ABSTRACT
Stroke remains a leading cause of morbidity and mortality worldwide, necessitating the development of rapid and reliable diagnostic tools for early detection and management. This manuscript presents an overview of innovative biosensors designed for the detection of key stroke biomarkers, including N‐terminal pro B‐type natriuretic peptide (NT‐proBNP), C‐reactive protein (CRP), D‐dimer, cardiac troponins, S100B protein, glial fibrillary acidic protein (GFAP), neurofilament light chain (NfL), matrix metalloproteinases (MMPs), interleukins and fibrinogen. We discuss the principles of operation, sensitivity, specificity and the technological advancements that have enabled the development of these biosensors, including electrochemical, optical and microfluidic platforms. The integration of nanomaterials and advanced signal amplification techniques has significantly enhanced the performance of these biosensors, allowing for the detection of biomarkers at low concentrations in complex biological samples. Furthermore, we explore the clinical implications of these biosensors in the context of stroke diagnosis, prognosis and monitoring, highlighting their potential to facilitate timely therapeutic interventions. By providing a comprehensive discussion on the current state of biosensor technology for stroke biomarker detection, this manuscript aims to underscore the importance of these tools in improving patient outcomes and advancing stroke research. Future directions for biosensor development and the challenges that remain in translating these technologies into clinical practice are also addressed.
Keywords: biosensors, clinical applications, nanomaterials, stroke, therapeutic interventions
1. Introduction
Stroke is a critical global health issue, representing one of the foremost causes of disability and death worldwide [1, 2]. According to the World Health Organization (WHO), approximately 15 million people suffer a stroke each year, with nearly 5 million resulting in death, and another 5 million experiencing permanent disability [1, 2]. The urgency of timely diagnosis and effective management of stroke is underscored by the need for rapid intervention to minimize neurological damage and improve patient outcomes [3, 4]. Traditional diagnostic methods, while effective, often lack the speed and sensitivity required for early detection, leading to delays in treatment that can have devastating consequences [3, 4]. In recent years, the emergence of innovative biosensors has revolutionized the landscape of stroke diagnostics, offering the potential for rapid, accurate and non‐invasive detection of biomarkers associated with stroke [5, 6]. Biosensors are analytical devices that convert a biological response into an electrical signal, enabling the detection of specific biomolecules with high sensitivity and specificity [5, 6]. The identification of key stroke biomarkers, such as N‐terminal pro B‐type natriuretic peptide (NT‐proBNP), C‐reactive protein (CRP), D‐dimer, and various proteins associated with neuronal injury, has paved the way for the development of these advanced diagnostic tools. The integration of cutting‐edge technologies, including electrochemical, optical and microfluidic platforms, has further enhanced the capabilities of biosensors, allowing for the detection of biomarkers at low concentrations within complex biological matrices. This study aims to provide a comprehensive overview of the current state of biosensor technology for stroke biomarker detection. We will discuss the principles of operation, performance metrics, and the technological advancements that have facilitated the evolution of these devices. In addition, we will explore the clinical implications of biosensors in stroke diagnosis, prognosis and monitoring, emphasizing their potential to enable timely therapeutic interventions. By highlighting the significance of these innovative tools in improving patient outcomes and advancing stroke research, we aim to underscore the critical role of biosensors in the future of stroke management. Finally, we will address the challenges that remain in translating these technologies into clinical practice and outline future directions for biosensor development in the context of stroke care.
2. Overview of Stroke
Stroke is a medical emergency characterized by the sudden onset of neurological deficits due to the interruption of blood supply to the brain [7, 8]. It is classified into two main types: ischaemic stroke, which accounts for approximately 87% of all cases and occurs when a blood vessel supplying blood to the brain is obstructed, and haemorrhagic stroke, which results from the rupture of a blood vessel, leading to bleeding in or around the brain [7, 8]. The consequences of stroke can be devastating, often resulting in long‐term disability or death, making it a leading cause of morbidity and mortality worldwide [9, 10]. The pathogenesis of stroke involves complex biological processes that vary between ischaemic and haemorrhagic types. In ischaemic stroke, the primary mechanism is the formation of a thrombus (blood clot) or embolus (a clot that travels from another part of the body) that obstructs blood flow to a specific region of the brain [9, 10]. This obstruction leads to a cascade of events, including neuronal cell death due to lack of oxygen and glucose, the release of excitotoxic neurotransmitters, and the activation of inflammatory pathways [11]. The area surrounding the core of the infarct, known as the penumbra, may remain viable for a limited time and is a critical target for therapeutic intervention [11]. In contrast, haemorrhagic stroke occurs when a blood vessel in the brain ruptures, leading to bleeding that can compress surrounding brain tissue and disrupt normal blood flow [7, 12]. The pathogenesis of haemorrhagic stroke often involves conditions such as hypertension, arteriovenous malformations or aneurysms. The resultant hematoma can cause direct neuronal injury and trigger inflammatory responses, further exacerbating brain damage [12, 13]. The risk factors for stroke are multifactorial and include both non‐modifiable factors, such as age, gender and family history, and modifiable factors, such as hypertension, diabetes, smoking, obesity and high cholesterol levels. The incidence of stroke increases with age, and it is more prevalent in individuals with pre‐existing cardiovascular conditions. According to the WHO, approximately 15 million people experience a stroke each year, with nearly 5 million resulting in death and another 5 million living with permanent disabilities [14, 15]. The clinical presentation of stroke varies depending on the area of the brain affected and the extent of damage. Common symptoms include sudden weakness or numbness on one side of the body, difficulty speaking or understanding speech, vision problems, loss of balance or coordination, and severe headache [14, 15]. The rapid recognition of these symptoms is crucial, as timely medical intervention can significantly improve outcomes. The ‘golden hour’ refers to the critical time frame within which treatment, such as thrombolysis for ischaemic stroke, can be most effective. Despite advancements in treatment and rehabilitation, stroke remains a significant public health challenge. The development of innovative diagnostic tools, such as biosensors for stroke biomarkers, is essential for improving early detection and management. These tools can facilitate timely therapeutic interventions, ultimately reducing the burden of stroke on individuals and healthcare systems. As research continues to evolve, understanding the underlying mechanisms of stroke and enhancing prevention strategies will be vital in addressing this global health crisis.
3. Stroke Blood Biomarkers
The identification and utilization of stroke biomarkers in blood represent a significant advancement in the field of stroke diagnosis and management [16, 17]. As stroke remains a leading cause of disability and mortality globally, the ability to rapidly and accurately assess the risk and presence of stroke through biomarker analysis is crucial [16, 17]. This discussion explores the implications of various stroke biomarkers, their mechanisms and their potential roles in clinical practice. NT‐proBNP has emerged as a critical biomarker, particularly in patients with concurrent heart failure [18, 19]. Elevated levels of NT‐proBNP not only indicate cardiac stress but also correlate with an increased risk of stroke, suggesting a complex interplay between cardiovascular health and cerebrovascular events [18, 19]. This relationship underscores the importance of a holistic approach to patient assessment, where cardiovascular and neurological evaluations are integrated. CRP serves as a general marker of inflammation, which is increasingly recognized as a contributing factor in the pathophysiology of stroke [20, 21]. Elevated CRP levels have been associated with both ischaemic and haemorrhagic strokes, indicating that systemic inflammation may play a role in stroke risk and outcomes [20, 21]. The challenge lies in distinguishing between CRP elevations due to stroke and those resulting from other inflammatory conditions, necessitating further research to clarify its specificity and sensitivity in stroke diagnosis. Cardiac troponins (I and T) are well‐established markers of myocardial injury, and their elevation in acute ischaemic stroke patients suggest a significant overlap between cardiac and cerebral events [22]. This finding highlights the need for comprehensive cardiovascular assessment in stroke patients, as myocardial infarction can complicate stroke management and influence treatment decisions [22]. Markers of brain injury, such as S100B protein and glial fibrillary acidic protein (GFAP), have shown promise in indicating neuronal damage and glial activation, respectively [23]. Their elevation in stroke patients reflects the underlying pathophysiological processes and may serve as indicators of stroke severity and prognosis [23]. Neurofilament light chain (NfL) has also gained attention as a sensitive marker of neuronal damage, with studies suggesting its potential to predict outcomes in various neurological disorders, including stroke. Matrix metalloproteinases (MMPs) are involved in the remodelling of the extracellular matrix and have been implicated in blood–brain barrier disruption during stroke [24]. Their elevation may indicate ongoing neuroinflammatory processes and could serve as a target for therapeutic intervention. Similarly, interleukins such as IL‐6 and IL‐1β, which are cytokines associated with inflammation, have been studied for their roles in stroke pathophysiology [25]. Elevated levels of these interleukins may reflect the inflammatory response following a stroke and could provide insights into the timing and nature of therapeutic interventions [25]. Fibrinogen, a key clotting factor, is another biomarker that has been associated with stroke risk. Elevated fibrinogen levels can indicate a hypercoagulable state, which is a significant risk factor for thromboembolic strokes [26, 27]. Understanding the role of fibrinogen in stroke pathophysiology may lead to improved risk stratification and management strategies. While the potential of these biomarkers is evident, their clinical utility remains variable [26, 27]. Many of these biomarkers are still under investigation, and their integration into routine clinical practice requires further validation through large‐scale studies. In addition, the complexity of stroke pathophysiology necessitates a multifaceted approach to biomarker assessment, where a panel of biomarkers may provide more comprehensive insights than individual markers alone. In conclusion, the exploration of stroke biomarkers in blood offers promising avenues for enhancing stroke diagnosis, risk assessment and management. Continued research is essential to establish standardized protocols for biomarker use in clinical settings, ultimately aiming to improve patient outcomes and advance our understanding of stroke mechanisms. As technology progresses, the development of rapid and reliable biosensors for these biomarkers may facilitate their integration into routine clinical practice, paving the way for more effective stroke care. The rapid and sensitive detection of stroke blood biomarkers is crucial for timely diagnosis and intervention, significantly impacting patient outcomes. Early identification of stroke through biomarker analysis allows for prompt therapeutic strategies, such as the administration of thrombolytics in acute ischaemic stroke, which can dramatically reduce morbidity and mortality. Moreover, sensitive detection methods can facilitate the differentiation between stroke subtypes, guiding tailored treatment approaches and improving prognostic assessments. As stroke often presents with overlapping symptoms and can progress rapidly, the ability to quickly and accurately measure biomarkers in blood not only enhances clinical decision‐making but also supports the efficient allocation of healthcare resources, ultimately leading to better management of stroke patients and improved recovery trajectories.
4. Conventional Methods in Detection of Stroke Blood Biomarkers
The detection of stroke blood biomarkers has traditionally relied on a variety of conventional laboratory methods, each with its own strengths and limitations [28, 29]. These methods are essential for diagnosing stroke, assessing its severity and guiding treatment decisions [28, 29]. The most commonly used conventional techniques include enzyme‐linked immunosorbent assays (ELISA), Western blotting, and immunohistochemistry (IHC), among others. ELISA is one of the most widely employed techniques for quantifying specific biomarkers in blood samples [30]. This method utilizes antigen–antibody interactions to detect and measure the concentration of biomarkers such as CRP, NT‐proBNP and various cytokines. ELISA is favoured for its sensitivity, specificity and ability to process multiple samples simultaneously [30]. However, it requires specialized equipment and trained personnel, and the turnaround time can be several hours to days, which may delay critical clinical decisions in acute stroke scenarios [31]. Western blotting is another conventional method used to detect specific proteins in a sample. This technique involves the separation of proteins by gel electrophoresis, followed by transfer to a membrane and probing with specific antibodies [32]. Western blotting is particularly useful for confirming the presence of biomarkers such as S100B protein and GFAP. While it provides high specificity and can detect low‐abundance proteins, the method is labour‐intensive and time‐consuming, often taking several hours to complete [31]. In addition, it requires careful optimization of conditions, which can introduce variability in results. IHC is a technique that allows for the visualization of specific antigens in tissue sections using labelled antibodies. While primarily used in research settings, IHC can provide valuable insights into the expression of biomarkers in brain tissue following a stroke [32]. This method can help correlate biomarker levels with histopathological changes, enhancing our understanding of stroke mechanisms. However, IHC is not typically used for routine blood biomarker detection due to its complexity and the need for tissue samples, which are not always available in acute clinical settings. Mass spectrometry (MS) has emerged as a powerful analytical tool for biomarker discovery and validation. While not a conventional method in the strictest sense, it has been increasingly applied in clinical laboratories for the analysis of complex biological samples. MS can provide detailed information about the molecular weight and structure of biomarkers, allowing for the identification of multiple biomarkers simultaneously. However, the high cost of equipment and the need for specialized expertise can limit its widespread use in routine clinical practice. Despite the utility of these conventional methods, they also have notable limitations. The time required for sample processing and analysis can hinder timely decision‐making in acute stroke situations, where every minute counts. In addition, the need for specialized equipment and trained personnel can restrict access to these diagnostic tools, particularly in resource‐limited settings. Furthermore, the variability in assay performance and the potential for cross‐reactivity can affect the reliability of results. In conclusion, while conventional methods for detecting stroke blood biomarkers have played a pivotal role in advancing our understanding of stroke pathophysiology and improving patient care, their limitations highlight the need for more rapid, sensitive and accessible diagnostic tools. The development of novel biosensors and point‐of‐care (POC) testing technologies holds promise for overcoming these challenges, enabling timely and accurate detection of stroke biomarkers in clinical practice. As research continues to evolve, integrating these advanced methodologies with conventional techniques may pave the way for more effective stroke management and improved patient outcomes (see Tables 1 and 2).
TABLE 1.
Summarizing the key stroke biomarkers along with their conventional detection methods, advantages and disadvantages.
| Biomarker | Conventional detection methods | Advantages | Disadvantages |
|---|---|---|---|
| N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) | ELISA, Western blotting | High sensitivity and specificity; useful in cardiac assessment. | Requires specialized equipment; turnaround time can be lengthy. |
| C‐reactive protein (CRP) | ELISA, high‐sensitivity CRP assays | Rapid results; widely available; indicates systemic inflammation. | Non‐specific; elevated in various conditions, not just stroke. |
| D‐dimer | ELISA, latex agglutination | Quick detection; useful for assessing thromboembolic events. | Elevated in many conditions; limited specificity for stroke. |
| Cardiac troponins (I and T) | ELISA, immunoassays | High specificity for myocardial injury; important in acute settings. | Time‐consuming; requires careful handling and processing. |
| S100B protein | ELISA, Western blotting | Sensitive to brain injury; correlates with stroke severity. | Labor‐intensive; variability in results based on assay conditions. |
| Glial fibrillary acidic protein (GFAP) | ELISA, Western blotting | Indicates glial activation; useful for assessing brain injury. | Requires specialized techniques; not routinely used in acute settings. |
| Neurofilament light chain (NfL) | ELISA, mass spectrometry | Sensitive marker of neuronal damage; potential for prognostic use. | High cost of mass spectrometry; requires specialized expertise. |
| Matrix metalloproteinases (MMPs) | ELISA, zymography | Provides insight into blood–brain barrier integrity; relevant in neuroinflammation. | Complex assay procedures; variability in expression levels. |
| Interleukins (e.g., IL‐6, IL‐1β) | ELISA, multiplex cytokine assays | Can assess multiple cytokines simultaneously; indicates inflammatory response. | Time‐consuming; may require specialized equipment for multiplex assays. |
| Fibrinogen | ELISA, clauss method | Important for assessing coagulation status; widely used. | Elevated in various conditions; may not be specific to stroke. |
TABLE 2.
Biosensor classification.
| Classification criteria | Type of biosensor | Description |
|---|---|---|
| Biological recognition element | Enzyme‐based biosensors | Utilize enzymes to detect specific substrates. |
| Antibody‐based biosensors | Use antibodies to bind to specific antigens for detection. | |
| Nucleic acid‐based biosensors | Employ DNA/RNA probes for genetic testing and pathogen detection. | |
| Cell‐based biosensors | Utilize living cells to monitor responses or detect analytes. | |
| Transducer mechanism | Electrochemical biosensors | Measure electrical signal changes in response to analyte binding. |
| Optical biosensors | Use light‐based methods to detect changes in light properties. | |
| Mass‐based biosensors | Detect changes in mass or frequency using technologies like QCM. | |
| Thermal biosensors | Measure changes in temperature or heat during biochemical reactions. | |
| Application area | Medical diagnostics | Used for disease detection and monitoring of biomarkers and drug levels. |
| Environmental monitoring | Detect pollutants and pathogens in environmental samples. | |
| Food safety | Monitor food quality and detect contamination. | |
| Bioprocess monitoring | Monitor fermentation processes and other biotechnological applications. |
Due to the limitations of conventional methods for detecting stroke blood biomarkers—such as lengthy turnaround times, the need for specialized equipment, and issues with specificity—there has been a significant shift towards the development of nanomaterial‐based methods, particularly biosensors, over the past decade. These innovative biosensors leverage the unique properties of nanomaterials to enhance sensitivity, specificity, and speed of biomarker detection, making them particularly well‐suited for the rapid assessment required in acute stroke situations. The integration of nanotechnology into biosensor design has enabled the creation of devices that can detect low concentrations of biomarkers in complex biological samples, facilitating timely diagnosis and intervention. The next section of this article will focus on the advancements in stroke blood biomarker biosensors, discussing their design, functionality, and the potential impact they hold for improving clinical outcomes in stroke management. Through this exploration, we aim to highlight the transformative role that these technologies can play in the future of stroke diagnosis and treatment.
5. Nanomaterial Based Biosensors
Nanomaterial‐based biosensors have emerged as a revolutionary technology in the medical field, offering rapid and sensitive detection of a wide range of disease‐related biomarkers and medications [33, 34]. These biosensors leverage the unique properties of nanomaterials, such as their high surface area, enhanced reactivity, and ability to facilitate electron transfer, to improve the performance of traditional biosensing platforms [33, 34]. Biosensors typically consist of a biological recognition element (such as enzymes, antibodies, or nucleic acids) and a transducer (Figure 1) that converts the biological response into a measurable signal.
FIGURE 1.
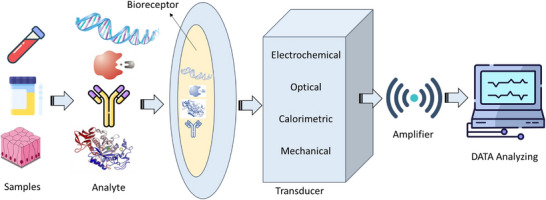
Schematic illustration of biosensors.
Nanomaterials, including nanoparticles, nanowires and nanotubes, can be integrated into these systems to enhance sensitivity and specificity. For instance, gold nanoparticles can amplify signals due to their plasmonic properties, while carbon nanotubes can improve electron transfer rates [35, 36]. Biosensors have a wide range of applications in medical diagnostics, particularly in the detection of disease biomarkers, drug monitoring and pathogen detection [35, 36]. Nanomaterial‐based biosensors are especially valuable for the early detection of diseases such as cancer, diabetes and infectious diseases. They can identify low concentrations of biomarkers, including proteins, nucleic acids and metabolites that indicate disease states [37]. For instance, biosensors utilizing graphene oxide have been developed to detect cancer biomarkers like carcinoembryonic antigen (CEA) and prostate‐specific antigen (PSA) [37]. In addition to disease detection, these biosensors play a crucial role in therapeutic drug monitoring, ensuring that patients receive the correct dosages of medications. Nanomaterial‐based sensors can measure drug levels in real‐time, facilitating personalized medicine approaches. Furthermore, rapid detection of pathogens is essential for controlling infectious diseases. Nanobiosensors can identify bacterial and viral pathogens with high sensitivity and specificity, enabling timely diagnosis and treatment. For example, biosensors that incorporate silver nanoparticles have shown promise in detecting pathogens such as E. coli and SARS‐CoV‐2 [38, 39]. The importance of rapid and sensitive detection in medical diagnostics cannot be overstated. Early diagnosis significantly improves treatment outcomes, and nanomaterial‐based biosensors can provide results in minutes, allowing for timely medical intervention. These biosensors can also be designed for POC applications, making testing accessible in remote or resource‐limited settings, which enhances healthcare delivery and disease management [38, 39]. In addition, by enabling rapid and accurate diagnostics, nanomaterial‐based biosensors can reduce the need for expensive laboratory tests and hospital visits, ultimately lowering healthcare costs. The ability to monitor biomarkers and drug levels in real‐time supports personalized treatment plans, thereby enhancing patient care and outcomes. Nanomaterial‐based biosensors represent a significant advancement in medical diagnostics, offering rapid, sensitive and specific detection of a wide range of biomarkers and medications [40, 41]. Their applications in disease detection, drug monitoring and pathogen identification highlight their importance in modern healthcare. As research continues to evolve, these biosensors hold the potential to transform medical diagnostics, leading to improved patient outcomes and more efficient healthcare systems. The classification of biosensors can be organized into three main criteria: biological recognition elements, transducer mechanisms and application areas [40, 41]. Under biological recognition elements, biosensors can be enzyme‐based, antibody‐based, nucleic acid‐based or cell‐based, each utilizing specific biological components to detect target analytes. In terms of transducer mechanisms, biosensors can be categorized as electrochemical, optical, mass‐based, or thermal, depending on how they convert the biological response into a measurable signal. Finally, biosensors are applied across various fields, including medical diagnostics for disease detection and biomarker monitoring, environmental monitoring for pollutant detection, food safety for quality assessment, and bioprocess monitoring in industrial applications. This classification underscores the versatility and significance of biosensors in advancing diagnostic and monitoring technologies across multiple domains.
The classification of biosensors highlights the diversity of these devices and their applications across various fields. Understanding the different types of biosensors and their mechanisms is crucial for selecting the appropriate technology for specific diagnostic or monitoring needs. As technology advances, the development of new biosensors continues to expand their potential applications and improve their performance.
6. Stroke Biosensors
6.1. NT‐proBNP Biosensors
The N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) is recognized as a crucial blood biomarker for stroke. A novel fibre optic nanogold‐linked immunosorbent assay (FONLISA) has been developed for the rapid, sensitive and cost‐effective detection of NT‐proBNP. This method employs a sandwich immunoassay strategy utilizing two monoclonal antibodies specific to NT‐proBNP: a capture antibody (AbC) and a detection antibody (AbD) [42]. A general method for creating multianalyte sensing platforms for the detection of BNP‐32 has been established. This involves the development of aptamer‐based electrochemical sensors specifically for brain natriuretic peptide (BNP‐32) and cardiac troponin I (cTnI). To achieve this, commercial gold‐based screen‐printed electrodes were modified electrophoretically using films of polyethyleneimine and reduced graphene oxide [43]. A highly sensitive dual‐mode competitive electrochemical immunosensor for detecting B‐type natriuretic peptide (BNP) has been successfully developed, utilizing both differential pulse voltammetry (DPV) and amperometric i–t curve response modes. Polyaniline (PAN) and tin dioxide (SnO2) were incorporated onto graphene sheets (GS), significantly enhancing the electron transfer process, which in turn amplified the current signal and improved the sensitivity of the immunosensor [44]. A novel ultra‐sensitive immunosensor has been developed for the direct detection of NT‐proBNP in human artificial saliva (AS), a promising biological matrix that may contain a wealth of biomarkers. This immunosensor aims to improve detection sensitivity, decrease measurement time and facilitate the simultaneous detection of multiple biomarkers. The biosensor, which utilizes gold working microelectrodes (WEs), was biofunctionalized with 4‐carboxymethyl aryl diazonium (CMA) to effectively immobilize anti‐NT‐proBNP antibodies [45]. A label‐free aptasensor was developed utilizing a liquid‐gated field‐effect transistor (FET) that incorporates a monolayer film of reduced graphene oxide (rGO) with an immobilized aptamer specific to NT‐proBNP. Findings revealed that the ionic strength of the tested solutions influenced the correlation levels in the electrical responses of the rGO‐FET aptasensor, specifically in terms of shifts in the Dirac point and changes in transconductance [46]. A highly responsive and specific electrochemical biosensor was developed using Ag–Au bimetallic nanoparticles for the measurement of BNP, a key indicator of heart failure. The sensor was created by modifying a glassy carbon electrode with Ag–Au bimetallic nanoparticles (average diameter: 20 ± 3 nm) and immobilizing anti‐BNP antibodies. It demonstrated a wide linear range, acceptable limits of detection (LOD), and excellent specificity for BNP, with minimal interference from other proteins [47]. A single‐chain variable fragment (scFv) that specifically targets the biomarker NT‐proBNP was created using biopanning techniques. This developed electrochemical biosensor serves as an alternative diagnostic tool. From the screening process, 10 clones were identified that bound to the target peptide, with two clones—scFv‐16 and scFv‐36—chosen for further analysis [48]. A ratiometric electrochemical immunosensor featuring a dual‐signal readout mode was developed using well‐dispersed hierarchical PtCoNi hollow multi‐branches (HMBs) combined with ferrocene‐grafted ionic liquid (Fc‐g‐IL, synthesized through a Schiff‐base reaction) and Co–N–C nanosheets (NSs, produced via a straightforward pyrolysis method) for signal amplification. The PtCoNi HMBs were synthesized through a solvothermal treatment and subsequently etched with acetic acid, see Figure 2 [49].
FIGURE 2.
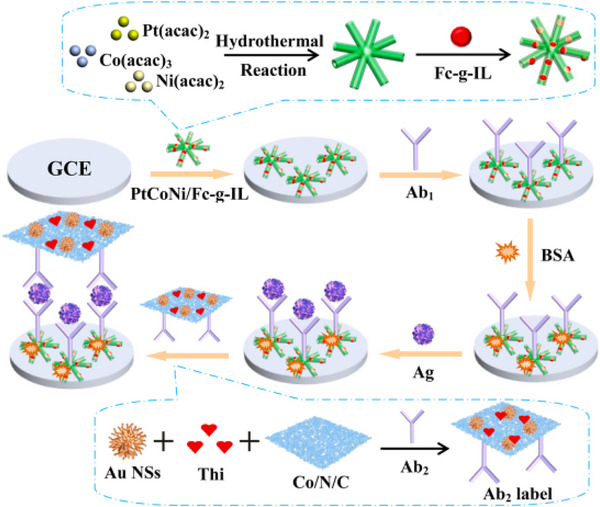
The schematic diagram for constructing the NT‐proBNP immunoassay [49].
Photoelectrochemical (PEC) sensors have demonstrated significant potential across various fields due to their high sensitivity, rapid response and ease of use. However, traditional PEC sensing methods depend on cumbersome external light sources and bulky electrodes, which impede miniaturization and implantation, thus limiting their application in real‐time disease monitoring. To address these challenges, a nanoencapsulated optical fibre (OF)‐based PEC microelectrode was developed. This microelectrode incorporates TiO2/CdS nanocrystals and layers of bis(2,2′‐bipyridine) (10‐methylphenanthroline [3,2‐a:2′3′‐c] pyridine ruthenium(II) dichloride ([Ru(bpy)2dppz]2+) @dsDNA/Au@epigallocatechin gallate nanoparticle (EGCG NP). Its application for detecting N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), a biomarker for cardiovascular diseases, was investigated, achieving an extensive linear range of 1–5000 pg/mL and a low detection limit of 0.36 pg/mL [50]. Additional data of NT‐proBNP biosensors are summarized in Table 3.
TABLE 3.
NT‐proBNP biosensors.
| Type | Technique | Electrode | NPs | Sample/matrix | Linear range | LOD | Refs. |
|---|---|---|---|---|---|---|---|
| Optical/immunosensors | SPR | — | AuNPs | Real | 0.50–5000 pg/mL | 058 pg/mL | [42] |
| EL/genosensor | — | Gold | Au/rGO | Plasma | 1 pg/mL to 1 µg/mL | 1 µg/mL | [43] |
| EL/immunosensors | CVs, DPVs | CNT | ZnCo2O4/N‐CNTs | Biological | 0.01 pg/mL–1 ng/mL | 3.4 fg/mL | [44] |
| EL/immunosensors | EIS/CVs | Gold | AuNPs | Salvia | 1–20 pg/mL | 0.03 pg/mL | [45] |
| EL/genosensor | FET | Gold | rGO‐FET | Salvia | 10 fg/mL–1 pg/mL | 41 fg/mL | [46] |
| EL/immunosensors | XDR, UVis, CVs | GCE | Ag–Au bimetallic | Spiked serum | 0.0001–100 ng/mL | 0.03 pg/mL | [47] |
| EL/immunosensors | SPR/SWV | SPCE/GO | — | Serum | 0–225 pg/mL | 69.09 pg/mL | [48] |
| EL/immunosensors | CVs, DPVs | GCE | Au‐Thi‐Co‐N‐C NSs | Serum | 0.001–10.0 ng/mL | 0.35 pg/mL | [49] |
| Optical/immunosensors | PEC | Micro E | EGCG NP | Biological | 1–5000 pg/mL | 0.36 pg/mL | [50] |
Abbreviation: EGCG NP, epigallocatechin gallate nanoparticle.
6.2. C‐Reactive Protein Biosensors
A cost‐effective experimental platform was developed to measure CRP concentrations in samples of buffer and enriched plasma. This system utilizes label‐free antigenic probes, traditional electrochemical techniques and open‐source hardware. The prototype is a portable, affordable device designed for POC use, featuring a user‐friendly interface that allows healthcare professionals to operate it wirelessly via a mobile phone or laptop, Figure 3 [51].
FIGURE 3.
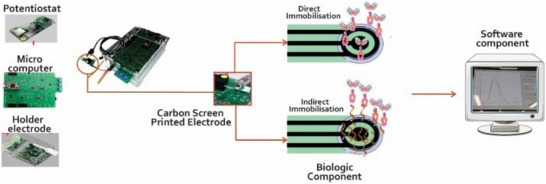
Cost‐effective experimental platform was developed to measure CRP [51].
A label‐free functionalized aptamer sensor was integrated with an electrode modified with gold nanoparticles and carboxylated graphene oxide (AuNPs/GO‐COOH) to enable sensitive measurements of CRP levels. Gold nanoparticles were chosen for their exceptional stability, high electrical conductivity and biocompatibility. In addition, carboxylated graphene oxide was employed to enhance the attachment of inducer molecules and improve detection accuracy [52]. A novel electrochemical aptamer biosensor was developed using Ti3C2Tx MXene and in‐situ reduced gold nanoparticles for the immobilization of thiolated RNA aptamers and the detection of CRP protein, utilizing Fc(COOH) as the signal probe. The sensor's performance for CRP detection was optimized by adjusting various working conditions, including incubation times and pH levels [53]. A highly sensitive MIPs biosensor was developed by integrating conductive and biocompatible graphdiyne (GDY) nanosheets with antifouling and specific MIPs to enable the precise recognition of human CRP. Dopamine was selected as the functional monomer due to its straightforward polymerization process and the proven low fouling characteristics of polydopamine, allowing it to form stable complexes with the template molecule through hydrogen bonding and multiple electrostatic interactions [54]. A dual‐channel electrochemical biosensor was designed for the concurrent detection of LPS and CRP. When operating under optimal conditions, it exhibited excellent linearity and acceptable LOD for CRP, consistent with clinical evaluation criteria [55]. A microfluidic immuno‐biosensor detection system is presented, consisting of micro‐spectrometer and a microfluidic spectrum chip for the rapid POC detection and quantification of high‐sensitivity C‐reactive protein (hs‐CRP) in urine. The detection process employs a highly specific ELISA method, where capture antibodies and detection antibodies are pre‐coated on the microchip substrate to create an immune complex with the target antigen [56]. An electrochemical biosensor designed to detect CRP, a biomarker for neonatal sepsis, employs a MIP that is applied to an electrode surface. To improve the sensor's surface area and catalytic properties, gold‐platinum bimetallic nanomaterials were coated onto the screen‐printed carbon electrode. Subsequently, the CRP imprinted polymer was applied to the electrode's surface [57]. One study proposed the use of hybrid plasmonic materials for OF sensor applications to sensitively monitor CRP concentrations based on hybrid material particles. Specifically, Au@Ag NPs are coated onto the surface of the OF through a self‐assembled monolayer using an NH2 bridge. CRP antigens are subsequently immobilized on the surface of the Au@Ag nanoparticles to selectively detect CRP and enhance the sensor's sensitivity [58]. A cost‐effective, highly sensitive and versatile POC testing method for various dimensional biomarkers is based on the target‐mediated catalytic reduction suppression of double‐sequence aptamer‐modified gold nanoparticles (dsApts@AuNPs) and NaBH4 in the presence of organic dyes (T‐AAND) [59]. The first PEC nanoimmunosensor utilizing a plasmonic graphene and gold nanostar (AuNS) heterojunction, excited by 765 nm red light, is introduced for the label‐free detection of CRP, an important biomarker for inflammation. This platform takes advantage of the distinctive localized surface plasmon resonance effect of AuNSs, combined with in situ generated graphene, to improve photoelectrical conversion efficiency when exposed to 765 nm monochromatic light [60]. Additional data of CRP biosensors are summarized in Table 3.
6.3. Cardiac Troponins Biosensors
A cost‐effective thin film gold electrode array was utilized alongside economical ELISA antibodies and reagents to create two amperometric immunosensors for cTnI. The HRP‐labelled sandwich immunocomplex was formed on the surface of the gold electrode, and chronoamperometry was employed to indirectly measure cTnI by assessing the quantity of TMB+ generated at the electrode's surface [61]. A sandwich signal‐on biosensor for cTnI has been created by employing a structure that sandwiches the antibody, cTnI, and an aptamer, utilizing a metal–organic framework (MOF) composed of Fe(III)‐2‐amino terephthalic acid (Fe‐ATA) as a dual‐function nanocarrier. This framework facilitates the incorporation of the signalling molecule dopamine and the aptamer recognition element. Initially, the cTnI antibody was immobilized onto an electrode modified with electro‐synthesized AuNPs [62]. A heterojunction of bismuth‐doped tin oxide and tin disulphide (Bi–SnOS) was synthesized using a straightforward one‐step hydrothermal technique and employed as a highly sensitive platform. By incorporating copper sulphide‐coated nano‐gold (Au@CuS) in a yolk‐shell nanocomposite structure as a dual quenching probe, an effective PEC biosensor was developed for the detection of cTnI through a sandwich immunorecognition approach [63]. Magnetic fluorescent nanoparticles have shown significant benefits as versatile tools in multifunctional immunoassays for lateral flow biosensors (LFB), improving the adaptability and usability of these systems. A newly developed type of flower‐shaped magnetic fluorescent nanoparticles (FMNP@QDs) exhibits enhanced fluorescence emission. The unique core/shell structure of FMNP@QDs features a core with high saturation magnetization, surrounded by oleic acid‐modified Fe3O4, while the outer shell consists of polymer colloids with quantum dots (QDs) evenly distributed on the surface [64]. A highly sensitive electrochemical biosensor was developed for the analysis of cTnI, utilizing simply synthesized mesoporous nanopolyhedras (MNPs) made of Pd@PdPtCo as signal enhancers. The mesoporous polyhedral design of these Pd@PdPtCo MNPs provides a greater specific surface area and more active sites, along with a synergistic effect from the combination of multiple metal components. These features collectively enhance the electrocatalytic efficiency of the Pd@PdPtCo MNPs in the effective oxidation of hydroquinone (HQ) to benzoquinone (BQ), see Figure 4 [65].
FIGURE 4.
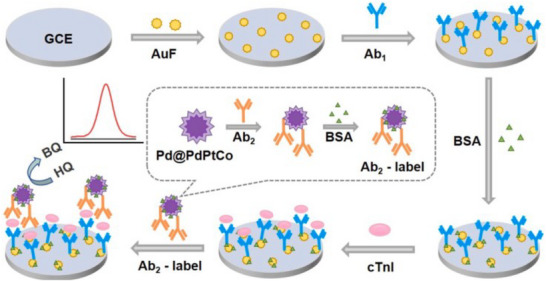
Illustration of an electrochemical biosensor utilizing Pd@PdPtCo MNPs for the detection of cardiac troponin I (cTnI) [65].
An ultra‐sensitive assay has been developed for detecting cTnI utilizing an electrical double layer gated high field AlGaN/GaN HEMT biosensor. This innovative gating approach addresses the issue of charge screening commonly encountered in conventional FET‐based biosensors, enabling the identification of target proteins in physiological solutions without the need for prior sample processing. The assay employs specific antibodies and aptamers that target troponin I as its receptors [66]. A highly sensitive unlabelled ratiometric biosensor has been created for the detection of cTnI. This biosensor utilizes a glassy carbon electrode coated with graphene oxide, which serves as a platform integrated with nitrogen and zinc co‐doped graphene QDs (N, Zn‐GQDs). The N, Zn‐GQDs were successfully synthesized from graphite powder and characterized. cTnI antibodies were attached to the surface of the N, Zn‐GQDs, functioning as a nanoprobe through amide bond formation. In the presence of cTnI, the signals from ECL and DPV were observed to decrease, indicating that the signal‐generating substance moved further away from the electrode [67]. An electrochemical biosensor has been developed that employs magnetic metal–organic framework (MMOF) nanocatalysts and DNA nanotetrahedron (NTH) based dual‐aptamer probes for the nonenzymatic detection of cTnI, which is a key biomarker for the early diagnosis of acute myocardial infarction (AMI). Initially, the NTH‐assisted dual‐aptamer capture probes (Tro4 and Tro6) were immobilized onto a screen‐printed gold electrode (SPGE) to significantly enhance the capture of the target cTnI, providing stable support and an optimized interface density [68] (see Tables 4, 5, 6, 7).
TABLE 4.
C‐reactive protein (CRP) biosensors.
| Type | Technique | Electrode | NPs | Sample/matrix | Linear range | LOD | Refs. |
|---|---|---|---|---|---|---|---|
| EC/ immunosensor | LSV, CV, PSV | Gold | CSPE‐COOH‐AuNPs | Serum | 0–100 µg/mL | 0058 µg/mL | [51] |
| EC/genosensor | CVs, DPV | SPE | AuNPs/GO‐COOH | Serum | 0.001–100 ng/mL | 2.941 × 10−4 | [52] |
| EC/genosensor | CVs, DPV | CNT | Ti3C2T x /Au | Serum | 0.05–80.0 ng/mL | 26 pg mL−1 | [53] |
| EC/immunosensor | MIP | — | graphdiyne (GDY) | Serum | 10−5–103 ng/mL | 0.41 × 10−5 ng/mL | [54] |
| EC/immunosensor | _ | — | AuNPs | Biological | 0.1–20 µg/mL | 0.05 µg/mL | [55] |
| EC/immunosensor | Microfluidic | — | — | Urine | — | 0.1 ng/mL | [56] |
| EC/immunosensor | MIP | SPCE | Au–Pt NMs/SPCE | Biological | 0.1–500 nM | 0.1 nM | [57] |
| EC/immunosensor | LSPR | — | Au@Ag NPs | Biological | 1.0–3.0 µg/mL | 2.4 × 10−5 mg/L | [58] |
| EC/genosensor | CVs, DPVs | — | dsApts@AuNPs | Biological | — | 79.7 pg/mL | [59] |
| EC/immunosensor | PEC | CNT | AuNS | Clinical | 25–800 pg/mL | 13.3 pg/mL | [60] |
TABLE 5.
Cardiac troponins biosensors.
| Type | Technique | Electrode | NPs | Sample/Matrix | Linear range | LOD | Refs. |
|---|---|---|---|---|---|---|---|
| EC/immunosensor | Amperometric | Gold | EC/ Immunosensor | Clinical | — |
233 pg/mL |
[61] |
| EC/aptasensor | — | GCE | Fe‐ATA MOF | Serum | 10 fg/mL to 100 ng/mL | 0.92 fg/mL | [62] |
| EC/immunosensor | PEC | — | Au@CuS | — | 0.1 pg/mL–5.0 ng/mL | 44.7 fg/mL | [63] |
| EC/optical | LFB | — | FMNP@QDs | Clinical | — | 0.0097 ng/mL | [64] |
| EC/immunosensor | DPVs | GCE | Pd@PdPtCo | Serum | 1.0 × 10−4−200 ng/mL | 0.031 pg/mL | [65] |
| EC/immunosensor | HEMT | — | AlGaN/GaN | Physiological | 0.006–148 ng/mL | 2 µL | [66] |
| ECL/immunosensor | DPV | GCE | N, Zn‐GQDs | Serum | 10–106 pg/L | 4.59 pg/L | [67] |
| EC/aptasensor | LBL | SPGE | Cu@Au | Clinical | 0.05–100 ng/mL | 16 pg/mL | [68] |
TABLE 6.
S100B protein biosensors.
| Type | Technique | Electrode | NPs | Sample/matrix | Linear range | LOD | Refs. |
|---|---|---|---|---|---|---|---|
| EC/immunosensor | Immunochromatography | — | Colloidal gold | Serum | 0.01–2 ng/mL | 4.6 pg/mL | [71] |
| EC/immunosensor | FTIR/EIS/SAM | Gold | Au | Plasma | 10–1000 pg/mL | 18 pg/mL | [72] |
| EC/immunosensor | DPV | Microchip | PMMA | Clinical | 0.1–100 pg/mL | 0.1 pg/mL | [73] |
| EC/immunosensor | EIS | Gold | Au | Plasma | 31 and 316 pg/mL | 35.73 pg/mL | [74] |
| EC/immunosensor | OSWV | Gold | — | Plasma | 10 nM Aβ1‐40 | 0.52 pM | [75] |
| EC/immunosensor | PEC | ITO | Au | Serum | 0.25–10 ng/mL | 0.15 pg/mL | [76] |
Abbreviation: OSWV, osteryoung square‐wave voltammetry.
TABLE 7.
MMPs protein biosensors.
| Type | Technique | Electrode | NPs | Sample/matrix | Linear range | LOD | Refs. |
|---|---|---|---|---|---|---|---|
| Optical | Fluorometric/colorimetric | — | AuNPs@gelatin/AuNCs | Serum | — | 2 ng/mL | [77] |
| Optical | ECL | GCE | AuNPs | Serum | 10–150 ng/mL | 8.0 ng/mL | [78] |
| Electrochemical | CVs | — | PAMAM | Tissue | 1.0 × 10−8 to 1.0 mg/L | 1.10 fg/mL | [79] |
| Electrochemical | SPR | — | AuNPs | Blood | 1.0–100.0 ng/mL | 3.9 ng/mL | [80] |
| Electrochemical | ECL | Gold | ((dfppy)2Ir(dcbpy)PF6, Ir1) | Clinical | 5 ng/mL–10 pg/mL | 5 ng/mL | [81] |
| Electrochemical | Genosensor, | Gold | CK11‐Fc | Clinical | 0.2 ng L–0.9 ng/L | 0.1 ng/L | [82] |
Abbreviations: ECL, electrogenerated chemiluminescence; PAMAM, polyamidoamine dendrimer.
6.4. S100B Protein Biosensors
Traumatic brain injury (TBI) has emerged as a significant public health concern. The timely identification, diagnosis and treatment of such injuries are crucial for patient outcomes, making the discovery of highly sensitive and specific biochemical markers essential for clinical practice [69, 70]. Among the various markers studied, the S100B protein stands out as the most researched and promising [69, 70]. A recent study developed a rapid quantitative biosensor for S100B utilizing colloidal gold labelling and a double antibody (8C10‐6B8) sandwich immunochromatography technique. This biosensor can measure S100B levels in just 15 min and demonstrates no cross‐reactivity with S100A, GFAP, (PGP9.5) and NSE. The reported detection limit and linear range are considered satisfactory [71]. A zero‐length cross‐linking method was utilized on a cysteamine self‐assembled monolayer (SAM) to attach anti‐S100B monoclonal antibodies to both planar gold electrodes (AuEs) and interdigitated gold electrodes (AuIDEs) through carbonyl bonding. The surface modifications were analysed using atomic force microscopy (AFM) and specular‐reflectance Fourier‐transform infrared spectroscopy (FTIR) at each stage of the functionalization process [72]. A highly sensitive microchip‐based electrochemical immunosensor has been created to measure low concentrations of S100B, a crucial biomarker for neurological disorders. Initially, poly(ethyleneimine) (PEI), which contains NH2 groups, was applied to the surface of the poly(methyl methacrylate) (PMMA) microchannel to capture monoclonal anti‐S100B antibodies. Subsequently, S100B was linked to a second polyclonal anti‐S100 antibody through specific antigen–antibody interactions, and alkaline phosphatase conjugates (ALP‐conjugates) were attached to the second antibodies within the PMMA microchannel [73]. A completely open‐source, modular, wireless and battery‐operated potentiostat has been developed, which is controlled via smartphone and is low‐cost. This device is designed to perform electrochemical impedance spectroscopy for the detection of the S100B protein, which is captured on an anti‐S100B functionalized thin‐film gold interdigitated electrode platform. This innovation aims to aid in the diagnosis and treatment of traumatic brain injuries [74]. A biosensor utilizing electrochemical methods was created to measure the S100B protein levels. This sensor employed His‐tagged VC1 domains from receptors for advanced glycation end (RAGE) products as the active analytical components. These domains were covalently attached to a monolayer formed from a thiol derivative of pentetic acid (DPTA) that was complexed with Cu(II) and applied to the surface of a gold electrode [75]. A sandwich‐style PEC immunoassay has been developed to detect the S100ß protein, a biomarker associated with Alzheimer's disease located in brain astrocytes. The antibody targeting S100ß (anti‐S100ß) was tagged with CdS QDs, serving as a secondary antibody in the assay. The properties of the labelled antibody were analysed using FTIR, ultraviolet‐visible spectroscopy and fluorescence spectroscopy [76].
6.5. Matrix Metalloproteinases Biosensors
MMPs are a group of proteolytic enzymes that play a crucial role in various biological processes, and their elevated levels are linked to the advancement of certain cancers. In this study, we introduced a new and highly sensitive technique for detecting MMP‐9 by utilizing its gelatinase activity. Researchers synthesized green‐emitting gold nanoclusters (AuNCs) using a gelatin substrate, and subsequently created a nanocomposite structure by coating gold nanoparticles (AuNPs) with the gelatin/AuNCs [77]. An ultra‐sensitive and specific biosensor utilizing electrogenerated chemiluminescence (ECL) was created to detect MMP‐3 in serum. This method relies on the cleavage of an oligopeptide triggered by the target enzyme, see Figure 5 [78].
FIGURE 5.
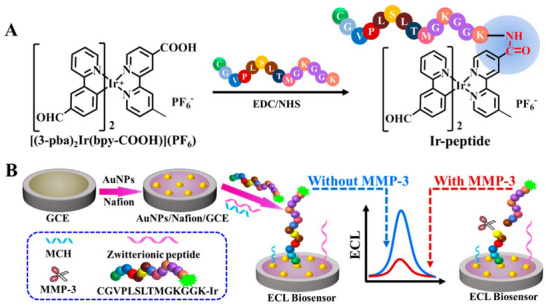
The representation graphs for the synthesis of Ir‐peptide (A) and the creation of the peptide‐based ECL biosensor for the detection of MMP‐3 (B) [78].
A cost‐effective, straightforward and quick approach was developed for the simultaneous detection of three MMPs (MMP‐1, MMP‐2 and MMP‐9). The sensor matrix was designed using a G2 polyamidoamine dendrimer (PAMAM) that incorporates amino, carboxyl and sulfhydryl functional groups [79]. A novel, label‐free surface plasmon resonance imaging biosensor was developed, offering high selectivity for the quantitative measurement of MMP‐2. The biosensor utilized ARP 101, a specific inhibitor of MMP‐2, as its receptor, which effectively captured the enzyme from the sample [80]. A specialized biosensor utilizing highly sensitive ECL has been developed for the concurrent detection of MMPs. This biosensor was easily constructed by co‐immobilizing peptides labelled with Ir1 and Ru1 onto a gold electrode through a self‐assembly method [81]. A peptide‐based electrochemical biosensor has been developed for the detection of MMP‐14. This approach relies on the cleavage of a specific peptide, which contains a ferrocene group (CK11‐Fc) that occurs in response to MMP‐14 activity and is anchored on a gold electrode [82].
7. Conclusion and Future Directions
In conclusion, the development of innovative biosensors for the detection of stroke biomarkers represents a significant advancement in the field of stroke diagnosis and management. The ability to rapidly and reliably identify key biomarkers such as NT‐proBNP, CRP, D‐dimer and others through advanced biosensing technologies has the potential to transform clinical practice by enabling timely therapeutic interventions and improving patient outcomes. The integration of nanomaterials and sophisticated signal amplification techniques has further enhanced the sensitivity and specificity of these biosensors, allowing for the detection of biomarkers at low concentrations in complex biological matrices. Despite these advancements, several challenges remain in the translation of biosensor technologies from the laboratory to clinical practice. Future directions should focus on addressing these challenges, including the need for standardization of biosensor protocols, validation of biosensor performance in diverse patient populations, and the establishment of regulatory pathways for clinical implementation. In addition, further research is needed to explore the integration of biosensors into POC settings, which could facilitate immediate decision‐making in emergency situations. Moreover, the exploration of multiplexed biosensing platforms that can simultaneously detect multiple biomarkers may provide a more comprehensive assessment of stroke risk and progression. The incorporation of machine learning and artificial intelligence in data analysis could also enhance the predictive capabilities of biosensors, leading to more personalized treatment strategies. In summary, while the current state of biosensor technology for stroke biomarker detection is promising, ongoing research and collaboration among scientists, clinicians and regulatory bodies will be essential to overcome existing barriers and fully realize the potential of these innovative tools in improving stroke care and outcomes.
Author Contributions
Ahmad Mobed, and Saeid Charsouei served as supervisors and corresponding authors. Ghazal Koohkansaadi and Arash Mohagheghi were responsible for writing and editing the original draft.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Consent
The authors have nothing to report.
Acknowledgements
This study was supported by the Physical Medicine and Rehabilitation Research Center, Aging Research Institute, Faculty of Medicine, Tabriz University of Medical Sciences, Iran.
Koohkansaadi G., Mohagheghi A., Mobed A., and Charsouei S., “Emerging Biosensor Technologies for Stroke Biomarker Detection: A Comprehensive Overview.” Analytical Science Advances 6, no. 2 (2025): 6, e70035. 10.1002/ansa.70035
Funding: The authors received no specific funding for this work.
Contributor Information
Ahmad Mobed, Email: Mobed1980@gmail.com, Email: Mobeda@tbzmed.ac.ir.
Saeid Charsouei, Email: Scharsouei@gmail.com.
Data Availability Statement
The authors have nothing to report.
References
- 1. Shil E. R., “Functional and Disability Status of Persons With Stroke in a Specialized Rehabilitation Centre” (Bachelor's diss.), Bangladesh Health Professions Institute, 2020. [Google Scholar]
- 2. Ma H. H., “Mechanical Thrombectomy in Acute Ischemic Stroke Management: An Extensive Examination of Worldwide Patterns, Lithuanian Medical Practices, and the Method Used in Hospital of Lithuanian University of Health Sciences Kaunas Clinics Between 2018 and 2022” (master's thesis, Lithuanian University of Health Sciences, 2024). [Google Scholar]
- 3. Wiyarta E., Fisher M., Kurniawan M., et al., “Global Insights on Prehospital Stroke Care: A Comprehensive Review of Challenges and Solutions in Low‐ and Middle‐Income Countries,” Journal of Clinical Medicine 13, no. 16 (2024): 4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li X., He Y., Wang D., and Rezaei M. J., “Stroke Rehabilitation: From Diagnosis to Therapy,” Frontiers in Neurology 15 (2024): 1402729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen H. and Luk K‐M, “Detection Capability Enhanced Biosensor Antenna for Portable Electromagnetic Stroke Diagnostic Systems,” IEEE Transactions on Biomedical Circuits and Systems 18, no. 1 (2023): 145–159. [DOI] [PubMed] [Google Scholar]
- 6. Sayad A., Uddin S. M., Yao S., et al., “A Magnetoimpedance Biosensor Microfluidic Platform for Detection of Glial Fibrillary Acidic Protein in Blood for Acute Stroke Classification,” Biosensors and Bioelectronics 211 (2022): 114410. [DOI] [PubMed] [Google Scholar]
- 7. Raggi A., Serretti A., and Ferri R., “A Comprehensive Overview of Post‐Stroke Depression Treatment Options,” International Clinical Psychopharmacology 39, no. 3 (2024): 127–138. [DOI] [PubMed] [Google Scholar]
- 8. Yi L., Huang L., Chen R., Zhan S., Huang H., and Yue Z., “Acupuncture for Post‐Stroke Spasticity: An Overview of Systematic Reviews,” Complementary Therapies in Medicine 80 (2024): 103024. [DOI] [PubMed] [Google Scholar]
- 9. Hall P., Lawrence M., Blake C., and Lennon O., “Interventions for Behaviour Change and Self‐Management of Risk in Stroke Secondary Prevention: An Overview of Reviews,” Cerebrovascular Diseases 53, no. 1 (2024): 1–13. [DOI] [PubMed] [Google Scholar]
- 10. Tan B. Y.‐Q., Tan S. M. L., Ng M. B. J., and Chan B. P., “Ischaemic Stroke in Young Adults: An Overview of the Classification, Risk Factors, Aetiologies and Outcomes,” Singapore Medical Journal (2025). 10.4103. [DOI] [PubMed] [Google Scholar]
- 11. Bondos B., Niewiadomska J., Stępień A., et al., “How to Reduce the Risk of Ischemic Stroke? Overview of Recommendations and Their Effectiveness,” Journal of Education, Health and Sport 75 (2024): 56337. [Google Scholar]
- 12. Syukriyah S. R. Z. M. D. and Al Fauzi A., “Hypertension as a Risk Factor in Stroke: An Overview,” World Journal of Advanced Research and Reviews 21, no. 1 (2024): 2370–2372. [Google Scholar]
- 13. Abujaber A. A., Imam Y., Nashwan A., Own A. M., and Akhtar N., “Stroke in Qatar: A Decade of Insights From a National Registry,” Neurological Research 46, no. 10 (2024): 893–906. [DOI] [PubMed] [Google Scholar]
- 14. Parry‐Jones A. R., Krishnamurthi R., Ziai W. C., et al., “World Stroke Organization (WSO): Global Intracerebral Hemorrhage Factsheet 2025,” International Journal of Stroke 20 (2025): 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsimpiris A., Tsolianos I., Grigoriadis A., Tsimtsiou Z., Goulis D. G., and Grigoriadis N., “Association of Chronic Periodontitis With Hemorrhagic Stroke: A Systematic Review and Meta‐Analysis,” European Journal of Dentistry 19 (2024): 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. García‐Carmona J., Conesa‐García E., Vidal‐Mena D., et al., “Increased Plasma Levels of N‐Terminal Pro‐B‐Type Natriuretic Peptide as Biomarker for the Diagnosis of Cardioembolic Ischaemic Stroke,” Neurologia 39, no. 6 (2024): 496–504. [DOI] [PubMed] [Google Scholar]
- 17. Shiroto H. and Hagii J., “Biomarkers for the Detection of Covert Atrial Fibrillation After Ischemic Stroke: NT‐proBNP or BNP?,” Journal of Stroke and Cerebrovascular Diseases 34 (2025): 108239. [DOI] [PubMed] [Google Scholar]
- 18. Patel J. and Bhaskar S. M., “Diagnostic Utility of N‐Terminal Pro‐B‐Type Natriuretic Peptide in Identifying Atrial Fibrillation Post‐Cryptogenic Stroke: A Systematic Review and Meta‐Analysis,” Pathophysiology 31, no. 3 (2024): 331–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manoj A. G., Prabhakar K., and Shashidhar K., “N‐Terminal Pro Brain Natriuretic Peptide (NT‐proBNP) as a Marker for Risk Stratification and Prediction of Functional Outcome in Acute Ischaemic Stroke,” Journal of Clinical & Diagnostic Research 18, no. 3 (2024): OC09–OC13. [Google Scholar]
- 20. Liu W., Sahin C., Güner Sak N., et al., “C‐Reactive Protein Expression in Acute Ischemic Stroke Blood Clots: Implications for Etiology,” European Stroke Journal (2025): 23969873251315636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sabbah A., Elsayed M., Helmy H., Mostafa R. H., and Hatem G., “The Role of Blood Protein Biomarkers in Acute Ischemic Stroke Prognosis,” Egyptian Journal of Neurology, Psychiatry and Neurosurgery 60, no. 1 (2024): 55. [Google Scholar]
- 22. Rosso M., Ramaswamy S., Mulatu Y., et al., “Rising Cardiac Troponin: A Prognostic Biomarker for Mortality After Acute Ischemic Stroke,” Journal of the American Heart Association 13, no. 4 (2024): e032922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anogianakis G., Daios S., Topouzis N., et al., “Current Trends in Stroke Biomarkers: The Prognostic Role of S100 Calcium‐Binding Protein B and Glial Fibrillary Acidic Protein,” Life 14, no. 10 (2024): 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rachman P. U., Juli C., and Wibisono Y., “Matrix Metalloproteinases as Biomarkers for Ischemic Stroke: A Systematic Review,” Bioscientia Medicina: Journal of Biomedicine and Translational Research 8, no. 11 (2024): 5407–5419. [Google Scholar]
- 25. Matys P., Mirończuk A., Starosz A., et al., “Expanding Role of Interleukin‐1 Family Cytokines in Acute Ischemic Stroke,” International Journal of Molecular Sciences 25, no. 19 (2024): 10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wei C., Zhai W., Zhao P., and Sun L., “Plasma Fibrinogen as a Potential Biomarker of Cognitive Impairment After Acute Ischemic Stroke,” Scientific Reports 14, no. 1 (2024): 32120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X., Du H., Song Z., et al., “Association Between Fibrinogen Levels and Stroke‐Associated Pneumonia in Acute Ischemic Stroke Patients,” BMC Neurology 24, no. 1 (2024): 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Makris K., Haliassos A., Chondrogianni M., and Tsivgoulis G., “Blood Biomarkers in Ischemic Stroke: Potential Role and Challenges in Clinical Practice and Research,” Critical Reviews in Clinical Laboratory Sciences 55, no. 5 (2018): 294–328. [DOI] [PubMed] [Google Scholar]
- 29. Sharma R., Macy S., Richardson K., Lokhnygina Y., and Laskowitz D. T., “A Blood‐Based Biomarker Panel to Detect Acute Stroke,” Journal of Stroke and Cerebrovascular Diseases 23, no. 5 (2014): 910–918. [DOI] [PubMed] [Google Scholar]
- 30. Medeiros R., Rossi S., López E., Miraballes I., and Borthagaray G., “Development and Application of Novel ELISA‐Based Analytical Tools for Assessing Nitroxidative Distress Biomarkers in Ischemic Stroke: Implications for Improved Diagnosis and Clinical Management,” Journal of Immunoassay and Immunochemistry 45, no. 2 (2024): 122–149. [DOI] [PubMed] [Google Scholar]
- 31. Liao C‐W, Fan C‐K, Kao T‐C, et al., “Brain Injury‐Associated Biomarkers of TGF‐beta1, S100B, GFAP, NF‐L, tTG, AbetaPP, and Tau Were Concomitantly Enhanced and the UPS Was Impaired During Acute Brain Injury Caused by Toxocara Canis in Mice,” BMC Infectious Diseases 8 (2008): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rossi R., Douglas A., Gil S. M., et al., “S100b in Acute Ischemic Stroke Clots Is a Biomarker for Post‐Thrombectomy Intracranial Hemorrhages,” Frontiers in Neurology 13 (2023): 1067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mobed A., Darvishi M., Alivirdiloo V., et al., “Cost‐Effective Nanosensor Solutions for Ultra‐Sensitive Detection of Metronidazole,” Analytical Science Advances 6, no. 1 (2025): e70000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rajaei S. N., Darvish M., Zare A., et al., “Advances in Nanobiosensors for Rapid and Sensitive Detection of Dengue Virus Biomarkers by Using Clinical Laboratory,” Journal of Clinical Laboratory Analysis 39 (2025): e70012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hassanzadeh‐Khanmiri M., Moshari A., Kheradmand R., et al., “Nanomedicine: A Cost‐Effective and Powerful Platform for Managing Neurodegenerative Diseases,” Metabolic Brain Disease 40, no. 3 (2025): 142. [DOI] [PubMed] [Google Scholar]
- 36. Wang L., Piao J., Qi H., et al., “Cuproptosis Nanomedicine in Cancer Therapy: Novel Insights and Future Perspectives,” Coordination Chemistry Reviews 523 (2025): 216284. [Google Scholar]
- 37. Sharma S., Gupta S., Saini A. K., Saini R. V., and Kaushal A., “Electrochemical Nanosensors: Revolutionizing Vitamin Detection,” Talanta 291 (2025): 127830. [DOI] [PubMed] [Google Scholar]
- 38. Apriyani F., Sari S. R., Petrus H. T. B. M., et al., “A Fluorescence Nanosensor Based on Modified Sustainable Silica for Highly Sensitive Detection of the SARS‐CoV‐2 IgG Antibody,” Nanoscale 17, no. 9 (2025): 5438–5446. [DOI] [PubMed] [Google Scholar]
- 39. Santra S., Johnson D., and Banerjee T., “BPS2025‐Membrane Fusion Interactions of Enveloped Viruses Using Lipid‐Coated Iron Oxide Nanosensors,” Biophysical Journal 124, no. 3 (2025): 498a. [Google Scholar]
- 40. Javaid M., Haleem A., Singh R. P., Rab S., and Suman R., “Exploring the Potential of Nanosensors: A Brief Overview,” Sensors International 2 (2021): 100130. [Google Scholar]
- 41. Adam T. and Gopinath S. C., “Nanosensors: Recent Perspectives on Attainments and Future Promise of Downstream Applications,” Process Biochemistry 117 (2022): 153–173. [Google Scholar]
- 42. Liu H‐L, Tseng Y‐T, Lai M‐C, and Chau L‐K, “Ultrasensitive and Rapid Detection of N‐Terminal Pro‐B‐Type Natriuretic Peptide (NT‐proBNP) Using Fiber Optic Nanogold‐Linked Immunosorbent Assay,” Biosensors 12, no. 9 (2022): 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grabowska I., Sharma N., Vasilescu A., et al., “Electrochemical Aptamer‐Based Biosensors for the Detection of Cardiac Biomarkers,” ACS Omega 3, no. 9 (2018): 12010–12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li X., Liu L., Dong X., et al., “Dual Mode Competitive Electrochemical Immunoassay for B‐Type Natriuretic Peptide Based on GS/SnO2/Polyaniline‐Au and ZnCo2O4/N‐CNTs,” Biosensors and Bioelectronics 126 (2019): 448–454. [DOI] [PubMed] [Google Scholar]
- 45. Baraket A., Ghedir E. K., Zine N., et al., “Electrochemical Immunosensor Prototype for N‐Terminal Natriuretic Peptide Detection in Human Saliva: Heart Failure Biomedical Application,” Chemosensors 13, no. 2 (2025): 66. [Google Scholar]
- 46. Jarić S., Kudriavtseva A., Nekrasov N., et al., “Femtomolar Detection of the Heart Failure Biomarker NT‐proBNP in Artificial Saliva Using an Immersible Liquid‐Gated Aptasensor With Reduced Graphene Oxide,” Microchemical Journal 196 (2024): 109611. [Google Scholar]
- 47. Zhang Y. and Sun M., “Ag‐Au Bimetallic Nanoparticle‐Based Electrochemical Sensing Platform for Quantification of B‐Type Natriuretic Peptide,” International Journal of Electrochemical Science 19, no. 8 (2024): 100703. [Google Scholar]
- 48. Wongjard S., Aiemderm P., Monkhang K., et al., “Selection, Alkaline Phosphatase Fusion, and Application of Single‐Chain Variable Fragment (scFv) Specific to NT‐proBNP as Electrochemical Immunosensor for Heart Failure,” Heliyon 9, no. 9 (2023): e19710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen Y., Wang X‐Y, Wang A‐J, et al., “Ultrasensitive Ratiometric Electrochemical Immunoassay of N‐Terminal Pro‐B‐Type Natriuretic Peptide Based on Three‐Dimensional PtCoNi Hollow Multi‐Branches/Ferrocene‐Grafted‐Ionic Liquid and CoNC Nanosheets,” Sensors and Actuators B: Chemical 326 (2021): 128794. [Google Scholar]
- 50. Zou H., Jiang Z., Bian E., et al., “Nanoencapsulated Optical Fiber‐Based PEC Microelectrode: Highly Sensitive and Specific Detection of NT‐proBNP and Its Implantable Performance,” Analytical Chemistry 97, no. 5 (2025): 2724–2735. [DOI] [PubMed] [Google Scholar]
- 51. Guillem P., Bustos R‐H, Garzon V., Munoz A., and Juez G., “A Low‐Cost Electrochemical Biosensor Platform for C‐Reactive Protein Detection,” Sensing and Bio‐Sensing Research 31 (2021): 100402. [Google Scholar]
- 52. Gao H., Bai Y., He B., and Tan C. S., “A Simple Label‐Free Aptamer‐Based Electrochemical Biosensor for the Sensitive Detection of C‐Reactive Proteins,” Biosensors 12, no. 12 (2022): 1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Z., Liu S., Shi Z., Lu D., Li Z., and Zhu Z., “Electrochemical Biosensor Based on RNA Aptamer and Ferrocenecarboxylic Acid Signal Probe for C‐Reactive Protein Detection,” Talanta 277 (2024): 126318. [DOI] [PubMed] [Google Scholar]
- 54. Cui M., Che Z., Gong Y., Li T., Hu W., and Wang S., “A Graphdiyne‐Based Protein Molecularly Imprinted Biosensor for Highly Sensitive Human C‐Reactive Protein Detection in Human Serum,” Chemical Engineering Journal 431 (2022): 133455. [Google Scholar]
- 55. Lu T‐C, Yang Y‐J, Zhong Y., et al., “Simultaneous Detection of C‐Reactive Protein and Lipopolysaccharide Based on a Dual‐Channel Electrochemical Biosensor for Rapid Gram‐Typing of Bacterial Sepsis,” Biosensors and Bioelectronics 243 (2024): 115772. [DOI] [PubMed] [Google Scholar]
- 56. Chen S‐J, Lu S‐Y, Tseng C‐C, Huang K‐H, Chen T‐L, and Fu L‐M, “Rapid Microfluidic Immuno‐Biosensor Detection System for the Point‐Of‐Care Determination of High‐Sensitivity Urinary C‐Reactive Protein,” Biosensors 14, no. 6 (2024): 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Balayan S., Chauhan N., Chandra R., and Jain U., “Electrochemical Based C‐Reactive Protein (CRP) Sensing Through Molecularly Imprinted Polymer (MIP) Pore Structure Coupled With Bi‐Metallic Tuned Screen‐Printed Electrode,” Biointerface Research in Applied Chemistry 6 (2022): 38. [Google Scholar]
- 58. Tran Truc Phuong N., Anh D. T., Hoang Nhat Thao N., et al., “Application of Hybrid Au@Ag Nanostructures in Fiber Optic Biosensor for Rapid Detection of C‐Reactive Protein,” Optical Materials 143 (2023): 114184. [Google Scholar]
- 59. Zhang R., Shao H., Hu X., et al., “Universal, Sensitive, and Visual Sandwich‐Type Biosensor Based on Nanogold‐Catalyzed Reduction and Its Application for Detecting C‐Reactive Protein in Serum by a Portable Colorimeter,” Sensors and Actuators B: Chemical 431 (2025): 137408. [Google Scholar]
- 60. Monsalve Y., Cruz‐Pacheco A. F., and Orozco J., “Plasmonic Graphene–Gold Nanostar Heterojunction for Red‐Light Photoelectrochemical Immunosensing of C‐Reactive Protein,” ACS Sensors 10, no. 2 (2025): 1493–1504. [DOI] [PubMed] [Google Scholar]
- 61. Docherty N., Collins L., Pang S., Fu Y., Milne S., and Corrigan D., “Cost‐Effective Amperometric Immunosensor for Cardiac Troponin I as a Step Towards Affordable Point‐Of‐Care Diagnosis of Acute Myocardial Infarction,” Sensing and Bio‐Sensing Research 47 (2025): 100725. [Google Scholar]
- 62. Li C., Yang H., Song Z., Gao F., Niu L., and Wang Q., “Rational Utilization of Fe(III)‐2‐Amino Terephthalic Acid MOF as a Bifunctional Nanocarrier of Dopamine and Aptamer for Constructing Ultrasensitive Sandwich Biosensor of Cardiac Troponin I,” Sensors and Actuators B: Chemical 411 (2024): 135746. [Google Scholar]
- 63. Guo J., Kuang G., Luo D., Yu W., Chen L., and Fu Y., “High‐Performance Assaying Cardiac Troponin I Using Bi‐Doped Tin‐Based Heterojunction in Photoelectrochemical Biosensing With the Quencher of Yolk‐Shell Nanostructure,” Talanta 277 (2024): 126342. [DOI] [PubMed] [Google Scholar]
- 64. Liu G., Chen M., Wang J., et al., “Flower‐Shaped Magnetic Fluorescent Nanoparticles Based Lateral Flow Biosensor for Highly Sensitive Detection of Cardiac Troponin I,” Sensors and Actuators B: Chemical 414 (2024): 135921. [Google Scholar]
- 65. Wang M., Sun H‐N, Liu X‐Y, Liu M., and Li S‐S, “A Sensitive Electrochemical Biosensor Based on Pd@PdPtCo Mesoporous Nanopolyhedras as Signal Amplifiers for Assay of Cardiac Troponin I,” Bioelectrochemistry 161 (2025): 108838. [DOI] [PubMed] [Google Scholar]
- 66. Sarangadharan I., Regmi A., Chen Y‐W, et al., “High Sensitivity Cardiac Troponin I Detection in Physiological Environment Using AlGaN/GaN High Electron Mobility Transistor (HEMT) Biosensors,” Biosensors and Bioelectronics 100 (2018): 282–289. [DOI] [PubMed] [Google Scholar]
- 67. Liu M., Jiang R., Zheng M., et al., “A Sensitive Ratiometric Biosensor for Determination Cardiac Troponin I of Myocardial Infarction Markers Based on N, Zn‐GQDs,” Talanta 249 (2022): 123577. [DOI] [PubMed] [Google Scholar]
- 68. Sun D., Luo Z., Lu J., et al., “Electrochemical Dual‐Aptamer‐Based Biosensor for Nonenzymatic Detection of Cardiac Troponin I by Nanohybrid Electrocatalysts Labeling Combined With DNA Nanotetrahedron Structure,” Biosensors and Bioelectronics 134 (2019): 49–56. [DOI] [PubMed] [Google Scholar]
- 69. National Academies of Sciences, Engineering, and Medicine, Traumatic Brain Injury: A Roadmap for Accelerating Progress (National Academies Press, 2022). [PubMed] [Google Scholar]
- 70. Poislane P‐A, Papin M., Masson D., et al., “Diagnostic Performance of S100B Assay for Intracranial Hemorrhage Detection in Patients With Mild Traumatic Brain Injury Under Antiplatelet or Anticoagulant Therapy,” Scientific Reports 15, no. 1 (2025): 5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ye L., Xu L., Kuang H., Xu X., and Xu C., “Colloidal Gold‐Based Immunochromatographic Biosensor for Quantitative Detection of S100B in Serum Samples,” Nanoscale Horizons 8, no. 9 (2023): 1253–1261. [DOI] [PubMed] [Google Scholar]
- 72. Rodríguez A., Burgos‐Flórez F., Posada J. D., et al., “Electrochemical Immunosensor for the Quantification of S100B at Clinically Relevant Levels Using a Cysteamine Modified Surface,” Sensors 21, no. 6 (2021): 1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu Y., Wang H., Chen J., et al., “A Sensitive Microchip‐Based Immunosensor for Electrochemical Detection of Low‐Level Biomarker S100B,” Electroanalysis 25, no. 4 (2013): 1050–1055. [Google Scholar]
- 74. Burgos‐Flórez F., Rodríguez A., Cervera E., Zucolotto V., Sanjuán M., and Villalba P. J., “TBISTAT: An Open‐Source, Wireless Portable, Electrochemical Impedance Spectroscopy Capable Potentiostat for the Point‐Of‐Care Detection of S100B in Plasma Samples,” PLoS ONE 17, no. 2 (2022): e0263738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mikuła E., Wysłouch‐Cieszyńska A., Zhukova L., et al., “Voltammetric Detection of S100B Protein Using His‐Tagged Receptor Domains for Advanced Glycation End Products (RAGE) Immobilized Onto a Gold Electrode Surface,” Sensors 14, no. 6 (2014): 10650–10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tabrizi M. A., Ferré‐Borrull J., Kapruwan P., and Marsal L. F., “A Photoelectrochemical Sandwich Immunoassay for Protein S100β, a Biomarker for Alzheimer's Disease, Using an ITO Electrode Modified With a Reduced Graphene Oxide‐Gold Conjugate and CdS‐Labeled Secondary Antibody,” Microchimica Acta 186, no. 2 (2019): 117. [DOI] [PubMed] [Google Scholar]
- 77. Dadmehr M., Mortezaei M., and Korouzhdehi B., “Dual Mode Fluorometric and Colorimetric Detection of Matrix Metalloproteinase MMP‐9 as a Cancer Biomarker Based on AuNPs@Gelatin/AuNCs Nanocomposite,” Biosensors and Bioelectronics 220 (2023): 114889. [DOI] [PubMed] [Google Scholar]
- 78. Qian M., Zeng Y., Li M., Gao Q., Zhang C., and Qi H., “Electrogenerated Chemiluminescence Biosensor for Quantization of Matrix Metalloproteinase‐3 in Serum via Target‐Induced Cleavage of Oligopeptide,” Biosensors 14, no. 4 (2024): 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kowalczyk A., Nisiewicz M. K., Bamburowicz‐Klimkowska M., et al., “Effective Voltammetric Tool for Simultaneous Detection of MMP‐1, MMP‐2, and MMP‐9; Important Non‐Small Cell Lung Cancer Biomarkers,” Biosensors and Bioelectronics 229 (2023): 115212. [DOI] [PubMed] [Google Scholar]
- 80. Tokarzewicz A., Romanowicz L., Sveklo I., Matuszczak E., Hermanowicz A., and Gorodkiewicz E., “SPRI Biosensors for Quantitative Determination of Matrix Metalloproteinase‐2,” Analytical Methods 9, no. 16 (2017): 2407–2414. [Google Scholar]
- 81. Gao H., Dang Q., Xia S., et al., “Highly Selective Electrogenerated Chemiluminescence Biosensor for Simultaneous Detection of Matrix Metalloproteinase‐2 and Matrix Metalloproteinase‐7 in Cell Secretions,” Sensors and Actuators B: Chemical 253 (2017): 69–76. [Google Scholar]
- 82. Sun L., Chen Y., Chen F., and Ma F., “Peptide‐Based Electrochemical Biosensor for Matrix Metalloproteinase‐14 and Protein‐Overexpressing Cancer Cells Based on Analyte‐Induced Cleavage of Peptide,” Microchemical Journal 157 (2020): 105103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors have nothing to report.


