TMZ, enhanced cytosolic DNA-PK-HSAP8 signaling and induced a delayed but more robust type I IFN response; (A) Representative IF images of dsDNA in sh-Control and sh-NSUN5#2 U251, exposed to TMZ (50 µM) or not; scale bar = 50 μm; (B) Western blot images of key proteins in STING-dependent and -independent pathways in U251; (C) Representative IF images of DNA-PK in U251; scale bar = 50 μm; (D) Representative IF images of HSPA8 in U251 cells; scale bar = 50 μm; (E-F) qRT-PCR data of (E) type I IFN genes and (F) IFN-stimulated genes (ISGs) in U251 cells at indicated time points after TMZ treatment (50 µM)
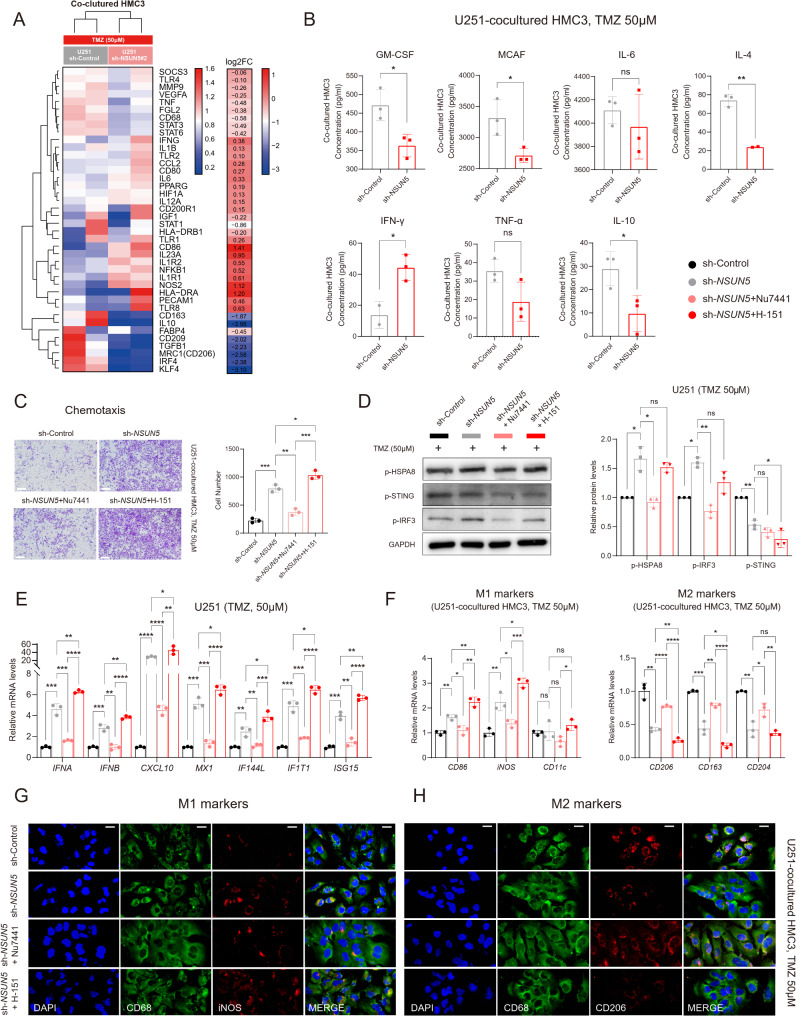
In the context of TMZ treatment, NSUN5 loss facilitated microglial M2-to-M1 polarization and chemotaxis, with this effect attenuated by DNA-PK Inhibition and augmented by STING Inhibition
Glioma-associated microglial/macrophages (GAMs) are pivotal non-tumor cells in the GBM microenvironments, influencing tumor immunity [38]. Using a Transwell co-culture system with U251 GBM cells and HMC3 microglial cells, we found that NSUN5 knockdown in GBM cells promoted M2-to-M1 polarization of HMC3 cells, as evidenced by the upregulation of M1-like markers (e.g., CD86, NOS2) and cytokines (e.g., IFN-γ), and the downregulation of M2-like markers (e.g., MRC1, CD163, CD209) and cytokines (e.g., GM-CSF, MCAF, IL-10, IL-4) (Fig. 6A-B and Supplementary Figure S11). Additionally, NSUN5 knockdown enhanced HMC3 chemotaxis towards GBM cells (Fig. 6C and Supplementary Figure S11). These effects were reversed by the DNA-PK inhibitor Nu7441, which also inhibited HSPA8-IRF3 signaling and downstream type I IFN production (Fig. 6D-E and Supplementary Figure S11). Conversely, NSUN5 overexpression in A172 cells promoted microglial M1-to-M2 polarization and inhibited their chemotaxis (Supplementary Figure S12). Our findings suggest that tumor-intrinsic NSUN5 deficiency may augment microglial-mediated anti-tumor immunity. Furthermore, despite further repressing p-STING activity (Fig. 6D and Supplementary Figure S11), the STING inhibitor H-151 amplified the type I IFN response in NSUN5-silenced GBM cells and enhanced microglial M2-to-M1 polarization and chemotaxis (Fig. 6E-H and Supplementary Figure S11). Overall, these findings indicate that cGAS-STING activation may counteract the immunostimulatory effects induced by DNA-PK-HSAP8 activation.
Fig. 6.
Loss of NSUN5, in cooperation with TMZ, promoted microglial M2-to-M1 polarization and chemotaxis, which was reversed by DNA-PK inhibition but amplified by STING inhibition; (A) Macrophage differentiation PCR array of microglial HMC3 co-cultured with sh-Control and sh-NSUN5#2 U251 exposed to TMZ (50 µM); (B) ELISA assay of M1/M2 macrophage cytokines in supernatants of microglial HMC3 co-cultured with sh-Control and sh-NSUN5#2 U251 exposed to TMZ (50 µM); (C) Migration assay of HMC3 co-cultured with sh-Control and sh-NSUN5#2 U251 exposed to TMZ (50 µM), and treated with DNA-PK inhibitor (Nu7441), STING inhibitor (H-151) or vehicle; scale bar = 200 μm; (D) Western blot images of key proteins involved in STING-dependent and -independent signaling, and corresponding statistical results in U251; (E) qRT-PCR data of type I IFN genes and ISGs in sh-Control and sh-NSUN5#2 U251 exposed to TMZ (50 µM), and treated with DNA-PK inhibitor (Nu7441), STING inhibitor (H-151) or vehicle; (E) qRT-PCR data and representative IF images of (G) M1 and (H) M2 macrophage markers in microglial HMC3 co-cultured with sh-Control and sh-NSUN5#2 U251 exposed to TMZ (50 µM), and treated with DNA-PK inhibitor (Nu7441), STING inhibitor (H-151) or vehicle; scale bar = 20 μm
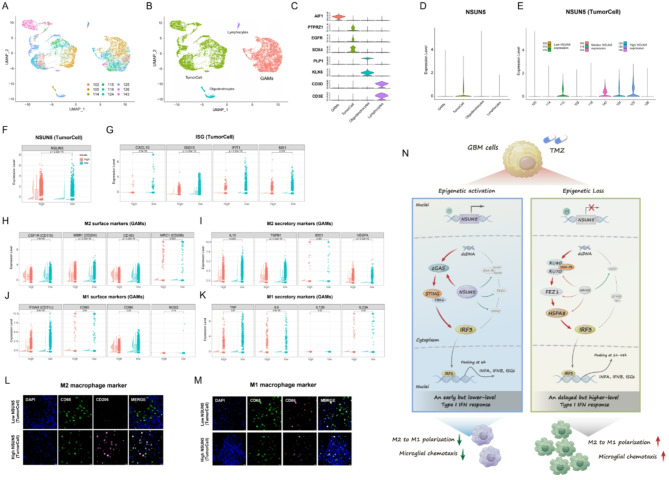
Intrinsic NSUN5 expression within GBM cells correlated with tumor cell-derived type I IFN production and polarization of GAMs in clinical samples
Single-cell RNA-seq analysis of nine IDHwt GBM samples revealed that NSUN5 was predominantly expressed in tumor cells (Fig. 7A-D). Based on NSUN5 expression levels, samples were stratified into the NSUN5low, NSUN5median, and NSUN5high groups (Fig. 7E, F). Tumor-specific ISG expression was higher in the NSUN5low group, while GAMs in NSUN5low samples exhibited lower expression of M2-like markers, with no significant changes in M1-like markers (Fig. 7G-K). IF staining confirmed these findings (Fig. 7L, M). Overall, these results support an association between NSUN5 expression and tumor-intrinsic type I IFN production as well as GAM functions in clinical samples.
Fig. 7.
Single-cell RNA-Seq data analysis and IF staining in primary GBM samples; Single-cell transcriptome dataset is downloaded from GEO (GSE131928) (A) Nine samples are integrated and cells from each sample are indicated by different colors; (B) Cells are classified into four cell types, including tumor cells, lymphocytes, oligodendrocytes and GAMs; (C) The violin plots of the marker gene expression in the four cell types; (D) The violin plots of NSUN5 expression in the four cell types; (E) The violin plots of NSUN5 expression in tumor cells across the nine samples; based on median NSUN5 expression, GBMs were classified into NSUN5low, NSUN5median, and NSUN5high samples; The violin plots, boxplots and scatter plots of (F) NSUN5 expression in tumor cells, (G) ISG expression in tumor cells, (H) expression of M2 surface markers in GAMs, (I) expression of M2 secretory markers in GAMs, (J) expression of M1 surface markers in GBMs, and (K) expression of M2 secretory markers in GAMs between NSUN5low and NSUN5high samples; (L-M) Representative IF images of M1 and M2 markers in local GBM samples with low and high NSUN5 expression defined by IHC scores; scale bar = 20 μm. (N) Schematic Illustrations for NSUN5 as a Key Modulator of Tumor-Intrinsic Cytosolic DNA-Sensing Modes Influencing Microglial Polarization and Chemotaxis
Discussion
The dysregulation of the DDR machinery has gained significant attention in tumor immunology, particularly in GBMs [2, 6]. Olivieri et al. (2020) [3] presented an unbiased genetic map of the DDR in human cells, encompassing 842 core DDR genes responsive to 27 genotoxic agents. In this study, we first revealed a strong correlation between global DNA methylation variations in these DDR genes and he prognosis of diffuse gliomas. We then identified a five-CpG panel within these variations, delineating novel glioma subtypes with distinct prognostic outcomes and local immune profiles. Furthermore, we developed and validated a risk-score signature based on these five CpGs for predicting the prognosis of the most aggressive non-G-CIMP GBM subtype. Bioinformatic analyses underscored the association between the DDR epigenetic signature and unique GBM immunophenotypes. These findings suggest that both global and selective DNA methylation variations in DDR genes significantly influence glioma prognosis and immunity, although the underlying mechanisms remain to be elucidated.
In our biological experiments, we focused on the CpG-gene pair cg01251255 and NSUN5, a human ribosome RNA methyltransferase, due to their consistent correlation in DNA methylation and gene expression. Correlation analysis and in vitro cell experiments supported that NSUN5 may be regulated by DNA methylation, which aligns with previous studies [11, 39]. Beyond DNA methylation, NSUN5 is likely controlled by a multilayered epigenetic network involving histone modifications and chromatin remodeling. Activating histone marks, such as H3K4me3 and H3K27ac, may promote NSUN5 transcription by recruiting methyltransferase complexes (e.g., MLL/SET) or enhancing distal enhancer activity [40]. Conversely, repressive marks like H3K27me3 and H3K9me3, mediated by Polycomb repressive complex 2 (PRC2) or heterochromatin formation, could suppress NSUN5 expression [39]. Chromatin remodelers, including SWI/SNF and NuRD complexes, also play a bidirectional role: SWI/SNF facilitates promoter accessibility, while NuRD suppresses expression through deacetylation and nucleosome repositioning [41]. Additionally, three-dimensional chromatin architecture, such as looping or topologically associating domains (TADs), may spatially link distal regulatory elements to the NSUN5 promoter, determining its activation or repression [42]. Metabolites (e.g., α-KG/2-HG) and non-coding RNAs could further modulate NSUN5 expression by influencing epigenetic enzyme activity or chromatin localization [39]. It is important to note that the proposed epigenetic regulatory mechanisms are largely speculative and require experimental validation. However, exploring and validating these mechanisms could provide deeper insights into the epigenetic regulation of NSUN5 and its potential as a therapeutic target.
NSUN5 is crucial for maintaining normal protein synthesis [11, 39]. In gliomas, DNA methylation-associated silencing of NSUN5 leads to an unmethylated status at the C3782 position of 28 S rRNA, restricting overall protein synthesis but enhancing translation of stress-responsive genes [11]. Moreover, NSUN5 exhibits tumor-suppressor characteristics in nude mouse xenograft models [11]. Although CRISPR-Cas9 screens [11] identified NSUN5 as a core DDR player, its DDR function is rarely reported. We found that NSUN5 knockdown enhanced TMZ resistance in GBM cells, possibly due to the stress-adaptive translational program resulting from NSUN5 deficiency, which enhances the translation of DNA damage repair-related genes (e.g., XRCC5, XRCC6) [11]. These findings suggest that NSUN5 may act as a tumor suppressor in vitro. However, NSUN5 is also reported as an unfavorable prognostic biomarker for GBMs, as its expression correlates with poorer OS in clinical samples [11, 39, 43]. This dual role of NSUN5 is consistent with its reported bidirectional effects in multiple cancers. In its pro-tumorigenic role, NSUN5 promotes tumor progression by catalyzing m5C modifications of 28 S rRNA and mRNA, enhancing ribosomal function and protein synthesis, and facilitating tumor proliferation, migration, and immune evasion [40, 44]. It also recruits WDR5 to induce H3K4me3 modifications at gene promoters, activating Wnt/β-catenin and SMAD3 signaling to drive tumor invasion [40, 45]. Additionally, NSUN5 upregulates cell cycle proteins (e.g., CDK4/6, ACC1) and inhibits ferroptosis, further supporting malignant progression [45–47]. Conversely, under specific conditions, NSUN5 exhibits tumor-suppressive effects. It catalyzes m5C modifications of immune-related proteins (e.g., GPX4), activating the cGAS-STING pathway to enhance type I IFN responses and macrophage M1 polarization, thereby promoting anti-tumor immunity [48]. It also induces β-catenin degradation via TET2/RBFOX2-dependent 5hmC modifications and activates the p53 pathway to induce apoptosis, inhibiting tumor growth [39, 49]. Functional paradoxes for the same gene are not uncommon in cancer research, as exemplified by IDH mutations [50]. Revisiting the current evidence, we observed that NSUN5’s tumor-suppressor-like functions were primarily demonstrated in experimental models lacking an immune microenvironment, whereas its pro-tumor characteristics were observed in clinical samples with a complete immune microenvironment. This suggests that tumor-immune interactions may underlie the functional paradox of NSUN5 in glioma biology. To further clarify this duality, future studies should focus on elucidating the complex and context-dependent molecular interactions of NSUN5, particularly in the presence of an immune microenvironment.
RNA-Seq analysis directed our attention to the impact of the DDR gene NSUN5 on cytosolic DNA-sensing pathways, a pivotal intersection of cellular DDR systems and tumor immunity [51, 52]. Tumor cells harness various cytosolic DNA-sensing pathways, notably the cGAS-STING axis, to orchestrate innate immune responses against damaged or exogenous DNA [53]. Recently, STING-independent DNA-sensing pathways have emerged, yet their roles in tumor immunity and interplay with the cGAS-STING axis remain elusive [54, 55]. Our findings suggest that NSUN5 may switch tumor-intrinsic cytosolic DNA-sensing modes in tumor cells. Specifically, NSUN5 knockdown enhances DNA-PK-HSPA8-mediated STING-independent signaling while repressing the cGAS-STING axis in GBM cells, potentially driven by differential regulation of translation efficiency. Previous studies showed that epigenetic loss of NSUN5 globally inhibits protein synthesis while promoting a selective translational program for stress adaptation [11, 43]. We found that NSUN5 depletion represses cGAS translation but enhances the translation of HSPA8, KU70 [5], KU80 [6], and FEZ1. Upon exposure to the DNA-damaging drug TMZ, NSUN5 loss shifts cytosolic DNA-sensing from cGAS-STING-dominant to DNA-PK-HSAP8-dominant, generating a delayed but stronger type I IFN response in GBM cells, a key contributor to tumor-killing immunity [56] (Fig. 7N). GAMs, comprising microglia and bone marrow-derived macrophages (BMDMs), are abundant non-tumor cells in GBM (30–50% of tumor mass) [38] and key immune effectors responding to type I IFN [57, 58]. Co-culture with NSUN5-silenced GBM cells promotes microglial chemotaxis and M1 polarization. Single-cell RNA-Seq and histological staining reveal an association between low NSUN5 expression, high type I IFN production in GBM cells, and a high proportion of M2 GAMs. Overall, our findings suggest that tumor-intrinsic NSUN5 loss generates a higher level of type I IFN response and promotes microglial-mediated anti-tumor immunity, indicating an immunosuppressive role of tumor-intrinsic NSUN5 in GBMs. The observed functional paradox of NSUN5 may be partially explained by its immunosuppressive effects on the tumor microenvironment outweighing its tumor-suppressor characteristics within tumor cells.
The intricacies of cytosolic DNA-sensing pathways in cancer immunity extend beyond current understanding. Among these pathways, the cGAS-STING axis is the most extensively studied for its role in tumor immunity [13]. Activation of this pathway holds promise in enhancing antitumor immunity and improving immunotherapy effectiveness [13]. However, suboptimal, chronic, or unregulated activation can exacerbate tumor metastasis or undermine sustained antitumor immunity [13]. The interplay between the cGAS-STING pathway and other cytosolic DNA sensors has emerged as a crucial determinant of the intensity and direction of dsDNA-triggered immune responses [54]. Taffoni et al. [55] noted that in the absence of cGAS, the DNA-PK complex alone serves as a cytosolic DNA sensor, eliciting a type I IFN response in GBM cells. Co-expression with cGAS initially promotes cGAS-STING activation but ultimately fosters an uncontrolled pro-tumorigenic inflammatory response [55]. Prognostic stratification based on DNA-PK and cGAS expression levels revealed insightful correlations: patients with high DNA-PK but low cGAS expression exhibited longer survival, while those with high expression of both had shorter survival [55]. In our study, NSUN5 depletion enhanced the activation of the DNA-PK-HSAP8-mediated, STING-independent pathway while repressing cGAS-STING activation in GBM cells. These altered DNA-sensing modes resulted in a delayed yet potent type I IFN response in NSUN5-silenced GBM cells and enhanced microglial-mediated antitumor immunity. DNA-PK inhibition reversed these immunostimulatory effects, implicating the DNA-PK-mediated DNA sensing pathway as the primary contributor to the augmented antitumor response. Notably, STING inhibition further increased tumor-intrinsic type I IFN production and microglial activation, highlighting the antagonistic effects of cGAS-STING activation on DNA-PK-HSPA8-mediated anti-tumor immunity. Overall, our study suggests that in tumor cells, the DNA-PK-HSAP8 pathway primarily contributes to dsDNA-induced anti-tumor immune responses. However, when cGAS-STING and DNA-PK-HSAP8 signals coexist, their interaction may promote pro-tumor inflammations, aligning with previous research [55]. In summary, our findings, alongside others [12, 54, 55], yield several implications for DNA sensing-based immunotherapy. Firstly, while multiple cytosolic DNA-sensing pathways can elicit type I IFN responses, their outcomes, either singly or in combination, can diverge and even conflict, possibly due to the intensity, spatial-temporal dynamics of the response, and tumor heterogeneity. Secondly, the coexistence of DNA-PK and cGAS, leading to pro-tumor inflammation, may contribute to the suboptimal response to cGAS/STING agonists in clinical trials. Thirdly, DNA-PK inhibition, noted for its cytotoxicity (by inhibiting DNA repair) and immunostimulatory effects (by inducing cytosolic DNA accumulation) [59], may paradoxically hinder type I IFN response and anti-tumor immunity in tumors dominated by DNA-PK-mediated sensing. Fourthly, targeted therapy for DNA-sensing pathways should be tailored to the interplay of sensing modes within a tumor, with potential benefits from unconventional regimens like cGAS/STING inhibitors and DNA-PK agonists. Lastly, modulating the dominance of cytosolic DNA sensing modes, perhaps through epigenetic therapy, presents a novel immunomodulatory strategy to augment cancer immunotherapy.
Study limitations
Our study has several limitations that merit consideration. First, while in vitro data demonstrate NSUN5-mediated shifts between STING-dependent and DNA-PK-HSPA8 DNA sensing pathways, the lack of in vivo validation—due to species-specific divergence in DNA-PK signaling (human vs. murine) [37, 39]—limits translational relevance. Humanized models or patient-derived organoids are needed to address this issue. Second, focusing on CpGs shared between 450k and 27k arrays restricted genomic coverage, potentially overlooking novel loci detectable via EPIC arrays or whole-genome bisulfite sequencing. Third, while our DDR CpG-based prognostic model showed clinical promise, its reliance on microarray data necessitates validation through targeted assays (e.g., pyrosequencing) and prospective trials. Fourth, the regulatory roles of non-methylation epigenetic mechanisms (e.g., histone modifications) on NSUN5 remain unaddressed, and DDR CpGs with weak gene expression correlations may act on distal regulatory elements (e.g., enhancers) or non-coding RNAs, requiring multi-omics approaches to elucidate their functional relevance. Fifth, the tumor microenvironment’s complexity was incompletely addressed; stromal cells (e.g., microglia) may modulate NSUN5’s dual roles in tumor suppression and immune evasion, necessitating spatial transcriptomics for cell-type-specific insights. Finally, clinical heterogeneity from mixed IDH-mutant and wild-type gliomas might obscure subtype-specific DDR CpG effects. Stratified analyses by molecular subtypes (IDH status, 1p/19q codeletion) could refine prognostic precision. These limitations underscore the need for mechanistic studies, advanced models, and clinical optimization to harness DNA sensing pathways for glioma immunotherapy.
Conclusions
This study underscores the profound influence of global DNA methylation variation within DDR genes on glioma prognosis and immunity. A panel of five selected CpGs may delineate novel, biologically and clinically relevant glioma subtypes. Utilizing these CpGs, a risk-score signature was validated for predicting the outcome of the most aggressive non-G-CIMP subtype. NSUN5, regulated by one of these CpGs, illustrates the biological significance of DDR CpGs. NSUN5 depletion shifts cytosolic DNA sensing from cGAS-STING to DNA-PK-HSPA8, promoting a robust type I IFN response in GBM cells and enhancing microglial M1 polarization and chemotaxis, despite its tumor-promoting role in an immune-free environment. This may clarify the functional paradox of NSUN5 between experimental and clinical settings. Ultimately, this study reveals the intricate interplay among DNA methylation, DDR machinery, cytosolic DNA sensing, and tumor immunity, inspiring novel strategies for DNA-sensing-based immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully acknowledge all patients who agreed to participate in this study and those who provided their medical care. We gratefully acknowledge Dr. Menei, Dr. Le Reste, Dr. Vauleon, Dr. Quillien, and the Tumor Banks from Angers and Rennes for their constant support in the collection, processing, and histological analysis of tumor samples. We also gratefully thank those willing to share valuable scientific data (Dr. Rose K Lai and Prof. Jill Barnholtz-Sloan). The corresponding author (AAY) wants to thank his daughters (Jiayan Yin and Xiyan Yin) and his wife (Dr. Yu Dong) for their great support. The results published here are partly based on data generated by the China Glioma Genome Atlas, The Cancer Genome Atlas, the German Cancer Research Center, and the research teams mentioned in this study.
Author contributions
Conception and design of the study: KC, YMW, and AAY. Provision of study material or patients: YY, YFL, YSJ, WL, YLH, AE, MA, and JM. Acquisition and assembly of data: YY, YFL, YSJ, TCM, and WHG. Project administration, software, methodology: WL, YLH, KC, YMW, and AAY. Manuscript writing: All authors. Final approval of the manuscript: All authors.
Funding
This work was partially funded by grants from the National Natural Science Foundation of China (No. 81402049, 81802486, 81671476, 31570906), Shandong Province Natural Science Foundation (No. ZR2020QH0233), Shaanxi Province Natural Science Foundation (No.2023-JCYB-641), the Key Research and Development Plan in Shaanxi (No. 2019SF-059, 2020SF-204), the Key Innovative Project in Shaanxi (No. 2021ZDLSF02-02), Grants from the Brittany Region (France), and FEDER (Europe).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request or public databases; TCGA: https://tcga-data.nci.nih.gov; https://xena.ucsc.edu/public/; GEO: https://www.ncbi.nlm.nih.gov/geo/; CGGA: http://www.cgga.org.cn/.
Declarations
Ethics approval and consent to participate
Informed consent was obtained from all participants from the Neurosurgery Departments of Rennes and Angers University Hospitals and the Department of Neurosurgery, Xijing Hospital. This study was approved by the Institutional Review Board at Xijing Hospital, Air Force Medical University (No. KY20223039-1).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An-an Yin, Yan Yao, Yi-feng Liu and Yu-sha Ji have contributed equally to this work and share first authorship.
Contributor Information
Yuan-ming Wu, Email: wuym_fmmu@163.com, Email: wuym@fmmu.edu.cn.
Kun Chen, Email: chenkun@fmmu.edu.cn, Email: chenkun45@126.com.
References
- 1.Pouyan A, Ghorbanlo M, Eslami M, et al. Glioblastoma multiforme: insights into pathogenesis, key signaling pathways, and therapeutic strategies. Mol Cancer. 2025;24(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman R, Shi DD, Reitman ZJ, et al. Dna damage response in brain tumors: a society for neuro-oncology consensus review on mechanisms and translational efforts in neuro-oncology. Neuro Oncol. 2024;26(8):1367–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olivieri M, Cho T, Alvarez-Quilon A, et al. A genetic map of the response to Dna damage in human cells. Cell. 2020;182(2):481–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matarrese P, Signore M, Ascione B, Fanelli G, Paggi MG, Abbruzzese C. Chlorpromazine overcomes Temozolomide resistance in glioblastoma by inhibiting cx43 and essential Dna repair pathways. J Transl Med. 2024;22(1):667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornepati A, Rogers CM, Sung P, Curiel TJ. The complementarity of Ddr, nucleic acids and anti-tumour immunity. Nature. 2023;619(7970):475–86. [DOI] [PubMed] [Google Scholar]
- 6.Maleki DP, Sadoughi F, Mirzaei H, Asemi Z, Yousefi B. Dna damage response and repair in the development and treatment of brain tumors. Eur J Pharmacol. 2022;924:174957. [DOI] [PubMed] [Google Scholar]
- 7.Yin A, Shang Z, Etcheverry A, et al. Integrative analysis identifies an immune-relevant epigenetic signature for prognostication of non-g-cimp glioblastomas. Oncoimmunology. 2021;10(1):1902071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bomsztyk K, Mar D, Denisenko O, et al. Analysis of Dna methylation in gliomas: assessment of preanalytical variables. Lab Invest. 2024;104(12):102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bady P, Kurscheid S, Delorenzi M, et al. The Dna methylome of Ddr genes and benefit from Rt or Tmz in Idh mutant low-grade glioma treated in Eortc 22033. Acta Neuropathol. 2018;135(4):601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunez FJ, Mendez FM, Kadiyala P et al. Idh1-r132h acts as a tumor suppressor in glioma via epigenetic up-regulation of the Dna damage response. Sci Transl Med; 11(479)(2019). [DOI] [PMC free article] [PubMed]
- 11.Janin M, Ortiz-Barahona V, de Moura MC, et al. Epigenetic loss of rna-methyltransferase nsun5 in glioma targets ribosomes to drive a stress adaptive translational program. Acta Neuropathol. 2019;138(6):1053–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camfield S, Chakraborty S, Dwivedi S, Pramanik PK, Mukherjee P, Bhattacharya R. Secrets of Dna-pkcs beyond Dna repair. NPJ Precis Oncol. 2024;8(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samson N, Ablasser A. The cgas-sting pathway and cancer. Nat Cancer. 2022;3(12):1452–63. [DOI] [PubMed] [Google Scholar]
- 14.Tian Z, Zeng Y, Peng Y, Liu J, Wu F. Cancer immunotherapy strategies that target the cgas-sting pathway. Front Immunol. 2022;13:996663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceccarelli M, Barthel FP, Malta TM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai RK, Chen Y, Guan X, et al. Genome-wide methylation analyses in glioblastoma multiforme. PLoS ONE. 2014;9(2):e89376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in h3f3a and idh1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–37. [DOI] [PubMed] [Google Scholar]
- 19.Kurscheid S, Bady P, Sciuscio D, et al. Chromosome 7 gain and Dna hypermethylation at the hoxa10 locus are associated with expression of a stem cell related hox-signature in glioblastoma. Genome Biol. 2015;16(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. Mgmt-stp27 methylation status as predictive marker for response to Pcv in anaplastic oligodendrogliomas and oligoastrocytomas. A report from Eortc study 26951. Clin Cancer Res. 2013;19(19):5513–22. [DOI] [PubMed] [Google Scholar]
- 21.Horvath S, Garagnani P, Bacalini MG, et al. Accelerated epigenetic aging in down syndrome. Aging Cell. 2015;14(3):491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iorio F, Knijnenburg TA, Vis DJ, et al. A landscape of Pharmacogenomic interactions in cancer. Cell. 2016;166(3):740–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Yan W, You G, et al. Genome-wide Dna methylation profiling identifies aldh1a3 promoter methylation as a prognostic predictor in g-cimp- primary glioblastoma. Cancer Lett. 2013;328(1):120–5. [DOI] [PubMed] [Google Scholar]
- 24.Etcheverry A, Aubry M, de Tayrac M, et al. Dna methylation in glioblastoma: impact on gene expression and clinical outcome. BMC Genomics. 2010;11:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gravendeel LA, Kouwenhoven MC, Gevaert O, et al. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 2009;69(23):9065–72. [DOI] [PubMed] [Google Scholar]
- 26.Gusev Y, Bhuvaneshwar K, Song L, Zenklusen JC, Fine H, Madhavan S. The Rembrandt study, a large collection of genomic data from brain cancer patients. Sci Data. 2018;5:180158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang K, Liu X, Li G, et al. Clinical management and survival outcomes of patients with different molecular subtypes of diffuse gliomas in China (2011–2017): a multicenter retrospective study from Cgga. Cancer Biol Med. 2022;19(10):1460–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–27. [DOI] [PubMed] [Google Scholar]
- 29.Du P, Zhang X, Huang CC, et al. Comparison of beta-value and m-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin AA, He YL, Etcheverry A, et al. Novel predictive epigenetic signature for Temozolomide in non-g-cimp glioblastomas. Clin Epigenetics. 2019;11(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanzelmann S, Castelo R, Guinney J. Gsva: gene set variation analysis for microarray and rna-seq data. BMC Bioinformatics. 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang P, Gu S, Pan D, et al. Signatures of t cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hothorn T, Zeileis A. Generalized maximally selected statistics. Biometrics. 2008;64(4):1263–9. [DOI] [PubMed] [Google Scholar]
- 36.Malikov V, Meade N, Simons LM, Hultquist JF, Naghavi MH. Fez1 phosphorylation regulates hspa8 localization and interferon-stimulated gene expression. Cell Rep. 2022;38(7):110396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burleigh K, Maltbaek JH, Cambier S et al. Human Dna-pk activates a sting-independent Dna sensing pathway. Sci Immunol; 5(43)(2020). [DOI] [PMC free article] [PubMed]
- 38.Khan F, Pang L, Dunterman M, Lesniak MS, Heimberger AB, Chen P. Macrophages and microglia in glioblastoma: heterogeneity, plasticity, and therapy. J Clin Invest; 133(1)(2023). [DOI] [PMC free article] [PubMed]
- 39.Wu R, Sun C, Chen X, et al. Nsun5/tet2-directed chromatin-associated Rna modification of 5-methylcytosine to 5-hydroxymethylcytosine governs glioma immune evasion. Proc Natl Acad Sci U S A. 2024;121(14):e1973356175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han H, Zhang C, Shi W, et al. Nsun5 facilitates hepatocellular carcinoma progression by increasing smad3 expression. Adv Sci (Weinh). 2025;12(2):e2404083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad K, Brahma S, Henikoff S. Epigenetic pioneering by Swi/snf family remodelers. Mol Cell. 2024;84(2):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng S, Feng Y, Pauklin S. 3d chromatin architecture and transcription regulation in cancer. J Hematol Oncol. 2022;15(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, Kong YS, Vincent KM, et al. Rna cytosine methyltransferase nsun5 promotes protein synthesis and tumorigenic phenotypes in glioblastoma. Mol Oncol. 2023;17(9):1763–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui Y, Hu Z, Zhang C. Rna methyltransferase nsun5 promotes esophageal cancer via 5-methylcytosine modification of mettl1. Mol Carcinog. 2025;64(3):399–409. [DOI] [PubMed] [Google Scholar]
- 45.Su Y, Liu J, Zheng Z, et al. Nsun5-fth1 axis inhibits ferroptosis to promote the growth of gastric cancer cells. Cell Biochem Biophys. 2023;81(3):553–60. [DOI] [PubMed] [Google Scholar]
- 46.Jiang Z, Li S, Han MJ, Hu GM, Cheng P. High expression of nsun5 promotes cell proliferation via cell cycle regulation in colorectal cancer. Am J Transl Res. 2020;12(7):3858–70. [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Chen XN, Zhang H, et al. Cdk13 promotes lipid deposition and prostate cancer progression by stimulating nsun5-mediated m5c modification of acc1 Mrna. Cell Death Differ. 2023;30(12):2462–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen B, Hong Y, Zhai X, et al. M6a and m5c modification of gpx4 facilitates anticancer immunity via Sting activation. Cell Death Dis. 2023;14(12):809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Li M, Zheng J, Li Z, Chen X. Knocking down nsun5 inhibits the development of clear cell renal cell carcinoma by inhibiting the p53 pathway. Aging. 2023;15(11):4757–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruda R, Horbinski C, van den Bent M, Preusser M, Soffietti R. Idh Inhibition in gliomas: from preclinical models to clinical trials. Nat Rev Neurol. 2024;20(7):395–407. [DOI] [PubMed] [Google Scholar]
- 51.Pilger D, Seymour LW, Jackson SP. Interfaces between cellular responses to Dna damage and cancer immunotherapy. Genes Dev. 2021;35(9–10):602–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reislander T, Groelly FJ, Tarsounas M. Dna damage and cancer immunotherapy: a Sting in the Tale. Mol Cell. 2020;80(1):21–8. [DOI] [PubMed] [Google Scholar]
- 53.Kwon J, Bakhoum SF. The cytosolic dna-sensing cgas-sting pathway in cancer. Cancer Discov. 2020;10(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taffoni C, Schussler M, Vila IK, Laguette N. Harnessing the Cooperation between dna-pk and Cgas in cancer therapies: the Cooperation between dna-pk and Cgas shapes tumour immunogenicity: the Cooperation between dna-pk and Cgas shapes tumour immunogenicity. BioEssays. 2023;45(7):e2300045. [DOI] [PubMed] [Google Scholar]
- 55.Taffoni C, Marines J, Chamma H, et al. Dna damage repair kinase dna-pk and Cgas synergize to induce cancer-related inflammation in glioblastoma. Embo J. 2023;42(7):e111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu R, Zhu B, Chen D. Type i interferon-mediated tumor immunity and its role in immunotherapy. Cell Mol Life Sci. 2022;79(3):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu C, Xiao M, Li X, et al. Origin, activation, and targeted therapy of glioma-associated macrophages. Front Immunol. 2022;13:974996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Zhou X, Hao H. Macrophage phenotype-switching in cancer. Eur J Pharmacol. 2022;931:175229. [DOI] [PubMed] [Google Scholar]
- 59.Damia G. Targeting dna-pk in cancer. Mutat Res. 2020;821:111692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request or public databases; TCGA: https://tcga-data.nci.nih.gov; https://xena.ucsc.edu/public/; GEO: https://www.ncbi.nlm.nih.gov/geo/; CGGA: http://www.cgga.org.cn/.