Abstract
Chronic atrophic gastritis and intestinal metaplasia (IM) are gastric precancerous conditions (GPCs) associated with an increased risk of gastric cancer. Early detection and accurate characterization of GPC are therefore crucial for risk stratification and the implementation of preventive strategies. In the absence of clear mucosal changes observed through white-light imaging (WLI) or virtual chromoendoscopy, endocytoscopy can help unveil the presence of GPC by enabling in vivo assessment of nuclear and cellular structures at ultra-high magnification. Endocytoscopy is typically performed using WLI following dye-based staining of the mucosa. In this case, we demonstrate that combining endocytoscopy with the texture and color enhancement imaging (TXI) mode substantially improves the assessment of the gastric mucosa. In a 61-year-old man undergoing esophagogastroduodenoscopy, WLI showed multifocal erythema in the stomach, without clearly visible lesions on either WLI or narrow-band imaging. Conventional endocytoscopy revealed multiple small spots of IM with characteristic changes in glandular structures, which were even more evident when using the TXI mode. Histological analysis of targeted biopsies confirmed small foci of IM in both the antrum and corpus. The patient was enrolled in a surveillance program because of his clinical background. The combination of endocytoscopy with the TXI mode significantly enhances the delineation of mucosal and cellular architecture, supporting a more accurate optical diagnosis.
Keywords: endocytoscopy, stomach, preneoplastic lesions, intestinal metaplasia, gastric atrophy, gastric cancer
Figure 1.
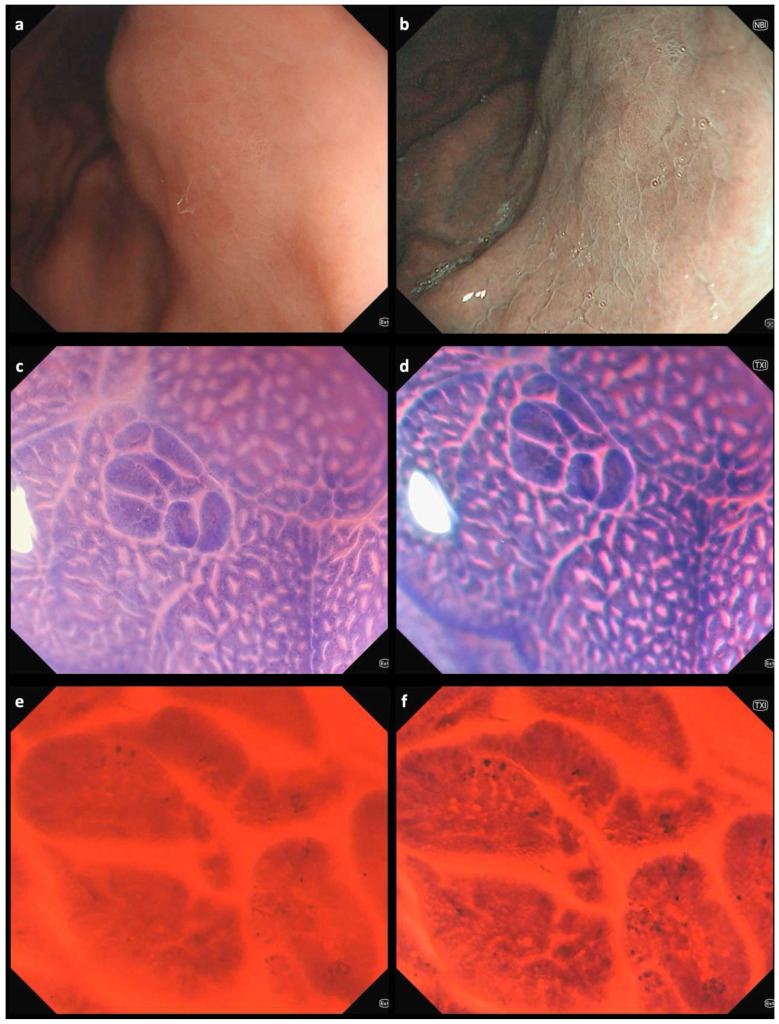
Endoscopic images of a gastric area in the stomach corpus using white-light imaging (WLI) (a) and narrow-band imaging (NBI) (b), showing mild multifocal erythema and edema. The pit pattern reveals an uneven distribution of gastric pits and the subepithelial capillary network [1,2]; collecting venules are not visible. However, no clearly delineable lesions are observed. Endocytoscopic images from the same gastric area obtained using WLI (left) and texture and color enhancement imaging (TXI) (right) modes at different magnifications: (c,d) 150×; (e,f) 520×. Endocytoscopic assessment of defined lesions is usually performed after double staining with methylene blue and crystal violet [3,4]. In the stomach, repeated staining following mucus removal using mucolytic and defoaming agents may be necessary to obtain clear endocytoscopic images [5]. In our case of a 61-year-old man referred for esophagogastroduodenoscopy due to dyspeptic symptoms, endocytoscopy facilitated the detection of intestinal metaplasia. Characteristic changes observed through endocytoscopy include narrowing of glandular lumens, increased dye uptake, and the presence of goblet cells [6,7]. These features were even more clearly visualized when endocytoscopy was combined with TXI mode (Video S1), which enhances the visualization of structure, color tone, and brightness, thus facilitating the detection of subtle mucosal changes often missed by standard WLI [8]. The patient, who also had a positive family history of gastric cancer, was included in a three-year surveillance program in accordance with current guidelines [9]. Extensive atrophic and metaplastic changes involving both the stomach antrum and corpus are associated with the highest risk of gastric cancer development [10]. These conditions are generally identifiable using modern high-resolution white-light endoscopy combined with image-enhanced modalities such as TXI, NBI, blue laser imaging (BLI), or iScan. TXI is a readily accessible feature on modern Olympus endoscopy systems, activated similarly to NBI by simply pressing a dedicated button, which enhances mucosal texture and color contrast in real time—improving the detection of subtle lesions without requiring additional equipment or significant changes to the endoscopic workflow. Endocytoscopy may serve as a complementary tool in selected cases—particularly when subtle changes remain unclear or when targeted biopsies are required in areas without obvious mucosal alterations, especially in high-risk individuals. The transition from probe-based to fourth-generation endocytoscopy has led to significant technical advancements. In the current system, the endocytoscope is fully integrated into the distal end of a standard gastroscope or colonoscope, eliminating the need for a separate probe [3]. However, despite this progress, several limitations continue to restrict the widespread adoption of the technology. These include the need for dedicated training to obtain high-quality images—typically after a minimum of 30–50 procedures—as well as the requirement for dye-based chromoendoscopy, which can be time-consuming and particularly challenging in the stomach due to mucus interference. Further development is necessary to make endocytoscopy more practical and user-friendly. In this regard, the integration of artificial intelligence is likely to play an important role in enhancing image interpretation and clinical applicability, as suggested by preliminary studies [11,12].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics15151925/s1, Video S1: Staining and endocytoscopic assessment of an area of intestinal metaplasia in the stomach corpus comparing white-light imaging and texture and color enhancement imaging modes.
Author Contributions
Conceptualization, R.V. and C.S.; Methodology, R.V.; Software, R.V. and J.R.W.; Validation, R.V. and C.S.; Formal Analysis, R.V. and C.S.; Investigation, R.V.; Resources, C.S.; Data Curation, R.V. and J.R.W.; Writing—Original Draft Preparation, R.V.; Writing—Review and Editing, C.S.; Visualization, R.V. and J.R.W.; Supervision, C.S.; Project Administration, R.V.; Funding Acquisition, R.V. and C.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the ethics committee of the Ludwig-Maximilians-Universität München (Project Number 20-875) and was conducted in accordance with current Good Clinical Practice guidelines and the Declaration of Helsinki.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This study was performed in the context of the ERANET Bavaria and Helicopredict projects (German clinical trials register—DRKS-ID: DRKS00028629) and was in part funded by the Deutsches Zentrum für Infektionsforschung, Partner Site Munich, Germany (TTU 06.715_00), as well as by the Bavarian Ministry of Science and the Arts within the framework of the Bavarian Research Network “New Strategies Against Multi-Resistant Pathogens by Means of Digital Networking—bayresq.net”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Uchima H., Yao K. Endoscopic microanatomy of the normal gastrointestinal mucosa with narrow band technology and magnification. Gastroenterol. Y Hepatol. 2019;42:117–126. doi: 10.1016/j.gastrohep.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Kurtcehajic A., Zerem E., Bokun T., Alibegovic E., Kunosic S., Hujdurovic A., Tursunovic A., Ljuca K. Could near focus endoscopy, narrow-band imaging, and acetic acid improve the visualization of microscopic features of stomach mucosa? World J. Gastrointest. Endosc. 2024;16:157–167. doi: 10.4253/wjge.v16.i3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abad M.R.A., Shimamura Y., Fujiyoshi Y., Seewald S., Inoue H. Endocytoscopy: Technology and clinical application in upper gastrointestinal tract. Transl. Gastroenterol. Hepatol. 2020;5:28. doi: 10.21037/tgh.2019.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasapolli R., Neuhaus L., Schirra J., Neumann J., Mayerle J., Malfertheiner P., Schulz C. Microscopic alterations of the gastric mucosa in preneoplastic lesions as assessed by new-generation endocytoscopy. Endoscopy. 2023;55:E998–E1000. doi: 10.1055/a-2119-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misawa M., Kudo S.-E., Takashina Y., Akimoto Y., Maeda Y., Mori Y., Kudo T., Wakamura K., Miyachi H., Ishida F., et al. Clinical Efficacy of Endocytoscopy for Gastrointestinal Endoscopy. Clin. Endosc. 2021;54:455–463. doi: 10.5946/ce.2021.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato H., Inoue H., Hayee B., Ikeda H., Sato C., Phalanusitthepha C., Santi E.G.R., Kobayashi Y., Kudo S.-E. In vivo histopathology using endocytoscopy for non-neoplastic changes in the gastric mucosa: A prospective pilot study (with video) Gastrointest. Endosc. 2015;81:875–881. doi: 10.1016/j.gie.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Chiu P.W.Y., Ng E.K.W., To K.F., Teoh A.Y.B., Lam C.C.H., Chan F.K.L., Sung J.J.Y., Lau J.Y.W. Recognition of goblet cells upon endocytoscopy indicates the presence of gastric intestinal metaplasia. Dig. Endosc. 2014;26:52–56. doi: 10.1111/den.12050. [DOI] [PubMed] [Google Scholar]
- 8.Sato T., Schmid M. TXI: Texture and Color Enhancement Imaging for Endoscopic Image Enhancement. J. Healthc. Eng. 2021;2021:5518948. doi: 10.1155/2021/5518948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinis-Ribeiro M., Libânio D., Uchima H., Spaander M.C., Bornschein J., Matysiak-Budnik T., Tziatzios G., Santos-Antunes J., Areia M., Chapelle N., et al. Management of epithelial precancerous conditions and early neoplasia of the stomach (MAPS III): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG) and European Society of Pathology (ESP) Guideline update 2025. Endoscopy. 2025;57:504–554. doi: 10.1055/a-2529-5025. [DOI] [PubMed] [Google Scholar]
- 10.Benites-Goñi H., Cabrera-Hinojosa D., Latorre G., Hernandez A.V., Uchima H., Riquelme A. OLGA and OLGIM staging systems on the risk assessment of gastric cancer: A systematic review and meta-analysis of prospective cohorts. Ther. Adv. Gastroenterol. 2025;18:17562848251325461. doi: 10.1177/17562848251325461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noda H., Kaise M., Higuchi K., Koizumi E., Yoshikata K., Habu T., Kirita K., Onda T., Omori J., Akimoto T., et al. Convolutional neural network-based system for endocytoscopic diagnosis of early gastric cancer. BMC Gastroenterol. 2022;22:237. doi: 10.1186/s12876-022-02312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H., Yang X., Tao Y., Zhang X., Huang X., Tseng C.-W. Diagnostic accuracy of endocytoscopy via artificial intelligence in colorectal lesions: A systematic review and meta-analysis. PLoS ONE. 2023;18:e0294930. doi: 10.1371/journal.pone.0294930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.