Abstract
BACKGROUND
Targeted therapy combined with anti-programmed cell death 1 immunotherapy (TP) and trifluridine/tipiracil (TAS-102) combined with bevacizumab (TB) are two common therapies for patients with late-line therapy in microsatellite stable (MSS) metastatic colorectal cancer (mCRC). However, it is still unclear which therapy can bring better prognosis.
AIM
To evaluate the effectiveness and safety of TP vs TB as the late-line regimen for MSS mCRC in the real world.
METHODS
This is a dual-center retrospective cohort study conducted in Peking University First Hospital and Jilin Cancer Hospital. Patients with MSS mCRC who had received at least the second line treatment were eligible. Propensity score (PS) would be calculated to balance the baseline characteristics of two cohorts. Progression-free survival (PFS) was set as the primary endpoint. The Kaplan-Meier method and Cox proportional hazard model were used to evaluate PFS and to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Landmark analysis was performed to create segmented survival curves, studying the impact of treatment regimen on prognosis during different follow-up periods.
RESULTS
Between July 2019 and March 2025 (data cutoff), 127 eligible patients were enrolled, with 88 and 39 patients assigned to the TP and TB cohorts, respectively, based on treatment allocation. At a global median follow-up of 9.73 months, the crude median PFS was 3.9 months (95%CI: 3.03-5.53) in the TP cohort vs 4.17 months (95%CI: 2.87-5.6) in the TB cohort, yielding a nonsignificant HR of 1.43 (95%CI: 0.94-2.18, P = 0.092; TB as reference). Multivariate Cox regression analysis, adjusted for sex, age > 60 years, Eastern Cooperative Oncology Group performance status, RAS mutation, primary tumor location (left vs right), number of metastatic organs (liver/lung), and treatment line (≥ 3rd line), demonstrated an adjusted HR of 1.23 (95%CI: 0.80-1.88, P = 0.348). PS-based analyses using three methodologies: Inverse probability weighting, PS matching (post-matching n = 55 vs 30), and PS-adjusted multivariate Cox regression. These analyses revealed consistent nonsignificant trends favoring TB, with HRs for TP of 1.26 (95%CI: 0.76-2.10, P = 0.077), 1.42 (95%CI: 0.87-2.34, P = 0.164), and 1.26 (95%CI: 0.76-2.10, P = 0.367), respectively. Notably, landmark PFS analyses at 90, 120, and 150 days demonstrated a significantly higher proportion of TP patients maintaining disease control beyond these timepoints (P = 0.048, 0.031, and 0.035, respectively), suggesting sustained clinical benefits in TP responders.
CONCLUSION
TP and TB demonstrated similar PFS in both crude and PS-adjusted analyses. However, patients who derived benefits from TP therapy exceeding 90 days showed more sustained clinical advantages compared to TB. Our study suggests that for patients with MSS mCRC who respond to TP therapy in later-line treatments, this regimen could provide additional prolonged clinical benefits, which warrants further validation through large-scale cohort investigations.
Keywords: Microsatellite stable, Metastatic colorectal cancer, Immune checkpoint inhibitors, Targeted therapy, Programmed cell death 1, Trifluridine/tipiracil
Core Tip: Targeted therapy combined with anti-programmed cell death 1 immunotherapy (TP) and trifluridine/tipiracil combined with bevacizumab (TB) are two common therapies for patients with late-line therapy in microsatellite stable (MSS) metastatic colorectal cancer (mCRC). However, it is still unclear which therapy can bring better prognosis. This study aims to evaluate the effectiveness and safety of TP vs TB as the late-line regimen for MSS mCRC in the real world.
INTRODUCTION
Colorectal cancer (CRC) is a prevalent malignant tumor of the digestive system. According to the latest data, CRC is projected to account for over 150000 new cases and more than 50000 deaths in the United States in 2025, solidifying its position as the third most common cancer and the third leading cause of cancer-related mortality. Notably, its incidence is rising annually, with an increasing trend toward younger onset, posing significant threats to global health[1]. Recent statistics from China’s National Cancer Center rank CRC as the second most common malignancy and the fourth leading cause of cancer-related deaths[2]. Approximately 20% CRC patients present with metastatic disease at initial diagnosis, exhibiting a dismal 5-year survival rate of 5%-15%[3,4]. Patients with microsatellite stable (MSS) metastatic CRC (mCRC) typically receive first- and second-line therapies, including fluorouracil-based chemotherapy (often combined with oxaliplatin or irinotecan), vascular endothelial growth factor (VEGF)-targeted agents [primarily bevacizumab (TB)], and epidermal growth factor receptor (EGFR) therapies (for RAS wild-type tumors)[5,6]. Disease progression following these regimens defines refractory mCRC. However, many patients retain good performance status, warranting further therapeutic interventions[7]. Third- and fourth-line options for refractory mCRC include reintroduction of oxaliplatin-based chemotherapy[8,9], re-challenge with EGFR inhibitors in RAS wild-type cases[10-12], and molecularly targeted therapies for actionable genomic alterations[13-15]. In the third-line setting, regorafenib and trifluridine/tipiracil (TAS-102) have demonstrated improved progression-free survival (PFS) and overall survival (OS)[16-21]. TAS-102, an oral combination of trifluridine (a thymidine analog) and tipiracil (a thymidine phosphorylase inhibitor), exhibits favorable activity and tolerability[22,23]. The SUNLIGHT trial recently established TAS-102 plus TB as a standard third-line regimen[21].
Therapeutic efficacy remains suboptimal in late-line mCRC (≥ 3rd line), with limited drug options. Preliminary clinical evidence supports the potential of anti-angiogenic/programmed cell death 1 (PD-1) inhibitor combinations in mCRC[24], a regimen increasingly adopted in clinical practice. Notably, the 2023 American Society of Clinical Oncology Annual Meeting highlighted results from the SUNLIGHT trial[25], which compared TB vs TAS-102 monotherapy in advanced mCRC. The combination significantly improved OS and objective response rates (ORRs), establishing its preferential use in eligible patients without contraindications. Building on these advances, both targeted therapy combined with anti-PD-1 immunotherapy (TP) and TB regimen demonstrate clinical benefits in late-line mCRC. However, direct comparisons between these strategies remain unexplored. To address this gap, our team has initiated a dual-center retrospective cohort study evaluating the real-world effectiveness and safety of TP vs TB regimens in MSS mCRC.
Based on the aforementioned evidence, both TP and TB regimen have demonstrated promising efficacy in late-line treatment of MSS mCRC. However, no direct comparative studies have yet established the superiority of either therapeutic strategy. To address this critical knowledge gap, our team has initiated a dual-center retrospective cohort study to systematically compare the clinical outcomes of these two regimens in real-world settings. This investigation aims to elucidate differential effectiveness, safety profiles, and survival benefits, thereby providing evidence-based guidance for clinical decision-making in late-line MSS mCRC management.
MATERIALS AND METHODS
Study design and participants
This study employed a dual-center retrospective cohort research design. Patients with MSS mCRC who were treated at Peking University First Hospital and Jilin Cancer Hospital between July 2019 and March 2025 were enrolled. The TP group consisted of patients who received targeted therapy combined with anti-PD-1 immunotherapy, while the TB group comprised patients who received TAS-102 with TB.
The inclusion criteria were: (1) Histologically or cytologically confirmed unresectable mCRC with measurable lesions based on the RECIST criteria; (2) Proficient mismatch repair (pMMR) or MSS, BRAF wild-type; (3) Patients who had received at least the second line treatment were eligible; (4) Having not undergone radiotherapy or having completed radiotherapy more than 4 weeks ago; and (5) Eastern Cooperative Oncology Group (ECOG) score ≤ 2.
The exclusion criteria were: (1) Patients with deficient mismatch repair or microsatellite instability-high, or BRAF mutations; (2) Presence of symptomatic brain metastases; (3) Uncontrolled active infection; (4) Dysphagia, intractable vomiting, or known drug absorption disorders; and (5) Patients with symptomatic or high-risk obstruction, bleeding, perforation, or those who have undergone intestinal stent placement to relieve intestinal obstruction.
In most cases, the targeted therapy regimen comprised fruquintinib and regorafenib. Anti-PD-1 immunotherapy included penpulimab, pembrolizumab, sintilimab, tislelizumab, and toripalimab. The anti-angiogenic agent was TB. The flowchart of patient selection was shown in Figure 1.
Figure 1.
Flow chart of patient inclusion. MSS: Microsatellite stable; TAS-102: Trifluridine/tipiracil.
Procedures
Follow-up data were collected through hospital records, telephone interviews, outpatient visits, and rehospitalizations. The data included age, sex, height, weight, ECOG status, primary tumor location, number of metastatic sites, tumor differentiation grade, percentage reduction in tumor volume, PFS, OS, follow-up duration, and survival status. The last follow-up date was in May 2025. PFS was the primary outcome, defined as the time from enrollment to the first documented disease progression according to RECIST version 1.1, or death from any cause, whichever occurred first. Secondary outcomes included OS, ORR, disease control rate (DCR), and safety evaluation. OS was calculated from the date of enrollment to the date of death from any cause, with censored cases defined by the last available follow-up. ORR was defined as the proportion of patients with a best objective response of complete response (CR) or partial response (PR) according to RECIST criteria (version 1.1). DCR was defined as the proportion of patients with CR, PR, or stable disease according to RECIST criteria (version 1.1). Treatment-related adverse events were evaluated according to the Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
Categorical variables were compared using the χ2 test or Fisher’s exact test, while continuous variables were assessed using the Mann-Whitney test. Kaplan-Meier estimates were obtained to compare the actuarial survival and efficacy endpoints between the two groups. Independent prognostic factors, hazard ratios (HRs), and 95% confidence intervals (CIs) were evaluated using the Cox proportional hazards model. Landmark analysis was performed to create segmented survival curves, studying the impact of treatment regimen on prognosis during different follow-up periods. A two-sided P value of < 0.050 was considered statistically significant. All statistical analyses in our study were performed using R software (version 4.4.2).
Sensitivity analysis
To mitigate potential confounding biases, three sensitivity analyses were conducted. First, propensity score matching (PSM) was performed using the nearest-neighbor method and a variable matching ratio (1:1 to 1:4) to balance baseline characteristics, including age, sex, ECOG performance status, metastatic sites, and tumor location, via the MatchIt package[26]. Second, propensity score (PS)-adjusted multivariable Cox proportional hazards regression was employed to further control for residual confounding factors. Third, inverse probability of treatment weighting was applied to evaluate treatment effects while adjusting for baseline covariates. Fourth, to evaluate the robustness of the results, we incorporated prior treatments into the Cox model and restricted the TP group to patients receiving regorafenib-based or fruquintinib-based therapies to focus our analysis.
RESULTS
Patients
From July 2019 to March 2025, 127 eligible patients in total were enrolled. Based on the treatment they received, 88 and 39 patients were respectively assigned to the TP cohort and the TB cohort. The cohort comprised 80 males (63.0%) and 47 females (37.0%), with 76 patients (59.8%) aged over 60 years. Clinicopathological characteristics revealed 31 cases (24.4%) of right-sided colon cancer, 119 patients (93.7%) who had undergone primary tumor resection, and 99 patients (78.0%) with metastases involving two or more organs. Molecular analysis showed that all patients exhibited pMMR, while RAS mutations were detected in 69 cases (54.3%). In the TP group, 41 patients received sintilimab treatment, and 46 patients received regorafenib (Supplementary Table 1). Detailed baseline characteristics are presented in Table 1.
Table 1.
Demographic and clinical characteristics of the patients at baseline, n (%)
|
Characteristics
|
Levels
|
n
|
| Age | ≤ 60 | 51 (40.2) |
| > 60 | 76 (59.8) | |
| Gender | Male | 80 (63.0) |
| Female | 47 (37.0) | |
| ECOG | 0-1 | 110 (86.6) |
| 2 | 17 (13.4) | |
| Primary tumor location | Right colon | 31 (24.4) |
| Left colon and rectum | 96 (75.6) | |
| Primary tumor surgery | No | 8 (6.3) |
| Yes | 119 (93.7) | |
| Number of metastatic organs | 1 | 28 (22.0) |
| ≥ 2 | 99 (78.0) | |
| Liver metastasis | No | 48 (37.8) |
| Yes | 79 (62.2) | |
| Lung metastasis | No | 47 (37.0) |
| Yes | 80 (63.0) | |
| RAS | Wild-type | 34 (26.8) |
| KRAS mutation | 62 (48.8) | |
| NRAS mutation | 7 (5.5) | |
| Unknown | 24 (18.9) | |
| Treatment regimen | Targeted drug + PD-1 | 88 (69.3) |
| TAS-102 + bevacizumab | 39 (30.7) | |
| BRAF | Wild-type | 91 (71.7) |
| Mutation | 6 (4.7) | |
| Unknown | 30 (23.6) | |
| Prior fluorouracil | No | 78 (61.4) |
| Yes | 49 (38.6) | |
| Prior oxaliplatin | No | 19 (15.0) |
| Yes | 108 (85.0) | |
| Prior irinotecan | No | 19 (15.0) |
| Yes | 108 (85.0) | |
| Prior capecitabine | No | 26 (20.5) |
| Yes | 101 (79.5) | |
| Prior raltitrexed | No | 86 (67.7) |
| Yes | 41 (32.3) | |
| Prior bevacizumab | No | 18 (14.2) |
| Yes | 109 (85.8) | |
| Prior cetuximab | No | 95 (74.8) |
| Yes | 32 (25.2) | |
| Treatment line | 3 Line | 69 (54.3) |
| > 3 Line | 58 (45.7) |
ECOG: Eastern Cooperative Oncology Group; PD-1: Programmed cell death 1; TAS-102: Trifluridine/tipiracil.
Effectiveness
With a median follow-up duration of 9.73 months (as of May 2025), the overall cohort demonstrated a median PFS of 4.1 months. The crude PFS was 3.9 (95%CI: 3.03-5.53) months in TP cohort vs 4.17 (2.87-5.6) months in TB cohort. However, this difference in PFS did not reach statistical significance (HR = 1.4322, 95%CI: 0.9406- 2.181, P = 0.092) (Figure 2). Similarly, no significant difference was observed in OS between groups (HR = 1.9989, 95%CI: 0.4991-8.005, P = 0.328) (Supplementary Figure 1). Multivariate Cox regression analysis, adjusted for sex, age > 60 years, ECOG performance status, RAS mutation, primary tumor location (left vs right), number of metastatic organs (liver/Lung), and treatment line (≥ 3rd line), demonstrated an adjusted HR of 1.23 (95%CI: 0.80-1.88, P = 0.348) in PFS (Table 2) and HR of 1.6023 (95%CI: 0.3900-6.582, P = 0.5132) in OS (Supplementary Table 2). Regarding response rates, the TP group demonstrated an ORR of 5.45% vs 3.33% in the TB group (P = 1.00), while the DCR reached 58.19% and 56.67%, respectively (P = 0.6711) (Table 3). Collectively, these results suggest comparable efficacy between the two treatment approaches without statistically significant differences in survival outcomes or response rates.
Figure 2.
Kaplan-Meier curve of original cohort (progression-free survival). TP: Targeted therapy combined with anti-programmed cell death 1 immunotherapy; TB: Bevacizumab.
Table 2.
Univariate and multivariate Cox analysis of the effect of prognostic factors in the original cohort (progression-free survival), n (%)
|
Dependent: Survival (PFS/30, status PFS)
|
All
|
HR (univariable)
|
HR (multivariable)
|
|
| Gender | Male | 80 (63.0) | ||
| Female | 47 (37.0) | 1.11 (0.74-1.66, P = 0.619) | ||
| Age | ≤ 60 | 51 (40.2) | ||
| > 60 | 76 (59.8) | 0.87 (0.58-1.31, P = 0.514) | ||
| ECOG | 0-1 | 110 (86.6) | ||
| 2 | 17 (13.4) | 0.54 (0.28-1.01, P = 0.055) | 0.56 (0.30-1.07, P = 0.079) | |
| Number of metastatic organs | 1 | 28 (22.0) | ||
| ≥ 2 | 99 (78.0) | 2.22 (1.34-3.68, P = 0.002) | 2.14 (1.28-3.57, P = 0.004) | |
| Primary tumor location | Right colon | 31 (24.4) | ||
| Left colon and rectum | 96 (75.6) | 0.94 (0.59-1.49, P = 0.800) | ||
| Liver metastasis | No | 48 (37.8) | ||
| Yes | 79 (62.2) | 1.39 (0.91-2.11, P = 0.126) | ||
| Lung metastasis | No | 47 (37.0) | ||
| Yes | 80 (63.0) | 1.01 (0.67-1.52, P = 0.966) | ||
| RAS | Wild-type | 34 (26.8) | ||
| Mutation | 69 (54.3) | 1.02 (0.65-1.59, P = 0.932) | ||
| Unknown | 24 (18.9) | 0.63 (0.33-1.20, P = 0.159) | ||
| Treatment regimen | Targeted drug + PD-1 | 88 (69.3) | ||
| TAS102 + bevacizumab | 39 (30.7) | 1.43 (0.94-2.18, P = 0.094) | 1.23 (0.80-1.88, P = 0.348) | |
n = 127, events = 100, likelihood ratio test = 16 on 3 (P = 0.001). HR: Hazard ratio; ECOG: Eastern Cooperative Oncology Group; PD-1: Programmed cell death 1; PFS: Progression-free survival; TAS-102: Trifluridine/tipiracil.
Table 3.
Comparison of short-term efficacy between two groups after propensity score matching (ratio = 4)
|
Group
|
Number
|
PR
|
SD
|
PD
|
ORR (%)
|
DCR (%)
|
| 1TP | 55 | 3 | 29 | 23 | 5.45 | 58.19 |
| 2TB | 30 | 1 | 16 | 13 | 3.33 | 56.67 |
| χ2 value | 0 | 0.1803 | 0.1803 | |||
| P value | 13 | 1 | 0.6711 | 13 | 0.6711 |
Received targeted therapy combined with anti-programmed cell death 1 immunotherapy.
Received trifluridine/tipiracil combined with bevacizumab.
Fisher’s exact test.
PR: Partial response; SD: Stable disease; PD: Progressive disease; ORR: Objective response rate; DCR: Disease control rate.
Sensitivity analysis
Using PS, we conducted three statistical methods, namely PS matching (the sample size was 55 vs 30 after matching) (Supplementary Figure 2), additional adjustment for PS with multivariate Cox regression and inverse probability weighting. After performing 1:4 PSM, we obtained a final cohort of 85 patients, with 55 in the TP group and 30 in the TB group. The baseline variables remained well-balanced between the two groups (Supplementary Figure 3A). In subgroup analysis, the TP group achieved a median PFS of 3.9 months compared to 3.87 months in the TB group. However, this difference in PFS did not reach statistical significance (HR = 1.422, 95%CI: 0.866- 2.335, P = 0.164) (Figure 3A). Adjusting the PSM ratio did not alter the final outcomes of PFS (Figure 3B-D). Additional adjustment for PS with multivariate cox regression, the HR of TP were 1.26 (95%CI: 0.76-2.10, P = 0.367) in PFS (Table 4) and the HR of TP were 1.14 (95%CI: 0.20-6.43, P = 0.886) in OS (Supplementary Table 3). Furthermore, the inverse probability of treatment weighting analysis corroborated this finding for PFS (P = 0.2398) (Figure 3E) and after inverse probability of treatment weighting, the baseline variables remained well-balanced between the two groups (Supplementary Figure 3B). Similarly, when prior treatments were incorporated into the Cox model, restricting the TP group to patients receiving regorafenib-based or fruquintinib-based therapies did not alter the study conclusions (Supplementary Tables 4-6).
Figure 3.
Propensity score-matching analysis in progression-free survival. A: After propensity score-matching analysis in progression-free survival (PFS) (ratio = 4); B: After propensity score-matching analysis in PFS (ratio = 3); C: After propensity score-matching analysis in PFS (ratio = 2); D: After propensity score-matching analysis in PFS (ratio = 1); E: After inverse probability of treatment weighting analysis (PFS). TP: Targeted therapy combined with anti-programmed cell death 1 immunotherapy; TB: Bevacizumab.
Table 4.
Propensity score-adjusted multivariable Cox regression analysis of prognostic factors associated with progression-free survival in the original cohort, mean ± SD/n (%)
|
Dependent: Survival (PFS/30, status PFS)
|
All
|
HR (multivariable)
|
|
| Gender | Male | 80 (63.0) | |
| Female | 47 (37.0) | 0.99 (0.62-1.58, P = 0.969) | |
| Age | ≤ 60 | 51 (40.2) | |
| > 60 | 76 (59.8) | 0.95 (0.62-1.47, P = 0.834) | |
| ECOG | 0-1 | 110 (86.6) | |
| 2 | 17 (13.4) | 0.51 (0.26-1.00, P = 0.051) | |
| Number of metastatic organs | 1 | 28 (22.0) | |
| ≥ 2 | 99 (78.0) | 2.40 (1.31-4.41, P = 0.005) | |
| Primary tumor location | Right colon | 31 (24.4) | |
| Left colon and rectum | 96 (75.6) | 1.23 (0.72-2.10, P = 0.450) | |
| Liver metastasis | No | 48 (37.8) | |
| Yes | 79 (62.2) | 0.91 (0.56-1.49, P = 0.721) | |
| Lung metastasis | No | 47 (37.0) | |
| Yes | 80 (63.0) | 0.99 (0.64-1.56, P = 0.982) | |
| RAS | Wild-type | 34 (26.8) | |
| Mutation | 69 (54.3) | 0.81 (0.50-1.34, P = 0.415) | |
| Unknown | 24 (18.9) | 0.67 (0.34-1.29, P = 0.228) | |
| Treatment regimen | Targeted drug + PD-1 | 88 (69.3) | |
| TAS-102 + bevacizumab | 39 (30.7) | 1.26 (0.76-2.10, P = 0.367) | |
| PS | 0.3 ± 0.2 | 0.85 (0.23-3.18, P = 0.805) | |
n = 127, events = 100, likelihood ratio test = 19.13 on 11 (P = 0.059). HR: Hazard ratio; ECOG: Eastern Cooperative Oncology Group; PD-1: Programmed cell death 1; PFS: Progression-free survival; TAS-102: Trifluridine/tipiracil; PS: Propensity score.
Landmark survival analyses
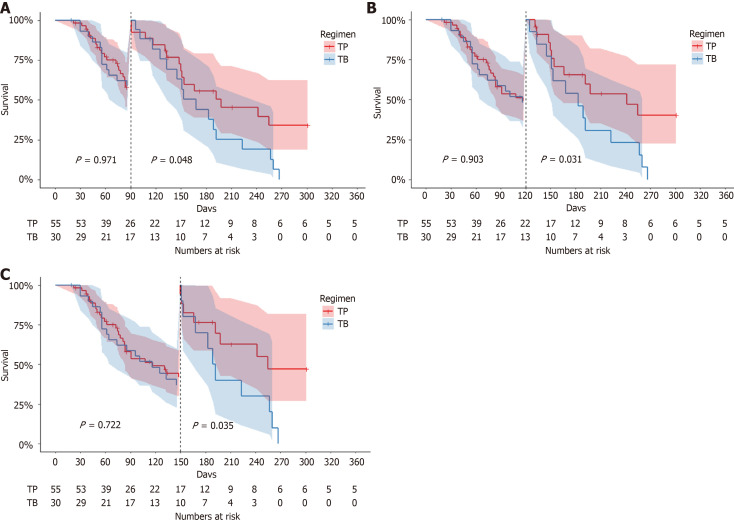
Following PSM, the survival curves of the two groups exhibited delayed divergence (Figure 2). Landmark analysis revealed no statistically significant difference in PFS between the cohorts at ≤ 90 days (P = 0.971) (Figure 4A). Subsequent landmark analyses stratified by progression-free status at 90 (Figure 4A), 120 (Figure 4B), and 150 days (Figure 4C) demonstrated that a significantly higher proportion of TP patients maintained disease control beyond these timepoints, conferring sustained PFS advantages (P = 0.048, 0.031, and 0.035, respectively; Figure 4). These patients were comparable at baseline (Supplementary Tables 7 and 8).
Figure 4.
Kaplan-Meier curve of landmark analysis in progression-free survival. A: Survival curves stratified by a 90-day landmark timepoint; B: Survival curves stratified by a 120-day landmark timepoint; C: Survival curves stratified by a 150-day landmark timepoint. TP: Targeted therapy combined with anti-programmed cell death 1 immunotherapy; TB: Bevacizumab.
Safety
The safety analysis demonstrated comparable toxicity profiles between the treatment arms, with no statistically significant differences in overall adverse event incidence. Although the TB group showed numerically higher rates of grade 3-4 neutropenia compared to the TP group (9.09% vs 26.67%, P = 0.055), this difference did not achieve statistical significance. Grade 1-2 adverse events occurred at similar frequencies across both groups (Table 5).
Table 5.
Treatment-emergent adverse events in the 85 patients of the matched dataset, n (%)
|
Toxicities
|
TP group
|
TB group
|
P value for grade 1-2
|
P value for grade 3-4
|
||||
|
Grade 0
|
Grade 1-2
|
Grade 3-4
|
Grade 0
|
Grade 1-2
|
Grade 3-4
|
|||
| n = 55 | n = 30 | |||||||
| Anemia | 37 (67.3) | 17 (30.9) | 1 (1.8) | 21 (70.0) | 7 (23.3) | 2 (6.7) | 0.62461 | 0.28332 |
| Neutropenia | 41 (74.5) | 9 (16.4) | 5 (9.1) | 17 (56.7) | 5 (16.7) | 8 (26.7) | 12 | 0.055132 |
| Leukocytopenia | 33 (60.0) | 18 (32.7) | 4 (7.3) | 18 (60.0) | 11 (36.7) | 1 (3.3) | 0.89921 | 0.65212 |
| Thrombocytopenia | 40 (72.7) | 15 (27.3) | 0 (0) | 17 (56.7) | 12 (40.0) | 1 (3.3) | 0.33671 | 0.35292 |
| Proteinuria | 39 (70.9) | 16 (29.1) | 0 (0) | 23 (76.7) | 7 (23.3) | 0 (0) | 0.75231 | 12 |
| Aspartate transaminase increased | 36 (65.5) | 18 (32.7) | 1 (1.8) | 23 (76.7) | 6 (20.0) | 1 (3.3) | 0.32041 | 12 |
| Alanine transaminase increased | 41 (74.5) | 14 (25.5) | 0 (0) | 20 (66.7) | 8 (26.7) | 2 (6.7) | 11 | 0.35292 |
| Alkaline phosphatase increased | 40 (72.7) | 13 (23.6) | 2 (3.6) | 21 (70.0) | 9 (30.0) | 0 (0) | 0.70321 | 0.53782 |
| Blood bilirubin increased | 39 (70.9) | 16 (29.1) | 0 (0) | 24 (80.0) | 5 (16.7) | 1 (3.3) | 0.31441 | 0.35292 |
| Triglycerides increased | 36 (65.5) | 17 (30.9) | 2 (3.6) | 24 (80.0) | 4 (13.3) | 2 (6.7) | 0.11332 | 0.61132 |
| Nausea | 40 (72.7) | 15 (27.3) | 0 (0) | 23 (76.7) | 6 (20.0) | 1 (3.3) | 0.63141 | 12 |
| Vomiting | 49 (89.1) | 6 (10.9) | 0 (0) | 21 (70.0) | 8 (26.7) | 1 (3.3) | 0.11741 | 0.35292 |
| Fatigue | 42 (76.4) | 13 (23.6) | 0 (0) | 20 (66.7) | 9 (30.0) | 1 (3.3) | 0.70321 | 0.35292 |
| Fever | 49 (89.1) | 6 (10.9) | 0 (0) | 26 (86.7) | 4 (13.3) | 0 (0) | 0.73671 | 12 |
| Diarrhea | 47 (85.5) | 8 (14.5) | 0 (0) | 25 (83.4) | 4 (13.3) | 1 (3.3) | 11 | 0.35292 |
| Hypertension | 48 (87.3) | 6 (10.9) | 1 (1.8) | 24 (80.0) | 3 (10.0) | 3 (10.0) | 12 | 0.12382 |
P values were calculated by χ2 test.
P values were calculated by Fisher’s exact test.
TP: Targeted therapy combined with anti-programmed cell death 1 immunotherapy; TB: Bevacizumab.
Subgroup analysis
To further investigate survival disparities between the regimens in the balanced cohort, subgroup analyses stratified by baseline characteristics including age, sex, primary tumor location, number of metastatic sites, presence of liver metastases, presence of lung metastases, RAS mutation status, and treatment line were performed (Figure 5). No statistically significant differences in PFS were observed across any subgroups.
Figure 5.
Forest plots depict the hazard ratios and 95% confidence intervals for progression-free survival by subgroup. HR: Hazard ratio; CI: Confidence interval.
DISCUSSION
In the third-line setting, conventional chemotherapy has demonstrated limited efficacy with rare tumor regression observed[27]. Nevertheless, many patients with mCRC retain good performance status at this stage, underscoring the urgent need for novel therapeutic agents. For those with chemotherapy-refractory disease, treatment goals focus on delaying disease progression and prolonging survival while preserving quality of life. Both regorafenib and TAS-102 have shown significant improvements in PFS and OS compared to placebo in patients who have exhausted standard therapies[16-21]. While systemic therapy remains the cornerstone of late-line mCRC management, some patients may benefit from combined local interventions, making ORR a critical efficacy metric. Achieving tumor response prior to local therapy could optimize clinical outcomes, a consideration highly prioritized in practice. Historically, single-agent anti-angiogenic therapies exhibited low DCR[25]. In contrast, both TP and TB regimens in our study achieved DCRs approaching 60%, offering a promising strategy to address this clinical challenge.
Emerging evidence suggests VEGF receptor (VEGFR) inhibitors may enhance tumor responsiveness to immunotherapy[28]. The combination of fruquintinib (a VEGFR inhibitor) with immune checkpoint inhibitors has emerged as an investigational approach for MSS mCRC. For instance, a 2022 retrospective study involving 52 advanced mCRC patients reported an ORR of 15.38%, DCR of 57.69%, and median PFS of 108 days with fruquintinib plus sintilimab (anti-PD-1)[29]. A phase Ib/II trial in 2023 further demonstrated promising efficacy in MSS mCRC patients, with an ORR of 20.0%, median PFS of 6.9 months, and OS of 20.0 months for the combination regimen[30]. These findings indicate TP regimen may confer survival benefits in MSS mCRC. Notably, a study presented at the 2023 American Society of Clinical Oncology Gastrointestinal Cancers Symposium evaluated fruquintinib plus anti-PD-1 therapy vs fruquintinib monotherapy in ≥ 2nd-line MSS mCRC. The combination arm showed significant PFS prolongation, providing additional clinical benefits[31]. Collectively, these advancements offer new hope for immunotherapy-resistant MSS mCRC patients, positioning fruquintinib-immune checkpoint inhibitor regimens as a potential therapeutic option.
The phase III international RECOURSE trial established TAS-102 as a standard therapy for refractory mCRC[17]. In this study, 800 patients previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based regimens (including TB and anti-EGFR agents for RAS wild-type tumors) were randomized to TAS-102 or placebo. TAS-102 significantly improved median OS (OS: 7.1 vs 5.3 months) and PFS (PFS: 2.0 vs 1.7 months) vs placebo. Notably, 17% TAS-102 recipients had prior regorafenib exposure. Subgroup analyses confirmed consistent efficacy across baseline characteristics, though ORR remained low (2%). Similar OS benefits were observed in the Asian phase III TERRA trial, with safety profiles aligning with RECOURSE findings[32]. Post-marketing studies further demonstrated that TAS-102 preserves health-related quality of life and delays ECOG performance status deterioration[33-35]. The recent SUNLIGHT trial[21] established TB as a new third-line standard, showing comparable PFS to TP in both crude and PS-adjusted analyses. However, landmark analyses revealed sustained PFS advantages for TP beyond 90 days (90-day: P = 0.048; 120-day: P = 0.031; 150-day: P = 0.035), with a higher proportion of TP patients maintaining long-term disease control.
Unlike conventional chemotherapy, immune-oncology therapies often exhibit delayed efficacy and prolonged survival benefits, characterized by late separation of Kaplan-Meier curves. Studies suggest that longer duration of response correlates with pronounced survival tail effects, highlighting the unique value of immunotherapy and its synergistic potential with chemotherapy[36,37]. Consistent with the delayed efficacy patterns of immune-oncology therapies, our landmark analyses demonstrated sustained PFS benefits for the TP regimen beyond critical timepoints: 90-day (P = 0.048), 120-day (P = 0.031), and 150-day (P = 0.035).
Our dual-center retrospective cohort study has several limitations that warrant consideration. Firstly, the retrospective design with a small sample size inherently limits data robustness. The maturity of PFS and OS data in the retrospective analysis was relatively low, and patient management was also relatively poor, introducing certain biases. Secondly, a critical gap in our analysis was the inability to assess PD-L1 expression levels, precluding any evaluation of their potential correlation with anti-PD-1 immunotherapy efficacy. This biomarker information could have provided valuable insights into treatment response variability. Third, therapeutic heterogeneity may have impacted our findings. The study incorporated two targeted therapy regimens (fruquintinib and regorafenib) and five different anti-PD-1 agents, creating substantial variability that could obscure true efficacy comparisons between treatment approaches.
CONCLUSION
TP and TB demonstrated similar PFS in both crude and PS-adjusted analyses. However, patients who derived benefits from TP therapy exceeding 90 days showed more sustained clinical advantages compared to TB. Our study suggests that for patients with MSS mCRC who respond to TP therapy in later-line treatments, this regimen could provide additional prolonged clinical benefits, which warrants further validation through large-scale cohort investigations.
ACKNOWLEDGEMENTS
We are grateful to the patients and their families for supporting the study.
Footnotes
Institutional review board statement: This study has been approved by the Ethics Committee of Peking University First Hospital (approval No. 2025R0190-0001) and Jilin Cancer Hospital (approval No. 202501-003-01).
Informed consent statement: Consent to participate not required.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade A, Grade A, Grade B, Grade B, Grade B
Novelty: Grade A, Grade A, Grade B, Grade B, Grade C
Creativity or Innovation: Grade A, Grade A, Grade B, Grade B, Grade C
Scientific Significance: Grade A, Grade A, Grade B, Grade B, Grade B
P-Reviewer: Chen YZ; Liu QL; Wu F S-Editor: Fan M L-Editor: A P-Editor: Yu HG
Contributor Information
Zhao Gao, Department of Medical Oncology, Peking University First Hospital, Beijing 100034, China.
Xiao-Yan Wang, Department of Pharmacy, Jilin Cancer Hospital, Changchun 130012, Jilin Province, China.
Tao Song, Department of Pharmacy, Jilin Cancer Hospital, Changchun 130012, Jilin Province, China.
Zhi-Gang Shen, Department of Pharmacy, Jilin Cancer Hospital, Changchun 130012, Jilin Province, China.
Xiao-Yun Wang, Department of Medical Oncology, Peking University First Hospital, Beijing 100034, China.
Shi-Kai Wu, Department of Medical Oncology, Peking University First Hospital, Beijing 100034, China.
Xuan Jin, Department of Medical Oncology, Peking University First Hospital, Beijing 100034, China. jinxuanbdyy@outlook.com.
Data sharing statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75:10–45. doi: 10.3322/caac.21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 5.Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10–32. doi: 10.1016/j.annonc.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Benson AB, Venook AP, Adam M, Chang G, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Haste P, Hecht JR, Hoffe S, Hunt S, Hussan H, Johung KL, Joseph N, Kirilcuk N, Krishnamurthi S, Malla M, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Shogan B, Skibber JM, Sofocleous CT, Tavakkoli A, Willett CG, Wu C, Gurski LA, Snedeker J, Jones F. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2024;22:e240029. doi: 10.6004/jnccn.2024.0029. [DOI] [PubMed] [Google Scholar]
- 7.Ciracì P, Studiale V, Taravella A, Antoniotti C, Cremolini C. Late-line options for patients with metastatic colorectal cancer: a review and evidence-based algorithm. Nat Rev Clin Oncol. 2025;22:28–45. doi: 10.1038/s41571-024-00965-0. [DOI] [PubMed] [Google Scholar]
- 8.Mauri G, Gori V, Bonazzina E, Amatu A, Tosi F, Bencardino K, Ruggieri L, Patelli G, Arena S, Bardelli A, Siena S, Sartore-Bianchi A. Oxaliplatin retreatment in metastatic colorectal cancer: Systematic review and future research opportunities. Cancer Treat Rev. 2020;91:102112. doi: 10.1016/j.ctrv.2020.102112. [DOI] [PubMed] [Google Scholar]
- 9.Amatu A, Mauri G, Tosi F, Bencardino K, Bonazzina E, Gori V, Ruggieri L, Arena S, Bardelli A, Marsoni S, Siena S, Sartore-Bianchi A. Efficacy of Retreatment with Oxaliplatin-Based Regimens in Metastatic Colorectal Cancer Patients: The RETROX-CRC Retrospective Study. Cancers (Basel) 2022;14:1197. doi: 10.3390/cancers14051197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cremolini C, Rossini D, Dell'Aquila E, Lonardi S, Conca E, Del Re M, Busico A, Pietrantonio F, Danesi R, Aprile G, Tamburini E, Barone C, Masi G, Pantano F, Pucci F, Corsi DC, Pella N, Bergamo F, Rofi E, Barbara C, Falcone A, Santini D. Rechallenge for Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer With Acquired Resistance to First-line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol. 2019;5:343–350. doi: 10.1001/jamaoncol.2018.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sartore-Bianchi A, Pietrantonio F, Lonardi S, Mussolin B, Rua F, Crisafulli G, Bartolini A, Fenocchio E, Amatu A, Manca P, Bergamo F, Tosi F, Mauri G, Ambrosini M, Daniel F, Torri V, Vanzulli A, Regge D, Cappello G, Marchiò C, Berrino E, Sapino A, Marsoni S, Siena S, Bardelli A. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat Med. 2022;28:1612–1618. doi: 10.1038/s41591-022-01886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinelli E, Martini G, Famiglietti V, Troiani T, Napolitano S, Pietrantonio F, Ciardiello D, Terminiello M, Borrelli C, Vitiello PP, De Braud F, Morano F, Avallone A, Normanno N, Nappi A, Maiello E, Latiano T, Falcone A, Cremolini C, Rossini D, Santabarbara G, Pinto C, Santini D, Cardone C, Zanaletti N, Di Liello A, Renato D, Esposito L, Marrone F, Ciardiello F. Cetuximab Rechallenge Plus Avelumab in Pretreated Patients With RAS Wild-type Metastatic Colorectal Cancer: The Phase 2 Single-Arm Clinical CAVE Trial. JAMA Oncol. 2021;7:1529–1535. doi: 10.1001/jamaoncol.2021.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738–746. doi: 10.1016/S1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 14.Tabernero J, Grothey A, Van Cutsem E, Yaeger R, Wasan H, Yoshino T, Desai J, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Elez E, Gollerkeri A, Maharry K, Christy-Bittel J, Kopetz S. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J Clin Oncol. 2021;39:273–284. doi: 10.1200/JCO.20.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siena S, Di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, Yamaguchi K, Nishina T, Fakih M, Elez E, Rodriguez J, Ciardiello F, Komatsu Y, Esaki T, Chung K, Wainberg Z, Sartore-Bianchi A, Saxena K, Yamamoto E, Bako E, Okuda Y, Shahidi J, Grothey A, Yoshino T DESTINY-CRC01 investigators. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22:779–789. doi: 10.1016/S1470-2045(21)00086-3. [DOI] [PubMed] [Google Scholar]
- 16.Yoshino T, Mizunuma N, Yamazaki K, Nishina T, Komatsu Y, Baba H, Tsuji A, Yamaguchi K, Muro K, Sugimoto N, Tsuji Y, Moriwaki T, Esaki T, Hamada C, Tanase T, Ohtsu A. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13:993–1001. doi: 10.1016/S1470-2045(12)70345-5. [DOI] [PubMed] [Google Scholar]
- 17.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Kim TW, Shen L, Sriuranpong V, Pan H, Xu R, Guo W, Han SW, Liu T, Park YS, Shi C, Bai Y, Bi F, Ahn JB, Qin S, Li Q, Wu C, Ma D, Lin D, Li J. Results of a Randomized, Double-Blind, Placebo-Controlled, Phase III Trial of Trifluridine/Tipiracil (TAS-102) Monotherapy in Asian Patients With Previously Treated Metastatic Colorectal Cancer: The TERRA Study. J Clin Oncol. 2018;36:350–358. doi: 10.1200/JCO.2017.74.3245. [DOI] [PubMed] [Google Scholar]
- 19.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L, Yeh KH, Bi F, Cheng Y, Le AT, Lin JK, Liu T, Ma D, Kappeler C, Kalmus J, Kim TW CONCUR Investigators. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 21.Ayala-de Miguel C, Jiménez-Castro J, Sánchez-Vegas A, Díaz-López S, Chaves-Conde M. Third-line treatment and beyond in metastatic colorectal cancer: What do we have and what can we expect? Crit Rev Oncol Hematol. 2024;202:104454. doi: 10.1016/j.critrevonc.2024.104454. [DOI] [PubMed] [Google Scholar]
- 22.Andersen SE, Andersen IB, Jensen BV, Pfeiffer P, Ota T, Larsen JS. A systematic review of observational studies of trifluridine/tipiracil (TAS-102) for metastatic colorectal cancer. Acta Oncol. 2019;58:1149–1157. doi: 10.1080/0284186X.2019.1605192. [DOI] [PubMed] [Google Scholar]
- 23.Peeters M, Cervantes A, Moreno Vera S, Taieb J. Trifluridine/tipiracil: an emerging strategy for the management of gastrointestinal cancers. Future Oncol. 2018;14:1629–1645. doi: 10.2217/fon-2018-0147. [DOI] [PubMed] [Google Scholar]
- 24.Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603) J Clin Oncol. 2020;38:2053–2061. doi: 10.1200/JCO.19.03296. [DOI] [PubMed] [Google Scholar]
- 25.Prager GW, Taieb J, Fakih M, Ciardiello F, Van Cutsem E, Elez E, Cruz FM, Wyrwicz L, Stroyakovskiy D, Pápai Z, Poureau PG, Liposits G, Cremolini C, Bondarenko I, Modest DP, Benhadji KA, Amellal N, Leger C, Vidot L, Tabernero J SUNLIGHT Investigators. Trifluridine-Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N Engl J Med. 2023;388:1657–1667. doi: 10.1056/NEJMoa2214963. [DOI] [PubMed] [Google Scholar]
- 26.Ho DE, Imai K, King G, Stuart EA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Soft. 2011;42 [Google Scholar]
- 27.Nielsen DL, Palshof JA, Larsen FO, Jensen BV, Pfeiffer P. A systematic review of salvage therapy to patients with metastatic colorectal cancer previously treated with fluorouracil, oxaliplatin and irinotecan +/- targeted therapy. Cancer Treat Rev. 2014;40:701–715. doi: 10.1016/j.ctrv.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Patel SA, Nilsson MB, Le X, Cascone T, Jain RK, Heymach JV. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin Cancer Res. 2023;29:30–39. doi: 10.1158/1078-0432.CCR-22-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gou M, Qian N, Zhang Y, Yan H, Si H, Wang Z, Dai G. Fruquintinib in Combination With PD-1 Inhibitors in Patients With Refractory Non-MSI-H/pMMR Metastatic Colorectal Cancer: A Real-World Study in China. Front Oncol. 2022;12:851756. doi: 10.3389/fonc.2022.851756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y, Zhang W, Ying J, Zhang Y, Pan Y, Qiu W, Fan Q, Xu Q, Ma Y, Wang G, Guo J, Su W, Fan S, Tan P, Wang Y, Luo Y, Zhou H, Li J. Phase 1b/2 trial of fruquintinib plus sintilimab in treating advanced solid tumours: The dose-escalation and metastatic colorectal cancer cohort in the dose-expansion phases. Eur J Cancer. 2023;181:26–37. doi: 10.1016/j.ejca.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 31.An T, Lian Y, Zhou Q, Zhao C, Wang Z, Zhao R. Fruquintinib with PD-1 inhibitors versus fruquintinib monotherapy in late-line mCRC: A retrospective cohort study based on propensity score matching. J Clin Oncol. 2024;42:139–139. [Google Scholar]
- 32.Dasari A, Sobrero A, Yao J, Yoshino T, Schelman W, Yang Z, Chien C, Kania M, Tabernero J, Eng C. FRESCO-2: a global Phase III study investigating the efficacy and safety of fruquintinib in metastatic colorectal cancer. Future Oncol. 2021;17:3151–3162. doi: 10.2217/fon-2021-0202. [DOI] [PubMed] [Google Scholar]
- 33.Weiss L, Karthaus M, Riera-Knorrenschild J, Kretzschmar A, Welslau M, Vehling-Kaiser U, Pelz H, Ettrich TJ, Hess J, Reisländer T, Klein A, Heinemann V TALLISUR study group. Efficacy, safety and quality-of-life data from patients with pre-treated metastatic colorectal cancer receiving trifluridine/tipiracil: results of the TALLISUR trial. ESMO Open. 2022;7:100391. doi: 10.1016/j.esmoop.2022.100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachet JB, Wyrwicz L, Price T, Cremolini C, Phelip JM, Portales F, Ozet A, Cicin I, Atlan D, Becquart M, Vidot L, Mounedji N, Van Cutsem E, Taieb J, Falcone A. Safety, efficacy and patient-reported outcomes with trifluridine/tipiracil in pretreated metastatic colorectal cancer: results of the PRECONNECT study. ESMO Open. 2020;5:e000698. doi: 10.1136/esmoopen-2020-000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taieb J, Price T, Vidot L, Chevallier B, Wyrwicz L, Bachet JB. Safety and efficacy of trifluridine/tipiracil in previously treated metastatic colorectal cancer: final results from the phase IIIb single-arm PRECONNECT study by duration of therapy. BMC Cancer. 2023;23:94. doi: 10.1186/s12885-022-10489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, Pluzanski A, Arrieta O, Frontera OA, Chiari R, Butts C, Wójcik-Tomaszewska J, Coudert B, Garassino MC, Ready N, Felip E, García MA, Waterhouse D, Domine M, Barlesi F, Antonia S, Wohlleber M, Gerber DE, Czyzewicz G, Spigel DR, Crino L, Eberhardt WEE, Li A, Marimuthu S, Brahmer J. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol. 2021;39:723–733. doi: 10.1200/JCO.20.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thummalapalli R, Ricciuti B, Bandlamudi C, Muldoon D, Rizvi H, Elkrief A, Luo J, Alessi JV, Pecci F, Lamberti G, Di Federico A, Hong L, Zhang J, Heymach JV, Gibbons DL, Plodkowski AJ, Ravichandran V, Donoghue MTA, Vanderbilt C, Ladanyi M, Rudin CM, Kris MG, Riely GJ, Chaft JE, Hellmann MD, Vokes NI, Awad MM, Schoenfeld AJ. Clinical and Molecular Features of Long-term Response to Immune Checkpoint Inhibitors in Patients with Advanced Non-Small Cell Lung Cancer. Clin Cancer Res. 2023;29:4408–4418. doi: 10.1158/1078-0432.CCR-23-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.