Abstract
The gut-liver axis represents a complex, bidirectional communication network between the gastrointestinal tract and the liver, playing a central role in maintaining metabolic homeostasis. In diabetes, disruption of this axis, mediated by gut microbiota dysbiosis, impaired intestinal barrier function, and pro-inflammatory signaling, contributes significantly to insulin resistance, hepatic steatosis, and systemic metabolic dysfunction. This review explores the underlying mechanisms by which microbial alterations, increased gut permeability, and inflammatory pathways influence hepatic insulin resistance and glucose metabolism. In addition to established mechanisms, emerging pathways involving neuroendocrine circuits, microbial metabolites, and immune mediators are discussed, offering deeper insight into gut-liver interactions in metabolic disease. The review also outlines therapeutic strategies targeting the gut-liver axis, including microbiota modulation, barrier function enhancement, and anti-inflammatory interventions, emphasizing their potential in advancing diabetes management. A conceptual framework is proposed to integrate these components into a precision medicine approach for metabolic regulation. Key challenges in clinical translation, including patient heterogeneity and the absence of reliable biomarkers to guide treatment decisions are also discussed to inform future research. By linking mechanistic understanding with therapeutic innovation, the review highlights the gut-liver axis as a promising target for personalized diabetes care.
Keywords: Diabetes, Microbiota, Diabetes management, Gut-liver axis, Anti-inflammatory, Intestinal permeability, Metabolic homeostasis
Core Tip: Disruption of the gut-liver axis plays a critical role in the pathophysiology of diabetes by linking gut microbiota alterations, intestinal barrier dysfunction, and inflammatory pathways to hepatic insulin resistance and impaired glucose metabolism. Understanding these mechanisms opens new therapeutic opportunities, including microbiota modulation, gut barrier reinforcement, and targeted anti-inflammatory strategies. These approaches have the potential to significantly improve diabetes management and metabolic outcomes.
INTRODUCTION
Diabetes mellitus is a complex metabolic disorder characterized by chronic hyperglycemia, insulin resistance (IR), and progressive β-cell dysfunction[1]. Over the past decade, growing evidence has established the gut-liver axis as a pivotal contributor to diabetes pathogenesis, offering novel insights into disease mechanisms and therapeutic opportunities. The gut-liver axis forms an integrated communication network linking metabolic, immune, and endocrine signals between the gastrointestinal tract and the liver[2]. This dynamic crosstalk is mediated by gut microbiota composition, intestinal barrier integrity, microbial metabolites, and inflammatory signaling pathways[3-5]. Disruption of this system contributes to IR, hepatic dysfunction, and systemic metabolic dysregulation, core features of diabetes[5].
Globally, the burden of diabetes has reached alarming levels, with an estimated 828 million adults affected in 2022, an increase of more than 630 million cases since 1990. This surge is closely linked to lifestyle changes, including increased consumption of ultra-processed foods, reduced physical activity, and escalating obesity rates, all of which also influence gut microbiota composition and liver health[6].
Non-alcoholic fatty liver disease (NAFLD), recently redefined as metabolic dysfunction-associated steatotic liver disease (MASLD), has emerged as a key comorbidity of type 2 diabetes (T2D). A meta-analysis of nearly 7 million individuals with NAFLD and over 1 million with MAFLD found that the pooled global prevalence of T2D among these populations was 28.3% and 26.2%, respectively, with incidence densities of 24.6 and 26.9 cases per 1000 person-years[7]. These findings confirm that liver fat accumulation is not only a consequence of metabolic dysfunction but also a strong predictor of diabetes onset. Conversely, diabetes accelerates the progression of steatotic liver disease to more advanced stages, including non-alcoholic steatohepatitis, fibrosis, and cirrhosis. This bidirectional relationship between liver disease and diabetes underscores the pivotal role of the gut-liver axis in metabolic disease and highlights the need for integrative treatment strategies targeting both organs.
Gut dysbiosis, an imbalance in microbial composition and function, has been implicated as a central factor in diabetes pathophysiology[8,9]. Individuals with diabetes often exhibit altered microbial profiles, increased intestinal permeability (“leaky gut”), systemic inflammation, and hepatic IR[10,11]. Moreover, disruptions in microbial metabolites such as short-chain fatty acids (SCFAs) and bile acid derivatives further impair metabolic function[12,13], positioning gut dysbiosis as a promising therapeutic strategy.
While most studies focus on T2D, emerging data also implicate gut-liver axis disruptions in type 1 diabetes (T1D) and type 3 diabetes (T3D). In T1D, an autoimmune condition involving pancreatic β-cell destruction, gut microbiota alterations, and increased gut permeability may contribute to immune dysregulation[14-16]. A loss of butyrate-producing bacteria, critical for maintaining immune tolerance, is commonly observed in individuals with T1D[17,18]. Similarly, in T3D, a term used to describe IR and metabolic dysfunction associated with Alzheimer’s disease, gut-liver axis disruptions have been linked to cognitive decline[19-21]. Hepatic IR and MASLD are recognized risk factors for neurodegeneration, with gut-derived inflammation implicated in promoting amyloid-beta accumulation and tau hyperphosphorylation[22-25].
Thus, the gut-liver axis stands at the crossroads of metabolic and neurodegenerative diseases, offering a unifying framework for understanding complex disease interactions. In the context of diabetes, unravelling this axis could unlock innovative therapies to restore metabolic homeostasis. Building on this perspective, the present review provides a comprehensive analysis of the gut-liver axis in diabetes, focusing on its mechanistic foundations and therapeutic relevance. Specifically, the objectives are to: (1) Describe the molecular and cellular mechanisms connecting gut microbiota, intestinal permeability, and hepatic metabolism; (2) Highlight emerging pathways involving neuroendocrine, immune, and microbial signaling; (3) Evaluate current and potential interventions targeting these mechanisms; and (4) Identify key knowledge gaps and translational challenges. Through this integrative approach, the review aims to offer a framework to inform future research and support more effective strategies for diabetes care.
THE GUT-LIVER AXIS: PHYSIOLOGICAL OVERVIEW
The gut-liver axis is a highly coordinated, bidirectional communication system that plays a critical role in maintaining metabolic equilibrium, immune regulation, and nutrient processing[5,26]. This connection is anatomically and functionally centered on the portal vein, which delivers nutrient- rich and microbe-derived blood directly from the intestine to the liver[27]. As a result, the liver continuously samples intestinal inputs, serving as both a metabolic hub and immune sentinel[28].
At the core of this axis lies the intestinal barrier, a multilayered defense system composed of mucus, epithelial cells joined by tight junctions, and mucosal immune components[29]. Its primary function is to allow nutrient absorption while preventing the translocation of pathogens and toxins into the bloodstream. Disruption of this barrier, often due to inflammation, poor diet, or dysbiosis, allows microbial products such as lipopolysaccharides (LPS) to enter the portal circulation, triggering hepatic immune responses that promote inflammation and metabolic dysfunction[29-32].
The gut microbiota, a diverse ecosystem of trillions of microorganisms, plays a central role in modulating gut-liver communication[3,8,33]. Microbial fermentation of dietary fibers produces SCFAs, including butyrate, acetate, and propionate, which serve as energy sources for colonocytes and influence both intestinal and hepatic immune function[34,35]. SCFAs also regulate gluconeogenesis, lipid metabolism, and insulin sensitivity via G-protein-coupled receptors and through epigenetic modifications such as histone acetylation[36-38].
Bile acids, synthesized in the liver and modified by gut microbiota, also act as key signaling molecules[39,40]. Secondary bile acids generated by microbial metabolism are reabsorbed and recirculated back to the liver via the enterohepatic circulation[41,42]. These bile acids activate nuclear receptors such as the farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5 (TGR5), thereby modulating lipid metabolism, glucose homeostasis, and inflammatory signaling pathways[43,44].
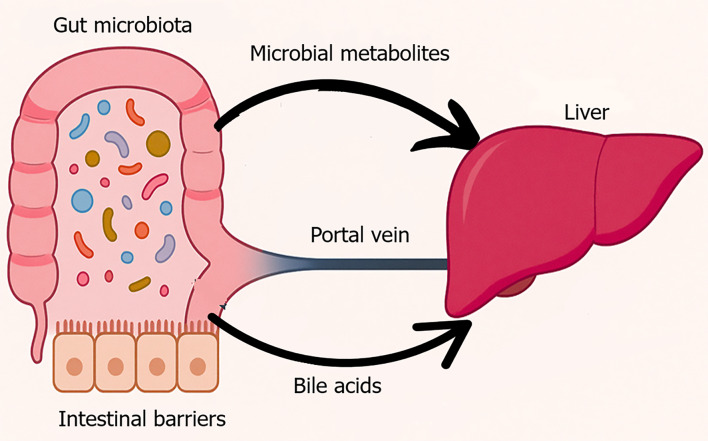
Additionally, gut microbes convert dietary choline and carnitine into trimethylamine-N-oxide (TMAO), a metabolite associated with IR, hepatic steatosis, and atherosclerosis. Elevated TMAO levels exacerbate oxidative stress and alter cholesterol metabolism, further linking gut microbial activity to liver dysfunction[45-47]. A visual representation of these physiological processes is provided in Figure 1.
Figure 1.
Physiological gut liver axis. This figure visually represents the anatomical and functional connectivity between the gut and liver, commonly referred to as the gut-liver axis. In a healthy state, a diverse and balanced gut microbiota resides within the intestinal lumen, supported by an intact epithelial barrier. Microbial metabolites such as short-chain fatty acids and secondary bile acids enter the portal circulation and interact with the liver to regulate glucose and lipid metabolism, immune tolerance, and bile acid recycling. This bidirectional communication maintains metabolic and immune homeostasis.
Together, the intestinal barrier, gut microbiota, microbial metabolites, and hepatic receptors form a dynamic network essential for maintaining systemic metabolic health. Disruption of this network impairs insulin signaling, promotes hepatic steatosis, and contributes to the development of T2D.
Building on this physiological framework, recent studies have identified specific molecular mediators and microbial signatures that mechanistically link gut-liver axis disruption to IR and T2D. The next section outlines these key mediators, including the roles of immune activation, receptor signaling, and microbial metabolites driving metabolic dysfunction.
KEY MEDIATORS OF THE GUT–LIVER AXIS IN DIABETES AND IR
Pro-inflammatory cytokines and immune signaling
A hallmark of gut-liver axis disruption in T2D is the upregulation of inflammatory signaling. Pro-inflammatory signaling. Elevated levels of pro-inflammatory cytokines, including interleukin-6, tumor necrosis factor-α, and interleukin-1beta (IL-1β) are consistently observed in insulin-resistant states. These cytokines impair insulin signaling by inhibiting the phosphorylation of insulin receptor substrate-1 (IRS-1), thereby disrupting downstream phosphatidylinositol 3-kinase-protein kinase B pathways[48-51].
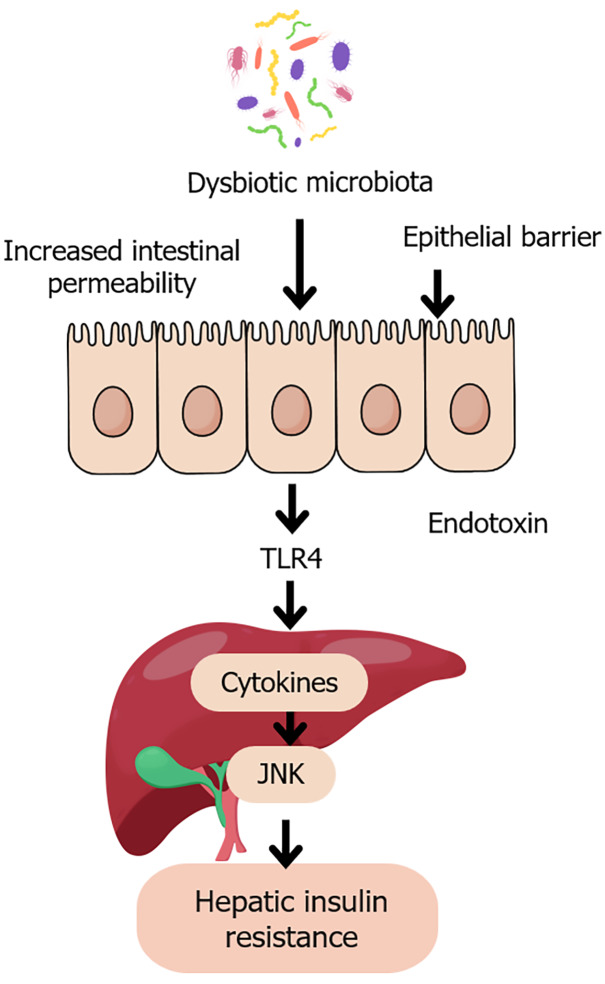
A key driver of this inflammation is LPS, an endotoxin produced by Gram-negative gut bacteria. When intestinal barrier integrity is compromised, LPS translocates into the portal circulation and binds to Toll-like receptor 4 (TLR4) on hepatic Kupffer cells. This interaction activates the nuclear factor-kappa B (NF-κB) and c-Jun N-terminal kinase (JNK) pathways, promoting hepatic inflammation and worsening IR[29,52-57]. The TLR4–JNK–NF-κB axis thus forms a critical molecular bridge between microbial dysbiosis and hepatic metabolic dysfunction[58-62], as depicted in Figure 2.
Figure 2.
Gut dysbiosis and hepatic insulin resistance. This figure is a schematic illustration of the pathological pathway linking dysbiotic gut microbiota to hepatic insulin resistance. Disruption of microbial balance leads to increased intestinal permeability and epithelial barrier dysfunction, allowing the translocation of endotoxins such as lipopolysaccharide into the portal circulation. These endotoxins activate toll-like receptor 4 on hepatic Kupffer cells, triggering pro-inflammatory cytokine release and activation of c-Jun N-terminal kinase signaling. This inflammatory cascade impairs insulin receptor signaling, contributing to hepatic insulin resistance, a key feature of type 2 diabetes. JNK: C-Jun N-terminal kinase; TLR4: Toll-like receptor 4.
Bile acid-activated receptors: FXR and TGR5
Bile acid signaling further exemplifies this interplay. The nuclear receptor FXR and the membrane-bound receptor TGR5 are activated by bile acids and exert dual metabolic and anti-inflammatory roles. FXR activation suppresses hepatic gluconeogenesis and lipogenesis while attenuating NF-κB- mediated inflammation. In the intestine, FXR induces fibroblast growth factor (FGF) 19, which acts hormonally to inhibit hepatic glucose production. Similarly, TGR5 activation on intestinal L-cells promotes glucagon-like peptide-1 (GLP-1) secretion and suppresses hepatic inflammatory responses. Dysbiosis-induced alterations in bile acid pools can attenuate FXR/TGR5 signaling, further worsening metabolic dysregulation[63-65].
Microbial dysbiosis
The composition and function of gut microbiota are essential for maintaining intestinal barrier integrity and regulating host metabolism. Individuals with T2D often exhibit reduced microbial diversity and depletion of beneficial commensals such as Faecalibacterium prausnitzii, Roseburia intestinalis, and Akkermansia muciniphila. These microbes are key producers of SCFAs, which help preserve epithelial integrity, regulate immune responses, and enhance insulin sensitivity. Their loss compromises tight junctions and mucin layers, increasing intestinal permeability and promoting a pro-inflammatory gut environment[66-69].
Concurrently, pathobionts such as Escherichia coli, Prevotella copri, and Bacteroides vulgatus become more prevalent in T2D. These microbes produce LPS, contributing to elevated systemic LPS levels and inducing metabolic endotoxemia[8,70-72]. Notably, Prevotella copri is associated with increased production of branched-chain amino acids (BCAAs), particularly leucine and valine. These BCAAs activate the mechanistic target of rapamycin-ribosomal protein S6 kinase 1 signaling pathway, which phosphorylates IRS-1 at inhibitory sites and disrupts of insulin signaling in the liver and muscle[73,74].
In addition, Akkermansia muciniphila, a mucin- degrading bacterium vital for tight junction function is often depleted in T2D. Its absence is linked to increased gut permeability and liver inflammation[75,76]. This enhanced permeability enables microbial products such as LPS to enter the portal circulation, activating hepatic immune responses and worsening systemic IR[11,77]. Restoration of Akkermansia muciniphila through prebiotics or supplementation has shown promise in improving metabolic parameters in both preclinical and early clinical studies[78,79].
Microbial and host-derived metabolites
Several gut and host-derived metabolites act as key modulators of gut-liver axis regulation and contribute to IR in T2D. Among them, LPS produced by Gram-negative bacteria, remains a central trigger of endotoxin-driven inflammation by activating hepatic immune pathways[54,59,60]. Secondary bile acids, formed through microbial conversion of primary bile acids, modulate FXR and TGR5 signaling. Altered bile salt hydrolase activity and disrupted bile acid profiles impair receptor activation, contributing to glucose dysregulation and hepatic lipotoxicity[39,43,64,65].
Another key metabolite is imidazole propionate (ImP), derived from microbial histidine metabolism. ImP interferes with insulin signaling via activation of p38γ and mechanistic target of rapamycin complex 1, and its elevated plasma levels have been linked to both prediabetes and T2D, suggesting its value as a biomarker and therapeutic target[80-82].
Tryptophan-derived indole metabolites, including indole-3-propionic acid and 5-hydroxyindole-3-acetic acid (5-HIAA), interact with the aryl hydrocarbon receptor (AhR) to modulate hepatic inflammation and insulin sensitivity. Reduced levels of these metabolites are associated with impaired glucose tolerance and elevated systemic inflammation in T2D[83].
Other significant microbial metabolites include phenylacetylglutamine, a co-metabolite of dietary phenylalanine that activates adrenergic receptors and promotes systemic inflammation[84]; hydrogen sulfide, which exhibits anti-inflammatory properties at low concentrations but becomes cytotoxic at higher levels, impairing mitochondrial function and compromising intestinal integrity[85]; and microbial succinate, a metabolite that signals through SUCNR1 to drive hepatic fibrosis and inflammation[86].
Additionally, endogenous ethanol (EE), produced by microbes such as Klebsiella pneumoniae and Candida spp., contributes to oxidative stress, hepatic steatosis, and mitochondrial dysfunction. Elevated EE levels have been observed in individuals with MASLD even in the absence of alcohol intake, identifying EE as a gut-derived metabolic toxin in diabetes[87-89].
A particularly novel mechanism involves bacterial extracellular vesicles (BEVs), nano-sized vesicles secreted by both commensal and pathogenic bacteria. BEVs can cross intact epithelial barriers and deliver microbial molecules, such as LPS and DNA, directly to hepatic immune cells. This enables microbial communication with the liver even without overt barrier disruption, providing a distinct pathway for inducing hepatic inflammation and IR[90,91].
Secretory immunoglobulin A and mucosal immune tolerance
Secretory immunoglobulin A (sIgA) plays a vital role in maintaining mucosal immune homeostasis and intestinal barrier integrity. It neutralizes pathogens, prevents their adherence to epithelial surfaces, and promotes a tolerogenic immune environment by modulating antigen presentation and dendritic cell function[92-94]. In T2D, impaired sIgA production or transport has been linked to reduced microbial diversity, enrichment of pro-inflammatory taxa, and increased gut permeability[92,95,96]. Experimental models show that sIgA deficiency facilitates microbial antigen translocation across the epithelium, triggering hepatic Kupffer cell activation and metabolic inflammation[97,98]. Clinically, decreased sIgA levels are observed in individuals with metabolic syndrome and correlate with markers of IR and systemic inflammation, underscoring sIgA’s role in gut-liver immune crosstalk[99].
Paneth and goblet cell dysfunction
Paneth and goblet cells are specialized intestinal epithelial cells essential for maintaining gut barrier integrity through the secretion of antimicrobial peptides and mucins, respectively[100,101]. In diabetic states, both cell types exhibit functional impairments[15]. Rodent models of IR show reduced expression of mucin and antimicrobial peptide genes, thinning of the mucus layer, and architectural changes in intestinal crypts[102]. In humans with diabetes, downregulation of transcripts such as MUC2 and DEFA5 indicates secretory epithelial exhaustion[15]. Interestingly, some studies report increased Paneth cell numbers in early diabetes, albeit with defective granules, suggesting a potential compensatory or biphasic response[103]. These findings highlight the need for stage-specific profiling of epithelial alterations. Given that dysfunction of these secretory cells facilitates microbial encroachment and antigen translocation, their impairment may act as an early trigger for gut-liver immune activation and metabolic disruption.
Circadian rhythm disruption
Circadian rhythm disruption is increasingly recognized as a systemic stressor affecting both gut and liver physiology. Intestinal epithelial clock genes regulate tight junction expression, epithelial turnover, and nutrient absorption in a time-dependent manner[104]. Experimental models show that circadian misalignment, induced by sleep fragmentation or altered light-dark cycle, can reshape the gut microbiome, impairs barrier integrity, and increases susceptibility to metabolic endotoxemia[105]. Concurrent disruption of hepatic circadian rhythms impairs bile acid metabolism and glucose homeostasis, contributing to steatosis and IR[106]. However, these effects are not strictly unidirectional. Time-restricted feeding has shown partial restoration of metabolic function even under circadian disruption, and not all core clock gene deletions result in metabolic liver disease[107]. These complexities suggest that circadian misalignment may amplify pre-existing gut-liver axis dysfunction in diabetes rather than initiate it independently.
Autophagy and epithelial stress
Autophagy, a cellular degradation and recycling process, is essential for maintaining epithelial resilience and barrier function. Secretory epithelial cells, including goblet and Paneth cells, depend on intact autophagy pathways to support granule secretion and mitigate endoplasmic reticulum stress[108]. In diabetes, persistent metabolic stress impairs autophagic flux, leading to epithelial apoptosis, mucus depletion, and tight junction disruption. Experimental studies support this link, showing that high-glucose conditions in intestinal organoids elevate oxidative stress and promote barrier breakdown, while interventions enhancing autophagy, such as adenosine 5’-monophosphate-activated protein kinase activators, restore barrier proteins and reduce inflammation[109]. Nonetheless, human data remain limited, and it is unclear whether autophagy disruption is a direct consequence of IR or a secondary effect of dysbiosis and inflammation[110]. Despite these uncertainties, autophagy appears to serve as a key molecular checkpoint for epithelial stress adaptation, and its impairment likely contributes to the breakdown of gut homeostasis and hepatic inflammation observed in T2D[111].
These interconnected mechanisms reflect the multifaceted disruption of the gut-liver axis in diabetes. Table 1 and Figure 3 provide an integrated overview of these pathways.
Table 1.
Key mediators of the gut-liver axis relevant to diabetes and insulin resistance
|
Mediator
|
Mechanism of action
|
Clinical relevance in T2D
|
Ref.
|
| Pro-inflammatory cytokines (IL-6, TNF-α, IL-1β) | Impair insulin signaling via IRS-1 inhibition; Activate hepatic inflammation through NF-κB/JNK pathways | Elevated in T2D, targets for anti-inflammatory therapies | Lara-Guzmán et al[48]; Rodrigues et al[49]; Liu et al[50]; D'Alessandris et al[51] |
| Lipopolysaccharides (LPS) and TLR4-JNK-NF-κB pathway | Trigger hepatic inflammation and insulin resistance following microbial translocation | LPS-driven endotoxemia links dysbiosis to metabolic dysfunction | Li and Wu[58]; Li et al[59]; Li et al[60]; Tanti and Jager[61]; Li et al[62] |
| FXR and TGR5 receptors | Regulate bile acid metabolism, inhibit gluconeogenesis, promote GLP-1 secretion, and suppress inflammation | Dysregulated in T2D; Therapeutic targets (e.g., FXR agonists) | Grüner and Mattner[39]; Kumari et al[43]; Bertolini et al[64]; Evangelakos et al[65] |
| Loss of SCFA-producing bacteria (e.g., Faecalibacterium prausnitzii) | Reduces gut barrier integrity, lowers SCFA and GLP-1 production, enhances intestinal permeability | Restoration improves insulin sensitivity and gut-liver communication | Garcia-Gutierrez et al[66]; He et al[67]; Verhoog et al[68]; Moran-Ramos et al[69] |
| Expansion of pathobionts (e.g., Prevotella copri) | Elevates LPS and BCAA production; activates mTOR-S6K1 pathway impairing insulin signaling | Associated with metabolic endotoxemia, systemic inflammation, and worsened glucose control | Murugesan et al[8]; Leite et al[70]; Gong et al[72] |
| Microbial metabolites (imidazole propionate, IPA, H2S, succinate, EE, PAGln) | Modulate insulin signaling, oxidative stress, hepatic lipotoxicity, and inflammatory cascades | Emerging biomarkers and therapeutic targets for metabolic dysfunction | Zeng et al[80]; Koh et al[81]; Koh et al[82]; Cussotto et al[83]; Yang et al[84]; Munteanu et al[85]; Huang et al[86]; Xue et al[87]; Chen et al[88]; Drda and Smith[89] |
| Bacterial extracellular vesicles | Transfer microbial molecules (e.g., LPS, DNA) across intact barriers to hepatic immune cells, triggering inflammation | Represent a novel barrier-independent mechanism contributing to hepatic insulin resistance | Melo-Marques et al[90]; Butcko et al[91] |
| Innate lymphoid cells (ILC3-IL-22 signaling) | Maintain epithelial barrier integrity and mucosal immune balance; Regulate gut homeostasis | ILC dysregulation associated with intestinal permeability defects and systemic inflammation in T2D | Wang et al[122]; Yin et al[123]; Horn and Sonnenberg[127] |
| Endocannabinoid system (ECS) | Modulates intestinal permeability, immune activation, hepatic lipid metabolism, and inflammatory tone | Dysregulated ECS signaling contributes to obesity, insulin resistance, and steatohepatitis | Bazwinsky-Wutschke et al[133]; Liu et al[134]; Cuddihey et al[135]; Lipina et al[136]; Roser et al[137]; O'Sullivan et al[138]; Ellermann[139] |
IL-1β: Interleukin-1 beta; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-α; TLR4: Toll-like receptor 4; JNK: C-Jun N-terminal kinase; NF-κB: Nuclear factor kappa B; LPS: Lipopolysaccharide; FXR: Farnesoid X receptor; TGR5: Takeda G protein-coupled receptor 5; SCFAs: Short-chain fatty acids; IPA: Indole-3-propionic acid; H2S: Hydrogen sulfide; EE: Endogenous ethanol; PAGln: Phenylacetylglutamine; ILC3: Group 3 innate lymphoid cell; ECS: Endocannabinoid system; IRS-1: Insulin receptor substrate-1; GLP-1: Glucagon-like peptide-1; BCAA: Branched-chain amino acids; S6K1: Ribosomal protein S6 kinase 1; mTOR: Mechanistic target of rapamycin; T2D: Type 2 diabetes.
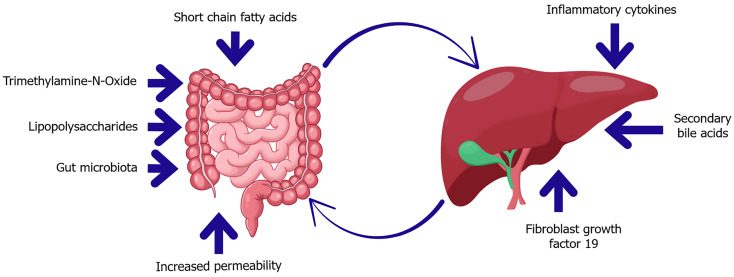
Figure 3.
Key mediators of the gut-liver axis in diabetes and insulin resistance. This diagram highlights bidirectional communication between the gut and liver mediated by microbial and host-derived factors. In dysbiosis, microbial metabolites such as trimethylamine-N-oxide and lipopolysaccharides increase gut permeability, enabling translocation of endotoxins into the portal circulation. These signals promote hepatic inflammation and insulin resistance. Conversely, the liver influences gut homeostasis through the secretion of secondary bile acids and fibroblast growth factor 19, which regulate microbial composition and barrier integrity. Short-chain fatty acids also play a protective role, supporting epithelial health and metabolic balance.
EMERGING MECHANISMS LINKING THE GUT–LIVER AXIS TO DIABETES
Recent studies have identified novel gut-liver communication pathways that contribute to the pathophysiology of T2D. Three principal axes emerged, involving distinct signals modes, neural, hormonal, and microbial that link gut sensing to hepatic and systemic metabolism.
Gut-liver-brain neuroendocrine crosstalk
This pathway involves gut derived hormones regulating hepatic glucose homeostasis via vagal nerve signaling. GLP-1, secreted by intestinal L-cells in response to nutrient intake, stimulates vagal afferents projecting to the hindbrain. This enhances postprandial insulin secretion and promotes satiety. However, obesity and high-fat diets induce an “enteric GLP-1 resistance”, characterized by reduced nitric oxide signaling and impaired vagal activation. This blunts the gut-brain insulinotropic axis, contributing to hyperglycemia and hepatic IR in T2D[112].
Similarly, FGF19, secreted by ileal cells in response to bile acid-FXR activation, acts on the hindbrain to suppress hepatic glucose production[113]. Impaired FGF19 signaling, along with GLP-1 resistance, exacerbates dysfunction in this axis[114,115].
FGF21, predominantly synthesized by the liver, further bridges the gut-liver-adipose axis. Its expression is regulated by bile acid-FXR signaling. Pharmacologic induction of FGF21 suppresses hepatic CYP7A1 expression, alters bile acid composition, and supports expansion of SCFA-producing gut microbiota[116]. These shifts enhance GLP-1 secretion, strengthen gut barrier function, and improve insulin sensitivity. FGF21 also stimulates adiponectin release, thermogenesis, and lipid clearance in peripheral tissues, thereby improving glucose homeostasis[117]. Clinical trials evaluating FGF21 analogs have demonstrated promising reductions in liver fat and glycemic parameters, underscoring its therapeutic relevance[118,119].
Tryptophan-AhR axis
Emerging evidence highlights the tryptophan-AhR axis as a key microbial-host signaling mechanism in T2D. Gut commensals metabolize dietary tryptophan into indole derivatives, including indole-3-acetic acid, indole-3-aldehyde, and 5-HIAA, which act as natural ligands for the AhR. Activation of AhR in hepatic macrophages and hepatocytes exerts anti-inflammatory and metabolic effects by suppressing NF-κB and STAT1 signaling and enhancing the IL-10-STAT3 axis[120]. Moreover, AhR activation also downregulates hepatic lipogenic pathways through inhibition of sterol regulatory element-binding protein 1c and its downstream targets, such as acetyl-CoA carboxylase and fatty acid synthase[120].
Notably, supplementation with 5-HIAA has been shown to ameliorate glucose intolerance and hepatic IR in experimental models of obesity. This effect is mediated through AhR-dependent upregulation of TSC2 and subsequent suppression of mechanistic target of rapamycin complex 1[121]. Collectively, these findings position the gut microbial-AhR axis as a critical modulator of hepatic inflammation and glucose metabolic in T2D[121].
Innate lymphoid cells and endocannabinoid signaling
Beyond systemic hormonal and microbial pathways, recent findings implicate additional immune and metabolic regulators in gut-liver axis dysfunction, particularly innate lymphoid cells (ILCs) and the endocannabinoid system (ECS). These pathways add further complexity to the immune-metabolic interplay between the intestine and liver in T2D[122-126].
ILCs: ILCs are a heterogeneous group of immune cells primarily located at mucosal surfaces, including the intestinal lamina propria, where they contribute to barrier integrity, immune surveillance, and tissue repair[122]. Among these, group 3 ILCs (ILC3s) are particularly important in gut-liver communication. ILC3s secrete interleukin-22 (IL-22), a cytokine that supports epithelial tight junction integrity, stimulates antimicrobial peptide production, and facilitates mucosal healing[127,128].
In metabolic disease models, reduced IL-22 production correlates with increased intestinal permeability, endotoxemia, hepatic inflammation, and IR[[129,130]. Conversely, therapeutic augmentation of IL-22 signaling has been to shown to restore intestinal barrier integrity, decrease portal LPS translocation, and improve hepatic steatosis and insulin sensitivity[131,132]. These findings position ILC3-derived IL-22 as a promising target for restoring gut–liver axis homeostasis in diabetes.
ECS: The ECS consists of cannabinoid receptors (CB1 and CB2), endogenous ligands such as anandamide, 2-arachidonoylglycerol, and associated metabolic enzymes. ECS plays a significant role in gut-liver communication by regulating intestinal permeability, immune responses, microbiota composition, and hepatic metabolism[126].
In diabetes and obesity, CB1 hyperactivation in the gut and liver contributes to dysbiosis, barrier dysfunction, adipogenesis, hepatic steatosis, and IR[133,134]. Pharmacologic blockade of CB1 receptors has been shown to reverse these effects, restoring gut barrier function and improving hepatic insulin sensitivity in animal models[135,136]. However, central nervous system side effects have limited the clinical utility of systemic CB1 antagonists. Current strategies focus on peripherally restricted CB1 blockers that offer metabolic benefits without neuropsychiatric risks[137,138].
Additionally, gut-derived endocannabinoid-like molecules (“endocannabinoidome”) interact with microbial metabolites and bile acid pathways, suggesting a complex tri-directional interplay between microbiota, ECS, and host metabolism[139].
Together, these emerging mechanisms highlight the intricate interplay between gut-derived signals, whether neural (vagal afferents), hormonal (FGF19, FGF21), immune-mediated (ILC3-IL-22 axis), microbial (indole metabolites), or metabolic (endocannabinoid signaling) and hepatic metabolic regulation. Disruption of any component within this network, such as GLP-1 resistance, impaired FGF or IL-22 signaling, loss of beneficial tryptophan metabolites, or dysregulated endocannabinoid activity, can drive hepatic IR, steatosis, and systemic metabolic dysfunction in T2D. Importantly, the emerging recognition of ILCs and the ECS as key modulators of the gut–liver axis expands the therapeutic landscape. Future interventions may combine microbiota-targeted therapies, barrier reinforcement strategies, and molecular modulators of immune and endocannabinoid pathways to synergistically restore gut-liver homeostasis and improve metabolic outcomes in diabetes.
THERAPEUTIC TARGETING OF THE GUT-LIVER AXIS IN DIABETES
Restoring gut-liver axis integrity has emerged as a promising frontier for improving metabolic outcomes in diabetes. Multiple strategies target intestinal barrier reinforcement, immune modulation, and microbiota reshaping. An overview of current and experimental interventions is provided in Table 2 with a graphical summary provided in Figure 4.
Table 2.
Key therapeutic strategies targeting gut-liver axis in diabetes
|
Strategy
|
Mechanism of action
|
Examples
|
Clinical tatus
|
Ref.
|
| Probiotics | Modulate microbiota composition; Enhance SCFA production; Reinforce gut barrier integrity | Lactobacillus, Bifidobacterium strains | Approved adjuncts; Variable efficacy | Grylls et al[156]; Zhang et al[160]; Memon et al[162]; McLoughlin et al[163] |
| Prebiotics | Promote growth of beneficial microbes; Increase SCFA levels; Reduce gut permeability and inflammation | Inulin, resistant starch, fructooligosaccharides | Clinically validated for glycemic improvement | McLoughlin et al[163]; Luzzi et al[165]; Jayedi et al[171] |
| Synbiotics | Synergistic effect of probiotics and prebiotics; Improve glycemia and lipid profiles | Probiotic + fiber combinations | Emerging evidence; Under clinical study | McLoughlin et al[163]; Luzzi et al[165]; Jayedi et al[171] |
| Postbiotics | Deliver microbial metabolites (e.g., SCFAs) directly to host tissues to modulate metabolism and immunity | SCFA supplements (e.g., acetate, butyrate infusions) | Experimental | McLoughlin et al[163]; Fang et al[164]; Luzzi et al[165] |
| Fecal microbiota transplantation | Reconstitute healthy microbiome diversity; Restore SCFA and bile acid metabolism | Donor stool capsules or infusions | Experimental; Some success in T2D trials | Wu et al[167]; Yadegar et al[168] |
| Zonulin inhibitors | Prevent tight junction disassembly; Restore intestinal barrier integrity | Larazotide acetate (AT-1001) | Phase III for celiac; Early-stage for T2D | Choi et al[73]; Górecka et al[143]; Yonker et al[145]; Tajik et al[146]; Jayashree et al[178]; Yuan et al[179] |
| Dietary interventions | Enrich SCFA-producing bacteria; Upregulate tight junction proteins; Reduce systemic inflammation | High-fiber and polyphenol-rich diets (berries, teas) | Clinically recommended adjunct therapy | Verhoog et al[68]; Mazhar et al[147]; Han et al[169] |
| GLP-1 receptor agonists | Enhance insulin secretion; Reduce hepatic and gut inflammation; Improve barrier function | Liraglutide, semaglutide | Approved for T2D and obesity | Zhang et al[141]; Alharbi[149] |
| SGLT2 inhibitors | Improve glycemic control; Reduce systemic and hepatic inflammation | Empagliflozin, dapagliflozin | Approved for T2D and cardiovascular protection | Theofilis et al[150]; Zhang et al[151] |
| FXR agonists | Regulate bile acid metabolism; Restore barrier function; Suppress liver fibrosis and inflammation | Obeticholic acid | Approved for PBC; Under investigation for NASH | Zhang et al[152] |
| TLR4 antagonists | Block LPS signaling to prevent endotoxin-driven inflammation | Eritoran | Experimental | Liang et al[170] |
| Cytokine inhibitors | Suppress pro-inflammatory cytokines (e.g., IL-1β) to reduce hepatic and systemic inflammation | Canakinumab, anakinra | Under investigation | Everett et al[157]; Howard et al[158] |
| AhR agonists | Activate anti-inflammatory pathways; Stabilize tight junctions | Indole derivatives (e.g., FICZ, 5-HIAA) | Experimental; Preclinical promising | Cussotto et al[83]; Zheng et al[120]; Du et al[121]; Pernomian et al[159] |
| Gut-targeted biologics/probiotics | Modulate mucosal immunity; Reduce pro-inflammatory responses locally | Engineered probiotics, oral cytokine blockers | Preclinical and early-phase trials | Zhang et al[160] |
This table summarizes current and emerging interventions aimed at modulating the gut microbiota, enhancing intestinal barrier integrity, reducing inflammation, and restoring gut-liver metabolic signaling. GLP-1: Glucagon-like peptide-1; SCFAs: Short-chain fatty acids; SGLT2: Sodium-glucose cotransporter-2; FXR: Farnesoid X receptor; TLR4: Toll-like receptor 4; AhR: Aryl hydrocarbon receptor; T2D: Type 2 diabetes; LPS: Lipopolysaccharide; PBC: Primary biliary cirrhosis; NASH: Non-alcoholic steatohepatitis; IL-1β: Interleukin-1 beta; FICZ: 6-formylindolo[3,2-b]carbazole; 5-HIAA: 5-hydroxyindole-3-acetic acid.
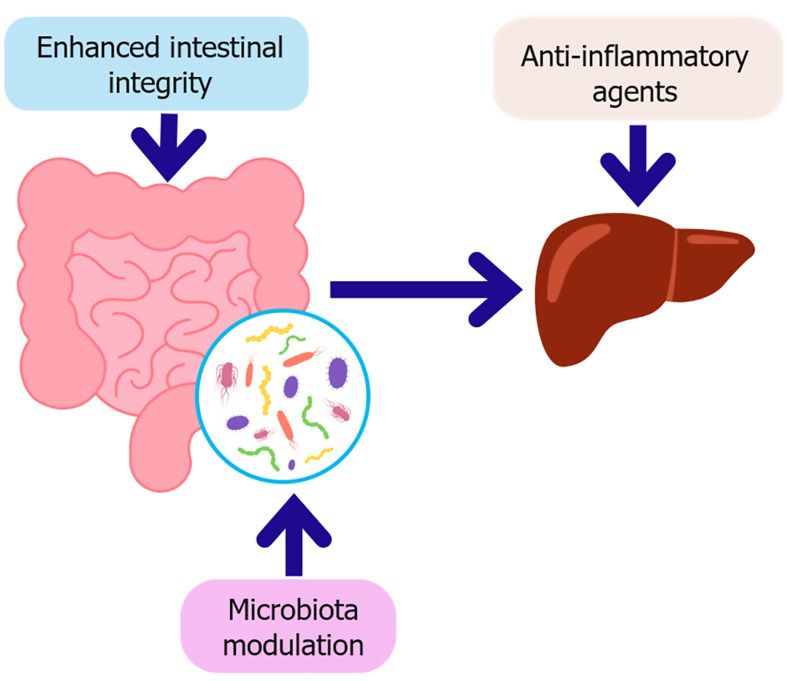
Figure 4.
Key therapeutic strategies targeting the gut-liver axis in diabetes. This schematic illustrates therapeutic strategies aimed at restoring gut-liver axis homeostasis in the context of type 2 diabetes. Microbiota modulation, through probiotics, prebiotics, synbiotics, or fecal microbiota transplantation which enhances intestinal integrity by strengthening the epithelial barrier and promoting beneficial metabolites such as short-chain fatty acids. Improved barrier function reduces microbial translocation, subsequently decreasing hepatic inflammation. In parallel, anti-inflammatory agents directly target hepatic immune activation, contributing to improved insulin sensitivity and metabolic regulation.
Enhancing intestinal barrier integrity
Reinforcing the intestinal barrier is a central therapeutic goal in diabetes management[140]. Tight junction proteins, claudins, occludin, and zonula occludens-1, seal the paracellular space between enterocytes. Their disruption increases permeability, microbial translocation, and hepatic inflammation[141-143]. Pharmacological interventions, such as zonulin inhibitors (e.g., larazotide acetate) stabilize tight junctions, reduce endotoxemia, and restore barrier function in preclinical models[144-146]. Dietary interventions such as soluble fibers promote SCFA production, particularly butyrate, which strengthens tight junction, while polyphenol-rich diets and omega-3 fatty acids provide antioxidative and anti-inflammatory support to the epithelium[147,148].
Anti-inflammatory interventions
Inflammation plays a pivotal role in gut-liver axis dysfunction. GLP-1 agonists (GLP-1 RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2i) offer both metabolic and anti-inflammatory benefits[141,149,150]. GLP-1 RAs improve glycemic control, reduce hepatic inflammation, and enhance barrier repair, while SGLT2i therapy decreases C-reactive protein and interleukin-6[151]. Furthermore, FXR agonists, such as obeticholic acid, modulate bile acid metabolism, improve intestinal integrity, and suppress liver fibrosis[152,153].
Bile acid sequestrants (BASs) such as colesevelam and colestimide also modulate enterohepatic signaling. A systematic review and meta-analysis of 17 randomized controlled trials involving 2950 patients showed significant glycated hemoglobin (HbA1c) reductions. These effects are attributed to delayed bile acids reabsorption, enhanced GLP-1 secretion, and FXR/TGR5 modulation. Though long-term safety data are limited, BASs are recommended as adjunctive therapy in T2D[154].
Emerging therapies include TLR4 antagonists, cytokine inhibitors such as canakinumab targeting IL-1β, and AhR agonists derived from microbial tryptophan metabolites, which enhance mucosal immunity and barrier function[155-159]. Engineered probiotics and oral anti-cytokine therapies are under investigation to restore mucosal balance without systemic immunosuppression[160,161].
Microbiota modulation
Modulating gut microbiota is another therapeutic cornerstone. Probiotics, particularly Lactobacillus and Bifidobacterium demonstrate modest improvements in glycemic and lipid profiles, especially with metformin[162]. Prebiotics such as inulin and resistant starch support SCFA-producing bacteria, improving barrier function and reducing inflammation[163]. Synbiotics, which combine probiotics and prebiotics, demonstrate synergistic benefits, while postbiotics (direct delivery of SCFAs) offer a novel therapeutic approach[164-166].
Fecal microbiota transplantation (FMT) enhances microbial diversity and insulin sensitivity, although donor variability and standardization challenges remain[167,168]. Additionally, high-fiber and polyphenol-rich diets effectively modulate the microbiome, increase SCFA production, and strengthen barrier function, contributing to improved metabolic outcomes[68,147,169].
LIMITATIONS OF CURRENT EVIDENCE AND KNOWLEDGE GAPS
Despite extensive research on the gut-liver axis in T2D, several limitations hinder the translation of mechanistic insights into clinically effective therapies. One major challenge is the gap between preclinical and clinical findings. While interventions such as FXR agonists, TLR4 antagonists, and indole-derived AhR ligands demonstrate robust metabolic and anti-inflammatory effects in rodent models, human trials often yield inconsistent, modest, or inconclusive results. For instance, the TLR4 antagonists eritoran failed to show significant clinical efficacy in metabolic or inflammatory conditions, possibly due to species-specific differences in innate immune pathways, genetic polymorphisms in TLR genes, or differences in gut microbial composition[170].
Moreover, while probiotic and prebiotic therapies show promise in experimental models, their outcomes in humans remain variable. Meta-analyses often reveal only modest and statistically insignificant changes in HbA1c or insulin sensitivity. Efficacy appears to depend on multiple factors, including strain selection, dosage, treatment duration, and baseline host microbiota profiles[171]. This variability reflects the complexity of host-microbe interactions and the need for personalized therapeutic approaches.
Furthermore, many mechanistic links remain correlative rather than causative. For example, alterations in microbial composition or metabolite levels such as ImP or SCFAs are frequently observed in T2D, but whether these changes are drivers of disease or markers of metabolic dysregulation remain unclear[172].
Similarly, while preclinical data support roles of microbial EVs, endocannabinoid signaling, and mucosal immune mediators such as IL-22 in regulating metabolic and inflammatory pathways, their specific functions in human T2D and gut-liver axis disorders are not well established. The mechanisms by which microbial EVs influence hepatic immunity, the context-dependent effects of cannabinoid receptor, and the dual roles of IL-22 still require validation in human studies and clinical trials[2,133,173,174].
A further challenge is the lack of longitudinal studies, large-scale trials, and standardized methodologies. Many existing studies are limited by small sample sizes and lack of stratification by key variables such as microbiota composition, diet, genetics, and comorbidities, complicating interpretation and reproducibility[175].
In summary, while the gut-liver axis presents substantial therapeutic potential, advancing the field requires moving beyond associative evidence to establish causality and therapeutic relevance through rigorous, human-centered research. Addressing these limitations is essential for developing safe, effective, and personalized interventions in T2D management.
CHALLENGES AND FUTURE DIRECTIONS
Despite advances in understanding the gut-liver axis, several challenges continue to impede the clinical translation of related therapies. One significant barrier is the high degree of microbial heterogeneity among individuals, which complicates therapeutic standardization and highlights the need for precision medicine approaches[176].
A major limitation is the lack of validated biomarkers to stratify patients and monitor therapeutic responses[177]. While serum markers such as LPS, zonulin, and ImP, are under investigation, they require further validation before being adopted in routine clinical practice[178,179]. Furthermore, regulatory hurdles, concerns over long-term safety, and complexities in manufacturing limit the clinical use of advanced interventions like FMT and engineered probiotics[180,181].
Future strategies should integrate multi-omics profiling, including microbiome, metabolome, transcriptome, and host genomic data, to build individualized therapeutic models. These datasets can inform stratified clinical trials and enable biomarker-driven intervention tailored to specific patient subtypes[182,183]. Moreover, novel tools such as gut organoids, humanized microbiota animal models, and artificial intelligence hold promise for bridging the translational gap between experimental studies and human applications[184].
Emerging insights into the gut-liver-brain axis also indicate that interventions targeting the gut may offer benefits beyond metabolic regulation, potentially improving neurocognitive outcomes in diabetes. Integrating metabolic and neurocognitive strategies could lead to more holistic approaches in managing T2D[185-187].
Together, these challenges and opportunities highlight a pivotal turning point in gut-liver research. To fully realize the therapeutic potential of this axis, future efforts must balance mechanistic complexity with clinical precision and personalized care models.
PROPOSED INTEGRATIVE FRAMEWORK FOR GUT–LIVER CROSSTALK IN T2D
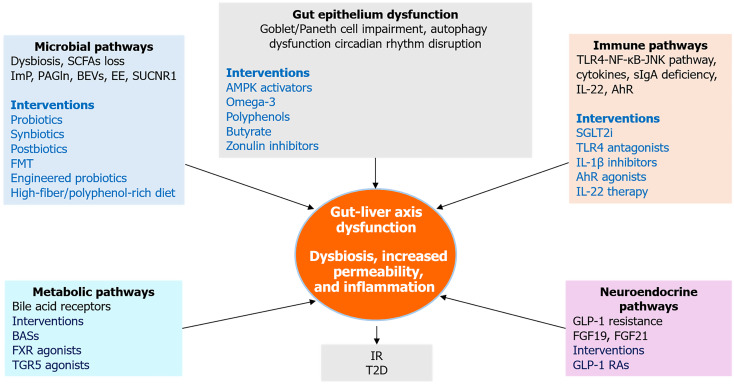
To advance conceptual clarity and support translational progress, this review proposes an integrative framework that synthesizes microbial, immune, metabolic, and neuroendocrine pathways implicated in the gut-liver crosstalk in the pathogenesis of T2D. This model maps the convergence of dysbiotic microbial signals, inflammatory mediators, hormonal feedback loops, and neural circuits to explain how disruptions across this network contribute to hepatic IR and systemic metabolic dysfunction.
The framework also informs therapeutic innovation by aligning key pathogenic mechanisms with corresponding intervention strategies. These include microbial modulation through probiotics and FMT, intestinal barrier reinforcement using dietary fibers, SCFAs and zonulin inhibitors, immune regulation via agents targeting IL-22 and AhR signaling, and restoration of enterohepatic signaling via GLP-1 receptor agonists and FXR/TGR5 modulators. By integrating mechanistic insight with intervention targets, the model supports a precision medicine approach aimed at tailoring therapies to individual microbial, immune, and metabolic profiles. The proposed conceptual framework is visually represented in Figure 5.
Figure 5.
Integrative therapeutic and mechanistic pathways linking gut and liver in type 2 diabetes. This integrative framework illustrates how five key mechanistic domains, microbial dysbiosis, gut epithelial dysfunction, immune activation, neuroendocrine disruption, and metabolic signaling which converge to drive gut-liver axis dysfunction in type 2 diabetes. Each domain includes representative molecular mediators and therapeutic interventions. The central node highlights dysbiosis, increased permeability, and inflammation as core features linking upstream disruptions to downstream insulin resistance and hepatic steatosis. SCFAs: Short-chain fatty acids; ImP: Imidazole propionate; PAGln: Phenylacetylglutamine; BEVs: Bacterial extracellular vesicles; EE: Endogenous ethanol; FMT: Fecal microbiota transplantation; BASs: Bile acid sequestrants; FXR: Farnesoid X receptor; TGR5: Takeda G protein-coupled receptor 5; AMPK: Adenosine 5’-monophosphate-activated protein kinase; IR: Insulin resistance; T2D: Type 2 diabetes; TLR4: Toll-like receptor 4; JNK: C-Jun N-terminal kinase; NF-κB: Nuclear factor kappa B; sIgA: Secretory immunoglobulin A; IL-1β: Interleukin-1 beta; IL-22: Interleukin-22; AhR: Aryl hydrocarbon receptor; SGLT2i: Sodium-glucose cotransporter-2 inhibitors; FGF19: Fibroblast growth factor 19; FGF21: Fibroblast growth factor 21; GLP-1: Glucagon-like peptide-1; GLP-1 RAs: Glucagon-like peptide-1 receptor agonists.
CONCLUSION
The gut-liver axis plays a central role in the development and progression of T2D by integrating microbiota, immune, barrier, and metabolic signals. Disruption of this axis contributes to IR, hepatic steatosis, and systemic metabolic dysfunction. Therapeutic strategies that aim to restore gut-liver homeostasis through microbiota modulation, enhancement of barrier integrity, anti-inflammatory interventions, and regulation of gut-derived signaling pathways offer promising opportunities to improve clinical outcomes. However, realizing this potential requires a personalized approach that incorporates individual microbial, immune, and metabolic profiles. Advances in microbiome research, multi-omics profiling, and precision medicine tools are rapidly expanding this therapeutic landscape. Despite such progress, several challenges remain, including the lack of validated biomarkers, variability in treatment responses, and limited long-term safety data. Furthermore, a deeper understanding of the gut-liver-brain network may help address complications beyond metabolism, including cognitive decline. Moving forward, combining gut-liver axis therapies with existing treatments may support a more holistic, durable, and individualized approach to diabetes management.
Footnotes
Conflict-of-interest statement: The author has no conflict of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Malaysia
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade B
Novelty: Grade A, Grade B, Grade B
Creativity or Innovation: Grade B, Grade B, Grade C
Scientific Significance: Grade A, Grade A, Grade D
P-Reviewer: Mohib MM; Pal B S-Editor: Fan M L-Editor: A P-Editor: Yu HG
References
- 1.Hudish LI, Reusch JE, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest. 2019;129:4001–4008. doi: 10.1172/JCI129188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mo W, Peng Y, Zheng Y, Zhao S, Deng L, Fan X. Extracellular vesicle-mediated bidirectional communication between the liver and other organs: mechanistic exploration and prospects for clinical applications. J Nanobiotechnology. 2025;23:190. doi: 10.1186/s12951-025-03259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yakut A. Gut microbiota in the development and progression of chronic liver diseases: Gut microbiota-liver axis. World J Hepatol. 2025;17:104167. doi: 10.4254/wjh.v17.i3.104167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brescia P, Rescigno M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol Med. 2021;27:844–855. doi: 10.1016/j.molmed.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhou M, Lv J, Chen X, Shi Y, Chao G, Zhang S. From gut to liver: Exploring the crosstalk between gut-liver axis and oxidative stress in metabolic dysfunction-associated steatotic liver disease. Ann Hepatol. 2025;30:101777. doi: 10.1016/j.aohep.2025.101777. [DOI] [PubMed] [Google Scholar]
- 6.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet. 2024;404:2077–2093. doi: 10.1016/S0140-6736(24)02317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao L, An Y, Liu H, Jiang J, Liu W, Zhou Y, Shi M, Dai W, Lv Y, Zhao Y, Lu Y, Chen L, Xia Y. Global epidemiology of type 2 diabetes in patients with NAFLD or MAFLD: a systematic review and meta-analysis. BMC Med. 2024;22:101. doi: 10.1186/s12916-024-03315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murugesan R, Kumar J, Leela KV, Meenakshi S, Srivijayan A, Thiruselvam S, Satheesan A, Chaithanya V. The role of gut microbiota and bacterial translocation in the pathogenesis and management of type 2 diabetes mellitus: Mechanisms, impacts, and dietary therapeutic strategies. Physiol Behav. 2025;293:114838. doi: 10.1016/j.physbeh.2025.114838. [DOI] [PubMed] [Google Scholar]
- 9.Arhire AI, Ioacara DS, Papuc T, Parcalibioru GG, Fica S. Exploring the severity and early onset of familial type 1 diabetes in Romania: genetic and microbiota insights. Arch Clin Cases. 2024;11:29–33. doi: 10.22551/2024.42.1101.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho J, Nicolucci AC, Virtanen H, Schick A, Meddings J, Reimer RA, Huang C. Effect of Prebiotic on Microbiota, Intestinal Permeability, and Glycemic Control in Children With Type 1 Diabetes. J Clin Endocrinol Metab. 2019;104:4427–4440. doi: 10.1210/jc.2019-00481. [DOI] [PubMed] [Google Scholar]
- 11.Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2024;19:275–293. doi: 10.1007/s11739-023-03374-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Domínguez Á, Savolainen O, Domínguez-Riscart J, Landberg R, Lechuga-Sancho A, González-Domínguez R. Probing erythrocytes as sensitive and reliable sensors of metabolic disturbances in the crosstalk between childhood obesity and insulin resistance: findings from an observational study, in vivo challenge tests, and ex vivo incubation assays. Cardiovasc Diabetol. 2024;23:336. doi: 10.1186/s12933-024-02395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Q, Wedick NM, Pan A, Townsend MK, Cassidy A, Franke AA, Rimm EB, Hu FB, van Dam RM. Gut microbiota metabolites of dietary lignans and risk of type 2 diabetes: a prospective investigation in two cohorts of U.S. women. Diabetes Care. 2014;37:1287–1295. doi: 10.2337/dc13-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Chierico F, Rapini N, Deodati A, Matteoli MC, Cianfarani S, Putignani L. Pathophysiology of Type 1 Diabetes and Gut Microbiota Role. Int J Mol Sci. 2022;23:14650. doi: 10.3390/ijms232314650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo Conte M, Cosorich I, Ferrarese R, Antonini Cencicchio M, Nobili A, Palmieri V, Massimino L, Lamparelli LA, Liang W, Riba M, Devecchi E, Bolla AM, Pedone E, Scavini M, Bosi E, Fasano A, Ungaro F, Diana J, Mancini N, Falcone M. Alterations of the intestinal mucus layer correlate with dysbiosis and immune dysregulation in human Type 1 Diabetes. EBioMedicine. 2023;91:104567. doi: 10.1016/j.ebiom.2023.104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harbison JE, Roth-Schulze AJ, Giles LC, Tran CD, Ngui KM, Penno MA, Thomson RL, Wentworth JM, Colman PG, Craig ME, Morahan G, Papenfuss AT, Barry SC, Harrison LC, Couper JJ. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: A prospective cohort study. Pediatr Diabetes. 2019;20:574–583. doi: 10.1111/pedi.12865. [DOI] [PubMed] [Google Scholar]
- 17.Mokhtari P, Metos J, Anandh Babu PV. Impact of type 1 diabetes on the composition and functional potential of gut microbiome in children and adolescents: possible mechanisms, current knowledge, and challenges. Gut Microbes. 2021;13:1–18. doi: 10.1080/19490976.2021.1926841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Groot PF, Nikolic T, Imangaliyev S, Bekkering S, Duinkerken G, Keij FM, Herrema H, Winkelmeijer M, Kroon J, Levin E, Hutten B, Kemper EM, Simsek S, Levels JHM, van Hoorn FA, Bindraban R, Berkvens A, Dallinga-Thie GM, Davids M, Holleman F, Hoekstra JBL, Stroes ESG, Netea M, van Raalte DH, Roep BO, Nieuwdorp M. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: a randomised controlled trial. Diabetologia. 2020;63:597–610. doi: 10.1007/s00125-019-05073-8. [DOI] [PubMed] [Google Scholar]
- 19.Navalón-Monllor V, Soriano-Romaní L, Silva M, de Las Hazas ML, Hernando-Quintana N, Suárez Diéguez T, Esteve PM, Nieto JA. Microbiota dysbiosis caused by dietetic patterns as a promoter of Alzheimer's disease through metabolic syndrome mechanisms. Food Funct. 2023;14:7317–7334. doi: 10.1039/d3fo01257c. [DOI] [PubMed] [Google Scholar]
- 20.Michailidis M, Moraitou D, Tata DA, Kalinderi K, Papamitsou T, Papaliagkas V. Alzheimer's Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer's Disease and Type 2 Diabetes. Int J Mol Sci. 2022;23:2687. doi: 10.3390/ijms23052687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdalla MMI. Insulin resistance as the molecular link between diabetes and Alzheimer's disease. World J Diabetes. 2024;15:1430–1447. doi: 10.4239/wjd.v15.i7.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meroni M, Longo M, Paolini E, Dongiovanni P. A narrative review about cognitive impairment in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Another matter to face through a holistic approach. J Adv Res. 2025;68:231–240. doi: 10.1016/j.jare.2024.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Wang W, Hou X, Wu J, Wang Y, Fan J, Zhang Z, Yuan Z, Sun C, Lu B, Zheng J. Metabolic-associated steatotic liver disease and risk of Alzheimer's disease: a real-world retrospective cohort study. Front Endocrinol (Lausanne) 2024;15:1451908. doi: 10.3389/fendo.2024.1451908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan X, Hu Y, Wang B, Wang S, Zhang X. Metabolic Dysregulation Contributes to the Progression of Alzheimer's Disease. Front Neurosci. 2020;14:530219. doi: 10.3389/fnins.2020.530219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullins RJ, Diehl TC, Chia CW, Kapogiannis D. Insulin Resistance as a Link between Amyloid-Beta and Tau Pathologies in Alzheimer's Disease. Front Aging Neurosci. 2017;9:118. doi: 10.3389/fnagi.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Wang X, Zhuo E, Chen B, Chan S. Gut-liver axis in liver disease: From basic science to clinical treatment (Review) Mol Med Rep. 2025;31:10. doi: 10.3892/mmr.2024.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pabst O, Hornef MW, Schaap FG, Cerovic V, Clavel T, Bruns T. Gut-liver axis: barriers and functional circuits. Nat Rev Gastroenterol Hepatol. 2023;20:447–461. doi: 10.1038/s41575-023-00771-6. [DOI] [PubMed] [Google Scholar]
- 28.Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh SS, Wang J, Yannie PJ, Ghosh S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J Endocr Soc. 2020;4:bvz039. doi: 10.1210/jendso/bvz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valitutti F, Mennini M, Monacelli G, Fagiolari G, Piccirillo M, Di Nardo G, Di Cara G. Intestinal permeability, food antigens and the microbiome: a multifaceted perspective. Front Allergy. 2024;5:1505834. doi: 10.3389/falgy.2024.1505834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wicha K, Brzozowska M, Wardal W, Furlepa N, Rzenno R, Wojciechowska K, Matuszewska M, Sidz N, Tomaszewska M, Jedlikowska W. Is There a Connection Between Intestinal Barrier Disruption, Dysbiosis, and the Development of Type 2 Diabetes Mellitus? - a literature review. J Educ Health Sport. 2025;79:58355. [Google Scholar]
- 32.Mishra S, Jain S, Agadzi B, Yadav H. A Cascade of Microbiota-Leaky Gut-Inflammation- Is it a Key Player in Metabolic Disorders? Curr Obes Rep. 2025;14:32. doi: 10.1007/s13679-025-00624-0. [DOI] [PubMed] [Google Scholar]
- 33.Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, Gmizic I, Stevanovic O, Djordjevic V, Lekic N, Russo E, Amedei A. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int J Mol Sci. 2019;20:395. doi: 10.3390/ijms20020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali Q, Ma S, La S, Guo Z, Liu B, Gao Z, Farooq U, Wang Z, Zhu X, Cui Y, Li D, Shi Y. Microbial short-chain fatty acids: a bridge between dietary fibers and poultry gut health - A review. Anim Biosci. 2022;35:1461–1478. doi: 10.5713/ab.21.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, Wichienchot S, He X, Fu X, Huang Q, Zhang B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci Tech. 2019;88:1–9. [Google Scholar]
- 36.He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, Zhao Y, Bai L, Hao X, Li X, Zhang S, Zhu L. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int J Mol Sci. 2020;21:6356. doi: 10.3390/ijms21176356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikeda T, Nishida A, Yamano M, Kimura I. Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol Ther. 2022;239:108273. doi: 10.1016/j.pharmthera.2022.108273. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu H, Ohue-Kitano R, Kimura I. Regulation of host energy metabolism by gut microbiota-derived short-chain fatty acids. Glycative Stress Res. 2019;6:181–191. [Google Scholar]
- 39.Grüner N, Mattner J. Bile Acids and Microbiota: Multifaceted and Versatile Regulators of the Liver-Gut Axis. Int J Mol Sci. 2021;22:1397. doi: 10.3390/ijms22031397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun R, Xu C, Feng B, Gao X, Liu Z. Critical roles of bile acids in regulating intestinal mucosal immune responses. Therap Adv Gastroenterol. 2021;14:17562848211018098. doi: 10.1177/17562848211018098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmann AF. The enterohepatic circulation of bile acids in mammals: form and functions. Front Biosci (Landmark Ed) 2009;14:2584–2598. doi: 10.2741/3399. [DOI] [PubMed] [Google Scholar]
- 42.Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann Hepatol. 2017;16:S21–S26. doi: 10.5604/01.3001.0010.5672. [DOI] [PubMed] [Google Scholar]
- 43.Kumari A, Pal Pathak D, Asthana S. Bile acids mediated potential functional interaction between FXR and FATP5 in the regulation of Lipid Metabolism. Int J Biol Sci. 2020;16:2308–2322. doi: 10.7150/ijbs.44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Xu H, Zhou X, Chen W, Zhou H. Dysregulated bile acid homeostasis: unveiling its role in metabolic diseases. Med Rev (2021) 2024;4:262–283. doi: 10.1515/mr-2024-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canyelles M, Tondo M, Cedó L, Farràs M, Escolà-Gil JC, Blanco-Vaca F. Trimethylamine N-Oxide: A Link among Diet, Gut Microbiota, Gene Regulation of Liver and Intestine Cholesterol Homeostasis and HDL Function. Int J Mol Sci. 2018;19:3228. doi: 10.3390/ijms19103228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randrianarisoa E, Lehn-Stefan A, Wang X, Hoene M, Peter A, Heinzmann SS, Zhao X, Königsrainer I, Königsrainer A, Balletshofer B, Machann J, Schick F, Fritsche A, Häring HU, Xu G, Lehmann R, Stefan N. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early Atherosclerosis in Humans. Sci Rep. 2016;6:26745. doi: 10.1038/srep26745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benson TW, Conrad KA, Li XS, Wang Z, Helsley RN, Schugar RC, Coughlin TM, Wadding-Lee C, Fleifil S, Russell HM, Stone T, Brooks M, Buffa JA, Mani K, Björck M, Wanhainen A, Sangwan N, Biddinger S, Bhandari R, Ademoya A, Pascual C, Tang WHW, Tranter M, Cameron SJ, Brown JM, Hazen SL, Owens AP 3rd. Gut Microbiota-Derived Trimethylamine N-Oxide Contributes to Abdominal Aortic Aneurysm Through Inflammatory and Apoptotic Mechanisms. Circulation. 2023;147:1079–1096. doi: 10.1161/CIRCULATIONAHA.122.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lara-Guzmán ÓJ, Arango-González ÁM, Álvarez-Quintero R, Escobar JS, Muñoz-Durango K, Sierra JA. Circulating hs-CRP, IL-18, Chemerin, Leptin, and Adiponectin Levels Reflect Cardiometabolic Dysfunction in Adults with Excess Weight. Int J Mol Sci. 2025;26:1176. doi: 10.3390/ijms26031176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodrigues KF, Pietrani NT, Bosco AA, Campos FMF, Sandrim VC, Gomes KB. IL-6, TNF-α, and IL-10 levels/polymorphisms and their association with type 2 diabetes mellitus and obesity in Brazilian individuals. Arch Endocrinol Metab. 2017;61:438–446. doi: 10.1590/2359-3997000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine. 2016;86:100–109. doi: 10.1016/j.cyto.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 51.D'Alessandris C, Lauro R, Presta I, Sesti G. C-reactive protein induces phosphorylation of insulin receptor substrate-1 on Ser307 and Ser 612 in L6 myocytes, thereby impairing the insulin signalling pathway that promotes glucose transport. Diabetologia. 2007;50:840–849. doi: 10.1007/s00125-006-0522-y. [DOI] [PubMed] [Google Scholar]
- 52.Guerville M, Boudry G. Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation. Am J Physiol Gastrointest Liver Physiol. 2016;311:G1–G15. doi: 10.1152/ajpgi.00098.2016. [DOI] [PubMed] [Google Scholar]
- 53.Nakamoto N, Kanai T. Role of toll-like receptors in immune activation and tolerance in the liver. Front Immunol. 2014;5:221. doi: 10.3389/fimmu.2014.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mak KM, Shekhar AC. Lipopolysaccharide, arbiter of the gut-liver axis, modulates hepatic cell pathophysiology in alcoholism. Anat Rec (Hoboken) 2025;308:975–1004. doi: 10.1002/ar.25562. [DOI] [PubMed] [Google Scholar]
- 55.Cheru L, Saylor CF, Lo J. Gastrointestinal Barrier Breakdown and Adipose Tissue Inflammation. Curr Obes Rep. 2019;8:165–174. doi: 10.1007/s13679-019-00332-6. [DOI] [PubMed] [Google Scholar]
- 56.Riedel S, Pheiffer C, Johnson R, Louw J, Muller CJF. Intestinal Barrier Function and Immune Homeostasis Are Missing Links in Obesity and Type 2 Diabetes Development. Front Endocrinol (Lausanne) 2021;12:833544. doi: 10.3389/fendo.2021.833544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Massier L, Blüher M, Kovacs P, Chakaroun RM. Impaired Intestinal Barrier and Tissue Bacteria: Pathomechanisms for Metabolic Diseases. Front Endocrinol (Lausanne) 2021;12:616506. doi: 10.3389/fendo.2021.616506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021;6:291. doi: 10.1038/s41392-021-00687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li W, Yang GL, Zhu Q, Zhong XH, Nie YC, Li XH, Wang Y. TLR4 promotes liver inflammation by activating the JNK pathway. Eur Rev Med Pharmacol Sci. 2019;23:7655–7662. doi: 10.26355/eurrev_201909_18889. [DOI] [PubMed] [Google Scholar]
- 60.Li X, Xu M, Shen J, Li Y, Lin S, Zhu M, Pang Q, Tan X, Tang J. Sorafenib inhibits LPS-induced inflammation by regulating Lyn-MAPK-NF-kB/AP-1 pathway and TLR4 expression. Cell Death Discov. 2022;8:281. doi: 10.1038/s41420-022-01073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanti JF, Jager J. Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol. 2009;9:753–762. doi: 10.1016/j.coph.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Li A, Lin C, Xie F, Jin M, Lin F. Berberine Ameliorates Insulin Resistance by Inhibiting IKK/NF-κB, JNK, and IRS-1/AKT Signaling Pathway in Liver of Gestational Diabetes Mellitus Rats. Metab Syndr Relat Disord. 2022;20:480–488. doi: 10.1089/met.2022.0017. [DOI] [PubMed] [Google Scholar]
- 63.Chávez-Talavera O, Haas J, Grzych G, Tailleux A, Staels B. Bile acid alterations in nonalcoholic fatty liver disease, obesity, insulin resistance and type 2 diabetes: what do the human studies tell? Curr Opin Lipidol. 2019;30:244–254. doi: 10.1097/MOL.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 64.Bertolini A, Fiorotto R, Strazzabosco M. Bile acids and their receptors: modulators and therapeutic targets in liver inflammation. Semin Immunopathol. 2022;44:547–564. doi: 10.1007/s00281-022-00935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evangelakos I, Heeren J, Verkade E, Kuipers F. Role of bile acids in inflammatory liver diseases. Semin Immunopathol. 2021;43:577–590. doi: 10.1007/s00281-021-00869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Gutierrez E, O'Mahony AK, Dos Santos RS, Marroquí L, Cotter PD. Gut microbial metabolic signatures in diabetes mellitus and potential preventive and therapeutic applications. Gut Microbes. 2024;16:2401654. doi: 10.1080/19490976.2024.2401654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He H, Sun Y, Zhang S, Zhang H, Su W, Guo Z, Zhang Y, Wen J, Li X, Hu J, Nie S. Arabinogalactan, Bifidobacterium longum, and Faecalibacterium prausnitzii improve insulin resistance in high‐fat diet‐induced C57BL/6J mice. eFood. 2022;3 [Google Scholar]
- 68.Verhoog S, Taneri PE, Roa Díaz ZM, Marques-Vidal P, Troup JP, Bally L, Franco OH, Glisic M, Muka T. Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A Systematic Review. Nutrients. 2019;11:1565. doi: 10.3390/nu11071565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moran-Ramos S, Macias-Kauffer L, López-Contreras BE, Villamil-Ramírez H, Ocampo-Medina E, León-Mimila P, Del Rio-Navarro BE, Granados-Portillo O, Ibarra-Gonzalez I, Vela-Amieva M, Tovar AR, Torres N, Gomez-Perez FJ, Aguilar-Salinas C, Canizales-Quinteros S. A higher bacterial inward BCAA transport driven by Faecalibacterium prausnitzii is associated with lower serum levels of BCAA in early adolescents. Mol Med. 2021;27:108. doi: 10.1186/s10020-021-00371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leite AZ, Rodrigues NC, Gonzaga MI, Paiolo JCC, de Souza CA, Stefanutto NAV, Omori WP, Pinheiro DG, Brisotti JL, Matheucci Junior E, Mariano VS, de Oliveira GLV. Detection of Increased Plasma Interleukin-6 Levels and Prevalence of Prevotella copri and Bacteroides vulgatus in the Feces of Type 2 Diabetes Patients. Front Immunol. 2017;8:1107. doi: 10.3389/fimmu.2017.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li WZ, Stirling K, Yang JJ, Zhang L. Gut microbiota and diabetes: From correlation to causality and mechanism. World J Diabetes. 2020;11:293–308. doi: 10.4239/wjd.v11.i7.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gong J, Zhang Q, Hu R, Yang X, Fang C, Yao L, Lv J, Wang L, Shi M, Zhang W, Ma S, Xiang H, Zhang H, Hou DX, Yin Y, He J, Peng L, Wu S. Effects of Prevotella copri on insulin, gut microbiota and bile acids. Gut Microbes. 2024;16:2340487. doi: 10.1080/19490976.2024.2340487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi BS, Daniel N, Houde VP, Ouellette A, Marcotte B, Varin TV, Vors C, Feutry P, Ilkayeva O, Ståhlman M, St-Pierre P, Bäckhed F, Tremblay A, White PJ, Marette A. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377. doi: 10.1038/s41467-021-23782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao H, Zhang F, Sun D, Wang X, Zhang X, Zhang J, Yan F, Huang C, Xie H, Lin C, Liu Y, Fan M, Yan W, Chen Y, Lian K, Li Y, Zhang L, Wang S, Tao L. Branched-Chain Amino Acids Exacerbate Obesity-Related Hepatic Glucose and Lipid Metabolic Disorders via Attenuating Akt2 Signaling. Diabetes. 2020;69:1164–1177. doi: 10.2337/db19-0920. [DOI] [PubMed] [Google Scholar]
- 75.Zeng Z, Chen M, Liu Y, Zhou Y, Liu H, Wang S, Ji Y. Role of Akkermansia muciniphila in insulin resistance. J Gastroenterol Hepatol. 2025;40:19–32. doi: 10.1111/jgh.16747. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Ni Y, Qian L, Fang Q, Zheng T, Zhang M, Gao Q, Zhang Y, Ni J, Hou X, Bao Y, Kovatcheva-Datchary P, Xu A, Li H, Panagiotou G, Jia W. Decreased Abundance of Akkermansia muciniphila Leads to the Impairment of Insulin Secretion and Glucose Homeostasis in Lean Type 2 Diabetes. Adv Sci (Weinh) 2021;8:e2100536. doi: 10.1002/advs.202100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020;9:F1000 Faculty Rev–F1000 Faculty R69. doi: 10.12688/f1000research.20510.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aggarwal V, Sunder S, Verma SR. Disease-associated dysbiosis and potential therapeutic role of Akkermansia muciniphila, a mucus degrading bacteria of gut microbiome. Folia Microbiol (Praha) 2022;67:811–824. doi: 10.1007/s12223-022-00973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao Y, Yang H, Wu P, Yang S, Xue W, Xu B, Zhang S, Tang B, Xu D. Akkermansia muciniphila: A promising probiotic against inflammation and metabolic disorders. Virulence. 2024;15:2375555. doi: 10.1080/21505594.2024.2375555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeng Y, Wu Q, Guo M, Teng F, Jiang C, Chen J, Tan X, Zeng C, Long Y, Law BY, Xu Y. Gut microbiota-derived imidazole propionate: an emerging target for the prevention and treatment of cardiometabolic diseases. Front Endocrinol (Lausanne) 2025;16:1409119. doi: 10.3389/fendo.2025.1409119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koh A, Mannerås-Holm L, Yunn NO, Nilsson PM, Ryu SH, Molinaro A, Perkins R, Smith JG, Bäckhed F. Microbial Imidazole Propionate Affects Responses to Metformin through p38γ-Dependent Inhibitory AMPK Phosphorylation. Cell Metab. 2020;32:643–653.e4. doi: 10.1016/j.cmet.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koh A, Molinaro A, Ståhlman M, Khan MT, Schmidt C, Mannerås-Holm L, Wu H, Carreras A, Jeong H, Olofsson LE, Bergh PO, Gerdes V, Hartstra A, de Brauw M, Perkins R, Nieuwdorp M, Bergström G, Bäckhed F. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell. 2018;175:947–961.e17. doi: 10.1016/j.cell.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 83.Cussotto S, Delgado I, Anesi A, Dexpert S, Aubert A, Beau C, Forestier D, Ledaguenel P, Magne E, Mattivi F, Capuron L. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front Immunol. 2020;11:557. doi: 10.3389/fimmu.2020.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang H, Wang T, Qian C, Wang H, Yu D, Shi M, Fu M, Liu X, Pan M, Rong X, Xiao Z, Chen X, Yeerken A, Wu Y, Zheng Y, Yang H, Zhang M, Liu T, Qiao P, Qu Y, Lin Y, Huang Y, Jin J, Liu N, Wen Y, Sun N, Zhao C. Gut microbial-derived phenylacetylglutamine accelerates host cellular senescence. Nat Aging. 2025;5:401–418. doi: 10.1038/s43587-024-00795-w. [DOI] [PubMed] [Google Scholar]
- 85.Munteanu C, Onose G, Rotariu M, Poștaru M, Turnea M, Galaction AI. Role of Microbiota-Derived Hydrogen Sulfide (H(2)S) in Modulating the Gut-Brain Axis: Implications for Alzheimer's and Parkinson's Disease Pathogenesis. Biomedicines. 2024;12:2670. doi: 10.3390/biomedicines12122670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang H, Li G, He Y, Chen J, Yan J, Zhang Q, Li L, Cai X. Cellular succinate metabolism and signaling in inflammation: implications for therapeutic intervention. Front Immunol. 2024;15:1404441. doi: 10.3389/fimmu.2024.1404441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xue G, Feng J, Zhang R, Du B, Sun Y, Liu S, Yan C, Liu X, Du S, Feng Y, Cui J, Gan L, Zhao H, Fan Z, Cui X, Xu Z, Fu T, Li C, Huang L, Zhang T, Wang J, Yang R, Yuan J. Three Klebsiella species as potential pathobionts generating endogenous ethanol in a clinical cohort of patients with auto-brewery syndrome: a case control study. EBioMedicine. 2023;91:104560. doi: 10.1016/j.ebiom.2023.104560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen X, Zhang Z, Li H, Zhao J, Wei X, Lin W, Zhao X, Jiang A, Yuan J. Endogenous ethanol produced by intestinal bacteria induces mitochondrial dysfunction in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35:2009–2019. doi: 10.1111/jgh.15027. [DOI] [PubMed] [Google Scholar]
- 89.Drda JC, Smith JP. Endogenous Alcohol and Auto-Brewery Syndrome Complicating Liver Transplantation: A Case Report and Literature Review. Livers. 2025;5:13. [Google Scholar]
- 90.Melo-Marques I, Cardoso SM, Empadinhas N. Bacterial extracellular vesicles at the interface of gut microbiota and immunity. Gut Microbes. 2024;16:2396494. doi: 10.1080/19490976.2024.2396494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Butcko AJ, Putman AK, Mottillo EP. The Intersection of Genetic Factors, Aberrant Nutrient Metabolism and Oxidative Stress in the Progression of Cardiometabolic Disease. Antioxidants (Basel) 2024;13:87. doi: 10.3390/antiox13010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tomasi TB. The discovery of secretory IgA and the mucosal immune system. Immunol Today. 1992;13:416–418. doi: 10.1016/0167-5699(92)90093-M. [DOI] [PubMed] [Google Scholar]
- 93.Inamine T, Schnabl B. Immunoglobulin A and liver diseases. J Gastroenterol. 2018;53:691–700. doi: 10.1007/s00535-017-1400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diana J, Moura IC, Vaugier C, Gestin A, Tissandie E, Beaudoin L, Corthésy B, Hocini H, Lehuen A, Monteiro RC. Secretory IgA induces tolerogenic dendritic cells through SIGNR1 dampening autoimmunity in mice. J Immunol. 2013;191:2335–2343. doi: 10.4049/jimmunol.1300864. [DOI] [PubMed] [Google Scholar]
- 95.Guo J, Han X, Huang W, You Y, Jicheng Z. Interaction between IgA and gut microbiota and its role in controlling metabolic syndrome. Obes Rev. 2021;22:e13155. doi: 10.1111/obr.13155. [DOI] [PubMed] [Google Scholar]
- 96.Luck H, Khan S, Kim JH, Copeland JK, Revelo XS, Tsai S, Chakraborty M, Cheng K, Tao Chan Y, Nøhr MK, Clemente-Casares X, Perry MC, Ghazarian M, Lei H, Lin YH, Coburn B, Okrainec A, Jackson T, Poutanen S, Gaisano H, Allard JP, Guttman DS, Conner ME, Winer S, Winer DA. Gut-associated IgA(+) immune cells regulate obesity-related insulin resistance. Nat Commun. 2019;10:3650. doi: 10.1038/s41467-019-11370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kadaoui KA, Corthésy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment. J Immunol. 2007;179:7751–7757. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- 98.Rifai A, Mannik M. Clearance of circulating IgA immune complexes is mediated by a specific receptor on Kupffer cells in mice. J Exp Med. 1984;160:125–137. doi: 10.1084/jem.160.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Catanzaro JR, Strauss JD, Bielecka A, Porto AF, Lobo FM, Urban A, Schofield WB, Palm NW. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory IgM. Sci Rep. 2019;9:13574. doi: 10.1038/s41598-019-49923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 101.Gustafsson JK, Johansson MEV. The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol. 2022;19:785–803. doi: 10.1038/s41575-022-00675-x. [DOI] [PubMed] [Google Scholar]
- 102.Gueddouri D, Caüzac M, Fauveau V, Benhamed F, Charifi W, Beaudoin L, Rouland M, Sicherre F, Lehuen A, Postic C, Boudry G, Burnol AF, Guilmeau S. Insulin resistance per se drives early and reversible dysbiosis-mediated gut barrier impairment and bactericidal dysfunction. Mol Metab. 2022;57:101438. doi: 10.1016/j.molmet.2022.101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lerkdumnernkit N, Sricharoenvej S, Lanlua P, Niyomchan A, Baimai S, Chookliang A, Plaengrit K, Pianrumluk S, Manoonpol C. The Effects of Early Diabetes on Duodenal Alterations in the Rats. Int J Morphol. 2022;40:389–395. [Google Scholar]
- 104.Kyoko OO, Kono H, Ishimaru K, Miyake K, Kubota T, Ogawa H, Okumura K, Shibata S, Nakao A. Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis. PLoS One. 2014;9:e98016. doi: 10.1371/journal.pone.0098016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu L, Li G, Shu Y, Hou X, Yang L, Jin Y. Circadian dysregulation induces alterations of visceral sensitivity and the gut microbiota in Light/Dark phase shift mice. Front Microbiol. 2022;13:935919. doi: 10.3389/fmicb.2022.935919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo X, Wang J, Xu H, Wang Y, Cao Y, Wen Y, Li J, Liu Y, Wang K, Wang J, Zhong X, Sun C, Zhang Y, Xu J, Li C, Mu P, Xu L, Xie C. Obesity induced disruption on diurnal rhythm of insulin sensitivity via gut microbiome-bile acid metabolism. Biochim Biophys Acta Mol Cell Biol Lipids. 2024;1869:159419. doi: 10.1016/j.bbalip.2023.159419. [DOI] [PubMed] [Google Scholar]
- 107.Chaix A, Lin T, Le HD, Chang MW, Panda S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019;29:303–319.e4. doi: 10.1016/j.cmet.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Naama M, Telpaz S, Awad A, Ben-Simon S, Harshuk-Shabso S, Modilevsky S, Rubin E, Sawaed J, Zelik L, Zigdon M, Asulin N, Turjeman S, Werbner M, Wongkuna S, Feeney R, Schroeder BO, Nyska A, Nuriel-Ohayon M, Bel S. Autophagy controls mucus secretion from intestinal goblet cells by alleviating ER stress. Cell Host Microbe. 2023;31:433–446.e4. doi: 10.1016/j.chom.2023.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jatana S, Abbadi A, West GA, Ponti AK, Braga-Neto MB, Smith JL, Marino-Melendez A, Willard B, Nagy LE, Motte C. Hyperglycemic environments directly compromise intestinal epithelial barrier function in an organoid model and hyaluronan (~35 kDa) protects via a layilin dependent mechanism. Matrix Biol. 2024;133:116–133. doi: 10.1016/j.matbio.2024.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao J, Duan L, Li J, Yao C, Wang G, Mi J, Yu Y, Ding L, Zhao Y, Yan G, Li J, Zhao Z, Wang X, Li M. New insights into the interplay between autophagy, gut microbiota and insulin resistance in metabolic syndrome. Biomed Pharmacother. 2024;176:116807. doi: 10.1016/j.biopha.2024.116807. [DOI] [PubMed] [Google Scholar]
- 111.Foerster EG, Mukherjee T, Cabral-Fernandes L, Rocha JDB, Girardin SE, Philpott DJ. How autophagy controls the intestinal epithelial barrier. Autophagy. 2022;18:86–103. doi: 10.1080/15548627.2021.1909406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wachsmuth HR, Weninger SN, Duca FA. Role of the gut-brain axis in energy and glucose metabolism. Exp Mol Med. 2022;54:377–392. doi: 10.1038/s12276-021-00677-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wean JB, Smith BN. FGF19 in the Hindbrain Lowers Blood Glucose and Alters Excitability of Vagal Motor Neurons in Hyperglycemic Mice. Endocrinology. 2021;162:bqab021. doi: 10.1210/endocr/bqab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yan M, Man S, Sun B, Ma L, Guo L, Huang L, Gao W. Gut liver brain axis in diseases: the implications for therapeutic interventions. Signal Transduct Target Ther. 2023;8:443. doi: 10.1038/s41392-023-01673-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cyphert HA, Ge X, Kohan AB, Salati LM, Zhang Y, Hillgartner FB. Activation of the farnesoid X receptor induces hepatic expression and secretion of fibroblast growth factor 21. J Biol Chem. 2012;287:25123–25138. doi: 10.1074/jbc.M112.375907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Q, Cheng W, Zhang J, Chi C, Lin M, He C, Liao Z, Gong F. Fibroblast growth factor 21 improves insulin sensitivity by modulating the bile acid-gut microbiota axis in type Ⅱ diabetic mice. Free Radic Biol Med. 2024;224:600–617. doi: 10.1016/j.freeradbiomed.2024.09.017. [DOI] [PubMed] [Google Scholar]
- 117.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Véniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chui ZSW, Shen Q, Xu A. Current status and future perspectives of FGF21 analogues in clinical trials. Trends Endocrinol Metab. 2024;35:371–384. doi: 10.1016/j.tem.2024.02.001. [DOI] [PubMed] [Google Scholar]
- 119.Yadav P, Khurana A, Bhatti JS, Weiskirchen R, Navik U. Glucagon-like peptide 1 and fibroblast growth factor-21 in non-alcoholic steatohepatitis: An experimental to clinical perspective. Pharmacol Res. 2022;184:106426. doi: 10.1016/j.phrs.2022.106426. [DOI] [PubMed] [Google Scholar]
- 120.Zheng W, Liu M, Lv X, He C, Yin J, Ma J. AhR governs lipid metabolism: the role of gut microbiota. Front Microbiol. 2025;16:1442282. doi: 10.3389/fmicb.2025.1442282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Du W, Jiang S, Yin S, Wang R, Zhang C, Yin BC, Li J, Li L, Qi N, Zhou Y, Ye BC. The microbiota-dependent tryptophan metabolite alleviates high-fat diet-induced insulin resistance through the hepatic AhR/TSC2/mTORC1 axis. Proc Natl Acad Sci U S A. 2024;121:e2400385121. doi: 10.1073/pnas.2400385121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang R, Cui W, Yang H. The interplay between innate lymphoid cells and microbiota. mBio. 2023;14:e0039923. doi: 10.1128/mbio.00399-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yin G, Zhao C, Pei W. Crosstalk between macrophages and innate lymphoid cells (ILCs) in diseases. Int Immunopharmacol. 2022;110:108937. doi: 10.1016/j.intimp.2022.108937. [DOI] [PubMed] [Google Scholar]
- 124.Painter JD, Akbari O. Type 2 Innate Lymphoid Cells: Protectors in Type 2 Diabetes. Front Immunol. 2021;12:727008. doi: 10.3389/fimmu.2021.727008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suriano F, Manca C, Flamand N, Depommier C, Van Hul M, Delzenne NM, Silvestri C, Cani PD, Di Marzo V. Exploring the endocannabinoidome in genetically obese (ob/ob) and diabetic (db/db) mice: Links with inflammation and gut microbiota. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867:159056. doi: 10.1016/j.bbalip.2021.159056. [DOI] [PubMed] [Google Scholar]
- 126.Cani PD, Plovier H, Van Hul M, Geurts L, Delzenne NM, Druart C, Everard A. Endocannabinoids--at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol. 2016;12:133–143. doi: 10.1038/nrendo.2015.211. [DOI] [PubMed] [Google Scholar]
- 127.Horn V, Sonnenberg GF. Group 3 innate lymphoid cells in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2024;21:428–443. doi: 10.1038/s41575-024-00906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coorens M, Rao A, Gräfe SK, Unelius D, Lindforss U, Agerberth B, Mjösberg J, Bergman P. Innate lymphoid cell type 3-derived interleukin-22 boosts lipocalin-2 production in intestinal epithelial cells via synergy between STAT3 and NF-κB. J Biol Chem. 2019;294:6027–6041. doi: 10.1074/jbc.RA118.007290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Couturier-Maillard A, Froux N, Piotet-Morin J, Michaudel C, Brault L, Le Bérichel J, Sénéchal A, Robinet P, Chenuet P, Jejou S, Dumoutier L, Renauld JC, Iovanna J, Huber S, Chamaillard M, Quesniaux V, Sokol H, Chamaillard M, Ryffel B. Interleukin-22-deficiency and microbiota contribute to the exacerbation of Toxoplasma gondii-induced intestinal inflammation. Mucosal Immunol. 2018;11:1181–1190. doi: 10.1038/s41385-018-0005-8. [DOI] [PubMed] [Google Scholar]
- 130.Gu J, Zhou P, Liu Y, Xu Q, Chen X, Chen M, Lu C, Qu C, Tong Y, Yu Q, Lu X, Yu C, Liu Z. Down-regulating Interleukin-22/Interleukin-22 binding protein axis promotes inflammation and aggravates diet-induced metabolic disorders. Mol Cell Endocrinol. 2022;557:111776. doi: 10.1016/j.mce.2022.111776. [DOI] [PubMed] [Google Scholar]
- 131.Zai W, Chen W, Liu H, Ju D. Therapeutic Opportunities of IL-22 in Non-Alcoholic Fatty Liver Disease: From Molecular Mechanisms to Clinical Applications. Biomedicines. 2021;9:1912. doi: 10.3390/biomedicines9121912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ge Y, Sun H, Xu L, Zhang W, Lv J, Chen Y. The amelioration of alcohol-induced liver and intestinal barrier injury by Lactobacillus rhamnosus Gorbach-Goldin (LGG) is dependent on Interleukin 22 (IL-22) expression. Bioengineered. 2022;13:12650–12660. doi: 10.1080/21655979.2022.2070998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bazwinsky-Wutschke I, Zipprich A, Dehghani F. Endocannabinoid System in Hepatic Glucose Metabolism, Fatty Liver Disease, and Cirrhosis. Int J Mol Sci. 2019;20:2516. doi: 10.3390/ijms20102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu J, Zhou L, Xiong K, Godlewski G, Mukhopadhyay B, Tam J, Yin S, Gao P, Shan X, Pickel J, Bataller R, O'Hare J, Scherer T, Buettner C, Kunos G. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology. 2012;142:1218–1228.e1. doi: 10.1053/j.gastro.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cuddihey H, Cavin JB, Keenan CM, Wallace LE, Vemuri K, Makriyannis A, MacNaughton WK, Sharkey KA. Role of CB(1) receptors in the acute regulation of small intestinal permeability: effects of high-fat diet. Am J Physiol Gastrointest Liver Physiol. 2022;323:G219–G238. doi: 10.1152/ajpgi.00341.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lipina C, Vaanholt LM, Davidova A, Mitchell SE, Storey-Gordon E, Hambly C, Irving AJ, Speakman JR, Hundal HS. CB1 receptor blockade counters age-induced insulin resistance and metabolic dysfunction. Aging Cell. 2016;15:325–335. doi: 10.1111/acel.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roser P, Vollenweider FX, Kawohl W. Potential antipsychotic properties of central cannabinoid (CB1) receptor antagonists. World J Biol Psychiatry. 2010;11:208–219. doi: 10.3109/15622970801908047. [DOI] [PubMed] [Google Scholar]
- 138.O'Sullivan SE, Yates AS, Porter RK. The Peripheral Cannabinoid Receptor Type 1 (CB(1)) as a Molecular Target for Modulating Body Weight in Man. Molecules. 2021;26:6178. doi: 10.3390/molecules26206178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ellermann M. Emerging mechanisms by which endocannabinoids and their derivatives modulate bacterial populations within the gut microbiome. Adv Drug Alcohol Res. 2023;3:11359. doi: 10.3389/adar.2023.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Y, Zhu X, Yu X, Novák P, Gui Q, Yin K. Enhancing intestinal barrier efficiency: A novel metabolic diseases therapy. Front Nutr. 2023;10:1120168. doi: 10.3389/fnut.2023.1120168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rochoń J, Kalinowski P, Szymanek-Majchrzak K, Grąt M. Role of gut-liver axis and glucagon-like peptide-1 receptor agonists in the treatment of metabolic dysfunction-associated fatty liver disease. World J Gastroenterol. 2024;30:2964–2980. doi: 10.3748/wjg.v30.i23.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Awad WA, Hess C, Hess M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins (Basel) 2017;9:60. doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Górecka A, Jura-Półtorak A, Koźma EM, Szeremeta A, Olczyk K, Komosińska-Vassev K. Biochemical Modulators of Tight Junctions (TJs): Occludin, Claudin-2 and Zonulin as Biomarkers of Intestinal Barrier Leakage in the Diagnosis and Assessment of Inflammatory Bowel Disease Progression. Molecules. 2024;29:4577. doi: 10.3390/molecules29194577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Slifer ZM, Krishnan BR, Madan J, Blikslager AT. Larazotide acetate: a pharmacological peptide approach to tight junction regulation. Am J Physiol Gastrointest Liver Physiol. 2021;320:G983–G989. doi: 10.1152/ajpgi.00386.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yonker LM, Swank Z, Gilboa T, Senussi Y, Kenyon V, Papadakis L, Boribong BP, Carroll RW, Walt DR, Fasano A. Zonulin Antagonist, Larazotide (AT1001), As an Adjuvant Treatment for Multisystem Inflammatory Syndrome in Children: A Case Series. Crit Care Explor. 2022;10:e0641. doi: 10.1097/CCE.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tajik N, Frech M, Schulz O, Schälter F, Lucas S, Azizov V, Dürholz K, Steffen F, Omata Y, Rings A, Bertog M, Rizzo A, Iljazovic A, Basic M, Kleyer A, Culemann S, Krönke G, Luo Y, Überla K, Gaipl US, Frey B, Strowig T, Sarter K, Bischoff SC, Wirtz S, Cañete JD, Ciccia F, Schett G, Zaiss MM. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;11:1995. doi: 10.1038/s41467-020-15831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mazhar M, Zhu Y, Qin L. The Interplay of Dietary Fibers and Intestinal Microbiota Affects Type 2 Diabetes by Generating Short-Chain Fatty Acids. Foods. 2023;12:1023. doi: 10.3390/foods12051023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lo Conte M, Antonini Cencicchio M, Ulaszewska M, Nobili A, Cosorich I, Ferrarese R, Massimino L, Andolfo A, Ungaro F, Mancini N, Falcone M. A diet enriched in omega-3 PUFA and inulin prevents type 1 diabetes by restoring gut barrier integrity and immune homeostasis in NOD mice. Front Immunol. 2022;13:1089987. doi: 10.3389/fimmu.2022.1089987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alharbi SH. Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications. Ther Adv Endocrinol Metab. 2024;15:20420188231222367. doi: 10.1177/20420188231222367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis K, Tousoulis D. The impact of SGLT2 inhibitors on inflammation: A systematic review and meta-analysis of studies in rodents. Int Immunopharmacol. 2022;111:109080. doi: 10.1016/j.intimp.2022.109080. [DOI] [PubMed] [Google Scholar]
- 151.Zhang R, Xie Q, Lu X, Fan R, Tong N. Research advances in the anti-inflammatory effects of SGLT inhibitors in type 2 diabetes mellitus. Diabetol Metab Syndr. 2024;16:99. doi: 10.1186/s13098-024-01325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang DY, Zhu L, Liu HN, Tseng YJ, Weng SQ, Liu TT, Dong L, Shen XZ. The protective effect and mechanism of the FXR agonist obeticholic acid via targeting gut microbiota in non-alcoholic fatty liver disease. Drug Des Devel Ther. 2019;13:2249–2270. doi: 10.2147/DDDT.S207277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ding C, Wang Z, Dou X, Yang Q, Ning Y, Kao S, Sang X, Hao M, Wang K, Peng M, Zhang S, Han X, Cao G. Farnesoid X receptor: From Structure to Function and Its Pharmacology in Liver Fibrosis. Aging Dis. 2024;15:1508–1536. doi: 10.14336/AD.2023.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hansen M, Sonne DP, Mikkelsen KH, Gluud LL, Vilsbøll T, Knop FK. Bile acid sequestrants for glycemic control in patients with type 2 diabetes: A systematic review with meta-analysis of randomized controlled trials. J Diabetes Complications. 2017;31:918–927. doi: 10.1016/j.jdiacomp.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 155.Płóciennikowska A, Hromada-Judycka A, Borzęcka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2015;72:557–581. doi: 10.1007/s00018-014-1762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grylls A, Seidler K, Neil J. Link between microbiota and hypertension: Focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed Pharmacother. 2021;137:111334. doi: 10.1016/j.biopha.2021.111334. [DOI] [PubMed] [Google Scholar]
- 157.Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, Glynn RJ, Libby P, Ridker PM. Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes. J Am Coll Cardiol. 2018;71:2392–2401. doi: 10.1016/j.jacc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 158.Howard C, Noe A, Skerjanec A, Holzhauer B, Wernsing M, Ligueros-Saylan M, Thuren T. Safety and tolerability of canakinumab, an IL-1β inhibitor, in type 2 diabetes mellitus patients: a pooled analysis of three randomised double-blind studies. Cardiovasc Diabetol. 2014;13:94. doi: 10.1186/1475-2840-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pernomian L, Duarte-Silva M, de Barros Cardoso CR. The Aryl Hydrocarbon Receptor (AHR) as a Potential Target for the Control of Intestinal Inflammation: Insights from an Immune and Bacteria Sensor Receptor. Clin Rev Allergy Immunol. 2020;59:382–390. doi: 10.1007/s12016-020-08789-3. [DOI] [PubMed] [Google Scholar]
- 160.Zhang T, Zhang J, Duan L. The Role of Genetically Engineered Probiotics for Treatment of Inflammatory Bowel Disease: A Systematic Review. Nutrients. 2023;15:1566. doi: 10.3390/nu15071566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Han K, Xu J, Xie F, Crowther J, Moon JJ. Engineering Strategies to Modulate the Gut Microbiome and Immune System. J Immunol. 2024;212:208–215. doi: 10.4049/jimmunol.2300480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Memon H, Abdulla F, Reljic T, Alnuaimi S, Serdarevic F, Asimi ZV, Kumar A, Semiz S. Effects of combined treatment of probiotics and metformin in management of type 2 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2023;202:110806. doi: 10.1016/j.diabres.2023.110806. [DOI] [PubMed] [Google Scholar]
- 163.McLoughlin RF, Berthon BS, Jensen ME, Baines KJ, Wood LG. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106:930–945. doi: 10.3945/ajcn.117.156265. [DOI] [PubMed] [Google Scholar]
- 164.Fang H, Rodrigues E-Lacerda R, Barra NG, Kukje Zada D, Robin N, Mehra A, Schertzer JD. Postbiotic Impact on Host Metabolism and Immunity Provides Therapeutic Potential in Metabolic Disease. Endocr Rev. 2025;46:60–79. doi: 10.1210/endrev/bnae025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Luzzi A, Briata IM, Di Napoli I, Giugliano S, Di Sabatino A, Rescigno M, Cena H. Prebiotics, probiotics, synbiotics and postbiotics to adolescents in metabolic syndrome. Clin Nutr. 2024;43:1433–1446. doi: 10.1016/j.clnu.2024.04.032. [DOI] [PubMed] [Google Scholar]
- 166.Bhatia A, Sharma D, Mehta J, Kumarasamy V, Begum MY, Siddiqua A, Sekar M, Subramaniyan V, Wong LS, Mat Rani NNI. Probiotics and Synbiotics: Applications, Benefits, and Mechanisms for the Improvement of Human and Ecological Health. J Multidiscip Healthc. 2025;18:1493–1510. doi: 10.2147/JMDH.S501056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu Z, Zhang B, Chen F, Xia R, Zhu D, Chen B, Lin A, Zheng C, Hou D, Li X, Zhang S, Chen Y, Hou K. Fecal microbiota transplantation reverses insulin resistance in type 2 diabetes: A randomized, controlled, prospective study. Front Cell Infect Microbiol. 2022;12:1089991. doi: 10.3389/fcimb.2022.1089991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yadegar A, Bar-Yoseph H, Monaghan TM, Pakpour S, Severino A, Kuijper EJ, Smits WK, Terveer EM, Neupane S, Nabavi-Rad A, Sadeghi J, Cammarota G, Ianiro G, Nap-Hill E, Leung D, Wong K, Kao D. Fecal microbiota transplantation: current challenges and future landscapes. Clin Microbiol Rev. 2024;37:e0006022. doi: 10.1128/cmr.00060-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Han D, Wu Y, Lu D, Pang J, Hu J, Zhang X, Wang Z, Zhang G, Wang J. Polyphenol-rich diet mediates interplay between macrophage-neutrophil and gut microbiota to alleviate intestinal inflammation. Cell Death Dis. 2023;14:656. doi: 10.1038/s41419-023-06190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liang H, Sathavarodom N, Colmenares C, Gelfond J, Espinoza SE, Ganapathy V, Musi N. Effect of acute TLR4 inhibition on insulin resistance in humans. J Clin Invest. 2022;132:e162291. doi: 10.1172/JCI162291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jayedi A, Aletaha A, Zeraattalab-Motlagh S, Shahinfar H, Mohammadpour S, Mirrafiei A, Jibril AT, Soltani A, Shab-Bidar S. Comparative efficacy and safety of probiotics, prebiotics, and synbiotics for type 2 diabetes management: A systematic review and network meta-analysis. Diabetes Metab Syndr. 2024;18:102923. doi: 10.1016/j.dsx.2023.102923. [DOI] [PubMed] [Google Scholar]
- 172.Cherta-Murillo A, Pugh JE, Alaraj-Alshehhi S, Hajjar D, Chambers ES, Frost GS. The effects of SCFAs on glycemic control in humans: a systematic review and meta-analysis. Am J Clin Nutr. 2022;116:335–361. doi: 10.1093/ajcn/nqac085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wei HX, Wang B, Li B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front Immunol. 2020;11:1315. doi: 10.3389/fimmu.2020.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen J, Lodi R, Zhang S, Su Z, Wu Y, Xia L. The double-edged role of IL-22 in organ fibrosis. Immunopharmacol Immunotoxicol. 2020;42:392–399. doi: 10.1080/08923973.2020.1799388. [DOI] [PubMed] [Google Scholar]
- 175.Morand C. How to better consider and understand interindividual variability in response to polyphenols in clinical trials. Front Nutr. 2024;11:1522516. doi: 10.3389/fnut.2024.1522516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shukla V, Singh S, Verma S, Verma S, Rizvi AA, Abbas M. Targeting the microbiome to improve human health with the approach of personalized medicine: Latest aspects and current updates. Clin Nutr ESPEN. 2024;63:813–820. doi: 10.1016/j.clnesp.2024.08.005. [DOI] [PubMed] [Google Scholar]
- 177.Drucker E, Krapfenbauer K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J. 2013;4:7. doi: 10.1186/1878-5085-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, Mohan V, Balasubramanyam M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem. 2014;388:203–210. doi: 10.1007/s11010-013-1911-4. [DOI] [PubMed] [Google Scholar]
- 179.Yuan JH, Xie QS, Chen GC, Huang CL, Yu T, Chen QK, Li JY. Impaired intestinal barrier function in type 2 diabetic patients measured by serum LPS, Zonulin, and IFABP. J Diabetes Complications. 2021;35:107766. doi: 10.1016/j.jdiacomp.2020.107766. [DOI] [PubMed] [Google Scholar]
- 180.Merrick B, Allen L, Masirah M Zain N, Forbes B, Shawcross DL, Goldenberg SD. Regulation, risk and safety of Faecal Microbiota Transplant. Infect Prev Pract. 2020;2:100069. doi: 10.1016/j.infpip.2020.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Merenstein D, Pot B, Leyer G, Ouwehand AC, Preidis GA, Elkins CA, Hill C, Lewis ZT, Shane AL, Zmora N, Petrova MI, Collado MC, Morelli L, Montoya GA, Szajewska H, Tancredi DJ, Sanders ME. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes. 2023;15:2185034. doi: 10.1080/19490976.2023.2185034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Khalsa J, Duffy LC, Riscuta G, Starke-Reed P, Hubbard VS. Omics for Understanding the Gut-Liver-Microbiome Axis and Precision Medicine. Clin Pharmacol Drug Dev. 2017;6:176–185. doi: 10.1002/cpdd.310. [DOI] [PubMed] [Google Scholar]
- 184.Liu T, Li X, Li H, Qin J, Xu H, Wen J, He Y, Zhang C. Intestinal organoid modeling: bridging the gap from experimental model to clinical translation. Front Oncol. 2024;14:1334631. doi: 10.3389/fonc.2024.1334631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Clemente-Suárez VJ, Martín-Rodríguez A, Curiel-Regueros A, Rubio-Zarapuz A, Tornero-Aguilera JF. Neuro-Nutrition and Exercise Synergy: Exploring the Bioengineering of Cognitive Enhancement and Mental Health Optimization. Bioengineering (Basel) 2025;12:208. doi: 10.3390/bioengineering12020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liang J, Liu B, Dong X, Wang Y, Cai W, Zhang N, Zhang H. Decoding the role of gut microbiota in Alzheimer's pathogenesis and envisioning future therapeutic avenues. Front Neurosci. 2023;17:1242254. doi: 10.3389/fnins.2023.1242254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aderinto N, Olatunji G, Abdulbasit M, Ashinze P, Faturoti O, Ajagbe A, Ukoaka B, Aboderin G. The impact of diabetes in cognitive impairment: A review of current evidence and prospects for future investigations. Medicine (Baltimore) 2023;102:e35557. doi: 10.1097/MD.0000000000035557. [DOI] [PMC free article] [PubMed] [Google Scholar]