Abstract
Purpose:
Although autistic adults have demonstrated weaker memory performance on lab-based measures in comparison to neurotypical peers, research on self-reported memory abilities in this group is scarce. Shedding light on memory abilities of autistic adults is important, as memory difficulties may impact daily living outcomes, an area of difficulty for autistic individuals. Thus, the current study aimed to characterize the self-reported memory complaints of a large sample of autistic adults in comparison to neurotypical adults.
Methods:
396 young autistic adults and 396 young neurotypical adults between the ages of 18 and 39 years and with similar background characteristics participated. Autistic adults were recruited from the Simons Powering Autism Research (SPARK) participant registry, and data from neurotypical adults were obtained from a large-scale internet study. The responses to the Prospective and Retrospective Memory Questionnaire were compared by diagnostic group, and within the autistic group, relations between memory complaints and real-world outcomes (activities of daily living [ADLs] and postsecondary employment and educational activities) were examined.
Results:
The results indicated that young autistic adults reported greater memory problems than their neurotypical peers, regardless of memory type, and autistic adults reported a comparable pattern of complaints to their neurotypical peers (i.e., greater prospective memory complaints in comparison to retrospective memory complaints). Additionally, we found a negative association between memory complaints and outcomes (i.e., greater memory complaints were associated with less independence in ADLs and less favorable postsecondary outcomes).
Conclusion:
The significant association between subjective memory complaints and real-world outcomes suggests that memory abilities are an important contributor to daily living skills and post-secondary outcomes among autistic adults and could represent a target for future intervention development.
Keywords: Autism, prospective memory, retrospective memory, activities of daily living
Introduction
Autism Spectrum Disorder (ASD), a neurodevelopmental condition characterized by social-communication challenges and the presence of restricted, repetitive behaviors (American Psychiatric Association, 2013), is associated with a range of cognitive strengths and difficulties (Charman et al., 2011; Takayanagi et al., 2022), including greater difficulties on some aspects of memory functioning relative to neurotypical (NT) populations (for review see Desaunay et al., 2020). Neuropsychological conceptualizations of memory indicate that it is a multicomponent system (Schacter & Tulving, 1994; Tulving, 1995) that can be divided into retrospective memory (RM) and prospective memory (PM) components. RM is the act of recalling previously learned information (Squire, 1987), whereas PM involves remembering to carry out a future action (Ceci et al., 1988).
Research findings of memory abilities of autistic adults without intellectual disability have been considerably variable and depend on the type of memory being assessed. Regarding RM, studies have suggested that performance of autistic individuals tends to be worse than NT peers when tasks are uncued or unstructured (Boucher et al., 2012; Gaigg et al., 2008; Landsiede et al., 2017); however, fewer difficulties are reported when recognition is probed. Consistent with this finding, Desaunay and colleagues’ (2020) meta-analysis of memory abilities of autistic children and adults (ages 8 to 50 years old) indicated that regardless of the task modality (i.e., verbal or nonverbal) or delay length (i.e., short or long delays), autistic individuals tend to have stronger recognition (i.e., cued) than recall (i.e., uncued) abilities (Desaunay et al., 2020). However, research examining only autistic adults has provided mixed findings of visual (Minshew & Goldstein, 2001; Grainger et al., 2014; Lever & Geurts, 2016; Tse et al., 2019; Torenviliet et al., 2022) and verbal recall (Williams et al., 2017; Minshew & Goldstein, 2001; Tse et al., 2019; Torenvliet et al., 2022). The general inconsistency of the literature reflects a greater need for further examination of RM abilities in this population, including replication studies. Moreover, many studies examining memory abilities in autistic adults have focused on middle to older adulthood (Lever & Geurts, 2016; Tse et al., 2019; Torenviliet et al., 2022) and less is known about memory abilities of autistic adults during young adulthood.
Research examining PM abilities of autistic adults without intellectual disability suggests that performance varies depending on the type of memory cue. Cues can be categorized as “event-based”, such as remembering to give a friend an item when you see them. Cues may also be considered “time-based”, such as remembering to take your medication at noon. These time-based cues are often less salient than event-based cues (Einstein & McDaniel, 1990). When performance-based PM tasks involve an event-based cue, some studies have shown that autistic adults have comparable performance to their NT peers (Altgassen et al., 2010; Altgassen & Koch, 2014; Williams et al., 2014). However, other studies have suggested that on naturalistic PM tasks that reflect real-world situations (versus lab-based performance tasks) with event-based cues, autistic adults show poorer performance than NT adults (Altgassen et al., 2012; Dehnavi & Khan, 2023; Kretschmer et al., 2014). On PM tasks with time-based cues, studies have indicated autistic adults have significantly weaker performance than NT adults (Altgassen et al., 2012; Dehnavi & Khan, 2023; Kretschmer et al., 2014).
Of note, research examining autistic adults has not compared RM abilities to PM abilities on performance-based tasks. However, one small study has examined self-reported memory problems of autistic adults (n=17) in comparison to age and IQ-matched NT adults (Williams et al., 2014) using the Prospective and Retrospective Memory Questionnaire (PRMQ; Crawford et al., 2003). The results indicated greater PM problems in comparison to RM problems within the autistic group and the autistic group had more memory complaints overall than the NT group (Williams et al., 2014).
Past research has suggested that the memory difficulties reported for autistic individuals are due to executive functioning weaknesses during encoding, rather than retrieval difficulties (Southwick et al., 2011). Executive functioning difficulties are a hallmark of the ASD cognitive profile and include difficulties in cognitive flexibility (Corbett et al., 2009; Wallace et al., 2016), working memory (Bennetto et al., 1996; McGonigle-Chalmers et al., 2008), inhibitory control (Corbett et al., 2009), as well as planning, organization, and integration (Geurts et al., 2004; Sinzig et al., 2008).
Working memory, or the ability to mentally manipulate, maintain, and track information from multiple sources, can impact the amount of information individuals can initially learn and cognitively store. List-learning studies have shown that while autistic adults remember the middle and ends of the lists as well as NT adults, they are more likely to forget information at the beginning of the list (Bowler et al., 2009). Additionally, poor inhibitory control can impact an individual’s ability to learn multiple pieces of information or large amounts of information at once. Research suggests that autistic adults can learn and recall one word list as well as NT adults yet struggle to learn a second list of words immediately after learning the first list (Bowler et al., 2010). Therefore, autistic adults may find it difficult to inhibit the previously learned information in order to learn the new information. In addition, autistic adults may find it challenging to mentally plan and organize information to aid their learning and recall in comparison to NT adults. In particular, autistic adults are less likely than NT adults to utilize strategies of sorting and clustering information to support verbal learning and recall (Bowler et al., 1997; Bowler et al., 2008). Autistic adults also demonstrate greater recall when provided with cued recall formats, such as multiple choice or yes/no choices, in comparison to free recall formats (Bowler et al., 2004; Bowler et al., 2009; Bowler et al., 2015), suggesting that they may benefit from being provided with structure to enhance memory function.
Moreover, executive functioning difficulties of this population include poor integration of information, as autistic individuals are more likely to process information using a discrete, detail-oriented approach (Happé & Frith, 2006; Stevenson et al., 2014; Roberts & Baron-Cohen, 2017). Research has shown that autistic adults recall discrete pieces of information as well as their cognitively matched and chronological age matched NT peers (Minshew & Goldstein, 2001; Bowler et al., 2009; Geurts & Vissers, 2012). However, the discrete processing approach can negatively impact learning and recall of categorically related information and episodic information for autistic adults. In fact, in comparison to their NT peers, autistic adults are more likely to have poorer recall of items presented within a visual scene (Cooper et al., 2015; Ring et al., 2015) and verbal information presented in an episodic story format (Minshew & Goldstein, 2001). Additionally, autistic adults do not use relational, or categorical memory aids as effectively as their NT peers to recall associated items (Bowler et al., 2008; Gaigg et al., 2008; Ring et al., 2016). In summary, autistic adults demonstrate a relative weakness when recalling contextual information, whereas their discrete processing approach likely aids their recall of rote information.
Memory and Real-World Outcomes
It is important to understand the memory abilities of autistic adults, as memory abilities may impact independence in young adulthood. In fact, memory abilities are significantly associated with independence outcomes for non-autistic adults with cognitive impairment (Mograbi et al., 2014; Njegovan et al., 2001) and children with developmental disabilities (Liss et al., 2001; Edgin et al., 2010). Independence can be achieved through developing activities of daily living (ADLs; Burton et al., 2006; Bal et al., 2015), which are tasks related to personal care, time management, financial management, household chores, and functioning within one’s community (Sparrow & Cicchetti, 1995; Sparrow et al., 2005). In addition to ADLs, (e.g., financial) independence in adulthood can also be facilitated through employment. The potential association between memory and independence skills is of interest within this population because independence skills have been a notable area of weakness (Shattuck et al., 2012) and young autistic adults have explicitly expressed a desire for increased independence (Cheak-Zamora et al., 2022; Sosnowy et al., 2018). Research has shown that ADLs are more challenging and employment rates are lower for autistic individuals without intellectual disability in comparison to NT populations and other developmental disability groups (Klin et al., 2007; Liss et al., 2001; Roux et al., 2015; Shattuck et al., 2012), and these difficulties can negatively impact their level of independence (Farley et al., 2009). Given that memory difficulties have been linked to independence and real-world outcomes in other clinical populations, it is possible that memory difficulties are associated with ADLs and employment for autistic adults. However, research has not yet examined the relation between memory abilities and real-world outcomes within the autistic adult population.
The Current Study
Although studies have demonstrated weaker memory performance on lab-based tasks for autistic individuals compared to NT individuals, research examining self-reported memory complaints among autistic adults is scarce. Subjective memory complaints are particularly important because they provide an ecologically relevant measure of memory problems in daily life. Research examining the association between NT adults’ self-reported memory complaints and performance-based memory task outcomes has been mixed (Arnold & Bayen, 2019; Horn et al., 2018; John et al., 2020; Kliegel & Jager, 2006). Nonetheless, greater self-reported memory complaints of older adults with and without cognitive impairment are associated with ADL impairments and poorer quality of life (Mentejo et al., 2012; Ryu et al., 2016). Therefore, regardless of their association with performance-based laboratory measures, self-reported memory measures are associated with real-world difficulties and thus, these measures offer clinical utility. Despite this, only one study of which we are aware, has examined self-reported memory complaints of autistic adults (Williams et al., 2014). Williams and colleagues (2014) found that autistic adults reported greater PM problems and had weaker performance on a time-based PM task in comparison to IQ-matched neurotypical adults. However, this study included a small sample size (autistic n=17), necessitating further research with a larger sample. Thus, the current study aimed to examine and further characterize the self-reported memory complaints of young autistic adults using the Prospective and Retrospective Memory Questionnaire and examine the association of memory complaints with ADLs and postsecondary outcomes (both education and vocational activities).
Methods
Participants
Young autistic adults (ages 18 to 39 years) were recruited from the Simons Powering Autism Research (SPARK; The SPARK Consortium, 2018) participant registry (via the Research Match service, Project Number: RM0045Wallace1839). These adults were identified as “independent” by SPARK, indicating that they are able to consent for themselves, and therefore they are unlikely to have an intellectual disability. Additionally, none of the participants reported a co-occurring diagnosis of intellectual disability on a health history questionnaire that was also completed. A self-disclosed community-based professional diagnosis of ASD was required for inclusion in the current study. While SPARK does not confirm diagnosis through independent measures, SPARK recruits from expert autism clinics, which increases the likelihood that participants have received an ASD diagnosis from a clinical professional (The SPARK Consortium, 2018). Corroborating this is a recently completed study in which ~99% of 254 autistic individuals sampled from SPARK had their ASD diagnosis independently confirmed based on electronic health records (Fombonne et al., 2022). Autistic participants in the current study also completed the AQ-28 (Hoekstra et al., 2011) and 92% of the sample in the current study met the AQ-28 cut-off (i.e., >65). The age group of 18 to 39 years was chosen due to the importance of adaptive skill development and securing employment during young adulthood (which developmental theories suggest occurs between the age of majority (i.e., 18 years) and late thirties or forty years of age; e.g., Erikson, 1982). Additionally, limiting our investigation to this younger age range reduced the likelihood of recruiting participants who had retired (which would obscure our investigation of the relationship between subjective memory complaints and vocational outcomes).
A total of 415 autistic adults completed or partially completed the PRMQ, the key measure for the current study (see below). Those with partial responses to the PRMQ (n=5), missing demographic data (n=1), or with a history of traumatic brain injury (n=13) were excluded. This resulted in a sample of 396 autistic adults with a mean age of 29 years who are the focus of the current research.
Data from NT adults were obtained from a large-scale internet study completed by Logie and colleagues in collaboration with the British Broadcasting Corporation (BBC; https://osf.io/ak5pb/; Johnson et al., 2010; Logie & Maylor, 2009) in which self-report data were collected from 408,938 participants, ages 8 to 90 years. Henceforth, we refer to this as the BBC dataset. For the purposes of the current study, only individuals in the BBC dataset from countries with English as a primary language, between the ages of 18 and 39 years who completed the PRMQ and reported their level of education were selected. This resulted in 176,626 NT adults who served as the pool from which the current study’s comparison sample was selected.
In order to a) obtain a NT comparison sample with similar background characteristics to the autistic sample and b) enhance the robustness of planned parametric statistical analyses, the data were preprocessed using non-parametric matching methods (Ho et al., 2007). Specifically, optimal (1:1) matching without replacement was implemented using the MatchIt package in R (Ho et al., 2011), which calls functions from the optmatch package (Hansen & Klopfer, 2006). Optimal 1:1 matching selects a single “control case” for each “study case” while minimizing the absolute pairwise distances between the “study” and matched samples (Hansen & Klopfer, 2006). Participants in the BBC dataset (n=176,626) were selected to be similar to those in the SPARK dataset (n=396) on the following characteristics: age, sex, and educational attainment.
Measures of balance (e.g., the mean difference between datasets) were examined and confirmed improved matching (i.e., smaller mean difference values) between autistic and NT samples when the NT sample of 396 was considered relative to the complete pool of NT (n=176,626) participants. Additionally, Q-Q and Love plots for each variable on which participants were matched were generated to visualize balance in the datasets before and after matching. Scrutiny of these plots also indicated improved balance after matching. The resulting matched samples did not significantly differ for birth-sex (χ2(1)=0.91, p=0.34) or educational attainment (χ2(4)=8.09, p=0.09). SPARK participants were younger than the NT comparison sample from the BBC dataset. Although this difference was statistically significant (t(790)=−4.32, p=0.00002), the effect was small in magnitude (d=0.3). To be conservative, analyses in which PRMQ scores of the autistic and NT groups were compared were run with age covaried. See Results for details. Table 1 provides demographic characteristics of the two groups, and Supplemental Materials provide additional details regarding BBC participant selection and demographics.
Table 1.
Demographic characteristics of SPARK & BBC samples.
| Empty Cell | SPARK N = 396 | BBC Matching 1:1 BBC N = 396 |
Statistics |
|---|---|---|---|
|
Age, years
Mean (SD) Median (Range) |
29 (5.9) 29.2 (18.17–39.42) |
27.11 (6.45) 26 (18–39) |
t(790) = −4.32, p = .00002 |
|
Sex, n (%)
Female Male |
239 (60.4 %) 157 (39.6 %) |
253 (63.9 %) 143 (36.1 %) |
χ2(1) = 0.91, p = .34 |
|
Education, n (%)
Some High School High School Technical/Vocational Bachelor’s Degree Graduate Degree |
11 (2.8 %) 101 (25.5 %) 159 (40.15 %) 84 (21.2 %) 41 (10.35 %) |
9 (2.3 %) 100 (25.2 %) 127 (32.1 %) 108 (27.3 %) 52 (13.1 %) |
χ2(4) = 8.09, p = .09 |
Note: Higher scores denote greater memory complaints.
Measures
Demographic and Health History Form.
Autistic participants completed a questionnaire regarding their medical history and demographics, including age, sex assigned at birth, gender, race, ethnicity, level of education, and parent’s level of education. Similarly, NT adults recruited through the BBC provided their age, sex, and highest level of education. Education for autistic adults was on a 9-point scale, whereas NT adults provided their education level on a 7-point scale. The two scales were harmonized and converted to a 5-level scale which included: 1=did not attend high school, some high school; 2=secondary or high school graduate; 3=technical or vocational college, associate degree, completed some college; 4=bachelor’s degree; 5=graduate or professional degree. This harmonized scale was used only to match participants based on educational attainment. However, primary analyses within the autistic group, in which education was used as a covariate, dichotomized participants as those who had received a bachelor’s degree versus those who had not. This dichotomization was chosen because of the significance a bachelor’s degree has on postsecondary economic and employment outcomes (Carnevale, 2020; Carnevale et., al 2016; Hayward et al., 2019; Kaya et al., 2016), which was a primary interest of this study. We could not use a continuous measure of educational attainment (i.e., years of education obtained) as a covariate in analyses, as this information was not available. Thus, the presence of a bachelor’s degree was deemed the most appropriate variable for analyses in which educational level was covaried.
Autism Spectrum Quotient (AQ-28).
The AQ-28 (Hoekstra et al., 2011) is a questionnaire measuring self-reported autistic traits using a 4-point Likert scale (ranging from 1=definitely agree to 4=definitely disagree). Total scores range from 28 to 112 with higher scores reflecting greater self-reported autistic traits. The AQ-28 has shown acceptable to good internal consistency, and good validity and reliability within samples of autistic adults (Hoekstra et al., 2011). This questionnaire was used to characterize the autistic sample.
The Prospective and Retrospective Memory Questionnaire (PRMQ).
To assess self-report of memory problems, the PRMQ was used (Crawford et al., 2003). The PRMQ includes 16 items rated on a Likert scale ranging from 1 (Never) to 5 (Very Often). Of note, item level data for NT adults used the scale of 1 (Very Often) to 5 (Never) and thus, was reverse scored for the purposes of this study. Additionally, a scale ranging from 1 through 6 was used for the SPARK data set (i.e., 1=Very rarely; 2=Rarely; 3=Occasionally; 4=Somewhat often; 5=Often; 6=Very often) and was recoded to be on a 5-point scale by collapsing responses of 4’s and 5’s together and recoding responses of 6’ into 5’s. Items probe different types of memory and vary with regard to whether they evaluated prospective or retrospective memory (e.g.., prospective: “Do you decide to do something in a few minutes and then forget to do it?”; retrospective: “Do you fail to recognize a place you visited before?), whether they involved a cue or not (e.g., cue:, “Do you forget to buy something you planned to buy, even when you see the shop?”; no cue: “Do you forget something you were told a few minutes before?”) and whether the recall followed a short or long delay (e.g., short delay: “Do you look at something without realizing you have seen it moments before?”; long delay “Do you forget what you watched on television the previous day?”). Items were evenly categorized (i.e., 8 items in each category) as referring to either prospective or retrospective memory, including an event-based cue or no recall cue (i.e., “self cue”), and having a long delay or a short delay. Total scores were created for the entire scale (PRMQ Total) as well as for prospective memory and retrospective memory item sets, and higher scores indicated greater memory problems.
Taylor Vocational Index (TVI).
Autistic participants provided information regarding their post-secondary activities including employment or post-secondary educational pursuits. Data were scored using the Taylor Vocational Index (TVI; Taylor & Seltzer, 2012), which categorizes post-secondary activities, including educational and employment activities, based on the level of independence in completing these activities and hours of engagement. An examination of the data indicated that the vast majority of participants (n=366; 92%) received a score of 1 (i.e., no vocational/educational activities) or 9 (i.e., postsecondary degree seeking educational program or employment in the community without supports greater than 10 hours per week;). Therefore, when relations between the PRMQ and the TVI were examined (using logistic regression), a dichotomous outcome variable for the TVI was used that only included scores of 1 or 9.
Waisman Activities of Daily Living scale (W-ADL).
An adapted self-report version of the W-ADL (which was originally developed as an informant-report measure) was used to assess daily living skills (Bishop-Fitzpatrick et al., 2017). Seventeen items assess activities of daily living and are rated on a Likert scale measuring independence level (i.e., 0=does not do at all, 1=does with help, 2=independent or does on own). The items assess independence on tasks of self-care (i.e., hygiene, bathing, dressing), household chores, cooking, and financial management. Higher scores on the W-ADL indicate greater levels of independence.
Procedure
Data Collection
Autistic adults were recruited via SPARK (The SPARK Consortium, 2018) and completed a battery of online self-report questionnaires between December of 2019 and January of 2020. Participants provided informed consent in accordance with the Declaration of Helsinki. The study was approved by George Washington University. Neurotypical participants were recruited between May 2006 and April 2009 in collaboration with the BBC and via the BBC Science website. Data are publicly available via the Center for Open Science website (https://osf.io/ak5pb/; Johnson et al., 2010; Logie & Maylor, 2009).
Analytic Method
To examine diagnostic group differences on the PRMQ, the ezANOVA function from the ez package in R (R Core Team, 2021) was used to complete a multifactorial ANOVA. The mixed ANOVA included one between-subjects factor of diagnostic group (autistic, NT), and three within-subjects factors including (1) memory type (PM, RM), (2) cue presence (no event cue, event cue) and (3) delay type (short delay, long delay).
To further characterize the degree of PM versus RM complaints, a difference score was created by subtracting the RM total score from the PM total score for each participant. Thus, a positive difference score indicated greater PM problems (in comparison to RM problems), whereas a negative difference score indicated greater RM problems (in comparison to PM problems). One independent samples t-test compared the autistic to the NT group. Then, two one-sample t-tests were conducted examining each diagnostic group’s difference score relative to 0, as 0 represented no difference between PM and RM complaints.
For the autistic group only, a 3-step hierarchical linear regression was completed in SPSS (version 28.0; IBM, 2021) to examine the relation between the PRMQ and the W-ADL. Specifically, the W-ADL score served as the dependent variable, and age, sex, and education level (i.e., whether or not the participant indicated having at least a bachelor’s degree) were included in the first step as covariates. The PRMQ total score was included in the second step, and the PRMQ (PM-RM) difference score was included in the third step. Of note, ceiling effects on select W-ADL items were observed. Specifically, >= 90% of participants indicated independence (i.e., rated themselves as “2”) on items 6 (i.e., washing), 7 (i.e., grooming), 8 (i.e., dressing), 9 (i.e., toileting), 10 (i.e., preparing simple foods), 14 (i.e., drinking from a cup) and 15 (i.e., eating from a plate). Thus, an adjusted W-ADL total score was created, excluding these 7 items, and a second hierarchical regression was completed using the adjusted W-ADL score to ensure that study findings were robust to possible ceiling effects.
Lastly, for the autistic group only, a logistic regression was completed in SPSS (version 28.0; IBM, 2021) to examine the relation between the PRMQ and the TVI. Because 92% (n=366) of participants received vocational rankings that indicated the highest or the lowest level of post-secondary independence (i.e., TVI scores of 1 or 9), a logistic regression was used to predict these two post-secondary outcomes with age and sex included as covariates. (Note that education could not be included as a covariate, as education is considered when calculating the TVI.)
Results
Prior to conducting analyses, correlations were examined between variables. Within the autistic adult group, the PM score was highly correlated with the RM score (r=0.87). The W-ADL was moderately associated with the PM (r=−0.30) and the RM (r=−0.35) scores. Lastly, the TVI demonstrated small associations with the PM score (r=−0.13) and RM score (r=−0.12).
A 2×2×2×2 mixed ANCOVA with one between-subjects factor (Diagnostic Group: autistic, NT), three within-subjects factors (memory type, event cue presence, delay type), and age covaried, was conducted to examine differences in PRMQ ratings between the two diagnostic groups. A main effect of diagnostic group was found with a medium effect size (F(1,790)=33.08, p<0.05, ηp2=0.04), such that the autistic group had higher PRMQ scores (indicating more subjective memory complaints) than the NT group. A main effect of memory type was also significant (F(1,790)=423.96, p<0.05, ηp2=0.35), such that the PM total score was significantly higher than the RM total score, indicating greater PM complaints regardless of diagnostic group (See Figures 1a and 1b). Additionally, a main effect of event cue presence (F(1,790)=310.46, p<0.05, ηp2=0.28) was found, indicating greater reported memory problems for tasks without an event cue compared to those with an event cue. Lastly, a main effect of delay type (F(1,790)=176.84, p<0.05, ηp2=0.18) was found indicating greater reported problems for tasks with a short delay than those with a long delay. There were no significant interactions between diagnostic group and memory type, event cue presence, or delay type. See Figure 1.
Fig. 1.
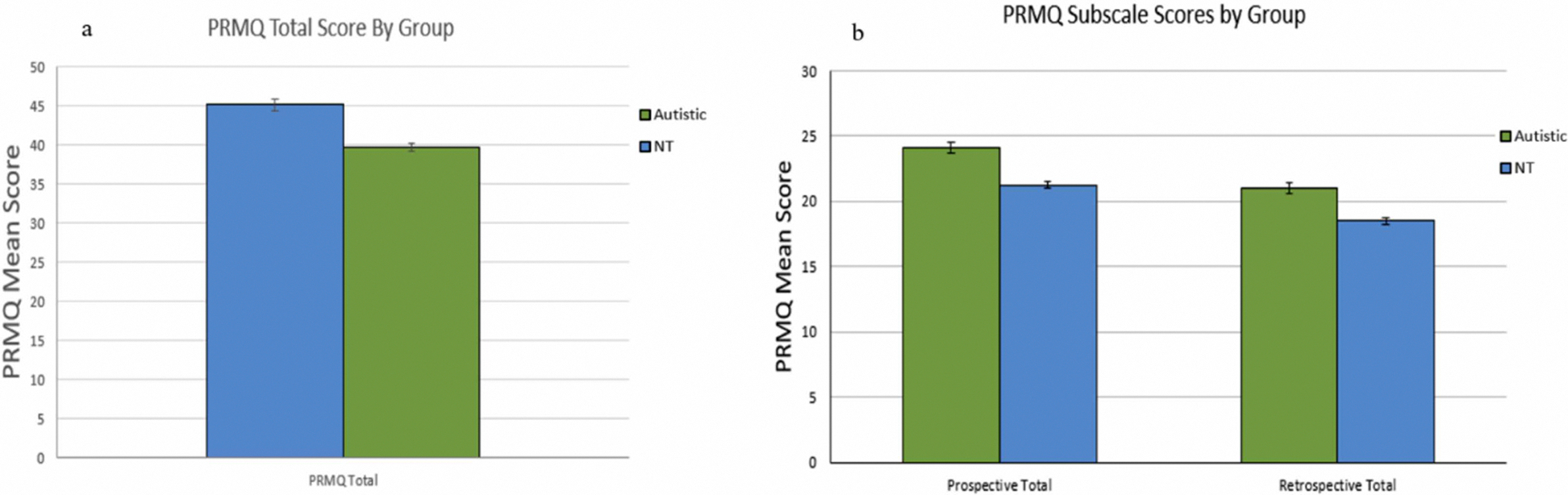
1a. Mean PRMQ Total Scores by group. 1b. Mean PM and RM Subscale Scores by Group.
Next, an independent samples t-test compared the PRMQ difference scores of the autistic and NT groups. There was no significant difference between the groups’ difference scores (autistic M=3.1 7; NT M=2.65; t(790)=1.40, p=0.16, d=0.10). Two one-sample t-tests, one for each diagnostic group, revealed that both groups difference scores were significantly greater than 0 (autistic: (t(395)=15.39, p<0.001, d=0.77; NT: (t(395)=13.71, p<0.001, d=0.69), indicating relatively greater PM than RM difficulties for both groups. Results of additional analyses examining group differences on the PRMQ are provided in the Supplemental Materials.
Within the autistic group only, a 3-step hierarchical linear regression was completed to examine if the PRMQ significantly predicted W-ADL scores, accounting for age, sex, and education in step 1 of the model. At step 2, the PRMQ total score was entered, and at step 3, the PRMQ difference score was entered. The hierarchical linear regression indicated that at step 1, age, sex, and education contributed significantly to the model (FΔ(3,392)=8.065, p<0.001, R2 Δ=0.06). Introducing the PRMQ total score improved prediction significantly (FΔ(1,391)=46.21, p<0.001, R2 Δ =0.10). Lastly, introducing the PRMQ difference score improved prediction significantly as well (FΔ(1,390)=8.13, p=0.005, R2Δ =0.02), and indicated that greater PRMQ difference scores (i.e., fewer RM problems) were associated with higher W-ADL scores (i.e., greater independence in ADLs). See Table 2.
Table 2.
Hierarchical linear regression examining the W-ADL performance.
| Empty Cell | B | SE B | t-value |
|---|---|---|---|
| Model 1 (covariates only): | |||
| Age | −0.001 | 0.002 | −0.559 |
| Sex | 0.026 | 0.028 | 0.924 |
| Education | 0.147 | 0.030 | 4.852 * * |
| Model 2: | |||
| Age | 0.000 | 0.002 | 0.028 |
| Sex | −0.008 | 0.027 | −0.293 |
| Education | 0.126 | 0.029 | 4.354 * * |
| PRMQ Total Score | −0.006 | 0.001 | −6.798 * * |
| Model 3 (all predictors): | |||
| Age | 0.000 | 0.002 | −0.112 |
| Sex | −0.007 | 0.027 | −0.276 |
| Education | 0.136 | 0.029 | 4.729 * * |
| PRMQ Total Score | −0.006 | 0.001 | −7.256 * * |
| PRMQ Difference Score | 0.009 | 0.003 | 2.852 * |
p < 0.01
p < .001
Due to ceiling effects observed on some items of the W-ADL, a second 3-step hierarchical linear regression was completed using an adjusted W-ADL total score, which omitted items with ceiling effects. Again, age, sex, and education were entered at step 1 of the regression to control for these variables, the PRMQ total score was entered at step 2, and the PRMQ difference score was entered in Step 3. Overall results remained unchanged. Specifically, the hierarchical linear regression indicated that at step 1, age, sex, and education contributed significantly to the model (FΔ(3,392)=9.335, p<0.001, R2Δ=0.07). Introducing the PRMQ total score to the model improved prediction significantly (FΔ(1,391)=43.91, p<0.001, R2Δ=0.09). Finally, introducing the PRMQ difference score significantly improved prediction as well (FΔ(1,390)=6.230, p=0.013, R2Δ =0.01).
Next, a logistic regression was completed to predict post-secondary outcome group membership (i.e., TVI scores of 1, least independent, versus 9, most independent), using the PRMQ total score and difference score. The baseline model, which included covariates of age and sex, resulted in 60.7% prediction accuracy of the TVI group membership. When the PRMQ total score was added, the model accuracy improved significantly (though the amount of additional variance explained was small with an increase of 0.2% from the model that only included covariates). An evaluation of the standardized beta coefficients and odds ratio values in the model indicated that the PRMQ total score added significant unique variance to the prediction of the post-secondary outcome group membership. In contrast, the PRMQ Difference Score did not add significant unique variance. See Table 3.
Table 3.
Logistic Regression Results for the PRMQ Predicting (Concurrent) Post-Secondary Outcomes.
| Empty Cell | B | SE B | p-value | Odds Ratio | 95 % CI of Odds Ratio |
|---|---|---|---|---|---|
| Model 1 (covariates only): | Empty Cell | Empty Cell | Empty Cell | Empty Cell | Empty Cell |
| Age | −0.010 | 0.018 | 0.597 | 0.990 | 0.955 – 1.027 |
| Sex | −0.384 | 0.222 | 0.085 | 0.681 | 0.441 – 1.054 |
| Model 2: | |||||
| Age | −0.007 | 0.019 | 0.696 | 0.993 | 0.957 – 1.030 |
| Sex | −0.309 | 0.226 | 0.171 | 0.734 | 0.471 – 1.143 |
| PRMQ Total Score | −0.017 | 0.007 | 0.024 * | 0.983 | 0.969 – 0.998 |
| Model 3 (all predictors): | |||||
| Age | −0.007 | 0.019 | 0.708 | 0.993 | 0.957 – 1.030 |
| Sex | −0.312 | 0.226 | 0.168 | 0.732 | 0.470 – 1.140 |
| PRMQ Total Score | −0.016 | 0.008 | 0.031 * | 0.984 | 0.969 – 0.999 |
| PRMQ Difference Score | −0.013 | 0.027 | 0.637 | 0.998 | 0.937 – 1.040 |
p < .05
To account for overall reported memory problems, a proportional difference score was also created, calculated by dividing the raw difference score (PM-RM) by RM ratings. The proportional difference score was highly correlated (r=0.92) with the raw difference score. Finally, the logistic regressions, including those examining W-ADL and TVI scores, were re-run using the proportional difference score (i.e., [PM-RM]/RM) and the results did not differ from those obtained using the raw difference score.
Discussion
Little is known about self-reported memory problems of autistic adults. The existing literature suggests autistic adults experience greater memory problems in certain domains in comparison to NT adults and that among autistic individuals, greater PM problems have been noted relative to RM problems (Williams et al., 2014). Despite this knowledge, only one small study has examined subjective report of memory difficulties among autistic adults (n=17) and no previous studies, of which we are aware, have examined whether memory abilities of autistic adults are associated with real-world outcomes, including daily living skills and post-secondary activities (i.e., work and educational activities). Thus, the current study sought to examine self-reported memory complaints of a large sample of young autistic adults and to examine the relation between self-reported memory abilities and both daily living skills and post-secondary activities.
Consistent with past research, the results of the current study found that young autistic adults reported greater memory problems than NT adults who were matched on sex and education level. These findings were true regardless of the memory type (e.g., RM, PM). These results confirm the findings of the only previous study that examined self-reported memory problems among autistic adults (Williams et al., 2014), though in the context of a considerably larger sample. Second, our results indicate that young autistic and NT adults did not differ in their pattern of complaints reported. Specifically, both groups reported significantly more PM problems than RM problems in daily life.
Moreover, our findings show that young autistic adults have greater memory complaints in comparison to NT peers, regardless of whether an event-based cue is present. Previous research on performance-based measures has generally suggested that autistic adults have benefitted from cues and have comparable memory performance to NT peers when provided with an event-based cue (Altgassen et al., 2010; Altgassen & Koch, 2014; Williams et al., 2014). However, as previously mentioned, two past studies found that when completing naturalistic tasks, rather than lab-based memory assessments, autistic adults did not benefit from the presence of an event-based cue (Altgassen et al., 2012; Kretschmer et al., 2014). Adding to this previous research, our results suggest that even when given a cue, young autistic adults perceive greater memory problems in comparison to their NT peers during naturalistic tasks.
Lastly, we evaluated the association between subjective memory complaints and real-world outcomes, including ADLs and post-secondary activities, in young autistic adults for the first time. No study of which we are aware has examined the association between memory abilities and real-world outcomes of autistic adults, despite known difficulties in both areas in this population (Baxter et al., 2015; Desaunay et al., 2020; Klin et al., 2007; Laxman et al., 2019; Liss et al., 2001; Magiati et al., 2014; Shattuck et al., 2011). The results showed a significant negative association between self-reported memory complaints and self-reported ADLs, even after accounting for age, sex, and education level. Additionally, there was a positive association between the PRMQ difference score and self-reported ADLs, suggesting that greater RM complaints in comparison to PM complaints are associated with poorer ADLs, above and beyond general memory complaints. Of note, the majority (i.e., 90% or more) of the autistic adult participants reported that they could complete basic ADLs independently (comprising a subset of W-ADL items), including tasks of hygiene, dressing, toileting, and feeding. Yet even after excluding these items from the analysis, our findings remained robust and indicated a significant association between self-reported memory problems and diminished ADL independence. Moreover, overall memory complaints were concurrently predictive of engagement in postsecondary activities, indicating that autistic adults who have greater memory problems may have more difficulty in obtaining and maintaining postsecondary educational placements or paid employment. Therefore, memory complaints of autistic adults may be indicative of suboptimal real-world outcomes, including diminished independence in ADLs and vocational pursuits after high school. Given these significant associations, it will be important for caregivers and educators to take note of memory complaints, as they could possibly indicate that greater support for daily living activities is needed.
Considering the increased memory complaints of young autistic adults in comparison to their NT peers, future research should examine the efficacy of interventions that support memory abilities for autistic adults. Past research examining memory interventions for non-autistic adults indicated improved PM when taught compensatory skills for PM tasks. For example, when taught to independently plan (Kliegel et al., 2007) and implement cues or reminders (Cicerone et al., 2019; Henry et al., 2012), adults’ PM performance improved. Additionally, non-autistic adults with cognitive impairment improved on RM tasks after participating in cognitive rehabilitation with training on memory strategies and compensatory strategies (Cicerone et al., 2019, Miller & Radford, 2014; O’Neil-Pirozzi et al., 2010). While studies have not yet examined memory interventions for autistic adults, autistic children have improved on memory recall tasks after being taught organizational strategies for learning and recall tasks (Bebko et al., 2021). In light of the successful outcomes of memory training for non-autistic adult populations and for autistic children, research is needed to examine the efficacy of memory interventions and supports for autistic adults. Of note, autistic adults have reported the need for reminders in order to successfully carry out daily living tasks and build independence (Cheak-Zamora et al., 2022). Thus, memory interventions that teach compensatory strategies (e.g., using cues and reminders) could result in downstream improvements in ADLs among autistic adults. Nonetheless, future research is needed to examine whether memory interventions and supports would be effective for autistic adults.
The current study is not without its limitations. First, a measure of intellectual functioning was not available for the participants of this study. However, education level was used as a proxy measurement for IQ and incorporated into the analyses both when comparing the young autistic adults to neurotypical adults and when examining relations between memory complaints and ADLs. In addition, it is useful to note that despite not matching on intellectual functioning, our findings of greater memory complaints for autistic adults in comparison to NT adults were consistent with findings of a previous study that matched participants on IQ (Williams et al., 2014). Second, participants’ ASD diagnoses were not confirmed through independent assessment and the AQ-28 measure was used, which has not consistently been shown to be a reliable predictor of ASD diagnosis (Ashwood et al., 2016). While this could increase the potential risk for including participants without an ASD diagnosis if used in isolation, a recent study completed using participants sampled from SPARK indicated that almost all (i.e., approximately 99%) of a subset of participants’ ASD diagnoses were confirmed when compared to their medical records. Third, the current study used self-report measures, rather than objective performance-based measures. Given that self-report measures are inherently subjective, use of such measures has the potential to result in biases. For example, individuals may be overconfident in their memory abilities or unaware of their memory difficulties, and thus inadvertently under or overreport memory problems. While this remains a possibility, past studies of NT adults have shown significant associations between self-report of memory problems and memory performance on lab-based measures (Horn et al., 2018; John et al., 2020). Moreover, the use of self-report measures increases ecological validity through the evaluation of everyday memory difficulties experienced by the individual that often cannot be captured in the laboratory or clinic setting using performance-based instruments (Chaytor & Schmitter-Edgecombe, 2003). Nonetheless, future research examining memory and ADL abilities and postsecondary activities among autistic adults is needed to verify and expand upon the current study, as these self-reported problems have significant real-world implications for autistic individuals.
In summary, the current study provided a unique examination of self-reported memory challenges among young autistic adults. Consistent with prior research on lab-based memory task performance, we found young autistic adults report greater memory problems than NT adults with similar background characteristics, although the pattern of complaints (i.e., PM greater than RM complaints) was similar. In addition, these self-reported memory challenges are related to real-world functioning, as greater memory complaints were associated with less independence in ADLs and post-secondary activities among these young autistic adults. Considering the functional outcomes associated with memory difficulties, interventions that incorporate training on memory strategies and compensatory techniques could serve to improve daily living skills and postsecondary educational and employment outcomes for young autistic adults.
Supplementary Material
Highlights.
Young autistic adults reported greater memory problems than their neurotypical peers.
In young autistic adults greater memory complaints were associated with less independence in daily living skills and less favorable postsecondary outcomes.
These results suggest that memory abilities of young autistic adults could impact daily living skills and post-secondary outcomes.
Acknowledgements
We are grateful for the autistic adults who participated in the study via SPARK, the SPARK clinical sites, and SPARK staff. We appreciate obtaining access to recruit participants through the SPARK Research Match. Additionally, this research was supported by an Autism Speaks Postdoctoral Fellowship (Grant ID 11808) to G.A.M, and by start-up funds from The George Washington University to G.L.W. Additional support was provided by the National Institutes of Health to G.A.M. (under grant K01MH129622), G.L.W. (under grants R01MH100028; R21HD106164; R21MH129777; R01MH133838), and N.R.L. (under grants R21HD100997; R21HD106164).
Footnotes
Conflict of Interest
The authors have no conflict of interest to disclose.
References
- Altgassen M, Schmitz-Hübsch M, & Kliegel M (2010). Event-based prospective memory performance in autism spectrum disorder. Journal of Neurodevelopmental Disorders, 2(1), 2–8. 10.1007/s11689-009-9030-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altgassen M, Koban N & Kliegel M (2012). Do Adults with Autism Spectrum Disorders Compensate in Naturalistic Prospective Memory Tasks? J Autism Dev Disord 42, 2141–2151. 10.1007/s10803-012-1466-3 [DOI] [PubMed] [Google Scholar]
- Altgassen M & Koch A (2014). Impact of inhibitory load on remembering delayed intentions in autism, International Journal of Developmental Disabilities, 60(3), 198–204, DOI: 10.1179/2047387714Y.0000000042 [DOI] [Google Scholar]
- American Occupational Therapy Association (2014). Occupational therapy practice framework: Domain & procss 3rd edition. American Journal of Occupational Therapy. 10.1002/oti.1363 [DOI] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. 5. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Arnold NR, & Bayen UJ (2019). Prospective memory: Comparing self- and proxy-reports with cognitive modeling of task performance. Journal of Applied Research in Memory and Cognition, 8(2), 244–254. https://doi-org.proxy1.library.jhu.edu/10.1016/j.jarmac.2019.04.001 [Google Scholar]
- Ashwood KL, Gillan N, Horder J, Hayward H, Woodhouse E, McEwen FS, … Simonoff E (2016). Predicting the diagnosis of autism in adults using the Autism-Spectrum Quotient (AQ) questionnaire. Psychological Medicine, 46(12), 2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal VH, Kim SH, Cheong D, & Lord C (2015). Daily living skills in individuals with autism spectrum disorder from 2 to 21 years of age. Autism, 19(7), 774–784. 10.1177/1362361315575840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebko JM, Dahary H, Goldstein G, Porthukaran A, & Ferland M (2021). Organizational memory strategy training with children with autism spectrum disorder. Canadian Journal of Behavioural Science / Revue canadienne des sciences du comportement, 53(1), 1–11. 10.1037/cbs0000183 [DOI] [Google Scholar]
- Bennetto L, Pennington BF, & Rogers SJ (1996). Intact and impaired memory functions in autism. Child Development, 67(4), 1816–1835. 10.2307/1131734 [DOI] [PubMed] [Google Scholar]
- Bishop-Fitzpatrick L, Minshew NJ, Mazefsky CA, & Eack SM (2017). Perception of life as stressful, not biological response to stress, is associated with greater social disability in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(1), 1–16. 10.1007/s10803-016-2910-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J, Mayes A, & Bigham S (2012). Memory in autistic spectrum disorder. Psychological Bulletin, 138(3), 458–496. doi: 10.1037/a0026869. [DOI] [PubMed] [Google Scholar]
- Bowler DM, Matthews NJ, & Gardiner JM (1997). Asperger’s syndrome and memory: Similarity to autism but not amnesia. Neuropsychologia, 35(1), 65–70. 10.1016/S0028-3932(96)00054-1 [DOI] [PubMed] [Google Scholar]
- Bowler DM, Gardiner JM, & Berthollier N (2004). Source memory in adolescents and adults with Asperger’s syndrome. Journal of autism and developmental disorders, 34(5), 533–542. 10.1007/s10803-004-2548-7 [DOI] [PubMed] [Google Scholar]
- Bowler DM, Gaigg SB, & Gardiner JM (2008). Effects of related and unrelated context on recall and recognition by adults with high-functioning autism spectrum disorder. Neuropsychologia, 46(4), 993–999. 10.1016/j.neuropsychologia.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Bowler DM, Limoges E, & Mottron L (2009). Different verbal learning strategies in autism spectrum disorder: evidence from the Rey Auditory Verbal Learning Test. Journal of autism and developmental disorders, 39(6), 910–915. 10.1007/s10803-009-0697-4 [DOI] [PubMed] [Google Scholar]
- Bowler DM, Gaigg SB & Gardiner JM (2010). Multiple List Learning in Adults with Autism Spectrum Disorder: Parallels with Frontal Lobe Damage or Further Evidence of Diminished Relational Processing?. J Autism Dev Disord 40, 179–187. 10.1007/s10803-009-0845-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler DM, Gaigg SB, & Gardiner JM (2015). Brief report: The role of task support in the spatial and temporal source memory of adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(8), 2613–2617. 10.1007/s10803-015-2378-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Strauss E, Hultsch DF, Hunter MA (2006). Cognitive functioning and everyday problem solving in older adults. The Clinical Neuropsychologist, 20(3):432–452. [DOI] [PubMed] [Google Scholar]
- Carter AS, Volkmar FR, Sparrow SS, Wang JJ, Lord C, Dawson G, Fombonne E, Loveland K, Mesibov G, & Schopler E (1998). The Vineland Adaptive Behavior Scales: supplementary norms for individuals with autism. Journal of autism and developmental disorders, 28(4), 287–302. 10.1023/a:1026056518470 [DOI] [PubMed] [Google Scholar]
- Charman T, Jones CRG, Pickles A, Simonoff E, Baird G, Happe F (2011). Defining the cognitive phenotype of autism. Brain Research, 1380 (22), 10–21. 10.1016/j.brainres.2010.10.075 [DOI] [PubMed] [Google Scholar]
- Carnevale AP (2020). Who’s Working From Home: The Education Divide. Georgetown University Center on Education and the Workforce. https://medium.com/georgetown-cew/whos-working-from-home-the-education-divide-5422ce774c9d [Google Scholar]
- Carnevale AP, Jayasundera T, & Gulish A (2016). America’s Divided Recovery: College Haves and Have-Nots. https://cew.georgetown.edu/cew-reports/americas-divided-recovery/
- Chaytor N, Schmitter-Edgecombe M (2003). The ecological validity of neuropsychological tests: a review of the literature on everyday cognitive skills. Neuropsychology Review 13(4),181–97. doi: 10.1023/b:nerv.0000009483.91468.fb. [DOI] [PubMed] [Google Scholar]
- Cheak-Zamora N, Tait A & Coleman A (2022). Assessing and Promoting Independence in Young Adults with Autism Spectrum Disorder. Journal of Developmental & Behavioral Pediatrics, 43 (3), 130–139. doi: 10.1097/DBP.0000000000001021. [DOI] [PubMed] [Google Scholar]
- Clarke EB, McCauley JB, & Lord C (2020). Post-high school daily living skills outcomes in autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 10.1016/j.jaac.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RA, Plaisted K, Hannula DE, Ranganath C, Baron-Cohen S, & Simons J (2015). Impaired recollection of visual scene details in adults with autism spectrum conditions.. J Abnorm Psychol, 24 565–575. 10.1037/abn0000070 [DOI] [PubMed] [Google Scholar]
- Crawford JR, Smith G, Maylor EA, Della Sala S, & Logie RH (2003). The Prospective and Retrospective Memory Questionnaire (PRMQ): Normative data and latent structure in a large non-clinical sample. Memory (Hove, England), 11(3), 261–275. 10.1080/09658210244000027 [DOI] [PubMed] [Google Scholar]
- Ceci SJ, Baker JG, & Bronfenbrenner U (1988). Prospective remembering, temporal calibration, and context. In Gruneberg MM, Morris PE, & Sykes RN (Eds.), Practical aspects of memory: Current research and issues, Vol. 1. Memory in everyday life (pp. 360–365). John Wiley & Sons. [Google Scholar]
- Cicerone K, Goldin Y, Ganci K, Rosebaum A, Weather J, Harley JP (2019). Evidence-based cognitive rehabilitation: systematic review of the literature from 2009 to 2014. Activities of Physical Medicine and Rehabilitation, 100(8), 1515–1533 [DOI] [PubMed] [Google Scholar]
- Corbett B, Constantine L, Hendren R, Rocke D, & Ozonoff S (2009). Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Research, 166(2–3), 210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehnavi F, & Khan A (2023). Time-Based and Event-Based Prospective Memory in Adults with Autism Spectrum Disorder: A Virtual Week Investigation. Journal of Autism & Developmental Disorders https://doi-org.proxy1.library.jhu.edu/10.1007/s10803-023-05975-y [DOI] [PubMed] [Google Scholar]
- Desaunay P, Briant AR, Bowler DM, Ring M, Gérardin P, Baleyte JM, Guénolé F, Eustache F, Parienti JJ, & Guillery-Girard B (2020). Memory in autism spectrum disorder: A meta-analysis of experimental studies. Psychological bulletin, 146(5), 377–410. [DOI] [PubMed] [Google Scholar]
- Duncan A, Liddle M, & Stark L (2021). Iterative Development of Daily Living Skills Intervention for Adolescents with Autism Without an Intellectual Disability. Clinical Child and Family Psychology Review. 10.1007/s10567-021-00360-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A, Meinzen-Derr J, Ruble LA, Fassler C, Stark LJ (2022). A Pilot Randomized Controlled Trial of a Daily Living Skills Intervention for Adolescents with Autism. Journal of Autism and Developmental Disorders 52(2):938–949. doi: 10.1007/s10803-021-04993-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgin JO, Pennington BF, & Mervis CB (2010). Neuropsychological components of intellectual disability: the contributions of immediate, working, and associative memory. 54(5), 406–417. doi: 10.1111/j.1365-2788.2010.01278.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein GO, & McDaniel MA (1990). Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 16(4), 717–726. [DOI] [PubMed] [Google Scholar]
- Farley MA, McMahon WM, Fombonne E, Jenson WR, Miller J, Gardner M, Block H, Pingree CB, & Ritvo RA (2009). Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Research, 2(2), 109–118. 10.1002/aur.69 [DOI] [PubMed] [Google Scholar]
- Fombonne E, Coppola L, Mastel S, & O’Roak BJ (2022). Validation of Autism Diagnosis and Clinical Data in the SPARK Cohort. Journal of Autism and Developmental Disorders, 52, 3383–3398. 10.1007/s10803-021-05218-y. [DOI] [PubMed] [Google Scholar]
- Gaigg SB, Gardiner JM, & Bowler DM (2008). Free recall in autism spectrum disorder: The role of relational and item-specific encoding. Neuropsychologia, 46(4), 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, Verté S, Oosterlaan J, Roeyers H, & Sergeant JA (2004). How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? Journal of child psychology and psychiatry, 45(4), 836–854. 10.1111/j.1469-7610.2004.00276.x [DOI] [PubMed] [Google Scholar]
- Geurts HM, & Vissers ME (2012). Elderly with autism: executive functions and memory. Journal of autism and developmental disorders, 42(5), 665–675. 10.1007/s10803-011-1291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger C, Williams DM, & Lind SE (2014). Metacognition, metamemory, and mindreading in high-functioning adults with autism spectrum disorder. Journal of Abnormal Psychology, 123(3), 650–659. 10.1037/a0036531. [DOI] [PubMed] [Google Scholar]
- Gray K, Keating C, Taffe J, Brereton A, Einfeld S, Reardon T, & Tonge B (2014). Adult outcomes in autism: community inclusion and living skills. Journal of Autism and Developmental Disorders 44, 3006–3015. [DOI] [PubMed] [Google Scholar]
- Happe F, & Frith U (2006). The weak coherence account: detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders, 36, 5–25. [DOI] [PubMed] [Google Scholar]
- Hansen BB, & Klopfer SO (2006). Optimal full matching and related designs via network flows. Journal of Computational and Graphical Statistics, 15(3), 609–627. 10.1198/106186006X137047 [DOI] [Google Scholar]
- Hayward S, McVilly K, Stokes M (2019). Autism and employment: What works. Research in Autism Spectrum Disorders. 60, 48–58. 10.1016/j.rasd.2019.01.006. [DOI] [Google Scholar]
- Henry JD, Rendell PG, Phillips LH, Dunlop L & Kliegel M (2012). Prospective memory reminders: A laboratory investigation of initiation source and age effects. Quarterly Journal of Experimental Psychology, 65(7), 1274–1287. [DOI] [PubMed] [Google Scholar]
- Ho DE, Imai K, King G, & Stuart EA (2011). MatchIt : Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software, 42(8), 1–28. 10.18637/jss.v042.i08 [DOI] [Google Scholar]
- Ho DE, Imai K, King G, Stuart EA (2007). Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit. Anal. 15, 199–236. 10.1093/pan/mpl013 [DOI] [Google Scholar]
- Hoekstra RA, Vinkhuyzen AA, Wheelwright S, Bartels M, Boomsma DI, Baron-Cohen S, Posthuma D, & van der Sluis S (2011). The construction and validation of an abridged version of the autism-spectrum quotient (AQ-Short). Journal of autism and developmental disorders, 41(5), 589–596. 10.1007/s10803-010-1073-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn MM, Kennedy KM, & Rodrigue KM (2018). Association between subjective memory assessment and associative memory performance: Role of ad risk factors. Psychology and aging, 33(1), 109–118. 10.1037/pag0000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SE, Evans SA, Hanfelt J, Loring DW, & Goldstein FC (2020). Subjective Memory Complaints in White and African American Participants. Journal of geriatric psychiatry and neurology, 33(3), 135–143. 10.1177/0891988719868305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W, Logie RH, & Brockmole JR (2010). Working memory tasks differ in factor structure across age cohorts: Implications for dedifferentiation. Intelligence, 38(5), 513–528. 10.1016/j.intell.2010.06.005 [DOI] [Google Scholar]
- Kaya C, Chan F, Rumrill P, Hartman E, Wehman P, Iwanaga K, Pai C, & Avellone L (2016). Vocational rehabilitation services and competitive employment for transition-age youth with autism spectrum disorders. Journal of Vocational Rehabilitation, 45(1), 73–83. https://doi-org.proxy1.library.jhu.edu/10.3233/JVR-160812 [Google Scholar]
- Klin A, Saulnier CA, Sparrow S, Cicchetti DV, Volkmar FR, & Lord C (2007). Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: The Vineland and the ADOS. Journal of Autism and Developmental Disorders, 37, 748–759. doi: 10.1007/s10803-006-0229-4. [DOI] [PubMed] [Google Scholar]
- Kliegel Matthias; Jäger Theodor (2006). Can the prospective and retrospective memory questionnaire (PRMQ) predict actual prospective memory performance? Current Psychology, 25(3):182–191. DOI: 10.1007/s12144-006-1002-8 [DOI] [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, Einstein GO, & Moor C (2007). Realizing complex delayed intentions in young and old adults: the role of planning aids. Memory & Cognition, 35(7), 1735–1746. doi: 10.3758/Bf03193506. [DOI] [PubMed] [Google Scholar]
- Kretschmer A, Altgassen M, Rendell PG, & Bölte S (2014). Prospective memory in adults with high-functioning autism spectrum disorders: exploring effects of implementation intentions and retrospective memory load. Research in developmental disabilities, 35(11), 3108–3118. [DOI] [PubMed] [Google Scholar]
- Lever AG, & Geurts HM (2016). Age-related differences in cognition across the adult lifespan in autism spectrum disorder. Autism Research, 9, 666–676. [DOI] [PubMed] [Google Scholar]
- Landsiedel J, Williams D, Abbot-Smith K (2017). A meta-analysis and critical review of prospective memory in autism spectrum disorder. Journal of Autism and Developmental Disorders, 47, 646–666 DOI 10.1007/s10803-016-2987-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsiedel J, & Williams DM (2020). Increasing extrinsic motivation improves time-based prospective memory in adults with autism: Relations with executive functioning and mentalizing. Journal of Autism and Developmental Disorders, 50(4), 1133–1146. 10.1007/s10803-019-04340-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxman D, Taylor J, DaWalt L, Greenberg J, & Mailick M (2019). Loss in services precedes high school exit for teens with autism spectrum disorder: a longitudinal study. Autism Res. 12, 911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss M, Harel B, Fein D, Allen D, Dunn M, Feinstein C, Morris R, Waterhouse L, & Rapin I (2001). Predictors and correlates of adaptive functioning in children with developmental disorders. Journal of autism and developmental disorders, 31(2), 219–230. 10.1023/a:1010707417274 [DOI] [PubMed] [Google Scholar]
- Logie RH, & Maylor EA (2009). An Internet study of prospective memory across adulthood. Psychology and Aging, 24(3), 767–774. 10.1037/a0015479 [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Smith LE, Hong J, Makuch R, Greenberg JS, & Mailick MR (2013). Evaluation of an activities of daily living scale for adolescents and adults with developmental disabilities. Disability and health journal, 6(1), 8–17. 10.1016/j.dhjo.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiati I, Tay XW, & Howlin P (2014). Cognitive, language, social and behavioural outcomes in adults with autism spectrum disorders: A systematic review of longitudinal follow-up studies in adulthood. Clinical Psychology Review, 34(1), 73–86. 10.1016/j.cpr.2013.11.002 [DOI] [PubMed] [Google Scholar]
- McGonigle-Chalmers M, Bodner K, Fox-Pitt A, & Nicholson L (2008). Size sequencing as a window on executive control in children with autism and Asperger’s syndrome. Journal of autism and developmental disorders, 38(7), 1382–1390. 10.1007/s10803-007-0396-y [DOI] [PubMed] [Google Scholar]
- McQuaid G, Weiss C, Said AJ, Pelphrey K, Lee NR, Wallace GL (2022). Increased perceived stress is negatively associated with activities of daily living and subjective quality of life in younger, middle, and older autistic adults. Autism Research, 15(8), 1535–1549. 10.1002/aur.2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentejo P, Montenegro M, Fenandez M, & Maestu F (2012). Memory complaints in the elderly: quality of life and daily living activities. A population based study. Archives of gerontology and geriatrics, 54(2), 298–304. [DOI] [PubMed] [Google Scholar]
- Miller LA, & Radford K (2014). Testing the effectiveness of group-based memory rehabilitation in chronic stroke patients. Neuropsychological Rehabilitation 24,721–737. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, & Goldstein G (2001). The pattern of intact and impaired memory functions in autism. Journal of child psychology and psychiatry, 42(8), 1095–1101. 10.1111/1469-7610.00808 [DOI] [PubMed] [Google Scholar]
- Mograbi DC, Faria C, Fichman HC, Paradela EM, & Lourenço RA (2014). Relationship between activities of daily living and cognitive ability in a sample of older adults with heterogeneous educational level. Annals of Indian Academy of Neurology, 17(1), 71–76. 10.4103/0972-2327.128558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njegovan V, Man-Son-Hing M, Mitchell SL, Molnar FJ. The hierarchy of functional loss associated with cognitive decline in older persons. The Journals of Gerontology, Series A. Biological Sciences and Medical Sciences. 2001;56:M638Y–M643. [DOI] [PubMed] [Google Scholar]
- O’Neil-Pirozzi TM, Strangman GE, Goldstein R et al. (2010). A controlled treatment study of internal memory strategies (I-MEMS) following traumatic brain injury. Journal of Head Trauma Rehabilitation, 25, 43–51 [DOI] [PubMed] [Google Scholar]
- R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Ring M, Gaigg SB & Bowler DM (2015). Object-location memory in adults with Autism spectrum disorder. Autism Research 8, 609–619. [DOI] [PubMed] [Google Scholar]
- Ring M, Gaigg SB & Bowler DM (2016). Relational memory processes in adults with Autism spectrum disorder. Autism Research, 9(1), pp. 97–106. doi: 10.1002/aur.1493 [DOI] [PubMed] [Google Scholar]
- Robertson CE, & Baron-Cohen S (2017). Sensory perception in autism. Nat Rev Neurosci, 18, 671–684. [DOI] [PubMed] [Google Scholar]
- Roux AM, Shattuck PT, Rast JE, Rava JA, & Anderson KA (2015). National Autism Indicators Report: Transition into Young Adulthood. Philadelphia, PA: Life Course Outcomes Research Program, A.J. Drexel Autism Institute, Drexel University. [Google Scholar]
- Ryu S, Lee S, Kim T, & Lee T (2016). Subjective memory complaints, depressive symptoms and instrumental activities of daily living in mild cognitive impairment. International Psychogeriatrics, 28(3), 487–494. doi: 10.1017/S1041610215001945 [DOI] [PubMed] [Google Scholar]
- Schacter DL, & Tulving E (1994). What are the memory systems of 1994? In Schacter DL & Tulving E (Eds.), Memory systems 1994 (pp. 1–38). Cambridge, MA: MIT Press. [Google Scholar]
- Shattuck PT, Narendorf SC, Cooper B, Sterzing PR, Wagner M, & Taylor JL (2012). Postsecondary education and employment among youth with an autism spectrum disorder. Pediatrics, 129(6), 1042–1049. 10.1542/peds.2011-2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck P, Wagner M, Narendorf S, Sterzing P, Hensley M (2011). Post–high school service use among young adults with an autism spectrum disorder. Arch Pediatr Adolesc Med. 165, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Maenner M, & Seltzer M (2012). Developmental trajectories in adolescents and adults with autism. The case of daily living skills. J Am Acad Child Adolesc Psychiatry. 51(6), 622–31. 10.1016/j.jaac.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnowy C, Silverman C, & Shattuck P (2018). Parents’ and young adults’ perspectives on transition outcomes for young adults with autism. Autism, 22, 29–39. [DOI] [PubMed] [Google Scholar]
- Southwick JS, Bigler ED, Froehlich A, DuBray MB, Alexander AL, Lange N, & Lainhart JE (2011). Memory functioning in children and adolescents with autism. Neuropsychology, 25, 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Cicchetti D, Balla D (2005). Vineland Adaptive Behavior Scales. 2nd ed. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Sparrow S, & Cicchetti D (1985). Diagnostic uses of the Vineland Adaptive Behavior Scales. J Pediatr Psychol, 10, 215–225. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Woynaroski TG, Schneider BC, Eberly HE, Camarata SM, & Wallace MT (2014). Evidence for diminished multisensory integration in autism spectrum disorders. Journal of autism and developmental disorders, 44(12), 3161–3167. 10.1007/s10803-014-2179-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR (1987). The organization and neural substrates of human memory. International Journal of Neurology, 21–22, 218–222 [PubMed] [Google Scholar]
- Takayanagi M, Kawasaki Y, Shinomiya M et al. (2022). Review of Cognitive Characteristics of Autism Spectrum Disorder Using Performance on Six Subtests on Four Versions of the Wechsler Intelligence Scale for Children. J Autism Dev Disord 52, 240–253. 10.1007/s10803-021-04932-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Center V, & Mailick M (2014) A longitudinal examination of 10-year change in vocational and educational activities for adults with autism spectrum disorders. Dev Psychol. 50, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, & Seltzer MM (2012). Developing a vocational index for adults with autism spectrum disorders. Journal of autism and developmental disorders, 42(12), 2669–2679. 10.1007/s10803-012-1524-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E (1995). Organization of memory: Quo vadis? In Gazzaniga MS (Ed.), The cognitive neurosciences (pp. 839–853). The MIT Press. [Google Scholar]
- Torenvliet C, Groenman AP, Radhoe TA, Agelink van Rentergem JA, Van der Putten WJ, & Geurts HM (2022). Parallel age-related cognitive effects in autism: A cross-sectional replication study. Autism research: Official journal of the International Society for Autism Research, 15(3), 507–518. 10.1002/aur.2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Kenworthy L, Pugliese C, Popal H, White E, Brodsky E, Martin A (2016). Real-world executive functions in adults with autism spectrum disorder: Profiles of impairment and associations with adaptive functioning and co-morbid anxiety and depression. Journal of Autism and Developmental Disorders, 46(3), 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Jarrold C, Grainger C, & Lind SE (2014). Diminished time-based, but undiminished event-based, prospective memory among intellectually high-functioning adults with autism spectrum disorder: relation to working memory ability. Neuropsychology, 28(1), 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Minshew NJ, Goldstein G, & Mazefsky CA (2017). Long-term memory in older children/adolescents and adults with autism spectrum disorder. Autism Research, 10(9), 1523–1532. 10.1002/aur.1801 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


