Abstract
Background
Tongue muscles contain a much greater number of residual adipocytes than other muscles do, which makes them susceptible to obesity-induced muscle fat remodeling. Tongue fat remodeling leads to obesity-induced obstructive sleep apnea (OSA), which is a common sleep disorder characterized by repeated episodes of upper airway collapse during sleep, resulting in fragmented sleep and oxygen deprivation. Although the obstructive role of fat remodeling in tongue muscles for OSA has been confirmed, the cellular and molecular mechanisms regulating fat remodeling in tongue and its impact on tongue muscles have not been well explored.
Methods
To study the impact of obesity on adipocytes and neuromuscular junctions (NMJs) in tongue muscles, we used a high-fat diet (HFD)-induced obese preclinical model.
Results
The results demonstrated hypertrophy of adipocytes and denervation at NMJs in tongue muscles by a HFD. Mechanistically, we revealed that a HFD repressed the expression of growth differentiation factor 10 (GDF10), which is expressed mainly in fibroadipogenic progenitors (FAPs) in skeletal muscles, repressing adipogenesis and maintaining the integrity of neuromuscular connections. We identified sex differences and muscle specificity of Gdf10 mRNA expression in FAPs. To understand how a HFD significantly reduces the level of Gdf10 mRNA expression in FAPs of the tongue, we investigated the epigenetic regulation of Gdf10. We found that a HFD increases miR-144-3p in tongue FAPs, which interferes with Gdf10 mRNA expression and induces adipogenesis. GDF10 overexpression by viral delivery effectively prevented HFD-induced fat remodeling of tongue and limb muscles.
Conclusion
These findings provide important insight into the role of FAP-derived GDF10 in the interplay between fat contents and tongue muscles in response to obesity and suggest potential therapeutic targets for OSA treatment.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13395-025-00389-z.
Keywords: Tongue muscle, Fibroadipose progenitors, Denervation, Obesity, High-fat diet, Growth differentiation factor 10, miRNA-144-3p
Statement of significance (120)
Obesity is the largest contributor to OSA risk. Obesity increases fat accumulation in the tongue, which narrows airway structures and increases the risk of airway collapse. Tongue muscles are particularly susceptible to obesity due to the high number of resident adipocytes in muscles compared to other muscles. Understanding the molecular processes by which fat infiltration of tongue muscles, which contributes to airway collapse, is crucial to developing potential therapeutics for OSA. We provide the role of growth differentiation factor 10 (GDF10) in limiting fat infiltration in tongue muscles using diet-induced obese mouse models and suggest high-fat diet-induced miRNA-regulated GDF10 expression in fibroadipogenic progenitors in tongue muscles. Our study indicates the potential therapeutic value of GDF10 to treat OSA.
Introduction
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder that affects nearly 1 billion adults globally [1]. It is linked to severe cardiovascular consequences, such as hypertension, heart failure, and stroke [2, 3]. Obesity is a significant risk factor for OSA. The previous clinical study shows that weight loss over a year resulted in significant and clinically relevant improvements in OSA [4]. Excessive fat deposition at the tongue base is thought to be a possible link between OSA and obesity. A clinical MRI study of OSA patients demonstrated that excessive fat deposition at the tongue base, adjacent to the upper airway, causes physical enlargement of the tongue and corresponding upper airway narrowing [3]. Although previous clinical studies have established a strong correlation between obesity and OSA through tongue fat deposits and muscle tone [3, 5, 6], the cellular and molecular mechanism underlying this connection remains unknown, mainly due to the lack of preclinical OSA models to study the causes of OSA. Most animal models used in OSA research have focused on observing the consequences of the condition, such as monitoring phenotypes after intermittent hypoxia, rather than exploring the cause of OSA [7]. While these studies have underscored the importance of OSA treatment, the lack of understanding of the disease's cellular and molecular mechanisms limits the development of potential therapeutics for OSA. Therefore, a preclinical model for investigating the mechanism of OSA is a critical gap in OSA research.
Fibro/adipogenic progenitors (FAPs) have been acknowledged as crucial for maintaining muscles in the limbs and craniofacial regions [8, 9]. FAPs play a significant role in supporting neuromuscular junctions (NMJs) and muscle mass, which can be affected by muscle atrophy and NMJ degeneration if there is a depletion of FAPs [8, 10]. One key molecular mechanism responsible for FAP-regulated muscle maintenance is FAPs-derived growth differentiation factor 10 (GDF10), a transforming growth factor-β superfamily member. GDF10 has been identified as having a function in forming the head in embryos and may be involved in various aspects of skeletal development [11]. GDF10 is highly expressed in FAPs and maintains Schwann cell stability [10]. Additionally, GDF10 has a negative effect on adipocyte hypertrophy. Studies have shown that overexpression of GDF10 in adipose tissues protects against high-fat diet-induced obesity [12], while its deficiency is linked with adipocyte hypertrophy [13]. Furthermore, GDF10 overexpression has been found to reduce fat infiltration in limb muscles [10, 14]. However, the role of GDF10 in fat deposits and neural connection in tongue muscles remains unknown.
This study used a mouse model to investigate the impact of a high-fat diet (HFD) on fat deposits and the integrity of NMJ in tongue muscles. We investigate if HFD feeding changes fat infiltration and NMJ integrity of tongue muscles and correlates with GDF10 expression in tongue FAPs. We reveal how HFD regulates the Gdf10 mRNA expression in tongue FAPs using miRNAs and determine whether the overexpression of GDF10 reverses adipocyte hypertrophy and denervation by HFD. The animal model established by this study provides a platform to explore OSA's cellular and molecular mechanisms and offers opportunities to develop therapeutic interventions for this condition.
Methods
Animals
C57BL/6 J mice (Jax000664), Pdgfra-EGFP mice (B6.129S4-Pdgfratm11(EGFP)Sor/J (Jax007669)), Pdgfra-Cre/ERT mice (B6N.Cg-Tg(Pdgfra-cre/ERT)467Dbe/J (Jax018280), tdTomato flox/flox mice (B6.Cg-Gt(ROSA)26Sortm14(CAG−tdTomato)Hze/J (Jax007914)) were purchased from Jackson Laboratories (Bar Harbor, ME; www.jax.org). Animals were maintained in pathogen-free housing with a 12-h light–dark cycle and ad libitum food and water. Mice received a normal chow diet (ND; Laboratory Rodent Diet 5001), which contains 13.5% of calories from fat until two months of age. Control mice continued on ND while experimental mice switched to a high-fat diet (HFD; Envigo, TD.88137 Adjusted calories diet) containing 42% calories from fat. Mice consumed ND or HFD for six months before analyses. HFD-induced obesity was monitored by monthly body weight measurement. After 6 months of ND or HFD, mice (8-month-old) were euthanized for tissue sampling. To induce Cre-dependent tdTomato expression in Pdgfra+ cells, we injected Tamoxifen (100 mg/kg) for 5 consecutive days at 10 weeks old and euthanized mice 3 months later. Experiments were carried out in accordance with authorized rules and ethical approval from Emory University's Institutional Animal Care and Use Committee, as well as in accordance with the National Institutes of Health.
Muscle tissue preparation
All muscle tissues were fresh frozen in Tissue-Tek® O.C.T. Compound (Sakura Finetek, Torrance, CA, USA) on N2-chilled methyl butane and stored at −80 °C. For muscles carrying genetically labeled fluorescent protein, we fixed muscle tissues with 4% paraformaldehyde for 2 h in room temperature and incubated in 30% sucrose in PBS overnight at 4 °C to preserve fluorescent signals. Then tissues were frozen in Tissue-Tek® O.C.T. Compound on N2-chilled methyl butane and stored at −80 °C. Cross-sections (10 μm thickness) of tongue base were prepared to capture adipocytes in the vertical and transverse muscles of the tongue or longitudinal sections (30 μm thickness) of tongue tip to capture neuromuscular junctions in genioglossus muscles. Tissue sections were collected every 200 μm using CryoStar NX50 Cryostat (Fisher Scientific, Waltham, MA).
Immunohistochemistry/immunofluorescence
Muscle sections were stained with hematoxylin and eosin (H&E) to observe histology and measure the cross-sectional area of myofibers. Muscle sections were fixed with 4% paraformaldehyde for 10 min and washed for 5 min 3 times with PBS. Then, sections were permeabilized with 0.5% Triton-X and blocked with 1% bovine serum albumin (BSA) and 5% donkey serum (blocking buffer) for 1 h and labeled with primary antibodies (anti-perilipin antibody (Sigma Aldrich (P1873), 1:100) and anti-neurofilament (SMI-32) antibody (Abcam (ab72996), 1:100) overnight at 4 °C in 10% diluted blocking buffer in 0.2% Tween-20 in PBS (washing buffer). The following day, sections were washed three times with washing buffer and incubated with fluorescence probe-conjugated secondary antibodies (Jackson ImmunoReserach) for 1 h at room temperature. Lipid droplets were stained with HCS LipidTOX™ Green neutral lipid stain (Invitrogen) for 30 min with 1:200 dilution in PBS. Acetylcholine receptors were labeled with fluorophore-conjugated α-bungarotoxin (αBTX) (1 μg/mL, Invitrogen) fluorescent for 30 min. Nuclei were stained with 1 µg/ml 4′,6-diamidino-2-phenylindole (DAPI). Before imaging, the stained sections were mounted with Vectashield (Vector Labs, www.vectorlabs.com, Burlingame, CA).
Image analysis
For fat infiltration of muscle analysis, images of perilipin-1 immunostained tongue cross-sectioned muscles were captured using the Nikon AX/AX R Confocal Microscope System (Nikon), and stitched images using the Nikon NIS-Elements platform (Nikon). Fat infiltration was quantified by counting adipocytes and measuring adipocyte area. Quantification of adipocyte area was performed using Image J.
For neuromuscular junction (NMJ) denervation analysis, Z-stack images of 30 μm thick longitudinal tongue sections were captured using the Nikon AX/AX R Confocal Microscope System (Nikon), and reconstructed images were displayed by maximum intensity projection using the Nikon NIS-Elements platform (Nikon). For denervation assessment, we analyzed 100–150 NMJs located in the genioglossus muscle per tongue muscle. The numbers of completely denervated, partially denervated, and innervated NMJs were counted using Image J.
FAPs isolation and fluorescence activated cell sorting
To obtain purified FAPs, primary cells were isolated as described previously with small modifications [15]. Dissected tongue and gastrocnemius muscles were minced and digested using 0.2% collagenase II (Gibco, Carlsbad, CA) in Dulbecco’s modified Eagle’s medium (DMEM) at 37 °C while shaking at 65 rpm for 90 min. Digested fiber suspension was washed and further digested with 0.2% collagenase II (Gibco, Carlsbad, CA) and 2.5 U/ml Dispase II (Gibco, Carlsbad, CA) at 37 °C while shaking at 65 rpm for 30 min. Digested muscles were then rinsed with the same volume of Hanks'Balanced Salt Solution (HBSS) containing 20% FBS and 100 μg/ml penicillin/streptomycin (P/S) to inactivate enzymes. Mononucleated cells were dissociated with a 10-gauged syringe and filtered with a 40 μm cell strainer (Thermo Fisher Scientific, Waltham, MA). To label FAPs, they were subsequently labeled with the following antibodies: 1:400 CD31-PE (clone 390; eBiosciences, San Diego, CA), 1:400 CD45-PE (clone 30-F11; BD Biosciences, San Jose, CA), and 1:4000 Sca-1-PE-Cy7 (clone D7; BD Biosciences, Vancouver, Canada). Fluorescence-activated cell sorting (FACS) was performed using a BD FACSAria II cell sorter (Becton–Dickinson, http://www.bd.com, Franklin Lakes, NJ) at the Emory University School of Medicine Core Facility for Flow Cytometry. Analyses of flow cytometry data were performed using FACSDiva (BD version 8.0.1).
FAPs culture and differentiation
FACS-purified FAPs were plated at 30,000 to 60,000 cells per well in a 6-well plate coated with collagen and cultured in DMEM supplemented with 20% FBS, 1% P/S, and 2.5 ng/ml bFGF (BioLegend, #579,604) inside an incubator with 5% CO2 (HERAcell VIOS 160i CO2 incubator, Thermo Fisher Scientific, Waltham, MA) until the cells reached 70% confluency. Medium was changed every 2–3 days. For spontaneous differentiation, FAPs were maintained for 10 days after 100% confluency with DMEM supplemented with 20% FBS and 1% P/S. For adipogenic differentiation, FAPs with 100% confluency were exposed to DMEM with 10% FBS, 0.5 mM IBMX (Sigma Aldrich, #I5879), 0.25 µM dexamethasone (Sigma Aldrich, #D2915), and 10 µg/ml insulin (Sigma Aldrich, #I0516) for 10 days. Medium was changed every 2–3 days.
Transfection of miRNA in FAPs
When FAPs reached 70% confluency, miR-144-3p or miR-139-5p inhibitors (100 nM) or mimics (100 nM) were transfected with Lipofectamine 2000 (Invitrogen, #11,668,030) following manufacturer’s instruction. Control miR mimics were used as control. Two days after transfection, FAPs were collected for RNA isolation.
Luciferase assay
HEK293 cells (80% confluency) were transfected with a luciferase reporter plasmid regulated by GDF10 3’-UTR (Active Motif #S806395) or GDF10-promoter (Active Motif #S708376) and their respective control vectors as control. At the same time, miR-144-3p or miR-139-5p mimics (100 nM) were transfected using Lipofectamine 2000 (Invitrogen, #11,668,030) following the manufacturer’s instruction. Control miR mimics were used as control. Luciferase assay was performed a day after transfection following the manufacturer’s instructions (LightSwitch™ Luciferase Assay Kit (Active Motif, #32,031) with a luminometer.
For the mutagenesis study, we generated mutations with Q5® Site-Directed Mutagenesis Kit (New England Biolabs). Mutated sequences were confirmed by Sanger sequencing. HEK293 cells were seeded at a density of 1.0 × 104 cells per well in 96-well plates (Day 0). The following day (Day 1), cells were co-transfected with 100 ng of a luciferase reporter plasmid containing either the wild-type (WT: 3'-AUACUGUA−5') or mutant (Mut: 3'-AUAAAGUA−5') seed sequences of miR-144-3p on GDF10 3'-UTR sequence along with 30 nM of miR-144-3p mimic, using Lipofectamine 2000. Twenty-four hours after transfection (Day 2), luciferase activity was measured using the LightSwitch™ Luciferase Assay Kit (Active Motif, #32,031) according to the manufacturer’s instructions. Luminescence was detected using a BioTek Synergy 2 microplate reader.
Gene expression analysis by qPCR
FAPs of tongue muscles were analyzed for the expression of related markers using comparative qPCR. RNA from samples was extracted using Trizol (Invitrogen) according to the manufacturer’s instructions. Isolated RNA (250 ng) was reverse transcripted into complementary DNA (cDNA) using random hexamers or miRNA adaptor primers and M-MLV reverse transcriptase (Invitrogen) and then analyzed by real-time qPCR. The cDNA was amplified using Power SYBR® Green Master Mix (Applied Biosystems, Waltham, MA) and 2.5 μM of each primer. All primer sequences are listed in Supplemental Table 1. PCR reactions were performed for 35 cycles under the following conditions: denaturation at 95 °C for 15 s and annealing at 60 °C for 1 min. Quantitative levels for all mRNAs were normalized to endogenous Gapdh expression and levels for all miRNAs were normalized to miR-16 [16]. Fold change of gene expression was determined using the ∆∆Ct method [17].
GDF10 overexpression
Five days after FAPs differentiation into adipocytes, cells were treated with ssAAV.CMV.Gdf10.P2A.mCherry.WPRE.SV40pA (1 × 1010 GC/well) (Packgene) or ssAAV.CMV.mCherry.WPRE.SV40pA (1 × 1010 GC/well) (empty control mCherry AAV, Packgene) for 3 days. Transduction was confirmed by RFP expression and qRT-PCR.
Mice were anesthetized with 3% isoflurane in a sealed chamber and maintained anesthesia via nose cone with 2.5% isoflurane. Hairs were shaved on digastric or tibialis anterior muscles with hair removal lotion. Skin was wiped with alcohol swabs before injection. Then 10 μl (1 × 1010 GC) of ssAAV.CMV.Gdf10.P2A.mCherry.WPRE.SV40pA (Packgene) or ssAAV.CMV.mCherry.WPRE.SV40pA (empty control mCherry AAV, Packgene) was injected intramuscularly. To deliver AAV throughout the tongue, we injected needles with very shallow angles (10 degrees) from surface of digastric muscles, which are located under the tongue base.
To observe the impact of GDF10 overexpression in glycerol-injured muscles. Three weeks after glycerol injury, 10 µl of AAV8-GDF10-RFP virus (ssAAV.CMV.Gdf10.P2A.mCherry.WPRE. SV40pA, 1X1011 GC) or control AAV8-RFP virus (ssAAV.CMV.mCherry.WPRE. SV40pA, 1X1011 GC) was injected into both TA muscles via the same injection protocol as glycerol injury.
Glycerol-induced muscle injury
We induced intramuscular adipogenesis in limb muscles via a glycerol injury model, with a focus on the tibialis anterior (TA) muscle. The mice were anesthetized with 3% isoflurane in a sealed chamber, and anesthesia was maintained via the nose corn with 2.5% isoflurane. To alleviate any potential discomfort, we administered 0.05 mg/kg buprenorphine-sustained release through subcutaneous injections for analgesia. We injected both TA muscles longitudinally with 25 µl of 50% glycerol in PBS solution via a syringe.
Grip strength measurement
The muscle grip strength of each mouse was measured two days before dissection to assess the effect of the treatment on muscle function. Hindlimb strength was determined by subtracting the average forelimb strength force from the average full-limb strength force. We obtained a minimum of seven valid grip strength values for each measurement method. For the forelimb measurement (17), a T-shaped grip bar was attached to the grip strength meter, which was set up perpendicular to the floor. The mouse could grip the bar with its forepaws while keeping its torso perpendicular to the floor. The mice were pulled downward to ensure that they maintained a firm grip with both forepaws. The grip strength meter recorded the maximal grip strength. For the combined forelimb, a 4 × 6 grid was attached to the grip strength meter, allowing the mouse to grip the grid with four limbs while keeping its torso perpendicular to the floor. The tail was gently pulled downward, with the force gradually increasing. A valid measurement was achieved by maintaining this pulling force for a few seconds, ensuring that the mouse maintained a firm grip on the grid. The meter recorded the maximum force.
Statistical Analyses
Statistical analysis was performed using Prism 10.0. Statistical testing was performed using the unpaired two-tailed Welch's t-test to compare two groups or 1- or 2- way ANOVA, depending on data structure, as stated in the figure legends. P-value < 0.05 is considered statistically significant. Error bars represent the mean ± SEM
Results
Hypertrophy of adipocytes causes fatty tongue in HFD-induced obese mice
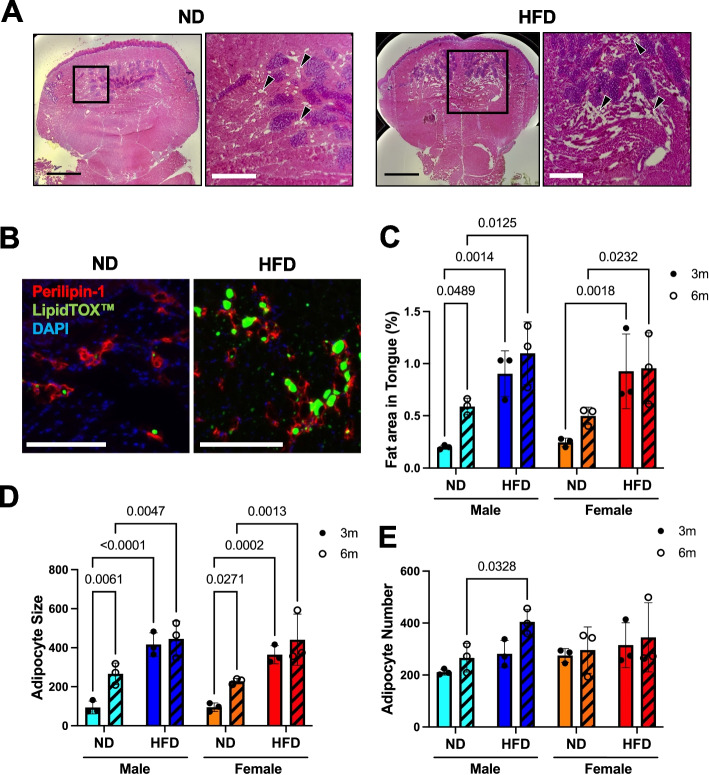
We investigated the role of a high-fat diet (HFD) in promoting fat accumulation in the tongue, a phenomenon observed in patients with OSA [3]. We placed 2-month-old wild-type (WT) male and female mice on a six-month diet-induced obesity course and observed marked weight gain (Supplementary Fig. 1). We then examined the adiposity of the tongue base in both normal diet (ND) and HFD groups and found unilocular adipocytes, particularly in the vertical and transverse muscles of the tongue (Fig. 1A). We confirmed their identity by immunostaining of perilipin-1, a mature adipocyte marker [18], and lipidTOX, a neutral lipid droplet marker [19] (Fig. 1B). We found that in both males and females, there was an increase in the percentage of tongue cross-sectional area (CSA) occupied by adipocytes (Fig. 1C). To determine whether the increased fat area was caused by the increased size of adipocytes (hypertrophy) or the increased number of adipocytes (hyperplasia), we measured the size and number of adipocytes in tongue sections. The average single adipocyte CSA was two times larger in HFD groups compared to ND groups, indicating hypertrophy of adipocytes by HFD (Fig. 1D); however, there was no significant difference in adipocyte number between ND and HFD groups except for the male group with 6-month HFD, indicating potential sex differences in adipocyte hyperplasia by HFD (Fig. 1E). Since OSA is more prevalent in males than females in humans, we analyzed if adipocyte hypertrophy shows sex differences in the 6-month HFD-induced obesity mouse model. We performed a 2-way ANOVA analysis, which revealed that diet (column factor p = 0.0121), but not sex (row factor p = 0.2622), was the main factor affecting adipocyte hypertrophy and fatty tongue in obese mice. Together, these results indicate that the primary factor contributing to the development of fatty tongue in obese mice is HFD-induced hypertrophy of adipocytes without sex differences in mice.
Fig. 1.
Hypertrophy of adipocytes causes fatty tongue in HFD-induced obese mice. (A) Hematoxylin and Eosin (H&E) staining of tongue cross-sections of male mice fed with ND or HFD for 6 months. The left panel shows the entire tongue section, and the right panel shows the magnified image of the boxed region. Arrowheads indicate adipocytes. Scale bar in left panel is 1 mm and the right panel is 400 µm. (B) Immunostaining of tongue cross-sections of ND and HFD-fed male mice for perilipin-1 (red), lipidTOX (green), and DAPI. Scale bar = 170 μm. (C-E) Quantification of B. Red perilipin-stained adipocyte number was counted, and the area was measured. (C) The percentage of fat area in tongue was determined by adding up the total area occupied by adipocytes and dividing it by the total area of the tongue. (D) The adipocyte size area was calculated by adding up the total area occupied by adipocytes and dividing it by the total adipocyte number. (E) Averaged adipocyte number in tongue section of each group. Cyan- and blue-colored bars indicate the male group, and orange- and red-colored bars indicate the female group. The hatched bar indicates the HFD group. The dots present the individual data of the group. n = 3. Statistical significance was determined by the 2-way ANOVA and Fisher’s LSD test as a post-hoc multiple comparison test. The p-values are shown above the horizontal line above the bars. Error bars represent the mean ± SEM
HFD-induced obesity compromises tongue muscle NMJ integrity
While an enlarged tongue is a known contributing factor [3], reduced tongue muscle tone during sleep can also lead to tongue collapse and OSA. To investigate the impact of HFD on the NMJ integrity of the tongue, we examined the integration of NMJs in the genioglossus muscle (Fig. 2A, white dotted line), a main muscle responsible for tongue protrusion and positioning during sleep. Using immunostaining for presynaptic (neurofilament) and postsynaptic regions (a-bungarotoxin (BTX) binding acetylcholine receptor) (Fig. 2B), we observed compromised NMJ integrity, such as denervation and fragmented post synaptic area, in the tongue muscle of mice fed HFD for six months (Fig. 2C). This effect was significant in males and females, with a higher percentage of denervated NMJs compared to those fed an ND (Fig. 2C and 2D). Interestingly, the denervation rate was significantly lower in females compared to male with HFD, which may explain male prevalence of OSA. Our findings suggest that HFD-induced obesity impairs neuromuscular connection in the tongue muscle, contributing to tongue collapse and potentially OSA.
Fig. 2.
HFD-induced obesity compromises tongue muscle NMJ integrity. (A) The genioglossus muscle (lined area) in H&E stained tongue longitudinal sections of female mice fed with ND or HFD for 6 months. Scale bar = 750 µm. (B) Immunofluorescence staining of genioglossus muscles in tongue longitudinal sections of female mice fed with ND or HFD for 6 months for neurofilament (NF) and acetylcholine receptor (αBTX). Scale bar = 20 µm. (C) Percentage of completely denervated (red), partially denervated (green), and innervated (yellow) NMJ were calculated. More than 100 NMJs were analyzed per muscle. All NMJs from 3 replicates were combined. (D) Percentage of completely denervated NMJs in 6-month ND and HFD-fed males. n = 3. Statistical significance for (D) was determined by 2-way ANOVA and Fisher’s LSD test as a post-hoc multiple comparison test. The p-values are shown above the horizontal line above the bars. Error bars represent the mean ± SEM
To monitor the functional consequences of increased fat and denervation of NMJs on tongue movement, we measured lick speed (tongue protrusions/second) in WT mice that were placed on a 6-month HFD feeding. Results showed that only female mice exhibited a slightly decreased lick speed, while males did not show significant changes (Supplementary Fig. 2 A). While the denervation rate in genioglossus muscles was increased in HFD, the size of genioglossus muscle fibers of HFD-fed mice remained similar to ND-fed mice (Supplementary Fig. 2B-2C). This suggests that HFD-induced obesity slightly impedes tongue motion but not muscle mass maintenance, which could have implications for the development of OSA in humans.
Next, we wonder whether HFD-induced adipogenesis and NMJ integrity are exclusive tongue muscles. Unlike tongue muscles, limb muscles rarely contain adipocytes. However, fat infiltration is elevated in limb muscles with aging [20] and chronic diseases, such as peripheral artery diseases and muscular dystrophy [21, 22]. To induce fat infiltration in limb muscles, we injected 50% glycerol in saline into tibialis anterior (TA) muscles [23]. Next, we fed mice either a ND or HFD for 4 weeks, during which HFD-fed mice gained more weight (Supplementary Fig. 3 A) and completed muscle regeneration. This allowed us to assess whether HFD influences FAP-driven adipogenesis, specifically adipocyte number and size, as well as NMJ integrity following regeneration. Similar to tongue muscles, we found that HFD caused increased fat area, adipocyte numbers, and size in glycerol-injected TA muscles (Supplementary Fig. 3B-F). Also, the denervation of NMJs in the TA muscles of HFD-fed mice was significantly higher than that in the TA muscles of ND-fed mice, and grip strength of HFD-fed mice was lowered as well (Supplementary Fig. 3G-I). Taken together, HFD induces hypertrophy and hyperplasia of resident adipocytes and interferes with neuromuscular connections and muscle strength in limb muscles.
HFD reduces GDF10 expression in FAPs and sex differences of gdf10 transcription
To explore the mechanism underlying the simultaneous enlargement of adipocytes and denervation of NMJs in the tongue of obese mice, we focused on the potential involvement of GDF10, which is highly expressed in FAPs and a secreted factor from FAPs. GDF10 deficiency has been linked to muscle mass loss and NMJ degeneration [10]. Studies have also shown that overexpression of GDF10 can protect against HFD-induced adipocyte hypertrophy [12], while a deficiency of Gdf10 is associated with adipocyte hypertrophy [13]. Therefore, we hypothesized that HFD induces Gdf10 mRNA decline in FAPs, which could be responsible for both adipocyte hypertrophy and NMJ degeneration via paracrine manner in the tongue of obese mice. To test this hypothesis, we analyzed the expression of Gdf10 mRNA in FAPs isolated from the tongue muscles of ND and HFD-fed mice. Our results revealed that Gdf10 expression was significantly lower in tongue FAPs in HFD-fed male and female mice. Also, Gdf10 expression was lower in male mice than female mice (Fig. 3A). We confirmed similar expression patterns of Gdf10 mRNA in FAPs of the gastrocnemius (GA) muscles (Fig. 3B). To understand sex differences in Gdf10 mRNA expression, we performed GDF10 promoter activity assay with sex hormones. We transfected HEK293 cells with plasmids containing GDF10 promoter-dependent luciferase and incubated HEK293 cells with various sex hormones. Compared to other sex hormones, β-estradiol significantly induced GDF10 promoter activity utilizing luciferase assay (Fig. 3C). We also confirmed that β-estradiol increased Gdf10 mRNA expression levels in tongue FAPs of male mice with a ND (Fig. 3D). These results suggest that Gdf10 mRNA expression shows sex differences, which are influenced by β-estradiol.
Fig. 3.
The expression of Gdf10 mRNA in FAPs is influenced by diet, sex, and anatomical muscle site. (A-B) Gdf10 mRNA expression was significantly decreased in both tongue (TG) and gastrocnemius (GA) FAPs of HFD-fed obese mice. Gdf10 mRNA expression was lower in males compared to females in both tongue and GA. n = 3. (C) GDF10 promoter activity was measured by luminescence with incubation of various sex hormones in HEK293 cells transfected with a plasmid containing GDF10 promoter-controlled luciferase. (D) Gdf10 mRNA expression was increased with β-estradiol treatment (10 μM, 48 h) compared to vehicle (ethanol) treatment in tongue FAPs (male, ND) FAPs. n = 4. Statistical significance for (A), (B), and (D) was determined by 2-way ANOVA and Fisher’s LSD test as a post-hoc multiple comparison test. Statistical significance for (C) was determined by 1-way ANOVA and Tukey test as a post-hoc multiple comparison test. The p-values are shown above the horizontal line, which is positioned above the bars. Error bars represent the mean ± SEM
High GDF10 expression shifts tongue FAPs to fibrogenic lineages and Pdgfra+ cells do not contribute to adipocyte homeostasis in adult tongue
GDF10 suppresses the adipogenic program of CD142+ interstitial cells (Lin−, Pdgfra+ and Sca1+), which shared surface markers with FAPs, of dystrophic muscles[24]. Interestingly, we observed that Gdf10 mRNA expression is more than 3,000 to 10,000 times higher in tongue FAPs compared to gastrocnemius FAPs (Fig. 4A). Given the inhibitory role of GDF10 in adipogenesis, we tested the adipogenic differentiation of tongue FAPs. As expected, tongue FAPs failed to differentiate into adipocytes, while gastrocnemius FAPs differentiated into adipocytes spontaneously after 10 days of cultures (Fig. 4B). We confirmed adipogenic marker gene (Pparg) was increased in gastrocnemius muscle FAPs but not in tongue FAPs after 10 days of culture. Instead, tongue FAPs induced the fibrogenic marker gene (Col1a1) (Fig. 4C). To investigate the role of GDF10 in differentiation lineage of FAPs, we overexpressed Gdf10 in gastrocnemius FAPs using AAV8-RFP virus, which known to transduce mononucleated cells, including FAPs, in skeletal muscles [25] (Fig. 4D). Compared to control AAV8-RFP virus infected GA FAPs, AAV8-Gdf10-RFP virus infected GA FAPs were less differentiated into adipocytes, which is visualized by Oil-Red-O neutral fat staining (Fig. 4D) and qRT-PCR of adipogenic marker gene expression (Fig. 4E). These findings imply that elevated GDF10 directs the differentiation potential of FAPs to the fibrogenic lineage rather than the adipogenic lineage. Also, those secreted GDF10 may regulate other cell types, such as adipocytes or motor neurons, in a paracrine manner.
Fig. 4.
Tongue FAPs are prone to fibrogenic lineage differentiation and do not contribute to adipocyte homeostasis in adult tongue. (A) Gdf10 mRNA expression was higher in tongue FAPs compared to gastrocnemius FAPs. n = 3. (B) Representative image of tongue and gastrocnemius FAPs after 10 days-culture. As a positive control, gastrocnemius FAPs were differentiated into adipocytes. (C) Pparg and Col1a1 mRNA expressions in tongue and gastrocnemius FAPs after 10 days-culture. Tongue FAPs before differentiation were used as a negative control. n = 5–7. Scale bar = 45 µm. (D) Representative Oil-Red-O image of gastrocnemius FAPs after 10 days-culture with AAV8-RFP-virus (Con) or AAV8-Gdf10-RFP-virus (GDF10). The AAV8-virus was treated at day 5 during adipogenic differentiation. Scale bar = 45 µm. (E) Gdf10, Pparg and Ap2 mRNA expressions in gastrocnemius FAPs after 10 days-culture with AAV8-RFP-virus (Con) or AAV8-Gdf10-RFP-virus (GDF10). n = 4. (F) Illustrated fluorescent images of Pdgfra+ cell-derived adipocyte in Pdgfra-H2B-EGFP mice. (G) Immunofluorescence staining of cross-sectioned tongue muscle isolated from Pdgfra-H2B-EGFP mice (3 months old) for adipocyte (perilipin-1, red) and Pdgfra cells with EGFP+ nuclei. Scale bar = 70 µm. (H) Quantification of Pdgfra-derived adipocytes (adipocytes with EGFP+ nuclei). n = 3 (I) Illustrated fluorescent images of Pdgfra+ cell-derived adipocyte in PdgfraCreERT-tdTomato mice. Tamoxifen was injected at 3 months old to label adult Pdgfra + cells with tdTomato. Tissues were isolated at 6 months old. (J) Immunofluorescence staining of cross-sectioned tongue muscle isolated from PdgfraCreERT-tdTomato mice (6 months old) for adipocyte (perilipin-1, green) and Pdgfra cells with tdTomato+ cytoplasm. Scale bar = 70 µm. (H) Quantification of Pdgfra-derived adipocytes (adipocytes with tdTomato+ cytoplasm). n = 3. Statistical significance for (A) was determined by 2-way ANOVA and Fisher’s LSD test as a post-hoc multiple comparison test. Statistical significance for (C) was determined by 1-way ANOVA and Tukey test as a post-hoc multiple comparison test. Statistical significance for (E) was determined by Welch’s t-test. The p-values are shown above the horizontal line, which is positioned above the bars. Error bars represent the mean ± SEM
The limited differentiation potential of tongue FAPs to adipocytes led us to investigate the role of FAPs in fat infiltration of tongue muscles. Unlike other muscles, the tongue muscle contains a large number of adipocytes [3]. Based on differentiation potential of tongue FAPs, adipocytes in tongue may be derived from preadipocytes not FAPs. We found a study using PdgfraCreERT mice for lineage tracing and deletion showed that Pdgfra+ cells are the embryonic origin of adipocytes in adipose tissues but are not responsible for adult adipocyte maintenance, even with HFD [26]. However, another study testing adipocyte origin in skeletal muscles using PdgfraCreERT mice, glycerol-injury derived adipocytes in adult skeletal muscles are generated from Pdgfra+ cells [27]. To test whether Pdgfra+ cells are development and homeostasis of adipocytes in tongue muscles, we first utilized Pdgfra-H2B-EGFP mice, which express EGFP in the nucleus of Pdgfra+ cells constitutively, to determine whether Pdgfra+ cells are the developmental origin of adipocytes in tongue muscles. Similar to the previous research [26], 72% of tongue adipocytes of Pdgfra-H2B-EGFP mice contain GFP+ nuclei (Fig. 4F-4H), indicating PDGFRα⁺ cells represent the embryonic origin of adipocytes in tongue muscles. The remaining 28% of adipocytes may also contain EGFP⁺ nuclei, but our imaging analysis could underestimate this due to muscle section thickness, which might capture only part of the adipocyte. To examine the contribution of Pdgfra+ cells in adult adipocyte homeostasis in tongue, we used Pdgfra CreERT-tdTomato mouse to label Pdgfra+ cells at 10 weeks by tamoxifen injection to trace for 3 months (Fig. 4I). However, tdTomato+ cytoplasmic adipocytes were rarely found (1%) in tongue muscle sections (Fig. 4J-4K). Taken together, tongue FAPs may not be in adult tongue muscles.
HFD-induced miR-144-3p interferes with Gdf10 expression in tongue FAPs
To study how HFD reduces Gdf10 mRNA expression in tongue FAPs, we focused on the epigenetic regulation of Gdf10 by microRNAs. Using miRdb.org and TargetScan.com, online database to predict miRNAs and their target genes based on complementary sequence matching, we identified the top two miRNA candidates binding to 3’-UTR of Gdf10 gene: miR-144-3p and miR-139-5p (Fig. 5A and supplementary Fig. 4 A). Both miRNAs can bind to conserved sequence of 3’-UTR of human and mouse Gdf10 mRNA. We performed a GDF10 3’-UTR luciferase assay in HEK293 cells with those miRNAs and confirmed both miR-144-3p and miR-139-5p reduced the activity of GDF10 3’-UTR (Fig. 5B and Supplementary Fig. 4B), indicating a negative effect of both miRNAs on GDF10 mRNA expression via 3’-UTR. To determine the specific binding of miR-144-3p on GDF10 3’-UTR, we generated mutations on the middle of the seeding sequences of miR-144-3p on GDF10 3’-UTR of GDF10 3’-UTR luciferase plasmids as follows: 5’-AUGUCAUA-3’ to 5’-AUGAAAUA-3’ (marked ** in Fig. 5A). We confirmed that the activity of GDF10 3’-UTR was twice increased with mutations on seeding sequences compared to wild-type sequences using GDF10 3’-UTR luciferase assays in the presence of miR-144-3p mimics (Fig. 5C). Therefore, we concluded that miR-144-3p interferes with Gdf10 mRNA expression via 3’-UTR binding.
Fig. 5.
HFD induces miR-144-3p that regulates GDF10 mRNA expression and adipogenesis of tongue FAPs. (A) Schematic binding sites between Gdf10 mRNA and miR-144-3p. ** indicates target sequences to generate mutations for GDF10 3’-UTR luciferase assay in (C). (B) The 3’-UTR activity of GDF10 was measured by luciferase assay with control miR or miR-144-3p in HEK293 cells transfected with a plasmid containing GDF10 3’-UTR-controlled luciferase. n = 3 (C) The 3’-UTR activity of GDF10 was measured by luciferase assay with miR-144-3p in HEK293 cells transfected with a plasmid containing luciferase, which is controlled by GDF10 3’-UTR with wild-type or mutant seed sequences. n = 3 (D) miR-144-3p expression was significantly induced in tongue FAPs of HFD-fed obese mice compared to ND-fed lean mice. miR-144-3p mRNA expression was lower in males compared to females. n = 3. (E) miR-144-3p expressions were reduced with miR-144-3p inhibitor treatment in tongue FAPs from HFD-fed female mice. n = 3. (F) Gdf10 mRNA expressions were induced with miR-144-3p inhibitor treatment in tongue FAPs from HFD-fed female mice. n = 3. (G) miR-144-3p expressions were induced with miR-144-3p mimic treatment in tongue FAPs from ND-fed male mice. n = 3. (H) Gdf10 mRNA expressions were reduced with miR-144-3p mimic treatment in tongue FAPs from ND-fed male mice. n = 3. Control miR inhibitor or control miR mimic was used as a negative control for (E–H). (I) Representative Oil-Red-O image of gastrocnemius FAPs from ND-fed male mice after 10 days-culture with control miR inhibitor or miR-144-3p inhibitor. miR inhibitors (30 nM) were treated at day 0, 2, and 4 days during adipogenic differentiation. Scale bar = 100 µm. (J) Gdf10, miR-144-3p, Pparg and Ap2 mRNA expressions in gastrocnemius FAPs after 10 days-culture with control miR inhibitor or miR-144-3p inhibitor. n = 4. (K) Representative Oil-Red-O image of tongue FAPs from ND-fed male mice after 10 days-culture with control miR mimic or miR-144-3p mimic. miR mimic (30 nM) were treated at day 0, 2, and 4 days during adipogenic differentiation. Scale bar = 100 µm. (L) Gdf10, miR-144-3p, Pparg and Ap2 mRNA expressions in tongue FAPs after 10 days-culture with control miR mimic or miR-144-3p mimic. n = 4. Statistical significance for (B) was determined by 1-way ANOVA and Tukey test as a post-hoc multiple comparison test. Statistical significance for (D) was determined by 2-way ANOVA and Fisher’s LSD test as a post-hoc multiple comparison test. Statistical significance for (C, E–H, J, L) was determined by Welch’s t-test. The p-values are shown above the horizontal line, positioned above the bars. Error bars represent the mean ± SEM
Next, we investigate whether the expression of both miRNAs in tongue FAPs is changed by HFD. Expression of miR-144-3p is increased by HFD in tongue FAPs of both sexes and female FAPs contain more miR-144-3p than male FAPs by HFD (Fig. 5D). However, miR-139-5p is only increased in male FAPs, not in female FAPs, by HFD (Supplementary Fig. 4D), which led us to focus on miR-144-3p for further study. To probe if miR-144-3p regulates Gdf10 mRNA expression in tongue FAPs, miR-144-3p mimic or inhibitor was transfected to tongue FAPs from ND or HFD-fed mice, respectively. To inhibit high expression of miR-144-3p in HFD, miR-144-3p inhibitors were transfected to tongue FAPs of females with HFD (Fig. 5E). As expected, Gdf10 mRNA expression is induced by decreased miR-144-3p inhibitor (Fig. 5F). In contrast, miR-144-3p mimics reduced Gdf10 mRNA expression in tongue FAPs of ND-fed male mice (Fig. 5G-5H). These results provide valuable insights into the molecular mechanisms underlying HFD-induced miR-144-3p changes that regulate Gdf10 mRNA expression, which affects fatty muscle remodeling in the tongues of obese subjects. To determine whether miR-144-3p affects adipogenesis of FAPs, we treated GA FAPs with miR-144-3p inhibitor during adipogenic differentiation. miR-144-3p inhibitor induced Gdf10 mRNA expression but reduced adipogenesis via Oil-Red-O staining and expression of adipogenic marker genes, including peroxisome proliferator-activated receptor gamma (Pparg) and adipocyte protein 2 (Ap2) (Fig. 5I-5J). Reversely, we treated TG FAPs with miR-144-3p mimic during adipogenic differentiation to see if it induces more adipogenesis. As expected miR-144-3p mimic caused slightly increased adipogenesis and adipogenic marker gene expressions (Fig. 5K-5L). Taken together, miR-144-3p regulates adipogenic potential of FAPs via post-transcriptional regulation of Gdf10.
GDF10 overexpression reduces fat infiltration in tongue and limb muscles with HFD
Lastly, we investigated whether GDF10 overexpression in muscle can reverse fat infiltration and denervation by HFD. Mice were fed HFD for 5 months and injected AAV8-Gdf10-RFP virus or AAV8-empty-RFP virus into tongue muscles. AAV8-virus has been used to overexpress protein of interest in fibroblasts as well as skeletal muscles by intramuscular injection [25, 28]. Then, mice were fed HFD for 1 month more before tissue sampling (Fig. 6A). We confirmed the overexpression of Gdf10 mRNA by qRT-PCR (Fig. 6B). We found that HFD-induced fat infiltration in tongue muscle is reduced by GDF10 overexpression due to reduced hypertrophy and hyperplasia of adipocytes (Fig. 6C-6F).
Fig. 6.
Overexpression of GDF10 restricted HFD-induced adipocyte hypertrophy and hyperplasia in the tongue and limb muscles. (A) Body weight of HFD-fed male mice. The virus was injected into tongue muscles at 5 months of the HFD regimen and mice were euthanized at 6 months post-HFD feeding. (B, I) Gdf10 mRNA expression was significantly increased in tongue or tibialis anterior (TA) muscles of HFD-fed obese mice after Gdf10 virus injection. Control virus was used as a negative control. n = 3–4. (C, J) Immunostaining of tongue or TA cross-sections of ND and HFD-fed mice for perilipin-1 (green or cyan). Scale bar = 125 μm. (D, K) The percentage of fat area in the muscle was determined by adding up the total area occupied by adipocytes and dividing it by the total area of the muscle. n = 3–4. (E, L) The adipocyte size area was calculated by adding up the total area occupied by adipocytes and dividing it by the total adipocyte number. n = 3–4. (F, M) Adipocyte number in muscle section of each group. n = 3–4. (G) Experimental scheme for glycerol injury, HFD and virus injection into TA muscles. (H) Hind limb grip strength normalized by body weight for control and Gdf10 virus-treated mice. n = 4. Statistical significance was determined by Welch’s t-test. The p-values are shown above the horizontal line above the bars. Error bars represent the mean ± SEM
To test if GDF10 overexpression reduced adipogenesis in limb muscles with fatty remodeling, we used glycerol injury with HFD to induce adipogenesis in tibialis anterior (TA) muscles (Fig. 6G). Four weeks post-injury and 2 weeks post-AAV8-Gdf10 virus injection, we observed increased hindlimb grip strength with GDF10 overexpression. We confirmed the overexpression of Gdf10 mRNA by qRT-PCR (Fig. 6H). The GDF10 overexpression also reduced fat infiltration in TA muscles after glycerol injury with HFD (Fig. 6J-6M). Unfortunately, we cannot determine if GDF10 overexpression reduces HFD-induced denervation due to high denervation after AAV8 injection (Supplementary Fig. 5). Taken together, GDF10 can reduce fat accumulation in tongue and limb muscles.
Discussion
In recent years, the prevalence of OSA has been increasing [29, 30], but the cellular and molecular mechanisms causing OSA have yet to be researched. Obesity-induced fat deposition in the tongue of OSA patients has been proposed as a link between obesity and OSA [3, 6], and our study provides the regulatory role of GDF10 in response to obesity in tongue muscle. Our results showed HFD-induced denervation at NMJs and hypertrophy of adipocytes in the tongue muscles of mice. We found that FAPs-derived GDF10 can be the driving factor behind these changes. Our study provides new insights into the underlying mechanism of HFD-induced Gdf10 regulation by miR-144-3p in tongue FAPs, which regulate fat infiltration and NMJ integrity in obese subjects. This sheds light on the complex interplay between muscle and adipocytes in the tongue in response to obesity. By understanding the regulatory roles of GDF10 in tongue muscle tissue, we may be able to identify new therapeutic targets for treating obesity-induced OSA and other pathophysiological conditions related to obesity-induced muscle dysfunction.
FAPs are responsible for fat infiltration in skeletal muscles [31]. GDF10 has been reported as anti-adipogenic factors secreted FAPs [24]. We revealed that tongue FAPs’ adipogenic potential is limited in tongue due to very high level of GDF10 in tongue FAPs. This led us to investigate the origin of abundant adipocytes in tongue muscles. Our results with lineage tracing with PDGFRa-Cre H2B-EGFP mice and PDGFRa Cre-ERT (Tg(Pdgfra-cre/ERT)467Dbe/J) mice indicated that Pdgfra+ cells are differentiated into adipocytes during development but not adulthood adipocyte homeostasis in tongue muscles with a normal diet, which is consistent with the previous studies on Pdgfra+ cell contribution to white adipose tissues (WAT) during postnatal development with lineage tracing [26, 32]. These studies used Tg(Pdgfra-cre/ERT2)1Wdr, which was a gift from William Richardson (University College London) [33], and fed these mice with a 60% high-fat diet for 8 weeks. Combined the results from both studies show that Pdgfra+ cells mostly contribute to adipose tissues before adulthood, such as 90% contribution to WAT during postnatal day 10 (p10)- p70 [26], 25% contribution to WAT during p30-p90 [32], and rare contribution to WAT during p60-p12026, and their contribution is stimulated by a high-fat diet during postnatal development. However, the role of PDGFRa+ cells in adult adipocyte homeostasis is controversial due to contradictory results. Cattaneo et al. [34] used Pdgfra-MerCreMer (Pdgfratm1.1(cre/Esr1*)Nshk), which is considered more stringent and less prone to leakage than Cre-ERT2 due to two mutated estrogen receptor domains [35], and generated by Dr. Hiroshi Kataoka [36]. Cattaneo et al. group injected Tamoxifen into Pdgfra-MerCreMer mice at 8 weeks old (young adult), and also fed them with 60% high-fat diet for 8 weeks. Opposite to the rare incorporation of Pdgfra+ cells in P60 in both normal and high-fat diets in a previous study [26], a 20% and 30% contribution of Pdgfra+ cells in WAT by normal and high-fat diets, respectively. The impact of a high-fat diet was much less pronounced in this mouse model, such as 10% additional contribution of Pdgfra + cells in perigonadal WAT but no impact in inguinal WAT. Given controversial reports regarding the role of Pdgfra+ cells for adulthood adipocyte homeostasis, differences between our mouse model and previous mouse models and the absence of adipogenesis stimulation, such as a high-fat diet, in our lineage tracing experimental design, we cannot dismiss their potential contribution to adipocytes in tongue muscles.
Tongue muscles contain a much higher number of residual adipocytes than other muscles [3], which makes them susceptible to obesity-induced muscle fat remodeling. In our study, we showed that HFD induces hypertrophy of residual adipocytes in tongue muscles, which likely explains the reduced fat portion in the tongue and OSA symptoms by weight loss [3, 6]. Also, we showed HFD-induced denervation of tongue genioglossus muscles, which are responsible for tongue collapse during sleep in OSA patients. To explain these phenomena of HFD-induced obesity, we investigate the role of the FAPs-derived secreted factor called GDF10. GDF10 is not only known to improve neuromuscular junctional connection by stabilizing Schwann cells in aged muscles, but is also known to limit adipocyte hypertrophy [24]. Interestingly, Gdf10 mRNA expression of tongue FAPs is reduced by HFD, which connects how HFD induces denervation and fat hypertrophy in tongue muscles. Furthermore, we elucidated the mechanism of how HFD decreases Gdf10 mRNA expression via miRNA-mediated mRNA stability regulation. Up-regulation of two miRNA candidates interacting with Gdf10 mRNA 3’-UTR has been reported in animals with HFD. miR-144-3p is induced during adipogenesis [37] and in the liver and myocardium after HFD [38, 39]. Also, miR-139-5p also increased in the pancreatic islets by HFD [40]. In our study, we identified increased miR-144-3p and miR-139-5p in tongue FAPs by HFD, which explains how HFD regulates Gdf10 expression. Lastly, we proved miR-144-3p regulates adipogenesis of FAPs. Previously, miR-144-3p has been reported to promote adipogenesis via inducing C/EBPa activity and its downstream adipogenic genes [37]. Although we provide the molecular mechanism of HFD-induced tongue remodeling, further research investigating FAPs temporal and spatial dynamics in tongue muscles with HFD and confirming this mechanism with OSA patient samples will be warranted to develop potential therapeutic targets for OSA treatment.
OSA is known to be more common in men than women, with male dominance of up to three-fold [41]. Moreover, research indicates that male mice are more vulnerable to the long-term effects of an HFD on NMJ damage than female mice [42]. Our data aligns with these findings, as we have discovered that male mice had higher denervation but lower levels of Gdf10 expression in FAPs from tongue muscles compared to female mice, which suggests that there may be sex differences in the molecular mechanisms underlying the denervation of NMJs in response to obesity, further male prevalence of OSA. Although we do not fully understand how larger tongue muscle denervation of male mice were not reflected in function decline, 10% denervation may not be enough to decline muscle function in males but may induce functional decline in females.
We found that β-estradiol potentially explains the higher Gdf10 mRNA expression in females. Interestingly, Gdf10 targeting miRNAs, including miR-144-3p and miR-miR-139-3p, also showed sex differences. While miR-144-3p is increased by HFD in both male and female FAPs, female FAPs contain more miR-144-3p than male FAPs in ND and HFD conditions. However, we found the expression of miR-144-3p was not regulated by direct incubation with β-estradiol in tongue FAPs (Data not shown). Thus higher expression of miR-144-3p in female tongue FAPs may be independent on presence of β-estradiol. In addition, miR-139-5p is only increased in male FAPs by HFD and miR-139-5p levels in both sexes with ND are similar. Although the mechanism of sex differences in miRNA regulation of FAPs is unclear, these differences likely affect several biological pathways in male and female FAPs, considering multiple target mRNAs of each miRNA. For example, depleted miR-144-3p protects males, but not females, from atherosclerosis [43]. However, sex differences in adipocyte hypertrophy by HFD in tongue muscles were not observed except increased adipocyte numbers in males with 6-month HFD. Further investigating these differences may provide valuable insights into sex-specific therapeutic development for OSA.
Our study presents an innovative approach to investigating the pathophysiology of OSA by creating an animal model of obesity-induced fatty tongue in mice, which offers several advantages. First, our model provides a tool to study the cause of fatty and denervated tongue muscles as a tissue model of OSA, which cannot be explored by mimicking OSA by intermittent hypoxia [7] in animals that only provide consequences of OSA. Second, the diet-induced obese model is physiologically relevant and more valuable compared to genetic models of obesity, such as the db/db or ob/ob model, as it more closely mimics the human behavior of becoming obese in the absence of genetic modification. However, the application of our study to the OSA field is still limited because we only examined the tongue muscle, while other upper airway muscles, such as the pharyngeal muscles or soft palate, also contribute to OSA. Lastly, we failed to determine whether GDF10 overexpression can rescue HFD-induced denervation in tongue muscles. We noticed that the control virus-injected muscle had unusually high denervation (about 20%), so we decided viral vector-induced GDF10 overexpression was not suited to our purpose. It may be achieved by transgenic mice overexpressing GDF10 in FAPs [14]. Overall, our findings provide important insights into the mechanisms underlying fatty tongue development in obesity and offer a new perspective for developing therapeutic interventions for OSA.
In conclusion, our study established the HFD-induced high-fat tongue as a valuable preclinical tissue model to investigate OSA. Our findings provided new insights into the adipogenic and neuromuscular remodeling of the tongue muscle in response to HFD-induced obesity, implicating the involvement of FAPs-derived GDF10 in developing OSA. Our study proposed the underlying mechanisms of HFD regulation of GDF10 expression by miR-144-3p and tested the potential therapeutic molecule GDF10 in treating fat infiltration and denervation in tongue muscles and other obesity-induced muscle dysfunction. Overall, our research contributes to a better understanding of the complex interplay between HFD and FAPs for muscle remodeling, fat deposition, and denervation in the tongue muscles, providing a foundation for developing more effective treatment strategies for OSA.
Supplementary Information
Acknowledgements
Amanda Kim, Christopher Zeuthen, and Aislinn Trejo Romeo assisted with experiments and data analysis. We appreciate Dr. Youngmok Jang for providing Pdgfra-EGFP mice.
Authors’ contributions
SAK, CZ, and KK were involved in designing the study, conducting experiments, analyzing data, creating figures, and writing the manuscript. XX conducted experiments, analyzing data and creating figures. YD conducted experiments and data analysis. HJC was involved in designing the study, analyzing data, creating figures, writing the manuscript, and providing funds.
Funding
The work was supported by the National Institutes of Arthritis and Musculoskeletal and Skin Diseases R01AR071397 grant and the Department of Defense USAMRAA W81XW2211040 grant.
Data availability
All data to support the conclusions of this manuscript are included in the main text and supplementary materials.
Declarations
Competing interests
The authors state that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Seung Hyun Kim, Christina Xu and Kyungmin Kim Co-first author.
References
- 1.Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. 2017;(1558–3597 (Electronic)) [DOI] [PMC free article] [PubMed]
- 2.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. 2019;(2213–2619 (Electronic)) [DOI] [PMC free article] [PubMed]
- 3.Kim AM, Keenan BT, Jackson N, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37(10):1639–48. 10.5665/sleep.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169(17):1619–26. 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim AM, Keenan BT, Jackson N, et al. Tongue fat and its relationship to obstructive sleep apnea. (1550–9109 (Electronic)) [DOI] [PMC free article] [PubMed]
- 6.Wang SH, Keenan BT, Wiemken A, et al. Effect of Weight Loss on Upper Airway Anatomy and the Apnea-Hypopnea Index. The Importance of Tongue Fat. Am J Respir Crit Care Med. Mar 15 2020;201(6):718–727. 10.1164/rccm.201903-0692OC [DOI] [PMC free article] [PubMed]
- 7.Trzepizur W, Cortese R, Gozal D. Murine models of sleep apnea: functional implications of altered macrophage polarity and epigenetic modifications in adipose and vascular tissues. Metabolism. Jul2018;84:44–55. 10.1016/j.metabol.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim E, Wu F, Lim D, et al. Fibroadipogenic Progenitors Regulate the Basal Proliferation of Satellite Cells and Homeostasis of Pharyngeal Muscles via HGF Secretion. Front Cell Dev Biol. 2022;10: 875209. 10.3389/fcell.2022.875209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall NS, Wong Kk Fau - Liu PY, Liu Py Fau - Cullen SRJ, Cullen Sr Fau - Knuiman MW, Knuiman Mw Fau - Grunstein RR, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. 2019;(0161–8105 (Print)) [PMC free article] [PubMed]
- 10.Uezumi A, Ikemoto-Uezumi M, Zhou H, et al. Mesenchymal Bmp3b expression maintains skeletal muscle integrity and decreases in age-related sarcopenia. J Clin Invest. Jan 4 2021;131(1)10.1172/jci139617 [DOI] [PMC free article] [PubMed]
- 11.Hino J, Kangawa K, Matsuo H, Nohno T, Nishimatsu S. Bone morphogenetic protein-3 family members and their biological functions. Front Biosci. 2004;9:1520–9. 10.2741/1355. [DOI] [PubMed] [Google Scholar]
- 12.Hino J, Nakatani M, Arai Y, et al. Overexpression of bone morphogenetic protein-3b (BMP-3b) in adipose tissues protects against high-fat diet-induced obesity. Int J Obes (Lond). Apr2017;41(4):483–8. 10.1038/ijo.2017.15. [DOI] [PubMed] [Google Scholar]
- 13.Martí-Pàmies Í, Thoonen R, Seale P, et al. Deficiency of bone morphogenetic protein-3b induces metabolic syndrome and increases adipogenesis. Am J Physiol Endocrinol Metab. 2020;319(2):E363-e375. 10.1152/ajpendo.00362.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurosawa T, Minato K, Ikemoto-Uezumi M, Hino J, Tsuchida K, Uezumi A. Transgenic Expression of Bmp3b in Mesenchymal Progenitors Mitigates Age-Related Muscle Mass Loss and Neuromuscular Junction Degeneration. Int J Mol Sci. Sep 23 2021;22(19)10.3390/ijms221910246 [DOI] [PMC free article] [PubMed]
- 15.Cheung TH, Quach NL, Charville GW, et al. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482(7386):524–8. 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange T, Stracke S, Rettig R, et al. Identification of miR-16 as an endogenous reference gene for the normalization of urinary exosomal miRNA expression data from CKD patients. PLoS ONE. 2017;12(8): e0183435. 10.1371/journal.pone.0183435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. Dec2001;25(4):402–8. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266(17):11341–6. [PubMed] [Google Scholar]
- 19.Miggitsch C, Meryk A, Naismith E, et al. Human bone marrow adipocytes display distinct immune regulatory properties. EBioMedicine. Aug2019;46:387–98. 10.1016/j.ebiom.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. Jul2004;7(4):405–10. 10.1097/01.mco.0000134362.76653.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald G, Turiel G, Gorski T, et al. MME(+) fibro-adipogenic progenitors are the dominant adipogenic population during fatty infiltration in human skeletal muscle. Commun Biol. Jan 27 2023;6(1):111. 10.1038/s42003-023-04504-y [DOI] [PMC free article] [PubMed]
- 22.Veeger TTJ, van Zwet EW, Al Mohamad D, et al. Muscle architecture is associated with muscle fat replacement in Duchenne and Becker muscular dystrophies. Muscle Nerve. Nov2021;64(5):576–84. 10.1002/mus.27399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahdy MAA. Glycerol-induced injury as a new model of muscle regeneration. Cell Tissue Res. Nov2018;374(2):233–41. 10.1007/s00441-018-2846-6. [DOI] [PubMed] [Google Scholar]
- 24.Camps J, Breuls N, Sifrim A, et al. Interstitial Cell Remodeling Promotes Aberrant Adipogenesis in Dystrophic Muscles. Cell Rep. May 5 2020;31(5):107597. 10.1016/j.celrep.2020.107597 [DOI] [PubMed]
- 25.McGowan TJ, Lewerenz N, Maino E, Thurkauf M, Jorin L, Ruegg MA. AAV capsids target muscle-resident cells with different efficiencies-A comparative study between AAV8, AAVMYO, and AAVMYO2. Mol Ther Methods Clin Dev. Jun 12 2025;33(2):101451. 10.1016/j.omtm.2025.101451 [DOI] [PMC free article] [PubMed]
- 26.Shin S, Pang Y, Park J, et al. Dynamic control of adipose tissue development and adult tissue homeostasis by platelet-derived growth factor receptor alpha. Elife. Jun 19 2020;910.7554/eLife.56189 [DOI] [PMC free article] [PubMed]
- 27.Kopinke D, Roberson EC, Reiter JF. Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis. Cell. Jul 13 2017;170(2):340–351 e12. 10.1016/j.cell.2017.06.035 [DOI] [PMC free article] [PubMed]
- 28.Greig JA, Peng H, Ohlstein J, et al. Intramuscular injection of AAV8 in mice and macaques is associated with substantial hepatic targeting and transgene expression. PLoS ONE. 2014;9(11): e112268. 10.1371/journal.pone.0112268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. Aug2015;7(8):1311–22. 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14. 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. Feb2010;12(2):153–63. 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15(4):480–91. 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivers LE, Young KM, Rizzi M, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. Dec2008;11(12):1392–401. 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cattaneo P, Mukherjee D, Spinozzi S, et al. Parallel Lineage-Tracing Studies Establish Fibroblasts as the Prevailing In Vivo Adipocyte Progenitor. Cell Rep. Jan 14 2020;30(2):571–582 e2. 10.1016/j.celrep.2019.12.046 [DOI] [PubMed]
- 35.Verrou C, Zhang Y, Zurn C, Schamel WW, Reth M. Comparison of the tamoxifen regulated chimeric Cre recombinases MerCreMer and CreMer. Biol Chem. Dec1999;380(12):1435–8. 10.1515/BC.1999.184. [DOI] [PubMed] [Google Scholar]
- 36.Ding G, Tanaka Y, Hayashi M, Nishikawa S, Kataoka H. PDGF receptor alpha+ mesoderm contributes to endothelial and hematopoietic cells in mice. Dev Dyn. Mar2013;242(3):254–68. 10.1002/dvdy.23923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen L, Li Q, Wang J, et al. miR-144-3p Promotes Adipogenesis Through Releasing C/EBPalpha From Klf3 and CtBP2. Front Genet. 2018;9:677. 10.3389/fgene.2018.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Xin X, Yu H, et al. microRNA Expression Profiles in Myocardium of High-Fat Diet-Induced Obesity Rat. Diabetes Metab Syndr Obes. 2020;13:1147–59. 10.2147/DMSO.S248948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niitsu Y, Komiya C, Takeuchi A, et al. Increased serum extracellular vesicle miR-144-3p and miR-486a-3p in a mouse model of adipose tissue regeneration promote hepatocyte proliferation by targeting Txnip. PLoS ONE. 2023;18(5): e0284989. 10.1371/journal.pone.0284989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang K, Zhang Y, Chen C, et al. miR-139-5p mediates the palmitate-induced inhibition of insulin secretion by targeting neuronal pentraxin 1 in INS-1 cells. Acta Biochim Biophys Sin (Shanghai). 2021;53(8):1017–26. 10.1093/abbs/gmab082. [DOI] [PubMed] [Google Scholar]
- 41.Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. Oct2004;98(10):984–9. 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Pena YVI, Akaaboune M. The disassembly of the neuromuscular synapse in high-fat diet-induced obese male mice. Mol Metab. Jun2020;36: 100979. 10.1016/j.molmet.2020.100979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng J, Cheng A, Clifford BL, et al. MicroRNA-144 Silencing Protects Against Atherosclerosis in Male, but Not Female Mice. Arterioscler Thromb Vasc Biol. Feb2020;40(2):412–25. 10.1161/ATVBAHA.119.313633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data to support the conclusions of this manuscript are included in the main text and supplementary materials.