Abstract
Optoretinography (ORG) is the optical measurement of changes in the retina in response to light stimulation. Adaptive optics optical coherence tomography (AOOCT) records photoreceptor ORGs by measuring the physical changes in their outer segment lengths in response to light stimulation. The main difficulty in recording these nanometer-scale changes is constant eye motion. Typically, fast volume acquisitions are used with offline spatial registration to compensate for the effect of eye motion. Here, we present an alternate solution whereby an adaptive optics scanning light ophthalmoscope (AOSLO) is used to measure the eye motion and actively guide the AOOCT beam to compensate for eye motion in real time. This system’s cellular-scale tracking offers unparalleled control over scanning raster size and shape, allowing for high-speed (up to 100 kHz) ORG acquisition from targeted locations. We validate the method by comparing cone classifications against those made with an established ORG approach.
1. Introduction
The pursuit to better understand retinal function has led to impressive growth in the technology used to characterize its neural activity. Fluorescent probes that are used to reveal neuronal activity are increasingly fast and sensitive [1] and the imaging systems to observe them are correspondingly improving [2]. Higher density electrode arrays in two and three dimensions are increasingly available [3], and combined optical and electrical recording is also now possible [4]. However, as useful as these technologies are for elucidating neural function, their translation to human use faces barriers from ethical and regulatory bodies due to their invasiveness and potential toxicity [5]. Instead, an emerging technology called optoretinography (ORG) fills the growing demand for high-sensitivity, non-invasive, and in vivo cellular recording [6,7]. ORG involves the use of optical methods to measure physiological signals from human retinal neurons, ensuring both high spatial and temporal accuracy while minimizing invasiveness [8].
In recent years, optical coherence tomography (OCT) has attracted the most interest as a tool for ORG. The technology is perfectly suited for imaging the weakly scattering, mostly transparent tissue of the retina and enables cross-sectional and three-dimensional imaging. Since OCT is interferometric and fundamentally measures the complex electric field of light, it has the ability to measure the phase and amplitude of scattered light in depth and can therefore quantify the functional response of retinal neurons by measuring their physical and optical changes in response to light stimulation [9–15]. The amplitude component is used to reconstruct the tomogram (also called the intensity image), while the phase component enables the measurement of several other kinds of contrast, notably blood flow, birefringence, and strain [16–18]. Through phase measurements, OCT can track changes in optical path length on the order of nanometers, its sensitivity improving with signal-to-noise ratio (SNR) [19,20].
1.1. Adaptive optics
Adaptive optics (AO), a technology to measure and compensate for aberrations in the eye which can be used to achieve diffraction-limited point spread functions (PSFs) and resolve retinal cells, is a perfect complement to OCT as it offers improved lateral resolution to match the axial resolution of OCT. AO for ophthalmic imaging was pioneered in the late 1990s, beginning with flood-illumination, full-field fundus imaging [21], and has been effectively integrated into all types of ophthalmoscopes, including scanning light ophthalmoscopy [22] and OCT [23–25]. Adaptive optics scanning light ophthalmoscopy (AOSLO) and adaptive optics optical coherence tomography (AOOCT) in particular have become the technologies of choice for studying the retina at the cellular scale, and the two have been combined to exploit the advantages of each technology [24,26,27].
1.2. Photoreceptor ORGs
Cone photoreceptor outer segments have been shown to change their reflectivity profile [28–30] and optical path length (OPL) [14,31] in response to light stimulation. This OPL change occurs in multiple phases: a fast, contractile response on the millisecond scale first, then at least two, slower, length-change components of hundreds of nanometers over several seconds [32]. The majority of this OPL change occurs between the inner segment/outer segment junction (IS/OS) and the cone outer segment tip (COST), whose high reflectivities allow the OCT to easily track their relative displacement over time and thus acquire an ORG signal [15,31]. This elongation is proportional to the light dose and the cone’s spectral sensitivity to the light stimulus. Taking advantage of this fact, the three cone spectral subtypes have been mapped by observing ORG responses of individual cones to specific stimulation wavelengths [14].
1.3. Active eye movement correction
One of the challenges of using ORG in a living human is the presence of constant eye movements, which occur even during steady fixation [33]. Even drift eye movements pose a problem for ORG systems with high resolution but small field-of-view. To extract ORG measurements, most groups have relied on careful post-processing of data to correct for eye movements [13,34–36].
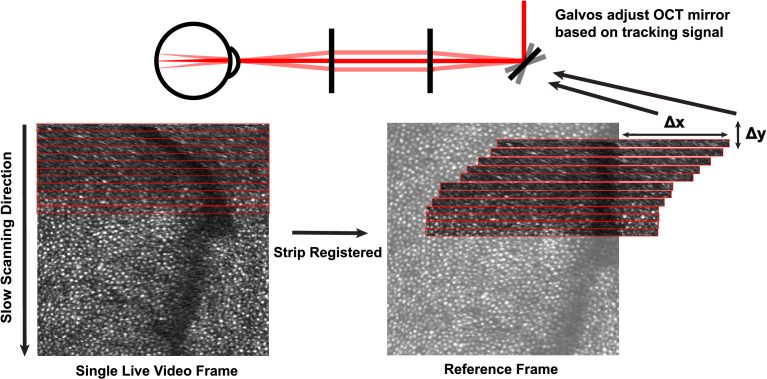
An alternate approach is to correct for eye movements in real time. Because high accuracy is needed, the most suitable eye trackers for this purpose are those that track the motion of the retina directly. This has been attempted using retinal-feature tracking methods [37], but is more commonly done using scanning light imaging systems like the AOSLO. As depicted in Fig. 1, a live AOSLO image can be registered strip by strip to a reference image, and the offset of the strips is scaled, then output to the AOOCT as a tracking signal [38]. This signal then provides offsets to the AOOCT scanners such that they follow the same retinal patch during fixational eye movement. This method has been developed and employed in several systems, including (i) active eye motion correction using a non-AO tracking scanning light ophthalmoscope (TSLO) [39] coupled with an OCT (also non-AO) [40,41], and (ii) active eye motion correction using a TSLO in an AOSLO [42].
Fig. 1.
Strip based eye tracking. As each AOSLO frame is scanned, it is compared, strip-by-strip to a reference frame, and the OCT scanners offsets are adjusted in real time, based on the registration. The reporting rate for eye position is the AOSLO frame rate multiplied by the number of strips per frame. In the current system the rate is 30 (fps) 32 (strips per frame) = 960 Hz.
In this article, we demonstrate cone photoreceptor ORGs using AOOCT with real-time eye motion correction. We show that by actively stabilizing an AOOCT probe using an AOSLO for high fidelity retinal tracking, we can record phase-sensitive ORGs at highly repeatable targeted retinal locations with minimal post processing. We first show ORGs using point and line rasters to demonstrate the system’s ability to image with high temporal resolution and fields of view too small to be registered. Then, larger ORG volumes are taken, and the imaged cones are classified by their spectral subtypes. The classifications are compared to ones from an independent measurement as a validation of our approach.
2. Methods
2.1. Design of the combined AOSLO-AOOCT system
The system is described in previous publications [43,44], and a schematic is provided in Fig. 2. Here, we present only a summary of the main characteristics. The system is a combination of two independent setups that are optically combined with a dichroic mirror and electronically synchronized by using the resonant scanner H-sync signal in the AOSLO. For the AOSLO, light from a supercontinuum source (NKT Photonics, Birkerød, Denmark) is split into four narrow-band channels that are used for imaging (840 nm), wavefront detection (940 nm), and stimulation (650 nm, 543 nm). The maximum laser powers used for the 543 nm, 650 nm, 840 nm, and 940 nm channels were measured to be approximately 0.096 mW, 1 mW, 0.16 mW, and 0.043 mW, respectively. A 16 kHz resonant scanner (SC-30, EOPC, Ridgewood, USA) and a galvanometer scanner (6210h, Cambridge Technology, Bedford, USA) control the fast and slow scans, respectively, to create a raster scan on the retina. The descanned light is detected by a photomultiplier tube (Hamamatsu, San Jose, USA) whose signal is digitized to create a 512 512 pixel image over an area of 1 1 degree at a frame rate of 30 Hz. The wavefront is obtained with a custom-built Shack-Hartmann wavefront sensor, which operates at 30 Hz. A deformable mirror (DM97-08, ALPAO, Montbonnot-Saint-Martin, France) is used to correct the ocular aberrations.
Fig. 2.
Schematic of the combined AOSLO-AOOCT system. Red trace indicates the AOSLO optical path, blue trace indicates the AOOCT optical path and green indicates where the two optical paths are common. More details on the system are described in other publications [43,44].
The AOOCT system is based on swept-source OCT technology [45]. The laser has a central wavelength of 1040 nm, 100 nm of bandwidth, and an A-scan rate of 100 kHz. The power at the cornea is roughly 0.65 mW. In the sample arm, an off-axis parabolic mirror (RC04APC-F01, Thorlabs Inc, Newton, NJ) collimates the light exiting the fiber to create a 4 mm diameter beam. Given the large bandwidth of the OCT radiation, a custom achromatizing lens was designed to compensate for the eye’s longitudinal chromatic aberration (LCA). A Badal telescope in a trombone assembly allows for correction of the relative LCA between the AOOCT and AOSLO wavelengths to ensure a common focus for both systems, but also to allow for independent focus differences between the AOOCT and the AOSLO [38]. The scanning is done using a pair of high-speed galvo scanners positioned at the pupil conjugate planes. The current scanning speed of the galvo scanner is set at a 242 Hz B-scan rate, equivalent to an 8-fold multiple of the AOSLO V-sync signal [44]. The OCT beam is coupled and co-aligned with the AOSLO just prior to the deformable mirror. As such, the OCT uses the AO correction that is informed by the AOSLO path.
2.2. AOOCT performance
The specifications of the system, including resolution, sensitivity, and motion correction performance, have been thoroughly described in a previous publication [44]. However, as phase sensitivity plays a crucial role in ORG experiments we have taken meticulous measurements and determinations of the phase sensitivity specifically for this study. Following the work by Braaf et al. [46,47], we separated the fundamental system phase noise from the contributions of the scanner. Given the considerable optical path length in air of our system (about 10 m), we used reflections from the front and back surface of a cover slip as a sample to reference the phase measurement within the sample itself, reducing the influence of mechanical motion in the setup. To test the influence of scanning we measured under two different conditions on the same scattering sample: a stationary beam and a beam scanning in one dimension, also called A-mode and B-mode as defined in the following Section 2.3. The near shot-noise limited phase sensitivities for a static cover slip was measured to be 4.8 mrad and 8.9 mrad or 0.29 nm and 0.54 nm for A- and B- modes, respectively. Both the front and back surface reflections had SNRs of 65.5 dB. A-mode phase sensitivity was found by first recording for 3.57 seconds and dividing the recording into segments of 1000 scans. Then, we took the standard deviation of the measured time-varying phase differences of each segment and averaged the results. B-mode phase sensitivity was found by taking the standard deviation of time-varying phase differences in the central pixel of each B-scan.
The phase sensitivities for scattering samples within the eye were also measured using A-mode. The pre-stimulus segment (first 0.5s) of A-mode recordings were analyzed. Since imaging quality gradually degrades as the tear film breaks, a short video better represents the best possible phase sensitivity within the eye. Two calculations were made on A-mode recordings. First, we referenced two adjacent pixels within the IS/OS layer. This represents an idealized measurement under biological conditions, since two pixels in the same layer are assumed to be physically fixed relative to each other. We found a best phase sensitivity of 44.8 mrad, or 2.7 nm. Then, we referenced one pixel from the IS/OS and one from the COST. This represents a real experimental measurement under biological conditions. We found a best phase sensitivity of 80.5 mrad, or 4.9 nm. The IS/OS and COST layers had SNRs of 53.0 dB and 52.8 dB, respectively.
2.3. AOOCT scanning protocol
To begin imaging, we first corrected for any aberrations using a deformable mirror in a control loop with the wavefront sensor in the AOSLO channel. This correction was continually updated throughout the imaging session in a closed-loop fashion. We then acquired an AOSLO reference frame for eye tracking by registering and averaging a short video. The AOOCT was then focused on the correct retinal layer by adjusting the position of the Badal retroreflector in the sample arm. A reference position was set for the pupil via an IR pupil camera. A consistent pupil location is essential to maintain a constant transverse chromatic aberration offset between the AOOCT and AOSLO beams [48]. Polarization was optimized using polarization controllers to remove interferometric artifacts and maximize signal. Finally, active eye tracking was engaged and the user could initiate AOOCT acquisition using a protocol of their choosing. OCT scanning protocols were programmed using Labview (National Instruments, Austin TX). To ensure consistency of timing, OCT acquisition is triggered and timed using the AOSLO resonant scanner H-sync signal [44].
The AOOCT system has a baseline acquisition rate of 100 kHz. These data points can be freely spatially distributed by programming different scan patterns. Here, we performed experiments using three different scan patterns: A-mode, B-mode, and C-mode. For all three modes, an area encompassing a square patch was first scanned to establish a contextual understanding of the imaged region by the AOOCT beam (structural scan). This area was sampled using 400 B-scans, with 350 A-lines per B-scan, at a B-scan rate of 242 Hz. Then, scans of different sizes were taken for each mode:
A-mode: 356832 A-scans are taken at a single location across 3.57 seconds.
B-mode: 864 B-scans, 30 arcminutes long, are taken at a single location across 3.57 seconds. Each B-scan contains 350 A-scans. A small pause in data acquisition between each B-scan, corresponding to 63 A-scans, is reserved for the flyback period of the scanner. A flyback period allows for linear scanning patterns for postprocessing ease.
C-mode: 72 C-scans, measuring 3 30 arcminutes, are taken across 3.57 seconds. Each C-scan consists of 12 B-scans, forming a rectangular volume. Each B-scan is identical to the one described in B-mode.
Note that for consistency, we define a "scan" as a single unit of each recording – for example, one B-scan for B-mode or one A-scan for A-mode.
Figure 3 depicts the scanned areas overlaid on an AOSLO image. The blue, red, and green lines indicate the areas repeatedly imaged for the A-, B- and C-mode ORG-scans. The black box represents the area of the aforementioned structural scan volume that was frequently recorded just prior to the ORG recordings. A typical acquisition sequence takes 5.22 seconds: 1.65 seconds for the structural scan (400 B-scans) and 3.57 seconds for the ORG scan. Full details for each scan mode are listed in Table 1.
Fig. 3.
AOOCT scanning location and sizes relative to the AOSLO reference frame. The black box denotes the structural scan and the blue, red, and green boxes denote the repeated A-, B-, and C-mode ORG scans respectively.
Table 1. Scanning details for each ORG mode.
| Protocol | ORG Scan | |||||
|---|---|---|---|---|---|---|
| ORG Sampling rate (Hz) | Scan size | B-scans per scan | Total no. scans | Stimulus onset (s) | Scan time (s) | |
| A-mode | 100k | N/A | N/A | 356832 | 0.59 | 3.57 |
| B-mode | 242 | 30 arcmin | 1 | 864 | ||
| C-mode | 20 | 3 30 arcmin | 12 | 72 | ||
2.4. Light stimulation
Light stimulation for all modes were via the AOSLO scanning raster and were delivered over a single AOSLO frame. Due to the raster scan, the stimulus over the tested area was delivered line-by-line but over total durations that were only a few (∼2) msec. First, for A- and B-mode, a patch of green light at 543 nm that roughly covers the desired cone(s) was targeted then delivered to the retina [49] 0.59 seconds after the start of the ORG scan. The wavelength of 543 nm was selected to maximally stimulate the L and M cone subtypes. The light delivery software controlled an acousto-optic modulator to correct for the non-uniform irradiance that would normally result from the changing speed of the sinusoidal resonant scanner. The bleach percentage was computed to be 90% for both L and M cones. For C-mode, a full-field, single-frame flash of red light was used to stimulate the cones. The light delivery software did not correct for the non-uniform irradiance for this wavelength, since we bypassed the acousto-optic modulator in order to get sufficient energy over a single AOSLO frame. To minimize the effect of non-uniform stimulation, we made sure the targeted region was centered in the central, most linear section of the AOSLO scan field prior to recording. The wavelength of 650 nm was used to ensure that L, M, and S cone responses were sufficiently different for cone classification purposes. Bleach percentage was ∼100% for L cones and 67% for M cones. An identical flash of red light was used in conjunction with A-mode to classify a few cones.
2.5. AOOCT data processing
We first applied a suite of standard OCT processing steps, including background subtraction, chromatic dispersion compensation, zero-padding, and Fourier transform. For B- and C-mode, we eliminated bulk axial motion by axially registering each B-scan to the average B-scan of the 144 pre-stimulus B-scans (this comprises 12 pre-scan volumes in C-mode). For A-mode, we axially registered the A-scans in sets of 413, using the average of the first 144 sets as a reference.
For ORG extraction, each scan captured by AOOCT was referenced to the mean of all pre-stimulus ORG scans. This reference ensured the cancellation of arbitrary phase variations at each pixel. Then, by multiplying the complex conjugate of each pixel value in the COST layer with the IS/OS layer, we were able to obtain phase differences between the two layers and track this phase change over time. The phase is then converted to displacement using:
| (1) |
where is the OCT central wavelength, is the phase change at time , and is the refractive index of the tissue. B-mode and C-mode were further spatially averaged. B-mode was averaged across the entire B-scan for each scan, while C-mode data were averaged across the aperture of a single cone.
For C-mode, an additional filtering step was taken. To account for cone misidentification in the comparison process, a source of noise that cannot be averaged out, we employed template filtering. We first generated template curves, or ORG traces that represent a characteristic "L", "M", and "S" response under our specific experimental conditions. Then, traces from each trial were compared to the templates and assigned a label of L, M, or S based on weighted Euclidean distance. When all traces had been compared and labeled, the traces corresponding to the most frequently occurring label (L, M, or S) were averaged, and traces corresponding to the other two labels were discarded. This "majority rules" method removes not only trials with misidentified cones, but also uncorrected eye motion, incorrectly unwrapped traces, and excessively noisy data. Note that this was only used for cone classification using C-mode; A- and B-modes did not require this step.
2.6. Human subjects
The University of California Berkeley Institutional Review Board approved this research, and subjects signed an informed consent form before participation. Mydriasis and cycloplegia were achieved with 1 tropicamide and 2.5 phenylephrine ophthalmic solutions before each experimental session. Subjects bit into a dental impression mount affixed to an XYZ translation stage to align and stabilize the eye and head position.
Two subjects were recruited for this experiment. Subject 10003 was a 58 y.o. adult Caucasian male with no known visual defects. Subject 20255 was a 23 y.o. adult Asian male with no known visual defects. Subject 10003 was imaged at 1.7 degrees temporal in the left eye. Subject 20255 was imaged at 2.5 degrees temporal and superior and 2.9 degrees temporal in the left eye. Each mode has at least 10 data acquisition sequences, or trials.
3. Results
3.1. Imaging with actively corrected AOOCT
First, the precision of real-time eye tracking was qualitatively observed in human eyes. Figure 4(b) depicts a typical time series of C-mode ORG volume en face images taken with AOSLO real time eye stabilization. Across 3.57 seconds of imaging, the cones remain individually distinguishable and round enough to be visually identified. Equally importantly, the probe successfully fixed on the same patch of cones across the 5-second imaging period. This is in stark contrast to Fig. 4(c), where real-time eye tracking is turned off. Despite the subject’s best attempt to fixate, drifts and small saccades are inevitable, leading to wildly different cone locations across consecutive frames. Figure 4(c) is comparable to conventional AOOCT results, where B-scans typically need to be registered in post-processing. However, real-time eye tracking can also add a high frequency periodic jitter to images, and this is visible in the structural scan portion of Fig. 4(b). This is because any minor distortions in the reference frame will result in a minor tracking error at 30 Hz (the AOSLO frame rate) and higher harmonics. This jitter is quantified in Section S1. To further confirm the eye tracking efficacy, we acquired a densely-scanned C-mode sequence of a single cone at 10 Hz for 3.57 seconds. As shown in Fig. 4(a), the cone remains centered in the image across the scanning period, granting us confidence that our A-mode probe also remains stably locked onto the target cone.
Fig. 4.
a) ORG scan of single cell using C-mode with a small field of view and eye tracking. b) AOOCT structural (top) and ORG scans (bottom) in C-mode with and c) without active eye motion correction. The green box in the ORG scans denote the size and shape of a single ORG scan, and corresponds to the green box in the structural scan. All scale bars are 10 arcmin. A video sequence of the stabilized ORG frames can be found in Visualization 1 (561.7KB, avi)
3.2. A-mode ORGs with 543-nm stimulation
Two sets of A-mode ORGs were collected in total. The first set was collected on Subject 10003 at 1.7 degrees temporal, and the second was collected on 20255 at 2.5 degrees temporal and superior. Figure 5(a1) shows a representative A-scan structural time series with the IS/OS and COST layers labeled. The consistency in the appearance of the A-scan structure across time lends confidence that our AOOCT probe was indeed localized onto the center of a single cone. Figure 5(a2) depicts both subjects’ ORG traces averaged across the ten trials. The fast contractile phase of the ORG response is clearly visible immediately following the stimulation [31]. The temporal resolution of this trace represents the maximum A-scan speed of our system, entirely dedicated to imaging a single cone.
Fig. 5.
a1) Representative A-mode A-scan time series from Subject 20255. Red and blue lines indicate the approximate location of the IS/OS and COST respectively. a2) Averaged A-mode ORG traces from Subject 10003 (orange) and 20255 (blue). The transparent traces are raw data, and the solid traces have been smoothed via moving window average of 3000 samples (or 0.03s) to better show the contractile response. b1) Representative B-mode en face time series from subject 20255 and b2) B-mode averaged trace. The trace was averaged both across multiple trials and spatially across a B-scan. Again, the transparent traces are raw data and the solid traces are smoothed with a moving window average of 10 samples (or 0.04s). Vertical green lines indicate 543 nm stimulus onset.
The ORG response from Subject 10003 was consistently higher than Subject 20255 across multiple trials. This may be due to differences in scanning location. Cone ORG responses have been shown to decrease with increased eccentricity [50], which would explain Subject 20255’s slightly lower ORG response. However, this is still speculative. Without knowledge of the cone type and a full characterization of both retinas, it is difficult to confidently attribute a single cause to this difference.
3.3. B-mode ORGs with 543-nm stimulation
Two sets of B-mode ORGs were collected in total. The first set was collected on Subject 10003 at 1.7 degrees temporal, and the second was collected on 20255 at 2.5 degrees temporal and superior. We spatially averaged the phase changes in each B-scan to achieve a single final trace. As such, a B-mode ORG is not a response from any single cone. Rather, it is an aggregate response that averages the ORG signal from a small area, including many cells and some intercellular space. Attempting to get ORG traces from individual cones in the B-scan is problematic as the narrow B-scan does not line up with the centers of many cones. Figure 5(b1) and (b2) show a representative time series of B-scan en faces and both ORG traces, respectively. The OCT probe remained consistently locked onto a single row of cones, and the fast contractile response is once again visible in the averaged traces. A B-mode en face appears as parallel bright and dark bars formed by the B-scans as the same location is scanned over time, as shown in Fig. 5(b1). Any failure or disruption of eye tracking will manifest as an obvious break in the parallel bars. As such, B-mode en faces also serve as visual indicators of tracking efficacy.
3.4. Classification validation
A set of C-mode ORGs of a classified retinal patch with 101 cones was taken in Subject 10003 at 1.7 degrees temporal. Figure 6(a) shows resulting ORG traces. The traces are colored by the subtype classes assigned for another study by collaborators at the University of Washington [51]. After the post-processing steps described in Section 2.5, the traces readily separate into L, M, and S classes in a manner expected of the 650 nm stimulus, resulting in assigned cone classes that are 90.1% consistent with our collaborator’s classifications (Fig. 6(b)).
Fig. 6.
a) ORG traces of cones in Subject 10003’s classified region (1.7 degrees temporal), where the colors represent our collaborator’s assigned cone classes: red for L cones, green for M cones, and blue for S cones. b) Comparison of experimental class assignments and collaborator assignments. c) ORG traces from an unclassified region of Subject 20255 (2.9 degrees temporal). Vertical red line indicates 650 nm stimulus onset. d) Retinal images of both subjects with cone class labels overlaid. Scale bars are 10 arcmin.
3.5. De novo classification
A set of C-mode ORGs of an unclassified retinal patch with 95 cones was taken in Subject 20255 at 2.9 degrees temporal. Figure 6(c) shows the ORG traces after post-processing. Again, the traces readily separate into characteristic L, M, and S subtypes. Subject 20255 was completely unclassified, so no comparison to previous studies could be made. However, the L, M, and S cone type ratios fall well within normal ranges [52,53], with an L:M ratio of 1.1:1 and an S-cone percentage of 5.2%.
3.6. A-mode classification
Since A-mode and C-mode ORGs work off the same principles, A-mode can also be used to classify cones. 3 randomly selected cones were classified using A-mode in Subject 10003 at 1.7 degrees temporal. The same 650 nm stimulus was used. All 3 classifications were consistent with the subtype classes assigned by our collaborators [51]. Figure 7(b) shows the resulting ORG traces, which reveal cone responses that match the C-mode classifications. The high temporal resolution enabled by real time tracking allowed successful, unambiguous classifications in as little as a single trial with minimal post processing. Though the same high frequency jitter described in Section 3.1 is visible in Fig. 7(a), it did not prevent a successful cone classification.
Fig. 7.
a) OCT en face image of Subject 10003. Three classified cones are colored based on collaborator cone subtype assignments. Scale bar is 5 arcmin. b) Resulting ORG traces. Colors are again based on collaborator assignments. The transparent traces are raw data, and the solid traces have been smoothed via moving window average of 3000 samples (or 0.03s). Vertical red line indicates 650 nm stimulus onset.
4. Discussion
In this article, eye tracking technology for obtaining ORGs via AOOCT was demonstrated by taking A-mode and B-mode ORGs, which do not have fields of view for post hoc registration. The system’s capabilities were validated using C-mode ORGs to classify multiple patches of cones into the three subtypes, then confirming the results with a comparison to past classifications done by collaborators. For all three modes, our protocol allowed us to follow the OPL time profile of individual or groups of photoreceptors over 3.57 seconds and over distinct acquisitions. These results show the potential of AOSLO-based active eye tracking technology to obtain targeted functional information from specific structures within the retina.
To verify the efficacy of our eye tracking, three key methods were used. First, a small-field-of-view C-mode (Fig. 4(a)) structural scan of a single cone was taken over 3.57 seconds. The cone remained centered and distinguishable across the scanning period. Then, a B-mode en face time series (Fig. 5(b1)) was also observed to be stable across 3.57 seconds. A B-mode en face readily reveals where tracking fails, which would appear as warped or inconsistent patterns rather than neat, repeated, bright and dark lines. As such, a stable pattern free of major disruption is reliable assurance of tracking efficacy. Finally, cone classifications of multiple retinal patches, some of which have been previously independently classified, were conducted.
The main advantage of the system comes from its flexible field of view, as demonstrated by A- and B-mode results. An active tracking AOOCT can bypass conventional OCT requirements such as a large field of view and high-speed hardware acquisition, both of which are necessary to cancel out the effects of eye motion post hoc. This novel system can still take traditional C-mode volumes, but also single-line rasters, A-lines, or extremely small volumes to oversample specific structures. Though we only demonstrate one shape (long rectangle), the C-mode raster can be easily reshaped into rectangles of any dimension. Once a raster size and shape is determined, the experimenter can freely target a specific location for scanning. This can be particularly useful for, for example, selectively targeting an S-cone or middle of a retinal ganglion cell for measurement. Note that the benefits of adaptive optics are diminished if the ORG raster is significantly displaced from or larger than the AOSLO scanning region, due to anisoplanataism. This is a theoretical constraint on the ORG size and scanning location.
A-mode, with the greatest time resolution, holds the greatest promise for basic science while C-mode best matches the ORG capabilities of conventional, non-tracking OCT systems. However, in its current form, B-mode may be the closest to practical application. Its unique shape captures both the structure and ORG response of a small slice of retina, allowing for spatial averaging without significantly compromising temporal resolution. Spatial averaging results in extremely reliable ORG signals in just a single trial. This combination of temporal resolution, spatial specificity, and reliability means it could potentially find use as a supplement to C-mode ORGs in clinical settings, where quick result turnaround is important.
Our future ambition is to take full advantage of the system’s flexibility by imaging retinal structures whose ORG responses may be significantly smaller and faster than that of a cone. On the forefront are retinal ganglion cells, whose deformations are suggested to be on the order of single nanometer and single milliseconds during action potentials [54]. The AOSLO’s tracking signals can be simultaneously used for targeted stimulation of specific cones [49], granting an additional degree of control that can be utilized to, for example, pair up cones with their downstream retinal ganglion cells.
Despite its novel capabilities, the system is not without limitations. Eye tracking will occasionally fail, leading to phase discontinuities that cannot be resolved in post processing. These failures often occur during larger microsaccades brought on by the fatigue of long imaging sessions, but can also be caused by smaller factors such as torsion, where the retina no longer matches the previously acquired reference frame. In practice, some jitter is unavoidable, as minor imperfections in the reference image will almost always lead to a small periodic error at 30 Hz, the AOSLO frame rate [40]. Trials with large tracking errors simply need to be filtered out and re-recorded. Reference frames may also occasionally be retaken if the discrepancy caused by torsion is unreasonably high.
5. Conclusion
We successfully implemented an actively stabilized phase-sensitive AOOCT system to measure the optoretinogram of a spatially targeted location. We show that the AOOCT was able to lock onto a set of select cone photoreceptors and reliably record the optical path length of the cell across multiple trials. We were able to successfully quantify the response of individual photoreceptors to light stimuli to obtain the optoretinogram for confident classification of a set of cones within a single imaging session with minimal post-processing.
Supplemental information
Acknowledgments
The authors thank Pavan Tiruveedhula for electronics and software support, Francesco LaRocca and Sanam Mozaffari for support with development and operation of the optical systems and Ramkumar Sabesan and Vimal Pandiyan from the University of Washington for providing the classified cone maps for one of our subjects.
Funding
National Eye Institute 10.13039/100000053 ( U01 EY032055, T32 EY007043); The Weill Neurohub Next Great Ideas Program.
Disclosures
The authors have no financial disclosures that are relevant to this work. Feroldi is currently employed at Carl Zeiss Meditec. Hosseinaee is currently employed at Kodiak Sciences.
Data availability
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the authors upon reasonable request.
Supplemental document
See Supplement 1 (2.2MB, pdf) for supporting content.
References
- 1.Deo C., Lavis L. D., “Synthetic and genetically encoded fluorescent neural activity indicators,” Curr. Opin. Neurobiol. 50, 101–108 (2018). 10.1016/j.conb.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 2.Yang W., Yuste R., “In vivo imaging of neural activity,” Nat. Methods 14(4), 349–359 (2017). 10.1038/nmeth.4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong G., Lieber C. M., “Novel electrode technologies for neural recordings,” Nat. Rev. Neurosci. 20(6), 330–345 (2019). 10.1038/s41583-019-0140-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vázquez-Guardado A., Yang Y., Bandodkar A. J., et al. , “Recent advances in neurotechnologies with broad potential for neuroscience research,” Nat. Neurosci. 23(12), 1522–1536 (2020). 10.1038/s41593-020-00739-8 [DOI] [PubMed] [Google Scholar]
- 5.Roorda A., “Optoretinography is coming of age,” Proc. Natl. Acad. Sci. 118(51), e2119737118 (2021). 10.1073/pnas.2119737118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulligan J. B., MacLeod D. I., Statler I. C., “In search of an optoretinogram,” in Vision Science and Its Application Topical Meeting, (1994). [Google Scholar]
- 7.Jonnal R. S., “Toward a clinical optoretinogram: a review of noninvasive, optical tests of retinal neural function,” Ann. Transl. Med. 9(15), 1270 (2021). 10.21037/atm-20-6440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams D. R., Burns S. A., Miller D. T., et al. , “Evolution of adaptive optics retinal imaging,” Biomed. Opt. Express 14(3), 1307–1338 (2023). 10.1364/BOE.485371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonnal R. S., Rha J., Zhang Y., et al. , “In vivo functional imaging of human cone photoreceptors,” Opt. Express 15(24), 16141–16160 (2007). 10.1364/OE.15.016141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonnal R. S., Kocaoglu O. P., Wang Q., et al. , “Phase-sensitive imaging of the outer retina using optical coherence tomography and adaptive optics,” Biomed. Opt. Express 3(1), 104–124 (2012). 10.1364/BOE.3.000104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillmann D., Spahr H., Pfäffle C., et al. , “In vivo optical imaging of physiological responses to photostimulation in human photoreceptors,” Proc. Natl. Acad. Sci. 113(46), 13138–13143 (2016). 10.1073/pnas.1606428113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocaoglu O. P., Turner T. L., Liu Z., et al. , “Adaptive optics optical coherence tomography at 1 MHz,” Biomed. Opt. Express 5(12), 4186–4200 (2014). 10.1364/BOE.5.004186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azimipour M., Zawadzki R. J., Gorczynska I., et al. , “Intraframe motion correction for raster-scanned adaptive optics images using strip-based cross-correlation lag biases,” PLoS One 13(10), e0206052 (2018). 10.1371/journal.pone.0206052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F., Kurokawa K., Lassoued A., et al. , “Cone photoreceptor classification in the living human eye from photostimulation-induced phase dynamics,” Proc. Natl. Acad. Sci. 116(16), 7951–7956 (2019). 10.1073/pnas.1816360116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandiyan V. P., Jiang X., Maloney-Bertelli A., et al. , “High-speed adaptive optics line-scan OCT for cellular-resolution optoretinography,” Biomed. Opt. Express 11(9), 5274–5296 (2020). 10.1364/BOE.399034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C.-L., Wang R. K., “Optical coherence tomography based angiography,” Biomed. Opt. Express 8(2), 1056–1082 (2017). 10.1364/BOE.8.001056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Boer J. F., Hitzenberger C. K., Yasuno Y., “Polarization sensitive optical coherence tomography–a review,” Biomed. Opt. Express 8(3), 1838–1873 (2017). 10.1364/BOE.8.001838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larin K. V., Sampson D. D., “Optical coherence elastography–OCT at work in tissue biomechanics,” Biomed. Opt. Express 8(2), 1172–1202 (2017). 10.1364/BOE.8.001172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park B. H., Pierce M. C., Cense B., et al. , “Real-time fiber-based multi-functional spectral-domain optical coherence tomography at 1.3 μm,” Opt. Express 13(11), 3931–3944 (2005). 10.1364/OPEX.13.003931 [DOI] [PubMed] [Google Scholar]
- 20.Yazdanfar S., Yang C., Sarunic M. V., et al. , “Frequency estimation precision in Doppler optical coherence tomography using the Cramer-Rao lower bound,” Opt. Express 13(2), 410–416 (2005). 10.1364/OPEX.13.000410 [DOI] [PubMed] [Google Scholar]
- 21.Liang J., Williams D. R., Miller D. T., “Supernormal vision and high-resolution retinal imaging through adaptive optics,” J. Opt. Soc. Am. A 14(11), 2884–2892 (1997). 10.1364/JOSAA.14.002884 [DOI] [PubMed] [Google Scholar]
- 22.Roorda A., Romero-Borja F., Donnelly III W. J., et al. , “Adaptive optics scanning laser ophthalmoscopy,” Opt. Express 10(9), 405–412 (2002). 10.1364/OE.10.000405 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Rha J., Jonnal R. S., et al. , “Adaptive optics parallel spectral domain optical coherence tomography for imaging the living retina,” Opt. Express 13(12), 4792–4811 (2005). 10.1364/OPEX.13.004792 [DOI] [PubMed] [Google Scholar]
- 24.Merino D., Dainty C., Bradu A., et al. , “Adaptive optics enhanced simultaneous en-face optical coherence tomography and scanning laser ophthalmoscopy,” Opt. Express 14(8), 3345–3353 (2006). 10.1364/OE.14.003345 [DOI] [PubMed] [Google Scholar]
- 25.Torti C., Považay B., Hofer B., et al. , “Adaptive optics optical coherence tomography at 120,000 depth scans/s for non-invasive cellular phenotyping of the living human retina,” Opt. Express 17(22), 19382–19400 (2009). 10.1364/OE.17.019382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zawadzki R. J., Jones S. M., Pilli S., et al. , “Integrated adaptive optics optical coherence tomography and adaptive optics scanning laser ophthalmoscope system for simultaneous cellular resolution in vivo retinal imaging,” Biomed. Opt. Express 2(6), 1674–1686 (2011). 10.1364/BOE.2.001674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azimipour M., Jonnal R. S., Werner J. S., et al. , “Coextensive synchronized SLO-OCT with adaptive optics for human retinal imaging,” Opt. Lett. 44(17), 4219–4222 (2019). 10.1364/OL.44.004219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grieve K., Roorda A., “Intrinsic signals from human cone photoreceptors,” Invest. Ophthalmol. Vis. Sci. 49(2), 713–719 (2008). 10.1167/iovs.07-0837 [DOI] [PubMed] [Google Scholar]
- 29.Cooper R. F., Brainard D. H., Morgan J. I., “Optoretinography of individual human cone photoreceptors,” Opt. Express 28(26), 39326–39339 (2020). 10.1364/OE.409193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bizheva K., Pflug R., Hermann B., et al. , “Optophysiology: depth-resolved probing of retinal physiology with functional ultrahigh-resolution optical coherence tomography,” Proc. Natl. Acad. Sci. 103(13), 5066–5071 (2006). 10.1073/pnas.0506997103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandiyan V. P., Maloney-Bertelli A., Kuchenbecker J. A., et al. , “The optoretinogram reveals the primary steps of phototransduction in the living human eye,” Sci. Adv. 6(37), eabc1124 (2020). 10.1126/sciadv.abc1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandiyan V. P., Nguyen P. T., Pugh Jr E. N., et al. , “Human cone elongation responses can be explained by photoactivated cone opsin and membrane swelling and osmotic response to phosphate produced by RGS9-catalyzed GTPase,” Proc. Natl. Acad. Sci. 119(39), e2202485119 (2022). 10.1073/pnas.2202485119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Conde S., Macknik S. L., Hubel D. H., “The role of fixational eye movements in visual perception,” Nat. Rev. Neurosci. 5(3), 229–240 (2004). 10.1038/nrn1348 [DOI] [PubMed] [Google Scholar]
- 34.Li H., Tan B., Pandiyan V. P., et al. , “Phase-restoring subpixel image registration: enhancing motion detection performance in Fourier-domain optical coherence tomography,” J. Phys. D: Appl. Phys. 58(14), 145102 (2024). 10.1088/1361-6463/adb3b4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z., Pandiyan V. P., Maloney-Bertelli A., et al. , “Correcting intra-volume distortion for AO-OCT using 3D correlation based registration,” Opt. Express 28(25), 38390–38409 (2020). 10.1364/OE.410374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurokawa K., Crowell J. A., Do N., et al. , “Multi-reference global registration of individual a-lines in adaptive optics optical coherence tomography retinal images,” J. Biomed. Opt. 26(1), 016001 (2021). 10.1117/1.JBO.26.1.016001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kocaoglu O. P., Ferguson R. D., Jonnal R. S., et al. , “Adaptive optics optical coherence tomography with dynamic retinal tracking,” Biomed. Opt. Express 5(7), 2262–2284 (2014). 10.1364/BOE.5.002262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mozaffari S., Feroldi F., LaRocca F., et al. , “Actively stabilized AOOCT with independent focus adjustment for optimal AOSLO-based eye tracking,” Investigative Ophthalmology Visual Science 62, 10 (2021). [Google Scholar]
- 39.Sheehy C. K., Yang Q., Arathorn D. W., et al. , “High-speed, image-based eye tracking with a scanning laser ophthalmoscope,” Biomed. Opt. Express 3(10), 2611–2622 (2012). 10.1364/BOE.3.002611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vienola K. V., Braaf B., Sheehy C. K., et al. , “Real-time eye motion compensation for OCT imaging with tracking SLO,” Biomed. Opt. Express 3(11), 2950–2963 (2012). 10.1364/BOE.3.002950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braaf B., Vienola K. V., Sheehy C. K., et al. , “Real-time eye motion correction in phase-resolved OCT angiography with tracking SLO,” Biomed. Opt. Express 4(1), 51–65 (2013). 10.1364/BOE.4.000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheehy C. K., Tiruveedhula P., Sabesan R., et al. , “Active eye-tracking for an adaptive optics scanning laser ophthalmoscope,” Biomed. Opt. Express 6(7), 2412–2423 (2015). 10.1364/BOE.6.002412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mozaffari S., LaRocca F., Jaedicke V., et al. , “Wide-vergence, multi-spectral adaptive optics scanning laser ophthalmoscope with diffraction-limited illumination and collection,” Biomed. Opt. Express 11(3), 1617–1632 (2020). 10.1364/BOE.384229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mozaffari S., Feroldi F., LaRocca F., et al. , “Retinal imaging using adaptive optics optical coherence tomography with fast and accurate real-time tracking,” Biomed. Opt. Express 13(11), 5909–5925 (2022). 10.1364/BOE.467634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yun S.-H., Tearney G. J., de Boer J. F., et al. , “High-speed optical frequency-domain imaging,” Opt. Express 11(22), 2953–2963 (2003). 10.1364/OE.11.002953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braaf B., Vermeer K. A., Sicam V. A. D., et al. , “Phase-stabilized optical frequency domain imaging at 1-μm for the measurement of blood flow in the human choroid,” Opt. Express 19(21), 20886–20903 (2011). 10.1364/OE.19.020886 [DOI] [PubMed] [Google Scholar]
- 47.Braaf B., Vermeer K. A., Vienola K. V., et al. , “Angiography of the retina and the choroid with phase-resolved OCT using interval-optimized backstitched B-scans,” Opt. Express 20(18), 20516–20534 (2012). 10.1364/OE.20.020516 [DOI] [PubMed] [Google Scholar]
- 48.Harmening W. M., Tiruveedhula P., Roorda A., et al. , “Measurement and correction of transverse chromatic offsets for multi-wavelength retinal microscopy in the living eye,” Biomed. Opt. Express 3(9), 2066–2077 (2012). 10.1364/BOE.3.002066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabesan R., Schmidt B. P., Tuten W. S., et al. , “The elementary representation of spatial and color vision in the human retina,” Sci. Adv. 2(9), e1600797 (2016). 10.1126/sciadv.1600797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang X., Liu T., Pandiyan V. P., et al. , “Coarse-scale optoretinography (COORG) with extended field-of-view for normative characterization,” Biomed. Opt. Express 13(11), 5989–6002 (2022). 10.1364/BOE.473475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greene M. J., Boehm A. E., Vanston J. E., et al. , “Unique yellow shifts for small and brief stimuli in the central retina,” Journal of Vision 24(6), 2 (2024). 10.1167/jov.24.6.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabesan R., Hofer H., Roorda A., “Characterizing the human cone photoreceptor mosaic via dynamic photopigment densitometry,” PLoS One 10(12), e0144891 (2015). 10.1371/journal.pone.0144891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hofer H., Carroll J., Neitz J., et al. , “Organization of the human trichromatic cone mosaic,” J. Neurosci. 25(42), 9669–9679 (2005). 10.1523/JNEUROSCI.2414-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ling T., Boyle K. C., Zuckerman V., et al. , “High-speed interferometric imaging reveals dynamics of neuronal deformation during the action potential,” Proc. Natl. Acad. Sci. 117(19), 10278–10285 (2020). 10.1073/pnas.1920039117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the authors upon reasonable request.