Highlights
-
•
Cross-sectional study on dietary niacin intake and epilepsy association.
-
•
Inverse association found between dietary niacin intake and epilepsy prevalence.
-
•
Higher dietary niacin intake may protect against epilepsy in U.S. adults.
Keywords: Cross-sectional study, Adults, Dietary niacin, Epilepsy, NHANES
Abstract
This study aimed to examine the association between dietary niacin intake and the prevalence of epilepsy. We conducted a cross-sectional analysis of 14,236 participants from the National Health and Nutrition Examination Survey (NHANES) 2013–2018. Multivariable logistic regression, generalized additive models, and subgroup analyses were employed to assess the relationship. An inverse association was found between dietary niacin intake and the prevalence of epilepsy: for every 10 mg/day increase, the odds of having epilepsy were reduced by 15 % (OR = 0.85, p = 0.0266). Participants in the highest quintile of niacin intake had a significantly lower odds of epilepsy compared to those in the lowest quintile (OR = 0.45, p = 0.0187). This negative association remained consistent across groups with diverse demographic characteristics, medical conditions, and lifestyle choices. Our findings suggest a possible inverse association between dietary niacin intake and the prevalence of epilepsy.
1. Introduction
Epilepsy is a common neurological disorder that causes frequent seizures due to irregular electrical discharges in the brain. These seizures may manifest as transient loss of consciousness, muscle spasms, or other abnormal behaviors [1]. The etiology of epilepsy is multifactorial and includes genetic susceptibility, central nervous system infections, brain injuries, and metabolic disturbances [2]. Several studies have indicated that energy metabolism, oxidative stress, neuroinflammation, and imbalance of the intestinal microbiome are associated with the pathogenesis of seizures, epilepsy, and epileptogenesis [[3], [4], [5]]. Epilepsy affects millions of people globally, and many sufferers are deeply disturbed by it [6]. The impact of epilepsy extends beyond the occurrence of seizures, as the unpredictability of these events can significantly disrupt daily life. Individuals living with epilepsy often experience heightened anxiety stemming from the fear of having a seizure at any moment, which can result in limitations on their social activities, work, and personal relationships [7]. Additionally, the unpredictability of seizures can impair a person’s sense of independence, as they may need to rely on others for support in various situations, further affecting their overall quality of life [8]. Although antiepileptic drugs are the primary treatment option, about 30 % of patients are refractory to pharmacological therapy and may require surgery or other interventions [9]. For these patients, the failure of antiepileptic drugs to control seizures significantly impacts their quality of life. As a result, alternative treatment options are necessary. Recent research indicates that the ketogenic diet might contribute to brain health and lower seizure rates [10], providing a promising direction for non-pharmacological treatment strategies for epilepsy.
Niacin serves as a vital precursor for many necessary coenzymes, which play crucial roles in a variety of physiological functions, particularly in energy metabolism [11]. Niacin can be obtained from foods such as yeast, milk, meat, tortillas, and cereal grains [12]. The maximum pharmacological dosage of niacin is up to 2000 mg/day [13]. Previous studies have shown that socio-economic factors, such as income and education, are closely associated with dietary intake and nutritional status, with individuals from lower socio-economic backgrounds often reporting inadequate intake of dietary niacin [14,15]. Dietary deficiency of niacin may hinder oxidative phosphorylation and disturb mitochondrial respiration, which in turn affects intracellular energy production processes [16]. Some studies suggest that niacin can change the body's inflammatory state, and its possible antioxidative qualities may also affect inflammation, reduce inflammatory processes, and improve metabolic health in general [17,18]. Additionally, niacin has been reported to regulate gut microbiota [[19], [20], [21], [22]]. Niacin may be a promising treatment for a number of neurological conditions, according to emerging evidence [23]. Nevertheless, as no relevant extensive studies have been carried out across the entire population, the association between dietary niacin intake and epilepsy continues to be inconclusive.
Therefore, we executed a cross-sectional study utilizing the NHANES database to investigate the association between dietary niacin intake and the prevalence of epilepsy among U.S. adults. We hypothesized that higher dietary niacin consumption correlates with a lower prevalence of epilepsy.
2. Methods
2.1. Survey description
NHANES, administered by the U.S. Centers for Disease Control and Prevention, is an extensive program designed to assess the health and nutritional status of the population, providing valuable insights that help shape public health policies and programs. This survey uses a sophisticated sampling design, including multistage and stratified probability sampling methods, to collect health and nutrition data representative of the American population [24]. The National Center for Health Statistics' Institutional Review Board examined and authorized the study, and participants or their legal guardians were required to provide written informed consent, confirming their voluntary participation in the study [25]. All NHANES data and detailed survey design information are publicly available online at the NHANES website, making it a transparent and accessible resource for scientific and public health research.
2.2. Study population
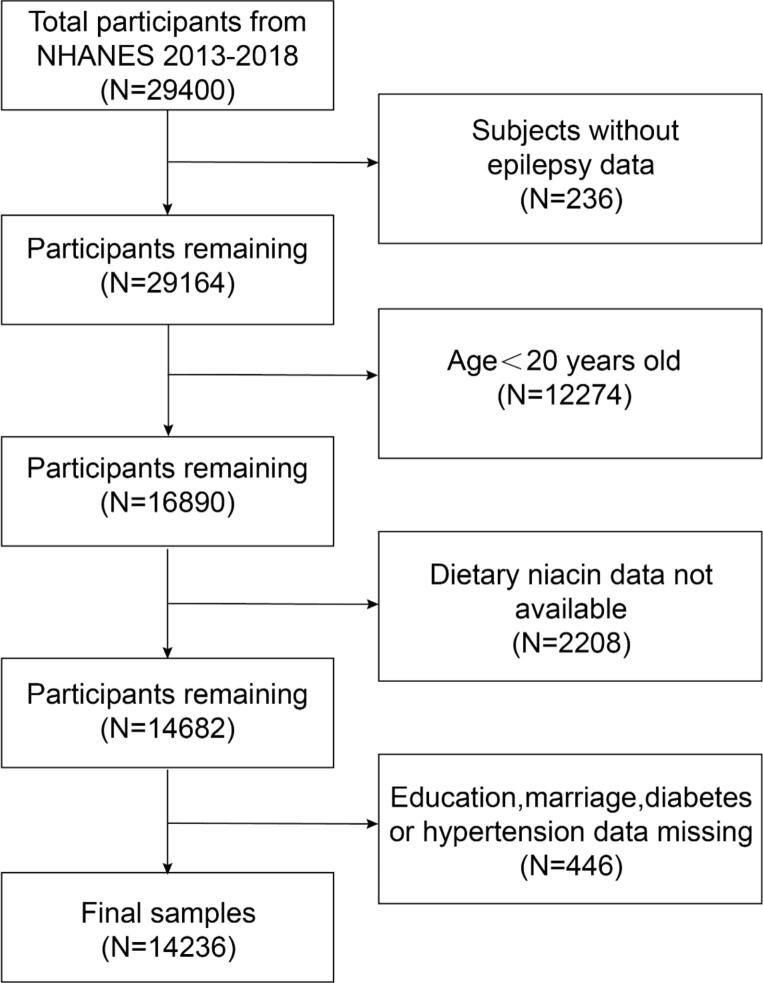
The NHANES 2013–2018 datasets served as the source of our study participants. 29,400 people in all finished the survey during this time. Individuals were removed from the study if they had incomplete data regarding their use of anti-epileptic medications (N = 236), were under the age of 20 (N = 12,274), lacked dietary niacin data (N = 2,208), or had missing information on covariates such as education, marital status, diabetes, and hypertension (N = 446). Ultimately, as shown in Fig. 1, the final analysis contained 14,236 qualified individuals.
Fig. 1.
The sample selection process.
2.3. Evaluation of dietary niacin intake
The collection of dietary data was conducted by well-trained health technicians at the Mobile Examination Centre (MEC), and the data were recorded in the Total Nutrient Intake files. For this study, we selected dietary niacin intake (mg) from the total nutrient intake recorded on Day 1. The first 24-hour dietary recollection interview, conducted in a face-to-face setting at the MEC, provided this information.
2.4. Definition of epilepsy
Epilepsy-related data were gathered from the “Prescription Drugs” segment of the NHANES questionnaire. Participants were asked about their use of any medications over the last 30 days, specifically for the purpose of treating “epilepsy and recurrent seizures.” Participants were considered to have epilepsy if they reported using such drugs [26]. Given that the sensitivity, specificity, and positive predictive value of self-reported epilepsy diagnoses are 84.2 %, 99.2 %, and 73.5 %, respectively, the questionnaire is considered suitable for identifying epilepsy at the population level [27]. This definition of epilepsy was deemed appropriate for the purposes of this study.
2.5. Covariates
Informed by previous research and clinical considerations, our analysis incorporated a range of potential confounding variables that might influence the relationship between dietary niacin intake and epilepsy. These covariates included age, sex, race/ethnicity, education level, marital status, poverty-income ratio, body mass index (kg/m2), diabetes status (yes/no), and hypertension status (yes/no). Comprehensive information on the data collection methods and results for these variables can be accessed publicly at the NHANES website.
2.6. Statistical analysis
Participants were divided into quintiles depending on their dietary niacin intake, with Quintile 1 representing the lowest intake and Quintile 5 representing the highest. Missing data for poverty-income ratio and body mass index were filled in using the median values for these variables. Quantitative data were reported as means with standard errors, whereas qualitative data were presented as percentages. To compare individuals across dietary niacin quintiles, the Student’s t-test was applied for quantitative data, and the chi-square test was applied for qualitative data. Multivariable logistic regression was performed to explore the relationship between dietary niacin intake and epilepsy using three distinct models. To amplify the effect size of the results, niacin intake values were divided by 10 (unit: 10 mg/d) when treated as quantitative data. In Model 1, no covariates were adjusted. Model 2 included adjustments for age, sex, and race/ethnicity. Model 3 adjusted for all covariates, including age, sex, race/ethnicity, education level, marital status, poverty-income ratio, body mass index, diabetes status, and hypertension status. The association between dietary niacin intake and epilepsy was further investigated with the use of a generalized additive model and smooth curve fitting. To look into any heterogeneity in the association across various categories, stratified multivariable regression was used for subgroup analysis. Statistical significance was predetermined at p < 0.05. R (v4.4.1) and EmpowerStats (v2.0) facilitated all statistical computations.
3. Results
3.1. Baseline characteristics of participants
This study included 14,236 participants, comprising 48.16 % males and 51.84 % females, as presented in Table 1. Participants' average niacin consumption was 25.55 ± 16.79 mg/d, and their mean age was 49.57 ± 17.59 years. The cohort included 124 epilepsy patients (0.87 %). Dietary niacin intake (mg/day) across Quintiles 1–5 was distributed as follows: <13.78, 13.78–19.45, 19.45–25.17, 25.17–34.08, and ≥ 34.08. Baseline characteristics differed significantly across quintiles of dietary niacin intake. Participants in the higher quintile of dietary niacin intake were generally younger, predominantly male, had a higher poverty-income ratio, and exhibited lower prevalence rates of hypertension and diabetes compared to those in the lower quintile.
Table 1.
Baseline characteristics of participants by quintiles of dietary niacin intake.
| Variables | Total (N = 14236) |
Quintiles of dietary niacin intake (mg/d) |
P-value | ||||
|---|---|---|---|---|---|---|---|
|
Q1(N = 2847) <13.78 |
Q2(N = 2847) 13.78–19.45 |
Q3(N = 2848) 19.45–25.17 |
Q4(N = 2846) 25.17–34.08 |
Q5(N = 2848) ≥34.08 |
|||
| Dietary niacin (mg/d), Mean ± SD | 25.55 ± 16.79 | 9.69 ± 3.00 |
16.68 ± 1.65 |
22.20 ± 1.65 |
29.17 ± 2.55 |
50.02 ± 20.86 |
<0.001 |
| Age (years), Mean ± SD | 49.57 ± 17.59 | 52.51 ± 17.89 | 51.56 ± 17.92 | 50.71 ± 17.64 | 48.68 ± 17.16 | 44.41 ± 16.09 | <0.001 |
| Sex (n, %) | <0.001 | ||||||
| Male | 6856 (48.16 %) | 816 (28.66 %) |
1082 (38.00 %) |
1316 (46.21 %) |
1565 (54.99 %) |
2077 (72.93 %) |
|
| Female | 7380 (51.84 %) | 2031 (71.34 %) |
1765 (62.00 %) |
1532 (53.79 %) |
1281 (45.01 %) |
771 (27.07 %) |
|
| Race/ethnicity (n, %) | <0.001 | ||||||
| Mexican American | 2091 (14.69 %) | 384 (13.49 %) |
401 (14.09 %) |
437 (15.34 %) |
421 (14.79 %) |
448 (15.73 %) |
|
| Other Hispanic | 1496 (10.51 %) | 314 (11.03 %) |
326 (11.45 %) |
282 (9.90 %) |
271 (9.52 %) |
303 (10.64 %) |
|
| Non-Hispanic White | 5446 (38.26 %) | 1001 (35.16 %) |
1063 (37.34 %) |
1142 (40.10 %) |
1149 (40.37 %) |
1091 (38.31 %) |
|
| Non-Hispanic Black | 3040 (21.35 %) | 703 (24.69 %) |
608 (21.36 %) |
575 (20.19 %) |
571 ( 20.06 %) |
583 (20.47 %) |
|
| Other | 2163 (15.19 %) | 445 (15.63 %) |
449 (15.77 %) |
412 (14.47 %) |
434 (15.25 %) |
423 (14.85 %) |
|
| Education (n, %) | <0.001 | ||||||
| Less than high school | 2943 (20.67 %) | 686 (24.10 %) |
603 (21.18 %) |
593 (20.82 %) |
515 (18.10 %) |
546 (19.17 %) |
|
| High school or equivalent | 3282 (23.05 %) | 683 (23.99 %) |
678 (23.81 %) |
625 (21.95 %) |
636 (22.35 %) |
660 (23.17 %) |
|
| More than high school | 8011 (56.27 %) | 1478 (51.91 %) |
1566 (55.01 %) |
1630 (57.23 %) |
1695 (59.56 %) |
1642 (57.65 %) |
|
| Marital status (n, %) | <0.001 | ||||||
| Married/Living with partner | 8513 (59.80 %) | 1565 (54.97 %) |
1697 (59.61 %) |
1716 (60.25 %) |
1793 (63.00 %) |
1742 (61.17 %) |
|
| Divorced/Separated/Widowed | 3069 (21.56 %) | 790 (27.75 %) |
682 (23.96 %) |
641 (22.51 %) |
506 (17.78 %) |
450 (15.80 %) |
|
| Never married | 2654 (18.64 %) | 492 (17.28 %) |
468 (16.44 %) |
491 (17.24 %) |
547 (19.22 %) |
656 (23.03 %) |
|
| Poverty-income ratio, Mean ± SD | 2.48 ± 1.55 | 2.25 ± 1.48 | 2.49 ± 1.54 | 2.53 ± 1.56 | 2.60 ± 1.57 | 2.53 ± 1.56 | <0.001 |
| Body mass index (kg/m2), Mean ± SD | 29.48 ± 7.12 | 29.73 ± 7.41 | 29.44 ± 6.93 | 29.50 ± 6.93 | 29.58 ± 7.29 | 29.13 ± 7.04 | 0.019 |
| Diabetes (n, %) | |||||||
| Yes | 2102 (14.77 %) | 504 (17.70 %) |
448 (15.74 %) |
436 (15.31 %) |
409 (14.37 %) |
305 (10.71 %) |
|
| No | 12,134 (85.23 %) | 2343 (82.30 %) |
2399 (84.26 %) |
2412 (84.69 %) |
2437 (85.63 %) |
2543 (89.29 %) |
|
| Hypertension (n, %) | <0.001 | ||||||
| Yes | 5227 (36.72 %) | 1173 (41.20 %) |
1120 (39.34 %) |
1089 (38.24 %) |
980 (34.43 %) |
865 (30.37 %) |
|
| No | 9009 (63.28 %) | 1674 (58.80 %) |
1727 (60.66 %) |
1759 (61.76 %) |
1866 (65.57 %) |
1983 (69.63 %) |
|
| Epilepsy (n, %) | 0.054 | ||||||
| Yes | 124 (0.87 %) |
32 (1.12 %) |
28 (0.98 %) |
30 (1.05 %) |
20 (0.70 %) |
14 (0.49 %) |
|
| No | 14,112 (99.13 %) | 2815 (98.88 %) |
2819 (99.02 %) |
2818 (98.95 %) |
2826 (99.30 %) |
2834 (99.51 %) |
|
3.2. Association between dietary niacin intake and epilepsy
Table 2 shows that a lower prevalence of epilepsy may be associated with higher dietary niacin intake. Both Model 1 and Model 2 indicated a negative correlation between dietary niacin intake and epilepsy (Model 1: OR = 0.84, p = 0.0135; Model 2: OR = 0.83, p = 0.0136). In the fully adjusted model, this negative association remained robust (OR = 0.85, p = 0.0266). Dietary niacin intake was further categorized into quintiles for analysis. Following complete adjustment, individuals in Quintile 5 had a statistically significant 55 % lower incidence of having epilepsy compared to those in Quintile 1 (OR = 0.45, p = 0.0187).
Table 2.
Multivariable logistic regression analysis of dietary niacin intake and epilepsy.
| Variable | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| OR (95 %CI) P-value | OR (95 %CI) P-value | OR (95 %CI) P-value | |
| Dietary niacin intake (10 mg/d) | 0.84 (0.72,0.96) 0.0135 | 0.83 (0.71,0.96) 0.0136 | 0.85 (0.73, 0.98) 0.0266 |
|
Quintiles of dietary niacin intake (mg/d) | |||
| Q1 < 13.78 | Reference | Reference | Reference |
| Q2 13.78–19.45 | 0.87 (0.52,1.45) 0.6039 | 0.85 (0.51,1.42) 0.5312 | 0.93 (0.56, 1.56) 0.7940 |
| Q3 19.45–25.17 | 0.94 (0.57,1.55) 0.7974 | 0.89 (0.54,1.48) 0.6534 | 0.99 (0.60, 1.65) 0.9698 |
| Q4 25.17–34.08 | 0.62 (0.36,1.09) 0.0979 | 0.59 (0.33,1.05) 0.0712 | 0.67 (0.38, 1.18) 0.1654 |
| Q5 ≥ 34.08 | 0.43 (0.23,0.82) 0.0095 | 0.41 (0.21,0.79) 0.0076 | 0.45 (0.23, 0.88) 0.0187 |
Model 1: no covariates were adjusted.
Model 2: adjusted for age, sex, and race/ethnicity.
Model 3: adjusted for age, sex, race/ethnicity, education level, marital status, poverty-income ratio, body mass index, diabetes status, and hypertension status.
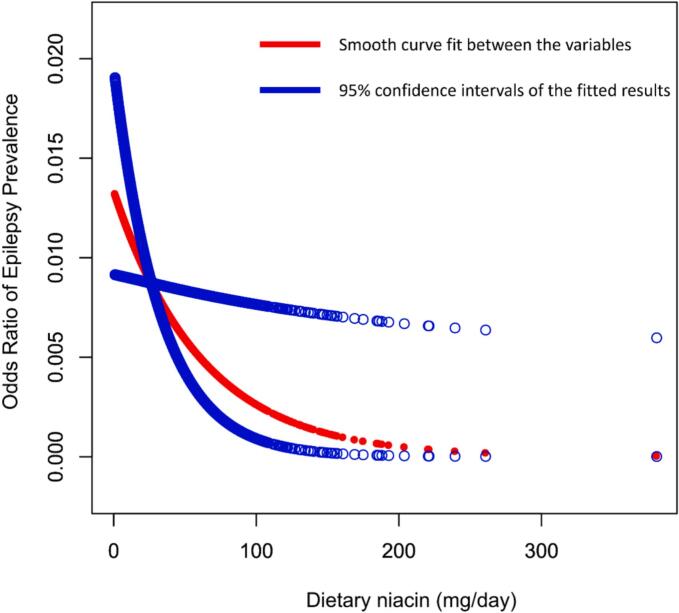
Additionally, as presented in Fig. 2, a generalized additive model and smooth curve fitting were applied to investigate the connection between dietary niacin intake and epilepsy. This analysis provided further evidence for the negative association.
Fig. 2.
Association between dietary niacin intake and epilepsy.
3.3. Subgroup analysis
In order to evaluate whether the association is consistent across different population settings, subgroup analysis was performed. Table 3 demonstrates that the negative association between dietary niacin intake and epilepsy was not significantly modified by stratifications such as age, sex, race/ethnicity, education level, hypertension status, and diabetes status (P for interaction > 0.05 for all variables). According to these results, there was a consistent and stable negative association between dietary niacin intake and epilepsy across groups with diverse demographic characteristics, medical conditions, and lifestyle choices.
Table 3.
Subgroup analysis of the association between dietary niacin intake and epilepsy.
| Subgroups | OR (95 % CI) | P -value | P for interaction |
|---|---|---|---|
| Age | 0.7349 | ||
| <40 | 0.989 (0.967, 1.011) | 0.3199 | |
| 40–60 | 0.976 (0.951, 1.001) | 0.0594 | |
| ≥60 | 0.985 (0.960, 1.010) | 0.2249 | |
| Sex | 0.9664 | ||
| male | 0.983 (0.966, 1.001) | 0.0616 | |
| female | 0.984 (0.959, 1.010) | 0.2236 | |
| Race/ethnicity | 0.6945 | ||
| Mexican American | 0.991 (0.952, 1.032) | 0.6572 | |
| Other Hispanic | 0.980 (0.935, 1.026) | 0.3849 | |
| Non-Hispanic White | 0.973 (0.951, 0.996) | 0.0206 | |
| Non-Hispanic Black | 0.996 (0.967, 1.026) | 0.7908 | |
| Other | 0.998 (0.962, 1.036) | 0.9294 | |
| Education | 0.9382 | ||
| Less than high school | 0.981 (0.954, 1.010) | 0.2019 | |
| High school or equivalent | 0.982 (0.958, 1.007) | 0.1590 | |
| More than high school | 0.987 (0.965, 1.009) | 0.2549 | |
| Diabetes | 0.1411 | ||
| Yes | 1.004 (0.977, 1.032) | 0.7875 | |
| No | 0.979 (0.962, 0.995) | 0.0121 | |
| Hypertension | 0.6971 | ||
| Yes | 0.986 (0.967, 1.006) | 0.1694 | |
| No | 0.981 (0.961, 1.001) | 0.0684 | |
4. Discussion
We analyzed NHANES data from 2013 to 2018 to examine the association between dietary niacin intake and epilepsy in U.S. adults. In this cross-sectional study involving 14,236 participants, an inverse association between dietary niacin intake and epilepsy was observed. This association remained largely unchanged even when groups were characterized by diverse demographic characteristics, medical conditions, and lifestyle choices.
To our knowledge, this is the first large-scale cross-sectional study to examine the association between dietary niacin intake and epilepsy. Our results are in accordance with previous research, suggesting that a lower prevalence of epilepsy may be associated with higher dietary niacin intake. Previous studies have reported similar associations using different epidemiological methods and target populations [28,29]. For example, a case-control study in northeastern Poland involving 130 participants, conducted by Szałwińska et al. indicated that individuals with epilepsy consumed less dietary niacin than the control group [28]. Similarly, a cross-sectional study in Egypt involving 150 epilepsy patients, as reported by Ismail et al., showed that dietary niacin intake among epilepsy patients was insufficient relative to the recommended dietary allowance [29].
The mechanistic basis of this adverse association remains to be established. Drawing from prior research and existing literature, we hypothesize that niacin may contribute to the processes of epilepsy through a range of potential mechanisms. To begin with, oxidative stress and disturbances in energy metabolism play a critical pathobiological role in epilepsy [3,[30], [31], [32], [33]]. It is thought that processes like lipid peroxidation, deoxyribonucleic acid damage, mitochondrial dysfunction, reactive oxygen species production, and disruption of the antioxidant defense system contribute to the onset of epilepsy [30]. In experiments on Sod2-/+ mice, Liang et al. found that mitochondrial oxidative stress can elevate neuronal excitability, which in turn triggers seizures [31]. Several studies have shown that brain regions ipsilateral to the epileptogenic focus often exhibit hypometabolism [32,33]. Disruption of astrocyte function, as these cells are vital for neuronal energy homeostasis, could cause excitation-inhibition equilibrium within the central nervous system, ultimately triggering seizures [3]. Niacin has been shown to improve energy metabolism and mitigate oxidative stress [16,17]. Niacin deficiency may impair mitochondrial respiratory function, as it is a critical coenzyme for energy metabolism [16], thereby disrupting energy production [17]. Motawi et al. found that niacin could alleviate disease-related oxidative stress by increasing the levels of antioxidants such as glutathione and superoxide dismutase [34].
Second, neuroinflammation is also associated with the pathogenesis of epilepsy. Inflammatory factors, activated neuroinflammatory cells, and corresponding inflammatory markers are significantly increased in the nervous system of individuals with epilepsy [[35], [36], [37]]. Increased neuroinflammation leads to astrocyte dysfunction, culminating in neuronal hyperexcitability and excitotoxicity, which subsequently result in seizures [38]. Niacin has been found to modulate the body's inflammatory response [39]. In an animal experiment conducted by Zhang et al., niacin treatment was shown to reduce the number of inflammatory infiltrates and improve neurological deficits [39]. Zhou et al. found that metabolites of niacin inhibited neuroinflammation and thus neuroprotection [18].
Additionally, substantial research has confirmed the association between gut microbiota imbalance and epilepsy. Epilepsy patients and healthy controls differ significantly in gut microbiota [40,41]. The gut microbiota can affect brain function through multiple pathways and systems, thereby influencing epileptic activity [5]. Dai et al. found that berberine reduces seizures by altering the gut microbial profile and protecting hippocampal neurons and related mechanisms in a mouse experiment [42]. According to Ding et al., gut microbes can alter the state of microglia to improve seizure progression [43]. Zou et al. concluded that intestinal flora improves ciprofloxacin-induced seizures in rats [44]. Notably, niacin has been shown to regulate gut microbiota composition, as evidenced by Zafar and Saier Jr.'s conclusion that the co-administration of extended-release niacin significantly increased the abundance of Bacteroidetes [21], which are widely regarded as beneficial microorganisms [22]. This finding suggests that the composition of the gut bacteria may be improved by niacin administration.
In summary, we speculate that niacin may influence the pathogenesis of epilepsy through multiple neuroprotective mechanisms, including improving energy metabolism, alleviating oxidative stress, reducing neuroinflammation, and regulating gut microbiota composition.
The NHANES dataset used in this study is representative of the national population and encompasses a substantial sample size. As such, our findings are partially generalizable to the adult population in the United States. Nonetheless, several limitations of this study must be addressed. First, as a cross-sectional study, it does not allow for the establishment of a causal relationship. Second, epilepsy was defined primarily based on participants' prescription medication history, without considering the duration or age of onset of epilepsy. While this definition is widely used in clinical practice, it may lead to misclassification of participants. For example, some individuals with epilepsy might be excluded, while non-epileptic patients who use anti-epileptic drugs to treat other conditions may be incorrectly categorized as having epilepsy. This classification approach may not fully reflect the clinical features and progression of epilepsy, which could influence our understanding of the relationship between dietary niacin intake and epilepsy. Therefore, future research should consider incorporating the age of onset, duration, and other relevant clinical variables of epilepsy in order to provide a more accurate analysis of epilepsy characteristics and reduce classification errors. Third, the inherent limitations of the single-day dietary recall technique may have compromised the reliability of the data, particularly in analyzing the relationship between micronutrient intake and disease. Using multi-day dietary records in future research could enhance the precision of dietary data. Fourth, while multiple covariates were adjusted for, the potential influence of unmeasured confounding factors cannot be entirely ruled out. Furthermore, variations in dietary habits, lifestyles, genetic backgrounds, and environmental exposures limit the applicability of these findings to populations in other countries or regions.
To overcome these limitations, further basic mechanistic research is essential, integrating multi-omics approaches such as metabolomics, transcriptomics, and microbiomics to clarify the specific pathways through which dietary niacin intake may influence epilepsy. Moreover, large-scale prospective studies are vital to establish a causal relationship between niacin intake and epilepsy risk. Future research directions should include conducting randomized controlled trials to assess the effects of niacin supplementation on seizure frequency and severity in epilepsy patients, as well as exploring specific populations, such as individuals with genetic susceptibility to epilepsy or drug-resistant epilepsy, to evaluate the therapeutic potential of niacin interventions. Furthermore, similar studies conducted in diverse countries or regions with varying dietary patterns and genetic backgrounds are necessary to validate the broader applicability of these results.
5. Conclusion
Our findings suggest a possible inverse association between dietary niacin intake and the prevalence of epilepsy, highlighting the potential of dietary strategies as adjunctive measures for epilepsy prevention and control.
Ethics statement
This study is based on the analysis of publicly available NHANES data. The NCHS Research Ethics Review Board approved the NHANES data collection. Use of the public-use datasets requires neither an Institutional Review Board review nor an exempt determination.
CRediT authorship contribution statement
Ken Ling: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Xinghui He: Writing – review & editing, Funding acquisition, Formal analysis, Conceptualization. Zhiquan Yang: Writing – review & editing, Funding acquisition, Conceptualization.
Funding
This work was supported by National Natural Science Foundation of China (82371463).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/default.aspx.
References
- 1.Neligan A., Hauser W.A., Sander J.W. The epidemiology of the epilepsies. Handb Clin Neurol. 2012;107:113–133. doi: 10.1016/B978-0-444-52898-8.00006-9. [DOI] [PubMed] [Google Scholar]
- 2.Beghi E., Giussani G., Sander J.W. The natural history and prognosis of epilepsy. Epileptic Disord. 2015;17:243–253. doi: 10.1684/epd.2015.0751. [DOI] [PubMed] [Google Scholar]
- 3.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Parsons A.L.M., Bucknor E.M.V., Castroflorio E., Soares T.R., Oliver P.L., Rial D. The interconnected mechanisms of oxidative stress and neuroinflammation in epilepsy. Antioxidants (Basel) 2022;11 doi: 10.3390/antiox11010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding M., Lang Y., Shu H., Shao J., Cui L. Microbiota-gut-brain axis and epilepsy: a review on mechanisms and potential therapeutics. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.742449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.G. B. D. Epilepsy Collaborators Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:357–375. doi: 10.1016/S1474-4422(18)30454-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobau R., Cui W., Kadima N., Zack M.M., Sajatovic M., Kaiboriboon K., et al. National Health Interview Survey. Epilepsy Behav. 2010;41(2014):66–73. doi: 10.1016/j.yebeh.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abetz L., Jacoby A., Baker G.A., McNulty P. Patient-based assessments of quality of life in newly diagnosed epilepsy patients: validation of the NEWQOL. Epilepsia. 2000;41:1119–1128. doi: 10.1111/j.1528-1157.2000.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 9.Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Cross J.H., Elger C.E., et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 10.Zarnowska I.M. Therapeutic use of the ketogenic diet in refractory epilepsy: what we know and what still needs to be learned. Nutrients. 2020;12 doi: 10.3390/nu12092616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkland J.B., Meyer-Ficca M.L. Niacin. Adv Food Nutr Res. 2018;83:83–149. doi: 10.1016/bs.afnr.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Mayo Clinic, Niacin, (2023). https://www.mayoclinic.org/drugs-supplements-niacin/art-20364984?utm_source (accessed February 16, 2025).
- 13.A.C. Goldberg, Clinical trial experience with extended-release niacin (Niaspan): dose-escalation study, Am J Cardiol 82 (1998) 35U-38U; discussion 39U-41U. https://doi.org/10.1016/s0002-9149(98)00952-7. [DOI] [PubMed]
- 14.Li L., Zhang B., Wang H.J., Ouyang Y.F., Huang F.F., Wang Y., et al. Sociodemographic factors associated with dietary intake of Thiamine, Riboflavin, and Niacin among Chinese adults in 2015. Biomed Environ Sci. 2020;33:660–669. doi: 10.3967/bes2020.087. [DOI] [PubMed] [Google Scholar]
- 15.Dapi L.N., Hörnell A., Janlert U., Stenlund H., Larsson C. Energy and nutrient intakes in relation to sex and socio-economic status among school adolescents in urban Cameroon, Africa. Public Health Nutr. 2011;14:904–913. doi: 10.1017/S1368980010003150. [DOI] [PubMed] [Google Scholar]
- 16.Pirinen E., Auranen M., Khan N.A., Brilhante V., Urho N., Pessia A., et al. Niacin cures systemic NAD(+) deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metab. 2020;31:1078–1090 e5. doi: 10.1016/j.cmet.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Wakade C., Giri B., Malik A., Khodadadi H., Morgan J.C., Chong R.K., et al. Niacin modulates macrophage polarization in Parkinson’s disease. J Neuroimmunol. 2018;320:76–79. doi: 10.1016/j.jneuroim.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y., Wu J., Sheng R., Li M., Wang Y., Han R., et al. Reduced nicotinamide adenine dinucleotide phosphate inhibits MPTP-induced neuroinflammation and neurotoxicity. Neuroscience. 2018;391:140–153. doi: 10.1016/j.neuroscience.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Blacher E., Bashiardes S., Shapiro H., Rothschild D., Mor U., Dori-Bachash M., et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572:474–480. doi: 10.1038/s41586-019-1443-5. [DOI] [PubMed] [Google Scholar]
- 20.Fangmann D., Theismann E.M., Türk K., Schulte D.M., Relling I., Hartmann K., et al. Targeted microbiome intervention by microencapsulated delayed-release niacin beneficially affects insulin sensitivity in humans. Diabetes Care. 2018;41:398–405. doi: 10.2337/dc17-1967. [DOI] [PubMed] [Google Scholar]
- 21.Zafar H., Saier M.H. Gut Bacteroides species in health and disease. Gut Microbes. 2021;13:1–20. doi: 10.1080/19490976.2020.1848158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wuerch E., Urgoiti G.R., Yong V.W. The Promise of Niacin in Neurology. Neurotherapeutics. 2023;20:1037–1054. doi: 10.1007/s13311-023-01376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtin L.R., Mohadjer L.K., Dohrmann S.M., Kruszon-Moran D., Mirel L.B., Carroll M.D., et al. National Health and Nutrition Examination Survey: sample design, 2007-2010. Vital Health Stat. 2013;2:1–23. [PubMed] [Google Scholar]
- 25.Fan Y., Zhao L., Deng Z., Li M., Huang Z., Zhu M., et al. Non-linear association between Mediterranean diet and depressive symptom in U.S. adults: a cross-sectional study, Front. Psychiatry. 2022;13 doi: 10.3389/fpsyt.2022.936283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ran L., Xu M., Zhang Z., Zeng X. The association of nutrient intake with epilepsy: a cross-sectional study from NHANES, 2013-2014. Epilepsy Res. 2024;200 doi: 10.1016/j.eplepsyres.2024.107297. [DOI] [PubMed] [Google Scholar]
- 27.Brooks D.R., Avetisyan R., Jarrett K.M., Hanchate A., Shapiro G.D., Pugh M.J., et al. Validation of self-reported epilepsy for purposes of community surveillance. Epilepsy Behav. 2012;23:57–63. doi: 10.1016/j.yebeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Szałwińska K., Cyuńczyk M., Kochanowicz J., Witkowska A.M. Dietary and lifestyle behavior in adults with epilepsy needs improvement: a case-control study from northeastern Poland. Nutr J. 2021;20:62. doi: 10.1186/s12937-021-00704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ismail R.S., Kishk N.A., Rizk H.I., El-Kholy T., Abd El-Maoula L.M., Ibrahim El-Desoky O., et al. Nutritional intake and its impact on patients with epilepsy: an analytical cross-sectional study. Nutr Neurosci. 2022;25:1813–1822. doi: 10.1080/1028415X.2021.1905371. [DOI] [PubMed] [Google Scholar]
- 30.Patel M.N. Oxidative stress, mitochondrial dysfunction, and epilepsy. Free Radic Res. 2002;36:1139–1146. doi: 10.1080/1071576021000016391. [DOI] [PubMed] [Google Scholar]
- 31.Liang L.P., Waldbaum S., Rowley S., Huang T.T., Day B.J., Patel M. Mitochondrial oxidative stress and epilepsy in SOD2 deficient mice: attenuation by a lipophilic metalloporphyrin. Neurobiol Dis. 2012;45:1068–1076. doi: 10.1016/j.nbd.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taussig D., Petrescu A.M., Aghakhani N., Herbrecht A., Dorfmüller G., Ferrand-Sorbets S., et al. (18) F-FDG-PET hypometabolic pattern reveals multifocal epileptic foci despite limited unique stereotyped seizures. Epilepsy Res. 2021;172 doi: 10.1016/j.eplepsyres.2021.106589. [DOI] [PubMed] [Google Scholar]
- 33.Tatekawa H., Uetani H., Hagiwara A., Hotta M., Stern J.M., Engel J., et al. Association of hypometabolic extension of (18)F-FDG PET with diffusion tensor imaging indices in mesial temporal lobe epilepsy with hippocampal sclerosis. Seizure. 2021;88:130–137. doi: 10.1016/j.seizure.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Motawi T.K., Sadik N.A.H., Hamed M.A., Ali S.A., Khalil W.K.B., Ahmed Y.R. Potential therapeutic effects of antagonizing adenosine A(2A) receptor, curcumin and niacin in rotenone-induced Parkinson’s disease mice model. Mol Cell Biochem. 2020;465:89–102. doi: 10.1007/s11010-019-03670-0. [DOI] [PubMed] [Google Scholar]
- 35.Ravizza T., Gagliardi B., Noé F., Boer K., Aronica E., Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Aronica E., Boer K., van Vliet E.A., Redeker S., Baayen J.C., Spliet W.G., et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol Dis. 2007;26:497–511. doi: 10.1016/j.nbd.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Crespel A., Coubes P., Rousset M.C., Brana C., Rougier A., Rondouin G., et al. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 2002;952:159–169. doi: 10.1016/s0006-8993(02)03050-0. [DOI] [PubMed] [Google Scholar]
- 38.Vezzani A., Balosso S., Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15:459–472. doi: 10.1038/s41582-019-0217-x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J., Chen J., Li Y., Cui X., Zheng X., Roberts C., et al. Niaspan treatment improves neurological functional recovery in experimental autoimmune encephalomyelitis mice. Neurobiol Dis. 2008;32:273–280. doi: 10.1016/j.nbd.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong X., Liu X., Chen C., Lin J., Li A., Guo K., et al. Alteration of gut microbiota in patients with epilepsy and the potential index as a biomarker. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.517797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kundu S., Nayak S., Rakshit D., Singh T., Shukla R., Khatri D.K., et al. The microbiome-gut-brain axis in epilepsy: pharmacotherapeutic target from bench evidence for potential bedside applications. Eur J Neurol. 2023;30:3557–3567. doi: 10.1111/ene.15767. [DOI] [PubMed] [Google Scholar]
- 42.Dai W.-T., Zhu Y., Jiang Z.-M., Xiang Y., Mao X.-Y., Liu Z.-Q. Berberine alleviates kainic acid-induced acute epileptic seizures in mice via reshaping gut microbiota-associated lipid metabolism. CNS Neurosci Ther. 2025;31 doi: 10.1111/cns.70253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding X., Zhou J., Zhao L., Chen M., Wang S., Zhang M., et al. Intestinal flora composition determines microglia activation and improves epileptic episode progress. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.835217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou S., Li Y., Zou Q., Yang M., Li H., Niu R., et al. Gut microbiota and serum metabolomic alterations in modulating the impact of fecal microbiota transplantation on ciprofloxacin-induced seizure susceptibility. Front Microbiol. 2024;15 doi: 10.3389/fmicb.2024.1403892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/default.aspx.