Abstract
Global plastic waste production remains a critical environmental issue. Microplastics (MPs), plastic particles less than 5 mm, are now pervasive across ecosystems. Humans are exposed to MPs via ingestion, inhalation, and dermal contact raising concerns about their health impacts. This systematic review investigates the influence of MPs on the human gut microbiome, focusing on changes in microbial composition, diversity, and metabolic pathways based on 12 studies identified through Scopus and PubMed following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Findings show that exposure to MPs such as polyethylene (PE), polystyrene (PS), polyethylene terephthalate (PET), polyvinyl chloride (PVC), and polylactic acid (PLA), induces gut dysbiosis, marked by a loss of beneficial genera, and enrichment of pathogenic species. MPs also impair short-chain fatty acid (SCFA) production, alter metabolic functions, and modulate immune pathways, contributing to intestinal diseases, metabolic syndrome, and chronic inflammation. The extent of disruption is influenced by MP-specific properties such as type, size, and concentration. These results suggest that MPs are emerging environmental risk factors with tangible implications for human health. To fully understand the health concerns associated with MPs long-term, human-relevant studies with standardized methodologies are urgently needed to define safe exposure levels and guide policies aimed at reducing MP-related health risks.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-025-04140-2.
Keywords: Microplastics, Gut Microbiome, Microbial diversity, Dysbiosis, Intestinal health
Introduction
The unceasing production of plastic waste remains a serious threat to environmental sustainability for decades since the invention of plastics. Despite the introduction of biodegradable plastics as a solution to reduce pollution, their incomplete degradation regrettably results in greater amount of microplastics (MPs), which adds to their persistence in the environment [1]. MPs, plastic particles less than 5 mm in diameter, are considered persistent pollutants due to their ability to accumulate in organisms over time and capacity for long-range transport [2]. Nanoplastics (NPs), potentially derived from the further breakdown of MPs and typically range from 1 to 100 nm in diameter are thought to exhibit more distinct physicochemical behaviors due to their smaller size and larger surface area.
Both MPs and NPs are found in various environments, including air, water, soil, household products, and food leading to unavoidable human exposure through ingestion, inhalation, and skin contact [3]. Alarmingly, MPs and NPs are known to be present and accumulated in human tissues including the lungs, placenta, feces, and blood [4, 5]. These findings raise urgent concerns about their potential impact on human health. In fact, this widespread exposure has been associated with the increased risk of several acute and chronic health conditions, including inflammatory bowel disease (IBD), infertility, diabetes, and neurodegenerative disorders. Among the known exposure routes, food consumption is considered the primary source of MPs. Moreover, MPs contamination in drinking water systems is widespread. Due to its high daily intake volume and consistent contamination across both tap and bottled sources, it represents a critical and unavoidable pathway for chronic MP exposure in humans [6, 7]. Once ingested, MPs can be absorbed through the gastrointestinal (GI) tract, disrupting gut microbiota by altering dietary habits, microbial metabolism, and immune responses [8].
The human microbiome is a diverse community of microorganisms residing in different parts of the body sites, including the skin, oral cavity, reproductive system, and gut. Due to its critical role in health and disease, the gut microbiome has drawn the most scientific attention [9]. The gut microbiome plays a crucial role in human physiology, regulating immune responses, metabolism, and intestinal integrity. Disruptions in microbial composition also known as dysbiosis, have been linked to intestinal barrier dysfunction and metabolic disorders. One essential role of the gut microbiome is the fermentation of dietary components, which leads to the production of key metabolites such as short-chain fatty acids (SCFAs) and aryl hydrocarbon receptor (AhR) ligands s [10, 11]. These metabolites are known to support intestinal barrier integrity and modulate immune responses, providing evidence for its influence on GI and systemic health [12, 13].
As aforementioned, ingested MPs could interact with the intestinal environment before excretion, potentially causing toxic effects on the gut [14].While several studies have reported the detection of MPs in human feces [4, 5], a critical lack remains in synthesizing and analyzing how MPs affect hut microbiota composition, diversity and function specifically in humans. Existing reviews have largely centered on environmental contamination or findings from animal models, without addressing human-relevant microbiome alterations. The studies in mammals and aquatic organisms consistently demonstrated that MPs, such as polystyrene (PS), polyethylene (PE), polyvinyl chloride (PVC), and polyethylene terephthalate (PET), accumulate in the intestinal tract, leading to conditions such as IBD, irritable bowel syndrome (IBS), and colitis [1, 15]. PS MPs, mainly has also been shown to disrupt gut microbial composition and induce hepatic lipid metabolism disorders in mice, suggesting gut-level toxicity [16]. Segment-specific intestinal damage especially in the colon has been linked to, reduced microbiota diversity and impairing nutrient metabolism [17]. Exposure to MPs worsened colitis, leading to shorter colon length, increased inflammation, reduced mucus secretion, thereby increasing the risk of colorectal cancer. MPs have been increasing suggested as potential environmental drivers of rising early-onset colorectal cancer rates [18, 19]. Despite this growing concern, no study to date has analyzed how specific types of MPs, affect the human gut microbiome and there is also a lack of synthesized evidence on the biological mechanisms by which MPs influence microbial communities.
This systematic review aims to examine the effects of MPs on the human gut microbiome, focusing on changes in microbial composition at the phylum, family, and genus levels, along with the alterations in microbial diversity and richness. It also looks into how MPs disrupt microbial functions and metabolic pathways. It highlights the health implications of MP-induced dysbiosis and identifies consistent microbial patterns. Furthermore, the review assesses how MP-specific properties such as size, shape, and polymer type influence microbiome responses, emphasizing the importance of additional research to address gaps in existing studies, providing a critical foundation for future risk assessment and targeted research for more understanding of MPs’ impact on human health.
Methodology
This systematic review was conducted in adherence to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework. PRISMA offers a structured and transparent approach to identifying, selecting, and synthesizing relevant research, ensuring the reliability and generalizability of the findings.
The literature search was conducted in July 2024 using two major scientific databases, Scopus and PubMed using the following search string “(microplastic OR nanoplastic) AND (human) AND (microbiome* OR microbiota* OR microbiomic*)”, designed to identify relevant studies, pertaining to the impact of micro- and nano-plastics on the human gut microbiome. No publication date restrictions were imposed, thereby ensuring the inclusion of all pertinent publications. The initial search resulted in a total of 320 records, with 235 from Scopus and 85 from PubMed, covering studies published between 2021 and May 2024.
Predefined inclusion and exclusion criteria were applied to ensure an unbiased screening process. To focus on original data and findings, only primary research articles were included. This excluded secondary publications such as reviews and book chapters to avoid duplication of findings that are potentially already identified in those secondary publications. Second, the language of publication was restricted to English to mitigate language barriers and potential bias associated with translation. Finally, all studies included had to directly address the impact of MPs and nanoplastics (NPs) on the human gut microbiome. Hence, studies outside the scope of this specific human health concern or those using animal models were excluded.
To expedite the screening process and ensure efficiency, initial filtering was conducted within each database, i.e. Scopus and PubMed, using their built-in filters to eliminate non-primary research and non-English studies. Following this initial filter, the search results from both databases were exported to EndNote 21, a reference management software, to identify and remove duplicate entries. Next, titles and abstracts were screened against the predefined inclusion and exclusion criteria. This ensured only English-language primary research studies that investigating the effects of MPs and NPs on the human gut microbiome were advanced for full-text evaluation. A total of 18 studies met these criteria and were retrieved for full-text evaluation. A full-text review of these 18 studies resulted in the exclusion of 6 additional studies that were either review articles or utilised animal models.
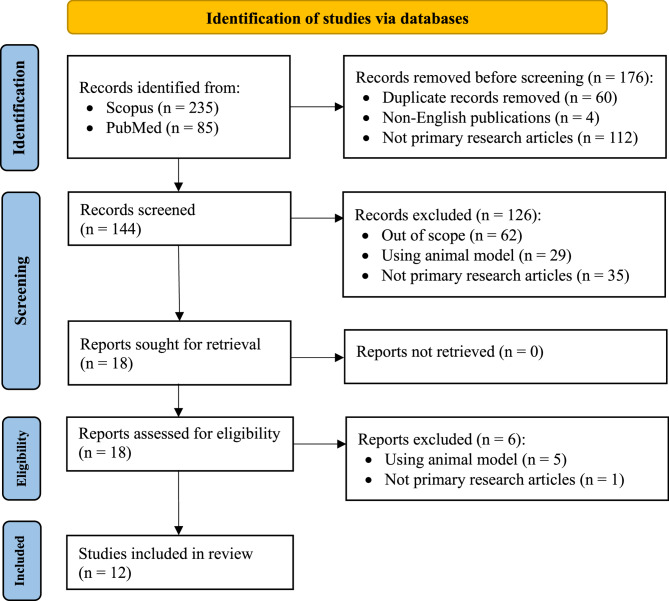
In the selection process, human cohort studies due to their higher external validity and direct relevance were prioritized. In vitro and in silico models were included only when they offered mechanistic insights that complemented findings of human studies. The quality of observational studies was evaluated using the Risk Of Bias In Non-randomized Studies– of Interventions (ROBINS-I) tool. Formal risk-of-bias assessments were not applied to in vitro or in silico studies; however, their methodological limitations were acknowledged and considered during the synthesis and interpretation of results. Ultimately, this selection process yielded 12 high-quality studies for inclusion in this systematic review. These comprised cross-sectional observational studies (n = 5) and in vitro simulated digestion models using human-derived samples (n = 7). Given the involvement of human participants or their biological materials, ethical approvals and informed consent were reported in most studies (n = 8). Two studies stated that ethical approval was not required under their national regulations for sample use (n = 2), while two studies (n = 2) did not provide information regarding ethical oversight. Figure 1 represents a flow diagram using PRISMA protocol that summarizes our screening processes to obtain the 12 studies for our systematic review.
Fig. 1.
PRISMA flow diagram of this systematic review. The diagram illustrates the study selection process in accordance with the PRISMA 2020 guidelines. The flowchart presents the number of records (n = 320) identified through database searching and other sources, the number of duplicates removed, the records screened, assessed for eligibility, and the final number of studies (n = 12) included in the qualitative and/or quantitative synthesis. The reasons for exclusion at each stage are documented to ensure transparency of the selection process
Results and discussion
Numerous studies have shown the harmful effects of MPs exposure in tissue cultures and animal models. However, direct evidence of its impact on human health remains lacking. Recent research has outlined the presence of MPs in various parts of the human body [20]. Extensive animal studies have shown that MPs exposure disrupts gut microbiota and causes dysbiosis [15].
This systematic review examines the impact of MPs exposure on microbiota composition at the phylum, family, and genus levels. Most of the studies reviewed utilize fecal samples, which are subsequently analyzed in in vitro models designed to mimic the human gastrointestinal system [1, 21–24]. A smaller number of studies focus on meconium samples [25]. The final included studies that met the inclusion criteria, particularly those focusing on human subjects or using human-derived samples, were geographically limited. Most studies originated from a small number of countries, including China (n = 7), followed by Spain (n = 2), France (n = 2), and Indonesia (n = 1), reflecting the narrow geographic distribution of current human-relevant research on MP-microbiome interactions. China’s prominence in MP research is closely tied to its status as a major plastics producer and its central role in global plastic waste management. Studies frequently report high MP levels in China’s inland waters, particularly in densely populated and industrialized regions, likely driving the volume of research output in this area [26, 27]. The key characteristics of the included studies analyzing the impact of MPs on the human gut microbiome are summarized in Table 1.
Table 1.
Summary of Article analyses from studies included in this systematic review on impact of MPs on the human gut microbiome
| Study (Reference No.) | Country of study origin | Study design | Participants and settings | Type of samples analyzed | Type of plastics involved | Key Findings | Microbiome analysis method |
|---|---|---|---|---|---|---|---|
| [1] | China | In vitro simulated digestion and fermentation | 6 healthy human volunteers (4 males, 2 females, aged 20–27, not ingested antibiotics at least 3 months); Laboratory-based simulated gastrointestinal digestion and colonic fermentation setup | Fecal samples |
PCL, ~ 150 nm; PLA, ~ 75 nm |
• PLA MPs & PCL MPs decreased gut microbiota richness and diversity. • PLA MPs & PCL MPs disrupted microbial community structure by suppressing beneficial genera (e.g., Bifidobacterium, Faecalibacterium) and enriched pathogenic species (e.g., Prevoltella). • PLA MPs caused greater metabolic disruption, while PCL more strongly reduced SCFAs. |
16 S rRNA sequencing (PacBio) |
| [8] | China | Quasi-experimental human study | 60 postgraduate students from Ningxia Medical University (Exposure group (n = 30), 3 hot meals/day in disposable plastic tableware (DPT); control group (n = 30), 3 hot meals/day in non-DPT; post-exposure group) | Fecal samples (2 per participant) | Disposable plastic tableware (not specified) |
• Alpha diversity significantly decreased in the post-exposure groups. • Beta diversity changes indicate delayed dysbiosis. • No significant species-level changes were observed post-exposure. • At the phylum level, Bacteroidota decreased and Actinobacteriota increased. • At the genus level, Faecalibacterium decreased and Blautia increased. |
16 S rRNA gene sequencing (V3–V4 region) |
| [22] | France | In vitro simulated toddler mucosal artificial colon (Tm-ARCOL model) | 4 healthy infants (2 boys, aged 30–20 months; 2 girls, aged 25–22 months); Fermentation of fecal samples in Tm-ARCOL model | Fecal samples | PE, 1–10 μm; dose = 21 mg/day for 14 days |
• PE MPs disrupted microbiota structure including families - ↑ Enterobacteriaceae, Dethiosulfovibrionaceae, Oscillospiraceae and ↓ Tannerellaceae, Rikenellaceae, Monoglobaceae. • Metabolic activity was altered: SCFAs - ↑ acetate, ↓ propionate & butyrate. AhR activation potential was reduced. Volatile organic compound profile changed significantly, with 26 discriminatory VOCs identified. |
16 S rRNA gene sequencing (V3–V4 region, Illumina MiSeq) |
| [14] | China | Cross-sectional observational study | 69 preschool children, aged 3–6 years, kindergarten in Xiamen, China | Fecal samples (2 per participant) | PVC, Polyethylene PET, PE, PA6 |
• PE, PVC, PET, and PA6 were detected in 85.5% of participants’ stool samples. • Dominant genera included Bacteroides, Faecalibacterium, Escherichia-Shigella, Bifidobacterium, Prevotella. |
16 S rRNA gene sequencing |
| [21] | France | In vitro simulated adult mucosal artificial colon (M-ARCOL model) | 4 healthy adults (2 males, 2 females, aged 23–52); Fermentation of fecal samples in M-ARCOL model | Fecal samples | PE, 1–10 μm; dose = 21 mg/day for 14 days |
• Dominant families included Bacteroidaceae, Lachnospiraceae, Ruminococcaceae, Veillonellaceae, Akkermansiaceae, Rikenellaceae. • PE MPs promoted pathobionts (Desulfovibrionaceae, Enterobacteriaceae) and reduced beneficial taxa (Christensenellaceae, Akkermansiaceae). • Metabolic activity: SCFA not significantly impacted, 5 5 discriminatory VOCs identified. AhR activity decreased. |
16 S rRNA gene sequencing (V3–V4 region, Illumina MiSeq) |
| [23] | Spain | In vitro simulation of INFOGEST static digestion + simgi® dynamic colon model | 3 healthy adult volunteers | Fecal samples | PLA MPs: 2 types - PLAg (millimetric, ~ 5 mm) and PLAm (micrometric, 240 ± 65 μm) | • PLAm exposure enriched the steroid degradation pathway and induction of xenobiotic metabolism. | 16 S rRNA gene sequencing (V3–V4 region, Illumina MiSeq) |
| [20] | Indonesia | Cross-sectional observational study |
22 healthy adult participants (Coastal group from Kenjeran, Surabaya, Indonesia (n = 11); highland group from Pacet, Mojokerto, Indonesia (n = 11)) |
Fecal samples | HDPE, PP, PS |
• No significant difference in gut microbiota composition, diversity, or gene abundance between with and without MP (HDPE, PP, PS) contamination. • Genes encoding plastic-degrading enzymes (feaB) were present in stool samples, suggesting microbial adaptation potential. |
Shotgun metagenomic sequencing (Illumina NovaSeq 6000) |
| [25] | China | Prospective observational pilot study |
18 mother-infant pairs from a hospital in Shanghai, China Samples collected immediately after birth in a plastic-free and sterile protocol |
Meconium samples | 16 types detected; major ones include PA, PU, PE, PET, PS, PVC, 20–500 μm |
• Dominated phylum included Proteobacteria, Firmicutes, and Bacteroidota. • Increased alpha diversity • MP type-specific correlations were observed: PA and PU positively associated with Escherichia-Shigella and Treponema. • Particle size (100–150 μm) positively correlated with Rikenellaceae and alpha diversity in meconium. |
16 S rRNA gene sequencing (V3–V4 region) |
| [28] | China | Cross-sectional observational study |
40 healthy participants (High-exposure group (n = 20), who lived and worked near a plastic factory; low-exposure group (n = 20) who lived and worked within 1 km of the Huanhuaxi Park, Chendu) |
Fecal samples | Mainly PU, also detected: SR, EVA, PE, 20–500 μm | • High exposure increased Bifidobacterium, Streptococcus, and Sphingomonas. and decreased Ruminococcus torques group, Dorea, Fusobacterium, and Coprococcus. | 16 S rRNA gene sequencing (Illumina NovaSeq6000 platform) |
| [24] | China | In vitro simulation of mucosal simulator of human intestinal microbial ecosystem (M-SHIME model) | 1 healthy adult male, aged 23, not taken antibiotics in the 6 months before the study | Fecal samples | PET, 100–300 μm |
• Luminal microbiota was more affected by MPs than mucosal microbiota. • MPs exposure reduced SCFA (acetate, butyrate, total) levels. • Functional pathway analysis showed ↑ energy metabolism and xenobiotic-related pathways, and ↓ lipid, carbohydrate, amino acid metabolism, and immune-related pathways. |
16 S rRNA gene sequencing (V4–V5 region) |
| [29] | Spain | In vitro simulation of static upper gastrointestinal digestion and dynamic colonic fermentation (simgi® model) | 2 healthy adult participants | Fecal samples, digestion fluid, colonic content from ascending (AC), transverse (TC), and descending (DC) colon compartments | PET, 160 ± 110 μm |
• PET MPs decreased Bacteroides, Parabacteroides, and Alistipes across all compartments. • Increases observed in pro-inflammatory genera Escherichia/Shigella, Bilophila, Cloacibacillus, Eisenbergiella, Megasphaera, and Oscillibacter. • Caused shifts in phyla: ↑ Firmicutes, Desulfobacterota, Synergistetes; ↓ Bacteroidetes, particularly in TC and DC compartments. |
16 S rRNA gene sequencing (V3–V4 region, Illumina MiSeq) |
| [11] | China | In vitro simulation of human gut using Caco-2 cells |
10 healthy volunteers (5 males, 5 females, aged 20–30, not treated for antibiotics for at least 3 months) |
Fecal samples | PE, 30–140 μm |
• PE MPs slightly increased Bacteroides. • TBBPA + PEMPs inhibited pentose phosphate, fructose/mannose metabolism, and bile acid synthesis. |
16 S rRNA gene sequencing (V3–V4 region, Illumina MiSeq/Hiseq) |
Note: “Study” refers to the included studies identified in the systematic review, cited by their reference numbers. The arrows ↑ and ↓ indicate increased and decreased abundance or levels, respectively
Impact of microplastics on microbiota composition
At the phylum level, gut bacterial composition varies across intestinal compartments, as outlined in Table 2. Microbiome analysis from fecal samples, conducted using simulated in vitro GI models, show distinct increases and decreases in specific phyla. PET were associated with shifts in bacterial communities, with increases in phyla such as Firmicutes, Synergistetes, and Desulfobacterota, alongside reductions in Proteobacteria and Bacteroidetes in all compartments - ascending colon (AC), descending colon (DC) and transverse colon (TC) compartments [29]. Moreover, exposure to a mixture of MPs was associated with an increase in Actinobacteria and a decrease in Bacteroidetes [8]. Notably in human, Firmicutes and Bacteroidetes are the main constituents of gut microbiota, along with smaller proportions of Proteobacteria and Actinobacteria. The disproportion of these microbial groups could potentially lead to gastrointestinal issues, immune disorders, neurological conditions and disruptions in energy metabolism [30]. MPs may exacerbate this imbalance by as vectors for pathogens and pollutants that accumulate in the GI tract, disturbing the microbial communities [31].
Table 2.
Effects of MPs exposure on human gut Microbiome at the phylum level
| Type of MPs | MP concentration/ size |
Sample/ Model |
Effect on bacteria | References | |
|---|---|---|---|---|---|
| Increase | Decrease | ||||
| PET | 166 mg/intake | Fecal (in vitro GI simulator model) |
AC compartment: Firmicutes Desulfobacterota TC compartment: Synergistetes Proteobacteria Desulfobacterota DC compartment: Desulfobacterota Synergistetes |
AC compartment: Proteobacteria Bacteroidetes TC compartment: Bacteroidetes DC compartment: Bacteroidetes |
[29] |
| MPs (not specified) |
Detected concentration - Exposure group: 24.650 items/g Control group: 19.645 items/g Post-exposure: 9.805 items/g (particle size − 20–500 μm) |
Fecal | Actinobacteria | Bacteroidetes | [8] |
The Firmicutes/Bacteroidetes ratio, with increased Firmicutes and reduced Bacteroidetes have been found in obesity, and other metabolic diseases [30, 32]. However, Firmicutes play role in fermenting dietary fibers into SCFAs, strengthening the intestinal barrier and reducing inflammation [33], whereas the reduction of Bacteroidetes potentially lead to reduced SCFA production, and adverse effects on gut health [34]. Moreover, a decrease in Bacteroidetes has been observed in individuals with IBD [35]. As Bacteroidetes are significant producers of SCFAs, and their reduction can compromise the functions of SCFA, leading to increased intestinal permeability and inflammation, that potentially develop into IBD [36]. Both phyla are important for immunomodulation, as their decline in numbers will likely result in systemic health issues, such as metabolic syndrome and autoimmune disorders. Another phylum of emerging interest, Synergistetes are part of the healthy gut and vaginal microbiome, their overgrowth may transition into opportunistic pathogens [37]. Desulfobacterota are known for their ability to produce hydrogen sulfide (H2S), which in controlled amounts supports intestinal health. However, increased proportion of various phyla could alter microbial ecosystems by consuming hydrogen and influence metabolic pathways, which could potentially be implicated in inflammation and childhood nutrition [38]. Increased H2S has resulted in various GI issues, including constipation, diarrhea and IBS [39].
At the family level, MP exposure significantly alters gut bacteria with specific effects depending on the type of MP. For PE, at a concentration of 21 mg of MPs per day, studies using Tm-ARCOL (Toddler mucosal artificial colon) and adult M-ARCOL models revealed increased abundance of families such as Acidaminococcaceae, Dethiosulfovibrionaceae, Enterobacteriaceae, Moraxellaceae, Oscillospiraceae, and Planococcaceae in both luminal and mucosal phases. In contrast, families like Monoglobaceae, Rikenellaceae, Tannerellaceae, and Lachnospiraceae showed decreasing trends [21, 22]. Exposure to polycaprolactone (PCL) led to an increase in Selenomonadaceae, while families such as Bifidobacteriaceae, Oscillospiraceae, Lactobacillaceae, Lachnospiraceae, Peptostreptococcaceae, Enterococcaceae, and Desulfovibrionaceae were significantly reduced. Similarly, exposure to polylactic acid (PLA) MPs resulted in an increase in Prevotellaceae, but reductions were observed in families like Bifidobacteriaceae, Oscillospiraceae, Lactobacillaceae, and Rikenellaceae [1]. The findings are also summarized in Table 3.
Table 3.
Effects of MPs exposure on human gut Microbiome at the family level
| Type of MPs | MP concentration/size | Sample/ Model |
Effect on bacteria | References | |
|---|---|---|---|---|---|
| Increase | Decrease | ||||
| PE | 21 mg/day for 14days | Fecal (Tm-ARCOL model) |
Luminal phase: Acidaminococcaceae Dethiosulfovibrionaceae Enterobacteriaceae Moraxellaceae Oscillospiraceae Planococcaceae Mucosal phase: Acidaminococcaceae Dethiosulfovibrionaceae Planococcaceae Oscillospiraceae |
Luminal phase: Monoglobaceae Rikenellaceae Tannerellaceae Mucosal phase: disappearance of Lachnospiraceae Monoglobaceae |
[22] |
| 21 mg/day for 14days | Fecal (M-ARCOL model) |
Both luminal and mucosal phase: Desulfovibrionaceae Enterobacteriaceae |
Mucosal phase: Christensenellaceae Akkermansiaceae |
[21] | |
| PCL | 166 mg/intake (size − 150 μm) | Fecal (in vitro simulated digestion and fermentation models) | Selenomonadaceae |
Bifidobacteriaceae Oscillospiraceae Lactobacillaceae Lachnospiraceae Peptostreptococcaceae Enterococcaceae Veillonellaceae Rikenellaceae Sutterellaceae Desulfovibrionaceae |
[1] |
| PLA | 166 mg/intake (particle size − 75 μm) | Fecal (in vitro simulated digestion and fermentation models) | Prevotellaceae |
Bifidobacteriaceae Oscillospiraceae Lactobacillaceae Lachnospiraceae Peptostreptococcaceae Enterococcaceae Veillonellaceae Rikenellaceae Sutterellaceae Desulfovibrionaceae Streptococcaceae |
[1] |
Based on the findings from our literature compilation, many of the bacterial families affected by MP exposure, such as Acidaminococcaceae, Lactobacillaceae, Lachnospiraceae, Peptostreptococcaceae, and Oscillospiraceae, belong to the phylum Firmicutes [40]. Enterobacteriaceae, Moraxellaceae, and Sutterellaceae are part of the phylum Proteobacteria [40–42]. Families such as Prevotellaceae, Rikenellaceae, and Tannerellaceae belong to the phylum Bacteroidetes [43]. Lastly, Desulfovibrionaceae and Dethiosulfovibrionaceae are part of the phylum Desulfobacterota [38]. The changes observed in bacterial families upon MP exposure all belong to phylum groups, which also exhibited shifts at the phylum groups.
Among the bacterial families, certain families such as Desulfovibrionaceae, Dethiosulfovibrionaceae and Enterobacteriaceae are recognized as pathobionts [21, 22]. The Dethiosulfovibrionaceae family has been associated with colorectal cancer, while Enterobacteriaceae, which includes well-known enteric pathogens of Escherichia, Shigella, Campylobacter, and Salmonella, has been associated with IBS and IBDs, such as Crohn’s disease and ulcerative colitis [44]. It contributes to intestinal inflammation by producing pro-inflammatory molecules and disrupting gut barrier function [42, 45]. Interestingly, Enterobacteriaceae are the major carriers of antibiotic resistance genes, one particular is the resistance to a major group of antibiotics, the carbapenems [46]. The relationship between IBD and antibiotic resistance genes still remain a subject of intrigue for the researchers.
At the genus level, MPs exposure induces changes in bacterial composition, with genera showing increases and decreases depending on the type. It has been observed that increases in Bacteroides and Clostridium has been associated with the exposure to most types of MPs detected across fecal and meconium samples [20, 25]. Clostridium can degrade PE, suggesting its ability to adapt and thrive in its presence. However, excessive growth of Clostridium can result in diarrhea and the production of intestinal toxins, posing serious health risks [11]. Studies also report an increased abundance of genera associated with inflammatory responses, including Streptococcus, Treponema, Escherichia-Shigella, Bilophila, and Porphyromonas. Meconium samples exposed to polyamide (PA) MPs, showed a rise in Treponema, Escherichia-Shigella, Bacteroides, and Clostridium [25]. The observed rise in in Bacteroidetes species following exposure to PA MPs may be attributed to the specific physicochemical properties of PA, although the exact mechanisms remain ambiguous. In this case, the meconium samples were collected immediately after birth using a plastic-free and sterile protocol, suggesting that MPs likely entered the fetal environment through transplacental transfer from the maternal circulation [47, 48]. This may be explained by the fact MPs elicit inflammatory responses and oxidative stress in maternal tissues, causing the increased placental permeability could facilitate the translocation of MPs, along with any adsorbed pathogens or pro-inflammatory mediators [48]. MPs have been observed to accumulate in fetal lungs, as reflected in meconium [49], which may be reflected in meconium aspiration syndrome (MAS), although this association warrants further investigation. Escherichia-Shigella and Bilophila, particularly Bilophila wadsworthia, are associated with pro-inflammatory effects, as B. wadsworthia promotes T-helper type 1 (TH1)-mediated immune response and potentially exacerbates colitis. These bacteria have been implicated in the pathogenesis of diseases such as IBD, colorectal cancer, and coronary artery diseases [29].
MP exposure in meconium samples also increases the prevalence of Porphyromonas and Streptococcus, both of which are often implicated in pathogenic conditions [25]. A similar effect was observed in fecal samples exposed to PET MPs, abundance of Bilophila elevated across all intestinal compartments (AC, DC and TC) examined [29]. MPs exposure also led to an increase in Faecalibacterium and Bifidobacterium genera which are typically considered the markers of gut health. Under a balanced condition, these bacteria have the potential to mitigate inflammatory and metabolic diseases [14, 23]. However, an overgrowth of Faecalibacterium contributes to the development of numerous gut-related disorders, including Crohn’s disease in children [50].
In contrast, MPs exposure has been shown to reduce beneficial probiotic bacteria, notably Lactobacillus. Lactobacillus species exhibit properties through the production of antimicrobial compounds, including organic acids, hydrogen peroxide, diacetyl, and bacteriocins. Studies have demonstrated the efficacy of Lactobacillus against a range of pathogens, such as Staphylococcus aureus, Listeria monocytogenes, Shigella flexneri, Klebsiella pneumoniae [11]. Reductions in other health-promoting genera, such as Blautia, Parabacteroides, Alistipes and Pelomonas have also been observed following MP exposure [14, 25, 29]. Parabacteroides and Alistipes are of particular interest due to their reported protective roles against inflammation, metabolic disorders, colitis, and IBS [33, 51]. A summary of the observed changes in bacterial composition, including shifts in specific genera and their abundance in response to MPs is presented in Table 4.
Table 4.
Effects of mpsexposure on human gut Microbiome at the genus level
| Type of MPs | MP concentration/size |
Sample/ Model |
Effect on bacteria | References | ||
|---|---|---|---|---|---|---|
| Increase | Decrease | |||||
| PCL | 166 mg/intake (size − 150 μm) | Fecal (in vitro simulated digestion and fermentation models) | Megamons |
Blautia Bifidobacterium Romboutsia Veillon |
[1] | |
| PLA | 166 mg/intake | Fecal (in vitro GI simulator model) | Bifidobacterium | |||
| 166 mg/intake (particle size − 75 μm) | Fecal (in vitro simulated GI digestion and colonic fermentation models) | Prevotella |
Ruminococcus Blautia Weissella |
[1] | ||
| PA | 24.9 particles/g (particle size − 20–500 μm (majority − 20–50 μm)) | Meconium |
Treponema Escherichia − Shigella Bacteroides Prevotellaceae_NK3B31_group Clostridia_UCG_014 |
Pelomonas | [25] | |
| PE | 1000 mg/L (particle size − 30 to 140 μm ) | Fecal (in vitro GI simulator model) |
Bacteroides Clostridium |
Lactobacillus Enterococcus |
[11] | |
| Detected concentration– 206.2 µg/g dry weight (estimated daily intake EDI– 231.9 µg/kg-body weight/day) | Fecal |
Parabacteroides [Eubacterium] _coprostanoligenes_group Lachnospiraceae_NK4A136_group |
[14] | |||
| HDPE (High-density PE) | Fecal | Bacteroides | [20] | |||
| PET | 166 mg/intake | Fecal (in vitro GI simulator model) |
AC compartment: Bilophila Eisenbergiella Megasphaera Oscillibacter TC and DC compartment: Bilophila Escherichia-Shigella Cloacibacillus |
All compartments: Alistipe Bacteroides Parabacteroides |
[29] | |
| Detected concentration − 299 µg/g dry weight (estimated daily intake EDI– 382.3 µg/kg-body weight/day) | Fecal | Agathobacter | [14] | |||
| 2 g/day (particle size– 100–300 μm) | Fecal (mucosal-simulator of the human intestinal microbial ecosystem (M-SHIME)) | Morganella | Acidaminococcus | [24] | ||
|
PP (Polypropylene) |
- | Fecal |
Bacteroides Roseburia Clostridium |
Prevotella copri | [20] | |
| PVC | Detected concentration − 317.4 µg/g dry weight (estimated daily intake EDI– 335.8 µg/kg-body weight/day) | Fecal | Feacalibacterium |
Alistipes [Eubacterium]_coprostanoligenes_group |
[14] | |
|
PU (Polyurethane) |
10.7 particles/g (particle size − 20–500 μm (majority − 20–50 μm)) | Meconium |
Streptococcus Treponema Prevotellaceae_NK3B31_group |
Pelomonas | [25] | |
| PS | (particle size − 20–500 μm (majority − 20–50 μm)) | Meconium |
Porphyromonas Haemophilus |
[Eubacterium]_coprostanoligenes_group Treponema |
[25] | |
| - | Fecal |
Bacteroides Prevotella copri |
Roseburia Clostridium |
[20] | ||
|
EVA (Ethylene Vinyl Acetate) |
(particle size − 20–500 μm (majority − 20–50 μm)) | Meconium | Parabacteroides | [25] | ||
| MPs (not specified) | 54.1 particles/g (particle size − 20–500 μm (majority − 20–50 μm)) | Meconium |
Streptococcus Treponema Prevotellaceae_NK3B31_group Clostridia_UCG-014, |
Pelomonas | [25] | |
| MPs (not specified) |
Detected concentration - Exposure group: 24.650 items/g Control group: 19.645 items/g Post-exposure: 9.805 items/g (particle size − 20–500 μm) |
Fecal | Blautia | Faecalibacterium | [8] | |
|
Mixture of 31 types of MPs (majority - PU, EVA, PE) |
- | Intestinal secretions |
Bifidobacterium Streptococcus Sphingomonas |
Dorea Fusobacterium Coprococcus [Ruminococcus]_torques_group |
[28] | |
|
Mixture of MPs (PE PVC PET PA6) |
- | Fecal | Feacalibacterium | [14] | ||
Impact of microplastics on microbiota diversity and richness
Our findings reveal that the MPs exert varying effects on the α-diversity and richness of microbiota with different types of MPs. PET MPs reduce α-diversity indices, such as Observed species and Shannon index, indicating a decline in microbial diversity [29]. PVC MPs were also negatively correlated with Chao1 and Observed species indices [14]. PS MPs, particularly in meconium microbiota, were negatively related to the Chao1 index [25]. Similarly, PCL and PLA MPs significantly reduced gut microbiota α-diversity [1]. This trend of decrease in α-diversity has been previously observed in animal models following exposure to MPs [52, 53].
PE MPs, in contrast, increase α-diversity indices (Chao1, observed species, Simpson, and Shannon) and mitigated the decline caused by tetrabromobisphenol A (TBBPA), which higher concentrations negatively impacted diversity [11]. Fournier et al. (2023) reported an increase in α-diversity, particularly in the luminal microbiota [22]. The luminal microbiota is posited to be more sensitive to MPs compared to the mucosal microbiota. To make matters worse, the phthalate esters (PAEs) released from MPs tend to induce more disturbances in the diversity. The likely explanation for the higher sensitivity of the lumen for MP damages is that the mucus promotes microbial colonization by enhancing attachment and stability, thereby reducing the disruption in the microbiota caused by MP exposures [54].
Moreover, there is an observable individual variation with the changes in microbiota diversity patterns [22, 23, 29]. In the Tm-ARCOL model using the stools of toddlers, the initial variation has been observed in donor stools, and the donor effect emerged as the primary factor influencing β-diversity [22]. In response to PET MPs, the differences in microbial responses were seen across individuals and intestinal compartments [29]. Certain conditions, for instance, thermal exposure to MPs, affect microbiome diversity with alterations in both α- and β-diversity [8]. Low α- diversity is linked to conditions such as obesity, IBD and chronic diseases [55].
Impact of microplastics on microbial metabolic function and pathways
MPs significantly impact metabolic functions of microbiota, which also contribute to intestinal and metabolic diseases [11]. The major metabolic function disrupted by MPs exposure is SCFA production, with specific changes depending on the type of MP (Table 5). Exposure to MPs of PE increased acetate levels but decreased propionate and butyrate [22]. Conversely, PET exposure results in the reduction of acetate (acetic acid) and butyrate levels [24]. PLA and PCL exposure is met with an increased levels of propionic acid and reductions in acetic acid as well as i- and n-valeric acids. PCL MPs show more inhibition of SCFA production compared to PLA MPs that paradoxically enhance propionic acid levels. This phenomenon is likely due to the abundance of Prevotella that promotes propionic acid production as a result of trigger from the PLA MPs [1].
Table 5.
Effects of MPs on microbial metabolic function and pathways
| Type of MPs | Metabolic Activity | Effects | References |
|---|---|---|---|
| PE | SCFA production |
Increased acetate Decreased Propionate and butyrate |
[22] |
| AhR activity | Less activation | [21] | |
| Volatolomics (VOCs production) |
Overproduced 7 hydrocarbon compounds (1) Pentane, 3-methyl- (2) Cyclopentane, 1,1,3-trimethyl- (3) 1-heptene, 2, 4-dimethyl- (4) Heptane, 2,3-dimethyl- (5) Methyl C9-alkane - RI 814 (6) Methyl C9-alkane - RI 821 (7) Methyl C9-alkane - RI 861 2 ketones (1) 2-heptanone (2) 2-heptanone, 4-methyl- 2 alcohols (1) 1-hexanol (2) 1-heptanol 2 aldehydes (1) Heptanal (2) Octanal Underproduced 8 esters of propanoic acid, ethyl ester and butanoic acid |
||
|
Increased abundance of 3 hydrocarbons (1) pentane, 3-methyl- (2) cyclopentane, 1,1,3- trimethyl- (3) 1-heptene, 2,4-dimethyl- 1 alcohol (1) 3-nonyn-2-ol 1 Nitrogen compound (1) Indole, 3-methyl- |
[21] | ||
| Carbohydrate metabolism |
Decreased pentose phosphate pathway Inhibited fructose and mannose metabolism |
||
| Bile acid synthesis | Decreased | ||
| PET | SCFA production | Decreased acetate, butyrate and total SCFAs | [24] |
| Amino acid metabolism | Increased | ||
| Energy metabolism | Increased | ||
| Carbohydrate metabolism | Decreased | ||
| Lipid metabolism | Decreased | ||
| PLA | SCFA production |
Decreased acetic acid Increased propionic acid |
[1] |
| Steroid degradation and xenobiotic degradation | More activation | [23] | |
| Vitamin B6 metabolism | Upregulated | [1] | |
| The citric acid cycle | Upregulated | ||
| Ubiquinone and terpenoid-quinone biosynthesis | Upregulated | ||
| Pentose and glucoronate interconversions | Upregulated | ||
| Terpenoid backbone biosynthesis | Upregulated | ||
| Protein processing in the ER | Upregulated | ||
| Cell cycle-Caulobacter | Upregulated | ||
| PCL | SCFA production |
Decreased acetic acid, i-valeric acid and n- valeric acid Increased propionic acid |
|
| Pentose and glucoronate interconversions | Upregulated | ||
| Terpenoid backbone biosynthesis | Upregulated | ||
| Amino acid metabolism | Upregulated histidine metabolism | ||
| Epithelial cell signaling in Helicobacter pylori infection | Upregulated | ||
| Protein processing in the ER | Upregulated | ||
| MPs (not specified) | Amino acid metabolism | Increased tyrosine metabolism | [8] |
The groups with the most significant changes at the phylum level, Firmicutes and Proteobacteria, are strongly correlated with SCFA production, metabolites produced through bacterial fermentation in the gut. These SCFAs not only influence pH of the intestinal environment but also exert critical physiological effects by maintaining the gut barrier integrity [13]. They enhance mucus production and strengthen intestinal epithelial tight junctions, thereby protecting against pathogens [12]. The observed reduction in bacteria responsible for fiber fermentation and SCFA production may increase susceptibility to the risk of IBD. SCFAs provide immunosuppressive function by reducing inflammation through interactions with G-protein-coupled receptors (GPCRs) and modulation of T-cell activity [56, 57]. Furthermore, SCFAs serve as substrates in lipid metabolism, influencing host energy balance and lipid regulation [58].
PE MPs has been shown to reduce AhR activity [21, 22]. AhR functions as a biosensor, responding to both endogenous ligands such as microbial metabolites, and exogenous ligands, including environmental toxins [59]. In a previous study [10], MPs exposure alters gut microbial composition, reducing the abundance of Akkermansia and Parabacteroides, which produce tryptophan derivatives that activate AhR. This decrease in AhR activity allows bacteria such as Enterobacteriaceae and Desulfovibrionaceae to proliferate, leading to conditions like leaky gut and systemic inflammation [10]. Moreover, PE exposure led to the overproduction of volatine organic compounds (VOCs) such as hydrocarbons, ketones, alcohols and aldehydes and underproduction of esters [21, 22]. Certain VOCs, particularly hydrocarbons and aldehydes such as benzene and formaldehyde are recognized carcinogens, and chronic exposure has been linked to increased cancer risks [60]. VOC may impair immune responses by inducing inflammatory pathways, which increase susceptibility to infections and chronic diseases [61].
Exposure to MPs significantly disrupts the metabolism of major biomolecules, including carbohydrates, lipids, and amino acids. PE and PET exposure was shown to inhibit carbohydrate metabolism, potentially impairing host energy production [21, 24]. In addition, PET also decreased lipid metabolism, however, increased energy metabolism, suggesting a shift in microbial energy utilization [24]. In mice, the interference of lipid metabolism by MP is explained by altering gut-liver axis which lead to hepatic lipid accumulation and dysregulation of cholesterol and bile acid homeostasis. MPs disrupt bile acid synthesis and metabolism, potentially causing cholestasis and altered bile acid profiles [62]. Amino acid metabolism was significantly increased with MPs exposure [8, 24]. This shift could be a compensatory response to energy deficits caused by impaired carbohydrate and lipid metabolism [63]. Moreover, the enrichment pathway analysis of fecal samples exposed to PLA and PCL MPs, revealed upregulation of many metabolic pathways, such as pentose and glucuronate interconversions, steroid degradation, vitamin B6 metabolism, ubiquinone and terpenoid-quinone biosynthesis, proteins synthesis for processing in endoplasmic reticulum (ER), and signaling pathways involved in H.pylori infection [1]. However, the exact mechanisms by which MPs interfere with these enriched metabolic pathways remain unclear.
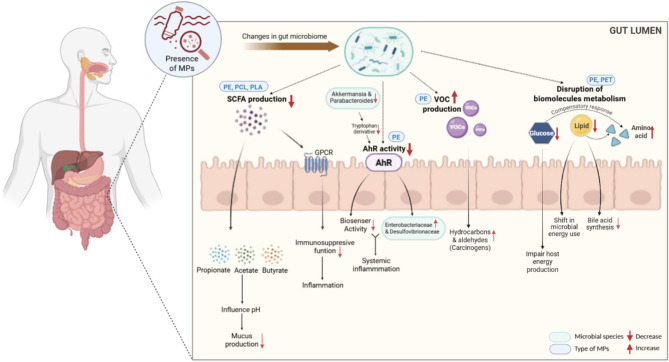
Figure 2 presents a graphical summary of changes in the microbial metabolic functions and signaling pathways caused by MPs exposure.
Fig. 2.
Summary of the impact of MPs on gut microbial metabolic pathways nad functions, emphasizing the type-specific impact of MPs and their downstream physiological consequences. The figure illustrates the downstream effects of MPs (e.g. PE, PCL, PLA, PET) in the GI tract following ingestion. [Red arrows (↑/↓) indicate increases or decreases in microbial species, metabolic output, or host response. Blue rounded boxes label specific MP types associated with observed effects. Green rounded boxes represent specific microbial taxa that are either increased or decreased in response to MP exposure.] (Fig. 2 was created using Biorender)
Impact of microplastics’ properties on microbiome
MPs exposure on microbiota diversity is strongly influenced by plastic-specific properties, including type, size and shape, in addition to the extent of exposure [64]. MP size in particular plays a critical role in determining bioaccumulation, toxicity, and its effects on gut microbiota shifts. Lei reported that size poses more significant impact on the toxicity than those of the concentration [65]. Peng et al., (2024) found that when exposed at the same concentration (166 mg per intake), for both PCL and PLA MPs, their sizes differed with 150 μm for PCL and 70 μm for PLA, resulting in distinct changes in bacterial diversity at both the genus and family levels, as well as in metabolic pathways of the bacteria [1]. However, the specific concentrations of MPs detected in fecal or intestinal samples have not been normalized across studies and more importantly, the threshold level of MPs that induces toxic effects has yet to be determined.
This size-dependent impact of PS has been reported in co-cultures of colon cancer cells, Caco-2 cells with HT29-MTX-E12 cells, highlighting that the toxicity of MPs is strongly dependent on their properties [66]. While small particle size MPs easily accumulate, they exhibit minimal impact on microbial growth. Conversely, larger particles, show lower toxicity, likely due to their inability to cross cell membranes, thus limiting their interaction with biological systems [67].
Conclusion
This systematic review highlights the growing concern over MP exposure as an emerging environmental risk factor in human gut microbiome health. Across studies reviewed, MPs were consistently associated with microbial dysbiosis, reduced microbial diversity, impaired SCFA production, and altered immune and metabolic pathways. These disruptions carry significant implications for intestinal health, potentially contributing to chronic inflammation, metabolic disorders, and increased disease susceptibility.
Despite these important findings, several limitations constrain the generalizability of current evidence to the preliminary state of research in this emerging issue. There is a lack of MP dosing across studies, and substantial variation exists in experimental parameters including the polymer type, particle size and duration of exposure. This lack of uniformity limits comparability and the synthesis of consistent conclusions. Furthermore, most available studies rely on in vitro simulations using human fecal samples rather than clinical data from human subjects, restricting the direct applicability of results. It is worth mentioning that the limited number of included studies, primarily conducted in China with a few from other countries, restricts the ability to draw comprehensive population-level or region-specific conclusions.
Moving forward, a more comprehensive understanding of MPs’ effects requires careful attention to methodological approaches. Studies are encouraged to adopt standardized exposure protocols that reflect realistic environmental concentrations rather than relying solely on estimated daily intake levels. The long-term cumulative effects of MPs remain poorly understood, as existing studies predominantly focus on short-term exposure. Another focus should lie in the absence of well-defined threshold levels of MP toxicity, which are essential for determining safe exposure limits. This is particularly concerning given the chronic and pervasive nature of MP contamination. Researchers should also consider real-world exposure scenarios, such as the combined effects of MPs with other environmental pollutants, dietary habits and host-specific variables. While the findings highlight the significant disruption of the gut microbiome caused by MP exposure, more comprehensive studies are required to fill these gaps and develop strategies to mitigate the rising threat of MP contamination.
Supplementary Information
Acknowledgements
The authors would like to express their sincere gratitude to Sunway University for their academic support throughout the course of this research. We would like to acknowledge Tan Yi Huan, a PhD candidate at Sunway University, for her valuable assistance in the initial stages of this study.
Abbreviations
- MP
Microplastic
- IBD
Inflammatory bowel disease
- GI
Gastrointestinal
- SCFA
Short-chain fatty acid
- AhR
Aryl hydrocarbon receptor
- PS
Polystyrene
- PE
Polyethylene
- PVC
Polyvinyl chloride
- PET
Polyethylene terephthalate
- IBS
Irritable bowel syndrome
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- ROBINS-I
Risk Of Bias In Non-randomized Studies– of Interventions
- NP
Nanoplastic
- PCL
Poly(ε-caprolactone)
- PLA
Polylactic acid
- PA6
Polyamide 6
- HDPE
High-density polyethylene
- PP
Polypropylene
- PS
Polystyrene
- PU
Polyurethane
- SR
Silicone resin
- EVA
Ethylene-vinyl acetate
- AC
Ascending colon
- DC
Descending colon
- TC
Transverse colon
- H2S
Hydrogen sulfide
- Tm-ARCOL
Toddler mucosal artificial colon
- M-ARCOL
Mucosal artificial colon
Authors’ contributions
ZST: Methodology, investigation, formal analysis, data curation, writing - original draft, review & editing, JC: Validation, supervision, writing – review & editing, TYYO: Validation, writing – review & editing, RARA: Supervision, funding acquisition, writing – review & editing, LTG: Conceptualization, methodology, validation, supervision, funding acquisition. writing – review & editing. All authors reviewed and approved the final manuscript.
Funding
This research was funded by Fundamental Research Grant Scheme (Grant no.: FRGS/1/2023/SKK10/SYUC/01/1) awarded by Ministry of Higher Education (MOHE) Malaysia.
Data availability
The majority of the data generated or analysed during this study are included in this published article and the PRISMA statement lists. All the protocols and studies analyzed for this systematic review were included in the figures and tables in the manuscript.The rest of the datasets used and/or analysed are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors reviewed and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peng Y, Lu J, Fan L, Dong W, Jiang M. Simulated Gastrointestinal digestion of two different sources of biodegradable microplastics and the influence on gut microbiota. Food Chem Toxicol. 2024;185:114474. [DOI] [PubMed] [Google Scholar]
- 2.Senathirajah K, Attwood S, Bhagwat G, Carbery M, Wilson S, Palanisami T. Estimation of the mass of microplastics ingested–A pivotal first step towards human health risk assessment. J Hazard Mater. 2021;404:124004. [DOI] [PubMed] [Google Scholar]
- 3.Kannan K, Vimalkumar K. A review of human exposure to microplastics and insights into microplastics as obesogens. Front Endocrinol. 2021;12:724989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alimba CG, Faggio C, Sivanesan S, Ogunkanmi AL, Krishnamurthi K. Micro(nano)-plastics in the environment and risk of carcinogenesis: insight into possible mechanisms. J Hazard Mater. 2021;416:126143. [Google Scholar]
- 5.Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, et al. Detection of various microplastics in human stool: a prospective case series. Ann Intern Med. 2019;171(7):453–7. [DOI] [PubMed] [Google Scholar]
- 6.Junjie Z, Yubin L, Liang Z, Chu P, Lei W. Microplastics and nanoplastics in drinking water and beverages: occurrence and human exposure. J Environ Exposure Assess. 2024;3(4):24. [Google Scholar]
- 7.Chaturvedi S, Kumar A, Min L, Kumar R. A perspective on green solutions and future research paths for microplastic and nanoplastic contamination in drinking water. CLEAN–Soil Air Water. 2025;53(1):e202400104. [Google Scholar]
- 8.Zhang X, He X, Pan D, Shi L, Wu Y, Yang Y, et al. Effects of thermal exposure to disposable plastic tableware on human gut microbiota and metabolites: A quasi-experimental study. J Hazard Mater. 2024;462:132800. [DOI] [PubMed] [Google Scholar]
- 9.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournier E, Denis S, Dominicis A, Van de Wiele T, Alric M, Mercier-Bonin M, et al. A child is not an adult: development of a new in vitro model of the toddler colon. Appl Microbiol Biotechnol. 2022;106(21):7315–36. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Yin H, Yang Y, Jin L, Lu G, Dang Z. Influence of the co-exposure of microplastics and tetrabromobisphenol A on human gut: simulation in vitro with human cell Caco-2 and gut microbiota. Sci Total Environ. 2021;778:146264. [DOI] [PubMed] [Google Scholar]
- 12.Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, et al. Short chain fatty acids in human gut and metabolic health. Beneficial Microbes. 2020;11(5):411–55. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Cai X, Fei W, Ye Y, Zhao M, Zheng C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. 2022;62(1):1–12. [DOI] [PubMed] [Google Scholar]
- 14.Ke D, Zheng J, Liu X, Xu X, Zhao L, Gu Y, et al. Occurrence of microplastics and disturbance of gut microbiota: a pilot study of preschool children in Xiamen. China EBioMedicine. 2023;97:104828. [DOI] [PMC free article] [PubMed]
- 15.Djouina M, Vignal C, Dehaut A, Caboche S, Hirt N, Waxin C, et al. Oral exposure to polyethylene microplastics alters gut morphology, immune response, and microbiota composition in mice. Environ Res. 2022;212:113230. [DOI] [PubMed] [Google Scholar]
- 16.Lu L, Wan Z, Luo T, Fu Z, Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ. 2018;631:449–58. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Chen J, Zhang X, Xu M, Zhang X, Zhao W, et al. Effects of microplastics and Tetracycline on intestinal injury in mice. Chemosphere. 2023;337:139364. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Keenan JI, Shaw IC, Frizelle FA. Could microplastics be a driver for early onset colorectal cancer? Cancers. 2023;15(13):3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo T, Wang D, Zhao Y, Li X, Yang G, Jin Y. Polystyrene microplastics exacerbate experimental colitis in mice tightly associated with the occurrence of hepatic inflammation. Sci Total Environ. 2022;844:156884. [DOI] [PubMed] [Google Scholar]
- 20.Nugrahapraja H, Pramudya Wisnu Wicaksono S, Balqis Qonita P, Ni’matuzahroh F, Huang L, et al. Effects of microplastic on human gut microbiome: detection of Plastic-Degrading genes in human gut exposed to microplastics—Preliminary study. Environments. 2022;9(11):140. [Google Scholar]
- 21.Fournier E, Leveque M, Ruiz P, Ratel J, Durif C, Chalancon S, et al. Microplastics: what happens in the human digestive tract? First evidences in adults using in vitro gut models. J Hazard Mater. 2023;442:130010. [DOI] [PubMed] [Google Scholar]
- 22.Fournier E, Ratel J, Denis S, Leveque M, Ruiz P, Mazal C, et al. Exposure to polyethylene microplastics alters immature gut Microbiome in an infant in vitro gut model. J Hazard Mater. 2023;443:130383. [DOI] [PubMed] [Google Scholar]
- 23.Jiménez-Arroyo C, Tamargo A, Molinero N, Reinosa JJ, Alcolea-Rodríguez V, Portela R, et al. Simulated Gastrointestinal digestion of polylactic acid (PLA) biodegradable microplastics and their interaction with the gut microbiota. Sci Total Environ. 2023;902:166003. [DOI] [PubMed] [Google Scholar]
- 24.Yan Z, Zhang S, Zhao Y, Yu W, Zhao Y, Zhang Y. Phthalates released from microplastics inhibit microbial metabolic activity and induce different effects on intestinal luminal and mucosal microbiota. Environ Pollut. 2022;310:119884. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Liu X, Guo J, Yang R, Wang H, Sun Y, et al. The association between microplastics and microbiota in placentas and meconium: the first evidence in humans. Environ Sci Technol. 2022;57(46):17774–85. [DOI] [PubMed] [Google Scholar]
- 26.Zhang K, Shi H, Peng J, Wang Y, Xiong X, Wu C, et al. Microplastic pollution in china’s inland water systems: A review of findings, methods, characteristics, effects, and management. Sci Total Environ. 2018;630:1641–53. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Li B, Zou X, Wang Y, Li Y, Xu Y, et al. Emission of primary microplastics in Mainland china: invisible but not negligible. Water Res. 2019;162:214–24. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Wang H, Peng S, Kang J, Xie Z, Tang R, et al. Effect of microplastics on nasal and intestinal microbiota of the high-exposure population. Front Public Health. 2022;10:1005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamargo A, Molinero N, Reinosa JJ, Alcolea-Rodriguez V, Portela R, Bañares MA, et al. PET microplastics affect human gut microbiota communities during simulated Gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci Rep. 2022;12(1):528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12(5):1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bora SS, Gogoi R, Sharma MR, Anshu, Borah MP, Deka P, et al. Microplastics and human health: unveiling the gut Microbiome disruption and chronic disease risks. Front Cell Infect Microbiol. 2024;14:2024. [DOI] [PMC free article] [PubMed]
- 32.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3. [DOI] [PubMed] [Google Scholar]
- 33.Andoh A. Physiological role of gut microbiota for maintaining human health. Digestion. 2016;93(3):176–81. [DOI] [PubMed] [Google Scholar]
- 34.Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, et al. The effects of vegetarian and vegan diets on gut microbiota. Front Nutr. 2019;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8(11):1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin Y, Han S, Kwon J, Ju S, Choi TG, Kang I et al. Roles of Short-Chain fatty acids in inflammatory bowel disease. Nutrients. 2023;15(20):4466. [DOI] [PMC free article] [PubMed]
- 37.Marchandin H, Damay A, Roudière L, Teyssier C, Zorgniotti I, Dechaud H, et al. Phylogeny, diversity and host specialization in the phylum Synergistetes with emphasis on strains and clones of human origin. Res Microbiol. 2010;161(2):91–100. [DOI] [PubMed] [Google Scholar]
- 38.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proceedings of the national academy of sciences. 2013;110(33):13582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh SB, Lin HC. Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms. 2015;3(4):866–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francini E, Orlandoni P, Sparvoli D, Jukic Peladic N, Cardelli M, Recchioni R, et al. Possible role of Tauroursodeoxycholic acid (TUDCA) and antibiotic administration in modulating human gut microbiota in home enteral nutrition therapy for the elderly: A case report. Int J Mol Sci. 2024;25(13):7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kersters K, De Vos P, Gillis M, Swings J, Vandamme P, Stackebrandt E. Introduction to the Proteobacteria. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The prokaryotes: volume 5: proteobacteria: alpha and Beta subclasses. New York, NY: Springer New York; 2006. pp. 3–37. [Google Scholar]
- 42.Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: a common factor in human diseases. Biomed Res Int. 2017;2017(1):9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoyles L, McCartney AL. What do we mean when we refer to Bacteroidetes populations in the human Gastrointestinal microbiota? FEMS Microbiol Lett. 2009;299(2):175–83. [DOI] [PubMed] [Google Scholar]
- 44.Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97–108. [DOI] [PubMed] [Google Scholar]
- 45.Khorsand B, Asadzadeh Aghdaei H, Nazemalhosseini-Mojarad E, Nadalian B, Nadalian B, Houri H. Overrepresentation of Enterobacteriaceae and Escherichia coli is the major gut Microbiome signature in crohn’s disease and ulcerative colitis; a comprehensive metagenomic analysis of IBDMDB datasets. Front Cell Infect Microbiol. 2022;12:1015890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017;215(suppl1):S28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu S, Guo J, Liu X, Yang R, Wang H, Sun Y, et al. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: A pilot prospective study. Sci Total Environ. 2023;854:158699. [DOI] [PubMed] [Google Scholar]
- 48.Zurub RE, Cariaco Y, Wade MG, Bainbridge SA. Microplastics exposure: implications for human fertility, pregnancy and child health. Front Endocrinol (Lausanne). 2023;14:1330396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anifowoshe AT, Akhtar MN, Majeed A, Singh AS, Ismail TF, Nongthomba U. Microplastics: A threat to fetoplacental unit and reproductive systems. Toxicol Rep. 2025;14:101938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Assa A, Butcher J, Li J, Elkadri A, Sherman PM, Muise AM, et al. Mucosa-Associated ileal microbiota in New-Onset pediatric crohn’s disease. Inflamm Bowel Dis. 2016;22(7):1533–9. [DOI] [PubMed] [Google Scholar]
- 51.Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A. The genus alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front Immunol. 2020;11:906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ. 2019;649:308–17. [DOI] [PubMed] [Google Scholar]
- 53.Zhu D, Chen Q-L, An X-L, Yang X-R, Christie P, Ke X, et al. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol Biochem. 2018;116:302–10. [Google Scholar]
- 54.Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kers JG, Saccenti E. The power of Microbiome studies: some considerations on which alpha and beta metrics to use and how to report results. Front Microbiol. 2022;12:796025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50(2):432–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21(17):6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hao N, Whitelaw ML. The emerging roles of AhR in physiology and immunity. Biochem Pharmacol. 2013;86(5):561–70. [DOI] [PubMed] [Google Scholar]
- 60.Lyu X, Guo H, Wang Y, Zhang F, Nie K, Dang J, et al. Hazardous volatile organic compounds in ambient air of China. Chemosphere. 2020;246:125731. [DOI] [PubMed] [Google Scholar]
- 61.Bessonneau V, Mosqueron L, Berrubé A, Mukensturm G, Buffet-Bataillon S, Gangneux JP, et al. VOC contamination in hospital, from stationary sampling of a large panel of compounds, in view of healthcare workers and patients exposure assessment. PLoS ONE. 2013;8(2):e55535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen J, Sun H, Yang B, Song E, Song Y, Jiang G. Environmentally relevant concentrations of microplastic exposure cause cholestasis and bile acid metabolism dysregulation through a Gut-Liver loop in mice. Environ Sci Technol. 2024;58(4):1832–41. [DOI] [PubMed] [Google Scholar]
- 63.Niu H, Liu S, Jiang Y, Hu Y, Li Y, He L, et al. Are microplastics toxic?? A review from Eco-Toxicity to effects on the gut microbiota. Metabolites. 2023;13(6):739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stock V, Böhmert L, Coban G, Tyra G, Vollbrecht M-L, Voss L, et al. Microplastics and nanoplastics: size, surface and dispersant–what causes the effect? Toxicol in Vitro. 2022;80:105314. [DOI] [PubMed] [Google Scholar]
- 65.Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, et al. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci Total Environ. 2018;619:1–8. [DOI] [PubMed] [Google Scholar]
- 66.Hesler M, Aengenheister L, Ellinger B, Drexel R, Straskraba S, Jost C, et al. Multi-endpoint toxicological assessment of polystyrene nano-and microparticles in different biological models in vitro. Toxicol in Vitro. 2019;61:104610. [DOI] [PubMed] [Google Scholar]
- 67.Fackelmann G, Sommer S. Microplastics and the gut microbiome: how chronically exposed species May suffer from gut dysbiosis. Mar Pollut Bull. 2019;143:193–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The majority of the data generated or analysed during this study are included in this published article and the PRISMA statement lists. All the protocols and studies analyzed for this systematic review were included in the figures and tables in the manuscript.The rest of the datasets used and/or analysed are available from the corresponding author on reasonable request.