Abstract
The fungal component of microbiota, known as the mycobiome, inhabits different body niches such as the skin and the gastrointestinal, respiratory, and genitourinary tracts. Much information has been gained on the bacterial component of the human microbiota, but the mycobiome has remained somewhat elusive due to its sparsity, variability, susceptibility to environmental factors (e.g., early life colonization, diet, or pharmacological treatments), and the specific in vitro culture challenges. Functionally, the mycobiome is known to play a role in modulating innate and adaptive immune responses by interacting with microorganisms and immune cells. The latter elicits anti-fungal responses via the recognition of specific fungal cell-wall components (e.g., β-1,3-glucan, mannan, and chitin) by immune system receptors. These receptors then regulate the activation and differentiation of many innate and adaptive immune cells including mucocutaneous cell barriers, macrophages, neutrophils, dendritic cells, natural killer cells, innate-like lymphoid cells, and T and B lymphocytes. Mycobiome disruptions have been correlated with various diseases affecting mostly the brain, lungs, liver and pancreas. This work reviews our current knowledge on the mycobiome, focusing on its composition, research challenges, conditioning factors, interactions with the bacteriome and the immune system, and the known mycobiome alterations associated with disease.
Keywords: mycobiome, immune cells, fungal receptors, cancer, infection, inflammation, autoimmunity
1. Introduction
The human body is a complex ecosystem hosting symbiotic, commensal, and pathogenic microbial relationships. The human microbiota, defined as the consortium of living microorganisms that reside in the human body, differs between individuals and body sites. It is subject to constant change, as its diversity is influenced by the environment of each body compartment. The microbiome refers to the collection of microbial genomes, metabolites, and structural elements within the microbiota [1]. So, each body compartment, such as the skin and the respiratory, gastrointestinal (GI), and genitourinary tracts, presents its own specific microbiome profile [2]. Most studies focus on the bacterial communities that constitute the majority of the human microbiota, but other lesser-studied microorganisms, such as fungi, archaea, viruses, and protists, also participate in that ecosystem with an impact on health and physiological homeostasis. In this context, the mycobiome, namely the fungal component of the microbiome, has recently gained much interest for its implications in metabolic disorders, autoimmunity, and cancer.
This review explores the mycobiome-immune axis with a particular focus on the immunomodulatory properties of fungal communities, the underlying mechanisms, and the impact of this interaction in health, disease, and in fungal-based therapeutic strategies.
2. Components of a Healthy Mycobiome
The current lack of consensus on what constitutes a healthy mycobiome is due to its sparsity compared to the bacteriome, its susceptibility to external factors, and its temporal, interpersonal, and intrapersonal variability [3,4]. This, together with specific methodological challenges, results in under-exploration and limited data at hand on the human mycobiome. This section will deal with the mycobiome details we have so far for the different human fungal niches. A summary of the most frequent taxa in each body niche is displayed in Table 1.
2.1. Gastrointestinal Tract
The GI microbiota has been extensively studied. Gut microbiota colonization varies throughout the GI tract in a continuum of increasing microbial densities, with the stomach and the duodenum being less populated segments, gradually increasing in the jejunum and ileum, and culminating with the highest concentration in the colon [5].
The Human Microbiome Project (HMP) [6] investigated mycobiome composition in a large cohort of healthy individuals through fungal Internal Transcribed Spacer 2 (ITS2) and 18S rRNA sequencing, revealing fungi mainly from the Ascomycota and Basidiomycota phyla in fecal samples [4]. It was suggested that both phyla, which are also dominant in skin and vaginal microbiota, posed an advantage in mammalian hosts. Additionally, the project showed that the most prevalent fungal genera included Saccharomyces, Malassezia, Candida, Cyberlindnera, Penicillium, Cladosporium, Aspergillus, Debaryomyces, Pichia, and Clavispora, with a remarkable overall dominance of the Saccharomyces genus. Other studies, including smaller or disease-specific cohorts, also identified Cryptococcus, Trichosporon, and Galactomyces as common mycobiome participants in the gut [7]. Most studies are carried out on readily available fecal samples, but samples from the intestinal mucosa exhibit a more stable fungal community. The species Rhodotorula mucilaginosa, Galactomyces geotrichum, Candida albicans, Candida dubliensi, Septoria epambrosiae, Cladosporium carnescens, Cladosporium cladosporioides, Bulleracrocea spp., Raciborskiomyces longisetosum, and Penicillium ialicum are also detectable in this setting [8].
2.2. Oral Cavity
The oral cavity mycobiome, one of the most versatile microbiomes, holds significant importance since disruption of the oral microbiome often underlies oral disease, especially in immunocompromised individuals [9]. The predominant fungal phyla in the oral cavity include Ascomycota, Basidiomycota, Glomeromycota, and Mucoromycota [10], with a high prevalence of the genus Candida from the phylum Ascomycota showing, furthermore, an age-dependent abundance. Most studies also include Cladosporium, Aureobasidium, and Saccharomycetales as frequently identified taxa with Aspergillus, Fusarium, and Cryptococcus present in lower frequencies [11].
2.3. Respiratory Tract
In the respiratory tract mycobiome, the conducting segment (from nose to bronchi) is constitutively colonized, whilst the respiratory portion (from bronchioles to alveoli) remains predominantly sterile or with minimal microbial load [2]. The lung mycobiome from healthy individuals predominantly comprises Aspergillus species, and this presence is strongly associated with environmental factors [12]. Other predominant genera include Cladosporium, Eurotium, and Penicillium [13,14], but in other cohorts, Candida and Saccharomyces are also highlighted as relevant groups [15].
2.4. Skin
The skin is colonized by numerous commensal microorganisms, of which 10% correspond to fungal species [16]. The composition of the skin mycobiome varies depending on gender, age (changing greatly during puberty), and body site (due to relative humidity variations) [2,10]. As in the GI tract, Ascomycota and Basidiomycota are the main resident phyla, with the basidiomycetous yeast Malassezia being the most recurrent skin colonizer, located at the inner side of the elbows, back, outer auditory canal, nostril, and some facial areas [17,18].
2.5. Genitourinary Tract
The urinary tract of healthy individuals is predominantly sterile except for the urethra, which is significantly more colonized in women [10]. Preliminary characterization of urinary fungal communities from healthy volunteers showed vast fungal interindividual variability, rendering the identification of a single dominant taxon inviable [19]. Further research is henceforth required to establish a specific profile of the urinary mycobiome beyond the more commonly known urinary tract infections (UTIs).
Conversely, the vaginal microbiome is largely assembled by bacterial and fungal species, mostly Lactobacillus and C. albicans, respectively. Whereas C. albicans commonly colonizes the vaginal lumen asymptomatically, symptomatic infection can result from overgrowth, leading to vulvovaginal candidiasis (VVC), the most prevalent human Candida infection [20]. Other taxa such as Saccharomycetales, Davidiellaceae, Cladosporium, and Pichia are also present in the vaginal mycobiome [21,22].
Table 1.
Most frequent taxa present on each body compartment.
| Body Niche | Frequently Reported Fungal Taxa | Relative Abundance | Study |
|---|---|---|---|
| Gastrointestinal tract | Saccharomyces cerevisiae | 13.9% | [23] |
| Candida spp. | 0.5% | ||
| Penicillium | <1% | ||
| Aspergillus | 25.7% | ||
| Debaryomyces udenii | 19.9% | ||
| Cladosporium | 6.1% | [24] | |
| Pichia | 18.9% | ||
| Oral cavity | Malassezia spp. | 37.7% | [25] |
| Epicoccum spp. | 33.8% | ||
| Candida/Pichia spp. | 9.6% | ||
| Cladosporium/Davidiella spp. | 3.0% | ||
| Alternaria spp. | 1.9% | ||
| Respiratory tract | Saccharomyctes | 48.1% | [12] |
| Microsporidia | 23.6% | ||
| Dothideomycetes | 16.6% | ||
| Cryptomycota | 3.0% | ||
| Chytridiomyctes | 3.0% | ||
| Skin | Malassezia restricta | 89.5% | [26] |
| Malassezia globosa | 2.90% | ||
| Vaginal tract | Candida albicans | 63.50% | [27] |
| Leptosphaerulina chartarum | 9.90% | ||
| Clavispora lusitaniae | 9.10% |
3. Methods and Limitations in Mycobiome Research
The significance of the human mycobiome cannot be understated, but admittedly, it has received limited research attention compared to bacterial communities. This is illustrated by the fact that less than 3% of the total microbiome research deals specifically with its mycobiome component [28], which is, in turn, a direct consequence of the limitations inherent to the taxonomic methods routinely applied.
Traditionally, fungal composition has been studied with culture-dependent methods such as biochemical assays, microscopy, and growth observations in cultures [29]. This allowed comprehensive phenotypic characterization and the isolation of strains of interest, but it was severely limited by the so-called “culturability bias”, resulting in a significant underestimation of true fungal diversity and an overrepresentation of fast-growing, culturable species that were viable in the chosen growth conditions (media, temperature, oxygen) [30]. This approach was also labor-intensive and time-consuming, limiting the number of samples that could be analyzed. The advent of molecular techniques, particularly DNA sequencing, revolutionized mycobiome studies by enabling culture-independent assessment of fungal diversity. PCR-based amplicon sequencing of the Internal Transcribed Spacer (ITS) region of the ribosomal RNA (rRNA) gene cluster is the most widely adopted approach [31], together with 18S rRNA sequencing. The ITS approach detects both culturable and unculturable fungi, providing a more comprehensive view of the fungal diversity in a sample. Next-generation sequencing (NGS) and bioinformatics analysis have enabled large-scale studies and the simultaneous analysis of thousands of samples. However, there are no truly universal primers for ITS. Existing primer pairs are designed to amplify a broad range of fungi, but they still exhibit biases, preferentially amplifying certain taxa over others, leading to an inaccurate representation of community composition. A particular problem is that the ITS region varies in length and copy number across fungal species, complicating accurate quantification and taxonomic assignment. The resolution provided by ITS sequencing is adequate at the genus level, but the accuracy is inherently dependent on the quality and completeness of reference databases (e.g., UNITE, NCBI GenBank). Additionally, the identification of cryptic species, defined as species that are indistinguishable in terms of morphology but genetically different [32], represents a major challenge for ITS. These sibling or cryptic species usually differ in only a few nucleotide positions, and these small differences can be wrongly attributed to intragenomic variation, leading to misidentification [33]. Thanks to recent drops in sequencing costs and advanced bioinformatics tools, a larger number of studies now use whole-genome shotgun (WGS) sequencing that interrogate a complete DNA set in a sample. This approach provides a more unbiased quantification and offers the potential to detect the presence of all microorganisms in a sample, including fungi, alongside bacteria, archaea, protists, and viruses. Furthermore, it offers better taxonomic resolution and enables the inference of functional capabilities. However, the low fungal content in the human microbiome continues to be a hurdle, requiring deeper sequencing to obtain sufficient fungal reads for robust analysis, making WGS sequencing less accessible for large-scale mycobiome profiling compared to amplicon sequencing. Analyzing large WGS datasets is computationally complex and labor-intensive, requiring substantial computing power and specialized bioinformatics pipelines [34]. A particularly challenging limitation in mycobiome WGS is the incomplete fungal species reference databases, far less comprehensive than bacterial ones [35]. This hinders accurate taxonomic assignment of novel or less-studied fungi and limits the functional annotation of their genes.
Accurate characterization of the mycobiome also hinges critically on our ability to extract and quantify fungal DNA. The presence of a thick fungal cell wall composed of chitin, glucans, and mannoproteins constitutes a barrier for DNA extraction [36], often requiring specific DNA extraction protocols to ensure efficient and unbiased fungal DNA. Inadequate cell lysis can result in an overall underrepresentation of fungi with respect to other microbes, or to certain fungal taxa. For instance, DNA extraction protocols may vary in yield efficiency on yeast or filamentous fungi, leading to a skewed perception of community composition and diversity. Another important challenge is the typically scarce fungal proportion of the total microbial biomass in the human microbiome. This means that fungal DNA is often present in minute quantities relative to bacterial or host DNA, which means fungal DNA is subject to higher stochastic variations and is harder to quantify precisely. To mitigate these challenges, a combination of mechanical, enzymatic, and chemical lysis methods is typically employed. Mechanical lysis, such as bead-beating with appropriately sized and dense beads (e.g., zirconia/silica beads), is often crucial for physically disrupting tough fungal cell walls. Optimization of bead-beating parameters (speed, duration, number of cycles) and enzyme concentrations is critical for maximizing yield and minimizing bias. The use of commercial kits can streamline the process, but even these often require user-specific adaptations and rigorous quality control, including the use of positive (mock communities) and negative controls, to ensure accurate representation of the fungal community [37].
The mycobiome composition is likewise affected by factors such as age, diet, host immunity, genetics, and interactions with other microorganisms, leading to diversity and stability variations [29]. In this sense, discriminating between foodborne fungi contaminants and commensal fungi that colonize the gut would benefit from specific tools and biomarkers (e.g., procalcitonin and neutrophil count) [29] beyond a clinical criterion that recognizes colonizers as asymptomatic.
4. Factors Influencing Mycobiome Composition
Many factors continuously shape mycobiome composition, including diet, antibiotics, antifungals, and other drugs, as well as host factors such as individual genetics, age, or immune status. Subsequently, fungal analyses also need to be studied in a sociodemographic population context [38].
Gut mycobiota colonization starts at birth with an increase in fungal diversity during childhood, which decreases at the start of adulthood [28] (Table 2). Neonatal gut mycobiome colonization is mainly determined by the delivery mode. Vaginally delivered infants acquire vaginal fungal colonizers (e.g., Candida and Pleosporales) and a more diverse mycobiome, whilst those delivered by caesarean section acquire skin-related fungi (e.g., Malassezia) [39,40]. The feeding method also modulates colonization, as detectable levels of Malassezia, Davidiella, Sistotrema, and Penicillium have been commonly found in breast milk [38]. The influence of breastfeeding on mycobiota composition has recently become an active area of research.
Table 2.
Predominant age-dependent gastrointestinal fungal species.
| Age Group | Predominant Fungal Species | Study |
|---|---|---|
| 10 days to 3 months after birth | Rhodotorula mucilaginosa and Debaryomyces hansenii (from breast milk or formula) | [41] |
| >1–2 years | Ascomycota, Zygomycota, and Basidiomycota, with Candida species and Saccharomyces cerevisiae of Ascomycota being the most abundant due to the introduction of solid foods. | [41] |
| Adults | Candida spp., Saccharomyces, and Cladosporium | [3] |
| Elders (>65 years) | Penicillium, Candida, Aspergillus, and Saccharomyces | [42,43] |
After colonization and in the span of a lifetime, different factors shape the mycobiome, with an important role attributed to dietary habits [44]. In this context, high levels of Candida correlate with carbohydrate intake, whereas Aspergillus spp. becomes scarce following short-chain acid consumption [45]. It has also been reported that plant-based diets lead to increased Candida spp., whereas animal-based ones correlate with enriched Debaryomces spp. and Penicillum spp. [46].
In terms of sex differences, the increased abundance and diversity of fungal isolates in females, as assessed by culture-based techniques [47], are corroborated by separate mycobiome clusters from female and male samples following metagenomic analysis, suggesting a sex bias in fungal communities that may reflect hormonal or diet-based influences.
In line with general microbiota, other factors in addition to hormones or diet, such as body mass index (BMI), occupation, autoimmune diseases, and the use of probiotics, are known to impact mycobiome composition [44].
5. The Mycobiome’s Interactions
The microbiota can be seen as an intricate system where all its components, including the mycobiota, engage in continuous interactions among themselves and with the host to maintain homeostasis. A disruption to this delicate balance may lie behind a number of diseases, as discussed in Section 6. But let us first look at the nature of the mycobiome interactions with bacteria and the immune system.
5.1. Interactions Between Fungi and Bacteria
Studying the interactions between fungi and bacteria generally involves the induction of gut dysbiosis by antifungal or antibacterial treatments. The mouse model of colitis induced by dextran-sulfate-sodium serves as a good example of the impact of these relationships, where antifungal drugs reduce fungal diversity while promoting pathogenic bacteria and exacerbating inflammation [48]. Contrary to this, when an antibiotic is used to induce dysbiosis, commensal fungi, including C. albicans and S. cerevisiae, functionally replace the bacteriome [49].
The bacteriome–mycobiome relationship can also provide health benefits. This is illustrated by the secretion of enzymes from Saccharomyces boulardii that inhibit Clostridium difficile [50] and Escherichia coli toxins [51]. Similarly, fatty acid metabolites secreted by commensal bacteriome impair the TOR pathway that normally activates C. albicans hyphae formation and colon invasion [52]. It is also widely acknowledged that lactic acid production by Lactobacillus species can protect from vaginal candidiasis [53,54,55].
Interactions between fungi and bacteria can also impact metabolic disorders, which occasionally exert negative effects on the host, as would be the case for the survival of Helicobacter pylori in the stomach’s acidic pH by residing within vacuoles in C. albicans cells [56].
The case of mixed fungal–bacterial biofilms (MFBBs) illustrates a different type of interaction that occurs in infectious keratitis between Staphylococcus aureus and Aspergillus fumigatus [57], when the fungus serves as a carrier to S. aureus, facilitating epithelial penetration [58]. Other MFBBs occur in the oral cavity between C. albicans and Streptococcus, typically associated with dental caries and more severe oropharyngeal pathologies [59], where SspA and SspB streptococcal adhesins interact with the Als3 protein from C. albicans to promote colonization [60]. Mutualistic relationships in MFBBs examples are abundant, including in vitro formation of biofilms between Trichosporon asahii and Staphylococcus simulans, C. albicans, and Citrobacter freundii [61] or Candida tropicalis, Serratia marcescens, and E. coli [62], where bacterial lipopolysaccharide production enhances fungal biofilm maturation [63,64].
5.2. Mycobiome Interactions with the Immune System
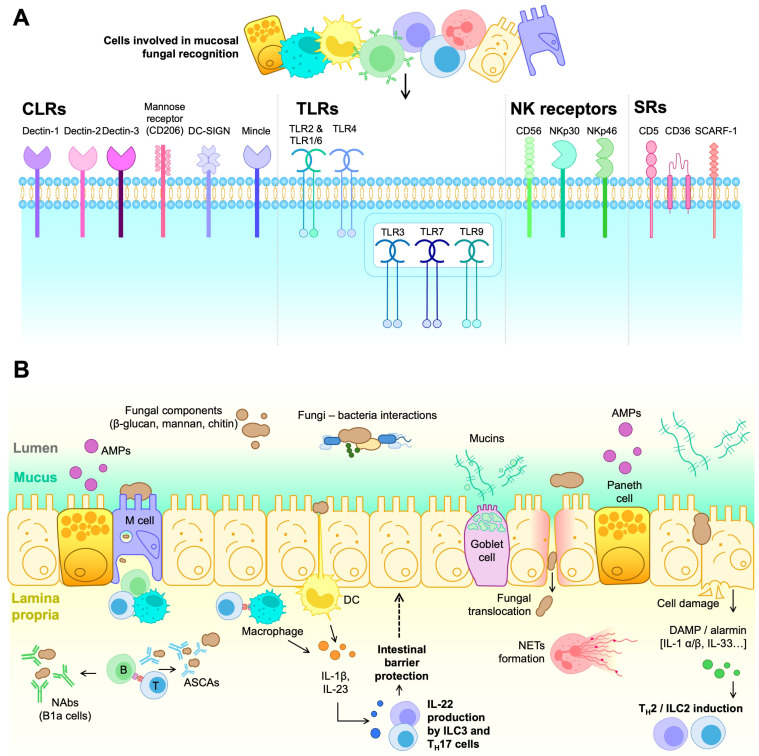
A significant part of the mycobiome’s impact relies on specific interactions with the immune system, leading to beneficial or deleterious effects in the host, as schematically depicted in Figure 1.
Figure 1.
Immune cell receptors and cell-mediated responses contributing to fungal recognition and overgrowth control in the intestinal mucosa. (A) Structurally and functionally diverse families of pattern recognition receptors (PRRs) expressed by different innate and adoptive immune cell types well-represented in the intestinal mucosa and involved in fungal recognition (i.e., CLRs, TLRs, NKRs, and SRs). (B) Cell-mediated responses against fungi first involve different cell types from the intestinal mucosal barrier, including enterocytes, Goblet cells, Paneth cells, and M cells involved in alarmin release, mucin production, AMP production, and transcytosis processes (sample and transport antigens/pathogens from the luminal surface to the sub-epithelium), respectively. In the lamina propria, DCs and macrophages can engage in fungal antigen presentation to T cells and participate in the antifungal response via cytokine secretion and phagocytosis of harmful fungal particles. In this context, TH17 and ILC3 responses can be triggered, ultimately leading to IL-22 release that can contribute to the integrity of the intestinal barrier. On the other hand, alarmins (e.g., IL-1β, IL-33) and DAMPs released in response to epithelial cell aggression or damage can trigger TH2 and ILC2 responses. Additionally, exposure to whole fungal cells and or particles can engage antifungal responses involving neutrophil activation (NETs formation) as well as T cell-dependent and independent B cell activation (production of ASCAs and NAbs by conventional or innate-type B1a cells, respectively).
In general, fungal recognition by the immune system relies on germ-line encoded receptors, the so-called pattern recognition receptors (PRRs). PRRs bind to conserved structures shared by different microbes and absent from the host. The PRR-ligand complex triggers immediate inflammatory responses (danger signals), contributing to the subsequent activation of innate and adaptive immune responses. The best-characterized PRRs in antifungal immunity are the C-type lectin receptors (CLRs), primarily expressed in monocytes, macrophages, dendritic cells (DCs), and granulocytes [65]. In this context, Dectin-1 stands out, as it specifically recognizes the β-1,3-glucans in fungal cell walls [66]. Particularly, Dectin-1 co-localizes and interacts with the Toll-like receptor 2 (TLR2), another membrane-bound PRR, synergistically over-activating both receptors to turn on tyrosine phosphorylation [67,68]. Additionally, Dectin-1 associates with Galectin-3 (a soluble PRR) in macrophages, which mediates discrimination between pathogenic strains such as C. albicans and non-pathogenic ones such as S. cerevisiae [69].
Mannose polymers are recognized by CLRs such as Dectin-2, Mannose receptor (MR), Mannose-binding lectin (MBL), Mincle, and DC-SIGN (for Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin) [70]. In this context, Dectin-2 also heterodimerizes with Dectin-3 to bind α-mannans more effectively during fungal infections [71].
Downstream of several C-type lectin receptors (CLRs), we can find caspase recruitment domain-containing protein 9 (CARD9), a cytosolic adaptor molecule critical in antifungal immunity. Upon receptor engagement, CARD9 promotes the activation of the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways, which in turn promote the production of pro-inflammatory cytokines and reactive oxygen species (ROS), as well as the formation of neutrophil extracellular traps (NETs) [72]. NETs contain neutrophil nuclear DNA and histones that trap and kill extracellular pathogens by phagocytosis. Additionally, CLR’s signaling regulates T cell activation and differentiation through various processes: (i) antigen recognition and presentation to prime naïve CD4+ T cells via class II major histocompatibility complex (MHC-II) molecules; (ii) induction of co-stimulatory molecules (e.g., CD40, CD80 and CD86) that support peptide-MHC/TCR-mediated activation; and (iii) release of cytokines and chemokines that promote CD4+ T cell differentiation into specific effector helper (TH) and regulatory (Treg) T cell subsets [73].
Toll-like receptors (TLRs) constitute another structurally different group of PRRs, some of which recognize fungal structures and trigger intracellular signals through adaptor proteins such as myeloid differentiation primary response 88 (MyD88) and TIR-domain-containing adapter-inducing IFN-β (TRIF) [74]. TLRs are broadly distributed across tissues and can be found in innate immune cells (e.g., monocytes, macrophages, neutrophiles, DCs, natural killer cells (NK), mast cells, eosinophils), non-hematopoietic cells (epithelial and endothelial cells, adipocytes, fibroblasts), and adaptive immune cells (T and B lymphocytes) [75]. TLR’s anti-fungal immune responses depend on the contact with fungal components and indirect association with other PRRs. For example, TLR3, TLR7, and TLR9 sense fungal RNA and DNA, leading to neutrophil recruitment, cytokine production, and clearance of pathogenic fungi [76], whilst TLR2 and TLR4 sense zymosan (a β-glucan- and mannan-rich particle derived from S. cerevisiae) and glucuronoxylomannan (GXM) from C. neoformans’s capsule, respectively, and couple with the MyD88 pathway and activate NF-kB and mitogen-activated protein kinase (MAPK), which induce IL-12, TNF-α, TGF-β, and IFN-γ production [77].
Beyond the roles of CLRs and TLRs in fungal recognition, other immune receptors contribute to antifungal defense. NK cells’ CD56, NKp30, and NKp46 receptors sense fungal components to initiate immune responses. The CD56 molecule interacts with A. fumigatus through galactosaminogalactan recognition [78], while the NKp30 and NKp46 members of the natural cytotoxicity receptor (NCR) family recognize β-1,3-glucans and fungal adhesins, respectively [79,80]. This interaction activates NK cells, cytotoxicity, and cytokine production to eliminate fungal pathogens, complementing the function of CLRs and TLRs in maintaining host defense.
Scavenger receptors (SRs), another group of PRRs, recognize fungi alongside TLRs, triggering phagocytosis, intracellular signaling, and inflammatory cytokine and chemokine production. Specifically, the CD5 SR on all T cells and some B cells, macrophages, and DC subsets, interacts with β-1,3-glucans in the fungal wall with a similar affinity to Dectin-1 [81]. The immunotherapeutic use of soluble human CD5 (shCD5) in mouse models of C. albicans and Cryptococcus neoformans fungal sepsis increased survival, concomitantly with reduced fungal loads, increased IFN-γ expression, and leukocyte infiltration in target organs (namely, spleen and kidney for C. albicans, lung and brain for C. neoformans) [82]. Moreover, the addition of shCD5 to ex vivo C. albicans-splenocyte co-cultures increased IFN-γ and TNF-α production, activating macrophage and neutrophil antifungal cytotoxic responses [82].
CD36 and SCARF1 (for Scavenger receptor class F member 1; also named SREC-1 or SR-F1) are type I transmembrane SRs that recognize multiple endogenous and exogenous ligands such as modified low-density lipoproteins (LDLs). They are mainly expressed in macrophages, DCs, and endothelial cells and act as co-receptors for TLR2 signaling in antifungal responses [83]. Notably, both molecules are essential for C. neoformans and C. albicans infection to mediate cytokine response and macrophage binding [84].
Fungal components first encounter PRRs at the mucocutaneous epithelial barrier and associated lymphoid system. In the skin, the epidermal layer, made up of dead keratinocytes and inert hydrophobic components, maintains skin homeostasis [85]. This layer is underlined by the stratum granulosum, characterized by tight junctions between keratinocytes renewed by the stem cells hosted at the stratum basale. The dermal extracellular matrix harbors nerve fibers and vascularization to enable immune cell infiltration during fungal infection. In addition to acting as a physical barrier, epithelial cells engage in antifungal responses by producing both anti-microbial peptides (AMPs) with fungitoxic capacity and pro-inflammatory cytokines [86], including IL-1α, IL-1β, IL-8, G-CSF, and GM-CSF in responses against C. albicans. Additionally, Langerhans cells present in the epithelial layer function as antigen-presenting cells (APCs) to generate antigen-specific TH17 cells upon C. albicans infection [87]. Dermis-resident immune cells include macrophages, mast cells, and γδ and αβ T cells. Specifically, γδ T cells have a precise role as the source of IL-17A in response to infection by the commensal Malassezia yeast [88].
In the case of mucosae from GI, urogenital, and respiratory tracts, AMPs and mucins secreted by goblet cells act as the first line of defense at the epithelial barrier [89]. Additionally, Microfold (M) cells—a highly specialized epithelium in luminal antigen phagocytosis and transcytosis—act as the entry portal for C. albicans into the intestinal epithelial barrier by specifically invading these cells [90]. Despite not showing specific degranulation upon fungal stimuli [91], Paneth cells can engage in intestinal fungal recognition by releasing specific AMPs, such as the YY peptide for Candida spp. inhibition [92,93].
However, the key drivers of fungal immunity in the mucosae are mononuclear phagocytes (MNPs), especially those expressing CX3CR1 involved in TH17 and IgA responses, and infiltrating neutrophils, which engage in phagocytosis and neutrophil extracellular trap (NET) formation [94]. Fungal antigen recognition by macrophages via Dectin-3 maintains homeostasis with the intestinal commensal mycobiota. Specifically, mice lacking Dectin-3 present an increased fungal burden that induces glycolysis in macrophages, subsequently leading to IL-7 secretion. IL-7 overproduction enhances IL-22 secretion by group 3 Innate Lymphoid Cells (ILC3s), which ultimately promote colon cancer, highlighting eubiosis and optimal fungal recognition in disease [95]. Conversely, IL-22 may benefit fungal response and homeostasis. Specifically, mucosa-associated fungi (MAF) reduce intestinal permeability and protect against intestinal injury thanks to IL-22 secretion by TH17 and ILC3 cells [96]. The role of ILCs in mucosal immunity has been described [97]. While ILCs include several subtypes (i.e., ILC1, ILC2, and ILC3), only ILC3 is involved in fungal responses. Thus, IL-23 and IL-1β secretion by CD11c+ myeloid DCs leads to the production of IL-22 by ILC3, which helps maintain intestinal barrier integrity [98].
In any case, failed PRR responses lead to cell destruction, with damage-associated molecular patterns (DAMPs) and alarmins signaling other responses and amplifying inflammation. These alarmins include protein and non-protein structures such as IL-1β and IL-1α, essential in various invasive fungal infections [99,100]. Additionally, the alarmin IL-33 is known to stimulate IL-4, IL-5, and IL-13 production by ILC2 cells, contributing to fungal-derived allergic inflammation [101].
Fungi like S. cerevisiae and C. albicans notably regulate the immune response by inducing trained immunity (innate immune memory) and reprogramming monocytes, all of which maintain gut homeostasis and protect against invading pathogens [29]. Specifically, C. albicans is recognized by macrophages via CLRs and stimulates TH17 responses, inducing TH17 cells and circulating IL-17-reactive neutrophils [76]. This response helps memory T cell formation that cross-reacts with species that share common T cell receptor (TCR) sequences, such as Candida and S. aureus, consequently blocking the invasion by exogenous forms of these microorganisms. In addition, TH17 cells induced by C. albicans in the gut can be expanded in the lungs through cross-reactivity to A. fumigatus [73].
Similarly, the mycobiome can modulate B cell activity to recognize fungal particles in a T cell-dependent manner (via BCR and CD40/CD40L co-stimulation) or in a T cell-independent manner via TLR recognition [102]. S. cerevisiae and/or C. albicans induce anti-S. cerevisiae IgA and IgG antibody production (ASCAs), that mediate fungal pathogen clearance via mannan recognition [103]. Additionally, B cells promote MHC-II-restricted antifungal TH17 responses in an antibody-independent manner [104]. Interestingly, CD5-expressing B1 cells produce polyreactive natural antibodies (NAbs) against fungal molecules and engage in macrophage-induced opsonization [105].
6. The Gut Mycobiome in Health and Disease
The gut mycobiome determines protection or susceptibility to disease by helping configure the host’s immune system and therefore, dampening or promoting inflammatory responses in a niche-dependent mode.
6.1. GI Tract Diseases
Inflammatory bowel diseases (IBDs), including UC and Crohn’s disease (CD), have been associated with gut mycobiota dysbiosis. As previously stated, C. albicans modulates TH17 responses, which are normally limited to the intestines and the skin under homeostatic conditions, thus contributing to local inflammatory disorders (e.g., IBD) when dysregulated [106].
In fecal samples from IBD patients, the C. albicans abundance doubled in relation to controls, increasing concomitantly with disease flares and reversed during remissions, suggesting an inter-dependent co-relationship with the microorganism [107]. Other findings highlight the connection between IBD and mycobiome dysbiosis. High levels of antibodies against fungal cell wall sugars and reduced IgG responses were observed in CD carriers of a mutated form of the chemokine receptor CX3CR1, and Debariomyces hansenii expansion in the mucosal wounds of these patients impaired their healing [108]. Moreover, single-nucleotide polymorphisms (SNPs) of genes involved in fungal recognition in humans (e.g., CLEC7A encoding Dectin-1 and CARD9, both involved in TH1 and TH17 cell differentiation) [109], and genetic ablation of CX3CR1 in mouse mononuclear phagocytes (needed for initiating antifungal immune responses) were correlated with worse UC [110]. Furthermore, the fungal/bacterial diversity ratio increases in samples from CD patients, suggesting that the GI environment of these patients is better suited to fungal colonization [111,112]. CARD9 protects against pathogenic fungi and also regulates the normal balance of the mycobiome. This adaptor molecule has been involved in the control of gut microbiota metabolism, and its deficiency leads to shifts in microbiota composition and impaired immune signaling, affecting susceptibility to inflammatory bowel diseases (IBD) [113].
The presence of mycotoxins, secondary metabolites produced by fungal species such as Aspergillus, Penicillium, and Fusarium, has also been related to alterations in gut microbiota composition, often leading to dysbiosis and promoting IBD pathogenesis [114].
It is worth noting that S. cerevisiae relieves gastroenteritis and adherent-invasive E. coli-induced colitis in mice and also alleviates IBD abdominal pain in humans [115,116].
Celiac disease is also associated with mycobiome dysbiosis. C. albicans hyphal wall protein resembles the T cell gliadin epitopes and is a transglutaminase substrate [117], so its presence increases the production of anti-gliadin and anti-transglutaminase antibodies, becoming a potential causal trigger for the disease under chronic inflammation conditions. However, when homeostasis is reconstituted, C. albicans interaction with mast cells can alleviate celiac symptomatology [118].
6.2. Neurological Disorders
Multiple neurological disorders have been associated with the mycobiome, with research suggesting a potential link between mycobiome dysbiosis and schizophrenia, autism, and multiple sclerosis.
Gut microbiota influences brain functions through the autonomic nervous system, cytokines, metabolic products (e.g., tryptophan, GABA, and acetylcholine), and by modulating the hypothalamus-pituitary-adrenal axis. This gut–brain connection has been correlated to depression, autism, Parkinson’s disease, Alzheimer’s disease, and eating disorders [119].
Some findings, like Candida’s ability to regulate this axis [120], lead to the hypothesis that gut fungi could favor mental disease progression. In this regard, Candida kefyr alleviated experimentally induced autoimmune encephalomyelitis in mice [121], schizophrenia patients presented changes in gut mycobiome composition and diversity [122,123], and neurons containing fungal cells have been observed in patients with Alzheimer’s disease [124].
6.3. Lung Disorders
Gut mycobiome dysbiosis has been widely associated with allergic airway disease and pulmonary pathologies [125,126]. The link between the gut mycobiome and the lungs relates to cell migrations between the two organs of either fungi-primed immune cells (via Th2 and TH17 responses) or gut fungi. Additionally, antibiotic-induced dysbiosis can lead to Candida overgrowth in the gut in an opportunistic manner, inducing TH17 responses and macrophage differentiation in the airways, all of which increase lung and airway inflammation [103,108].
Gut mycobiome disequilibrium has been correlated with Cystic Fibrosis (CF) and infection by Influenza A virus, in which C. albicans and/or S. cerevisiae intestinal colonization protected against respiratory epithelium cell invasion [127].
There is growing concern about mucormycosis, a severe infection caused by members of the Mucorales order [128]. Emerging infections by these fungi, mainly in immunocompromised individuals, show high mortality rates (>40%), with higher incidence following the SARS-CoV-2 pandemic [129]. Mucormycosis is mainly caused by mucorales species, of which Rhizopus species account for >70% of all cases, but Mucor and Lichtheimia species are also frequent etiologic agents [130]. There is much preoccupation with mucormycosis in patients on antifungal treatment as a result of hypervirulent strains following epigenetic modifications [131,132].
6.4. Systemic Infections
Patients experiencing chronic critical illness (CCI) show an altered gut mycobiome profile up to two weeks after experiencing sepsis [133]. Relevant relationships between gut mycobiome composition and viral infections have been described. For instance, Geotrichum species are more represented in SARS-CoV-2 patients than in controls [134], C. albicans or S. cerevisiae can reverse susceptibility of Influenza A infection upon antibiotic treatment in mice [49], and in the case of Human immunodeficiency virus type 1 (HIV-1)/Hepatitis C virus (HCV) co-infected patients show decreased fungal alpha diversity, and co-infection was specifically associated with a more dysbiotic mycobiome composition [135].
Invasive pulmonary aspergillosis, caused by Aspergillus, has been recognized as an important opportunistic infection in patients with viral pneumonia [136]. This condition, which affects critically ill patients after ICU admission, has been related to two specific viral conditions: influenza-associated pulmonary aspergillosis (IAPA) and COVID-19-associated pulmonary aspergillosis (CAPA). IAPA presents a variable incidence (10 to 30%), and its appearance on patients occurs within a short time after ICU admission (median of 3 days). However, despite the CAPA incidence being similar (4–35%), its appearance mostly occurs 7 days after the ICU admission [137,138]. Both present high rates of mortality (40–60% and 60–70%, respectively), and both share airway epithelial damage caused by the viral infection [136]. Little is known about the alterations that these conditions produce in gut mycobiota, but COVID-19 and H1N1 influenza patients present lower gut fungal α-diversity and microbial diversity [139,140].
Finally, Candida auris is a multidrug-resistant infectious fungus that represents a current threat to public health. Its virulence relies on its cell polymorphism, environmental adaptation, and endurance on surfaces, as well as on its immune evasion mechanisms [141]. Specifically, innate immunity modulation has been associated with C. auris invasive candidiasis, showing enhanced resistance towards neutrophil activation and NET formation, macrophage evasion, and lower pro-inflammatory mediators production (IL-1β, IL-6, tumor necrosis factor-α, CXCL1/KC, and CXCL2/MIP2) [142].
6.5. Liver Disorders
Mycobiome dysbiosis has been linked to alcohol-associated liver disease [143] (F. Li et al., 2019). These patients show poor fungal diversity and richness, a correlation with Candida overgrowth, high expression of candidalysin (a hepatocyte-damaging C. albicans exotoxin), and high levels of serum ASCAs, suggesting increased systemic exposure to intestinal fungi or their products [144].
It is uncertain whether gut mycobiome alterations are a cause or a consequence of obesity, but some studies report diminished fungal diversity in obese patients, with S. cerevisiae and C. albicans reduced in obese children [145,146] and Mucor being more prevalent in non-obese subjects [144]. In this manner, mycobiome composition may be involved in the pathogenesis of non-alcoholic fatty liver disease, currently associated with obesity.
In light of the above, primary sclerosing cholangitis, another chronic liver disease, may be linked to the gut mycobiome, as it is frequently associated with IBD [147] and higher ASCA serum levels [148].
6.6. Pancreatic Disorders
The mycobiome may have a pathogenetic role in diabetes mellitus (DM), as type 1 and 2 DM (T1DM and T2DM) patients are largely colonized by C. albicans, and those with T1DM also present increased fungal diversity [149,150].
Furthermore, both bacteria and fungi are increased in the pancreas of patients with pancreatic ductal adenocarcinoma (e.g., Malassezia), perhaps as a consequence of their migration through Oddi’s sphincter [151]. The mycobiome may also signal fungal cell wall glycan (MBL and complement cascade), but it is unclear whether this represents the cause or consequence of malignant progression.
6.7. Skin Disorders
Skin mycobiome diversity tends to be low, but multiple studies found pathological relationships between these communities and dermatologic disorders [152]. In this context, Malassezia species stands out, being common in human skin as a commensal microorganism. In a study focused on atopic dermatitis (AD), these patients showed a different mycobiome composition with increased abundance of Cladosporium, Malassezia, Candida, Diplodia, Saccharomyces, Penicillium, and Aspergillus [153]. Interestingly, it was demonstrated that patients exposed to air-conditioning showed a mycobiome profile closer to that of AD rather than a healthy mycobiome.
On the other hand, the skin mycobiome profile of psoriasis patients receiving IL-23 inhibitors revealed increased species diversity after treatment [154]. Additionally, the abundance of Malassezia species decreased after the treatment. Outside this therapeutic context, the role of Malassezia in psoriatic patients has been widely discussed, but no definitive conclusion is in place. While some historic studies demonstrated patient improvement after antifungal treatment [155] or proved that M. ovalis fragments could induce scalp lesions mimicking psoriasis symptomatology [156], others demonstrated that there was no difference in scalp psoriasis development upon itraconazole treatment [157] or, more recently, that the relative abundance of Malassezia species is increased in healthy individuals compared to psoriasis patients [158]. On the contrary, in the case of pityriasis versicolor or tinea versicolor, it is well-known that Malassezia species are causative, with M. furfur, M. globosa, and M. sympodialis being the most common species [159].
6.8. Genitourinary Disorders
While urinary tract infections (UTIs) are usually associated with bacterial species, Candida infections represent 5–10% of diagnosed UTIs [160], and other genera, such as Cryptococcus, Aspergillus, Histoplasma, Mucoraceae, Blastomyces, and Coccidioides, can also be identified as the causative agents [161]. Candiduria, defined as the presence of C. albicans in urine, may occur in both asymptomatic and symptomatic UTIs [162].
Vulvovaginal candidiasis (VVC) caused by C. albicans is a prevalent disease of the lower female reproductive tract, where the microorganism exists in the mucosa asymptomatically [20]. While C. albicans is responsible for over 90% of VVC diagnoses, other non-albicans species can act as causal agents, such as C. glabrata, C. krusei, C. parapsilosis, or C. tropicalis. Interestingly, episodes of VVC are more common in the luteal phase of the menstrual cycle, when the levels of estrogen increase, inhibiting fungal opsonization and phagocytosis via complement impairment [163,164].
6.9. Cancer
The digestive system harbors the most diverse microbiome and mycobiome, so current fungal studies in tumor contexts are focused on this niche. The pancreas, considered both part of the digestive and the endocrine system, has gained interest thanks to the work of Aykut and colleagues [151]. These authors demonstrated that the infiltrating fungal composition of pancreatic ductal adenocarcinoma (PDA) was enriched for Malassezia species, and that oral antifungal treatment protected from tumor progression, suggesting a pivotal role of fungal species for both the nature and treatment of PDA. It was later demonstrated that mycobiome-driven tumorigenesis occurred via modulation of IL-33 secretion, which enhances TH2 and ILC2 recruitment and leads to enhanced tumor growth [165]. PDA has also been studied in association with the oral mycobiome, identifying Aspergillus and Cladosporium as putative biomarkers to distinguish PDAC patients when compared to healthy controls [166].
Gut mycobiome has also been extensively studied for colorectal cancer (CRC), showing an altered fungal profile for these patients, with increased presence of Candida and Malassezia and decreased presence of S. cerevisiae [167], together with a higher Basidiomycota:Ascomycota ratio [168]. Also, additional research highlighted the possible role of CARD9, a protein involved in Dectin-1 and Dectin-3 downstream signaling, in the mycobiome-CRC association. It was shown that Card9−/− mice displayed higher tumor burden and heavier fecal fungal load, and this was highly influenced by the housing conditions, suggesting a strong influence of horizontal mycobiome transfer [169].
In the case of gastric cancer, these patients show a specific disease signature, including decreased diversity and richness, higher proportion of opportunistic fungi, and increased Basidiomycota:Ascomycota ratio in concordance with CRC studies [170].
As for hepatocellular carcinoma, fungal aflatoxin (produced by Aspergillus) acts as a carcinogen by promoting mutations in the p53 tumor suppressor gene [171].
Mycobiome studies on salivary samples of oral squamous cell carcinoma (OSCC) patients in Sudan demonstrated that these individuals showed increased abundance of Candida, Malassezia, Saccharomyces, Aspergillus, and Cyberlindnera [172]. Specifically, whereas Candida was associated with poorer overall survival (OS), Malassezia pointed to favorable outcomes, to the extent of becoming an independent prognostic marker for OSCC. Another study confirmed the pivotal role of C. albicans in these patients and additionally identified other common non-albicans Candida (NAC) species in OSCC salivary samples, including C. dubliniensis, C. tropicalis, C. glabrata, C. parapsilosis, C. sake, C. krusei, and C. guilliermondii [173].
Enrichment of the Blastomyces genus was detected in lung cancer samples in a pan-cancer mycobiome analysis [174]. The analysis of the intratumor lung adenocarcinoma mycobiome detected a decrease in Candida and an increase in Saccharomyces in these patients [175]. Interestingly, it was later proven that a specific tumor-resident species, Aspergillus sidowii, mediated lung adenocarcinoma progression by modulating the activity of myeloid-derived suppressor cells (MDSC) [176]. It was shown that A. sydowii specifically promoted an immunosuppressing environment by stimulating IL-1β secretion via the β-glucan/Dectin-1/CARD-9 pathway, acting as a possible opportunistic causative agent.
6.10. Autoimmune Disorders
The relationship between mycobiome and pathological settings mostly relies on the mycobiome-immune axis. Thus, dysbiosis-derived imbalance of the immune system, or conversely, immune-derived dysbiosis, can be studied in the context of autoimmune diseases.
Central nervous system (CNS) samples from multiple sclerosis (MS) patients contained Trichosporum mucoides, otherwise absent from healthy controls, suggesting a link between CNS mycosis and MS-associated neuroinflammation [177]. Furthermore, when the gut mycobiome of MS patients was characterized, these subjects showed higher alpha diversity and an over-representation of Saccharomyces and Aspergillus [147]. Interestingly, while they found a positive association between Aspergillus and activated CD16+ DCs, Saccharomyces was positively associated with circulating basophiles and negatively with regulatory B (Breg) cells.
A study on fecal mycobiome profiles of systemic lupus erythematosus (SLE), undifferentiated connective tissue diseases (UCTDs), and rheumatoid arthritis (RA) patients demonstrated that the mycobiome composition varied widely between these patients and healthy controls [178]. Additionally, SLE patients displayed dysbiosis, specifically showing enrichment of the Pezizales, Cantharellales, and Pseudaleuria groups.
As for Sjögren syndrome, patients with a Candida infection had a higher risk of developing the disease in a Taiwanese cohort, suggesting that this factor should be taken into account for early diagnosis [179].
7. Clinical Applications of Mycobiome Modulation
Mycobiome composition is usually studied in a disease-focused manner, specifically aiming to describe increased risks of a defined pathology. However, the study of fungal communities may also contribute to the active manipulation of the mycobiome in therapeutic approaches.
One example is the use of fungal-derived immunomodulatory molecules as an attractive or complementary alternative to aggressive antitumor therapies. These strategies consist of the administration of fungal substances, triggering an immune-reactive state in tumor settings [180]. β-glucan administration has been widely studied as an antitumoral therapy, and current knowledge points to macrophage modulation via Dectin-1 interaction as its main mechanism of action [181,182]. Specifically, this molecule can reprogram immunosuppressive M2 macrophages into inflammatory M1 macrophages and thus favor tumor attack. There are numerous clinical trials on the effectiveness of β-glucan administration [182,183], mostly as an adjuvant agent, including neuroblastoma [184], lung cancer [185,186], non-Hodgkin’s lymphoma [187,188], and colorectal cancer [189], among others.
Probiotic supplements are extensively associated with bacterial communities, but fungi also engage in gut homeostasis and can thus be included in these formulations. Specifically, Saccharomyces cerevisiae var. boulardii supplementation induces therapeutic improvement for inflammatory bowel disease (IBD) [190], diarrhea [191,192] and ulcerative colitis (UC) settings [193,194] by pro-inflammatory cytokine inhibition via NF-κB and ERK/1 pathway impairment, and by directly suppressing bacteria overgrowth and, as previously mentioned, cleavage of C. difficile toxins [195].
It is worth mentioning that other Saccharomyces cerevisiae strains have been proposed as a probiotic agent for IBD [196], colitis [197], or enterohemorrhagic E. coli infections [198]. S. cerevisiae has even been studied as a part of a post-biotic preparation, meaning a preparation where S. cerevisiae is used to obtain fermentation-derived bioactive compounds, mainly fungal cell wall components (e.g., β-glucans and mannans) [199,200].
Even after sustained research on new fungal-derived probiotics, limited species have shown potential as alternative probiotic agents [201]. Some of these include D. hansenii [202,203], Kluyveromyces marxianus [204], Yarrowiali polytica [205], Pichia kudriavzevii [206], Torulaspora delbrueckii [207], but their current application still relies on the need for more biosafety studies and regulatory requirements.
The gut mycobiome also holds significant importance in the case of fecal microbiota transplantation (FMT), specifically applied in the treatment of C. difficile [208]. Notably, over-representation of C. albicans was associated with decreased FMT success and, conversely, patients classified as “responders” showed increased relative abundance of Saccharomyces and Aspergillus. Interestingly, in UC patients, higher C. albicans abundance prior to FMT was associated with responsiveness [209], but a higher presence of the microorganism decreased response in a post-FMT setting. This led to the conclusion that C. albicans contributed to a supporting niche suitable for FMT engraftment.
Current knowledge on cancer-associated fungi shows that the mycobiome communities can act as predictive biomarkers [210]. As previously stated, multiple studies have highlighted the diagnostic potential of the mycobiome composition, as is the case with the Ascomycota/Basidiomycota ratio for CRC [210] or the A. sidowii presence in lung adenocarcinoma [176]. Additionally, the mycobiome composition can also represent a useful tool in the era of precision medicine [211]. For instance, the ASCAs and the perinuclear antineutrophil cytoplasmic antibodies (pANCAs) levels serve as a diagnostic tool to distinguish between Crohn’s disease and UC [212]. The study of the prognostic value of fungi composition can lead to surprising observations, such as the fact that the mycobiome may have a predictive role in abdominal aortic aneurysm (AAA), as it was shown that AAA patients display an increase in Candida species and a reduction of Saccharomyces cerevisiae [25,213].
The current scientific attention and research directed at immunotherapy, especially at immune checkpoint inhibitors (ICIs) and chimeric antigen receptors (CARs), reinforces the importance of the immune-mycobiome axis, but few studies have focused on the specific role of fungi in immunotherapy. There is limited information that associates the expression of immune checkpoint molecules with the presence of the fungal receptor Dectin-1 [210]. Specifically, dectin-1 upregulates PD-L1 surface expression upon neutrophil exposure to β-glucans [214], and CTLA-4 expression by Tregs also increases upon Dectin-1 activation [215]. Other studies have evaluated the effect of mycobiota dysbiosis in ICI blockade therapy, such as Szóstak and colleagues, who associated Saccharomyces paradoxus with better responses in anti-PD-1 treatment, whereas high levels of Malassezia restricta and C. albicans led to worse responses [216]. A multi-kingdom gut microbiota study identified several biomarkers for ICI blockade responses, especially when combining fungi and bacterial data [217]. Interestingly, they identified Schizosaccharomyces octosporus as a central hub in responder patients for its short-chain fatty acid production. Beyond this, the role of the gut mycobiome in anti-PD-1 and anti-PD-L1 therapy has been studied in the context of FMT, showing that donors can be stratified into enterotypes based on mycobiome composition, distinguishing a favorable and an unfavorable profile that significantly differ in terms of their immune, metabolic, and bacteriome profiles [218]. Other studies performing a similar approach for hepatocellular carcinoma [219] or including multiple solid tumors [220] also show promising results, suggesting that further research in this field will lead to improved treatments.
8. Concluding Remarks
There is a crucial need to further our knowledge on the composition and roles of the human mycobiome in health and disease to enable a complete functional scenario of the bacteriome. Future research should consider eco-systemic approaches, recognizing and highlighting the intricate interactions between the different components of the human microbiome and its host. This implies reaching beyond the fungal component dealt with in this review, to embrace also protists, archaea, and viruses that co-inhabit these ecological niches, in constant interaction, leading to tolerance or response by the immune system and their impact on specific diseases.
A deep understanding of the mycobiome physiology precedes the way to innovative clinical approaches in many disease categories and tailored antifungal therapies.
Author Contributions
Conceptualization, M.V.-d.A. and F.L.; writing—original draft preparation, L.C.-S., J.L.-T., V.P.-R. and M.V.-d.A.; writing—review and editing, L.C.-S., V.P.-R., L.A.-S., M.I., T.G., M.V.-d.A. and F.L.; visualization, F.L. and L.C.-S.; supervision, F.L. and M.V.-d.A.; project administration, F.L.; funding acquisition, F.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
F.L. is the founder and ad honorem scientific advisor of Sepsia Therapeutics S.L. M.I. is a founding partner of Sepsia Therapeutics S.L. The rest of the authors declare no conflicts of interest.
Funding Statement
Work is supported by projects PID2022-140932OB-I00 (funded by MCIN-AEI-10.13039-501100011033-FEDER, UE) and 2021-SGR-0113 (funded by AGAUR) to F.L. L.C.-S., V.P.-R., and L.A.-S are recipients of fellowships PRE2020-093993, FPU21-06217, and PREP2022-000394, respectively.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Berg G., Rybakova D., Fischer D., Cernava T., Vergès M.-C.C., Charles T., Chen X., Cocolin L., Eversole K., Corral G.H., et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome. 2020;8:103. doi: 10.1186/s40168-020-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dekaboruah E., Suryavanshi M.V., Chettri D., Verma A.K. Human Microbiome: An Academic Update on Human Body Site Specific Surveillance and Its Possible Role. Arch. Microbiol. 2020;202:2147–2167. doi: 10.1007/s00203-020-01931-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallen-Adams H.E., Kachman S.D., Kim J., Legge R.M., Martínez I. Fungi Inhabiting the Healthy Human Gastrointestinal Tract: A Diverse and Dynamic Community. Fungal Ecol. 2015;15:9–17. doi: 10.1016/j.funeco.2015.01.006. [DOI] [Google Scholar]
- 4.Nash A.K., Auchtung T.A., Wong M.C., Smith D.P., Gesell J.R., Ross M.C., Stewart C.J., Metcalf G.A., Muzny D.M., Gibbs R.A., et al. The Gut Mycobiome of the Human Microbiome Project Healthy Cohort. Microbiome. 2017;5:153. doi: 10.1186/s40168-017-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieterich W., Schink M., Zopf Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018;6:116. doi: 10.3390/medsci6040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proctor L.M., Creasy H.H., Fettweis J.M., Lloyd-Price J., Mahurkar A., Zhou W., Buck G.A., Snyder M.P., Strauss J.F., Weinstock G.M., et al. The Integrative Human Microbiome Project. Nature. 2019;569:641–648. doi: 10.1038/s41586-019-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallen-Adams H.E., Suhr M.J. Fungi in the Healthy Human Gastrointestinal Tract. Virulence. 2017;8:352–358. doi: 10.1080/21505594.2016.1247140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ott S.J., Kühbacher T., Musfeldt M., Rosenstiel P., Hellmig S., Rehman A., Drews O., Weichert W., Timmis K.N., Schreiber S. Fungi and Inflammatory Bowel Diseases: Alterations of Composition and Diversity. Scand. J. Gastroenterol. 2008;43:831–841. doi: 10.1080/00365520801935434. [DOI] [PubMed] [Google Scholar]
- 9.Ptasiewicz M., Grywalska E., Mertowska P., Korona-Głowniak I., Poniewierska-Baran A., Niedźwiedzka-Rystwej P., Chałas R. Armed to the Teeth—The Oral Mucosa Immunity System and Microbiota. Int. J. Mol. Sci. 2022;23:882. doi: 10.3390/ijms23020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belvoncikova P., Splichalova P., Videnska P., Gardlik R. The Human Mycobiome: Colonization, Composition and the Role in Health and Disease. J. Fungi. 2022;8:1046. doi: 10.3390/jof8101046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandara H.M.H.N., Panduwawala C.P., Samaranayake L.P. Biodiversity of the Human Oral Mycobiome in Health and Disease. Oral Dis. 2019;25:363–371. doi: 10.1111/odi.12899. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen L.D.N., Viscogliosi E., Delhaes L. The Lung Mycobiome: An Emerging Field of the Human Respiratory Microbiome. Front. Microbiol. 2015;6:89. doi: 10.3389/fmicb.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuthbertson L., Felton I., James P., Cox M.J., Bilton D., Schelenz S., Loebinger M.R., Cookson W.O.C., Simmonds N.J., Moffatt M.F. The Fungal Airway Microbiome in Cystic Fibrosis and Non-Cystic Fibrosis Bronchiectasis. J. Cyst. Fibros. 2021;20:295–302. doi: 10.1016/j.jcf.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver D., Gago S., Bromley M., Bowyer P. The Human Lung Mycobiome in Chronic Respiratory Disease: Limitations of Methods and Our Current Understanding. Curr. Fungal Infect. Rep. 2019;13:109–119. doi: 10.1007/s12281-019-00347-5. [DOI] [Google Scholar]
- 15.Ali N.A.B.M., Ivan F.X., Mac Aogáin M., Narayana J.K., Lee S.Y., Lim C.L., Chotirmall S.H. The Healthy Airway Mycobiome in Individuals of Asian Descent. Chest. 2021;159:544–548. doi: 10.1016/j.chest.2020.09.072. [DOI] [PubMed] [Google Scholar]
- 16.Oh J., Freeman A.F., Park M., Sokolic R., Candotti F., Holland S.M., Segre J.A., Kong H.H. The Altered Landscape of the Human Skin Microbiome in Patients with Primary Immunodeficiencies. Genome Res. 2013;23:2103–2114. doi: 10.1101/gr.159467.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Findley K., Oh J., Yang J., Conlan S., Deming C., Meyer J.A., Schoenfeld D., Nomicos E., Park M., Kong H.H., et al. Topographic Diversity of Fungal and Bacterial Communities in Human Skin. Nature. 2013;498:367–370. doi: 10.1038/nature12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H., Goh B.N., Teh W.K., Jiang Z., Goh J.P.Z., Goh A., Wu G., Hoon S.S., Raida M., Camattari A., et al. Skin Commensal Malassezia Globosa Secreted Protease Attenuates Staphylococcus aureus Biofilm Formation. J. Investig. Dermatol. 2018;138:1137–1145. doi: 10.1016/j.jid.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Ackerman A.L., Underhill D.M. The Mycobiome of the Human Urinary Tract: Potential Roles for Fungi in Urology. Ann. Transl. Med. 2017;5:31. doi: 10.21037/atm.2016.12.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willems H.M.E., Ahmed S.S., Liu J., Xu Z., Peters B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi. 2020;6:27. doi: 10.3390/jof6010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esposito M.M., Patsakos S., Borruso L. The Role of the Mycobiome in Women’s Health. J. Fungi. 2023;9:348. doi: 10.3390/jof9030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra K., Bukavina L., Ghannoum M. Symbiosis and Dysbiosis of the Human Mycobiome. Front. Microbiol. 2021;12:636131. doi: 10.3389/fmicb.2021.636131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raimondi S., Amaretti A., Gozzoli C., Simone M., Righini L., Candeliere F., Brun P., Ardizzoni A., Colombari B., Paulone S., et al. Longitudinal Survey of Fungi in the Human Gut: ITS Profiling, Phenotyping, and Colonization. Front. Microbiol. 2019;10:1575. doi: 10.3389/fmicb.2019.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabwe M.H., Vikram S., Mulaudzi K., Jansson J.K., Makhalanyane T.P. The Gut Mycobiota of Rural and Urban Individuals Is Shaped by Geography. BMC Microbiol. 2020;20:257. doi: 10.1186/s12866-020-01907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupuy A.K., David M.S., Li L., Heider T.N., Peterson J.D., Montano E.A., Dongari-Bagtzoglou A., Diaz P.I., Strausbaugh L.D. Redefining the Human Oral Mycobiome with Improved Practices in Amplicon-Based Taxonomy: Discovery of Malassezia as a Prominent Commensal. PLoS ONE. 2014;9:e90899. doi: 10.1371/journal.pone.0090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung M.H.Y., Chan K.C.K., Lee P.K.H. Skin Fungal Community and Its Correlation with Bacterial Community of Urban Chinese Individuals. Microbiome. 2016;4:46. doi: 10.1186/s40168-016-0192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehtoranta L., Hibberd A.A., Yeung N., Laitila A., Maukonen J., Ouwehand A.C. Characterization of Vaginal Fungal Communities in Healthy Women and Women with Bacterial Vaginosis (BV); a Pilot Study. Microb. Pathog. 2021;161:105055. doi: 10.1016/j.micpath.2021.105055. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L., Zhan H., Xu W., Yan S., Ng S.C. The Role of Gut Mycobiome in Health and Diseases. Ther. Adv. Gastroenterol. 2021;14:17562848211047130. doi: 10.1177/17562848211047130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin V.K., Yong V.C., Chong P.P., Amin Nordin S., Basir R., Abdullah M. Mycobiome in the Gut: A Multiperspective Review. Mediat. Inflamm. 2020;2020:9560684. doi: 10.1155/2020/9560684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naranjo-Ortiz M.A., Gabaldón T. Fungal Evolution: Diversity, Taxonomy and Phylogeny of the Fungi. Fungi. Biol. Rev. Camb. Philos. Soc. 2019;94:2101–2137. doi: 10.1111/brv.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tedersoo L., Bahram M., Zinger L., Nilsson R.H., Kennedy P.G., Yang T., Anslan S., Mikryukov V. Best Practices in Metabarcoding of Fungi: From Experimental Design to Results. Mol. Ecol. 2022;31:2769–2795. doi: 10.1111/mec.16460. [DOI] [PubMed] [Google Scholar]
- 32.Steenwyk J.L., Knowles S., Bastos R.W., Balamurugan C., Rinker D., Mead M.E., Roberts C.D., Raja H.A., Li Y., Colabardini A.C., et al. Evolutionary Origin and Population Diversity of a Cryptic Hybrid Pathogen. Nat. Commun. 2024;15:8412. doi: 10.1038/s41467-024-52639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kauserud H. ITS Alchemy: On the Use of ITS as a DNA Marker in Fungal Ecology. Fungal Ecol. 2023;65:101274. doi: 10.1016/j.funeco.2023.101274. [DOI] [Google Scholar]
- 34.Avershina E., Qureshi A.I., Winther-Larsen H.C., Rounge T.B. Challenges in Capturing the Mycobiome from Shotgun Metagenome Data: Lack of Software and Databases. Microbiome. 2025;13:66. doi: 10.1186/s40168-025-02048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khannous-lleiffe O., Gabaldón T. B-GUT Reference Genome Database Improves Biomarker Discovery and Fungal Identification in Gut Metagenomes. Res. Sq. 2025 doi: 10.21203/rs.3.rs-6766778/v1. [DOI] [Google Scholar]
- 36.Gow N.A.R., Latge J.P., Munro C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017;5:267–292. doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rittenour W.R., Park J.H., Cox-Ganser J.M., Beezhold D.H., Green B.J. Comparison of DNA Extraction Methodologies Used for Assessing Fungal Diversity via ITS Sequencing. J. Environ. Monit. 2012;14:766–774. doi: 10.1039/c2em10779a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boix-Amorós A., Puente-Sánchez F., du Toit E., Linderborg K.M., Zhang Y., Yang B., Salminen S., Isolauri E., Tamames J., Mira A., et al. Mycobiome Profiles in Breast Milk from Healthy Women Depend on Mode of Delivery, Geographic Location, and Interaction with Bacteria. Appl. Environ. Microbiol. 2019;85:e02994-18. doi: 10.1128/AEM.02994-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward T.L., Knights D., Gale C.A. Infant Fungal Communities: Current Knowledge and Research Opportunities. BMC Med. 2017;15:30. doi: 10.1186/s12916-017-0802-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu T., Duan Y.-Y., Kong F.-Q., Galzote C., Quan Z.-X. Dynamics of Skin Mycobiome in Infants. Front. Microbiol. 2020;11:1790. doi: 10.3389/fmicb.2020.01790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schei K., Avershina E., Øien T., Rudi K., Follestad T., Salamati S., Ødegård R.A. Early Gut Mycobiota and Mother-Offspring Transfer. Microbiome. 2017;5:107. doi: 10.1186/s40168-017-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmad H.F., Castro Mejia J.L., Krych L., Khakimov B., Kot W., Bechshøft R.L., Reitelseder S., Højfeldt G.W., Engelsen S.B., Holm L., et al. Gut Mycobiome Dysbiosis Is Linked to Hypertriglyceridemia Among Home Dwelling Elderly Danes. biorXiv. 2020 doi: 10.1101/2020.04.16.044693. [DOI] [Google Scholar]
- 43.Wu L., Zeng T., Deligios M., Milanesi L., Langille M.G.I., Zinellu A., Rubino S., Carru C., Kelvin D.J. Age-Related Variation of Bacterial and Fungal Communities in Different Body Habitats across the Young, Elderly, and Centenarians in Sardinia. mSphere. 2020;5:e00558-19. doi: 10.1128/mSphere.00558-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szóstak N., Handschuh L., Samelak-Czajka A., Tomela K., Schmidt M., Pruss Ł., Milanowska-Zabel K., Kozlowski P., Philips A. Host Factors Associated with Gut Mycobiome Structure. mSystems. 2023;8:e0098622. doi: 10.1128/msystems.00986-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffmann C., Dollive S., Grunberg S., Chen J., Li H., Wu G.D., Lewis J.D., Bushman F.D. Archaea and Fungi of the Human Gut Microbiome: Correlations with Diet and Bacterial Residents. PLoS ONE. 2013;8:e66019. doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strati F., Di Paola M., Stefanini I., Albanese D., Rizzetto L., Lionetti P., Calabrò A., Jousson O., Donati C., Cavalieri D., et al. Age and Gender Affect the Composition of Fungal Population of the Human Gastrointestinal Tract. Front. Microbiol. 2016;7:1227. doi: 10.3389/fmicb.2016.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu X., Zhang F., Yang X., Wu N., Jiang W., Li X., Li X., Liu Y. Changes in the Composition of Intestinal Fungi and Their Role in Mice with Dextran Sulfate Sodium-Induced Colitis. Sci. Rep. 2015;5:10416. doi: 10.1038/srep10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang T.T., Shao T.Y., Ang W.X.G., Kinder J.M., Turner L.H., Pham G., Whitt J., Alenghat T., Way S.S. Commensal Fungi Recapitulate the Protective Benefits of Intestinal Bacteria. Cell Host Microbe. 2017;22:809–816.e4. doi: 10.1016/j.chom.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castagliuolo I., Riegler M.F., Valenick L., LaMont J.T., Pothoulakis C. Saccharomyces boulardii Protease Inhibits the Effects of Clostridium Difficile Toxins A and B in Human Colonic Mucosa. Infect. Immun. 1999;67:302–307. doi: 10.1128/IAI.67.1.302-307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czerucka D., Dahan S., Mograbi B., Rossi B., Rampal P. Saccharomyces boulardii Preserves the Barrier Function and Modulates the Signal Transduction Pathway Induced in Enteropathogenic Escherichia coli-Infected T84 Cells. Infect. Immun. 2000;68:5998–6004. doi: 10.1128/IAI.68.10.5998-6004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.García C., Tebbji F., Daigneault M., Liu N.-N., Köhler J.R., Allen-Vercoe E., Sellam A. The Human Gut Microbial Metabolome Modulates Fungal Growth via the TOR Signaling Pathway. mSphere. 2017;2:e00555-17. doi: 10.1128/mSphere.00555-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das S., Konwar B.K. Inhibiting Pathogenicity of Vaginal Candida albicans by Lactic Acid Bacteria and MS Analysis of Their Extracellular Compounds. APMIS. 2024;132:161–186. doi: 10.1111/apm.13365. [DOI] [PubMed] [Google Scholar]
- 54.Lan J., Chen C. The Role of Lactic Acid Bacteria in Maintaining Vaginal Internal Environment Homeostasis in Patients with Infertility. Microb. Pathog. 2023;176:106004. doi: 10.1016/j.micpath.2023.106004. [DOI] [PubMed] [Google Scholar]
- 55.Osset J., García E., Bartolomé R.M., Andreu A. Role of Lactobacillus as Protector against Vaginal Candidiasis. Med. Clin. 2001;117:285–288. doi: 10.1016/S0025-7753(01)72089-1. [DOI] [PubMed] [Google Scholar]
- 56.Hui W.W., Emerson L.E., Clapp B., Sheppe A.E., Sharma J., del Castillo J., Ou M., Maegawa G.H.B., Hoffman C., Larkin J., et al. The Cross-Kingdom Interaction between Helicobacter Pylori and Candida albicans. PLoS Pathog. 2021;17:e1009515. doi: 10.1371/JOURNAL.PPAT.1009515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Granillo A.R., Canales M.G.M., Espíndola M.E.S., Rivera M.A.M., De Lucio V.M.B., Tovar A.V.R. Antibiosis Interaction of Staphylococccus Aureus on Aspergillus fumigatus Assessed In Vitro by Mixed Biofilm Formation. BMC Microbiol. 2015;15:33. doi: 10.1186/s12866-015-0363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters B.M., Jabra-Rizk M.A., Scheper M.A., Leid J.G., Costerton J.W., Shirtliff M.E. Microbial Interactions and Differential Protein Expression in Staphylococcus aureus–Candida albicans Dual-Species Biofilms. FEMS Immunol. Med. Microbiol. 2010;59:493–503. doi: 10.1111/j.1574-695X.2010.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koo H., Andes D.R., Krysan D.J. Candida–Streptococcal Interactions in Biofilm-Associated Oral Diseases. PLoS Pathog. 2018;14:e1007342. doi: 10.1371/journal.ppat.1007342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silverman R.J., Nobbs A.H., Vickerman M.M., Barbour M.E., Jenkinson H.F. Interaction of Candida albicans Cell Wall Als3 Protein with Streptococcus Gordonii SspB Adhesin Promotes Development of Mixed-Species Communities. Infect. Immun. 2010;78:4644–4652. doi: 10.1128/IAI.00685-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalan L., Loesche M., Hodkinson B.P., Heilmann K., Ruthel G., Gardner S.E., Grice E.A. Redefining the Chronic-Wound Microbiome: Fungal Communities Are Prevalent, Dynamic, and Associated with Delayed Healing. mBio. 2016;7:e01058-16. doi: 10.1128/mBio.01058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoarau G., Mukherjee P.K., Gower-Rousseau C., Hager C., Chandra J., Retuerto M.A., Neut C., Vermeire S., Clemente J., Colombel J.F., et al. Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn’s Disease. mBio. 2016;7:e01250-16. doi: 10.1128/mBio.01250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Brucker K., Tan Y., Vints K., De Cremer K., Braem A., Verstraeten N., Michiels J., Vleugels J., Cammue B.P.A., Thevissen K. Fungal β-1,3-Glucan Increases Ofloxacin Tolerance of Escherichia coli in a Polymicrobial E. coli/Candida albicans Biofilm. Antimicrob. Agents Chemother. 2015;59:3052–3058. doi: 10.1128/AAC.04650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kong E.F., Tsui C., Kucharíková S., Andes D., Van Dijck P., Jabra-Rizk M.A. Commensal Protection of Staphylococcus aureus against Antimicrobials by Candida albicans Biofilm Matrix. mBio. 2016;7:e01365-16. doi: 10.1128/mBio.01365-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatinguais R., Willment J.A., Brown G.D. C-type Lectin Receptors in Antifungal Immunity: Current Knowledge and Future Developments. Parasite Immunol. 2022;45:e12951. doi: 10.1111/pim.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huysamen C., Brown G.D. The Fungal Pattern Recognition Receptor, Dectin-1, and the Associated Cluster of C-Type Lectin-like Receptors. FEMS Microbiol. Lett. 2008;290:121–128. doi: 10.1111/j.1574-6968.2008.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li M., Vultorius C., Bethi M., Yu Y. Spatial Organization of Dectin-1 and TLR2 during Synergistic Crosstalk Revealed by Super-Resolution Imaging. J. Phys. Chem. B. 2022;126:5781–5792. doi: 10.1021/acs.jpcb.2c03557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li W., Yan J., Yu Y. Geometrical Reorganization of Dectin-1 and TLR2 on Single Phagosomes Alters Their Synergistic Immune Signaling. Proc. Natl. Acad. Sci. USA. 2019;116:25106–25114. doi: 10.1073/pnas.1909870116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esteban A., Popp M.W., Vyas V.K., Strijbis K., Ploegh H.L., Fink G.R. Fungal Recognition Is Mediated by the Association of Dectin-1 and Galectin-3 in Macrophages. Proc. Natl. Acad. Sci. USA. 2011;108:14270–14275. doi: 10.1073/pnas.1111415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson A., da Fonseca D.M., Walker L., Griffiths J.S., Taylor P.R., Gow N.A.R., Orr S.J. Dependence on Mincle and Dectin-2 Varies with Multiple Candida Species During Systemic Infection. Front. Microbiol. 2021;12:633229. doi: 10.3389/fmicb.2021.633229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu L.L., Zhao X.Q., Jiang C., You Y., Chen X.P., Jiang Y.Y., Jia X.M., Lin X. C-Type Lectin Receptors Dectin-3 and Dectin-2 Form a Heterodimeric Pattern-Recognition Receptor for Host Defense against Fungal Infection. Immunity. 2013;39:324–334. doi: 10.1016/j.immuni.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 72.Drummond R.A., Franco L.M., Lionakis M.S. Human CARD9: A Critical Molecule of Fungal Immune Surveillance. Front. Immunol. 2018;9:1836. doi: 10.3389/fimmu.2018.01836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Speakman E.A., Dambuza I.M., Salazar F., Brown G.D. T Cell Antifungal Immunity and the Role of C-Type Lectin Receptors. Trends Immunol. 2020;41:61–76. doi: 10.1016/j.it.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hatinguais R., Willment J.A., Brown G.D. PAMPs of the Fungal Cell Wall and Mammalian PRRs. Curr. Top. Microbiol. Immunol. 2020;425:187–223. doi: 10.1007/82_2020_201. [DOI] [PubMed] [Google Scholar]
- 75.Duan T., Du Y., Xing C., Wang H.Y., Wang R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022;13:812774. doi: 10.3389/fimmu.2022.812774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salazar F., Brown G.D. Antifungal Innate Immunity: A Perspective from the Last 10 Years. J. Innate Immun. 2018;10:373–397. doi: 10.1159/000488539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang D., Wang Y., Shen S., Hou Y., Chen Y., Wang T. The Mycobiota of the Human Body: A Spark Can Start a Prairie Fire. Gut Microbes. 2020;11:655–679. doi: 10.1080/19490976.2020.1731287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heilig L., Natasha F., Trinks N., Aimanianda V., Wong S.S.W., Fontaine T., Terpitz U., Strobel L., Mauff F.L., Sheppard D.C., et al. CD56-Mediated Activation of Human Natural Killer Cells Is Triggered by Aspergillus fumigatus Galactosaminogalactan. PLoS Pathog. 2024;20:e1012315. doi: 10.1371/journal.ppat.1012315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li S.S., Ogbomo H., Mansour M.K., Xiang R.F., Szabo L., Munro F., Mukherjee P., Mariuzza R.A., Amrein M., Vyas J.M., et al. Identification of the Fungal Ligand Triggering Cytotoxic PRR-Mediated NK Cell Killing of Cryptococcus and Candida. Nat. Commun. 2018;9:751. doi: 10.1038/s41467-018-03014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vitenshtein A., Charpak-Amikam Y., Yamin R., Bauman Y., Isaacson B., Stein N., Berhani O., Dassa L., Gamliel M., Gur C., et al. NK Cell Recognition of Candida Glabrata through Binding of NKp46 and NCR1 to Fungal Ligands Epa1, Epa6, and Epa7. Cell Host Microbe. 2016;20:527–534. doi: 10.1016/j.chom.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vera J., Fenutria R., Cañadas O., Figueras M., Mota R., Sarrias M.R., Williams D.L., Casals C., Yelamos J., Lozano F. The CD5 Ectodomain Interacts with Conserved Fungal Cell Wall Components and Protects from Zymosan-Induced Septic Shock-like Syndrome. Proc. Natl. Acad. Sci. USA. 2009;106:1506–1511. doi: 10.1073/pnas.0805846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Velasco-de-Andrés M., Català C., Casadó-Llombart S., Simões I., Zaragoza O., Carreras E., Lozano F. The Lymphocyte Scavenger Receptor CD5 Plays a Nonredundant Role in Fungal Infection. Cell Mol. Immunol. 2021;18:498–500. doi: 10.1038/s41423-020-0434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Means T.K., Mylonakis E., Tampakakis E., Colvin R.A., Seung E., Puckett L., Tai M.F., Stewart C.R., Pukkila-Worley R., Hickman S.E., et al. Evolutionarily Conserved Recognition and Innate Immunity to Fungal Pathogens by the Scavenger Receptors SCARF1 and CD36. J. Exp. Med. 2009;206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Means T.K. Fungal Pathogen Recognition by Scavenger Receptors in Nematodes and Mammals. Virulence. 2010;1:37–41. doi: 10.4161/viru.1.1.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruchti F., LeibundGut-Landmann S. New Insights into Immunity to Skin Fungi Shape Our Understanding of Health and Disease. Parasite Immunol. 2022;45:e12948. doi: 10.1111/pim.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swidergall M., Filler S.G. Oropharyngeal Candidiasis: Fungal Invasion and Epithelial Cell Responses. PLoS Pathog. 2017;13:e1006056. doi: 10.1371/journal.ppat.1006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Igyártó B.Z., Haley K., Ortner D., Bobr A., Gerami-Nejad M., Edelson B.T., Zurawski S.M., Malissen B., Zurawski G., Berman J., et al. Skin-Resident Murine Dendritic Cell Subsets Promote Distinct and Opposing Antigen-Specific T Helper Responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruchti F., Tuor M., Mathew L., McCarthy N.E., LeibundGut-Landmann S. Γδ T Cells Respond Directly and Selectively to the Skin Commensal Yeast Malassezia for IL-17-Dependent Fungal Control. PLoS Pathog. 2024;20:e1011668. doi: 10.1371/journal.ppat.1011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jensen O., Trujillo E., Hanson L., Ost K.S. Controlling Candida: Immune Regulation of Commensal Fungi in the Gut. Infect. Immun. 2024;92:e00516–e00523. doi: 10.1128/iai.00516-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albac S., Schmitz A., Lopez-Alayon C., d’Enfert C., Sautour M., Ducreux A., Labruère-Chazal C., Laue M., Holland G., Bonnin A., et al. Candida albicans Is Able to Use M Cells as a Portal of Entry across the Intestinal Barrier In Vitro. Cell Microbiol. 2016;18:195–210. doi: 10.1111/cmi.12495. [DOI] [PubMed] [Google Scholar]
- 91.Ayabe T., Satchell D.P., Wilson C.L., Parks W.C., Selsted M.E., Ouellette A.J. Secretion of Microbicidal α-Defensins by Intestinal Paneth Cells in Response to Bacteria. Nat. Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 92.Pierre J.F., Peters B.M., Torre D.L., Sidebottom A.M., Tao Y., Zhu X., Cham C.M., Wang L., Kambal A., Harris K.G., et al. Peptide YY: A Paneth Cell Antimicrobial Peptide That Maintains Candida Gut Commensalism. Science. 2023;381:502–508. doi: 10.1126/science.abq3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elphick D.A., Mahida Y.R. Paneth Cells: Their Role in Innate Immunity and Inflammatory Disease. Gut. 2005;54:1802–1809. doi: 10.1136/gut.2005.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alonso-Monge R., Gresnigt M.S., Román E., Hube B., Pla J., Jarosz D. Candida albicans colonization of the gastrointestinal tract: A double-edged sword. PLoS Pathog. 2021;17:e1009710. doi: 10.1371/journal.ppat.1009710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Y., Shi T., Lu X., Xu Z., Qu J., Zhang Z., Shi G., Shen S., Hou Y., Chen Y., et al. Fungal-induced Glycolysis in Macrophages Promotes Colon Cancer by Enhancing Innate Lymphoid Cell Secretion of IL-22. EMBO J. 2021;40:e105320. doi: 10.15252/embj.2020105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leonardi I., Gao I.H., Lin W.Y., Allen M., Li X.V., Fiers W.D., De Celie M.B., Putzel G.G., Yantiss R.K., Johncilla M., et al. Mucosal Fungi Promote Gut Barrier Function and Social Behavior via Type 17 Immunity. Cell. 2022;185:831–846.e14. doi: 10.1016/j.cell.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo Y., Liu Y., Rui B., Lei Z., Ning X., Liu Y., Li M. Crosstalk between the Gut Microbiota and Innate Lymphoid Cells in Intestinal Mucosal Immunity. Front. Immunol. 2023;14:1171680. doi: 10.3389/fimmu.2023.1171680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Castleman M.J., Dillon S.M., Purba C.M., Cogswell A.C., Kibbie J.J., McCarter M.D., Santiago M.L., Barker E., Wilson C.C. Commensal and Pathogenic Bacteria Indirectly Induce IL-22 but Not IFNγ Production from Human Colonic ILC3s via Multiple Mechanisms. Front. Immunol. 2019;10:649. doi: 10.3389/fimmu.2019.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Griffiths J.S., Camilli G., Kotowicz N.K., Ho J., Richardson J.P., Naglik J.R. Role for IL-1 Family Cytokines in Fungal Infections. Front. Microbiol. 2021;12:633047. doi: 10.3389/fmicb.2021.633047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vonk A.G., Netea M.G., Van Krieken J.H., Iwakura Y., Van Der Meer J.W.M., Kullberg B.J. Endogenous Interleukin (IL)-1α and IL-1β Are Crucial for Host Defense against Disseminated Candidiasis. J. Infect. Dis. 2006;193:1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- 101.Zheng Y., Dang E.V. Novel Mechanistic Insights Underlying Fungal Allergic Inflammation. PLoS Pathog. 2023;19:e1011623. doi: 10.1371/journal.ppat.1011623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferreira-Gomes M., Wich M., Böde S., Hube B., Jacobsen I.D., Jungnickel B. B Cell Recognition of Candida albicans Hyphae via TLR 2 Promotes IgG1 and IL-6 Secretion for TH17 Differentiation. Front. Immunol. 2021;12:698849. doi: 10.3389/fimmu.2021.698849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu X., Xia Y., He F., Zhu C., Ren W. Intestinal Mycobiota in Health and Diseases: From a Disrupted Equilibrium to Clinical Opportunities. Microbiome. 2021;9:60. doi: 10.1186/s40168-021-01024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li R., Rezk A., Li H., Gommerman J.L., Prat A., Bar-Or A., Canadian B Cells in MS Team Antibody-Independent Function of Human B Cells Contributes to Antifungal T Cell Responses. J. Immunol. 2017;198:3245–3254. doi: 10.4049/jimmunol.1601572. [DOI] [PubMed] [Google Scholar]
- 105.Tian R., Fu M., Zhang Z., Ren J., An J., Liu Y., Li W. In Situ IgM Production and Clonal Expansion of B-1 Cells in Peritoneal Cavity Promote Elimination of C. Albicans Infection in IgH Transgenic Mice with VH Derived from a Natural Antibody. PLoS ONE. 2013;8:e60779. doi: 10.1371/annotation/bedab014-d393-4fad-aa07-0df1efb59038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen L., Ruan G., Cheng Y., Yi A., Chen D., Wei Y. The Role of Th17 Cells in Inflammatory Bowel Disease and the Research Progress. Front. Immunol. 2023;13:1055914. doi: 10.3389/fimmu.2022.1055914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chehoud C., Albenberg L.G., Judge C., Hoffmann C., Grunberg S., Bittinger K., Baldassano R.N., Lewis J.D., Bushman F.D., Wu G.D. Fungal Signature in the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015;21:1948–1956. doi: 10.1097/MIB.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gutierrez M.W., van Tilburg Bernardes E., Changirwa D., McDonald B., Arrieta M.C. “Molding” Immunity—Modulation of Mucosal and Systemic Immunity by the Intestinal Mycobiome in Health and Disease. Mucosal Immunol. 2022;15:573–583. doi: 10.1038/s41385-022-00515-w. [DOI] [PubMed] [Google Scholar]
- 109.Iliev I.D., Funari V.A., Taylor K.D., Nguyen Q., Reyes C.N., Strom S.P., Brown J., Becker C.A., Fleshner P.R., Dubinsky M., et al. Interactions between Commensal Fungi and the C-Type Lectin Receptor Dectin-1 Influence Colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leonardi I., Li X., Semon A., Li D., Doron I., Putzel G., Bar A., Prieto D., Rescigno M., McGovern D.P.B., et al. CX3CR1+, Mononuclear Phagocytes Control Immunity to Intestinal Fungi. Science. 2018;359:232–236. doi: 10.1126/science.aao1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kumamoto C.A. Cell Host and Microbe. Cell Press; Cambridge, MA, USA: 2016. The Fungal Mycobiota: Small Numbers, Large Impacts; pp. 750–751. [DOI] [PubMed] [Google Scholar]
- 112.Sokol H., Leducq V., Aschard H., Pham H.P., Jegou S., Landman C., Cohen D., Liguori G., Bourrier A., Nion-Larmurier I., et al. Fungal Microbiota Dysbiosis in IBD. Gut. 2017;66:1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luo P., Yang Z., Chen B., Zhong X. The Multifaceted Role of CARD9 in Inflammatory Bowel Disease. J. Cell. Mol. Med. 2019;24:34–39. doi: 10.1111/jcmm.14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gasperini A.M., Faccenda D., Garcia-Cela E. Unravelling the Impact of Mycotoxins on Gut Health: Implications for Inflammatory Bowel Disease. Curr. Opin. Food Sci. 2025;64:101316. doi: 10.1016/j.cofs.2025.101316. [DOI] [Google Scholar]
- 115.Richard M.L., Sokol H. The Gut Mycobiota: Insights into Analysis, Environmental Interactions and Role in Gastrointestinal Diseases. Nat. Rev. Gastroenterol. Hepatol. 2019;16:331–345. doi: 10.1038/s41575-019-0121-2. [DOI] [PubMed] [Google Scholar]
- 116.Sivignon A., De Vallée A., Barnich N., Denizot J., Darcha C., Pignède G., Vandekerckove P., Darfeuille-Michaud A. Saccharomyces cerevisiae CNCM I-3856 Prevents Colitis Induced by AIEC Bacteria in the Transgenic Mouse Model Mimicking Crohn’s Disease. Inflamm. Bowel Dis. 2015;21:276–286. doi: 10.1097/MIB.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 117.Renga G., Bellet M.M., Stincardini C., Pariano M., Oikonomou V., Villella V.R., Brancorsini S., Clerici C., Romani L., Costantini C. To Be or Not to Be a Pathogen: Candida albicans and Celiac Disease. Front. Immunol. 2019;10:2844. doi: 10.3389/fimmu.2019.02844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Renga G., Pariano M., D’onofrio F., Pieraccini G., Di Serio C., Villella V.R., Abbate C., Puccetti M., Giovagnoli S., Stincardini C., et al. The Immune and Microbial Homeostasis Determines the Candida–Mast Cells Cross-Talk in Celiac Disease. Life Sci. Alliance. 2024;7:e202302441. doi: 10.26508/lsa.202302441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gwak M.G., Chang S.Y. Gut-Brain Connection: Microbiome, Gut Barrier, and Environmental Sensors. Immune Netw. 2021;21:e20. doi: 10.4110/in.2021.21.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Markey L., Hooper A., Melon L.C., Baglot S., Hill M.N., Maguire J., Kumamoto C.A. Colonization with the Commensal Fungus Candida albicans Perturbs the Gut-Brain Axis through Dysregulation of Endocannabinoid Signaling. Psychoneuroendocrinology. 2020;121:104808. doi: 10.1016/j.psyneuen.2020.104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takata K., Tomita T., Okuno T., Kinoshita M., Koda T., Honorat J.A., Takei M., Hagihara K., Sugimoto T., Mochizuki H., et al. Dietary Yeasts Reduce Inflammation in Central Nerve System via Microflora. Ann. Clin. Transl. Neurol. 2014;2:56–66. doi: 10.1002/acn3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yuan X., Li X., Hei G., Zhang X., Song X. Intestinal Mycobiota Dysbiosis Associated Inflammation Activation in Chronic Schizophrenia. Behav. Brain Res. 2024;472:115149. doi: 10.1016/j.bbr.2024.115149. [DOI] [PubMed] [Google Scholar]
- 123.Zhou K., Baranova A., Cao H., Sun J., Zhang F. Gut Microbiome and Schizophrenia: Insights from Two-Sample Mendelian Randomization. Schizophrenia. 2024;10:75. doi: 10.1038/s41537-024-00497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tiew P.Y., Mac Aogain M., Ali N.A.B.M., Thng K.X., Goh K., Lau K.J.X., Chotirmall S.H. The Mycobiome in Health and Disease: Emerging Concepts, Methodologies and Challenges. Mycopathologia. 2020;185:207–231. doi: 10.1007/s11046-019-00413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kanj A.N., Skalski J.H. Gut Mycobiome and Asthma. J. Fungi. 2024;10:192. doi: 10.3390/jof10030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sey E.A., Warris A. The Gut-Lung Axis: The Impact of the Gut Mycobiome on Pulmonary Diseases and Infections. Oxf. Open Immunol. 2024;5:iqae008. doi: 10.1093/oxfimm/iqae008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Soret P., Vandenborght L.E., Francis F., Coron N., Enaud R., Avalos M., Schaeverbeke T., Berger P., Fayon M., Thiebaut R., et al. Respiratory Mycobiome and Suggestion of Inter-Kingdom Network during Acute Pulmonary Exacerbation in Cystic Fibrosis. Sci. Rep. 2020;10:3589. doi: 10.1038/s41598-020-60015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liang M., Xu J., Luo Y., Qu J. Epidemiology, Pathogenesis, Clinical Characteristics, and Treatment of Mucormycosis: A Review. Ann. Med. 2024;56:2396570. doi: 10.1080/07853890.2024.2396570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hoenigl M., Seidel D., Carvalho A., Rudramurthy S.M., Arastehfar A., Gangneux J.P., Nasir N., Bonifaz A., Araiza J., Klimko N., et al. The Emergence of COVID-19 Associated Mucormycosis: A Review of Cases from 18 Countries. Lancet Microbe. 2022;3:e543. doi: 10.1016/S2666-5247(21)00237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alqarihi A., Kontoyiannis D.P., Ibrahim A.S. Mucormycosis in 2023: An Update on Pathogenesis and Management. Front. Cell. Infect. Microbiol. 2023;13:1254919. doi: 10.3389/fcimb.2023.1254919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Axell-House D.B., Wurster S., Jiang Y., Kyvernitakis A., Lewis R.E., Tarrand J.J., Raad I.I., Kontoyiannis D.P. Breakthrough Mucormycosis Developing on Mucorales-Active Antifungals Portrays a Poor Prognosis in Patients with Hematologic Cancer. J. Fungi. 2021;7:217. doi: 10.3390/jof7030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gullì S.P., Hallur V., Kale P., Menezes G.A., Russo A., Singla N. From Spores to Solutions: A Comprehensive Narrative Review on Mucormycosis. Diagnostics. 2024;14:314. doi: 10.3390/diagnostics14030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park G., Munley J.A., Kelly L.S., Kannan K.B., Mankowski R.T., Sharma A., Upchurch G., Casadesus G., Chakrabarty P., Wallet S.M., et al. Gut Mycobiome Dysbiosis after Sepsis and Trauma. Crit. Care. 2024;28:18. doi: 10.1186/s13054-023-04780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Krivonos D.V., Fedorov D.E., Klimina K.M., Veselovsky V.A., Kovalchuk S.N., Pavlenko A.V., Yanushevich O.O., Andreev D.N., Sokolov F.S., Fomenko A.K., et al. Gut Mycobiome Changes During COVID-19 Disease. J. Fungi. 2025;11:194. doi: 10.3390/jof11030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yin Y., Tuohutaerbieke M., Feng C., Li X., Zhang Y., Xu Q., Tu J., Yang E., Zou Q., Shen T. Characterization of the Intestinal Fungal Microbiome in HIV and HCV Mono-Infected or Co-Infected Patients. Viruses. 2022;14:1811. doi: 10.3390/v14081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Feys S., Carvalho A., Clancy C.J., Gangneux J.P., Hoenigl M., Lagrou K., Rijnders B.J.A., Seldeslachts L., Vanderbeke L., van de Veerdonk F.L., et al. Influenza-Associated and COVID-19-Associated Pulmonary Aspergillosis in Critically Ill Patients. Lancet Respir. Med. 2024;12:728–742. doi: 10.1016/S2213-2600(24)00151-6. [DOI] [PubMed] [Google Scholar]
- 137.Lamoth F., Lewis R.E., Walsh T.J., Kontoyiannis D.P. Navigating the Uncertainties of COVID-19-Associated Aspergillosis: A Comparison with Influenza-Associated Aspergillosis. J. Infect. Dis. 2021;224:1631–1640. doi: 10.1093/infdis/jiab163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dewi I.M., Janssen N.A., Rosati D., Bruno M., Netea M.G., Brüggemann R.J., Verweij P.E., van de Veerdonk F.L. Invasive Pulmonary Aspergillosis Associated with Viral Pneumonitis. Curr. Opin. Microbiol. 2021;62:21–27. doi: 10.1016/j.mib.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 139.Lv L., Gu S., Jiang H., Yan R., Chen Y., Chen Y., Luo R., Huang C., Lu H., Zheng B., et al. Gut Mycobiota Alterations in Patients with COVID-19 and H1N1 Infections and Their Associations with Clinical Features. Commun. Biol. 2021;4:480. doi: 10.1038/s42003-021-02036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maurer H.C., Schult D., Koyumdzhieva P., Reitmeier S., Middelhoff M., Rasch S., List M., Janssen K.P., Steiger K., Protzer U., et al. Gut Microbial Disruption in Critically Ill Patients with COVID-19-Associated Pulmonary Aspergillosis. J. Fungi. 2022;8:1265. doi: 10.3390/jof8121265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dakalbab S., Hamdy R., Holigová P., Abuzaid E.J., Abu-Qiyas A., Lashine Y., Mohammad M.G., Soliman S.S.M. Uniqueness of Candida Auris Cell Wall in Morphogenesis, Virulence, Resistance, and Immune Evasion. Microbiol. Res. 2024;286:127797. doi: 10.1016/j.micres.2024.127797. [DOI] [PubMed] [Google Scholar]
- 142.Holt A.M., Nett J.E. Innate Immune Response to Candida auris. Curr. Opin. Microbiol. 2024;80:102510. doi: 10.1016/j.mib.2024.102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li F., McClain C.J., Feng W. Microbiome dysbiosis and alcoholic liver disease. Liver Res. 2019;3:218–226. doi: 10.1016/j.livres.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jiang L., Stärkel P., Fan J.G., Fouts D.E., Bacher P., Schnabl B. The Gut Mycobiome: A Novel Player in Chronic Liver Diseases. J. Gastroenterol. 2020;56:1–11. doi: 10.1007/s00535-020-01740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Borgo F., Verduci E., Riva A., Lassandro C., Riva E., Morace G., Borghi E. Relative Abundance in Bacterial and Fungal Gut Microbes in Obese Children: A Case Control Study. Child. Obes. 2017;13:78–84. doi: 10.1089/chi.2015.0194. [DOI] [PubMed] [Google Scholar]
- 146.Mar Rodríguez M., Pérez D., Javier Chaves F., Esteve E., Marin-Garcia P., Xifra G., Vendrell J., Jové M., Pamplona R., Ricart W., et al. Obesity Changes the Human Gut Mycobiome. Sci. Rep. 2015;5:14600. doi: 10.1038/srep14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shah A., MacDonald G.A., Morrison M., Holtmann G. Targeting the Gut Microbiome as a Treatment for Primary Sclerosing Cholangitis: A Conceptional Framework. Am. J. Gastroenterol. 2020;115:814–822. doi: 10.14309/ajg.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Muratori P., Muratori L., Guidi M., Maccariello S., Pappas G., Ferrari R., Gionchetti P., Campieri M., Bianchi F.B. Anti-Saccharomyces cerevisiae Antibodies (ASCA) and Autoimmune Liver Diseases. Clin. Exp. Immunol. 2003;132:473–476. doi: 10.1046/j.1365-2249.2003.02166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gosiewski T., Salamon D., Szopa M., Sroka A., Malecki M.T., Bulanda M. Quantitative Evaluation of Fungi of the Genus Candida in the Feces of Adult Patients with Type 1 and 2 Diabetes—A Pilot Study. Gut Pathog. 2014;6:43. doi: 10.1186/s13099-014-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Soyucen E., Gulcan A., Aktuglu-Zeybek A.C., Onal H., Kiykim E., Aydin A. Differences in the Gut Microbiota of Healthy Children and Those with Type 1 Diabetes. Pediatr. Int. 2014;56:336–343. doi: 10.1111/ped.12243. [DOI] [PubMed] [Google Scholar]
- 151.Aykut B., Pushalkar S., Chen R., Li Q., Abengozar R., Kim J.I., Shadaloey S.A., Wu D., Preiss P., Verma N., et al. The Fungal Mycobiome Promotes Pancreatic Oncogenesis via MBL Activation. Nature. 2019;574:264–267. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nguyen U.T., Kalan L.R. Forgotten Fungi: The Importance of the Skin Mycobiome. Curr. Opin. Microbiol. 2022;70:102235. doi: 10.1016/j.mib.2022.102235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Żychowska M., Bakuła Z., Decewicz P., Hryncewicz-Gwóźdź A., Dyląg M., Jankowska-Konsur A., Gawor J., Gromadka R., Żaczek A., Jagielski T. The Skin Mycobiome of Patients With Atopic Dermatitis and Healthy Volunteers: A Case-Control Study. Exp. Dermatol. 2025;34:e70085. doi: 10.1111/exd.70085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Koike Y., Kuwatsuka S., Motooka D., Murota H. Dysbiosis of the Human Skin Mycobiome in Patients Receiving Systemic IL-23 Inhibitors. Allergol. Int. 2025;74:72–77. doi: 10.1016/j.alit.2024.06.003. [DOI] [PubMed] [Google Scholar]
- 155.Carr M.M., Pryce D.M., Ive F.A. Treatment of Seborrhoeic Dermatitis with Ketoconazole: I. Response of Seborrhoeic Dermatitis of the Scalp to Topical Ketoconazole. Br. J. Dermatol. 1987;116:213–216. doi: 10.1111/j.1365-2133.1987.tb05814.x. [DOI] [PubMed] [Google Scholar]
- 156.Lober C.W. Patch Tests with Killed Sonicated Microflora in Patients with Psoriasis. Arch. Dermatol. 1982;118:322–325. doi: 10.1001/archderm.1982.01650170036019. [DOI] [PubMed] [Google Scholar]
- 157.Jury C.S., McHugh L., Shankland G.S., Burden A.D. A Randomized, Placebo-Controlled Trial of Oral Itraconazole in Scalp Psoriasis. J. Dermatol. Treat. 2000;11:85–89. doi: 10.1080/09546630050517469. [DOI] [Google Scholar]
- 158.Liu X., Cai Q., Yang H., Gao Z., Yang L. Distribution of Malassezia Species on the Skin of Patients with Psoriasis. J. Mycol. Med. 2021;31:101111. doi: 10.1016/j.mycmed.2021.101111. [DOI] [PubMed] [Google Scholar]
- 159.Łabędź N., Navarrete-Dechent C., Kubisiak-Rzepczyk H., Bowszyc-Dmochowska M., Pogorzelska-Antkowiak A., Pietkiewicz P. Pityriasis Versicolor—A Narrative Review on the Diagnosis and Management. Life. 2023;13:2097. doi: 10.3390/life13102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gajdács M., Dóczi I., Ábrók M., Lázár A., Burián K. Epidemiology of Candiduria and Candida Urinary Tract Infections in Inpatients and Outpatients: Results from a 10-Year Retrospective Survey. Cent. Eur. J. Urol. 2019;72:209–214. doi: 10.5173/CEJU.2019.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wehedy E., Murugesan S., George C.R., Shatat I.F., Al Khodor S. Characterization of the Urinary Metagenome and Virome in Healthy Children. Biomedicines. 2022;10:2412. doi: 10.3390/biomedicines10102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Behzadi P., Behzadi E., Ranjbar R. Urinary Tract Infections and Candida albicans. Cent. Eur. J. Urol. 2015;68:96–101. doi: 10.5173/ceju.2015.01.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fidel P.L., Cutright J., Steele C. Effects of Reproductive Hormones on Experimental Vaginal Candidiasis. Infect. Immun. 2000;68:651–657. doi: 10.1128/IAI.68.2.651-657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kumwenda P., Cottier F., Hendry A.C., Kneafsey D., Keevan B., Gallagher H., Tsai H.J., Hall R.A. Estrogen Promotes Innate Immune Evasion of Candida albicans through Inactivation of the Alternative Complement System. Cell Rep. 2022;38:110183. doi: 10.1016/j.celrep.2021.110183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Alam A., Levanduski E., Denz P., Villavicencio H.S., Bhatta M., Alhorebi L., Zhang Y., Gomez E.C., Morreale B., Senchanthisai S., et al. Fungal Mycobiome Drives IL-33 Secretion and Type 2 Immunity in Pancreatic Cancer. Cancer Cell. 2022;40:153–167.e11. doi: 10.1016/j.ccell.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wei A., Zhao H., Cong X., Wang L., Chen Y., Gou J., Hu Z., Hu X., Tian Y., Li K., et al. Oral Mycobiota and Pancreatic Ductal Adenocarcinoma. BMC Cancer. 2022;22:1251. doi: 10.1186/s12885-022-10329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qin X., Gu Y., Liu T., Wang C., Zhong W., Wang B., Cao H. Gut Mycobiome: A Promising Target for Colorectal Cancer. Biochim. Biophys. Acta Rev. Cancer. 2021;1875:188489. doi: 10.1016/j.bbcan.2020.188489. [DOI] [PubMed] [Google Scholar]
- 168.Coker O.O., Nakatsu G., Dai R.Z., Wu W.K.K., Wong S.H., Ng S.C., Chan F.K.L., Sung J.J.Y., Yu J. Enteric Fungal Microbiota Dysbiosis and Ecological Alterations in Colorectal Cancer. Gut. 2019;68:654–662. doi: 10.1136/gutjnl-2018-317178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Malik A., Sharma D., Malireddi R.K.S., Guy C.S., Chang T.C., Olsen S.R., Neale G., Vogel P., Kanneganti T.D. SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer. Immunity. 2018;49:515–530.e5. doi: 10.1016/j.immuni.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang P., Zhang X., Xu R., Adeel K., Lu X., Chen M., Shen H., Li Z., Xu Z. Fungal Microbiota Dysbiosis and Ecological Alterations in Gastric Cancer. Front. Microbiol. 2022;13:889694. doi: 10.3389/fmicb.2022.889694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Johannesburg W., Kew M.C. AAatoxins as a Cause of Hepatocellular Carcinoma. J. Gastrointest. Liver Dis. 2013;22:305–310. doi: 10.3748/wjg.v19.i10.1508. [DOI] [PubMed] [Google Scholar]
- 172.Mohamed N., Litlekalsøy J., Ahmed I.A., Martinsen E.M.H., Furriol J., Javier-Lopez R., Elsheikh M., Gaafar N.M., Morgado L., Mundra S., et al. Analysis of Salivary Mycobiome in a Cohort of Oral Squamous Cell Carcinoma Patients from Sudan Identifies Higher Salivary Carriage of Malassezia as an Independent and Favorable Predictor of Overall Survival. Front. Cell. Infect. Microbiol. 2021;11:673465. doi: 10.3389/fcimb.2021.673465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mäkinen A., Nawaz A., Mäkitie A., Meurman J.H. Role of Non-Albicans Candida and Candida albicans in Oral Squamous Cell Cancer Patients. J. Oral. Maxillofac. Surg. 2018;76:2564–2571. doi: 10.1016/j.joms.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 174.Dohlman A.B., Klug J., Mesko M., Gao I.H., Lipkin S.M., Shen X., Iliev I.D. A Pan-Cancer Mycobiome Analysis Reveals Fungal Involvement in Gastrointestinal and Lung Tumors. Cell. 2022;185:3807–3822.e12. doi: 10.1016/j.cell.2022.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu Q., Zhang W., Pei Y., Tao H., Ma J., Li R., Zhang F., Wang L., Shen L., Liu Y., et al. Gut Mycobiome as a Potential Non-Invasive Tool in Early Detection of Lung Adenocarcinoma: A Cross-Sectional Study. BMC Med. 2023;21:409. doi: 10.1186/s12916-023-03095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu N.N., Yi C.X., Wei L.Q., Zhou J.A., Jiang T., Hu C.C., Wang L., Wang Y.Y., Zou Y., Zhao Y.K., et al. The Intratumor Mycobiome Promotes Lung Cancer Progression via Myeloid-Derived Suppressor Cells. Cancer Cell. 2023;41:1927–1944.e9. doi: 10.1016/j.ccell.2023.08.012. [DOI] [PubMed] [Google Scholar]
- 177.Alonso R., Fernández-Fernández A.M., Pisa D., Carrasco L. Multiple sclerosis and mixed microbial infections. Direct identification of fungi and bacteria in nervous tissue. Neurobiol. Dis. 2018;117:42–61. doi: 10.1016/j.nbd.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 178.Li B.Z., Wang H., Li X.B., Zhang Q.R., Huang R.G., Wu H., Wang Y.Y., Li K.D., Chu X.J., Cao N.W., et al. Altered Gut Fungi in Systemic Lupus Erythematosus—A Pilot Study. Front. Microbiol. 2022;13:1031079. doi: 10.3389/fmicb.2022.1031079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen C.L., Chang F.C., Hung Y.M., Chou M.C., Yip H.T., Chang R., Wei J.C.C. Candida Infection as an Early Sign of Subsequent Sjögren’s Syndrome: A Population-Based Matched Cohort Study. Front. Med. 2022;8:796324. doi: 10.3389/fmed.2021.796324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pathak M.P., Pathak K., Saikia R., Gogoi U., Ahmad M.Z., Patowary P., Das A. Immunomodulatory Effect of Mushrooms and Their Bioactive Compounds in Cancer: A Comprehensive Review. Biomed. Pharmacother. 2022;149:112901. doi: 10.1016/j.biopha.2022.112901. [DOI] [PubMed] [Google Scholar]
- 181.Liu M., Luo F., Ding C., Albeituni S., Hu X., Ma Y., Cai Y., McNally L., Sanders M.A., Jain D., et al. Dectin-1 Activation by a Natural Product β-Glucan Converts Immunosuppressive Macrophages into an M1-like Phenotype. J. Immunol. 2015;195:5055–5065. doi: 10.4049/jimmunol.1501158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Varnosfaderani S.M.N., Ebrahimzadeh F., Oryani M.A., Khalili S., Almasi F., Heris R.M., Payandeh Z., Li C., Afjadi M.N., Bahrami A.A. Potential Promising Anticancer Applications of β-Glucans: A Review. Biosci. Rep. 2024;44:BSR20231686. doi: 10.1042/bsr20231686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Steimbach L., Borgmann A.V., Gomar G.G., Hoffmann L.V., Rutckeviski R., de Andrade D.P., Smiderle F.R. Fungal Beta-Glucans as Adjuvants for Treating Cancer Patients—A Systematic Review of Clinical Trials. Clin. Nutr. 2021;40:3104–3113. doi: 10.1016/j.clnu.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 184.Kushner B.H., Cheung I.Y., Modak S., Kramer K., Ragupathi G., Cheung N.K.V. Phase i Trial of a Bivalent Gangliosides Vaccine in Combination with β-Glucan for High-Risk Neuroblastoma in Second or Later Remission. Clin. Cancer Res. 2014;20:1375–1382. doi: 10.1158/1078-0432.CCR-13-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Engel-Riedel W., Lowe J., Mattson P., Richard Trout J., Huhn R.D., Gargano M., Patchen M.L., Walsh R., Trinh M.M., Dupuis M., et al. A Randomized, Controlled Trial Evaluating the Efficacy and Safety of BTH1677 in Combination with Bevacizumab, Carboplatin, and Paclitaxel in First-Line Treatment of Advanced Non-Small Cell Lung Cancer. J. Immunother. Cancer. 2018;6:16. doi: 10.1186/s40425-018-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Geng Q., Lu Y., Li D., Qin L., Qi C., Pu X., Zhuang Y., Zhu Y., Zha Q., Wang G., et al. β-Glucan Combined with Envafolimab and Endostar as Immune Rechallenge for Metastatic Non-Small Cell Lung Cancer. BMC Immunol. 2024;25:60. doi: 10.1186/s12865-024-00651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Modak S., Koehne G., Vickers A., O’Reilly R.J., Cheung N.K.V. Rituximab Therapy of Lymphoma Is Enhanced by Orally Administered (1 → 3),(1 → 4)-D-β-Glucan. Leuk. Res. 2005;29:679–683. doi: 10.1016/j.leukres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 188.Zent C.S., Call T.G., Bowen D.A., Conte M.J., LaPlant B.R., Witzig T.E., Ansell S.M., Weiner G.J. Early Treatment of High Risk Chronic Lymphocytic Leukemia with Alemtuzumab, Rituximab and PGG Beta Glucan Is Well Tolerated and Achieves High Complete Remission Rates. Leuk. Lymphoma. 2015;56:2373–2378. doi: 10.3109/10428194.2015.1016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Segal N.H., Gada P., Senzer N., Gargano M.A., Patchen M.L., Saltz L.B. A Phase 2 Efficacy and Safety, Open-Label, Multicenter Study of Imprime PGG Injection in Combination with Cetuximab in Patients with Stage IV KRAS-Mutant Colorectal Cancer. Clin. Color. Cancer. 2016;15:222–227. doi: 10.1016/j.clcc.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dalmasso G., Cottrez F., Imbert V., Lagadec P., Peyron J.F., Rampal P., Czerucka D., Groux H. Saccharomyces boulardii Inhibits Inflammatory Bowel Disease by Trapping T Cells in Mesenteric Lymph Nodes. Gastroenterology. 2006;131:1812–1825. doi: 10.1053/j.gastro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 191.Mourey F., Sureja V., Kheni D., Shah P., Parikh D., Upadhyay U., Satia M., Shah D., Troise C., Decherf A. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of Saccharomyces boulardii in Infants and Children With Acute Diarrhea. Pediatr. Infect. Dis. J. 2020;39:e347. doi: 10.1097/INF.0000000000002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pal B.B., Bandagi R.V., Pebbili K.K., Rathod R., Kotak B., Dhanaki G., Shah S. Effectiveness of Saccharomyces boulardii CNCM I-745 in Adult Indian Patients with Diarrhoea: A Real-World, Multicentre, Retrospective, Comparative Study. Drugs Real World Outcomes. 2024;11:309–316. doi: 10.1007/s40801-024-00424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Guslandi M., Giollo P., Testoni P.A. A Pilot Trial of Saccharomyces boulardii in Ulcerative Colitis. Eur. J. Gastroenterol. Hepatol. 2003;15:697–698. doi: 10.1097/00042737-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 194.Heavey M.K., Hazelton A., Wang Y., Garner M., Anselmo A.C., Arthur J.C., Nguyen J. Targeted Delivery of the Probiotic Saccharomyces boulardii to the Extracellular Matrix Enhances Gut Residence Time and Recovery in Murine Colitis. Nat. Commun. 2024;15:3784. doi: 10.1038/s41467-024-48128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pothoulakis C. Review Article: Anti-Inflammatory Mechanisms of Action of Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2008;30:826–833. doi: 10.1111/j.1365-2036.2009.04102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pineton de Chambrun G., Neut C., Chau A., Cazaubiel M., Pelerin F., Justen P., Desreumaux P. A Randomized Clinical Trial of Saccharomyces cerevisiae versus Placebo in the Irritable Bowel Syndrome. Dig. Liver Dis. 2015;47:119–124. doi: 10.1016/j.dld.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 197.Lee J.E., Lee E. The Probiotic Effects of the Saccharomyces cerevisiae 28-7 Strain Isolated from Nuruk in a DSS-Induced Colitis Mouse Model. J. Microbiol. Biotechnol. 2022;32:877–884. doi: 10.4014/jmb.2206.06035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Etienne-Mesmin L., Livrelli V., Privat M., Denis S., Cardot J.M., Alric M., Blanquet-Diot S. Effect of a New Probiotic Saccharomyces cerevisiae Strain on Survival of Escherichia Coli O157:H7 in a Dynamic Gastrointestinal Model. Appl. Environ. Microbiol. 2011;77:1127–1131. doi: 10.1128/AEM.02130-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Duysburgh C., Miclotte L., Green J.B., Watts K.T., Sardi M.I., Chakrabarti A., Khafipour E., Marzorati M. Saccharomyces cerevisiae Derived Postbiotic Alters Gut Microbiome Metabolism in the Human Distal Colon Resulting in Immunomodulatory Potential in Vitro. Front. Microbiol. 2024;15:1358456. doi: 10.3389/fmicb.2024.1358456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Poloni V.L., Pérez M.E., Escobar F., Luna J., Pereyra Y., Cristofolini A., Magnoli A., Cavaglieri L. Postbiotics from Saccharomyces cerevisiae RC016 Cell Wall (Formerly Classified as a Prebiotic): Exploring In Vitro and In Vivo Benefits. Probiotics Antimicrob. Proteins. 2025 doi: 10.1007/s12602-025-10492-8. [DOI] [PubMed] [Google Scholar]
- 201.Tullio V. Probiotic Yeasts: A Developing Reality? J. Fungi. 2024;10:489. doi: 10.3390/jof10070489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Angulo M., Ramos A., Reyes-Becerril M., Guerra K., Monreal-Escalante E., Angulo C. Probiotic Debaryomyces hansenii CBS 8339 yeast enhanced immune responses in mice. 3 Biotech. 2022;13:28. doi: 10.1007/s13205-022-03442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ochangco H.S., Gamero A., Smith I.M., Christensen J.E., Jespersen L., Arneborg N. In Vitro Investigation of Debaryomyces Hansenii Strains for Potential Probiotic Properties. World J. Microbiol. Biotechnol. 2016;32:141. doi: 10.1007/s11274-016-2109-1. [DOI] [PubMed] [Google Scholar]
- 204.Ullah M., Rizwan M., Raza A., Xia Y., Han J., Ma Y., Chen H. Snapshot of the Probiotic Potential of Kluveromyces marxianus DMKU-1042 Using a Comparative Probiogenomics Approach. Foods. 2023;12:4329. doi: 10.3390/foods12234329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Keskin P., Kılıç Kanak E., Öztürk Yılmaz S. Assessment of the Probiotic Properties of Yarrowia Lipolytica Isolated from Cold-Pressed Olive Oil. Microorganisms. 2024;12:1905. doi: 10.3390/microorganisms12091905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ganapathiwar S., Bhukya B. In Vitro Assessment for the Probiotic Potential of Pichia Kudriavzevii. Bioinformation. 2023;19:441–444. doi: 10.6026/97320630019441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Helmy E.A., Abdel-Fadeel R.H., Yosri M., Hassan E. Does Torulaspora Delbrueckii Has Some Probiotic Capabilities? In Vitro and in Vivo Assessment. Nutrire. 2024;49:15. doi: 10.1186/s41110-024-00258-7. [DOI] [Google Scholar]
- 208.Zuo T., Wong S.H., Cheung C.P., Lam K., Lui R., Cheung K., Zhang F., Tang W., Ching J.Y.L., Wu J.C.Y., et al. Gut Fungal Dysbiosis Correlates with Reduced Efficacy of Fecal Microbiota Transplantation in Clostridium Difficile Infection. Nat. Commun. 2018;9:3663. doi: 10.1038/s41467-018-06103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Leonardi I., Paramsothy S., Doron I., Semon A., Kaakoush N.O., Clemente J.C., Faith J.J., Borody T.J., Mitchell H.M., Colombel J.F., et al. Fungal Trans-Kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe. 2020;27:823–829.e3. doi: 10.1016/j.chom.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li T., Wu X., Li X., Chen M. Cancer-Associated Fungi: An Emerging Powerful Player in Cancer Immunotherapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer. 2025;1880:189287. doi: 10.1016/j.bbcan.2025.189287. [DOI] [PubMed] [Google Scholar]
- 211.El Jaddaoui I., Sehli S., Al Idrissi N., Bakri Y., Belyamani L., Ghazal H. The Gut Mycobiome for Precision Medicine. J. Fungi. 2025;11:279. doi: 10.3390/jof11040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Quinton J.F., Sendid B., Reumaux D., Duthilleul P., Cortot A., Grandbastien B., Charrier G., Targan S.R., Colombel J.F., Poulain D. Anti-Saccharomyces cerevisiae Mannan Antibodies Combined with Antineutrophil Cytoplasmic Autoantibodies in Inflammatory Bowel Disease: Prevalence and Diagnostic Role. Gut. 1998;42:788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yao G., Zhang X., Zhang T., Jin J., Qin Z., Ren X., Wang X., Zhang S., Yin X., Tian Z., et al. The Role of Dysbiotic Gut Mycobiota in Modulating Risk for Abdominal Aortic Aneurysm. Microbiol. Spectr. 2024;12:e0177624. doi: 10.1128/spectrum.01776-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yu Y., Wang R.R., Miao N.J., Tang J.J., Zhang Y.W., Lu X.R., Yan P.Y., Wang J., Jia X.M. PD-L1 Negatively Regulates Antifungal Immunity by Inhibiting Neutrophil Release from Bone Marrow. Nat. Commun. 2022;13:6857. doi: 10.1038/s41467-022-34722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Turnbull C., Bones J., Stanley M., Medhavy A., Wang H., Lorenzo A.M.D., Cappello J., Shanmuganandam S., Pandey A., Seneviratne S., et al. DECTIN-1: A Modifier Protein in CTLA-4 Haploinsufficiency. Sci. Adv. 2023;9:eadi9566. doi: 10.1126/sciadv.adi9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Szostak N., Handschuh L., Samelak-Czajka A., Tomela K., Pietrzak B., Schmidt M., Galus Ł., Mackiewicz J., Mackiewicz A., Kozlowski P., et al. Gut Mycobiota Dysbiosis Is Associated with Melanoma and Response to Anti–PD-1 Therapy. Cancer Immunol. Res. 2024;12:427–439. doi: 10.1158/2326-6066.CIR-23-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Huang X., Hu M., Sun T., Li J., Zhou Y., Yan Y., Xuan B., Wang J., Xiong H., Ji L., et al. Multi-Kingdom Gut Microbiota Analyses Define Bacterial-Fungal Interplay and Microbial Markers of Pan-Cancer Immunotherapy across Cohorts. Cell Host Microbe. 2023;31:1930–1943.e4. doi: 10.1016/j.chom.2023.10.005. [DOI] [PubMed] [Google Scholar]
- 218.Hu M., Zhu X., Huang X., Hua L., Lin X., Zhang H., Hu Y., Tong T., Li L., Xuan B., et al. Optimizing Anti-PD-1/PD-L1 Therapy Efficacy and Fecal Microbiota Transplantation Donor Selection through Gut Mycobiome-Based Enterotype. Cell Rep. 2025;44:115589. doi: 10.1016/j.celrep.2025.115589. [DOI] [PubMed] [Google Scholar]
- 219.Zhu C., Zhang C., Wang S., Xun Z., Zhang D., Lan Z., Zhang L., Chao J., Liang Y., Pu Z., et al. Characterizations of Multi-Kingdom Gut Microbiota in Immune Checkpoint Inhibitor-Treated Hepatocellular Carcinoma. J. Immunother. Cancer. 2024;12:e008686. doi: 10.1136/jitc-2023-008686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lin A., Huang L., Jiang A., Zhu L., Mou W., Li Y., Zhang C., Liu Z., Zhang J., Cheng Q., et al. Microbiota Boost Immunotherapy? A Meta-Analysis Dives into Fecal Microbiota Transplantation and Immune Checkpoint Inhibitors. BMC Med. 2025;23:341. doi: 10.1186/s12916-025-04183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]