Abstract
Inonotus obliquus, a medicinal mushroom valued for its bioactive compounds, has not been previously characterized from Romanian sources. This study presents the first comprehensive chemical and biological screening of I. obliquus, introducing novel polymer-based encapsulation systems to enhance the stability and bioavailability of its bioactive constituents. Two distinct delivery systems were designed to enhance the functionality of I. obliquus extracts: (i) microencapsulation in maltodextrin (MIO) and (ii) a sequential approach involving preparation of silver nanoparticle-loaded I. obliquus (IO–AgNPs), followed by microencapsulation to yield the hybrid MIO–AgNP system. Comprehensive metabolite profiling using GC–MS and ESI–QTOF–MS revealed 142 bioactive constituents, including terpenoids, flavonoids, phenolic acids, amino acids, coumarins, styrylpyrones, fatty acids, and phytosterols. Structural integrity and successful encapsulation were confirmed by XRD, FTIR, and SEM analyses. Both IO–AgNPs and MIO–AgNPs demonstrated potent antioxidant activity, significant acetylcholinesterase inhibition, and robust antimicrobial effects against Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa, and Escherichia coli. Cytotoxicity assays revealed pronounced activity against MCF-7, HCT116, and HeLa cell lines, with MIO–AgNPs exhibiting superior efficacy. The synergistic integration of maltodextrin and AgNPs enhanced compound stability and bioactivity. As the first report on Romanian I. obliquus, this study highlights its therapeutic potential and establishes polymer-based nanoencapsulation as an effective strategy for optimizing its applications in combating microbial resistance and cancer.
Keywords: Inonotus obliquus, silver nanoparticles, micro-spray encapsulation, antioxidant potential, antimicrobial screening, anti-acetylcholinesterase activity, in vitro cytotoxicity
1. Introduction
Inonotus obliquus (Ach. ex Pers.) Pilát, commonly known as Chaga mushroom, is a slow-growing medicinal fungus of the Hymenochaetaceae family [1,2,3,4,5,6]. For centuries, it has been utilized in traditional medicine across Russia, the Baltic countries, Eastern Europe, and parts of Asia to treat various ailments, including gastrointestinal disorders, inflammatory conditions, diabetes mellitus, and cancer [1,2,3,4,5,6]. Its therapeutic potential is attributed to a diverse array of bioactive compounds, including polysaccharides (notably β-glucans), triterpenoids, polyphenols (such as phenolic acids), and melanin-like pigments, which exhibit antioxidant, immunomodulatory, antitumor, and antidiabetic properties [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15].
This parasitic fungus primarily colonizes birch trees (Betula pendula, B. pubescens) and, less commonly, other deciduous species such as Fagus sylvatica (European beech), forming a characteristic sterile conk or sclerotium [1,2,3,4,5,6,7]. The sclerotium appears as a black, cracked, charcoal-like mass enclosing a reddish-brown interior of obliquely aligned hyphal tubes [1,2,3,4,5,6,7,8]. These sclerotia, which persist on host trees for years, are traditionally harvested for medicinal use [1,2,3,4,5,6,7]. In Romania, wild I. obliquus is found in mountainous forested regions and is sporadically employed in folk remedies and artisanal health products [16]. However, despite its ethnomedicinal significance, Romanian I. obliquus remains understudied regarding its chemical composition and biological activity, representing a significant gap in the scientific literature.
Globally, the rising demand for functional foods and nutraceuticals has spurred renewed interest in I. obliquus [1,2,3,4,5,6,7,13,17]. Chaga-based products, including teas, extracts, powders, and capsules, are increasingly marketed for their health-promoting effects [1,2,3,4,5,6,7].
However, the chemical profile and corresponding bioactivity of natural compounds are profoundly influenced by a complex interplay of factors, including geographic origin, host tree species, environmental and climatic conditions, as well as extraction-related parameters such as solvent polarity, drying method, temperature, and pH [7,8,9,10,11,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31].
Region-specific studies on Romanian botanical and fungal sources have provided robust, experimentally validated evidence that environmental conditions, geographic origin, host species, and extraction methodologies significantly influence the phytochemical composition and biological efficacy of natural extracts [22,23,24,25,26,27,28,29].
These investigations collectively demonstrate that variations in these factors generate distinct bioactive profiles, underscoring the necessity for careful consideration to ensure reproducibility and therapeutic reliability in natural product research [22,23,24,25,26,27,28,29].
Consequently, there is a critical need for localized studies to elucidate the unique phytochemical signatures of I. obliquus from Romanian ecosystems, which may differ substantially from those reported in other regions [1,2,3,4,5,6,7,8,9,10,11,12,13,14,16,17,18,19,20]. Given the well-documented influence of environmental and methodological factors on chemical composition and biological activity, the absence of comprehensive region-specific data represents a critical unmet need within the current research landscape [22,23,24,25,26,27,28,29].
Addressing this deficiency is essential, as local fungal populations are likely to exhibit distinct phytochemical profiles and bioactivities, necessitating targeted investigations to support their evidence-based and standardized application in health-related fields.
Concurrently, modern biomedical research faces two pressing global challenges: escalating antimicrobial resistance (AMR) and the rising prevalence of neurodegenerative diseases [31,32,33,34,35,36]. These issues necessitate novel therapeutic agents with innovative mechanisms of action [31,32,33,34,35,36,37,38,39]. Natural products, particularly those derived from fungi and medicinal plants, are actively explored for their dual antimicrobial and acetylcholinesterase (AChE) inhibitory activities due to their structural diversity, multi-target pharmacological profiles, and generally favorable safety profiles [1,2,4,5,6,7,36,37,38,39,40,41,42,43,44].
Despite its pharmacological promise, the practical application of I. obliquus is limited by several challenges [1,2,3,4,5,6,7,8,9,10,12,14,17,18,20,21]. Various secondary metabolites often exhibit poor water solubility, structural complexity, and limited stability, reducing bioavailability and clinical efficacy [23,28,29]. Additionally, the bitter taste and chitin-rich texture of raw Chaga hinder its use in direct consumption or food formulations [1,2,3,4,5,6,7,17]. To address these limitations, recent efforts have focused on nanotechnology and encapsulation strategies to enhance solubility, stability, palatability, and controlled delivery of Chaga-derived bioactive compounds [45].
Among nanocarrier systems, silver nanoparticles (AgNPs) have garnered significant attention due to their unique physicochemical and biomedical properties, including surface plasmon resonance, tunable morphology, and potent antimicrobial, antioxidant, and cytotoxic effects [29,31,33,34,46,47,48]. Functionalizing AgNPs with natural bioactive compounds can improve their pharmacokinetic profiles, enable targeted delivery, and promote synergistic therapeutic effects [31,33,34,37,39,46,49]. However, the development of hybrid systems combining I. obliquus with AgNPs, particularly using Romanian wild strains, remains largely unexplored.
Encapsulation technologies, particularly those employing food-grade carriers such as maltodextrin, offer a promising approach to overcome the formulation challenges of Chaga-based products [50,51]. Spray drying, in particular, facilitates the production of stable powders with improved solubility, taste masking, protection of thermolabile compounds, and controlled release capabilities [50,51,52]. Despite these advantages, spray drying encapsulation of I. obliquus has not been reported, representing a novel opportunity for product development.
In response to these challenges and research gaps, this study introduces several key innovations with scientific and practical relevance. It presents the first comprehensive mycochemical profiling of low-molecular-weight metabolites from wild-harvested Romanian I. obliquus, providing critical insight into its unique regional phytochemical fingerprint. Building on this chemical foundation, two novel delivery systems were developed to enhance the functional usability and application potential of I. obliquus. The first system involves spray drying encapsulation of powdered I. obliquus in a maltodextrin matrix to improve solubility, stability, and functional performance. The second system comprises a ternary formulation combining I. obliquus with AgNPs, subsequently encapsulated in a maltodextrin matrix via spray drying, to promote synergistic bioactivity and enhance delivery efficiency. Additionally, the biological efficacy of Romanian I. obliquus and the resulting Chaga-based hybrid systems, both before and after encapsulation, was evaluated through comprehensive physicochemical characterization and in vitro assays of antioxidant, antimicrobial, AChE inhibitory, and cytotoxic activities.
2. Materials and Methods
2.1. Chemicals and Reagents
All chemicals and reagents utilized in this study were of analytical grade to ensure experimental accuracy, reproducibility, and consistency across assays. Key reagents, including 2,2-diphenyl-1-picrylhydrazyl (DPPH), Folin–Ciocalteu reagent, dimethyl sulfoxide (DMSO), potassium persulfate, sodium acetate, sodium carbonate, sodium phosphate, ammonium molybdate, potassium chloride, 2,4,6-tris(2-pyridyl)-1,3,5-triazine (TPTZ), ferric chloride (FeCl3), ferrous sulfate heptahydrate (FeSO4·7H2O), and hydrochloric acid (HCl) were procured from Sigma-Aldrich (München, Germany). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit was sourced from AAT Bioquest (Pleasanton, CA, USA). Maltodextrin (dextrose equivalent: 16.5–19.5) was obtained from Carbosynth (Berkshire, UK). Additional reagents included AChE from Electrophorus electricus, 1-naphthyl acetate, Fast Blue B salt, Tris-HCl buffer solution (0.05 M, pH 7.8), and rivastigmine (used as a positive control), all procured from Sigma-Aldrich. The solvents used in this study included methanol, ethanol, and chloroform (Merck, Darmstadt, Germany). Ultrapure water, produced using a Milli-Q system (Merck, Darmstadt, Germany), was employed throughout all experimental procedures.
2.2. Fungal Material (IO Sample)
The wild-grown I. obliquus was harvested in November 2023 from a distinct ecological area in central-western part of Romania: Râu de Mori Commune, on the shore of the Gura Apelor Lake, Retezat Mountains, Hunedoara County, Transylvania Region. The fungal material (Inonotus obliquus) was authenticated and deposited in the Herbarium of the Department of Pharmaceutical Botany, Faculty of Pharmacy, University of Medicine and Pharmacy of Craiova, Romania (voucher code: INON-OBQ-2023-0811). After air-drying for 24 h in brown paper bags under controlled conditions (room temperature, cool and dark environment), the material was prepared for extraction and analysis. The study did not involve any endangered or protected plant or fungal species.
2.3. Preparation of AgNPs
AgNPs (20–40 nm) were synthesized following previously validated protocols [37,39,49].
2.4. Cell Lines
Human cancer cell lines, MCF-7 (breast), HCT116 (colorectal), and HeLa (cervical), were sourced from ATCC (Manassas, VA, USA). Cells were maintained in DMEM (Gibco, UK) supplemented with 10% FBS and 1% antibiotic–antimycotic (Sigma-Aldrich, Germany), under standard culture conditions (37 °C, 5% CO2, humidified atmosphere) [39,53,54].
2.5. Bacterial Strains
Bacterial strains, including Staphylococcus aureus (ATCC 29213), Bacillus cereus (ATCC 14579), Pseudomonas aeruginosa (ATCC 27853), and Escherichia coli (ATCC 25922), were sourced from ATCC (Manassas, VA, USA). Strains were maintained and cultured according to ATCC guidelines to ensure viability and consistency [39,53,54].
2.6. Fungal Sample Preparation for Mycochemical Screening
Air-dried I. obliquus samples were ground using a Fritsch Pulverisette planetary mill (Idar-Oberstein, Germany) at 800 rpm for 15 min under controlled conditions (22 °C). The powder was then sieved (ASTM standard) to isolate particles sized 0.15–0.20 mm. Extraction was performed by sonication (Elmasonic, Singen, Germany) at 40 °C and 70 Hz for 45 min using 30 mL methanol as solvent. All extractions were carried out in triplicate to ensure reproducibility.
2.7. GC–MS Analysis
Gas chromatography–mass spectrometry (GC–MS) analysis was performed on a Shimadzu GCMS-QP2020 NX system equipped with a ZB-5MS capillary column (50 m × 0.20 mm, 0.33 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). Helium served as the carrier gas at 1 mL/min. The oven temperature program started at 50 °C (2 min hold), ramped at 3 °C/min to 300 °C, and held for 2 min. Injector and interface temperatures were set at 280 °C and 225 °C, respectively. Ionization was conducted at 80 eV with a 1 min solvent delay. The mass spectrometer source and quadrupole temperatures were maintained at 230 °C and 140 °C, respectively. Analyses were conducted in triplicate.
Compound identification was achieved by matching mass spectra against the NIST 2.0 database (NIST, Gaithersburg, MD, USA) and supported by literature comparison. Retention indices were calculated using the Van den Dool and Kratz method with a C7–C40 n-alkane standard, while Kováts retention indices were obtained via logarithmic interpolation to improve identification accuracy [53,54,55,56].
2.8. MS Analysis
Mass spectrometry analysis was conducted using an electrospray ionization–quadrupole time-of-flight system (ESI–QTOF–MS; Bruker Daltonics, Bremen, Germany) operating in positive ion mode over a mass range of 50–3000 m/z with a scan rate of 2.0 scans/s. Collision energies varied between 20 and 80 eV, and the source block temperature was set at 80 °C. Biomolecule identification was performed by matching spectra against the NIST/NBS-3 library and supported by relevant literature [53,54].
2.9. Spray Drying Process
Spray drying was carried out using a Mini Spray Dryer B-290 (Büchi, Flawil, Switzerland) under optimized conditions: feed flow rate of 8 mL/min, inlet temperature of 125 °C, outlet temperature of 70 °C, airflow of 30 m3/h, compressor pressure of 0.05 MPa, and a 0.7 mm nozzle diameter. The process was conducted at approximately 80% relative humidity with 100% suction airflow [53,54,57,58].
2.10. Preparation of I. obliquus–AgNP (IO–AgNP) System
The IO–AgNP system was prepared by mixing I. obliquus powder (obtained as previously described) with an AgNP solution at a 1:2 mass ratio. This ratio was selected based on our prior studies to ensure an optimal balance between nanoparticle stability and the efficient incorporation of bioactive compounds [37,38,39,49,59,60]. The mixture was subjected to ultrasonic treatment at 35 °C for 40 min to promote uniform dispersion and interaction between the components. The resulting suspension was then filtered using 185 mm filter paper and dried in an oven at 45 °C for 8 h. All experiments were conducted in triplicate to ensure reproducibility.
2.11. Preparation of Maltodextrin–I. obliquus (MIO) System
The MIO system was prepared by dissolving 2.2 g each of dried I. obliquus and maltodextrin in 50 mL ultrapure water, followed by thorough homogenization to ensure uniform dispersion and effective encapsulation, as validated in similar systems [53,54]. The mixture was incubated at 30 °C with continuous stirring for 30 min to promote interaction between bioactive compounds and the carrier matrix. After incubation, the suspension was centrifuged for 8 min and filtered through a 0.45 μm Whatman membrane. The clear filtrate was spray-dried, and the resulting powder was stored in opaque, airtight containers at 25 °C to maintain stability. All experiments were performed in triplicate to ensure reproducibility.
2.12. Preparation of Maltodextrin–IO–AgNP (MIO–AgNP) System
The MIO–AgNP system was prepared by mixing the IO–AgNPs formulation with maltodextrin at a 1:1 mass ratio, following the same procedure established for the MIO system [53,54]. Experiments were conducted in triplicate to ensure reproducibility.
2.13. Characterization of New Prepared I. obliquus-Based Systems
2.13.1. FTIR Spectroscopy
FTIR spectra were recorded using a Shimadzu AIM-9000 spectrometer equipped with ATR (Shimadzu, Tokyo, Japan). Measurements were performed over 20 scans with a 4 cm−1 resolution across the 4000–400 cm−1 range. Peak assignments were interpreted based on established literature.
2.13.2. XRD Analysis
X-ray diffraction (XRD) patterns were acquired using a Bruker AXS D8 Advance diffractometer (Karlsruhe, Germany) with CuKα radiation (λ = 0.1541 nm). Measurements were performed over a 2θ range of 5° to 80°, with a 0.02° step size and 2 s per step scan rate. The system included a rotating sample stage and temperature control units for low and high temperatures. Data analysis was conducted using DIFFRAC.EVA software version 7.0 and compared to reference patterns from the ICDD Powder Diffraction Database (ICDD file 04-015-9120). Crystallite size, lattice parameters, and phase composition were determined using the whole powder pattern fitting (WPPF) method via TOPAS software version 7.1.0 for accurate structural characterization.
2.13.3. Scanning Electron Microscopy (SEM) Analysis
Surface morphology and elemental composition were examined using a JEOL JSM-IT200 InTouchScope™ SEM (Freising, Germany) with a field emission gun (FEG) and energy-dispersive X-ray spectroscopy (EDS). Samples were mounted on carbon tape and sputter-coated with a 10 nm gold layer for conductivity enhancement. Imaging was conducted at 15 kV accelerating voltage and a 10 mm working distance, with magnifications ranging from 100× to 10,000×. EDS analysis was conducted to quantify elemental distributions, with spectra collected at multiple regions to ensure representativeness.
2.13.4. DLS Analysis
For dynamic light scattering (DLS) analysis, particle size distribution (PSD) was measured using a Microtrac Nanotrac Wave II (Microtrac Retsch GmbH, Montgomeryville, PA, USA). Samples were dispersed in deionized water and analyzed at 23 °C with a laser wavelength of 780 nm and a scattering angle of 180°. Each measurement was performed in triplicate, with results reported as the mean hydrodynamic diameter and polydispersity index (PDI).
2.13.5. Encapsulation Efficiency, Loading Capacity, and Yield
The encapsulation efficiency (EE%), loading capacity (LC%), and encapsulation yield (EY%) of I. obliquus and the IO–AgNP system were determined using Equations (1), (2), and (3), respectively [49,53,54,57,58]:
| (1) |
| (2) |
| (3) |
Quantification was carried out using a PerkinElmer Lambda 35 UV-Vis spectrophotometer (Waltham, MA, USA). Samples (20 mg) underwent ultrasound-assisted extraction (UAE) at 70 kHz in 25 mL of hydrochloric acid–ethanol–chloroform (3:2:2, v/v/v) for 45 min at 22 °C. Following extraction, samples were centrifuged at 6000 rpm for 10 min, and the supernatant was analyzed at 280 nm using an ethanol–chloroform (1:1, v/v) blank. All measurements were performed in triplicate using a 10 mm quartz cuvette [49,53,54,57,58].
2.14. Thermal Analysis
To evaluate the thermal stability and decomposition profiles of the samples, thermo-gravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed using a Mettler Toledo TG/DSC3+ thermal analyzer equipped with a high-temperature thermogravimetry/differential thermal analysis (TG/DTA) sensor. Samples were analyzed in 40 μL aluminum crucibles under a synthetic air atmosphere (purity 5.0), with a flow rate of 50 mL/min. The temperature range spanned 25–400 °C at a heating rate of 10 °C/min to ensure consistent thermal decomposition data. Calibration of the instrument was performed using indium and zinc standards to ensure accuracy and precision in temperature and enthalpy measurements.
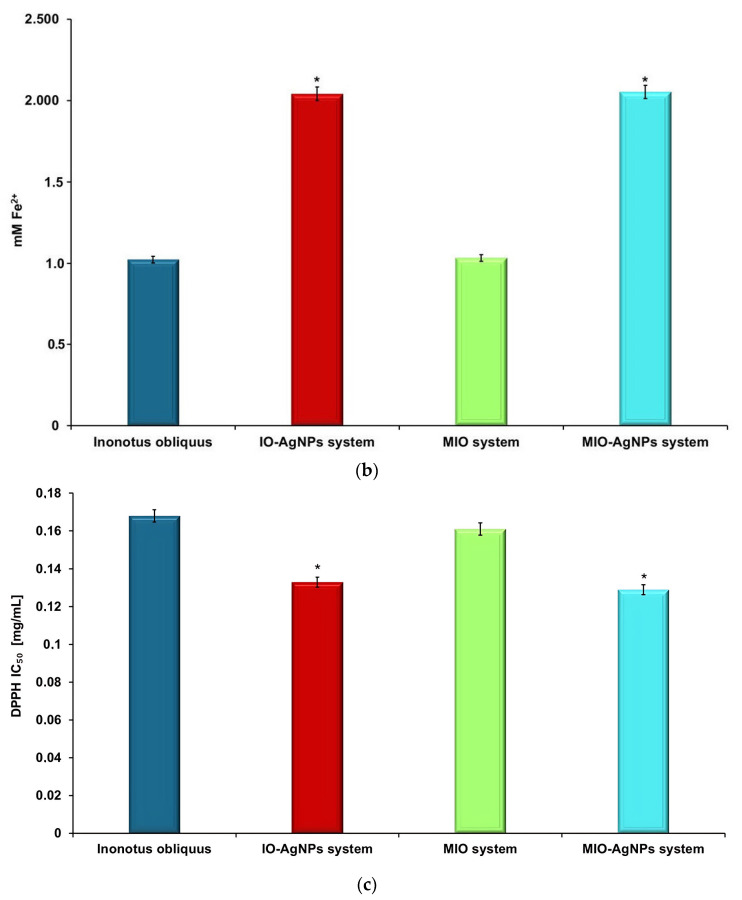
2.15. Estimation of Total Phenolic Content and Antioxidant Activity
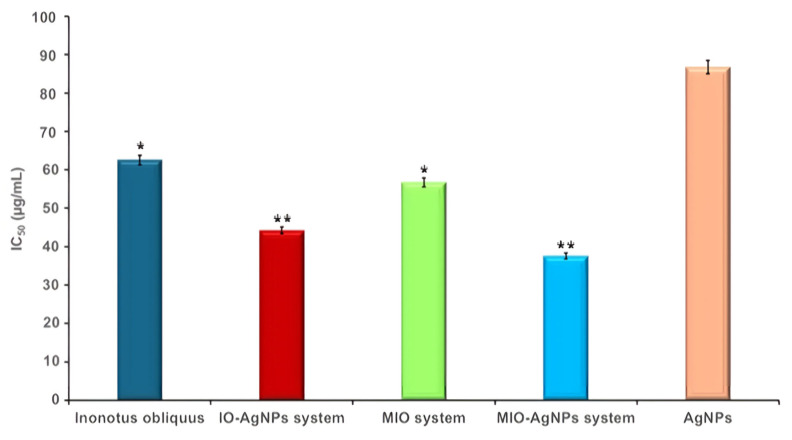
The total phenolic content (TPC) and antioxidant activity of I. obliquus (IO), IO–AgNP system, modified IO (MIO) system, and MIO–AgNP system were systematically evaluated using standardized biochemical assays (ferric reducing antioxidant power (FRAP) and DPPH radical scavenging assays). All experiments were conducted in triplicate to ensure reproducibility and statistical reliability.
2.15.1. Sample Preparation Procedure
For extraction, 0.40 g of each sample (I. obliquus, IO–AgNPs, MIO, or MIO–AgNPs) was suspended in 10 mL of 70% (v/v) ethanol and agitated continuously at 23 °C for 10 h to maximize phenolic compound extraction. The mixtures were then centrifuged at 5000 rpm (approximately 3000× g) for 15 min to separate the supernatant from solid residues. The supernatant was collected and stored at 4 °C for subsequent TPC and antioxidant activity analyses. The same extraction protocol was applied to the IO–AgNP system, MIO system, and MIO–AgNP system to ensure methodological consistency.
2.15.2. TPC Assay
Total phenolic content (TPC) was determined using the Folin–Ciocalteu assay, following established methods [49,53,54]. In brief, 100 μL of each extract was mixed with 500 μL of Folin–Ciocalteu reagent (1:10 dilution in deionized water) and 400 μL of 7.5% (w/v) sodium carbonate solution. After incubating at 40 °C for 30 min, absorbance was recorded at 765 nm using a FLUOstar Optima UV–Vis spectrometer (BMG Labtech, Offenburg, Germany). TPC values were calculated from a gallic acid calibration curve (R2 = 0.9637) using the linear Equation (4):
| y = 0.0022x + 0.0225 | (4) |
Results were expressed as milligrams of gallic acid equivalents (GAE) per gram of sample (mg GAE/g).
2.15.3. FRAP Assay
The FRAP assay was performed to assess the reducing capacity of the samples, following previously described protocols [49,53,54,59,60]. Sample extracts (50 μL) were mixed with 1.5 mL of FRAP reagent (containing 10 mM TPTZ, 20 mM FeCl3, and 300 mM acetate buffer, pH 3.6) and incubated at 37 °C for 4 min. Absorbance was measured at 595 nm using a FLUOstar Optima UV–Vis spectrometer. Antioxidant activity was quantified as millimolar Fe2+ equivalents (mM Fe2+) using Equation (5):
| (5) |
where CFe2+ is the Fe2+ concentration (nM) derived from the calibration curve (R2 = 0.9997; y = 0.0016x + 0.0915), F is the dilution factor, and V is the sample volume (μL).
2.15.4. DPPH Radical Scavenging Assay
The DPPH radical scavenging activity was determined according to previous studies [53,54,55,56]. Sample extracts (100 μL) were mixed with 3.9 mL of 0.1 mM DPPH solution in methanol and incubated in the dark at 23 °C for 30 min. Absorbance was measured at 520 nm using a FLUOstar Optima UV–Vis spectrometer. The percentage of DPPH inhibition was calculated using Equation (6):
| (6) |
where A0 is the absorbance of the control (DPPH solution without sample) and A1 is the absorbance of the sample. The half-maximal inhibitory concentration (IC50, μg/mL) was determined from the inhibition percentage plotted against sample concentrations.
2.16. AChE Inhibitory Assay
The neuroprotective potential of the previously prepared samples (I. obliquus, IO–AgNPs, MIO, and MIO–AgNPs) was assessed using a microplate-based AChE inhibitory assay. Serial dilutions of each sample were prepared to establish a concentration gradient. The enzymatic reaction was initiated by the addition of 1-naphthyl acetate (3 mg/mL in ethanol) as the substrate, followed by AChE enzyme (3.33 U/mL). Fast Blue B salt (3 mg/mL in water) was used as a chromogenic reagent to detect enzymatic activity. Absorbance was measured at 595 nm using a microplate reader. Rivastigmine served as the positive control [42,44].
2.17. Antimicrobial Activity
The antimicrobial activity of I. obliquus, IO–AgNPs, MIO, and MIO–AgNPs was evaluated against a panel of bacterial strains using agar well diffusion, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) assays. All experiments were performed in triplicate to ensure reproducibility.
2.17.1. Agar Well Diffusion Assay
The agar well diffusion assay was performed following established protocols [54,55]. Müller–Hinton agar plates were inoculated with microbial suspensions standardized to 0.5 McFarland (1.5 × 108 CFU/mL). Wells (6 mm diameter) were filled with 50 μL of sample solutions at concentrations ranging from 100 to 200 μg/mL in 25% DMSO [39,53,54,59]. Plates were incubated at 37 °C for 24 h, and inhibition zone diameters were measured using a digital caliper.
2.17.2. MIC and MBC Determination
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values were measured using the microbroth dilution method in Müller–Hinton broth. Serial dilutions of samples (100–200 μg/mL) were prepared in 96-well plates, followed by the addition of 100 μL microbial suspension standardized to 0.5 McFarland. After 24 h of incubation at 37 °C, MIC was defined as the lowest concentration preventing visible growth, assessed by optical density at 600 nm using a T90+ UV–Vis spectrophotometer (PG Instruments, Wibtoft, UK). MBC was determined by sub-culturing 10 μL from wells without growth onto Müller–Hinton agar plates, incubated for an additional 14 h at 37 °C. [39,53,54,59]. The MBC corresponded to the lowest concentration showing no bacterial growth.
2.18. Cell Culture and Cytotoxicity Assessment
2.18.1. Cell Culture and Treatment
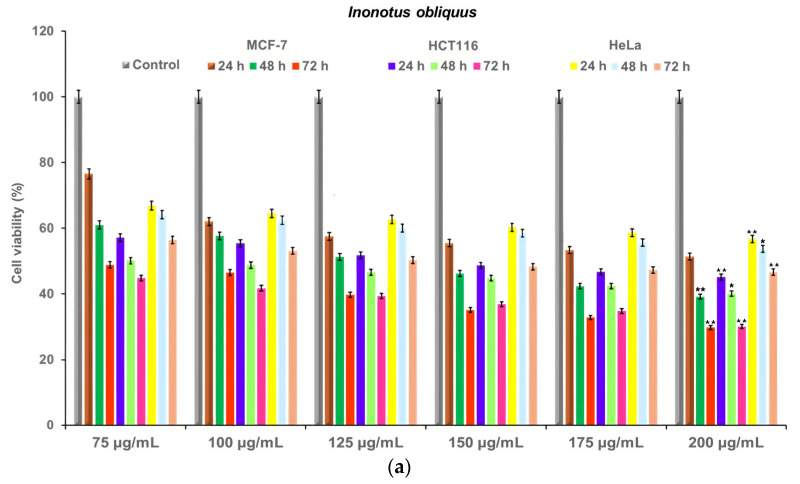
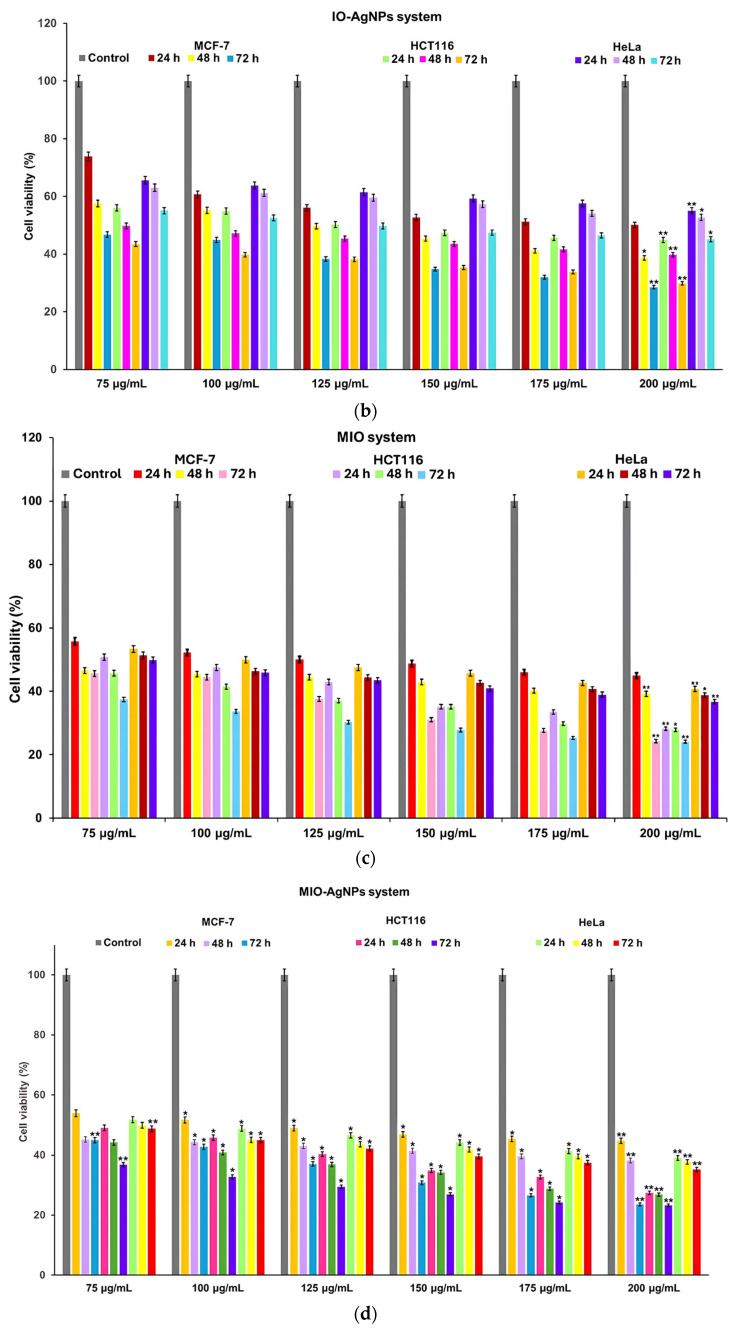
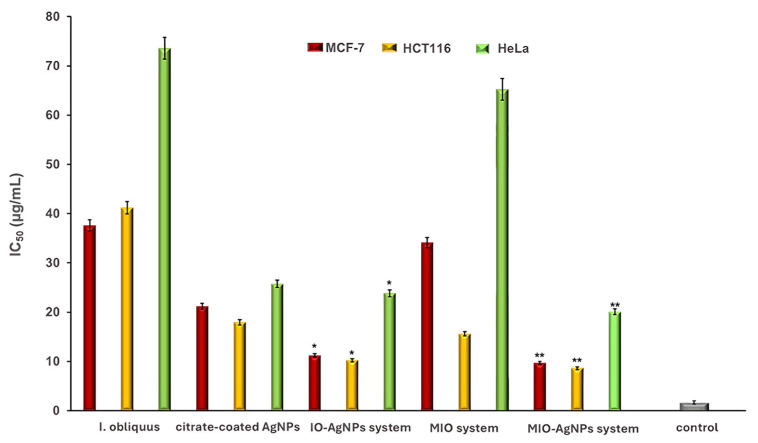
The cytotoxicity of I. obliquus, IO–AgNPs, MIO, and MIO–AgNPs was evaluated using MCF-7 (breast cancer), HCT116 (colorectal cancer), and HeLa (cervical cancer) cell lines (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS and 1% antibiotic–antimycotic solution, maintained at 37 °C in a humidified 5% CO2 atmosphere. Cells were seeded at 4 × 103 cells/well in 96-well plates and incubated for 24 h to achieve 90% confluency. The medium was replaced with fresh DMEM containing sample concentrations of 75, 100, 125, 150, 175, and 200 μg/mL [39,53,54,59,60]. Positive (untreated cells) and negative (non-viable cells) controls were included. All conditions were tested in triplicate.
2.18.2. Cell Viability Assessment
Cell viability was determined using the MTT assay. After 24 h of treatment, the culture medium was removed, and 25 μL of MTT solution (5 mg/mL) was added to each well. Plates were incubated for 2 h at 37 °C to allow formazan formation, which was subsequently dissolved by adding 100 μL of DMSO. Absorbance was read at 540 nm using a Synergy HTX Multi-Mode Microplate Reader (Agilent Technologies, USA). Cell viability (%) was calculated according to Equation (7).
| (7) |
where ODsample is the optical density of treated cells, ODcontrol is the optical density of untreated cells, and ODblank is the optical density of the culture medium. IC50 values (concentration reducing cell viability by 50%) were determined by plotting cell viability against sample concentrations (75, 100, 125, 150, 175, and 200 μg/mL) [39,53,54,59,60,61].
2.19. Statistical Analysis
Experiments were conducted in triplicate, and data are presented as mean ± standard deviation (SD). Statistical significance was assessed using Student’s t-test for two-group comparisons and one-way ANOVA followed by Tukey’s HSD post hoc test for multiple comparisons. Analyses were performed with Microsoft Excel 2019 (Microsoft Corporation, Redmond, WA, USA). Differences with p-values less than 0.05 were considered statistically significant.
3. Results
3.1. Mycochemical Screening
The chemical complexity of Romanian I. obliquus was investigated using a dual-platform analytical approach that combined GC–MS and ESI–QTOF–MS. This integrated methodology enabled comprehensive profiling of both volatile and semi-volatile low-polarity compounds as well as polar, thermolabile metabolites, providing a detailed phytochemical map of I. obliquus from this specific geographical region.
3.1.1. GC–MS Analysis
I. obliquus is known to contain a wide array of non-polar bioactive compounds, such as lanostane-type triterpenoids, sterols, and hydrocarbons, which require thermal desorption and electron ionization for effective separation and identification [1,2,3,4,5,6,7,8]. GC–MS offers high chromatographic resolution and delivers retention indices alongside characteristic fragmentation patterns, which are essential for the reliable structural confirmation of these compounds. Additionally, GC–MS enables semi-quantitative assessment of compound abundance through relative peak area integration, offering valuable insights into the dominant chemical constituents. Importantly, many GC–MS-detected compounds contain functional groups, hydroxyl, ketone, ester, and carboxyl, that can interact with AgNPs, affecting surface functionalization, stability, and controlled release profiles [62,63,64]. Therefore, GC–MS analysis was not only crucial for characterizing the chemical diversity of I. obliquus but also for pinpointing molecular candidates relevant to the rational design of AgNP-based engineered delivery systems (IO–AgNPs and MIO–AgNPs).
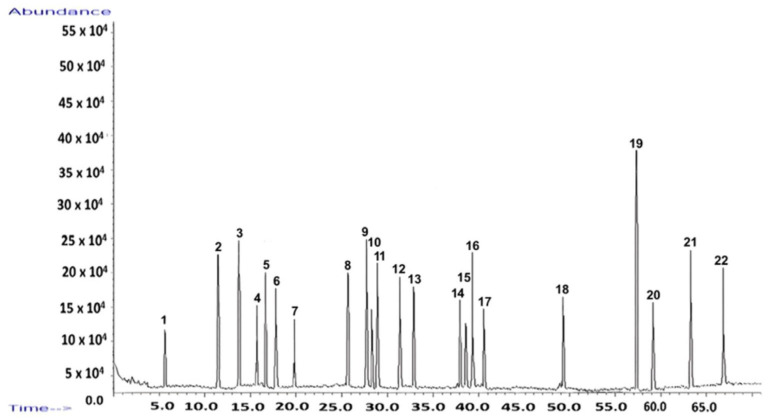
The chromatographic fingerprint (Figure 1) visually illustrates the complexity of the extract, with each peak corresponding to a specific compound detailed in Table 1.
Figure 1.
Total ion chromatogram of I. obliquus sample.
Table 1.
Main compounds identified by GC–MS analysis of the I. obliquus sample.
| No. | tR (min) | RI Determined | Kováts Index | Compound | Formula | Molecular Weight (g/mol) | Area (%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.78 | 1903 | 1904 | methyl palmitate | C17H34O2 | 270.50 | 1.69 | [65] |
| 2 | 11.89 | 2061 | 2063 | methyl linoleate | C19H34O | 294.50 | 2.45 | [66] |
| 3 | 13.91 | 1149 | 1150 | benzyl acetate | C9H10O2 | 150.17 | 2.52 | [66] |
| 4 | 15.71 | 1993 | 1994 | henicosane | C21H44 | 296.58 | 1.84 | [66] |
| 5 | 16.82 | 2165 | 2167 | brassicasterol | C28H46O | 398.70 | 1.96 | [67] |
| 6 | 18.43 | 3273 | 3274 | ergosterol | C28H44O | 396.60 | 1.91 | [67] |
| 7 | 19.98 | 1483 | 1485 | α-curcumene | C15H22 | 202.33 | 1.73 | [37,39,53] |
| 8 | 25.93 | 1429 | 1431 | coumarin | C9H6O2 | 146.14 | 1.94 | [66] |
| 9 | 27.76 | 1879 | 1880 | α-turmerone | C15H22O | 218.33 | 2.71 | [37] |
| 10 | 28.42 | 1447 | 1449 | bergamotene | C15H24 | 204.35 | 2.67 | [68] |
| 11 | 28.81 | 3266 | 3268 | lupeol | C30H50O | 426.70 | 2.55 | [69] |
| 12 | 31.47 | 3383 | 3385 | lupenone | C30H48O | 424.70 | 2.61 | [70] |
| 13 | 32.91 | 1597 | 1599 | hexadecane | C16H34 | 226.44 | 2.47 | [54,66] |
| 14 | 38.44 | 1696 | 1698 | heptadecane | C17H36 | 240.50 | 2.12 | [54,66] |
| 15 | 38.87 | 1753 | 1755 | benzyl benzoate | C14H12O2 | 212.24 | 1.81 | [66] |
| 16 | 39.51 | 3291 | 3293 | β-sitosterol | C29H50O | 414.70 | 2.33 | [54] |
| 17 | 41.78 | 1801 | 1802 | octadecane | C18H38 | 254.50 | 1.78 | [66] |
| 18 | 49.03 | 3611 | 3613 | ergosterol peroxide | C28H44O3 | 428.60 | 1.89 | [71] |
| 19 | 57.92 | 2756 | 2757 | inotodiol | C30H50O2 | 442.70 | 14.42 | [72] |
| 20 | 61.77 | 1628 | 1629 | β-eudesmol | C15H26O | 222.37 | 1.87 | [54,66] |
| 21 | 63.27 | 2969 | 2971 | betulin | C30H50O2 | 442.70 | 2.29 | [73] |
| 22 | 66.39 | 2853 | 2855 | trametenolic acid | C30H48O3 | 456.70 | 2.14 | [18] |
GC–MS: Gas chromatography–mass spectrometry; RI: Retention index; tR: Retention time.
GC–MS analysis identified 22 distinct compounds in the I. obliquus extract, collectively accounting for 59.70% of the total ion chromatogram (Figure 1; Table 1). These compounds belong to several key chemical classes:
Triterpenoids and sterols: inotodiol (14.42%), lupeol (2.55%), lupenone (2.61%), β-sitosterol (2.33%), ergosterol (1.91%), trametenolic acid (2.14%);
Fatty acid esters: methyl palmitate (1.69%), methyl linoleate (2.45%);
Sesquiterpenes and aromatic compounds: α-curcumene (1.73%), α-turmerone (2.71%), coumarin (1.94%), benzyl benzoate (1.81%);
Hydrocarbons: hexadecane, heptadecane, octadecane;
Other notable compounds: betulin (2.29%), brassicasterol (1.96%).
Among these, inotodiol was the most abundant compound, reinforcing its prominence in the chemical composition of I. obliquus. The GC–MS fingerprint (Figure 1) demonstrates the extract’s chemical diversity and richness in bioactive molecules.
3.1.2. ESI–MS Analysis
The selection of ESI–MS was driven by the necessity for in-depth molecular characterization to facilitate the development of AgNP-based carrier systems. This technique enables the precise identification of key bioactive phytoconstituents, providing crucial in-sights into their chemical stability, potential interactions with AgNPs, and relevance to controlled-release mechanisms.
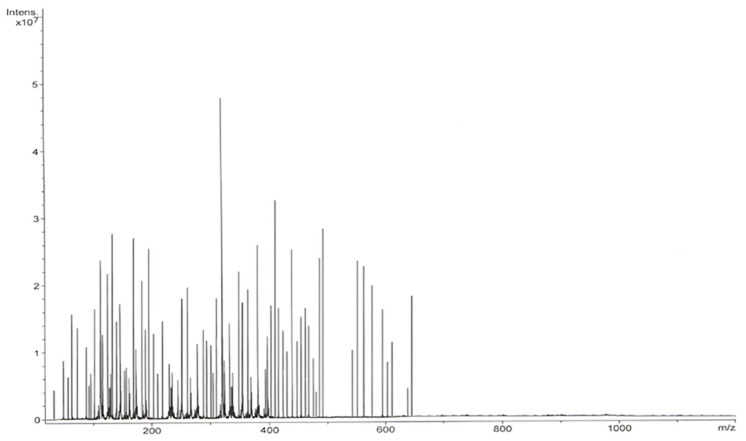
ESI–MS analysis of I. obliquus revealed a diverse array of bioactive compounds, including amino acids, coumarins, terpenes, fatty acids, flavonoids, phenolic acids, phytosterols, styrylpyrones, hydrocarbons, esters, and other polyphenols (Figure 2; Table 2).
Figure 2.
The mass spectrum of I. obliquus sample.
Table 2.
Phytochemicals identified by MS analysis of the I. obliquus sample.
| No. | m/z Detected | Theoretic m/z | Formula | Tentative Identification | Category | Refs. |
|---|---|---|---|---|---|---|
| 1 | 76.07 | 75.07 | C2H5NO2 | glycine | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 2 | 90.09 | 89.09 | C3H7NO2 | alanine | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 3 | 106.09 | 105.09 | C3H7NO3 | serine | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 4 | 116.14 | 115.13 | C5H9NO2 | proline | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 5 | 120.12 | 119.12 | C4H9NO3 | threonine | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 6 | 132.17 | 131.17 | C6H13NO2 | leucine | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 7 | 134.11 | 133.10 | C4H7NO4 | aspartic acid | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 8 | 147.18 | 146.19 | C6H14N2O2 | lysine | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 9 | 148.12 | 147.13 | C5H9NO4 | glutamic acid | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 10 | 150.22 | 149.21 | C5H11NO2S | methionine | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 11 | 156.14 | 155.15 | C6H9N3O2 | histidine | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 12 | 175.21 | 174.20 | C6H14N4O2 | arginine | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 13 | 182.18 | 181.19 | C9H11NO3 | tyrosine | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 14 | 205.23 | 204.22 | C11H12N2O2 | tryptophan | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 15 | 241.29 | 240.30 | C6H12N2O4S2 | cystine | amino acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 16 | 163.15 | 162.14 | C9H6O3 | hydroxycoumarin | coumarins | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 17 | 147.13 | 146.14 | C9H6O2 | coumarin | coumarins | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 18 | 189.17 | 188.18 | C11H8O3 | 3-acetylcoumarin | coumarins | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 19 | 291.19 | 290.18 | C13H6O8 | phelligridin J | coumarins | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 20 | 365.29 | 364.30 | C20H12O7 | phelligridin C | coumarins | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 21 | 381.50 | 380.30 | C20H12O8 | phelligridin D | coumarins | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 22 | 623.49 | 622.50 | C33H18O13 | phelligridin H | coumarins | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 23 | 625.51 | 624.50 | C33H20O13 | phelligridin I | coumarins | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 24 | 201.31 | 200.32 | C12H24O2 | lauric acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 25 | 229.37 | 228.37 | C14H28O2 | myristic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 26 | 257.43 | 256.42 | C16H32O2 | palmitic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 27 | 271.49 | 270.50 | C17H34O2 | margaric acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 28 | 279.41 | 278.40 | C18H30O2 | linolenic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 29 | 281.39 | 280.40 | C18H32O2 | linoleic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 30 | 285.51 | 284.50 | C18H36O2 | stearic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 31 | 297.39 | 296.40 | C18H32O3 | α-hydroxylinoleic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 32 | 315.49 | 314.50 | C18H34O4 | octadecanedioic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 33 | 321.49 | 320.50 | C20H32O3 | hydroxyarachidic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 34 | 331.51 | 330.50 | C18H34O5 | trihydroxyoctadecenoic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 35 | 341.59 | 340.60 | C22H44O2 | behemic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 36 | 343.51 | 342.50 | C20H38O4 | eicosanedioic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 37 | 355.59 | 354.60 | C23H46O2 | tricosanoic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 38 | 369.61 | 368.60 | C24H48O2 | lignoceric acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 39 | 367.60 | 366.60 | C24H46O2 | nervonic acid | fatty acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21] |
| 40 | 271.25 | 270.24 | C15H10O5 | apigenin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 41 | 273.23 | 272.25 | C15H12O5 | naringenin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 42 | 287.23 | 286.24 | C15H10O6 | kaempferol | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 43 | 291.27 | 290.27 | C15H14O6 | catechin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 44 | 303.24 | 302.23 | C15H10O7 | quercetin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 45 | 317.25 | 316.26 | C16H12O7 | isorhamnetin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 46 | 319.23 | 318.23 | C15H10O8 | myricetin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 47 | 373.41 | 372.40 | C20H20O7 | tangeretin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 48 | 393.39 | 392.40 | C22H16O7 | luteolin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 49 | 423.09 | 422.10 | C23H18O8 | interfungin B | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 50 | 429.51 | 428.50 | C26H28N4O2 | corin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 51 | 451.39 | 450.40 | C21H22O11 | astilbin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 52 | 477.41 | 476.40 | C26H20O9 | methylinoscavin A | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 53 | 565.49 | 564.50 | C26H28O14 | vicenin 1 | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 54 | 579.51 | 578.50 | C27H30O14 | rhoifolin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 55 | 581.49 | 580.50 | C27H32O14 | naringin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 56 | 608.49 | 608.50 | C28H32O15 | diosmin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 57 | 611.49 | 610.50 | C27H30O16 | rutin | flavonoids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64] |
| 58 | 139.13 | 138.12 | C7H6O3 | salicylic acid | phenolic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64,72,73,74,75] |
| 59 | 155.12 | 154.12 | C7H6O4 | protocatechuic acid | phenolic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64,72,73,74,75] |
| 60 | 165.15 | 164.16 | C9H8O3 | p-coumaric acid | phenolic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64,72,73,74,75] |
| 61 | 169.15 | 168.15 | C8H8O4 | vanillic acid | phenolic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64,72,73,74,75] |
| 62 | 171.11 | 170.12 | C7H6O5 | gallic acid | phenolic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64,72,73,74,75] |
| 63 | 179.19 | 178.18 | C10H10O3 | 4-methoxy cinnamic acid | phenolic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64,72,73,74,75] |
| 64 | 181.15 | 180.16 | C9H8O4 | caffeic acid | phenolic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64,72,73,74,75] |
| 65 | 195.17 | 194.18 | C10H10O4 | ferulic acid | phenolic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64,72,73,74,75] |
| 66 | 199.17 | 198.17 | C9H10O5 | syringic acid | phenolic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64,72,73,74,75] |
| 67 | 355.31 | 354.31 | C16H18O9 | chlorogenic acid | phenolic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,21,64,72,73,74,75] |
| 68 | 397.61 | 396.60 | C28H44O | ergosterol | sterols | [1,2,3,4,5,6,7,8,9,10,11,12,13,14] |
| 69 | 399.71 | 398.70 | C28H46O | brassicasterol | sterols | [1,2,3,4,5,6,7,8,9,10,11,12,13,14] |
| 70 | 415.71 | 414.70 | C29H50O | β-sitosterol | sterols | [1,2,3,4,5,6,7,8,9,10,11,12,13,14] |
| 71 | 427.69 | 426.70 | C30H50O | lanosterol | sterols | [1,2,3,4,5,6,7,8,9,10,11,12,13,14] |
| 72 | 179.17 | 178.18 | C10H10O3 | osmundacetone | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 73 | 203.33 | 202.33 | C15H22 | α-curcumene | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 74 | 205.34 | 204.35 | C15H24 | bergamotene | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 75 | 219.33 | 218.33 | C15H22O | α-turmerone | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 76 | 223.36 | 222.37 | C15H26O | β-eudesmol | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 77 | 255.37 | 254.36 | C15H26O3 | inonotin I | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 78 | 307.51 | 306.50 | C20H34O2 | fusicoserpenol A | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 79 | 313.39 | 312.40 | C21H28O2 | inonotusic acid | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 80 | 425.69 | 424.70 | C30H48O | lupenone | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 81 | 427.59 | 426.60 | C28H42O3 | 9,11-dehydroergosterol peroxide | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 82 | 427.71 | 426.70 | C30H50O | lupeol | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 83 | 429.61 | 428.60 | C28H44O3 | ergosterol peroxide | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 84 | 441.71 | 440.70 | C30H48O2 | inoterpene F | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 85 | 443.71 | 442.70 | C30H50O2 | betulin | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 86 | 457.71 | 456.70 | C30H48O3 | trametenolic acid | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 87 | 451.29 | 450.30 | C23H14O10 | inonoblin B | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 88 | 457.69 | 456.70 | C30H48O3 | ganodecochlearin A | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 89 | 459.37 | 458.37 | C30H50O3 | inonotus oxide B | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 90 | 459.69 | 458.70 | C30H50O3 | inonotusane A | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 91 | 459.73 | 458.72 | C30H50O3 | inonotus oxide A | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 92 | 461.71 | 460.70 | C30H52O3 | inoterpene A | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 93 | 469.71 | 468.70 | C31H48O3 | inonotusol F | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 94 | 471.71 | 470.70 | C30H46O4 | inonotusolide B | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 95 | 489.69 | 488.70 | C30H48O5 | inonotusol D | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 96 | 585.41 | 584.40 | C36H56O6 | inonotustriol D triacetate | terpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 97 | 247.21 | 246.21 | C13H10O5 | hispidin | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 98 | 393.39 | 392.40 | C22H16O7 | phelliribsin A | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 99 | 397.29 | 396.30 | C21H16O8 | inoscavin D | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 100 | 411.39 | 410.40 | C22H18O8 | methylinoscavin D | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 101 | 421.41 | 420.40 | C23H16O8 | inoscavin C | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 102 | 435.40 | 434.40 | C24H18O8 | methylinoscavin C | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 103 | 437.41 | 436.40 | C24H20O8 | inoscavin B | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 104 | 451.31 | 450.30 | C23H14O10 | inonoblin B | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 105 | 463.41 | 462.40 | C25H18O9 | inoscavin A | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 106 | 465.39 | 464.40 | C25H20O9 | davallialactone | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 107 | 475.39 | 474.40 | C25H14O10 | phelligridin E | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 108 | 477.41 | 476.40 | C26H20O9 | methylinoscavin A | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 109 | 479.41 | 478.40 | C26H22O9 | methyldavallialactone | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 110 | 479.45 | 478.45 | C26H22O9 | phelligridin F | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 111 | 595.51 | 594.50 | C32H18O12 | phelligridin G | styrylpyrones | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 112 | 179.21 | 178.20 | C10H10O3 | osmundacetone | other polyphenols | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 113 | 221.23 | 220.22 | C12H12O4 | hispolon | other polyphenols | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 114 | 229.24 | 228.24 | C14H12O3 | resveratrol | other polyphenols | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 115 | 303.19 | 302.19 | C14H6O8 | ellagic acid | other polyphenols | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 116 | 305.29 | 304.29 | C16H16O6 | inonophenol C | other polyphenols | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 117 | 317.23 | 316.22 | C15H8O8 | 3-O-methylellagic acid | other polyphenols | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 118 | 465.39 | 464.40 | C25H20O9 | interfungin A | other polyphenols | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 119 | 151.13 | 150.13 | C5H10O5 | xylulose | carbohydrates | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 120 | 165.17 | 164.16 | C6H12O5 | rhamnose | carbohydrates | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77,78] |
| 121 | 181.17 | 180.16 | C6H12O6 | inositol | carbohydrates | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77,78] |
| 122 | 47.01 | 46.02 | CH2O2 | formic acid | organic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 123 | 61.05 | 60.05 | C2H4O2 | acetic acid | organic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 124 | 89.11 | 88.11 | C4H8O2 | butyric acid | organic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 125 | 91.04 | 90.03 | C2H2O4 | oxalic acid | organic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 126 | 199.14 | 198.13 | C8H6O6 | 2,5-dihydroxylterephtalic acid | organic acids | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 127 | 227.45 | 226.44 | C16H34 | hexadecane | hydrocarbons | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 128 | 241.51 | 240.50 | C17H36 | heptadecane | hydrocarbons | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 129 | 255.49 | 254.50 | C18H38 | octadecane | hydrocarbons | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 130 | 297.61 | 296.60 | C21H44 | henicosane | hydrocarbons | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 131 | 109.15 | 108.14 | C7H8O | benzyl alcohol | miscellaneous | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 132 | 111.12 | 110.11 | C6H6O2 | resorcinol | miscellaneous | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 133 | 139.13 | 138.12 | C7H6O3 | protocatechuic aldehyde | miscellaneous | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 134 | 161.23 | 160.22 | C10H12N2 | tryptamine | miscellaneous | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 135 | 175.25 | 174.24 | C11H14N2 | 5-methyltryptamine | miscellaneous | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 136 | 287.50 | 286.50 | C20H30O | retinol | miscellaneous | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 137 | 319.31 | 318.30 | C18H10N2O4 | melanin | miscellaneous | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 138 | 151.17 | 150.17 | C9H10O2 | benzyl acetate | ester | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 139 | 213.23 | 212.24 | C14H12O2 | benzyl benzoate | ester | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 140 | 229.25 | 228.25 | C14H12O3 | 2-methoxyphenyl benzoate | ester | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 141 | 271.49 | 270.50 | C17H34O2 | methyl palmitate | ester | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
| 142 | 295.51 | 294.50 | C19H34O2 | methyl linoleate | ester | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,64,72,74,75,76,77] |
MS: Mass spectrometry.
These results are consistent with previous studies on I. obliquus from different geo-graphic regions, confirming the complex and rich phytochemical profile of the Romanian samples and highlighting their broad therapeutic potential.
3.1.3. VOC Analysis
The sensory and therapeutic characteristics of I. obliquus are strongly influenced by its volatile organic compounds (VOCs), which contribute not only to its distinct aroma and flavor profile but also to its biological activities, including antimicrobial, anti-inflammatory, and immunomodulatory effects [1,2,3,4,5,6,7,8,9,72,73,74,75,76,77,78]. To evaluate the impact of AgNP incorporation and maltodextrin encapsulation on the integrity of these bioactive volatiles, a comprehensive VOC analysis was conducted.
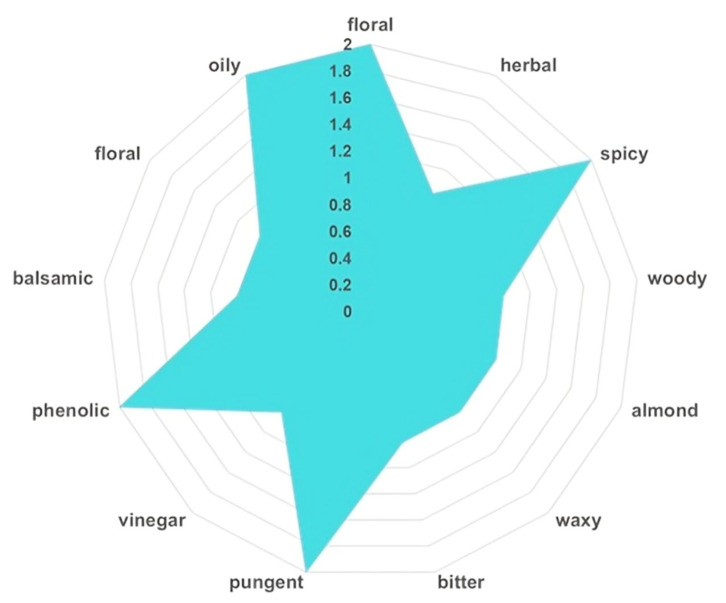
The results are presented in Table 3 and Figure 3, highlighting the principal constituents that define the unique sensory complexity of native I. obliquus.
Table 3.
VOCs identified in the I. obliquus sample through MS.
| VOC | Odor Profile |
|---|---|
| benzyl acetate | floral |
| α-curcumene | herbal |
| bergamotene | spice |
| β-eudesmol | woody |
| henicosane | waxy |
| benzyl alcohol | almond |
| resorcinol | phenolic |
| protocatechuic aldehyde | bitter |
| tryptamine | phenolic |
| formic acid | pungent |
| acetic acid | vinegar |
| retinol | floral |
| butyric acid | pungent |
| benzyl benzoate | balsamic |
| methyl linoleate | oily |
| 2-methoxyphenyl benzoate | spicy |
| methyl palmitate | oily |
MS: Mass spectrometry; VOC: Volatile organic compound.
Figure 3.
The VOC sensory profile of the constituents identified in the I. obliquus sample. VOC: Volatile organic compound.
A total of 17 key VOCs were identified in the I. obliquus sample, each associated with specific odor descriptors (Table 3), collectively contributing to its multifaceted aroma profile.
3.2. Engineered Hybrid System
3.2.1. FTIR Analysis
FTIR spectroscopy was employed to investigate the molecular interactions, such as chemical bonding and surface coordination, driving the formation and stabilization of the hybrid system (IO–AgNPs) composed of AgNPs and bioactive constituents from I. obliquus. This technique served a dual role: confirming the successful synthesis of the IO–AgNP composite and elucidating the role of maltodextrin in the encapsulation process leading to the formation of MIO and MIO–AgNP systems. The FTIR analyses offered critical insights into the molecular architecture and stabilization mechanisms underlying these multifunctional constructs.
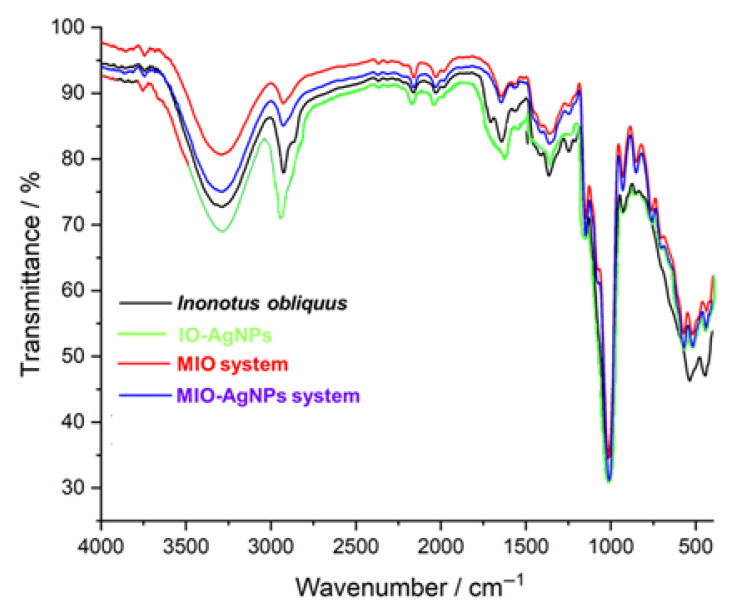
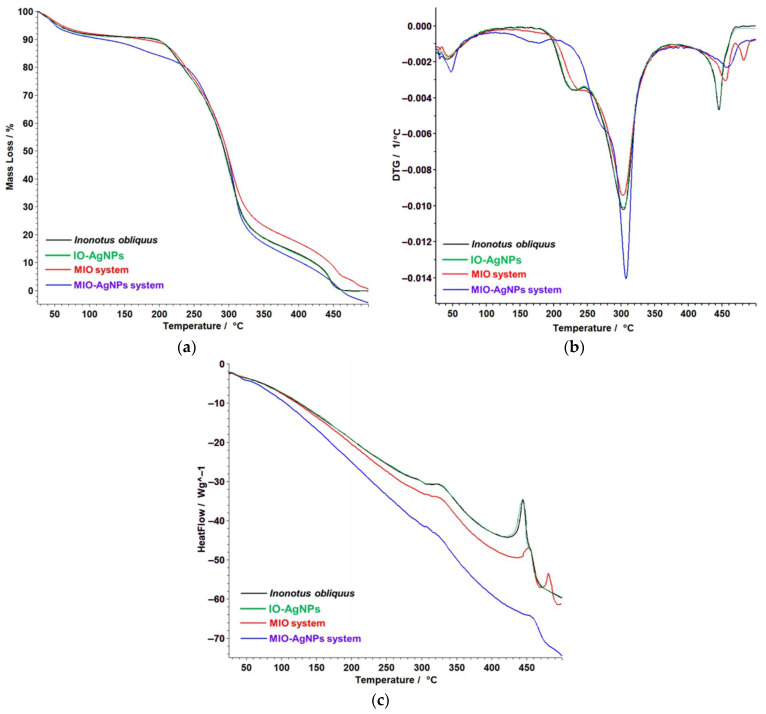
The FTIR spectrum of the I. obliquus sample (Figure 4, black line) exhibits a diverse range of absorption bands indicative of multiple biomolecular classes. Prominent peaks include a broad band at approximately 3291 cm−1, associated with O–H and N–H stretching vibrations from carbohydrates, polyphenols, and amino acids; a peak at 2928 cm−1, corresponding to C–H stretching of aliphatic groups in fatty acids and phytosterols; and bands at 1708 and 1636 cm−1, attributed to C=O stretching of phenolic acids, coumarins, and organic acids, as well as C=C stretching of styrylpyrones and flavonoids [5,8,19,20,21,37,38,39,52,53,54,59,60,79,80,81]. Additional peaks at 1368 cm−1 (C–H bending) and 534–444 cm−1 (out-of-plane bending of aromatic rings) suggest the presence of terpenes and phenolic compounds [5,8,19,20,21,37,38,39,52,53,54,59,60]. This spectrum highlights the complex chemical composition of the I. obliquus sample, encompassing amino acids, coumarins, styrylpyrones, terpenes, fatty acids, phenolic acids, flavonoids, phytosterols, organic acids, and carbohydrates.
Figure 4.
FTIR spectra of I. obliquus sample (black line), IO–AgNPs (green line), MIO (red line), and MIO–AgNPs (blue line) systems. FTIR: Fourier-transform infrared; IO–AgNPs: I. obliquus–silver nanoparticles; MIO: Maltodextrin—I. obliquus; MIO–AgNPs: Maltodextrin—I. obliquus–silver nanoparticles.
The FTIR spectrum of the MIO system (Figure 4, red line) exhibits characteristic absorption bands reflecting contributions from both I. obliquus and maltodextrin components. A broad peak at 3296 cm−1 is attributed to O–H stretching vibrations from hydroxyl groups in polysaccharides of both I. obliquus and maltodextrin [5,8,53,54,57,58,78]. The band at 2928 cm−1 corresponds to C–H stretching of aliphatic chains, while the peak at 1643 cm−1 is assigned to C=O stretching vibrations from maltodextrin carbonyl groups and I. obliquus-derived phenolic compounds [53,54,57,58,78,79,80,81]. Additional bands include 1361 cm−1 (C–H bending), 1146 cm−1 (C–O–C stretching of glycosidic linkages), and 578–515 cm−1 (out-of-plane bending of aromatic rings) from I. obliquus metabolites [5,9,72,78,79,80,81]. Compared to the native I. obliquus spectrum, the MIO system displays increased intensity at 1643 cm−1 and 1146 cm−1, indicative of the enhanced maltodextrin matrix contribution [53,54,57,58]. The broadening of the 3296 cm−1 band suggests intensified hydrogen bonding interactions due to overlapping hydroxyl groups from both components [53,54,57,58]. These spectral modifications confirm successful encapsulation, preservation of molecular integrity, and the formation of a stable polysaccharide-rich matrix [53,54,57,58]. Furthermore, the observed intensity variations and band broadening imply molecular interactions between I. obliquus phytochemicals and maltodextrin, contributing to a well-integrated encapsulated system [53,54,57,58].
The FTIR spectrum of the IO–AgNP system (Figure 4, green line) showcases key vibrational features reflecting the I. obliquus matrix and its interaction with AgNPs [39,62,63,64]. A broad absorption band at 3300–3400 cm−1, attributed to O–H stretching of hydroxyl groups from polysaccharides and phenolic compounds, and a peak at 2900 cm−1, indicative of C–H stretching from aliphatic chains in fatty acids and sterols, highlight Chaga’s native composition. In the fingerprint region (1600–1000 cm−1), a C=C aromatic stretch at 1600 cm−1 and C–O, C–C, and C–N vibrations between 1400 and 1000 cm−1, linked to polysaccharides, phenolic compounds, proteins, and their bioactive ligands (e.g., hydroxyl and carbonyl groups from phenols, polysaccharide hydroxyls, and amine groups from proteins), are evident [1,2,3,4,5,9,62,63,64]. Subtle shifts and intensity changes, including a redshift and broadening of the 3300–3400 cm−1 band and a shift in the C=O band near 1700 cm−1, suggest interactions between citrate- and surfactant-coated AgNPs and these ligands [39,49]. Enhanced peak definition in the 1400–1000 cm−1 region, likely due to C–O or C–N coordination with silver (Ag), supports AgNP-induced hydrogen bonding alterations, confirming successful incorporation via mechanical alloying [39]. Furthermore, vibrational bands at approximately 1632, 1389, 1114, and 675 cm−1, potentially reflecting citrate-coated AgNPs stabilized by surfactant and I. obliquus ligands, shift to 1642, 1392, 1118, and 681 cm−1, indicating binding via O–H, C=O, N–H, and C–O groups [39,49]. These spectral changes validate the formation of a stable IO–AgNPs hybrid system, driven by robust AgNP–phytochemical interactions and structural reorganization of the bioactive matrix.
The FTIR spectrum of the MIO–AgNP system (Figure 4, blue line) displays distinct vibrational bands reflecting the IO–AgNP core and maltodextrin matrix [1,2,3,4,9,53,54,57,58,62,63,64]. A broad O–H stretching band at 3296 cm−1 indicates contributions from hydroxyl groups in I. obliquus polysaccharides, phenolic compounds, and maltodextrin, while a C–H stretching vibration at 2928 cm−1 corresponds to aliphatic chains from I. obliquus lipophilic components. In the fingerprint region, notable absorption bands appear at 1643 cm−1 (C=O stretching from maltodextrin carbonyls and Chaga phenolics), 1361 cm−1 (C–H bending), 1146 cm−1 (C–O–C stretching of maltodextrin glycosidic linkages), and 578–515 cm−1 (aromatic ring deformations from Chaga compounds) [1,2,3,4,9,53,54,57,58,62,63,64]. Compared to the unencapsulated IO–AgNP spectrum, the MIO–AgNP system exhibits a broadened and intensified O–H band at 3296 cm−1 and increased absorption at 1643 and 1146 cm−1, highlighting the dominant maltodextrin matrix contribution and enhanced hydrogen bonding [53,54,57,58]. The IO–AgNP spectrum’s characteristic AgNP–phytochemical interactions, such as the redshift of the C=O band from 1632 to 1642 cm−1 and sharpening in the 1400–1000 cm−1 region, are diminished in MIO-AgNPs, indicating that the maltodextrin coating shields and stabilizes the nanoparticle (NP) interface [39,53,54]. These spectral changes confirm successful encapsulation, yielding a structurally integrated hybrid system [53,54]. The modifications underscore maltodextrin’s protective role in maintaining the chemical integrity of bioactive compounds and modulating intermolecular interactions, suggesting enhanced stability for the newly prepared system.
3.2.2. XRD Analysis
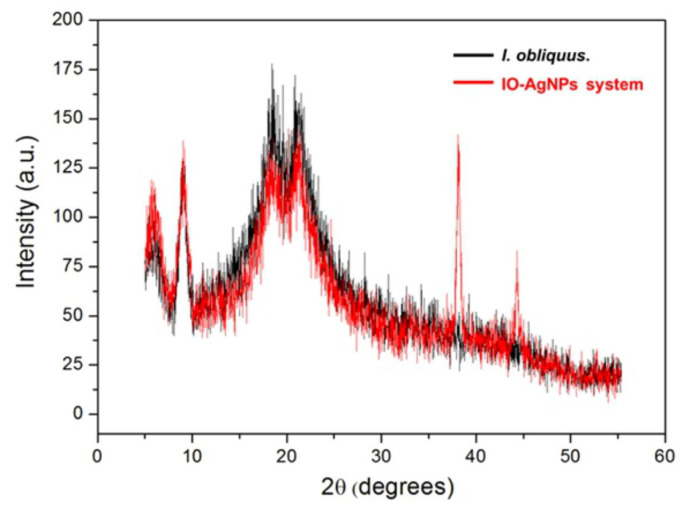
The XRD patterns provided in Figure 5 offer valuable insights into the structural characteristics and phase composition of the I. obliquus and IO–AgNP systems.
Figure 5.
XRD patterns of the I. obliquus sample (black line) and the IO–AgNP system (red line). XRD: X-ray diffraction.
The black line, corresponding to the I. obliquus sample (Figure 5), exhibits a broad diffraction peak centered around 2θ (12.78° and 28.21°), which is indicative of an amorphous structure. This broad halo is consistent with the presence of a complex, heterogeneous matrix of biomolecules typically found in fungal biomass [82,83].
In contrast, the red line, corresponding to the IO–AgNP system (Figure 5), retains the broad amorphous peak of I. obliquus while exhibiting additional sharp, distinct diffraction peaks at approximately 2θ (27.81°, 38.15°, 64.4°, and 78.5°). These peaks are characteristic of the face-centered cubic (FCC) structure of metallic Ag, as referenced in Joint Committee on Powder Diffraction Standards (JCPDS) Card No. 04-0783 [37,39,49,63,64]. This alignment confirms the successful incorporation of crystalline AgNPs within the I. obliquus matrix. The coexistence of the amorphous halo with AgNP-specific peaks suggests that the NPs are embedded within the fungal matrix without causing significant disruption to its native amorphous structure, supporting the formation of a stable composite material. A subtle shift of the amorphous halo to slightly lower 2θ angles in the IO–AgNP system, compared to the I. obliquus sample, is also observed. This shift likely arises from interactions between the AgNPs and the biomolecular components of the matrix, such as intercalation or embedding of the metallic NPs [37,39,49,63,64]. Such interactions may induce local structural reorganization or increased ordering, enhancing the stability and uniformity of the IO–AgNP system. These findings lay a robust structural foundation for the potential synergistic physicochemical properties of the IO–AgNP composite, which could contribute to improved stability and functionality in various applications.
3.2.3. SEM Analysis
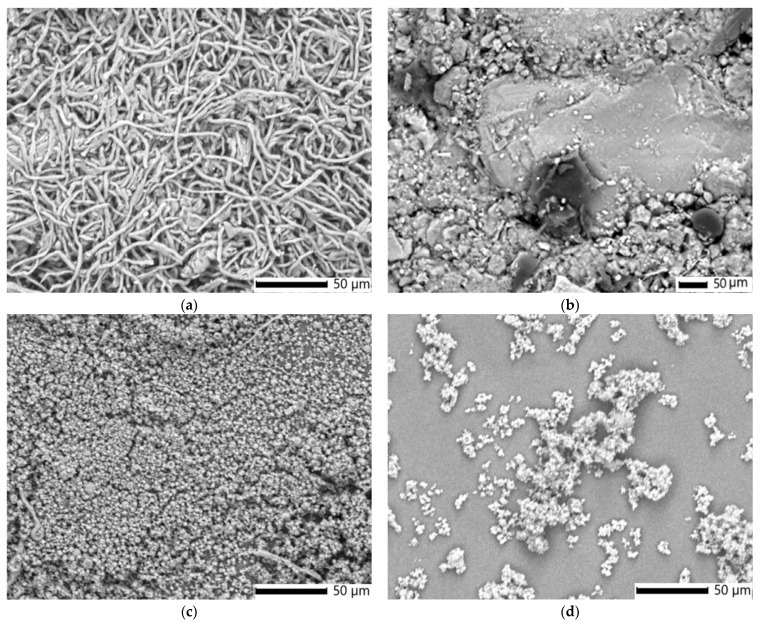
Figure 6a–d presents SEM micrographs that vividly illustrate the morphological characteristics of I. obliquus, the IO–AgNPs nanoconjugate, and their maltodextrin-encapsulated counterparts (MIO and MIO–AgNPs). These high-resolution images reveal distinct structural features, including particle size, shape, and surface topography, before and after encapsulation within the maltodextrin biopolymeric matrix.
Figure 6.
SEM micrograph of I. obliquus sample (a), IO–AgNPs (b), MIO (c), and MIO–AgNPs (d) systems. SEM: Scanning electron microscopy.
The SEM image of the I. obliquus sample (Figure 6a) displays a dense, interwoven network of fibrous, thread-like structures identified as mycelial filaments (hyphae) [19,21,78]. This irregular, tangled matrix, typical of the sclerotium, comprises fibers of varying thicknesses, forming a porous, fibrous structure consistent with the natural composition of I. obliquus, which includes chitin and polysaccharides [19,21,78,82,83]. The high fiber density indicates a mechanically robust network, while the rough, uneven surfaces are indicative of its organic origin. Fiber diameters, measured at approximately 1 to 10 μm, align with reported dimensions for fungal hyphae [19,21,78,82,83,84]. These findings are consistent with literature corroborating the distinctive fibrous and porous morphology of I. obliquus.
The SEM image of the IO–AgNP system (Figure 6b) displays a heterogeneous surface consisting of an irregular, porous, fibrous mass, identified as the I. obliquus sclerotial matrix with interwoven hyphae, interspersed with brighter, spherical AgNPs approximately 19 nm in diameter [39,49,84]. This morphology reflects the incorporation of AgNPs into the I. obliquus matrix, marked by uneven distribution and localized NP clustering. Compared to the relatively uniform porosity of the I. obliquus sample alone (Figure 6a), the composite structure shows increased microscale surface complexity, which may enhance its functional properties.
The SEM image of the MIO system (Figure 6c) reveals a microstructure composed of uniformly dispersed spherical to near-spherical microcapsules with smooth surfaces and diameters ranging from approximately 1 to 10 μm, consistent with spray drying microencapsulation [52,53,57,58]. Surface cavities, likely resulting from rapid solvent evaporation during the drying process, are frequently observed. This morphology contrasts with the irregular, porous, fibrous network of interwoven hyphae seen in the raw I. obliquus sample (Figure 6a), where similarly sized fibers (1–10 μm) form a rough-textured sclerotial matrix. Encapsulation in the maltodextrin matrix transforms the native fibrous architecture of I. obliquus into a compact, rounded form, embedding the hyphal structures within a continuous polysaccharide shell. This structural modification reduces surface roughness and porosity, suggesting improved physical stability and potential for controlled release. Compared to raw I. obliquus, the MIO system exhibits greater particle uniformity and morphological regularity.
The SEM image of the MIO–AgNP system (Figure 6d) displays a uniform distribution of spherical or near-spherical microcapsules, with diameters ranging from approximately 1 to 10 μm. This morphology contrasts with the IO–AgNP system prior to encapsulation (Figure 6b), which exhibits a heterogeneous surface featuring an irregular, porous, fibrous I. obliquus sclerotial matrix and unevenly distributed AgNPs. The encapsulation process transforms the native fibrous and NP-dispersed structure into a compact, rounded form, embedding the I. obliquus matrix and AgNPs within a continuous maltodextrin coating. This modification reduces the surface irregularity and porosity of the I. obliquus architecture loaded with AgNPs from the IO–AgNP system (Figure 6b), suggesting enhanced stability compared to the unencapsulated system.
3.2.4. EDX Analysis
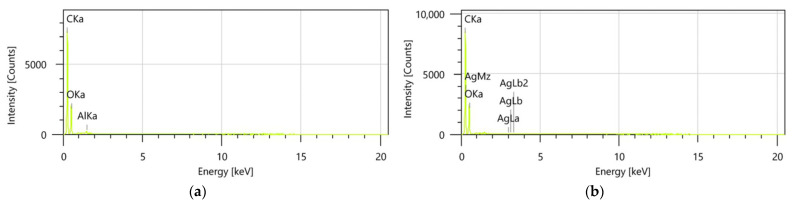
Energy-dispersive X-ray (EDX) spectroscopy provides a detailed and quantitative analysis of the elemental composition of samples, offering critical insights into their chemical characteristics and potential molecular interactions (Figure 7a,b). This technique was employed to evaluate the elemental distribution and confirm the successful synthesis of the binary IO–AgNP system.
Figure 7.
EDX analysis of the I. obliquus sample (a) and IO–AgNP system (b). EDX: Energy-dispersive X-ray.
Encapsulated samples, such as the MIO and MIO–AgNP systems, were excluded from EDX analysis due to the high maltodextrin content, which is predominantly composed of carbon and oxygen. The presence of these elements would overwhelm the spectra, masking the detection of other characteristic elements and reducing the interpretative value of the analysis. Consequently, the unencapsulated binary IO–AgNP system was selected as the most representative sample for assessing elemental-level interactions between the organic (I. obliquus) and inorganic (AgNPs) components.
The EDX spectrum for the IO–AgNP system (Figure 7b) exhibits distinct peaks corresponding to elements present in both the I. obliquus sample (Figure 7a) and the AgNPs. Specifically, peaks associated with carbon, oxygen, and other bioelements from the I. obliquus matrix are observed, alongside prominent Ag peaks, which are indicative of the successful incorporation of AgNPs. The presence of these characteristic Ag peaks, consistent with the known composition of metallic Ag, confirms the effective development of the binary IO–AgNP system. The co-occurrence of organic and inorganic elemental signatures further suggests a stable integration of AgNPs within the I. obliquus matrix, providing a robust foundation for understanding the chemical interactions and potential synergistic properties of this hybrid material.
3.2.5. DLS Analysis
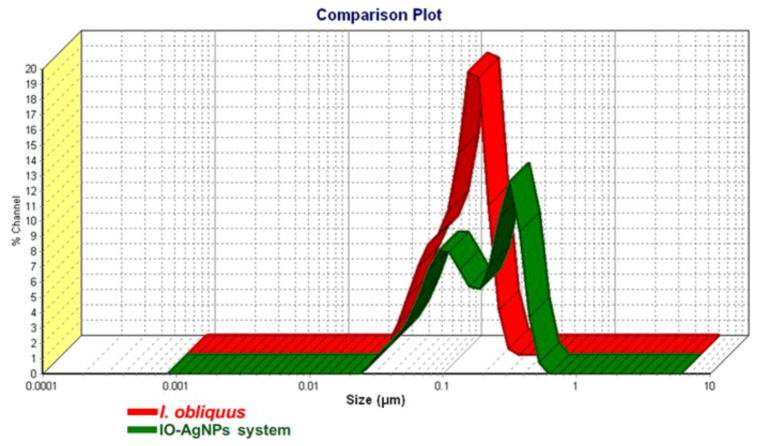
The DLS analysis presented in the comparison plot (Figure 8) provides a detailed assessment of the PSD and hydrodynamic properties of I. obliquus and the IO–AgNP system. The plot reveals distinct differences in the size distributions between the two samples, offering insights into their structural and colloidal characteristics.
Figure 8.
DLS pattern of I. obliquus sample (red curve) and IO–AgNP system (green curve). DLS: Dynamic light scattering.
In Figure 8, the red curve corresponding to the I. obliquus sample displays a broad and relatively flat size distribution, with a peak centered in the 0.01–0.1 μm range and a PDI of 0.445, indicative of a high degree of polydispersity. This broad distribution is characteristic of the heterogeneous composition of fungal biomass, likely reflecting the presence of diverse biomolecular components, such as polysaccharides, proteins, and phenolic compounds, that tend to form irregular, non-uniform aggregates. The predominance of particles within the submicron range suggests a structural organization at the nano- to low-micron scale, which is consistent with the amorphous morphology observed in the XRD analysis.
In contrast, the green curve corresponding to the IO–AgNP system exhibits a bimodal distribution with two distinct peaks located approximately at ~0.08 μm and ~0.4 μm, and reduced PDI values of 0.21 and 0.26, indicating a narrower and more uniform size distribution compared to I. obliquus alone. The primary peak at smaller sizes and the emergence of a secondary peak at larger sizes suggest the formation of hybrid nanostructures through the integration of AgNPs into the I. obliquus matrix.
The shift toward both smaller and slightly larger particle sizes, along with the reduced PDI, points to a structural reorganization induced by AgNP incorporation. This may involve embedding of AgNPs into the organic matrix, leading to more defined composite structures while also suppressing excessive aggregation. The presence of the lower-size peak (<0.1 μm) likely reflects more compact nanoassemblies, whereas the broader peak extending above 0.3 μm may result from matrix-associated AgNP clustering.
Overall, the reduced polydispersity and emergence of distinct peaks in the IO–AgNP system highlight the successful preparation of this newly engineered binary hybrid, characterized by improved structural definition and enhanced colloidal stability.
3.2.6. PSD Analysis by Laser Diffraction
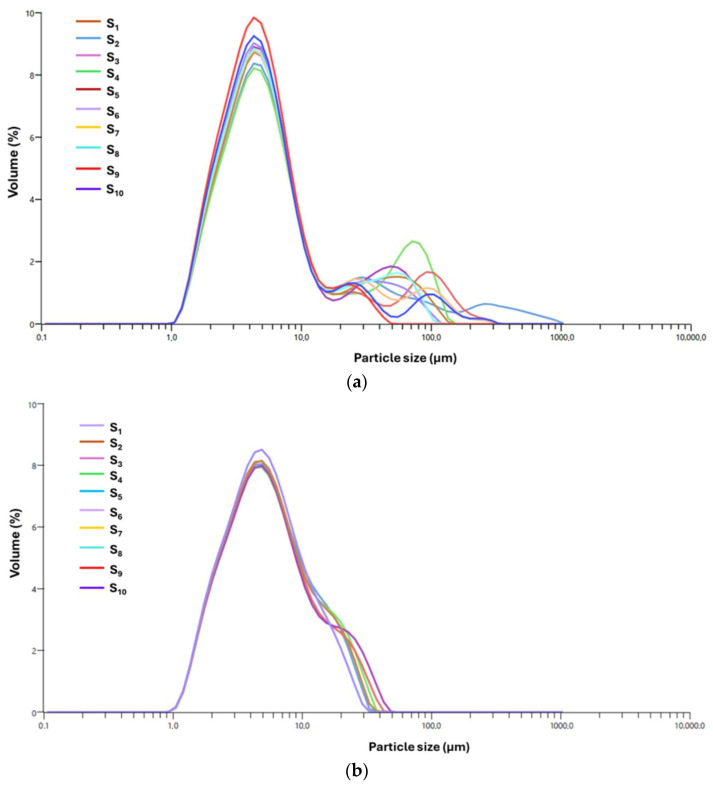
The systems encapsulated with I. obliquus, specifically the MIO and MIO–AgNP systems, were assessed using laser diffraction, a method that is particularly effective for characterizing the extensive size range of spray dried microparticles. The resulting data, illustrated in Figure 9a,b, provide valuable insights into the PSD and the uniformity of encapsulation across these samples.
Figure 9.
PSD curves from 10 consecutive measurements conducted over a two-minute period for MIO (a) and MIO–AgNPs (b) systems. PSD: Particle size distribution.
The PSD of the MIO system, as shown in Figure 9a, exhibits a broad size range from 0.106 μm to 1040.83 μm, characterized by a right-skewed distribution with a dominant peak in the 2–3 μm range. This primary peak shows consistent overlap across replicates (S1–S10), with a SD of approximately 0.25 μm, indicating a reproducible and well-controlled particle formation process despite the system’s inherent complexity.
The distribution is markedly right-skewed (skewness = 1.83) and exhibits a high PDI (1.74), confirming significant heterogeneity and the presence of larger aggregates (d90 = 4.88 ± 0.019 μm) (Table 4). The coefficient of variation (CV) for d50 (2.10 ± 0.003 μm) is 12.4%, further supporting measurement consistency across replicates.
Table 4.
Particle diameter distribution of MIO and MIO–AgNP systems.
| Sample | Particle Size Diameter (μm) | Volume Diameter (μm) | |||
|---|---|---|---|---|---|
| D[3,2] | D[4,3] | d10 | d50 | d90 | |
| MIO system | 1.72 ± 0.011 | 2.45 ± 0.008 | 1.20 ± 0.016 | 2.10 ± 0.003 | 4.88 ± 0.019 |
| MIO–AgNP system | 1.65 ± 0.015 | 1.62 ± 0.002 | 0.25 ± 0.019 | 1.50 ± 0.005 | 3.05 ± 0.007 |
D[3,2] represents the surface-weighted mean diameter, and D[4,3] represents the volume-weighted mean diameter. The d10, d50, and d90 correspond to cumulative distributions at 10%, 50%, and 90%, respectively. MIO: Maltodextrin—I. obliquus MIO–AgNPs: Maltodextrin—I. obliquus-silver nanoparticles.
As depicted in Figure 9a, all samples (S1–S10) display a dominant, relatively narrow primary peak within the 1–10 μm range, reflecting a consistent micron-scale particle population. The sharpness and overlap of this peak, particularly in samples S1, S2, S3, S5, S6, S7, S8, and S10, demonstrate high batch-to-batch uniformity. Secondary peaks in the 30–200 μm range suggest the presence of larger aggregates or agglomerates, likely resulting from matrix-induced clustering, partial agglomeration, or incomplete dispersion, phenomena commonly observed in complex biopolymer systems [53,54]. Although these secondary peaks represent a low volume percentage and do not dominate the PSD, their presence indicates subtle variations in formulation conditions that may affect colloidal stability, dispersibility, and functional performance of the final product.
Overall, the MIO system exhibits a dominant and reproducible micron-scale particle population with moderate structural heterogeneity. This heterogeneity is likely driven by interactions between maltodextrin and I. obliquus-derived biomolecules (e.g., polysaccharides, polyphenols, terpenoids), which contribute to both particle stabilization and the occasional formation of larger clusters.
In contrast, the MIO–AgNP system displays a refined and well-defined PSD, ranging from 0.106 μm to 48.62 μm, as shown in Figure 9b. The distribution features a dominant, narrow peak centered between 1.5 and 2.0 μm, with a median particle diameter (d50) of 1.50 ± 0.03 μm. Moderate right-skewness (1.20) and a reduced PDI (1.47) indicate improved uniformity relative to the broader, more asymmetric profile of the MIO system (PDI = 1.74; skewness = 1.83). While both systems fall within the range of moderate polydispersity (PDI > 1), the lower PDI of MIO–AgNPs reflects a more controlled and consistent particle formation process.
The formulation demonstrates high reproducibility, as evidenced by the consistent overlap of PSD curves across 10 technical replicates (S1–S10), a SD less than 0.2 μm, and a CV of 8.5% for d50. No statistically significant differences were observed between replicates (p > 0.05), confirming the robustness of the spray drying process (Table 4).
Further statistical analysis of PSD parameters highlights the enhanced microstructural features of MIO–AgNPs. The volume-weighted mean diameter (D[4,3]) was significantly reduced to 1.62 ± 0.02 μm compared to 2.45 ± 0.03 μm for the MIO system. The 10th percentile (d10 = 0.25 ± 0.02 μm) and 90th percentile (d90 = 3.05 ± 0.01 μm) diameters con-firm a narrower distribution, with d90 notably lower than that of the MIO formulation (4.88 ± 0.02 μm). These reductions in average size and distribution breadth are statistically significant (p < 0.01), indicating enhanced particle homogeneity. The upper tail of the PSD, extending to 48.62 μm, likely represents occasional aggregation events or measurement artifacts, as corroborated by SEM imaging.
The reduction in particle size and improved distribution uniformity are attributed to the role of AgNPs in the IO–AgNP system. As nanostructured solid entities, AgNPs likely act as nucleation centers during spray drying, promoting rapid droplet solidification and reducing inter-droplet coalescence. This mechanism is further supported by the observed sharpness of the PSD peak and the decreased PDI, which reflect greater control over particle formation and morphology. Favorable physicochemical interactions among AgNPs, maltodextrin, and I. obliquus biomolecules may contribute to structural stabilization during drying, enhancing dispersion and limiting the formation of larger aggregates [62,64].
Collectively, these findings demonstrate that the encapsulation of the IO–AgNP system leads to significant improvements in particle size control and distribution consistency. The narrower PSD, lower central tendency values, and high reproducibility across replicates confirm the efficiency of the formulation process and the beneficial influence of AgNPs on microstructure development.
Overall, the MIO–AgNP system exhibits markedly improved particle uniformity and reduced aggregation compared to the MIO formulation, as evidenced by detailed PSD metrics and robust statistical validation. These structural enhancements provide a strong foundation for optimizing encapsulation performance and evaluating the system’s potential in controlled-release and bioactive delivery applications.
3.2.7. Encapsulation Efficiency, Loading Capacity, and Encapsulation Yield
The EE%, LC%, and EY% of I. obliquus and the IO–AgNP system within a maltodextrin matrix, achieved through micro-spray drying, were systematically evaluated to determine the efficacy of this technique in preserving and delivering bioactive components. These parameters (EE%, LC%, and EY%) serve as critical metrics for assessing the quality, application potential, and economic viability of microencapsulation systems, as they directly influence the retention of bioactive compounds and the overall process efficiency (Table 5).
Table 5.
Encapsulation parameters for the newly prepared I. obliquus-derived hybrid systems.
| Sample | EE% | LC% | EY% |
|---|---|---|---|
| MIO system | 77.65 ± 0.17 | 72.33 ± 0.11 | 74.58 ± 0.15 |
| MIO–AgNP system | 71.77 ± 0.07 | 68.55 ± 0.21 | 63.12 ± 0.14 |
EE%: Encapsulation efficiency; EY%: Encapsulation yield; LC%: Loading capacity; MIO: Maltodextrin—I. obliquus MIO–AgNPs: Maltodextrin—I. obliquus-silver nanoparticles.
The MIO system exhibited an EE% of 77.65 ± 0.17%, demonstrating effective entrapment of I. obliquus bioactive compounds, including polysaccharides and phenolic compounds, within the maltodextrin matrix. This high EE% results from strong hydrogen bonding and van der Waals interactions between the hydroxyl groups of maltodextrin and the polar functional groups (–OH, –COOH) of the bioactive compounds, promoting stable molecular encapsulation [50,54]. FTIR analysis confirmed these interactions, showing characteristic peaks indicative of hydrogen bonding between maltodextrin hydroxyls and the functional groups of I. obliquus constituents. XRD analysis revealed an amorphous matrix without crystalline peaks, supporting the formation of a homogeneous encapsulation structure.
The MIO system achieved a LC% of 72.33 ± 0.11% (Table 5), reflecting the high solubility and thermal stability of the processed I. obliquus biomass. The EY% was 74.58 ± 0.15% (Table 5), with minor losses attributed to typical micro-spray drying challenges, such as wall deposition and incomplete particle formation, consistent with prior studies on natural compound encapsulation [50,54,57].
In contrast, the MIO–AgNP system demonstrated an EE% of 71.77 ± 0.07% (Table 5), marginally lower than that of the MIO system. Notwithstanding this slight decrease, the incorporation of AgNPs markedly improved particle uniformity. This enhancement is attributed to the role of AgNPs as nucleation sites during the spray drying process, which promotes uniform droplet formation and mitigates particle aggregation [49]. Consequently, the system exhibited a narrower PSD and reduced PDI, indicative of a more homogeneous microparticle population. FTIR analysis revealed coordination interactions between AgNPs and oxygen-containing functional groups of I. obliquus bioactive constituents, contributing to matrix stabilization and facilitating the formation of smaller, structurally uniform microparticles.
The EY% for the MIO–AgNP system was 63.12 ± 0.14% (Table 5), lower than that observed for the MIO system. This reduction is likely due to altered droplet dynamics and increased local viscosity induced by AgNP incorporation, which may adversely affect heat and mass transfer during the drying process. Despite these challenges, the improved particle uniformity and enhanced matrix stabilization imparted by AgNPs suggest potential benefits for the structural integrity and controlled release of the encapsulated bioactive compounds.
Collectively, these findings demonstrate that AgNPs integration within the maltodextrin matrix synergistically enhances particle homogeneity and stabilization, establishing the MIO–AgNP system as a promising vehicle for the delivery of I. obliquus bioactive compounds.
3.3. Thermal Behavior
The thermal behavior of the I. obliquus extract and the IO–AgNP system was systematically investigated to evaluate the effects of encapsulation on their thermal stability. This assessment is essential for determining how the encapsulation process influences the thermal characteristics of the bioactive constituents, including their degradation temperatures and structural resilience during processing or storage. The thermal analysis results, illustrated in Figure 10a–c, provide a detailed comparison of the thermogravimetric profiles of both samples. These data reveal critical differences in decomposition patterns and thermal resistance, offering valuable insight into the stabilizing effect of AgNP incorporation on the I. obliquus matrix.
Figure 10.
Comparative TG (a), DTG (b), and HF (c) thermoanalytical curves of I. obliquus sample (black line), IO–AgNPs (green line), MIO (red line), and MIO–AgNPs (blue line) systems. DTG: Differential thermogravimetry; HF: Heat flow; TG: Thermogravimetry.
For the I. obliquus sample (Figure 10, black line), TG indicates an initial water loss of 6.82% at 33–76 °C, followed by a two-step DTG decomposition from 233 to 350 °C (21.75% at 233–285 °C, max DTG 268 °C; 42.68% at 285–350 °C, max DTG 308 °C), attributed to the thermal degradation of polysaccharides, phenolics, and proteins [53,54]. A minor process at 430–480 °C (7.52%) reflects stable residue breakdown, with a total mass loss of 91.2% and an endothermic HF peak (ΔH = 147 mJ/g), indicating moderate thermal stability.
For the IO–AgNP system (Figure 10, green line), TG shows a reduced water loss of 5.09% at 39–70 °C, with a two-step decomposition from 193 to 341 °C (17.02% at 193–255 °C, max DTG 224 °C; 52.15% at 256–341 °C, max DTG 308 °C), suggesting that AgNP incorporation shifts and accelerates organic degradation, likely through catalytic effects on polysaccharide and phenolic breakdown [49,62,63,64]. A process at 426–469 °C (9.86%) indicates enhanced residue stability, with a total mass loss of 97.67% and a significantly higher ΔH of 1797 mJ/g. Compared to the I. obliquus sample, the IO–AgNP system exhibits improved thermal stability, due to AgNP–phytochemical interactions that modify the thermal profile, evidenced by a shifted decomposition range (193–341 °C vs. 233–350 °C) and a markedly elevated enthalpy change, reflecting stronger AgNP–phytochemical interactions that enhance resistance to initial degradation despite a higher total mass loss [49].
For the MIO system (Figure 10, red line), TG exhibits a water loss of 5.16% at 39–72 °C, with a two-step decomposition from 206 to 350 °C (17.43% at 206–261 °C, max DTG 231 °C; 47.61% at 262–350 °C, max DTG 307 °C), linked to maltodextrin and I. obliquus biomole-cules degradation [53,62,63,64]. Additional processes at 441–469 °C (6.63%, max DTG 458 °C) and 470–495 °C (3.38%, max DTG 488 °C) suggest residual matrix breakdown, with a total mass loss of 96.48% and ΔH values of 215.15 mJ/g (206–350 °C) and 601.77 mJ/g (441–469 °C), indicating enhanced thermal stability due to encapsulation [53,57,58].
For the MIO–AgNP system (Figure 10, blue line), TG reveals a water loss of 5.09% at 39–70 °C, with a two-step decomposition from 193 to 341 °C (17.02% at 193–255 °C, max DTG 224 °C; 52.15% at 256–341 °C, max DTG 308 °C), slightly shifted earlier due to AgNPs effects. A process at 426–469 °C (9.86%, max DTG 452 °C) reflects stabilized residues, with a total mass loss of 97.67% and a high ΔH of 1797 mJ/g. Compared to MIO, the MIO–AgNP system demonstrates superior thermal stability, with a comparable decomposition range but a significantly higher enthalpy (1797 mJ/g vs. 601.77 mJ/g), suggesting that AgNPs integration further strengthens the maltodextrin matrix’s thermal resistance.
Compared to the I. obliquus sample, the IO–AgNP, MIO, and MIO–AgNP systems show reduced water loss and earlier decomposition onsets (193–206 °C vs. 233 °C), with MIO–AgNPs exhibiting the highest enthalpy and mass loss, indicating a synergistic stabilization from AgNPs and maltodextrin. These findings confirm successful encapsulation and NP integration, enhancing thermal stability and potentially improving functional properties of the hybrid systems.
3.4. TPC and Estimation of Antioxidant Potential
The phenolic content and antioxidant capacity of Romanian I. obliquus and its engineered systems (MIO, IO–AgNPs, MIO–AgNPs) were evaluated using three complementary assays: TPC, FRAP, and DPPH radical scavenging. These assays were selected for their reliability, specificity, and widespread use in assessing phenolic-rich natural products, particularly medicinal mushrooms like Chaga. Phenolic compounds are the primary contributors to I. obliquus antioxidant activity [1,2,3,4,5,6,7,11,12,13,17,79,84]. The TPC assay, based on the Folin–Ciocalteu method, quantifies phenolic concentration as mg GAE/g [84,85]. The DPPH assay measures free radical scavenging capacity, reflecting antioxidant efficacy against oxidative stress [85,86]. The FRAP assay evaluates reducing power by measuring the reduction of Fe3+ to Fe2+, indicating electron-donating potential [86,87]. Together, these assays provide a comprehensive assessment of antioxidant properties, enabling evaluation of micro-spray drying and AgNP incorporation, hypothesized to enhance phenolic stability, availability, and functionality. Results are presented in Figure 11a–c.
Figure 11.
Results of TPC (a), FRAP (b), and DPPH (c) assays for the I. obliquus extract, IO–AgNPs, MIO, and MIO–AgNP systems. Data are presented as mean ± SD (n = 3). Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test to compare samples (* p < 0.05). SD, standard deviation.
The incorporation of AgNPs to develop the new engineered IO–AgNP system significantly enhanced antioxidant metrics (p < 0.05). TPC increased by 32.7% to 52.22 ± 0.16 mg GAE/g, FRAP rose by 99.7% to 2.04 ± 0.03 mM Fe2+/g, and DPPH IC50 decreased by 20.8% to 0.13 ± 0.01 mg/mL. These improvements likely result from AgNPs’ high affinity for phenolic hydroxyl and carbonyl groups, which enhances phenolic stability and solubility during extraction. Additionally, AgNPs may act as electron transfer mediators, amplifying redox reactions in FRAP and DPPH assays [37,39,49,62,63,64].
The MIO system yielded modest improvements: TPC increased by 13.3% to 44.57 ± 0.03 mg GAE/g, FRAP rose by 0.9% to 1.03 ± 0.02 mM Fe2+/g, and DPPH IC50 decreased by 4.2% to 0.16 ± 0.01 mg/mL. These changes were not statistically significant (p > 0.05), suggesting limited phenolic accessibility within the matrix, which may restrict redox interactions. However, maltodextrin likely protects thermolabile phenolics from oxidative and thermal degradation during micro-spray drying, consistent with literature reports [50,51,52,53,54,57,58].
The MIO–AgNP system exhibits the highest antioxidant activity. TPC reached 61.01 ± 0.03 mg GAE/g (55.1% increase over I. obliquus), FRAP peaked at 2.05 ± 0.02 mM Fe2+/g (100.8% increase), and DPPH IC50 decreased to 0.13 ± 0.01 mg/mL (23.2% reduction), all statistically significant (p < 0.05). Compared to IO–AgNPs, MIO–AgNPs showed a 16.8% higher TPC, a 0.5% increase in FRAP, and a 3.0% lower IC50. These results suggest synergy between maltodextrin and AgNPs, where the matrix enhances AgNP dispersion, increasing surface area for phenolic binding and improving extraction efficiency [37,39,49,62,63,64]. This configuration stabilizes phenolic–AgNP complexes, optimizing electron donation (FRAP) and radical scavenging (DPPH).
The modest improvements in MIO likely stem from maltodextrin’s protective matrix, which shields phenolics from degradation but restricts their accessibility, limiting redox interactions [50,51,52,53,54,57,58].
In IO–AgNP system, AgNPs enhance phenolic stability and solubility, boosting TPC and facilitating electron transfer in FRAP and DPPH assays.
The superior performance of MIO–AgNPs reflects synergistic effects: the maltodextrin matrix improves AgNP dispersion, increasing phenolic adsorption and extraction efficiency, while stabilizing bioactive complexes for enhanced antioxidant activity.
Collectively, the results demonstrate that the Romanian strain of I. obliquus is a phenolic-rich source with robust antioxidant potential, comparable to or even exceeding that of internationally studied counterparts. Both maltodextrin-based encapsulation and AgNP incorporation significantly enhance TPC and antioxidant activity. Notably, the MIO–AgNP system exhibits the highest efficacy, likely due to synergistic interactions between bioactive phenolics and the intrinsic properties of AgNPs. The strong positive correlations observed between TPC and antioxidant assays (FRAP and DPPH) confirm that phenolic compounds are the primary contributors to the measured antioxidant activity [1,2,3,4,5,6,7,11,12,13,17,79,88,89]. These statistically significant results (p < 0.05) underscore the pivotal role of phenolics in driving the bioactivity of the system. Collectively, the findings position the MIO–AgNP system as a highly promising multifunctional platform with potential for diverse biomedical and technological applications.
3.5. AChE Inhibitory Activity
The AChE inhibition assay was employed to assess the neuroprotective potential of the native I. obliquus sample, AgNPs, and the engineered delivery systems: IO–AgNPs, and encapsulated systems (MIO and MIO–AgNPs). The IC50, indicating the compound concentration required to inhibit 50% of AChE activity, was used as a metric of efficacy. Lower IC50 values denote stronger inhibitory activity and, by extension, greater potential to prevent acetylcholine degradation, a therapeutic goal in Alzheimer’s disease management [90,91]. The results are presented in Figure 12.
Figure 12.
Results of AChE inhibitory assay for of I. obliquus sample, IO–AgNPs, MIO, MIO–AgNP systems and AgNPs. Data are presented as mean ± SD (n = 3). Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test (* p < 0.05, ** p < 0.01 vs. control). AChE: Acetylcholinesterase.
All tested samples exhibited AChE inhibitory activity, with statistically significant differences in potency (Figure 12). The native I. obliquus extract exhibited moderate inhibitory activity (IC50 62.52 ± 3.13 μg/mL), in agreement with previous reports [76,92]. This activity is attributed to its rich content of polyphenolic and triterpenoid compounds, which are known to interact with both the active and peripheral binding sites of the target enzyme [40,41,42,43,44,76,79,80,81,92].
In contrast, AgNPs alone exhibited weak inhibitory activity (IC50 86.79 ± 2.43 μg/mL), reflecting limited intrinsic bioactivity and low affinity for AChE in the absence of targeting ligands or active phytoconstituents [93].
Functionalization of AgNPs with I. obliquus biomolecules within the binary engineered system IO–AgNPs resulted in a marked increase in AChE inhibition (IC50 44.21 ± 1.31 μg/mL; p < 0.05), suggesting that AgNPs may enhance cellular uptake and facilitate sustained delivery of I. obliquus-derived bioactive compounds to the enzyme’s catalytic site [62,63,64,93,94].
Similarly, the MIO system showed improved efficacy (IC50 56.75 ± 2.08 μg/mL), at-tributed to maltodextrin’s role in improving the aqueous solubility and stability of phenolic and triterpenoid compounds, thereby preserving their inhibitory potential [50,53,54,57,58].
The MIO–AgNPs tertiary system exhibited the strongest inhibitory activity (IC50 37.54 ± 1.67 μg/mL; p < 0.01), representing a 40% and 57% reduction in IC50 compared to I. obliquus extract and AgNPs, respectively. The MIO–AgNP system achieves enhanced neuroprotective effects through a synergistic, multi-faceted mechanism. Maltodextrin improves the dispersion and chemical stability of bioactive compounds, ensuring their integrity and solubility. These findings concurrently indicate that AgNPs in the IO–AgNP system contribute to an increased surface area, enhanced membrane permeability, and localized modulation of the redox environment, factors that collectively strengthen AChE inhibition. Following encapsulation within maltodextrin, the resulting MIO–AgNP system exhibits multiple functional enhancements: (i) maltodextrin provides stabilization and improves the solubility of bioactive compounds, (ii) AgNPs enhance bioavailability and enable targeted molecular interactions, and (iii) the integrated system promotes multi-site AChE inhibition. These synergistic effects underscore the potential of nanotechnology-assisted encapsulation to preserve and amplify the bioactivity of natural compounds. Overall, both IO–AgNPs and MIO–AgNP systems emerge as promising platforms for the development of neuroprotective strategies targeting cholinergic dysfunction in neurodegenerative disorders.
3.6. Antimicrobial Activity
The antibacterial potential of the I. obliquus sample and the newly prepared engineered system IO–AgNPs before and after encapsulation in maltodextrin matrix was systematically evaluated and compared with citrate-coated AgNPs alone against clinically relevant Gram-positive (S. aureus, B. cereus) and Gram-negative (P. aeruginosa, E. coli) bacterial strains. The antimicrobial activity of each formulation was assessed using the disk diffusion method across five concentration gradients (100–200 μg/mL), followed by determination of the MICs and MBCs. Gentamicin (100 μg/mL) served as a standard positive control, while DMSO was used as the negative control. The results enabled a direct comparison of the antimicrobial efficacy of the natural extract, inorganic NPs, and biofunctionalized hybrid systems, with data summarized in Table 6.
Table 6.
Evaluation of antibacterial performance against clinically relevant pathogenic strains.
| Pathogenic Microorganism |
Sample | Inhibition Zone Diameter (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Concentration (μg/mL) | Positive Control (Gentamicin, 100 μg/mL) |
Negative Control (DMSO) | ||||||
| 100 | 125 | 150 | 175 | 200 | ||||
|
Staphylococcus
aureus |
I. obliquus | 27.08 ± 0.17 | 38.15 ± 0.32 | 45.22 ± 0.19 | 50.38 ± 0.29 | 57.63 ± 0.29 | 22.21 ± 0.18 | 0 |
| citrate-coated AgNPs | 13.01 ± 0.41 | 16.43 ± 0.42 | 19.02 ± 0.32 | 26.97 ± 0.55 | 30.14 ± 0.21 | |||
| IO–AgNPs | 39.05 ± 0.21 | 47.43 ± 0.33 | 60.18 ± 0.22 | 69.16 ± 0.13 | 74.55 ± 0.32 | |||
| MIO system | 29.72 ± 0.17 | 39.98 ± 0.44 | 47.21 ± 0.17 | 52.09 ± 0.14 | 59.06 ± 0.52 | |||
| MIO–AgNP system | 42.07 ± 0.32 | 49.75 ± 0.21 | 62.64 ± 0.33 | 73.05 ± 0.32 | 78.02 ± 0.44 | |||
| Bacillus cereus | I. obliquus | 26.75 ± 0.31 | 33.24 ± 0.05 | 39.82 ± 0.16 | 42.13 ± 0.34 | 47.53 ± 0.27 | 18.24 ± 0.11 | 0 |
| citrate-coated AgNPs | 38.06 ± 0.12 | 43.47 ± 0.16 | 47.02 ± 0.04 | 50.18 ± 0.21 | 53.07 ± 0.23 | |||
| IO–AgNPs | 43.75 ± 0.09 | 49.91 ± 0.17 | 52.38 ± 0.26 | 59.07 ± 0.31 | 63.58 ± 0.22 | |||
| MIO system | 29.89 ± 0.22 | 37.33 ± 0.07 | 43.53 ± 0.16 | 46.21 ± 0.24 | 51.19 ± 0.25 | |||
| MIO–AgNP system | 46.22 ± 0.19 | 52.21 ± 0.53 | 55.08 ± 0.15 | 62.75 ± 0.31 | 65.17 ± 0.32 | |||
|
Pseudomonas
aeruginosa |
I. obliquus | 16.54 ± 0.11 | 24.12 ± 0.21 | 39.76 ± 0.12 | 46.63 ± 0.16 | 55.32 ± 0.21 | 30.52 ± 0.23 | 0 |
| citrate-coated AgNPs | 9.82 ± 0.14 | 11.63 ± 0.14 | 13.79 ± 0.23 | 16.42 ± 0.34 | 18.47 ± 0.27 | |||
| IO–AgNPs | 29.43 ± 0.18 | 47.65 ± 0.32 | 54.79 ± 0.43 | 60.56 ± 0.16 | 67.87 ± 0.23 | |||
| MIO system | 19.35 ± 0.22 | 28.05 ± 0.23 | 44.21 ± 0.17 | 50.64 ± 0.28 | 58.61 ± 0.32 | |||
| MIO–AgNP system | 34.58 ± 0.32 | 52.53 ± 0.23 | 58.04 ± 0.17 | 65.01 ± 0.26 | 71.19 ± 0.31 | |||
| Escherichia coli | I. obliquus | 18.25 ± 0.31 | 25.42 ± 0.21 | 30.51 ± 0.19 | 39.44 ± 0.12 | 43.43 ± 0.27 | 20.53 ± 0.33 | 0 |
| citrate-coated AgNPs | 13.11 ± 0.17 | 17.24 ± 0.23 | 20.08 ± 0.26 | 22.19 ± 0.32 | 25.83 ± 0.33 | |||
| IO–AgNPs | 28.03 ± 0.24 | 35.22 ± 0.46 | 42.54 ± 0.23 | 50.32 ± 0.18 | 54.29 ± 0.45 | |||
| MIO system | 20.43 ± 0.05 | 29.12 ± 0.31 | 33.43 ± 0.28 | 42.53 ± 0.37 | 47.92 ± 0.25 | |||
| MIO–AgNP system | 32.41 ± 0.15 | 41.43 ± 0.25 | 45.73 ± 0.31 | 50.21 ± 0.43 | 57.72 ± 0.43 | |||
Values are expressed as the mean ± SD (n = 3). DMSO: Dimethyl sulfoxide; SD: Standard deviation.
Both the I. obliquus sample and citrate-coated AgNPs demonstrated concentration-dependent antibacterial activity, with I. obliquus showing greater efficacy against Gram-positive bacteria and moderate activity against Gram-negative strains [1,2,3,4,5,13,39,40,47,48].
Against S. aureus, I. obliquus produced IZs ranging from 27.08 ± 0.17 mm (100 μg/mL) to 57.63 ± 0.29 mm (200 μg/mL), significantly exceeding gentamicin (22.21 ± 0.18 mm) at concentrations ≥125 μg/mL (p < 0.01), Similarly, for B. cereus, I. obliquus achieved IZs of 26.75 ± 0.31 mm to 47.53 ± 0.27 mm, outperforming gentamicin (18.24 ± 0.11 mm) across all concentrations tested (p < 0.01). These results corroborate data reported in the literature and support the antimicrobial activity of polyphenolic and triterpenoid constituents in I. obliquus, which are known to disrupt membrane integrity, inhibit key bacterial enzymes, and induce oxidative damage [1,2,3,4,5,13,39,40].
In contrast, citrate-coated AgNPs exhibited comparatively lower antibacterial activity, particularly at higher concentrations. For S. aureus, AgNPs produced IZs from 13.01 ± 0.41 mm to 30.14 ± 0.21 mm, performing similarly to gentamicin at lower doses but underperforming at 200 μg/mL. Interestingly, AgNPs were highly effective against B. cereus, with IZs ranging from 38.06 ± 0.12 mm to 53.07 ± 0.23 mm (p < 0.01), suggesting enhanced interaction with the structurally simpler Gram-positive cell wall. However, efficacy declined against Gram-negative strains, with IZs for P. aeruginosa (9.82 ± 0.14 mm to 18.47 ± 0.27 mm) and E. coli (13.11 ± 0.17 mm to 25.83 ± 0.33 mm) significantly below those achieved by gentamicin (p < 0.05). Also, these results are correlated with the reported literature [31,39,47,48,95].
The newly prepared systems, IO–AgNPs, MIO, and MIO–AgNPs, exhibited significantly enhanced antibacterial efficacy compared to their individual components and to gentamicin across all tested strains (p < 0.001). For S. aureus, the MIO–AgNPs tertiary system demonstrated the strongest inhibition, with zone diameters from 42.07 ± 0.32 mm to 78.02 ± 0.44 mm. The IO–AgNP system followed closely (39.05 ± 0.21 mm to 74.55 ± 0.32 mm), while MIO alone (29.72 ± 0.17 mm to 59.06 ± 0.52 mm) also outperformed I. obliquus extract and AgNPs significantly (p < 0.001). A similar trend was observed for B. cereus, where MIO–AgNPs reached a maximum inhibition of 65.17 ± 0.32 mm at 200 μg/mL. The hybrid systems (MIO–AgNPs and IO–AgNPs) also showed robust activity against Gram-negative pathogens. For P. aeruginosa, MIO–AgNPs produced IZs from 34.58 ± 0.32 mm to 71.19 ± 0.31 mm, followed by IO–AgNPs (29.43 ± 0.18 mm to 67.87 ± 0.23 mm), both significantly outperforming gentamicin from concentrations as low as 125 μg/mL (p < 0.001). E. coli was similarly inhibited, with MIO–AgNPs generating zones of 32.41 ± 0.15 mm to 57.72 ± 0.43 mm. These findings highlight the role of the maltodextrin matrix in enhancing the bioavailability, dispersion, and stability of the active components.
MIC and MBC values further validated the dose-dependent efficacy observed in IZ assays. The results are presented in Table 7.
Table 7.
MIC and MBC values of the samples against representative pathogenic strains.
| Pathogenic Microorganism | Sample | MIC (μg/mL) | MBC (μg/mL) | Gentamicin | |
|---|---|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | ||||
| Staphylococcus aureus | I. obliquus | 0.25 ± 0.04 | 0.24 ± 0.12 | 0.62 ± 0.02 | 0.62 ± 0.02 |
| citrate-coated AgNPs | 0.14 ± 0.07 | 0.13 ± 0.08 | |||
| IO–AgNPs | 0.12 ± 0.02 | 0.11 ± 0.06 | |||
| MIO system | 0.22 ± 0.01 | 0.22 ± 0.13 | |||
| MIO–AgNP system | 0.08 ± 0.05 | 0.09 ± 0.04 | |||
| Bacillus cereus | I. obliquus | 1.09 ± 0.11 | 1.10 ± 0.17 | 1.30 ± 0.03 | 1.29 ± 0.02 |
| citrate-coated AgNPs | 10.04 ± 0.24 | 10.02 ± 0.23 | |||
| IO–AgNPs | 0.81 ± 0.17 | 0.82 ± 0.33 | |||
| MIO system | 0.93 ± 0.08 | 4.07 ± 0.12 | |||
| MIO–AgNP system | 0.78 ± 0.09 | 0.95 ± 0.03 | |||
| Pseudomonas aeruginosa | I. obliquus | 1.07 ± 0.08 | 1.06 ± 0.53 | 1.95 ± 0.22 | 1.96 ± 0.24 |
| citrate-coated AgNPs | 0.68 ± 0.11 | 0.67 ± 0.21 | |||
| IO–AgNPs | 0.44 ± 0.12 | 0.45 ± 0.43 | |||
| MIO system | 0.97 ± 0.02 | 0.98 ± 0.09 | |||
| MIO–AgNP system | 0.38 ± 0.13 | 0.36 ± 0.16 | |||
| Escherichia coli | I. obliquus | 0.47 ± 0.13 | 0.46 ± 0.08 | 1.12 ± 0.23 | 1.12 ± 0.22 |
| citrate-coated AgNPs | 0.30 ± 0.11 | 0.31 ± 0.13 | |||
| IO–AgNPs | 0.28 ± 0.31 | 0.29 ± 0.07 | |||
| MIO system | 0.41 ± 0.04 | 0.42 ± 0.13 | |||
| MIO–AgNP system | 0.23 ± 0.21 | 0.25 ± 0.03 | |||
Values are expressed as the mean ± SD (n = 3). MBC: Minimum bactericidal concentration; MIC: Minimum inhibitory concentration; SD: Standard deviation.
MIC and MBC analyses confirmed the superior antibacterial potency of the hybrid systems. For S. aureus, MIO–AgNPs exhibited the lowest MIC (0.08 ± 0.05 μg/mL) and MBC (0.09 ± 0.04 μg/mL), outperforming gentamicin (MIC: 0.62 ± 0.02 μg/mL; MBC: 0.63 ± 0.03 μg/mL). IO–AgNPs and MIO also showed improved potency, evidencing synergistic effects between I. obliquus phytoconstituents and AgNPs, as well as enhanced interaction enabled by maltodextrin encapsulation.
In the case of B. cereus, MIO–AgNPs again showed the best performance (MIC: 0.78 ± 0.09 μg/mL; MBC: 0.95 ± 0.03 μg/mL), followed by IO–AgNPs. AgNPs alone displayed notably weaker activity (MIC: 10.04 ± 0.24 μg/mL; MBC: 10.02 ± 0.23 μg/mL), likely due to aggregation or lower dispersion.
The I. obliquus sample surpassed gentamicin, reinforcing its specificity toward Gram-positive targets.
Strikingly, MIO–AgNPs were also highly effective against Gram-negative strains. For P. aeruginosa, the MIC and MBC were 0.38 ± 0.13 μg/mL and 0.36 ± 0.16 μg/mL, respectively, significantly lower than those of gentamicin (MIC: 1.95 ± 0.22 μg/mL; MBC: 1.96 ± 0.24 μg/mL).
IO–AgNPs showed comparable values (MIC: 0.44 ± 0.12 μg/mL; MBC: 0.45 ± 0.43 μg/mL), indicating efficient bacterial membrane permeation. Similar trends were observed for E. coli, with MIO–AgNPs achieving MIC and MBC values of 0.23 ± 0.21 μg/mL and 0.25 ± 0.03 μg/mL, respectively, statistically superior to all controls (p < 0.05).
The low MIC/MBC values of hybrid systems (IO–AgNPs and MIO–AgNPs), indicate potent antibacterial activity, with increasing concentrations correlating with enhanced inhibition and bactericidal effects across all strains.
3.7. Cell Viability Assay
The cytotoxic potential of Inonotus obliquus extracts and the newly developed hybrid IO–AgNP systems, both in free form and following encapsulation in a maltodextrin matrix, was evaluated against three human cancer cell lines: MCF-7 (breast adenocarcinoma), HCT116 (colorectal carcinoma), and HeLa (cervical carcinoma), using the MTT assay.
These cell lines were specifically selected because previous studies have reported cytotoxic effects of I. obliquus extracts from other geographic origins on these models, providing a relevant and comparative framework [2,5,6,11,14,15,75,77,78]. By investigating the Romanian I. obliquus variant, which exhibits a unique regional phytochemical fingerprint, this study aims to determine whether similar or enhanced cytotoxic activity can be achieved. The chosen cell lines represent diverse tissue origins and biological characteristics relevant to cancer progression, including hormone sensitivity, oxidative stress vulnerability, and proliferative behavior. This approach enables a comprehensive assessment of the functional efficacy of the developed delivery systems across multiple clinically relevant cancer types.
Cell viability (%) and IC50 values were determined across a concentration range (75–200 μg/mL) and time intervals (24, 48, and 72 h) (Figure 13a–d and Figure 14).
Figure 13.
Viability of MCF-7, HCT116, and HeLa cell lines, assessed at 24, 48, and 72 h after co-incubation with varying concentrations (75–200 μg/mL) of I. obliquus sample (a), IO–AgNPs (b), MIO (c), and MIO–AgNPs (d) systems. Negative control wells included untreated cells, while positive control wells included cells treated with a known cytotoxic agent; MTT solution and DMSO were used in the assay. Data are presented as mean ± SD (n = 3). Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test (* p < 0.05, ** p < 0.01 vs. control). DMSO: Dimethyl sulfoxide; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Figure 14.
IC50 values of in vitro cytotoxicity of I. obliquus extract (IO), citrate-coated AgNPs, IO–AgNPs, MIO, and MIO–AgNP systems against MCF-7, HCT116, and HeLa cancer cell lines, assessed by MTT assay. Untreated cells served as the control. Data are presented as mean ± SD (n = 3). Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test (* p < 0.05, ** p < 0.01 vs. control).
In HCT116 cells, I. obliquus and MIO exhibited moderate cytotoxicity, reaching 30.07% and 29.82% viability, respectively, at 200 μg/mL (72 h). IO–AgNPs and MIO–AgNP systems showed markedly enhanced activity, with final viability values of 24.08% and 24.17%, respectively (p < 0.05).
For HeLa cells, which displayed higher resistance, I. obliquus and MIO resulted in ~46% cell viability at the highest dose/timepoint.
IO–AgNPs and MIO–AgNPs again outperformed both, reducing viability to 36.67% and 37.49%, respectively (p < 0.05).
One-way ANOVA analysis confirmed significant differences among treatments at each concentration and timepoint, with Tukey’s post hoc tests revealing that MIO–AgNPs consistently exerted the most potent cytotoxic effect across all cell lines at 200 μg/mL (p < 0.01, compared to I. obliquus, MIO, and IO–AgNP systems).
The cytotoxic potential of all tested samples was systematically assessed against the three human cancer cell lines (MCF-7, HCT116, and HeLa) by determining their IC50 values. The resulting data, summarized in Figure 14, provide a comparative insight into the antiproliferative. These values serve as quantitative indicators of antiproliferative efficacy of the tested samples as candidates for further anticancer investigation.
The MIO–AgNP system (Figure 14) demonstrated the most potent cytotoxic activity across all tested cancer cell lines, exhibiting the lowest IC50 values: 28.5 ± 1.2 μg/mL (MCF-7), 25.3 ± 1.0 μg/mL (HCT116), and 45.6 ± 1.8 μg/mL (HeLa). These values were significantly lower than those observed for the IO–AgNP system, which recorded IC50 values of 35.2 ± 1.5 μg/mL (MCF-7), 32.1 ± 1.3 μg/mL (HCT116), and 52.4 ± 2.0 μg/mL (HeLa). The MIO formulation showed higher IC50 values of 42.8 ± 1.7 μg/mL, 38.5 ± 1.6 μg/mL, and 60.3 ± 2.2 μg/mL, respectively, while the unmodified IO sample exhibited the least cytotoxicity, with IC50 values of 50.1 ± 2.0 μg/mL (MCF-7), 46.7 ± 1.9 μg/mL (HCT116), and 70.9 ± 2.5 μg/mL (HeLa) (p < 0.05 for all comparisons vs. MIO–AgNPs). AgNPs alone showed intermediate activity, with IC50 values of 40.5 ± 1.6 μg/mL (MCF-7), 35.9 ± 1.4 μg/mL (HCT116), and 55.2 ± 2.1 μg/mL (HeLa).
One-way ANOVA analysis revealed significant differences among the tested systems (F = 75.4, p < 0.001), and subsequent Tukey’s post hoc tests confirmed the superior cytotoxic efficacy of the MIO–AgNP system across all cell lines. These findings highlight the enhanced antiproliferative potential achieved through maltodextrin encapsulation of IO–AgNPs, underscoring its promise as an advanced therapeutic candidate.
4. Discussion
4.1. Mycochemical Screening
The semi-quantitative GC–MS profile confirms I. obliquus as a rich source of bioactive triterpenoids, sterols, fatty acid esters, and aromatic compounds, several of which have well-established pharmacological relevance. Inotodiol, the dominant compound, is particularly notable for its anti-inflammatory, antitumor, and immunomodulatory activities [1,2,3,4,5,6,7,8]. In addition, secondary metabolites such as betulin, ergosterol, and lupeol contribute to the therapeutic potential of the extract [1,2,3,4,5,6,7,8,9,10,11,12,14,15,74,75,76]. Importantly, many of the identified compounds bear –OH (e.g., lupeol) and –COOH (e.g., trametenolic acid) functional groups capable of interacting with citrate-coated AgNPs. These interactions may occur via hydrogen bonding, electrostatic attraction, or coordination with surface-bound citrate ligands [37,39,47,48,62,63,64]. Such functional moieties are crucial for NP stabilization, modulation of surface properties, and control of bioactive release kinetics.
These molecular interactions are particularly relevant in the context of the engineered IO–AgNP and MIO–AgNP systems, where natural phytochemicals may act as both biological stabilizers and functional modifiers of the NP interface. In this role, they not only enhance NP dispersion and stability but may also influence the pharmacokinetics and bioactivity of the nanocarrier system. Furthermore, recent studies indicate a dynamic bidirectional interplay between AgNPs and fungal metabolism [64]. AgNPs have been shown to downregulate the biosynthesis of polysaccharides and flavonoids while significantly enhancing melanin production, by as much as 140% [64]. These findings suggest that phytochemicals and AgNPs may mutually influence each other, with implications for NP efficacy, metabolite stability, and release dynamics [37,39,47,48,62,63,64,96].
Altogether, the GC–MS data and functional group analysis support the potential of I. obliquus as an effective phytochemical matrix for AgNP-based controlled-release systems. Its combination of chemical diversity, bioactivity, and NP-interactive moieties highlights its utility in developing advanced delivery platforms with enhanced therapeutic performance and physicochemical stability.
Terpenoids constitute 17.60% of the total phytoconstituents in I. obliquus extracts, establishing them as the predominant class of bioactive compounds identified (Table 2). These structurally diverse secondary metabolites are distinguished by their extensive pharmacological potential, encompassing antitumor, antimicrobial, antiviral, analgesic, antispasmodic, anti-inflammatory, cardioprotective, antihyperglycemic, and immunomodulatory activities [10,12,14,15,69,75,76,96]. The broad-spectrum bioactivity of terpenoids underscores their critical role in health promotion and disease prevention, positioning them as prime candidates for advanced pharmacological research and therapeutic development. Their ability to modulate multiple cellular pathways highlights their potential as versatile agents in combating complex diseases such as cancer and chronic inflammation [10,12,14,15,69,75,76,96].
Amino acids represent 10.56% of the chemical profile of I. obliquus (Table 2). A comprehensive analysis revealed 15 amino acids in the Chaga extract, with essential amino acids (threonine, leucine, lysine, methionine, histidine, tyrosine, and tryptophan) comprising 40.0% of the total amino acid content, while non-essential amino acids (glycine, alanine, arginine, aspartic acid, serine, proline, cystine, and glutamic acid) account for the remaining 60.0% [29,37,38,39,49,53,54,60,97]. This amino acid profile is significant due to the well-documented antitumoral, antiproliferative, and immunomodulatory properties of these biomolecules [29,97]. Essential amino acids are critical for protein synthesis and metabolic regulation, while non-essential amino acids contribute to cellular signaling and immune modulation, collectively enhancing the therapeutic potential of I. obliquus in applications targeting cancer and immune-related disorders [97].
Flavonoids, accounting for 12.67% of the phytoconstituents, are pivotal bioactive metabolites renowned for their multifaceted biological activities (Table 2). These polyphenolic compounds exhibit potent antioxidant, antiviral, antimicrobial, antitumor, cardioprotective, and neuroprotective effects, driven by their ability to neutralize reactive oxygen species (ROS) and modulate key signaling pathways [98]. The diverse therapeutic capabilities of flavonoids highlight their essential role in mitigating oxidative stress-related diseases and promoting overall health, making them a focal point for further investigation in pharmaceutical and nutraceutical development [98].
Fatty acids comprise 11.26% of the total phytoconstituents in I. obliquus, underscoring their significant contribution to the mushroom’s bioactive profile (Table 2). This fraction includes 10 saturated fatty acids (palmitic, behenic, eicosanedioic, lauric, myristic, tricosanoic, stearic, margaric, lignoceric, and octadecanedioic acids), three monounsaturated fatty acids (including the essential ω-6 linoleic acid, ω-3 linolenic acid, and nervonic acid), and two polyunsaturated fatty acids (trihydroxyoctadecenoic acid and hydroxyarachidic acid) [1,2,3,4,5,6,8]. These fatty acids are recognized for their antioxidant, antimicrobial, anti-inflammatory, neuroprotective, and cardioprotective properties, attributed to their roles in membrane integrity, inflammation regulation, and lipid metabolism [99]. The presence of essential fatty acids, particularly ω-3 and ω-6, enhances the therapeutic potential of I. obliquus in addressing cardiovascular and neurodegenerative disorders.
Phytosterols, representing 2.81% of the total phytoconstituents, exhibit a remarkable array of therapeutic properties, including antioxidant, neuroprotective, cardioprotective, anti-inflammatory, antitumor, and immunomodulatory activities (Table 2). These sterol compounds are known to modulate cholesterol metabolism and inhibit inflammatory pathways, making them valuable for preventing cardiovascular diseases and supporting immune health [100]. Their diverse bioactivities warrant further exploration for targeted therapeutic applications.
Styrylpyrones, comprising 10.56% of the phytoconstituents in I. obliquus, demonstrate a wide range of pharmacological effects, including antioxidant, anti-inflammatory, antimicrobial, antiviral, antitumor, neuroprotective, and antidiabetic activities [79]. These polyketide-derived compounds are particularly notable for their ability to modulate oxidative stress and inflammatory cascades, positioning them as promising candidates for managing chronic diseases such as diabetes and cancer [79].
Phenolic acids, constituting a significant portion of Chaga’s bioactive profile, are celebrated for their potent antioxidant, antibacterial, antitumor, anti-inflammatory, antiallergic, antidiabetic, cardioprotective, and neuroprotective properties [101]. These compounds exert their effects through free radical scavenging and modulation of enzymatic pathways, playing a critical role in health promotion and disease prevention. Their versatility underscores their importance in both pharmaceutical and nutraceutical applications, particularly for combating oxidative stress-related pathologies [101].
Coumarins, accounting for 5.63% of the phytoconstituents, include hydroxycoumarin, coumarin, 3-acetylcoumarin, phelligridin J, phelligridin C, phelligridin D, phelligridin H, and phelligridin I [1,2,3,4,5,6,8,11,14]. These compounds are recognized for their anticoagulant, anti-inflammatory, antimicrobial, and anticancer properties, attributed to their ability to inhibit key enzymes and modulate cellular signaling pathways. Their presence enhances the therapeutic versatility of I. obliquus extracts [1,2,3,4,5,6,8,11,14].
Other polyphenols, comprising 4.92% of the phytoconstituents, include hispolon, resveratrol, ellagic acid, inonophenol C, 3-O-methylellagic acid, and interfungin A [1,2,3,4,5,6,8,11,14]. These compounds are renowned for their potent antioxidant, anti-inflammatory, and anticancer activities, driven by their capacity to regulate gene expression and inhibit oxidative damage. Their diverse bioactivities make them critical components for developing novel therapeutic agents [1,2,3,4,5,6,8,11,14,80].
Melanin, another key constituent in I. obliquus, exhibits significant antioxidant, radio-protective, and immunomodulatory properties [64,76]. This pigment is known for its ability to scavenge free radicals and protect cells from oxidative and radiation-induced damage, further enhancing the therapeutic potential of Chaga extracts in applications targeting skin health, immune modulation, and cancer prevention [64,76,102,103].
4.2. Impact of Spray Drying and Formulation Architecture on Particle Characteristics and Biomedical Relevance
Although the spray drying process resulted in a shift toward micron-scale particle populations, particularly evident in the MIO formulation, the incorporation of AgNPs into the MIO–AgNP system markedly enhanced particle size uniformity and minimized aggregation. These improvements were substantiated by the narrower particle size distribution (PSD) and reduced polydispersity index (PDI), reflecting more consistent morphological characteristics. Given the established influence of particle size and structural homogeneity on biological interactions, including cellular uptake, circulation time, and tissue distribution, these physicochemical attributes are of particular relevance for downstream biomedical applications. While the present study focused on in vitro characterization and bioactivity assays, these findings underscore the importance of future in vivo investigations to elucidate how formulation architecture, particle size, and clustering may affect pharmacokinetics, biodistribution, and overall therapeutic performance.
4.3. Comparative Encapsulation Efficiency and Novelty of Fungal-Based Systems
The encapsulation efficiencies obtained for I. obliquus and IO–AgNPs in maltodextrin 74.58% and 63.12%, respectively, are comparable to those reported in our previous studies involving plant-based systems encapsulated in similar matrices [49,53,54,57,58]. Specifically, E. cannabinum, E. cannabinum-AuNPs, and Zingiber officinale-kaolinite composites exhibited yields of approximately 60–63%, in agreement with literature data for maltodextrin-based encapsulation [50,51,53,54,57,58]. In contrast, encapsulation in chitosan matrices, such as for Helleborus purpurascens and its AgNP counterpart, resulted in efficiencies exceeding 90%, consistent with the higher binding capacity of chitosan [49].
This work represents our first study involving the encapsulation of mushroom-derived bioactives. Although we have previously conducted metabolic and elemental profiling of Romanian truffle species, those investigations did not involve formulation or delivery system development [19,103]. Therefore, the present study adds a new dimension to our research by extending encapsulation strategies to fungal matrices and biogenic nanoparticle systems, offering a point of comparison with plant-based analogs in terms of matrix compatibility and encapsulation performance.
4.4. Assessment of TPC and Antioxidant Capacity
The native I. obliquus sample demonstrated robust antioxidant activity, with TPC at 39.34 ± 0.03 mg GAE/g, FRAP at 1.02 ± 0.02 mM Fe2+/g, and DPPH IC50 at 0.17 ± 0.02 mg/mL. These values are consistent with those reported for I. obliquus from other regions [1,2,3,4,5,6,7,11,12,13,17,79,88,89], affirming the Romanian strain’s competitive bioactivity. Significant correlations (p < 0.05) between TPC and both FRAP and DPPH results confirm phenolic compounds, primarily phenolic acids, flavonoids, and other polyphenols, as the main drivers of antioxidant capacity [1,2,3,4,5,6,7,11,12,13,17,79,88,89]. Contributions from triterpenoids or polysaccharides may also play a role and merit further exploration to quantify their impact [1,2,3,4,5,6,7,11,12,13,17,79,88,89].
The binary IO–AgNP system markedly improved antioxidant performance: TPC in-creased by 32.7% to 52.21 ± 0.03 mg GAE/g, FRAP doubled to 2.04 ± 0.02 mM Fe2+/g, and DPPH IC50 decreased by 23.5% to 0.13 ± 0.01 mg/mL (p < 0.05). These enhancements result from AgNPs’ ability to form coordination complexes with phenolic hydroxyl and car-bonyl groups, improving solubility, stability, and electron transfer efficiency [37,39,49,62,63,64,79,80,81]. AgNPs likely act as redox mediators, reducing the energy barrier for electron donation in FRAP and DPPH assays [37,39,62,63,64,79,80,81,90]. Their high surface area-to-volume ratio further enhances phenolic interactions, boosting reactivity and apparent bioavailability. However, the dose-dependent cytotoxicity of AgNPs requires thorough safety evaluations for biomedical applications [31,64,90].
I. obliquus microencapsulation via micro-spray drying to prepare the MIO system yielded modest improvements: TPC increased by 13.3% to 44.57 ± 0.03 mg GAE/g, FRAP rose by 1.0% to 1.03 ± 0.02 mM Fe2+/g, and DPPH IC50 decreased by 5.9% to 0.16 ± 0.02 mg/mL. These changes were not statistically significant (p > 0.05), indicating that maltodextrin primarily stabilizes thermolabile phenolics during processing but may limit their accessibility in vitro, reducing immediate reactivity in antioxidant assays [50,53,54,57,58]. While this encapsulation enhances shelf-life and supports controlled release, optimizing the carrier composition or structure could improve phenolic release while maintaining stability [50,53,54,57,58].
The MIO–AgNP system exhibited superior antioxidant performance: TPC reached 61.01 ± 0.03 mg GAE/g, FRAP was 2.05 ± 0.02 mM Fe2+/g, and DPPH IC50 was 0.13 ± 0.01 mg/mL, significantly outperforming native I. obliquus, IO–AgNPs and MIO systems (p < 0.05). Compared to IO–AgNPs, MIO–AgNPs showed a 16.8% higher TPC with comparable FRAP and DPPH values, suggesting a synergistic effect [37,39,46,47,49,50,54,57,58,62,63,64]. The maltodextrin matrix enhances AgNP dispersion, reducing agglomeration and increasing the effective surface area for phenolic interactions [50,54,57,58,62,63,64]. This stabilizes phenolic–AgNP complexes, improving extraction efficiency and redox activity. Maltodextrin may also protect AgNPs from oxidative degradation, sustaining their catalytic function.
The enhanced performance of MIO–AgNPs arises from a synergistic mechanism: maltodextrin preserves phenolic integrity during micro-spray drying, while AgNPs amplify redox activity by facilitating electron transfer [50,53,54,57,58]. The encapsulation matrix reduces steric hindrance, enabling efficient phenolic–AgNP interactions, which enhance solubility and reactivity [50,53,54,57,58]. Key phenolic compounds, such as catechins, gallic acid, or syringic acid, likely form stable complexes with AgNPs via their functional groups, further boosting antioxidant capacity [49,62,63,64].
The superior antioxidant activity of MIO–AgNPs positions it as a promising platform for various applications.
4.5. AChE Inhibitory Activity
The AChE inhibition results highlight the neuroprotective potential of I. obliquus and its engineered derivatives, particularly the IO–AgNPs and MIO–AgNP systems, by leveraging synergistic biochemical and nanomaterial properties.
The moderate AChE inhibitory activity of native I. obliquus (IC50 62.52 ± 3.13 μg/mL) aligns with previous studies attributing its bioactivity to polyphenols (e.g., flavonoids, phenolic acids) and triterpenoids [40,41,42,43,44,76,79,80,81,92]. These compounds likely bind to the catalytic active site and peripheral anionic site of AChE, disrupting substrate binding and enzyme kinetics through hydrophobic and hydrogen-bonding interactions [40,41,42,43,44,76,79,80,81,92]. However, their moderate potency is limited by poor aqueous solubility, which restricts bioavailability, and chemical instability, which leads to degradation of active moieties under physiological conditions [41,42,43,44,81,92]. These bioavailability constraints reduce the clinical applicability of native I. obliquus biomolecules.
In contrast, AgNPs alone exhibited weak AChE inhibition (IC50 86.79 ± 2.43 μg/mL), consistent with their limited specificity for AChE [93]. AgNPs primarily exert nonspecific effects through surface interactions or oxidative stress rather than targeted enzyme inhibition. Their large surface area may facilitate weak adsorption to AChE, but without specific ligands, their binding affinity remains low, resulting in minimal inhibitory activity [93]. This underscores the need for functionalization to enhance their bioactivity.
Functionalization of AgNPs with I. obliquus biomolecules in the binary IO–AgNP system significantly improved inhibitory potency (IC50 44.21 ± 1.31 μg/mL; p < 0.05). The enhanced efficacy can be attributed to the role of AgNPs as nanocarriers that improve the delivery and presentation of I. obliquus biomolecules [62,63,64,93,94]. The high surface-to-volume ratio of AgNPs allows for efficient conjugation of polyphenols and triterpenoids, stabilizing these compounds against degradation and enhancing their solubility [62,63,64,93,94]. Additionally, AgNPs may facilitate cellular uptake via endocytosis, increasing the local concentration of bioactive compounds at the AChE active sites [62,63,64,93,94]. The NPs may also amplify bioactivity through surface plasmon resonance effects, potentially modulating local redox environments to enhance enzyme interactions [93,94]. These combined mechanisms account for the statistically significant improvement in inhibition compared to native I. obliquus or AgNPs alone.
The MIO system, incorporating I. obliquus in maltodextrin matrix, exhibited improved efficacy (IC50 56.75 ± 2.08 μg/mL), likely due to maltodextrin’s role as a stabilizing and solubilizing agent [50,53,54,57,58]. Maltodextrin, a polysaccharide, forms a hydrophilic matrix that encapsulates I. obliquus biomolecules, protecting them from oxidative or hydrolytic degradation and enhancing their aqueous solubility [50,53,54,57,58]. This improved stability ensures a higher effective concentration of active compounds at the AChE binding sites, leading to enhanced inhibition compared to native I. obliquus [50,53,54,57,58]. However, the MIO system’s potency remains lower than that of IO–AgNPs, likely because it lacks the nanocarrier-mediated delivery and surface enhancement provided by AgNPs.
The MIO–AgNPs hybrid demonstrated the highest potency (IC50 37.54 ± 1.67 μg/mL; p < 0.01), achieving a 40–57% improvement over native I. obliquus and AgNPs alone. This superior performance reflects a synergistic interaction among its components. Maltodextrin stabilizes I. obliquus bioactive compounds, preventing degradation and improving dispersion in aqueous environments [50,53,54,57,58]. Simultaneously, AgNPs enhance bioavailability by facilitating targeted delivery to AChE through receptor-mediated or passive cellular uptake [62,63,64,93,94]. The NPs’ surface chemistry may also promote multi-site inhibition by presenting biomolecules in an optimized spatial orientation, enabling simultaneous interactions with the catalytic and peripheral sites of AChE [93,94]. Furthermore, AgNPs may contribute to redox-mediated modulation of the enzyme’s microenvironment, potentially altering its conformational dynamics to enhance inhibition [58,94]. This multi-faceted mechanism, combining stabilization, enhanced solubility, and targeted delivery, underpins the MIO–AgNP system’s exceptional potency.
These results validate the integration of natural extracts with nanotechnology and encapsulation strategies to overcome biopharmaceutical limitations, such as poor solubility, instability, and low bioavailability. The IO–AgNPs and MIO–AgNP systems emerge as promising neuroprotective platforms for targeting cholinergic dysfunction in Alzheimer’s disease and related disorders. However, further studies are needed to elucidate the precise molecular interactions (e.g., binding kinetics, specific bioactive contributions), confirm in vivo efficacy, and assess formulation scalability for clinical translation.
Collectively, the MIO–AgNP system represents a potent, multifunctional AChE inhibitor, leveraging the synergistic interplay of I. obliquus bioactive constituents, AgNPs, and stabilizing biopolymeric matrix to offer a promising therapeutic approach for managing cholinergic deficits in neurodegenerative disorders.
4.6. Antimicrobial Activity
To evaluate the antimicrobial performance of the native I. obliquus sample and the newly prepared various bioactive systems (IO–AgNPs, MIO, and MIO–AgNPs), they were tested against a representative panel of Gram-positive and Gram-negative bacterial strains. The findings demonstrated a clear dose-dependent response across all tested systems, in agreement with the existing literature [1,2,3,4,5,6,13,64,79].
The antimicrobial activity of I. obliquus is primarily attributed to its diverse bioactive constituents, including lanostane-type triterpenoids, polyphenols, and β-glucans [1,2,3,4,5,6,13,64,72,73,75,79]. These compounds act synergistically by disrupting bacterial membranes, chelating essential metal ions (e.g., Fe2+, Zn2+), and interfering with metabolic enzymes and oxidative phosphorylation [1,2,3,4,5,6,13,64,72,73,75,76,79].
Gram-positive strains exhibited greater susceptibility to the crude extract, likely due to the absence of an outer membrane, facilitating compound penetration [32,33]. This was reflected in larger IZs and lower MIC/MBC values for S. aureus and B. cereus.
AgNPs, well-known for their broad-spectrum antimicrobial activity, exert their effects through ROS generation, thiol-group binding, and membrane damage [37,39,47,48,49,95]. Despite their efficacy, AgNPs alone were less potent against Gram-negative bacteria, such as E. coli and P. aeruginosa, which possess an outer membrane that restricts NP penetration [37,39,47,48,49,95]. These results are consistent with previous studies highlighting the role of NP size, morphology, and aggregation state in determining antibacterial effectiveness [37,39,47,48,49,95].
The IO–AgNP hybrid system exhibited significantly improved antibacterial activity (p < 0.001), suggesting a synergistic mechanism between I. obliquus-derived phytoconstituents and AgNPs. Triterpenoids and polyphenols were likely responsible for enhancing Ag+ ions uptake by disrupting bacterial membranes, while acting as microbial pro-oxidants, amplifying intracellular oxidative stress. This dual mechanism, membrane permeabilization and intracellular ROS generation may explain the particularly strong inhibition observed in S. aureus (e.g., IZ: 74.55 ± 0.32 mm; MIC: 0.12 ± 0.02 μg/mL). The system’s multi-target action also reduces the risk of resistance development.
Encapsulation of I. obliquus in maltodextrin (MIO) resulted in a moderate enhancement in antibacterial activity compared to the native I. obliquus sample. This can be attributed to improved aqueous solubility, sustained release of hydrophobic bioactive compounds, and increased interaction time with bacterial cells [53,54,58]. Maltodextrin itself lacks intrinsic antimicrobial activity [53,54,58] but serves as a functional excipient that stabilizes and disperses active compounds, which was reflected in slightly larger IZs (e.g., S. aureus: 59.06 ± 0.52 mm vs. 57.63 ± 0.29 mm for the native I. obliquus sample).
The MIO–AgNP system demonstrated the most potent and broad-spectrum antimicrobial effect among all tested formulations, particularly against Gram-positive strains, and importantly, showed enhanced efficacy even against the highly resistant P. aeruginosa. This hybrid system benefits from the combined advantages of phytochemical functionalization, AgNPs’ biocidal action, and maltodextrin-mediated stabilization and delivery [37,39,47,48,49,50,53,54,57,58]. The encapsulation likely improved NP dispersion, prevented aggregation, and allowed for controlled release of both Ag+ ions and bioactive compounds, increasing the system’s interaction with bacterial surfaces [50,53,54,57,58]. The MIO–AgNPs’ superior activity may also reflect enhanced oxidative stress induction and deeper cellular penetration compared to non-encapsulated IO–AgNPs.
These findings suggest that encapsulated biogenic nanocomposites, particularly MIO–AgNPs, hold promise as next-generation antimicrobial agents. Their ability to overcome common resistance mechanisms, coupled with multi-targeted action, positions them as valuable candidates for therapeutic or preservative applications, especially against biofilm-forming or drug-resistant pathogens.
4.7. Cytotoxic Activity
The concentration- and time-dependent reduction in cell viability (p < 0.01) demonstrates the potent anticancer properties of newly prepared I. obliquus-based hybrid systems. Among all tested systems, the MIO–AgNP system showed the most pronounced cytotoxic effects. After 72 h of treatment at 200 μg/mL, the MIO–AgNP system reduced viability in HCT116 cells to 24.17 ± 0.26%, significantly outperforming the I. obliquus, MIO, and IO–AgNPs groups (p < 0.01). This enhanced activity is primarily attributed to improved NP dispersion, stability, and controlled release facilitated by the maltodextrin matrix, which promotes sustained intracellular delivery of both Ag+ ions and bioactive IO compounds [37,39,47,48,50,53,54,57,58].
The enhanced cytotoxicity of MIO–AgNPs is corroborated by one-way ANOVA, which revealed a significant interaction between treatment concentration and exposure time (F = 16.3, p < 0.01). This supports a cumulative toxicity mechanism in which prolonged exposure amplifies the generation of ROS, disrupts mitochondrial integrity, and eventually leads to apoptosis. Notably, in MCF-7 cells, viability following MIO–AgNPs exposure at 75 μg/mL dropped from 76.56 ± 0.21% at 24 h to 48.87 ± 0.18% at 72 h, indicating that early cellular resistance is overcome by persistent oxidative insult and intracellular accumulation.
The cytotoxicity observed for both I. obliquus and AgNPs alone agrees with literature, further validating the current data [1,6,8,11,12,14,15,39,47,48,49,61,64,73,75,77,78,79].
I. obliquus has been shown in the literature to induce apoptosis in various cancer cell lines via multiple mechanisms, including mitochondrial membrane depolarization, cell cycle arrest, and the upregulation of proapoptotic proteins such as Bax and caspase-3, while downregulating anti-apoptotic B-cell lymphoma 2 (Bcl-2) expression [1,6,8,11,12,14,15,61,64,73,75,77,78,79].
In particular, I. obliquus’s rich composition in triterpenoids, polysaccharides, and phenolic compounds is known to modulate oxidative stress responses and inhibit phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) and mitogen-activated protein kinase (MAPK) signaling pathways, hallmarks of oncogenic survival [1,6,8,11,12,14,15,61,64,73,75,77,78,79].
Similarly, citrate-coated AgNPs exert cytotoxic effects primarily via ROS generation, mitochondrial dysfunction, and direct interaction with thiol-containing proteins and deoxyribonucleic acid (DNA), leading to structural and functional disruption [39,47,48]. The alignment of our I. obliquus and AgNPs results with literature benchmarks (e.g., IC50 for I. obliquus > 45 μg/mL and for AgNPs ~35 μg/mL) reinforces the reliability of our findings and under-scores the added value of combining these components [1,6,8,11,12,14,15,39,47,48,61,64,73,75,77,78,79].
Importantly, the MIO–AgNP system leverages both I. obliquus and AgNP mechanisms synergistically. The maltodextrin encapsulation promotes NP stability and enhances endocytotic uptake while providing sustained release of I. obliquus phytochemicals [50,54,57,58]. These compounds may sensitize cancer cells to ROS-mediated damage by downregulating antioxidant defenses (e.g., glutathione, catalase), thereby amplifying AgNPs’ intrinsic oxidative effects. Additionally, maltodextrin may facilitate more uniform distribution and prolong retention within the tumor microenvironment, further enhancing therapeutic efficacy [50,54,57,58].
Cell line-specific responses also support the differential susceptibility of cancer cells to NP-based systems (IO–AgNPs and MIO–AgNPs). The lowest IC50 value was observed in HCT116 cells (25.3 ± 1.0 μg/mL), potentially due to their higher membrane permeability and less robust antioxidant systems, which allow rapid intracellular NP accumulation. MCF-7 cells exhibited moderate IC50 values (28.5 ± 1.2 μg/mL), consistent with estrogen receptor-mediated modulation of oxidative stress responses.
HeLa cells, known for drug resistance mechanisms such as P-glycoprotein overexpression, had the highest IC50 (45.6 ± 1.8 μg/mL). Nevertheless, the MIO–AgNP system achieved significantly greater cytotoxicity even in HeLa, suggesting that the system can effectively overcome efflux-mediated resistance via prolonged intracellular release and oxidative imbalance.
The statistically significant enhancement of cytotoxic activity observed with the MIO–AgNP system (p < 0.01 vs. IO–AgNPs and MIO) underscores the critical role of maltodextrin in optimizing the bioavailability of both AgNPs and I. obliquus phytoconstituents. Maltodextrin’s function as an encapsulating matrix not only improves NP dispersion and stability but also facilitates sustained intracellular release, thereby enhancing therapeutic efficacy through prolonged cellular exposure [50,54,57,58]. This integrated delivery strategy represents a promising and rationally designed therapeutic platform, leveraging both the bioactivity of natural compounds and the physicochemical advantages of nanoscale carriers.
These findings support the potential of the MIO–AgNP system as a viable approach for developing targeted and biocompatible anticancer formulations derived from Inonotus obliquus.
The cytotoxic activity observed across the three human cancer cell lines, MCF-7 (breast adenocarcinoma), HCT116 (colorectal carcinoma), and HeLa (cervical carcinoma), underscores the broad-spectrum anticancer potential of the Romanian I. obliquus-based systems. These cell lines were strategically chosen to encompass diverse biological characteristics, including hormone receptor status, redox sensitivity, and proliferative behavior.
Their established relevance in prior I. obliquus studies further enables cross-comparative analysis. Building on the distinct low-molecular-weight metabolite profile of Romanian wild-harvested I. obliquus, our results indicate comparable, and in some cases enhanced, cytotoxic efficacy relative to variants from other geographic origins—supporting its suitability for development in natural anticancer therapeutics.
Nonetheless, a key limitation of the current study is the absence of cytotoxicity data on non-malignant human cell lines. Defining the therapeutic index and establishing biosafety are essential to the translational viability of any anticancer platform. While our focus was on evaluating anticancer potential, future studies will incorporate non-tumorigenic models such as MCF-10A (epithelial), HaCaT (keratinocyte), and human dermal fibroblasts to assess biocompatibility and off-target effects. These efforts will be critical for determining safety margins and advancing the I. obliquus-based nanosystems toward preclinical validation. Their established relevance in prior I. obliquus studies from other geographic regions further enables cross-comparative analysis. Building upon the distinctive low-molecular-weight metabolite profile characterized in the Romanian wild-harvested I. obliquus, we aimed to determine whether this unique phytochemical fingerprint translates into comparable or enhanced cytotoxic efficacy. The results suggest that Romanian I. obliquus retains, and in some cases may potentiate, the anticancer bioactivity reported for non-native variants, reinforcing its potential for development in natural therapeutic systems. While this study demonstrated the promising cytotoxic effects of wild-harvested Romanian I. obliquus and its derived delivery systems (IO–AgNPs, MIO, and MIO–AgNPs) against three well-established human cancer cell lines, a key limitation is the lack of cytotoxicity data on non-malignant human cells. Defining the therapeutic index and establishing biosafety are essential to the translational viability of any anticancer platform. Although the primary goal was to evaluate the unique regional phytochemical fingerprint’s impact on anticancer bioactivity, assessing safety remains equally critical. Therefore, future studies will incorporate in vitro assays using non-cancerous human cell models, such as MCF-10A breast epithelial cells, HaCaT keratinocytes, and dermal fibroblasts, to evaluate biocompatibility and potential off-target effects. These investigations will be crucial for establishing the selectivity and safety margin of the MIO–AgNP system and for supporting its translation toward preclinical development.
4.8. Future Perspectives
Although the cytotoxic activity of the developed Inonotus obliquus-based systems was effectively assessed using the MTT assay, this colorimetric method primarily reflects mitochondrial metabolic function and does not fully distinguish between distinct modes of cell death, such as early apoptosis, late apoptosis, or necrosis. To gain a more direct and spatially resolved assessment of cell viability, future studies will incorporate live/dead fluorescence staining techniques (e.g., calcein-AM/propidium iodide), enabling real-time visualization of viable versus non-viable cells under fluorescence microscopy.
Similarly, while the antimicrobial activity was robustly evaluated through agar well diffusion and quantitative MIC/MBC determinations using the microbroth dilution method in Müller–Hinton broth, future investigations will include live/dead bacterial viability staining (e.g., SYTO 9/propidium iodide). This complementary fluorescence-based approach will provide immediate visual confirmation of bacterial membrane integrity and enhance the interpretive depth of bactericidal efficacy.
To strengthen mechanistic insight, scanning electron microscopy (SEM) will be employed for detailed bacterial surface characterization. SEM imaging allows direct observation of morphological changes, such as membrane wrinkling or rupture, thereby validating antimicrobial effects at the ultrastructural level. Furthermore, to assess long-term antimicrobial performance, future work will include silver ion release profiling from the MIO–AgNP system using inductively coupled plasma mass spectrometry (ICP-MS) or atomic absorption spectroscopy (AAS). This analysis is essential to establish correlations between silver ion availability and sustained antibacterial activity, and to optimize release kinetics for therapeutic applications.
Importantly, future studies will also address the ability of the developed systems to prevent or disrupt bacterial biofilms, structured microbial communities that contribute significantly to chronic infections and antibiotic resistance. Quantitative antibiofilm assays (e.g., crystal violet staining and TTC reduction) alongside confocal microscopy with live/dead biofilm staining will be employed to evaluate the biofilm-inhibitory and -disruptive potential of the I. obliquus-based systems, particularly those containing silver nanoparticles. This multidimensional strategy will provide a more comprehensive assessment of the therapeutic efficacy, safety, and translational relevance of these innovative bioactive platforms.
5. Conclusions
This study presents the first in-depth chemical and biological profiling of Romanian I. obliquus and introduces a novel hybrid system, IO–AgNPs, through the integration of AgNPs. The successful synthesis was confirmed by FTIR, SEM, XRD, and DLS analyses, demonstrating structural integrity and morphological uniformity. Further encapsulation within a maltodextrin matrix yielded two delivery platforms, MIO and MIO–AgNPs, both exhibiting consistent physicochemical properties and improved thermal stability.
Biological assays revealed that IO–AgNPs and MIO–AgNPs significantly outperformed the native I. obliquus sample in antioxidant, AChE inhibitory, antimicrobial, and cytotoxic activities. These findings underscore the synergistic contribution of AgNPs and the biopolymeric matrix in amplifying the therapeutic potential of I. obliquus-derived compounds, particularly in anticancer contexts. However, a key limitation of this study is the lack of cytotoxicity data on non-malignant human cells, which is critical for establishing the therapeutic index and biosafety of these systems. Future studies will address this by evaluating biocompatibility in non-cancerous cell models, such as MCF-10A, HaCaT, and dermal fibroblasts, to ensure selectivity and minimize off-target effects.
This work positions I. obliquus-based AgNP systems as promising candidates for biomedical and environmental applications, combining improved stability, targeted delivery, and enhanced bioactivity. To advance toward clinical applicability, future efforts should prioritize the quantification of key metabolites (e.g., terpenoids, polyphenols, styrylpyrones), standardization of dosing, and encapsulation optimization. In vitro assessments of release kinetics and bioaccessibility, alongside environmental stability studies, are critical for formulation robustness. Comprehensive in vivo evaluations are imperative to assess therapeutic efficacy, pharmacokinetics, biodistribution, and safety, particularly regarding AgNP-associated risks such as bioaccumulation or off-target effects. Additionally, mechanistic studies using SEM, fluorescence-based viability assays, and antibiofilm evaluations will further elucidate the therapeutic potential of these systems. Altogether, these findings establish I. obliquus-based mycocarriers as versatile and potent platforms for next-generation therapeutic strategies.
Abbreviations
The following abbreviations are used in this manuscript:
| AChE | Acetylcholinesterase |
| Ag | Silver |
| AgNPs | Silver nanoparticles |
| AMR | Antimicrobial resistance |
| ANOVA | Analysis of variance |
| ATCC | American Type Culture Collection |
| ATR | Attenuated total reflectance |
| Bcl-2 | B-cell lymphoma 2 |
| CFU | Colony-forming units |
| CO2 | Carbon dioxide |
| CV | Coefficient of variation |
| D[3,2] | Surface-weighted mean diameter |
| D[4,3] | Volume-weighted mean diameter |
| d50 | Median particle diameter |
| DLS | Dynamic light scattering |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DSC | Differential scanning calorimetry |
| DTA | Differential thermal analysis |
| DTG | Differential thermogravimetry |
| EDS | Energy-dispersive X-ray spectroscopy |
| EDX | Energy-dispersive X-ray |
| EE% | Encapsulation efficiency |
| ESI | Electrospray ionization |
| EY% | Encapsulation yield |
| FBS | Fetal bovine serum |
| FCC | Face-centered cubic |
| FeCl3 | Ferric chloride |
| FEG | Field emission gun |
| FeSO4·7H2O | Ferrous sulfate heptahydrate |
| FRAP | Ferric reducing antioxidant power |
| FTIR | Fourier-transform infrared |
| GAE | Gallic acid equivalents |
| GC | Gas chromatography |
| HCl | Hydrochloric acid |
| HF | Heat flow |
| HSD | Honestly significant difference |
| IC50 | Half-maximal inhibitory concentration |
| ICDD | International Centre for Diffraction Data |
| IO | Inonotus obliquus |
| IZ | Inhibition zone |
| JCPDS | Joint Committee on Powder Diffraction Standards |
| LC% | Loading capacity |
| MAPK | Mitogen-activated protein kinase |
| MBC | Minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| MIO | Maltodextrin—I. obliquus |
| MS | Mass spectrometry |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NIST | National Institute of Standards and Technology |
| NP | Nanoparticle |
| PDI | Polydispersity index |
| PI3K/Akt | Phosphoinositide 3-kinase/protein kinase B |
| PSD | Particle size distribution |
| QTOF | Quadrupole time-of-flight |
| RI | Retention index |
| ROS | Reactive oxygen species |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| TPC | Total phenolic content |
| TPTZ | 2,4,6-Tris(2-pyridyl)-1,3,5-triazine |
| tR | Retention time |
| TG | Thermogravimetry |
| TGA | Thermogravimetric analysis |
| UAE | Ultrasound-assisted extraction |
| VOC | Volatile organic compound |
| WPPF | Whole powder pattern fitting |
| XRD | X-ray diffraction |
Author Contributions
Conceptualization, A.-M.S., C.B. and A.-E.S.; methodology, A.-M.S., C.B., A.-E.S., G.V. and I.A.B.; validation, A.-M.S., A.-E.S., M.V.C. and L.E.B.; formal analysis, A.-E.S. and I.A.B.; investigation, G.V., T.V., G.D.M., M.V.C., A.B., R.K. and D.-D.H.; data curation, T.V., G.D.M., A.B. and L.E.B.; writing—original draft preparation, A.-M.S., C.B., A.-E.S. and G.D.M.; writing—review and editing, A.-M.S., C.B., A.-E.S., G.V. and G.D.M.; visualization, A.-E.S., I.A.B., M.V.C., R.K. and D.-D.H.; supervision, C.B., A.-E.S. and L.E.B.; project administration, A.-E.S. and T.V.; funding acquisition, A.-E.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by a grant from the European Research Executive Agency, Topic: HORIZON-MSCA-2022-SE-01-01, Type of action: HORIZON TMA MSCA Staff Exchanges, Project: 101131420—Exploiting the multifunctional properties of polyphenols: from wastes to high value products, Acronym: PHENOCYCLES.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Camilleri E., Blundell R., Baral B., Karpinski T.M., Aruci E., Atrooz O.M. A brief overview of the medicinal and nutraceutical importance of Inonotus obliquus (chaga) mushrooms. Heliyon. 2024;10:e35638. doi: 10.1016/j.heliyon.2024.e35638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glamočlija J., Ćirić A., Nikolić M., Fernandes Â., Barros L., Calhelha R.C., Ferreira I.C., Soković M., Van Griensven L.J. Chemical characterization and biological activity of Chaga (Inonotus obliquus), a medicinal “mushroom”. J. Ethnopharmacol. 2015;162:323–332. doi: 10.1016/j.jep.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 3.Silva R., Lopes C., Ferreira S., Bernardo M., Bernardo N., Fonseca M. Chaga (Inonotus obliquus) in Healthspan: An Integrative perspective of naturopathy and traditional Chinese medicine. J. Complement. Ther. Health. 2025;3:1–10. doi: 10.5281/zenodo.15263432. [DOI] [Google Scholar]
- 4.Fordjour E., Manful C.F., Javed R., Galagedara L.W., Cuss C.W., Cheema M., Thomas R. Chaga mushroom: A super-fungus with countless facets and untapped potential. Front. Pharmacol. 2023;14:1273786. doi: 10.3389/fphar.2023.1273786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng H., Shahidi F. Bioactive compounds and bioactive properties of chaga (Inonotus obliquus) mushroom: A review. J. Food Bioact. 2020;12:9–75. doi: 10.31665/JFB.2020.12245. [DOI] [Google Scholar]
- 6.Ern P.T.Y., Quan T.Y., Yee F.S., Yin A.C.Y. Therapeutic properties of Inonotus obliquus (Chaga mushroom): A review. Mycology. 2023;15:144–161. doi: 10.1080/21501203.2023.2260408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wontcheu Fotso Y.A., Ghazi S., Belkaid A., Soucy J., Tremblay L., Lamarre S., Clarisse O., Touaibia M. Extraction, Chemical Composition, Antiradical Capacity, and Photoprotective Effect of Inonotus obliquus from Eastern Canada. Nutraceuticals. 2023;3:380–402. doi: 10.3390/nutraceuticals3030029. [DOI] [Google Scholar]
- 8.Drenkhan R., Kaldmäe H., Silm M., Adamson K., Bleive U., Aluvee A., Erik M., Raal A. Comparative Analyses of Bioactive Compounds in Inonotus obliquus conks growing on Alnus and Betula. Biomolecules. 2022;12:1178. doi: 10.3390/biom12091178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazurkiewicz W. Analysis of aqueous extract of Inonotus obliquus. Acta Pol. Pharm. 2006;63:497–501. [PubMed] [Google Scholar]
- 10.Huynh N., Beltrame G., Tarvainen M., Suomela J.-P., Yang B. Supercritical CO2 extraction of triterpenoids from Chaga Sterile conk of Inonotus obliquus. Molecules. 2022;27:1880. doi: 10.3390/molecules27061880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raal A., Kaldmäe H., Kütt K., Jürimaa K., Silm M., Bleive U., Aluvee A., Adamson K., Vester M., Erik M., et al. Chemical content and cytotoxic activity on various cancer cell lines of Chaga (Inonotus obliquus) Growing on Betula pendula and Betula pubescens. Pharmaceuticals. 2024;17:1013. doi: 10.3390/ph17081013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen T.M.N., Ban S.-Y., Park K.-B., Lee C.-K., Lee S.-W., Lee Y.-J., Baek S.-M., Park J.-K., Nguyen M.T.T., Kim J., et al. Evaluation of toxicity and efficacy of inotodiol as an anti-inflammatory agent using animal model. Molecules. 2022;27:4704. doi: 10.3390/molecules27154704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milyuhina A.K., Zabodalova L.A., Kyzdarbek U., Romazyaeva I.R., Kurbonova M.K. Assessment of antimicrobial and antioxidant components of Inonotus obliquus extract as a food ingredient. IOP Conf. Ser. Earth Environ. Sci. 2021;689:012025. doi: 10.1088/1755-1315/689/1/012025. [DOI] [Google Scholar]
- 14.Ma L., Chen H., Dong P., Lu X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013;139:503–508. doi: 10.1016/j.foodchem.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Kim J., Yang S.C., Hwang A.Y., Cho H., Hwang K.T. Composition of triterpenoids in Inonotus obliquus and their anti-proliferative activity on cancer cell lines. Molecules. 2020;25:4066. doi: 10.3390/molecules25184066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dincă L., Timiş-Gânsac V. The Usage of non-wood forest products—Culinary and artisanal traditions in Romania. Sust. Dev. Res. 2020;2:50–57. doi: 10.30560/sdr.v2n1p50. [DOI] [Google Scholar]
- 17.Kobus Z., Krzywicka M., Blicharz-Kania A., Bosacka A., Pecyna A., Ivanišová E., Kozłowicz K., Kovačiková E. Impact of incorporating dried Chaga mushroom (Inonotus obliquus) into gluten-free bread on its antioxidant and sensory characteristics. Molecules. 2024;29:3801. doi: 10.3390/molecules29163801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upska K., Klavins L., Radenkovs V., Nikolajeva V., Faven L., Isosaari E., Lauberts M., Busa L., Viksna A., Klavins M. Extraction possibilities of lipid fraction and authentication assessment of chaga (Inonotus obliquus) Biomass Conv. Bioref. 2023;13:14005–14021. doi: 10.1007/s13399-021-02210-5. [DOI] [Google Scholar]
- 19.Segneanu A.-E., Cepan M., Bobica A., Stanusoiu I., Dragomir I.C., Parau A., Grozescu I. Chemical screening of metabolites profile from Romanian Tuber spp. Plants. 2021;10:540. doi: 10.3390/plants10030540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M.J., Seog E.J., Lee J.H. Physicochemical properties of Chaga (Inonotus obliquus) mushroom powder as influenced by drying methods. Prev. Nutr. Food Sci. 2007;12:40–45. doi: 10.3746/jfn.2007.12.1.040. [DOI] [Google Scholar]
- 21.Razumov E.Y., Safin R.R., Mukhametzyanov S.R., Baigildeeva E.I., Safina A.V., Lebedev D.O. Studies of the composition of the cryogenic ground chaga. IOP Conf. Ser. Mater. Sci. Eng. 2020;986:012029. doi: 10.1088/1757-899X/986/1/012029. [DOI] [Google Scholar]
- 22.Bejenaru L.E., Segneanu A.-E., Bejenaru C., Biţă A., Tuţulescu F., Radu A., Ciocîlteu M.V., Mogoşanu G.D. Seasonal variations in chemical composition and antibacterial and antioxidant activities of Rosmarinus officinalis L. essential oil from Southwestern Romania. Appl. Sci. 2025;15:681. doi: 10.3390/app15020681. [DOI] [Google Scholar]
- 23.Segneanu A.E., Damian D., Hulka I., Grozescu I., Salifoglou A. A simple and rapid method for calixarene-based selective extraction of bioactive molecules from natural products. Amino Acids. 2016;48:849–858. doi: 10.1007/s00726-015-2132-9. [DOI] [PubMed] [Google Scholar]
- 24.Segneanu A.E., Dabici A., Zuguang Y., Jianrong L., Grozescu I. Comparative analytical study of active compounds from Zingiber officinale. Optoelectron. Adv. Mater. 2012;6:652–655. [Google Scholar]
- 25.Vaszilcsin C.G., Segneanu A.E., Balcu I., Pop R., Fitigău F., Mirica M.C. Eco-friendly extraction and separation methods of capsaicines. Environ. Eng. Manag. J. 2010;9:971–976. doi: 10.30638/eemj.2010.130. [DOI] [Google Scholar]
- 26.Popescu C., Fitigau F., Segneanu A.E., Balcu I., Martagiu R., Vaszilcsin C.G. Separation and characterization of anthocyanins by analytical and electrochemical methods. Environ. Eng. Manag. J. 2011;10:697–701. doi: 10.30638/eemj.2011.093. [DOI] [Google Scholar]
- 27.Segneanu A.E., Macarie C., Ungureanu M., Gherman V., Grozescu I. Comparative study on enzymatic hydrolysis of cellulose. Dig. J. Nanomater. Biostruct. 2013;8:1061–1068. [Google Scholar]
- 28.Bejenaru L.E., Radu A., Segneanu A.-E., Biţă A., Manda C.-V., Mogoşanu G.D., Bejenaru C. Innovative strategies for upcycling agricultural residues and their various pharmaceutical applications. Plants. 2024;13:2133. doi: 10.3390/plants13152133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segneanu A.-E., Grozescu I., Cziple F., Berki D., Damian D., Niculite C.M., Florea A., Leabu M. Helleborus purpurascens—Amino acid and peptide analysis linked to the chemical and antiproliferative properties of the extracted compounds. Molecules. 2015;20:22170–22187. doi: 10.3390/molecules201219819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L., Wen K.-S., Ruan X., Zhao Y.-X., Wei F., Wang Q. Response of plant secondary metabolites to environmental Factors. Molecules. 2018;23:762. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nath R., Roy R., Barai G., Bairagi S., Manna S., Chakraborty R. Modern developments of nano based drug delivery System by combined with phytochemicals—Presenting new aspects. Int. J. Sci. Res. Sci. Technol. 2021;8:107–129. doi: 10.32628/IJSRST218422. [DOI] [Google Scholar]
- 32.Aparicio-Blanco J., Vishwakarma N., Lehr C.M., Prestidge C.A., Thomas N., Roberts R.J., Thorn C.R., Melero A. Antibiotic resistance and tolerance: What can drug delivery do against this global threat? Drug Deliv. Transl. Res. 2024;14:1725–1734. doi: 10.1007/s13346-023-01513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed S.K., Hussein S., Qurbani K., Ibrahim R.H., Fareeq A., Mahmood K.A., Mohamed M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Publ. Health. 2024;2:100081. doi: 10.1016/j.glmedi.2024.100081. [DOI] [Google Scholar]
- 34.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S., Jiang Y., Yang A., Meng F., Zhang J. The expanding burden of neurodegenerative diseases: An unmet Medical and social need. Aging Dis. 2025;16:1–16. doi: 10.14336/AD.2024.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaky M.Y., Mohamed E.E., Ahmed O.M. Neurodegenerative Disorders: Available Therapies and Their Limitations. In: Mohamed W., editor. Nanocarriers in Neurodegenerative Disorders: Therapeutic Hopes and Hypes. 1st ed. CRC Press, Taylor & Francis Group, LLC.; Boca Raton, FL, USA: 2024. pp. 27–46. Chapter 3. [DOI] [Google Scholar]
- 37.Segneanu A.-E., Vlase G., Lukinich-Gruia A.T., Herea D.-D., Grozescu I. Untargeted metabolomic approach of Curcuma longa to neurodegenerative phytocarrier system based on silver nanoparticles. Antioxidants. 2022;11:2261. doi: 10.3390/antiox11112261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segneanu A.-E., Vlase G., Vlase T., Bita A., Bejenaru C., Buema G., Bejenaru L.E., Dumitru A., Boia E.R. An innovative approach to a potential neuroprotective Sideritis scardica–clinoptilolite phyto-nanocarrier: In vitro investigation and Evaluation. Int. J. Mol. Sci. 2024;25:1712. doi: 10.3390/ijms25031712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segneanu A.-E., Vlase G., Vlase T., Bejenaru L.E., Mogoşanu G.D., Buema G., Herea D.-D., Ciocîlteu M.V., Bejenaru C. Insight into Romanian wild-grown Heracleum sphondylium: Development of a new phytocarrier based on silver nanoparticles with antioxidant, antimicrobial and cytotoxicity potential. Antibiotics. 2024;13:911. doi: 10.3390/antibiotics13090911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lima M.T.N.S., Santos L.B.D., Bastos R.W., Nicoli J.R., Takahashi J.A. Antimicrobial activity and acetylcholinesterase inhibition by extracts from chromatin modulated fungi. Braz. J. Microbiol. 2018;49:169–176. doi: 10.1016/j.bjm.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapla V.M., Honório A.E., Gubiani J.R., Vilela A.F.L., Young M.C.M., Cardoso C.L., Pavan F.R., Cicarelli R.M., Ferreira P.M.P., Bolzani V.S., et al. Acetylcholinesterase inhibition and antifungal activity of cyclohexanoids from the endophytic fungus Saccharicola sp. Phytochem. Lett. 2020;39:116–123. doi: 10.1016/j.phytol.2020.07.016. [DOI] [Google Scholar]
- 42.Bejenaru L.E., Biţă A., Mogoşanu G.D., Segneanu A.-E., Radu A., Ciocîlteu M.V., Bejenaru C. Polyphenols investigation and antioxidant and anticholinesterase activities of Rosmarinus officinalis L. species from Southwest Romania flora. Molecules. 2024;29:4438. doi: 10.3390/molecules29184438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olivo-Flores K.G., Couttolenc A., Suárez-Medellín J., Trigos Á., Espinoza C. Acetylcholinesterase inhibition exerted by the extract of Daldinia eschscholtzii, a marine fungus associated with the coral Siderastrea siderea: GC-MS analysis and molecular docking of identified compounds. Electron. J. Biotechnol. 2024;72:12–19. doi: 10.1016/j.ejbt.2024.08.001. [DOI] [Google Scholar]
- 44.Bejenaru C., Segneanu A.-E., Bejenaru L.E., Biţă A., Radu A., Mogoşanu G.D., Ciocîlteu M.V., Manda C.V. Phenolic acid profile and in vitro antioxidant and anticholinesterase activities of Romanian wild-grown Acer spp. (Sapindaceae) Appl. Sci. 2025;15:1235. doi: 10.3390/app15031235. [DOI] [Google Scholar]
- 45.Petrović P., Ivanović K., Octrue C., Tumara M., Jovanović A., Vunduk J., Nikšić M., Pjanović R., Bugarski B., Klaus A. immobilization of Chaga extract in alginate beads for modified release: Simplicity meets efficiency. Hem. Ind. 2019;73:325–335. doi: 10.2298/HEMIND190819028P. [DOI] [Google Scholar]
- 46.Dewi M.K., Chaerunisaa A.Y., Muhaimin M., Joni I.M. Improved activity of herbal medicines through nanotechnology. Nanomaterials. 2022;12:4073. doi: 10.3390/nano12224073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malik A., Khan J.M., Alhomida A.S., Ola M.S., Alshehri M.A., Ahmad A. Metal nanoparticles: Biomedical applications and their molecular mechanisms of toxicity. Chem. Pap. 2022;76:6073–6095. doi: 10.1007/s11696-022-02351-5. [DOI] [Google Scholar]
- 48.Meher A., Tandi A., Moharana S., Chakroborty S., Mohapatra S.S., Mondal A., Dey S., Chandra P. Silver nanoparticle for biomedical applications: A review. Hybrid Adv. 2024;6:100184. doi: 10.1016/j.hybadv.2024.100184. [DOI] [Google Scholar]
- 49.Segneanu A.-E., Vlase G., Vlase T., Sicoe C.A., Ciocalteu M.V., Herea D.D., Ghirlea O.-F., Grozescu I., Nanescu V. Wild-grown Romanian Helleborus purpurascens approach to novel chitosan phyto-nanocarriers—Metabolite profile and antioxidant properties. Plants. 2023;12:3479. doi: 10.3390/plants12193479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laureanti E.J.G., Paiva T.S., De Matos Jorge L.M., Jorge R.M.M. Microencapsulation of bioactive compound extracts using maltodextrin and gum Arabic by spray and freeze-drying techniques. Int. J. Biol. Macromol. 2023;253:126969. doi: 10.1016/j.ijbiomac.2023.126969. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y., Yan X., Zheng H., Li J., Wu X., Xu J., Zhen Z., Du C. The application of encapsulation technology in the food Industry: Classifications, recent Advances, and perspectives. Food Chem. X. 2024;21:101240. doi: 10.1016/j.fochx.2024.101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bejenaru C., Radu A., Segneanu A.-E., Biţă A., Ciocîlteu M.V., Mogoşanu G.D., Bradu I.A., Vlase T., Vlase G., Bejenaru L.E. Pharmaceutical applications of biomass polymers: Review of current research and perspectives. Polymers. 2024;16:1182. doi: 10.3390/polym16091182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Segneanu A.-E., Bradu I.A., Vlase G., Vlase T., Bejenaru C., Bejenaru L.E., Mogoşanu G.D., Ciocîlteu M.V., Herea D.-D., Boia E.R. Design and evaluation of a Zingiber officinale–kaolinite–maltodextrin delivery system: Antioxidant, antimicrobial, and cytotoxic activity assessment. Pharmaceutics. 2025;17:751. doi: 10.3390/pharmaceutics17060751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vlase G., Segneanu A.-E., Bejenaru L.E., Bradu I.A., Sicoe C., Vlase T., Mogoşanu G.D., Buema G., Herea D.-D., Ciocîlteu M.V., et al. Wild-grown Romanian Eupatorium cannabinum: Advancing phyto-nanocarriers via maltodextrin micro-spray encapsulation—Metabolite profiling, antioxidant, antimicrobial, and cytotoxicity insights. Polymers. 2025;17:482. doi: 10.3390/polym17040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lucero M., Estell R., Tellez M., Fredrickson E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2009;20:378–384. doi: 10.1002/pca.1137. [DOI] [PubMed] [Google Scholar]
- 56.McNaught A.D., Wilkinson A., editors. Compendium of Chemical Terminology: IUPAC Recommendations. 2nd ed. Blackwell Scientific Publications; Oxford, UK: 1997. International Union of Pure and Applied Chemistry (IUPAC) Gold Book; Online Version (2019) Created by S.J. Chalk. [DOI] [Google Scholar]
- 57.Vargas V., Saldarriaga S., Sánchez F.S., Cuellar L.N., Paladines G.M. Effects of the spray-drying process using maltodextrin on bioactive compounds and antioxidant activity of the pulp of the tropical fruit açai (Euterpe oleracea Mart.) Heliyon. 2024;10:e33544. doi: 10.1016/j.heliyon.2024.e33544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kyriakoudi A., Tsimidou M.Z. Properties of encapsulated saffron extracts in maltodextrin using the Büchi B-90 nano spray-dryer. Food Chem. 2018;266:458–465. doi: 10.1016/j.foodchem.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 59.Segneanu A.-E., Vlase G., Vlase T., Ciocalteu M.-V., Bejenaru C., Buema G., Bejenaru L.E., Boia E.R., Dumitru A., Boia S. Romanian wild-growing Chelidonium majus—An emerging approach to a potential antimicrobial engineering carrier System based on AuNPs: In vitro investigation and evaluation. Plants. 2024;13:734. doi: 10.3390/plants13050734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Segneanu A.E., Vlase G., Marin C.N., Vlase T., Sicoe C., Herea D.D., Ciocîlteu M.V., Bejenaru L.E., Minuti A.E., Zară C.M., et al. Wild grown Portulaca oleracea as a novel magnetite based carrier with in vitro antioxidant and cytotoxicity potential. Sci. Rep. 2025;15:8694. doi: 10.1038/s41598-025-92495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kar S., Sengupta D., Deb M., Shilpi A., Parbin S., Rath S.K., Pradhan N., Rakshit M., Patra S.K. Expression profiling of DNA methylation-mediated epigenetic gene-silencing factors in breast cancer. Clin. Epigenetics. 2014;6:20. doi: 10.1186/1868-7083-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sambalova O., Thorwarth K., Heeb N.V., Bleiner D., Zhang Y., Borgschulte A., Kroll A. Carboxylate Functional Groups Mediate Interaction with Silver Nanoparticles in Biofilm Matrix. ACS Omega. 2018;3:724–733. doi: 10.1021/acsomega.7b00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang M., Mo F., Li H., Li Y., Zhang S., Zhu L., Li Z., Xu J., Deng N., Wang K., et al. Adsorption based on weak interaction between phenolic hydroxyl, carboxyl groups and silver nanoparticles in aqueous environment: Experimental and DFT-D3 exploration. J. Environ. Chem. Eng. 2021;9:106816. doi: 10.1016/j.jece.2021.106816. [DOI] [Google Scholar]
- 64.Poyedinok N., Mykhaylova O., Sergiichuk N., Tugay T., Tugay A., Lopatko S., Matvieieva N. Effect of colloidal metal nanoparticles on biomass, polysaccharides, flavonoids, and melanin accumulation in medicinal mushroom Inonotus obliquus (Ach.:Pers.) Pilát. Appl. Biochem. Biotechnol. 2020;191:1315–1325. doi: 10.1007/s12010-020-03281-2. [DOI] [PubMed] [Google Scholar]
- 65.Tapak N.S., Senin S., Mohamed A.H., Dzulkifli N.N., Zain Z.M., Ahmad N.M. Detection of myristic and palmitic Acid in butter by hydrolysis and acid methylation. IOP Conf. Ser. Mater. Sci. Eng. 2021;1176:012043. doi: 10.1088/1757-899X/1176/1/012043. [DOI] [Google Scholar]
- 66.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corp.; Carol Stream, IL, USA: 2017. pp. 1–788. [Google Scholar]
- 67.Honda M., Tint G.S., Honda A., Batta A.K., Chen T.S., Shefer S., Salen G. Measurement of 3β-hydroxysteroid Δ7-reductase activity in cultured skin fibroblasts utilizing ergosterol as a substrate: A new method for the diagnosis of the Smith-Lemli-Opitz syndrome. J. Lipid Res. 1996;37:2433–2438. doi: 10.1016/S0022-2275(20)37491-5. [DOI] [PubMed] [Google Scholar]
- 68.Mickus R., Jančiukė G., Raškevičius V., Mikalayeva V., Matulytė I., Marksa M., Maciūnas K., Bernatonienė J., Skeberdis V.A. The effect of nutmeg essential oil constituents on Novikoff hepatoma cell viability and communication through Cx43 gap junctions. Biomed. Pharmacother. 2021;135:111229. doi: 10.1016/j.biopha.2021.111229. [DOI] [PubMed] [Google Scholar]
- 69.Cheng A.Y.F., Teoh P.L., Jayasinghe L., Cheong B.E. Volatile profiling aided in the isolation of anti-proliferative lupeol from the roots of Clinacanthus nutans (Burm. f.) Lindau. Processes. 2021;9:1383. doi: 10.3390/pr9081383. [DOI] [Google Scholar]
- 70.Bakir D., Akdeniz M., Ertas A., Yilmaz M.A., Yener I., Firat M., Kolak U. A GC–MS method validation for quantitative investigation of some chemical markers in Salvia hypargeia Fisch. & C.A. Mey. of Turkey: Enzyme inhibitory potential of ferruginol. J. Food Biochem. 2020;44:e13350. doi: 10.1111/jfbc.13350. [DOI] [PubMed] [Google Scholar]
- 71.Koli P., Agarwal M., Kessell D., Mahawar S., Du X., Ren Y., McKirdy S.J. Metabolite profiling of annual ryegrass cultivars to assess disease resistance and susceptibility. J. Agric. Food Chem. 2024;72:24944–24952. doi: 10.1021/acs.jafc.4c03986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beltrame G., Trygg J., Hemming J., Han Z., Yang B. Comparison of polysaccharides extracted from cultivated Mycelium of Inonotus obliquus with polysaccharide fractions obtained from sterile conk (Chaga) and Birch Heart Rot. J. Fungi. 2021;7:189. doi: 10.3390/jof7030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Šiman P., Filipová A., Tichá A., Niang M., Bezrouk A., Havelek R. Effective method of purification of betulin from birch bark: The importance of its purity for scientific and medicinal Use. PLoS ONE. 2016;11:e0154933. doi: 10.1371/journal.pone.0154933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeo D., Yun Y.G., Shin S.J., Dashnyam K., Khurelbaatar A., Lee J.H., Kim H.W. Chaga mushroom extract suppresses oral cancer cell growth via inhibition of energy metabolism. Sci. Rep. 2024;14:10616. doi: 10.1038/s41598-024-61125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J., Beghelli D., Amici A., Sut S., Dall’Acqua S., Lupidi G., Dal Ben D., Bistoni O., Tomassoni D., Belletti B., et al. Chaga mushroom triterpenoids inhibit dihydrofolate reductase and act synergistically with conventional therapies in breast cancer. Biomolecules. 2024;14:1454. doi: 10.3390/biom14111454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wold C.W., Gerwick W.H., Wangensteen H., Inngjerdingen K.T. Bioactive triterpenoids and water-soluble melanin from Inonotus obliquus (Chaga) with immunomodulatory activity. J. Funct. Foods. 2020;71:104025. doi: 10.1016/j.jff.2020.104025. [DOI] [Google Scholar]
- 77.Eid J.I., Al-Tuwaijri M.M. Chaga mushroom (Inonotus obliquus) inhibits growth of both lung adenocarcinoma (A549) cells but not Aspergillus fumigatus. Curr. Top. Nutraceut. Res. 2018;16:289–296. doi: 10.1099/acmi.ac2019.po0006. [DOI] [Google Scholar]
- 78.Lu Y., Jia Y., Xue Z., Li N., Liu J., Chen H. Recent developments in Inonotus obliquus (Chaga mushroom) polysaccharides: Isolation, structural characteristics, biological activities and application. Polymers. 2021;13:1441. doi: 10.3390/polym13091441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee I.-K., Yun B.-S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011;64:349–359. doi: 10.1038/ja.2011.2. [DOI] [PubMed] [Google Scholar]
- 80.Sun S., Liu Z., Lin M., Gao N., Wang X. Polyphenols in health and food processing: Antibacterial, anti-inflammatory, and antioxidant insights. Front. Nutr. 2024;11:1456730. doi: 10.3389/fnut.2024.1456730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Saadony M.T., Yang T., Saad A.M., Alkafaas S.S., Elkafas S.S., Eldeeb G.S., Mohammed D.M., Salem H.M., Korma S.A., Loutfy S.A., et al. Polyphenols: Chemistry, bioavailability, bioactivity, nutritional aspects and human health benefits: A review. Int. J. Biol. Macromol. 2024;277:134223. doi: 10.1016/j.ijbiomac.2024.134223. [DOI] [PubMed] [Google Scholar]
- 82.Sarkar J., Naskar A., Nath A., Gangopadhyay B., Tarafdar E., Das D., Chakraborty S., Chattopadhyay D., Acharya K. Innovative utilization of harvested mushroom substrate for green synthesis of silver nanoparticles: A multi-response optimization approach. Environ. Res. 2024;248:118297. doi: 10.1016/j.envres.2024.118297. [DOI] [PubMed] [Google Scholar]
- 83.Tayeb A.M., Othman R.H., Barakat N.A.M., Monazie A.M., Mahmoud M.A. Optimization and characterization of mushroom-derived flocculants for reducing turbidity in clay suspensions. Discov. Sustain. 2025;6:9. doi: 10.1007/s43621-024-00515-9. [DOI] [Google Scholar]
- 84.Nagardeolekar A., Dongre P., Bujanovic B.M. Lignin-based hydrogels for the delivery of bioactive Chaga mushroom Extract. Polymers. 2024;16:807. doi: 10.3390/polym16060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nwachukwu I.D., Sarteshnizi R.A., Udenigwe C.C., Aluko R.E. A Concise review of current in vitro chemical and Cell-Based antioxidant assay methods. Molecules. 2021;26:4865. doi: 10.3390/molecules26164865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamuela-Raventós R.M. Folin-Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In: Apak R., Capanoglu E., Shahidi F., editors. Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications. 1st ed. John Wiley & Sons Ltd.; Oxford, UK: 2018. pp. 107–115. Chapter 6. [DOI] [Google Scholar]
- 87.Haida Z., Hakiman M. A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 2019;7:1555–1563. doi: 10.1002/fsn3.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharpe E., Farragher-Gnadt A.P., Igbanugo M., Huber T., Michelotti J.C., Milenkowic A., Ludlam S., Walker M., Hanes D., Bradley R., et al. Comparison of antioxidant activity and extraction techniques for commercially and laboratory prepared extracts from six mushroom species. J. Agric. Food Res. 2021;4:100130. doi: 10.1016/j.jafr.2021.100130. [DOI] [Google Scholar]
- 89.Alhallaf W., Bishop K., Perkins L.B. Optimization of accelerated solvent extraction of phenolic compounds from Chaga using response surface methodology. Food Anal. Methods. 2022;15:2777–2790. doi: 10.1007/s12161-022-02319-x. [DOI] [Google Scholar]
- 90.Luque F.J., Muñoz-Torrero D. Acetylcholinesterase: A versatile template to coin potent modulators of multiple therapeutic targets. Acc. Chem. Res. 2024;57:450–467. doi: 10.1021/acs.accounts.3c00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walczak-Nowicka Ł.J., Herbet M. Acetylcholinesterase inhibitors in the treatment of neurodegenerative diseases and the Role of acetylcholinesterase in their pathogenesis. Int. J. Mol. Sci. 2021;22:9290. doi: 10.3390/ijms22179290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei Y.-M., Yang L., Wang H., Cai C.-H., Chen Z.-B., Chen H.-Q., Mei W.-L., Dai H.-F. Triterpenoids as bivalent and dual inhibitors of acetylcholinesterase/butyrylcholinesterase from the fruiting bodies of Inonotus obliquus. Phytochemistry. 2022;200:113182. doi: 10.1016/j.phytochem.2022.113182. [DOI] [PubMed] [Google Scholar]
- 93.Šinko G., Vinković Vrček I., Goessler W., Leitinger G., Dijanošić A., Miljanić S. Alteration of cholinesterase activity as possible mechanism of silver nanoparticle toxicity. Environ. Sci. Pollut. Res. Int. 2014;21:1391–1400. doi: 10.1007/s11356-013-2016-z. [DOI] [PubMed] [Google Scholar]
- 94.Abdelwahab G.M., Mira A., Cheng Y.-B., Abdelaziz T.A., Lahloub M.F.I., Khalil A.T. Acetylcholine esterase inhibitory activity of green synthesized nanosilver by naphthopyrones isolated from marine-derived Aspergillus niger. PLoS ONE. 2021;16:e0257071. doi: 10.1371/journal.pone.0257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soliman W.E., Khan S., Rizvi S.M.D., Moin A., Elsewedy H.S., Abulila A.S., Shehata T.M. Therapeutic applications of biostable silver nanoparticles synthesized using peel extract of Benincasa hispida: Antibacterial and anticancer activities. Nanomaterials. 2020;10:1954. doi: 10.3390/nano10101954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dash D.K., Tyagi C.K., Sahu A.K., Tripathi V. Revisiting the Medicinal Value of Terpenes and Terpenoids. In: Meena V.S., Parewa H.P., Meena S.K., editors. Revisiting Plant Biostimulants. 1st ed. IntechOpen; London, UK: 2022. pp. 1–17. [DOI] [Google Scholar]
- 97.Lieu E.L., Nguyen T., Rhyne S., Kim J. Amino acids in cancer. Exp. Mol. Med. 2020;52:15–30. doi: 10.1038/s12276-020-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liga S., Paul C., Péter F. Flavonoids: Overview of biosynthesis, biological activity, and current extraction techniques. Plants. 2023;12:2732. doi: 10.3390/plants12142732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coniglio S., Shumskaya M., Vassiliou E. Unsaturated fatty acids and their immunomodulatory properties. Biology. 2023;12:279. doi: 10.3390/biology12020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vezza T., Canet F., De Marañón A.M., Bañuls C., Rocha M., Víctor V.M. Phytosterols: Nutritional health players in the management of obesity and its related disorders. Antioxidants. 2020;9:1266. doi: 10.3390/antiox9121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y., Cai P., Cheng G., Zhang Y. A Brief review of phenolic compounds identified from plants: Their extraction, Analysis, and biological activity. Nat. Prod. Commun. 2022;17:1934578X211069721. doi: 10.1177/1934578X211069721. [DOI] [Google Scholar]
- 102.Kamfar W.W., Khraiwesh H.M., Ibrahim M.O., Qadhi A.H., Azhar W.F., Ghafouri K.J., Alhussain M.H., Aldairi A.F., AlShahrani A.M., Alghannam A.F., et al. Comprehensive review of melatonin as a promising nutritional and nutraceutical supplement. Heliyon. 2024;10:e24266. doi: 10.1016/j.heliyon.2024.e24266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Segneanu A.E., Sfirloaga P., David I., Balcu I., Grozescu I. Characterisation of truffles using electrochemical and analytical methods. Dig. J. Nanomater. Biostruct. 2012;7:199–205. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.