Abstract
Stachys tibetica, a significant medicinal plant was collected from Ladakh (India) and subjected to extraction. The extract was titrated with AgNO3 at 50ºC to obtain silver nanoparticles with plant extract acts as a reducing and capping agent. The eco-friendly synthetic procedure utilizes the phytochemicals in Stachys tibetica, ensuring a sustainable and non-toxic approach to nanoparticle production. The characterization of the silver nanoparticles was conducted using various techniques, including Fourier-transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), and dynamic light scattering (DLS). Field emission scanning electron microscopy (FE-SEM) was utilized to examine the surface morphology, revealing the irregular and rugged spherical shape of the synthesized nanoparticles. Stachys tibetica-derived silver nanoparticles (AgNPs) act as a versatile and robust multifunctional material with proficient bioactivity and catalytic properties. The synthesized silver nanoparticles displayed effective antioxidant and antimicrobial properties against Bacillus Cereus, Pseudomonas, Escherichia coli, and S.Aureus. Additionally, the green nanoparticles degraded Rhodamine-B under sunlight irradiation within 1 h at a rate constant of 0.066 min− 1.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-92507-6.
Keywords: Stachys Tibetica, Silver nanoparticles, FE-SEM, XRD, Rhodamine-b
Subject terms: Environmental social sciences, Materials science
Introduction
Nanotechnology an emerging multidisciplinary field having roots in basic sciences such as physics, chemistry, and biology uses science to manipulate matter at the molecular level and create nanoparticles of varying sizes, shapes, and chemical compositions for their potential applications to human benefit1–4. Physical, chemical, and environmentally friendly processes are used to create nanoparticles5,6. In recent years, researchers have focused on developing efficient green chemistry procedures that use natural reducing capping and stabilizing chemicals to make metal nanoparticles with the desired morphology and size. Scientists have been successful in increasing the efficacy of these materials by reducing particle size, because of their sub-microscopic size they have unique material characteristics and so synthesized nanoparticles are used in various areas, such as engineering, medicine, environmental remediation, and catalysis7. Environmental and toxicity concerns involved in some of synthetic methodologies involving the use of organic solvents, toxic reducing substances used for the reduction of metals, etc. have led for the demand of environmentally friendly, clean, reliable and biologically appropriate approaches. Nanoparticle synthesis from plant extracts and different types of plant biomass not only uses time-conserving and environmentally friendly procedures but also these nanoparticles involve very simple and easy handling procedures8.
Stachys is a genus of 350–400 species of plants belonging to the family Lamiaceae, also called as mint family. Plants belonging to this family are found worldwide. Most members of this family are either perennial or annual herbs although some species of this family are shrubs or subshrubs9. Extensive phytochemical and pharmacological studies have been carried out on Stachys species, proving their ethnopharmacological benefits. Anti-inflammatory, antioxidant, analgesic, Renoprotective, anxiolytic, and depressive activities are some of the prominent biological activities carried out on these species10–13. The phytochemical content of these species is linked to a wide spectrum of medicinal benefits. As a result, the genus Stachys has garnered a lot of interest for the isolation and bioprospection of bioactive secondary metabolites of plant. In total, more than 200 compounds from this genus have been isolated, including terpenes (e.g., iridoids triterpenes, diterpenes, ), polyphenols (e.g., lignans, flavone derivatives, phenylethanoid glycosides, ), and phenolic acids etc10,14–18.
Very efficient metallic nanoparticles like Iron, nickel, Copper, and Silver have been synthesized by researchers from the plants of the Lamiaceae family e.g. Nanoparticles synthesized from Mentha Pulegium, exhibited potential antibacterial, cytotoxic, antioxidant and antifungal activities19–21. Besides being used as antibacterial agents nanoparticles also play the role of a catalyst for the degradation of various environmentally hazardous chemicals. Saliva officinalis mediated silver nanoparticles degrade Congo red dye very efficiently22. similarly, AgNPs from endostemon viscosus degrade methylene blue and crystal violet dyes23.
Keeping these findings in view, the aim of our study is the green synthesis of silver nanoparticles from Stachys tibetica (locally known as yaktse), which is not reported in the literature yet. we were further motivated to carry out antibacterial, anti-oxidant and dye degradation properties of these silver nanoparticles. The sample plant for this study was collected from Minjhi, Ladakh, and Silver nanoparticles were synthesized from the extract of this plant using a 20 mM AgNO3 solution. The bactericidal effect of these nanoparticles was checked against four bacterial strains namely Bacillus cereus, E.Coli, S.Aeureus, and Pseudomonas. The synthesized nanoparticles were further utilized as a catalyst in the degradation of environmentally hazardous dye Rhodamine-B with the use of a UV-Vis spectrophotometer. The absorbance spectrum of the solution was examined at various wavelengths. The absorbance value at 550–560 nm depicted dye concentration throughout degradation.
Materials and methods
Devices
The ATR-FTIR Spectrophotometer (Agilent Technologies, USA) was used to study the structural properties of dried plant extract and silver nanoparticles at a constant temperature of 25 °C. The wavenumber range was 500–4000 cm− 1. FE-SEM was used to analyze the surface morphology of nanoparticles (FEI Quanta 200, USA) operated at 200 V. XRD (Ultima-IV (Rigaku Corporation, Tokyo, Japan) uses Cu Kα radiation. The samples were scanned in a 2θ range of 10 − 80° at a scan rate of 5 min− 1.
Materials
Merck (Mumbai, India) supplied the analytical grade, high purity silver nitrate (AgNO3) and rhodamine-b used in this work. The glassware used in the experiments was cleaned with aqua regia and washed with deionized water. Double-distilled deionized water was used to prepare all aqueous solutions and reagents.
Collection of plant material
The aerial parts of Stachys tibetica plant were collected from Minjhi region of Ladakh India, after obtaining due permission from the concerned authorities. The proper identification of material was done by curator Akhter Ahmed Malik of the Department of Taxonomy, Kashmir University with voucher no [9062-(KASH)]. A herbarium of the plant specimen was deposited at Department of Taxonomy, Kashmir University, Srinagar.
Extract preparation
The collected material was shade-dried for ten days. 30 g of ground plant material was soaked in distilled water and kept on standing for 5 days. The final solution was filtered through Whatman filter paper No.1 to obtain a uniform yellowish aqua extract of Stachys tibetica for further experimentation24–26.
Methods
Preparation of silver nanoparticle
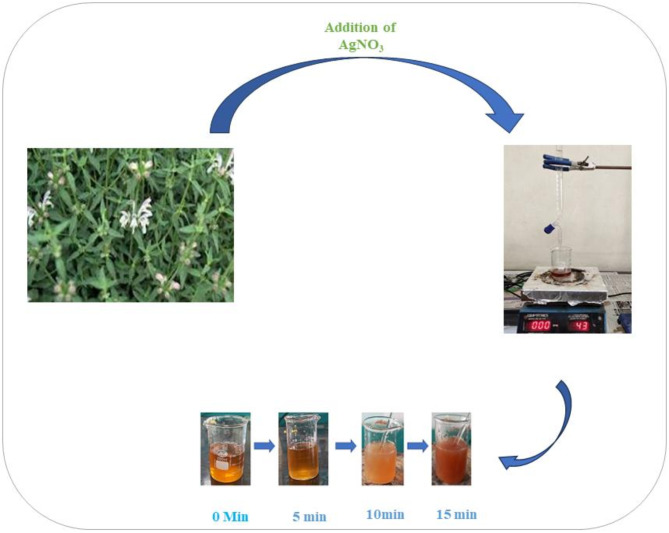
Stachtys tibetica extract-based nanoparticles were synthesized as per the green synthetic methodology reported for the synthesis of nanoparticles27. The aqueous extract of Stachys tibetica was dried on a water bath till the solution became deeply yellow. 20 mmol solution of AgNO3 was filled in the burette and added to aqueous extract of Stachys tibetica dropwise the solution was continuously stirred on a hotplate set at a temperature of 40 ºC. Silver nitrate solution was continuously added till the color of the solution changes from yellowish to deep brown which marked the end point of the reaction. The final solution was centrifuged at 8000 rpm for 15 min. The final nanoparticle was obtained by discarding the supernatant. The silver nanoparticle obtained was washed with distilled water 3–4 times to obtain a pure mass of nano scale particles which was further dried in oven at 50ºC to obtain a powdery mass of nanoparticle28–31.
Scheme 1.
Green synthesis of Silver nanoparticle from Aqueous extract of S. Tibetica.
Antibacterial activity
A panel of four bacterial strains namely Staphylococcus aureus, Bacillus cereus, Pseudomonas and E. coli, obtained from Microbial type culture collection (MTCC), Institute of Microbial Technology, Chandigarh, India. These bacterial strains were cultivated on nutrient agar plates and kept on agar slants at 37ºC. Microorganism cell suspension in 0.9% NaCl was adjusted to 0.5 Mc Farland to obtain approximately 106 cfu/ml32,33.
Antioxidant properties
The free radical scavenging potential of AgNPs, plant extract, and standard ascorbic acid was checked against DPPH(2,2-Diphenyl-1-Picrylhydrazyl) using a modified approach proposed by W brand Williams et al.34. In different test tubes solutions with concentrations (20,40,60,80,100,120,140,160,180,200 µg/ml) of AgNPs, plant extract, and ascorbic acid were prepared). To each solution, DPPH(1mM) prepared in methanol was added and mixed thoroughly. The solution obtained after the addition of DPPH was incubated in the dark for 30 min at room temperature before being measured for UV absorbance at 517 nm with a UV-Vis spectrophotometer (perkin-Elmer Lambda 950:UK). The DPPH solution obtained without the addition of a sample was used as a control to measure radical scavenging activity. The Free radical scavenging activity of all samples was calculated using the formula;
Radical scavenging activity = 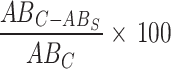
Where ABC is the absorbance of methanol + DPPH, and ABS is the absorbance of sample solution + DPPH35,36.
Degradation of rhodamine-B dye
10 mg of Rhodamine-b dye was typically added to 1000 mL of double distilled water as a stock solution. A control of dye and NaBH4(0.06 M) without the inclusion of silver nanoparticles was also kept. 5 ml freshly prepared NaBH4 solution was added to 20 ml of rhodamine B solution and subsequently, 5 ml of nanocatalyst of a definite concentration in double-distilled deionized water was added to it. Prior to irradiation, the reaction suspension was thoroughly mixed to ensure that the working solution was clearly balanced. Following that, the dispersion was exposed to visible light and continuously monitored. Aliquots of 2–3 mL solution were filtered and utilized to analyze the photocatalytic degradation of dye at particular time intervals. The absorbance spectrum of the solution was examined with a UV-Vis spectrophotometer at various wavelengths. The absorbance value at 550–560 nm depicted dye concentration throughout degradation37,38.
Results and discussion
Nanoparticles serve an effective scientific and research tools having an excellent catalytic efficiency due to their large surface area. Silver nanoparticles synthesized through green approach act as functional materials that have been explored for curbing of various modern biological, industrial and environmental challenges. These nanoparticles synthesized from the aqueous extract of Stachys tibetica were characterized using various techniques. Initial screening was carried out through FT-IR analysis, that revealed the presence of various functional groups. P-XRD revealed the crystallinity, and the morphology was screened using SEM analysis. The synthesised nanoparticles were explored in various directions ranging from bioactivity, antioxidant mitigation to water pollution remediation. The extensive discussions are addressed in various sections.
FTIR analysis of synthesized nanoparticle
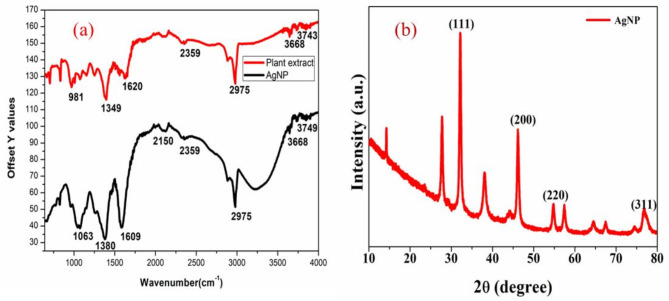
The FTIR (Fig. 1a) spectrum of produced AgNPs was carried out to determine the presence of different functional groups in the synthesized nanoparticles. The IR spectrum of silver nanoparticles shows absorption bands at 2975 cm− 1 corresponding to N-H Stretching, 2150 cm− 1 corresponding to C-N double bond stretching, 1609 cm− 1 corresponding to N-H bending, 1380 cm− 1 corresponding to N-O stretching, the IR bands of AgNPs confirm the presence of elements like Nitrogen (N), Oxygen (O) responsible for the reduction of silver. Plant extract absorbs at 2945 cm− 1,2041 cm− 1,1609 cm− 1,1392 cm− 1. On comparison of IR spectra of plant extract and nanoparticles, we observe a shift in the position of some bands besides some bands of plant extract are highly diminished in nanoparticles which may be due to the covalent and noncovalent interactions between the extract phytoconstituents and elemental silver.
Fig. 1.
(a) FTIR spectra of plant extract and silver nanoparticle (b) XRD pattern of AgNP.
XRD analysis
The XRD pattern (Fig. 1b) of silver nanoparticles shows strong peaks at 2θ = 33.21º, 38.47º, 47.37º and 77º which correspond to the (1 1 1), (2 0 0), (2 2 0), (3 1 1), Bragg’s reflections of the face-centered cubic structure of silver. In addition, unassigned peaks that were weaker than those of silver could be related to the bioorganic chemicals forming on the surface of the AgNPs39. The crystal size of the nanoparticles was determined by using Scherrer formula calculations40,41.
D = 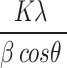
Where D = Crystal size in nanometres(nm), K = Scherrer constant (0.9), λ = X-Ray wavelength (1.54 Aº), β = Full width at half maximum (FWHM) of diffraction peaks(radians). θ = Diffraction angle.
Upon θ = 14º and β = 0.5, D = 16.7 nm. So, from Scherrer formula calculations, the crystallite size of the nanoparticles is found to be 16.7 nm.
SEM and EDS analysis of synthesized nanoparticle
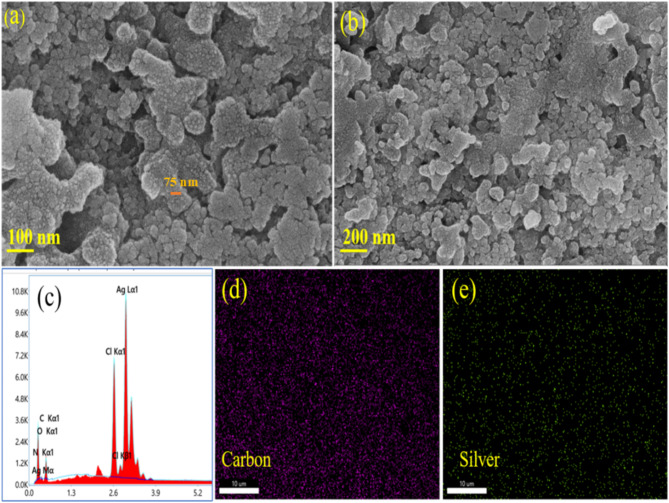
Field-Emission Scanning electron Microscope (FE-SEM) was used to analyze the surface topography and morphology of S.Tibetica based AgNPs. It is apparent from the SEM monographs that the material has developed into regular nanospheres with a rugged surface which increases the surface area of the catalyst Fig. 2a and b. The existence of silver metal in the produced nanoparticles was confirmed using energy dispersive X-ray spectroscopy (EDS). As seen in Fig. 2(C). A sharp peak at 3 KeV established the existence of elemental silver in the nanoparticles. The EDS examination of produced AgNPs revealed presence of elements like Carbon(C), Nitrogen(N), and Oxygen(O), etc. besides metallic silver because the substance generated via biological method had a minor fraction of Carbon, Nitrogen and Oxygen, etc42. , the same is also clear from the elemental mapping images of nanoparticles. A homogenous distribution of carbon and silver is seen in the elemental mapping images as seen in Fig. 2 (d, e) and supporting information (S1to S3).
Fig. 2.
(a,b) SEM images of Ag nanoparticles at 100 nm and 200 nm. (c) EDX of the nanoparticle. (d and e) Elemental mapping images of carbon and silver respectively revealing a uniform distribution of carbon and silver.
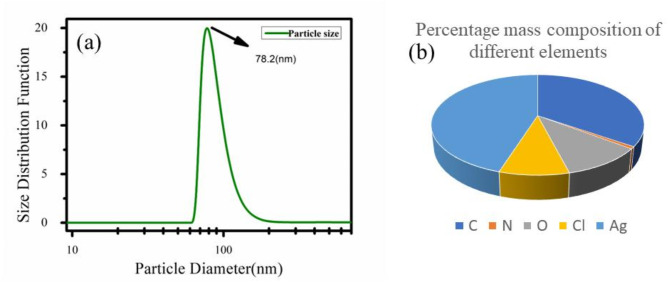
Dynamic light scattering (DLS) a common technique used to determine the particle size was employed to find out the size of the synthesized nanoparticles. The average particle size as measured from DLS measurements was found out to be 78.2 nm Fig. 3a. A pie chart representation of composition of different elements present in nanoparticle by weight percentages is depicted in Fig. 3b.
Fig. 3.
(a) Average particle size as obtained from DLS measurements. (b) Percentage weight composition of different elements.
Antibacterial properties
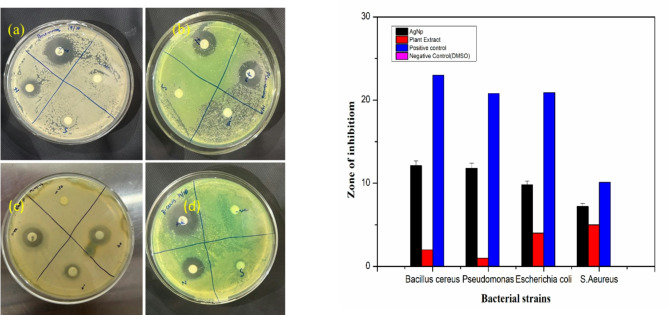
The MIC value of biosynthesized AgNPs to inhibit bacterial growth was determined by testing zone formation with dilutions ranging from 5 to 40 µg/ml. The synthesized AgNP has a MIC of 10 µg/ml and 20 µg/ml, for E. coli and C. aureus respectively (S-5). The antibacterial activity of AgNPs, Stachys tibetica plant extract, and Streptomycin was tested at a level of 10 µg/ml, as both pathogens demonstrated sensitivity at this concentration. The freshly prepared samples were tested for antibacterial activity against two gram-positive and two gram-negative strains namely Bacillus cereus, Pseudomonas, Escherichia coli, and Staphylococcus aureus. AgNPs (10 µg/ml) have shown highest zone of inhibition of about 12.1 mm for Bacillus Cereus followed by Pseudomonas (11.8 mm), E. coli (9.8 mm) and Staphylococcus aureus (7.2 mm). The zone of inhibition observed for plant extract was less as compared to zone of inhibition obtained for AgNPs as shown in Fig. 4a and S6. A bar graph representation of zones of inhibition of positive standard (Streptomycin), AgNPs and plant extract is shown in Fig. 4b.
Fig. 4.
Antibacterial bacterial activity against four bacterial strains (a) Bacillus cereus (b) Pseudomonas (c) E. coli (d) S. aureus (e) Bar graph plot of zone of inhibition of four bacterial strains.
This study developed the synergistic antibiotic activity of AgNPs, which is greater than the individual antibacterial activity of plant extract when used together43. AgNP behaves as a positively charged centre that interacts with the negatively charged centres of bacterial cells44. Recent research has found that the methods of action of AgNPs against microbes are dependent on the NPs’ size, shape, stability, and affinity45. Literature has cited that the bactericidal effect of AgNPs is due to the interaction of silver with nucleosides of nucleic acids and the creation of subsequent silver compounds with bactericidal potential25. The organic layer-coated silver nanoparticle has shown the highest bactericidal effect towards Bacillus cereus (12.1 ± 1 mm) which can be inferred from the relatively simple cell wall structure of G(+) bacterial species and the presence of a thick PG layer of 30 nm46. The reason of bactericidal behaviour of AgNPs towards Gram negative G(-) can be inferred from the fact that G(-) bacteria have a small PG layer of 1–5 nm between the exterior layer and the cytoplasmic film that makes these species prone to bactericidal effects47.
Antioxidant properties
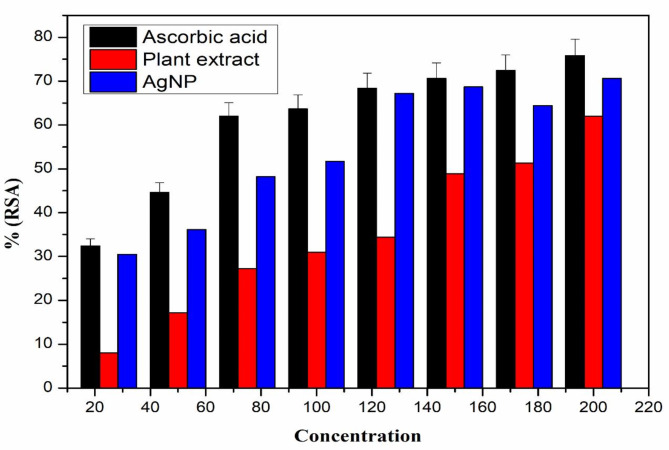
DPPH has hydrogen acceptor capability to antioxidants48.DPPH can easily accept electron from antioxidant compounds and during this process the color of DPPH changes from violet to yellow. Thus, DPPH is a free radical that accepts a free electron from antioxidants and becomes a stable diamagnetic molecule. The DPPH radical scavenging tends to increase with increasing the concentration of AgNPs, showing a maximum at higher concentrations. At concentrations 20–200 µg/mL, AgNPs showed 32.4–75.8%, Plant extract showed 8–62%, and standard ascorbic acid showed 34.5– 89.6% radical scavenging activity. the radical scavenging activity of plant extract and AgNp was lower than that of standard ascorbic acid. The results obtained for the antioxidant activity of AgNP, plant extract, and standard ascorbic acid at different concentrations are represented in Fig. 5 and S4.
Fig. 5.
Percentage radical scavenging activity of ascorbic acid, plant extract and AgNP.
Dye degradation
Dye degradation studies were performed using AgNPs as catalyst. The following formula was used to calculate the percentage of dye degradation:
%Degradation = 100× ( - C) /
- C) /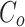
where Cο is the initial concentration of dye solution and C is the concentration of dye solution following photocatalytic degradation. The PFO model was found to be the optimal choice in determining the rate constant and rate constant was found out to be 0.06667 min− 1 using the following rate equation:
 |
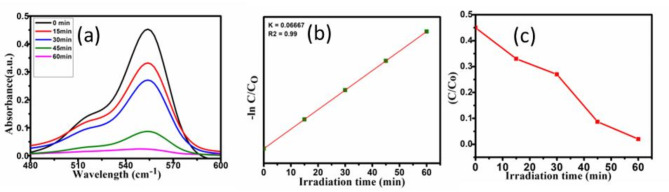
The catalytic efficiency of AgNPs was accessed from the λmax peak at 550–560 nm at different intervals of irradiation. In the presence of AgNPs, the primary absorption peak of dye at 550 nm decreased slowly with increasing time of exposure of dye to visible light in the presence of AgNp. The decrease in intensity of peak at 550 nm depicted the photocatalytic degradation of Rhodamine-b dye in the presence of AgNPs Fig. 6a–c. In the Dye degradation reaction, NaBH4 helps in the reduction of dye, the Ag nanoparticles act as an electron relay from BH4−(donor) to dye(acceptor). The plausible mechanism of dye degradation is believed to proceed with the decomposition of NaBH4 into BH4− (nucleophilic) and subsequently produces H-Ag and Ag-BH3 as reaction intermediates in presence of AgNPS. The intermediate Ag-H is responsible for the degradation of dye(electrophilic) into harmless degradation products49.
Fig. 6.
(a) The gradual decrease in intensity of absorption peak at 550 nm with irradiation time. (b) The plot of - 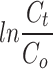 versus time and value of rate constant is found to be 0.06667 min− 1 (c) Gradual decrease in the concentration of Rhodamine-b with time.
versus time and value of rate constant is found to be 0.06667 min− 1 (c) Gradual decrease in the concentration of Rhodamine-b with time.
Studies on photocatalytic studies demonstrated that the photocatalytic activity of metallic nanoparticles is dependent on their crystallographic structure, shape, and size50. J Kadam et al. employed AgNPs produced from Cauliflower waste to photodegrade methylene blue in 152 min51,52, whereas the current investigation exhibited 100% Rh-B degradation in just 1 h As a result, this study demonstrated the efficient photodegradation capacity of AgNPs generated using S. Tibetica in visible light.
Conclusion
The green synthesis of silver nanoparticles from Stachys tibetica extract has been carried out in the present study, whose characterization revealed the face-centered cubic structure. The synthesized nanoparticles not only act as good catalyst for the degradation of rhodamine-b but also exhibited significant antibacterial and anti-oxidant properties. Therefore the versatile applicability of AgNPs could pave the way for the future applications of S.tibetica-mediated AgNPs for the prevention of microbial infection, mitigation of free radicals, and degradation of harmful chemicals like rhodamine-b. The multifunctional characteristics of these NPs are also consistent with the United Nations Development Goals, particularly SDG 6 (Clean Water and Sanitation), which facilitates water purification, and SDG 12 (Responsible Consumption and Production), which promotes environmentally friendly synthesis. Thus, Stachys tibetica derived AgNPs provide a sustainable solution for environmental remediation and green technology applications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Department of Chemistry, university of Kashmir, and NIT Srinagar for providing the necessary facilities to carry out biological activity and characterization during the work.
Author contributions
Doctor syed wajaht amin shah conceptualized, drafted, and supervised the work . Mr Naseer Ahmad Dar carried out synthesis and characterization of the synthesized nanoparticles . Dr. Parvaiz wrote and thoroughly checked the manuscript. Dr Mahpara carried out biological properties and communicated the manuscript.
Data availability
The data underlying this study are available in the manuscript and its supplementary material.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fardood, S. T., Ramazani, A. & Moradi, S. Green synthesis of Ni–Cu–Mg ferrite nanoparticles using tragacanth gum and their use as an efficient catalyst for the synthesis of polyhydroquinoline derivatives. J. Solgel Sci. Technol.82, 432–439 (2017). [Google Scholar]
- 2.Albrecht, M. A., Evans, C. W. & Raston, C. L. Green chemistry and the health implications of nanoparticles. Green Chem.8, 417–432 (2006). [Google Scholar]
- 3.Khaghani, S., Ghanbari, D. & Khaghani, S. Green synthesis of iron oxide-palladium nanocomposites by pepper extract and its application in removing of colored pollutants from water. J. Nanostruct.7, 175–182 (2017). [Google Scholar]
- 4.Fardood, S. T., Atrak, K. & Ramazani, A. Green synthesis using tragacanth gum and characterization of Ni–Cu–Zn ferrite nanoparticles as a magnetically separable photocatalyst for organic dyes degradation from aqueous solution under visible light. J. Mater. Sci.: Mater. Electron.28, 10739–10746 (2017). [Google Scholar]
- 5.Fardood, S. T., Ramazani, A. & Moradi, S. A novel green synthesis of nickel oxide nanoparticles using Arabic gum. Chem. J. Moldova, 115–118 (2017).
- 6.Hosseini, S. A. & Moalemzade, P. CuFe 2 – x Lu X O 4 nanoparticles: synthesis through a green approach and its photocatalyst application. J. Mater. Sci. Mater. Electron.27, 8802–8806 (2016). [Google Scholar]
- 7.Adeyemi, J. O., Oriola, A. O., Onwudiwe, D. C. & Oyedeji, A. O. Plant extracts mediated metal-based nanoparticles: synthesis and biological applications. Biomolecules12, 627 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangula, A., Podila, R., Karanam, L., Janardhana, C. & Rao, A. M. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from Breynia rhamnoides. Langmuir27, 15268–15274 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Manikandan, D. B. et al. Green fabrication, characterization of silver nanoparticles using aqueous leaf extract of Ocimum americanum (Hoary Basil) and investigation of its in vitro antibacterial, antioxidant, anticancer and photocatalytic reduction. J. Environ. Chem. Eng.9, 104845 (2021). [Google Scholar]
- 10.Tundis, R., Peruzzi, L. & Menichini, F. Phytochemical and biological studies of Stachys species in relation to chemotaxonomy: A review. Phytochemistry102, 7–39 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Khanavi, M., Sharifzadeh, M., Hadjiakhoondi, A., Shafiee, A. & Koch Phytochemical investigation and anti-inflammatory activity of aerial parts of Stachys byzanthina C. J. Ethnopharmacol., 97 463–468. (2005). [DOI] [PubMed] [Google Scholar]
- 12.Jahani, R. et al. Evaluation and comparison of the antidepressant-like activity of Artemisia dracunculus and Stachys Lavandulifolia ethanolic extracts: an in vivo study. Res. Pharm. Sci.14, 544 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadeghi, H. et al. Antioxidant and protective effect of Stachys pilifera Benth against nephrotoxicity induced by cisplatin in rats. J. Food Biochem.44, e13190 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Marin, P. D., Grayer, R. J., Grujic-Jovanovic, S., Kite, G. C. & Veitch, N. C. Glycosides of Tricetin Methyl ethers as chemosystematic markers in Stachys subgenus Betonica. Phytochemistry65, 1247–1253 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Karioti, A., Bolognesi, L., Vincieri, F. F. & Bilia, A. R. Analysis of the constituents of aqueous preparations of Stachys recta by HPLC–DAD and HPLC–ESI-MS. J. Pharm. Biomed. Anal.53, 15–23 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Meremeti, A., Karioti, A., Skaltsa, H., Heilmann, J. & Sticher, O. Secondary metabolites from Stachys Ionica. Biochem. Syst. Ecol.32, 139–151 (2004). [Google Scholar]
- 17.Piozzi, F. & Bruno, M. Diterpenoids from roots and aerial parts of the genus Stachys. Records Nat. Prod.5, 1 (2011). [Google Scholar]
- 18.Goren, A. C. Use of Stachys species (mountain tea) as herbal tea and food. Records Nat. Prod.8, 71 (2014). [Google Scholar]
- 19.Dousari, A. S., Hosseininasab, S. S., Akbarizadeh, M. R., Naderifar, M. & Satarzadeh, N. Mentha pulegium as a source of green synthesis of nanoparticles with antibacterial, antifungal, anticancer, and antioxidant applications. Sci. Hort.320, 112215 (2023). [Google Scholar]
- 20.ShekoftehNarm, T., Hamidinezhad, H., Sabouri, Z. & Darroudi, M. Plant-based synthesis of Selenium-doped silver/magnesium oxide/zinc oxide nanocomposite using Mentha Pulegium to investigate their photocatalytic and biological properties. J. Mol. Struct., 139678 (2024).
- 21.Rad, S. S., Sani, A. M. & Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L). Microb. Pathog.131, 239–245 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Albeladi, S. S. R., Malik, M. A. & Al-thabaiti, S. A. Facile biofabrication of silver nanoparticles using Salvia officinalis leaf extract and its catalytic activity towards congo red dye degradation. J. Mater. Res. Technol.9, 10031–10044 (2020). [Google Scholar]
- 23.Chinnasamy, R. et al. Eco-friendly synthesis of Ag-NPs using endostemon viscosus (Lamiaceae): antibacterial, antioxidant, larvicidal, photocatalytic dye degradation activity and toxicity in zebrafish embryos. Environ. Res.218, 114946 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Logeswari, P., Silambarasan, S. & Abraham, J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc.19, 311–317 (2015). [Google Scholar]
- 25.Ahmed, S., Ahmad, M., Swami, B. L. & Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res.7, 17–28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee, P., Satapathy, M., Mukhopahayay, A. & Das, P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess.1, 1–10 (2014). [Google Scholar]
- 27.Vanlalveni, C. et al. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv.11, 2804–2837 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alharbi, N. S., Alsubhi, N. S. & Felimban, A. I. Green synthesis of silver nanoparticles using medicinal plants: characterization and application. J. Radiation Res. Appl. Sci.15, 109–124 (2022). [Google Scholar]
- 29.Prasad, R. Synthesis of silver nanoparticles in photosynthetic plants. J. Nanopart.2014, 963961 (2014). [Google Scholar]
- 30.Ahmad, N. et al. Rapid synthesis of silver nanoparticles using dried medicinal plant of Basil. Colloids Surf. B81, 81–86 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Jain, S. & Mehata, M. S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep.7, 15867 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah, M. Z. et al. Synthesis of silver nanoparticles using Plantago lanceolata extract and assessing their antibacterial and antioxidant activities. Sci. Rep.11, 20754 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Urnukhsaikhan, E., Bold, B. E., Gunbileg, A. & Sukhbaatar, N. Mishig-Ochir, antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep.11, 21047 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brand-Williams, W., Cuvelier, M. E. & Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol.28, 25–30 (1995). [Google Scholar]
- 35.Ansar, S. et al. Eco friendly silver nanoparticles synthesis by Brassica oleracea and its antibacterial, anticancer and antioxidant properties. Sci. Rep.10, 18564 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arshad, H., Sami, M. A., Sadaf, S. & Hassan, U. Salvadora persica mediated synthesis of silver nanoparticles and their antimicrobial efficacy. Sci. Rep.11, 5996 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mavaei, M., Chahardoli, A., Shokoohinia, Y., Khoshroo, A. & Fattahi, A. One-step synthesized silver nanoparticles using isoimperatorin: evaluation of photocatalytic, and electrochemical activities. Sci. Rep.10, 1762 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarina, S., Waclawik, E. R. & Zhu, H. Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem.15, 1814–1833 (2013). [Google Scholar]
- 39.Ajitha, B., Reddy, Y. A. K. & Reddy, P. S. Biogenic nano-scale silver particles by Tephrosia purpurea leaf extract and their inborn antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.121, 164–172 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Nazir, I. et al. Electrochemical detection and catalytic reduction of nitrobenzene using a bimetallic NiS 2/fe 3 S 4 magnetic heterostructure: an innovative approach for environmental remediation. New J. Chem.48, 4909–4921 (2024). [Google Scholar]
- 41.Nazir, I. et al. Synergistic Sb2S3-NiS2 heterostructure: A robust electrocatalyst for electrochemical sensing of hg (II), as (III) ions and carbendazim fungicide. J. Environ. Chem. Eng.12, 112793 (2024). [Google Scholar]
- 42.Oves, M. et al. Green synthesis of silver nanoparticles by Conocarpus lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci.29, 460–471 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raja, S., Ramesh, V. & Thivaharan, V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab. J. Chem.10, 253–261 (2017). [Google Scholar]
- 44.Cao, R., Jin, C. A. & Mirkin DNA-modified core – shell Ag/Au nanoparticles. J. Am. Chem. Soc.123, 7961–7962 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Park, S. B. et al. Silver-coated magnetic nanocomposites induce growth Inhibition and protein changes in foodborne bacteria. Sci. Rep.9, 17499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh, M., Singh, S., Prasad, S. & Gambhir, I. Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Digest J. Nanomater. Biostruct.3, 115–122 (2008). [Google Scholar]
- 47.Guzman, M., Dille, J. & Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med.8, 37–45 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Safaepour, M., Shahverdi, A. R., Shahverdi, H. R., Khorramizadeh, M. R. & Gohari, A. R. Green synthesis of small silver nanoparticles using geraniol and its cytotoxicity against fibrosarcoma-wehi 164. Avicenna J. Med. Biotechnol.1, 111 (2009). [PMC free article] [PubMed] [Google Scholar]
- 49.Blosi, M. et al. Green and easily scalable microwave synthesis of noble metal nanosols (Au, Ag, Cu, Pd) usable as catalysts. New J. Chem.38, 1401–1409 (2014). [Google Scholar]
- 50.Balázs, N. et al. The effect of particle shape on the activity of nanocrystalline TiO2 photocatalysts in phenol decomposition. Appl. Catal. B. 84, 356–362 (2008). [Google Scholar]
- 51.Kadam, J., Dhawal, P., Barve, S. & Kakodkar, S. Green synthesis of silver nanoparticles using cauliflower waste and their multifaceted applications in photocatalytic degradation of methylene blue dye and hg 2 + biosensing. SN Appl. Sci.2, 1–16 (2020). [Google Scholar]
- 52.Roy, K., Ghosh, C. K. & Sarkar, C. K. Degradation of toxic textile dyes and detection of hazardous Hg2 + by low-cost bioengineered copper nanoparticles synthesized using Impatiens balsamina leaf extract. Mater. Res. Bull.94, 257–262 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the manuscript and its supplementary material.