Abstract
Background
Tsetse flies are vectors of African trypanosomiasis, a disease that affects both humans and animals. Trypanosomiasis remains a threat to lives and it is an impediment to socio-economic development in sub-Saharan Africa. In spite of decades of chemotherapy and vector control, the disease has not been eradicated. Parasitic drug resistance has been developed to existing drugs, while vector control strategies are expensive and unsustainable. Therefore, there is a need to explore other control approaches, such as the transformation of tsetse fly endosymbionts to render the fly refractory to trypanosome infection. This research focused on investigating the prevalence and triparty association of infection of trypanosomes with some endosymbionts of tsetse flies from Yankari Game Reserve.
Methods
Tsetse flies were captured using biconical traps, identified morphologically, dissected and their entire guts were isolated and used for DNA extraction. Polymerase Chain Reaction (PCR) was used in confirming the identity of the tsetse flies by amplifying the cytochrome C oxidase-1 gene. PCR was also used to screen for the presence of endosymbionts (Sodalis glossinidius, Wolbachia, and Spiroplasma sp.) and trypanosomes.
Results
Glossina tachinoides was the only vector species identified. Trypanosome infection rate was 10.70% with Trypanosoma grayi being the most prevalent (9.78%) amongst the three trypanosome species detected. The prevalence of Wolbachia and Spiroplasma species were 2.80% and 40.8% respectively in flies. Sodalis glossinidius was not detected. There was an association between the presence of trypanosomes and Wolbachia, while no association was depicted between trypanosomes and Spiroplasma.
Conclusion
It has been observed from this study that the presence of Wolbachia seems to favour trypanosome infections. Investigation on the Wolbachia genetic polymorphism in tsetse could help to better understand this association.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-025-04959-7.
Keywords: Trypanosoma, Spiroplasma, Wolbachia, Sodalis glossinidius, Glossina Tachinoides, Yankari game reserve
Introduction
Tsetse flies are viviparous obligate hematophagous insects found in sub-Saharan Africa serving as vectors of African trypanosomiasis, a parasitic disease known as Human African Trypanosomiasis (HAT) in humans and Animal African Trypanosomiasis (AAT) in animals [1]. AAT affects wild fauna, domestic animals (goats, dogs, sheep and pigs) and domesticated livestock like camels, donkeys and horses [2]. Economic retardation due AAT is estimated at 4.75 billion dollars annually [3]. Vector control and drug administration are being used to control AAT. Drugs presently used in the management of trypanosome infections in animals are less efficient due to the increasing number of drug resistant strains, high cost, toxicity, and less availability [4]. Trapping, use of insecticides and sterile insect control is used for vector control. However, the drawbacks of these techniques are their field application and sustainability which include limited resources for trapping and development of resistance to insecticides. In addition, most insecticides are not environmentally friendly. Sterile insect technique is specific to a particular species of tsetse flies [5].
Investigations of endosymbionts that could be implicated in the vector competence of tsetse flies have been undertaken in different tsetse species [6]. Sodalis glossinidius have been genetically transformed to express anti-trypanosome molecules within tsetse flies making them refractory to trypanosomes, a technique known as paratransgenesis [7]. Mitigating vector competence will require complete interruption of disease transmission. These could be achieved using genetic engineering to generate transgenic insects capable of blocking the biological and cyclical transmission of the parasites [8]. The microbial community influences several aspects of Tsetse’s physiology, including nutrition, fecundity, and vector competence [9, 10]. Selected strains of this microbial community prevent the vector from transmitting diseases by either blocking pathogen development or reducing the life span of the vector [11]. Tsetse flies harbour three maternally transmitted symbiotic bacteria: Wolbachia, Sodalis glossinidius and Wigglesworthia glossinidia [10, 12]. Recently, Spiroplasma was reported as an endosymbiont of tsetse flies [13].
W. glossinidia is an obligate symbiotic bacterium found in all tsetse species. It provides food supplements to maintain fecundity. It is also important in the maturation of the immune system of the fly [6], however, not relevant for this current study. S. glossinidius is involved in tsetse fly vector competence by favouring parasite fixing in the insect midgut via a biochemical mechanism involving the production of N-acetyl glucosamine [12]. The colonisation of the tsetse’s midgut and spread of the trypanosome correlate positively with the presence of S. glossinidius [14]. Wolbachia is an intracellular alpha proteobacteria that is trans-ovarially transmitted and occur in about 65% of arthropods, associated with reproductive tissues and cause reproductive abnormalities such as cytoplasmic incompatibility (CI) and parthenogenesis [15]. Spiroplasma is a genus of wall-less bacteria belonging to the class Mollicutes and has been shown to protect Drosophila neotestacea from nematode infection [16] against fungi in the pea aphid (Acyrthosiphon pisum), and against a parasitoid wasp in Drosophila hydei [17]. Reports are available on the prevalence of S. glossinidius, Wolbachia, and Spiroplasma in tsetse species from different communities across the globe highlighting an association between the presence of the endosymbiont S. glossinidius and the aptness of tsetse flies to harbour trypanosomes [18]. Despite these reports, data is lacking on the association between endosymbionts and trypanosomes in G. tachinoides from Yankari Game Reserve, Nigeria, a region with tourism and agriculture as some major income-generating activities. Therefore, this study aimed to investigate the prevalence and association between trypanosomes and endosymbionts in tsetse flies from Yankari Game Reserve.
Methods
Study area
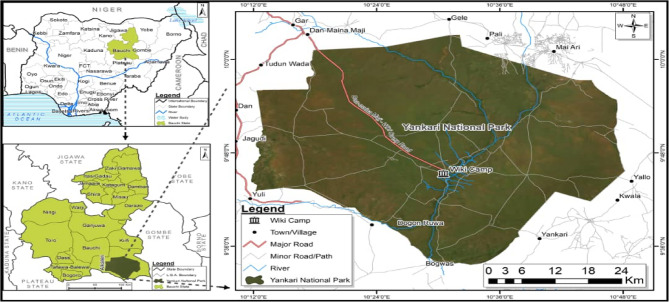
This study was done in Yankari Game Reserve (Fig. 1). Yankari Game Reserve is known to have a lot of tsetse flies and also harbour wildlife that could act as reservoirs of African Trypanosomiasis. In addition, Yankari Game Reserve is a region with tourism and agriculture as some major income-generating activities. Thus, controlling trypanosomiasis is this region will minimise economic losses. The game reserve occupies an area of about 2244 km2. The park is centred at 9.50’N and 10.30’E in the South-central area of Bauchi State in North-eastern Nigeria [19]. The annual rainfall in the area ranges between 900 mm3 and 1,000 mm3, with the wet season starting in May and ending in September and the dry season starting in October and ending in April [20]. Its moderate climate, dense vegetation providing shelter, presence of slow-flowing streams, and wild animals make the environment suitable for tsetse flies. The rich diversity of animals in the reserve probably constitutes the main food source for the tsetse population.
Fig. 1.
Study area
Sampling and morphological identification
Trapping of tsetse flies was carried out in November using 11 biconical traps placed at suitable locations (cool, shady areas to avoid desiccation of flies) for three days consecutively. The coordinates of all traps were recorded using a Global Positioning System (GPS) device (GPSMAP® 60CSx Garmin). Collection of tsetse flies was once daily at 4 pm. The species of tsetse flies was identified immediately using morphological features such as size, colour and number of dark tarsal segments following the Food and Agricultural Organization (FAO) training manual for tsetse personnel volume 1 [21] while sex was determined based on the presence or absence of hypogeum [22]. The flies were dissected under a microscope in a drop of phosphate buffered saline (PBS) solution using sterile dissection tools. The tools were cleaned using 3% bleach (sodium hypochlorite), followed by 70% ethanol, and finally sterile distilled water after the dissection of each fly to prevent contamination. Guts and other body parts were separately transferred into cryotubes containing RNA Later® (Sigma-Aldrich) and kept at room temperature in the field, then at − 20 ℃ in the laboratory until required for use.
DNA extraction
Nucleic acid material (DNA) was isolated from the guts using AccuPrep Genomic DNA extraction kit (Bioneer, South Korea) according to the instructions of the manufacturer. The extracted DNA was quantified using a NanoDrop1000 C spectrophotometer (Thermo scientific, Germany) and stored at −20 ℃.
Molecular confirmation of tsetse fly species
To confirm the species of tsetse flies previously identified morphologically, a PCR was performed to amplify the mitochondrial cytochrome C oxidase 1 gene (COX1) using COX-1 primers (Table 1) adapted from Dyer et al. [23]. The PCR reaction was performed in a 20 µL volume containing 1X DreamTaq buffer, 1 U DreamTaq polymerase, 0.2 mM dNTPs, 2 µM of forward and 2 µM of reverse primers, 2 µL of DNA template, and the final volume was made up with nuclease-free water. The cycling conditions were; initial denaturation at 95 ℃ for 5 min, followed by 30 cycles of denaturation at 94 ℃ for 60 s, annealing at 55 ℃ for 60 s, and elongation at 72 ℃ for 2 min. The final extension was at 72 °C for 10 min.
Table 1.
Sequence of primers used for molecular identification of organisms
| Organism | Primer | Direction | Sequence | Reference |
|---|---|---|---|---|
| Glossina spp | COX1 | Forward | TTGATTTTTTGGTCATCCAGAAGT | [23] |
| Glossina spp | COX1 | Reverse | TGAAGCTTAAATTCATTGCACTAATC | [23] |
| Trypanosoma spp | ITS-1 | External Forward | TGCAATTATTGGTCGCGC | [24] |
| Trypanosoma spp | ITS-1 | External Reverse | CTTTGCTGCGTTCTT | [24] |
| Trypanosoma spp | ITS-1 | Internal Forward | AAGCCAAGTCATCCATCG | [24] |
| Trypanosoma spp | ITS-1 | Internal Reverse | TAGAGGAAGCAAAAG | [24] |
| Trypanosoma spp | gGAPDH | External Forward | TTYGCCGYATYGGYCGCATGG | [25] |
| Trypanosoma spp | gGAPDH | External Reverse | ACMAGRTCCACCACRCGGTG | [25] |
| Trypanosoma spp | gGAPDH | Internal Forward | GCSTAYCAGATGAAGTAC GAC | [26] |
| Trypanosoma spp | gGAPDH | Internal Reverse | GTTYTGCAGSGTCGCCTTGG | [25] |
| Sodalis | pSG2 | Forward | TGAAGTTGGGAATGTCG | [27] |
| Sodalis | pSG2 | Reverse | AGTTGTAGCACAGCGTGTA | [27] |
| Wolbachia | W-spec | Forward | CATACC TATTCGAAGGGATAG | [15] |
| Wolbachia | W-spec | Reverse | AGCTTCGAGTGAA ACCAATTC | [15] |
| Spiroplasma | 63 F | Forward | GCCTAATACATGCAAGTCGAAC | [13] |
| Spiroplasma | 63 F | Reverse | TAGCCGTGGCTTTCTGGTAA | [13] |
Identification of trypanosome species
A nested PCR using ITS-1 generic primers (Table 1) was performed according to Adams et al. [24]. The first round of PCR was performed in a 20 µL reaction volume containing 1X DreamTaq buffer, 1 U DreamTaq polymerase 0.2 mM dNTPs, 1 µM each of the outer primers (forward and reverse), and 3 µL of DNA template. The volume was made up using nuclease free-water. The cycling conditions were; initial denaturation at 95 ℃ for 3 min, followed by 35 cycles of denaturation at 94 ℃ for 30 s, annealing at 54 ℃ for 30 s, extension at 72 ℃ for 60 s, with a final extension at 72 ℃ for 5 min. The second round of PCR was carried out using ITS-1 internal primers (Table 1) and was performed in 20 µL reaction volume containing 1X DreamTaq buffer, 1 U DreamTaq polymerase, 0.2 mM dNTPs, 1 µM of each primer, 2 µL DNA (1/40 dilution of first round PCR product) and the volume was made up with nuclease free water. The cycling conditions were as follows: initial denaturation at 95 ℃ for 3 min, followed by 35 cycles of denaturation at 94 ℃ for 30 s, annealing at 55 ℃ for 30 s, extension at 72 ℃ for 60 s, and a final extension at 72 ℃ for 5 min. The expected band sizes for trypanosome species were: Trypanosoma congolense (640 bp), Trypanosoma grayi (240–380 bp), Trypanosoma vivax (400 bp), Trypanosoma simiae (400 bp), Trypanosoma brucei (500 bp) and Trypanosoma evansi (500 bp).
Another nested PCR targeting an 800 bp DNA fragment of the glycosomal Glyceraldehyde-3-phosphate dehydrogenase (gGAPDH) gene of trypanosomes was performed to confirm the trypanosomes positive tsetse guts using gGAPDH primers (Table 1) designed by Hamilton et al. [25] following the protocol of Weber et al. [26]. The first round PCR was performed in a 20 µL reaction volume containing 1X DreamTaq buffer, 1 U DreamTaq polymerase, 0.2 mM dNTPs, 1 µM of each gGAPDH external primer, 2 µL DNA template and the volume was made up using nuclease free water. The cycling conditions were; initial denaturation for 3 min at 95 °C, followed by 30 cycles of 60 s at 95 °C, 30 s at 55 °C and 60 s at 72 °C, and a final elongation at 72 °C for 10 min. The first PCR products were diluted 40-fold and 1 µL of this dilution was used for the second PCR round using gGAPDH internal primers, performed in a 20 µL reaction volume containing 1X DreamTaq buffer, 1 U DreamTaq polymerase, 0.2 mM dNTPs, 1 µM each of gGAPDH internal primer (Table 1), and the volume was made up using nuclease free water. The cycling conditions were; initial denaturation for 3 min at 95 °C, followed by 30 cycles of 60 s at 95 °C, 30 s at 52 °C and 60 s at 72 °C, and a final elongation at 72 °C for 10 min. Amplicons were resolved on 1.5% agarose gel. The double distilled water was used as negative control while trypanosome positive controls were obtained from Weber et al. [26] DNA positive samples.
Detection of endosymbionts
The presence of S. glossinidius was screened by PCR, amplifying a 120 bp DNA fragment of S. glossinidius extrachromosomal plasmid (pSG2) using pSG2 primers (Table 1) adapted from Darby et al. [27] in a total reaction volume of 20 µL containing 1X DreamTaq buffer, 1 U DreamTaq polymerase, 0.2 mM dNTPs, 1 µM each of primers, and 3 µL of DNA template. The volume was made up using nuclease-free water. The cycling conditions were: initial denaturation at 94 ℃ for 3 min, followed by 30 cycles of 94 ℃ for 30 s, 51 ℃ for 30 s, 72 ℃ for 30 s, and a final extension at 72 ℃ for 5 min. Wolbachia was detected by PCR using W-spec primers (Table 1) that amplify a 438 bp DNA fragment of the 16 S rRNA [15] in a 20 µL reaction volume containing 1X DreamTaq buffer, 0.15 mM dNTPs, 1 U DreamTaq polymerase, 0.5 µM each of primers, and 2 µL of template DNA. The volume was made up using nuclease-free water. The cycling conditions were: initial denaturation at 95 ℃ for 5 min, followed by 30 cycles of 95 ℃ for 30 s, 54 ℃ for 30 s, 72 ℃ for 60 s, and a final extension at 72 ℃ for 10 min. Spiroplasma was detected by amplifying a 455 bp DNA fragment of the 16 S rRNA gene of the bacterium using 63 F primers (Table 1) as described by Doudoumis et al. [13] in a 20 µL reaction volume consisting of 1X DreamTaq buffer, 0.15 mM dNTPs, 1 U DreamTaq polymerase, 0.25 µM each of primers, and 3 µL of template DNA. The cycling conditions were: initial denaturation at 95 ℃ for 5 min, followed by 30 cycles of 95 ℃ for 30 s, 59 ℃ for 30 s, 72 ℃ for 60 s, and a final extension at 72 ℃ for 10 min. All the PCR products were resolved on 1.5% agarose gel and visualised under UV illumination. The negative control for this study was a double distilled water while Sodalis, Wolbachia and Spiroplasma positive controls were DNA positive samples from a previous study by Mfopit et al. [28].
PCR product purification and sequencing
Amplicons of PCR were excised from the gel and purified using the GeneJET Gel Extraction Kit (Thermo Scientific) following the instructions of the manufacturer. The DNA purified was used for direct Sanger sequencing by a commercial provider (Microsynth SeqLab, Göttingen, Germany). The nucleotide sequences generated were analysed using the Bio-edit software, and a BLAST (Basic Local Alignment Search Tool) search was conducted using Nucleotide BLAST at the NCBI (National Centre for Biotechnology Information) database [29]. Nucleotide sequences isolated in this study were deposited in the NCBI database (GenBank).
Statistical analyses
Entomological data were expressed in terms of abundance of tsetse flies, estimated by apparent density of flies per trap per day (FTD) according to the following formula: FTD = Nc/TD (where Nc is the total number of captured tsetse flies, T is the total number of traps, and D is the total number of trapping days). Trypanosomes and symbionts hosted by tsetse flies were expressed in terms of prevalence. The Fisher test and the logistic regression model were used to analyse the association between the endosymbionts (Wolbachia and Spiroplasma) and trypanosome infection in tsetse flies.
Results and discussion
Entomological survey
A total of 2,742 tsetse flies were trapped from 5 locations, using 11 biconical traps in three days. These locations include; Magama, Salt-lake, Marshal cave, Kwan Kirya and Bokono (Table 2).
Table 2.
Relative abundance of Tsetse flies per collection site
| Location | Coordinate | Sex of tsetse fly | ||
|---|---|---|---|---|
| Male | Female | Total | ||
| Magama | 09°44.870’N/010°30.896’E | 375 | 918 | 1,293 |
| Salt Lake | 09°45.174’N/010°31.508’E | 153 | 370 | 523 |
| Marshall Cave | 09°46.631’N/010°32.296’E | 35 | 79 | 114 |
| Kwan Kirya | 09°45.401’N/010°31.760’E | 107 | 256 | 363 |
| Bokono Site | 09°46.602’N/010°32.361’E | 133 | 316 | 449 |
| 803 | 1,939 | 2,742 | ||
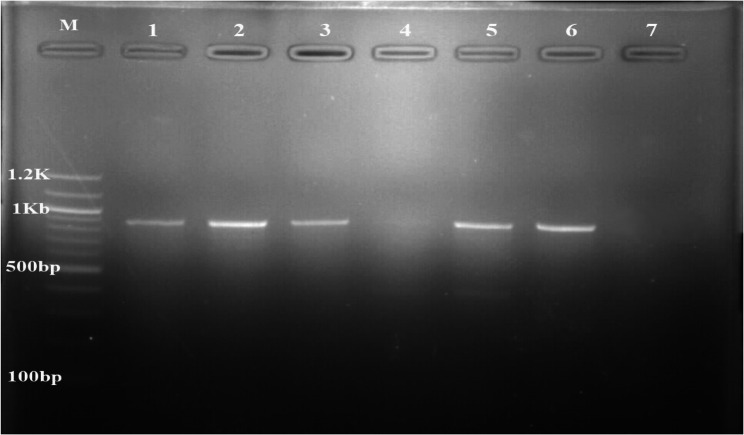
Out of 2,742 tsetse flies, 803 were males while 1,939 were females. The apparent fly density was 83.09 F/T/D. All the trapped flies were identified morphologically to be Glossina tachinoides. A total of 215 were dissected, and found to be teneral. The amplification (Fig. 2) and sequencing of the COX1 partial gene sequence confirmed the Glossina species to be G. tachinoides. The sequence of our amplicons was closely related (99.87% similarity) to other Glossina tachinoides COX1 sequences isolated in the same locality (MG234544, MG234547).
Fig. 2.
PCR amplification of Glossina cytochrome C oxidase 1 gene from gDNA. M: marker (100 bp), lane 1: positive control, 2, 3, 5 and 6: Glossina tachinoides samples and lane 4 and 7: negative controls
A relatively high apparent tsetse fly density of 83.09 F/T/D was determined. Previous studies reported apparent fly densities of 22.5 [30] and 128.03 F/T/D for G. tachinoides [31]. This variation in apparent fly density could be a result of variations in study periods. In our study, samples were collected at the start of the dry season (November), whereas in the other studies, sample collection was in March and August, respectively. We identified a single tsetse fly species (Glossina tachinoides), whereas a previous study reported two species: Glossina morsitans and Glossina tachinoides [30]. The same study reported Glossina tachinoides as the predominant species of tsetse flies in Yankari Game Reserve [30]. This difference in Glossina species may be due to variation in season and trap locations during the entomological survey.
Trypanosome infection rate
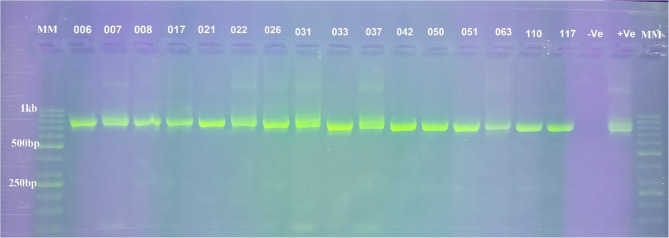
Out of 215 guts of Glossina tachinoides analysed by ITS-1 nested PCR (Fig. S1), only 23 (10.70%) were infected with at least one species of trypanosomes. Three species of trypanosomes were identified: Trypanosoma grayi, T. congolense, and T. vivax. The total number of flies infected by Trypanosoma grayi, Trypanosoma congolense, Trypanosoma vivax, Trypanosoma grayi/Trypanosoma congolense mixed infection and Trypanosoma grayi/Trypanosoma vivax mixed infection was: 12/215, 1/215, 1/215, 7/215 and 2/215 respectively (Table 3). Following the identification of trypanosomes using ITS-1 nested PCR, gGAPDH nested PCR (Fig. 3) was performed to confirm the trypanosome positive tsetse flies.
Table 3.
Trypanosome species isolated from midgut of Glossina Tachinoides
| Type of Infection | T. grayi | T. congolense | T. vivax | T. grayi/congolense | T. grayi/vivax |
|---|---|---|---|---|---|
| Infections (Out of 215) | 12/215 | 1/215 | 1/215 | 7/215 | 2/215 |
| Prevalence | 5.58% | 0.47% | 0.47% | 3.26% | 0.93% |
Overall prevalence of trypanosome infection = 23/215 (10.70%)
Fig. 3.
PCR amplification of trypanosome (gGAPDH gene) from genomic DNA of tsetse. MM: marker (50 bp), Lane 6, 8, 17, 21, 22, 26, 33, 42, 50, 51, 63, 117: T. grayi, Lane 7, 31 and 37: T. congolense. Lane 110: T. vivax, -Ve: negative control and + Ve: positive control
The trypanosome infection rate of 10.70% was similar to the 11.9% infection rate obtained in another study in the same location on Glossina tachinoides [31]. This study reported the presence of three species of trypanosomes: T. congolense, T. vivax, and T. grayi, which supports the work of Weber et al. [26], who reported the same species. No trypanosome species of the T. brucei complex was found, which is in agreement with Weber et al. [26] that reported the absence of T. brucei species. This could be as a result of low circulation of species of the Trypanosoma brucei complex. Trypanosoma vivax was found in only two tsetse fly samples. This low infection rate observed could be attributed to the fact that DNA was isolated only from the midgut of tsetse flies since the development of T. vivax is restricted to the mouthparts, though few reports indicate that this parasite can be detected from the midgut of tsetse flies up to four days after a blood meal infected with trypanosomes [32].
Symbiont occurrence rate
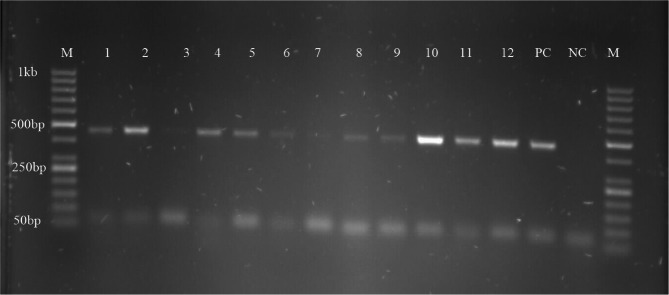
Out of 215 midgut samples screened for Spiroplasma, Wolbachia, and S. glossinidius (Fig. 4, Fig. S2), 87 harboured Spiroplasma, and 6 harboured Wolbachia while no sample was found to harbour S. glossinidius, giving endosymbiont infection rates of 40.47%, 2.80% and 0.00% respectively.
Fig. 4.
PCR amplification of Spiroplasma 16 S rRNA gene M: marker, lane 1 to 12: positive Spiroplasma samples, lane 3: Spiroplasma negative sample PC: positive control and NC: negative control
Our findings reported the presence of two Tsetse’s symbiotic bacteria: Spiroplasma and Wolbachia. The absence of Sodalis glossinidius in Glossina tachinoides agrees with the findings of Mfopit et al. [28], who reported a 0.00% prevalence of Sodalis in the same study location, but differs with 3.7% and 16% infection rates reported for G. austeni and G. pallidipes, respectively, in Shimba Hills National Reserve, Kenya [33]. Findings from our study also differ with the 37.0% Sodalis infection rate found in Glossina tachinoides captured in Cameroon [6]. The remarkable difference observed with our study could be attributed to differences in tsetse species and geographic location because the microbiota of tsetse flies vary with Glossina species and geographic location [14]. The low occurrence of Wolbachia (2.80%) corroborates with another study that reported the absence of Wolbachia in the Glossina palpalis group [34]. However, Kame-Ngasse et al. [6] reported a high prevalence of 68.1% in Glossina tachinoides from the Adamawa region, Cameroon. These observations suggest that the prevalence of Wolbachia may depend on the ecological conditions of the tsetse fly populations [35]. The high rate of Spiroplasma infection in Glossina tachinoides (40.47%) is consistent with other studies that reported 37.5% [13] and 44.5% infection rate of Spiroplasma in G. tachinoides captured from Burkina Faso [34].
Relationship between trypanosome infection and symbiont presence
Of the 87 tsetse flies harbouring Spiroplasma, 13 had at least one species of trypanosome (Table 4). Of the 128 tsetse flies not harbouring Spiroplasma, 10 were harbouring trypanosomes. There was no association (p = 0.116) between the presence of Spiroplasma and the trypanosome infection. Out of 6 flies harbouring Wolbachia, 5 had at least one species of trypanosome, while one was trypanosome negative. Of the 209 Wolbachia negative flies, 18 had trypanosome infection. The Fisher test (Table 4) shows a positive association between Wolbachia presence and trypanosome infection (p = 0.001).
Table 4.
Statistical association of Wolbachia and Spiroplasma endosymbionts with trypanosome DNA in Glossina Tachinoides
| Spiroplasma and trypanosome co-infection (N = 215) | Wolbachia and trypanosome co-infection (N = 215) | ||||
|---|---|---|---|---|---|
| T/S | S- | S+ | W/T | W- | W+ |
| T- | 118 | 74 | T- | 191 | 1 |
| T+ | 10 | 13 | T+ | 18 | 5 |
| p = 0.116 | p = 0.001 | ||||
T+/T-: Trypanosome positive/negative, W+/W-: Wolbachia positive/negative, Sp+/Sp-: Spiroplasma positive/negative
An association was not seen between Spiroplasma and trypanosome infection, indicating that Spiroplasma does not affect the establishment of trypanosomes in the midgut of Glossina tachinoides. An association was observed between Wolbachia and trypanosomes in tsetse flies, but this relationship could not be conclusive because only a few tsetse flies (6/215) were harbouring the bacterium. This study disagrees with the study of Kante et al. [36], who reported the absence of an association between Wolbachia and trypanosomes in Glossina palpalis palpalis populations from three sleeping sickness foci of southern Cameroon. The low prevalence of Wolbachia could be because of the circulation of another Wolbachia strain which could not be detected by the primers used for this study. The study of [37] used the Wsp primers targeting Wolbachia surface protein for the screening of Wolbachia while we used the W-spec primers targeting the Wolbachia 16 S rRNA gene. The study should be performed with different haplotypes of Wolbachia to have a clearer picture of the possible relationship existing between Wolbachia and the level of infection with trypanosomes in tsetse flies.
Conclusion
Our study found that Glossina tachinoides from Yankari Game Reserve are infected with trypanosomes, with a prevalence of 10.70%. The infection rates of Sodalis, Wolbachia and Spiroplasma were 0.00%, 2.80%, and 40.47%, respectively. An association was observed between Wolbachia and trypanosomes in tsetse flies, but no association was observed between Spiroplasma and trypanosomes. Findings from this study provide useful data on the microbiota of tsetse flies and could be further used to investigate and understand the role of these symbiotic bacteria on the physiology of tsetse flies, thus helping in the development of new disease control techniques.
Supplementary Information
Supplementary Material 1: Fig. S1. PCR amplification of trypanosome (ITS-1 gene) from genomic DNA of tsetse fly. MM: marker (50bp), lane 005: mixed infection of T. vivax and T. grayi, lane 007: T. congolense, lane 017: T. grayi, lane 22: mixed infection of T. grayi and T. congolense, lane 037: mixed infection of T. grayi and T. vivax NC: negative control and PC: positive control.
Supplementary Material 2: Fig. S2. PCR amplification of endosymbionts. A. Wolbachia 16S rRNA gene. M: marker, lane 1, 2, 3 and 4 are positive Wolbachia samples, NC: negative control and PC: positive control.
Acknowledgements
The authors sincerely thank Africa Centre of Excellence for Neglected Tropical Diseases and Forensic Biotechnology (ACENTDFB), Ahmadu Bello University, Zaria, Nigeria for sponsoring the research. We also thank the Centre for Biotechnology Research and Training (CBRT), Ahmadu Bello University for their technical assistance during laboratory work. Our sincere gratitude goes to the department of Biochemistry, Ahmadu Bello University for administrative and technical support in carrying out the study. We would also like to thank the following persons: Prof. Achukwi Daniel, Mr. Nsadztsen Gilbert Adzemye, and Mr. Ahmadu, a staff member of the Nigerian Institute of Trypanosomiasis Research.
Authors’ contributions
YMM conceived the study. ACMT, YMM, AA, GDC, and JK designed the protocol of the study. ACMT, YMM, ABY, PYM, EFG and GAA contributed to sample collection. ACMT and YMM carried out laboratory analyses. AA, GDC, MM and JK supervised laboratory analyses. YMM carried out data analyses. ACMT drafted the manuscript. All authors read, revised, and approved the manuscript.
Funding
This work was supported by the Africa Centre of Excellence for Neglected Tropical Diseases and Forensic Biotechnology (ACENTDFB), Ahmadu Bello University, Zaria, Nigeria; and the Deutsche Forschungsgemeinschaft (DFG project grant to GDC: Ke428/13 − 1).
Data availability
The datasets supporting the conclusions of this article are included within the article and its additional files. Nucleotide sequences are openly available in National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/), with following reference numbers: Glossina tachinoides: OQ653471; Trypanosoma congolense: OQ658682 and OQ658683; Trypanosoma vivax: OQ658688; Trypanosoma grayi: OQ658685, OQ658686, and OQ658687; Wolbachia: OQ658372 and Spiroplasma: OQ658371.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Attardo GM, Scolari F, Malacrida A. Bacterial Symbionts of Tsetse Flies: Relationships and Functional Interactions Between Tsetse Flies and Their Symbionts. In: Kloc M, editor. Symbiosis: Cellular, Molecular, Medical and Evolutionary Aspects. Cham: Springer International Publishing; 2020:pp. 497–536. (Results and Problems in Cell Differentiation; vol. 69). Available from: https://link.springer.com/10.1007/978-3-030-51849-3_19. Cited 2023 Feb 27. [DOI] [PubMed]
- 2.Okello I, Mafie E, Eastwood G, Nzalawahe J, Mboera LEG. African animal trypanosomiasis: a systematic review on prevalence, risk factors and drug resistance in sub-Saharan Africa. J Med Entomol. 2022;59(4):1099–143. [DOI] [PubMed] [Google Scholar]
- 3.Namangala B, Odongo S. Animal African Trypanosomosis in Sub-Saharan Africa and Beyond African Borders. In: Magez S, Radwanska M, editors. Trypanosomes and Trypanosomiasis. Vienna: Springer Vienna; 2014:pp. 239–60. Available from: https://link.springer.com/10.1007/978-3-7091-1556-5_10. Cited 2023 Feb 28.
- 4.Geerts S, Holmes PH, Eisler MC, Diall O. African bovine trypanosomiasis: the problem of drug resistance. Trends Parasitol. 2001;17(1):25–8. [DOI] [PubMed] [Google Scholar]
- 5.Kumar G, Baharia R, Singh K, Gupta SK, Joy S, Sharma A et al. Addressing challenges in vector control: a review of current strategies and the imperative for novel tools in India’s combat against vector-borne diseases. bmjph. 2024;2(1). Available from: https://bmjpublichealth.bmj.com/content/2/1/e000342. Cited 2025 Apr 27. [DOI] [PMC free article] [PubMed]
- 6.Kame-Ngasse GI, Njiokou F, Melachio-Tanekou TT, Farikou O, Simo G, Geiger A. Prevalence of symbionts and trypanosome infections in Tsetse flies of two villages of the Faro and déo division of the Adamawa region of Cameroon. BMC Microbiol. 2018;18(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simo G, Kanté ST, Madinga J, Kame G, Farikou O, Ilombe G, et al. Molecular identification of wolbachia and sodalis glossinidius in the midgut of Glossina fuscipes quanzensis from the Democratic Republic of congo. Parasite. 2019;26:5. 10.1051/parasite/2019005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rio RVM, Hu Y, Aksoy S. Strategies of the home-team: symbioses exploited for vector-borne disease control. Trends Microbiol. 2004;12(7):325–36. [DOI] [PubMed] [Google Scholar]
- 9.Balmand S, Lohs C, Aksoy S, Heddi A. Tissue distribution and transmission routes for the tsetse fly endosymbionts. J Invertebr Pathol. 2013;112(Suppl):S116-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Weiss BL, Aksoy S. Tsetse fly microbiota: form and function. Front Cell Infect Microbiol. 2013. 10.3389/fcimb.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mustafa MS, Rastogi V, Gupta RK, Jain S, Singh PMP, Gupta A. Wolbachia: the selfish Trojan horse in dengue control. Med J Armed Forces India. 2016;72(4):373–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dale C, Welburn SC. The endosymbionts of Tsetse flies: manipulating host–parasite interactions. Int J Parasitol. 2001;31(5–6):628–31. [DOI] [PubMed] [Google Scholar]
- 13.Doudoumis V, Blow F, Saridaki A, Augustinos A, Dyer NA, Goodhead I, et al. Challenging the wigglesworthia, sodalis, wolbachia symbiosis dogma in Tsetse flies: spiroplasma is present in both laboratory and natural populations. Sci Rep. 2017;7(1):4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farikou O, Njiokou F, Mbida Mbida JA, Njitchouang GR, Djeunga HN, Asonganyi T, et al. Tripartite interactions between tsetse flies, sodalis glossinidius and trypanosomes—an epidemiological approach in two historical human African trypanosomiasis foci in Cameroon. Infect Genet Evol. 2010;10(1):115–21. [DOI] [PubMed] [Google Scholar]
- 15.Werren JH, Windsor DM. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc R Soc Lond B Biol Sci. 2000;267(1450):1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science. 2010;329(5988):212–5. [DOI] [PubMed] [Google Scholar]
- 17.Xie J, Vilchez I, Mateos M. Spiroplasma Bacteria Enhance Survival of Drosophila hydei Attacked by the Parasitic Wasp Leptopilina heterotoma. Raine NE, editor. PLoS ONE. 2010;5(8):e12149. [DOI] [PMC free article] [PubMed]
- 18.Geiger A, Ravel S, Frutos R, Cuny G. Sodalis glossinidius (Enterobacteriaceae) and vectorial competence of Glossina palpalis gambiensis and Glossina morsitans morsitans for Trypanosoma congolense Savannah type. Curr Microbiol. 2005;51(1):35–40. [DOI] [PubMed] [Google Scholar]
- 19.Odunlami S. An assessment of the ecotourism potential of yankari national park Nigeria. In 2003. Available from: https://www.semanticscholar.org/paper/%22AN-ASSESSMENT-OF-THE-ECOTOURISM-POTENTIAL-OF-PARK%2C-Odunlami/b1f4929d2bbd0fb956149a9eb672419825958cee. Cited 2024 Sep 12.
- 20.Abdullahi M, Sanusi S, Abdul S, F.B.J. S. An assessment of the herbaceous species vegetation of Yankari game reserve. Bauchi Nigeria. 2009;6:20–5. [Google Scholar]
- 21.Pollock JN. Training manual for Tsetse control personnel: Tsetse biology, systematics and distribution; techniques. Food and Agriculture Organization of the United Nations; 1982. p. 280.
- 22.Gooding RH, Krafsur ES. TSETSE genetics: contributions to biology, systematics, and control of Tsetse flies. Annu Rev Entomol. 2005;50(1):101–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyer N, Lawton S, Ravel S, Choi K, Lehane M, Robinson A, et al. Molecular phylogenetics of tsetse flies (Diptera: Glossinidae) based on mitochondrial (COI, 16S, ND2) and nuclear ribosomal DNA sequences, with an emphasis on the palpalis group. Mol Phylogenet Evol. 2008;49(1):227–39. [DOI] [PubMed] [Google Scholar]
- 24.Adams ER, Malele II, Msangi AR, Gibson WC. Trypanosome identification in wild Tsetse populations in Tanzania using generic primers to amplify the ribosomal RNA ITS-1 region. Acta Trop. 2006;100(1–2):103–9. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton PB, Stevens JR, Gaunt MW, Gidley J, Gibson WC. Trypanosomes are monophyletic: evidence from genes for glyceraldehyde phosphate dehydrogenase and small subunit ribosomal RNA. Int J Parasitol. 2004;34(12):1393–404. [DOI] [PubMed] [Google Scholar]
- 26.Weber JS, Ngomtcho SCH, Shaida SS, Chechet GD, Gbem TT, Nok JA, et al. Genetic diversity of trypanosome species in tsetse flies (Glossina spp.) in Nigeria. Parasit Vectors. 2019;12(1):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darby AC, Lagnel J, Matthew CZ, Bourtzis K, Maudlin I, Welburn SC. Extrachromosomal DNA of the symbiont Sodalis glossinidius. J Bacteriol. 2005;187(14):5003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mfopit YM, Weber JS, Chechet GD, Ibrahim MAM, Signaboubo D, Achukwi DM, et al. Molecular detection of Sodalis glossinidius, Spiroplasma and Wolbachia endosymbionts in wild population of tsetse flies collected in Cameroon, Chad and Nigeria. Res Sq. 2023;rs.3.rs-2902767. [DOI] [PMC free article] [PubMed]
- 29.Syngai G, Barman P, Bharali R, Dey S. BLAST: an introductory tool for students to bioinformatics applications. Kenean J Sci. 2013;2:67–76. [Google Scholar]
- 30.Shaida SS, Weber JS, Gbem TT, Ngomtcho SCH, Musa UB, Achukwi MD, et al. Diversity and phylogenetic relationships of Glossina populations in Nigeria and the Cameroonian border region. BMC Microbiol. 2018;18(1): 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isaac C, Ciosi M, Hamilton A, Scullion KM, Dede P, Igbinosa IB, et al. Molecular identification of different trypanosome species and subspecies in tsetse flies of Northern Nigeria. Parasit Vectors. 2016;9(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ooi CP, Schuster S, Cren-Travaillé C, Bertiaux E, Cosson A, Goyard S, et al. The cyclical development of Trypanosoma vivax in the tsetse fly involves an asymmetric division. Front Cell Infect Microbiol. 2016;6. Available from: https://www.frontiersin.org/articles/10.3389/fcimb.2016.00115. Cited 2023 Feb 28. [DOI] [PMC free article] [PubMed]
- 33.Wamwiri FN, Alam U, Thande PC, Aksoy E, Ngure RM, Aksoy S, et al. Wolbachia, Sodalis and Trypanosome co-infections in natural populations of Glossina Austeni and Glossina pallidipes. Parasit Vectors. 2013;6(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Khamlichi S, Maurady A, Asimakis E, Stathopoulou P, Sedqui A, Tsiamis G. Detection and characterization of Spiroplasma and wolbachia in a natural population of Glossina Tachinoides. In: Kacprzyk J, Balas VE, Ezziyyani M, editors. Advanced intelligent systems for sustainable development (AI2SD’2020). Cham: Springer International Publishing; 2022. pp. 256–64. (Advances in Intelligent Systems and Computing). [Google Scholar]
- 35.Yun Y, Lei C, Peng Y, Liu F, Chen J, Chen L. Wolbachia strains typing in different geographic population spider, Hylyphantes graminicola (Linyphiidae). Curr Microbiol. 2011;62(1):139–45. [DOI] [PubMed] [Google Scholar]
- 36.Kante TS, Melachio Tanekou TT, Amih O, Njiokou F, Simo G. Detection of wolbachia and different trypanosome species in Glossina palpalis palpalis populations from three sleeping sickness foci of Southern Cameroon. Parasit Vectors. 2018;11:630. 10.1186/s13071-018-3229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Y, Xi Z, Liu X, Wang J, Guo Y, Ren D, et al. Identification and molecular characterization of wolbachia strains in natural populations of Aedes albopictus in China. Parasit Vectors. 2020;13(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Fig. S1. PCR amplification of trypanosome (ITS-1 gene) from genomic DNA of tsetse fly. MM: marker (50bp), lane 005: mixed infection of T. vivax and T. grayi, lane 007: T. congolense, lane 017: T. grayi, lane 22: mixed infection of T. grayi and T. congolense, lane 037: mixed infection of T. grayi and T. vivax NC: negative control and PC: positive control.
Supplementary Material 2: Fig. S2. PCR amplification of endosymbionts. A. Wolbachia 16S rRNA gene. M: marker, lane 1, 2, 3 and 4 are positive Wolbachia samples, NC: negative control and PC: positive control.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files. Nucleotide sequences are openly available in National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/), with following reference numbers: Glossina tachinoides: OQ653471; Trypanosoma congolense: OQ658682 and OQ658683; Trypanosoma vivax: OQ658688; Trypanosoma grayi: OQ658685, OQ658686, and OQ658687; Wolbachia: OQ658372 and Spiroplasma: OQ658371.