Abstract
Background
Among all the elderly patients with type 2 diabetes mellitus (T2DM) of different body compositions, patients with sarcopenic obesity exhibited the most severe degree of insulin resistance despite possessing a normal body weight. It is well-established that interventions encompassing resistance training alone or in combination with whey protein supplementation, referred to hereafter as muscle-building interventions, are effective for increasing muscle mass and function in the elderly population. However, the impact of these muscle-building interventions on glucose metabolism in elderly T2DM patients with sarcopenic obesity remains unclear. The objective of this study was to elucidate the effect of muscle-building interventions on blood glucose and insulin resistance in elderly T2DM patients with sarcopenic obesity.
Methods
The muscle-building interventions in this study included resistance training alone and resistance training combined with whey protein supplementation. In this randomized controlled trial, elderly T2DM patients with sarcopenic obesity were divided into three distinct groups: the control group, the resistance training group, and the resistance training combined with whey protein supplement group. The muscle-building interventions were conducted for 12 weeks.
Results
Implementing muscle-building interventions showed significant improvements in glycemic indices (HbA1c, OGTT 2-hour plasma glucose) and insulin resistance levels (HOMA-IR, Gutt index) for elderly T2DM patients with sarcopenic obesity. These muscle-building interventions enhanced muscle functional indices (handgrip strength, 5-time chair stand test). The combination of resistance training with whey protein supplementation did not significantly enhance the favorable effects on glucose metabolism compared to resistance exercise alone. Correlation analyses revealed that improvements in muscle strength were significantly associated with enhancements in glucose metabolism.
Conclusions
In elderly T2DM patients with sarcopenic obesity, muscle-building interventions (particularly resistance exercise) have been shown to significantly improve blood glucose control and insulin resistance.
Keywords: Sarcopenic obesity, Insulin resistance, Glucose metabolism, Type 2 diabetes mellitus, Muscle
Introduction
With advancing age, elderly Asian patients with type 2 diabetes mellitus (T2DM) are particularly susceptible to alterations in body composition, characterized by a greater decline in skeletal muscle mass compared to Western populations [1]. This age-related muscle reduction may even lead to the development of sarcopenia, a condition characterized by the loss of muscle mass and muscle strength. Notably, a subset of elderly T2DM patients now falls under the classification of sarcopenic obesity, which represents a high-risk geriatric syndrome marked by the coexistence of sarcopenia and obesity [2]. Sarcopenic obesity is considered the result of the convergence of two major epidemics: the aging population and the ongoing increase in obesity rates [3]. However, there is a deficiency in standardized diagnostic criteria for sarcopenic obesity currently. The prevalence worldwide varies between 5% and 10% based on diverse definitions and study populations [4]. In our previous research, the prevalence of sarcopenic obesity among elderly T2DM patients was found to be 14.5% (11.9% in males and 17.7% in females) [5].
Sarcopenic obesity is currently a prominent focus in the field of aging research. The coexistence of sarcopenia and obesity during the aging process results in dual metabolic pressures, amplifying the risk of various adverse health outcomes in older adults, including disability, falls, frailty, fractures, cardiovascular and metabolic diseases, depression, impaired mental health, cancer, hospitalization, and mortality [6]. This condition significantly impairs the quality of life for the elderly and imposes substantial burdens on families, society, and public health systems [7]. Our previous study found that the patients with sarcopenic obesity, defined by the coexistence of low muscle mass and high body fat, exhibited the most severe degree of insulin resistance among all the elderly T2DM patients of different body compositions, despite appearing to have normal body weight [5]. In addition, multiple studies also found similar results to our research, which suggested that individuals with sarcopenic obesity had the highest risk of insulin resistance among either T2DM patients or populations without diabetes [8–12]. However, developing effective glycemic management strategies for elderly T2DM patients with sarcopenic obesity remains a significant treatment challenge. The traditional weight loss programs including dietary energy restriction and aerobic exercise may be beneficial for improving insulin resistance and lowering blood glucose. However these approaches could potentially exacerbate muscle reduction, increasing the risk of disability, falls, and frailty in the elderly. Therefore, they may not be suitable as early interventions for this subset of elderly T2DM patients. Resistance training and adequate supplementation of high-quality protein are effective interventions to increase muscle mass and function in the elderly population [13–15]. Concurrently implementing a combined intervention demonstrates superior efficacy when compared to individual intervention modalities [16]. These muscle-building interventions (hereafter referring specifically to resistance training alone or in combination with whey protein supplementation) serve as the foundation for the prevention and treatment of sarcopenia in older adults. However, the impact of these muscle-building interventions on glucose metabolism in elderly T2DM patients with sarcopenic obesity remains unclear.
We employed a prospective study design to investigate the effects of muscle-building interventions (including both resistance training alone and resistance training combined with whey protein supplementation) on blood glucose levels and insulin resistance in elderly T2DM patients with sarcopenic obesity. Additionally, we aimed to explore the effects of these interventions on anthropometric measurements, body composition indicators, and muscle function in the patients.
Methods
Clinical study design and participants
This was a 12-week, non-blinded randomized controlled trial among the elderly T2DM patients with sarcopenic obesity. The study was approved by the Shanghai Jiaotong University School of Medicine, Renji Hospital Ethics Committee (Approval No. LY2022-039-A), performed in accordance with the Declaration of Helsinki, and was registered with the Chinese Clinical Trial Registry (ChiCTR2200065362). All participants provided written informed consent.
The inclusion criteria for the study population were as follows: (1) meeting the diagnostic criteria for type 2 diabetes mellitus; (2) HbA1c < 7.5% (this threshold was selected in accordance with the safety parameters established in the Guideline for the Prevention and Treatment of Diabetes Mellitus in China [17], and does not necessitate immediate pharmacological intervention) and no use of any glucose-lowering medications (including insulin sensitizer such as metformin, thiazolidinediones; insulin secretagogues such as sulfonylureas, glinides; alpha-glucosidase inhibitors; sodium-glucose co-transporter 2 inhibitors; incretin-based therapies such as glucagon-like peptide-1 receptor agonists, dipeptidyl peptidase-4 inhibitors; insulin) within the past three months (to eliminate pharmacological confounding of intervention effects); (3) age between 60 and 75 years, without gender restriction; (4) sarcopenic obesity, characterized by the presence of both reduced skeletal muscle mass and obesity. The definition of reduced skeletal muscle mass was determined using the skeletal muscle index (SMI) measured through bioelectrical impedance analysis, with a cutoff of < 7.0 kg/m2 for males and < 5.7 kg/m2 for females [13]. Obesity was defined as a percent body fat (PBF) of ≥ 25% for males and ≥ 35% for females [18]. Moreover, subjects with any of the following conditions were excluded from the study: type 1 diabetes or specific types of diabetes; a history of confirmed infection within one week prior to enrollment or at the time of enrollment (including respiratory, urinary, gastrointestinal, or other organ system infections); malignant tumors; rheumatologic or autoimmune diseases; impaired liver function (serum alanine aminotransferase level ≥ 2 times the upper limit of normal); renal insufficiency (creatinine clearance rate < 60 mL/min); heart failure (NYHA class ≥ III); uncontrolled hypertension; history of coronary heart disease; unstable cardiac arrhythmias; severe chronic obstructive pulmonary disease; neuromuscular, rheumatic, or genetic muscle diseases such as myasthenia gravis, dermatomyositis, or Duchenne muscular dystrophy; edema; creatine kinase levels above the upper limit of normal; abnormal thyroid function, including hyperthyroidism and hypothyroidism; use of medications within the past three months that affect blood glucose or muscle (including glucocorticoids, growth hormones, sex hormones, thyroid hormones, niacin, diuretics, phenytoin, gamma interferon, etc.); inability to consume food orally; malabsorption disorders such as inflammatory bowel disease, irritable bowel syndrome, gastrointestinal bleeding, chronic diarrhea, etc.; individuals with ongoing unresolved sports injuries and other conditions unsuitable for exercise, such as a documented history of cervical or lumbar disc herniation, spinal curvature, knee joint injury, diagnosed osteoarthritis, etc.; regular exercisers or individuals with a history of high-intensity exercise, such as fitness enthusiasts, marathon runners, etc.; history of substance abuse; history of alcohol or tobacco dependence; documented history of psychiatric disorders; any other conditions as determined by the researcher that could potentially affect study enrollment.
After sex stratification, all the participants were randomly allocated to three groups in a 1:1:1 ratio using a computer-generated random number sequence based on the intervention approach: (a) resistance training (RT) group, which received muscle-building intervention through resistance exercise; (b) resistance training combined with whey protein supplementation (RT + WPS) group, which received muscle-building intervention through resistance exercise combined with whey protein supplementation; (c) control (Con) group, which did not receive any muscle-building intervention. All participants received diabetes-specific dietary education from specialized nurses and were provided with educational handbooks on diet.
Baseline information collection
At baseline, participant characteristics including gender, age, dietary intake, and physical activity level were collected. Dietary intake was assessed through the completion of a 3-day consecutive dietary diary. Participants were instructed to maintain a self-recorded 3-day dietary diary, documenting meal occasions, food items with preparation methods, and food weight (grams). Researchers analyzed daily total energy intake and macronutrient composition (protein, fat, and carbohydrates) using the Booheehealth software, with mean daily intake values derived from the three-day records. Physical activity assessment was conducted by recording daily step counts. Participants wore the Polygon3D electronic pedometer during daily life to record step counts. Data were collected over three consecutive days, and the mean daily step count was calculated from the recorded values.
Interventions
The RT group and the RT + WPS group exercised 2 non-consecutive days per week for 12 weeks. The design of all resistance training movements followed the principles of safety, convenience, efficacy, and suitability for home-based exercise. The following exercises targeted large symmetrical muscle groups: biceps curls, triceps curls, bench press, shoulder press, bent-over rows, squats, deadlifts, and leg swings. Each set consisted of 6 to 12 repetitions. The participants engaged in 2–4 sets of exercise with an inter-set rest duration of 2–3 min. Each session had a 5-minute warm-up and cool-down period. The total duration of each training session was 45 min, with a minimum interval of 48 h between sessions. The intensity was set at 60% of the most recently determined one-repetition maximum, reassessed every 4 weeks.
Within the first month of participant enrollment, a total of three supervised training sessions were conducted on-site to ensure participants’ acquisition of correct exercise techniques. Additionally, one on-site training session was scheduled within the 5th and 9th weeks after the initiation of resistance training. The purpose of these sessions was to modify the exercise program, including exercise modalities, resistance levels, repetition counts, and frequency, based on individual changes in participants’ exercise capacity. This aimed to further optimize the exercise program. During non-site training periods, participants followed the prescribed resistance training program (recorded videos) for home-based training and documented their training completion through online check-ins. Participants were required to complete the resistance training program twice per week. Researchers provided weekly supervision, documentation, and follow-up on participants’ exercise progress via telephone.
The RT + WPS group incorporated whey protein supplementation (20 g/day) into their daily dietary intake. Whey protein was consumed within 30 min after resistance training sessions and during breakfast on non-resistance training days. Researchers also conducted weekly telephone monitoring and follow-up on the participants’ whey protein supplementation regimen.
Outcomes and measurement methods
Blood samples were obtained from each participant’s antecubital vein after an overnight fast at the baseline and the completion of the 12-week intervention period. The primary outcome was the change in glycated hemoglobin (HbA1c) from baseline at 12 weeks, which was assessed using high-performance liquid chromatography (HPLC) method. All participants underwent a 75 g oral glucose tolerance test (OGTT) and an insulin release test (IRT) before and after the 12-week intervention period. The glycemic parameters assessed in this study included fasting plasma glucose (FPG) and OGTT 2-hour plasma glucose (2hPG) which were measured using the glucose oxidase method; and fasting serum insulin (FINS) and 2-hour postprandial serum insulin (2hINS) which were measured using the chemiluminescent method. The insulin resistance indices included the homeostatic model assessment of insulin resistance (HOMA-IR) and the Gutt index, and the calculation formulas could refer to relevant literature [19]. Anthropometric measurements including body height, weight, waist circumference (WC) were measured according to guidelines by the World Health Organization (WHO) [20]. Body mass index (BMI) was calculated as body weight divided by the square of height (kg/m2). Body composition was analyzed using a bioelectrical impedance analysis device (InBody770, InBody, Seoul, Korea). The body composition indices included appendicular skeletal muscle mass (ASM), skeletal muscle index (SMI), body fat mass (BFM), and percent body fat (PBF). SMI was calculated by dividing ASM by the square of height (kg/m2). The muscle function assessment included handgrip strength, which was used to evaluate upper limb muscle strength and was measured by a hydraulic electronic hand dynamometer (Jamar Plus+, Performance Health, USA), and the 5-time chair stand test, which was used to assess lower limb muscle strength. In addition, safety indicators included complete blood count analysis, liver and renal function, creatine kinase levels, and electrocardiography.
Adverse events reporting
Participants were instructed to report any discomfort or injuries during exercise, such as mild muscle or joint pain, skin abrasions, or exercise-related injuries. Additionally, any discomfort arising from whey protein supplementation, primarily gastrointestinal reactions including nausea, vomiting, abdominal pain, bloating, or diarrhea, was to be reported. Furthermore, any abnormalities in safety indicators following the intervention were also to be promptly reported. The researchers would then provide appropriate management and intervention measures in response to these reported adverse events.
Sample size calculation
The sample size calculation was performed using PASS 15 software. The significance level (α) was set at 0.05, and the power (1-β) was set at 80%. The primary outcome of the study was the change in HbA1c. Based on preliminary experimental results, the mean differences before and after intervention were set as 0, -0.3, and − 0.3 for the three groups respectively, with a standard deviation of 0.25. Considering an anticipated dropout rate of 20% in each group, the calculated sample size was determined to be a total of 45 participants, with 15 participants allocated to each group.
Statistical analysis
The normality of distributions of variables was analyzed with the Kolmogorov–Smirnov normality test. Descriptive statistics for continuous variables were presented as mean ± standard deviation (SD) if variables were normally distributed. Group based differences of quantitative variables were compared by analysis of variance (ANOVA) following Turkey’s test for normally distributed data and Kruskal-Wallis H-test for skewed data. Categorical variables were compared by using Pearson’s chi-squared test. The changes in variables were compared among the groups using ANOVA. Correlation analysis was conducted using Pearson’s correlation coefficient. The incidence of adverse events was compared among the groups using Fisher’s exact test. P values < 0.05 were considered statistically significant. All statistical analyses were conducted utilizing SPSS, version 26.0 (SPSS Inc., Chicago, IL, USA).
Results
Workflow of the clinical study
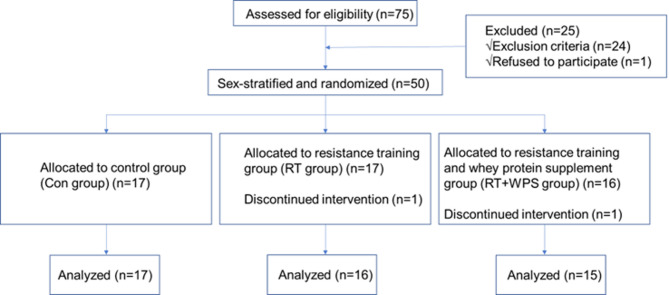
Between November 2022 and May 2023, 75 elderly T2DM patients with sarcopenic obesity who met the inclusion criteria were enrolled. Among them, 50 subjects were finally included in this study and were randomly assigned to three groups following sex stratification: Con group (n = 17), RT group (n = 17), RT + WPS group (n = 16). During the intervention period, two subjects withdrew from the study due to exercise-related discomfort (one from the RT group due to right knee-joint pain, and one from the RT + WPS group due to bilateral hip-joints pain). Consequently, a total of 48 subjects completed the study and were included in the final data analysis: Con group (n = 17), RT group (n = 16), RT + WPS group (n = 15). The flowchart of the clinical study is presented in Fig. 1.
Fig. 1.
Flowchart of the clinical study
Participants’ baseline characteristics
There were no significant statistical differences in age, gender, BMI, SMI, PBF, HbA1c, HOMA-IR, Gutt index, total daily energy intake, the content of the three major nutrients (protein, fat, carbohydrates) and physical activity among the three groups of patients (Table 1).
Table 1.
Participants’ baseline characteristics
| Variables | Con(n = 17) | RT(n = 16) | RT + WPS(n = 15) |
|---|---|---|---|
| Age(years) | 65.17 ± 4.51 | 64.69 ± 3.05 | 66.73 ± 3.65 |
| Sex(Male/Female) | 9/8 | 8/8 | 7/8 |
| BMI(kg/m2) | 22.78 ± 1.45 | 22.63 ± 1.63 | 22.79 ± 1.56 |
| SMI(kg/m2) | 6.12 ± 0.64 | 6.09 ± 0.63 | 6.05 ± 0.78 |
| PBF(%) | 33.56 ± 6.57 | 32.49 ± 6.42 | 33.72 ± 7.59 |
| HbA1c(%) | 6.91 ± 0.26 | 6.98 ± 0.35 | 6.88 ± 0.33 |
| HOMA-IR | 3.28 ± 0.67 | 2.88 ± 0.70 | 2.88 ± 0.69 |
| Gutt index | 38.50 ± 4.83 | 40.48 ± 6.67 | 39.98 ± 4.30 |
| Physical activity | |||
| Walk steps(steps/day) | 5376.16 ± 1759.04 | 5161.07 ± 1952.93 | 5856.21 ± 1640.72 |
| Dietary intake | |||
| Energy(kcal/day) | 1237.83 ± 165.13 | 1230.13 ± 142.96 | 1215.42 ± 133.16 |
| Protein(g/day) | 69.44 ± 11.53 | 69.92 ± 11.27 | 66.75 ± 9.23 |
| Fat(g/day) | 34.27 ± 4.45 | 32.86 ± 4.28 | 34.05 ± 4.63 |
| Carbohydrate(g/day) | 162.90 ± 22.65 | 163.68 ± 19.24 | 160.48 ± 17.21 |
Data were presented as mean ± standard deviation (SD)
BMI: Body mass index; SMI: Skeletal muscle index; PBF: Percent body fat; HbA1c: Glycated hemoglobin A1c; HOMA-IR: Homeostatic model assessment of insulin resistance
Effect of muscle-building interventions on glycemic parameters
When compared to the Con group, both the RT group and RT + WPS group exhibited statistically significant differences in the changes of HbA1c, 2hPG, HOMA-IR, and Gutt index (P < 0.05). Insulin resistance indices (HOMA-IR and Gutt index) and 2hPG were significantly improved after the muscle-building intervention in the RT group or the RT + WPS group compared with the Con group (P < 0.05). Moreover, in comparison to the RT group, the RT + WPS group showed no significant differences in the changes of HbA1c, 2hPG, HOMA-IR, and Gutt index. However, there was no significant statistical difference observed in the change of FPG between the RT group/RT + WPS group and the Con group regardless of gender. (Table 2)
Table 2.
Changes of indices in the groups during the intervention
| Variables | Group | Baseline | 12 weeks | Change (Δ 12 weeks) |
|---|---|---|---|---|
| Body Weight(kg) | Con | 60.07 ± 6.77 | 59.86 ± 6.70 | -0.21 ± 1.87 |
| RT | 58.69 ± 5.22 | 58.15 ± 6.14 | -0.55 ± 1.78 | |
| RT&WPS | 58.94 ± 4.90 | 59.14 ± 4.97 | 0.20 ± 1.21 | |
| WC(cm) | Con | 83.98 ± 4.20 | 83.70 ± 5.05 | -0.28 ± 2.18 |
| RT | 81.29 ± 4.04 | 80.99 ± 4.02 | -0.30 ± 1.64 | |
| RT&WPS | 83.42 ± 3.96 | 83.87 ± 4.45 | 0.45 ± 2.05 | |
| ASM(kg) | Con | 16.27 ± 3.00 | 16.08 ± 3.11 | -0.19 ± 0.35 |
| RT | 16.00 ± 3.18 | 16.05 ± 3.26 | 0.05 ± 0.40 | |
| RT&WPS | 15.82 ± 3.31 | 16.23 ± 3.42 | 0.41 ± 0.20a, b | |
| BFM(kg) | Con | 19.89 ± 2.94 | 19.81 ± 3.04 | -0.08 ± 0.63 |
| RT | 18.87 ± 3.00 | 18.29 ± 2.97 | -0.58 ± 0.56 | |
| RT&WPS | 19.80 ± 4.70 | 19.68 ± 4.74 | -0.12 ± 0.44 | |
| FPG(mmol/L) | Con | 6.80 ± 0.23 | 6.76 ± 0.32 | -0.04 ± 0.16 |
| RT | 6.91 ± 0.39 | 6.74 ± 0.39 | -0.17 ± 0.22 | |
| RT&WPS | 6.79 ± 0.38 | 6.61 ± 0.38 | -0.18 ± 0.22 | |
| 2hPG(mmol/L) | Con | 14.66 ± 1.67 | 14.59 ± 1.71 | -0.07 ± 1.56 |
| RT | 14.61 ± 1.74 | 11.16 ± 2.06a | -3.45 ± 1.09a | |
| RT&WPS | 14.71 ± 1.96 | 11.41 ± 1.33a | -3.30 ± 1.30a | |
| HbA1c(%) | Con | 6.92 ± 0.25 | 6.90 ± 0.30 | -0.02 ± 0.13 |
| RT | 6.97 ± 0.34 | 6.7 ± 0.35 | -0.27 ± 0.09a | |
| RT&WPS | 6.88 ± 0.32 | 6.58 ± 0.36 | -0.30 ± 0.17a | |
| HOMA-IR | Con | 3.28 ± 0.67 | 3.29 ± 0.64 | 0.01 ± 0.13 |
| RT | 2.88 ± 0.69 | 2.51 ± 0.62a | -0.36 ± 0.16a | |
| RT&WPS | 2.88 ± 0.69 | 2.42 ± 0.60a | -0.45 ± 0.16a | |
| Gutt index | Con | 38.50 ± 4.83 | 38.70 ± 4.39 | 0.20 ± 3.02 |
| RT | 40.48 ± 6.67 | 53.16 ± 8.48a | 12.68 ± 3.49a | |
| RT&WPS | 39.98 ± 4.30 | 55.02 ± 5.74a | 15.03 ± 3.35a | |
| Handgrip strength(kg) | Con | 23.83 ± 5.72 | 24.05 ± 5.90 | 0.22 ± 1.46 |
| RT | 24.88 ± 5.47 | 27.46 ± 6.38 | 2.58 ± 1.09a | |
| RT&WPS | 23.10 ± 5.73 | 26.13 ± 6.92 | 3.03 ± 1.36a | |
| Chair stand test(s) | Con | 10.95 ± 1.61 | 11.11 ± 1.75 | 0.15 ± 0.69 |
| RT | 10.48 ± 1.74 | 9.35 ± 1.57a | -1.13 ± 0.29a | |
| RT&WPS | 10.34 ± 1.97 | 8.56 ± 1.67a | -1.78 ± 0.38a, b |
Data were presented as mean ± standard deviation (SD)
WC: Waist circumference; ASM: Appendicular skeletal muscle mass; BFM: Body fat mass; FPG: Fasting plasma glucose; 2hPG: OGTT2h plasma glucose; HbA1c: Glycated hemoglobin A1c; HOMA-IR: Homeostatic model assessment of insulin resistance
aP<0.05, versus the Con group; bP<0.05, versus the RT group
Effect of muscle-building interventions on anthropometric measurements and body composition indicators
There were no statistically significant differences in the changes of body weight, waist circumference, and body fat content among the RT group, the RT + WPS group and the Con group. The change in appendicular skeletal muscle mass in the RT group showed no significant statistical difference compared to the Con group. However, the increase in appendicular skeletal muscle mass in the RT + WPS group was significantly higher than that in the Con group or the RT group (P < 0.05). (Table 2)
Effect of muscle-building interventions on skeletal muscle function
Compared to the Con group, there were statistically significant differences in the changes of handgrip strength and chair stand test time, as well as chair stand test time after muscle-building interventions, in both the RT group and the RT + WPS group (P < 0.05). In comparison to the RT group, the RT + WPS group showed further improvement in the change of chair stand test time (P < 0.05). (Table 2)
Correlation analysis of changes in muscle strength indices and glycemic metabolism indices before and after muscle-building interventions
The change in handgrip strength showed a negative correlation with changes in HbA1c (P < 0.01), 2hPG (P < 0.05) and HOMA-IR (P < 0.01), and a positive correlation with the change of Gutt index (P < 0.01). However, there was no correlation between the change of handgrip strength and the change of FPG. The change in chair stand test time showed a positive correlation with changes in HbA1c (P < 0.01), FPG (P < 0.05), 2hPG (P < 0.01), and HOMA-IR (P < 0.01), and a negative correlation with changes in Gutt index (P < 0.01). (Table 3).
Table 3.
Correlations between muscle strength indices and glycemic metabolism indices
| Variables | Δ Handgrip strength | Δ Chair stand test |
|---|---|---|
| Δ HbA1c | -0.483** | 0.493** |
| Δ FPG | -0.257 | 0.301* |
| Δ 2hPG | -0.350* | 0.603** |
| Δ HOMA-IR | -0.562** | 0.692** |
| Δ Gutt index | 0.479** | -0.776** |
HbA1c: Glycated hemoglobin A1c; FPG: Fasting plasma glucose; 2hPG: OGTT2h plasma glucose; HOMA-IR: Homeostatic model assessment of insulin resistance
*P < 0.05, **P < 0.01
Safety assessment
Adverse events reporting included: 2 patients in the RT group, one of whom withdrew from the study due to mild pain in the right knee that slightly affected walking, and the other had a mild increase in serum creatine kinase level (more than 10% above the normal upper limit of creatine kinase reference value) after 12 weeks of intervention implementation, but the patient completed the intervention and was included in the analysis; 2 patients in the RT + WPS group, one of whom withdrew from the study due to bilateral hip-joints pain, and the other reported mild pain in the bottom of the right foot that slightly affected walking at the last follow-up, and was included in the analysis due to completing the intervention. After completing relevant imaging examinations for these patients with adverse events, no abnormalities were suggested. No adverse reactions were reported during the administration of whey protein to the RT + WPS group. No serious adverse events were reported. The incidence of adverse events was not statistically different among the three groups.
Discussion
To the best of our knowledge, for the first time we targeted elderly T2DM patients with sarcopenic obesity, and discovered that a 12-week muscle-building intervention, whether conducted solely with moderate-intensity resistance exercise or in conjunction with whey protein supplementation, significantly improved HbA1c, postprandial blood glucose, and insulin resistance.
Over an extended period, weight management in T2DM patients has been a prominent focus [21]. The DiRECT study highlighted the significance of reducing body fat as a pivotal component in the prevention and treatment of T2DM [22, 23]. Some studies primarily investigated the impact of muscle-building interventions on insulin resistance and glucose metabolism during weight loss in elderly overweight/obesity T2DM patients. However, the findings from these studies were inconsistent. The study by Dunstan DW et al. demonstrated that resistance training could increase skeletal muscle content and function in overweight elderly individuals with T2DM, while also lowering HbA1c [24]. Subsequent research found that following a 6-month resistance training combined with weight loss at the gym, a transition to home-based resistance training combined with weight loss could effectively sustain the improvements in muscle strength and lean body mass, though it failed to maintain the glucose-lowering effects [25]. Furthermore, a meta-analysis revealed that resistance training significantly enhances glycemic control and skeletal muscle strength in elderly T2DM patients, though it shows no discernible effect on skeletal muscle mass [26]. Another systematic review demonstrated that resistance training was an effective strategy to decrease HbA1c in individuals with T2DM. Importantly, interventions producing larger training effects appeared more effective in reducing HbA1c compared with those generating medium and small effects [27]. It is worth noting that the concurrent use of antidiabetic medications served as a significant confounding factor, which could potentially influence the reliability of the study outcomes. The impact of whey protein, a pivotal nutritional supplement in muscle-building interventions, on blood glucose levels has yielded divergent research findings. A meta-analysis indicated that whey protein could lower fasting blood glucose levels in overweight and obese patients [28]. In contrast, a study conducted by Miller EG et al. suggested that for overweight/obese elderly T2DM individuals, six-month supplementation of whey protein combined with vitamin D failed to augment the beneficial effects of progressive resistance training on blood glucose control [29]. For normal-weight T2DM patients, Kobayashi Y et al. recently revealed that resistance training was superior to aerobic training for HbA1c reduction, and increased lean mass relative to decreased fat mass was an independent predictor of reduction in HbA1c level [30].
The body composition of sarcopenic obesity is recognized as a specific subset of elderly T2DM patients, which is more common in Asian populations. Our previous study has found that the patients with sarcopenic obesity, despite possessing a normal body weight, exhibited the most severe degree of insulin resistance among all the elderly patients with T2DM [5]. This study for the first time focused on this subset of elderly T2DM patients. We discovered that 12-week muscle-building interventions significantly improved HbA1c, postprandial blood glucose, and insulin resistance in these patients. Notably, the combination of resistance exercise with whey protein supplementation did not significantly enhance the favorable effects on glucose metabolism compared to resistance exercise alone. Furthermore, correlation analysis revealed that enhancement in muscle strength was significantly associated with improvement in blood glucose level (HbA1c, 2hPG) and insulin resistance indices (HOMA-IR, Gutt index). A growing body of clinical research has substantiated the association between diminished skeletal muscle strength and T2DM pathogenesis. Analysis of nationally representative data from the China Health and Retirement Longitudinal Study (CHARLS) by Chun Luo et al. revealed that possible sarcopenia (characterized by low muscle strength or reduced physical performance) correlated with elevated risks of new-onset T2DM in older adults, particularly among individuals with normal weight or aged ≤ 75 years [31]. Additionally, a complementary CHARLS-based investigation demonstrated that higher muscle strength was associated with decreased odds of diabetes progression [32]. Notably, the Men Androgen Inflammation Lifestyle Environment and Stress (MAILES) Study, encompassing a representative South Australian male cohort, identified reduced muscle strength (but not diminished muscle mass) as an independent risk factor for incident T2DM [33]. Furthermore, analysis of UK Biobank data in a large prospective cohort study established grip strength as a gender-independent predictor of T2DM incidence, with this association persisting after adjustment for key confounders including age, socioeconomic status, adiposity metrics, and lifestyle factors [34].
This study also had some limitations. Firstly, the 12-week duration of the muscle-building interventions represents a relatively short-term clinical observation period; therefore, longer-term investigations with extended follow-up periods are required to assess sustained effects. Secondly, the randomized controlled trial was conducted in an open-label design without the use of blinding, which may have introduced performance bias. Thirdly, the participants primarily originated from the Shanghai region of China, introducing a potential regional bias that could limit the generalizability of the findings. Fourthly, our study primarily focused on handgrip strength and lower limb muscle strength, but it did not assess a broader range of muscle groups that might provide a fuller picture of the impact of interventions on overall muscle function. Fifthly, although the sample size was statistically justified through a priori power analysis focused on the primary endpoint (HbA1c changes), the lack of sample size estimation based on all secondary endpoints represents a methodological limitation. While secondary outcomes like muscle strength changes were supported by preliminary estimates, others were not formally powered and should be interpreted with caution. Therefore, larger-scale, multi-center studies would enhance the reliability and external validity of the findings, particularly for secondary outcomes. Sixthly, taking into account the practicality of dietary regimen execution, the nutritional intervention in our study focused on whey protein supplementation, whereas a structured dietary intervention regimen accounting for total energy intake and macronutrient distribution was not incorporated. A more tailored dietary approach might further enhance the effects of the muscle-building interventions, which will be explored in future studies. Finally, although researchers included dietary maintenance reminders in their weekly telephone follow-ups, the lack of post-intervention dietary measurements prevented us from determining whether dietary adjustments influenced the results or whether the benefits observed were solely due to exercise. Future studies should integrate post-intervention dietary assessments to isolate exercise effects more robustly.
Conclusions
In elderly type 2 diabetic patients with sarcopenic obesity, muscle-building interventions, particularly moderate-intensity resistance exercise, have been shown to significantly improve blood glucose control and insulin resistance, thereby representing an effective and safe early-phase lifestyle intervention for this population.
Acknowledgements
The authors gratefully acknowledge the participants’ invaluable contributions to this study.
Author contributions
T.H. conceived the project; designed, performed, and analyzed data; and wrote the manuscript. X.L. was responsible for the patient recruitment, grouping, supervision, follow-up and data storage; and assisted analyzing data. S.S. and J.S. assisted providing supervision, documentation, and follow-up on exercise progress. H.L., M.Z., Y.W. and X.J. assisted recruiting the patients. H.C., N.C., Y.L., Z.W. assisted the patient follow-up and data storage. Y.H. initiated the project, supported the work and the analysis, and revised the manuscript. Y.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have reviewed and approved the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. U22A20287) and the Foundation from Shanghai Municipal Health Commission (No. 202440018).
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request(Email:amin1031@hotmail.com.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Shanghai Jiaotong University School of Medicine, Renji Hospital Ethics Committee (Approval No. LY2022-039-A). All participants provided written informed consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tingting Han and Xinyue Liang have contributed equally to this work and share first authorship.
References
- 1.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–40. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48. [DOI] [PubMed] [Google Scholar]
- 3.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12(6):887–8. [DOI] [PubMed] [Google Scholar]
- 4.Lee DC, Shook RP, Drenowatz C, Blair SN. Physical activity and sarcopenic obesity: definition, assessment, prevalence and mechanism. Future Sci OA. 2016;2(3):Fso127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han T, Yuan T, Liang X, Chen N, Song J, Zhao X, Weng Y, Hu Y. Sarcopenic obesity with normal body size May have higher insulin resistance in elderly patients with type 2 diabetes mellitus. Diabetes Metabolic Syndrome Obesity: Targets Therapy. 2022;15:1197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prado CM, Batsis JA, Donini LM, Gonzalez MC, Siervo M. Sarcopenic obesity in older adults: a clinical overview. Nat Reviews Endocrinol. 2024;20(5):261–77. [DOI] [PubMed] [Google Scholar]
- 7.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Reviews Endocrinol. 2018;14(9):513–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JA, Hwang SY, Chung HS, Kim NH, Seo JA, Kim SG, Kim NH, Choi KM, Baik SH, Yoo HJ. Proportion and characteristics of the subjects with low muscle mass and abdominal obesity among the newly diagnosed and Drug-Naïve type 2 diabetes mellitus patients. Diabetes Metabolism J. 2019;43(1):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National health and nutrition examination survey III. PLoS ONE. 2010;5(5):e10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TN, Park MS, Lim KI, Choi HY, Yang SJ, Yoo HJ, Kang HJ, Song W, Choi H, Baik SH, et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: the Korean sarcopenic obesity study. Clin Endocrinol. 2013;78(4):525–32. [DOI] [PubMed] [Google Scholar]
- 11.Chung JY, Kang HT, Lee DC, Lee HR, Lee YJ. Body composition and its association with cardiometabolic risk factors in the elderly: a focus on sarcopenic obesity. Arch Gerontol Geriatr. 2013;56(1):270–8. [DOI] [PubMed] [Google Scholar]
- 12.Habib SS, Alkahtani S, Alhussain M, Aljuhani O. Sarcopenia coexisting with high adiposity exacerbates insulin resistance and dyslipidemia in Saudi adult men. Diabetes Metabolic Syndrome Obesity: Targets Therapy. 2020;13:3089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–e307302. [DOI] [PubMed] [Google Scholar]
- 14.Beasley JM, Shikany JM, Thomson CA. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr Clin Practice: Official Publication Am Soc Parenter Enter Nutr. 2013;28(6):684–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage Whey. J Food Sci. 2015;80(Suppl 1):A8–15. [DOI] [PubMed] [Google Scholar]
- 16.Mori H, Tokuda Y. Effect of Whey protein supplementation after resistance exercise on the muscle mass and physical function of healthy older women: A randomized controlled trial. Geriatr Gerontol Int. 2018;18(9):1398–404. [DOI] [PubMed] [Google Scholar]
- 17.Chinese Diabetes Society. Guideline for the prevention and treatment of diabetes mellitus in China (2024 edition). Chin J Diabetes Mellitus. 2025;17(1):16–139. [Google Scholar]
- 18.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R, AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY COMPREHENSIVE CLINICAL PRACTICE GUIDELINES FOR MEDICAL CARE OF PATIENTS WITH OBESITY. Endocr Practice: Official J Am Coll Endocrinol Am Association Clin Endocrinologists. 2016;22(Suppl 3):1–203. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Han T, Gao J, Zhang Y, Zhao S, Sun R, Sun C, Niu Y, Li Y. Association of serum calcium and insulin resistance with hypertension risk: A prospective Population-Based study. J Am Heart Association. 2019;8(1):e009585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organization technical report series. 1995, 854:1-452. [PubMed]
- 21.8. Obesity management for the treatment of type 2 diabetes: standards of medical care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S89–97. [DOI] [PubMed] [Google Scholar]
- 22.Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet (London England). 2018;391(10120):541–51. [DOI] [PubMed] [Google Scholar]
- 23.Lean MEJ, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the direct open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7(5):344–55. [DOI] [PubMed] [Google Scholar]
- 24.Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, Zimmet P. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729–36. [DOI] [PubMed] [Google Scholar]
- 25.Dunstan DW, Daly RM, Owen N, Jolley D, Vulikh E, Shaw J, Zimmet P. Home-based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care. 2005;28(1):3–9. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Kim D, Kim C. Resistance training for glycemic control, muscular strength, and lean body mass in old type 2 diabetic patients: A Meta-Analysis. Diabetes Therapy: Res Treat Educ Diabetes Relat Disorders. 2017;8(3):459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansson AK, Chan LX, Lubans DR, Duncan MJ, Plotnikoff RC. Effect of resistance training on HbA1c in adults with type 2 diabetes mellitus and the moderating effect of changes in muscular strength: a systematic review and meta-analysis. BMJ Open Diabetes Res Care 2022, 10(2). [DOI] [PMC free article] [PubMed]
- 28.Wirunsawanya K, Upala S, Jaruvongvanich V, Sanguankeo A. Whey protein supplementation improves body composition and cardiovascular risk factors in overweight and obese patients: A systematic review and Meta-Analysis. J Am Coll Nutr. 2018;37(1):60–70. [DOI] [PubMed] [Google Scholar]
- 29.Miller EG, Nowson CA, Dunstan DW, Kerr DA, Menzies D, Daly RM. Effects of Whey protein plus vitamin D supplementation combined with progressive resistance training on glycaemic control, body composition, muscle function and cardiometabolic risk factors in middle-aged and older overweight/obese adults with type 2 diabetes: A 24-week randomized controlled trial. Diabetes Obes Metab. 2021;23(4):938–49. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y, Long J, Dan S, Johannsen NM, Talamoa R, Raghuram S, Chung S, Kent K, Basina M, Lamendola C, et al. Strength training is more effective than aerobic exercise for improving glycaemic control and body composition in people with normal-weight type 2 diabetes: a randomised controlled trial. Diabetologia. 2023;66(10):1897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo C, Liu RY, Zhang GW, Hu F, Jin YH, Liu BY. Possible sarcopenia and risk of new-onset type 2 diabetes mellitus in older adults in china: a 7-year longitudinal cohort study. BMC Geriatr. 2023;23(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu S, Cai X, Yuan Y, Xie B, Sun Z, Wang D, Wu T. Muscle strength and prediabetes progression and regression in middle-aged and older adults: a prospective cohort study. J Cachexia Sarcopenia Muscle. 2022;13(2):909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li JJ, Wittert GA, Vincent A, Atlantis E, Shi Z, Appleton SL, Hill CL, Jenkins AJ, Januszewski AS, Adams RJ. Muscle grip strength predicts incident type 2 diabetes: Population-based cohort study. Metab Clin Exp. 2016;65(6):883–92. [DOI] [PubMed] [Google Scholar]
- 34.Boonpor J, Parra-Soto S, Petermann-Rocha F, Ferrari G, Welsh P, Pell JP, Sattar N, Gill JMR, Ho FK, Gray SR et al. Associations between grip strength and incident type 2 diabetes: findings from the UK biobank prospective cohort study. BMJ Open Diabetes Res Care 2021, 9(1). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request(Email:amin1031@hotmail.com.