Abstract
Objective
Computer-assisted surgery (CAS) and patient-specific instrumentation (PSI) are digital techniques to improve the accuracy of implant positioning in total knee arthroplasty (TKA), but their effects on clinical outcomes are still in dispute. The objective of this trial is to evaluate the efficacy and safety of CAS and PSI compared to conventional instrumentation (CI) in TKA.
Methods
A prospective randomized controlled trial was conducted. A total of 135 patients undergoing TKA were randomized into CAS group, PSI group and CI group with 45 patients in each group. Primary outcome is the coronal mechanical axis of lower extremity. Secondary outcomes include Femoral Rotation Angle (FRA) of the femoral prosthesis, operation time, perioperative blood loss, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Forgotten Joint Score (FJS) and complications.
Results
Outliers of Hip-Knee-Ankle angle (HKA) were 24.4% in CI group, 17.8% in CAS group and 31.1% in PSI group respectively, and there was no significant difference among these 3 groups (P > 0.05). Outliers of FRA were 13.3% in CI group, 26.7% in CAS group and 11.1% in PSI group respectively with no significant difference (P > 0.05). Operation time was (66.67 ± 12.85)min, (81.67 ± 12.31)min and (52.78 ± 8.62)min in CI, CAS and PSI group. Operation time in CI was longer than PSI and shorter than CAS with significant difference (P < 0.01). There was no significant difference in comparison of blood loss, transfusion rate, postoperative WOMAC and FJS (P > 0.05).
Conclusion
CAS and PSI, compared with CI, did not significantly improve clinical outcomes including lower limb alignment, rotation of femoral prosthesis, blood loss, transfusion rate, and function scores. However, CAS was associated with prolonged operation time, whereas PSI resulted in a reduced operation time.
Level of evidence
Level II.
Trial registration
ChiCTR-INR-17,012,881 (registration date: 03/10/2017).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-025-08980-7.
Keywords: Computer-assisted surgery, Patient-specific instrumentation, Total knee arthroplasty
Introduction
Total knee arthroplasty (TKA) is widely regarded as one of the most effective treatments of severe knee joint diseases such as osteoarthritis [1]. However, deviations in postoperative lower limb alignment or component positioning from the normal range can severely compromise the recovery of knee function and survival of the prosthesis [2–4]. Therefore, restoring proper limb alignment and achieving precise component positioning remain primary goals of joint surgeons.
To enhance alignment and accuracy in TKA, computer-assisted surgery (CAS) and patient-specific instrumentation (PSI) have been introduced into TKA [5–10]. Nevertheless, the application of these digital techniques remains controversial. Some studies suggest that CAS or PSI can effectively improve the accuracy of TKA [11–14] and reduce perioperative blood loss [15, 16], supporting their broader application. Conversely, other researches indicate that there is no significant improvement in lower limb alignment or blood loss in CAS or PSI compared to conventional instrumentation (CI) [17–20], and highlight their potential in increasing operation time [21, 22], raising concerns about their routine use.
Furthermore, the comparison between CAS and PSI is also in dispute. Some researchers report that CAS takes advantages in recovering lower limb alignment over PSI [12, 18], while others hold the point that PSI is as effective as CAS in lower limb alignment or even better [11, 17]. In terms of operation time, most researchers agreed that CAS has longer operation time than PSI [11, 21]. Only a few researches compare blood loss in CAS and PSI, but existing results suggest no significant difference [19].
At present, there is still a lack of high-quality studies concentrating on comparison of CAS, PSI and CI in TKA. Most previous researches focus solely on lower limb alignment without discussing operation time and blood loss. Therefore, we conduct this prospective randomized controlled trial in order to evaluate the clinical outcomes of these two techniques in Chinese patients and offer advice for clinical practice.
Methods
Study design
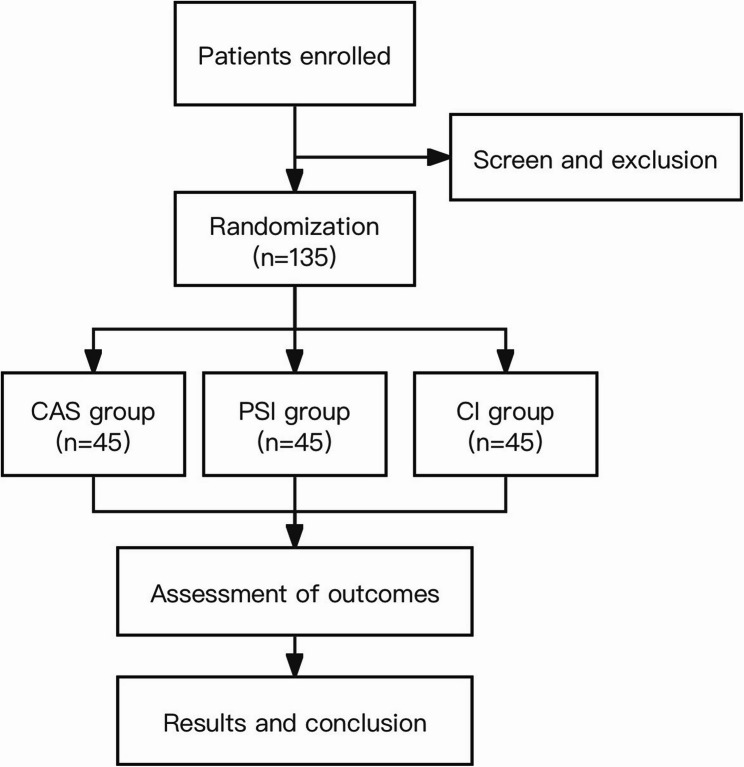
This is a prospective single-blind randomized controlled trial adhering to CONSORT guidelines. A sample size of approximately 135 participants with primary knee osteoarthritis undergoing TKA were enrolled from October 2017 to June 2022 at Peking University Third Hospital. All the participants enrolled were randomly allocated to CAS, PSI, and CI group (45 patients in each group). This trial was approved by Peking University Third Hospital Medical Science Research Ethics Committee (IRB00006761-2016064). (Fig. 1).
Fig. 1.
Study flowchart. Flowchart of the study process
Eligibility criteria
Patients diagnosed with primary knee osteoarthritis undergoing unilateral primary knee arthroplasty were prospectively enrolled in this study. Exclusion criteria includes: (1) severe varus or valgus knee deformity (larger than 20°); (2) previous long-term consumption of anticoagulant drugs; (3) complicated with diseases including renal insufficiency, liver dysfunction, severe heart disease, severe respiratory system diseases, previous venous thromboembolism history or high-risk of thrombosis, blood coagulation dysfunction, the acquired thrombotic diseases, blood coagulation dysfunction, clinical history of stroke or malignant tumor; (4) refusal to sign the informed consent form for any reason [23].
Allocation, randomization and blinding
All participants enrolled were randomly assigned in a 1:1:1 ratio into the CAS group, PSI group and CI group respectively with the method of random envelope by an independent epidemiologist in our research team. Participants were blinded to the group allocation and the technique used in their surgery.
Trial registration, Ethics review and informed consent
This trial was registered at Chinese clinical trial registry (Registration number: ChiCTR-INR-17012881) and approved by Peking University Third Hospital Medical Science Research Ethics Committee (Registration number: IRB00006761-2016064). All the participants have signed the informed consent.
Operative and perioperative procedures
Participants were operated by the same surgeon in our research team who were experienced in CAS-assisted, PSI-assisted and conventional TKA. Posterior stabilized Columbus knee prosthesis system (Aesculap, Tuttlingen, Germany) and Aesculap navigation system was used in CAS group. Participants in PSI group was operated with posterior stabilized A3 prosthesis and 3D-printed osteotomy guide plate (AK Medical, Beijing, China). Participants in CI group were operated with Columbus prosthesis (Aesculap, Tuttlingen, Germany) by conventional instrumentation. Tourniquets were applied in all TKAs. Perioperative treatments including anticoagulation, hemostasis, analgesia, anti-inflammatory, rehabilitation and other therapeutic methods were the same in all participants.
Outcomes and measures
Preoperative measurements
Demographic information of all the patients were collected, including gender, age, height, weight and BMI. Baseline outcomes including hematocrit and hemoglobin level, preoperative Hip-Knee-Ankle angle (HKA) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) were evaluated before surgery but after randomization and allocation.
Primary outcome
Coronal mechanical alignment of lower limb (HKA) was the primary outcome in our study, which was measured before hospital discharge (positive for varus and negative for valgus). A deviation zone of ± 3° was considered to be acceptable.
Secondary outcomes
Postoperative femoral rotation angle.
The Femoral Rotation Angle (FRA) was measured before hospital discharge as the angle between the line connecting the posterior condyles of the femoral prosthesis and the transcondylar axis of the femur through computed tomography to evaluate the rotation of femoral prosthesis. A deviation zone of ± 2° was considered to be acceptable.
-
(2)
Operation time.
Operation time was defined as the time from skin cutting to the skin suture in TKA, which was accurate to the minute.
-
(3)
Blood loss and transfusion rate.
Total blood loss, intraoperative blood loss and postoperative blood loss (3 days after surgery) were separately evaluated. Hematocrit (Hct) level was tested before surgery and 3 days after surgery. Blood transfusion rates during hospitalization were also recorded.
Postoperative blood loss = total blood loss - intraoperative blood loss [23].
Intraoperative blood loss = intraoperative drainage volume - intraoperative irrigation volume + gauze infiltration volume [23].
Total blood loss = preoperative blood volume (PBV) × (preoperative Hct– postoperative Hct)/average Hct; average Hct=(preoperative Hct + postoperative Hct)/2 [23, 24].
PBV = K1× height (m)3+K2× weight (kg) + K3 (for male: K1 = 0.3669, K2 = 0.03219, K3 = 0.6041; for female, K1 = 0.3561, K2 = 0.03308, K3 = 0.1833) [23, 25].
-
(4)
Postoperative function scores.
WOMAC and Forgotten Joint Score (FJS) were planned to be recorded in the follow-up of 3 months and 1 year after TKA.
-
(5)
Complications.
Deep vein thrombosis (DVT) was evaluated by ultrasonography before hospital discharge. Other potential complications including periprosthetic joint infection (PJI) were recorded during hospitalization and in the follow-up of 3 months, 6 months and 1 year after surgery.
Statistical analysis and sample size considering
Continuous variables of normal distribution were presented as means and standard deviations. One factor ANOVA test and Kruskal-Wallis H test were used for comparison among three groups, and SNK or LSD test was used for intergroup comparison in ANOVA test. Continuous variables of non-normal distribution were presented as median and interquartile range. Wilcoxon rank sum test was used for comparison of groups in abnormal distribution. Categorical variables were presented as percentages, and they were analyzed by the Pearson’s chi-square test or Fisher’s exact test. All statistical analysis were conducted with the statistical package SPSS V26.0 (SPSS Inc). Statistical significance was defined as p < 0.05 with two-sided testing.
A pilot study was conducted with 6 patients in each group. Using postoperative HKA as the primary outcome for sample size calculation, the two groups with the smallest intergroup difference were selected. The absolute postoperative HKA was (2.33 ± 1.25)° in the CI group and (1.58 ± 1.13)° in the PSI group. Assuming a significance level of α = 0.05 and power (1–β) = 0.80, the required sample size was calculated using PASS 11.0 software, resulting in 41 participants per group and a total of 123 participants in all groups. Considering a drop-out rate of 10% in participants, a sample size of 135 participants could be adequate for this study.
Results
Preoperative information
A total number of 135 participants were enrolled in this study. There was no significant difference in gender, age, BMI and operation side in CI, CAS and PSI group (P > 0.05). Baseline information including preoperative WOMAC score and preoperative Hct level also showed no significant difference in these 3 groups (P > 0.05). (Table 1)
Table 1.
Preoperative information in CAS, PSI and CI group
| CI | CAS | PSI | F/χ2 | P | |
|---|---|---|---|---|---|
| gender(female/male) | 34/11 | 39/6 | 38/7 | 2.128b | 0.345 |
| age(years) | 69.44 ± 6.29 | 68.07 ± 6.41 | 68.53 ± 6.20 | 0.556a | 0.575 |
| BMI(kg/m2) | 27.99 ± 3.55 | 27.56 ± 3.52 | 27.87 ± 3.48 | 0.176a | 0.839 |
| operation side(L/R) | 23/22 | 17/28 | 25/20 | 3.086b | 0.214 |
| preoperative WOMAC (points) | 48.27 ± 9.99 | 48.80 ± 11.50 | 52.36 ± 8.14 | 2.236a | 0.111 |
| preoperative Hct | 0.40 ± 0.034 | 0.41 ± 0.034 | 0.40 ± 0.038 | 1.255a | 0.288 |
| preoperative HKA (°) | 9.79 ± 6.10 | 9.67 ± 5.63 | 10.90 ± 5.93 | 0.599a | 0.551 |
(a refers to F value, b refers to χ2 value)
Primary outcome
Postoperative HKA were 1.90(0.25, 2.75) °, 1.90(0.50, 2.90) ° and 2.50(0.85, 3.25) ° in CI, CAS and PSI group with no significant difference (P > 0.05). The overall outliers of HKA(varus/valgus > 3°) were 25.9% in all participants. Outliers were 24.4% in CI group, 17.8% in CAS group and 31.1% in PSI group respectively, and there was no significant difference among these 3 groups (P > 0.05). (Table 2)
Table 2.
Clinical outcomes in CAS, PSI and CI group
| CI | CAS | PSI | F/χ2/H | P | |
|---|---|---|---|---|---|
| outliers of postoperative HKA (%) | 24.4% | 17.8% | 31.1% | 2.166b | 0.362 |
| outliers of postoperative FRA (%) | 13.3% | 26.7% | 11.1% | 4.507 b | 0.105 |
| operation time (min) | 66.67 ± 12.85 | 81.67 ± 12.31 | 52.78 ± 8.62 | 70.830c | < 0.001 |
| total blood loss (ml) | 1097.44 ± 366.26 | 1108.13 ± 359.32 | 1101.74 ± 471.24 | 0.008a | 0.992 |
| intraoperative blood loss (ml) | 6.00 (5.00, 7.00) | 7.00 (5.00, 8.50) | 5.00 (5.00, 10.00) | 4.738c | 0.094 |
| postoperative blood loss (ml) | 1090.55 ± 364.41 | 1100.68 ± 360.28 | 1091.03 ± 470.95 | 0.009a | 0.991 |
| transfusion rate | 2.2% | 2.2% | 4.4% | 0.515b | 1.000 |
| postoperative WOMAC (points) | 8.29 ± 9.607 | 10.73 ± 7.297 | 12.53 ± 10.894 | 1.616a | 0.203 |
| postoperative FJS (points) | 7.26 ± 12.19 | 10.45 ± 10.65 | 8.66 ± 13.74 | 0.568a | 0.568 |
| DVT rate | 2.2% | 20% | 20% | 7.840b | 0.019 |
(a refers to F value, b refers to χ2 value, c refers to H value)
Secondary outcome
Postoperative FRA.
Postoperative FRA were 0.70(0.25, 1.40) °, 0.70(−0.15, 1.60) °, 0.70(0.00, 1.25) ° in CI, CAS and PSI group with no significant difference (P > 0.05). The overall outliers of FRA (> 2°or<−2°) were 17.04% in all participants. Outliers were 13.3% in CI group, 26.7% in CAS group and 11.1% in PSI group respectively with no significant difference (P > 0.05). (Table 2)
-
(2)
Operation time.
Operation time were (66.67 ± 12.85)min, (81.67 ± 12.31)min and (52.78 ± 8.62)min in CI, CAS and PSI group. Operation time in CI was longer than PSI and shorter than CAS with significant difference (P < 0.01). (Table 2)
-
(3)
Blood loss and transfusion rate.
Total blood loss was (1097.44 ± 366.26)ml, (1108.13 ± 359.32)ml, (1101.74 ± 471.24)ml in CI, CAS and PSI group with no significant difference (P > 0.05). Intraoperative blood loss were 6.00 (5.00, 7.00) ml, 7.00 (5.00, 8.50) ml, and 5.00 (5.00, 10.00) ml in the CI, CAS, and PSI groups, respectively, with no significant difference (P > 0.05). Postoperative blood loss was (1090.55 ± 364.41)ml, (1100.68 ± 360.28)ml and (1091.03 ± 470.95)ml in CI, CAS and PSI group with no significant difference (P > 0.05). Transfusion rates were 2.2%, 2.2% and 4.4% in CI, CAS and PSI group with no significant difference (P > 0.05). (Table 2)
-
(4)
Postoperative function scores.
Postoperative WOMAC scores were 8.29 ± 9.607, 10.73 ± 7.297, and 12.53 ± 10.894 in CI, CAS and PSI group with no significant difference (P > 0.05). Postoperative FJS scores were 7.26 ± 12.19, 10.45 ± 10.65 and 8.66 ± 13.74 in CI, CAS and PSI group with no significant difference (P > 0.05). (Table 2)
-
(5)
Complications.
There were 19 patients diagnosed with DVT. No patients encountered with PJI or prosthetic loosening in the follow-up. The rate of DVT were 2.2%, 20% and 20% in CI, CAS and PSI group. DVT rate in CAS and PSI group was significantly higher than that in CI group (OR = 11.0; P < 0.017). (Table 2)
Discussion
Lower limb alignment is considered critical to the outcomes of TKA. It is reported that deviation of lower limb alignment larger than 3° can lead to an increased risk of aseptic loosening [26]. As a result, digital techniques such as CAS and PSI are applied in TKA in order to improve lower limb alignment. However, there is still no consensus on clinical outcomes of CAS and PSI in comparison to CI.
CAS and PSI were both designed to realize enhanced accuracy in TKA, while some researchers questioned the improvement of postoperative lower limb alignment. Budhiparama et al. [27] reported conflicting evidence regarding whether CAS effectively reduces coronal-plane outliers or improves alignment. Hinloopen et al. [20] also suggest no clinically relevant advantages in PSI application. In this study, there was no statistically significant difference in HKA, FRA, or their respective outlier rates among the three groups. However, a trend was observed: CAS had the lowest rate of HKA outliers, while PSI had the lowest rate of FRA outliers. These findings suggest that CAS may offer better control over coronal plane alignment (HKA), whereas PSI may provide advantages in femoral rotational alignment (FRA). This implies that different navigation techniques may have distinct strengths in optimizing specific dimensions aspects of accuracy in TKA.
As for operation time, CAS were considered to have longer operation time than PSI and CI in previous studies [11, 22, 28]. However, opinions remained divided on whether PSI significantly differs from CI in terms of operative time. Vide et al. [29] found that PSI could significantly reduce operation time compared to CI, while Randelli et al. [30] found no significant difference between PSI and CI. Operation time in this study was significantly longer in CAS and shorter in PSI compared to CI. We owe it to the additional procedure of registration and instruments preparation in CAS and the simplification of intraoperative measurement in PSI.
CAS and PSI were believed to reduce perioperative blood loss due to the skipped step of reamer breakage in the femoral medulla, but some studies found no significant difference in blood loss among CAS, PSI and CI. In a cohort study conducted by Cundy et al. [19], no significant difference in blood loss were found among these three groups. Total blood loss, intraoperative blood loss, postoperative blood loss and transfusion rate were separately analyzed in this study and no significant difference was found in CAS, PSI and CI groups. The procedural steps omitted in CAS and PSI may not be substantial enough to significantly impact perioperative blood loss.
Improvement of knee function scores were also controversial in CAS and PSI. Yaffe et al. [17] found that patients in CAS and PSI groups had better function scores than CI, while Yan et al. [21] found no significant difference in KSS and OKS among these three groups. Based on our study, we found that CAS and PSI could not significantly improve knee function scores including postoperative WOMAC and FJS when compared with conventional TKA. We tend to attribute the consistently favorable functional scores in these groups to the satisfactory accuracy of implant positioning and functional recovery achieved by CAS, PSI, and CI.
Complications including prosthetic loosening and DVT were recorded and analyzed. No severe complication was found in CAS, PSI and CI groups. While, it was shown in this study that CI had significantly lower DVT incidence rate than CAS and PSI. It was considered to be a potential drawback of digital techniques such as CAS and PSI in TKA. Although the elevated incidence rate of DVT might correlate with factors such as prolonged operation time, increased blood loss, and delayed functional recovery, no significant correlation was observed between these variables and DVT occurrence in this study. Further researches with larger sample size are needed to validate these findings.
There are some limitations in this study. Firstly, no significant difference in HKA, FRA and their outliers are found among CAS, PSI and CI groups. More precise measurements or studies with larger sample size are needed in the future to evaluate alignment and implant positioning. Secondly, two types of prostheses from different manufacturers are used in this study, but all the prosthesis are widely used and posterior stabilized which makes the slight difference of prosthesis among groups acceptable. Thirdly, the follow-up rate was 82.96%, with an average follow-up duration of 4.14 years in postoperative function scores, which was partly limited and delayed by difficulties in conducting follow-up visits during the COVID-19 pandemic.
In conclusion, CAS and PSI, compared with CI, did not significantly improve lower limb alignment, rotation of femoral prosthesis, and other clinical outcomes including blood loss, transfusion rate, function scores and complication rate. However, CAS was associated with prolonged operation time, whereas PSI resulted in a reduced operation time. The increased incidence rate of DVT in the CAS and PSI groups is in need of further researches.
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributions
Ziyang Dong, Yang Li, and Yipu Zhang contributed equally to this article and are co-first authors. They were primarily responsible for designing the study, analyzing the data, and writing the main manuscript. Xiao Geng, Xinguang Wang, Yuhang Zheng, Junhao Feng, and Zhencan Han were mainly involved in patient follow-up, clinical data collection, and data processing. Hua Tian provided administrative support, overall supervision, and critical guidance throughout the study. All authors read and approved the final manuscript.
Funding
Key Clinical Projects of Peking University Third Hospital (BYSY2016010).
Clinical Cohort Construction Program of Peking University Third Hospital (BYSYDL2023007).
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study is conducted in accordance with the Declaration of Helsinki and is approved by Peking University Third Hospital Medical Science Research Ethics Committee (Registration number: IRB00006761-2016064). All the participants have signed the informed consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ziyang Dong, Yang Li and Yipu Zhang contributed equally to this article.
References
- 1.Dall’Oca C, Ricci M, Vecchini E, Giannini N, Lamberti D, Tromponi C, et al. Evolution of TKA design. Acta Biomed. 2017;88:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002. 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Miller MC, Berger RA, Petrella AJ, Karmas A, Rubash HE. Optimizing femoral component rotation in total knee arthroplasty. Clin Orthop Relat Res. 2001. 10.1097/00003086-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Olcott CW, Scott RD. The Ranawat award. Femoral component rotation during total knee arthroplasty. Clin Orthop Relat Res. 1999; (367):39–42. [PubMed]
- 5.Jones CW, Jerabek SA. Current role of computer navigation in total knee arthroplasty. J Arthroplasty. 2018;33:1989–93. [DOI] [PubMed] [Google Scholar]
- 6.Mathew KK, Marchand KB, Tarazi JM, Salem HS, DeGouveia W, Ehiorobo JO, et al. Computer-Assisted navigation in total knee arthroplasty. Surg Technol Int. 2020;36:323–30. [PubMed] [Google Scholar]
- 7.Matsumoto T, Nakano N, Lawrence JE, Khanduja V. Current concepts and future perspectives in computer-assisted navigated total knee replacement. Int Orthop. 2019;43:1337–43. [DOI] [PubMed] [Google Scholar]
- 8.Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong S, Xu W, Wang R, Wang Z, Wang B, Han L, et al. Patient-specific instrumentation improved axial alignment of the femoral component, operative time and perioperative blood loss after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2019;27:1083–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauzenberger L, Munz M, Brandl G, Frank JK, Heuberer PR, Laky B, et al. Patient-specific instrumentation improved three-dimensional accuracy in total knee arthroplasty: a comparative radiographic analysis of 1257 total knee arthroplasties. J Orthop Surg Res. 2019;14:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDessi SJ, Jang B, Harris IA, Wheatley E, Bryant C, Chen DB. A comparison of alignment using patient specific guides, computer navigation and conventional instrumentation in total knee arthroplasty. Knee. 2014;21:406–9. [DOI] [PubMed] [Google Scholar]
- 12.Lei K, Liu L, Chen X, Feng Q, Yang L, Guo L. Navigation and robotics improved alignment compared with PSI and conventional instrument, while clinical outcomes were similar in TKA: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2022;30:721–33. [DOI] [PubMed] [Google Scholar]
- 13.Koh Y-G, Nam J-H, Kim J-K, Suh D-S, Chung JH, Park KK, et al. Enhancing surgical efficiency and radiological outcomes through advances in Patient-Specific instrument design. J Clin Med. 2025;14:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim C-H, Park Y-B, Baek SH. Clinical and radiological outcomes of computer-assisted navigation in primary total knee arthroplasty for patients with extra-articular deformity: systematic review and meta-analysis. Clin Orthop Surg. 2024;16:430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnurr C, Csécsei G, Eysel P, König DP. The effect of computer navigation on blood loss and transfusion rate in TKA. Orthopedics. 2010;33:474. [DOI] [PubMed] [Google Scholar]
- 16.Cucchi D, Menon A, Zanini B, Compagnoni R, Ferrua P, Randelli P. Patient-specific instrumentation affects perioperative blood loss in total knee arthroplasty. J Knee Surg. 2019;32:483–9. [DOI] [PubMed] [Google Scholar]
- 17.Yaffe M, Luo M, Goyal N, Chan P, Patel A, Cayo M, et al. Clinical, functional, and radiographic outcomes following total knee arthroplasty with patient-specific instrumentation, computer-assisted surgery, and manual instrumentation: a short-term follow-up study. Int J Comput Assist Radiol Surg. 2014;9:837–44. [DOI] [PubMed] [Google Scholar]
- 18.Thienpont E, Fennema P, Price A. Can technology improve alignment during knee arthroplasty. Knee. 2013;20(Suppl 1):S21–28. [DOI] [PubMed] [Google Scholar]
- 19.Cundy WJ, Theodoulou A, Ling CM, Krishnan J, Wilson CJ. Blood loss in total knee arthroplasty. J Knee Surg. 2017;30:452–9. [DOI] [PubMed] [Google Scholar]
- 20.Hinloopen JH, Puijk R, Nolte PA, Schoones JW, de Ridder R, Pijls BG. The efficacy and safety of patient-specific instrumentation in primary total knee replacement: a systematic review and meta-analysis. Expert Rev Med Devices. 2023;20:245–52. [DOI] [PubMed] [Google Scholar]
- 21.Yan CH, Chiu KY, Ng FY, Chan PK, Fang CX. Comparison between patient-specific instruments and conventional instruments and computer navigation in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23:3637–45. [DOI] [PubMed] [Google Scholar]
- 22.Shi J, Wei Y, Wang S, Chen F, Wu J, Huang G, et al. Computer navigation and total knee arthroplasty. Orthopedics. 2014;37:e39–43. [DOI] [PubMed] [Google Scholar]
- 23.Dong Z, Li Y, Tao L, Tian H. The effect of tourniquet application on the morphology and function of quadriceps in patients undergoing total knee arthroplasty: study protocol for a single-blind randomized controlled trial. Trials. 2022;23:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58:277–80. [DOI] [PubMed] [Google Scholar]
- 25.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–32. [PubMed] [Google Scholar]
- 26.Longstaff LM, Sloan K, Stamp N, Scaddan M, Beaver R. Good alignment after total knee arthroplasty leads to faster rehabilitation and better function. J Arthroplasty. 2009;24:570–8. [DOI] [PubMed] [Google Scholar]
- 27.Budhiparama NC, Lumban-Gaol I, Ifran NN, Parratte S, Nelissen R. Does accelerometer-based navigation have any clinical benefit compared with conventional TKA? A systematic review. Clin Orthop Relat Res. 2019;477:2017–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiengwittayaporn S, Kanjanapiboonwong A, Junsee D. Midterm outcomes of electromagnetic computer-assisted navigation in minimally invasive total knee arthroplasty. J Orthop Surg Res. 2013;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vide J, Freitas TP, Ramos A, Cruz H, Sousa JP. Patient-specific instrumentation in total knee arthroplasty: simpler, faster and more accurate than standard instrumentation-a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25:2616–21. [DOI] [PubMed] [Google Scholar]
- 30.Randelli PS, Menon A, Pasqualotto S, Zanini B, Compagnoni R, Cucchi D. Patient-specific instrumentation does not affect rotational alignment of the femoral component and perioperative blood loss in total knee arthroplasty: a prospective, randomized, controlled trial. J Arthroplasty. 2019;34:1374-e13811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.