Abstract
Although prescription opioids may be necessary to manage severe and persistent pain, many factors including concern for opioid abuse and misuse have led to restricted availability of these analgesics. Opioid abuse-deterrent formulations (ADFs) were developed to enhance resistance to tampering yet retain analgesic efficacy. US Food and Drug Administration (FDA) approval for the ADF designation is based on prespecified preclinical (category 1), pharmacokinetic (category 2), and/or clinical (category 3) evidence demonstrating abuse-deterrent properties. Currently, 4 opioid formulations carry the ADF designation: XTAMPZA® ER (oxycodone), OXYCONTIN® (oxycodone hydrochloride), HYSINGLA™ ER (hydrocodone bitartrate), and ROXYBOND™ (oxycodone hydrochloride). The FDA requires that ADFs undergo postapproval evaluation to assess their impact on meaningful reductions in abuse, misuse, and related clinical outcomes. An additional designation is available based on FDA assessment of these postmarket studies (category 4). However, none of the 4 opioid ADFs have yet attained this additional category 4 labeling. The impact of opioid ADFs on abuse, misuse, and related clinical outcomes is unclear. The objectives of this narrative review are 1) to describe the benefits of and need for ADFs; 2) to provide an overview of the FDA guidance for ADFs, with a focus on category 4 postmarketing requirements; and 3) to summarize select postmarketing studies of the ADF prescription opioids currently available in the US. We identified key postmarketing publications for these ADFs via PubMed searches and investigation of literature cited in relevant publications. Three opioid ADFs (XTAMPZA ER, OXYCONTIN, and HYSINGLA ER) currently report postmarketing research, generally demonstrating reduced nonoral abuse or misuse compared with non-ADFs or other ADFs. Of note, XTAMPZA ER has shown sustained lower levels of nonoral abuse or misuse compared with other ADFs, despite a substantial increase in dispensed prescriptions since its launch in 2016. Additional postmarketing research is needed, especially for HYSINGLA ER and ROXYBOND.
Keywords: ADF categories, oxycodone, hydrocodone, postmarketing research
Introduction
Chronic pain is a serious global health issue,1,2 affecting approximately 1.9 billion people worldwide.3 In 2021 in the US, 20.9% of noninstitutionalized adults, representing 40 to 50 million Americans, were affected by chronic pain.1–4 As a leading cause of disability, chronic pain negatively impacts mental, physical, and social functioning.5,6 Opioid therapy is one option considered in appropriate patients as a component of the multimodal management of severe and persistent pain.7–10 However, endogenous opioids affect many processes other than pain transmission.11,12 Consequently, the nonanalgesic effects of opioids (eg, constipation, pruritus, hypogonadism, behavioral effects) must be considered.13
Opioid receptors play a role in behavioral functions such as drug reward, tolerance, and dependence.7,10,14 Prescription opioids have been linked to opioid use disorder (OUD), diversion, and overdose deaths, posing serious and complex public health concerns to consider when prescribing opioids.2,10,14–16 Therefore, when weighing the risks and benefits of opioids for chronic pain, clinicians must additionally consider the potential for OUD and diversion12,17 as well as the risk of undertreating medically relevant chronic pain. Factors such as individual differences in pain tolerance or the lack of objective pain measures or biomarkers further complicate these decisions.8,12,13,18,19
The Department of Health and Human Services formally declared the opioid crisis a public health emergency on October 26, 2017.20 At that time, opioids were associated with approximately 68% of all drug overdose deaths, and 35% of opioid deaths involved prescription opioids.21 US public health–related regulatory agencies launched several initiatives aimed at preventing opioid overdose, which included the following key strategies: improving opioid prescribing, preventing and treating OUD, reducing overdose and death, educating on safe opioid disposal, and establishing guiding principles for opioid use in clinical practice. Multiple guidelines have suggested that short- and long-term opioid therapy be restricted to only those individuals for whom other alternative approaches have not been helpful or would otherwise not be appropriate.10,12,19 Not all opioid medications are the same, varying in chemical entity in addition to their ease of abuse or misuse.
These initiatives also led to the advent of opioid abuse-deterrent formulations (ADFs) per guidance from the US Food and Drug Administration (FDA).22 Misuse of oral opioids via the intranasal or intravenous routes can quickly deliver increased drug levels to the brain, heightening the risk of severe medical outcomes (including death) compared with oral routes of opioid administration.15,23,24 ADFs developed to date target routes of administration that facilitate drug abuse, such as crushing for inhalation or dissolving for injection. However, currently available ADF technologies do not prevent all forms of opioid misuse, especially via the oral route (eg, swallowing higher doses), which remains the most common for opioid misuse or abuse.15,23
Rates of prescription opioid–involved deaths have fluctuated marginally since 2010,25 when the first opioid ADF (OXYCONTIN® [oxycodone hydrochloride]) was approved.15,24,26,27 The FDA requires products with ADF labeling to undergo postmarket research to evaluate meaningful reductions in measures of abuse, misuse, and related clinical outcomes. However, optimal outcome measures are still evolving in the context of current ADF limitations, such as continued oral abuse and mixed evidence on overdose reduction. Therefore, the true impact of opioid ADFs is unclear. Here, we searched PubMed and the bibliographies of relevant publications to identify select, recent postmarketing data for opioids with FDA-approved ADF designations. This narrative review aims to provide an overview of FDA guidance for ADFs, with a focus on formal postmarketing requirements, and to summarize key postmarketing studies of the ADF prescription opioids currently available in the US.
ADF Categories and Key Requirements
In 2015, the FDA published ADF guidance for industry15,22,24 that describes 7 deterrence methods: 1) physical/chemical barriers, 2) combinations of agonist/antagonist, 3) aversion, 4) delivery systems, 5) prodrugs or new molecular entities, 6) a combination of some or all of these methods, or 7) novel and yet undefined approaches to target expected routes of abuse and hinder extraction of their active ingredients and/or limit bioavailability.22 To date, only physical/chemical barriers have been utilized for ADFs currently in market.24 Implementation of ADFs may allow patients with chronic pain to receive necessary analgesia while decreasing the opportunity for OUD, overdose, and other drug-related adverse events.28
To date, FDA guidance outlines 4 mutually exclusive ADF study categories (Table 1),22 which have prespecified results demonstrating abuse-deterrent properties for the potential ADF opioid, including preclinical, laboratory-based research (category 1); pharmacokinetic (PK) studies (category 2); clinical research (category 3); and postapproval evaluation of the impact of ADF on meaningful reductions in abuse, misuse, and related clinical outcomes (category 4).22
Table 1.
ADF Study Categories and Descriptions
| ADF Study Category | ADF Study Descriptions |
|---|---|
| 1 | Results from laboratory-based in vitro manipulation and extraction studies demonstrating abuse-deterrent properties by evaluating the ease with which the potentially abuse-deterrent properties of a formulation can be defeated or compromised |
| 2 | Results from pharmacokinetic studies demonstrating abuse-deterrent properties by comparing the pharmacokinetic profiles of the manipulated formulation with the intact formulation and with manipulated and intact formulations of the comparator drugs through one or more routes of administration |
| 3 | Results from clinical abuse-potential studies demonstrating abuse deterrent properties by evaluating subjective responses predictive of the likelihood of abuse |
| 4 | Results from postmarket studies evaluating whether the marketing of a product with abuse-deterrent properties results in meaningful reductions in abuse, misuse, and related adverse clinical outcomes, including addiction, overdose, and death in the postapproval setting |
Abbreviation: ADF, abuse-deterrent formulation.
The focus of ADF categories 1 through 3 is to assess potential abuse-deterrent properties under controlled settings, whereas the focus of category 4 is to ascertain whether ADFs are associated with meaningful reductions in clinical outcomes (eg, abuse, misuse, addiction, overdose, death) in the real world using postmarket evaluations.22 Category 4 is the most difficult designation to achieve, and none of the available prescription opioids currently have category 4 ADF designation from the FDA. The low market volume of ADF products severely limits the availability of postmarket data that are adequately powered to meet category 4 study design elements. Currently, ADF products are only a small portion of the overall prescription opioid market; their impact on public health may not be fully realized until they are more widely utilized. There are also challenges with the detection of OUD in intended patient populations compared with those who are abusing or misusing diverted medications. Determining causal relationships to the many interventions that have been launched to reduce these outcomes is difficult. Another complication stems from the lack of a strict definition of “meaningful reduction”, rendering data interpretation subjective. Finally, the related clinical outcomes of addiction, overdose, and death most often involve polysubstance use, and determining a causal relationship to a specific product is impossible.
The 2 types of postmarket evaluations of abuse deterrence that may be submitted for ADF category 4 approval are formal studies and supportive information. Specifically, formal studies must 1) follow good epidemiological practices and utilize outcomes that provide meaningful measures for hypothesis-driven, observational, population-based evaluations; 2) capture at least 1 outcome that can be used in the assessment of meaningful reductions in abuse, addiction, misuse, overdose, and death; 3) produce abuse-related estimates and related clinical outcomes that are either nationally representative or can be reasonably generalized to the national level; 4) assess overall and route of administration–specific (eg, smoked, snorted, injected) alterations in abuse levels related to an abuse-deterrent product; and 5) be sufficiently powered statistically for the assessment of meaningful changes in drug abuse and be of sufficient duration to examine trends in abuse following the marketing of an ADF product. Supportive information can be used to provide additional context on behavioral, clinical, and societal aspects of abuse and abuse deterrence but may not serve as a substitute for formal studies.22
Recognizing the evolving opioid landscape, the FDA included multiple statements in the guidance regarding their commitment to taking a flexible, adaptive approach to evaluation and labeling of ADF opioids, noting the plasticity of methodologies and sources of data used for formal studies, the lack of established best practices, and the evolving science of identifying relevant end points for evaluation. The guidance was finalized in 2015 and could use updating based on the current opioid landscape and flexibility when evaluating current and potential future ADF products.22
Overview of ADF Prescription Opioids Currently Available in the US
There are 4 currently available prescription opioids with FDA-approved abuse-deterrence label claims based on premarket studies, each of which is orally administered and designed with mechanisms to deter abuse and potentially avoid intentional misuse (Table 2).29,30 Three of these products are extended-release (ER) opioid analgesics and 1 is an immediate-release (IR) formulation of oxycodone hydrochloride:15,24,30
XTAMPZA® ER (oxycodone) (Collegium Pharmaceutical, Inc., Stoughton, Massachusetts, USA) was approved as an ADF product in 2016 as oxycodone. Its deterrence mechanism utilizes DETERx technology, a microsphere-in-capsule formulation, which reduces physical manipulation or chemical extraction for intranasal and intravenous routes of administration.15,31 XTAMPZA ER maintains its ER pharmacokinetic profile even when chewed or inhaled after crushing.15,24,27,31,32 DETERx allows for alternative modes of administration for people who have difficulty swallowing or comorbid dysphagia.31
OXYCONTIN® (oxycodone hydrochloride) (Purdue Pharma LP, Stamford, Connecticut, USA) was the first FDA-approved ADF prescription opioid (upon reformulation in 2010). Its ADF deterrence mechanism uses physical and chemical properties to prevent manipulation (eg, crushing, chemical extraction) for intranasal and intravenous administration via Intac and RESISTEC technologies.15,24,26,27
HYSINGLA™ ER (hydrocodone bitartrate) (Purdue Pharma LP, Stamford, Connecticut, USA) was approved as an ADF product in 2014. Its ADF deterrence mechanism reduces physical manipulation or chemical extraction for intranasal and intravenous routes of administration via RESISTEC technology.15,24,27,33
ROXYBOND™ (oxycodone hydrochloride) (Protega Pharmaceuticals Inc; Princeton, New Jersey, USA) was the first and remains the only IR opioid analgesic FDA-approved with ADF labeling (in 2017). Its ADF deterrence mechanism utilizes physical and chemical properties (including inactive ingredients) to reduce physical manipulation or chemical extraction for intranasal and intravenous routes of administration via SentryBond technology.15,34
Table 2.
FDA-Approved Prescription Opioids with ADF Study Category 1 to 3 Labeling
| Compound / Active Moiety | ADF Approval | Formulation and Technology | Categories 1 to 3 Study Results |
|---|---|---|---|
| XTAMPZA® ER (oxycodone) | 2016 Most recent Prescribing Information update: 2023 |
|
|
| OXYCONTIN® (ER) (oxycodone hydrochloride) | 2010 Most recent Prescribing Information update: 2023 |
|
|
| HYSINGLA™ ER (hydrocodone bitartrate) | 2014 Most recent Prescribing Information update: 2023 |
|
|
| ROXYBONDTM IR (oxycodone hydrochloride) | 2017 Most recent Prescribing Information update: 2023 |
|
|
Abbreviations: ADF, abuse-deterrent formulation; Cat, category; ER, extended release; IR, immediate release; PK, pharmacokinetic.
Although each of these 4 ADF products may still be abused orally (eg, taking more tablets intact), they all utilize physical and chemical properties to deter abuse through nonoral routes. For example, each reduces intravenous abuse by becoming viscous when attempting to liquify.26,32–34 Of these, only XTAMPZA ER and ROXYBOND (IR) do not have a Boxed Warning on the potential exposure to a fatal dose of opioid when chewed or crushed.32,34 XTAMPZA ER is the only oxycodone product that currently has ADF labeling for the oral route of administration.
ER opioids are often preferred over IR formulations for nonmedical use and abuse due to their high drug content.31 Manipulation of these ER formulations can convert the ER mechanism to IR and, instead of releasing the dose over an extended period of time, the dose is released immediately, thereby increasing the risk of morbidity and mortality.31 To mitigate potential misuse and abuse, the ADF Category 2 label requires the evaluation of whether deliberate or accidental manipulations of an ADF opioid could increase its abuse potential by increasing the maximum concentration (Cmax) or reducing the time to achieve Cmax (Tmax) of the drug.22,31 As described in the prescribing information for XTAMPZA ER, the effects of physical manipulation on the oral PK profile of XTAMPZA ER were compared with that of OXYCONTIN and IR oxycodone; data revealed that crushing or chewing XTAMPZA ER prior to administration did not increase Cmax or total exposure (AUC0-INF) relative to intact product. Moreover, Cmax was lower and Tmax was longer for all XTAMPZA ER treatments relative to both IR oxycodone and crushed OXYCONTIN, findings that are consistent with an ER profile.31,32
ADF Category 4 – Postmarketing Findings
The focus of the ADF category 4 label requirements is to determine whether premarket findings from the first 3 categories, which are conducted under controlled settings, translate into meaningful reductions in clinical outcomes (eg, addiction, overdose, and death) using data from postmarketing surveillance.15,22,24,31 Of the 4 ADFs, OXYCONTIN has been approved for the longest period of time and, unsurprisingly, has more published postmarketing data than the other 3 products. Accordingly, OXYCONTIN postmarketing studies generally compare the ADF to its original non-ADF product (as reviewed by Gadd et al35). Several postmarketing studies on XTAMPZA ER have been published in the last 5 years.36–41 Many of these use other ADFs as comparators, including OXYCONTIN and HYSINGLA. Nevertheless, postmarketing data for HYSINGLA ER remain sparse, and none are currently published for ROXYBOND IR. A summary of these findings and an update to the body of published or presented postmarketing information on currently available ADF opioids are described below and in Table 3.
Table 3.
ADF Category 4 Postmarketing Study Findings
| ADF Product | Category 4 Postmarketing Studies | |||
|---|---|---|---|---|
| ASI-MV | RADARS | HEOR | Notes | |
| XTAMPZA® ER (oxycodone) |
|
|
|
|
| OXYCONTIN® (ER) (oxycodone hydrochloride) |
|
|
|
|
| HYSINGLA™ ER (hydrocodone bitartrate) |
|
|
||
| ROXYBONDTM IR (oxycodone hydrochloride) | Postmarketing data (category 4 studies) not yet available | |||
Abbreviations: ADF, abuse-deterrent formulation; ASI-MV, Addiction Severity Index-Multimedia Version; ER, extended release; HCUP, Healthcare Cost and Utilization Project; HEOR, health economics and outcomes research; IR, immediate release; NPDS, National Poison Data System; NMU, nonmedical use; OR, odds ratio; PK, pharmacokinetic; RADARS, Researched Abuse Diversion and Addiction-Related Surveillance.
XTAMPZA ER Postmarketing Studies
Postmarketing surveillance involves a vast array of different data sources. The Addiction Severity Index-Multimedia Version (ASI-MV) is a clinical tool used to evaluate substance abuse and nonmedical use (NMU) in a real-world setting, particularly in high-risk populations.38 Past 30-day NMU and routes of administration of XTAMPZA ER were compared with available US oxycodone products via a cross-sectional surveillance study conducted between 2016 (launch of XTAMPZA ER) and 2019.38 During this 3.5-year period, there were 192,810 assessments to the ASI-MV network; 42,279 (21.9%) reported NMU of ≥1 prescription opioid. Of these, <1% specified past 30-day XTAMPZA ER NMU (0.2%, n=73), whereas 9.0% (n=3802) specified other oxycodone ER NMU and 34.5% (n=14,579) specified oxycodone IR NMU.38 It should be noted that XTAMPZA ER launched in 2016 but was not added to the ASI-MV until the third quarter of 2017, a full year after the study began. Within the ASI-MV network, XTAMPZA ER accounted for 0.1% of all opioid prescriptions dispensed (831,552). Other oxycodone ER accounted for 1.5% of all opioids dispensed (9,891,591); oxycodone IR accounted for 23.0% of all opioids dispensed (150,629,158). When adjusted for units dispensed, past 30-day NMU for XTAMPZA ER ranged from 0.77 to 5.32. Other oxycodone ER past 30-day NMU (adjusted) ranged from 6.32 to 9.56, and oxycodone IR past 30-day NMU (adjusted) ranged from 2.33 to 4.86. With respect to XTAMPZA ER, all quarterly rates of past 30-day NMU (adjusted for volume of ASI-MV assessments) and almost all drug utilization adjusted rates were lower as compared with oxycodone ER and oxycodone IR, but most did not reach statistical significance likely because of the low volume of prescriptions and units dispensed during introduction of XTAMPZA to the market.38 A similar study presented at PainWEEK 2024 investigated 341,851 ASI-MV assessments completed between 2016 and 2023, of which 67,855 reported past 30-day NMU. During this 7-year period, quarterly NMU utilization adjusted rates of XTAMPZA ER were significantly lower than those of other ER oxycodone (between 2018 and 2023) and IR oxycodone (entire study duration).37
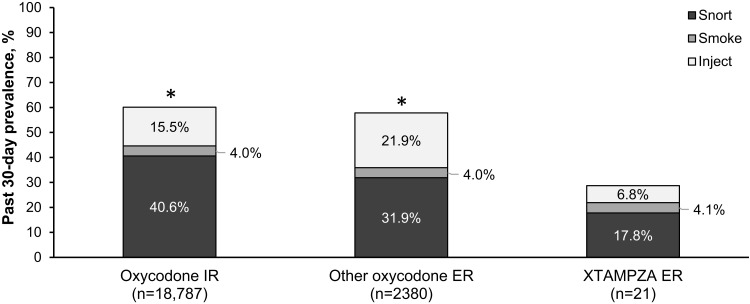
In the 3.5-year study, nonoral routes of administration were significantly higher for nonmedical users of oxycodone IR products (60.1%) and other oxycodone ER products (57.9%) than for XTAMPZA ER (28.8%, both P<0.001) (Figure 1). In particular, injecting and snorting were reported more frequently for other oxycodone ER (21.9% injecting and 31.9% snorting) and oxycodone IR (15.5% injecting and 40.6% snorting) than with XTAMPZA ER (6.8% injecting and 17.8% snorting).38 These trends for nonoral use were similar in the 7-year study (other oxycodone ER, 56.2%, oxycodone IR, 60.1%, XTAMPZA ER, 25.5%).37 Thus, these differences between XTAMPZA ER and other oxycodone opioids are still observed after a longer period on the market.
Figure 1.
Prevalence of past 30-day nonmedical use of opioid by route of administration (nonoral).38 ASI-MV assessments from July 1, 2016, through December 31, 2019, were analyzed and previously reported.38 Of 42,279 assessments reporting NMU of ≥1 prescription opioid by any route of administration (oral and nonoral), XTAMPZA ER accounted for 0.2% (n=73, reporting 73 total NMU mentions), other oxycodone ER for 9.0% (n=3802, reporting 4114 total NMU mentions), and oxycodone IR for 34.5% (n=14,579, reporting 31,281 total NMU mentions). Of the total NMU mentions for each group, nonoral routes (snorting, smoking, or injecting) are shown. *Statistically significant difference (P<0.001) by chi-square test for any nonoral route compared with XTAMPZA ER.
Abbreviations: ASI-MV, Addiction Severity Index-Multimedia Version; ER, extended release; IR, immediate release; NMU, nonmedical use.
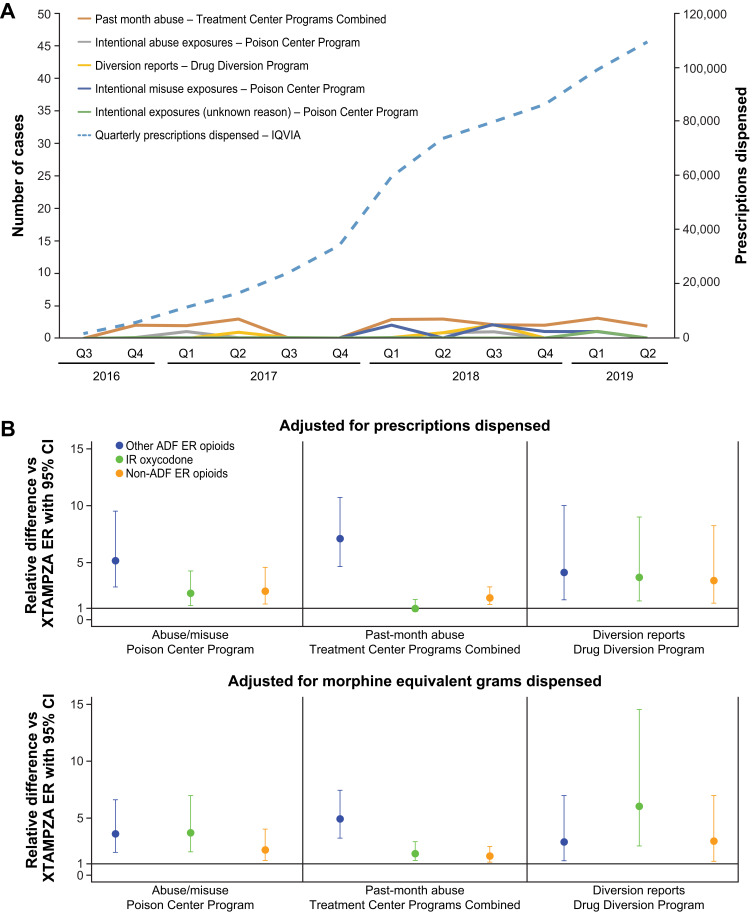
A data source commonly used for analysis of prescription drugs use is the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS®) System, which has been monitoring drug use since 2006.48 RADARS harnesses data from the US poison centers (National Poison Center Program [NPCP]) as well as several other programs (eg, Drug Diversion Program [DDP]) to provide product- and geographic-specific data on abuse, misuse and diversion of prescription drugs.23,41,48 As reported by Severtson in 2020, during a 3-year analysis period following initial marketing of XTAMPZA ER (July 1, 2016–June 30, 2019), cases of abuse, misuse, diversion, and death were stable despite the increased number of prescriptions for XTAMPZA ER (Figure 2).41 Based on data obtained from the US-based Longitudinal Patient Database, IQVIA® (Danbury, Connecticut, USA), the number of XTAMPZA ER prescriptions dispensed increased every quarter since launch, from 1,876 in quarter 2 of 2016 to 109,624 in quarter 2 of 2019, representing a more than 50-fold increase (Figure 2A). This increase was not accompanied by heightened levels of abuse or misuse, as the NPCP received only 10 cases involving XTAMPZA ER across the 3-year period: 6 intentional misuse cases, 3 intentional abuse exposures, and 1 intentional unknown case.41 Only 5 cases involving diversion of XTAMPZA ER were reported by the DDP versus 4,360 for IR oxycodone.41 There were no documented cases of abuse or misuse of XTAMPZA via injection or inhalation; all 10 cases reported by the NPCP involved the ingestion of multiple tablets.41 By contrast, abuse, misuse, and diversion of non-ADF ER opioids and other ADF ER opioids were significantly higher than XTAMPZA ER even after adjusting across 2 separate drug availability measures (prescriptions dispensed and morphine equivalent milligrams dispensed; Figure 2B).41
Figure 2.
RADARS system postmarketing data analysis of XTAMPZA ER vs other opioids (2016–2019). (A) Number of XTAMPZA ER prescriptions dispensed and reported cases of abuse, diversion, and misuse per quarter between its launch in 2016 and 2019. (B) Relative differences of commonly abused opioid analgesics vs XTAMPZA ER.
Note: Adapted from Severtson SG, Kreider SED, Amioka EC, Margolin ZR, Iwanicki JL, Dart RC. Postmarketing analysis of misuse, abuse, and diversion of Xtampza ER. Pain Med. 2020;21(12):3660–3668. Creative Commons.41
Abbreviations: ER, extended release; IR, immediate release; Q, quarter; RADARS, Researched Abuse, Diversion, and Addiction-Related Surveillance.
The RADARS system was also employed in a real-world tampering (defined as chewing, smoking, snorting, or injecting an opioid) study comparing XTAMPZA ER with IR oxycodone, an ADF formulation of ER oxycodone, and ER oxymorphone (Opana ER, discontinued in 2017 for failing to meet ADF standards).24,42 Using data collected by the Treatment Center Programs Combined program between quarter 3 of 2018 through quarter 3 of 2021, XTAMPZA ER demonstrated the lowest rate of tampering (38.5%; 95% CI 23.4, 55.4) followed by ER oxycodone (56.4%; 95% CI 53.9, 58.8) and ER oxymorphone (66.7%; 95% CI 62.1, 71.2); IR single-entity oxycodone had the highest rate of tampering (72.5%; 95% CI 69.9, 74.9).42 The frequency of tampering with XTAMPZA ER was significantly lower (77%) than tampering with IR oxycodone (OR 0.23; 95% CI 0.11, 0.50; P<0.0002) and 70% lower than ER oxymorphone (OR 0.30, 95% CI 0.14, 0.67; P<0.0038) but not significantly lower than that of the ADF ER oxycodone (OR 0.50; 95% CI 0.24, 1.03; P<0.0612).42 It should be noted that only 39 (1.7%) of the 2273 participants in the study represented the XTAMPZA ER group, while 1427 (62.8%) participants represented the ADF ER oxycodone group.42 Altogether, these RADARS system postmarket results provide real-world evidence that ADFs can deter individuals from tampering with and abusing prescription opioids.41,42
A third study utilizing the RADARS system was conducted to assess whether the previously reported low rates of XTAMPZA ER abuse, misuse, and unintended routes of exposure correlate with fewer severe outcomes as compared with other opioid analgesic exposures.40 Data were obtained via the NPCP from 2016 through 2021 that involved cases utilizing XTAMPZA ER, OXYCONTIN, HYSINGLA ER, and other ADF opioids that were approved and dispensed during the time of the study. Non-ADF ER opioids and single-entity IR oxycodone were also compared; notably, the ADF IR ROXYBOND (which was approved in 2017 but not launched until 2022) was not included.24,40,49 Compared with the other ADF and non-ADF ER opioids, XTAMPZA ER was involved in fewer intentional exposures and less severe outcomes (41.99% and 33.33% vs 25.4%, respectively). Over the 6-year study, there were no reports of a nonoral route of administration.40 By contrast, 8.10% of other ADF ER opioid cases and 2.01% of non-ADF ER opioid cases reported misuse via inhalation or injection.40 In general, these findings are consistent with reduced abuse-related outcomes noted with XTAMPZA ER than its ADF and non-ADF comparators in the postmarketing setting. These RADARS system results complement the real-world evidence from ASI-MV findings; together, they suggest that XTAMPZA ER can better deter tampering and nonoral NMU compared with other oxycodone ER and IR products.
Postmarketing surveillance for ADF can also include health economics and outcomes research (HEOR). Manipulation of many prescription ER opioid formulations can result in serious or fatal medical outcomes; the majority of the economic burden from opioid misuse or abuse is due to reduced health-related quality of life and the value of life lost caused by fatal opioid overdose.50 The contributions that ADF opioids provide to offset the economic burden are therefore important to assess. A US retrospective claims analysis was conducted to compare healthcare costs associated with 2 different ER ADFs of oxycodone: XTAMPZA ER and OXYCONTIN.39 US insurance claims data from individuals with noncancer chronic pain who had switched from IR oxycodone and were prescribed a 90-day supply of ADF opioid between June 1, 2015, and February 29, 2020, were obtained from IBM MarketScan Commercial Claims and Encounters Medicare Supplemental Databases.39 The analysis favored XTAMPZA ER over OXYCONTIN because of the potential significant cost savings.39 For individuals treated with XTAMPZA ER, total healthcare costs were significantly lower than for those treated with OXYCONTIN ($22,630 vs $28,386, P=0.005, respectively); this was primarily attributed to the lower ER opioid prescription cost for the XTAMPZA ER cohort ($2645 vs $3141, P<0.001).39
Significant differences in clinical outcomes and estimated healthcare costs of intentional misuse or abuse exposures between XTAMPZA ER, other ER oxycodone, and IR oxycodone were described using data accessed from the National Poison Data System (NPDS) and the Healthcare Cost and Utilization Project (HCUP) from 2019 through 2022.36 During the study period, NPDS reported 301 exposures to XTAMPZA ER, 2159 to other oxycodone ER, and 17,694 to oxycodone IR. When adjusted for drug utilization (via IQVIA data) and US population metrics (per 100,000 US population), relative risk analyses revealed significantly higher risk for other ER oxycodone and IR oxycodone vs XTAMPZA ER for all clinical outcomes observed. Specifically, the relative risk for other ER oxycodone and IR oxycodone, respectively, were 7.4 and 2.0 times more likely for intentional abuse/misuse, 25.9 and 7.2 times for major effect or death, 9.7 and 2.8 times for emergency department visits, and 14.3 and 4.0 times for hospital admission).36 Extrapolation of estimated costs of these emergency department visits involving intentional abuse or misuse was lowest for XTAMPZA ER ($1135.36) and highest for other ER oxycodone ($10,966.63). Similarly, extrapolated estimates for hospital admissions were lowest for XTAMPZA ER ($2815.77) and highest for IR oxycodone ($40,354.04).36
OXYCONTIN ADF Postmarketing Studies
OXYCONTIN entered the market in 1996 and was one of the leading opioids for the treatment of chronic pain in the US by 2001.24 In 2010, the FDA approved the replacement of the original ER formulation with ADF technology, as OXYCONTIN had become one of the most widely abused drugs in the US. In 2013, ADF labeling was granted based on the deterrence of abuse via the intravenous and intranasal routes; at that time, the FDA stated it would no longer approve applications for generic OXYCONTIN if ADF technology was not present.24
RADARS drug-seeking data from the nationwide Survey of Key Informants’ Patients (SKIP) Program revealed an initial, significant reduction of past-month abuse after the introduction of reformulated OXYCONTIN (from 45.1% [95% CI, 41.2%–49.1%] between January and June 2009 to 26.0% [95% CI, 23.6%–28.4%] between July and December 2012; P<0.001).44 However, this reduction leveled off to roughly 25% to 30% between 2012 and 2014, suggesting that participants either transitioned from nonoral routes to oral use (43%) or exclusively used the oral route regardless of ADF formulation (23%).44 Of note, over a third (34%) reported that they successfully defeated the ADF mechanism and continued inhaled or injected use.44
In a separate RADARS system SKIP Program study, the ADF of OXYCONTIN was highly effective in reducing nonoral abuse vs the non-ADF (from 91.4% before ADF to 47.9% after ADF) via both insufflation (78.0% to 28.8%) and injection (42.7% to 21.4%) routes.45 By contrast, while the reformulation of Opana ER, which utilized the same Intac foundation technology, was effective in reducing insufflation (80% to 37.1%), injection (60.0% to 51.4%), and overall nonoral abuse (94.3% to 77.1%), it showed no significant decrease over time.45 Of note, Opana ER never received ADF labeling despite its reformulation in 2012 to make it resistant to abuse by snorting or injecting (category 1). It was removed from the market in 2017 following the FDA review of postmarketing data demonstrating significant shifts in abuse route from nasal to injection and case reports of thrombotic microangiopathy secondary to improper injection of Opana.24,27,51
Petrilla et al evaluated the relationships between ADF opioid formulary coverage and risk for opioid abuse/overdose and related healthcare resource utilization by comparing ADF formulations of OXYCONTIN, HYSINGLA ER, and EMBEDA (morphine sulfate and naltrexone hydrochloride ER; discontinued).46,52 They found that broad formulary coverage inclusive of ADF opioids, which is currently at a low rate of <2%, was associated with reduced rates (15% to 25%) of opioid abuse– or overdose-related hospitalization rate and medical costs and concluded that shifting patients from traditional non-ADF drugs to ADF drugs can result in meaningful reductions in opioid misuse or abuse.46
OXYCONTIN was the topic of a joint FDA advisory meeting in 2020 to discuss its postmarketing evidence for meaningful reductions in abuse, misuse, and overdose (fatal and nonfatal) of the reformulation of OXYCONTIN relative to its original formulation.47 At that time, the committee determined that the evidence showed a meaningful reduction of abuse by nonoral routes (ie, intranasal and intravenous), but not in the overall risk of abuse and opioid overdose by all routes of administration.47
Since then, additional FDA-required postmarketing studies for OXYCONTIN have been published that compare data from pre-reformulated ER oxycodone to post-reformulated ER OXYCONTIN vs that of other opioid comparators.43,53 A retrospective claims study of the national Medicaid Analytic eXtract (MAX) database, IBM MarketScan database, and HealthCore Integrated Research Database (HIRD) evaluated unintentional fatal or nonfatal overdose rates in pre- vs post-reformulation of OXYCONTIN vs those of opioid comparators.53 The overdose incidence rate declined from pre- to post-OXYCONTIN reformulation [Medicaid adjusted rate ratios (aRRs), 0.93 (95% CI, 0.83–1.04); MarketScan/HIRD aRR, 0.86 (95% CI, 0.75–1.00)].53 However, no differences arose between the opioid comparators vs OXYCONTIN.53 An observational study using ASI-MV compared past 30-day nonoral abuse of pre-reformulated ER OxyContin (2-year period) to that of post-reformulated OXYCONTIN (4-year period) as well as other comparator opioids.43 Compared to the original ER oxycodone, the ADF OXYCONTIN demonstrated a significant decline in nonoral abuse, as measured by proportions (PR, −30.7% [95% CI, −46.9%, −9.5%]) and abuse report dispensing ratios (ARDR, −29.3% [95% CI, −37.5%, −20.1%]), analyzed via regression models.43 All comparator opioids (ER morphine, IR hydrocodone, and other Schedule II drugs) followed different trends with smaller reductions in abuse.43
HYSINGLA ER and ROXYBOND Postmarketing Studies
Relatively few postmarketing studies for HYSINGLA ER have been published thus far. HYSINGLA was one of 5 ADF ER formulations used for comparing misuse, abuse, and diversion of prescription opioids with XTAMPZA ER in the RADARS study described above.41 In that study, poison center exposures were greater for other ADF ER opioids (rate ratio, 5.2; P<0.001) and non-ADF ER opioids (rate ratio, 2.5; P=0.004) than for XTAMPZA ER. The combined past-month abuse prevalence for other ADF ER opioids (odds ratio, 7.4; P<0.001) and non-ADF ER opioids (odds ratio, 2.0; P=0.002) was greater than XTAMPZA ER.41
To our knowledge, no postmarketing data are yet available for ROXYBOND.
Discussion
Current guidelines recommend opioids be prescribed only when benefits for pain and function are expected to outweigh safety risks, but many healthcare professionals avoid prescribing opioids completely due to their perceived high potential for drug abuse, misuse, and criminal diversion.19,54 The implementation of ADFs for prescription opioids represents one of many interventions intended to curb the opioid crisis. ADFs serve as an opportunity to simultaneously address opioid abuse, misuse, and diversion while also providing pain control for people suffering from moderate to severe persistent pain.39 Overall, data suggest that ADFs have impacted prescriber and user behaviors, resulting in a reduction of abuse, misuse, and related clinical outcomes. A recent research report from the Institute for Clinical and Economic Review (ICER) evaluating the clinical effectiveness and economic impact of ADF opioids relative to non-ADF opioids stated that over a 5-year period, the use of ADF opioids prevented an additional 2300 new cases of opioid abuse. The ICER concluded that, although expensive, ADF opioids have the potential to prevent new cases of opioid abuse.55
In general, ADFs have been associated with reduced risk of abuse and misuse; however, some formulations may be more effective than others. We now have real-world data to differentiate between ADF formulations. The 4 currently available prescription opioids that have FDA-approved abuse-deterrence labeling have multiple premarket in vitro studies demonstrating resistance to physicochemical manipulation (category 1) and reduction of abuse via the intranasal route via human abuse-potential studies (category 3).15,22,24 However, FDA labeling for the oxycodone ER formulations of XTAMPZA ER and OXCONTIN differ in 2 important ways: 1) XTAMPZA ER is expected to reduce opioid abuse via oral administration in addition to nonoral routes and 2) crushed or chewed XTAMPZA ER retains bioequivalence to intact XTAMPZA ER, as demonstrated in human pharmacokinetic studies (category 2). By contrast, crushed OXYCONTIN does not retain bioequivalence to intact OXYCONTIN but instead to IR oxycodone.26,31,32,56 Of the 4 approved ADF opioids, only XTAMPZA ER has the category 2 ADF designation.30
A number of national, postmarketing, real-world setting studies on XTAMPZA ER have recently been published.36–41 Notably, the findings from the ASI-MV studies provide evidence for lower rates of nonmedical use for XTAMPZA vs other ADF ER oxycodone analgesics (primarily OXYCONTIN) that were maintained over a 7-year period; lower rates of nonoral manipulation (eg, inhalation, injection) were reported for XTAMPZA vs other ADF ER oxycodone analgesics.37,38 Several independent RADARS system studies also demonstrated lower rates of abuse, misuse, and diversion for XTAMPZA ER vs other ADF ER opioids (primarily OXYCONTIN) across nearly all programs utilized for the 3 years since its 2016 introduction to the market, despite the increase in XTAMPZA ER prescriptions; these findings persisted at least through 2021.40,41 Importantly, there were no cases of XTAMPZA ER being used by a nonoral route of administration reported to the RADARS Poison Center Program from 2016 through 2021 (6 years since its launch).40 Finally, HEOR-based analyses not only revealed that hospital admission rates (based on NPDS) were significantly lower with intentional misuse or abuse exposures of XTAMPZA ER but also reported significant cost advantages with XTAMPZA ER over other prescription opioids, including OXYCONTIN.36,39
The 2015 FDA guidance stresses the importance of including information about a product’s abuse-deterrent properties in labeling to inform healthcare professionals, the patient community, and the public, yet none of the extensive postmarket data has yet been approved to be included in a product label.24 During the past 9 years, we have learned more about the challenges in studying the original outcome measures (abuse, misuse, addiction, overdose, and death), and the flexible, adaptive approach promised by FDA is now warranted. Current ADF opioid formulations aim to deter manipulation of the product. Thus, the only direct measure of the effectiveness of an ADF is route of administration, as they cannot be chewed, snorted, smoked, or injected without physical manipulation. However, abuse, misuse, or nonmedical use can still be achieved without physical manipulation. Addiction, overdose, and death are all downstream surrogate measures riddled with confounders such as polysubstance use, lack of product-specific data, and by the fact that these can also occur without manipulation. The 2015 guidance also recognized that at the time, the epidemiologic methods and data sources for formal postmarket studies were evolving and best practices had not been established.24
Since 2015, numerous studies from diverse data sources employing a variety of study designs have been published, which describe strengths and limitations to take into consideration as the totality of evidence is evaluated. As the guidance states, labeling should reflect both the predictive quality of premarket studies as well as the results of relevant completed postmarket studies.24 Additional postmarketing studies are needed for HYSINGLA ER and ROXYBOND. The lack of category 4 labeling for any of the currently approved formulations, despite substantial postmarketing research for OXYCONTIN and XTAMPZA ER, underscores the need for continued refinements to measures of meaningful reduction in abuse or misuse.
Strengths and Limitations
Limitations of this article include those inherent in a narrative review. The studies cited herein were identified from PubMed literature searches and manual assessment of bibliographies from relevant articles rather than from a systematic approach. In addition, the postmarketing sections of our review focus heavily on XTAMPZA ER and OXYCONTIN due to the paucity of available postmarketing research for HYSINGLA ER and ROXYBOND. Valuable insights into real-world clinical practice and healthcare utilization can be obtained through postmarketing studies. Here, we summarize previous studies that utilized several independent databases. Many postmarketing surveillance results, such as data from the Poison Control Center, are based on voluntary reporting and may not encompass the full picture of opioid exposures; they represent only a snapshot in time and are subject to change. Finally, each data source used in individual studies has strengths and limitations. The purpose of this mosaic approach is to evaluate whether similar findings were discovered across different data sources, methods, outcomes, and populations. When the totality of evidence is consistent, convergent validity provides higher confidence that results are associated with the intervention and were less likely due to chance or confounding factors.
Conclusions
Balancing effective use of chronic opioid therapy while concomitantly decreasing the risk of opioid misuse and abuse remains challenging. The 4 currently FDA-approved and available long-acting ADF opioids exhibit their ADF properties in a heterogeneous manner with actual differences in outcomes across independent data sources. Real-world evidence postmarketing studies suggest that OXYCONTIN and, to a greater extent, XTAMPZA ER, deter tampering and nonoral, nonmedical use compared with other oxycodone ADF and non-ADF products. Despite some evidence for abuse deterrence, however, these ADFs do not fully address the multifaceted aspects of the opioid crisis. Future steps should include refinements to measures of meaningful reduction in abuse or misuse, as well as the development of new technologies and formulations that will reduce abuse and misuse without sacrificing analgesia for patients with chronic pain. The postmarketing studies summarized herein add to the body of evidence that support the use of ADF opioid medications when chronic opioid therapy is appropriate. In addition, these studies provide further evidence that, since the available ADF opioids are not equivalent with respect to abuse deterrence, prescribers should consider an ADF opioid with the most evidence for risk reduction when treating their patients.
Acknowledgments
Medical writing support for this manuscript was provided by Kristin Larsen, PhD, and Christi Alston, PhD, ELS, from Citrus Health Group, Inc. (Chicago, Illinois), in accordance with Good Publication Practices 2022 (GPP 2022), and was funded by Collegium Pharmaceutical, Inc. The authors retained full control over the content of this manuscript. Collegium Pharmaceutical, Inc. was not involved with any content generation, data collection, analysis, or data interpretation.
Funding Statement
This review was funded by Collegium Pharmaceutical, Inc. While the sponsor provided research support and reviewed the manuscript, all content of this article, data collection, analysis, and ultimate data interpretation were made by the authors without the sponsor’s influence.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors were involved in writing and reviewing the manuscript.
Disclosure
L.W.: Consultant for CognifiSense, Collegium, Elysium Pharmaceuticals, Ensysce Biosciences, Quivive Pharma, Salix Pharmaceuticals, Trevi Therapeutics. Advisory board participation for AdhereRx, Ensysce Biosciences, KemPharm, MedLogix. Travel expenses: AdhereRx, Elysium Pharmaceuticals, Ensysce Biosciences, PainScript. L.W. also reports personal fees for providing independent and evidence-based medical opinions, outside the submitted work. J.G.: Consultant for Acertis, Collegium, Ensysce, Hisamitsu, Kailo, Sanofi, Latigo, Xgene, and Tris. J.L.G.: Employee of Inflexxion, a division of Integrated Behavioral Health, which contracts with FDA and multiple sponsor companies with interests in some of the products included in the compounds evaluated for this article. J.L.G. is also a consultant for the Opioid Post-Marketing Requirement Consortium (OPC). C.E.A.: Grants and/or personal fees from AbbVie, Nevro Corp, Lilly, XGene Pharma, Lundbeck, Biohaven, Amgen, OPC, Collegium, Elsevier, Averitas, Scilex Holding, and Vertex. The authors report no other conflicts of interest in this work.
References
- 1.Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high-impact chronic pain in the United States. J Pain. 2019;20(2):146–160. doi: 10.1016/j.jpain.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults--United States, 2019-2021. MMWR Morb Mortal Wkly Rep. 2023;72(15):379–385. doi: 10.15585/mmwr.mm7215a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stark CW, Isaamullah M, Hassan SS, Dyara O, Abd-Elsayed A. A review of chronic pain and device interventions: benefits and future directions. Pain Ther. 2023;12(2):341–354. doi: 10.1007/s40122-022-00470-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi: 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–2097. doi: 10.1016/S0140-6736(21)00393-7 [DOI] [PubMed] [Google Scholar]
- 6.Goldsmith E, Koffel E, Ackland P, et al. VA evidence-based synthesis program reports. In: Implementation of Psychotherapies and Mindfulness-Based Stress Reduction for Chronic Pain and Chronic Mental Health Conditions: A Systematic Review. Washington (DC): Department of Veterans Affairs (US); 2021. [PubMed] [Google Scholar]
- 7.Altawili AA, Altawili MA, Alzarar AH, et al. Adverse events of the long-term use of opioids for chronic non-cancer pain: a narrative review. Cureus. 2024;16(1):e51475. doi: 10.7759/cureus.51475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan MJ, Gudin JA. The prescription opioid conundrum: 21st century solutions to a millennia-long problem. Postgrad Med. 2020;132(1):17–27. doi: 10.1080/00325481.2019.1677383 [DOI] [PubMed] [Google Scholar]
- 9.Malafoglia V, Ilari S, Vitiello L, et al. The interplay between chronic pain, opioids, and the immune system. Neuroscientist. 2022;28(6):613–627. doi: 10.1177/10738584211030493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webster LR. Risk factors for opioid-use disorder and overdose. Anesth Analg. 2017;125(5):1741–1748. doi: 10.1213/ANE.0000000000002496 [DOI] [PubMed] [Google Scholar]
- 11.Meske DS, Lawal OD, Elder H, Langberg V, Paillard F, Katz N. Efficacy of opioids versus placebo in chronic pain: a systematic review and meta-analysis of enriched enrollment randomized withdrawal trials. J Pain Res. 2018;11:923–934. doi: 10.2147/JPR.S160255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadeau SE, Wu JK, Lawhern RA. Opioids and chronic pain: an analytic review of the clinical evidence. Front Pain Res. 2021;2:721357. doi: 10.3389/fpain.2021.721357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pergolizzi JV Jr, LeQuang JA, Berger GK, Raffa RB. The basic pharmacology of opioids informs the opioid discourse about misuse and abuse: a review. Pain Ther. 2017;6(1):1–16. doi: 10.1007/s40122-017-0068-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeves KC, Shah N, Muñoz B, Atwood BK. Opioid receptor-mediated regulation of neurotransmission in the brain. Front Mol Neurosci. 2022;15:919773. doi: 10.3389/fnmol.2022.919773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler JA, Mallick-Searle T. An overview of abuse-deterrent opioids and recommendations for practical patient care. J Multidiscip Healthc. 2018;11:323–332. doi: 10.2147/JMDH.S166915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williamson C, Martin BJ, Argoff C, et al. Pain management and opioid therapy: persistent knowledge gaps among primary care providers. J Pain Res. 2021;14:3223–3234. doi: 10.2147/JPR.S316637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argoff CE, Viscusi ER. The use of opioid analgesics for chronic pain: minimizing the risk for harm. Am J Gastroenterol Suppl. 2014;2(1):3–8. doi: 10.1038/ajgsup.2014.3 [DOI] [PubMed] [Google Scholar]
- 18.Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. 2017;158(Suppl 1):S11–s18. doi: 10.1097/j.pain.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manchikanti L, Helm Ii S, Benyamin RM, Hirsch JA. Evolution of US health care reform. Pain Physician. 2017;20(3):107–110. doi: 10.36076/ppj.2017.110 [DOI] [PubMed] [Google Scholar]
- 20.US GAO. Opioid crisis: status of public health emergency authorities. Available from: https://www.gao.gov/products/gao-18-685r. Accessed October 14, 2024.
- 21.Boté SH. U.S. opioid epidemic: impact on public health and review of prescription drug monitoring programs (PDMPs). Online J Public Health Inform. 2019;11(2):e18. doi: 10.5210/ojphi.v11i2.10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Center for Drug Evaluation and Research (CDER). Abuse-Deterrent Opioids--Evaluation and Labeling: Guidance for Industry. Silver Spring, MD: Food and Drug Administration; 2015. [Google Scholar]
- 23.Green JL, Bucher Bartelson B, Le Lait MC, et al. Medical outcomes associated with prescription opioid abuse via oral and non-oral routes of administration. Drug Alcohol Depend. 2017;175:140–145. doi: 10.1016/j.drugalcdep.2017.01.039 [DOI] [PubMed] [Google Scholar]
- 24.Litman RS, Pagan OH, Cicero TJ. Abuse-deterrent opioid formulations. Anesthesiology. 2018;128(5):1015–1026. doi: 10.1097/ALN.0000000000002031 [DOI] [PubMed] [Google Scholar]
- 25.National Institute on Drug Abuse. US Overdose Deaths Involving Prescription Opioids. 1999-2022. Available from: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates. Accessed November 6, 2024.
- 26.OXYCONTIN (Oxycodone Hydrochloride) [Prescribing Information]. Stamford, CT, USA: Purdue Pharma LP; 2023. [Google Scholar]
- 27.US Food and Drug Administration. Timeline of selected FDA activities and significant events addressing substance use and overdose prevention. Available from: https://www.fda.gov/drugs/food-and-drug-administration-overdose-prevention-framework/timeline-selected-fda-activities-and-significant-events-addressing-substance-use-and-overdose. Accessed April 28, 2024.
- 28.Simon K, Worthy SL, Barnes MC, Tarbell B. Abuse-deterrent formulations: transitioning the pharmaceutical market to improve public health and safety. Ther Adv Drug Saf. 2015;6(2):67–79. doi: 10.1177/2042098615569726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration. Abuse-deterrent opioid analgesics. FDA. Available from: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/abuse-deterrent-opioid-analgesics. Accessed October 2, 2024.
- 30.Webster L, Gudin J. Review of opioid abuse-deterrent formulations: impact and barriers to access. J Pain Res. 2024;17:1989–2000. doi: 10.2147/JPR.S457982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudin J. Oxycodone DETERx(®): a novel abuse-deterrent, extended-release analgesic option for the treatment of patients with chronic pain. Pain Ther. 2016;5(2):171–186. doi: 10.1007/s40122-016-0062-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.XTAMPZA ER (Oxycodone) [Prescribing Information]. Stoughton, MA, USA: Collegium Pharmaceutical, Inc.; 2023. [Google Scholar]
- 33.HYSINGLA ER (Hydrocodone Bitartrate) [Prescribing Information]. Stamford, CT, USA: Purdue Pharma LP; 2023. [Google Scholar]
- 34.ROXYBOND (Oxycodone Hydrochloride) [Prescribing Information]. Princeton, NJ, USA: Protega Pharmaceuticals Inc; 2023. [Google Scholar]
- 35.Gadd S, Cox N, Samuelson J, Kenney A, Turner K, Cochran G. Abuse-deterrent opioid formulations and the opioid crisis: a pharmacist’s perspective. Ther Drug Monit. 2021;43(1):35–41. doi: 10.1097/FTD.0000000000000844 [DOI] [PubMed] [Google Scholar]
- 36.Green JL, Dailey-Govoni T, Vosburg SK. Opioid-related clinical outcomes and associated healthcare costs following abuse/misuse of oxycodone formulations: a HEOR analysis from real-world data. J Opioid Manag. 2024;20(4):281–288. doi: 10.5055/jom.0878 [DOI] [PubMed] [Google Scholar]
- 37.Green JL, Hartlage K, Dailey-Govoni T, Vosburg SK. Nonmedical use and route of administration of XTAMPZA® ER versus other oxycodone medications (extended- and immediate-release) in adults evaluated for substance use with the addiction severity index-multimedia version (ASI-MV®). Presented At: PainWEEK. September 3–6 2024; Las Vegas, NV, USA. [Google Scholar]
- 38.Green JL, Robbins RS, Dailey-Govoni T, Butler SF. Nonmedical use of Xtampza® ER and other oxycodone medications in adults evaluated for substance abuse treatment: real-world data from the addiction severity index-multimedia version (ASI-MV®). J Pain Res. 2021;14:1773–1783. doi: 10.2147/JPR.S304805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olatoke O, Zah V, Stanicic F, et al. A US retrospective claims analysis comparing healthcare costs of patients transitioning from immediate-release oxycodone to two different formulations of extended-release oxycodone: Xtampza ER or OxyContin. Clinicoecon Outcomes Res. 2022;14:119–128. doi: 10.2147/CEOR.S340290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Severtson SG, Haanschoten EE, Iwanicki J, Dart RC. Differences in severity of poison centers exposures involving XTAMPZA(®) ER versus other opioid analgesics. Pain Manag. 2023;13(9):519–527. doi: 10.2217/pmt-2022-0068 [DOI] [PubMed] [Google Scholar]
- 41.Severtson SG, Kreider SED, Amioka EC, Margolin ZR, Iwanicki JL, Dart RC. Postmarketing analysis of misuse, abuse, and diversion of Xtampza ER. Pain Med. 2020;21(12):3660–3668. doi: 10.1093/pm/pnaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jewell J, Black J, Ellis M, Olsen H, Iwanicki J, Dart R. A cross-sectional study of tampering in Xtampza ER, an abuse-deterrent formulation of an extended-release opioid, in a treatment center population. Clin Drug Investig. 2023;43(3):197–203. doi: 10.1007/s40261-023-01248-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez RD, Dailey Govoni T, Rajagopal V, Green JL. Evaluating the effectiveness of reformulated extended-release oxycodone with abuse-deterrent properties on reducing non-oral abuse among individuals assessed for substance abuse treatment with the addiction severity index-multimedia version (ASI-MV). Curr Med Res Opin. 2023;39(4):579–587. doi: 10.1080/03007995.2023.2178080 [DOI] [PubMed] [Google Scholar]
- 44.Cicero TJ, Ellis MS. Abuse-deterrent formulations and the prescription opioid abuse epidemic in the United States: lessons learned from oxycontin. JAMA Psychiatry. 2015;72(5):424–430. doi: 10.1001/jamapsychiatry.2014.3043 [DOI] [PubMed] [Google Scholar]
- 45.Cicero TJ, Ellis MS, Kasper ZA. A tale of 2 ADFs: differences in the effectiveness of abuse-deterrent formulations of oxymorphone and oxycodone extended-release drugs. Pain. 2016;157(6):1232–1238. doi: 10.1097/j.pain.0000000000000511 [DOI] [PubMed] [Google Scholar]
- 46.Petrilla A, Marrett E, Shen X, Kwong WJ, Pezalla E. Association between formulary coverage and use of abuse-deterrent prescription opioids, risk for abuse or overdose, and associated healthcare resource utilization. Am Health Drug Benefits. 2020;13(1):21–31. [PMC free article] [PubMed] [Google Scholar]
- 47.Center for Drug Evaluation and Research (CDER). Final Summary minutes of the Drug Safety and Risk Management Advisory Committee and the Anesthetic and Analgesic Drug Products Advisory Committee Joint Meeting. Washington, DC: Food and Drug Administration; 2020. [Google Scholar]
- 48.RMPDS Research. Drug use in the United States. Available from: https://public.tableau.com/app/profile/rmpds.research/viz/PublicDashboard_t2/Overview. Accessed October 14, 2024.
- 49.BioSpace. Protega Pharmaceuticals™ LLC Announces Commercialization of RoxyBond™ (Oxycodone Hydrochloride) Tablets CII in the U.S. Princeton, NJ, USA: BioSpace; 2023. [Google Scholar]
- 50.Florence C, Luo F, Rice K. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol Depend. 2021;218:108350. doi: 10.1016/j.drugalcdep.2020.108350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunt R, Yalamanoglu A, Tumlin J, et al. A mechanistic investigation of thrombotic microangiopathy associated with IV abuse of Opana ER. Blood. 2017;129(7):896–905. doi: 10.1182/blood-2016-08-736579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dasgupta N, Brown JR, Nocera M, Lazard A, Slavova S, Freeman PR. Abuse-deterrent opioids: a survey of physician beliefs, behaviors, and psychology. Pain Ther. 2022;11(1):133–151. doi: 10.1007/s40122-021-00343-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beachler DC, Hall K, Garg R, et al. An evaluation of the effect of the OxyContin reformulation on unintentional fatal and nonfatal overdose. Clin J Pain. 2022;38(6):396–404. doi: 10.1097/AJP.0000000000001034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain - United States, 2022. MMWR Recomm Rep. 2022;71(3):1–95. doi: 10.15585/mmwr.rr7103a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Institute for Clinical and Economic Review (ICER). Impact of Abuse Deterrent Formulations of Opioids in Patients with Chronic Pain in the United States: A Cost-Effectiveness Model. Boston, MA: ICER Analytics; 2024. [DOI] [PubMed] [Google Scholar]
- 56.Gudin J, Levy-Cooperman N, Kopecky EA, Fleming AB. Comparing the effect of tampering on the oral pharmacokinetic profiles of two extended-release oxycodone formulations with abuse-deterrent properties. Pain Med. 2015;16(11):2142–2151. doi: 10.1111/pme.12834 [DOI] [PubMed] [Google Scholar]