Abstract
The mammalian oviduct (also called the fallopian tube) is an essential organ for natural pregnancy. As one of its major functions, the oviduct transports preimplantation embryos to the uterus for implantation. This is a critical process, and abnormalities are responsible for a range of reproductive disorders, such as tubal ectopic pregnancy and infertility, whose etiologies are unclear. For transporting embryos, the oviduct is fundamentally a tubular mechanical pump with motile cilia lining the luminal epithelium and smooth muscle surrounding the mucosa wall. Although bidirectional movement of embryos has been observed during the transport process, how the oviduct produces this type of embryo movement remains unknown. Understanding this pumping mechanism is vital to identifying the functional causes of oviduct-related reproductive disorders, but answering this question requires dynamic imaging of the transport process in its native environment, which is difficult to achieve in mammalian models. Here, we use optical coherence tomography and apply in vivo dynamic 3D imaging of the mouse oviduct to uncover the oviduct pumping mechanism in transporting preimplantation embryos toward pregnancy. By inhibiting the oviduct smooth muscle contraction, we first show that the oviduct muscular activity drives the bidirectional embryo movement. We then present a quantitative assessment of the oviduct contraction wave. This analysis, together with the embryo movement information, indicates that the forward movement of embryos is produced by peristalsis, while the backward embryo movement is generated by a suction process driven by the oviduct relaxation at earlier contraction sites, showing a leaky peristaltic pump. Finally, we reveal how the net displacement of embryos is created under this pumping mechanism, which effectively transports embryos toward the uterus. This work elucidates, for the first time, the oviduct pumping mechanism in transporting preimplantation embryos, paving the way for understanding the biomechanics of the mammalian oviduct.
1. Introduction
In mammals, the oviduct (also called fallopian tube) plays an essential role in achieving natural pregnancy. It transports gametes, hosts fertilization, and transports preimplantation embryos to the uterus for implantation [1–3]. The function of the oviduct in transporting embryos is closely linked to the disorder of embryo retention inside the oviduct [4], which is a prerequisite of tubal ectopic pregnancy [5]. It is known that the motile cilia and smooth muscle of the oviduct enable its transport function [6,7]; however, their specific roles in orchestrating a successful transport process remain to be fully elucidated [8,9]. This is the primary reason underlying the unknown functional cause of tubal ectopic pregnancy that affects nearly 2% of all pregnancies and is life threatening [10]. A fundamental understanding of how the oviduct transports preimplantation embryos is key to improving the prediction, prevention, and early diagnosis of oviduct-related reproductive disorders.
In terms of its transport function, the oviduct can be considered a tubular mechanical pump equipped with forces provided by ciliary beating and muscular activities [11]. To determine the pumping mechanism, analyzing the temporal relationship between these dynamic forces and the embryo movement is indispensable [12]. Previous studies revealed bidirectional movement of embryos in the mouse oviduct [13,14], but the dynamics of the oviduct were largely missing from these analyses, and an assessment of its relationship with the embryo movement has not been reported. As a result, little is known about how this tubular mechanical pump works to transport embryos [11]; in particular, how the bidirectional embryo movement is produced by the oviduct remains unknown. Given the dynamic nature of the transport process as well as the complex mechanical environment in the oviduct, answering this question requires quantitative assessment of the oviduct dynamics in vivo in the mammalian model [15], which is difficult to achieve. This has acted as a technical hurdle for mechanistic studies.
In vivo high-resolution imaging of the mouse oviduct is possible through several approaches [16–19]. Among these, using optical coherence tomography (OCT) with an intravital window provides dynamic 3D imaging of the mouse oviduct [15], allowing for quantitative analysis. As a label-free imaging modality, OCT relies on the backscattering of light from tissue for imaging contrast and employs low-coherence interferometry to resolve depth [20]. In general, OCT has a microscale spatial resolution and a millimeter-level imaging depth, ideal for covering the entire tubular structure of the mouse oviduct and imaging the preimplantation embryos inside. With laser scanning, OCT can reach a hertz-level volume rate with decent spatial sampling, sufficient to capture both the oviduct dynamics and the embryo movement in 3D. Over the past decade, a series of OCT-based imaging methods have been developed for in vivo quantitative assessment of the mouse oviduct and the reproductive events in it [13,14,21–25]. This includes the detailed 3D morphology of mucosa folds [25], the cilia beat frequency and its metachronal wave [24,26], the smooth muscle contraction and its wave propagation [23], the velocimetry of embryos [14], the stage of preimplantation development [22], the dynamic movement pattern of eggs and embryos [13], and the sperm behaviors at the native site of fertilization [21]. These works have led to an exciting time of understanding the specific roles of the mammalian oviduct in fertilization and pregnancy, both dynamically and in vivo, which has broad and significant implications for female reproductive health and fertility treatment.
Here, we applied the OCT-based in vivo imaging methods in the mouse model to uncover the oviduct pumping mechanism in transporting preimplantation embryos toward the uterus. Our experiments revealed that the oviduct muscular activity drives the embryo movement in the oviduct isthmus, the longest portion of the oviduct hosting preimplantation development. Furthermore, our observations and analyses indicate a leaky peristaltic pumping process, where the peristalsis creates the forward embryo movement, while the suction caused by the relaxation of earlier contraction sites generates the backward embryo movement. We further characterized the net displacement of embryos with repeated imaging sessions and showed an unexpected role of the luminal constriction at the oviduct turning points in producing the effective embryo transport toward the uterus over a prolonged period. This work provides the first biomechanical understanding of how the mammalian oviduct transports preimplantation embryos and paves the way for elucidating the functional cause of embryo retention inside the oviduct in the female reproductive disorders.
2. Materials and methods
2.1. OCT system
A lab-built spectral domain OCT system was used in this study. The details of the system were described in our previous work [14]. With a central wavelength of 852 nm, a full bandwidth of 146 nm (a -3 dB bandwidth of 96 nm), and 2048 pixels in the spectrometer, the OCT system has an axial resolution of ∼6 µm with a depth range of ∼2.5 mm, both in air. The difference between the measured axial resolution and the theoretical one is primarily due to the dispersion mismatch between the reference and sample arms and the non-Gaussian shape of the spectrum. The transverse resolution of the system is ∼9 µm with a galvanometer-mirror-based 2D laser scanning that provides a large transverse field of view on the scale of millimeters. The system was operated at an A-scan rate of 100 kHz. For 2D imaging of the oviduct cilia beat frequency, the B-scan rate was set as 100 Hz. For acquiring an individual volume that has a large transverse field of view of the oviduct, the data acquisition time for the volume was set as 18 seconds. For timelapse 3D imaging of the embryo transport process in the oviduct, the volume rate was set as 2 Hz, with a B-scan rate of 250 Hz.
2.2. In vivo imaging setup
All animal procedures were approved by the Institutional Animal Care and Use Committee at Stevens Institute of Technology, and all the experiments followed the approved guidelines and regulations.
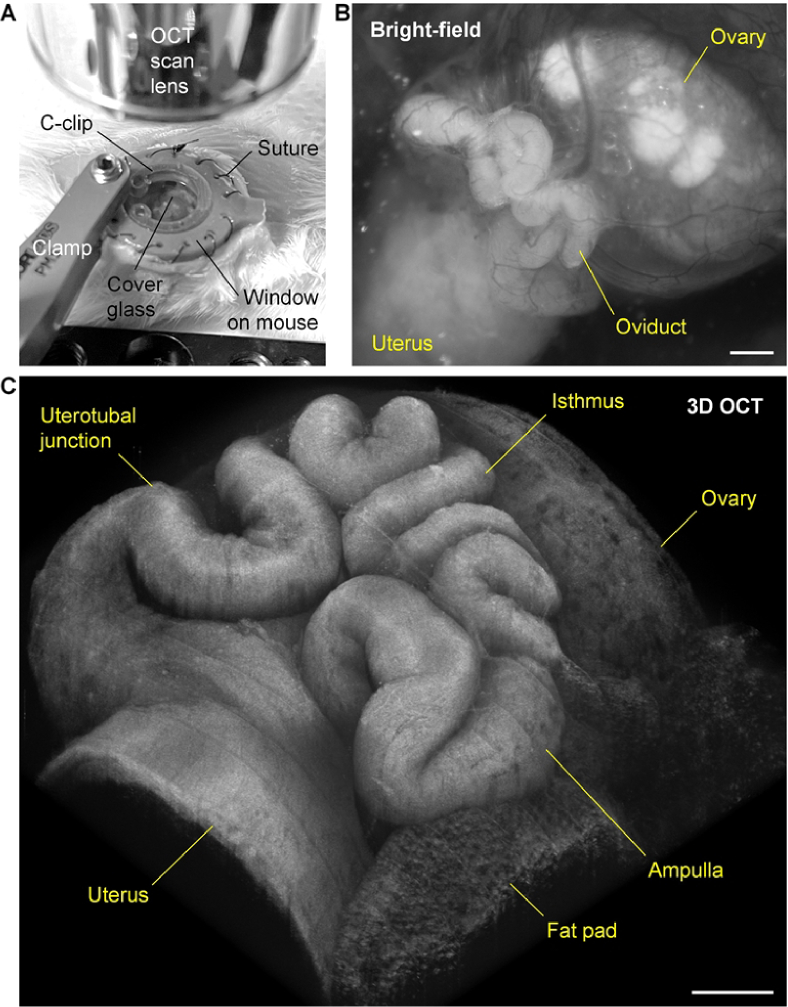
In vivo OCT imaging of the mouse oviduct relied on a dorsal intravital window to bypass the skin and muscle layers [13]. The window was 3D printed with grey resin using the Form 3 printer (Formlabs, USA). Implantation of the window to the mouse was performed through surgery. Briefly, with the mouse under isoflurane anesthesia and placed on a 37 °C platform, a circular piece of skin on the right dorsal side was replaced by the window frame via suturing. Through a tiny incision on the muscle layer, the reproductive organs, including the ovary, the oviduct, and a portion of the uterus were gently pulled out of the body cavity. Tissue adhesive was used to attach the fat pad of the ovary to the tissue holders of the window in the way that the oviduct faced up for imaging. The window was then filled with 1X PBS at 37 °C, and a piece of circular cover glass was used to close the window, which could be secured by a C-clip that was also 3D printed. During imaging, the mouse was kept under anesthesia and on a 37 °C platform, and a stabilized clamp was used to hold the window frame to minimize motion effects from the mouse breathing. This in vivo imaging setup is shown in Fig. 1(A). The intravital window provides a clear in vivo optical access to the oviduct (Fig. 1(B)), and by using OCT, the major portions of the mouse oviduct, including the ampulla (where fertilization occurs), the isthmus (which hosts the preimplantation development), and the uterotubal junction can all be imaged in 3D (Fig. 1(C)).
Fig. 1.
In vivo 3D imaging of the mouse oviduct using OCT through an intravital window. (A) In vivo imaging setup with a clamp stabilizing the intravital window implanted on the right dorsal side of the mouse. (B) In vivo bright-field image of the oviduct as well as the ovary and a portion of the uterus through the intravital window. (C) In vivo 3D OCT image of the oviduct through the intravital window showing its 3D morphology and structure. Scale bars are 500 µm.
2.3. In vivo experiments
All experiments from this study were performed in vivo with adult female mice of CD-1 strain (Strain Code 022, Charles River Laboratories, USA). Male adult mice of the same strain were used for mating. Our results were obtained from a total of 12 female mice that were imaged in vivo.
Timed matings were set up, and vaginal plugs were checked daily in the morning, with the day of finding a plug counted as 0.5 days post coitum (dpc). For studying the oviduct pumping mechanism in transporting preimplantation embryos, the implantation of intravital window and in vivo imaging were conducted at 1.5 dpc, when fertilization was complete and preimplantation embryos at two-cell or four-cell stage were expected in the oviduct isthmus during the transport toward the uterus for implantation. This expectation is based on previous studies [15,22].
Prifinium bromide, or 3-(diphenylmethylene)-1,1-diethyl-2-methylpyrrolidinium bromide, an anticholinergic drug, which has been shown to inhibit the mouse oviduct contraction ex vivo [27], was utilized in this study for in vivo inhibition of the oviduct muscular activity. Prifinium bromide was purchased in solid form (MilliporeSigma, USA), and its solution at 2 mg/ml in 1X PBS at 37 °C was used to fill the implanted intravital window as a topical treatment for the oviduct. This treatment was conducted by temporarily opening the cover glass on the window, removing the existing 1X PBS from the window, and replacing it with the prifinium bromide solution.
We performed a number of in vivo imaging experiments, including 1) determining the effect of prifinium bromide on the oviduct muscular contraction, ciliary beating, and the movement of embryos, 2) defining the pumping mechanism by analyzing the propagation of contraction waves and its temporal relationship with the embryo movement, and 3) assessing the way that the oviduct generates the net displacement of embryos toward the uterus with the bidirectional embryo movement. Our results from these experiments explicitly answered the question of how the mammalian oviduct produces a timed transport of preimplantation embryos in the isthmus for successful pregnancy.
2.4. Image-based measurement and analysis
Visualizations of 3D and 4D (3D + time) OCT data were performed with Open Chrono-Morph Viewer (OCMV) [28] and Imaris (Oxford Instruments, United Kingdom). The clipping spline function in OCMV [29] was employed to reveal the coiled mouse oviduct in a single view when needed, and the spot function in Imaris was used to label the embryos as necessary.
The oviduct cilia beat frequency was measured based on 2D OCT images taken in vivo over time, which had a sampling frequency of 100 Hz and a duration of 1.28 seconds. Details of the method can be found in the previous work [24]. Briefly, the averaged 2D structure image over time was used for a binary mask to eliminate the background in the image, and the dB intensity from each pixel over time was analyzed via a fast Fourier transform in MATLAB (MathWorks, USA). The amplitude of the dominant peak in the frequency domain was used to determine the location of motile cilia, and the corresponding frequency of the peak was extracted as the cilia beat frequency. The frequency values of ciliary beating at similar locations of the oviduct before and after the prifinium bromide treatment were first compared within each mouse, with a total of five mice. The median of the data was then selected to represent each mouse, due to the non-Gaussian data distribution (confirmed by Kolmogorov-Smirnov test). A statistical comparison before and after the prifinium bromide treatment across all the five mice was performed with Wilcoxon signed-rank test (non-Gaussian data distribution under each condition confirmed by Kolmogorov-Smirnov test).
The dynamics of the oviduct lumen was assessed with 4D OCT data. Cross-sectional images of the oviduct perpendicular to the tubular axis were saved, and Fiji [30] was used to manually measure the luminal area over time. A three-point moving-average filter was utilized to smooth the temporal profile of the luminal area, and the gradient at each time point (approximated as the first-order derivative) was calculated to show the area changing rate, both of which were performed in MATLAB (MathWorks, USA). To further reveal the contraction wave, the temporal profiles were normalized for the start around 0 and the minimum at -1. The oviduct contraction wave speed was estimated through manually measuring the distance between two locations along the oviduct tube and obtaining the time difference for the peak area changing rate during contraction between the two locations. Characterizations of contraction wave speed and peak area changing rate were performed on four mice with the speed measurement from at least two oviduct locations and the measurement of area changing rate from at least four oviduct locations, covering the ampulla and the isthmus in both characterizations. The direction of the embryo movement was manually determined by inspecting the 4D OCT data.
3. Results
3.1. Oviduct muscular activity drives preimplantation embryo movement
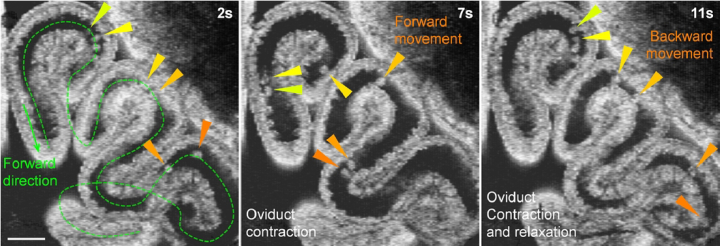
Timelapse in vivo 3D imaging of the mouse oviduct at 1.5 dpc shows bidirectional movement of preimplantation embryos in the oviduct isthmus (Fig. 2 and Visualization 1 (5.5MB, mov) ). Specifically, embryos in all parts of the isthmus have the same bidirectional movement pattern, though not fully synchronized in time ( Visualization 1 (5.5MB, mov) ). Such movements take place while the contraction wave propagates and the relaxation occurs in the oviduct (Fig. 2). It is known that there are few motile cilia covering the luminal epithelium of the isthmus, representing a significantly reduced cilia coverage compared with the oviduct ampulla [24]. As such, it has been speculated that the muscular activity, rather than the beating of cilia, drives the bidirectional embryo movement in the isthmus [13]; however, this hypothesis has not been directly tested, and in theory, there is a possibility that the ciliary beating in the ampulla remotely affects the fluid flow in the isthmus. Therefore, we performed a targeted pharmaceutical inhibition of the oviduct muscular activity to determine its role in driving the embryo movement.
Fig. 2.
In vivo 3D OCT imaging of the preimplantation embryo movement and the oviduct contraction and relaxation. The embryo movement is bidirectional in all regions of the oviduct isthmus and coincides with the oviduct muscular activity shown as contraction and relaxation ( Visualization 1 (5.5MB, mov) ). Triangles of the same color point at the same embryo at different time points. Scale bar is 300 µm.
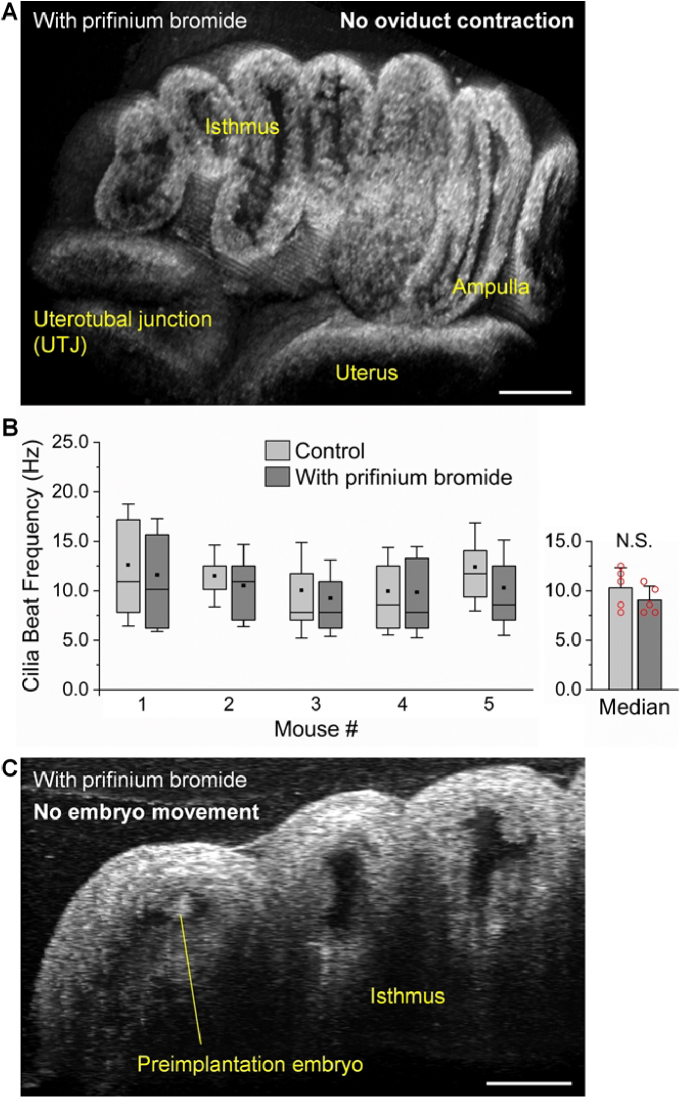
Topical administration of 2 mg/ml prifinium bromide to the mouse oviduct in vivo rapidly inhibits the muscular activity within 10 minutes, completely silencing the oviduct contraction in both the isthmus and the ampulla (Fig. 3(A) and Visualization 2 (19.5MB, mov) ). Notably, this treatment does not affect the ciliary beating, and our measurements show similar values of cilia beat frequency before and after the treatment in vivo from selected locations in the ampulla (Fig. 3(B)). With the muscular activity inhibited but the ciliary beating remaining normal, our results show that the embryos in the isthmus become stationary relative to the oviduct, with no movement observed, even at the microscale (Fig. 3(C) and Visualization 3 (12.8MB, mov) ). This indicates that the muscular activity, not the ciliary beating, drives the bidirectional embryo movement in the oviduct isthmus.
Fig. 3.
The movement of preimplantation embryos in the oviduct isthmus is driven by the oviduct muscular activity. (A) No oviduct contraction in vivo after topical administration of prifinium bromide to the oviduct, inhibiting the oviduct muscular activity ( Visualization 2 (19.5MB, mov) ). (B) The oviduct motile cilia remain beating at the similar rates in vivo after topical administration of prifinium bromide to the oviduct. Box plots with square dots for the mean and whiskers for the standard deviation. Bar plot shows the mean and standard deviation with data points representing the median value from each mouse. N.S. indicates not significant with p = 0.125 from Wilcoxon signed-rank test. Legend and vertical axis label apply to both box and bar plots. (C) There is no movement of preimplantation embryos in vivo after topical administration of prifinium bromide to the oviduct ( Visualization 3 (12.8MB, mov) ). Scale bars are 400 µm in (A) and 200 µm in (C).
3.2. Spatiotemporal characterization of oviduct contraction wave propagation
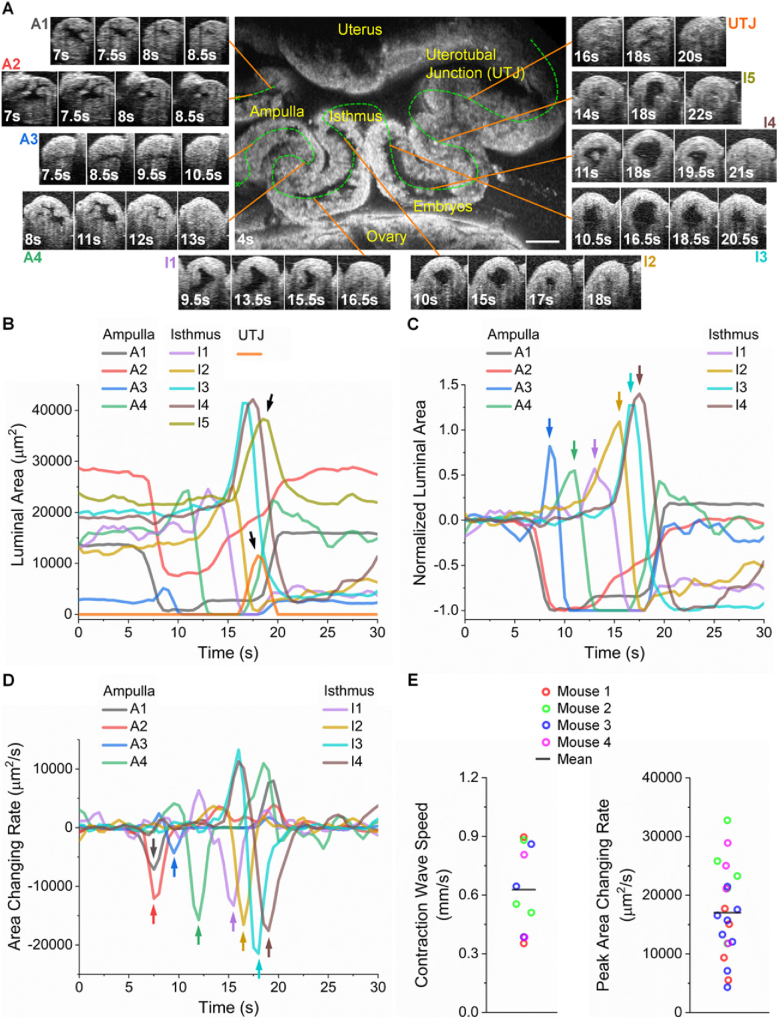
Detailed 3D dynamics of the oviduct contraction wave propagation in vivo are shown in Fig. 4(A) and Visualization 4 (24MB, mov) . The contraction wave initiates in the ampulla, with positions A1 and A2 contracting nearly synchronously. This contraction then propagates to positions A3 and A4, entering the isthmus, and stops in the lower isthmus, as positions I5 and UTJ only show luminal expansions with no contractions observed. This is clearly seen from the temporal profiles of the luminal areas from all selected locations in Fig. 4(B), where arrows point at the expansion only, without a contraction prior to it. These area measurements also show that the lumens along the oviduct are of a range of sizes and that the contractions at certain positions in both the ampulla and isthmus do not generate a completely closed lumen.
Fig. 4.
In vivo 3D dynamic imaging and quantitative assessment of the oviduct contraction wave propagation from the ampulla to the isthmus. (A) Cross-sectional images from selected locations of the ampulla, isthmus, and uterotubal junction (UTJ) showing the contraction process over time at different locations along the oviduct ( Visualization 4 (24MB, mov) ). (B) Plot of the luminal areas over time from all the locations in the ampulla, isthmus, and UTJ. Arrows point at the luminal expansion without a prior contraction. (C) Plot of the normalized luminal areas over time from the locations in the ampulla and isthmus. The normalization is for the curves to start around zero and have the minimum at negative one. Color arrows point at the luminal expansion right before contractions. Such luminal expansions do not exist at the contraction initiation sites. (D) Plot of the luminal area changing rate from the locations in the ampulla and isthmus. Color arrows point at the peak of changing rates during contractions. Locations I5 and UTJ are not included in (C) and (D) due to no contractions. (E) Measurements of the oviduct contraction wave propagation speed and the peak luminal area changing rate from four mice. The data points for each mouse represent different measurement locations on the oviduct. Scale bar is 300 µm, and all the cross-sectional timelapse images of the oviduct lumen share the same sale as the center image in (A).
With the normalized temporal profiles of the luminal area, it can be seen from Fig. 4(C) that there exist clear time delays of the contraction processes from A1&A2 to A4, to I1 and to I4, indicating the propagation of the contraction wave. Unlike the contraction, the relaxations from different locations of the oviduct do not follow a clear temporal order, with some positions even presenting a two-phase relaxation process (e.g., position A1). Furthermore, the relaxation from earlier contraction sites takes place while the contraction wave propagates. Interestingly, except for the wave initiation positions (A1 and A2), the other positions (A3–I4) all experience luminal expansions prior to their contractions (color arrows in Fig. 4(C)). This is due to the expected high viscosity of the oviduct fluid at this 1.5 dpc stage [31] and the oviduct wall being elastic so that the temporary fluid accumulation caused by the nearby contractions enlarges the lumen.
The plot of the luminal area changing rate (Fig. 4(D)) highlights the contraction process with the negative peak (color arrows), which were used as a measure of the oviduct contraction, and the temporal locations of these peaks were taken to quantify the wave speed. These parameters span a large range (Fig. 4(E)), indicating the heterogeneity of oviduct contraction across different mice as well as at different oviduct locations of the same mouse. Furthermore, the ampulla and the isthmus do not have the same contraction parameters, and notably, although the isthmus is known to have a thicker smooth muscle layer than the ampulla [23,24], the peak area changing rate or the contraction wave propagation speed is not always higher in the isthmus.
3.3. Oviduct is a leaky peristaltic pump in transporting preimplantation embryo
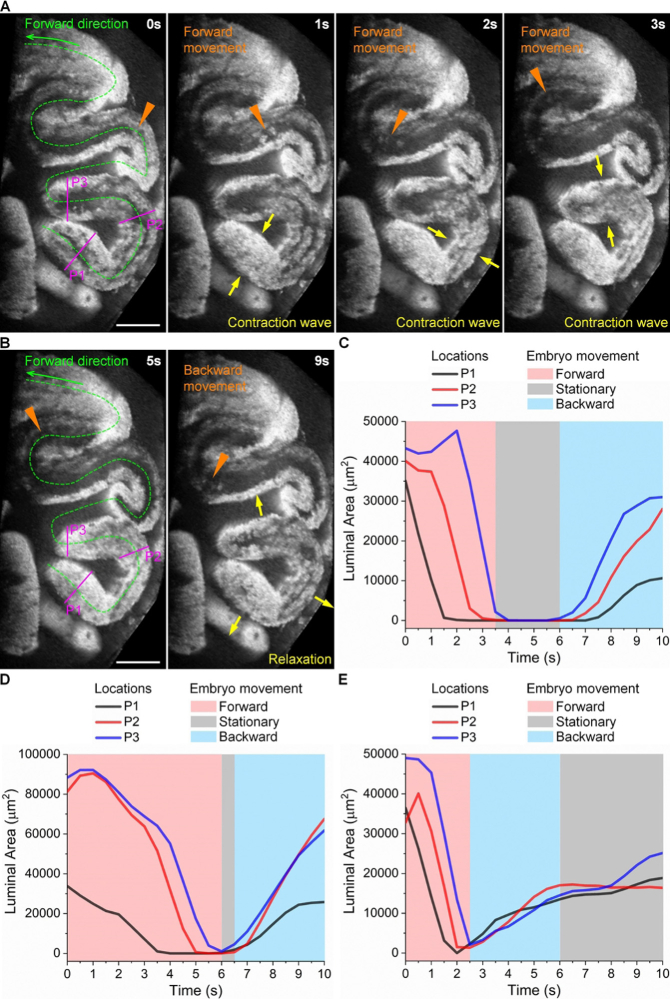
With the oviduct contraction wave propagation from the ampulla to the isthmus, it was expected that the forward embryo movement (toward the uterus) would be produced by peristalsis. Our analysis of the temporal relationship between the oviduct dynamics and the embryo movement clearly revealed this process and confirmed the peristaltic pumping (Fig. 5(A) and Visualization 5 (2.4MB, mov) ). In contrast, how the oviduct generates the backward embryo movement was not apparent, since a typical peristalsis does not produce backward fluid flows. From our temporal analysis, we found that such a backward embryo movement takes place during the oviduct relaxation at earlier contraction sites (Fig. 5(B) and Visualization 5 (2.4MB, mov) ), indicating that the relaxation creates a negative pressure gradient pulling embryos back via a suction process. This is made possible by the fact that the oviduct lumen does not always maintain a completely closed point during the propagation of a contraction wave, which is different from a technical peristaltic pump, thus providing the opportunity for the suction process to happen. Therefore, we named this a leaky peristaltic pump. This pumping process can be directly seen from the plot of the luminal areas over time on top of the embryo movement direction (Fig. 5(C)), which shows that the backward embryo movement occurs right after the relaxations initiate. In Fig. 5(D) and Fig. 5(E), we show such plots from another two mice, where the oviduct contraction dynamics are not the same as the one in Fig. 5(C) in terms of the transition from the contraction to relaxation. Specifically, the contraction-to-relaxation transition is faster in Fig. 5(D) than that in Fig. 5(C), leading to a shorter period of embryos being stationary, and the transition shown in Fig. 5(E) is instantaneous, with no stationary period of embryos. Despite such differences in the oviduct contraction dynamics, the backward movement of embryos takes place right after the relaxation initiates (Fig. 5(D) and Fig. 5(E)). This is consistent with our observations from Fig. 5(C). These further demonstrate the suction process and indicate the leaky peristaltic pump.
Fig. 5.
The forward embryo movement is driven by the oviduct contraction wave, while the backward movement of embryos is driven by the oviduct relaxation ( Visualization 5 (2.4MB, mov) ). (A) Images of the oviduct contraction wave propagation and the forward embryo movement show a peristaltic pumping process. (B) Images of the oviduct relaxation and the backward movement of embryos show a suction process, indicating a leaky peristaltic pump. Triangles point at the embryo location, and arrows show contraction or relaxation. (C) Plot of the luminal areas over time at three locations (P1–P3) overlapping with the embryo movement direction for data in (A) and (B). (D, E) Plots of the luminal areas over time together with embryo movement direction from two additional mice showing the leaky peristaltic pump. Scale bars are 400 µm.
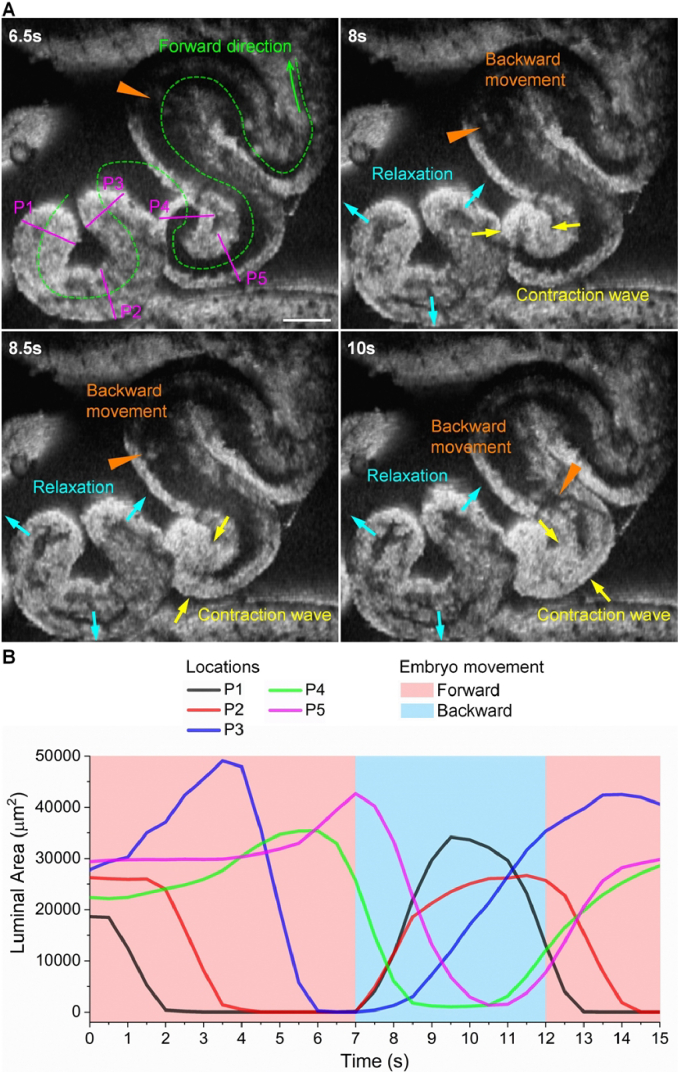
Furthermore, we show that the suction process can occur at the same time as the contraction wave propagating right in front of the backward embryo movement (Fig. 6(A) and Visualization 6 (7.3MB, mov) ). This suggests that the relaxation of oviduct can generate a higher pressure gradient than the contraction wave, overcoming the peristalsis to produce the backward embryo movement. This process can be clearly seen from the plot of the temporal luminal areas from the five locations overlapping the embryo moving direction (Fig. 6(B)).
Fig. 6.
The backward embryo movement driven by the relaxation of the oviduct at earlier contraction sites can take place while the contraction wave propagation continues right in front of the moving embryos in the backward direction. (A) Images of the backward embryo movement with the oviduct relaxation from earlier contraction sites while the contraction wave continues propagating against the embryo movement ( Visualization 6 (7.3MB, mov) ). Triangles point at the embryo locations, yellow arrows show the contraction wave, and cyan arrows show the relaxation. (B) Plot of the luminal areas over time from locations P1–P5 in (A) overlapping with the embryo movement direction. Scale bar is 300 µm.
3.4. Luminal constriction at the oviduct turning points enables net displacement of embryo
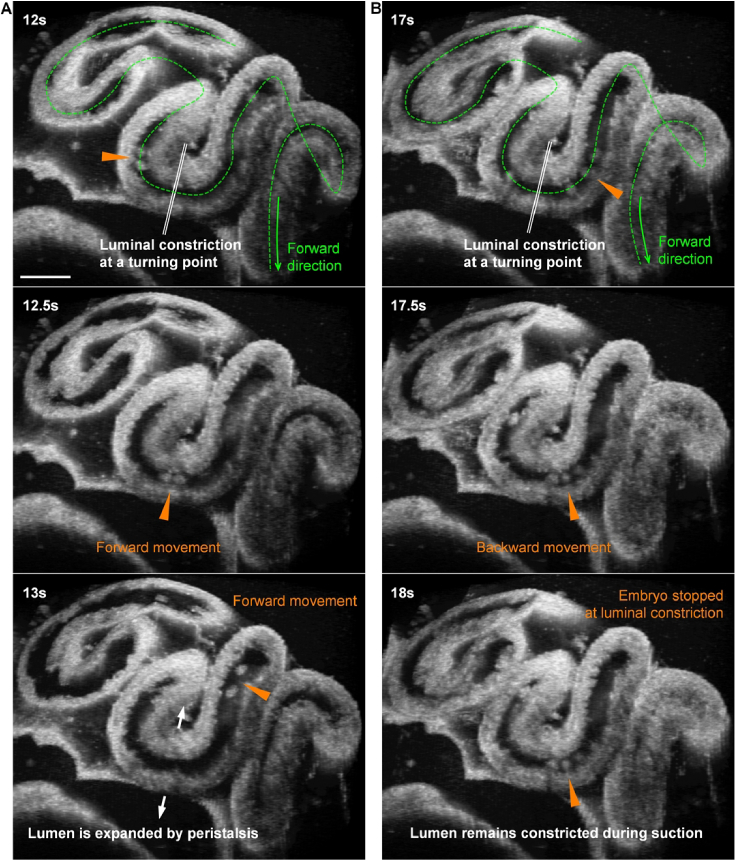
In our pumping mechanism analysis, and from the selected cycles of bidirectional movements, the leaky peristaltic pumping process itself did not appear to generate the net displacement of embryos toward the uterus. Thus, following the pumping analysis, we assessed how the oviduct, under this particular pumping mechanism, effectively transports embryos forward over a longer period of time. By examining continuous cycles of the embryo movement in prolonged imaging sessions, we found that the constricted lumens at the oviduct turning points can act as stoppers at times, enabling the net displacement of embryos in the forward direction. Specifically, in the forward movement, because of the peristalsis and the luminal expansion prior to the contraction (Fig. 4(C)), the constricted lumen at the turning point is expanded, allowing the embryo to freely pass through (Fig. 7(A)); in contrast, in the backward movement, the suction process does not expand the constricted lumen at the turning point, and the moving embryos can thus be stopped by the constricted lumen (Fig. 7(B)), leading to a shorter distance of the backward movement in comparison to the forward one, which produces the net forward displacement of embryos. This entire process can be clearly seen from Visualization 7 (8.9MB, mov) .
Fig. 7.
Dynamics of the luminal constriction at an oviduct turning point during the forward and backward movements of preimplantation embryos ( Visualization 7 (8.9MB, mov) ). (A) In the forward embryo movement driven by peristalsis, the constricted lumen at the turning point is expanded, allowing the embryo to easily pass through. (B) In the backward embryo movement driven by a suction process, the constricted lumen at the turning point remains constricted and can stop the embryo from moving through. Triangles point at the embryo locations. Scale bars are 300 µm.
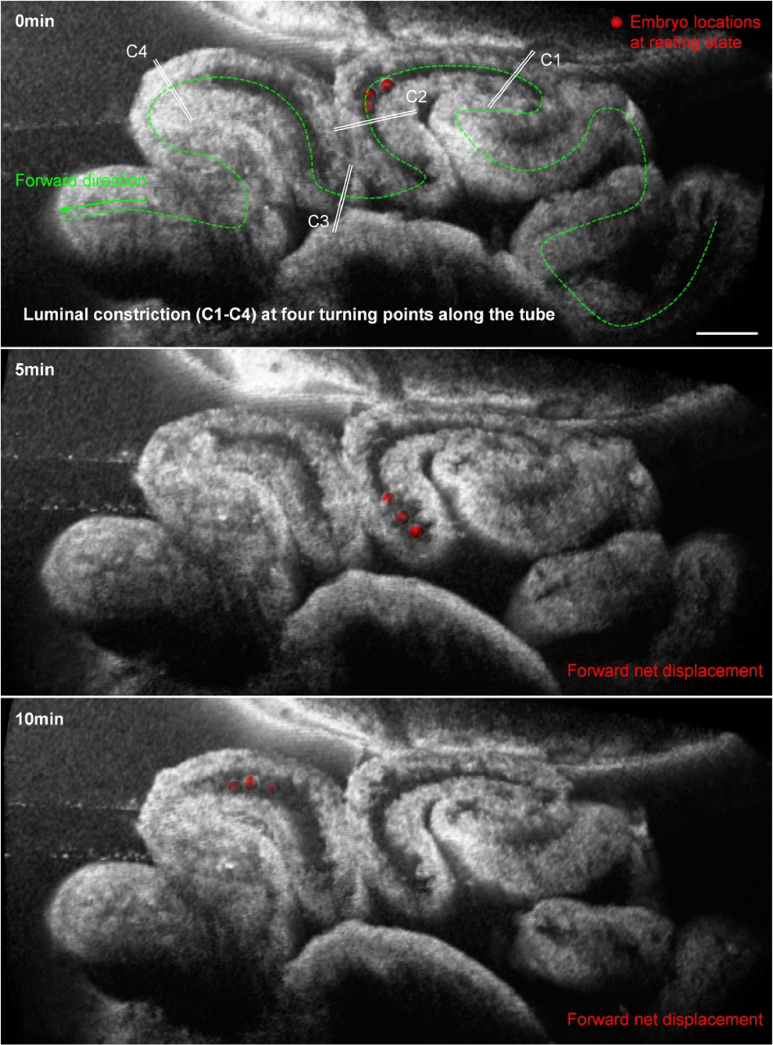
With this mechanism, the oviduct isthmus can be divided into sections based on the turning points. While embryos can move across several sections in a single cycle of their bidirectional movement, their oscillation tends to center around one particular location, which we termed the embryo location at the resting state. Through repeated imaging, we found that such locations at the resting state can shift over adjacent sections in the forward direction over minutes (Fig. 8), which represents the forward net displacement of embryos toward the uterus.
Fig. 8.
The net displacement of embryos in the forward direction over time. Images of the preimplantation embryos (labeled as red spheres) and the luminal constrictions (C1–C4) at the oviduct turning points showing the net forward displacement of embryos relative to the turning points over 10 minutes. Scale bar is 300 µm.
4. Discussion
This work is a progression from technical advancements to biological insights. Specifically, we applied the methodological innovations in optical imaging of the mouse oviduct to uncover the oviduct pumping mechanism in transporting preimplantation embryos toward pregnancy. This mechanistic insight into the working principle of the oviduct as a mechanical pump is a critical understanding that has broad implications. In terms of basic knowledge, it answers fundamental questions on the organ-level biomechanics of the mammalian oviduct: how it works to transport embryos, what the driving force is, why the movement of embryos is bidirectional, and how it produces the net forward displacement of embryos with this pattern of movement. With respect to clinical significance, it points to the direction for identifying the functional cause of embryo retention inside the oviduct, which is imperative for improving the clinical management of tubal ectopic pregnancy and is also a major step forward in unveiling the etiology of oviduct-related infertility.
Whether it is the ciliary beating or the muscular activity that drives the oviduct transport of eggs and embryos remains debatable [8], with growing evidence on both sides [6,7,32–37]. In our view, and based on our in vivo observations from the mouse model, the roles of cilia and muscle are spatiotemporally dependent [15], closely linked to the location of eggs/embryos in the oviduct and the stage of the transport process. For embryos in the oviduct isthmus, this work explicitly shows that the muscular activity is the driver of the transport process. This is not only demonstrated by the inhibition of smooth muscle contraction while preserving normal beating of motile cilia but is also indicated from the analysis of the temporal relationship between the oviduct contraction/relaxation and the embryo movement. Based on these results, we conclude that the oviduct muscular activity is responsible for the embryo transport process in the isthmus. This conclusion is also supported by the recent finding in the mouse model that motile cilia are essential for picking up oocytes upon ovulation but are not required for transporting sperm and preimplantation embryos [7]. It is worth noting that this conclusion specifically applies to the oviduct transport of embryos inside the isthmus and does not contradict the discoveries of other studies on the essential roles of motile cilia in enabling the oviduct transport functions in other regions of the oviduct.
The oviduct contraction is a result of its muscular activity that is initiated and propagated by the oviduct pacemaker cells, the interstitial cells of Cajal, distributed along the oviduct [38]. Regarding the contraction wave propagation, our observations also indicate that the contraction waves initiated in the ampulla do not always propagate throughout the entire oviduct. From an analysis over selected time periods across three mice, the contraction waves propagating only halfway occurred nearly twice as often as those propagating over the full length of the oviduct (7 versus 4 occurrences, 13 versus 7, and 5 versus 3). Understanding this pattern of contraction wave propagation requires further in vivo imaging of the conduction wave in the mouse oviduct (similar to that for the tubular embryonic heart [39]), for which the imaging technique remains to be established. In our observations, the contraction wave propagation direction at 1.5 dpc is mostly ovary-to-uterus; nevertheless, we have also seen, rarely, contraction wave propagations in the uterus-to-ovary direction in the lower isthmus. The role of this type of wave propagation is a focus of our current work, and we speculate that it functions to regulate the embryo transport in the uterotubal junction.
Our analyses show that the contraction wave propagation drives forward embryo movement through peristalsis, while the relaxation at earlier contraction sites generates backward embryo movement through suction. This functions as a leaky peristaltic pump producing bidirectional movement of embryos in the isthmus. Our results further suggest that the net displacement of embryos toward the uterus is enabled by the mechanistic difference between the peristalsis and the suction in combination with the luminal constriction at the oviduct turning points. Here, we provide a qualitative explanation of this. Considering pumping fluid in a tube, peristalsis has the driving pressure acting on the back of the flow, but suction has the driving pressure acting at the front of the flow. Because the oviduct fluid is viscous [31], the fluid displacement is not instantaneous. In the case of pressure on the back of the flow, fluid accumulation occurs while the pressure propagates, which expands the constricted lumen, allowing embryos freely passing through. However, with the pressure at the front of the flow, the fluid is constantly removed from the front of the flow to the relaxing part of the oviduct, which is then replaced by the fluid behind it. With this pressure propagating against the flow, the fluid only accumulates into the relaxing region of the oviduct, and the other parts of the oviduct do not have fluid accumulation. Thus, the constricted lumen at the oviduct turning point remains constricted, which can prevent embryos directly passing through during their backward movement. It is worth noting that this stopping of the backward movement occurs occasionally, not in every cycle of the bidirectional movement, since both the embryo and the oviduct wall are elastic and the embryo can squeeze through the constricted lumen. This leads to the net displacement of embryos taking place on a longer time scale, on the level of minutes, than the period of the bidirectional movement, which is on the level of seconds.
During the transport process in the isthmus, embryos undergo their critical preimplantation development and become ready for their implantation in the uterus [40]. In terms of delivering embryos to the uterus. the uncovered leaky peristaltic pumping process with the bidirectional embryo movement is highly inefficient; however, this particular way of pumping and transport provides time for the developmental process, enabling successful pregnancy. In addition to this benefit on the temporal aspect, the pumping dynamics with the oscillation of embryos is thought to play an important role in generating mechanical cues that support or even regulate cellular activities during the preimplantation development, which we will study in our future work.
In summary, we applied OCT-based in vivo imaging to study the oviduct transport function in the mouse model, and we uncovered the pumping mechanism of the oviduct in transporting preimplantation embryos toward the uterus. Specifically, we determined the indispensable role of the oviduct muscular activity in driving the embryo movement in the isthmus; our analyses indicate a leaky peristaltic pumping process that provides the first biomechanical explanation as to how the oviduct produces bidirectional embryo movement; and our imaging results further reveal how the oviduct achieves the net displacement of embryos in the forward direction under such pumping dynamics. This work opens the avenue for identifying the functional causes of the oviduct-related reproductive disorders.
Supplemental information
Funding
National Institutes of Health 10.13039/100000002 ( R35GM142953).
Disclosures
The authors declare no conflicts of interest.
Data availability
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the authors upon reasonable request.
References
- 1.Avilés M., Coy P., Rizos D., “The oviduct: A key organ for the success of early reproductive events,” Animal Frontiers 5(1), 25–31 (2015). 10.2527/af.2015-0005 [DOI] [Google Scholar]
- 2.Li S., Winuthayanon W., “Oviduct: roles in fertilization and early embryo development,” J. Endocrinol. 232(1), R1–R26 (2017). 10.1530/JOE-16-0302 [DOI] [PubMed] [Google Scholar]
- 3.Pérez-Cerezales S., Ramos-Ibeas P., Acuña O. S., et al. , “The oviduct: from sperm selection to the epigenetic landscape of the embryo†,” Biol. Reprod. 98(3), 262–276 (2018). 10.1093/biolre/iox173 [DOI] [PubMed] [Google Scholar]
- 4.Croxatto H. B., “Physiology of gamete and embryo transport through the Fallopian tube*,” Reprod. BioMed. Online 4(2), 160–169 (2002). 10.1016/S1472-6483(10)61935-9 [DOI] [PubMed] [Google Scholar]
- 5.Shaw J. L. V., Dey S. K., Critchley H. O. D., et al. , “Current knowledge of the aetiology of human tubal ectopic pregnancy,” Hum. Reprod. Update 16(4), 432–444 (2010). 10.1093/humupd/dmp057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi E., Sun Y., Almansa-Ordonez A., et al. , “Control of oviductal fluid flow by the G-protein coupled receptor Adgrd1 is essential for murine embryo transit,” Nat. Commun. 12(1), 1251 (2021). 10.1038/s41467-021-21512-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan S., Wang Z., Peng H., et al. , “Oviductal motile cilia are essential for oocyte pickup but dispensable for sperm and embryo transport,” Proc. Natl. Acad. Sci. U.S.A. 118(22), e2102940118 (2021). 10.1073/pnas.2102940118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward S. M., Hwang S. J., Yan W., et al. , “Intrinsic pacemaker activity and propulsive forces provided by the myosalpinx are necessary for egg and embryo transport in the oviduct,” Biol. Reprod. 106(1), 4–5 (2022). 10.1093/biolre/ioab209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezzati M., Djahanbakhch O., Arian S., et al. , “Tubal transport of gametes and embryos: a review of physiology and pathophysiology,” J Assist Reprod Genet 31(10), 1337–1347 (2014). 10.1007/s10815-014-0309-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanitha N. S., Duncan W. C., Emma K., et al. , “Diagnosis and management of ectopic pregnancy,” J Fam Plann Reprod Health Care 37(4), 231–240 (2011). 10.1136/jfprhc-2011-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elad D., Jaffa A. J., Grisaru D., “Biomechanics of Early Life in the Female Reproductive Tract,” Physiology 35(2), 134–143 (2020). 10.1152/physiol.00028.2019 [DOI] [PubMed] [Google Scholar]
- 12.Seraj H., Nazari M. A., Atai A. A., et al. , “A Review: Biomechanical Aspects of the Fallopian Tube Relevant to its Function in Fertility,” Reprod. Sci. 31(6), 1456–1485 (2024). 10.1007/s43032-024-01479-x [DOI] [PubMed] [Google Scholar]
- 13.Wang S., Larina I. V., “In vivo dynamic 3D imaging of oocytes and embryos in the mouse oviduct,” Cell Rep. 36(2), 109382 (2021). 10.1016/j.celrep.2021.109382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang T., Han H., Sun J., et al. , “Three-dimensional particle streak velocimetry based on optical coherence tomography for assessing preimplantation embryo movement in mouse oviduct in vivo,” Biomed. Opt. Express 15(4), 2466–2480 (2024). 10.1364/BOE.519595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S., Larina I. V., “Dynamics of gametes and embryos in the oviduct: what can in vivo imaging reveal?” Reproduction 165(2), R25–R37 (2023). 10.1530/REP-22-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muro Y., Hasuwa H., Isotani A., et al. , “Behavior of Mouse Spermatozoa in the Female Reproductive Tract from Soon after Mating to the Beginning of Fertilization1,” Biol. Reprod. 94(4), 81–87 (2016). 10.1095/biolreprod.115.135368 [DOI] [PubMed] [Google Scholar]
- 17.Hino T., Yanagimachi R., “Active peristaltic movements and fluid production of the mouse oviduct: their roles in fluid and sperm transport and fertilization†,” Biol. Reprod. 101(1), 40–49 (2019). 10.1093/biolre/ioz061 [DOI] [PubMed] [Google Scholar]
- 18.Bochner F., Fellus-Alyagor L., Kalchenko V., et al. , “A Novel Intravital Imaging Window for Longitudinal Microscopy of the Mouse Ovary,” Sci. Rep. 5(1), 12446 (2015). 10.1038/srep12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S., Larina I. V., “Optical imaging of the mammalian oviduct in vivo,” in Imaging from Cells to Animals In Vivo, Barroso M., Intes X., eds. (CRC Press, 2020), Chap. 17. [Google Scholar]
- 20.Huang D., Swanson E. A., Lin C. P., et al. , “Optical coherence tomography,” science 254(5035), 1178–1181 (1991). 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S., Larina I. V., “In vivo three-dimensional tracking of sperm behaviors in the mouse oviduct,” Development 145(6), dev157685 (2018). 10.1242/dev.157685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore E. L., Wang S., Larina I. V., “Staging mouse preimplantation development in vivo using optical coherence microscopy,” J. Biophotonics 12(5), e201800364 (2019). 10.1002/jbio.201800364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S., Syed R., Grishina O. A., et al. , “Prolonged in vivo functional assessment of the mouse oviduct using optical coherence tomography through a dorsal imaging window,” J. Biophotonics 11(5), e201700316 (2018). 10.1002/jbio.201700316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S., Burton J. C., Behringer R. R., et al. , “In vivo micro-scale tomography of ciliary behavior in the mammalian oviduct,” Sci. Rep. 5(1), 13216 (2015). 10.1038/srep13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton J. C., Wang S., Stewart C. A., et al. , “High-resolution three-dimensional in vivo imaging of mouse oviduct using optical coherence tomography,” Biomed. Opt. Express 6(7), 2713–2723 (2015). 10.1364/BOE.6.002713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia T., Umezu K., Scully D. M., et al. , “In vivo volumetric depth-resolved imaging of cilia metachronal waves using dynamic optical coherence tomography,” Optica 10(11), 1439–1451 (2023). 10.1364/OPTICA.499927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa Y., Usui T., Yamashita M., et al. , “Surfing and Swimming of Ejaculated Sperm in the Mouse Oviduct1,” Biol. Reprod. 94(4), 81–89 (2016). 10.1095/biolreprod.115.135418 [DOI] [PubMed] [Google Scholar]
- 28.Faubert A. C., Wang S., “Open Chrono-Morph Viewer: visualize big bioimage time series containing heterogeneous volumes,” Bioinformatics 41(1), btae761 (2024). 10.1093/bioinformatics/btae761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faubert A. C., Wang S., “Clipping spline: interactive, dynamic 4D volume clipping and analysis based on thin plate spline,” Biomed. Opt. Express 16(2), 499–519 (2025). 10.1364/BOE.544231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schindelin J., Arganda-Carreras I., Frise E., et al. , “Fiji: an open-source platform for biological-image analysis,” Nat. Methods 9(7), 676–682 (2012). 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achinger L., Kluczynski D. F., Gladwell A., et al. , “The Known and Unknown About Female Reproductive Tract Mucus Rheological Properties,” BioEssays 47, e70002 (2025). 10.1002/bies.70002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scully D. M., Xia T., Musina G. R., et al. , “Region-specific roles of oviductal motile cilia in oocyte/embryo transport and fertility†,” Biol. Reprod. 112(4), 651–662 (2025). 10.1093/biolre/ioaf005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ning N., Luo D., Xia W., et al. , “Dysregulation of TMEM16A impairs oviductal transport of embryos,” American Journal of Physiology-Cell Physiology 325(3), C623–C632 (2023). 10.1152/ajpcell.00031.2023 [DOI] [PubMed] [Google Scholar]
- 34.McGlade E. A., Stephens K. K., Winuthayanon S., et al. , “Classical Estrogen Signaling in Ciliated Epithelial Cells of the Oviduct Is Nonessential for Fertility in Female Mice,” Endocrinology 165(1), bqad163 (2023). 10.1210/endocr/bqad163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrera G. G. B., Lierz S. L., Harris E. A., et al. , “Oviductal Retention of Embryos in Female Mice Lacking Estrogen Receptor α in the Isthmus and the Uterus,” Endocrinology 161(2), bqz033 (2020). 10.1210/endocr/bqz033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian J., Zhang Y., Qu Y., et al. , “Caffeine consumption during early pregnancy impairs oviductal embryo transport, embryonic development and uterine receptivity in mice†,” Biol. Reprod. 99(6), 1266–1275 (2018). 10.1093/biolre/ioy155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon R. E., Hwang S. J., Kim B. H., et al. , “Myosalpinx Contractions Are Essential for Egg Transport Along the Oviduct and Are Disrupted in Reproductive Tract Diseases,” in Smooth Muscle Spontaneous Activity: Physiological and Pathological Modulation , Hashitani H., Lang R. J., eds. (Springer; Singapore, 2019), pp. 265–294. [DOI] [PubMed] [Google Scholar]
- 38.Dixon R. E., Hwang S. J., Hennig G. W., et al. , “Chlamydia Infection Causes Loss of Pacemaker Cells and Inhibits Oocyte Transport in the Mouse Oviduct1,” Biol. Reprod. 80(4), 665–673 (2009). 10.1095/biolreprod.108.073833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu S., Wang Y. T., Ma P., et al. , “Mapping conduction velocity of early embryonic hearts with a robust fitting algorithm,” Biomed. Opt. Express 6(6), 2138–2157 (2015). 10.1364/BOE.6.002138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besenfelder U., Havlicek V., Brem G., “Role of the Oviduct in Early Embryo Development,” Reprod. Domest. Anim. 47(s4), 156–163 (2012). 10.1111/j.1439-0531.2012.02070.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the authors upon reasonable request.