Implications.
This paper presents recent research that provides insights into how tannins influence forage intake, rumen microbial growth, volatile fatty acid production, and grazing behavior in ruminants.
Including tannins in grazing diets presents a double-edged sword, offering potential benefits for ruminant growth efficiency, methane mitigation, and nutrient utilization while posing challenges to digestibility and feed intake.
Tannins, naturally occurring in plants, interact with ruminant ingestive behavior and rumen microbes, significantly affecting fermentation processes, nutrient absorption, and animal growth, thereby impacting environmental sustainability.
The key lies in balancing tannin levels and type (e.g., condensed, hydrolyzable, phlorotannins) to optimize their positive effects without compromising animal performance. The dual nature of tannins highlights their complex role in animal nutrition and health, as well as in the diversity of feeding responses exhibited by ruminant livestock.
Introduction
Tannins have long been used to preserve and tan leather. There are reports of tannin use dating back to the late Neolithic period (Falcão and Araujo, 2018), when aqueous extracts with plant parts were used to prevent the degradation of slaughtered animal skins, transforming them into leather. Tannins are important in leather tanning because their molecules bind to proteins, causing the fibers to break down. Chemically, tannins are polyphenolic compounds characterized by aromatic compounds linked to hydroxyl groups that bind to proteins through hydrogen bonds (Naumann et al., 2017; Tedeschi et al., 2021b). In addition to binding to proteins, tannins can also precipitate polysaccharides, alkaloids, and metal ions (Falcão and Araujo, 2018). They can have antioxidant capacity, serving as a substance that mitigates the negative effects of free radicals in oxidative stress (Mora et al., 2022).
There are many ways in which tannins can influence grazing and ruminant growth, making it impossible to complete this review. However, we will highlight the main effects of condensed tannins (CT) on forage intake and the ruminal environment of sheep and cattle. Figure 1 presents a summary of the main relationships between plant tannins and ruminants. We propose the hypothesis that plants accumulate CT as secondary compounds not only to constrain grazing or repel ruminants, but they are also to balance and attenuate environmental impacts through reductions in overgrazing and gastrointestinal methane emissions while helping ruminants maintain health, grow, and reproduce.
Figure 1.
Summary of the main relationships between plant tannins and ruminants. Source: Authors.
CT Biochemistry and Anatomical Distribution in Plants
According to Mora et al. (2022), tannins can be subdivided into three families: hydrolyzable tannins (HT), CT, and phlorotannins. Some plants, mainly dicotyledonous, produce HT and CT. Phorotannins are found only in marine re-brown algae. Plant tannins are considered secondary compounds, as there is currently no evidence that they participate in vital essential metabolic pathways. Secondary compounds are often specialized and are much more variable than primary ones (Pott et al., 2019). In plants, the formation of HT follows the metabolic pathway of shikimate, and the CT follows the metabolism of phenylpropanol (Monteiro et al., 2005).
CT have been extensively studied due to their benefits to ruminants and their greater presence in some forage plants than in others (Tontini et al., 2019). These molecules, called proanthocyanidins due to their reddish pigments (Monteiro et al., 2005), are polymers of flavan-3-ol and/or flavan-3,4-diol that present a wide diversity of compounds as a result of different chemical bonds. CT are known as a secondary compound that evolved to restrict the amount of plant tissue consumed by herbivores, which restricts ingestion in some insects and grazing in ruminants. However, they can be beneficial for the growth and health of ruminants due to their antioxidant properties, protection against protein degradation in the rumen, reduction of methane production, and control of gastrointestinal parasite infection (Mueller-Harvey et al., 2019).
In contrast, knowledge about the effects of HT in ruminant growth (Piluzza et al., 2014) is still limited. Although HT have oxygen-free radical absorption capacity and less effects on protein digestibility than CT (Hagerman et al., 1992), HT molecules can be toxic to ruminants. Reed (1995) explains that the degradation of HT by ruminal microorganisms can produce pyrogallol, a hepatotoxin and nephrotoxin compound for ruminants. Krumholz and Bryant (1986) and Lotfi (2010) comment that a concentration of pyrogallol above 10 mmol l−1 is toxic. In addition, Clifford and Scalbert (2000) mention that punicalagin, an ellagitannin (subclass of HT), can also cause liver necrosis in cattle and sheep. It is estimated that HT should not be consumed at levels exceeding 5 mg day−¹, suggesting that even small amounts can pose a toxicological risk.
Plant tannins can comprise up to 25%, on a dry matter (DM) basis, of ruminant-edible materials such as leaves, roots, bark, and reproductive structures (Iqbal and Poor, 2024). CT can contribute up to 14% of total above-ground DM in some forage legumes (Muir et al., 2008).
CT are generally labeled secondary metabolites because they accumulate primarily in reaction to or as mitigation of environmental and biotic stressors (Dehghanian et al., 2022). These stressors can include environmental extremes such as drought or temperature, but can also be organismal, from microbes to insects and ruminant herbivore attacks (Skovmand et al., 2023). These can be broadly described as limiting oxidative stress (Tong et al., 2022) and consist of fixing carbon dioxide through metabolic processes described in detail by Iqbal and Poor (2024). The prevalent hypothesis is that stressors stimulate CT accumulation to discourage herbivory or pathogenic attacks (Harding, 2019; Zhu et al., 2023).
For obvious plant-protection benefits, CT accumulate most readily in photosynthetically active materials, even though they do appear in other anatomical parts of the plant (Wang et al., 2023). Within the plant as a whole, CT content can change with ontogeny. Reproductive strategies are most likely involved (e.g., decreases when epizoochory becomes important, Cooper et al., 2014), but other evidence, such as dormancy during dry or cold seasons, can also be involved (Norris et al., 2023), or prior to abscission during leaf drop (Acero et al., 2010) can also be factors.
Dicotyledonous plants are far more likely to accumulate CT, while monocotyledonous plants rarely present these molecules in their tissues; these can be found in monocotyledonous grains, as in some sorghum cultivars (Kaufman et al., 2013). The biosynthesis and regulation of tannins in plants are governed by intricate molecular mechanisms, which are finely modulated by genetic, environmental, and developmental cues (Molnar et al., 2024). Hanlin et al. (2010), studying tannin in grapes for wine, relate the tannin to the plant cell wall composition. Our laboratories have determined that biologically active CT are more likely to appear in the general order forbs > bush > arboreal leaves. Further research or metanalyses are needed to substantiate these initial data. Likewise, our laboratory data sets indicate that dicotyledonous growth patterns that accumulate greater concentration of CT are generally long-term perennial > perennial > semi-perennial > annual reproductive strategies. Again, systematic meta-analyses are needed to confirm these initial findings.
Influence of Plant Tannins on Forage Intake by Ruminants
Tannins have been traditionally regarded as chemicals with dose-dependent impacts on forage intake by livestock (Barry and McNabb, 1999). For instance, while moderate concentrations of CT in feeds (e.g., 1.5% to 4% DM, depending on bioactivity) may not inhibit intake (Barry and McNabb, 1999; Henke et al., 2017), concentrations above 5% can depress consumption and negatively impact animal productivity (Hervás et al., 2003). However, this description of the influence of tannins on forage intake is restrictive, as their impacts on the foraging animal are multidimensional, rooted in the complexity of tannin chemistry, interactions with other biochemicals, concentrations in plant tissues, and the diverse impacts of tannins on livestock nutrition and health (Frutos et al., 2004; Waghorn, 2008; Mueller-Harvey et al., 2019; Yanza et al., 2021; da Silva Aguiar et al., 2023; Villalba et al., 2024). In fact, the influence of tannins on intake should be interpreted under the context of their bioreactivity, oro-sensorial and post-ingestive actions. This is because feed intake and preference in herbivores are determined by the interaction between the senses of taste and smell and post-ingestive feedback, influenced by an animal’s physiological condition and a food’s chemical characteristics (Provenza, 1995). Taste and smell enable animals to discriminate among foods and provide hedonic sensations associated with eating. Post-ingestive feedback calibrates these oro-sensorial dimensions as a function of a food’s homeostatic utility (Provenza, 1995).
This dynamic relationship between oro-sensorial experiences and post-ingestive feedback in mammals, that is, the “behavior by consequences” paradigm, coupled with differences in tannin chemistry, configuration, biochemical context, animal physiology, dose, and bioactivity, can help explain the multifaceted actions of tannins on the subsequent feeding behavior of grazing animals.
In addition to individual experiences, social learning is another important mechanism modulating forage intake and preference, particularly in complex grazing environments. Young animals often acquire dietary preferences and avoidance behaviors by observing and mimicking their mothers or peers (Thorhallsdottir et al., 1990; Bailey and Provenza, 2008). This socially mediated learning and early life experience can facilitate the acceptance of tannin-rich forages if influential individuals within the group include them in their diet (Distel and Villalba, 2018). Consequently, group structure and social dynamics may accelerate or buffer the individual response to the oro-sensorial and post-ingestive effects of tannins, promoting more flexible or resilient foraging strategies under variable dietary contexts.
Oro-sensorial effects of tannins
Tannins are known for their bitterness and astringency, which can reduce feed intake in ruminants (Naumann et al., 2017). These sensations are experienced immediately after feeds contact the buccal cavity, and thus, they exert a short-term effect on feed intake, whereas the post-ingestive impacts of tannins (e.g., decreased ruminal digestibility, malaise) entail longer-term effects on intake, because they require dilution and mixing of the ingested matter with gastrointestinal fluids, in addition to metabolites undergoing biodegradation, all processes which require more than minutes to be completed (Landau et al., 2000). Taste receptor cells are characterized by the expression of members of the TASTE Receptor type 2 (TAS2R) gene family, which encode different G-protein-coupled receptors, some with high affinity for either CT or HT (Soares et al., 2020). Upon binding of bitter compounds to TAS2Rs, a taste transduction response is triggered, culminating in the release of neurotransmitters that lead to the recognition of bitter compounds in the mouth. The molecular size, stereochemistry, and interflavonoid bonds of tannins play crucial roles in bitter taste perception (Peleg et al., 1999). Generally, lower molecular weight compounds are perceived as more bitter by mammals than those of greater molecular weight (Soares et al., 2020). Beyond the tannin chemical structure and activation of TAS2Rs, the perception and response to bitterness are not a simple or universal mechanism but rather a complex trait that has evolved differently across species based on their dietary habits and ecological niches.
In addition to bitterness, astringency promoted by phenolic compounds like tannins is a complex oral sensation involving multiple mechanisms, including (1) interaction with and precipitation of salivary proteins (Jones and Mangan, 1977), (2) impacts on epithelial cells that cause changes in their morphology and function, and (3) activation of trigeminal chemoreceptors in the oral cavity, all triggering astringent sensations associated with a dry, puckering sensation in the mouth (Huang and Xu, 2021). Salivary proline-rich proteins (PRPs) play a significant role in reducing astringency and protecting animals against tannins (i.e., maintaining feed intake) through their high affinity for these compounds and the formation of complexes, which reduce the amount of free tannins available to interact with other proteins in the oral tissues. Precipitation of tannin-PRP complexes effectively removes tannins from the oral environment, maintaining salivary lubrication (Boze et al., 2010). Moreover, due to their structure and conformation, PRPs inactivate tannins to a greater extent than dietary proteins, resulting in reduced nutritional losses, as PRPs contain nonspecific nitrogen and non-essential amino acids (Soares et al., 2020). On the contrary, for humans and ruminants, these proteins are constitutive and exist in amounts that appear to reflect the predictable level of tannins in the animals’ diets (Soares et al., 2020). Nevertheless, other salivary proteins have been detected in cattle, which are not rich in proline, and these proteins also have a high affinity for binding to tannins (Makkar and Becker, 1998). Additionally, sheep can adapt to tannin-rich diets by producing salivary proteins with a high-tannin-binding capacity (Vargas-Magaña et al., 2013).
Not all tannins bind protein equally or cause the same astringent stimulus; phenolic compounds with different types and structures show different astringency thresholds, suggesting a relationship between phenolic structures and perceived astringency (Huang and Xu, 2021). For instance, CTs in Desmodium paniculatum have a lower protein-binding affinity than Neptunia lutea or Lespedeza cuneata (Naumann et al., 2014). This difference may contribute to explain why some legumes with a relatively high concentration of CTs (i.e., Onobrychus viciifolia; 9% to 10%) are more palatable to sheep than legumes with much lower content of CTs (i.e., Lotus corniculatus; 2% to 4%) (Häring et al., 2008; Naumann et al., 2017).
Negative post-ingestive effects of tannins and intake
The negative impacts of tannins on herbivores extend beyond taste. CTs can cause post-ingestive discomfort in ruminants, leading to food aversions with substantial reductions in feed intake (Provenza et al., 1990). Conditioned food aversion learning is the process by which, after eating a specific food, a physiological event or a chemical causes nausea (Garcia, 1989; Provenza, 1995, 1996). Mammals learn to associate the flavor of the ingested harmful feed with nausea, leading to avoidance of that feed in future encounters (Provenza et al., 1990). This adaptive mechanism is critical for survival, enabling animals to minimize the risk of ingesting metabolites, such as tannins, that can trigger adverse physiological responses. Administration of a selective antagonist of serotonin receptors (Ondansetron) attenuated aversions to a plant secondary compound (jensenone, an acylphloroglucinol derivative found in the foliage of Eucalyptus jensenii) in possums (Lawler et al., 1998). This result shows experimentally that the antifeed effects of plant secondary compounds in herbivores are mediated by serotonin action on 5HT3 receptors through a conditioned food aversion triggered by nausea. Similarly, the high reactivity of tannins with animal tissues, such as the epithelial lining of the gastrointestinal tract (Hervás et al., 2003), as well as organs like the kidney and liver (Kumar and Singh, 1984), can cause direct tissue damage, leading to post-ingestive malaise and a subsequent reduction in feed intake. Serotonin receptors in gastrointestinal tissues may also trigger this mechanism, as they have also been shown to play a role in pain perception through the gut-brain axis (Gershon, 2004).
The affinity of tannins to bind proteins also causes multiple interactions with digestive enzymes, contributing to explain the negative impacts of tannins on feed intake (Landau et al., 2000). Complexation of tannins with digestive enzymes inhibits their activity (Kumar and Singh, 1984) and causes declines in food digestibility (Waghorn et al., 1994; Silanikove et al., 1997) through reductions in degradation of organic matter, protein, and plant cell walls in the rumen (Silanikove et al., 2001). In addition, tannins can reduce fiber digestion by forming complexes with lignocellulose, which prevents microbial attachment and degradation, or through their antibiotic actions on cellulolytic microorganisms (Hervás et al., 2003). A depression on digestibility constrains the rate of evacuation of digesta from the rumen (gut-fill effect), causing reductions in feed intake by ruminants (Kumar and Singh, 1984). This is because the digesta contents in the rumen are regulated at a constant level, and thus, increments in digestibility enhance the evacuation rate, allowing more food to be consumed. Beyond the rumen, tannins reaching the duodenum delay the passage of digesta through the intestine, and they may reduce the intestinal activity of pancreatic enzymes (i.e., trypsin and amylase) and amino acid absorption from the small intestine. The overall effect is a delayed transit of fluid and particulate matter throughout the entire gastrointestinal tract (Silanikove et al., 2001), resulting in reduced feed intake.
Finally, tannins are reactive molecules that can negatively impact forage intake (Delimont et al., 2017) by binding with chemicals other than protein or carbohydrates. In support of this, sheep and goats supplemented with a high-tannin-containing legume (Sericea lespedeza) showed reductions in serum concentration of Mo, Mn, Zn, and Se relative to animals receiving a control supplement without tannins (Burke et al., 2023).
Positive post-ingestive effects of tannins and intake
Certain tannin molecules, administered at appropriate doses, may also provide beneficial effects to livestock, including anti(endo)parasitic, antimicrobial, antioxidant, prebiotic, anti-inflammatory, and immunomodulatory effects (Hoste et al., 2015; Mueller-Harvey et al., 2019; Molino et al., 2022; Villalba et al., 2024). Research has demonstrated that the consistent consumption of low doses of plant-derived phenolics, such as tannins, can mitigate inflammation and oxidative stress in livestock (Gessner et al., 2017). These effects collectively contribute to enhanced animal performance, improved health outcomes, and superior product quality (Mueller-Harvey et al., 2019; Orzuna-Orzuna et al., 2021; VanVliet et al., 2023).
Gastro-intestinally parasitized ruminants demonstrate self-medication through selective consumption of tannin-rich diets. In a series of studies, lambs infected with Haemonchus contortus exhibited increased voluntary intake of quebracho tannin-containing feed (8% tannins) compared to their non-parasitized counterparts (Villalba et al., 2010; Juhnke et al., 2012). Notably, this differential intake pattern disappeared following antiparasitic treatment, suggesting a learned, adaptive behavior that aligns with the behavior-by-consequences paradigm described at the beginning of this section. This paradigm also suggests that ruminants may exhibit increased acceptance of medicinal bioactive, such as tannins, when experiencing illness (Villalba et al., 2024).
It is important to note that this self-medicative behavior is not only instinctive in the strict sense, nor does it result from conscious perception of illness. Rather, it is primarily acquired through associative learning based on post-ingestive feedback. Animals learn to associate the sensory properties of specific forages (e.g., bitterness, odor) with the relief of negative physiological states, such as parasitic infections or oxidative stress, reinforcing their preference for those feeds in future exposures (Provenza, 1995; Lisonbee et al., 2009a; Villalba et al., 2010). These learned associations are critical for the development of self-medication strategies and highlight the role of the animal’s physiological condition in modulating intake behavior (Poli et al., 2018). This phenomenon is particularly evident in bitter-tasting compounds, which are typically avoided by animals due to their aversive sensory properties (Glendinning, 1994), as discussed before. The risk-prone behavior exhibited by sick individuals, characterized by increased consumption of potentially toxic bitter feeds, may enhance the likelihood of ingesting therapeutic doses of medicinal bioactives, including tannins. Supporting this hypothesis, studies have demonstrated that naive parasitized lambs display a greater intake of bitter tannin-containing feeds compared to their healthy counterparts (Lisonbee et al., 2009b). Furthermore, parasitized sheep showed a heightened intake of rations containing bitter-tasting antioxidant polyphenols and terpenes (Poli et al., 2018). These findings suggest that tannin intake is also a function of an adaptive mechanism whereby animals modulate their feeding behavior in response to their physiological state, leveraging the medicinal properties of tannins to address health challenges.
In some instances, livestock may consume more and even prefer forages with greater concentrations of tannins. This phenomenon was observed in a study where both parasitized and non-parasitized lambs showed a preference for sainfoin (Onobrychis viciifolia) pellets with higher tannin content (4%) over those with lower tannin content (2%) after a conditioning period that involved 21 d of exposure to both types of pellets (Costes-Thiré et al., 2018). The unique tannin profile of sainfoin may explain why lambs showed increased intake and preference for the higher tannin pellets following a conditioning period, as they likely learned to associate the positive post-ingestive effects of sainfoin tannins with their sensory properties (Costes-Thiré et al., 2018).
Finally, plant secondary compounds can interact synergistically to influence feeding behavior in ruminants. For instance, saponins (plant-derived glycosidic compounds characterized by a steroid or triterpenoid core linked to sugar side chains) can bind to tannins in the gastrointestinal tract, mitigating their potential negative effects. Tannins and saponins form insoluble complexes in the intestine, effectively neutralizing their negative individual impacts (Freeland et al., 1985). Goats exhibit increased intake when offered a diversity of shrubs containing tannins or saponins relative to animals offered single shrubs (Rogosic et al., 2006). Similarly, lambs presented with choices between saponin- and tannin-containing feeds show greater DMI than lambs offered single feeds (Copani et al., 2013). These findings highlight the influence of the biochemical context (i.e., the presence of saponins in the diet) on the impact of plant tannins on feeding behavior and intake.
Tannin Impacts on Microbial Growth, Rumen Fermentation, and Volatile Fatty Acids
Tannins, naturally occurring polyphenolic compounds, are increasingly recognized for their role in modulating ruminant nutrition and improving the sustainability of livestock production. These compounds, particularly CT and HT, have a profound effect on microbial growth, rumen fermentation, and volatile fatty acid (VFA) production. Their ability to influence these processes is crucial to their application as natural feed additives, enhancing feed efficiency, reducing nitrogen losses, and mitigating methane emissions.
Modulation of rumen microbial ecology and fermentation
Tannins affect microbial growth primarily through their ability to bind proteins, carbohydrates, polysaccharides, alkaloids, and metal ions, forming complexes that are resistant to microbial degradation. This binding, facilitated by hydrogen bonding and hydrophobic interactions, reduces microbial proteolytic activity, ammonia production, and overall nitrogen availability, thereby altering the rumen nitrogen cycle rumen (Mueller-Harvey et al., 2019; Tedeschi et al., 2021b). This reduction in ammonia can influence the growth of ammonia-utilizing bacteria, further affecting the VFA profile (Besharati et al., 2022). The effects of tannins are not uniform across microbial populations; they selectively inhibit cellulolytic bacteria and methanogenic archaea while promoting tannin-resistant species, leading to significant shifts in microbial community composition and fermentation dynamics (Patra and Saxena, 2011; Salminen and Karonen, 2011; Salminen et al., 2011). High concentrations of tannins in fodder plants inhibit gastrointestinal bacteria and reduce ruminant performance, but including tannin-resistant bacteria in the rumen can mitigate these effects. The protective role of these bacteria may involve mechanisms such as tannin degradation, dissociation of tannin–substrate complexes, or membrane repair (Smith et al., 2005). HT degrade in the rumen and can release potentially toxic metabolites (including mostly pyrogallol, gallic acid, resorcinol, and phloroglucinol) when consumed in excess (Lotfi, 2020). By contrast, CT form insoluble complexes with proteins that dissociate in the acidic abomasum, enhancing post-ruminal protein absorption and improving nitrogen utilization (Salminen and Karonen, 2011; Salminen et al., 2011; Tedeschi et al., 2014).
Condensed tannins as methane mitigators
The effects of CT on rumen fermentation are closely tied to their impact on the profile of VFA. By altering the availability of substrates, tannins influence the metabolic pathways of rumen microbes. CT reduce the degradation of structural carbohydrates and proteins, resulting in a shift toward increased propionate production and decreased acetate formation (Tedeschi et al., 2014). This shift is particularly advantageous because propionate serves as a more efficient glucose precursor than acetate, thereby enhancing the energy efficiency of the animal. Additionally, reducing acetate production and hydrogen availability contributes to lower methane emissions, aligning with global efforts to reduce greenhouse gas emissions from livestock (Beauchemin et al., 2009; Jayanegara et al., 2012). Meta-analyses demonstrate that the inclusion of tannins in grazing diets can reduce methane emissions by 20–50%, depending on the type and dose of tannins used (Jayanegara et al., 2012).
Building on these insights, Berça et al. (2023) conducted a comprehensive meta-analysis that further elucidated the mechanisms through which tannins mitigate methane emissions. Their findings revealed that dietary inclusion of CT reduced CH4 emissions, expressed in liters per kilogram of dry matter intake (DMI), through direct and indirect mechanisms. Directly, CT inhibited the growth of methanogenic archaea, while indirectly, CT reduced hydrogen availability by altering the ruminal fermentation pathways. The study quantified that a dietary CT level of 3.9% DM could decrease methane production by approximately 16.7% in vivo and 6.3% in vitro. The study also highlighted an increase in total VFAs, particularly propionate and butyrate, in response to CT supplementation, emphasizing the potential of CT to enhance fermentability while mitigating methane emissions. Importantly, the findings identified critical interactions between CT and dietary crude protein (CP), where higher CP levels amplified the methane-mitigating effects of CT. These interactions offer a pathway for precision diet formulation targeting specific methane reduction goals. However, these reductions must be balanced against potential declines in digestibility or feed intake, particularly when tannin levels exceed thresholds considered safe for ruminant health and productivity. The meta-analysis indicated that optimal CT inclusion levels range between 2% and 4% of diet DM, depending on specific CT bioreactivity, beyond which adverse effects on digestibility and animal performance may occur.
These findings underscore the dual benefits of CT in improving the diet’s glucogenic potential while mitigating environmental impacts. Future strategies should focus on tailoring CT use to individual dietary compositions and animal needs, thereby maximizing both productivity and sustainability.
Impact on nutrient absorption and digestibility
CT achieve these effects through several mechanisms. Their protein-binding capacity reduces ruminal proteolysis and deamination, leading to lower ammonia concentrations and improved nitrogen utilization (Tedeschi et al., 2014). By directly inhibiting methanogenic archaea and protozoa, CT reduce the production of hydrogen—a key substrate for methane synthesis—thereby shifting metabolic pathways toward propionate formation (Salminen et al., 2011). Additionally, tannin metabolites act as hydrogen sinks, further curbing methane emissions (Tedeschi et al., 2021b). Recent studies have also highlighted the pro-oxidant activities of ellagitannins, which generate reactive oxygen species that disrupt microbial enzymatic functions and inhibit the growth of certain microbes (Salminen and Karonen, 2011). Smith et al. (2005) proposed that tannins inhibit gastrointestinal bacteria by complexing with polymers and minerals, while tannin-resistant microbes may utilize mechanisms such as tannin inactivation or sequestration of metal ions to overcome these effects.
Regarding fiber digestion, tannins can inhibit the activity of cellulolytic enzymes, resulting in lower neutral detergent fiber digestibility. However, strategic management of tannin sources and inclusion levels can mitigate these effects. For instance, feeding moderate levels of CT (2% to 4% of DM) has been shown to improve nitrogen retention and animal performance without impairing fiber utilization (Min et al., 2003).
The application of CT as feed additives is influenced by their chemical structure, concentration, and the dietary context in which they are used. For example, quebracho CT reduce methane emissions in roughage-based diets but exhibit inconsistent effects in concentrate-fed systems, highlighting the complexity of their interactions within the rumen environment (Tedeschi et al., 2014, Tedeschi et al., 2021b). Differences in tannin polymerization, hydroxylation, and galloylation patterns further modulate their biological activity and interactions with rumen microbes (Salminen and Karonen, 2011; Salminen et al., 2011).
Practical applications of tannins in ruminant diets extend beyond their effects on fermentation. Their application in ruminant diets offers promising opportunities for enhancing production efficiency and sustainability. By reducing protein degradation in the rumen, tannins increase the flow of bypass protein to the small intestine, enhancing post-ruminal protein absorption and supporting muscle growth and milk production (Waghorn, 2008; Tedeschi et al., 2014).
Practical Implications for Grazing Systems
Plant species, growth stage, and environmental conditions, such as drought or soil fertility, influence the concentration and composition of tannins in forages. This variability necessitates careful selection and monitoring of forage species in grazing systems. Incorporating CT-rich legumes, such as birdsfoot trefoil (L. corniculatus) or sainfoin (O. viciifolia), into pastures has been proposed as a practical approach to harnessing the benefits of tannins while minimizing their anti-nutritional effects. Understanding the mechanisms by which tannins inhibit bacteria and how rumen microbes overcome these effects can provide new insights into optimizing tannin use for sustainable livestock production (Smith et al., 2005).
Moreover, CT supplementation provides an environmentally friendly approach for reducing nitrogen excretion and methane emissions in grazing systems. As regulatory pressures to reduce greenhouse gas emissions intensify, tannins present a natural alternative to synthetic additives, aligning with consumer preferences for sustainable meat production. The integration of tannins, especially CT, as feed additives in grazing systems necessitates a deeper understanding of their structure-function relationships and the development of strategies to optimize their benefits. Current research focuses on elucidating the mechanisms underlying tannins’ effects on the rumen microbiome and exploring novel dietary approaches to maximize their utility while minimizing potential drawbacks (Salminen and Karonen, 2011; Salminen et al., 2011; Tedeschi et al., 2014).
Over the years, extensive research has deepened our understanding of tannins’ biochemical and chemical properties (Tedeschi and Fox, 2020a, 2020b). For example, tannins have demonstrated the ability to bind proteins and inhibit microbial attachment, altering rumen fermentation and nutrient absorption pathways. CT reduce methane emissions and nitrogen excretion, emphasizing their dual role in improving environmental sustainability and enhancing animal productivity. However, despite these advances, the variability in CT activity across plant species and environmental conditions underscores the challenge of predicting their effects with simple empirical models (Tedeschi and Fox, 2020a, 2020b).
Tedeschi et al. (2014) proposed a conceptual model that identifies five key mechanisms of CT action: methane reduction, protein sparing, prevention of frothy bloat, parasite suppression, and improvements in reproductive health. These mechanisms are interlinked through feedback loops that influence energy balance and nutrient utilization. This framework, when integrated with insights from existing mechanistic nutrition models (Tedeschi and Fox, 2020a, 2020b), highlights the potential of tannins to improve ruminant production systems while mitigating environmental impacts.
Despite substantial progress, significant knowledge gaps persist, particularly in quantifying the interactions of tannins with rumen microbes and their long-term effects on animal physiology. The variability in tannin concentration due to environmental factors—such as soil quality, drought, and plant maturity—complicates their inclusion in feed formulation and decision-support tools.
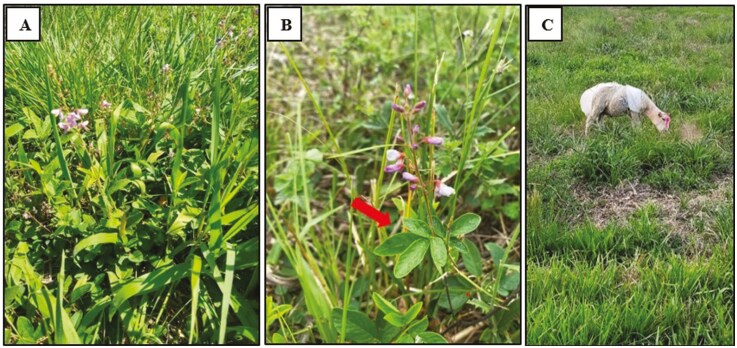
These challenges are especially pronounced in grazing systems, where ruminants select their diet from a diverse array of plant species at various phenological stages and under different forage conditions, including height, humidity, and nutritional content. Figure 2 illustrates a native grassland in southern Brazil, a heterogeneous pasture marked by considerable forage species diversity and tannin source. Unlike confined systems, where diet composition and intake can be closely controlled and monitored, pasture-based systems present an inherently dynamic and selection-rich environment. This heterogeneity, both spatial and temporal, makes it difficult to determine the exact quantity and quality of forage and, consequently, the tannins consumed by each individual. Furthermore, in grazing systems, it is unrealistic to assume that all animals will voluntarily consume the optimal amount of tannins to meet nutritional or medicinal needs. Even the provision of tannins through supplementation can be difficult to manage, as it is influenced not only by individual animal factors but also by herd behavioral dynamics. Dominant individuals may monopolize access to tannin-enriched supplements, while subordinate animals may consume subtherapeutic or excessive amounts, reducing efficacy and complicating management strategies (Neave et al., 2018). Although precision livestock farming technologies, such as Radio Frequency Identification (RFID) sensors for individual animal detection and automated supplementation, hold promise for individualized monitoring, their application remains limited in extensive grazing systems due to cost, technical constraints, and infrastructure requirements (Tedeschi et al., 2021a). Even the use of simpler technologies can be challenging due to pedoclimatic conditions and landscape features; nevertheless, incorporating tannin-rich legumes, such as sainfoin, into pastures represents a promising strategy. In rough terrains or nutrient-poor soils, establishing and maintaining these legumes can be technically and economically unfeasible (Frame et al., 1997). Addressing these gaps will require region-specific research and interdisciplinary collaboration to generate the data necessary for refining models and validating their predictions (Tedeschi et al., 2014; Tedeschi and Fox, 2020a, 2020b).
Figure 2.
Images illustrating a native grassland in southern Brazil, source of tannin and the primary forage resource for sheep and cattle in this region. In image A, the high floristic diversity characteristic of these grasslands is evident. Image B provides a detailed view of one of the main legumes found in these systems (Desmodium incanum, indicated by arrow), which, according to studies conducted by our laboratory, can contain more than 6% condensed tannins per kilogram of dry matter. The final image (C) shows a sheep grazing in a native pasture, equipped with a fecal collection bag used to estimate intake. Source: Authors.
Conclusions
Tannins are a diverse group of phenolic compounds characterized by complex structures, high reactivity ranges, and variable concentrations in plants, resulting in a wide range of potential biological activities. These activities span from anti-nutritional and toxic effects to medicinal and prophylactic benefits, influencing their role in animal nutrition, health, and environmental sustainability. Their chemical complexity and interactions with multiple molecules in plant tissues and livestock feeding behaviors also underlie their selective impacts on microbial growth and rumen fermentation profiles. Consequently, tannin research in ruminant ecosystems necessitates a targeted, case-by-case approach to optimize their benefits for livestock production and environmental sustainability while mitigating potential adverse effects.
Acknowledgments
We gratefully acknowledge the partial financial support provided by the United States Department of Agriculture—Foreign Agricultural Service (USDA/FAS) through award #FX21SX-10620R005, which supported Dr. Cesar Poli’s exchange program at Texas A&M University from April to July 2023, as well as the reciprocal visit of Drs. James Muir and Luis Tedeschi in Porto Alegre, Brazil, in November 2023. The authors also thank the National Council for Scientific and Technological Development of Brazil (CNPq) and the Coordination for the Improvement of Higher Education Personnel of Brazil (CAPES) for the research funding. The authors, Cesar Henrique Espírito Candal Poli and Jalise Fabíola Tontini, are members of the SISPEC (Intelligent and Sustainable Livestock Systems Network) funded by CYTED (ref. 125RT0167). This manuscript was invited for submission by the American Society of Animal Science. The views expressed in this publication are those of the author(s) and do not necessarily reflect the views or policies of the American Society of Animal Science, the journal, or the publisher.
Contributor Information
Cesar H E C Poli, Department of Animal Science, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Jalise F Tontini, Department of Animal Science, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Luiza R Jacondino, Department of Animal Science, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Juan J Villalba, Department of Wildland Resources, Utah State University, Logan, UT.
James P Muir, Texas A&M AgriLife Research, Stephenville, TX.
Luis O Tedeschi, Department of Animal Science, Texas A&M University, College Station, TX.
About the Authors

Cesar Henrique Espírito Candal Poli is a Full Professor in the Department of Animal Science at the Federal University of Rio Grande do Sul (UFRGS), Brazil. He holds a PhD in Animal Science from Massey University, New Zealand, where he studied the relationship between pasture characteristics and foraging behavior in grazing ruminants. He has completed postdoctoral fellowships in the United States, France, and Scotland, focusing on topics such as plant secondary compounds and meat traceability. His research focuses on pasture management, native and cultivated grasslands, ruminant nutrition, and meat quality. Dr. Poli coordinates interinstitutional research projects and leads the Center for Teaching and Research in Sheep Production (CEPOV) at UFRGS. He also contributes to regional and international initiatives related to sustainable livestock systems and small ruminant production. Dr. Poli is a member of the SISPEC (Intelligent and Sustainable Livestock Systems Network) funded by CYTED (ref. 125RT0167).

Jalise Fabíola Tontini is a postdoctoral researcher in Animal Science at the Federal University of Rio Grande do Sul (UFRGS), Brazil. She holds a PhD in Animal Science from UFRGS, with a research internship at Texas A&M University—AgriLife Research. With over 12 years of experience in ruminant production and nutrition, her work focuses on sustainable livestock systems in the Pampa biome. Her research includes improving meat quality through native pasture management, reducing methane emissions through the use of plant secondary compounds and nutritional strategies, and enhancing forage planning using cultivated pastures and feedlot finishing. She is currently a CNPq-funded postdoctoral fellow leading a project on the role of native pasture compounds in improving meat quality and reducing the environmental impact of livestock production. Dr. Tontini is a member of the SISPEC (Intelligent and Sustainable Livestock Systems Network) funded by CYTED (ref. 125RT0167).

Luiza Rodegheri Jacondino is a veterinarian with a background in large animal clinical practice and a PhD in Animal Science from the Federal University of Rio Grande do Sul (UFRGS), Brazil. She completed her veterinary degree at UFRGS and a residency in large animal medicine with an emphasis on ruminant care at the Federal University of Santa Maria (UFSM). Her graduate research focused on ruminant production systems and nutrition, with a particular emphasis on sheep production. As part of her doctoral studies, she participated in the CAPES-PrInt internationalization program, conducting research at Texas A&M University—AgriLife Research and Extension Center in Stephenville, Texas. Her research interests include sustainable sheep production, animal nutrition, and health management in pasture-based systems.

Juan J. Villalba is a Professor in the Department of Wildland Resources and Associate Dean for Research and Graduate Programs in the S.J. & Jessie E. Quinney College of Natural Resources at Utah State University, USA. He leads the USDA-NIFA Sustainable Agricultural Systems Multi-State Program, “Using Smart Foodscapes to Enhance the Sustainability of Western Rangelands.” With more than 35 years of experience in ruminant production systems, Dr. Villalba’s research focuses on the behavioral and physiological mechanisms underlying food selection and intake in herbivores. His work seeks to develop innovative and ecologically sound strategies for managing grazing in rangelands and pasturelands that enhance productivity, sustainability, and animal welfare. Dr. Villalba’s research integrates knowledge from animal behavior, plant–herbivore interactions, and environmental physiology to address challenges in livestock systems. His internationally recognized program has informed practices in sustainable grazing management and rangeland restoration, and his lab has mentored students and visiting scientists from across the globe.

James Pierre Muir is a professor and researcher at the Texas A&M AgriLife Research and Extension Center and teaches graduate courses at Tarleton State University in Stephenville, Texas, USA. His research focuses on grassland ecology, particularly at the plant–animal interface, with emphasis on legumes. His work includes grassland restoration, the domestication of native legumes, and the role of condensed tannins in ruminant systems. Dr. Muir is actively involved in research promoting sustainable forage and rangeland management. He also contributes to advancing knowledge on ecosystem services and the development of pasture-based livestock systems.

Luis Orlindo Tedeschi is a globally recognized expert in ruminant nutrition and systems modeling, currently serving as a Professor of Animal Science at Texas A&M University. His pioneering work integrates mechanistic nutrition models with artificial intelligence and machine learning to optimize livestock production, nutrient utilization, and environmental sustainability. He is the developer of several influential models, including the Ruminant Nutrition System (RNS), Large and Small Ruminant Nutrition Systems (LRNS, SRNS), and the Cattle Value Discovery System, widely adopted in academia and industry across the globe. Dr. Tedeschi served as the lead modeler and coauthor of the National Academies of Sciences, Engineering, and Medicine’s Nutrient Requirements of Beef Cattle (8th Revised Edition), a comprehensive update that reflects advances in ruminant nutrition and modeling. His research portfolio spans feed evaluation, methane mitigation, microbial protein synthesis, and precision livestock farming (PLF), with a recent focus on integrating hybrid mechanistic–machine learning models, synthetic data generation, and quantum computing for predictive livestock nutrition. He currently chairs both the Nutrition Modeling Committee and the Climate Smart Feed Management Committee of the National Animal Nutrition Program, where he leads national efforts to enhance modeling capacity and develop decision-support tools for sustainable animal production. Recognized for his scientific leadership and innovation, Dr. Tedeschi is helping redefine the role of digital and predictive tools in livestock production, bridging the gap between traditional nutrition science and the future of sustainable, precision animal agriculture.
Author Contributions
Cesar Poli (Conceptualization, Funding acquisition, Supervision, Writing—original draft, Writing—review & editing), Jalise Tontini (Conceptualization, Writing—original draft, Writing—review & editing), Luiza Jacondino (Writing—original draft, Writing—review & editing), Juan Villalba (Conceptualization, Writing—original draft, Writing—review & editing), James Muir (Conceptualization, Writing—original draft, Writing—review & editing), and Luis Orlindo Tedeschi (Conceptualization, Writing—original draft, Writing—review & editing)
Conflict of interest
The authors declare no real or perceived conflicts of interest.
References
- Acero, A., Muir J.P., and Wolfe R.M... 2010. Nutritional composition and CT concentration changes as leaves become litter in nine trees and vines. J. Sci. Food Agric. 90(15):2582–2585. doi: https://doi.org/ 10.1002/jsfa.4124 [DOI] [PubMed] [Google Scholar]
- Bailey, D.W., and Provenza F.D... 2008. Mechanisms determining large-herbivore distribution. In: Prins, H. H. T., and Van Langevelde F., editors. Resource ecology. Wageningen UR Frontis Series. Vol. 23. Dordrecht: Springer; p. 7–28. doi: https://doi.org/ 10.1007/978-1-4020-6850-8_2 [DOI] [Google Scholar]
- Barry, T.N., and McNabb W.C... 1999. The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. Brit. J. Nutr. 81:263–272. doi: https://doi.org/ 10.1017/S0007114599000501 [DOI] [PubMed] [Google Scholar]
- Beauchemin, K.A., McAllister T.A., and McGinn S.M... 2009. Dietary mitigation of enteric methane from cattle. CABI Rev. 1–18. doi: https://doi.org/ 10.1079/pavsnnr20094035 [DOI] [Google Scholar]
- Berça, A.S., Tedeschi L.O., da Silva Cardoso A., and Reis R.A... 2023. Meta-analysis of the relationship between dietary condensed tannins and methane emissions by cattle. Anim. Feed Sci. Technol. 298:115564. doi: https://doi.org/ 10.1016/j.anifeedsci.2022.115564 [DOI] [Google Scholar]
- Besharati, M., Maggiolino A., Palangi V., Kaya A., Jabbar M., Eseceli H., De Palo P., and Lorenzo J.M... 2022. Tannin in ruminant nutrition: review. Molecules 27(23):8273. doi: https://doi.org/ 10.3390/molecules27238273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boze, H., Marlin T., Durand D., Pérez J., Vernhet A., Canon F., Sarni-Manchado P., Cheynier V., and Cabane B... 2010. Proline-rich salivary proteins have extended conformations. Biophys. J. 99(2):656–665. doi: https://doi.org/ 10.1016/j.bpj.2010.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, J.M., Miller J.E., Terrill T.H., Orlik S.T., Garza J.J., Acharya M., and Wood E.L... 2023. Case study: Sericea lespedeza leaf meal fed to sheep and goats reduces serum concentrations of trace minerals. Sheep Goat Res. J. 38:1–10. [Google Scholar]
- Clifford, M.N., and Scalbert A... 2000. Ellagitannins—nature, occurrence and dietary burden. J. Sci. Food Agricul. 80:1118–1125. doi: https://doi.org/ [DOI] [Google Scholar]
- Cooper, C.M., Naumann H.D., Lambert B.D., Kattes D., and Muir J.P... 2014. Legume protein precipitable phenolic and nutrient concentrations responses to defoliation and ontogeny. J. Plant Interactions 9:468–477. doi: https://doi.org/ 10.1080/17429145.2013.860561 [DOI] [Google Scholar]
- Copani, G., Hall J.O., Miller J., Priolo A., and Villalba J.J... 2013. Plant secondary compounds as complementary resources: are they always complementary? Oecologia 172(4):1041–1049. doi: https://doi.org/ 10.1007/s00442-012-2551-1 [DOI] [PubMed] [Google Scholar]
- Costes-Thiré, M., Villalba J.J., Hoste H., and Ginane C... 2018. Increased intake and preference for tannin-rich sainfoin (Onobrychis viciifolia) pellets by both parasitized and non-parasitized lambs after a period of conditioning. Appl. Anim. Behav. Sci. 203:11–18. doi: https://doi.org/ 10.1016/j.applanim.2018.02.015 [DOI] [Google Scholar]
- da Silva Aguiar, F., Bezerra L.R., Cordão M.A., Cavalcante I.T.R., de Oliveira J.P.F., do Nascimento R.R., de Souza B.B., Oliveira R.L., Pereira E.S., and Filho J.M.P... 2023. Effects of increasing levels of total tannins on intake, digestibility, and balance of nitrogen, water, and energy in hair lambs. Animals 13(15):2497. doi: https://doi.org/ 10.3390/ani13152497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghanian, Z., Habibi K., Dehghanian M., Aliyar S., Lajayer B.A., Astatkie T., Minkina T., and Keswani C... 2022. Reinforcing the bulwark: unravelling the efficient applications of plant phenolics and tannins against environmental stresses. Heliyon 8:e09094. doi: https://doi.org/ 10.1016/j.heliyon.2022.e09094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delimont, N.M., Haub M.D., and Lindshield B.L... 2017. The impact of tannin consumption on iron bioavailability and status: a narrative review. Curr. Dev. Nutr. 1(2):1–12. doi: https://doi.org/ 10.3945/cdn.116.000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel, R.A., and Villalba J.J... 2018. Use of unpalatable forages by ruminants: the influence of experience with the biophysical and social environment. Animals 8(4):56. doi: https://doi.org/ 10.3390/ani8040056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcão, L., and Araújo M.E.M... 2018. Vegetable tannins used in the manufacture of historic leathers. Molecules 23(5):1081. doi: https://doi.org/ 10.3390/molecules23051081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame J., Charlton J.F.L., and Laidlaw A.S... 1997. Temperate forage legumes. CABI; Publishing. ISBN: 9780851992143 [Google Scholar]
- Freeland, W.J., Calcott P.H., and Anderson L.R... 1985. Tannins and saponin: interaction in herbivore diets. Biochem. Syst. Ecol. 13(2):189–193. doi: https://doi.org/ 10.1016/0305-1978(85)90078-x [DOI] [Google Scholar]
- Frutos, P., Hervas G., Giráldez F.J., and Mantecón A.R... 2004. Review. Tannins and ruminant nutrition. Span. J. Agric. Res. 2:191–202. doi: https://doi.org/ 10.5424/sjar/2004022-73 [DOI] [Google Scholar]
- Garcia, J. 1989. Food for Tolman: cognition and cathexis in concert. In: Archer, T. and Nilsson L., editors. Aversion, avoidance and anxiety. Hillsdale (NJ): Earlbaum; p. 45–85. [Google Scholar]
- Gershon, M.D. 2004. Review article: Serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 20(Suppl 7):3–14. doi: https://doi.org/ 10.1111/j.1365-2036.2004.02180.x [DOI] [PubMed] [Google Scholar]
- Gessner, D.K., Ringseis R., and Eder K... 2017. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. (Berl) 101(4):605–628. doi: https://doi.org/ 10.1111/jpn.12579 [DOI] [PubMed] [Google Scholar]
- Glendinning, J.I. 1994. Is the bitter rejection response always adaptive? Physiol. Behav. 56(6):1217–1227. doi: https://doi.org/ 10.1016/0031-9384(94)90369-7 [DOI] [PubMed] [Google Scholar]
- Hagerman, A.E., Robbins C.T., Weerasuriya Y., Wilson T.C., and McArthur C... 1992. Tannin chemistry in relation to digestion. J. Range Manag. 45(1):57–62. doi: https://doi.org/ 10.2307/4002526 [DOI] [Google Scholar]
- Hanlin, R.L., Hrmova M., Harbertson J.F., and Downey M.O... 2010. Review: condensed tannin and grape cell wall interactions and their impact on tannin extractability into wine. Aust. J. Grape Wine Res. 16(1):173–188. doi: https://doi.org/ 10.1111/j.1755-0238.2009.00068.x [DOI] [Google Scholar]
- Harding, S.A. 2019. CTs: arbiters of abiotic stress tolerance? Tree Physiol. 39(3):341–344. doi: https://doi.org/ 10.1093/treephys/tpz005 [DOI] [PubMed] [Google Scholar]
- Häring, D.A., Huber M.J., Suter D., Edwards P.J., and Lüscher A... 2008. Plant enemy-derived elicitors increase the foliar tannin concentration of Onobrychis viciifolia without a trade-off to growth. Ann. Bot. (Lond.) 102(6):979–987. doi: https://doi.org/ 10.1093/aob/mcn189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke, A., Dickhoefer U., Westreicher-Kristen E., Knappstein K., Molkentin J., Hasler M., and Susenbeth A... 2017. Effect of dietary Quebracho tannin extract on feed intake, digestibility, excretion of urinary purine derivatives and milk production in dairy cows. Arch. Anim. Nutr. 71(1):37–53. doi: https://doi.org/ 10.1080/1745039X.2016.1250541 [DOI] [PubMed] [Google Scholar]
- Hervás, G., Frutos P., Giráldez F.J., Mantecón R., and Del Pino M.C... 2003. Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Anim. Feed Sci. Technol. 109:65–78. doi: https://doi.org/ 10.1016/S0377-8401(03)00208-6 [DOI] [Google Scholar]
- Hoste, H., Torres-Acosta J.F.J., Sandoval-Castro C.A., Mueller-Harvey I., Sotiraki S., Louvandini H., Thamsborg S.M., and Terrill T.H... 2015. Tannin containing legumes as a model for nutraceuticals against digestive parasites in livestock. Vet. Parasitol. 212(1-2):5–17. doi: https://doi.org/ 10.1016/j.vetpar.2015.06.026 [DOI] [PubMed] [Google Scholar]
- Huang, R., and Xu C... 2021. An overview of the perception and mitigation of astringency associated with phenolic compounds. Compr. Rev. Food Sci. Food Saf. 20(1):1036–1074. doi: https://doi.org/ 10.1111/1541-4337.12679 [DOI] [PubMed] [Google Scholar]
- Iqbal, N., and Poor P... 2024. Plant protection by tannins depends on defence-related phytohormoes. J. Plant Growth Regul. 44(1):22–39. doi: https://doi.org/ 10.1007/s00344-024-11291-1 [DOI] [Google Scholar]
- Jayanegara, A., Leiber F., and Kreuzer M... 2012. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. (Berl) 96(3):365–375. doi: https://doi.org/ 10.1111/j.1439-0396.2011.01172.x [DOI] [PubMed] [Google Scholar]
- Jones, W.T., and Mangan J.L... 1977. Complexes of the CTs of sainfoin (Onobrychis viciifolia Scop.) with fraction 1 leaf protein and with submaxillary mucoprotein, and their reversal by polyethylene glycol and pH. J. Sci. Food Agr. 28:126–136. doi: https://doi.org/ 10.1002/jsfa.2740280204 [DOI] [Google Scholar]
- Juhnke, J., Miller J., Hall J.O., Provenza F.D., and Villalba J.J... 2012. Preference for CTs by sheep in response to challenge infection with Haemonchus contortus. Vet. Parasitol. 188(1–2):104–114. doi: https://doi.org/ 10.1016/j.vetpar.2012.02.015 [DOI] [PubMed] [Google Scholar]
- Kaufman, R.C., Herald T.J., Bean S.R., Wilson J.D., and Tuinstra M.R... 2013. Variability in tannin content, chemistry and activity in a diverse group of tannin containing sorghum cultivars. J. Sci. Food Agric. 93(5):1233–1241. doi: https://doi.org/ 10.1002/jsfa.5890 [DOI] [PubMed] [Google Scholar]
- Krumholz, L.R., and Bryant M... 1986. Eubacterium oxidoreducens sp. nov. requiring H2 or formate to degrade gallate, pyrogallol, phloroglucinol and quercetin. Arch. Microbiol. 144:8–14. doi: https://doi.org/ 10.1007/BF00454948 [DOI] [Google Scholar]
- Kumar, R., and Singh M... 1984. Tannins, their adverse role in ruminant nutrition. J. Agric. Food Chem. 32(3):447–453. doi: https://doi.org/ 10.1021/jf00123a006 [DOI] [Google Scholar]
- Landau, S., Silanikove N., Nitsan Z., Barkai D., Baram H., Provenza F.D., and Perevolotsky A... 2000. Short-term changes in eating patterns explain the effects of CTs on feed intake in heifers. Appl. Anim. Behav. Sci. 69(3):199–213. doi: https://doi.org/ 10.1016/s0168-1591(00)00125-8 [DOI] [PubMed] [Google Scholar]
- Lawler, I.R., Foley W.J., Pass G.J., and Eschler B.M... 1998. Administration of a 5HT3 receptor antagonist increases the intake of diets containing Eucalyptus secondary metabolites by marsupials. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 168(8):611–618. doi: https://doi.org/ 10.1007/s003600050183 [DOI] [PubMed] [Google Scholar]
- Lisonbee, L.D., Villalba J.J., and Provenza F.D... 2009a. Effects of tannins on selection by sheep of forages containing alkaloids, saponins, and tannins. J. Sci. Food Agric. 89(15):2668–2677. doi: https://doi.org/ 10.1002/jsfa.3772 [DOI] [Google Scholar]
- Lisonbee, L.D., Villalba J.J., Provenza F.D., and Hall J.O... 2009b. Tannins and self-medication: Implications for sustainable parasite control in herbivores. Behav. Process. 82(2):184–189. doi: https://doi.org/ 10.1016/j.beproc.2009.06.009 [DOI] [PubMed] [Google Scholar]
- Lotfi, R. 2010. Biological studies on the effect of Tanninferous feed on the Ruminal Microorganisms of Taleshi Sheep. Iran: Department of Animal Science, Tarbiat Modares University. [Google Scholar]
- Lotfi, R. 2020. A commentary on methodological aspects of hydrolysable tannins metabolism in ruminant: a perspective view. Lett. Appl. Microbiol. 71(5):466–478. doi: https://doi.org/ 10.1111/lam.13346 [DOI] [PubMed] [Google Scholar]
- Makkar, H.P.S., and Becker K... 1998. Adaptation of cattle to tannins: role of proline-rich proteins in oak-fed cattle. Anim. Sci. 67(2):277–281. doi: https://doi.org/ 10.1017/s1357729800010031 [DOI] [Google Scholar]
- Min, B.R., Barry T.N., Attwood G.T., and McNabb W.C... 2003. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Anim. Feed Sci. Technol. 106(1-4):3–19. doi: https://doi.org/ 10.1016/s0377-8401(03)00041-5 [DOI] [Google Scholar]
- Molino, S., Lerma-Aguilera A., Jiménez-Hernández N., Rufián Henares J., and Francino M.P... 2022. Evaluation of the effects of a short supplementation with tannins on the gut microbiota of healthy subjects. Front. Microbiol. 13:848611. doi: https://doi.org/ 10.3389/fmicb.2022.848611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar, M., Jakovljević Kovač M., and Pavić V... 2024. A comprehensive analysis of diversity, structure, biosynthesis and extraction of biologically active tannins from various plant-based materials using deep eutectic solvents. Molecules 29(11):2615. doi: https://doi.org/ 10.3390/molecules29112615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro, J.M., Albuquerque U.P.D., Araújo E.D.L., and Amorim E.L.C.D... 2005. Taninos: uma abordagem da química à ecologia. Quím. Nova 28:892–896. doi: https://doi.org/ 10.1590/S0100-40422005000500029 [DOI] [Google Scholar]
- Mora, J., Pott D.M., Osorio S., and Vallarino J.G... 2022. Regulation of plant of tannin synthesis in crop species. Fornt. Genet 13:870976. doi: https://doi.org/ 10.3389/fgene.2022.870976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Harvey, I., Bee G., Dohme-Meier F., Hoste H., Karonen M., Kölliker R., Lüscher A., Niderkorn V., Pellikaan W.F., Salminen J.-P.,. et al. 2019. Benefits of condensed tannins in forage legumes fed to ruminants: importance of structure, concentration, and diet composition. Crop Sci. 59(3):861–885. doi: https://doi.org/ 10.2135/cropsci2017.06.0369 [DOI] [Google Scholar]
- Muir, J.P., Bow J.R., Rodriguez W., and Patterson J.M... 2008. Defoliation of Panicled tick-clover, Tweedy’s tick-clover and Tall bush-clover: II. Herbage nutritive value and CT concentrations. Agron. J. 100(6):1635–1639. doi: https://doi.org/ 10.2134/agronj2008.0094 [DOI] [Google Scholar]
- Naumann, H.D., Hagerman A.E., Lambert B.D., Muir J.P., Tedeschi L.O., and Kothmann M.M... 2014. Molecular weight and protein-precipitating ability of CTs from warm-season perennial legumes. J. Plant Interact. 9:212–219. doi: https://doi.org/ 10.1080/17429145.2013.811547 [DOI] [Google Scholar]
- Naumann, H.D., Tedeschi L.O., Zeller W.E., and Huntley N.F... 2017. The role of condensed tannins in ruminant animal production: advances, limitations and future directions. Rev. Bras. Zootec. 46(12):929–949. doi: https://doi.org/ 10.1590/s1806-92902017001200009 [DOI] [Google Scholar]
- Neave, H.W., Weary D.M., and von Keyserlingk M. A. G... 2018. Review: individual variability in feeding behaviour of domesticated ruminants. Animal 12:s419–s430. doi: https://doi.org/ 10.1017/S1751731118001325 [DOI] [PubMed] [Google Scholar]
- Norris, A.B., Miller M.S., Muir J.P., Harp R.M., Kinman L.A., and Cherry N... 2023. Winter dynamics of white-tailed deer browse nutritive value in the southern cross timbers and prairies of Texas. Southwestern Nat. 67:27–38. doi: https://doi.org/ 10.1894/0038-4909-67.1.27 [DOI] [Google Scholar]
- Orzuna-Orzuna, J.F., Dorantes-Iturbide G., Lara-Bueno A., Mendoza-Martínez G.D., Miranda-Romero L.A., and Lee-Rangel H.A... 2021. Growth performance, meat quality and antioxidant status of sheep supplemented with tannins: a meta-analysis. Animals 11(11):3184. doi: https://doi.org/ 10.3390/ani11113184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra, A.K., and Saxena J... 2011. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 91(1):24–37. doi: https://doi.org/ 10.1002/jsfa.4152 [DOI] [PubMed] [Google Scholar]
- Peleg, H., Gacon K., Schlich P., and Noble A.C... 1999. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 79:1123–1128. doi:10.1002/(SICI)1097-0010(199906)79:8<1123::AID-JSFA336>3.0.CO;2-D [Google Scholar]
- Piluzza, G., Sulas L., and Bullitta S... 2014. Tannins in forage plants and their role in animal husbandry and environmental sustainability: a review. Grass Forage Sci. 69(1):32–48. doi: https://doi.org/ 10.1111/gfs.12053 [DOI] [Google Scholar]
- Poli, C.H.E.C., Thornton-Kurth K.J., Legako J.F., Bremm C., Hampel V.S., Hall J., Ipharraguerre I.R., and Villalba J.J... 2018. Self-selection of plant bioactive compounds by sheep in response to challenge infection with Haemonchus contortus. Physiol. Behav. 194:302–310. doi: https://doi.org/ 10.1016/j.physbeh.2018.06.013 [DOI] [PubMed] [Google Scholar]
- Pott, D.M., Osorio S., and Vallarino J.G... 2019. From central to specialized metabolism: an overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front. Plant Sci. 10:835. doi: https://doi.org/ 10.3389/fpls.2019.00835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenza, F.D. 1995. Postingestive feedback as an elementary determinant of food preference and intake in ruminants. J. Range Manag. 48(1):2–17. doi: https://doi.org/ 10.2307/4002498 [DOI] [Google Scholar]
- Provenza, F.D. 1996. Acquired aversions as the basis for varied diets of ruminants foraging on rangelands. J. Anim. Sci. 74(8):2010–2020. doi: https://doi.org/ 10.2527/1996.7482010x [DOI] [PubMed] [Google Scholar]
- Provenza, F.D., Burritt E.A., Clausen T.P., Bryant J.P., Reichardt P.B., and Distel R.A... 1990. Conditioned flavor aversion: a mechanism for goats to avoid CTs in blackbrush. Am. Nat. 136(6):810–828. doi: https://doi.org/ 10.1086/285133 [DOI] [Google Scholar]
- Reed, J.D. 1995. Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Anim. Sci. 73(5):1516–1528. doi: https://doi.org/ 10.2527/1995.7351516x [DOI] [PubMed] [Google Scholar]
- Rogosic, J., Estell R.E., Skobic D., Martinovic A., and Maric S... 2006. Role of species diversity and secondary compound complementarity on diet selection of Mediterranean shrubs by goats. J. Chem. Ecol. 32(6):1279–1287. doi: https://doi.org/ 10.1007/s10886-006-9084-1 [DOI] [PubMed] [Google Scholar]
- Salminen, J.-P., and Karonen M... 2011. Chemical ecology of tannins and other phenolics: we need a change in approach. Funct. Ecol. 25(2):325–338. doi: https://doi.org/ 10.1111/j.1365-2435.2010.01826.x [DOI] [Google Scholar]
- Salminen, J.-P., Karonen M., and Sinkkonen J... 2011. Chemical ecology of tannins: recent developments in tannin chemistry reveal new structures and structure–activity patterns. Chemistry 17(10):2806–2816. doi: https://doi.org/ 10.1002/chem.201002662 [DOI] [PubMed] [Google Scholar]
- Silanikove, N., Gilboa N., and Nitsan Z... 1997. Interactions among tannins, supplementation and polyethyleneglycol in goats given oak leaves: effects on digestion and food intake. Anim. Sci. 64(3):479–483. doi: https://doi.org/ 10.1017/s135772980001609x [DOI] [Google Scholar]
- Silanikove, N., Perevolotsky A., and Provenza F.D... 2001. Use of tannin-binding chemicals to assay for tannins and their negative postingestive effects in ruminants. Anim. Feed Sci. Technol. 91(1–2):69–81. doi: https://doi.org/ 10.1016/s0377-8401(01)00234-6 [DOI] [Google Scholar]
- Skovmand, L., O’Dea R.E., Greig K.A., Amato K.R., and Hendry A.P... 2023. Effects of leaf herbivory and autumn seasonality on plant secondary metabolites: a meta-analysis. Ecol. Evol. 14:e10912. doi: https://doi.org/ 10.1002/ece3.10912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A.H., Zoetendal E., and Mackie R.I... 2005. Bacterial mechanisms to overcome inhibitory effects of dietary tannins. Microb. Ecol. 50(2):197–205. doi: https://doi.org/ 10.1007/s00248-004-0180-x [DOI] [PubMed] [Google Scholar]
- Soares, S., Brandão E., Guerreiro C., Soares S., Mateus N., and De Freitas V... 2020. Tannins in food: Insights into the molecular perception of astringency and bitter taste. Molecules 25(11):2590. doi: https://doi.org/ 10.3390/molecules25112590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi, L.O., and Fox D.G... 2020a. The Ruminant nutrition system: An applied model for predicting nutrient requirements and feed utilization in Ruminants. Vol. 1. (3rd ed.) (3rd repr.). Dubuque (IA): Kendall Hunt. First published in 2020 by XanEdu, Ann Arbor, MI. [accessed February 7, 2025]. https://he.kendallhunt.com/product/ruminant-nutrition-system-vol-1. [Google Scholar]
- Tedeschi, L. O., and Fox D. G... 2020b. The ruminant nutrition system: Tables of equations and coding. Vol. 2. (3rd repr.). Dubuque (IA): Kendall Hunt. First published in 2020 by XanEdu, Ann Arbor, MI. [accessed February 7, 2025]. https://he.kendallhunt.com/product/ruminant-nutrition-system-vol-2. [Google Scholar]
- Tedeschi, L.O., Greenwood P.L., and Halachmi I... 2021a. Advancements in sensor technology and decision support intelligent tools to assist smart livestock farming. J. Anim. Sci. 99(2):1–11. doi: https://doi.org/ 10.1093/jas/skab038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi, L.O., Muir J.P., Naumann H.D., Norris A.B., Ramírez-Restrepo C.A., and Mertens-Talcott S.U... 2021b. Nutritional aspects of ecologically relevant phytochemicals in ruminant production. Front. Vet. Sci. 8:1–24. doi: https://doi.org/ 10.3389/fvets.2021.628445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi, L.O., Ramírez-Restrepo C.A., and Muir J.P... 2014. Developing a conceptual model of possible benefits of condensed tannins for ruminant production. Animal 8(7):1095–1105. doi: https://doi.org/ 10.1017/S1751731114000974 [DOI] [PubMed] [Google Scholar]
- Thorhallsdottir, A.G., Provenza F.D., and Balph D.F... 1990. Ability of lambs to learn about novel foods while observing or participating with social models. Appl. Anim. Behav. Sci. 25(1-2):25–33. doi: https://doi.org/ 10.1016/0168-1591(90)90066-m [DOI] [Google Scholar]
- Tong, Z., He W., Fan X., and Guo A... 2022. Biological function of plant tannin and its application in animal health. Front. Vet. Sci. 8:803657. doi: https://doi.org/ 10.3389/fvets.2021.803657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontini, J.F., Poli C.H.E.C., Hampel V.S., Minho A.P., and Muir J.P... 2019. Nutritional values and chemical composition of tropical pastures as potential sources of α-tocopherol and condensed tannin. Afr. J. Range For. Sci. 36:181–189. doi: https://doi.org/ 10.2989/10220119.2019.1679883 [DOI] [Google Scholar]
- Van Vliet, S., Blair A.D., Hite L.M., Cloward J., Ward R.E., Kruse C., van Wietmarchsen H.A., van Eekeren N., Kronberg S.L., and Provenza F.D... 2023. Pasture-finishing of bison improves animal metabolic health and potential health-promoting compounds in meat. J. Anim. Sci. Biotechnol. 14(1):49. doi: https://doi.org/ 10.1186/s40104-023-00843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Magaña, J.J., Aguilar-Caballero A.J., Torres-Acosta J.F.J., Sandoval-Castro C.A., Hoste H., and Capetillo-Leal C.M... 2013. Tropical tannin-rich fodder intake modifies saliva-binding capacity in growing sheep. Animal 7(12):1921–1924. doi: https://doi.org/ 10.1017/S1751731113001651 [DOI] [PubMed] [Google Scholar]
- Villalba, J.J., Provenza F.D., Hall J.O., and Lisonbee L.D... 2010. Selection of tannins by sheep in response to gastrointestinal nematode infection. J. Anim. Sci. 88(6):2189–2198. doi: https://doi.org/ 10.2527/jas.2009-2272 [DOI] [PubMed] [Google Scholar]
- Villalba, J.J., Ramsey R.D., and Athanasiadou S... 2024. Herbivory and the power of phytochemical diversity on animal health. Animal. 101287. doi: https://doi.org/ 10.1016/j.animal.2024.101287 [DOI] [PubMed] [Google Scholar]
- Waghorn, G. 2008. Beneficial and detrimental effects of dietary CTs for sustainable sheep and goat production—progress and challenges. Anim. Feed Sci. Technol. 147(1-3):116–139. doi: https://doi.org/ 10.1016/j.anifeedsci.2007.09.013 [DOI] [Google Scholar]
- Waghorn, G.C., Shelton I.D., and Mc Nabb W.C... 1994. Effects of CTs in Lotus pedunculatus on its nutritive value for sheep: I. Non-nitrogenous aspects. J. Agric. Sci. 123:99–107. doi: https://doi.org/ 10.1017/S0021859600067824 [DOI] [Google Scholar]
- Wang, M., Chen T., Wang Q., and Shi Y... 2023. Antioxidant, bacteriostatic and preservative effects of extractable CTs isolated from Longan pericarps and seeds. Plants 12(3):512. doi: https://doi.org/ 10.3390/plants12030512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanza, Y.R., Fitri A., Suwignyo B., Hidayatik N., Kumalasari N.R., Irawan A., and Jayanegara A... 2021. The utilisation of tannin extract as a dietary additive in ruminant nutrition: a meta-analysis. Animal 11:3317. doi: https://doi.org/ 10.3390/ani11113317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X., Huang H., Luo X., Wei Y., Du S., Yu J., Guo S., Chen K., and Chen L... 2023. CT accretions specifically distributed in mesophyll cells of non-salt secretor mangroves help in salt tolerance. Planta 258(5):100. doi: https://doi.org/ 10.1007/s00425-023-04254-5 [DOI] [PubMed] [Google Scholar]