Abstract
In order to solve the problem of loose meat quality and single flavor level of broiler chicken, the present study was conducted to improve the quality characteristics of chicken by incorporating unfermented (URH) and fermented Rumex hanus (FRH) in basal diets. URH supplementation significantly improved meat textural properties, evidenced by increased hardness, gumminess and chewiness. Histomorphological analysis demonstrated denser myofibrillar structures in URH-treated samples, concomitant with elevated immobilized water content and reduced free water fraction, contributing to enhanced tenderness and juiciness. Compared to CON, supplementation of FRH increased volatile organic compounds (48.97 %), notably increasing the content of nitrogen oxides and sulfur-containing compounds, as well as saltiness, umami and richness, with higher alcohol (wine aroma) and ester (fruity) contents. Liquid chromatography-mass spectrometry (LC-MS) detected the coclaurine and L-aspartic acid as molecular mimetic candidates. Molecular simulations revealed stable binding of flavor compounds to myosin without conformational perturbation, primarily mediated by intermolecular interactions with residues Lys273 and Tyr135. These findings highlight the potential of Rumex hanus to improve meat quality and flavor and also provide a theoretical framework for innovation in the field of feed.
Keywords: Rumex hanus, Meat quality, Volatile flavor compounds, Molecular docking analysis, Molecular dynamics simulations
Highlights
-
•
Rumex hanus improved meat quality, offering sustainable poultry nutrition.
-
•
FRH elevated esters, alcohols and ketones, enhancing fruity and creamy aromas.
-
•
Molecular simulation revealed stable binding of flavor compounds to myosin.
-
•
Key flavor compounds like ethyl acetate bound strongly to myosin for flavor stability.
1. Introduction
Rumex hanus, commonly referred to as “protein grass”, is emerging as a promising high-quality source of plant-based protein (Gao, Huang, Huang, & Ming, 2024) with the characteristics of wide distribution, cold resistance and strong environmental adaptability (Hu et al., 2023). Under the same planting area, its crude protein yield was 9 times and 14 times that of soybean and corn, respectively (Lou et al., 2021). Studies on Rumex hanus primarily focused on extracting and analyzing its proteins and active compounds in recent years. Spínola, Llorent-Martínez, and Castilho (2018) used ultrasound-assisted methods to extract polyphenols from Rumex hanus, which exhibited significant antioxidant activity, which could inhibit the oxidation of chicken lipids and delay the deterioration of meat quality. Protein sources are critical components of poultry diets (Belhadj Slimen, Yerou, Ben Larbi, M'Hamdi, & Najar, 2023). High protein content and rich supply of essential amino acids and minerals of Rumex hanus position it as a potential high-quality feed ingredient (Yang et al., 2024). Additionally, the use of fermented feed products has gained increasing attention in recent years due to their ability to enhance feed's nutritional and functional properties (Canibe & Jensen, 2012). For example, Li et al. (2025) found that replacing soybean meal with fermented Rumex hanus diet could improve the growth performance, slaughter performance and meat quality of sheep, probably due to the improvement of the intestinal flora by the fiber in it and the combined effect of organic and inorganic nutrients. In light of these considerations, this study aims to investigate whether incorporating fermented Rumex hanus into poultry diets yields favorable outcomes.
White feather broilers are commonly utilized to compare the growth performance of specialized diets against standard basic diets, owing to their rapid growth characteristics. Recently, there has been increasing interest in incorporating solid fermented feeds into broiler diets. Fermentation is known to enrich active ingredients, enhance taste and flavor, and reduce the content of toxic or anti-nutritional factors. Niu et al. (2020) reported that fermented cottonseed meal decreased fat deposition in white feather broilers by enhancing cecal microbiota and metabolite profiles. It was found that supplementing diets with palm kernel meal improved fatty acid composition and flavor profile in sheep (Ma et al., 2024). Studies have shown that fermented Rumex hanus diets can enhance the growth performance of finishing pigs, improve their blood parameters and carcass traits, and increase the percentage of lean meat (Lei et al., 2023). Few studies have examined the impact of unfermented and fermented Rumex hanus on the texture and flavor of meat. In this study, we investigated how unfermented and fermented Rumex hanus contribute to improving chicken meat quality.
Over the past few decades, the evaluation methodology for meat quality has progressively developed into a comprehensive and well-established system. Techniques such as headspace-solid phase microextraction gas chromatography–mass spectrometry (HS-SPME-GC–MS) and liquid chromatography-mass spectrometry (LC-MS) are commonly employed to identify volatile flavor compounds and differential metabolites. Their results often correlate with findings from electronic nose (E-nose) and electronic tongue (E-tongue) analyses. Fu, Fang, et al. (2024) reported adverse effects of chronic heat stress on the sensory properties of oysters based on E-nose, E-tongue, GC–MS, and LC-MS. Furthermore, molecular docking offers valuable insights into ligand-receptor binding sites and interaction patterns. However, molecular dynamics simulations address the limitations of molecular docking by illustrating the dynamic nature of these interactions, providing a more comprehensive understanding of complex molecular systems. Yu et al. (2023) demonstrated that appropriate or optimized heat treatment can increase the exposure of binding sites, thereby enhancing the interactions between myofibrillar proteins (MPs) and pyrazine compounds. Studies also have identified hydrogen bonding, van der Waals forces, and hydrophobic interactions as the primary forces driving the binding of MPs to various furan derivatives (Yin, Gao, Wang, Chen, & Kong, 2022). It is evident that MPs are frequently utilized as receptors to investigate the binding and release properties of flavor compounds. Additionally, myosin, the predominant protein in MPs, is commonly employed in molecular simulations. This approach provided more profound insights into the binding tightness and affinity of myosin to aroma-active compounds and metabolites.
This study aimed to investigate the effects of unfermented and fermented Rumex hanus on chicken breast meat quality and the molecular mechanisms underlying their interactions. The influence of Rumex hanus diets on moisture content, texture structure, antioxidant properties, and flavor compounds was evaluated using various advanced analytical techniques. These included chromatic aberration analysis, texture profile analysis (TPA), scanning electron microscopy (SEM), hematoxylin-eosin (HE) staining, low-field nuclear magnetic resonance (LF-NMR), E-nose, E-tongue, reactive oxygen species (ROS) measurements, HS-SPME-GC–MS, and LC-MS. Furthermore, molecular docking and molecular dynamics simulations were used to explore the interactions between selected flavor compounds and chicken myosin. This comprehensive approach provides valuable new insights and theoretical foundations for developing innovative broiler diets and improving chicken meat quality.
2. Materials and methods
2.1. Sampling and processing of Rumex hanus
Dried Rumex hanus leaves (moisture content about 15 %, and the growth cycle 1 month), sourced from Wuzhishan, Hainan, China. The leaves were ground into a fine powder (40 mesh sieve) and incorporated into standard powdered chicken feed at a 10 % inclusion rate to produce the unfermented Rumex hanus (URH) diet. The preparation of fermented Rumex hanus involved mixing 10 kg of Rumex hanus powder with purified water at a 1:10 ratio. The mixture was inoculated with 5 g of Lactiplantibacillus plantarum and a mixed culture of Lactiplantibacillus (≥1 × 1011 CFU/g, Chr. Hansen Co., Ltd.), then subjected to anaerobic solid-state fermentation at 38–42 °C for 72 h. After fermentation, the product was freeze-dried, resulting in the fermented Rumex hanus (FRH). The FRH was also added to chicken feed at a 10 % inclusion rate. The nutrient compositions of unfermented and fermented Rumex hanus are presented in (Table 1). The basal diet contains 58.50 % corn, 29.60 % soybean meal, 3.00 % soybean oil, 5.00 % peanut vine, 0.22 % salt, 0.1 % threonine, 0.18 % methionine, 1.40 % dicalcium phosphate, and 2.00 % premix.
Table 1.
Nutrient content of unfermented and fermented Rumex hanus.
| Items | Protein (g/100 g) | Fat (g/100 g) | Ash (g/100 g) | Crude fiber (%) |
|---|---|---|---|---|
| URH | 32.63 ± 0.13 | 4.47 ± 0.15 | 15.07 ± 0.07 | 8.13 ± 0.07 |
| FRH | 34.07 ± 0.09 | 4.33 ± 0.12 | 14.97 ± 0.03 | 8.20 ± 0.12 |
UFH = unfermented Rumex hanus; FRH = fermented Rumex hanus.
2.2. Experimental design and feeding management
The feeding method employed in this study was adapted from Wang et al. (2024) with slight modifications. A total of 120 healthy, 3-day-old white feather broiler male chicks (Yunnan Fengzhen Agriculture and Animal Husbandry Co., Ltd., Yunnan, China) were randomly allocated into three dietary groups, each consisting of five replicates of eight chicks in a cage. The stocking density did not exceed 30 kg/m2, and all chicks were adapted for 4 days. The three dietary treatments included a basal diet (CON), a basal diet supplemented with 10 % unfermented Rumex hanus (URH), and a basal diet supplemented with 10 % fermented Rumex hanus (FRH). The experimental chicken house was a semi-enclosed facility that allowed for manual control of temperature, humidity, and ventilation. The initial temperature was maintained at 32 °C, followed by a gradual reduction of 2–3 °C per week until stabilizing at 20 °C. The trial was conducted at the experimental chicken farm of the College of Animal Science and Technology, Yunnan Agricultural University (Ethics Review Number: 202209012).
2.3. Sample collection
The sampling method referred to GB/T 19478-2018 and Wang et al. (2024). Samples were collected on day 42nd after a 12-h fasting period. Following the fasting, the broiler was weighed, and automatic anaesthesia (30–50 V shock) was administered. The neck, just behind the lower forehead, was incised with a knife, and the trachea, blood vessels, and esophagus were removed. The bleeding duration ranged from 3 to 5 min, after which the sampling process was carried out. Following slaughter, the left breast muscle was carefully excised before freezing in the refrigerator at −80 °C for subsequent pH measurements, color differences, and other quality indicators. Appropriate samples were then used for various analyses, including texture profile analysis (TPA), histological examinations, E-nose and tongue evaluations, and assessments of both volatile and non-volatile differential metabolites. Replicates were prepared for each analysis to ensure data reliability.
2.4. Growth performance evaluation
The methods of the assessment referred to Zhang et al. (2021) with minor modifications. The weight of the chickens was recorded on a weekly basis. The average final body weight (BD) of each group was recorded, and key growth performance indicators, including average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (F/G), were calculated as follows:
ADG (g) = (final BD − initial BD)/number of test days.
ADFI (g) = average feed intake/number of test days.
F/G = average feed intake/total weight gain.
2.5. Meat quality determination
2.5.1. Determination of pH
The pH value of the left breast muscle was determined at one h and 24 h post-slaughter using a pH meter (pH-STAR, MATTHAUS, Eckelsheim, Germany) (Wang et al., 2024). The probe was inserted into the center of the chicken breast, and the puncture depth was more than 1 cm. The pH measurement was repeated three times for each sample to ensure accuracy and consistency.
2.5.2. Determination of flesh color
The color of the left breast muscle was evaluated using a colorimeter (CM-5, Minolta, Tokyo, Japan), measuring brightness (L⁎), redness (a⁎), and yellowness (b⁎) (Wang et al., 2024). The samples of chicken breast were placed snugly against the measuring mouth of the whiteboard-calibrated colorimeter, and three replicates were measured for each indicator.
2.6. Texture profile and histological analysis
The texture of chicken breast samples was evaluated using a cylindrical probe attached to a texture analyzer (FTC TMS-TOUCH, FTC, Washington, USA) (Guo et al., 2019). The test parameters were set to a test speed of 60 mm/min, a trigger force of 0.1 N, and a compression percentage of 50 %. For histological examination, samples were first fixed in universal tissue fixative (Wuhan Powerful Biotechnology Co., Ltd., No. B0038) for 24 h. Muscle tissue was stained using HE staining (the kit from Wuhan Powerful Biotechnology Co., Ltd., No. BH0001). After staining, the samples were dehydrated with a series of graded anhydrous ethanol (≥99.7 %, Sinopharm Chemical Reagent Co., Ltd., No. 100092683), followed by n-butanol (≥ 99.8 %, Sinopharm Chemical Reagent Co., Ltd., No. 10005208) and xylene (≥99.0 %, Sinopharm Chemical Reagent Co., Ltd., No. 10023418), before being imaged under a microscope. The optical microscope (NIKON ECLIPSE CI, Nikon, Tokyo, Japan) was used to assess the muscle structure further. Samples were dehydrated in graded ethanol solutions, sputter-coated with gold for 10 s, and examined under a scanning electron microscope.
2.7. Analysis of LF-NMR
Transverse relaxation data were collected from chicken breast samples subjected to various treatments using a MesoMR23-060H-I LF-NMR analyzer (Suzhou Newmai Analytical Instrument Co., Ltd., Suzhou, China). The measurement parameters were set as follows: a waiting time of 3500 ms, an echo time of 0.08 ms, a total of 18,000 echoes, and a sampling frequency of 250 kHz (Guo et al., 2020). Three replicates were measured.
2.8. ROS and antioxidant parameters
The antioxidant index was modified slightly according to the method used by Fu, Zheng, et al. (2024). The content of ROS in raw chicken breast was determined using a ROS assay kit (NO. E004-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Following digestion and centrifugation of muscle tissue, the cells were re-suspended in phosphate-buffered saline (PBS) and transferred to a 96-well plate. Fluorescence was measured at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Additionally, oxidant and antioxidant contents were further analyzed using a series of specialized assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). NADPH (No. A115-1-1) was assessed using the oxidized thiazole blue method at 570 nm after 0.10 g of tissue being tribulated, extracted, centrifuged protected from light, while glutathione (GSH, No. A006-1-1) levels were determined at 420 nm through a reaction with dithiodinitrobenzoic acid after 0.10 g of tissue being weighed and homogenized, centrifuged, and mixed with various reagents in the kit. Catalase (CAT, No. A007-1-1) activity was measured at 405 nm using the ammonium molybdate method after the tissue being mixed with normal saline at a ratio of 1:9, and the supernatant being taken after centrifugation. Malondialdehyde (MDA, No. A003-1) content were determined at 532 nm using the thiobarbituric acid (TBA) assay after 1.0 g of tissue being prepared into a 10 % homogenate, mixed with each reagent, taken by 95 °C water bath, centrifugation, and supernatant. Total superoxide dismutase (T-SOD, No. A001) activity was evaluated at 560 nm by measuring the conversion of hydroxylamine to nitrite via xanthine oxidase after the samples mixed with normal saline at a ratio of 1:9, homogenized, centrifuged, and supernatant mixed with the various reagents in the kit. Protein concentrations were normalized using a bicinchoninic acid (BCA, No. A045-3-2) protein assay kit to ensure measurement accuracy. The protein concentration of the sample was calculated by substituting the absorbance value of the sample into the standard curve. The antioxidant indicators above are calculated as follows:
2.9. Sensory property analysis of samples using E-nose and E-tongue
The olfactory characteristics of muscle samples were analyzed using a portable E-nose (PEN3, AIRSENSE, Schwerin, Germany) under direct headspace aspiration conditions (Banerjee, Roy, Tudu, Bandyopadhyay, & Bhattacharyya, 2019). Measurement settings included a sampling time of 1 s per group, a sensor self-cleaning time of 80 s, a sample injection time of 5 s, an injection flow rate of 400 mL/min, and a total analysis sampling time of 80 s. Taste characteristics were assessed with an E-tongue (SA402B, Insent, Atsugi, Japan) equipped with six sensors and three standard electrodes to measure five basic tastes—sour, sweet, bitter, salty, and umami—along with astringency. The testing protocol consisted of a 30-s measurement to obtain the initial taste values. After a brief cleaning with the reference solution for 3 s, the sensors were placed into a new reference solution for a subsequent 30-s aftertaste measurement. Each sample was analyzed in triplicate to ensure consistency and reliability.
2.10. Analysis of volatile compounds using HS-SPME-GC–MS
Volatile compounds were extracted from samples using headspace solid-phase microextraction (HS-SPME) (Li, Geng, et al., 2022). Deuterated n-hexanol was used as the internal standard, and the volatile aroma of the sample was sampled using SPME. The SPME fibers are inserted into the gas chromatography injector and the extracted analytes are thermally desorbed at 250 °C. Put the SPME fiber to chamber at 270 °C for 10 min after the injection step. Chromatography was performed using a GC × GC-TOF MS system (LECO Pegasus BT 4D, LECO, St. Joseph, MI, USA) consisting of an Agilent 8890 A GC (Agilent Technologies, Palo Alto, CA, USA) system equipped with a split/splitless injector, and dual-stage cryogenic modulator (LECO) coupled with a TOFMS detector (LECO). The separation system utilized a DB-Heavy Wax (30 m × 250 μm × 0.5 μm) column (Agilent, USA). The carrier gas was high-purity helium (>99.99 %) at a constant flow rate of 1.0 mL/min. The oven temperature program was as follows: initially, the temperature was held at 50 °C for 2 min, then increased to 230 °C at a rate of 5 °C/min, and maintained at 230 °C for 5 min.
Mass spectrometry analysis was conducted using a LECO Pegasus BT 4D. The transfer line and TOF MS ion source temperatures were maintained at 250 °C. Spectra were acquired at a frequency of 10 spectra per second. The mass spectrometer operated in electron impact (EI) mode at 70 eV, covering a mass-to-charge ratio (m/z) range of 35–550, with a detector voltage set to 1960 V. To evaluate the contribution of volatile compounds to the overall aroma profile, the relative odor activity value (ROAV) was calculated. Compounds with ROAV values greater than 1 were classified as key volatile components, while compounds with ROAV values between 0.1 and 1 were considered modifiers or potential volatile constituents (Yi, Li, Zhu, Liu, & Quan, 2021). The calculation formulas used to determine ROAV are provided below.
OAVmax represents the highest odor activity value among volatile compounds, and OAVi is the odor activity value of a particular volatile compound.
Ci represents the concentration percentage of a volatile compound in the sample, while Ti represents the odor threshold of the compound in water.
2.11. Determination of differential metabolites by LC-MS
The sample was accurately weighed 50.0 mg into a 2 mL centrifuge tube and add 1000 μL of tissue extract. Samples were ground, sonicated, centrifuged, and then concentrated and dried for LC-MS detection. Differential metabolites were analyzed by chromatography using a Thermo Vanquish ultra-high performance liquid chromatography system (Thermo Fisher Scientific, Waltham, USA) equipped with an ACQUITY UPLC HSS T3 column (2.1 × 100 mm, 1.8 μm (Zelena et al., 2009); Waters, Milford, MA, USA). The column was maintained at 40 °C. The flow rate and injection volume were set at 0.3 mL/min and 2 μL, respectively. For LC-ESI (+)-MS analysis, the mobile phases consisted of (B2) 0.1 % formic acid in acetonitrile (v/v) and (A2) 0.1 % formic acid in water (v/v). Separation was conducted under the following gradient: 0–1 min,10 % B2; 1–5 min, 10 %–98 % B2; 5–6.5 min, 98 % B2; 6.5–6.6 min, 98 %–10 % B2; 6.6–8 min, 10 % B2. For LC-ESI (−)-MS analysis, the analytes were carried out with (B3) acetonitrile and (A3) ammonium formate (5 mmol/L). Separation was conducted under the following gradient: 0–1 min, 10 % B3; 1–5 min, 10 %–98 % B3; 5–6.5 min, 98 % B3; 6.5–6.6 min, 98 %–10 % B3; 6.6–8 min, 10 % B3.
Mass spectrometric detection of metabolites was performed on Q Exactive Focus (Thermo Fisher Scientific, USA) with an ESI ion source. Simultaneous MS1 and MS/MS (Full MS-ddMS2 mode, data-dependent MS/MS) acquisition was used. The parameters were as follows: sheath gas pressure, 40 arb; aux gas flow, 10 arb; spray voltage, 3.50 kV and − 2.50 kV for ESI(+) and ESI(−), respectively; capillary temperature, 325 °C; MS1 range, m/z 100–1000; MS1 resolving power, 70000 FWHM; number of data dependant scans per cycle, 3; MS/MS resolving power, 17500 FWHM; normalized collision energy, 30 eV; dynamic exclusion time.
2.12. Homology modelling and molecular docking
Homology modelling was performed using the SWISS-MODEL website to identify a structural model with the highest sequence identity to the target myosin protein (PDB ID: 6YSY, obtained from the RCSB PDB website) (Li et al., 2024). Model quality and reliability were assessed using a Ramachandran plot. Compounds for molecular docking were selected based on LC-MS and GC–MS screening results. LC-MS data were evaluated using P values, VIP values, relative concentrations, and E-tongue taste profiles to identify key flavor contributors: the umami-enhancing compound L-aspartic acid and the bitter compound coclaurine. Volatile compounds were also chosen using ROAV values, highlighting ethyl acetate and 4-methyl-2-heptanone as the most impactful odorants. These critical flavor and odor compounds were subjected to molecular docking studies using PyRx (version 0.8) software. Binding site predictions and binding energy calculations were performed to evaluate the interactions between the selected compounds and myosin models.
2.13. Molecular dynamics simulations (MDS)
Molecular dynamics simulations were conducted using GROMACS software to analyze the stability and interactions of complexes obtained from molecular docking. The procedure followed Huang et al. (2025) with minor modifications. Ligand topologies were generated using Sobtop (version 1.0), while charge calculations were performed with Orca (version 5.0.4) and Multiwfn (version 3.8). The Amber99sb.ff force field was applied to the receptor, and the GAFF force field was used for the ligand. Sodium and chloride ions were introduced to neutralize the system's charge. The system was equilibrated for 200 ps before running production simulations for 100 ns. The analysis included several structural and interaction parameters: root mean square deviation (RMSD), root mean square fluctuation (RMSF), radius of gyration (Rg), hydrogen bond interactions, solvent-accessible surface area (SASA), and binding free energy.
2.14. Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) performed in SPSS (version 27). Duncan's multiple comparison tests were conducted to identify significant differences among groups. Results are expressed as mean ± standard deviation, and statistically significant differences were denoted by superscript letters (a–d) for p < 0.05. Data visualization was performed using Origin software (version 2021, OriginLab), with asterisks used to indicate levels of significance where * represents p < 0.05, ** represents p < 0.01, and *** represents p < 0.001.
3. Results and discussion
3.1. Growth performance and meat quality characteristics
The inclusion of URH or FRH in the basal diet did not result in significant changes (P > 0.05) in initial or final body weights (Table 2). Additionally, average daily feed intake (ADFI), average daily gain (ADG), and feed/gain ratio (F/G) showed no statistically significant differences among the groups (P > 0.05). However, some studies have shown that mixed diets with fermented Rumex hanus increased the growth performance of sheep (Li et al., 2025), which differs from the findings of this study and may be due to differences in the digestive systems of herbivorous sheep and chickens.
Table 2.
The growth status and meat quality changes of white feather broilers.
| Items | Group |
||
|---|---|---|---|
| CON | URH | FRH | |
| Original BW (g) | 130.61 ± 0.52a | 131.40 ± 0.60a | 130.18 ± 0.53a |
| Final BW (g) | 2091.33 ± 25.23a | 2047.72 ± 36.79a | 2068.86 ± 22.25a |
| ADFI (g) | 105.58 ± 2.87a | 96.65 ± 4.56a | 108.29 ± 5.18a |
| ADG (g) | 44.79 ± 1.15a | 42.42 ± 1.10a | 44.06 ± 1.98a |
| F/G (g/g) | 2.36 ± 0.10a | 2.28 ± 0.12a | 2.45 ± 0.23a |
| pH1h | 6.27 ± 0.028b | 6.26 ± 0.010b | 6.35 ± 0.015a |
| pH24h | 6.21 ± 0.005c | 6.24 ± 0.019b | 6.31 ± 0.010a |
| Shear force (N) | 5.42 ± 0.11b | 9.95 ± 0.18a | 4.62 ± 0.18c |
| L* | 29.51 ± 0.50a | 27.43 ± 0.15b | 26.55 ± 0.39b |
| a* | 3.21 ± 0.11c | 4.45 ± 0.14b | 5.34 ± 0.23a |
| b* | 9.26 ± 0.12c | 10.35 ± 0.13b | 10.88 ± 0.09a |
BW = body weight; ADFI = average daily feed intake; ADG = average daily gain; F/G = feed/gain ratio; L⁎: luminance; a⁎: redness; b⁎: yellowness.
CON = basal diet (BD); URH = BD + 10 % unfermented rumex hanu; FRH = BD + 10 %fermented rumex hanu.
a–c means the average value of its upper index has significant difference, that is, p < 0.05.
The pH measurements at 1-h post-slaughter showed that the CON and URH groups had lower pH values than the FRH group. After 24 h, the FRH group exhibited the highest pH levels, while the CON group had the lowest, with significant differences. This might provide a longer shelf life, as well as improved tenderness, associated with a higher pH value. Over time, the pH of all three groups decreased, which is generally attributed to the accumulation of lactic acid produced during muscle glycolysis. The addition of URH and FRH in the basal diet decreased the L* values of chicken breast meat, while a* and b* values increased significantly (p < 0.05). The L* value of the FRH group was 10.03 % lower than that of CON, and the higher L* was due to the increase in light reflection caused by water exudation in the meat (Jin et al., 2021). The increase in a* reflects an improvement in meat redness, which aligns with findings of Miao et al. (2024), who observed a rise in a* values in irradiated raw chicken. However, the accumulation of reactive oxygen species (ROS) can induce oxidative stress in ketone bodies, ultimately oxidizing myoglobin and negatively affecting meat color (Qi, Zhang, Yang, Luo, & Mao, 2023). This oxidative process often results in a decrease in a* values. Therefore, the observed enhancement in meat color may be associated with the production or increased levels of certain metabolites possessing antioxidant properties due to the feeding of Rumex hanus, which could mitigate myoglobin oxidation and improve overall meat appearance.
3.2. Analysis of TPA and histological properties
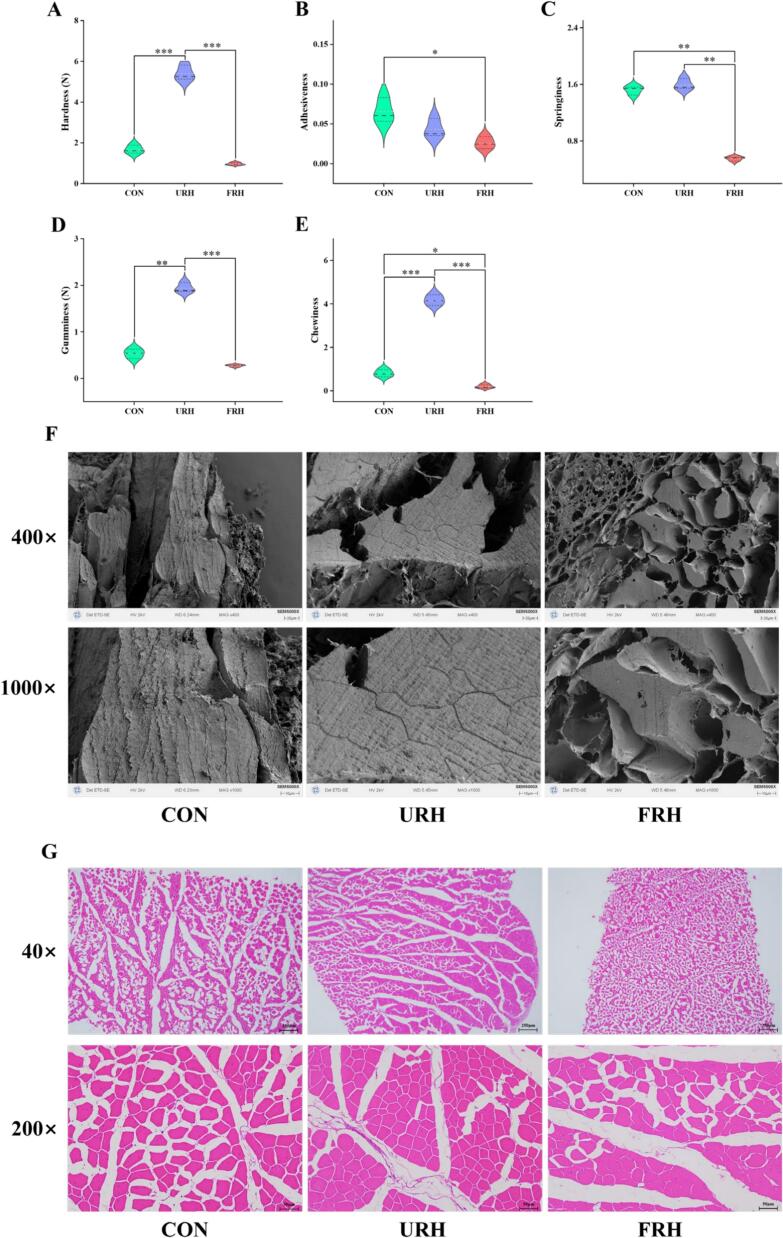
The Texture Profile Analysis (TPA) results are presented in (Fig. 1A-1E). The data show that hardness, gumminess, and chewiness exhibited the highest values observed in the URH group and the lowest in the FRH group, with significant differences. Specifically, the addition of URH to the basal diet enhanced the firmness and chewiness of the chicken, improving the overall texture. This may be attributed to the reduced oxidation levels, which help preserve the structural integrity of muscle proteins (MPs). A more stable three-dimensional network consequently results in improved texture of the chicken (Sun, Zhang, & Sun, 2024). The mild improvement in chewiness can give the loose meat a better taste in white feather broilers. Gu et al. (2021) found that small white feather broilers had higher shear force and more tender meat than large broilers. The springiness was slightly higher in the URH than in the CON group with no significant difference. However, softer but less springy chicken was found in the FRH group, which may be the result of differences in protein and fiber after fermentation of Rumex hanus affecting the myofiber network.
Fig. 1.
Texture profile analysis (TPA) of chicken left breast chicken meat (A-E). Scanning electron microscopy (SEM) under 400× and 1000× (F). Hematoxylin-eosin (HE) staining under 40× and 200× (G).
Microstructural differences among the three groups were observed through SEM (Fig. 1F). In the URH group, myofibers were tightly packed and regularly arranged, whereas the muscle fibers in the CON group displayed noticeable gaps. These gaps could facilitate the formation of water channels, potentially reducing the muscle's water retention capacity (Li, Guo, et al., 2022). The muscle fibers in the FRH group were loosely arranged and exhibited a porous structure, which could negatively impact the texture, aligning with the results from the TPA analysis. Histological examination using hematoxylin and eosin (HE) staining of muscle fiber cross-sections (Fig. 1G), revealed similar patterns. The URH group exhibited neatly organized, closely packed muscle fibers with smaller diameters, which indicates improved muscle structure. This finding is consistent with recent studies suggesting that broad bean diets enhance meat quality by reducing muscle fiber diameter and increasing fiber density, thereby improving hardness and elasticity in grass carp (Fu et al., 2024). These results further support the beneficial effect of adding URH to the diet in improving the texture of chicken meat.
3.3. Moisture distribution in chicken meat: LF-NMR analysis of water binding in response to URH and FRH supplementation
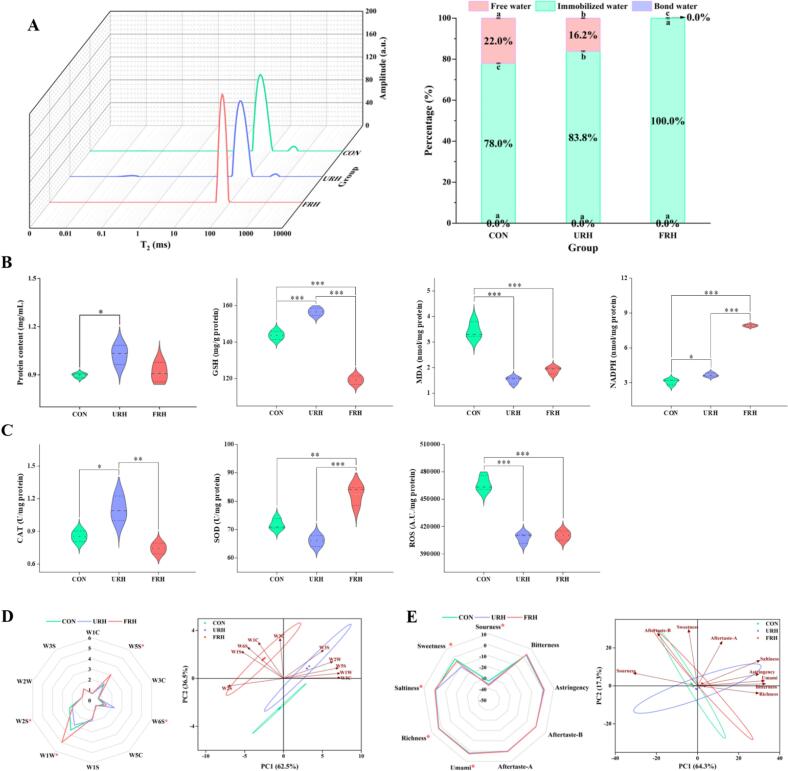
The LF-NMR analysis of moisture distribution is shown in (Fig. 2A). In LF-NMR, water molecules are classified based on their relaxation times into three categories: bound water (0.01–10 ms), which is tightly bound to other molecules; immobilized water (10–100 ms); and free water (100–1000 ms), which exhibits the weakest binding force (Wang, Yao, et al., 2023). The T2 peak analysis revealed that no bound water was detected in the CON group, while the FRH group lacked bound and free water. The water distribution in the three groups was characterized by the proportions of bound water (P2b), immobilized water (P21), and free water (P22). The results showed a significant increase in immobilized water in the URH and FRH groups, contributing to enhanced chicken breast tenderness. The transition from free water to immobilized water likely made the chicken tissue tender and juicier. This finding is consistent with Pearce, Rosenvold, Andersen, and Hopkins (2011), who reported that tender meat quality is often associated with a higher proportion of immobilized and lower amounts of free water. Additionally, this change in water distribution could be related to the increased protein content in the URH and FRH groups. The proteins likely cross-link to form a network that binds water molecules, reducing their mobility and enhancing their texture. Furthermore, the denser myofiber structure and reduced crevice formation observed in the muscle tissues of the URH and FRH groups may also contribute to these changes in moisture distribution and texture, supporting the findings from earlier analyses. It was also worth noting that the immobilized water in the FRH group was significantly increased compared to the other groups, close to 100.0 %. This finding could be explained by the findings of Li et al. (2025). They found that the Rumex hanus were fermented by mixed bacteria to degrade plant carbohydrates and other macromolecular substances in them, increasing the content of soluble fiber, and enhancing the body's protein synthesis and metabolism. Fermented crude proteins, as well as soluble fibers (e.g., gum and dextran, etc.), are exposed to more hydroxyl and carboxyl groups and can form a stronger hydrogen bond network with water molecules, which may explain the significantly increased immobilized water in the FRH group.
Fig. 2.
Low-field nuclear magnetic resonance relaxation time T2 spectrum 3D diagram and moisture ratio diagram (A). Protein content and glutathione (GSH), malondialdehyde (MDA), NADPH oxidation index (B). Catalase (CAT), superoxide dismutase (SOD) and reactive oxygen species (ROS) (C). Radar map and PCA score map of electronic nose (D). Radar map and PCA score map of electronic tongue (E).
3.4. Effects of URH and FRH supplementation on protein content and antioxidant properties of chicken meat
The protein content of chicken breast is shown in (Fig. 2B), where it is evident that the inclusion of URH and FRH in the feed significantly increased the protein content. This increase can likely be attributed to the high protein content of Rumex hanus, the primary ingredient in these supplements. Several oxidation-related biomarkers are presented in (Fig. 2B and C). GSH levels reflect the antioxidant capacity, while NADPH provides electrons to reduce oxidized glutathione (GSSG) back to GSH. Additionally, CAT and SOD are enzymes that act as scavengers of free radicals, helping to reduce oxidative stress. The results showed that GSH and CAT levels increased in the URH group. In contrast, NADPH and SOD levels were higher in the FRH group, indicating enhanced antioxidant capacity in both groups. In contrast, the CON group exhibited significantly elevated MDA and ROS levels, suggesting increased lipid peroxidation and oxidative stress, leading to oxidative damage. These findings align with those of Li et al. (2023), who demonstrated that Rumex hanus is rich in phenolic compounds and flavonoids, which contribute to its potent free radical scavenging activity. This result is consistent with the antioxidant improvements observed in the URH and FRH groups. Previous studies have demonstrated that moderate oxidation can enhance flavor, whereas excessive oxidation can negatively impact quality (Wang, Xiang, Wei, & Li, 2023). In the regulation of the gut-liver axis in chickens, fermented Rumex hanus can improve the intestinal microbiota, and its metabolites preferentially activate SOD and promote the production of NADPH by activating the pentose phosphate pathway and entering the liver (Lee, Kwon, Apostolidis, Shetty, & Kim, 2011). In contrast, due to the presence of anti-nutritional factors in unfermented Rumex hanus, it was easy to induce oxidative stress in the body, so as to remove H2O2 produced by stress by upregulating the content of GSH and CAT. At the same time, unfermented Rumex hanus might provide more metabolites needed for GSH synthesis.
3.5. Impact of URH and FRH supplementation on chicken meat quality: E-nose and E-tongue analysis
The E-nose simulates the human sense of smell by using various sensors to analyze the volatile compounds in samples (Chen et al., 2020). The E-nose data, presented with principal component analysis (PCA) (Fig. 2D), reveal significant differences in the responses of chicken samples from the three groups to the W5S (nitrogen oxides), W6S (hydrides), W1W (sulfur-containing compounds), and W2S (aromatic compounds and alcohols) sensors. However, no statistically significant responses (p > 0.05) were observed for the other six sensors. These results suggest that dietary modifications impact the levels of nitrogen oxides, such as furans, and sulfur-containing compounds in chicken (Han, Zhang, Fauconnier, & Mi, 2020). Specifically, the FRH group exhibited higher nitrogen oxides and sulfur-containing compounds, while the URH group generated more hydrides. PCA was further employed to analyze the distribution of volatile compounds in the chicken samples (Fig. 2D). The first principal component (PC1) and the second principal component (PC2) accounted for 99.0 % of the total variance, indicating that these two components contain the key information regarding the volatile profiles of the samples. This strongly reflects the overall distribution of volatile compounds across the three dietary treatments.
By simulating the mechanism of human taste perception, the E-tongue, which simulates human taste perception, evaluates the five basic tastes—sour, sweet, bitter, umami, and salty—along with astringency. This technology eliminates the subjectivity of human sensory evaluation (Jiang, Zhang, Bhandari, & Adhikari, 2018). Compared to CON, URH and FRH had lower sour and sweet flavors. Still, the FRH group demonstrated significantly improved response values for saltiness, umami, and richness, while the other flavors showed no statistically significant difference (Fig. 2E). Notably, both sourness and sweetness levels were below the tasteless threshold for human perception, suggesting that these flavors may not be detectable by the human tongue. A combination of bitterness, umami, richness, and saltiness characterized the overall taste profile of the chicken. Studies have shown that the most critical non-volatile substances in chicken are umami and salty substances (Yang, Ge, Ge, Zhao, Liang, & Xiao, 2025; Zheng, Zhang, Sun, Qiao, Sun, & Zhang, 2024), suggesting that FRH is more dominant in favorable tastes. The PCA plot of the E-tongue (Fig. 2E) indicated that PC1 and PC2 explained 64.3 % and 17.3 % of the total variance, respectively, contributing a total of 81.6 % and effectively capturing the differences among the samples. Notably, the data points of the three groups were tightly clustered, suggesting that the E-tongue responses across the groups were relatively similar.
The results from both the E-nose and E-tongue analyses revealed significant differences in the quality of chicken breast between the dietary groups. Specifically, the URH group generated more hydrides. In contrast, the FRH group produced more nitrogen oxides and sulfur-containing compounds than the CON group, and the FRH group had the largest radar area, indicating that the FRH group embraced the richest layers of flavor possible. The FRH group exhibited a significant increase in saltiness, umami, and richness, demonstrating great potential for food processing. These findings suggest that URH and FRH supplementation may lead to differences in metabolic pathways, further enhancing the flavor of chicken. At the same time, FRH reduced crude fiber and anti-nutritional factors through the procedure of fermentation and enrichment, improving the intestinal flora and digestion capacity of chickens, further enhancing the overall flavor of chicken in the FRH group.
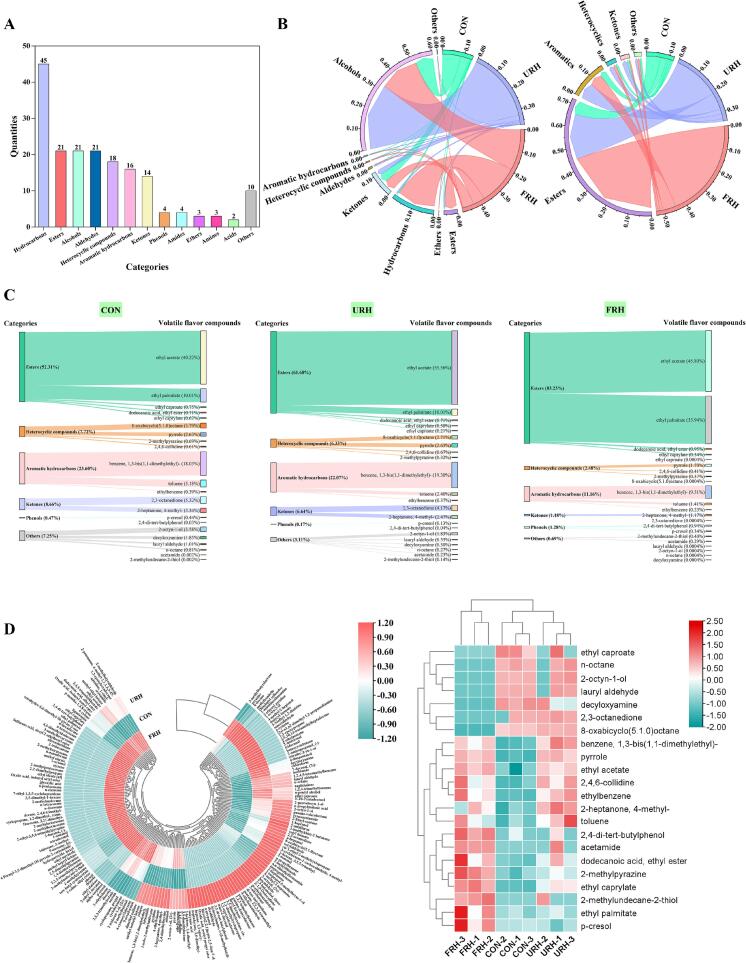
3.6. Assessing volatile metabolites by HS-SPME-GC–MS
Flavor is a critical sensory attribute of chicken that significantly influences consumer perception and preference. In this study, we identified 182 volatile compounds in chicken breast samples, categorized into 13 groups (Fig. 3A). As shown in Fig. 3B and Table S1, the results indicate that the FRH group contained the highest proportion of volatile organic compounds, accounting for 48.97 %, compared to only 13.95 % in the CON group. Alcohols dominated the volatile profile, comprising 60.29 % of the total, with both URH and FRH supplementation significantly increasing their content. Alcohols, with low odor thresholds, are known for their aromatic, plant-like scents, which contribute to the unique aroma of raw chicken (Huang, Ho, & Wang, 2021). This might be due to the fact that water-soluble sugars (glucose, fructose) and anti-nutritional factors (tannins, glucosinolates) in Rumex hanus were the main precursors for its production. Additionally, the FRH group exhibited increased levels of alkanes, which positively impacted the aroma and overall flavor of the chicken meat (Xu, Lu, Song, Zhang, & Guo, 2018).
Fig. 3.
Analysis of GC–MS. Types and number of volatile flavor compounds (A). The volatile proportion chord plot of the CON, URH, and FRH groups and the chord plot of the proportion of key volatile substances after screening (B). Sankey plots of the proportion of key volatile flavor compounds screened for each group (C). GC–MS heat map of all differential flavor compounds and key volatile substances (D).
Filter criteria: VIP > 1 as well as P < 0.05. CON: Basic diet (BD). URH: BD + 10 % unfermented rumex hanu. FRH = BD + 10 %fermented rumex hanu.
A clustered heat map was generated based on the concentration of all compounds to explore further the effects of different feeds on volatile flavor compounds (Fig. 3D). Significant differences were observed in the levels of primary volatile substances, including alcohols, hydrocarbons, and esters. Alcohols are responsible for their distinct aromas and contribute to forming esters, which impart floral and fruity scents during processing (Yao et al., 2022). These compounds enrich the flavor profile, playing a vital role in transitioning from aroma to flavor (Li et al., 2016). In addition to ethanol, the control (CON) group contained 2-aminopropanol (−), a compound associated with a fishy odor, which was absent in the URH and FRH groups. This substance was detected relatively high in the CON group, accounting for 10.92 % of the alcohols. Including URH and FRH in the diet effectively eliminated this undesirable odor from the chicken. Additionally, 18 heterocyclic compounds were identified, including pyrazines, pyridines, and pyrroles, all known for their low odor thresholds. Notably, five pyrazine compounds were detected in the FRH group, which are typically formed through non-enzymatic browning and provide a nutty aroma reminiscent of toasted bread with extremely low odor threshold (Kantono et al., 2021; Sidhu, Lund, Kotseridis, & Saucier, 2015). This was related to the fact that Rumex hanus was fermented to produce abundant free amino acids, which were condensed into a cyclic dipeptide, which is then converted into pyrazine compounds (Lei et al., 2018).
The analysis also revealed the presence of 12 ketones in the URH and FRH groups, with the FRH group showing the highest ketone content (44.58 %). Acetoin (3-hydroxy-2-butanone) and diacetyl (2,3-butanedione) dominated this group's ketone profile. Acetoin is known for its creamy flavor, commonly used as a flavor enhancer in dairy products like cream, cheese, and coffee (Zhen, 2015), contributing a unique milky aroma to the chicken. Diacetyl, with its low odor threshold, imparts a sweet aroma (Sun, Yu, et al., 2024), while other new ketones in the FRH group, such as 2-tridecanone and methyl heptyl ketone, are associated with creamy and fruity tastes, linked to β-ketoacid oxidation products from fatty acid degradation in fermented Rumex hanus (Sarnoski, O'Keefe, Jahncke, Mallikarjunan, & Flick, 2010).
Aldehydes, typically formed from lipid oxidative degradation, are associated with low odor thresholds and contribute significantly to the overall aroma (Sérot, Regost, Prost, Robin, & Arzel, 2001). In this study, the URH group exhibited high levels of (2E)-2-octenal, 4-ethylbenzaldehyde, and hexaldehyde, with the latter contributing a grassy aroma that enhances the chicken's flavor complexity.
The key volatile compounds, identified based on a VIP value greater than 1 and a P-value less than 0.05, are presented in Fig. 3B. Like the total volatile organic compound content, the FRH group contained the highest proportion (53.57 %) of key volatiles, with esters making up 72.09 % of the key volatile compounds. The relative content of esters increased progressively in the URH and FRH groups, with ethyl acetate and ethyl palmitate being the most significant esters (Fig. 3C). Both compounds have extremely low odor thresholds, with ethyl acetate's threshold reaching as low as 0.005 (Table S2), and they played a crucial role in providing fruity and creamy aromas in the FRH group. Additionally, 4-methyl-2-heptanone, a ketone-like substance with an ROAV value greater than 0.1, was also identified as a significant contributor to the flavor profile.
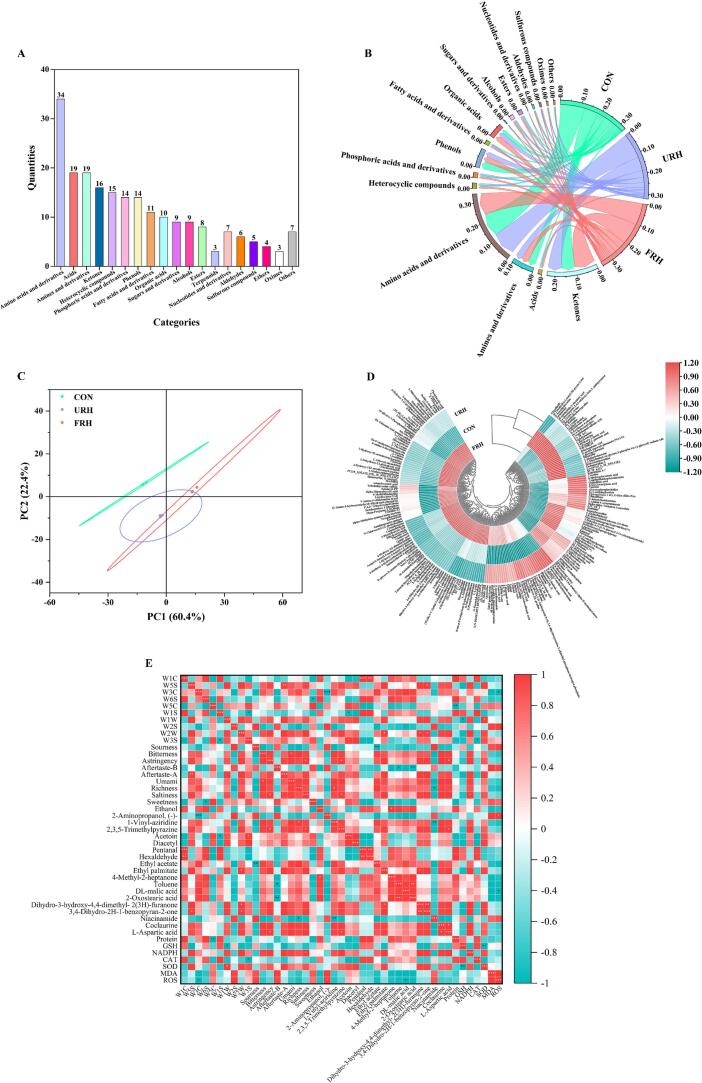
3.7. Assessing non-volatile metabolites by LC-MS
To investigate the differences in non-volatile metabolites in chicken breast, liquid chromatography-tandem mass spectrometry (LC-MS) was employed to detect differential metabolites, resulting in the identification of 1021 metabolites. To increase the accuracy and significance of the analysis, key differential metabolites were selected based on VIP > 1 and p < 0.05, leading to the identification of 213 key metabolites. These included 34 amino acids and derivatives, 19 acids, 19 amines and derivatives, 16 ketones, 15 heterocyclic compounds, and other categories of differential metabolites (Fig. 4A). Notably, the FRH group exhibited a significantly higher concentration of differential metabolites (35.16 %) than the other two groups (P > 0.05) (Fig. 4B). Principal component analysis (PCA), as shown in (Fig. 4C), revealed that PC1 and PC2 accounted for 82.8 % of the total variance, indicating that these two components captured the majority of the sample's variance and explained the main differences between the groups.
Fig. 4.
Analysis of LC-MS. Major differential metabolites after screening (VIP > 1, P < 0.05) (A). The contents of various types of substances in the CON, URH, FRH groups (B). PCA plot (C). Cluster heat map of major differential metabolites (D). Correlation analysis of electronic nose, electronic tongue, important volatile compounds and differential metabolites as well as oxidation indicators (E).
*: P < 0.05, **: P < 0.01, ***: P < 0.001. Red represents a positive correlation, blue represents a negative correlation, and the shade of color represents the strength of the correlation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The scattered distribution of the data points suggests notable differences in the differential metabolites among the three groups. Cluster analysis of the quantitative data for the selected differential metabolites (Fig. 4D) revealed specific variations. The organic acid profile, for instance, showed significant differences. DL-malic acid, known as a flavor enhancer and preservative (Marques et al., 2020), increased by 44.88 % in the URH group and contributed to the sour flavor often associated with ripe fruits. Another key organic acid, 2-oxostearic acid, increased by 50.87 % in the URH group, though its flavor impact remains undefined. Ketones, which are often associated with positive flavors, were found to be higher in the FRH group, with an increase of 47.59 %. Two compounds, dihydro-3-hydroxy-4,4-dimethyl-2(3H)-furanone and 3,4-dihydro-2H-1-benzopyran-2-one, accounted for more than 90 % of the rise in the FRH group, with increases of 94.72 % and 24.38 %, respectively. These compounds are known to impart sweet and vanilla-like flavors to the chicken. Additionally, niacinamide, a derivative found predominantly in the control group (72.07 %), was the most abundant amine derivative. Though niacinamide is not typically associated with flavor, it is a known vitamin in beef (Xu et al., 2023). At the same time, the significant increase in the content of umami amino acids (such as glutamic acid, aspartic acid and their derivatives) in the FRH group confirmed the considerable enhancement of the umami and richness response values of the E-tongue in the FRH group.
In conclusion, the supplementation of FRH in chicken diets significantly increased the content of favorable differential metabolites, contributing to a unique and desirable flavor profile in the chicken breast, allowing the chicken to concretely exhibit a richer umami, saltiness, and other pleasant flavors, increasing consumers' willingness to purchase.
3.8. Correlation analysis between E-nose, E-tongue, volatile compounds, and differential metabolites in broiler chicken meat
To better understand the relationship between the E-nose, E-tongue, key volatile compounds, and critical differential metabolites, a correlation heatmap was generated (Fig. 4E). The results revealed several noteworthy associations. The signal intensity of the W3C sensor, which detected aromatic odors, was found to be significantly inversely proportional to the content of 2-aminopropanol (−), further supporting the idea that this compound negatively impacts the overall aroma of the sample. Additionally, an inverse correlation was observed between Aftertaste-B and 2-oxostearic acid, suggesting that 2-oxostearic acid might reduce the retention time of bitterness in the mouth, despite lacking a distinct flavor. Furthermore, a negative correlation was found between richness and niacinamide, indicating that higher levels of niacinamide in the CON group might adversely affect the overall flavor profile of the chicken, possibly by influencing the umami aftertaste through specific metabolic pathways. In contrast, the relative content of 4-methyl-2-heptanone was positively correlated with ethanol, suggesting that increased ethanol levels may contribute to more intense grassy and fruity aromas (Zhang, Lu, Yu, Chen, & Xu, 2025), characteristic of 4-methyl-2-heptanone, which also provided strong evidence for the relevant test results of GC–MS. Notably, Asp, an umami amino acid, was positively correlated with 2,3,5-trimethylpyrazine, a pyrazine compound known for its pleasant odor, which may contribute to the umami flavor of the chicken. From this result, the FRH group showed a high level of pyrazine content in the GC–MS results, which was also mutually corroborated by the high response intensities of umami and richness in the E-nose results. Additionally, 2-aminopropanol (−) was positively correlated with ROS, highlighting that excessive oxidation can lead to oxidative damage and the formation of undesirable flavor compounds (Wang, Xiang, et al., 2023). In contrast, DL-malic acid, a common antioxidant, was found to reduce ROS levels, further supporting its role in scavenging free radicals, which is consistent with the findings of Guo, Chen, Hong, Jiang, and Zheng (2017). The compounds associated with favorable flavors, such as alcohols, aldehydes, pyrazines, and esters, showed positive correlations. In contrast, compounds detrimental to flavor, like 2-aminopropanol (−), were often linked to excessive oxidation and oxidative damage. Alcohols are typically linked to mellow, wine-like aromas, aldehydes and pyrazines to pleasant odors, and esters to fruity notes. Moderate oxidation in the body generally promotes the formation of favorable flavor compounds (Wang, Xiang, et al., 2023), which aligns with the findings of our study, as determined by GC–MS, LC-MS, and oxidation indicators.
3.9. Homology modelling and molecular docking analysis of volatile compounds and differential metabolites interactions with myofibrillar proteins in chicken meat
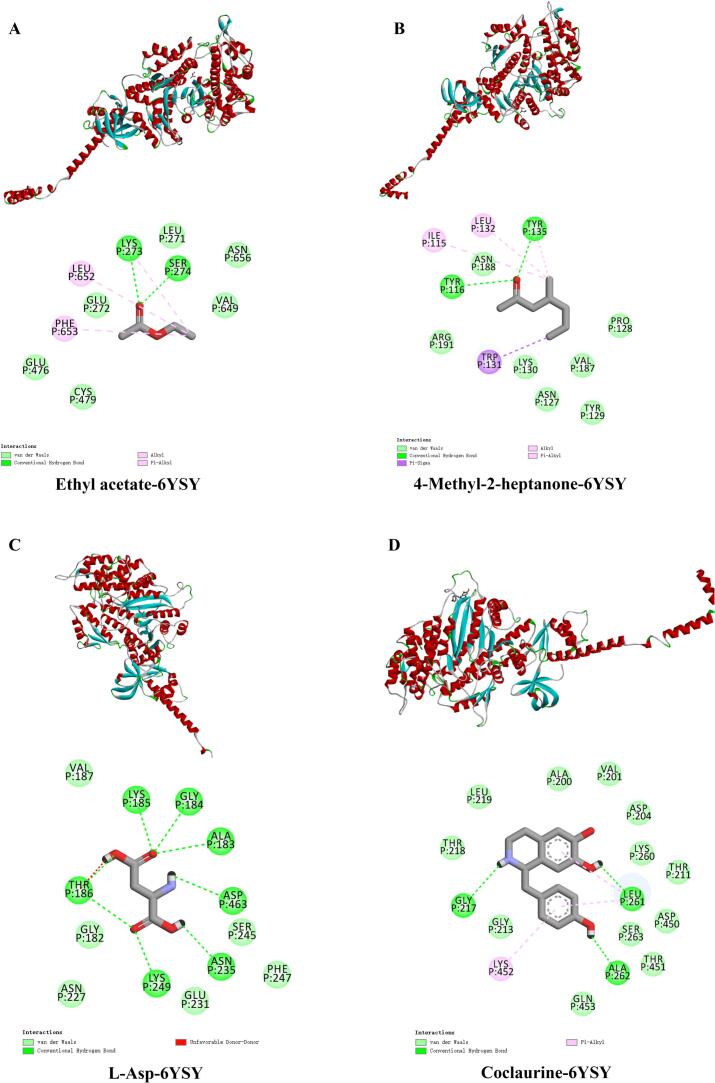
Molecular docking examined the interactions between volatile compounds, differential metabolites, and MPs in chickens. Homology modelling revealed that 98.5 % of the amino acid residues of the selected model were within the confidence region of the pull plot, confirming the high reliability of the model. A binding energy of less than −4 kcal/mol is typically considered indicative of stable binding, and the binding energies for the four complexes, along with the sites of hydrogen bonding and hydrophobic interactions (Table 3). The results indicated stable binding for all four complexes, with the binding between coclaurine and myosin being firm at −7.6 kcal/mol, demonstrating a high affinity between the two. Myosin interacts primarily with the four small-molecule ligands through hydrogen bonds, hydrophobic interactions, and van der Waals forces (Fig. 5). Hydrogen bonding was emphasised as crucial for forming stable complexes, with hydrophobic interactions also contributing to their stability. In forming Ethyl acetate-6YSY complex, hydrogen bonds were formed at residue sites Lys273 and Ser274, while hydrophobic interactions primarily involved Lys273, Leu652, and Phe653. Wu et al. (2023) reported that CAP and myosin formed two short, strong hydrogen bonds at Lys432 and Lys599, with van der Waals forces observed at several sites, including Lys273. Our findings reinforce the importance of Lys273 in forming both hydrogen bonds and hydrophobic interactions, suggesting it is a key site in complex formation. For the 4-methyl-2-heptanone-6YSY complex, hydrogen bonds formed at Tyr116 and Tyr135, while hydrophobic interactions were present at Tyr135, Leu132, Ile115, and Trp131. Notably, Tyr not only formed hydrogen bonds with ketone groups at two sites but also exhibited hydrophobic interactions with methyl groups at Tyr135, positioning it as a key active site for complex formation. Yin et al. (2022) also observed that furan derivatives formed extensive hydrophobic interactions at Tyr269 when bound to myosin, further confirming the critical role of Tyr in stabilizing these complexes. The formation of complexes between L-Asp and myosin primarily involved hydrogen bonds formed at various sites, supplemented by van der Waals forces that provided additional stability. Notably, the interaction at Thr186 was less favorable, Still, the complex's binding energy reached −5.5 kcal/mol, likely due to the formation of polyhydrogen bonds, with the ketone group of L-Asp playing a central role in hydrogen bonding. In the coclaurine-6YSY complex, hydrogen bonds at Leu261, Ala262, and Gly217, combined with hydrophobic interactions at Leu261 and Lys452, facilitated complex formation. The complex exhibited a high affinity, as reflected in the binding energy of −7.6 kcal/mol, with van der Waals forces contributing to its stability. Notably, Leu261 was involved in both hydrogen bonding with the hydroxyl group of coclaurine and hydrophobic interactions with the π-alkyl groups from the benzene rings. Wang et al. (2021) reported similar hydrophobic interactions at Leu141 and Leu347 when myosin bound to 1-heptanol, further underscoring the importance of Leu residues in stabilizing these complexes.
Table 3.
Binding energy and residues involved in hydrogen bonding and hydrophobic interactions for chicken myosin (6YSY) bonding to ethyl acetate, 4-methyl-2-heptanone, L-Aspartic acid and coclaurine.
| Complex | Binding energy (kcal/mol) | Hydrophobic interactions | Hydrogen bonding |
|---|---|---|---|
| Ethyl acetate-6YSY | −4.1 | Lys273, Leu652, Phe653 | Lys273, Ser274 |
| 4-Methyl-2-heptanone-6YSY | −4.8 | Tyr135, Leu132, Ile115, Trp131 | Tyr116, Tyr135 |
| L-Asp-6YSY | −5.5 | \ | Thr186, Lys185, Lys249, Asn235, Asp463, Ala183, Gly184 |
| Coclaurine-6YSY | −7.6 | Leu261, Lys452 | Leu261, Ala262, Gly217 |
Fig. 5.
Molecular docking of myosin protein (6YSY) with different volatile compounds and differential metabolites.
3.10. Molecular dynamics simulations (MDS) of myosin complexes: Stability and affinity of key volatile compounds and differential metabolites
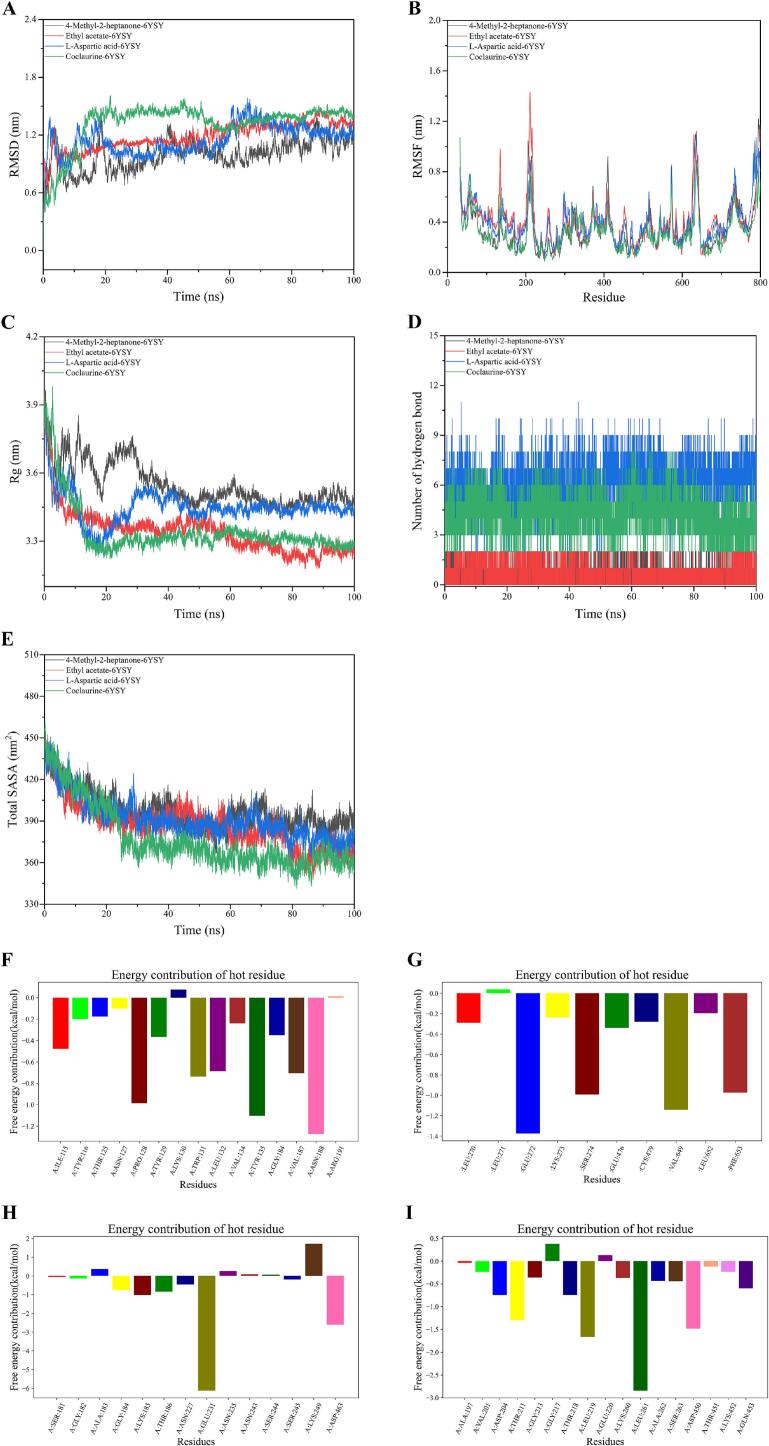
Molecular dynamics simulations (MDS) over 100 ns were employed to further assess the stability and affinity of the four complexes identified through molecular docking. The root mean square deviation (RMSD) was used to evaluate the stability of the system by quantifying the positional deviation of all atoms from their original configuration at any given time (Fateminasab, Bordbar, Shityakov, & Saboury, 2020). As shown in (Fig. 6A), all four complexes exhibited stable fluctuations between 0.6 and 1.5 nm, indicating strong system stability. The root mean square fluctuation (RMSF) was also analyzed to evaluate the flexibility of the amino acids, with high RMSF values indicating increased residue flexibility. As shown in Fig. 6B, the RMSF for all four complexes remained below 1.0 nm, signifying relative stability in most protein regions. However, amino acid residues 200 and 650 exhibited notable oscillations, suggesting that these regions exhibit higher flexibility. The radius of gyration (Rg) was employed to assess the compactness of protein structures (Zheng, Wang, Song, Jiao, & Wang, 2022). The Rg trends of the four complexes are shown in (Fig. 6C) which shows a decrease in compactness during the first 30 ns of simulation, followed by stabilization. This indicated that the complexes underwent initial densification without significant changes to protein conformation. Hydrogen bonding, a key factor in the stable binding of these complexes, was further analyzed (Fig. 6D) (Zhao et al., 2023). The number of hydrogen bonds in the L-Asp-6YSY and Coclaurine-6YSY complexes fluctuated between 3 and 9, indicating that hydrogen bonding was crucial for their stability. Conversely, the ethyl acetate-6YSY and 4-methyl-2-heptanone-6YSY complexes exhibited only 1–2 hydrogen bonds during the simulation, yet still displayed high affinity. This suggested that hydrophobic interactions played a more prominent role in stabilizing these complexes, as confirmed by molecular docking results (Sharma & Paul, 2016). The total solvent-accessible surface areas (SASA) of the complexes were simulated (Fig. 6E). A slight overall decrease was observed until 25 ns, after which stable fluctuations indicated that the tertiary structure of the protein remained essentially unchanged during the simulation, suggesting that the functional regions of the protein were well-protected from external interference. Further analysis of energy contributions from key residues revealed critical amino acid sites involved in complex stability. In the 4-methyl-2-heptanone-6YSY complex, Pro128, Tyr135, and Asn188 contributed significantly to the binding affinity. Similarly, Glu272, Ser274, Val649, and Phe653 were necessary for the ethyl acetate-6YSY complex, while Glu231 played a central role in the interaction with L-Asp. Notably, Leu residues were identified as key contributors to the formation of the coclaurine-6YSY complex (Al-Khafaji & Taskin Tok, 2020). The MMGBSA binding free energy analysis of the four complexes, presented in Table 4, showed that electrostatic energy was the primary factor driving the stability of the complexes by optimizing charge distribution. Van der Waals and gas-phase energy also contributed to the binding, highlighting the importance of hydrophobic interactions in complex stability. While nonpolar solvation energy counteracted binding to some extent, polar solvation energy and solvation energy favored complex stability. The negative free energy of binding confirmed that the formation of these complexes was thermodynamically favorable and tightly bound, with electrostatic interactions and hydrophobic effects being the primary driving forces. In conclusion, the molecular dynamics simulations demonstrated that the interactions between myosin and key volatile substances, as well as differential metabolites, affect the interaction force of the receptor protein but do not change its conformation. These interactions exhibited strong affinity and stability, contributing to a more stable binding of the chicken to the flavor compounds.
Fig. 6.
Analysis of molecular dynamics simulations. Root mean square deviation (RMSD) (A). Root mean square fluctuation (RMSF) (B). Radius of gyration (Rg) (C). Number of hydrogen bond (D). Total solvent accessible surface area (SASA) (E). The energy contribution of hot resides of 4 complexes (F–I).
Table 4.
Energy composition during molecular dynamics simulations.
| Complexs | Energy Component | Average | SD (Prop.) | SD | Standard error of mean (Prop.) | Standard error of mean |
|---|---|---|---|---|---|---|
| 4-Methyl-2-heptanone-6YSY | VDWAALS | −6090.6 | 30.59 | 30.59 | 9.67 | 9.67 |
| EEL | −58,543.46 | 112.84 | 112.84 | 35.68 | 35.68 | |
| EGB | −10,325.79 | 104.25 | 104.25 | 32.97 | 32.97 | |
| ESURF | 321.9 | 2.43 | 2.43 | 0.77 | 0.77 | |
| GGAS | −5654.64 | 160.7 | 150.9 | 50.82 | 47.72 | |
| GSOLV | −10,003.89 | 104.27 | 103.92 | 32.97 | 32.86 | |
| TOTAL | −15,658.53 | 191.56 | 84.15 | 60.58 | 26.61 | |
| Ethyl acetate-6YSY | VDWAALS | −6062.83 | 50.06 | 50.06 | 15.83 | 15.83 |
| EEL | −58,540.69 | 211.26 | 211.26 | 66.81 | 66.81 | |
| EGB | −10,371.28 | 97.4 | 97.4 | 30.8 | 30.8 | |
| ESURF | 324.84 | 5.2 | 5.2 | 1.65 | 1.65 | |
| GGAS | −5584.75 | 254.42 | 165.84 | 80.45 | 52.44 | |
| GSOLV | −10,046.44 | 97.54 | 93.95 | 30.84 | 29.71 | |
| TOTAL | −15,631.19 | 272.47 | 100.06 | 86.16 | 31.64 | |
| L-Aspartic acid-6YSY | VDWAALS | −6048.67 | 41.57 | 41.57 | 13.14 | 13.14 |
| EEL | −58,672.37 | 198.38 | 198.38 | 62.73 | 62.73 | |
| EGB | −10,272.24 | 154.78 | 154.78 | 48.95 | 48.95 | |
| ESURF | 325.6 | 2.38 | 2.38 | 0.75 | 0.75 | |
| GGAS | −5665.48 | 228.49 | 253.72534 | 72.25 | 80.24 | |
| GSOLV | −9946.64 | 154.8 | 154.15 | 48.95 | 48.75 | |
| TOTAL | −15,612.12 | 275.99 | 127.96 | 87.28 | 40.46 | |
| Coclaurine-6YSY | VDWAALS | −6046.05 | 34.28 | 34.28 | 10.84 | 10.84 |
| EEL | −58,400.3 | 115.96 | 115.96 | 36.67 | 36.67 | |
| EGB | −10,504.72 | 140.75 | 140.75 | 44.51 | 44.51 | |
| ESURF | 332.03 | 1.61 | 1.61 | 0.51 | 0.51 | |
| GGAS | −5434.29 | 178.12 | 154.01 | 56.33 | 48.7 | |
| GSOLV | −10,172.69 | 140.76 | 140.44 | 44.51 | 44.41 | |
| TOTAL | −15,606.98 | 227.02 | 120.35 | 71.79 | 38.06 |
SD-Sample standard deviation, SD (Prop.) and Standard error of mean (Prop.)-SD and standard error of mean obtained with propagation of uncertainty formula.
VDWAALS-Van der Waals energy, EEL-Electrostatic interactions, EGB-Polar solvation energy, ESURF-Nonpolar solvation energy, GGAS-Gas-phase energy, GSOLV-Solvation energy, TOTAL-Free energy of binding.
4. Conclusions
This study comprehensively evaluated the effects of URH and FRH on broiler chicken meat quality, flavor profile, antioxidant capacity, and molecular interactions. While no significant differences were observed in growth performance metrics such as ADG, ADFI, or F/G, substantial improvements were noted in meat quality traits and biochemical properties. URH significantly enhanced meat texture, increasing hardness, gumminess, and chewiness (p < 0.05), supported by denser myofiber arrangement and greater immobilized water content. In contrast, E-nose and E-tongue assessments confirmed enhanced sensory qualities in the FRH group, particularly in umami, richness, and saltiness. FRH supplementation improved flavor characteristics, evidenced by a 48.97 % increase in total volatile organic compounds, with alcohols and esters reaching 60.29 % and 72.09 %, respectively. GC–MS analysis revealed key flavor volatiles such as ethyl acetate and 4-methyl-2-heptanone, while LC-MS identified 213 differential metabolites, including umami-related amino acids and sweet-tasting ketones. Antioxidant analyses showed elevated NADPH, SOD, GSH, and CAT levels in both URH and FRH groups, accompanied by significantly reduced MDA and ROS, indicating improved oxidative stability. Molecular docking showed stable binding of key volatiles and metabolites—most notably coclaurine and L-aspartic acid—to chicken myosin, with binding energies as low as −7.6 kcal/mol. Molecular dynamics simulations further confirmed the stability of these complexes, revealing that the interactions primarily involved hydrogen bonds and hydrophobic forces, without altering the tertiary structure of myosin. Residues such as Tyr135, Leu261, and Lys273 were identified as critical for binding affinity. In conclusion, FRH supplementation not only enriches the flavor and nutritional profile of chicken meat but also enhances antioxidant defense mechanisms and molecular binding stability with flavor compounds. These findings provide strong scientific evidence for the development of functional poultry feed using Rumex hanus, supporting its application in improving meat quality and consumer sensory satisfaction in the poultry industry.
CRediT authorship contribution statement
Yuwei Guo: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis. Yaling Gu: Writing – review & editing, Investigation, Formal analysis, Data curation. Lijiu Yang: Resources, Methodology, Investigation, Data curation. Muhammad Aaqil: Validation, Software, Investigation, Data curation. Feng Zhang: Validation, Formal analysis, Data curation. Jingchuan Zheng: Investigation, Formal analysis, Data curation. Zhen Zhang: Investigation, Formal analysis, Data curation. Xiaolin Huang: Validation, Methodology, Investigation. Yewei Huang: Resources, Investigation, Formal analysis, Data curation. Ya Wang: Supervision, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Cunchao Zhao: Supervision, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Ethics statement
The animal care and use procedures were approved by the Animal Care Committee of the College of Animal Science and Technology, Yunnan Agricultural University (Ethics Review Number: 202209012).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This study was supported by Special Project for High-level Scientific and Technological Talents and Innovation Teams of Yunnan Province (202305AS350025).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2025.102858.
Contributor Information
Yewei Huang, Email: lichuangyewei100@163.com.
Ya Wang, Email: wangya9188@126.com.
Cunchao Zhao, Email: zcc272744372@163.com.
Appendix A. Supplementary data
Total ion chromatogram of GC–MS. CON group (A). URH group (B). FRH group (C).
Total ion chromatography of LC-MS. Positive mode (A). Negative mode (B).
Relative content of volatile compounds detected using HS-SPME-GC–MS.
The ROAV for each volatile compound based on the identified thresholds in each group.
Data availability
Data will be made available on request.
References
- Al-Khafaji K., Taskin Tok T. Molecular dynamics simulation, free energy landscape and binding free energy computations in exploration the anti-invasive activity of amygdalin against metastasis. Computer Methods and Programs in Biomedicine. 2020;195 doi: 10.1016/j.cmpb.2020.105660. [DOI] [PubMed] [Google Scholar]
- Banerjee M.B., Roy R.B., Tudu B., Bandyopadhyay R., Bhattacharyya N. Black tea classification employing feature fusion of E-nose and E-tongue responses. Journal of Food Engineering. 2019;244:55–63. doi: 10.1016/j.jfoodeng.2018.09.022. [DOI] [Google Scholar]
- Belhadj Slimen I., Yerou H., Ben Larbi M., M'Hamdi N., Najar T. Insects as an alternative protein source for poultry nutrition: A review. Frontiers in Veterinary Science. 2023;10 doi: 10.3389/fvets.2023.1200031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canibe N., Jensen B.B. Fermented liquid feed—Microbial and nutritional aspects and impact on enteric diseases in pigs. Animal Feed Science and Technology. 2012;173(1):17–40. doi: 10.1016/j.anifeedsci.2011.12.021. [DOI] [Google Scholar]
- Chen X., Chen H., Xiao J., Liu J., Tang N., Zhou A. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Research International. 2020;138 doi: 10.1016/j.foodres.2020.109717. [DOI] [PubMed] [Google Scholar]
- Fateminasab F., Bordbar A.K., Shityakov S., Saboury A.A. Molecular insights into inclusion complex formation between β- and γ-cyclodextrins and rosmarinic acid. Journal of Molecular Liquids. 2020;314 doi: 10.1016/j.molliq.2020.113802. [DOI] [Google Scholar]
- Fu B., Fang C., Li Z., Zeng Z., He Y., Chen S., Yang H. The effect of heat stress on sensory properties of fresh oysters: A comprehensive study using E-nose, E-tongue, sensory evaluation, HS–SPME–GC–MS, LC–MS, and transcriptomics. Foods. 2024;13 doi: 10.3390/foods13132004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B., Zheng M., Yang H., Zhang J., Li Y., Wang G.…Yu E. The effect of broad bean diet on structure, flavor and taste of fresh grass carp: A comprehensive study using E-nose, E-tongue, TPA. Food Chemistry. 2024;436 doi: 10.1016/j.foodchem.2023.137690. [DOI] [PubMed] [Google Scholar]
- Gao S., Huang W., Huang Z., Ming M. Study on the extraction process and properties of protein from edible dock. Food and Fermentation Industries. 2024;1-16 doi: 10.13995/j.cnki.11-1802/ts.038605. [DOI] [Google Scholar]
- Gu S., Xu Z., Sun C., Wu G., Li G., Yan Y.…Yang N. Comparation of muscle quality and nutritional components in different white feather Broilers'Breeds. China Poultry. 2021;43(02):21–27. doi: 10.16372/j.issn.1004-6364.2021.02.004. [DOI] [Google Scholar]
- Guo H., Chen H., Hong C., Jiang D., Zheng B. Exogenous malic acid alleviates cadmium toxicity in Miscanthus sacchariflorus through enhancing photosynthetic capacity and restraining ROS accumulation. Ecotoxicology and Environmental Safety. 2017;141:119–128. doi: 10.1016/j.ecoenv.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Guo L., Yu B., Wang S., Zhu Y., Li P., Wang B.…Sun J. Effect of ripening with penicillium roqueforti on texture, microstructure, water distribution and volatiles of chicken breast meat. International Journal of Food Science & Technology. 2019;54(5):1550–1557. doi: 10.1111/ijfs.14019. [DOI] [Google Scholar]
- Guo X., Tao S., Pan J., Lin X., Ji C., Liang H.…Li S. Effects of l-lysine on the physiochemical properties and sensory characteristics of salt-reduced reconstructed ham. Meat Science. 2020;166 doi: 10.1016/j.meatsci.2020.108133. [DOI] [PubMed] [Google Scholar]
- Han D., Zhang C.-H., Fauconnier M.-L., Mi S. Characterization and differentiation of boiled pork from Tibetan, Sanmenxia and duroc × (Landrac × Yorkshire) pigs by volatiles profiling and chemometrics analysis. Food Research International. 2020;130 doi: 10.1016/j.foodres.2019.108910. [DOI] [PubMed] [Google Scholar]
- Hu G., Liu Z., Zhang Y., Wang C., Wang J., Wang Q., Hao J. Nutritional components and application value of edible dock. Food Research and Development. 2023;44(16):208–212. https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8uevx0J090twFqvbyyal8BxtoIJJHcboufGQV_g1M1hxEGQvbibe3Z7zPyv-q4DI7qSIi7X9huBABKoWY7q21Yvmp8a3BRW-QD4Dsq1aDrKvfkqHOQktqpoJJvYzPZkaezhfQ54NAaGVYnz2Rg_jYJQT9PXqZTfD63Gpn__G8aTV9RXNOu5342mUpWdW4upJwf4=&uniplatform=NZKPT&language=CHS [Google Scholar]
- Huang L., Ho C.-T., Wang Y. Biosynthetic pathways and metabolic engineering of spice flavors. Critical Reviews in Food Science and Nutrition. 2021;61(12):2047–2060. doi: 10.1080/10408398.2020.1769547. [DOI] [PubMed] [Google Scholar]
- Huang P., Zhao X., Fan Q., Yang H., Ma S., Wang H.…Cui C. Decoding of novel umami peptides from corn fermented powder and its mechanism via multisensory techniques, virtual screening, and molecular simulation approaches. Food Chemistry. 2025;463 doi: 10.1016/j.foodchem.2024.141449. [DOI] [PubMed] [Google Scholar]
- Jiang H., Zhang M., Bhandari B., Adhikari B. Application of electronic tongue for fresh foods quality evaluation: A review. Food Reviews International. 2018;34:1–24. doi: 10.1080/87559129.2018.1424184. [DOI] [Google Scholar]
- Jin S., Pang Q., Yang H., Diao X., Shan A., Feng X. Effects of dietary resveratrol supplementation on the chemical composition, oxidative stability and meat quality of ducks (Anas platyrhynchos) Food Chemistry. 2021;363 doi: 10.1016/j.foodchem.2021.130263. [DOI] [PubMed] [Google Scholar]
- Kantono K., Hamid N., Chadha D., Ma Q., Oey I., Farouk M.M. Pulsed electric field (PEF) processing of chilled and frozen-thawed lamb meat cuts: Relationships between sensory characteristics and chemical composition of meat. Foods. 2021;10(5) doi: 10.3390/foods10051148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee O.H., Kwon Y.I., Apostolidis E., Shetty K., Kim Y.C. Rhodiola-induced inhibition of adipogenesis involves antioxidant enzyme response associated with pentose phosphate pathway. Phytotherapy Research. 2011;25(1):106–115. doi: 10.1002/ptr.3236. [DOI] [PubMed] [Google Scholar]
- Lei L., Zhang S., Zhang Y., Lu L., Li C., Liao Y.…Shen S. Effects of Total mixed protein grass fermented diet on growth performance, blood indexes and carcass traits of finishing pigs. Chinese Journal of Animal Nutrition. 2023;35(02):813–822. https://link.cnki.net/urlid/11.5461.s.20221222.1332.027 [Google Scholar]
- Lei Y., Xie S., Guan X., Song C., Zhang Z., Meng J. Methoxypyrazines biosynthesis and metabolism in grape: A review. Food Chemistry. 2018;245:1141–1147. doi: 10.1016/j.foodchem.2017.11.056. [DOI] [PubMed] [Google Scholar]
- Li H., Geng W., Haruna S.A., Zhou C., Wang Y., Ouyang Q., Chen Q. Identification of characteristic volatiles and metabolomic pathway during pork storage using HS-SPME-GC/MS coupled with multivariate analysis. Food Chemistry. 2022;373 doi: 10.1016/j.foodchem.2021.131431. [DOI] [PubMed] [Google Scholar]
- Li H., Liu J., Liang J., Gao J., Sun J., Huang M.…Sun B. Research on volatile compounds in 2 kinds of Gujinggong liquor. Journal of Food Science and Technology. 2016;34(01):55–65. https://link.cnki.net/urlid/11.4644.n.20160119.1346.018 [Google Scholar]
- Li J., Rao W., Sun Y., Zhou C., Xia Q., He J.…Du L. Structural and gel property changes in chicken myofibrillar protein induced by argon cold plasma-activated water: With a molecular docking perspective. Food Research International. 2024;197 doi: 10.1016/j.foodres.2024.115271. [DOI] [PubMed] [Google Scholar]
- Li R., Guo M., Liao E., Wang Q., Peng L., Jin W., Wang H. Effects of repeated freezing and thawing on myofibrillar protein and quality characteristics of marinated Enshi black pork. Food Chemistry. 2022;378 doi: 10.1016/j.foodchem.2021.131994. [DOI] [PubMed] [Google Scholar]
- Li X., He T., Yang F., Wang C., Zhou Y., Sha R., Mao J. Analysis of nutritional components, functional components and bioactivity of edible dock. Science and Technology of Food Industry. 2023;44(03):307–315. doi: 10.13386/j.issn1002-0306.2022040234. [DOI] [Google Scholar]
- Li Y., Wang Z., Zhao Q., Gao J., Wang R., Zhu J. Effects of Rumex hanus by. Fermented diet on growth performance, slaughter performance, meat quality, nutrient apparent digestibility, and serum biochemical indices in sheep. Feed. 2025;Research(06):7–12. doi: 10.13557/j.cnki.issn1002-2813.2025.06.002. [DOI] [Google Scholar]
- Lou M., Qu X., Zhang L., Liu Z., Hu Z., Wang Y. Safety evaluation of edible dock as a new food raw material. Journal of Food Safety and Quality. 2021;12(10):3919–3926. doi: 10.19812/j.cnki.jfsq11-5956/ts.2021.10.008. [DOI] [Google Scholar]
- Ma Y., Han L., Hou S., Gui L., Sun S., Yuan Z.…Yang B. Fatty acids and volatile flavor components of adipose tissue from local Tibetan sheep in Qinghai with dietary supplementation of palm kernel meal (PKM) Animals. 2024;14 doi: 10.3390/ani14142113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques C., Sotiles A.R., Farias F.O., Oliveira G., Mitterer-Daltoé M.L., Masson M.L. Full physicochemical characterization of malic acid: Emphasis in the potential as food ingredient and application in pectin gels. Arabian Journal of Chemistry. 2020;13(12):9118–9129. doi: 10.1016/j.arabjc.2020.10.036. [DOI] [Google Scholar]
- Miao C., Wen X., Lan B., Wu J., Luo P., Liang S. Effect of irradiation combined with compound preservative to preserve fresh chicken. Food Science and Technology. 2024;49(05):107–114. doi: 10.13684/j.cnki.spkj.2024.05.017. [DOI] [Google Scholar]
- Niu J., Zhang J., Wei L., Ma X., Zhang W., Nie C. Cottonseed meal fermented by Candida tropical reduces the fat deposition in white-feather broilers through cecum bacteria-host metabolic cross-talk. Applied Microbiology and Biotechnology. 2020;104(10):4345–4357. doi: 10.1007/s00253-020-10538-7. [DOI] [PubMed] [Google Scholar]
- Pearce K.L., Rosenvold K., Andersen H.J., Hopkins D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes — A review. Meat Science. 2011;89(2):111–124. doi: 10.1016/j.meatsci.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Qi T., Zhang Y., Yang X., Luo X., Mao Y. Recent Progress in research on the effect of oxidative stress on beef color and stability. Food Science. 2023;44(07):260–266. https://link.cnki.net/urlid/11.2206.TS.20220729.0934.022 [Google Scholar]
- Sarnoski P.J., O’Keefe S.F., Jahncke M.L., Mallikarjunan P., Flick G.J. Analysis of crab meat volatiles as possible spoilage indicators for blue crab (Callinectes sapidus) meat by gas chromatography–mass spectrometry. Food Chemistry. 2010;122(3):930–935. doi: 10.1016/j.foodchem.2010.03.069. [DOI] [Google Scholar]
- Sérot T., Regost C., Prost C., Robin J., Arzel J. Effect of dietary lipid sources on odour-active compounds in muscle of turbot (Psetta maxima) Journal of the Science of Food and Agriculture. 2001;81(14):1339–1346. doi: 10.1002/jsfa.950. [DOI] [Google Scholar]
- Sharma B., Paul S. Role of caffeine as an inhibitor in aggregation of hydrophobic molecules: A molecular dynamics simulation study. Journal of Molecular Liquids. 2016;224:930–939. doi: 10.1016/j.molliq.2016.10.086. [DOI] [Google Scholar]
- Sidhu D., Lund J., Kotseridis Y., Saucier C. Methoxypyrazine analysis and influence of viticultural and enological procedures on their levels in grapes, musts, and wines. Critical Reviews in Food Science and Nutrition. 2015;55(4):485–502. doi: 10.1080/10408398.2012.658587. [DOI] [PubMed] [Google Scholar]
- Spínola V., Llorent-Martínez E.J., Castilho P.C. Antioxidant polyphenols of Madeira sorrel (Rumex maderensis): How do they survive to in vitro simulated gastrointestinal digestion? Food Chemistry. 2018;259:105–112. doi: 10.1016/j.foodchem.2018.03.112. [DOI] [PubMed] [Google Scholar]
- Sun H., Zhang Y., Sun J. Dietary inulin supplementation improves the physicochemical and gel properties of duck myofibrillar protein: Insights into the effect of muscle fiber types. Food Hydrocolloids. 2024;150 doi: 10.1016/j.foodhyd.2023.109722. [DOI] [Google Scholar]
- Sun X., Yu Y., Wang Z., Akhtar K.H., Saleh A.S.M., Li W., Zhang D. Insights into flavor formation of braised chicken: Based on E-nose, GC–MS, GC-IMS, and UPLC-Q-Exactive-MS/MS. Food Chemistry. 2024;448 doi: 10.1016/j.foodchem.2024.138972. [DOI] [PubMed] [Google Scholar]
- Wang H., Xia X., Yin X., Liu H., Chen Q., Kong B. Investigation of molecular mechanisms of interaction between myofibrillar proteins and 1-heptanol by multiple spectroscopy and molecular docking methods. International Journal of Biological Macromolecules. 2021;193:672–680. doi: 10.1016/j.ijbiomac.2021.10.105. [DOI] [PubMed] [Google Scholar]
- Wang M., Sheng J., Zhang F., Tan C., Huang S., Mu H.…Zhao C. Effects of adding different sources of oil powder to the diet on the quality and cecal gut microbesof white feather broilers. Journal of Functional Foods. 2024;116 doi: 10.1016/j.jff.2024.106151. [DOI] [Google Scholar]
- Wang X., Xiang X., Wei S., Li S. Multi-omics revealed the formation mechanism of flavor in salted egg yolk induced by the stages of lipid oxidation during salting. Food Chemistry. 2023;398 doi: 10.1016/j.foodchem.2022.133794. [DOI] [PubMed] [Google Scholar]
- Wang X., Yao Y., Yu J., Cui H., Hayat K., Zhang X., Ho C.-T. Evolution of lean meat tenderness stimulated by coordinated variation of water status, protein structure and tissue histology during cooking of braised pork. Food Research International. 2023;171 doi: 10.1016/j.foodres.2023.113081. [DOI] [PubMed] [Google Scholar]
- Wu Z., Xu J., Ruan J., Chen J., Li X., Yu Y.…Li H. Probing the mechanism of interaction between capsaicin and myofibrillar proteins through multispectral, molecular docking, and molecular dynamics simulation methods. Food Chemistry: X. 2023;18 doi: 10.1016/j.fochx.2023.100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Lu Y., Song Y., Zhang Z., Guo M. Evaluation of aroma characteristic in various fruit wines by GC-FID, HS-SPME-GC-MS and electronic nose. Food and Fermentation Industries. 2018;44(12):229–236. doi: 10.13995/j.cnki.11-1802/ts.017964. [DOI] [Google Scholar]
- Xu L., He J., Duan M., Chang Y., Gu T., Tian Y.…Lu L. Effects of lactic acid bacteria-derived fermented feed on the taste and quality of duck meat. Food Research International. 2023;174 doi: 10.1016/j.foodres.2023.113679. [DOI] [PubMed] [Google Scholar]
- Yang C., Chen B., Zhou W., Chen D., Li Q., Zhang N.…Diao Q. Study on nutritional value and ruminal degradation characteristics of protein grasses in mutton sheep. Chinese Journal of Animal Nutrition. 2024;36(06):3789–3796. https://link.cnki.net/urlid/11.5461.s.20240604.1553.030 [Google Scholar]
- Yao W., Cai Y., Liu D., Chen Y., Li J., Zhang M.…Zhang H. Analysis of flavor formation during production of Dezhou braised chicken using headspace-gas chromatography-ion mobility spec-trometry (HS-GC-IMS) Food Chemistry. 2022;370 doi: 10.1016/j.foodchem.2021.130989. [DOI] [PubMed] [Google Scholar]
- Yi C., Li Y., Zhu H., Liu Y., Quan K. Effect of lactobacillus plantarum fermentation on the volatile flavors of mung beans. LWT. 2021;146 doi: 10.1016/j.lwt.2021.111434. [DOI] [Google Scholar]
- Yin X., Gao M., Wang H., Chen Q., Kong B. Probing the interaction between selected furan derivatives and porcine myofibrillar proteins by spectroscopic and molecular docking approaches. Food Chemistry. 2022;397 doi: 10.1016/j.foodchem.2022.133776. [DOI] [PubMed] [Google Scholar]
- Yu Y., Saleh A.S.M., Sun X., Wang Z., Lu Y., Zhang D., Zhang C. Exploring the interaction between myofibrillar proteins and pyrazine compounds: Based on molecular docking, molecular dynamics simulation, and multi-spectroscopy techniques. International Journal of Biological Macromolecules. 2023;253 doi: 10.1016/j.ijbiomac.2023.126844. [DOI] [PubMed] [Google Scholar]
- Zelena E., Dunn W.B., Broadhurst D., Francis-McIntyre S., Carroll K.M., Begley P.…Kell D.B. Development of a robust and repeatable UPLC−MS method for the long-term metabolomic study of human serum. Analytical Chemistry. 2009;81(4):1357–1364. doi: 10.1021/ac8019366. [DOI] [PubMed] [Google Scholar]
- Zhang J., Lu J., Yu X., Chen S., Xu Y. Characterization of aroma differences in Jiangxiangxing baijiu with varying ethanol concentrations: Emphasis on olfactory threshold changes of aroma compounds. Food Chemistry. 2025;469 doi: 10.1016/j.foodchem.2024.142506. [DOI] [PubMed] [Google Scholar]
- Zhang L., Hong Y., Liao Y., Tian K., Sun H., Liu X.…Lu Y. Dietary Lasia spinosa Thw. Improves growth performance in broilers. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.775223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Wang S., Jiang D., Lu Y., Chen Y., Tang Y.…Dong W. Unravelling the interaction between α-SOH and myofibrillar protein based on spectroscopy and molecular dynamics simulation. Food Chemistry: X. 2023;20 doi: 10.1016/j.fochx.2023.100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen D. On the current chemical synthesis techniques of acetoin flavor. Journal of Qiannan Normal University for Nationalities. 2015;35(04):121–124. https://kns.cnki.net/kcms2/article/abstract?v=-xbefZa1Cds3Ui1QCtjCY7KCAxVzWWr3R0JoPSKYqqRSYQw1B8R5g0DNZjBVQT4Yc-NsP1dqPv1sYNvV6-tBBdPpPieqj_iuVPr3y94-vaTqGAzn5V3-zdgEp2egeyuMbY9J9dTE1L0me9HOLN1UXTWiq-ChZCMbLTAaIAIldnWjsmnAoI8KWg8KuXdGxfW7LzCvl5cxKfI=&uniplatform=NZKPT&language=CHS [Google Scholar]
- Zheng J., Wang D., Song M., Jiao M., Wang H. Molecular dynamics simulation of effects of three accelerators on the microstructure of polyesters in non-aqueous solvents. Materials Today Communications. 2022;33 doi: 10.1016/j.mtcomm.2022.104404. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total ion chromatogram of GC–MS. CON group (A). URH group (B). FRH group (C).
Total ion chromatography of LC-MS. Positive mode (A). Negative mode (B).
Relative content of volatile compounds detected using HS-SPME-GC–MS.
The ROAV for each volatile compound based on the identified thresholds in each group.
Data Availability Statement
Data will be made available on request.